Abstract
The drastic decline in coral coverage has stimulated an interest in reef restoration, and various iterations of coral nurseries have been used to augment restoration strategies. Here we examine the growth of two species of Hawaiian Montipora that were maintained in mesocosms under either ambient or warmed annual bleaching conditions for two consecutive years prior to outplanting to determine whether preconditioning aided coral restoration efforts. Using coral trees to create a nearby ocean nursery, we examined whether: (1) previous ex situ mesocosm growth would mirror in situ coral tree nursery growth; and (2) thermal ex situ stress-hardening would predict future success during natural warming events in situ for corals moved from tanks to trees. For Montipora capitata, we found that variation in growth was explained primarily by genotype; growth rates in the mesocosms were similar to those in situ, irrespective of preconditioning. Variation in M. flabellata growth, however, was explained by both genotype and culture method such that an individual M. flabellata colony that grew well in the tanks did not necessarily perform as well on the coral trees. For both species, previous exposure to elevated temperatures in the mesocosms provided no benefit to either growth or survival during a warming event in the coral tree nursery compared to those grown in ambient temperatures. Overall, M. capitata performed better in the tree nursery with higher net growth, lower mortality, and was subject to less predation than M. flabellata. Our results show little benefit of the additional cost and time of stress-hardening these corals prior to outplanting because it is unlikely to aid resilience to future warming events. These results also suggest that selecting corals for restoration based on long-term genotype growth performance may be more effective for optimal outcomes but should be weighed against other factors, such as coral morphology, in situ nursery method, location, and other characteristics.
Keywords: Resilience, Coral nursery, Assisted evolution, Coral tree, Coral farm, Adaptive potential, Acclimatization, Bleaching, Acropora, Reef restoration
Introduction
Coral reefs around the world are in decline from combined global and local environmental stressors such as climate change, nutrient pollution and runoff, sedimentation, and destructive fishing practices (Fabricius, 2005; Hoegh-Guldberg et al., 2007; Hughes et al., 2017; Jackson et al., 2001; Zaneveld et al., 2016). When one or more of these stressors are alleviated, it is possible that an area of degraded reef might be a candidate for restoration (Anthony, 2016; Bahr, Jokiel & Toonen, 2015; Jury & Toonen, 2019; van Oppen et al., 2017). Classic coral reef restoration projects have typically used ‘fragments of opportunity’ (broken coral fragments collected after a storm, boat grounding, or other mechanical disturbance) or other sources of asexual fragments, which are then grown in an underwater nursery (‘coral garden’) and later transplanted to the reef (Epstein, Bak & Rinkevich, 2003; Rinkevich, 2000; Rinkevich, 2014; Shafir, Van Rijn & Rinkevich, 2006). Corals in these in situ nurseries have been grown on cinder blocks, wire or PVC table structures, rope lines, and floating platforms (Boström-Einarsson et al., 2020; Young, Schopmeyer & Lirman, 2012). More recently, restoration strategies have expanded to include using planulae and/or settled sexual recruits to seed the reef (Boström-Einarsson et al., 2020; Chamberland et al., 2015; dela Cruz & Harrison, 2017) or small ‘microfragments’ attached to substrate, grown ex situ in holding tanks, and transplanted to the reef when those individuals have fused into a larger colony (Forsman et al., 2015; Page, Muller & Vaughan, 2018). However, because holding and caring for a coral in captivity for long periods of time increases the cost-per-unit and reduces the scope of the restoration effort, high financial costs and the small scale of restoration programs can limit effectiveness at the ecological scale (Guest et al., 2014; Young, Schopmeyer & Lirman, 2012).
Coral nursery practitioners, particularly those in Florida and the Caribbean, continue to develop techniques that show promise for lower cost, large scale reef restoration projects that can be exported to other locations around the world. A relatively simple vertical PVC and horizontal fiberglass rod structure secured to the ocean floor–dubbed ‘coral trees’–allows corals to be grown in situ via suspension by a monofilament line in the water column. This method reduces the burden of threats and potential sources of mortality characteristic of typical coral benthic habitat (sedimentation, algal competition, and even some predation) that plague early sexual recruits and small asexual fragments (Nedimyer, Gaines & Roach, 2011). While coral trees have demonstrated success in a few locations, the technique needs to be examined in other areas, for not all reef environments are the same, and potential modifications might need to be explored and tested.
Often larger sized coral colonies have an increased chance of survival than smaller counterparts (Becker & Mueller, 2001; Pausch et al., 2015). For large scale reef restoration projects with multi-tiered components, sexual recruits or small fragments that have been settled or grown ex situ in holding tanks and stabilized after a short period of time might benefit from a grow-out period in an in situ nursery (e.g., on coral trees) to further increase in size prior to outplanting, potentially increasing survivorship while keeping costs manageable (Guest et al., 2014; Lirman & Schopmeyer, 2016). It has been demonstrated in both ex situ and in situ culture methods that some individuals of a species tend to calcify faster than conspecifics, suggesting that genotype plays an important role in growth performance (Bahr et al., 2020; Drury, Manzello & Lirman, 2017; Jury & Toonen, 2019; Lohr & Patterson, 2017; O’Donnell et al., 2017). What is unclear is whether the growth performance from individuals in the ex situ nursery will indicate future success when transferred to the in situ ocean nursery and, eventually, to the reef (Edmunds & Putnam, 2020).
Further complicating restoration initiatives, rising ocean temperatures are a recurring threat to the persistence of reefs (Hughes et al., 2018). Repeated bleaching events increase the susceptibility of some species to future warming (Grottoli et al., 2014), but there is some evidence for potential acclimatization or adaptation to increasing temperatures if corals survive a warming event (Bahr, Rodgers & Jokiel, 2017; Coles et al., 2018; Guest et al., 2012; Jury & Toonen, 2019; Maynard et al., 2008). Trying to draw upon and propagate this variation, assorted intervention strategies have been proposed to augment restoration programs (van Oppen et al., 2017). Some methods include selective breeding (Chan et al., 2018; Quigley, Bay & van Oppen, 2020), assisted gene flow (Hagedorn et al., 2021), microbiome manipulation (Damjanovic et al., 2019; Riegl et al., 2011; Rosado et al., 2019), and preconditioning (Morikawa & Palumbi, 2019; Putnam & Gates, 2015) which are designed to increase tolerance to environmental pressures, such as increasing thermal stress (Kleypas et al., 2021). The practicality of stress hardening corals to adapt to future climate conditions is an active area of interest within the field of conservation and restoration.
In the present study, we sought to examine if the growth performance differences identified among coral genotypes cultured in mesocosms were retained when they were transferred back to the ocean during a transition grow-out period on coral trees and if thermal preconditioning while in the mesocosms affected their subsequent responses during a natural warming event. Found in a diverse array of Hawaiian reef habitats, Montipora capitata is a dominant species that has both branching and plating morphologies, whereas M. flabellata displays an encrusting growth form that is found primarily in shallow areas of high wave action and irradiance (Fenner, 2005; Hunter & Evans, 1995; Rodgers et al., 2015). Using small fragments of M. capitata and M. flabellata from a prior 2-year mesocosm study (Bahr et al., 2020; McLachlan et al., 2022; Timmers et al., 2021), we tracked the growth of corals on the trees through an additional year and compared that to their previous performance in the mesocosm system. Additionally, because subsets of corals were also grown under present-day average and high temperature conditions as part of the original study, we investigated what effect, if any, the exposure to heat stress had on growth rate and survival of corals from the high temperature tanks compared to those from the ambient system when exposed to a natural warming event while growing on the coral trees. Finally, we compared the overall general performance on the coral trees of the two species.
Methods
Previous experiment and coral history
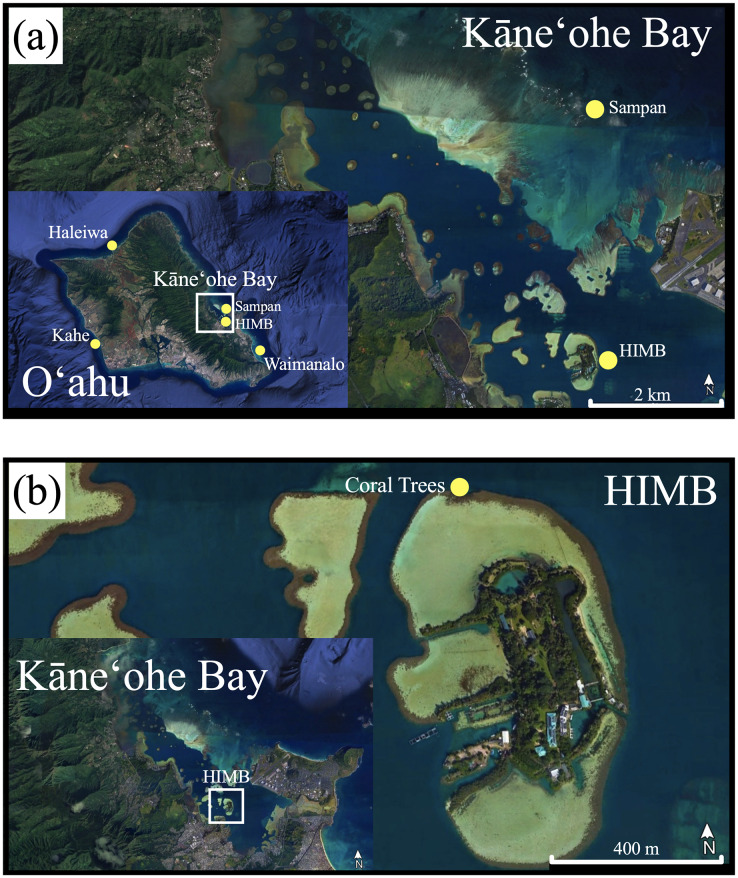
The corals used in this nursery experiment were obtained from a concluding study that were grown in mesocosms for approximately two years at the Hawai‘i Institute of Marine Biology in Kāne‘ohe Bay, Hawai‘i. Corals from the original ex situ mesocosm studies were collected from different locations around O‘ahu (Haleiwa, the reef around HIMB, Kahe, Sampan Channel, and Waimānalo; Fig. 1A) and housed in flow-through seawater tanks at HIMB. Over the 2-year mesocosm study, many of these corals grew into larger colonies from which fragments for the present study were derived (described in Bahr et al., 2020; McLachlan et al., 2022; Timmers et al., 2021).
