Abstract
Background and Purpose:
Delayed time to recanalization is associated with reduced recanalization success of mechanical thrombectomy (MT) and thrombolysis in acute ischemic stroke (AIS). The reasons for this are unclear. We hypothesized that alterations in thrombus structure and composition could be responsible for this.
Methods:
Retrieved thrombi from AIS patients who underwent MT less than 8 hours from symptom onset to groin puncture (SOGP) were evaluated. Patients were divided into early (≤4 hrs.) vs delayed (> 4 hrs.) groups based SOGP timing. Thrombi were histologically analysed using Martius Scarlett Blue and immunohistochemistry staining for von Willebrand Factor (vWF), anti-citrullinated H3 (H3Cit; NETs [neutrophil extracellular traps] marker). We used inferential statistics including, t-test, artificial neural network (ANN) to interpret the data.
Results:
A total of 137 thrombi were collected. The overall average percentage of red blood cells (RBC), white blood cells (WBC), platelet, fibrin, H3Cit, and vWF components in thrombi was 45.83%, 3.58%, 22.23%, 28.27%, 19.97% and 16.23% respectively. Delayed group had higher WBCs, (p=0.02), fibrin (p=0.02), H3Cit (p=0.04) and vWF (p=0.03) thrombus fractions compared to early group. Based on ANN model, the most important factors for predicting the number of passes required for successful recanalization are fibrin and RBC contents of the thrombus followed by vWF and H3Cit contents.
Conclusions:
Longer time to recanalization was associated with increased WBCs, fibrin, H3Cit and vWF fractions of thrombi reflecting possible in situ maturation of thrombus components. Increased fibrin, NETs and vWF composition may reduce likelihood of revascularization by altering thrombus mechanical properties.
Keywords: Delayed recanalization time, Thrombus composition, Mechanical thrombectomy, NETs, vWF
INTRODUCTION
The era of mechanical thrombectomy for treatment of acute ischemic stroke enables histopathological analysis of retrieved thrombus. (1). Prior quantitative analyses of retrieved thrombi have suggested that red blood cell and fibrin fractions may affect recanalization rates (2–4) and thrombi of different stroke etiologies have different composition and size that might underlie different recanalization time and passes (5). Additionally increasing number of passes has been associated with lower rate good neurological outcome, higher rate of vessel wall injury and increasing rate of endovascular device damage and emboli (6–9).
Recently, time to recanalization has been demonstrated to predict rate of recanalization success following endovascular thrombectomy for acute ischemic stroke (10). There are strong evidences suggesting that fibrinolytic agents depends on time of administration (11–13). The reasons for recanalization time and success depend on the cause of stroke; moreover the thrombus composition might also be influenced by the time from thrombus formation to embolization(12). for this are unclear and potential hypotheses include thrombus compaction or thrombus maturation We hypothesized that Thrombus composition may change with longer time to recanalization (longer time in situ within the artery), which may contribute to reported lower recanalization rates.
METHODS
Patients
This study comprised a retrospective review from October 2016 until November 2020 of patients included in a prospective registry examining thrombus histopathology at our institution. Institutional review board approval was received prior to review and a waiver of consent was granted. Inclusion criteria are a) adult patients, b) mechanical thrombectomy with retrieval of thrombus material within 8 hours from symptom onset to groin puncture and c) availability for pathologic analysis. Baseline clinical characteristics was gathered for each patients. Stroke etiology was determined according to the TOAST criteria (14). The result of compositional analysis of thrombi included in this study has been reported as a part of our multi-center registry.
Thrombectomy procedures
Mechanical thrombectomy was performed using direct aspiration, stent-retriever, combination technique, at the discretion of neurointerventionalist. Recanalization status was assessed on final cerebral angiogram; successful recanalization was defined as modified Thrombolysis in Cerebral Infarction (mTICI) grade 2b or greater.
Symptom onset to groin puncture (SOGP) was defined as the time from symptom onset to groin puncture. Time to recanalization was defined as the time from groin puncture to recanalization or the end of procedure.
Histopathological analysis
Retrieved thrombus material was immediately fixed in 10% phosphate-buffered formalin. Thrombus material was then cut into 3–5μm slices and representative serial sections were stained with Martius Scarlett Blue trichrome (MSB) for main thrombus component including red blood cells (RBCs), white blood cells (WBCs), fibrin, platelet and collagen. Immunohistochemistry (IHC) staining was also performed using Leica 3M autostainer and BOND Polymer Refine Red Detection kit for the following components: vWF (Dako anti-human von Willebrand Factor -M061601 clone F8/86, 1/200 dilution), Neutrophil Extracellular Traps (NETs): H3Cit (anti-citrulinated H3, Abcam ab1791, 1/1000 dilution). Positive component would be stained red to pink on IHC using our method.
