Abstract
We compared the efficacies of fluconazole (Flu), amphotericin B (AmB), and 5-fluorocytosine (5FC) monotherapies with the combination of Flu plus 5FC and Flu plus AmB in a rabbit model of Candida albicans endocarditis, endophthalmitis, and pyelonephritis. The dose of Flu used was that which resulted in an area under the concentration-time curve in rabbits equivalent to that seen in humans who receive Flu at 1,600 mg/day, the highest dose not associated with central nervous system toxicity in humans. Quantitative cultures of heart valve vegetations, the choroid-retina, vitreous humor, and kidney were conducted after 1, 5, 14, and 21 days of therapy. All untreated controls died within 6 days of infection; animals treated with 5FC monotherapy all died within 18 days. In contrast, 93% of animals in the other treatment groups appeared well and survived until they were sacrificed. At day 5, the relative decreases in CFU per gram in the vitreous humor were greater in groups that received Flu alone and in combination with 5FC or AmB than in groups receiving AmB or 5FC monotherapies (P < 0.005) but were similar thereafter. In the choroid-retina, 5FC was the least-active drug. However, there were no differences in choroidal fungal densities between the other treatment groups. On days 5 and 14 of therapy, fungal densities in kidneys of AmB recipients were lower than those resulting from the other therapies (P < 0.001 and P ≤ 0.038, respectively) and AmB-plus-Flu therapy was antagonistic; however, all therapies for fungal pyelonephritis were similar by treatment day 21. While fungal counts in cardiac valves of Flu recipients were similar to those of controls on day 5 of therapy and did not change from days 1 to 21, AmB therapy significantly decreased valvular CFUs versus Flu at days 5, 14, and 21 (P < 0.005 at each time point). 5FC plus Flu demonstrated enhanced killing in cardiac vegetations compared with Flu or 5FC as monotherapies (P < 0.03). Similarly, the combination of AmB and Flu was more active than Flu in reducing the fungal density in cardiac vegetations (P < 0.03). However, as in the kidney, AmB plus Flu demonstrated antagonism versus AmB monotherapy in the treatment of C. albicans endocarditis (P < 0.05, P = 0.036, and P < 0.008 on days 5, 14, and 21, respectively).
Endocarditis due to the fungus Candida albicans is associated with high mortality (21, 37, 41). Moreover, it can be complicated by endophthalmitis and renal abscesses (46). Amphotericin B (AmB) is often prescribed for the treatment of C. albicans infection at these sites; infective endocarditis also generally requires valve replacement (21, 37). However, the toxicities of AmB frequently limit its use (32, 42). Fluconazole (Flu) is a triazole antifungal agent that has activity against many fungal species, including C. albicans (2, 14, 19). It rarely causes toxicity (19). In addition, this drug achieves good concentrations in the vitreous humor, aqueous humor, and cerebrospinal fluid when it is administered orally and parenterally (10, 19, 33, 35). Previous studies have shown that Flu is less effective than AmB in the treatment of experimental endocarditis (13, 43, 50). However, the relative efficacies of AmB and Flu in the treatment of C. albicans pyelonephritis and endophthalmitis are unclear. Also unclear is whether or not the combinations of Flu plus 5-fluorocytosine (5FC) and Flu plus AmB offer improved outcome versus Flu and AmB monotherapies at these infection sites.
In this study we used a rabbit infection model to compare the efficacy of Flu, alone and in combination with 5FC, with those of AmB and 5FC monotherapies for the treatment of C. albicans endocarditis, endophthalmitis, and pyelonephritis. We also characterized the relative efficacies of the combination of AmB plus Flu and AmB and Flu monotherapies for the treatment of these infections.
Louie et al. (31) showed that for Flu the pharmacodynamic parameter that best predicts outcome is the ratio of the area under the serum concentration-time curve (AUC) to the MIC. Thus, to maximize the clinical relevance of our results, we used a dosage of Flu which resulted in a steady-state AUC in rabbits that was equivalent to the serum AUC achieved in humans who receive 1,600 mg of Flu daily (30). In humans, 1,600 mg/day is the highest dosage of Flu given that is not associated with neurologic side effects (4). For AmB, the pharmacodynamic parameter that defines efficacy is not known. Thus, we selected a dose of AmB that results in serum trough and 24-h AUC values similar to those seen in humans given 1 mg of this drug/kg of body weight/day (6, 12, 22).
(This study was presented in part at the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy, New Orleans, La., 15 to 18 September 1996.)