Figure 1. Original coral collection and tree nursery.
(A) Map of O‘ahu with original collection sites of corals grown in mesocosms for the original study. (B) Coral tree nursery location on the north side of the HIMB reef in Kāne‘ohe Bay. (Google Earth Pro v. 7.3.3.7786, www.earth.google.com).
Briefly, each mesocosm from the previous experiment experienced natural daily and seasonal fluctuations in light, seawater temperature, and carbonate chemistry with the temperature treatments set to either the present-day 2-week average of O‘ahu (hereafter referred to as ambient) or +2 °C elevated seawater temperatures (high temperature) that simulated future ocean conditions with approximately 24 degree heating weeks per year of bleaching stress (McLachlan et al., 2022; Timmers et al., 2021). Buoyant weight converted to dry weight was used to determine growth (Jokiel, Maragos & Franzisket, 1978). Corals were originally collected under Special Activity Permit numbers SAP 2015-17 and SAP 2016-69, and the coral tree nursery experiment was conducted under Site Plan Approval SPA OA-17-45. All permits were issued by the State of Hawai‘i Department of Land and Natural Resources.
Colony fragmentation and coral tree deployment
In total, 10 coral trees were secured near HIMB in Kāne‘ohe Bay (21°43′769″N, 157°78′962″W), anchored at a depth of 4 m, and were installed in a line along the reef with alternating species per tree: M. capitata trees 1, 3, 5, 7, 9; M. flabellata trees 2, 4, 6, 8, 10 (Figs. 1B, 2A). For both species, one colony of each genet (genotype) remaining in both ambient and higher temperature treatments in the original mesocosm study (McLachlan et al., 2022; Timmers et al., 2021) was selected to fragment into ten smaller ramets (replicate fragments) to be deployed to the coral trees. Care was taken to make ramets from each colony approximately the same size, with each initially no larger than ~7 × 10 cm. Each ramet was tagged using the same colony site location, number, and treatment from the preceding study with an additional identifier applied to track each ramet. To be comparable to the original mesocosm study, corals were then weighed using buoyant weight (Mettler PM2000 scale; Mettler-Toledo, Columbus, OH, USA) that was later converted to dry weight (Jokiel, Maragos & Franzisket, 1978) and photographed (Nikon D810 with 50 mm lens; Nikon Inc., Melville, NY, USA) (Fig. 2B).
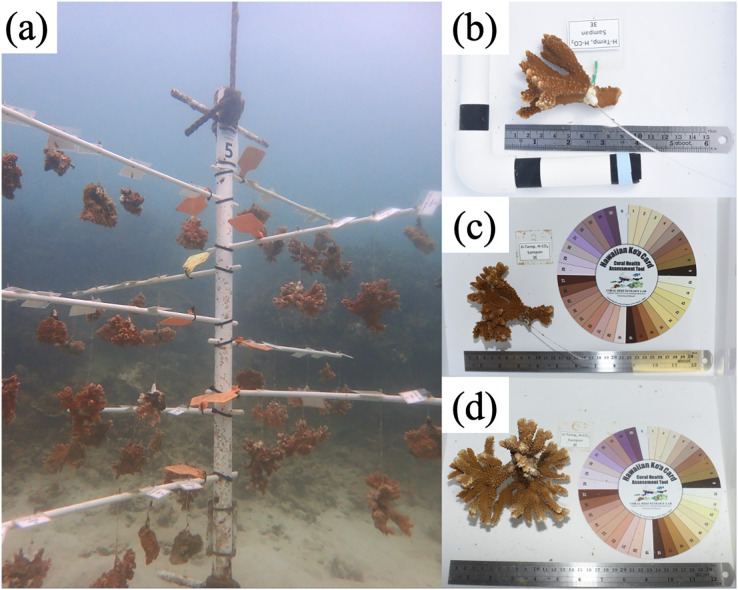
Figure 2. Coral tree and fragment growth.
(A) Coral tree at HIMB with tree number, branch number, coral ramet identification, and suspended corals characteristic of the method. Due to the shallow depth (4 m), the trees were shorter than typical coral trees anchored in deeper water and the number of corals per branch doubled from three to six. (B) Initial size of M. capitata ramet beginning in Jan/Feb 2019, (C) mid-point size Aug/Sept 2019, and (D) final size Feb/Mar 2020 of the same ramet. Note that the coral more than doubled in size as did its weight. Photo credits: author.
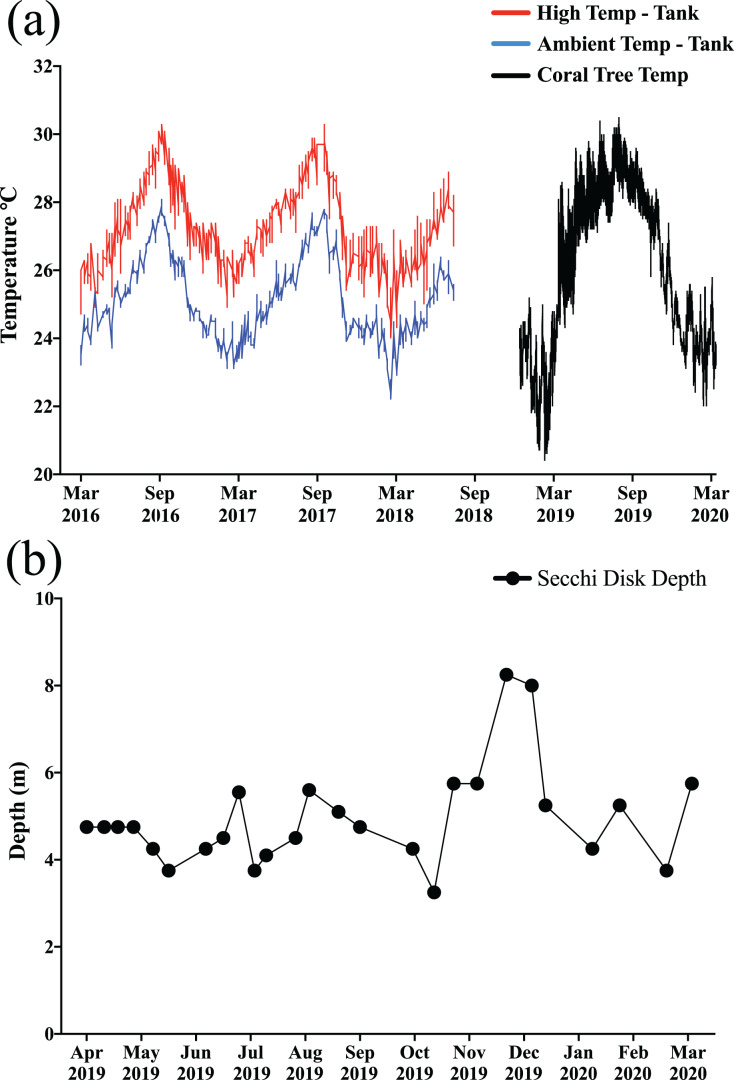
After weighing and photographing, ramets from each species were haphazardly selected and secured to coral tree branches (fiberglass rods) with monofilament line and crimp via the hanging arrangement per methods used by Nedimyer, Gaines & Roach (2011), resulting in a haphazard mixture of corals from each temperature treatment and mesocosm on each tree. In addition to each individual coral having a tag, each branch on each tree was also labeled, and the order of corals on each branch was recorded to ensure proper identification in the event that some tags were lost. Ramets of M. capitata (n = 216) were secured to the trees in late January through early February 2019 while those of M. flabellata (n = 290) were deployed the following month. The trees were inspected one to two times per month, and the few times fragments had fallen they were placed back on the tree. HOBO temperature loggers (HOBO Pendant© Temperature 64K Data Logger; Onset, Bourne, MA, USA) were used to record temperature, and Secchi disk measurements (Preisendorfer, 1986) were taken at least two to three times per month to monitor turbidity and water clarity (Figs. 3A and 3B).
Figure 3. Temperature and water clarity.
(A) The colored lines on the left side of the graph the portray the high (red) and ambient (blue) temperature profiles for the ex situ mesocosms (prior to these experiments), and the in situ temperatures (black) for the coral trees are on the right side of the graph. (B) Secchi disk turbidity readings near coral trees. The in situ exposure covered the entire range of temperatures that the corals experienced in their ex situ mesocosms prior to being transplanted onto the trees. Moreover, except for a few months during the winter, all of the trees experienced a relatively constant, but low light regime, due to turbidity in the bay.
At six months post deployment–throughout August and early September 2019–branches from individual trees were brought into and temporarily housed in tanks with flow-through seawater and 70% shade cloth. Corals were removed from the branches, photographed, and buoyant weights recorded. As part of the visual assessment, data were also recorded and estimated for tissue loss (partial/total mortality, if any), any paling or bleaching, and if there were signs of significant predation, approximately how much of the ramet was lost or if it had completely disappeared from the coral tree. Corals were redeployed to their respective location on each tree within four days of removal (Fig. 2C).
For many corals–particularly M. flabellata–there was a heavy infestation of oysters growing on portions of their skeleton where there was no live tissue (either from the initial deployment or if tissue had receded in situ). In many cases, the oysters would have artificially inflated the weight of a coral and were therefore removed prior to measurement. At 1-year (February/March 2020), the tree branches and corals were once again brought into the seawater system tanks and reassessed as described above (Fig. 2D). Since the corals on the trees were similar in size to the initial mesocosm fragments and were deployed for 1 year in the field, their buoyant weights were compared to those obtained during the first year of the mesocosm study, thereby helping to ensure comparability between studies.
Experiments and statistical analysis
Net growth (final weight–initial weight) of dry weight was used as the metric for calcification and normalized to the initial skeletal weight yielding a rate of mg g−1 day−1 (milligrams of skeletal weight increase per gram of initial skeletal weight per day). In order to test the question of growth performance in a land-based nursery as predictive of expected growth in ocean nurseries (ex situ vs in situ), only the corals from the ambient system were used for analysis. For M. capitata, 17 genotypes were compared across both the mesocosms and trees (N = 17, n = 50 mesocosm ramets, n = 81 tree ramets), and for M. flabellata there were 13 genotypes common to both culture methods (N = 13, n = 41 mesocosm ramets, n = 48 tree ramets). See Table S1 in Supplemental Information. A two-way ANOVA with culture method (tanks vs trees) and genotype as fixed factors followed by a Tukey’s HSD post hoc was fit for each species independently.