Slides were scanned at 20x magnification using Motic Slide Scanner. Orbit Image Analysis software (www.orbit.bio) was used for quantitative analysis of Thrombus components (15).
Statistical analysis
Baseline characteristics are summarized as mean (±standard deviation) for continuous variables or proportions for categorical variables. Patients were divided into two groups based SOGP timing. Group one (early group) included patients with less than 4 hours from SOGP and second group (delayed group) included patients in 4 to 8 hours window from SOGP. Independent sample t-test was used to compare the composition of Thrombus including CitH3, vWF, RBC, WBC, Fibrin, Platelet, and Collagen between two Groups. Baseline Characteristics was also compared between early and delayed group using independent T-test for continuous variables and Chi-Square Test for categorical variables. The Pearson correlation was used to evaluate the association between time to recanalization and significantly different components between early and delayed groups.
We also employed artificial neural network (ANN) to evaluate the importance of thrombus components, SOGP, IV-tPA and thrombectomy technique with the number of passes required for successful recanalization. For the machine learning model, an exploratory two-layer multiplayer perceptron (MLP) artificial neural network (ANN) model with a back propagation algorithm was constructed. In the exploratory ANN model, data were randomly assigned to training sample (70%) and test sample (30%). Hyberbolic tangent activation function was used in the hidden layer and sigmoid activation function was selected for output layers. Each hidden layer had 50 units. Scaled conjugate gradient was used for optimization algorithm. The initial Lambda was 0.0000005, initial Sigma was 0.00005 and the interval offset was ±0.5. We also employed batch mode as a type of training. Rescaling of covariates was performed with standardized mode. For constructing ANN we used continuous variables including contents H3Cit, vWF, RBC, fibrin and platelet for covariates and dummy-coded categorical variable including IV-tPA (Yes vs No), thrombectomy techniques ( direct aspiration vs stent retriever vs combination) , SOGP (early group vs delayed group) for factors. Our outcome of interest (dependent variable) was the number of passes required to achieve successful recanalization which was also categorized into three groups (single pass, 2–3 passes and 4 or more passes).
A p-value less than 0.05 was considered statistically significant and all statistical analyses were undertaken using SPSS 23.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Patient population
During the study period, 137 thrombi were retrieved from patients who underwent mechanical thrombectomy. Successful recanalization was achieved in 131 patients (95.6%). There was no statistically significant difference between two groups (early vs delayed SOGP groups) in terms of stroke etiology (P value: 0.06) and IV-tPA (P value: 0.62). Table 1 summarizes baseline characteristics of patients.
Table 1.
baseline characteristics of patients’ cohort
| Variable | Early Group (n=88) |
Delayed Group (n=49) |
P value |
|---|---|---|---|
| Age (mean ±SD) | 67.18(±14.17) | 64.42(±10.7) | 0.28 |
| Male (n, %) | 51 (58%) | 25 (51%) | 0.43 |
| Hypertension (n, %) | 39 (44%) | 19 (38%) | 0.52 |
| Diabetes (n, %), | 22 | 13 | 0.84 |
| NIHSS Score, Median(IQR) | 17(10.5–23) | 17.5 (7.25–21.5) | 0.39 |
| Premedication with Anti-platelets (n, %) | 52 (59%) | 26 (53%) | 0.49 |
| Premedication with Anti-coagulant (n,%) | 6 (%7) | 4 (%8) | 0.77 |
| Occlusion Site (n, %) | |||
| ICA | 16 (18%) | 13(26%) | |
| ICA Terminus | 13 (15%) | 7 (14%) | |
| M1 | 42 (48%) | 21 (43%) | |
| M2 | 22 (25%) | 13 (27%) | |
| A1 | 4 (5%) | 0 (0%) | |
| Vertebral | 1 (1%) | 1(2%) | |
| Basilar | 4 (5%) | 3(6%) | |
| P1 | 3 (3%) | 1 (2%) | 0.36 |
| IV-tPA (n, %) | |||
| Yes | 42 (48%) | 25 (51%) | |
| No | 46 (52%) | 24 (49%) | 0.62 |
| Etiology (n, %) | |||
| Large Artery atherosclerosis (LAA) | 46(52%) | 18 (37%) | |
| Cardioembolic | 11(13%) | 14 (29%) | |
| Unknown | 15 (17%) | 5 (10%) | |
| Other | 16 (18%) | 12 (24%) | 0.06 |
| Final TICI Score 2b-3 (n,%) | 84 (95%) | 47 (96%) | 0.48 |
| Number of passes required (Median) | 2.09 (±1.67) | 2.49 (±1.83) | 0.24 |
| Time to recanalization (minutes) | 24.32(±14.83) | 32.23 (±35.59) | 0.18 |
| Thrombectomy technique (n, %) | |||
| Aspiration | 54 (61%) | 35 (71%) | |
| Stent retriever | 8 (9%) | 3 (6%) | |
| Combination | 26 (30%) | 11 (22%) | 0.49 |
Thrombus composition change over time
The overall average percentage of RBCs, WBCs, platelet, fibrin, H3Cit, and vWF components in thrombi were 45.83%, 3.58%, 22.23%, 28.27%, 19.97% and 16.23% respectively (Figure 1). As shown in Table 2, when stratifying by the SOGP time, WBCs, fibrin, H3Cit and vWF thrombus fractions were significantly higher in the delayed group (Figure 2&3). Additionally, RBC content was lower in the delayed group but it did not reach statistical significance. (Table 2).
