Abstract
Background and purpose:
Flow diverters (FD) can cause rare but devastating delayed aneurysm ruptures due to a potential implication of matrix metalloproteinases (MMPs). Concomitant coiling or anti-inflammatory medications have been proposed to prevent the risk of delayed ruptures. The aims of this study were to evaluate concomitant coiling and cyclosporine to regulate the MMPs expression in FD treated aneurysms.
Materials and methods:
Elastase-induced aneurysms were created in 20 rabbits. Aneurysms were treated with 1) FD alone, 2) FD with concomitant coiling 3) FD and cyclosporine or 4) left untreated as controls. At sacrifice, MMPs levels were analyzed via zymography. Kruskal-Wallis one-way non-parametric ANOVA was performed for each enzyme. If significant results were observed for the Kruskal-Wallis test, pairwise group comparisons were performed using Dunn’s test with Bonferroni multiple-testing correction.
Results:
Significant differences were observed among groups for pro-MMP9 (p=.0337). Pairwise comparison demonstrated higher levels of pro-MMP9 with concomitant coiling compared to untreated aneurysms (p=.012), with higher though not significantly different levels of pro-MMP9 in FD with concomitant coiling versus FD alone. While not statistically significant, trends were noted regarding differences in active-MMP9 across groups with lower level of active-MMP9 with concomitant coiling compared to the other FD groups. No significant differences were observed for pro- or active-MMP2 across groups, nor for FD with cyclosporine compared to FD alone.
Conclusions:
FD implantation increases the level of pro-MMP9 expression in aneurysms. Provocative trends regarding modulation of active-MMP9 expression with concomitant coiling suggests the need for larger, confirmatory preclinical studies. Anti-inflammatory treatment with cyclosporine appears to have minimal biological effect.
Introduction
Flow diverters (FD) are now largely accepted as standard of care in treatment of selected aneurysms due to their high rates of angiographic occlusion and good clinical outcomes1–3. However, these devices may have rare but severe complications such as post-operative or delayed aneurysm rupture4–8. Despite the fact that numerous case series and case reports have reported on this complication, there continues to be controversy surrounding its origin with poor evidence regarding the risk factors and mechanisms of these hemorrhagic complications4, 6, 8–12. Prior studies have suggested a potential role of intra-aneurysmal thrombus in the pathophysiologic mechanism of aneurysm rupture7.
As previously described in abdominal aortic aneurysms, leukocytes trapped in the intra luminal thrombus are a source of storage, release and activation of various proteases such as metalloproteinases (MMP2 and MMP9) and serine proteases. These have high proteolytic activity which could participate in the degradation of structural components of the arterial wall and lead to aneurysm rupture7, 13–20. MMPs are secreted as inactive proforms (pro-MMP) and are activated by protein cleavage by other proteinases (active-MMP)20. The over expression of activated type IV collagenases MMP9 and MMP2 in cerebral ruptured aneurysms21 indicate that effective regulation of MMPs may result in improving clinical prognosis of cerebral aneurysms.
Some studies have already described the implication of MMPs in cerebral vascular diseases and aneurysms22–29. Previous studies have demonstrated a higher risk of rupture in giant aneurysms2, 6, 7, 12 and have recommended that giant aneurysms are treated with concomitant coiling and flow diverter treatment in order to protect the dome of the aneurysm in an attempt to prevent delayed ruptures4, 6, 11, 30, 31.
Cyclosporine A is an anti-inflammatory agent32 widely used to prevent organ transplant rejection or treat autoimmune disorders33. Cyclosporine has already been tested for its effect on MMPs levels in various models and disorders32–42. In a study about abdominal aortic aneurysms, cyclosporine decreased MMP9 and stabilized expanding aortic arteries38. However, cyclosporine has not been tested in intracranial aneurysms to regulate the MMPs expression.
The aims of our study were to evaluate the effect of associated coiling and cyclosporine to regulate the MMPs expression in flow diverter treated aneurysms in a rabbit model.