For the temperature stress-hardening analysis, only corals from the trees were used with genotypes that were common to both ambient and high temperature preconditioning histories from the previous experiment (M. capitata: N = 8 genotypes, n = 43 ambient temperature ramets, n = 45 high temperature ramets; M. flabellata: N = 8 genotypes, n = 32 ambient ramets, n = 26 high temperature ramets). See Table S2 in Supplemental Information. For the group (population) response comparison, the one-year net growth was split into two six-month increments, pre-heat stress (Jan/Feb 2019–Aug/Sept 2019) and post-heat stress (Aug/Sept 2019–Feb/Mar 2020). A two-way ANOVA with time period (pre- and post-heat stress) and temperature preconditioning treatment as fixed factors followed by a Tukey’s HSD post hoc was fit for each species independently. To assess if there was a response by genotype (individual) and previous temperature exposure, net growth for the whole year (Jan/Feb 2019–Feb/Mar 2020) was analyzed. For each species separately, a two-way ANOVA with genotype and temperature preconditioning treatment as fixed factors followed by a Tukey’s HSD post hoc was fit to test differences in growth, and a chi-square test was used to determine if there were differences in survivorship between ambient and high temperature groups.
At the midpoint and final assessments, corals were considered to be in good condition if they had normal looking coloration (not pale/bleached), had not experienced 25% (or more) tissue loss, and had not suffered predation of more than 25%. If there was evidence of predation or partial/whole ramet mortality at either assessment, those corals were excluded only from the net growth measurement portion of the analysis. For the ambient vs high temperature survival comparison, corals that died while on the trees were included. A between-species comparison of the overall number of corals surviving and remaining in good condition after a year on the trees was also examined with a chi square test. The calcification rate data for M. capitata culture method and temperature preconditioning were square root transformed to satisfy ANOVA assumptions; no data transformations were needed for M. flabellata. Normality assumptions were analyzed via diagnostic plots of the residuals and confirmed with a Shapiro-Wilk’s test. R version 3.5.3 (R Core Team, 2019) was used for ANOVAs and post hoc analyses, and chi-squares tests and graphics were made with GraphPad Prism 9 software (version 9.0.1; San Diego, CA).
Results
Mesocosm vs coral tree growth
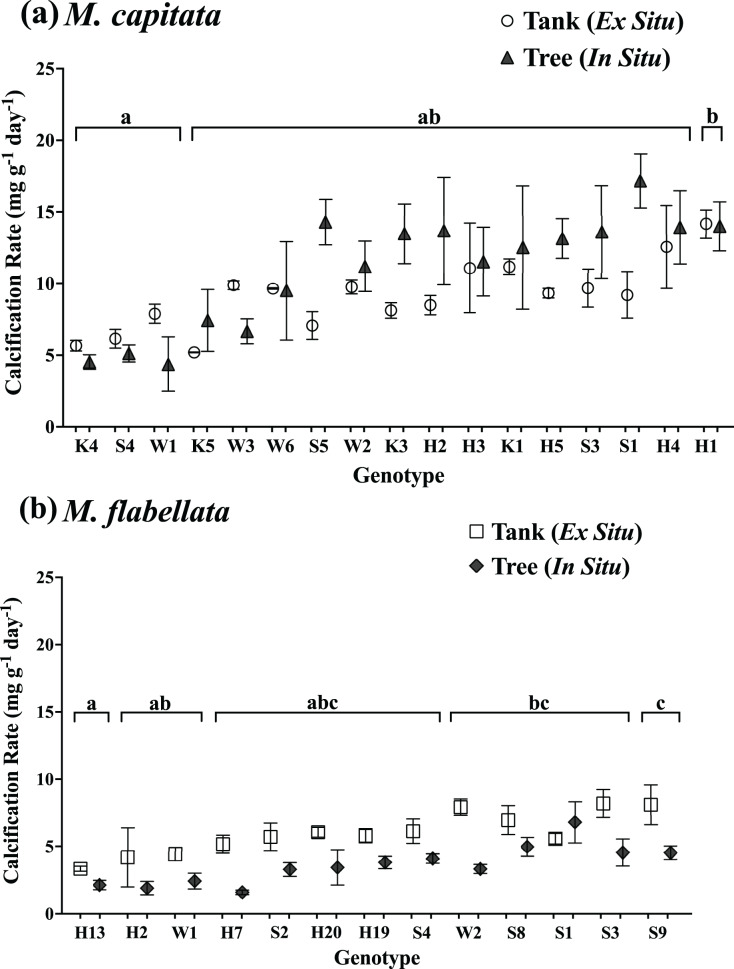
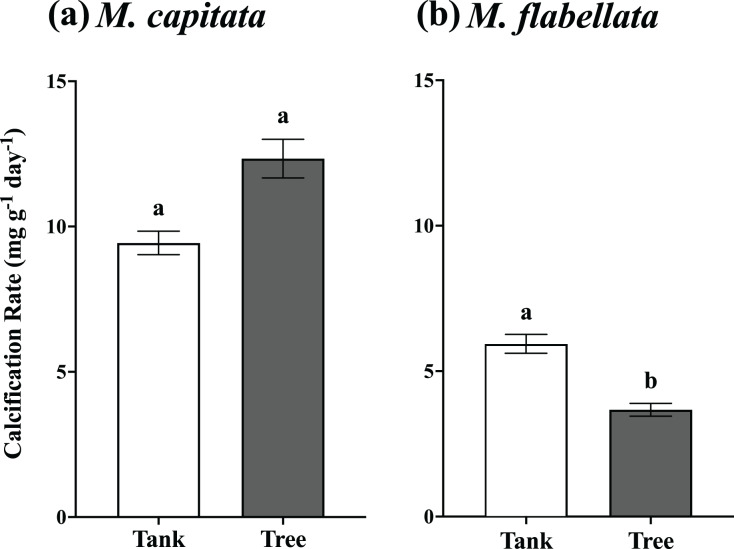
The buoyant weight of M. capitata and M. flabellata corals previously grown in mesocosms and then moved to a coral tree nursery was tracked in both systems. Differences in calcification rate for M. capitata were attributed primarily to genotype (F(16,97) = 3.1; p = 0.0003) rather than culture method. Neither effect of culture method (F(1,97) = 2.97; p = 0.09) nor the interaction of genotype with method (F(16,97) = 0.99; p = 0.476) were significant. A Tukey’s HSD post hoc test identified variation in growth among the genotypes (Fig. 4A; see Table S3 in Supplemental Information for ANOVA tables). When all the genotypes were combined, mean calcification rate on the trees for M. capitata (12.34 +/− 0.66 mg g−1 day−1) was trending greater than in the tanks (9.44 +/− 0.4 mg g−1 day−1), but it was not significantly greater (p = 0.09). See Table 1 and Fig. 5A.
Figure 4. Calcification rate of corals by genotype and culture method.
Comparison of calcification rate by coral genotype and method (tanks vs trees) after 1 year for (A) M. capitata: only genotype main effects were significant (p = 0.0003). (B) M. flabellata: the interaction of culture method and genotype was not significant (p = 0.0930), but the main effects of both genotype and method (p < 0.0001 for both) were. Variation in growth on the trees vs in mesocosms is explained primarily by genotype rather than culture method for M. capitata. For M. flabellata, growth was significantly impacted by culture method and genotype. Error bars are SE, and different letters above groups indicate significant difference in overall calcification genotypes.
Table 1. Growth (calcification rate) results of both species by culture method after 1 year in tanks (ex situ) vs on coral trees (in situ). The number of genotypes (N) and total number of pooled ramets (n) used for each analysis are provided.
| M. capitata | M. flabellata | ||||
|---|---|---|---|---|---|
| Tanks | Trees | Tanks | Trees | ||
| Culture method | Genotypes (N) Pooled ramets (n) |
N = 17 n = 50 |
N = 17 n = 81 |
N = 13 n = 41 |
N = 13 n = 48 |
| Calc. Rate (mg g−1 day−1) Mean ± SEM | 9.43 ± 0.404 | 12.34 ± 0.664 | 5.95 ± 0.308 | 3.72 ± 0.227 | |
Figure 5. One year mean calcification rate for all fragments, tanks vs trees.
(A) M. capitata: Calcification rate of all fragments on the trees is not significantly greater than tanks after 1 year but is trending toward significance (p = 0.088). (B) M. flabellata: Calcification rate of all fragments in the tanks is greater than on the trees after 1 year (p < 0.0001). The trees demonstrated marginally higher growth for M. capitata, while calcification rate for M. flabellata declined once on the trees. Error bars are SE, and different letters indicate significant difference.
For M. flabellata, both main effects, genotype (F(12,63) = 4.26; p < 0.0001) and culture method (F(1,63) = 61.1; p < 0.0001), were significant, but the interaction of genotype with method was not (F(12,63) = 1.68; p = 0.093). The post hoc analysis also identified significant differences in performance among various genotypes (Fig. 4B; see Table S3 Supplemental Information for ANOVA tables). When the genotypes were combined for M. flabellata, the corals grew better in the mesocosms after one year; mean calcification rate was greater in the tanks (5.95 +/− 0.31 mg g−1 day−1) than on the trees (3.72 +/− 0.23 mg g−1 day−1; p < 0.0001). See Table 1 and Fig. 5B (and Table S3 in Supplemental Information for ANOVA tables). For M. capitata, growth on the trees was relatively consistent with growth in the tanks, with variation explained mostly through genotype, whereas the growth of M. flabellata was significantly impacted by culture method as well as genotype.
Performance of outplanted, stress-hardened corals during a natural bleaching event
The maximum level of heat stress experienced by the corals in both experiments was relatively similar, reaching a peak of about 20–23 degree heating weeks (DHW, °C-weeks) in both the mesocosms and on the reef (Fig. 3A; Fig. S1 in Supplemental Information). While bleaching was observed in corals throughout the bay and on the HIMB reef where the trees were located during the course of this study, no M. capitata bleached and only one genotype of M. flabellata (Haleiwa-13) from the ambient temperature treatment history group visually paled in coloration. For both species, preconditioning the corals to high temperatures had no significant effect on growth in either species either before or after the natural bleaching event.