Fig. 1.
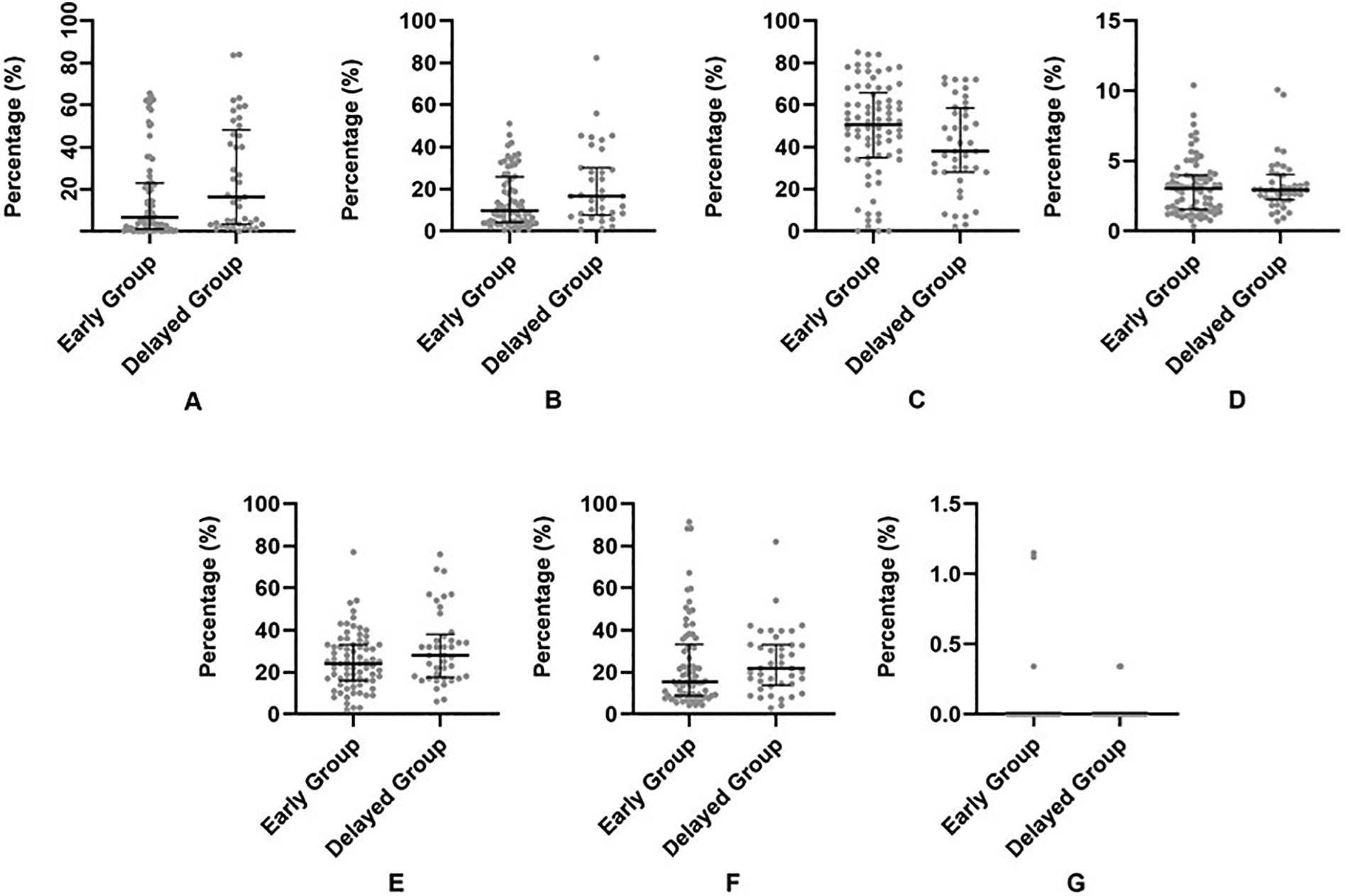
Scatter plot (with median and interquartile range) showing the comparison between SOGP less than 4 h (A, Early Group) and SOGP more than 4 h (B, Delayed Group) for each component of thrombus including H3Cit (A), vWF (B), RBCs (C), WBCs (D), Fibrin (E), Platelet (F) and Collagen (G). Independent sample t-test was used for comparison.
