Abstract
We propose an initial explanation for how myalgic encephalomyelitis / chronic fatigue syndrome (ME/CFS) could originate and perpetuate by drawing on findings from critical illness research. Specifically, we combine emerging findings regarding (a) hypoperfusion and endotheliopathy, and (b) intestinal injury in these illnesses with our previously published hypothesis about the role of (c) pituitary suppression, and (d) low thyroid hormone function associated with redox imbalance in ME/CFS. Moreover, we describe interlinkages between these pathophysiological mechanisms as well as “vicious cycles” involving cytokines and inflammation that may contribute to explain the chronic nature of these illnesses. This paper summarizes and expands on our previous publications about the relevance of findings from critical illness for ME/CFS. New knowledge on diagnostics, prognostics and treatment strategies could be gained through active collaboration between critical illness and ME/CFS researchers, which could lead to improved outcomes for both conditions.
Keywords: post-viral fatigue, hypoperfusion, endotheliopathy, gut permeability, endotoxemia, pituitary, non-thyroidal illness syndrome, myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)
Introduction
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a debilitating illness that affects millions of people worldwide (an estimated 800,000 to 2.5 million in the USA) (1, 2). Impaired function, post-exertional malaise, and unrefreshing sleep are core symptoms (1, 3, 4). At least one-quarter of ME/CFS patients are house- or bedbound at some point in their lives (1); the illness can be completely incapacitating (5). The etiology of the illness is unclear (6, 7) and peri-onset events include infection-related episodes, stressful incidents, and exposure to environmental toxins (8).
Critical illness refers to the physiological response to virtually any severe injury or infection, such as head injury, burns, cardiac surgery, SARS-CoV-2 infection and heat stroke (9). Researchers make a distinction between the acute phase of critical illness—in the first hours or days following severe trauma or infection; and the chronic or prolonged phase—in the case of patients who survive the acute phase but for unknown reasons do not start recovering and continue to require intensive care (10–13). Regardless of the initial injury or infection, these “chronic Intensive Care Unit (ICU) patients” experience profound muscular weakness, cognitive impairment, pain, vulnerability to infection, etc. (9, 11, 14). The treatment of prolonged critical illness is incomplete and remains an active area of research. Moreover, cognitive and/or physical disability can last for months or even years after treatment in ICUs (i.e., post intensive care syndrome, PICS) for as of yet unexplained reasons (15–17).
Drawing on findings from critical illness, we here propose an initial explanation for how ME/CFS could originate and perpetuate. Specifically, we combine emerging findings regarding (a) hypoperfusion and endotheliopathy, and (b) intestinal injury in these illnesses with our previously published hypothesis about the role of (c) pituitary suppression, and (d) low thyroid hormone function associated with redox imbalance in ME/CFS. Moreover, we describe interlinkages between these pathophysiological mechanisms as well as “vicious cycles” involving cytokines and inflammation that may contribute to explain the chronic nature of these illnesses. This explanation summarizes and expands on our previous publications about the relevance of findings from critical illness for ME/CFS (18–20) and builds on the work by Nacul et al. (21). The general lack of large high-quality ME/CFS studies (a reflection of the lack of funding in this field) poses a challenge for the assessment of overlaps between the two conditions.
Pathophysiological Mechanisms
In the following sections we describe four central pathophysiological mechanisms in critical illness, including their relationship to inflammation. We also provide initial arguments for suggesting that similar mechanisms may underlie ME/CFS. Readers are referred to our prior publications for additional details about these mechanisms in critical illness (including heat stroke) and possible lessons for understanding ME/CFS (18–20).
Hypoperfusion and Endotheliopathy
It has long been suggested that inadequate oxygen circulation is central to critical illness (22). Specifically, the redistribution of blood away from the splanchnic area to critical tissues is considered an adaptive androgenic response to physiological stress (23, 24). However, the resulting ischemia / reperfusion (I/R) can contribute to tissue injury driving sepsis and multi-organ dysfunction (25, 26). The relative importance of reduced blood flow, vasoconstriction (27), capillary flow disturbances (28) and impaired cellular oxygen utilization (29, 30) in driving critical illness continues to be debated.
Endothelial dysfunction appears to occur in parallel with circulation disturbances during critical illness. Probable drivers of distortions in the structure and function of endothelial lining (i.e., glycocalyx) are cytokines (31), inflammation, exposure to oxidative stress (28, 32) and/or sympatho-adrenal hyperactivation (33). Crucially, endothelial dysfunction during critical illness has been associated with altered cerebral blood flow (34, 35) and increased blood–brain barrier (BBB) permeability resulting in long-term cognitive impairment (36, 37). A leaky BBB could also contribute to increased intracranial pressure (38, 39). Finally, researchers have found that endotheliopathy and coagulation disorder bolster each other via inflammatory pathways (40). Coagulation abnormalities vary in critical illness, but coagulopathy is associated with unfavorable outcomes in prolonged critical illness (i.e., length of ICU stay and mortality) (41).
We propose that similar alterations of the vascular system in response to a physical, infectious and / or emotional stressor (i.e., physiological insult) may also contribute to explain the emergence of ME/CFS. This is consistent with recent hypotheses describing vasoconstriction in muscle and brain as a principal element of ME/CFS (42–46), and findings of cerebral hypoperfusion (47–49) and intracranial hypertension (50) in ME/CFS patients. It is also consistent with studies that have shown that endothelial function is impaired in ME/CFS (51, 52), both in large vessels and in the microcirculation (53, 54)—associated with redox imbalance (51). Finally, it is consistent with a new hypothesis for ME/CFS which suggests that endothelial senescence underpins ME/CFS by disrupting the intestinal barriers and BBBs (55), as well as with suggestions that leakage from dysfunctional blood vessels could explain many of the symptoms in ME/CFS (56).
Intestinal Injury
Critical illness researchers have found profound intestinal alterations within hours following a physiological insult: a dramatic shift in the composition and virulence of intestinal microbes (57–59), an erosion of the mucus barrier, an increase in the permeability of the gut (i.e., “leaky gut”) (60–62), and a disruption in gut motility (63). This intestinal injury is thought to be largely a consequence of local I/R and redox imbalance resulting from splanchnic hypoperfusion (58, 61, 64–67). Indeed, studies in the field of exercise immunology have shown that even relatively low levels of splanchnic hypoperfusion during exercise result in intestinal injury (68).
Critically, this intestinal injury may lead to bacterial translocation from the gut into circulation (i.e., endotoxemia) and/or the formation of toxic gut-derived lymph (57, 60). This in turn can induce pro-inflammatory cytokines and systemic inflammation (69, 70). Moreover, changes in the intestinal microbiome or the mucus barrier may also impact the immune system directly (57). Thus, researchers have long considered the gut “the motor of critical illness” driving sepsis and distant organ dysfunction (71). Some have suggested that a self-perpetuating vicious inflammatory cycle centered around intestinal injury can hinder recovery from critical illness (61, 72).
We propose that the sequence during critical illness—from splanchnic hypoperfusion to hypoxia, redox imbalance, altered gut microbiome, intestinal injury, gut-related endotoxemia, pro-inflammatory cytokines and systemic inflammatory—may also contribute to explain the emergence of ME/CFS following a physiological insult. Our proposal is in alignment with others' findings that intestinal injury and resulting inflammation are central to ME/CFS (73–81) and consistent with findings linking the gut microbiome to inflammation (82–85) and to fatigue symptoms in ME/CFS (86). If verified, the existence of a vicious inflammatory cycle centered around intestinal injury could contribute to explain the perpetuation of ME/CFS. Post-exertional malaise—a key symptom of ME/CFS—could be the manifestation of an accentuation in intestinal injury following exertion. Moreover, the translocation of gut microbes or toxin from the intestines to the brain (55) might contribute to explain central nervous system inflammation in ME/CFS (87–89). Finally, leaky gut is also associated with auto-immunity (90, 91)—an important factor in ME/CFS pathology (92–94).
Pituitary Suppression
Almost immediately after a physiological insult, endocrine axes experience profound alterations considered a vital response to severe stress or injury to allow for a shift in energy and resources to essential organs and repair (95–97). Whereas, in critically ill patients who begin to recover, endocrine axes essentially normalize within 28 days of illness, in cases of prolonged critical illness the pituitary's pulsatile secretion of tropic hormones (unexpectedly) remains suppressed.
Why and how this central suppression is maintained in prolonged critical illness continues to be debated. Inflammatory pathways likely play a role irrespective of the nature of the original injury or infection. For example, cytokines increase the abundance and affinity of glucocorticoid receptors (GR) at the level of the hypothalamus / pituitary, thereby enhancing the negative feedback loop of the hypothalamic-pituitary-adrenal (HPA) axis, and consequently suppressing pituitary release of adrenocorticotropic hormone (ACTH) (95, 98). Similarly, cytokines up-regulate deiodinase enzymes in the hypothalamus resulting in higher local levels of the active thyroid hormone (T3), thereby enhancing the hypothalamic-pituitary-thyroid (HPT) axis' negative feedback loop and consequently suppressing pituitary secretion of thyroid stimulating hormone (TSH) irrespective of circulating thyroid hormone concentrations (99–101). Cytokines may also suppress the release of TSH by the pituitary directly (102, 103) contributing to a virtual complete loss of pulsatile TSH secretion (96).
The loss of pulsatile pituitary secretions has important implications for the autonomic nervous system, metabolism, and the immune system. Without sufficient pulsatile stimulation by ACTH, adrenal glands begin to atrophy (104, 105), compromising patients' ability to cope with external stressors and permitting excessive inflammatory responses. Erratic rather than pulsatile pituitary production of growth hormone (GH) leads to an imbalance between catabolic and anabolic hormones, resulting in loss of muscle and bone mass, muscle weakness, and changes in glucose and fat metabolism (106–108). Finally, suppression of the HPT axis is associated with tiredness and other hypothyroid-like symptoms (109, 110).
We propose that the sequence during critical illness—from increased release of pituitary hormones during the acute phase to suppression of the pituitary gland's pulsatile secretion in the prolonged phase—could also contribute to explain the emergence of ME/CFS following a physiological insult. This proposal is consistent with descriptions of ME/CFS as a progression from a hypermetabolic to hypometabolic state (21). It also aligns with a recent hypothesis relating many of the symptoms in severe ME/CFS to impaired pituitary function (111). Further support for this proposal is provided by the many previous ME/CFS studies that have documented dysfunctions in the hypothalamic–pituitary–somatotropic (HPS) axis (112–114), the HPT axis (115–120), and the HPA axis (121–136)—notably associated with inflammation and oxidative & nitrosative stress (O&NS) (137–140). Strikingly, models relating the persistence of a suppressed HPA axis in ME/CFS to a change in central GRs concentrations resemble the explanations provided for pituitary suppression in critical illness (141–146). Moreover, suppression of ACTH release would explain why in a small study ME/CFS patients were found to have 50% smaller adrenals than controls (147), resembling adrenal atrophy in prolonged critical illness. However, the relationship between the pituitary's pulsatile secretions, physiological alterations and severity of illness—which proved revelatory in understanding prolonged critical illness—remains unexplored in ME/CFS.