MATERIALS AND METHODS
C. albicans isolate.
C. albicans ATCC 36082 (American Type Culture Collection, Manassas, Va.) was used. The fungal suspension was prepared as described by Witt and Bayer (50) with minor modifications. For each study, a few colonies of the fungus were inoculated into yeast nitrogen base broth (Difco, Detroit, Mich.) containing 1% dextrose and 0.15% l-asparagine (Calbiochem, San Diego, Calif.) and grown overnight at 35°C. After 18 h of incubation an aliquot of the suspension was inoculated into fresh yeast nitrogen broth and incubated for an additional 24 h. The organisms were washed once and resuspended to a concentration of 107 CFU/ml with 0.85% sterile saline by using a spectrophotometer. The concentration of the fungal suspension was confirmed by quantitative cultures.
Antifungal agents.
Flu powder was supplied by Pfizer, Inc. (Groton, Conn.). AmB-desoxycholate powder (Adria Laboratories, Columbus, Ohio) was purchased from the hospital pharmacy, and 5FC powder was purchased from Sigma Chemical Co. (St. Louis, Mo.). The limit of solubility of Flu in 5% dextrose in water (D5W) or normal saline was 3 mg/ml. Thus, the Flu solution was made in 7.5% absolute ethyl alcohol (EtOH) to a final concentration of 10 mg/ml since the volume of D5W needed to deliver the desired dose of Flu resulted in respiratory distress and death in 20% of animals. In vitro studies demonstrated that this concentration of EtOH did not affect the growth of C. albicans or the potency of Flu. Initial studies demonstrated that the fungal densities in the eyes, heart valve vegetations, and kidneys from animals that received Flu that was prepared in 7.5% EtOH or D5W were similar when treatment was continued for up to 21 days (data not shown). The concentration of EtOH used did not affect the in vivo and in vitro interactions between Flu plus AmB and Flu plus 5FC (data not shown). AmB was dissolved in sterile D5W to a final concentration of 0.2 mg/ml. 5FC was prepared in sterile normal saline at a concentration of 8 mg/ml. All antifungal agents were administered intravenously via a central venous catheter (see “Infection model”). For animals that received the combination of Flu plus 5FC or AmB plus Flu, the drugs were administered 4 h apart. For AmB-plus-Flu recipients, AmB was infused first.
In vitro susceptibility testing.
The MICs of Flu, AmB, and 5FC for the C. albicans isolate were determined by the macrodilution susceptibility testing method defined by the National Committee for Clinical Laboratory Standards (36). The MICs were determined after the yeast was incubated with antifungal drugs for 48 h. The MICs were 0.25 μg/ml for Flu, 0.125 μg/ml for AmB, and 1 μg/ml for 5FC.
Animals.
Male New Zealand White rabbits weighing 2.5 kg were used in this study. The animals were housed in individual cages and received food and water ad libitum. All animal procedures were approved by the Institutional Animal Care and Use Committee of Albany Medical College, a facility accredited by the Association for Assessment and Accreditation of International Laboratory Animal Care.
Infection model.
C. albicans endocarditis, endophthalmitis, and pyelonephritis were established in each animal by the methods of Durack et al. (15) and Witt and Bayer (50), with minor modifications. In our laboratory the procedure reproducibly resulted in aortic valve endocarditis, endogenous endophthalmitis, and renal abscesses (pyelonephritis) in 98, 100, and 100% of rabbits, respectively. Briefly, under general anesthesia, a sterile vinyl catheter (1.32-mm external diameter; Bolab Products, Lake Havasu City, Ariz.) was inserted through an incision in the carotid artery and threaded into the left ventricle. The catheter was secured in place. The deep fascial tissue layers in the neck and the skin incision were closed with sutures. During the same surgical session, a vinyl catheter (1.57-mm internal diameter; Bolab Products) was inserted into an external jugular vein by the method of Walsh et al. (47) and secured in place with sutures. The latter catheter was used for subsequent drug administrations. The venous catheter was flushed with 10 U of heparin (SoloPak Laboratories, Inc., Elk Grove Village, Ill.) at the time it was placed and then after each antifungal treatment was given. Both catheters were left in place for the duration of the study. Cefazolin (100 mg/kg) was administered intramuscularly daily for 3 days as prophylaxis against bacterial infection at the surgical site. Forty-eight hours after the catheters were placed, the animals were injected intravenously (i.v.) with 2 × 107 CFU of C. albicans, in 2 ml of normal saline, via the marginal ear vein.