METHODS
Aneurysm Creation and Treatment
The Institutional Animal Care and Use Committee approved all procedures before the initiation of this study. Some of the rabbits used in this study were originally employed as part of other investigations, where we investigated the gene expression between aneurysms treated with microcoils and flow diverters43. Elastase induced saccular aneurysms were created in 20 New Zealand White rabbits as previously described44. Three weeks after the aneurysm creation, rabbits were treated either with FD alone (n=5), FD with concomitant coiling (n=6), FD and cyclosporine (n=5) or left untreated (n=4)45, 46. The rabbits treated with FD and cyclosporine were given 10 mg Cyclosporine A/kg body weight by oral gavage once daily for 4 weeks.
Rabbits were sacrificed 4 weeks after the treatment procedure. At the time of sacrifice, animals were deeply anesthetized. The animals were then euthanized with a lethal injection of pentobarbital. Aneurysms were immediately harvested, and frozen in liquid nitrogen and kept at −70°C until used43.
MMPs Gelatin Zymography
Frozen samples were pulverized under liquid nitrogen and extracted in ice-cold lysis buffer (10 mmol/l sodium phosphate, pH 7.2, 150 mmol/l NaCl, 1% Triton X-100, 0.1% SDS, 0.5% sodium deoxycholate, and 0.2% sodium azide). After centrifugation at 10,000 g for 20 min at 4°C, the protein concentration of the supernatant was determined (Pierce Biotechnology). Samples were resolved by nonreducing 10% SDS-PAGE through gels containing 0.1% gelatin (Bio-Rad). Gels were washed with 2.5% Triton X-100 for 1 h, then incubated for 24 h at 37°C in developing buffer (50 mmol/l Tris-HCl, pH 8.5, 5 mmol/l CaCl2 and 0.5 mmol/l ZnCl2). MMPs-2 and -9 are collagenases, which act on the gelatin (a partial collagen digest) in the gel and produce gelatinolytic zones. Gelatinolytic zones, representing the activities of MMPs, were visualized after staining the gels with 0.5% Coomassie blue R-250. The gelatinolytic zones of MMP-2 and MMP-9 were analyzed densitometrically using Image-J software and converted to quantifiable data in the number of pixels47, 48. The intensities of the gelatinolytic bands reflect the activity of corresponding MMP.