Table 2.
Association of SOGP time with Thrombus composition
| Mean (± SD) | P value | ||
|---|---|---|---|
| Red Blood Cells* (%) | Early group | 48.52 ± 23.13 | 0.09 |
| Delayed group | 41.02 ± 22.04 | ||
| White Blood Cells* (%) | Early group | 3.21 ± 2.02 | 0.02 |
| Delayed group | 4.25 ± 2.5 | ||
| Fibrin* (%) | Early group | 25.62 ± 13.90 | 0.02 |
| Delayed group | 33.03 ± 18.09 | ||
| Platelets* (%) | Early group | 22.55 ± 20.76 | 0.81 |
| Delayed group | 21.68 ± 15.76 | ||
| Collagen* (%) | Early group | 0.07 ± 0.45 | 0.2 |
| Delayed group | 0.006 ± 0.03 | ||
| H3Cit* (%) | Early group | 16.31 ± 19.88 | 0.03 |
| Delayed group | 26.57 ± 25.9 | ||
| vWF* (%) | Early group | 13.94 ± 13.48 | 0.04 |
| Delayed group | 20.36 ± 20.61 |
Percentage is calculated over sectioned total thrombus area.
Fig. 2.
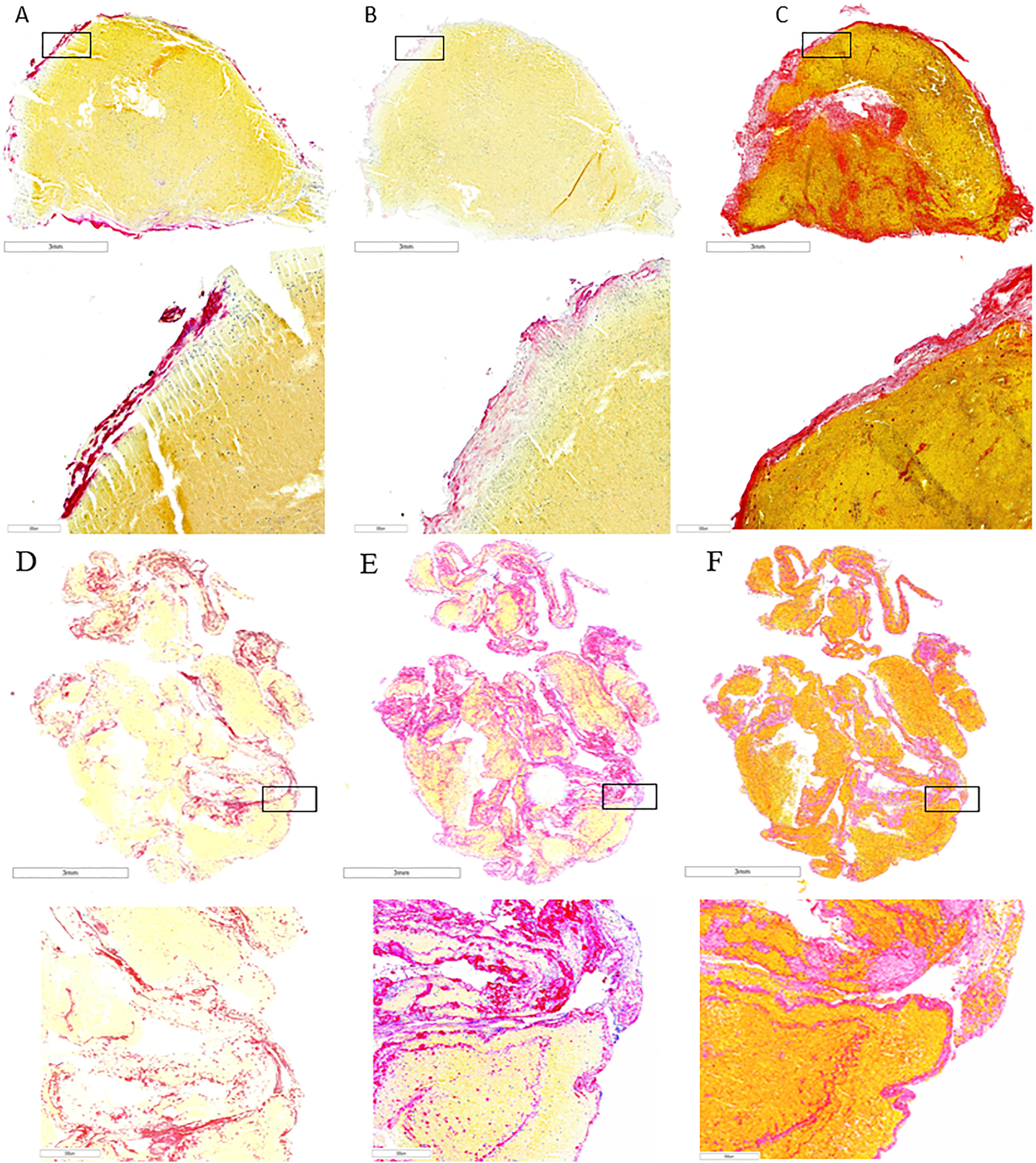
Representative case of thrombus retrieved from a patient in early SOGP group showing lower expression of H3Cit (A, red), vWF (B, pink) and fíbrin (C, Martius Scarlet Blue staining, red) compared to thrombi retrieved from a patient in delayed SOGP group depicting high expression of H3Cit (D, red) and vWF (E, pink) and high content of fíbrin (F, Martius Scarlet Blue staining, red).
Fig. 3.
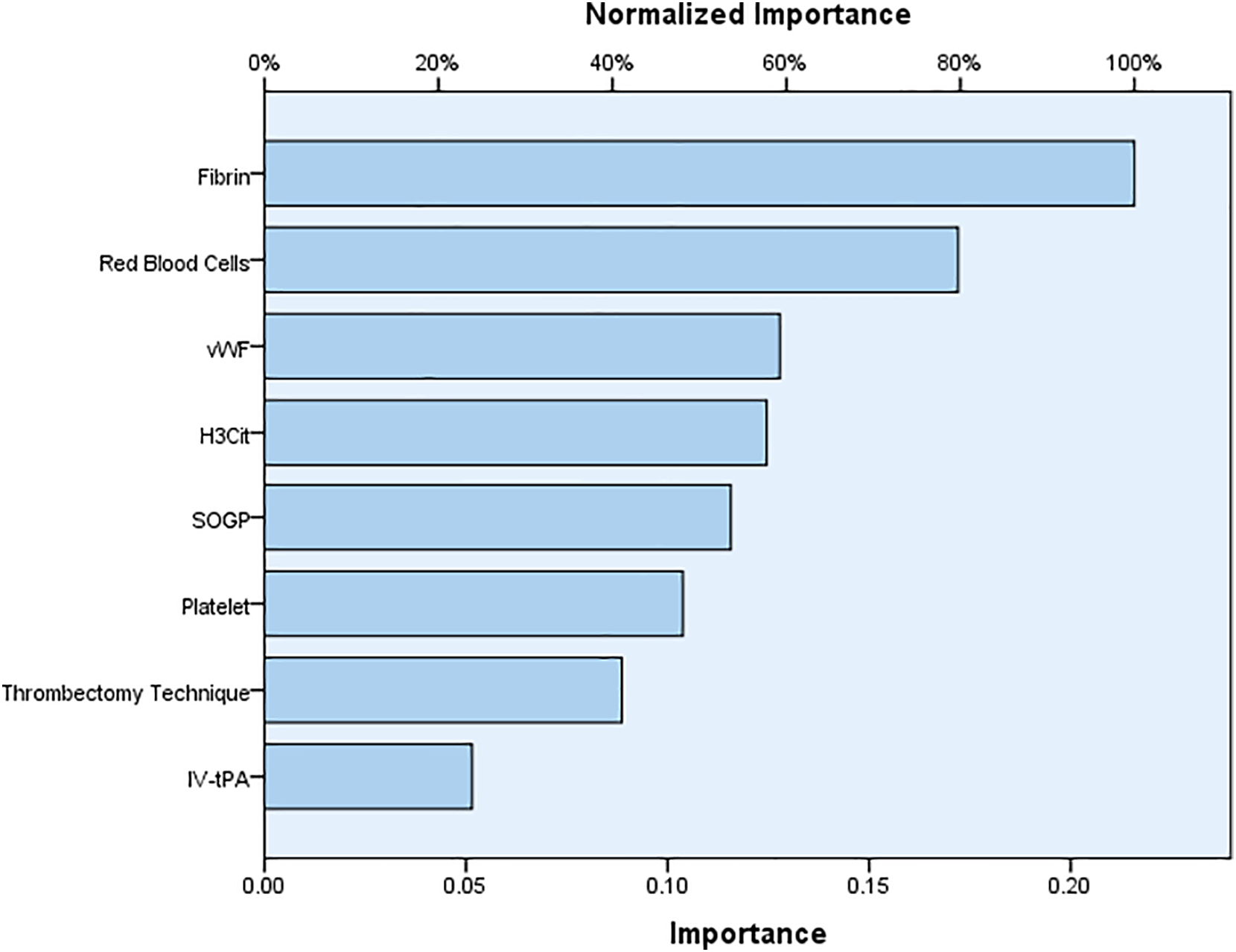
Normalized importance of factors in predicting number of passes required for achieving successful recanalization. Two-layer multiplayer perceptron (MLP) artificial neural network (ANN) model with a back propagation algorithm was used to calculate Normalized importance of factors.