The infected rabbits were divided into six groups. There were 20 to 30 animals per group. Antifungal therapy was initiated 24 h after fungal inoculation. Animals in group I received Flu (85.2 mg/kg i.v.) given in two divided doses. Those in group II received 5FC (50 mg/kg i.v.) daily. Rabbits in group III received Flu and 5FC i.v. in combination at the doses indicated above. Animals in group IV received AmB-desoxycholate (1 mg/kg i.v. per day), and those in group V received AmB in combination with Flu at the doses indicated above. Each day, the AmB was administered before Flu. Lastly, group VI received saline. All drugs and saline (placebo) were given i.v. via the previously placed external jugular vein catheter.
Animals received 1, 5, 14, or 21 days of treatment. Twenty-four hours after the last scheduled dose was given, a subset of animals from each group were sacrificed with a rapid i.v. infusion of 125 mg of pentobarbital/kg, followed by the induction of bilateral pneumothoraces. The location of the aortic catheter was confirmed. Aortic valve vegetations, a portion of the right kidney, and vitreous humor and choroid-retina samples were cultured quantitatively. Specifically, the cardiac vegetations from each animal were collected, pooled, and weighed. The samples were homogenized and serially diluted in sterile saline. The entire volume of the first tube of the dilution series and 200 μl of all the other tubes were plated onto Sabouraud dextrose agar. The entire vitreous humor body from each eye was collected, weighed, and homogenized. Then, the entire vitreous humor sample was inoculated onto Sabouraud dextrose agar. Kidney and choroid-retina samples were weighed, homogenized, and serially diluted in saline. Then 200 μl from each tube of the dilution series was inoculated onto nutrient agar. The culture plates were incubated at 37°C for 48 h prior to colony counting. Colony counts were described as the number of CFUs per gram of each specimen. Preliminary studies demonstrated that quantitative cultures of C. albicans prepared in vitreous humors, choroid-retinas, kidneys, and cardiac vegetations collected from noninfected rabbits 24 h after the animals received the fourth dose of any of the antifungal drug regimens described above did not affect the culture results. Thus, our data was not affected by drug carryover. The quantitative culture results for each sacrifice time and treatment group represent the cumulative results of at least three separate trials. In total, there were 20 to 30 rabbits in each experimental group.
Animals that lost 20% of their body weight were humanely euthanized with an i.v. injection of pentobarbital at 125 mg/kg followed by induction of bilateral pneumothoraces. This degree of weight loss was seen only in animals that received 5FC as monotherapy. Results of quantitative organ cultures of animals that died or were sacrificed within 24 h of the 1-, 5-, 14-, or 21-day time point were included for analysis with the closest time point. All other culture results were excluded from further analysis (i.e., 10 rabbits from the 5FC monotherapy group and 1 to 2 animals from each of the other treatment groups died outside these guidelines and were excluded from analysis).
All animals that received 5FC monotherapy died or were humanely sacrificed within 18 days of therapy. To determine if the outcome was influenced by 5FC-induced neutropenia, leukocyte (WBC) counts in a subset of eight animals that received 5FC as monotherapy were assessed immediately before they were humanely euthanized. Since massive hepatic necrosis has been reported to cause death in rabbits treated with ≥75 mg of 5FC/kg/day (23), serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, and total bilirubin levels in a subset (n = 7) of 5FC recipients were assessed prior to infection, after 5 days of therapy, and when rabbits were euthanized. These results were compared with serum ALT, AST, alkaline phosphatase, and total bilirubin values obtained from animals who received Flu monotherapy (n = 6) or Flu plus 5FC (n = 6) after 5 and 14 days of therapy. Finally, the effect of 5FC at 50 mg/kg/day on survival, WBC counts, and liver enzymes of uninfected rabbits (n = 5) was determined after 0, 5, 14, and 21 days of treatment.
Pharmacokinetics of AmB and Flu in serum and the vitreous humor.