Statistics
Kruskal-Wallis one-way non-parametric ANOVA was performed for each enzyme (pro-MMP2, active-MMP2, pro-MMP9, and active-MMP9) for each of the four treatment groups. Kruskal-Wallis p-values were not corrected for multiple testing. Post-hoc pairwise group comparisons were performed using Dunn’s test with Bonferroni multiple-testing correction49. Statistical analyses were performed in R (version 3.1.1; Vienna, Austria). Dunn’s test was performed using R package dunn.test (version 1.2.4). A value of α=0.05 was selected as the significance threshold.
RESULTS
Summary statistics are reported in Table 1 as medians and interquartile range (IQR). Figure 1 represents MMPs expression by aneurysm treatment with boxplots and individual data points.
Table 1:
Descriptive statistics for pixel counts by enzyme. Kruskal-Wallis tests.
| Untreated Aneurysm (N=4) |
FD alone (N=6) |
FD+ Coils (N=5) |
FD+ Cyclosporine (N=5)* |
p value | |
|---|---|---|---|---|---|
| Pro-MMP9 | 0.0337 | ||||
| Median | 428 | 2575 | 9774 | 3159 | |
| IQR | 90 – 1941 | 697 – 4917 | 7657 – 13562 | 1730 – 4150 | |
| Active-MMP9 | 0.126 | ||||
| Median | 483 | 2666 | 496 | 2410 | |
| IQR | 251 – 1897 | 1066 – 5291 | 420 – 1734 | 2099 – 3039 | |
| Pro-MMP2 | 0.779 | ||||
| Median | 3250 | 4644 | 4553 | 4628 | |
| IQR | 2950 – 6684 | 2403 – 7693 | 3962 – 5261 | 4066 – 8065 | |
| Active-MMP2 | 0.152 | ||||
| Median | 1294 | 3500 | 3653 | 3822 | |
| IQR | 1143 – 1666 | 2684 – 4476 | 2186 – 4360 | 2670 – 4068 |
Flow diverter + Cyclosporine Pro-MMP9 N=4
Figure 1:
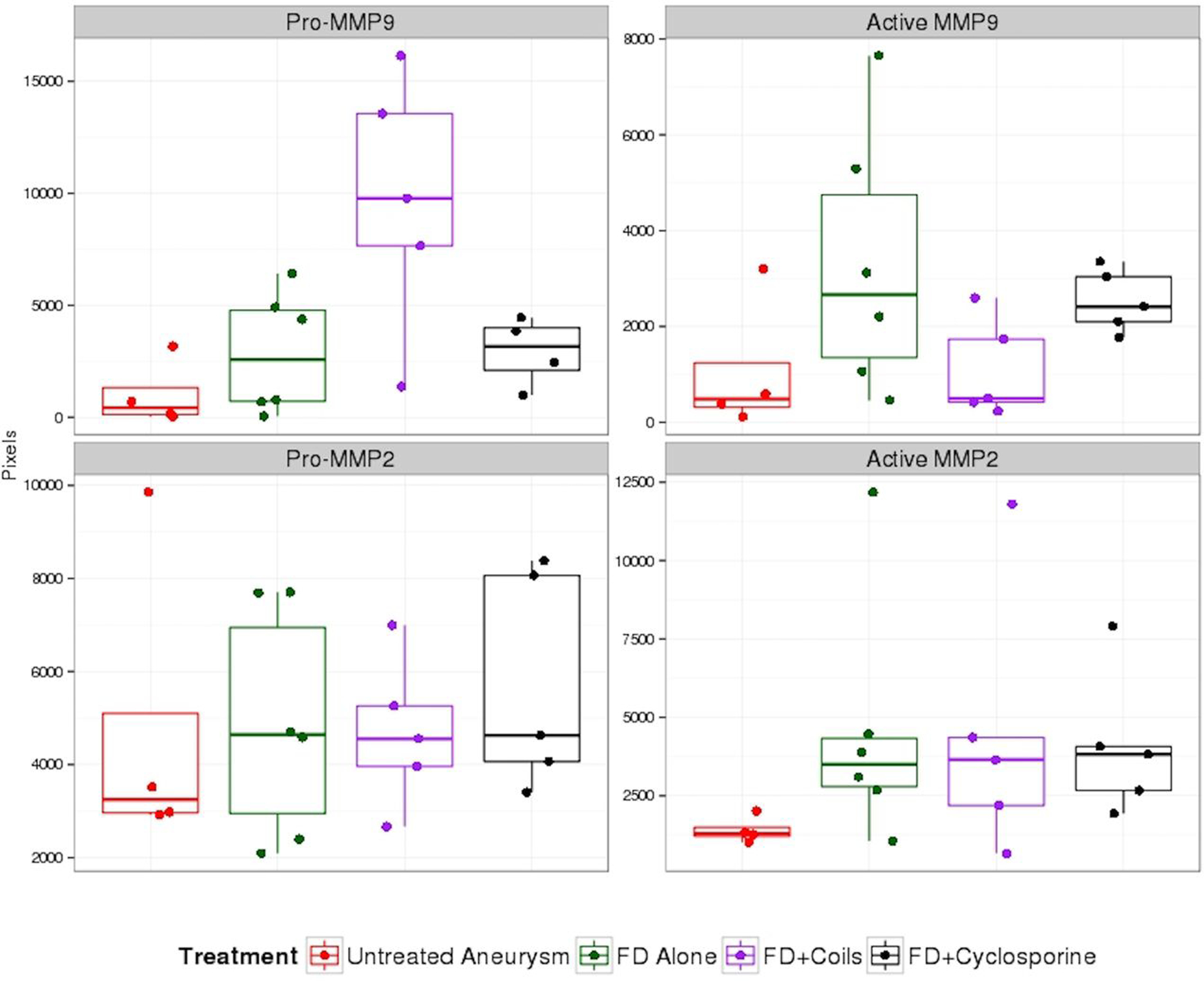
Boxplot of MMPs expression by aneurysm treatment. The individual data points for each treatment are also shown. FD=flow diverter
In all treatment groups, levels of MMPs in treated aneurysms appeared higher than in the untreated group, with the exception of the FD with concomitant coiling group for active MMP-9; however, significant differences between groups were only found for pro-MMP9. MMP2 levels were similar throughout the three different FD treatment groups.
