Abstract
Acute disseminated encephalomyelitis (ADEM) is an acute demyelinating disorder of the central nervous system that is ordinarily monophasic. ADEM can develop following infection or vaccination. Here, we present a 37 y/o male patient with progressive muscle weakness in all limbs along with dysphagia following COVID-19 vaccination. Brain magnetic resonance imaging (MRI) revealed typical imaging findings which presented as multifocal T2-FLAIR signal changes in the corticospinal tract, pons, and temporal lobe with diffusion restriction. Magnetic resonance spectroscopy (MRS) further confirmed the diagnosis by the typical elevation of the Choline and Myoinositol peaks. Neurologic impairments have been reported as the potential side effects of COVID-19 vaccines. Appropriate imaging modalities together with a thorough clinical examination are essential for making a correct diagnosis.
Keywords: Brain ,MRI ,MRS; ADEM; Demyelinating disease
Background
Acute disseminated encephalomyelitis (ADEM) is an intense disorder identifies by multifocal demyelinating white matter in the central nervous system (CNS). Dispersed neurologic signs in harmony with geographic distribution in the CNS, which are observed in imaging examination, confirm the condition [1], [2], [3]. There are some pieces of evidence that vaccinations may provoke autoimmune demyelination. Previous studies revealed that ADEM events were linked with immunization for several infectious agents such as influenza, varicella, measles, mumps, rubella, tetanus, diphtheria, pertussis, and hepatitis B [4], [5], [6]. Coronavirus 2019 infection (COVID-19) is an overwhelming pandemic, which has so far led to the death of over 4 million patients globally [7,8]. From December 2020, vaccination was approved as a safe and imminent solution for the completion of the critical condition and protected the individuals from the virus [9,10]. Besides all the positive aspects of the COVID-19 vaccines, some adverse effects were observed, that based on reports, ADEM is one of them [11], [12], [13], [14]. Here, we report a case with signs, and symptoms of ADEM diagnosed after COVID-19 vaccination.
Case presentation
A previously healthy Iranian 37 y/o male, municipal labor, referred the emergency department (ED) of a local hospital on the June 16 with progressive weakness of 4 limbs, dysphagia, drooling, nausea, and vomiting. On arrival to the ED, vital signs showed a temperature of 37°C, heart rate of 88 bpm, blood pressure of 115 of 80 mm Hg, and respiratory rate of 20/min. On neurologic examination, he was completely alert to the time, place, and person. He was completely alert to the time, place and person. He had signs, and symptoms of bilateral facial nerve paralysis. Pupils were 3 mm and reactive to light bilaterally. No sensory level was detectable in the examination. The muscle power was 2 of 5 in all 4 limbs. Extensor plantar response existed bilaterally. The patient had no known COVID-19 exposures, history of a recent viral illness, or other recognized risk factors. His COVID-19 PCR was negative. The only significant past medical history was that the patient had taken the first dose of the Sinopharm vaccine (Intramuscular in the left Deltoid muscle, 0.5 mL auto-disabled syringes with 23G × 1″ needle; manufactured in Beijing Institute of Biological Products with Lot No. 2021030257) 1 month before.
In the days after the injection of the first dose of the vaccine on the May 15, the patient became lethargic. His condition aggravated in the following days with intermittent myalgia, which was treated symptomatically with subsequent partial improvement. He was admitted to the hospital with suspicion of Guillain-Barre syndrome (GBS). The individual's electrocardiography (ECG) was without problem. The next day, with the probability of requiring plasmapheresis and receiving IVIG, he was transferred to the intensive care unit (ICU) of the tertiary referral hospital of the province.
Laboratory parameters in the primary workup revealed a hemoglobin level of 14.1 g/dL, white blood cell (WBC) count of 11,600/µL, a platelet (plt) count of 305,000/mm3, and prothrombin time (PT) of 17.5 seconds, partial thromboplastin time (PTT) of 37.2 seconds, and the international normalized ratio (INR) of 1.27. The erythrocyte sedimentation rate (ESR) of 23 mm/1st hour and C-reactive protein (CRP) were negative. Blood culture results were negative for any significant infection. Serum chemistry, including kidney and liver function tests, was within the normal range. Lumbar puncture for CSF analysis was conducted to rule out the infectious process, which demonstrated 2 WBCs, 32 RBCs, 56 mg/dL protein, and glucose of 97 mg/dL vs serum glucose of 159. At the same time, a search was made to find IgG oligoclonal bands that were not detected. There was no evidence of urinary tract infection confirmed by non–reactive urine analysis and urine culture.