The pharmacokinetics of Flu and AmB were determined at steady state in eight infected rabbits. Treatment started 24 h after fungal inoculation. AmB at 1 mg/kg/day was administered to four rabbits for four doses. The other four rabbits received 85.2 mg of Flu/kg/day in two divided doses for 4 days. Prior to the administration of the last dose of AmB or Flu, a 24-gauge angiocatheter was inserted into the central artery of each animal to obtain blood samples. For AmB recipients, 1 ml of blood was collected at 0.25, 0.50, 1.0, 2.0, 2.5, 3, 4, 6, 8, 12, and 24 h after drug administration. For Flu recipients, samples were collected at 0.25, 0.5, 1, 2, 4, 6, 8, 10, and 12 h after drug infusion. Two additional rabbits received Flu, and another two rabbits received AmB, as described above for 4 days. Prior to the administration of the last dose of the drug, these animals were anesthetized with a subcutaneous dose of urethane (1.62 g/kg of body weight). One milliliter of blood and 15 μl of vitreal fluid were simultaneously collected at the 0-, 0.5-, 1-, 2-, 4-, 6-, 8-, 10-, and 12-h time points for the respective drug. After the last samples were collected, all animals were sacrificed with 125 mg of pentobarbital/kg followed by induction of bilateral pneumothoraces. The serum was separated from the clot by centrifugation. Serum and vitreous humor samples were stored at −70°C.
To determine if drug therapy affected the clearance of Flu or AmB, serial serum creatinine, blood urea nitrogen (BUN), and trough drug levels in a different subset of animals were assessed. For infected animals that received Flu, AmB, AmB plus Flu, and Flu plus 5FC, four animals assigned to receive 21 days of antifungal therapy had blood drawn immediately before the antifungal drug(s) was administered on days 0, 5, 14, and 21 of therapy for serum creatinine and BUN measurements. Also, serum was collected from these animals at the last three time points for trough antifungal drug levels. In another three noninfected rabbits AmB levels in serum collected 24 h after these animals received a single dose of AmB (1 mg/kg/day) were measured; AmB concentrations measured in these animals served as the comparative controls for AmB levels measured at the other time points. Flu trough levels in the sera of three additional noninfected rabbits were assessed 12 h after they received the 10th dose of Flu at 85.2 mg/kg in two divided doses each day.
Penetration of AmB and Flu into the kidney and cardiac vegetation.
Six rabbits underwent surgical venous and arterial catheter placements and were infected as described above. Beginning 24 h after fungal inoculation, 3 animals were given 82.5 mg of Flu/kg i.v. in two divided doses per day for 4 days. The other three rabbits received AmB at 1 mg/kg i.v. each day for four doses. One milliliter of blood was collected from each animal 12 and 24 h after the last doses of Flu and AmB, respectively, were administered. These represented trough levels. The blood was allowed to clot on ice. The serum was separated by centrifugation and stored at −70°C. Immediately after the blood samples were collected the rabbits were sacrificed with 125 mg of pentobarbital/kg i.v. followed by induction of bilateral pneumothoraces. A sample of the right kidney was collected from each rabbit and weighed. The sample was homogenized and underwent quantitative culture analysis. Also, cardiac vegetations were collected and quickly rinsed with 15 ml of sterile D5W. Then the vegetations were placed in 15 ml of fresh D5W and manually shaken for 10 s. The vegetations were removed from the solution and weighed. Those collected from each rabbit were placed in 100 μl of D5W and were homogenized. The concentrations of AmB or Flu in kidneys and cardiac vegetations were measured by microbiological assays (see below).
To determine whether the concentration of drug measured in the vegetations obtained as part of the above study represented drug that was within (rather than coating) the vegetations, an additional six rabbits underwent surgical placement of the arterial and venous catheters and were infected as described above. However, these animals did not receive antifungal drugs. Four days after fungal inoculation, the vegetations were collected from sacrificed rabbits and weighed. The vegetations were placed in fresh rabbit serum containing either 0.45 μg of AmB/ml or 35 μg of Flu/ml for 10 s to coat the vegetations with drug while minimizing the penetration of drug into the vegetation. These concentrations corresponded to the trough concentrations of drug measured in serum at steady state. The vegetations were removed from the drug-containing sera, quickly washed with 15 ml of D5W, and then shaken in 15 ml of fresh D5W for 10 s. They were removed from the solution and weighed. Then they were homogenized in 100 μl of D5W, and the concentrations of drug in the homogenates were assessed.
Antifungal drug assays.