Low Thyroid Hormone Function
Peripheral mechanisms involving cytokines lead to the rapid depression of thyroid hormone activity following a severe physiological insult (148–152). This is termed “non-thyroidal illness syndrome” (NTIS), “euthyroid sick syndrome” or “low T3 syndrome” and is thought to be an adaptive response to conserve energy resources during critical illness (152–154). The mechanisms involved include alterations in the half-life of thyroid hormone in circulation (155–157); modifications in the uptake of thyroid hormone by cells (158, 159); down- and up-regulation of deiodinase enzymes that convert the thyroid hormone into active and inactive forms respectively (156, 160); and alterations in sensitivity of cells to thyroid hormones (161–163). These alterations can lead to important tissue-specific depression in thyroid hormone function (164, 165) which is, however, often missed altogether in clinical settings (166) because most of the alterations do not translate into changes in the blood concentrations of thyroid hormones (164, 167, 168). Indeed, the decrease in the ratio of the active form of thyroid hormone (T3) relative to the inactivated thyroid hormone (rT3) (150, 152, 169)—considered the most sensitive marker of NTIS—may be just the “tip of the iceberg” of the depressed thyroid hormone function in target tissues (120, 170).
While NTIS may be beneficial in the acute phase of critical illness, it is increasingly seen as maladaptive and hampering the recovery of patients in the case of prolonged critical illness (96, 101, 152, 169, 171–173). Low thyroid hormone function may hamper the function of organs (170) and the activity of immune cells, including natural killer cells (174–185). Immune dysfunctions might in turn explain other pathologies, such as viral reactivation observed in ICU patients (186–188). Some critical illness researchers have proposed a model that describes how NTIS is maintained by reciprocal relationships between inflammation (notably pro-inflammatory cytokines), O&NS and reduced thyroid hormone function, forming a “vicious cycle” (101, 173). This model can help to explain the perplexing failure to recover of some critically ill patients in ICUs that survive their initial severe illness or injury.
We propose that low thyroid hormone function could also contribute to explain the emergence of ME/CFS following a physiological insult. An immune-mediated loss of thyroid hormone function in ME/CFS has long been suspected (117). A recent study showed that the thyroid panel of ME/CFS patients resembles that of critical illness patients, including significantly lower ratio of T3 to rT3 hormones (120). Moreover, the other elements for a “vicious cycle” which researchers have suggested perpetuate a hypometabolic and inflammatory state in critical illness are also present in ME/CFS, including inflammation (140, 189), increased O&NS (190–192) and altered cytokine profiles (193, 194).
Discussion
Hypoperfusion and endotheliopathy, intestinal injury, pituitary suppression, and low thyroid hormone function are each central to prolonged critical illness regardless of the nature of the initial severe injury or infection (101, 173, 195, 196). We propose that, similarly, these mechanisms and their reciprocal relationships with inflammation could underlie ME/CFS regardless of the nature of the peri-onset event (i.e., infection, stressful incident, exposure to environmental toxins or other) (Table 1). Moreover, the severity of ME/CFS may be a function of the strength of these mechanisms.
Table 1.
Central pathophysiological mechanisms in prolonged critical illness, probable drivers and implications, and initial evidence suggesting similar mechanisms in ME/CFS.
| Pathophysiological mechanisms | In prolonged critical illness (Probable drivers and implications) | In ME/CFS (Initial evidence) |
|---|---|---|
| Hypoperfusion |
Drivers: • Redistribution of blood away from the splanchnic area to critical tissues (23, 24) • Reduced blood flow, vasoconstriction (27) • Capillary flow disturbances (28) • Additional: impaired cellular oxygen utilization (29, 30) Implications: • Ischemia / reperfusion (I/R) • Tissue injury driving sepsis and multi-organ dysfunction (25, 26) |
Initial evidence • Vasoconstriction in muscle and brain (42–45) • Cerebral hypoperfusion (47–49) • Intracranial hypertension (50) |
| Endotheliopathy |
Drivers: • Cytokines (31), Inflammation, exposure to oxidative stress (28, 32) • Sympatho-adrenal hyperactivation (33) Implications: • Altered cerebral blood flow (34, 35) • Increased blood–brain barrier (BBB) permeability (36, 37) • Increased intracranial pressure (38, 39). • (variable) Coagulation disorder (40) |
Initial evidence • Impaired endothelial function (51, 52), in large vessels and microcirculation (53, 54)—associated with redox imbalance (51) • Endothelial senescence disrupting the intestinal barriers and BBBs (55) • Redox imbalance |
| Intestinal injury |
Drivers: • Local I/R and redox imbalance resulting from splanchnic hypoperfusion (58, 61, 64–67) • Disruption in gut motility (63) • Shift in the composition and virulence of intestinal microbes (57–59) Implications: • Erosion of the mucus barrier, increase in the permeability of the gut (i.e., “leaky gut”) (60–62) • Bacterial translocation from the gut into circulation (i.e., endotoxemia) and/or the formation of toxic gut-derived lymph (57, 60) • Pro-inflammatory cytokines and systemic inflammation (69, 70) • Direct impacts on the immune system (57) • Vicious inflammatory cycle centered around intestinal injury (61, 72) • Decreased secretion of gastrointestinal hormones including ghrelin (63, 197) impacting pituitary activity |
Initial evidence • Intestinal injury and resulting inflammation (73–81) • Altered gut microbiome linked to inflammation (82–85). • Lack of beneficial gut bacteria linked to fatigue symptoms (86) • Endothelial senescence disrupting the intestinal barriers (55) • Auto-immunity (92–94) |
| Suppression of pulsatile pituitary function |
Drivers • Cytokines acting on abundance and affinity of glucocorticoid receptors (GR) at central level (95, 98) • Cytokines affecting deiodinase enzymes in the hypothalamus (99–101) • Direct action of cytokines on TSH release by the pituitary directly (102, 103) Implications • Loss of ACTH pulsatility: atrophy of adrenal glands (104, 105) compromising patients' ability to cope with external stressors and permitting excessive inflammatory responses • Loss of GH pulsatility: imbalance between catabolic and anabolic hormones, resulting in loss of muscle and bone mass, muscle weakness, and changes in glucose and fat metabolism (106–108). Alterations in deiodinase enzyme (D3) activity enabling low thyroid hormone function (96, 108, 198) • Loss of TSH pulsatility (109, 110) |
Initial evidence • Progression from a hypermetabolic to hypometabolic state (21) • Impaired pituitary function (hypothesis) (111) • Dysfunctions in HPS axis (112–114), HPT axis (115–120) and HPA axis (121–136) – associated with inflammation O&NS (137–140) • Changes in central GRs concentrations (models) (141–146) • Smaller adrenals (147) |
| Low thyroid hormone function |
Drivers • Alterations in the half-life of thyroid hormone in circulation (155–157) • Modifications in the uptake of thyroid hormone by cells (158, 159) • Down- and up-regulation of deiodinase enzymes that convert the thyroid hormone into active and inactive forms, respectively (156, 160) • Alternations in sensitivity of cells to thyroid hormones (161–163) Implications • Tissue-specific depression in thyroid hormone function (164–166) • Hampered function of organs (170) • Altered activity of immune cells, including natural killer cells (174–185) • Viral reactivation (186–188) • Vicious inflammatory cycle (101, 173) |
Initial evidence • Immune-mediated loss of thyroid hormone function in ME/CFS (suspected) (117) • Significantly lower ratio of T3 to rT3 hormones (120) |
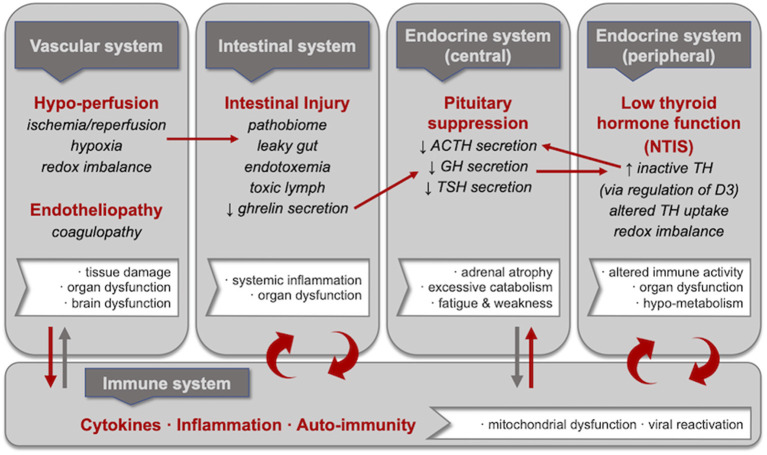
However, each of these pathological mechanisms has largely been studied in isolation and rarely have the linkages between them been explored. Yet, the aggregate of these mechanisms is likely necessary to fully explain the perpetuation of critical illness—and to inform the understanding of ME/CFS (Figure 1). Additional areas for inquiry thus include the following:
Figure 1.
Central pathophysiological mechanisms in critical illness including selected consequences and inter-linkages. Hypoperfusion and endotheliopathy, intestinal injury, pituitary suppression, and low thyroid hormone function are each central to prolonged critical illness regardless of the nature of the initial severe injury or infection. These pathophysiological mechanisms are in reciprocal relationships with inflammation; specifically, researchers have proposed vicious cycles involving intestinal injury and low thyroid hormone function. Moreover, linkages have been described between these pathophysiological mechanisms, including (i) hypoperfusion and intestinal injury (i.e., leaky gut resulting from ischemia/reperfusion, hypoxia and redox imbalance); (ii) intestinal injury and pituitary suppression (i.e., suppressed growth hormone release resulting from reduced ghrelin secretion by the intestines); (iii) pituitary suppression and low thyroid hormone function (i.e., increased inactivated thyroid hormone resulting from the upregulation of D3 deiodinase as a consequence of lower growth hormone); and (iv) low thyroid hormone function and pituitary suppression (i.e., decreased ACTH secretion resulting from lower levels of activated thyroid hormone). We propose that these mechanisms and the linkages between them—alongside reciprocal relationships with inflammation—could also underlie ME/CFS.
Linkages Between Intestinal Injury and Pituitary Suppression
Intestinal injury during critical illness results in decreased secretion of gastrointestinal hormones including ghrelin (63, 197). Decreased stimulation of the pituitary and hypothalamus by ghrelin during prolonged critical illness in turn results in lower secretion of GH by the pituitary (199). Researchers have found that the administration of an artificial ghrelin in chronic ICU patients reactivated the pulsatile secretion of GH by the pituitary and—when done in combination with thyrotropin-releasing hormones (TRH)—had beneficial metabolic effects (96, 108, 198). Similarly, the administration of ghrelin to the I/R rats “inhibited pro-inflammatory cytokine release, reduced neutrophil infiltration, ameliorated intestinal barrier dysfunction, attenuated organ injury, and improved survival” (200). The sequence between intestinal injury, ghrelin secretion and GH release by the pituitary could be particularly relevant for solving ME/CFS given that “several of the main typical symptoms in severe ME/CFS, such as fatigue, myalgia, contractility, delaying muscle recovery and function, exertional malaise, neurocognitive dysfunction, and physical disability may be related to severe GH deficiency” (111).