Compared to the untreated aneurysms, aneurysms treated with FD alone had higher levels of pro-MMP9 (2575, IQR: 697–4917; versus 428, IQR: 90–1941), active-MMP9 (2666, IQR: 1066–5291; versus 483, IQR: 251–1897), pro-MMP2 (4644, IQR: 2403–7693; versus 3250, IQR: 2950–6684) and active-MMP2 (3500, IQR: 2684–4476; versus 1294, IQR: 1143–1666) but those differences did not reach significant levels (Table 2).
Table 2:
Post-hoc pairwise group comparison p-values for Pro-MMP9 using Dunn’s method with Bonferroni correction.
| FD + Coils | FD alone | FD + Cyclosporine | |
|---|---|---|---|
| FD alone | 0.141 | --- | --- |
| FD + Cyclosporine | 0.414 | 1.00 | --- |
| Untreated Aneurysm | 0.012 | 0.783 | 0.561 |
In the group treated with FD and concomitant coiling the level of active-MMP9 was similar to the untreated group (496, IQR: 420–1734; versus 483, IQR: 251–1897). The level of pro-MMP9 was significantly higher in the FD with concomitant coiling group than in the untreated aneurysms group (9774, IQR: 7657–13562; versus 428, IQR: 90–1941; p value=0.012) (Table 2).
We did not observe any statistically significant difference or trends in difference when comparing the MMPs levels in aneurysms treated with FD + cyclosporine and FD alone.
DISCUSSION
This study demonstrates that treatment with FD affects MMPs levels in intracranial aneurysms. Specifically, aneurysms treated with FD and concomitant coiling demonstrate significant increased levels of pro-MMP9. In our current, relatively small study, we noted a trend toward decreased active-MMP9 with concomitant coiling compared to the other FD groups. Anti-inflammatory medications with cyclosporine did not significantly impact levels of MMPs. These findings suggest the need for larger, preclinical studies focused on MMP9 biology following treatment with FD.
By analogy to abdominal aneurysms, previous experimental and clinical studies have suggested that the intra-aneurysmal thrombus associated with FD could be a site of activation of MMPs and a potential cause of delayed ruptures7, 13–19. Furthermore, prior studies have suggested or demonstrated a higher risk of delayed ruptures after FD in giant aneurysms2, 6, 7, 12. Since giant aneurysms are generally more likely to have a larger intraluminal thrombus, it is probable that larger FD treated aneurysms have higher levels of MMPs expression. Based on our current findings, we believe that ongoing focus on MMP9 may provide important insights into FD-related complications.
The trend toward decreased active-MMP9 with concomitant coiling compared to other FD groups is of very high importance. Indeed, despite previous recommendations for concomitant coiling in aneurysms larger than 15 mm4, 6, 11, 30, 31, no study described its effect on MMPs levels. Our study shows that the effect of concomitant coiling is not only related to a mechanical effect of the coils to protect the aneurysms dome but, at least in part, related to a biological effect on MMPs expression. It suggests that concomitant coiling may reduce the level of active-MMP9 expression in the FD treated aneurysms by blocking the activation of pro-MMP9 in its active form with accumulation of its inactive proform, which could be a potential solution to prevent delayed aneurysms ruptures after FD.
Anti-inflammatory and immunosuppressive drugs such as cyclosporine A have proved to be beneficial on MMPs levels in abdominal aneurysms38, 50, 51. However, in our study, the expression levels of MMP2 and MMP9 in the group treated with FD and cyclosporine were comparable to aneurysms treated with FD alone with higher levels of active-MMP9. While it was hoped that cyclosporine could be used to control MMPs levels, it does not appear to have an effect on either MMP9 or MMP2. Further research could be done to explore the possibility of using other anti-inflammatory medications on MMPs expression. Additionally the role of pro-inflammatory mediators in aneurysm progression and rupture versus healing after treatment remains to be elucidated. Prior studies have demonstrated that MMPs and MCP-1 play key roles in formation and rupture with MCP-1 promoting MMP9, but post treatment expression also increases possibly due to aneurysm healing29, 52, 53.