The concentrations of Flu in the serum, vitreous humor samples, kidneys, and cardiac vegetations were determined by a well diffusion microbiological assay developed by Jorgensen et al. (24), with the modifications described by Madu et al. (33). Candida pseudotropicalis (ATCC 46764) was used as the assay organism. Pour plates of the fungus were prepared with synthetic fungal amino acid medium-molten agar and allowed to solidify at room temperature. Four-millimeter-diameter wells were made in the agar. Twenty-microliter aliquots of serum, vitreous humor, and homogenates of kidneys or valvular vegetations collected from rabbits or standards were dispensed into the wells, and the wells were kept at 4°C for 1 h and then incubated overnight for 16 h at 30°C in an ambient air incubator. For the pharmacokinetic studies of serum, the Flu standards were prepared in normal rabbit serum. For studies on the vitreous humor and valve vegetations, the standards were prepared in D5W. The diameters of inhibition for each serum, vitreous humor, and tissue homogenate sample and standards were measured with a vernier caliper to the nearest 0.1 mm. Antifungal drug concentrations in samples were calculated with the curves derived from Flu standards. The standard curve was linear for concentrations of Flu between 0.5 and 100 μg/ml of serum and D5W. For serum samples that resulted in diameters of inhibition that were greater than those associated with the linear portion of the standard curve, the serum and kidney samples were diluted 1:4 with saline and retested. The calculation of the concentration of the drug accounted for this. The intraday and interday coefficients of variation of the microbiological assay were 4.9 and 6.8%, respectively.
Concentrations of AmB in serum, vitreous humor, kidneys, and cardiac vegetations were determined by a microbiological assay described by Bannatyne and Cheung (8) and Granich et al. (18) with slight modifications. Paecilomyces variotii (ATCC 22319) was used as the assay organism. The fungus was grown on Sabouraud dextrose agar slants for 5 to 7 days at 35°C. Mature spores from these cultures were harvested with a sterile cotton-tipped applicator and placed in normal saline. The total concentration of spores was determined by hemocytometer. Spores were added to synthetic fungal amino acid medium-molten agar to a final concentration of 105 spores/ml. Pour plates of the fungal spores were made and allowed to solidify at room temperature. For assessment of AmB concentrations in serum, kidney, and cardiac vegetations, 10-mm-diameter wells were made in the agar and 100 μl of sample or standards was pipetted into wells. For assessment of AmB concentrations in the vitreous humor, 15 μl of sample or standards was added to 4-mm-diameter wells. After incubation for 24 h at 35°C, the diameters of zones of inhibited growth were measured to the nearest 0.1 mm with a vernier caliper. Antifungal drug concentrations in samples were calculated with the curves derived from AmB standards. The standard curve was linear from concentrations of 0.1 to 20 μg/ml. The intraday and interday coefficients of variation of the microbiological assay were 4.3 and 6.2%, respectively.
The trough levels of AmB and Flu in animals that received 5, 14, and 21 days of AmB or Flu were also measured by the above-described biological assays. For animals that received AmB and Flu in combination, AmB concentrations in serum were measured with a Flu-resistant C. albicans strain (B59630; gift from F. Odds, Janssen Research Foundation, Beerse, Belgium) in the biological assay. Initial studies demonstrated that serum that contained 0.1 to 4.0 μg of AmB/ml together with 1 to 150 μg of Flu/ml produced the same diameters of zones of growth inhibition as serum containing only AmB. Flu concentrations in sera of animals that received AmB plus Flu and Flu plus 5FC were determined by a high-pressure liquid chromatography (HPLC) method described elsewhere (33). Previously, we demonstrated that Flu concentrations measured by using the bioassay and HPLC were equivalent (33). The intraday and interday coefficients of variation of the HPLC at 1 μg/ml were 4.5 and 5.6%, respectively.
Pharmacokinetic analysis.
Pharmacokinetic analysis of the serum and vitreous humor samples for Flu and AmB concentration-time relationships were performed with the nonlinear least-squares regression program RSTRIP II (Micromath Scientific Software, Salt Lake City, Utah). The most appropriate pharmacokinetic models were determined by using model selection criteria based on a modified form of Akaike’s information criterion (1). Cmax was defined as the highest concentration of drug measured in serum after the drug was administered. The trough level was defined as the concentration of drug in serum that was collected just before the next dose of drug was administered. To determine the AUC in serum and the vitreous humor, the trapezoidal method was used for the data obtained from time zero to the last time point.
Statistical analysis.