Linkages Between Pituitary Suppression and Low Thyroid Hormone Function
There are several pathways linking the activity of the pituitary with that of thyroid hormones. Firstly, GH secreted by the pituitary co-regulates the activity of the deiodinase enzyme (D3) responsible for the conversion of thyroid hormones into inactive forms (i.e., rT3 and inactivate forms of T2) (106, 201). Researchers showed that normalization of the GH secretion in prolonged critically ill patients is necessary to inhibit the increase in plasma rT3 concentrations (96, 108, 198). In other words, dampened GH release by the pituitary during prolonged critical illness enables low thyroid hormone function. Secondly, the lack of stimulation of the adrenals by ACTH could (by causing an atrophy of adrenals) create the condition necessary for persistent inflammation which depresses the activity of thyroid hormones during critical illness (148–152). In other words, dampened ACTH release by the pituitary during prolonged critical illness might permit the vicious inflammatory cycles described above. Thirdly, there is evidence that thyroid hormone conversely also stimulates ACTH secretion (202, 203). In summary, the bi-directional relationships between the endocrine axes and thyroid hormone function (in addition to reciprocal relationships with inflammation) could contribute to explain the persistence of chronic ICU and ME/CFS.
Linkages Between Low Thyroid Hormone Function and Endothelial Function
Upon binding to specific receptors on endothelial cells, thyroid hormones (T3 and T4) activate the endothelial nitric oxide synthase (eNOS) responsible for nitric oxide (NO) production (204), which in turn impacts vasodilation and inflammation (205–207). A further line of inquiry may thus be the role of thyroid hormone function in endotheliopathy in ME/CFS, including as it relates to the new finding that plasma from ME/CFS patients inhibits eNOS and NO production in endothelial cells (208). Relatedly, critical illness researchers have found that serum from patients with NTIS inhibits the uptake of thyroid hormone (209, 210); the mechanisms remain unresolved (165).
Linkages to Mitochondrial Function
The impaired perfusion, redox imbalance, lower thyroid hormone function and inflammation appear to collectively affect mitochondrial activity in critical illness (via inhibition, damage, and/or decreased turnover of new mitochondrial protein) (30, 211–213). Mitochondrial activity may be similarly affected in ME/CFS (190). Some have suggested that this down-regulation of mitochondrial activity (and oxygen utilization) in critical illness may be an adaptive form of “hibernation” to protect cells from death pathways (30, 213). This suggestion echoes the hypothesis that ME/CFS is a form of “dauer” or “cell danger response” (214–216). Lower mitochondrial activity in turn affects the immune system and the gut endothelial “such that the host's immune response and physical barriers to infection are simultaneously compromised” (29).
Relevance of Critical Illness Treatment Trials for ME/CFS
Although prolonged critical illness remains unresolved, early treatment trials—such as the reactivation of the pituitary, or interruption of the vicious inflammatory cycles centered around either gut injury or low thyroid hormone function—may provide therapeutic avenues for ME/CFS (19). Longitudinal studies of (spontaneous) recovery from critical illness may also give clues about prerequisites for recovery from ME/CFS. Researchers have, for example, found that “supranormal TSH precedes onset of recovery” from prolonged critical illness (96) and that metabolic rate rises > 50% above normal in the recovery phase (213).
Commonality With Other Illnesses
Researchers have suggested commonality in the illnesses induced by physical, infectious, and / or emotional stressors (132, 217). These include heat stroke, fibromyalgia, ME/CFS, prolonged critical illness, PICS, cancer-related fatigue, post-viral fatigue, post-acute COVID-19 syndrome (PACS) and long-COVID. Specifically, it is necessary to explore whether the pathological mechanisms described above also underlie long COVID—a disease which resembles ME/CFS (218–228) and can arise even after mild COVID-19 cases.
Conclusion
Decades of research in the field of critical illness medicine have demonstrated that in response to the stress of severe infection or injury, the vascular system, intestines, endocrine axes and thyroid hormone function experience profound alterations. Self-reinforcing interlinkages between these pathophysiological mechanisms as well as “vicious cycles” involving cytokines and inflammation may perpetuate illness irrespective of the initial severe infection or injury. Without excluding possible predisposing genetic or environmental factors, we propose that the pathological mechanisms—and the interlinkages between them—that prevent recovery of some critically ill patients may also underlie ME/CFS. This initial proposal is in line with and complements several existing hypotheses of ME/CFS pathogenesis. If this hypothesis is validated, past treatment trials for critical illness may provide avenues for a cure for ME/CFS. Certainly, given the similarities described above, active collaboration between critical illness and ME/CFS researchers could lead to improved understanding of not only both conditions, but also PICS, long-COVID, PACS, and fibromyalgia.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
DS wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
The Open Medicine Foundation (JB) is acknowledged for support.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
Abbreviations
- BBB
Blood–brain barrier
- ACTH
Adrenocorticotropic hormone
- GH
Growth hormone
- GR
glucocorticoid receptors
- HPA
hypothalamus-pituitary-adrenal axis: “Adreno-cortical axis”
- HPS
Hypothalamic-pituitary-somatotropic axis: “Somatropic axis”
- HPT
Hypothalamic-pituitary-thyroid: “Thyrotropic axis”
- ICU
Intensive Care Unit
- I/R
Ischemia/reperfusion
- ME/CFS
Myalgic Encephalomyelitis/Chronic Fatigue Syndrome
- NO
Nitrox oxide
- NTIS
Non-thyroidal illness syndrome
- O&NS
oxidative and nitrosative stress
- PACS
Post-acute COVID-19 syndrome
- PICS
Post-intensive care syndrome
- TRH
Thyrotropin-releasing hormone
- TSH
Thyroid stimulating hormone.
References
- 1.Institute of Medicine . Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. Washington, DC: The National Academies Press; (2015). [PubMed] [Google Scholar]
- 2.Jason LA, Mirin AA. Updating the National Academy of Medicine ME/CFS prevalence and economic impact figures to account for population growth and inflation. Fatigue Biomed Health Behav. (2021) 9:9–13. 10.1080/21641846.2021.1878716 [DOI] [Google Scholar]
- 3.Open Medicine Foundation,. Symptoms of ME/CFS. (2020). Available online at: https://www.omf.ngo/symptoms-mecfs (accessed March 27, 2021).
- 4.Nacul L, Authier FJ, Scheibenbogen C, Lorusso L, Helland IB, Martin JA, et al. European Network on Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (EUROMENE): expert consensus on the diagnosis, service provision, and care of people with ME/CFS in Europe. Medicina. (2021) 57:510. 10.3390/medicina57050510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dafoe W. Extremely severe ME/CFSra personal account. Healthcare. (2021) 9:504. 10.3390/healthcare9050504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Komaroff AL. Advances in understanding the pathophysiology of chronic fatigue syndrome. JAMA. (2019) 322:499–500. 10.1001/jama.2019.8312 [DOI] [PubMed] [Google Scholar]
- 7.Komaroff AL. Myalgic encephalomyelitis/chronic fatigue syndrome: when suffering is multiplied. Healthcare. (2021) 9:919. 10.3390/healthcare9070919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu L, Valencia IJ, Garvert DW, Montoya JG. Onset patterns and course of myalgic encephalomyelitis/chronic fatigue syndrome. Front Pediatr. (2019) 7:12. 10.3389/fped.2019.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loss SH, Nunes DSL, Franzosi OS, Salazar GS, Teixeira C, Vieira SRR. Chronic critical illness: are we saving patients or creating victims? Rev Bras Ter Intensiva. (2017) 29:87–95. 10.5935/0103-507X.20170013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van den Berghe G. Novel insights into the neuroendocrinology of critical illness. Eur J Endocrinol. (2000) 143:1–13. 10.1530/eje.0.1430001 [DOI] [PubMed] [Google Scholar]
- 11.Nelson JE, Cox CE, Hope AA, Carson SS. Chronic critical illness. Am J Respir Crit Care Med. (2010) 182:446–44610espir C1164/rccm.201002-0210CI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van den Berghe GH. Acute and prolonged critical illness are two distinct neuroendocrine paradigms. Verh K Acad Geneeskd Belg. (1998) 60:487–518; discussion −20. [PubMed] [Google Scholar]
- 13.Vanhorebeek I, Van den Berghe G. The neuroendocrine response to critical illness is a dynamic process. Crit Care Clin. (2006) 22:1–15, v. 10.1016/j.ccc.2005.09.004 [DOI] [PubMed] [Google Scholar]
- 14.Vanhorebeek I, Latronico N, Van den Berghe G. ICU-acquired weakness. Intensive Care Med. (2020) 46:637–53. 10.1007/s00134-020-05944-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Aerde N, Van Dyck L, Vanhorebeek I, Van den Berghe G. Endocrinopathy of the Critically Ill. In: Preiser J-C, Herridge M, Azoulay E, editors. Post-Intensive Care Syndrome. Cham: Springer International Publishing; (2020). p. 125–43. [Google Scholar]
- 16.Rawal G, Yadav S, Kumar R. Post-intensive care syndrome: an overview. J Transl Int Med. (2017) 5:90–2. 10.1515/jtim-2016-0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith S, Rahman O. Post Intensive Care Syndrome. Treasure Island, FL: StatPearls Publishing; (2020). [Google Scholar]
- 18.Stanculescu D, Larsson L, Bergquist J. Hypothesis: mechanisms that prevent recovery in prolonged ICU patients also underlie Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Front Med. (2021) 8:628029. 10.3389/fmed.2021.628029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stanculescu D, Larsson L, Bergquist J. Theory: treatments for prolonged ICU patients may provide new therapeutic avenues for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Front Med. (2021) 8:672370. 10.3389/fmed.2021.672370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanculescu D, Sepulveda N, Lim CL, Bergquist J. Lessons from heat stroke for understanding Myalgic Encephalomyelitis / Chronic Fatigue Syndrome. Front Neurol. (2021) 12:789784. 10.3389/fneur.2021.789784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nacul L, O'Boyle S, Palla L, Nacul FE, Mudie K, Kingdon CC, et al. How Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) progresses: the natural history of ME/CFS. Front Neurol. (2020) 11:826. 10.3389/fneur.2020.00826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Broder G, Weil MH. Excess lactate: an index of reversibility of shock in human patients. Science. (1964) 143:1457–9. 10.1126/science.143.3613.1457 [DOI] [PubMed] [Google Scholar]