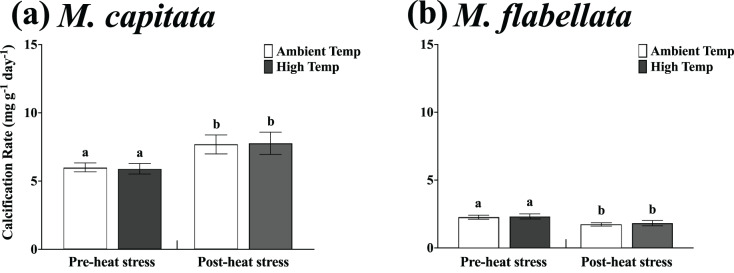
Specifically, the mean calcification rate of ambient and high temperature M. capitata corals on the trees from Jan-2019 to Aug-2019 (pre-heat stress) was 5.99 +/− 0.34 mg g−1 day−1 and 5.89 +/− 0.398 mg g−1 day−1, respectively. For the post-heat stress period (Aug-2019 to Feb-2020), the mean ambient and high temperature calcification rates were also not significantly different at 7.69 +/− 0.69 mg g−1 day−1 and 7.77 +/− 0.81 mg g−1 day−1, respectively (Table 2; Fig. 6A). A two-way ANOVA determined that there was no interaction of preconditioning with the pre- and post-heat stress time periods (F(1,172) = 0.02; p = 0.89), and ambient vs high temperature (preconditioning) treatment was not a significant factor (F(1,172) = 0.07; p = 0.79). The main effect for time period on the trees was significant, though near the threshold (F(1,172) = 4.29; p = 0.04), with calcification rate for both groups greater during the post-heat stress time period.
Table 2. Growth (calcification rate) and survival of temperature preconditioning groups while on coral trees (in situ only) pre- and post-heat stress. The number of genotypes (N) and total number of pooled ramets (n) used for each analysis are provided.
| M. capitata | M. flabellata | ||||
|---|---|---|---|---|---|
| Ambient temp | High temp | Ambient temp | High temp | ||
| Temperature group: Growth | Genotypes (N) Pooled ramets (n) |
N = 8 n = 43 |
N = 8 n = 45 |
N = 8 n = 32 |
N = 8 n = 26 |
| Calc. Rate (mg g−1 day−1) Mean ± SEM, Pre-heat stress |
5.99 ± 0.341 | 5.89 ± 0.398 | 2.26 ± 0.152 | 2.32 ± 0.191 | |
| Calc. Rate (mg g−1 day−1) Mean ± SEM, Post-heat stress |
7.69 ± 0.693 | 7.77 ± 0.814 | 1.71 ± 0.144 | 1.83 ± 0.195 | |
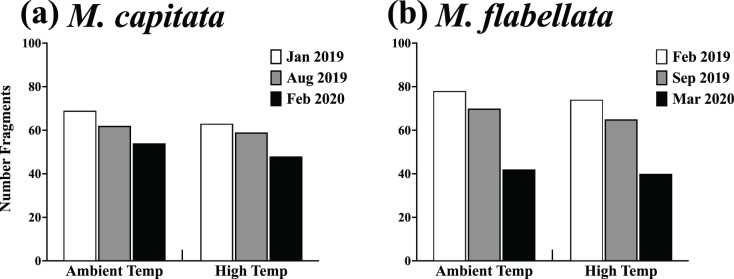
| Temperature group: Survival | Initial pooled ramets (n) Jan/Feb 2019 |
69 | 63 | 78 | 74 |
| Surviving pooled ramets (n) Aug/Sept 2019 |
62 | 59 | 70 | 65 | |
| Surviving pooled ramets (n) Feb/Mar 2020 |
54 | 48 | 42 | 40 | |
Figure 6. Calcification rate of ambient vs high temperature preconditioned corals on trees, pre- and post-heat stress.
Mean growth rate of each group of ambient and high temperature preconditioned corals on the trees in six-month increments (before and after a natural warming event) for (A) M. capitata and (B) M. flabellata. For both species there was no difference in growth between ambient or high temperature preconditioned populations for either time period; temperature preconditioning treatment while in the mesocosms did not benefit growth during an ex situ warming event (M. capitata: p = 0.792; M. flabellata: p = 0.604). The calcification rate of both groups of M. capitata was greater post-heat stress (p = 0.04) while the reverse was true for M. flabellata (p = 0.002). Error bars are SE, and different letters indicate significant difference.
For M. flabellata, the pre- heat stress mean calcification rate was 2.26 +/− 0.15 mg g−1 day−1 for ambient corals and 2.32 +/− 0.19 mg g−1 day−1 for high temperature fragments. The mean calcification rate declined during the post-heat stress period to 1.71 +/− 0.14 mg g−1 day−1 and 1.83 +/− 0.19 mg g−1 day−1 for the ambient and high treatment groups, respectively (Table 2; Fig. 6B). The two-way ANOVA determined that there was no interaction between preconditioning with the pre- and post-heat stress time periods (F(1,112) = 0.03; p = 0.856), and ambient versus high temperature (preconditioning) treatment, again, was also not a significant factor (F(1,112) = 0.27; p = 0.604). Time period on the trees, however, was significant (F(1,112) = 9.63; p = 0.002) indicating that the calcification rate for both groups significantly declined during the second six month, post-heat stress period compared to the first. For both species, growth of fragments was not influenced by previous temperature preconditioning treatment. The rate of calcification for both ambient and stress-hardened M. capitata increased post-heat stress, whereas M. flabellata had reduced calcification post-heat stress regardless of preconditioning, suggesting other factors impacted growth equally across treatments (Fig. 6). See Table S4 in Supplemental Information for two-way ANOVA details for both species.
There was also no difference in post-heat stress survival between preconditioning groups of either species. Specifically, a chi-square analysis for both M. capitata (Table 1; Fig. 7A, X2(2,355) = 0.066, p = 0.967) and M. flabellata (Table 1; Fig. 7B, X2(2,369) = 0.011, p = 0.994) determined that, for both species, there was no difference in survivorship between ambient and stress-hardened corals while on trees, despite the prior temperature conditions in the mesocosms and similar warming in the fall of 2019 (Fig. 3A; Fig. S1).
Figure 7. Survivorship of ambient and high temperature preconditioned corals on trees.
Results of a chi-square analysis of surviving fragments from ambient and high temperature preconditioned groups for both (A) M. capitata (X2(2,355) = 0.066, p = 0.967) and (B) M. flabellata X2(2,369) = 0.011, p = 0.994. For both species there was no difference in survivorship between ambient and high temperature preconditioned corals while on trees after a warming event. The exposure to increased ex situ temperatures while in the mesocosms did not appear to benefit the fragments of either species in situ during a time of elevated temperatures while on the coral trees.
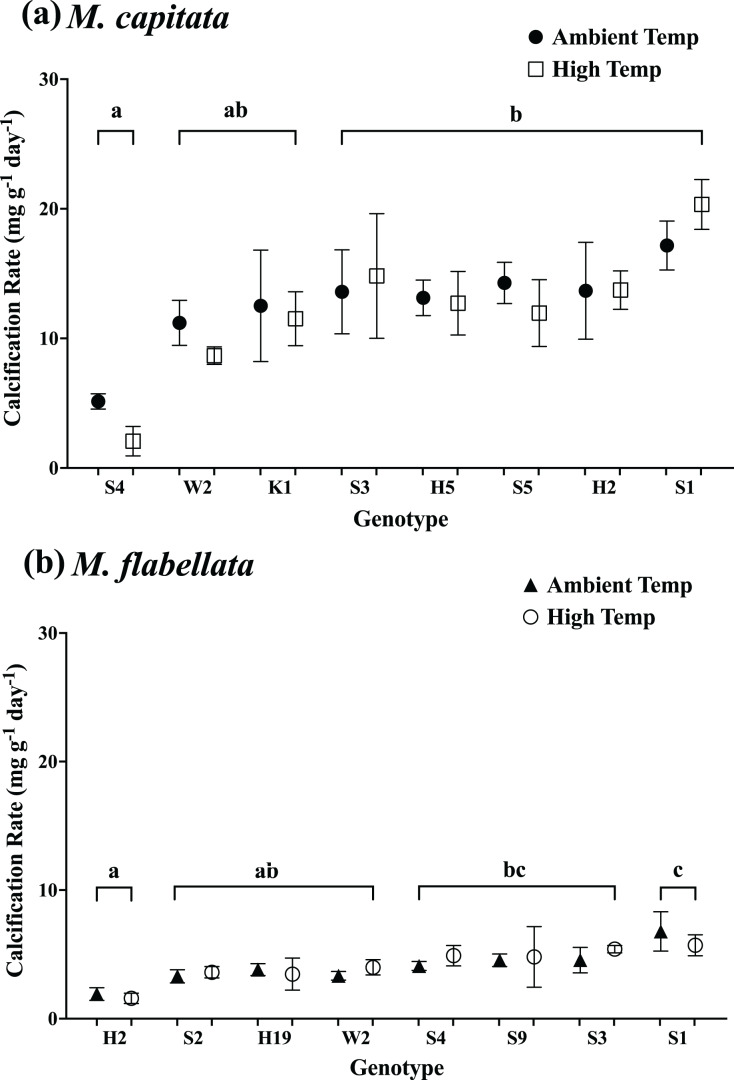
Additionally, for both species, when temperature preconditioning treatment was examined by genotype, the differences in growth between the ambient and high temperature corals were explained by genotype rather than temperature group. For M. capitata, neither the interaction of genotype with preconditioning temperature (F(7,72) = 0.36; p = 0.92) nor the temperature group main effects (F(1,72) = 0.05; p = 0.82) were significant, but genotype main effects were significant (F(7,72) = 4.44; p = 0.0004); a Tukey’s post hoc comparison identified a significant difference between genotypes (Fig. 8A; see Table S5 in Supplemental Information for ANOVA tables). Similarly, for M. flabellata there was no interaction of genotype with temperature preconditioning (F(7,42) = 0.39; p = 0.91), preconditioning main effects were not significant (F(1,42) = 0.48; p = 0.49), but genotype main effects were significant (F(7,42) = 5.4; p = 0.0002). The Tukey’s post hoc test found a significant difference between genotypes relative to the others (Fig. 8B; see Table S5 in Supplemental Information for ANOVA tables). For both species, stress-hardening had no effect on growth during a warming event in situ; the differences in growth were based upon genotype differences.