Time to recanalization and thrombus composition
There was no correlation between time to recanalization and any of significantly different components (early vs delayed group) including WBC (P value: 0.43), Fibrin (P value: 0.88), H3Cit (P value: 0.53) and vWF (P value: 0.45).
DISCUSSION
In this study we demonstrated that delayed recanalization times were associated with increased fibrin, H3Cit and vWF content of the thrombus. These phenomena are likely due to coagulation activation within the thrombus over time, thereby increasing the fibrin:RBC ratio of the thrombus. Thrombus composition influences its mechanical behaviour and thus this finding may have implications for technical success with either stent-retriever or aspiration MT. Fibrin-rich thrombi are importantly less viscoelastic, as the organization of fibrin mesh alters thrombus mechanical properties which could make MT more difficult (16, 17). Meta-analysis of the Highly Effective Reperfusion Using Multiple Endovascular devices (HERMES) data shows that increased in both times from computed tomography to arterial access and door to arterial access are associated with lower odds of achieving mTICI 2b/3 following thrombectomy (OR 0.78, 95% CI 0.64–0.95)(10). The reasons for this were somewhat unclear. Our data suggest that cellular composition of the embolus continues to evolve over this time as fibrin mesh entraps red blood cells within the thrombus. Neutrophil extracellular traps are additional important components of thrombi that increase with thrombus age and alter fibrin architecture (18, 19). A recent analysis of 43 retrieved thrombi did not detect any significant difference in time from symptom onset to recanalization between erythrocyte-rich and fibrin-rich thrombi (20). However, procedure time was negatively correlated with erythrocyte infiltration and positively correlated with fibrin infiltration within the thrombus (20). Our study differs from this previously published study due to the fact that we were able to include a larger number of patients as well as the fact that we were able to use machine-learning algorithms for thrombus characterization.
Few studies have directly correlated post-thrombectomy angiographic outcomes with thrombus composition. The likelihood of successful recanalization has been shown to be higher with RBC-rich thrombi(3). In an in vitro study, the coefficient of friction of Thrombus samples in a bovine vessel were shown to be inversely correlated with increasing RBC content, with fibrin-rich thrombi (0% RBC in their study) having a threefold higher coefficient of friction than whole blood (RBC-rich) thrombus (21). Aspiration thrombectomy in particular relies on the ability to overcome friction force between proximal Thrombus and catheter tip and thus reducing fibrin fraction with improved recanalization times may improve technical success. However, the ability to extrapolate these findings in vivo to human patients is unclear. Further recent work by Weafer and colleagues using ovine blood thrombi has demonstrated that fibrin-rich thrombi have greater compressive Thrombus stiffness and a concomitant reduced maximum indentation depth (22). Increased thrombi stiffness has additionally been shown to decrease engagement with stent-retriever device struts, in vitro, and fibrin-rich thrombi are more adherent to the vessel wall (23). These findings have implications for first-pass success with stent-retriever thrombectomy, a technique that relies on Thrombus engagement with device struts and radial force generation.