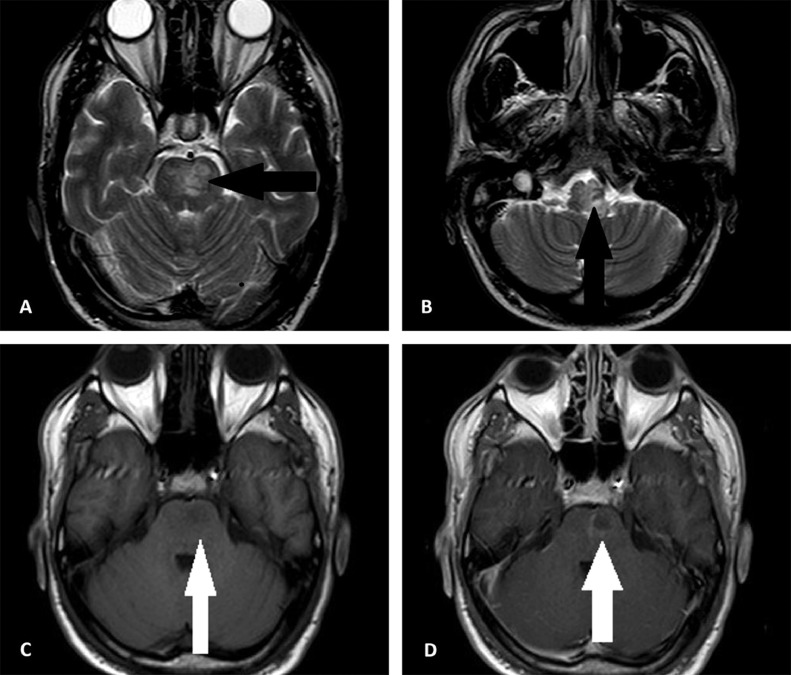
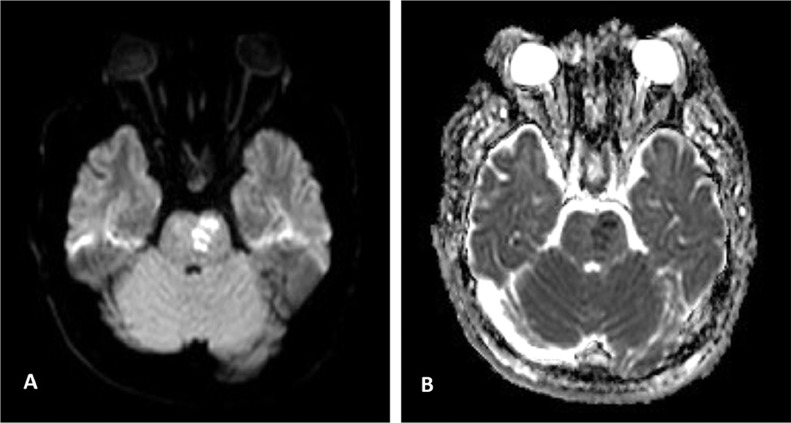
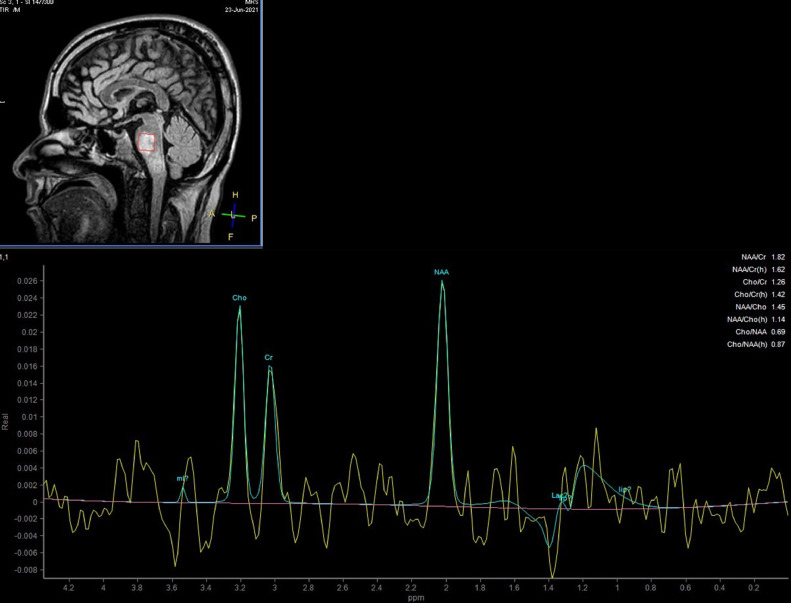
The next day, he was transferred to the intensive care unit (ICU) of the tertiary referral hospital of the province with the probability of requiring plasmapheresis, and receiving IVIG. Blood gas analysis on the first day of admission in ICU exhibited respiratory alkalosis. His oxygen saturation (O2 sat) was 79.4% without ancillary oxygen. Brain magnetic resonance imaging (MRI), performed on the second day of ICU admission, and revealed hyperintense T2- FLAIR foci within the left corticospinal tract in the left cerebral peduncle, right and left sides of the pons, and medulla. These lesions are poorly marginated in parts, and were slightly hyposignal on the T1 sequence. Post contrast T1 images revealed faint enhancement in some of the lesions (Fig. 1). Diffusion weighted imaging (DWI) demonstrated significant restriction at the level of the pons (Fig. 2). Magnetic resonance spectroscopy (MRS) confirmed the demyelination process by the presence of Myoinositol and Choline peaks (Fig. 3).Cervical spine MRI was unremarkable. Although, the patient remained afebrile during ICU care, antibiotic therapy commenced due to gradual increase in the WBC up to 19400/µL. Systemic autoimmune disease markers, vasculitis workup, and serum testing for viral markers was negative. He received Heparin, Pantoprazole, Clindamycin, Paracetamol, and Methylprednisolone (up to 7 gram) on hospitalization. He showed progressive recovery of his motor function, as his muscle power improved from 2 of 5 to 5 of 5, and was safely discharged on the tenth day of admission with an excellent general condition. He was followed up for 2 weeks for corticosteroid tapering, which reported a stable condition without difficulties reversing.
Fig. 1.
(A,B) Axial T2 weighted images demonstrate hypersignal foci in pons and medulla (black arrows). (C,D) Axial T1 image shows slight hyposignal lesion in left side of the pons with mild peripheral enhancement after injection of contrast.
Fig. 2.
(A,B) The pontine lesion is high signal on DWI and low on ADC map, confirming diffusion restriction.
Fig. 3.
Single voxel MR spectroscopy demonstrates mild increase in choline and myoinositol peaks with almost normal NAA/cr.