- 23.Halter JB, Pflug AE, Porte D. Jr. Mechanism of plasma catecholamine increases during surgical stress in man. J Clin Endocrinol Metab. (1977) 45:936–44. 10.1210/jcem-45-5-936 [DOI] [PubMed] [Google Scholar]
- 24.Zhang D, Li H, Li Y, Qu L. Gut rest strategy and trophic feeding in the acute phase of critical illness with acute gastrointestinal injury. Nutr Res Rev. (2019) 32:176–82. 10.1017/S0954422419000027 [DOI] [PubMed] [Google Scholar]
- 25.Rock P, Yao Z. Ischemia reperfusion injury, preconditioning and critical illness. Curr Opin Anaesthesiol. (2002) 15:139–46. 10.1097/00001503-200204000-00001 [DOI] [PubMed] [Google Scholar]
- 26.Schwarte L, Stevens M, Ince C. Splanchnic perfusion and oxygenation in critical illness. Intensive Care Med. (2006) 627–40. 10.1007/3-540-33396-7_58 [DOI] [Google Scholar]
- 27.Pastores SM, Katz DP, Kvetan V. Splanchnic ischemia and gut mucosal injury in sepsis and the multiple organ dysfunction syndrome. Am J Gastroenterol. (1996) 91:1697–6979 [PubMed] [Google Scholar]
- 28.Ostergaard L, Granfeldt A, Secher N, Tietze A, Iversen NK, Jensen MS, et al. Microcirculatory dysfunction and tissue oxygenation in critical illness. Acta Anaesthesiol Scand. (2015) 59:1246–59. 10.1111/aas.12581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crouser ED. Mitochondrial dysfunction in septic shock and multiple organ dysfunction syndrome. Mitochondrion. (2004) 4:729–41. 10.1016/j.mito.2004.07.023 [DOI] [PubMed] [Google Scholar]
- 30.Singer M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence. (2014) 5:66–72. 10.4161/viru.26907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang S, Kishimoto T. Interplay between interleukin-6 signaling and the vascular endothelium in cytokine storms. Exp Mol Med. (2021) 53:1116–23. 10.1038/s12276-021-00649-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cerny V, Astapenko D, Brettner F, Benes J, Hyspler R, Lehmann C, et al. Targeting the endothelial glycocalyx in acute critical illness as a challenge for clinical and laboratory medicine. Crit Rev Clin Lab Sci. (2017) 54:343–57. 10.1080/10408363.2017.1379943 [DOI] [PubMed] [Google Scholar]
- 33.Johansson PI, Stensballe J, Ostrowski SR. Shock induced endotheliopathy (SHINE) in acute critical illness - a unifying pathophysiologic mechanism. Crit Care. (2017) 21:25. 10.1186/s13054-017-1605-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slessarev M, Mahmoud O, McIntyre CW, Ellis CG. Cerebral blood flow deviations in critically ill patients: potential insult contributing to ischemic and hyperemic injury. Front Med. (2020) 7:615318. 10.3389/fmed.2020.615318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bowton DL, Bertels NH, Prough DS, Stump DA. Cerebral blood flow is reduced in patients with sepsis syndrome. Crit Care Med. (1989) 17:399–39989are Medo1097/00003246-198905000-00004 [DOI] [PubMed] [Google Scholar]
- 36.Hughes CG, Patel MB, Brummel NE, Thompson JL, McNeil JB, Pandharipande PP, et al. Relationships between markers of neurologic and endothelial injury during critical illness and long-term cognitive impairment and disability. Intensive Care Med. (2018) 44:345–55. 10.1007/s00134-018-5120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hughes CG, Morandi A, Girard TD, Riedel B, Thompson JL, Shintani AK, et al. Association between endothelial dysfunction and acute brain dysfunction during critical illness. Anesthesiology. (2013) 118:631–9. 10.1097/ALN.0b013e31827bd193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schizodimos T, Soulountsi V, Iasonidou C, Kapravelos N. An overview of management of intracranial hypertension in the intensive care unit. J Anesth. (2020) 34:741–57. 10.1007/s00540-020-02795-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naessens DMP, de Vos J, VanBavel E, Bakker E. Blood-brain and blood-cerebrospinal fluid barrier permeability in spontaneously hypertensive rats. Fluids Barriers CNS. (2018) 15:26. 10.1186/s12987-018-0112-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vallet B, Wiel E. Endothelial cell dysfunction and coagulation. Crit Care Med. (2001) 29:S36–41. 10.1097/00003246-200107001-00015 [DOI] [PubMed] [Google Scholar]
- 41.Winer LK, Salyer C, Beckmann N, Caldwell CC, Nomellini V. Enigmatic role of coagulopathy among sepsis survivors: a review of coagulation abnormalities and their possible link to chronic critical illness. Trauma Surg Acute Care Open. (2020) 5:e000462. 10.1136/tsaco-2020-000462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wirth KJ, Scheibenbogen C. Pathophysiology of skeletal muscle disturbances in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). J Transl Med. (2021) 19:162. 10.1186/s12967-021-02833-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wirth K, Scheibenbogen C A. Unifying hypothesis of the pathophysiology of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): recognitions from the finding of autoantibodies against ßc-adrenergic receptors. Autoimmun Rev. (2020) 19:102527. 10.1016/j.autrev.2020.102527 [DOI] [PubMed] [Google Scholar]
- 44.Malato J, Sotzny F, Bauer S, Freitag H, Fonseca A, Grabowska AD, et al. The SARS-CoV-2 receptor angiotensin-converting enzyme 2 (ACE2) in myalgic encephalomyelitis/chronic fatigue syndrome: a meta-analysis of public DNA methylation and gene expression data. Heliyon. (2021) 7:e07665. 10.1016/j.heliyon.2021.e07665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fluge O, Tronstad KJ, Mella O. Pathomechanisms and possible interventions in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J Clin Invest. (2021) 131:e150377. 10.1172/JCI150377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wirth KJ, Scheibenbogen C, Paul F. An attempt to explain the neurological symptoms of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J Transl Med. (2021) 19:471. 10.1186/s12967-021-03143-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campen C, Rowe PC, Visser FC. Orthostatic symptoms and reductions in cerebral blood flow in Long-Haul COVID-19 patients: similarities with myalgic encephalomyelitis/chronic fatigue syndrome. Medicina. (2021) 58:28. 10.3390/medicina58010028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Campen C, Visser FC. Psychogenic pseudosyncope: real or imaginary? Results from a case-control study in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) patients. Medicina. (2022) 58:98. 10.3390/medicina58010098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Campen CMC, Verheugt FWA, Rowe PC, Visser FC. Cerebral blood flow is reduced in ME/CFS during head-up tilt testing even in the absence of hypotension or tachycardia: A quantitative, controlled study using Doppler echography. Clinical Neurophysiol Pract. (2020) 5:50–8. 10.1016/j.cnp.2020.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bragee B, Michos A, Drum B, Fahlgren M, Szulkin R, Bertilson BC. Signs of intracranial hypertension, hypermobility, and craniocervical obstructions in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Front Neurol. (2020) 11:828. 10.3389/fneur.2020.00828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blauensteiner J, Bertinat R, Leon LE, Riederer M, Sepulveda N, Westermeier F. Altered endothelial dysfunction-related miRs in plasma from ME/CFS patients. Sci Rep. (2021) 11:10604. 10.1038/s41598-021-89834-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scherbakov N, Szklarski M, Hartwig J, Sotzny F, Lorenz S, Meyer A, et al. Peripheral endothelial dysfunction in myalgic encephalomyelitis/chronic fatigue syndrome. ESC Heart Fail. (2020) 7:1064–71. 10.1002/ehf2.12633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Newton DJ, Kennedy G, Chan KK, Lang CC, Belch JJ, Khan F. Large and small artery endothelial dysfunction in chronic fatigue syndrome. Int J Cardiol. (2012) 154:335–6. 10.1016/j.ijcard.2011.10.030 [DOI] [PubMed] [Google Scholar]
- 54.Sorland K, Sandvik MK, Rekeland IG, Ribu L, Smastuen MC, Mella O, et al. Reduced endothelial function in myalgic encephalomyelitis/chronic fatigue syndrome-results from open-label cyclophosphamide intervention study. Front Med. (2021) 8:642710. 10.3389/fmed.2021.642710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sfera A, Osorio C, Zapata Martin Del Campo CM, Pereida S, Maurer S, Maldonado JC, et al. Endothelial senescence and chronic fatigue syndrome, a COVID-19 based hypothesis. Front Cell Neurosci. (2021) 15:673217. 10.3389/fncel.2021.673217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lubell J. Letter: could endothelial dysfunction and vascular damage contribute to pain, inflammation and post-exertional malaise in individuals with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)? J Transl Med. (2022) 20:40. 10.1186/s12967-022-03244-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alverdy JC, Krezalek MA. Collapse of the Microbiome, Emergence of the Pathobiome, and the Immunopathology of Sepsis. Crit Care Med. (2017) 45:337–47. 10.1097/CCM.0000000000002172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Otani S, Coopersmith CM. Gut integrity in critical illness. J Intensive Care. (2019) 7:17. 10.1186/s40560-019-0372-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ojima M, Motooka D, Shimizu K, Gotoh K, Shintani A, Yoshiya K, et al. Metagenomic analysis reveals dynamic changes of whole gut microbiota in the acute phase of intensive care unit patients. Dig Dis Sci. (2016) 61:1628–34. 10.1007/s10620-015-4011-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mittal R, Coopersmith CM. Redefining the gut as the motor of critical illness. Trends Mol Med. (2014) 20:214–23. 10.1016/j.molmed.2013.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sertaridou E, Papaioannou V, Kolios G, Pneumatikos I. Gut failure in critical care: old school versus new school. Ann Gastroenterol. (2015) 28:309–22. [PMC free article] [PubMed] [Google Scholar]