Comparison of colony counts among the different treatment groups for each treatment duration was performed by using the Kruskal-Wallis test with multiple comparisons followed by Newman-Keuls analysis with the software program True Epistat, version 5.3 (Epistat Services, Richardson, Tex.). Choroid-retina and vitreous humor samples collected from each eye were considered individual samples. Preliminary studies demonstrated that we reliably detected ≥5 CFU/g of vitreous humor when the entire vitreous humor was cultured. Thus, for statistical analysis, a computer program randomly assigned culture-negative samples a number between 0 and 4 CFU/g. In preliminary studies, we reliably detected ≥50 CFU/g of kidney and choroid-retina after they were diluted 10-fold in sterile saline. Thus, for statistical calculations, the computer program randomly assigned a value between 0 and 49 CFU/g to specimens that were culture negative. Pooled vegetations from each animal frequently weighed less than 1 g. Thus, culture-negative vegetations were assigned a value for CFU per gram equal to the inverse of the weight of the sample. For example, a pooled sample weighing 0.05 g was assigned a value of 20 CFU/g and one weighing 0.1 g was given a value of 10 CFU/g. P < 0.05 was considered significant.
Differences in total WBC, liver enzyme, total bilirubin, BUN, and serum creatinine concentrations between time points among groups were determined by analysis of variance. P < 0.05 was considered significant.
Comparison of the proportions of vitreal, choroid-retinal, kidney, and valvular vegetations that were culture negative among the treatment groups on the various days of sacrifice was performed with a two-tailed Fisher exact test with Bonferroni’s correction for multiple comparisons. For day 5 of treatment, when a control group was evaluable, seven different pair-wise comparisons were made. The comparative groups were control versus AmB, control versus Flu, 5FC versus Flu, AmB versus Flu, AmB versus AmB plus Flu, Flu versus AmB plus Flu, and Flu versus Flu plus 5FC. Thus, P < 0.007 was considered significant. Since none of the controls and an insufficient number of animals in the 5FC monotherapy groups survived to treatment days 14 and 21, only four pair-wise comparisons were made at these time points. The comparative groups for these time points were AmB versus Flu, AmB versus AmB plus Flu, Flu versus AmB plus Flu, and Flu versus Flu plus 5FC. For day 14 and 21 analyses, P < 0.01 was considered significant.
Comparison of the rapidities with which AmB and the combination of AmB and Flu (and Flu versus Flu plus 5FC) sterilized kidneys and valvular vegetations was performed by Kaplan-Meier time-to-event analysis followed by the Wilcoxon test. For specimens associated with sterile cultures, the date of organ procurement was considered the date the tissue was sterilized. For specimens that yielded fungus on culture, the date the organ was collected was right censored. P < 0.05 was considered statistically significantly different.
RESULTS
Pharmacokinetics of Flu and AmB in serum and vitreous humor at steady state in infected rabbits; percent penetration of drug into the vitreous humor.
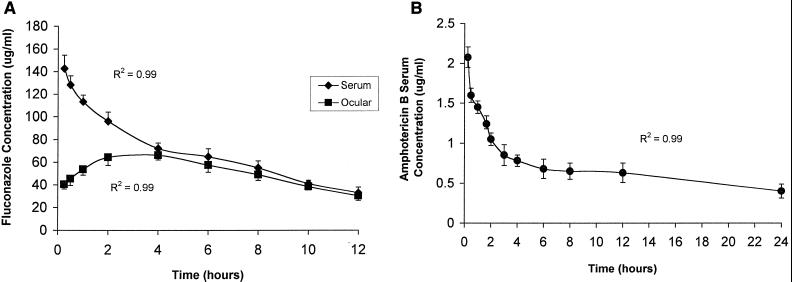
The time-concentration relationships for Flu and AmB at steady state are shown in Fig. 1. The animals were infected with C. albicans. Flu and AmB pharmacokinetics were best described by two-compartment and three-compartment models, respectively. At steady state, the serum Cmax and AUC from 0 to 24 h (AUC0–24) for Flu were 142.6 ± 12.3 μg/ml and 1,652 ± 34.6 μg · h/ml, respectively. The half-life at β phase (t1/2β) was 7.3 h, and the Cmin (trough concentration) was 34.4 ± 3.7 μg/ml. The serum Cmax and AUC0–24 for AmB were 2.19 ± 0.36 μg/ml and 16.56 ± 2.79 μg · h/ml, respectively. The t1/2β was 21.2 h and the Cmin at 24 h was 0.41 ± 0.20 μg/ml. In the vitreous humor, the Cmax and AUC0–24 for Flu were 66.1 ± 5.4 μg/ml and 1,229.26 ± 31.77 μg · h/ml, respectively (74.4% penetration). AmB was not measurable in the vitreous humor at any time (assay sensitivity: 0.1 μg/ml).
FIG. 1.