Discussion
Several neurologic complications have been reported following COVID-19 vaccination, many of which are of autoimmune nature. These include GBS, exacerbation of multiple sclerosis (MS), and other demyelinating disorders including ADEM, Transverse myelitis, and neuromyelitis spectrum disorders [[13], [15], [16], [17], [18], [19]].
We reported a patient with progressive weakness and dysphagia a few days after receiving is 1st dose of Sinopharm vaccine. The constellation of the clinical examination, laboratory findings, and imaging led to the diagnosis of ADEM. Although multiple differential diagnoses were proposed initially based on the primary clinical presentation, such as GBS, MS, autoimmune encephalitis, and stroke, further investigations ruled out diagnoses other than ADEM.
The preserved reflexes, absence of sensory deficits, and brain MRI abnormalities were against the diagnosis of GBS. The lack of oligoclonal bands of CSF, bilateral neurologic signs, and configuration of lesions in MRI were not in line with the diagnosis of MS. Autoimmune encephalitis has some key symptoms such as seizures, memory deficits, and psychiatric manifestations [20], none of which existed in this case. Lack of changes in the level of consciousness, gradual progression of symptoms, and clinical and imaging localization didn't match stroke.
ADEM is characterized by an acute onset of encephalopathy, often with multifocal rapidly progressive neurologic deficits. Neurologic signs and symptoms may be preceded by a prodromal phase with constitutional symptoms such as fever, irritability, headache, and vomiting [21]. The diagnosis is principally based on the history and clinical course together with supporting imaging features [21]. MRI plays a pivotal role in the primary diagnosis and follow up. T2 weighted and Flair images, as the main sequences, typically demonstrating multifocal, bilateral hypersignal foci. These have characteristic asymmetric distribution and are morphologically poorly marginated [22]. Typically, these lesions are in the deep white matter. However, involvement of cortical and deep white matter has also been reported and may aid in differentiating the condition from other differentials such as MS [21,23].