- 62.Doig CJ, Sutherland LR, Sandham JD, Fick GH, Verhoef M, Meddings JB. Increased intestinal permeability is associated with the development of multiple organ dysfunction syndrome in critically ill ICU patients. Am J Respir Crit Care Med. (1998) 158:444–51. 10.1164/ajrccm.158.2.9710092 [DOI] [PubMed] [Google Scholar]
- 63.Martinez EE, Fasano A, Mehta NM. Gastrointestinal function in critical illness—a complex interplay between the nervous and enteroendocrine systems. Pediatr Med. (2020) 3:26. 10.21037/pm-20-74 [DOI] [Google Scholar]
- 64.Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, et al. Intestinal permeability–a new target for disease prevention and therapy. BMC Gastroenterol. (2014) 14:189. 10.1186/s12876-014-0189-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aranow J, Fink M. Determinants of intestinal barrier failure in critical illness. Br J Anaesth. (1996) 77:71–81. 10.1093/bja/77.1.71 [DOI] [PubMed] [Google Scholar]
- 66.Jakob SM. Clinical review: splanchnic ischaemia. Crit Care. (2002) 6:306–12. 10.1186/cc1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stechmiller JK, Treloar D, Allen N. Gut dysfunction in critically ill patients: a review of the literature. Am J Crit Care. (1997) 6:204–9. 10.4037/ajcc1997.6.3.204 [DOI] [PubMed] [Google Scholar]
- 68.van Wijck K, Lenaerts K, van Loon LJ, Peters WH, Buurman WA, Dejong CH. Exercise-induced splanchnic hypoperfusion results in gut dysfunction in healthy men. PLoS ONE. (2011) 6:e22366. 10.1371/journal.pone.0022366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fink MP, Delude RL. Epithelial barrier dysfunction: a unifying theme to explain the pathogenesis of multiple organ dysfunction at the cellular level. Crit Care Clin. (2005) 21:177–96. 10.1016/j.ccc.2005.01.005 [DOI] [PubMed] [Google Scholar]
- 70.Holland J, Carey M, Hughes N, Sweeney K, Byrne PJ, Healy M, et al. Intraoperative splanchnic hypoperfusion, increased intestinal permeability, down-regulation of monocyte class II major histocompatibility complex expression, exaggerated acute phase response, and sepsis. Am J Surg. (2005) 190:393–400. 10.1016/j.amjsurg.2005.03.038 [DOI] [PubMed] [Google Scholar]
- 71.Meakins J, Marshall J. Multi-organ-failure syndrome. The gastrointestinal tract: the “motor” of MOF. Arch Surg. (1986) 121:196–208. 10.1001/archsurg.1986.01400020082010 [DOI] [PubMed] [Google Scholar]
- 72.Deitch EA. Gut-origin sepsis: evolution of a concept. Surgeon. (2012) 10:350–6. 10.1016/j.surge.2012.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maes M, Coucke F, Leunis JC. Normalization of the increased translocation of endotoxin from gram negative enterobacteria (leaky gut) is accompanied by a remission of chronic fatigue syndrome. Neuro Endocrinol Lett. (2007) 28:739–44. [PubMed] [Google Scholar]
- 74.Morris G, Berk M, Carvalho AF, Caso JR, Sanz Y, Maes M. The role of microbiota and intestinal permeability in the pathophysiology of autoimmune and neuroimmune processes with an emphasis on inflammatory bowel disease type 1 diabetes and chronic fatigue syndrome. Curr Pharm Des. (2016) 22:6058–75. 10.2174/1381612822666160914182822 [DOI] [PubMed] [Google Scholar]
- 75.Morris G, Maes M, Berk M, Puri BK. Myalgic encephalomyelitis or chronic fatigue syndrome: how could the illness develop? Metab Brain Dis. (2019) 34:385–415. 10.1007/s11011-019-0388-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maes M, Mihaylova I, Leunis JC. Increased serum IgA and IgM against LPS of enterobacteria in chronic fatigue syndrome (CFS): indication for the involvement of gram-negative enterobacteria in the etiology of CFS and for the presence of an increased gut-intestinal permeability. J Affect Disord. (2007) 99:237–40. 10.1016/j.jad.2006.08.021 [DOI] [PubMed] [Google Scholar]
- 77.Zhang ZT, Du XM, Ma XJ, Zong Y, Chen JK Yu CL, et al. Activation of the NLRP3 inflammasome in lipopolysaccharide-induced mouse fatigue and its relevance to chronic fatigue syndrome. J Neuroinflammation. (2016) 13:71. 10.1186/s12974-016-0539-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maes M, Leunis JC. Normalization of leaky gut in chronic fatigue syndrome (CFS) is accompanied by a clinical improvement: effects of age, duration of illness and the translocation of LPS from gram-negative bacteria. Neuro Endocrinol Lett. (2008) 29:902–10. [PubMed] [Google Scholar]
- 79.Missailidis D, Annesley SJ, Fisher PR. Pathological mechanisms underlying myalgic encephalomyelitis/chronic fatigue syndrome. Diagnostics. (2019) 9:80. 10.20944/preprints201907.0196.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anderson G, Maes M. Mitochondria and immunity in chronic fatigue syndrome. Prog Neuropsychopharmacol Biol Psychiatry. (2020) 103:109976. 10.1016/j.pnpbp.2020.109976 [DOI] [PubMed] [Google Scholar]
- 81.Shukla SK, Cook D, Meyer J, Vernon SD, Le T, Clevidence D, et al. Changes in gut and plasma microbiome following exercise challenge in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). PLoS ONE. (2015) 10:e0145453. 10.1371/journal.pone.0145453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Giloteaux L, Goodrich JK, Walters WA, Levine SM, Ley RE, Hanson MR. Reduced diversity and altered composition of the gut microbiome in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome. (2016) 4:30. 10.1186/s40168-016-0171-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lakhan SE, Kirchgessner A. Gut inflammation in chronic fatigue syndrome. Nutr Metab. (2010) 7:79. 10.1186/1743-7075-7-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Varesi A, Deumer U-S, Ananth S, Ricevuti G. The emerging role of gut microbiota in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): current evidence and potential therapeutic applications. J Clin Med. (2021) 10:5077. 10.3390/jcm10215077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.König RS, Albrich WC, Kahlert CR, Bahr LS, Löber U, Vernazza P, et al. the gut microbiome in Myalgic Encephalomyelitis (ME)/Chronic Fatigue Syndrome (CFS). Front Immunol. (2022) 12:628741. 10.3389/fimmu.2021.628741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guo C, Che X, Briese T, Allicock O, Yates RA, Cheng A, et al. Deficient butyrate-producing capacity in the gut microbiome of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome patients is associated with fatigue symptoms. [Preprint] medRxiv. (2021) Available at: https://www.medrxiv.org/content/10.1101/2021.10.27.21265575v1 (accessed February 21, 2021).
- 87.Nakatomi Y, Kuratsune H, Watanabe Y. [Neuroinflammation in the brain of patients with myalgic encephalomyelitis/chronic fatigue syndrome]. Brain Nerve. (2018) 70:19–25. 10.11477/mf.1416200945 [DOI] [PubMed] [Google Scholar]
- 88.Nakatomi Y, Mizuno K, Ishii A, Wada Y, Tanaka M, Tazawa S, et al. Neuroinflammation in patients with chronic fatigue syndrome/myalgic encephalomyelitis: an (1)(1)C-(R)-PK11195 PET study. J Nucl Med. (2014) 55:945–50. 10.2967/jnumed.113.131045 [DOI] [PubMed] [Google Scholar]
- 89.Mueller C, Lin JC, Sheriff S, Maudsley AA, Younger JW. Evidence of widespread metabolite abnormalities in Myalgic encephalomyelitis/chronic fatigue syndrome: assessment with whole-brain magnetic resonance spectroscopy. Brain Imaging Behav. (2020) 14:562–72. 10.1007/s11682-018-0029-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mu Q, Kirby J, Reilly CM, Luo XM. Leaky gut as a danger signal for autoimmune diseases. Front Immunol. (2017) 8:598. 10.3389/fimmu.2017.00598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fasano A. Leaky gut and autoimmune diseases. Clin Rev Allergy Immunol. (2012) 42:71–71 10.1007/s12016-011-8291-x [DOI] [PubMed] [Google Scholar]
- 92.Sotzny F, Blanco J, Capelli E, Castro-Marrero J, Steiner S, Murovska M, et al. Myalgic encephalomyelitis/chronic fatigue syndrome – evidence for an autoimmune disease. Autoimmun Rev. (2018) 17:601–9. 10.1016/j.autrev.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 93.Morris G, Berk M, Galecki P, Maes M. The emerging role of autoimmunity in myalgic encephalomyelitis/chronic fatigue syndrome (ME/cfs). Mol Neurobiol. (2014) 49:741–56. 10.1007/s12035-013-8553-0 [DOI] [PubMed] [Google Scholar]
- 94.Blomberg J, Gottfries CG, Elfaitouri A, Rizwan M, Rosén A. Infection elicited autoimmunity and myalgic encephalomyelitis/chronic fatigue syndrome: an explanatory model. Front Immunol. (2018) 9:229. 10.3389/fimmu.2018.00229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boonen E, Bornstein SR, Van den Berghe G. New insights into the controversy of adrenal function during critical illness. Lancet Diabetes Endocrinol. (2015) 3:805–15. 10.1016/S2213-8587(15)00224-7 [DOI] [PubMed] [Google Scholar]
- 96.Van den Berghe G. On the neuroendocrinopathy of critical illness. Perspectives for feeding and novel treatments. Am J Respir Crit Care Med. (2016) 194:1337–48. 10.1164/rccm.201607-1516CI [DOI] [PubMed] [Google Scholar]
- 97.Bergquist M, Huss F, Fredén F, Hedenstierna G, Hästbacka J, Rockwood AL, et al. Altered adrenal and gonadal steroids biosynthesis in patients with burn injury. Clin Mass Spectr. (2016) 1:19–26. 10.1016/j.clinms.2016.10.002 [DOI] [Google Scholar]
- 98.Marik PE. Mechanisms and clinical consequences of critical illness associated adrenal insufficiency. Curr Opin Crit Care. (2007) 13:363–9. 10.1097/MCC.0b013e32818a6d74 [DOI] [PubMed] [Google Scholar]
- 99.Boelen A, Kwakkel J, Thijssen-Timmer DC, Alkemade A, Fliers E, Wiersinga WM. Simultaneous changes in central and peripheral components of the hypothalamus-pituitary-thyroid axis in lipopolysaccharide-induced acute illness in mice. J Endocrinol. (2004) 182:315–23. 10.1677/joe.0.1820315 [DOI] [PubMed] [Google Scholar]
- 100.Joseph-Bravo P, Jaimes-Hoy L, Charli JL. Regulation of TRH neurons and energy homeostasis-related signals under stress. J Endocrinol. (2015) 224:R139–59. 10.1530/JOE-14-0593 [DOI] [PubMed] [Google Scholar]
- 101.Chatzitomaris A, Hoermann R, Midgley JE, Hering S, Urban A, Dietrich B, et al. Thyroid allostasis–adaptive responses of thyrotropic feedback control to conditions of strain, stress, and developmental programming. Front Endocrinol. (2017) 8:163. 10.3389/fendo.2017.00163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Harel G, Shamoun DS, Kane JP, Magner JA, Szabo M. Prolonged effects of tumor necrosis factor-alpha on anterior pituitary hormone release. Peptides. (1995) 16:641–5. 10.1016/0196-9781(95)00019-G [DOI] [PubMed] [Google Scholar]