Thrombus contraction is also another issue that may be associated with timing. It has been shown that thrombi undergo contraction driven by platelet-associated forces propagated by fibrin mesh network over time which could result in potentially reducing the occlusion and improving the blood flow past the thrombi. In contracted thrombus, erythrocytes are deformed and compacted into the tessellated arrays of polyhedral-like cells with fibrin and platelet on the surface of thrombus. Polyhedral structures help to prevent vascular obstruction but may result in resistance to thrombolysis (24). Thrombus contraction affects internal and external fibrinolysis. It has been shown that contraction doubles the rate of internal fibrinolysis but substantially impairs external fibrinolysis, likely affecting fibrinolytic recanalization rates (25). These observed differences suggest that the rate of thrombus lysis is controlled by the interplay between accessibility of fibrin mesh network to fibrinolytic molecules, including thrombus permeability, and spatial proximity of the fibrin strains that regulates the effects of the fibrinolytic enzymes. It is also noteworthy that thrombus contraction results in stiffer Thrombus and expulsion of serum from thrombus with consequent change in the content of pro and anti-fibrinolytic within the thrombus(26). In our study the older thrombi (more than 4 hours) were associated with higher amount of fibrin and CitH3 and vWF which could collectively indicate the contraction of thrombi over time and may also indirectly suggest that IV-tPA past four hours may not be able to dissolve or soften the thrombus.
This study has limitations, including its single-center, retrospective. Thrombus may not always have been removed en bloc during thrombectomy, and device manipulation during the procedure may cause clot fragmentation and potential embolization, affecting the composition of retrieved clots. Additionally the composition of thrombus before embolization; diverse underlying stroke etiology and different extraction rates may have some effect on the final composition thrombus available for histological analysis.
CONCLUSIONS
Longer times to recanalization are associated with increased WBCs, fibrin, H3Cit and vWF fraction of retrieved thrombi following endovascular thrombectomy for acute ischemic stroke possibly due to in vivo thrombus maturation.
Highlights.
Longer time to recanalization is associated with alteration in thrombus composition.
Longer time to recanalization was associated with increased WBCs, fibrin, H3Cit and vWF in thrombus.
Number of passes required for successful recanalization was influenced by fibrin and RBCs.
Source of Funding
This work was supported by the National Institutes of Health grant number (R01 NS105853)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
All authors have nothing to disclose
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- 1.Brouwer PA, Brinjikji W, De Meyer SF. Clot Pathophysiology: Why Is It Clinically Important? Neuroimaging clinics of North America. 2018;28(4):611–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gong L, Zheng X, Feng L, Zhang X, Dong Q, Zhou X, et al. Bridging Therapy Versus Direct Mechanical Thrombectomy in Patients with Acute Ischemic Stroke due to Middle Cerebral Artery Occlusion: A Clinical-Histological Analysis of Retrieved Thrombi. Cell transplantation. 2019:963689718823206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin JW, Jeong HS, Kwon H-J, Song KS, Kim J. High red blood cell composition in clots is associated with successful recanalization during intra-arterial thrombectomy. PloS one. 2018;13(5):e0197492–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abbasi M, Kvamme P, Layton KF, Hanel RA, Almekhlafi MA, Delgado JE, et al. Per pass analysis of thrombus composition retrieved by mechanical thrombectomy. Interventional Neuroradiology.0(0):15910199211009119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brinjikji W, Nogueira RG, Kvamme P, Layton KF, Almandoz JED, Hanel RA, et al. Association between clot composition and stroke origin in mechanical thrombectomy patients: analysis of the Stroke Thromboembolism Registry of Imaging and Pathology. Journal of neurointerventional surgery. 2021;13(7):594–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mereuta OM, Abbasi M, Fitzgerald S, Dai D, Kadirvel R, Hanel RA, et al. Histological evaluation of acute ischemic stroke thrombi may indicate the occurrence of vessel wall injury during mechanical thrombectomy. Journal of neurointerventional surgery. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arturo Larco J, Abbasi M, Liu Y, Madhani SI, Shahid AH, Kadirvel R, et al. Per-pass analysis of recanalization and good neurological outcome in thrombectomy for stroke: Systematic review and meta-analysis. Interventional Neuroradiology. 