Limitations
Our study has several limitations. We used the rabbit elastase model which has histological, morphological, biological, and hemodynamic similarities to humans and is stable in time with no spontaneous thrombosis54. However, this model is neither a model of spontaneous rupture nor a model of delayed aneurysm rupture after FD and some biological aspects may differ when considering rupture-prone aneurysms. To explore these mechanisms, it would be of interest to analyze levels of MMPs in new models for active aneurysms with inflamed aneurysms wall or bio-active thrombus55, 56. Also, the reported cases of delayed ruptures after FD occurred mostly in large or giant aneurysms but the aneurysms used in this study were less than 20 mm. Some of our results did not reach significant differences but our ability to detect differences between groups was limited by the size of the treatment groups. Further studies should be done with larger groups. For the effect of concomitant coiling, we did not do any analysis of the impact of the packing density. Perhaps a denser coils packing would increase the effect of MMPs regulation. Different anti-inflammatory drug would maybe have different results but we decided to use cyclosporine because rabbits are known to be extremely sensitive to steroids57–60. Further, we analyzed only one dose for cyclosporine administration and its effect may be different with higher doses. Only one time point was studied following treatment of the aneurysm and protein expression may change over time. Finally, only two MMPs were studied and other MMPs isoforms may play important roles in delayed aneurysm rupture61. Transformation growth factor (TGF)-β is a key factor for MMP down-regulation. However, we did not measure the level of TGF-β in this study38. It may be possible that other proteolytic enzymes such as cathepsin or other pathways lead to delayed aneurysm rupture.
Conclusion
FD implantation increases the level of pro-MMP9 expression in aneurysms. Provocative trends regarding modulation of active-MMP9 expression with concomitant coiling suggests the need for larger, confirmatory preclinical studies. Anti-inflammatory treatment with cyclosporine appears to have minimal biological effect.
Abbreviations:
- MMPs
Matrix Metalloproteinases
- FD
Flow Diverter
References
- 1.Arrese I, Sarabia R, Pintado R, Delgado-Rodriguez M. Flow-diverter devices for intracranial aneurysms: systematic review and meta-analysis. Neurosurgery 2013;73:193–199; discussion 199–200. [DOI] [PubMed] [Google Scholar]
- 2.Brinjikji W, Murad MH, Lanzino G, Cloft HJ, Kallmes DF. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke 2013;44:442–447. [DOI] [PubMed] [Google Scholar]
- 3.Wakhloo AK, Gounis MJ. Revolution in aneurysm treatment: flow diversion to cure aneurysms: a paradigm shift. Neurosurgery 2014;61 Suppl 1:111–120. [DOI] [PubMed] [Google Scholar]
- 4.Turowski B, Macht S, Kulcsar Z, Hanggi D, Stummer W. Early fatal hemorrhage after endovascular cerebral aneurysm treatment with a flow diverter (SILK-Stent): do we need to rethink our concepts? Neuroradiology 2011;53:37–41. [DOI] [PubMed] [Google Scholar]
- 5.Cebral JR, Mut F, Raschi M, et al. Aneurysm rupture following treatment with flow-diverting stents: computational hemodynamics analysis of treatment. AJNR Am J Neuroradiol 2011;32:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kulcsár ZSI. The ESMINT Retrospective Analysis of Delayed Aneurysm Ruptures after flow diversion (RADAR) study. The eJournal of the European Society of Minimally Invasive Neurological Therapy 2012. [Google Scholar]