- 103.Wassen FW, Moerings EP, Van Toor H, De Vrey EA, Hennemann G, Everts ME. Effects of interleukin-1 beta on thyrotropin secretion and thyroid hormone uptake in cultured rat anterior pituitary cells. Endocrinology. (1996) 137:1591–8. 10.1210/endo.137.5.8612490 [DOI] [PubMed] [Google Scholar]
- 104.Boonen E, Langouche L, Janssens T, Meersseman P, Vervenne H, De Samblanx E, et al. Impact of Duration of Critical Illness on the Adrenal Glands of Human Intensive Care Patients. J Clin Endocrinol Metab. (2014) 99:4214–22. 10.1210/jc.2014-2429 [DOI] [PubMed] [Google Scholar]
- 105.Téblick A, Peeters B, Langouche L, Van den Berghe G. Adrenal function and dysfunction in critically ill patients. Nat Rev Endocrinol. (2019) 15:417–27. 10.1038/s41574-019-0185-7 [DOI] [PubMed] [Google Scholar]
- 106.Weekers F, Van den Berghe G. Endocrine modifications and interventions during critical illness. Proc Nutr Soc. (2004) 63:443–50. 10.1079/PNS2004373 [DOI] [PubMed] [Google Scholar]
- 107.Baxter RC. Changes in the IGF–IGFBP axis in critical illness. Best Pract Res Clin Endocrinol Metab. (2001) 15:421–34. 10.1053/beem.2001.0161 [DOI] [PubMed] [Google Scholar]
- 108.Van den Berghe G, Wouters P, Weekers F, Mohan S, Baxter RC, Veldhuis JD, et al. Reactivation of pituitary hormone release and metabolic improvement by infusion of growth hormone-releasing peptide and thyrotropin-releasing hormone in patients with protracted critical illness. J Clin Endocrinol Metab. (1999) 84:1311–23. 10.1210/jc.84.4.1311 [DOI] [PubMed] [Google Scholar]
- 109.Litin SC. Mayo Clinic Family Health Book 5th Edition: Completely Revised and Updated. Rochester, MN: Mayo Clinic Press; (2018). [Google Scholar]
- 110.Hertoghe T. Atlas of Endocrinology for Hormone Therapy. Luxembourg: International Medical Books; (2010). [Google Scholar]
- 111.De Bellis A, Bellastella G, Pernice V, Cirillo P, Longo M, Maio A, et al. Hypothalamic-Pituitary autoimmunity and related impairment of hormone secretions in chronic fatigue syndrome. J Clin Endocrinol Metab. (2021) 106:e5147–55. 10.1210/clinem/dgab429 [DOI] [PubMed] [Google Scholar]
- 112.Berwaerts J, Moorkens G, Abs R. Secretion of growth hormone in patients with chronic fatigue syndrome. Growth Horm IGF Res. (1998) 8(Suppl. B):127–9. 10.1016/S1096-6374(98)80036-1 [DOI] [PubMed] [Google Scholar]
- 113.Moorkens G, Berwaerts J, Wynants H, Abs R. Characterization of pituitary function with emphasis on GH secretion in the chronic fatigue syndrome. Clin Endocrinol. (2000) 53:99–106. 10.1046/j.1365-2265.2000.01049.x [DOI] [PubMed] [Google Scholar]
- 114.Cleare AJ, Sookdeo SS, Jones J, O'Keane V, Miell JP. Integrity of the growth hormone/insulin-like growth factor system is maintained in patients with chronic fatigue syndrome. J Clin Endocrinol Metab. (2000) 85:1433–9. 10.1210/jc.85.4.1433 [DOI] [PubMed] [Google Scholar]
- 115.Teitelbaum J, Bird B. Effective Treatment of Severe Chronic Fatigue: A Report of a Series of 64 Patients. J Musculoskelet Pain. (1995) 3:91–110. 10.1300/J094v03n04_11 [DOI] [Google Scholar]
- 116.Holtorf K. Diagnosis and treatment of Hypothalamic-Pituitary-Adrenal (HPA) axis dysfunction in patients with chronic Fatigue Syndrome (CFS) and Fibromyalgia (FM). J Chronic Fatigue Syndr. (2008) 14:59–88. 10.1300/J092v14n03_06 [DOI] [Google Scholar]
- 117.Fuite J, Vernon SD, Broderick G. Neuroendocrine and immune network re-modeling in chronic fatigue syndrome: An exploratory analysis. Genomics. (2008) 92:393–9. 10.1016/j.ygeno.2008.08.008 [DOI] [PubMed] [Google Scholar]
- 118.Holtorf K. Thyroid hormone transport into cellular tissue. J Restor Med. (2014) 3:53–68. 10.14200/jrm.2014.3.0104 [DOI] [Google Scholar]
- 119.Holtorf K. Peripheral thyroid hormone conversion and its impact on TSH and metabolic activity. J Restor Med. (2014) 3:30–52. 10.14200/jrm.2014.3.0103 [DOI] [Google Scholar]
- 120.Ruiz-Núñez B, Tarasse R, Vogelaar EF, Janneke Dijck-Brouwer DA, Muskiet FAJ. Higher prevalence of “low T3 syndrome” in patients with chronic fatigue syndrome: a case–control study. Front Endocrinol. (2018) 9:97. 10.3389/fendo.2018.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Poteliakhoff A. Adrenocortical activity and some clinical findings in acute and chronic fatigue. J Psychosom Res. (1981) 25:91–5. 10.1016/0022-3999(81)90095-7 [DOI] [PubMed] [Google Scholar]
- 122.Demitrack MA, Dale JK, Straus SE, Laue L, Listwak SJ, Kruesi MJ, et al. Evidence for impaired activation of the hypothalamic-pituitary-adrenal axis in patients with chronic fatigue syndrome. J Clin Endocrinol Metab. (1991) 73:1224–34. 10.1210/jcem-73-6-1224 [DOI] [PubMed] [Google Scholar]
- 123.Scott LV, Medbak S, Dinan TG. Blunted adrenocorticotropin and cortisol responses to corticotropin-releasing hormone stimulation in chronic fatigue syndrome. Acta Psychiatr Scand. (1998) 97:450–7. 10.1111/j.1600-0447.1998.tb10030.x [DOI] [PubMed] [Google Scholar]
- 124.De Becker P, De Meirleir K, Joos E, Campine I, Van Steenberge E, Smitz J, et al. Dehydroepiandrosterone (DHEA) response to iv ACTH in patients with chronic fatigue syndrome. Horm Metab Res. (1999) 31:18–21. 10.1055/s-2007-978690 [DOI] [PubMed] [Google Scholar]
- 125.Cleare AJ, Miell J, Heap E, Sookdeo S, Young L, Malhi GS, et al. Hypothalamo-pituitary-adrenal axis dysfunction in chronic fatigue syndrome, and the effects of low-dose hydrocortisone therapy. J Clin Endocrinol Metab. (2001) 86:3545–54. 10.1210/jcem.86.8.7735 [DOI] [PubMed] [Google Scholar]
- 126.Gaab J, Huster D, Peisen R, Engert V, Heitz V, Schad T, et al. Hypothalamic-pituitary-adrenal axis reactivity in chronic fatigue syndrome and health under psychological, physiological, and pharmacological stimulation. Psychosom Med. (2002) 64:951–62. 10.1097/00006842-200211000-00012 [DOI] [PubMed] [Google Scholar]
- 127.Jerjes WK, Cleare AJ, Wessely S, Wood PJ, Taylor NF. Diurnal patterns of salivary cortisol and cortisone output in chronic fatigue syndrome. J Affect Disord. (2005) 87:299–304. 10.1016/j.jad.2005.03.013 [DOI] [PubMed] [Google Scholar]
- 128.Segal TY, Hindmarsh PC, Viner RM. Disturbed adrenal function in adolescents with chronic fatigue syndrome. J Pediatr Endocrinol Metab. (2005) 18:295–301. 10.1515/JPEM.2005.18.3.295 [DOI] [PubMed] [Google Scholar]
- 129.Van Den Eede F, Moorkens G, Hulstijn W, Van Houdenhove B, Cosyns P, Sabbe BG, et al. Combined dexamethasone/corticotropin-releasing factor test in chronic fatigue syndrome. Psychol Med. (2008) 38:963–73. 10.1017/S0033291707001444 [DOI] [PubMed] [Google Scholar]
- 130.Van Den Eede F, Moorkens G, Van Houdenhove B, Cosyns P, Claes SJ. Hypothalamic-pituitary-adrenal axis function in chronic fatigue syndrome. Neuropsychobiology. (2007) 55:112–20. 10.1159/000104468 [DOI] [PubMed] [Google Scholar]
- 131.Papadopoulos AS, Cleare AJ. Hypothalamic-pituitary-adrenal axis dysfunction in chronic fatigue syndrome. Nat Rev Endocrinol. (2011) 8:22–32. 10.1038/nrendo.2011.153 [DOI] [PubMed] [Google Scholar]
- 132.Craddock TJ, Fritsch P, Rice MA Jr, del Rosario RM, Miller DB, et al. A role for homeostatic drive in the perpetuation of complex chronic illness: Gulf War Illness and chronic fatigue syndrome. PLoS ONE. (2014) 9:e84839. 10.1371/journal.pone.0084839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gaab J, Engert V, Heitz V, Schad T, Schurmeyer TH, Ehlert U. Associations between neuroendocrine responses to the Insulin Tolerance Test and patient characteristics in chronic fatigue syndrome. J Psychosom Res. (2004) 56:419–24. 10.1016/S0022-3999(03)00625-1 [DOI] [PubMed] [Google Scholar]
- 134.Tomas C, Newton J, Watson S A. review of hypothalamic-pituitary-adrenal axis function in chronic fatigue syndrome. ISRN Neurosci. (2013) 2013:784520. 10.1155/2013/784520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Di Giorgio A, Hudson M, Jerjes W, Cleare AJ. 24-hour pituitary and adrenal hormone profiles in chronic fatigue syndrome. Psychosom Med. (2005) 67:433–40. 10.1097/01.psy.0000161206.55324.8a [DOI] [PubMed] [Google Scholar]
- 136.Pednekar DD, Amin MR, Fekri Azgomi H, Aschbacher K, Crofford LJ, Faghih RT. Characterization of cortisol dysregulation in fibromyalgia and chronic fatigue syndromes: a state-space approach. IEEE Trans Biomed Eng. (2020) 67:3163–72. 10.1109/TBME.2020.2978801 [DOI] [PubMed] [Google Scholar]
- 137.Morris G, Anderson G, Maes M. Hypothalamic-pituitary-adrenal hypofunction in Myalgic Encephalomyelitis (ME)/Chronic Fatigue Syndrome (CFS) as a consequence of activated immune-inflammatory and oxidative and nitrosative pathways. Mol Neurobiol. (2017) 54:6806–19. 10.1007/s12035-016-0170-2 [DOI] [PubMed] [Google Scholar]
- 138.Hatziagelaki E, Adamaki M, Tsilioni I, Dimitriadis G, Theoharides TC. Myalgic encephalomyelitis/chronic fatigue syndrome-metabolic disease or disturbed homeostasis due to focal inflammation in the hypothalamus? J Pharmacol Exp Ther. (2018) 367:155–67. 10.1124/jpet.118.250845 [DOI] [PubMed] [Google Scholar]
- 139.Jason LA, Porter N, Herrington J, Sorenson M, Kubow S. Kindling and oxidative stress as contributors to myalgic encephalomyelitis/chronic fatigue syndrome. J Behav Neurosci Res. (2009) 7:1–17. [PMC free article] [PubMed] [Google Scholar]
- 140.Morris G, Maes M. Oxidative and nitrosative stress and immune-inflammatory pathways in patients with Myalgic Encephalomyelitis (ME)/Chronic Fatigue Syndrome (CFS). Curr Neuropharmacol. (2014) 12:168–85. 10.2174/1570159X11666131120224653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gupta S, Aslakson E, Gurbaxani BM, Vernon SD. Inclusion of the glucocorticoid receptor in a hypothalamic pituitary adrenal axis model reveals bistability. Theor Biol Med Model. (2007) 4:8. 10.1186/1742-4682-4-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ben-Zvi A, Vernon SD, Broderick G. Model-based therapeutic correction of hypothalamic-pituitary-adrenal axis dysfunction. PLoS Comput Biol. (2009) 5:e1000273. 10.1371/journal.pcbi.1000273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sedghamiz H, Morris M, Craddock TJA, Whitley D, Broderick G. High-fidelity discrete modeling of the HPA axis: a study of regulatory plasticity in biology. BMC Syst Biol. (2018) 12:76. 10.1186/s12918-018-0599-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hosseinichimeh N, Rahmandad H, Wittenborn AK. Modeling the hypothalamus-pituitary-adrenal axis: A review and extension. Math Biosci. (2015) 268:52–65. 10.1016/j.mbs.2015.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Craddock TJ, Del Rosario RR, Rice M, Zysman JP, Fletcher MA, Klimas NG, et al. Achieving remission in gulf war illness: a simulation-based approach to treatment design. PLoS ONE. (2015) 10:e0132774. 10.1371/journal.pone.0132774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Morris MC, Cooney KE, Sedghamiz H, Abreu M, Collado F, Balbin EG, et al. Leveraging prior knowledge of endocrine immune regulation in the therapeutically relevant phenotyping of women with chronic fatigue syndrome. Clin Ther. (2019) 41:656–74 e4. 10.1016/j.clinthera.2019.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Scott LV, Teh J, Reznek R, Martin A, Sohaib A, Dinan TG. Small adrenal glands in chronic fatigue syndrome: a preliminary computer tomography study. Psychoneuroendocrinology. (1999) 24:759–68. 10.1016/S0306-4530(99)00028-1 [DOI] [PubMed] [Google Scholar]