Figure 8. Calcification rate of corals by genotype and previous temperature treatment.
Calcification rate by coral genotype and temperature preconditioning group (ambient vs high temperature) after 1 year while on trees during a natural warming event. For both species, only genotype main effects were significant (A) M. capitata (p = 0.0004) and (B) M. flabellata (p = 0.0002). Temperature preconditioning main effects were not significant (M. capitata p = 0.82; M. flabellata p = 0.49), and there was no significant interaction between genotype and temperature group (p = 0.92 and p = 0.91, respectively). For both species, exposure to elevated temperatures while in mesocosms did not benefit growth during a heat stress event in the ocean nursery. Variation in growth is explained by genotype but not previous temperature stress-hardening exposure. Error bars are SE, and different letters indicate significant difference among genotypes.
General coral tree performance
Finally, there were far more M. flabellata than M. capitata ramets lost or damaged during these experiments. To examine this, we compared M. flabellata initial and final coral numbers remaining and in good condition to that of M. capitata. Corals were not considered in good condition if, during the year on the tree, they experienced any significant tissue loss or signs of predation (more than ~25% for either) or if they had died or were lost from the tree. At the initial deployment, all M. capitata (n = 216) and M. flabellata (n = 290) ramets were in good condition. After 1 year, 61% of M. capitata corals had survived, with 39% lost or considered not in good condition. By comparison, 14% of M. flabellata had survived, with a significantly greater 86% of ramets that were lost or in poor condition (X2(1,679) = 60.33, p < 0.0001).
Discussion
The devastation to and decline of reefs in the recent past (De’ath et al., 2012; Gardner et al., 2003; Pandolfi & Jackson, 2006) has spurred many in the field of marine conservation to shift focus from habitat protection (more passive, classic conservation biology) to more active intervention management or restoration ecology (Anthony et al., 2017; Epstein, Bak & Rinkevich, 2003; van Oppen et al., 2017). This shift has fueled the development of a number of novel strategies, with variations, that exist under the umbrella of conservation and restoration, including land-based aquaria and ocean-based nurseries that utilize fragments (asexual reproduction) and larval recruits (sexual reproduction) as strategies for restoration (Chamberland et al., 2015; dela Cruz & Harrison, 2017; Forsman et al., 2018; Guest et al., 2014; Nedimyer, Gaines & Roach, 2011; Page, Muller & Vaughan, 2018; Rinkevich, 2000; Ware et al., 2020). The concept of ‘coral gardening’ within an ocean nursery has become a familiar method used in restoration projects that has shown promise, and the overwhelming majority of corals used in nursery restoration have typically been fast growing, branching species (Boström-Einarsson et al., 2020; Forsman et al., 2012; Rinkevich, 2014).
One of the initial measures of a successful restoration program is not only survival of the outplanted corals but also rapid growth after relocation to the ocean (Boström-Einarsson et al., 2020). It is reasonable for a culture facility to want to target those individual genotypes that have demonstrated previous success. However, the confines of a maintained ex situ culture facility are substantially different from the selective pressures in the open ocean, and growth in aquaria or among time points might not translate to success on the reef (Edmunds & Putnam, 2020). The present study examined genotype performance, survivorship, and the effectiveness of stress-hardening in situ of M. capitata and M. flabellata corals that had known growth histories from an ex situ mesocosm nursery. These experiments addressed questions surrounding reef restoration, specifically: (1) is previous growth in an ex situ mesocosm system a predictor of growth in an in situ ocean nursery; and (2) does preconditioning corals to thermal stress in an ex situ culture facility benefit them in situ during a natural bleaching event?
Our results indicate that variation in growth was better explained through genotype than culture method for one of the species (M. capitata, Fig. 4A), and this was consistent with other studies that have found genotype to be a predictor of growth success in a nursery setting (Kuffner et al., 2017; Lirman et al., 2014; Lohr & Patterson, 2017; Morikawa & Palumbi, 2019; O’Donnell et al., 2017) and in a reciprocal outplant study (Drury, Manzello & Lirman, 2017). This expected result for M. capitata was tempered somewhat by genotype and culture method both providing variation in growth for M. flabellata, which complicates overall predictions (Fig. 4B). In general, if a colony of M. capitata grew well in our mesocosm nursery, it could be expected to perform similarly well when moved to a coral tree ocean nursery. These growth rates were also about three times higher than those reported in previous mesocosm studies, suggesting that differences in ex situ culture conditions such as water flow (Jokiel et al., 2008) and irradiance (Jury & Toonen, 2019) can dramatically affect calcification rates. Although some genotypes of M. flabellata grew well in both a mesocosm setting and an ocean nursery, a different nursery design (other than coral trees) that accommodates the encrusting growth formation could result in more comparable growth rates between ex situ and in situ methods.
There is some optimism that individual acclimatization or population adaptation of corals to rising ocean temperatures will drive reef resilience to climate change (Coles et al., 2018; Majerova et al., 2021; Middlebrook, Hoegh-Guldberg & Leggat, 2008; Thomas et al., 2018), and while there are probable limits (Ainsworth et al., 2016; Hughes et al., 2017), various intervention strategies are attempting to direct that effort (Hagedorn et al., 2021; Hancock et al., 2021; van Oppen et al., 2017; van Oppen et al., 2015). Because Kāne‘ohe Bay again experienced elevated temperatures and subsequent bleaching throughout the bay and around HIMB (where the nursery trees were located) during the summer and fall of 2019, we examined the response between individual and groups of corals with and without a history of previous exposure to elevated temperatures.
For both species during a warming event while on the trees, the preconditioning to heat stress (simulated stress events) did not have any beneficial impact on calcification rate or survival compared to those previously maintained in ambient mesocosm temperatures. Corals throughout the bay, including colonies near the section of reef where the coral trees were located, visually bleached; there was no paling or bleaching of M. capitata on the trees, and only one genotype of M. flabellata bleached. However, for both ambient and high temperature preconditioned groups of M. capitata, the calcification rate was greater in the six-month post-bleaching event period than prior (Fig. 6A). The opposite was true for M. flabellata, where growth was reduced in both groups after bleaching compared to the initial period (Fig. 6B). In addition, temperature treatment had no impact on growth of individual genotypes for both species (Fig. 8). The lack of preconditioning benefit was true at the population level (treatment group) as well as at the individual level (genotype) for both species despite similar level and duration of heat stress (Fig. 3A; Fig. S1). It is possible that these results are due to increased heterotrophic feeding by M. capitata under thermal stress (Grottoli, Rodrigues & Palardy, 2006) while the coral tree design and location were not optimal for M. flabellata.
Similarly, Middlebrook et al. (2012) found Acropora millepora colonies that were experimentally preconditioned to warmer temperatures were not more bleaching tolerant during the following bleaching event (though their symbionts did show improvement), yet the reverse was true for A. aspera (Middlebrook, Hoegh-Guldberg & Leggat, 2008); some Caribbean species have shown mixed results to repeated warming events (Grottoli et al., 2014). While the preconditioning of Pocillopora acuta (formerly identified as P. damicornis) has conveyed thermal tolerance to larvae via transgenerational acclimatization (Putnam & Gates, 2015), that effect was lost among the adults (Jury, Delano & Toonen, 2019). Conversely, Morikawa & Palumbi (2019) were able to construct a multispecies nursery that experienced less bleaching during a warming event by identifying specific colonies that were thermally tolerant in naturally-occurring areas of the reef, similar to our finding that genotype was the predominant significant factor in determining coral responses to these experiments. While individual genotypes of some species might be resilient to repeated warming, experimentally subjecting corals to simulated stress events in order to stress harden them to future warming or to select those individuals most likely to be “winners” appears not to be a practical strategy. This conclusion is corroborated by Barott et al. (2021) who likewise recently found no alteration of bleaching response among preconditioned corals in a reciprocal transplant experiment in Kāne‘ohe Bay. Taken together, thermally preconditioning or stress hardening corals would likely also not transfer to future generations.
As previously stated, the calcification rate for M. capitata was greater on the trees than in the mesocosms (though the difference was not statistically significant), while the reverse was true for M. flabellata, and there were far more M. flabellata lost while on the trees. The overall better success on the trees for M. capitata might be explained by the growth formation, natural history of the two species, and tree location in the bay. With its branching and plating morphology, M. capitata is a common, wide-ranging species found across variable reef habitats and depths; M. flabellata, however, is an encrusting species restricted to more narrow bands of reefs with high wave action and irradiance (Fenner, 2005; Hunter & Evans, 1995; Rodgers et al., 2015). To date, the most commonly used corals in restoration projects have been those with branching morphologies (Boström-Einarsson et al., 2020). Additionally, the permitted location for the coral trees had abundant wild M. capitata colonies but was not an ideal habitat for M. flabellata (Bahr, Jokiel & Toonen, 2015; Hunter & Evans, 1995; Richards Donà, 2019).
Within a few weeks of deployment, the coral trees (PVC frame, fiberglass branches, rope anchors) were inundated with filter feeding organisms (oysters, tunicates, sponges) rather than filamentous algae, and these organisms, in particular the oysters, would additionally recruit onto areas of the coral that did not have live tissue. Unless a portion of the fragment died while on the tree, this was typically not an issue for M. capitata. For M. flabellata, however, its encrusting morphology allowed the underside of the fragment to be exposed, and there was substantial recruitment of filter feeders, primarily oysters, while hanging from the branches. Unlike M. capitata, this unnatural exposure of the underside appeared to lead to heavy predation on the fragments of M. flabellata throughout the study–large sections of fragments missing and even the shearing of monofilament line whereupon whole fragments disappeared from the trees. It is likely, then, that the poor performance of M. flabellata on the trees was due to a combination of less-than-ideal habitat location and ill-suited morphology for the coral tree design that led to an increased likelihood of predation. While fish predation has also been identified as an obstacle for massive corals in other restoration efforts (Koval et al., 2020), M. flabellata might have better success in a nursery location near a more oceanic setting and utilizing the flat plate design for the coral tree branches rather than the line suspension method.