- 7.Kulcsar Z, Houdart E, Bonafe A, et al. Intra-aneurysmal thrombosis as a possible cause of delayed aneurysm rupture after flow-diversion treatment. AJNR Am J Neuroradiol 2011;32:20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz JP, Chow M, O’Kelly C, et al. Delayed ipsilateral parenchymal hemorrhage following flow diversion for the treatment of anterior circulation aneurysms. AJNR Am J Neuroradiol 2012;33:603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saatci I, Yavuz K, Ozer C, Geyik S, Cekirge HS. Treatment of intracranial aneurysms using the pipeline flow-diverter embolization device: a single-center experience with long-term follow-up results. AJNR Am J Neuroradiol 2012;33:1436–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becske T, Kallmes DF, Saatci I, et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology 2013;267:858–868. [DOI] [PubMed] [Google Scholar]
- 11.Berge J, Biondi A, Machi P, et al. Flow-diverter silk stent for the treatment of intracranial aneurysms: 1-year follow-up in a multicenter study. AJNR Am J Neuroradiol 2012;33:1150–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kallmes DF, Hanel R, Lopes D, et al. International retrospective study of the pipeline embolization device: a multicenter aneurysm treatment study. AJNR Am J Neuroradiol 2015;36:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adolph R, Vorp DA, Steed DL, Webster MW, Kameneva MV, Watkins SC. Cellular content and permeability of intraluminal thrombus in abdominal aortic aneurysm. J Vasc Surg 1997;25:916–926. [DOI] [PubMed] [Google Scholar]
- 14.Rossignol P, Fontaine V, Meilhac O, Angles-Cano E, Jacob MP, Michel JB. [Physiopathology of aortic aneurysm]. Rev Prat 2002;52:1061–1065. [PubMed] [Google Scholar]
- 15.Carrell TW, Burnand KG, Booth NA, Humphries J, Smith A. Intraluminal thrombus enhances proteolysis in abdominal aortic aneurysms. Vascular 2006;14:9–16. [DOI] [PubMed] [Google Scholar]
- 16.Fontaine V, Jacob MP, Houard X, et al. Involvement of the mural thrombus as a site of protease release and activation in human aortic aneurysms. Am J Pathol 2002;161:1701–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houard X, Rouzet F, Touat Z, et al. Topology of the fibrinolytic system within the mural thrombus of human abdominal aortic aneurysms. J Pathol 2007;212:20–28. [DOI] [PubMed] [Google Scholar]
- 18.Michel JB, Rouer M, Alsac JM. Regarding “A multilayer stent in the aorta may not seal the aneurysm, thereby leading to rupture”. J Vasc Surg 2013;57:605. [DOI] [PubMed] [Google Scholar]
- 19.Frosen J, Piippo A, Paetau A, et al. Remodeling of saccular cerebral artery aneurysm wall is associated with rupture: histological analysis of 24 unruptured and 42 ruptured cases. Stroke; a journal of cerebral circulation 2004;35:2287–2293. [DOI] [PubMed] [Google Scholar]
- 20.Tulamo R, Frosen J, Hernesniemi J, Niemela M. Inflammatory changes in the aneurysm wall: a review. J Neurointerv Surg 2010;2:120–130. [DOI] [PubMed] [Google Scholar]
- 21.Jin D, Sheng J, Yang X, Gao B. Matrix metalloproteinases and tissue inhibitors of metalloproteinases expression in human cerebral ruptured and unruptured aneurysm. Surgical neurology 2007;68 Suppl 2:S11–16; discussion S16. [DOI] [PubMed] [Google Scholar]
- 22.Starke RM, Komotar RJ, Hwang BY, et al. Systemic expression of matrix metalloproteinase-9 in patients with cerebral arteriovenous malformations. Neurosurgery 2010;66:343–348; discussion 348. [DOI] [PubMed] [Google Scholar]
- 23.Aoki T, Kataoka H, Moriwaki T, Nozaki K, Hashimoto N. Role of TIMP-1 and TIMP-2 in the progression of cerebral aneurysms. Stroke; a journal of cerebral circulation 2007;38:2337–2345. [DOI] [PubMed] [Google Scholar]
- 24.Aoki T, Kataoka H, Morimoto M, Nozaki K, Hashimoto N. Macrophage-derived matrix metalloproteinase-2 and −9 promote the progression of cerebral aneurysms in rats. Stroke; a journal of cerebral circulation 2007;38:162–169. [DOI] [PubMed] [Google Scholar]