2021:15910199211028342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbasi M, Liu Y, Fitzgerald S, Mereuta OM, Larco JLA, Rizvi A, et al. Systematic review and meta-analysis of current rates of first pass effect by thrombectomy technique and associations with clinical outcomes. Journal of neurointerventional surgery. 2021;13(3):212–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abbasi M, Dai D, Liu Y, Fitzgerald S, Kadirvel R, Savastano L, et al. Iatrogenic Foreign Materials Associated with Retrieved Clot Tissue via Mechanical Thrombectomy. American Journal of Neuroradiology. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourcier R, Goyal M, Liebeskind DS, Muir KW, Desal H, Siddiqui AH, et al. Association of Time From Stroke Onset to Groin Puncture With Quality of Reperfusion After Mechanical Thrombectomy: A Meta-analysis of Individual Patient Data From 7 Randomized Clinical Trials. JAMA neurology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Investigators CASPR. Prioritizing interventions to improve rates of thrombolysis for ischemic stroke. Neurology. 2005;64(4):654–9. [DOI] [PubMed] [Google Scholar]
- 12.Jolugbo P, Ariëns RA. Thrombus composition and efficacy of thrombolysis and thrombectomy in acute ischemic stroke. Stroke. 2021;52(3):1131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. The Lancet. 2014;384(9958):1929–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. stroke. 1993;24(1):35–41. [DOI] [PubMed] [Google Scholar]
- 15.Fitzgerald S, Wang S, Dai D, Murphree DH Jr., Pandit A, Douglas A, et al. Orbit image analysis machine learning software can be used for the histological quantification of acute ischemic stroke blood clots. PLoS One. 2019;14(12):e0225841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gersh KC, Nagaswami C, Weisel JW. Fibrin network structure and clot mechanical properties are altered by incorporation of erythrocytes. Thrombosis and haemostasis. 2009;102(6):1169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brinjikji W, Mereuta OM, Dai D, Kallmes DF, Savastano L, Liu Y, et al. Mechanisms of fibrinolysis resistance and potential targets for thrombolysis in acute ischaemic stroke: lessons from retrieved stroke emboli. Stroke and Vascular Neurology. 2021:svn-2021–001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varju I, Longstaff C, Szabo L, Farkas AZ, Varga-Szabo VJ, Tanka-Salamon A, et al. DNA, histones and neutrophil extracellular traps exert anti-fibrinolytic effects in a plasma environment. Thrombosis and haemostasis. 2015;113(6):1289–98. [DOI] [PubMed] [Google Scholar]
- 19.Laridan E, Denorme F, Desender L, Francois O, Andersson T, Deckmyn H, et al. Neutrophil extracellular traps in ischemic stroke thrombi. Annals of neurology. 2017;82(2):223–32. [DOI] [PubMed] [Google Scholar]
- 20.Maekawa K, Shibata M, Nakajima H, Mizutani A, Kitano Y, Seguchi M, et al. Erythrocyte-Rich Thrombus Is Associated with Reduced Number of Maneuvers and Procedure Time in Patients with Acute Ischemic Stroke Undergoing Mechanical Thrombectomy. Cerebrovascular diseases extra. 2018;8(1):39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunning GM, McArdle K, Mirza M, Duffy S, Gilvarry M, Brouwer PA. Clot friction variation with fibrin content; implications for resistance to thrombectomy. Journal of neurointerventional surgery. 2018;10(1):34–8. [DOI] [PubMed] [Google Scholar]
- 22.Weafer FM, Duffy S, Machado I, Gunning G, Mordasini P, Roche E, et al. Characterization of strut indentation during mechanical thrombectomy in acute ischemic stroke clot analogs. Journal of neurointerventional surgery. 2019. [DOI] [PubMed] [Google Scholar]
- 23.Fennell VS, Setlur Nagesh SV, Meess KM, Gutierrez L, James RH, Springer ME, et al. What to do about fibrin rich ‘tough clots’? Comparing the Solitaire stent retriever with a novel geometric clot extractor in an in vitro stroke model. Journal of neurointerventional surgery. 2018;10(9):907–10. [DOI] [PubMed] [Google Scholar]
- 24.Cines DB, Lebedeva T, Nagaswami C, Hayes V, Massefski W, Litvinov RI, et al. Clot contraction: compression of erythrocytes into tightly packed polyhedra and redistribution of platelets and fibrin. Blood, The Journal of the American Society of Hematology. 2014;123(10):1596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tutwiler V, Peshkova AD, Le Minh G, Zaitsev S, Litvinov RI, Cines DB, et al. Blood clot contraction differentially modulates internal and external fibrinolysis. Journal of Thrombosis and Haemostasis. 2019;17(2):361–70. [DOI] [PubMed] [Google Scholar]
- 26.Blinc A, Keber D, Lahajnar G, Stegnar M, Zidanšek A, Demsar F. Lysing patterns of retracted blood clots with diffusion or bulk flow transport of plasma with urokinase into clots–a magnetic resonance imaging study in vitro. Thrombosis and haemostasis. 1992;68(12):667–71. [PubMed] [Google Scholar]