This study examined whether growth in an ex situ nursery could accurately predict in situ growth of fragments and whether there was a benefit of stress hardening corals prior to outplanting. We focused on growth and survival of corals in a coral tree ocean nursery and the role of genotype in nursery growth; however, there are more considerations for a nursery and restoration than growth alone. The Caribbean staghorn coral, Acropora cervicornis, is one of the most intensely studied species in restoration projects. Examinations of nursery and restored A. cervicornis have suggested the fastest growing genotypes of restored colonies might trade growth for thermal stress recovery (Ladd et al., 2017), a potential tradeoff of skeletal density with branch extension (Lohr & Patterson, 2017), and growth alone when moved from nursery to reef was not predictive of success (O’Donnell et al., 2018). Additionally, based on the outcome of this study and (Barott et al., 2021) the time, effort, and increased expense of thermal preconditioning to promote stress hardening is not warranted.
In a large, scaled-up restoration program, it may be cost prohibitive or logistically infeasible to track multiple metrics of every coral in the system. If restoration continues to be employed as a strategy for reef conservation, there is a need for rapid, low-cost and low-tech proxies for potential success to keep costs manageable (Guest et al., 2014; Morikawa & Palumbi, 2019; Young, Schopmeyer & Lirman, 2012), accompanied by more accurate and standardized metrics of growth and performance (Edmunds & Putnam, 2020). Baums et al. (2019) note that in addition to growth rate and genetic diversity, disease resistance, fecundity, partial tissue loss, rate of wound healing, symbiont variability, bleaching resistance/resilience, and other traits are all factors to consider. Reducing stressors must continue before significant population growth and recovery will occur (Ware et al., 2020). Even if fast-growing, acclimatized, or more thermally tolerant corals can be used in meaningful, large-scale successful restoration projects, such efforts will not justify complacency toward climate change mitigation.
Supplemental Information
Thermal stress experienced by corals in the high temperature mesocosms (ex situ tanks) and all corals while on the in situcoral trees expressed in degree heating weeks (DHW). Temperature stress reached a peak of approximately 20–23 DHW in both experiments. DHW are calculated using the mean monthly maximum (MMM) temperature baseline of 26.98 °C for the Main Hawaiian Islands from NOAA Coral Reef Watch with a nominal bleaching threshold of MMM+1 °C, per the methodology found in Skirving et al. (2020).
Acknowledgments
Thanks to Ken and Denise Nedimyer for their help with the coral tree training. Thank you to Kathy Wood, Katherine Hardy, and Riley Perry for their help with processing corals and deploying them into the field. Many thanks to committee members for their insight, review, and support. This publication is referenced as the Hawai‘i Institute of Marine Biology (HIMB) contribution number 1878.
Funding Statement
This research was supported by funds from the Smithsonian Conservation Biology Institute, the Hawai‘i Institute of Marine Biology, the Paul M. Angell Family Foundation, the FONZ Conservation Grant and Committee, Lord Scholarship Fund and HIMB Scholarship Committee, UH SOEST Denise B. Evans Fellowship, Roy and Patricia Disney Family Foundation, Volgenau Foundation, Roddenberry Foundation, Smithsonian Women’s Committee, Smithsonian Scholars Marine Afterschool Program, Smithsonian Youth Access Grant, and donations from Lou and Chosun Mastriani and Bob and Tamie Dewitt. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Coauthor Robert J. Toonen is an editor for PeerJ.
Author Contributions
E Michael Henley conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Jessica Bouwmeester performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Christopher P Jury conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Robert J. Toonen conceived and designed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Mariko Quinn performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Claire VA Lager performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Mary Hagedorn conceived and designed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw data is available in the Supplemental File.
References
- Ainsworth et al. (2016).Ainsworth TD, Heron SF, Ortiz JC, Mumby PJ, Grech A, Ogawa D, Eakin CM, Leggat W. Climate change disables coral bleaching protection on the Great Barrier Reef. Science. 2016;352(6283):338–342. doi: 10.1126/science.aac7125. [DOI] [PubMed] [Google Scholar]
- Anthony (2016).Anthony K. Coral reefs under climate change and ocean acidification: challenges and opportunities for management and policy. Annual Review of Environment and Resources. 2016;41(1):59–81. doi: 10.1146/annurev-environ-110615-085610. [DOI] [Google Scholar]
- Anthony et al. (2017).Anthony K, Bay LK, Costanza R, Firn J, Gunn J, Harrison P, Heyward A, Lundgren P, Mead D, Moore T, Mumby PJ, van Oppen MJH, Robertson J, Runge MC, Suggett DJ, Schaffelke B, Wachenfeld D, Walshe T. New interventions are needed to save coral reefs. Nature Ecology & Evolution. 2017;1(10):1420–1422. doi: 10.1038/s41559-017-0313-5. [DOI] [PubMed] [Google Scholar]
- Bahr, Jokiel & Toonen (2015).Bahr KD, Jokiel PL, Toonen RJ. The unnatural history of Kāne‘ohe Bay: coral reef resilience in the face of centuries of anthropogenic impacts. PeerJ. 2015;3(1):e950. doi: 10.7717/peerj.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr, Rodgers & Jokiel (2017).Bahr KD, Rodgers KS, Jokiel PL. Impact of three bleaching events on the reef resiliency of Kāne‘ohe Bay, Hawai‘i. Frontiers in Marine Science. 2017;4:398. doi: 10.3389/fmars.2017.00398. [DOI] [Google Scholar]
- Bahr et al. (2020).Bahr KD, Tran T, Jury CP, Toonen RJ. Abundance, size, and survival of recruits of the reef coral Pocillopora acuta under ocean warming and acidification. PLOS ONE. 2020;15(2):e0228168. doi: 10.1371/journal.pone.0228168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barott et al. (2021).Barott KL, Huffmyer AS, Davidson JM, Lenz EA, Matsuda SB, Hancock JR, Innis T, Drury C, Putnam HM, Gates RD. Coral bleaching response is unaltered following acclimatization to reefs with distinct environmental conditions. Proceedings of the National Academy of Sciences of the United States of America. 2021;118(22):e2025435118. doi: 10.1073/pnas.2025435118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baums et al. (2019).Baums IB, Baker AC, Davies SW, Grottoli AG, Kenkel CD, Kitchen SA, Kuffner IB, LaJeunesse TC, Matz MV, Miller MW, Parkinson JE, Shantz AA. Considerations for maximizing the adaptive potential of restored coral populations in the western Atlantic. Ecological Applications. 2019;29(8):e01978. doi: 10.1002/eap.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker & Mueller (2001).Becker L, Mueller E. The culture, transplantation and storage of Montastraea faveolata, Acropora cervicornis and Acropora palmata: what we have learned so far. Bulletin of Marine Science. 2001;69:881–896. [Google Scholar]
- Boström-Einarsson et al. (2020).Boström-Einarsson L, Babcock RC, Bayraktarov E, Ceccarelli D, Cook N, Ferse SCA, Hancock B, Harrison P, Hein M, Shaver E, Smith A, Suggett D, Stewart-Sinclair PJ, Vardi T, McLeod IM. Coral restoration–a systematic review of current methods, successes, failures and future directions. PLOS ONE. 2020;15(1):e0226631. doi: 10.1371/journal.pone.0226631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberland et al. (2015).Chamberland VF, Vermeij MJA, Brittsan M, Carl M, Schick M, Snowden S, Schrier A, Petersen D. Restoration of critically endangered elkhorn coral (Acropora palmata) populations using larvae reared from wild-caught gametes. Global Ecology and Conservation. 2015;4(7):526–537. doi: 10.1016/j.gecco.2015.10.005. [DOI] [Google Scholar]
- Chan et al. (2018).Chan WY, Peplow LM, Menéndez P, Hoffmann AA, van Oppen MJH. Interspecific hybridization may provide novel opportunities for coral reef restoration. Frontiers in Marine Science. 2018;5:1. doi: 10.3389/fmars.2018.00160. [DOI] [Google Scholar]
- Coles et al. (2018).Coles SL, Bahr KD, Rodgers KS, May SL, McGowan AE, Tsang A, Bumgarner J, Han JH. Evidence of acclimatization or adaptation in Hawaiian corals to higher ocean temperatures. PeerJ. 2018;6(3):e5347. doi: 10.7717/peerj.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damjanovic et al. (2019).Damjanovic K, van Oppen MJH, Menéndez P, Blackall LL. Experimental inoculation of coral recruits with marine bacteria indicates scope for microbiome manipulation in Acropora tenuis and Platygyra daedalea. Frontiers in Microbiology. 2019;10:3518. doi: 10.3389/fmicb.2019.01702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dela Cruz & Harrison (2017).dela Cruz DW, Harrison PL. Enhanced larval supply and recruitment can replenish reef corals on degraded reefs. Scientific Reports. 2017;7(1):13985. doi: 10.1038/s41598-017-14546-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De’ath et al. (2012).De’ath G, Fabricius KE, Sweatman H, Puotinen M. The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(44):17995–17999. doi: 10.1073/pnas.1208909109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury, Manzello & Lirman (2017).Drury C, Manzello D, Lirman D. Genotype and local environment dynamically influence growth, disturbance response and survivorship in the threatened coral, Acropora cervicornis. PLOS ONE. 2017;12(3):e0174000. doi: 10.1371/journal.pone.0174000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmunds & Putnam (2020).Edmunds PJ, Putnam HM. Science-based approach to using growth rate to assess coral performance and restoration outcomes. Biology Letters. 2020;16(7):20200227. doi: 10.1098/rsbl.2020.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein, Bak & Rinkevich (2003).Epstein N, Bak RPM, Rinkevich B. Applying forest restoration principles to coral reef rehabilitation. Aquatic Conservation: Marine and Freshwater Ecosystems. 2003;13(5):387–395. doi: 10.1002/aqc.558. [DOI] [Google Scholar]