Pharmacokinetics of Flu at 85.2 mg/kg twice a day in serum and vitreous humor (A) and AmB at 1 mg/kg/day in serum (B) at steady state in rabbits systemically infected with C. albicans. AmB could not be detected in the vitreous humor (sensitivity of bioassay: 0.1 μg/ml).
Serial serum creatinines and trough AmB and Flu concentrations.
The serum creatinines in animals after 0, 5, 14, and 21 days of therapy with AmB, Flu, AmB plus Flu, and Flu plus 5FC are shown in Table 1. The mean serum creatinine was 0.82 ± 0.05 mg/dl prior to infection. The serum creatinine significantly increased with time only in controls. Serum creatinines tended to increase with time in rabbits treated with AmB or AmB plus Flu by day 14 of therapy (not significant) but were unchanged in the other treatment groups. These results are consistent with those of Chemlal et al. (13), who reported the absence of nephrotoxity in rabbits treated with AmB at 5 mg/kg/day for 7 days and Lee et al. (26), who found that serum creatinine increased slightly after 14 days of treatment with AmB at 1 mg/kg/day. Trough serum concentrations of Flu and AmB in animals that were treated with AmB, Flu, AmB plus Flu, and Flu plus 5FC did not change with time (Table 2).
TABLE 1.
Serum creatinine and BUN levels in infected rabbits treated with various antifungal drug regimens for up to 21 days
| Drug(s) (n) | Mean serum creatinine/BUN levelsa ± 1 SD on day:
|
|||
|---|---|---|---|---|
| 0 | 5 | 14 | 21 | |
| Control (7) | 0.8 ± 0.1/19.4 ± 2.3 | 2.4 ± 0.7/44.5 ± 4.1 | ||
| 5FC (4) | 0.8 ± 0.1/20.2 ± 3.4 | 0.9 ± 1.0/28.2 ± 3.6 | ||
| AmB (4) | 0.9 ± 0.1/19.1 ± 1.8 | 0.8 ± 0.1/22.7 ± 2.4 | 1.1 ± 0.1/24.3 ± 2.6 | 1.2 ± 0.1/25.2 ± 2.2 |
| Flu (4) | 0.9 ± 0.1/20.3 ± 1.9 | 0.8 ± 0.2/25.4 ± 2.6 | 0.9 ± 0.1/24.6 ± 2.0 | 1.0 ± 0.0/24.7 ± 1.6 |
| AmB + Flu (4) | 0.8 ± 0.0/19.8 ± 2.3 | 0.9 ± 0.1/24.4 ± 2.3 | 1.0 ± 0.2/25.1 ± 2.6 | 1.1 ± 0.1/23.2 ± 2.2 |
| Flu + 5FC (4) | 0.8 ± 0.1/19.6 ± 2.8 | 0.8 ± 0.1/22.1 ± 2.8 | 0.8 ± 0.1/23.4 ± 2.2 | 0.9 ± 0.1/20.3 ± 1.8 |
On day 0, creatinine and BUN levels in serum collected from rabbits were measured before the rabbits received their first dose of antifungal drug(s). Serum creatinine and BUN concentrations in healthy, noninfected rabbits (n = 4) were 0.82 ± 0.05 and 20.4 ± 2.1 mg/dl, respectively.
TABLE 2.
Trough concentrations in the serum of infected rabbits after 5, 14, and 21 days of treatment with various antifungal drug regimensa
| Drug(s) (n) | Mean trough AmB/Flu concn ± 1 SD on day:
|
||
|---|---|---|---|
| 5 | 14 | 21 | |
| Flu (4) | NMb/35.4 ± 4.4 | NM/39.7 ± 3.6 | NM/36.1 ± 3.6 |
| AmB (4) | 0.41 ± 0.04/NM | 0.43 ± 0.03/NM | 0.44 ± 0.04/NM |
| AmB + Flu (4) | 0.42 ± 0.02/38.3 ± 7.2 | 0.44 ± 0.04/32.8 ± 5.5 | 0.40 ± 0.03/37.1 ± 4.5 |
| Flu + 5FC (4) | NM/34.4 ± 6.0 | NM/36.2 ± 4.2 | NM/32.2 ± 8.7 |
The trough serum Flu concentration of Flu in noninfected rabbits (n = 3) that received Flu at 85.2 mg/kg twice per day i.v. for 5 days was 34.4 ± 3.7 μg/ml, and that of AmB in noninfected rabbits (n = 3) given AmB at 1.0 mg/kg for one dose was 0.41 ± 0.20 μg/ml. Trough concentrations in serum collected 12 and 24 h after the Flu and AmB doses were given, respectively, were measured.