Involvement of posterior fossa structures and spinal cord may occur either with supratentorial brain or in isolation. Acute lesions may enhance following contrast injection. Although initial brain MRI may resemble that of MS in many cases, sparing of periventricular regions, involvement of gray matter, absence of Dawson finger configuration, indistinct margin of the lesions, and lack of T1 black holes are in favor the diagnosis of ADEM [21,23]. Moreover, the resolution of the lesions in the follow-up MRI is a supporting clue for the diagnosis of ADEM. Conversely, detecting new lesions is unlikely in monophasic ADEM, and may suggest other disorders such as multiphasic ADEM or MS [24]. Role of more advanced MR techniques such as DWI and MRS has also been studied previously [21,25,26]. These may increase the diagnostic power of MRI in the setting of demyelination and may be helpful in staging the disease [[26], [27]]. Diffusion restriction is more common in the acute stage of the disease, whereas subacute lesions show free diffusion [25,26]. Decrease in the N-acetyl aspartate has been reported in the subacute stage of the disease and elevated lactate and lipid indicate acute demyelination [26].
In conclusion, typical MR findings at presentation together with appropriate clinical history, rapid improvement of neurologic symptoms, and absence of new lesions on follow up MRI and can help establishing a definitive diagnosis in most cases.
Funding sources and/or Disclosures
Authors have reported no conflict of interest or funding sources.
Patient consent
The authors have obtained a written informed consent from the patient to publish his case (including publication of images).
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Alper G. Acute disseminated encephalomyelitis. J Child Neurol. 2012;27(11):1408–1425. doi: 10.1177/0883073812455104. [DOI] [PubMed] [Google Scholar]
- 2.Pohl D., Alper G., Van Haren K., Kornberg A.J., Lucchinetti C.F., Tenembaum S., et al. Acute disseminated encephalomyelitis: updates on an inflammatory CNS syndrome. Neurology. 2016;87(9 Supplement 2):S38–S45. doi: 10.1212/WNL.0000000000002825. [DOI] [PubMed] [Google Scholar]
- 3.Tenembaum S., Chitnis T., Ness J., Hahn J.S. Group ftIPMS. Acute disseminated encephalomyelitis. Neurology. 2007;68(16 suppl 2):S23–S36. doi: 10.1212/01.wnl.0000259404.51352.7f. [DOI] [PubMed] [Google Scholar]
- 4.Cole J., Evans E., Mwangi M., Mar S. Acute disseminated encephalomyelitis in children: an updated review based on current diagnostic criteria. Pediatr Neurol. 2019;100:26–34. doi: 10.1016/j.pediatrneurol.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 5.Garg R.K. Acute disseminated encephalomyelitis. Postgrad Med J. 2003;79(927):11–17. doi: 10.1136/pmj.79.927.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menge T., Hemmer B., Nessler S., Wiendl H., Neuhaus O., Hartung H.-P., et al. Acute disseminated encephalomyelitis: an update. Arch Neurol. 2005;62(11):1673–1680. doi: 10.1001/archneur.62.11.1673. [DOI] [PubMed] [Google Scholar]
- 7.Hannah Ritchie EM, Lucas Rodés-Guirao, Cameron Appel, Charlie Giattino, Esteban Ortiz- Ospina, Joe Hasell, et al. Coronavirus pandemic (COVID-19). Our World in Data. 2020.