- Fabricius (2005).Fabricius KE. Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Marine Pollution Bulletin. 2005;50(2):125–146. doi: 10.1016/j.marpolbul.2004.11.028. [DOI] [PubMed] [Google Scholar]
- Fenner (2005).Fenner DP. Corals of Hawai’i. A field guide to the hard, black, and soft corals of Hawai’i and the northwest Hawaiian Islands, including Midway. Honolulu: Mutual Publishing Company; 2005. [Google Scholar]
- Forsman et al. (2012).Forsman ZH, Kimokeo BK, Bird CE, Hunter CL, Toonen RJ. Coral farming: effects of light, water motion and artificial foods: coral reefs, the aquarium trade and the maritime industry. Journal of the Marine Biological Association of the United Kingdom. 2012;92(4):721–729. doi: 10.1017/S0025315411001500. [DOI] [Google Scholar]
- Forsman et al. (2018).Forsman ZH, Maurin P, Parry M, Chung A, Sartor C, Hixon MA, Hughes K, Rodgers KS, Knapp ISS, Gulko DA, Franklin EC, Del Rio Torres L, Chan NT, Wolke CS, Gates RD, Toonen RJ. The first Hawai’i workshop for coral restoration & nurseries. Marine Policy. 2018;96(12):133–135. doi: 10.1016/j.marpol.2018.08.009. [DOI] [Google Scholar]
- Forsman et al. (2015).Forsman ZH, Page CA, Toonen RJ, Vaughan D. Growing coral larger and faster: micro-colony-fusion as a strategy for accelerating coral cover. PeerJ. 2015;3(1):e1313. doi: 10.7717/peerj.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner et al. (2003).Gardner TA, Cote IM, Gill JA, Grant A, Watkinson AR. Long-term region-wide declines in Caribbean corals. Science. 2003;301(5635):958–960. doi: 10.1126/science.1086050. [DOI] [PubMed] [Google Scholar]
- Grottoli, Rodrigues & Palardy (2006).Grottoli AG, Rodrigues LJ, Palardy JE. Heterotrophic plasticity and resilience in bleached corals. Nature. 2006;440(7088):1186–1189. doi: 10.1038/nature04565. [DOI] [PubMed] [Google Scholar]
- Grottoli et al. (2014).Grottoli AG, Warner ME, Levas SJ, Aschaffenburg MD, Schoepf V, McGinley M, Baumann J, Matsui Y. The cumulative impact of annual coral bleaching can turn some coral species winners into losers. Global Change Biology. 2014;20(12):3823–3833. doi: 10.1111/gcb.12658. [DOI] [PubMed] [Google Scholar]
- Guest et al. (2012).Guest JR, Baird AH, Maynard JA, Muttaqin E, Edwards AJ, Campbell SJ, Yewdall K, Affendi YA, Chou LM. Contrasting patterns of coral bleaching susceptibility in 2010 suggest an adaptive response to thermal stress. PLOS ONE. 2012;7(3):e33353. doi: 10.1371/journal.pone.0033353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest et al. (2014).Guest JR, Baria MV, Gomez ED, Heyward AJ, Edwards AJ. Closing the circle: is it feasible to rehabilitate reefs with sexually propagated corals? Coral Reefs. 2014;33(1):45–55. doi: 10.1007/s00338-013-1114-1. [DOI] [Google Scholar]
- Hagedorn et al. (2021).Hagedorn M, Page CA, O’Neil KL, Flores DM, Tichy L, Conn T, Chamberland VF, Lager C, Zuchowicz N, Lohr K, Blackburn H, Vardi T, Moore J, Moore T, Baums IB, Vermeij MJA, Marhaver KL. Assisted gene flow using cryopreserved sperm in critically endangered coral. Proceedings of the National Academy of Sciences of the United States of America. 2021;118(38):e2110559118. doi: 10.1073/pnas.2110559118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock et al. (2021).Hancock JR, Barrows AR, Roome TC, Huffmyer AS, Matsuda SB, Munk NJ, Rahnke SA, Drury C. Coral husbandry for ocean futures: leveraging abiotic factors to increase survivorship, growth, and resilience in juvenile Montipora capitata. Marine Ecology Progress Series. 2021;657:123–133. doi: 10.3354/meps13534. [DOI] [Google Scholar]
- Hoegh-Guldberg et al. (2007).Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, Harvell CD, Sale PF, Edwards AJ, Caldeira K, Knowlton N, Eakin CM, Iglesias-Prieto R, Muthiga N, Bradbury RH, Dubi A, Hatziolos ME. Coral reefs under rapid climate change and ocean acidification. Science. 2007;318(5857):1737–1742. doi: 10.1126/science.1152509. [DOI] [PubMed] [Google Scholar]
- Hughes et al. (2018).Hughes TP, Anderson KD, Connolly SR, Heron SF, Kerry JT, Lough JM, Baird AH, Baum JK, Berumen ML, Bridge TC, Claar DC, Eakin CM, Gilmour JP, Graham NAJ, Harrison H, Hobbs J-PA, Hoey AS, Hoogenboom M, Lowe RJ, McCulloch MT, Pandolfi JM, Pratchett M, Schoepf V, Torda G, Wilson SK. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science. 2018;359(6371):80–83. doi: 10.1126/science.aan8048. [DOI] [PubMed] [Google Scholar]
- Hughes et al. (2017).Hughes TP, Kerry JT, Álvarez-Noriega M, Álvarez-Romero JG, Anderson KD, Baird AH, Babcock RC, Beger M, Bellwood DR, Berkelmans R, Bridge TC, Butler IR, Byrne M, Cantin NE, Comeau S, Connolly SR, Cumming GS, Dalton SJ, Diaz-Pulido G, Eakin CM, Figueira WF, Gilmour JP, Harrison HB, Heron SF, Hoey AS, Hobbs J-PA, Hoogenboom MO, Kennedy EV, C-y Kuo, Lough JM, Lowe RJ, Liu G, McCulloch MT, Malcolm HA, McWilliam MJ, Pandolfi JM, Pears RJ, Pratchett MS, Schoepf V, Simpson T, Skirving WJ, Sommer B, Torda G, Wachenfeld DR, Willis BL, Wilson SK. Global warming and recurrent mass bleaching of corals. Nature. 2017;543(7645):373–377. doi: 10.1038/nature21707. [DOI] [PubMed] [Google Scholar]
- Hunter & Evans (1995).Hunter CL, Evans CW. Coral reefs in Kaneohe Bay, Hawaii: two centuries of western influence and two decades of data. Bulletin of Marine Science. 1995;57:501–515. [Google Scholar]
- Jackson et al. (2001).Jackson JBC, Kirby MX, Berger WH, Bjorndal KA, Botsford LW, Bourque BJ, Bradbury RH, Cooke R, Erlandson J, Estes JA, Hughes TP, Kidwell S, Lange CB, Lenihan HS, Pandolfi JM, Peterson CH, Steneck RS, Tegner MJ, Warner RR. Historical overfishing and the recent collapse of coastal ecosystems. Science. 2001;293(5530):629–637. doi: 10.1126/science.1059199. [DOI] [PubMed] [Google Scholar]
- Jokiel, Maragos & Franzisket (1978).Jokiel PL, Maragos JE, Franzisket L. Coral growth: buoyant weight technique. In: Stoddart DR, Johannes RE, editors. Coral Reefs: Research Methods. Paris, France: UNESCO; 1978. pp. 529–541. [Google Scholar]
- Jokiel et al. (2008).Jokiel PL, Rodgers KS, Kuffner IB, Andersson AJ, Cox EF, Mackenzie FT. Ocean acidification and calcifying reef organisms: a mesocosm investigation. Coral Reefs. 2008;27(3):473–483. doi: 10.1007/s00338-008-0380-9. [DOI] [Google Scholar]
- Jury, Delano & Toonen (2019).Jury CP, Delano MN, Toonen RJ. High heritability of coral calcification rates and evolutionary potential under ocean acidification. Scientific Reports. 2019;9(1):20419. doi: 10.1038/s41598-019-56313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jury & Toonen (2019).Jury CP, Toonen RJ. Adaptive responses and local stressor mitigation drive coral resilience in warmer, more acidic oceans. Proceedings of the Royal Society B: Biological Sciences. 2019;286(1902):20190614. doi: 10.1098/rspb.2019.0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleypas et al. (2021).Kleypas J, Allemand D, Anthony K, Baker AC, Beck MW, Hale LZ, Hilmi N, Hoegh-Guldberg O, Hughes T, Kaufman L, Kayanne H, Magnan AK, McLeod E, Mumby P, Palumbi S, Richmond RH, Rinkevich B, Steneck RS, Voolstra CR, Wachenfeld D, Gattuso J-P. Designing a blueprint for coral reef survival. Biological Conservation. 2021;257:109107. doi: 10.1016/j.biocon.2021.109107. [DOI] [Google Scholar]
- Koval et al. (2020).Koval G, Rivas N, D’Alessandro M, Hesley D, Santos R, Lirman D. Fish predation hinders the success of coral restoration efforts using fragmented massive corals. PeerJ. 2020;8(2):e9978. doi: 10.7717/peerj.9978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffner et al. (2017).Kuffner IB, Bartels E, Stathakopoulos A, Enochs IC, Kolodziej G, Toth LT, Manzello DP. Plasticity in skeletal characteristics of nursery-raised staghorn coral, Acropora cervicornis. Coral Reefs. 2017;36(3):679–684. doi: 10.1007/s00338-017-1560-2. [DOI] [Google Scholar]
- Ladd et al. (2017).Ladd MC, Shantz AA, Bartels E, Burkepile DE. Thermal stress reveals a genotype-specific tradeoff between growth and tissue loss in restored Acropora cervicornis. Marine Ecology Progress Series. 2017;572:129–139. doi: 10.3354/meps12169. [DOI] [Google Scholar]
- Lirman & Schopmeyer (2016).Lirman D, Schopmeyer S. Ecological solutions to reef degradation: optimizing coral reef restoration in the Caribbean and Western Atlantic. PeerJ. 2016;4(2):e2597. doi: 10.7717/peerj.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lirman et al. (2014).Lirman D, Schopmeyer S, Galvan V, Drury C, Baker AC, Baums IB. Growth dynamics of the threatened caribbean staghorn coral Acropora cervicornis: influence of host genotype, symbiont identity, colony size, and environmental setting. PLOS ONE. 2014;9(9):e107253. doi: 10.1371/journal.pone.0107253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr & Patterson (2017).Lohr KE, Patterson JT. Intraspecific variation in phenotype among nursery-reared staghorn coral Acropora cervicornis (Lamarck, 1816) Journal of Experimental Marine Biology and Ecology. 2017;486:87–92. doi: 10.1016/j.jembe.2016.10.005. [DOI] [Google Scholar]