- 148.Boelen A, Platvoet-Ter Schiphorst MC, Wiersinga WM. Association between serum interleukin-6 and serum 3,5,3'-triiodothyronine in nonthyroidal illness. J Clin Endocrinol Metab. (1993) 77:1695–9. 10.1210/jcem.77.6.8263160 [DOI] [PubMed] [Google Scholar]
- 149.Davies PH, Black EG, Sheppard MC, Franklyn JA. Relation between serum interleukin-6 and thyroid hormone concentrations in 270 hospital in-patients with non-thyroidal illness. Clin Endocrinol. (1996) 44:199–205. 10.1046/j.1365-2265.1996.668489.x [DOI] [PubMed] [Google Scholar]
- 150.Warner MH, Beckett GJ. Mechanisms behind the non-thyroidal illness syndrome: an update. J Endocrinol. (2010) 205:1–13. 10.1677/JOE-09-0412 [DOI] [PubMed] [Google Scholar]
- 151.Wajner SM, Goemann IM, Bueno AL, Larsen PR, Maia AL. IL-6 promotes nonthyroidal illness syndrome by blocking thyroxine activation while promoting thyroid hormone inactivation in human cells. J Clin Invest. (2011) 121:1834–45. 10.1172/JCI44678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wajner SM, Maia AL. New insights toward the acute non-thyroidal illness syndrome. Front Endocrinol. (2012) 3:8. 10.3389/fendo.2012.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Carter JN, Eastman CJ, Corcoran JM, Lazarus L. Effect of severe, chronic illness on thyroid function. Lancet. (1974) 2:971–4. 10.1016/S0140-6736(74)92070-4 [DOI] [PubMed] [Google Scholar]
- 154.Van den Berghe G. Novel insights in the HPA-axis during critical illness. Acta Clin Belg. (2014) 69:397–406. 10.1179/2295333714Y.0000000093 [DOI] [PubMed] [Google Scholar]
- 155.Bartalena L, Farsetti A, Flink IL, Robbins J. Effects of interleukin-6 on the expression of thyroid hormone-binding protein genes in cultured human hepatoblastoma-derived (Hep G2) cells. Mol Endocrinol. (1992) 6:935–42. 10.1210/mend.6.6.1323058 [DOI] [PubMed] [Google Scholar]
- 156.Bartalena L, Bogazzi F, Brogioni S, Grasso L, Martino E. Role of cytokines in the pathogenesis of the euthyroid sick syndrome. Eur J Endocrinol. (1998) 138 6:603–14. 10.1530/eje.0.1380603 [DOI] [PubMed] [Google Scholar]
- 157.Afandi B, Vera R, Schussler GC, Yap MG. Concordant decreases of thyroxine and thyroxine binding protein concentrations during sepsis. Metabolism. (2000) 49:753–4. 10.1053/meta.2000.6239 [DOI] [PubMed] [Google Scholar]
- 158.Bartalena L, Robbins J. Variations in thyroid hormone transport proteins and their clinical implications. Thyroid. (1992) 2:237–45. 10.1089/thy.1992.2.237 [DOI] [PubMed] [Google Scholar]
- 159.Mebis L, Paletta D, Debaveye Y, Ellger B, Langouche L, D'Hoore A, et al. Expression of thyroid hormone transporters during critical illness. Eur J Endocrinol. (2009) 161:243. 10.1530/EJE-09-0290 [DOI] [PubMed] [Google Scholar]
- 160.Huang SA, Mulcahey MA, Crescenzi A, Chung M, Kim BW, Barnes C, et al. Transforming growth factor-beta promotes inactivation of extracellular thyroid hormones via transcriptional stimulation of type 3 iodothyronine deiodinase. Mol Endocrinol. (2005) 19:3126–36. 10.1210/me.2005-0173 [DOI] [PubMed] [Google Scholar]
- 161.Kwakkel J, Wiersinga WM, Boelen A. Interleukin-1beta modulates endogenous thyroid hormone receptor alpha gene transcription in liver cells. J Endocrinol. (2007) 194:257–65. 10.1677/JOE-06-0177 [DOI] [PubMed] [Google Scholar]
- 162.Rodriguez-Perez A, Palos-Paz F, Kaptein E, Visser TJ, Dominguez-Gerpe L, Alvarez-Escudero J, et al. Identification of molecular mechanisms related to nonthyroidal illness syndrome in skeletal muscle and adipose tissue from patients with septic shock. Clin Endocrinol. (2008) 68:821–7. 10.1111/j.1365-2265.2007.03102.x [DOI] [PubMed] [Google Scholar]
- 163.Lado-Abeal J, Romero A, Castro-Piedras I, Rodriguez-Perez A, Alvarez-Escudero J. Thyroid hormone receptors are down-regulated in skeletal muscle of patients with non-thyroidal illness syndrome secondary to non-septic shock. Eur J Endocrinol. (2010) 163:765–73. 10.1530/EJE-10-0376 [DOI] [PubMed] [Google Scholar]
- 164.Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, et al. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev. (2008) 29:898–938. 10.1210/er.2008-0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.De Groot LJ. The non-thyroidal illness syndrome. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dungan K, et al. editors. Endotext. South Dartmouth, MA: MDText.com, Inc. (2000). [PubMed] [Google Scholar]
- 166.Dietrich JW, Landgrafe-Mende G, Wiora E, Chatzitomaris A, Klein HH, Midgley JE, et al. Calculated parameters of thyroid homeostasis: emerging tools for differential diagnosis and clinical research. Front Endocrinol. (2016) 7:57. 10.3389/fendo.2016.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mendoza A, Hollenberg AN. New insights into thyroid hormone action. Pharmacol Ther. (2017) 173:135–45. 10.1016/j.pharmthera.2017.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cicatiello AG, Di Girolamo D, Dentice M. Metabolic effects of the intracellular regulation of thyroid hormone: old players, new concepts. Front Endocrinol. (2018) 9:474. 10.3389/fendo.2018.00474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.De Groot LJ. Dangerous dogmas in medicine: the nonthyroidal illness syndrome. J Clin Endocrinol Metab. (1999) 84:151–64. 10.1210/jcem.84.1.5364 [DOI] [PubMed] [Google Scholar]
- 170.Donzelli R, Colligiani D, Kusmic C, Sabatini M, Lorenzini L, Accorroni A, et al. Effect of hypothyroidism and hyperthyroidism on tissue thyroid hormone concentrations in rat. Eur Thyroid J. (2016) 5:27–34. 10.1159/000443523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Plikat K, Langgartner J, Buettner R, Bollheimer LC, Woenckhaus U, Scholmerich J, et al. Frequency and outcome of patients with nonthyroidal illness syndrome in a medical intensive care unit. Metabolism. (2007) 56:239–44. 10.1016/j.metabol.2006.09.020 [DOI] [PubMed] [Google Scholar]
- 172.Boelen A, Kwakkel J, Fliers E. Beyond low plasma T3: local thyroid hormone metabolism during inflammation and infection. Endocr Rev. (2011) 32:670–93. 10.1210/er.2011-0007 [DOI] [PubMed] [Google Scholar]
- 173.Mancini A, Di Segni C, Raimondo S, Olivieri G, Silvestrini A, Meucci E, et al. Thyroid hormones, oxidative stress, and inflammation. Mediators Inflamm. (2016) 2016:6757154. 10.1155/2016/6757154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Balazs C, Leovey A, Szabo M, Bako G. Stimulating effect of triiodothyronine on cell-mediated immunity. Eur J Clin Pharmacol. (1980) 17:19–23. 10.1007/BF00561672 [DOI] [PubMed] [Google Scholar]
- 175.Pillay K. Congenital hypothyroidism and immunodeficiency: evidence for an endocrine-immune interaction. J Pediatr Endocrinol Metab. (1998) 11:757–61. 10.1515/JPEM.1998.11.6.757 [DOI] [PubMed] [Google Scholar]
- 176.Klecha AJ, Genaro AM, Gorelik G, Barreiro Arcos ML, Silberman DM, Schuman M, et al. Integrative study of hypothalamus-pituitary-thyroid-immune system interaction: thyroid hormone-mediated modulation of lymphocyte activity through the protein kinase C signaling pathway. J Endocrinol. (2006) 189:45–55. 10.1677/joe.1.06137 [DOI] [PubMed] [Google Scholar]
- 177.Klein JR. The immune system as a regulator of thyroid hormone activity. Exp Biol Med. (2006) 231:229–36. 10.1177/153537020623100301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hans VH, Lenzlinger PM, Joller-Jemelka HI, Morganti-Kossmann MC, Kossmann T. Low T3 syndrome in head-injured patients is associated with prolonged suppression of markers of cell-mediated immune response. Eur J Trauma. (2005) 31:359–68. 10.1007/s00068-005-2068-y [DOI] [Google Scholar]
- 179.Hodkinson CF, Simpson EE, Beattie JH, O'Connor JM, Campbell DJ, Strain JJ, et al. Preliminary evidence of immune function modulation by thyroid hormones in healthy men and women aged 55-70 years. J Endocrinol. (2009) 202:55–63. 10.1677/JOE-08-0488 [DOI] [PubMed] [Google Scholar]
- 180.Straub RH, Cutolo M, Buttgereit F, Pongratz G. Energy regulation and neuroendocrine-immune control in chronic inflammatory diseases. J Intern Med. (2010) 267:543–60. 10.1111/j.1365-2796.2010.02218.x [DOI] [PubMed] [Google Scholar]
- 181.Jara EL, Munoz-Durango N, Llanos C, Fardella C, Gonzalez PA, Bueno SM, et al. Modulating the function of the immune system by thyroid hormones and thyrotropin. Immunol Lett. (2017) 184:76–83. 10.1016/j.imlet.2017.02.010 [DOI] [PubMed] [Google Scholar]
- 182.Bilal MY, Dambaeva S, Kwak-Kim J, Gilman-Sachs A, Beaman KD. A role for iodide and thyroglobulin in modulating the function of human immune cells. Front Immunol. (2017) 8:1573. 10.3389/fimmu.2017.01573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.van der Spek AH, Surovtseva OV, Jim KK, van Oudenaren A, Brouwer MC, Vandenbroucke-Grauls C, et al. Regulation of intracellular triiodothyronine is essential for optimal macrophage function. Endocrinology. (2018) 159:2241–52. 10.1210/en.2018-00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.De Vito P, Incerpi S, Pedersen JZ, Luly P, Davis FB, Davis PJ. Thyroid hormones as modulators of immune activities at the cellular level. Thyroid. (2011) 21:879–90. 10.1089/thy.2010.0429 [DOI] [PubMed] [Google Scholar]
- 185.De Luca R, Davis PJ, Lin HY, Gionfra F, Percario ZA, Affabris E, et al. Thyroid hormones interaction with immune response, inflammation and non-thyroidal illness syndrome. Front Cell Dev Biol. (2020) 8:614030. 10.3389/fcell.2020.614030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Textoris J, Mallet F. Immunosuppression and herpes viral reactivation in intensive care unit patients: one size does not fit all. Crit Care. (2017) 21:230. 10.1186/s13054-017-1803-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Coşkun O, Yazici E, Sahiner F, Karakaş A, Kiliç S, Tekin M, et al. Cytomegalovirus and Epstein–Barr virus reactivation in the intensive care unit. Med Klin Intensivmed Notfallmed. (2017) 112:239–45. 10.1007/s00063-016-0198-0 [DOI] [PubMed] [Google Scholar]