- 8.Yazdanpanah F, Hamblin MR, Rezaei N. The immune system and COVID-19: friend or foe? Life Sci. 2020;256 doi: 10.1016/j.lfs.2020.117900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Creech CB, Walker SC, Samuels RJ. SARS-CoV-2 vaccines. JAMA. 2021;325(13):1318–1320. doi: 10.1001/jama.2021.3199. [DOI] [PubMed] [Google Scholar]
- 10.Thanh Le T, Andreadakis Z, Kumar A, Gómez Román R, Tollefsen S, Saville M, et al. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19(5):305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 11.Keshavarz P, Yazdanpanah F, Rafiee F, Mizandari M. Lymphadenopathy following COVID-19 vaccination: imaging findings review. Acad Radiol. 2021;28(8):1058–1071. doi: 10.1016/j.acra.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim DD, Kung CS, Perez DL. Helping the public understand adverse events associated with COVID-19 vaccinations: lessons learned from functional neurological disorder. JAMA Neurol. 2021;78(7):789–790. doi: 10.1001/jamaneurol.2021.1042. [DOI] [PubMed] [Google Scholar]
- 13.Waheed S, Bayas A, Hindi F, Rizvi Z, Espinosa PS. Neurological complications of COVID-19: Guillain-Barre syndrome following Pfizer COVID-19 vaccine. Cureus. 2021;13(2) doi: 10.7759/cureus.13426. e13426-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsh EE, Frenck RW, Falsey AR, Kitchin N, Absalon J, Gurtman A, et al. Safety and immunogenicity of two RNA-based covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu L, Xiong W, Mu J, Zhang Q, Zhang H, Zou L, et al. The potential neurological effect of the COVID-19 vaccines: a review. Acta Neurol Scand. 2021;144(1):3–12. doi: 10.1111/ane.13417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vegezzi E, Ravaglia S, Buongarzone G, Bini P, Diamanti L, Gastaldi M, et al. Acute myelitis and ChAdOx1 nCoV-19 vaccine: casual or causal association? J Neuroimmunol. 2021;359 doi: 10.1016/j.jneuroim.2021.577686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao L, Ren L. Acute disseminated encephalomyelitis after severe acute respiratory syndrome coronavirus 2 vaccination: a case report. Acta Neurol Belg. 2021:1–3. doi: 10.1007/s13760-021-01608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kania K, Ambrosius W, Tokarz Kupczyk E, Kozubski W. Acute disseminated encephalomyelitis in a patient vaccinated against SARS-CoV-2. Ann Clin Transl Neurol. 2021;8(10):2000–2003. doi: 10.1002/acn3.51447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogrig A, Janes F, Gigli GL, Curcio F, Negro ID, D'Agostini S, et al. Acute disseminated encephalomyelitis after SARS-CoV-2 vaccination. Clin Neurol Neurosurg. 2021;208 doi: 10.1016/j.clineuro.2021.106839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391–404. doi: 10.1016/S1474-4422(15)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pohl D, Alper G, Van Haren K, Kornberg AJ, Lucchinetti CF, Tenembaum S, et al. Acute disseminated encephalomyelitis: updates on an inflammatory CNS syndrome. Neurology. 2016;87(9 Supplement 2):S38–S45. doi: 10.1212/WNL.0000000000002825. [DOI] [PubMed] [Google Scholar]
- 22.Tenembaum S, Chamoles N, Fejerman N. Acute disseminated encephalomyelitis: a long-term follow-up study of 84 pediatric patients. Neurology. 2002;59(8):1224–1231. doi: 10.1212/wnl.59.8.1224. [DOI] [PubMed] [Google Scholar]
- 23.Huynh W, Cordato DJ, Kehdi E, Masters LT, Dedousis C. Post-vaccination encephalomyelitis: literature review and illustrative case. J Clin Neurosci. 2008;15(12):1315–1322. doi: 10.1016/j.jocn.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Callen DJ, Shroff MM, Branson HM, Li DK, Lotze T, Stephens D, et al. Role of MRI in the differentiation of ADEM from MS in children. Neurology. 2009;72(11):968–973. doi: 10.1212/01.wnl.0000338630.20412.45. [DOI] [PubMed] [Google Scholar]
- 25.Tenembaum SN. Acute disseminated encephalomyelitis. Handb Clin Neurol. 2013;112:1253–1262. doi: 10.1016/B978-0-444-52910-7.00048-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balasubramanya KS, Kovoor JM, Jayakumar PN, Ravishankar S, Kamble RB, Panicker J, et al. Diffusion-weighted imaging and proton MR spectroscopy in the characterization of acute disseminated encephalomyelitis. Neuroradiology. 2007;49(2):177–183. doi: 10.1007/s00234-006-0164-2. [DOI] [PubMed] [Google Scholar]
- 27.Mader I, Wolff M, Nägele T, Niemann G, Grodd W, Küker W. MRI and proton MR spectroscopy in acute disseminated encephalomyelitis. Childs Nerv Syst. 2005;21(7):566-72. [DOI] [PubMed]