- Majerova et al. (2021).Majerova E, Carey FC, Drury C, Gates RD. Preconditioning improves bleaching tolerance in the reef-building coral Pocillopora acuta through modulations in the programmed cell death pathways. Molecular Ecology. 2021;00(14):1–15. doi: 10.1111/mec.15988. [DOI] [PubMed] [Google Scholar]
- Maynard et al. (2008).Maynard JA, Anthony KRN, Marshall PA, Masiri I. Major bleaching events can lead to increased thermal tolerance in corals. Marine Biology. 2008;155(2):173–182. doi: 10.1007/s00227-008-1015-y. [DOI] [Google Scholar]
- McLachlan et al. (2022).McLachlan RH, Price JT, Muñoz-Garcia A, Weisleder NL, Levas SJ, Jury CP, Toonen RJ, Grottoli AG. Physiological acclimatization in Hawaiian corals following a 22-month shift in baseline seawater temperature and pH. Scientific Reports. 2022;12(3712) doi: 10.1038/s41598-022-06896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrook et al. (2012).Middlebrook R, Anthony KRN, Hoegh-Guldberg O, Dove S. Thermal priming affects symbiont photosynthesis but does not alter bleaching susceptibility in Acropora millepora. Journal of Experimental Marine Biology and Ecology. 2012;432-433(14):64–72. doi: 10.1016/j.jembe.2012.07.005. [DOI] [Google Scholar]
- Middlebrook, Hoegh-Guldberg & Leggat (2008).Middlebrook R, Hoegh-Guldberg O, Leggat W. The effect of thermal history on the susceptibility of reef-building corals to thermal stress. Journal of Experimental Biology. 2008;211(7):1050–1056. doi: 10.1242/jeb.013284. [DOI] [PubMed] [Google Scholar]
- Morikawa & Palumbi (2019).Morikawa MK, Palumbi SR. Using naturally occurring climate resilient corals to construct bleaching-resistant nurseries. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(21):10586–10591. doi: 10.1073/pnas.1721415116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedimyer, Gaines & Roach (2011).Nedimyer K, Gaines K, Roach S. Coral Tree Nursery©: an innovative approach to growing corals in an ocean-based field nursery. AACL Bioflux. 2011;4:442–446. [Google Scholar]
- O’Donnell et al. (2017).O’Donnell KE, Lohr KE, Bartels E, Patterson JT. Evaluation of staghorn coral (Acropora cervicornis, Lamarck 1816) production techniques in an ocean-based nursery with consideration of coral genotype. Journal of Experimental Marine Biology and Ecology. 2017;487:53–58. doi: 10.1016/j.jembe.2016.11.013. [DOI] [Google Scholar]
- O’Donnell et al. (2018).O’Donnell KE, Lohr KE, Bartels E, Baums IB, Patterson JT. Acropora cervicornis genet performance and symbiont identity throughout the restoration process. Coral Reefs. 2018;37(4):1109–1118. doi: 10.1007/s00338-018-01743-y. [DOI] [Google Scholar]
- Page, Muller & Vaughan (2018).Page CA, Muller EM, Vaughan DE. Microfragmenting for the successful restoration of slow growing massive corals. Ecological Engineering. 2018;123:86–94. doi: 10.1016/j.ecoleng.2018.08.017. [DOI] [Google Scholar]
- Pandolfi & Jackson (2006).Pandolfi JM, Jackson JBC. Ecological persistence interrupted in Caribbean coral reefs. Ecology Letters. 2006;9(7):818–826. doi: 10.1111/j.1461-0248.2006.00933.x. [DOI] [PubMed] [Google Scholar]
- Pausch et al. (2015).Pausch RE, Miller M, Williams DE, Bright AJ. Effects of outplant size on Acropora palmata fragment survivorship, growth, and condition. 2015. Technical Report. Miami, FL: Protected Resources and Biodiversity Division, National Oceanic and Atmospheric Administration.
- Preisendorfer (1986).Preisendorfer RW. Secchi disk science: visual optics of natural waters. Limnology and Oceanography. 1986;31(5):909–926. doi: 10.4319/lo.1986.31.5.0909. [DOI] [Google Scholar]
- Putnam & Gates (2015).Putnam HM, Gates RD. Preconditioning in the reef-building coral Pocillopora damicornis and the potential for trans-generational acclimatization in coral larvae under future climate change conditions. The Journal of Experimental Biology. 2015;218(15):2365–2372. doi: 10.1242/jeb.123018. [DOI] [PubMed] [Google Scholar]
- Quigley, Bay & van Oppen (2020).Quigley KM, Bay LK, van Oppen MJH. Genome-wide SNP analysis reveals an increase in adaptive genetic variation through selective breeding of coral. Molecular Ecology. 2020;29(12):2176–2188. doi: 10.1111/mec.15482. [DOI] [PubMed] [Google Scholar]
- R Core Team (2019).R Core Team R: a language and environment for statistical computing. 2019. http://www.R-project.org/ http://www.R-project.org/ Vienna, Austria: R Foundation for Statistical Computing.
- Richards Donà (2019).Richards Donà A. Investigation into the functional role of chromoproteins in the physiology and ecology of the Hawaiian stony coral Montipora flabellata in Kāne‘ohe Bay, O‘ahuDoctoral Dissertation. 2019. University of Hawai‘i at Mānoa.
- Riegl et al. (2011).Riegl BM, Purkis SJ, Al-Cibahy AS, Abdel-Moati MA, Hoegh-Guldberg O. Present limits to heat-adaptability in corals and population-level responses to climate extremes. PLOS ONE. 2011;6(9):e24802. doi: 10.1371/journal.pone.0024802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkevich (2000).Rinkevich B. Steps towards the evaluation of coral reef restoration by using small branch fragments. Marine Biology. 2000;136(5):807–812. doi: 10.1007/s002270000293. [DOI] [Google Scholar]
- Rinkevich (2014).Rinkevich B. Rebuilding coral reefs: does active reef restoration lead to sustainable reefs? Current Opinion in Environmental Sustainability. 2014;7:28–36. doi: 10.1016/j.cosust.2013.11.018. [DOI] [Google Scholar]
- Rodgers et al. (2015).Rodgers KS, Jokiel PL, Brown EK, Hau S, Sparks R. Over a decade of change in spatial and temporal dynamics of hawaiian coral reef communities. Pacific Science. 2015;69(1):1–13. doi: 10.2984/69.1.1. [DOI] [Google Scholar]
- Rosado et al. (2019).Rosado PM, Leite DCA, Duarte GAS, Chaloub RM, Jospin G, Nunes da Rocha U, Saraiva PJ, Dini-Andreote F, Eisen JA, Bourne DG, Peixoto RS. Marine probiotics: increasing coral resistance to bleaching through microbiome manipulation. The ISME Journal. 2019;13(4):921–936. doi: 10.1038/s41396-018-0323-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafir, Van Rijn & Rinkevich (2006).Shafir S, Van Rijn J, Rinkevich B. Steps in the construction of underwater coral nursery, an essential component in reef restoration acts. Marine Biology. 2006;149(3):679–687. doi: 10.1007/s00227-005-0236-6. [DOI] [Google Scholar]
- Thomas et al. (2018).Thomas L, Rose NH, Bay RA, López EH, Morikawa MK, Ruiz-Jones L, Palumbi SR. Mechanisms of thermal tolerance in reef-building corals across a fine-grained environmental mosaic: lessons from Ofu, American Samoa. Frontiers in Marine Science. 2018;4:434. doi: 10.3389/fmars.2017.00434. [DOI] [Google Scholar]
- Timmers et al. (2021).Timmers MA, Jury CP, Vicente J, Bahr KD, Webb MK, Toonen RJ. Biodiversity of coral reef cryptobiota shuffles but does not decline under the combined stressors of ocean warming and acidification. Proceedings of the National Academy of Sciences of the United States of America. 2021;118(39):e2103275118. doi: 10.1073/pnas.2103275118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oppen et al. (2017).van Oppen MJH, Gates RD, Blackall LL, Cantin N, Chakravarti LJ, Chan WY, Cormick C, Crean A, Damjanovic K, Epstein H, Harrison PL, Jones TA, Miller M, Pears RJ, Peplow LM, Raftos DA, Schaffelke B, Stewart K, Torda G, Wachenfeld D, Weeks AR, Putnam HM. Shifting paradigms in restoration of the world’s coral reefs. Global Change Biology. 2017;23(9):3437–3448. doi: 10.1111/gcb.13647. [DOI] [PubMed] [Google Scholar]
- van Oppen et al. (2015).van Oppen MJH, Oliver JK, Putnam HM, Gates RD. Building coral reef resilience through assisted evolution. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(8):2307–2313. doi: 10.1073/pnas.1422301112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware et al. (2020).Ware M, Garfield EN, Nedimyer K, Levy J, Kaufman L, Precht W, Winters RS, Miller SL. Survivorship and growth in staghorn coral (Acropora cervicornis) outplanting projects in the Florida Keys National Marine Sanctuary. PLOS ONE. 2020;15(5):e0231817. doi: 10.1371/journal.pone.0231817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, Schopmeyer & Lirman (2012).Young CN, Schopmeyer S, Lirman D. A review of reef restoration and coral propagation using the threatened genus Acropora in the caribbean and western Atlantic. Bulletin of Marine Science. 2012;88(4):1075–1098. doi: 10.5343/bms.2011.1143. [DOI] [Google Scholar]
- Zaneveld et al. (2016).Zaneveld JR, Burkepile DE, Shantz AA, Pritchard CE, McMinds R, Payet JP, Welsh R, Correa AMS, Lemoine NP, Rosales S, Fuchs C, Maynard JA, Thurber RV. Overfishing and nutrient pollution interact with temperature to disrupt coral reefs down to microbial scales. Nature Communications. 2016;7(1):11833. doi: 10.1038/ncomms11833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Thermal stress experienced by corals in the high temperature mesocosms (ex situ tanks) and all corals while on the in situcoral trees expressed in degree heating weeks (DHW). Temperature stress reached a peak of approximately 20–23 DHW in both experiments. DHW are calculated using the mean monthly maximum (MMM) temperature baseline of 26.98 °C for the Main Hawaiian Islands from NOAA Coral Reef Watch with a nominal bleaching threshold of MMM+1 °C, per the methodology found in Skirving et al. (2020).
Data Availability Statement
The following information was supplied regarding data availability:
The raw data is available in the Supplemental File.