- 25.Nuki Y, Tsou TL, Kurihara C, Kanematsu M, Kanematsu Y, Hashimoto T. Elastase-induced intracranial aneurysms in hypertensive mice. Hypertension 2009;54:1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Starke RM, Chalouhi N, Jabbour PM, et al. Critical role of TNF-alpha in cerebral aneurysm formation and progression to rupture. J Neuroinflammation 2014;11:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makino H, Tada Y, Wada K, et al. Pharmacological stabilization of intracranial aneurysms in mice: a feasibility study. Stroke; a journal of cerebral circulation 2012;43:2450–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim SC, Singh M, Huang J, et al. Matrix metalloproteinase-9 in cerebral aneurysms. Neurosurgery 1997;41:642–666; discussion 646–647. [DOI] [PubMed] [Google Scholar]
- 29.Hoh BL, Hosaka K, Downes DP, et al. Monocyte chemotactic protein-1 promotes inflammatory vascular repair of murine carotid aneurysms via a macrophage inflammatory protein-1alpha and macrophage inflammatory protein-2-dependent pathway. Circulation 2011;124:2243–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Velioglu M, Kizilkilic O, Selcuk H, et al. Early and midterm results of complex cerebral aneurysms treated with Silk stent. Neuroradiology 2012;54:1355–1365. [DOI] [PubMed] [Google Scholar]
- 31.Largen E, Balt-Extrusion. Urgent Field Safety Notice Intracranial Stent “SILK”. Clarification of the Indications. [online]. Available at: https://www.swissmedic.ch/recalllists_dl/03071/Vk_20100309_06-e1.pdf. [Google Scholar]
- 32.Kim YH, Jung JC, Jung SY, Kim YI, Lee KW, Park YJ. Cyclosporine A Downregulates MMP-3 and MMP-13 Expression in Cultured Pterygium Fibroblasts. Cornea 2015;34:1137–1143. [DOI] [PubMed] [Google Scholar]
- 33.Gawronska-Kozak B, Kirk-Ballard H. Cyclosporin A reduces matrix metalloproteinases and collagen expression in dermal fibroblasts from regenerative FOXN1 deficient (nude) mice. Fibrogenesis Tissue Repair 2013;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shetty R, Ghosh A, Lim RR, et al. Elevated expression of matrix metalloproteinase-9 and inflammatory cytokines in keratoconus patients is inhibited by cyclosporine A. Invest Ophthalmol Vis Sci 2015;56:738–750. [DOI] [PubMed] [Google Scholar]
- 35.Kuo PJ, Tu HP, Chin YT, et al. Cyclosporine-A inhibits MMP-2 and −9 activities in the presence of Porphyromonas gingivalis lipopolysaccharide: an experiment in human gingival fibroblast and U937 macrophage co-culture. J Periodontal Res 2012;47:431–438. [DOI] [PubMed] [Google Scholar]
- 36.Chiu HC, Lu YT, Chin YT, et al. Cyclosporine A inhibits the expression of membrane type-I matrix metalloproteinase in gingiva. J Periodontal Res 2009;44:338–347. [DOI] [PubMed] [Google Scholar]
- 37.Bolzani G, Della Coletta R, Martelli Junior H, Graner E. Cyclosporin A inhibits production and activity of matrix metalloproteinases by gingival fibroblasts. J Periodontal Res 2000;35:51–58. [DOI] [PubMed] [Google Scholar]
- 38.Dai J, Michineau S, Franck G, et al. Long term stabilization of expanding aortic aneurysms by a short course of cyclosporine A through transforming growth factor-beta induction. PLoS One 2011;6:e28903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ha E, Mun KC. Effects of cyclosporine on metalloproteinase in endothelial cells. Transplant Proc 2012;44:991–992. [DOI] [PubMed] [Google Scholar]
- 40.Huang YH, Ma YL, Ma L, et al. Cyclosporine A improves adhesion and invasion of mouse preimplantation embryos via upregulating integrin beta3 and matrix metalloproteinase-9. Int J Clin Exp Pathol 2014;7:1379–1388. [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou WH, Du MR, Dong L, et al. Cyclosporin A increases expression of matrix metalloproteinase 9 and 2 and invasiveness in vitro of the first-trimester human trophoblast cells via the mitogen-activated protein kinase pathway. Hum Reprod 2007;22:2743–2750. [DOI] [PubMed] [Google Scholar]
- 42.Bianchi R, Rodella L, Rezzani R. Cyclosporine A up-regulates expression of matrix metalloproteinase 2 and vascular endothelial growth factor in rat heart. Int Immunopharmacol 2003;3:427–433. [DOI] [PubMed] [Google Scholar]