- 188.Walton AH, Muenzer JT, Rasche D, Boomer JS, Sato B, Brownstein BH, et al. Reactivation of multiple viruses in patients with sepsis. PLoS ONE. (2014) 9:e98819. 10.1371/journal.pone.0098819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pall M. The NO/ONOO-cycle mechanism as the cause of chronic fatigue syndrome/myalgia encephalomyelitis. In: Svoboda E, Zelenjcik K, editors. Chronic Fatigue Syndrome: Symptoms, Causes and Prevention. Hauppauge, NY: Nova Publishers; (2009). p. 27–56 [Google Scholar]
- 190.Morris G, Maes M. Mitochondrial dysfunctions in myalgic encephalomyelitis/chronic fatigue syndrome explained by activated immuno-inflammatory, oxidative and nitrosative stress pathways. Metab Brain Dis. (2014) 29:19–36. 10.1007/s11011-013-9435-x [DOI] [PubMed] [Google Scholar]
- 191.Armstrong CW, McGregor NR, Lewis DP, Butt HL, Gooley PR. Metabolic profiling reveals anomalous energy metabolism and oxidative stress pathways in chronic fatigue syndrome patients. Metabolomics. (2015) 11:1626–39. 10.1007/s11306-015-0816-5 [DOI] [Google Scholar]
- 192.Shungu DC, Weiduschat N, Murrough JW, Mao X, Pillemer S, Dyke JP, et al. Increased ventricular lactate in chronic fatigue syndrome. III Relationships to cortical glutathione and clinical symptoms implicate oxidative stress in disorder pathophysiology. NMR Biomed. (2012) 25:1073–87. 10.1002/nbm.2772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Montoya JG, Holmes TH, Anderson JN, Maecker HT, Rosenberg-Hasson Y, Valencia IJ, et al. Cytokine signature associated with disease severity in chronic fatigue syndrome patients. Proc Natl Acad Sci USA. (2017) 114:E7150–8. 10.1073/pnas.1710519114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hornig M, Montoya JG, Klimas NG, Levine S, Felsenstein D, Bateman L, et al. Distinct plasma immune signatures in ME/CFS are present early in the course of illness. Sci Adv. (2015) 1:e1400121. 10.1126/sciadv.1400121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Langouche L, Van den Berghe G. Hypothalamic-pituitary hormones during critical illness: a dynamic neuroendocrine response. Handb Clin Neurol. (2014) 124:115–26. 10.1016/B978-0-444-59602-4.00008-3 [DOI] [PubMed] [Google Scholar]
- 196.Boonen E, Van den Berghe G. Endocrine responses to critical illness: novel insights and therapeutic implications. J Clin Endocrinol Metab. (2014) 99:1569–82. 10.1210/jc.2013-4115 [DOI] [PubMed] [Google Scholar]
- 197.Deane A, Chapman MJ, Fraser RJ, Horowitz M. Bench-to-bedside review: the gut as an endocrine organ in the critically ill. Crit Care. (2010) 14:228. 10.1186/cc9039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Van den Berghe G, de Zegher F, Baxter RC, Veldhuis JD, Wouters P, Schetz M, et al. Neuroendocrinology of prolonged critical illness: effects of exogenous thyrotropin-releasing hormone and its combination with growth hormone secretagogues. J Clin Endocrinol Metab. (1998) 83:309–19. 10.1210/jc.83.2.309 [DOI] [PubMed] [Google Scholar]
- 199.Mesotten D, Van den Berghe G. Changes within the growth hormone/insulin-like growth factor I/IGF binding protein axis during critical illness. Endocrinol Metab Clin North Am. (2006) 35:793–805, ix–x. 10.1016/j.ecl.2006.09.010 [DOI] [PubMed] [Google Scholar]
- 200.Wu R, Dong W, Ji Y, Zhou M, Marini CP, Ravikumar TS, et al. Orexigenic hormone ghrelin attenuates local and remote organ injury after intestinal ischemia-reperfusion. PLoS ONE. (2008) 3:e2026. 10.1371/journal.pone.0002026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Debaveye Y, Ellger B, Mebis L, Darras VM, Van den Berghe G. Regulation of tissue iodothyronine deiodinase activity in a model of prolonged critical illness. Thyroid. (2008) 18:551–60. 10.1089/thy.2007.0287 [DOI] [PubMed] [Google Scholar]
- 202.Lizcano F, Rodríguez JS. Thyroid hormone therapy modulates hypothalamo-pituitary-adrenal axis. Endocr J. (2011) 58:137–42. 10.1507/endocrj.K10E-369 [DOI] [PubMed] [Google Scholar]
- 203.Sánchez-Franco F, Fernández L, Fernández G, Cacicedo L. Thyroid hormone action on ACTH secretion. Horm Metab Res. (1989) 21:550–2. 10.1055/s-2007-1009285 [DOI] [PubMed] [Google Scholar]
- 204.Hiroi Y, Kim HH, Ying H, Furuya F, Huang Z, Simoncini T, et al. Rapid nongenomic actions of thyroid hormone. Proc Natl Acad Sci USA. (2006) 103:14104–9. 10.1073/pnas.0601600103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sharma JN, Al-Omran A, Parvathy SS. Role of nitric oxide in inflammatory diseases. Inflammopharmacology. (2007) 15:252–9. 10.1007/s10787-007-0013-x [DOI] [PubMed] [Google Scholar]
- 206.Handa O, Stephen J, Cepinskas G. Role of endothelial nitric oxide synthase-derived nitric oxide in activation and dysfunction of cerebrovascular endothelial cells during early onsets of sepsis. Am J Physiol Heart Circ Physiol. (2008) 295:H1712–9. 10.1152/ajpheart.00476.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Gluvic ZM, Obradovic MM, Sudar-Milovanovic EM, Zafirovic SS, Radak DJ, Essack MM, et al. Regulation of nitric oxide production in hypothyroidism. Biomed Pharmacother. (2020) 124:109881. 10.1016/j.biopha.2020.109881 [DOI] [PubMed] [Google Scholar]
- 208.Bertinat R, Villalobos-Labra R, Hofmann L, Blauensteiner J, Sepúlveda N, Westermeier F. Decreased NO production in endothelial cells exposed to plasma from ME/CFS patients. Vascul Pharmacol. (2022) 148:106953. 10.1016/j.vph.2022.106953 [DOI] [PubMed] [Google Scholar]
- 209.Lim CF, Docter R, Visser TJ, Krenning EP, Bernard B, van Toor H, et al. Inhibition of thyroxine transport into cultured rat hepatocytes by serum of nonuremic critically ill patients: effects of bilirubin and nonesterified fatty acids. J Clin Endocrinol Metab. (1993) 76:1165–72. 10.1210/jcem.76.5.8496307 [DOI] [PubMed] [Google Scholar]
- 210.Vos RA, De Jong M, Bernard BF, Docter R, Krenning EP, Hennemann G. Impaired thyroxine and 3,5,3'-triiodothyronine handling by rat hepatocytes in the presence of serum of patients with nonthyroidal illness. J Clin Endocrinol Metab. (1995) 80:2364–70. 10.1210/jcem.80.8.7629231 [DOI] [PubMed] [Google Scholar]
- 211.Preiser JC, Ichai C, Orban JC, Groeneveld AB. Metabolic response to the stress of critical illness. Br J Anaesth. (2014) 113:945–54. 10.1093/bja/aeu187 [DOI] [PubMed] [Google Scholar]
- 212.McBride MA, Owen AM, Stothers CL, Hernandez A, Luan L, Burelbach KR, et al. The metabolic basis of immune dysfunction following sepsis and trauma. Front Immunol. (2020) 11:1043. 10.3389/fimmu.2020.01043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Singer M. Critical illness and flat batteries. Crit Care. (2017) 21:309. 10.1186/s13054-017-1913-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Naviaux RK, Naviaux JC Li K, Bright AT, Alaynick WA, Wang L, et al. Metabolic features of chronic fatigue syndrome. Proc Natl Acad Sci USA. (2016) 113:E5472–80. 10.1073/pnas.1607571113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Naviaux RK. Perspective: Cell danger response Biology—The new science that connects environmental health with mitochondria and the rising tide of chronic illness. Mitochondrion. (2020) 51:40–5. 10.1016/j.mito.2019.12.005 [DOI] [PubMed] [Google Scholar]
- 216.Naviaux RK. Metabolic features and regulation of the healing cycle-a new model for chronic disease pathogenesis and treatment. Mitochondrion. (2019) 46:278–97. 10.1016/j.mito.2018.08.001 [DOI] [PubMed] [Google Scholar]
- 217.Arnett SV, Clark IA. Inflammatory fatigue and sickness behaviour - lessons for the diagnosis and management of chronic fatigue syndrome. J Affect Disord. (2012) 141:130–42. 10.1016/j.jad.2012.04.004 [DOI] [PubMed] [Google Scholar]
- 218.Greenhalgh T, Knight M, A'Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. (2020) 370:m3026. 10.1136/bmj.m3026 [DOI] [PubMed] [Google Scholar]
- 219.Dani M, Dirksen A, Taraborrelli P, Torocastro M, Panagopoulos D, Sutton R, et al. Autonomic dysfunction in 'long COVID': rationale, physiology and management strategies. Clin Med. (2020) 21:e63–7. 10.7861/clinmed.2020-0896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. (2021) 397:220–32. 10.1016/S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Townsend L, Dyer AH, Jones K, Dunne J, Mooney A, Gaffney F, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS ONE. (2020) 15:e0240784. 10.1371/journal.pone.0240784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Komaroff AL, Bateman L. Will COVID-19 lead to myalgic encephalomyelitis/chronic fatigue syndrome? Front Med. (2021) 7:606824. 10.3389/fmed.2020.606824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wildwing T, Holt N. The neurological symptoms of COVID-19: a systematic overview of systematic reviews, comparison with other neurological conditions and implications for healthcare services. Ther Adv Chronic Dis. (2021) 12. 10.1177/2040622320976979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Proal AD, VanElzakker MB. Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol. (2021) 12:698169. 10.3389/fmicb.2021.698169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Mackay A A. Paradigm for post-Covid-19 fatigue syndrome analogous to ME/CFS. Front Neurol. (2021) 12:701419. 10.3389/fneur.2021.701419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Komaroff AL, Lipkin WI. Insights from myalgic encephalomyelitis/chronic fatigue syndrome may help unravel the pathogenesis of postacute COVID-19 syndrome. Trends Mol Med. (2021) 27:895–906. 10.1016/j.molmed.2021.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Comella PH, Gonzalez-Kozlova E, Kosoy R, Charney AW, Peradejordi IF, Chandrasekar S, et al. A Molecular network approach reveals shared cellular and molecular signatures between chronic fatigue syndrome and other fatiguing illnesses. [Preprint] medRxiv. (2021). Available at: https://www.medrxiv.org/content/10.1101/2021.01.29.21250755v1 (accessed February 21, 2021).
- 228.Paul BD, Lemle MD, Komaroff AL, Snyder SH. Redox imbalance links COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome. Proc Natl Acad Sci USA. (2021) 118:e2024358118. 10.1073/pnas.2024358118 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.