- 43.Puffer C, Dai D, Ding YH, Cebral J, Kallmes D, Kadirvel R. Gene expression comparison of flow diversion and coiling in an experimental aneurysm model. J Neurointerv Surg 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Altes TA, Cloft HJ, Short JG, et al. 1999 ARRS Executive Council Award. Creation of saccular aneurysms in the rabbit: a model suitable for testing endovascular devices. American Roentgen Ray Society. AJR American journal of roentgenology 2000;174:349–354. [DOI] [PubMed] [Google Scholar]
- 45.Kallmes DF, Ding YH, Dai D, Kadirvel R, Lewis DA, Cloft HJ. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke 2007;38:2346–2352. [DOI] [PubMed] [Google Scholar]
- 46.Kallmes DF, Helm GA, Hudson SB, et al. Histologic evaluation of platinum coil embolization in an aneurysm model in rabbits. Radiology 1999;213:217–222. [DOI] [PubMed] [Google Scholar]
- 47.Toth M, Sohail A, Fridman R. Assessment of gelatinases (MMP-2 and MMP-9) by gelatin zymography. Methods Mol Biol 2012;878:121–135. [DOI] [PubMed] [Google Scholar]
- 48.Toth M, Fridman R. Assessment of Gelatinases (MMP-2 and MMP-9 by Gelatin Zymography. Methods Mol Med 2001;57:163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dunn OJ. Multiple Comparisons Using Rank Sums. Technometrics 1964;6:241–252. [Google Scholar]
- 50.Yamaguchi T, Yokokawa M, Suzuki M, et al. The effect of immunosuppression on aortic dilatation in a rat aneurysm model. Surg Today 2000;30:1093–1099. [DOI] [PubMed] [Google Scholar]
- 51.Piechota-Polanczyk A, Demyanets S, Nykonenko O, et al. Decreased tissue levels of cyclophilin A, a cyclosporine a target and phospho-ERK1/2 in simvastatin patients with abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 2013;45:682–688. [DOI] [PubMed] [Google Scholar]
- 52.Aoki T, Kataoka H, Ishibashi R, Nozaki K, Egashira K, Hashimoto N. Impact of monocyte chemoattractant protein-1 deficiency on cerebral aneurysm formation. Stroke; a journal of cerebral circulation 2009;40:942–951. [DOI] [PubMed] [Google Scholar]
- 53.Kanematsu Y, Kanematsu M, Kurihara C, et al. Critical roles of macrophages in the formation of intracranial aneurysm. Stroke; a journal of cerebral circulation 2011;42:173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ding YH, Dai D, Lewis DA, et al. Long-term patency of elastase-induced aneurysm model in rabbits. AJNR American journal of neuroradiology 2006;27:139–141. [PMC free article] [PubMed] [Google Scholar]
- 55.Gounis MJ, van der Bom IM, Wakhloo AK, et al. MR imaging of myeloperoxidase activity in a model of the inflamed aneurysm wall. AJNR Am J Neuroradiol 2015;36:146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Delbosc S, Alsac JM, Journe C, et al. Porphyromonas gingivalis participates in pathogenesis of human abdominal aortic aneurysm by neutrophil activation. Proof of concept in rats. PLoS One 2011;6:e18679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosenthal KL. Therapeutic contraindications in exotic pets. Seminars in Avian and Exotic Pet Medicine 2004;13:44–48. [Google Scholar]
- 58.Borgmann AR, Bogle D, Robb CA, McDonald TO. Comparative toxicity of two-dexamethasone derivatives following topical ocular instillation to rabbits. II. Systemic histopathological changes. Toxicology 1976;6:77–84. [DOI] [PubMed] [Google Scholar]
- 59.Tennant BC, Balazs T, Baldwin BH, et al. Assessment of hepatic function in rabbits with steroid-induced cholestatic liver injury. Fundam Appl Toxicol 1981;1:329–333. [DOI] [PubMed] [Google Scholar]
- 60.Sheng FC, Freischlag JA, Backstrom B, Kelly D, Busuttil RW. The effect of dexamethasone in vivo on blood and peritoneal neutrophils (PMN) in rabbits with peritonitis. J Surg Res 1987;43:296–301. [DOI] [PubMed] [Google Scholar]
- 61.Zhang B, Dhillon S, Geary I, et al. Polymorphisms in matrix metalloproteinase-1, −3, −9, and −12 genes in relation to subarachnoid hemorrhage. Stroke 2001;32:2198–2202. [DOI] [PubMed] [Google Scholar]