NM, not measured.
Concentrations of Flu and AmB in the kidney and cardiac vegetations.
The concentrations of Flu and AmB measured in cardiac vegetations are shown in Table 3. The concentrations of Flu in cardiac vegetations were 5.88% of the serum trough level. AmB concentrations in cardiac vegetations were 6.98% of the serum trough level. However, the difference between the concentration of AmB in vegetations collected from treated, infected animals and that in “vegetation controls” is small. Thus, the percent penetration of AmB into cardiac vegetations must be viewed with caution.
TABLE 3.
Percent penetration of AmB and Flu into cardiac vegetations in rabbits treated intravenously with either AmB at 1.0 mg/kg per day or Flu at 85.2 mg/kg in divided doses for 4 days
| Drug tested | Mean trough serum drug concna (μg/ml) ± 1 SD | Mean drug concn (μg/ml) ± 1 SD in:
|
% Penetration of drug into cardiac vegetationsc | |
|---|---|---|---|---|
| Cardiac vegetations | Vegetations of controlsb | |||
| AmB | 0.43 ± 0.03 | 0.21 ± 0.03 | 0.18 ± 0.02 | 6.98 |
| Flu | 34.38 ± 6.51 | 2.02 ± 1.33 | Not detectable | 5.88 |
Trough levels were measured from serum samples that were collected from infected rabbits 24 h after they received their last dose of AmB or 12 h after they received their last dose of Flu. Cardiac vegetations were collected from sacrificed animals immediately thereafter.
Vegetation controls are vegetations collected from untreated, infected rabbits that were dipped in solutions of AmB at 0.45 μg/ml or Flu 35 at μg/ml and then washed with D5W before the vegetations were homogenized. They are used to determine the concentration of drug that coats the vegetations. The lower limits of sensitivity of the AmB and Flu bioassays were 0.1 and 0.5 μg/ml, respectively.
Percent penetration was calculated from the following formula: [(drug concentration in cardiac vegetations − drug concentration in vegetation controls)/trough serum drug concentration] × 100.
The concentration of Flu in the kidney was 38.3 ± 8.5 μg/ml (or 115% of the serum trough level of 34.4 μg/ml). The AmB concentration was 8.33 ± 2.46 μg/ml (or 1,900% of the serum trough level of 0.41 μg/ml).
Course of C. albicans infection in the eyes, kidneys, and heart valves of untreated rabbits.
All untreated rabbits that received 2 × 107 CFU of C. albicans died within 6 days of infection. Between days 1 and 5 of infection, the mean (±1 standard deviation [SD]) values of the log CFU per gram in vitreous humors, choroid-retinas, kidneys, and cardiac vegetations increased (Table 4). Of note, 2 × 107 CFU of C. albicans was given to all animals in the treatment studies (see below).
TABLE 4.
Fungal densities at various sites in rabbits inoculated with C. albicansa
| Fungal inoculum (CFU) | Site | Fungal density (log10 [CFU/g] ± 1 SD) on indicated day after fungal inoculation
|
|||
|---|---|---|---|---|---|
| 1 | 5 | 14 | 21 | ||
| 2 × 107 | Vitreous humor | 1.1 ± 0.3 | 2.4 ± 0.7 | ||
| Choroid | 3.3 ± 0.2 | 4.1 ± 0.3 | |||
| Kidney | 5.7 ± 0.2 | 6.3 ± 0.3 | |||
| Valve | 6.1 ± 0.1 | 6.5 ± 0.3 | |||
| 1 × 106 | Vitreous humor | 0.4 ± 0.1 | 1.4 ± 0.2 | 1.3 ± 0.3 | |
| Choroid | 2.0 ± 0.0 | 1.8 ± 0.4 | 1.9 ± 0.2 | ||
| Kidney | 3.7 ± 0.1 | 3.8 ± 0.7 | 3.2 ± 0.4 | ||
| Valve | 3.0 ± 0.1 | 6.3 ± 1.2 | 7.1 ± 1.1 | ||
The rabbits were not treated with antifungal agents. All animals that were infected with the higher fungal inoculum died within 6 days of inoculation.
Few animals that received treatment after infection with 2 × 107 CFU of C. albicans died. To prove that decrements in fungal densities in organs of treated animals were due solely to antifungal therapy (and not due to self-clearing), we defined the courses of C. albicans vitritis, choroid-retinitis, pyelonephritis, and endocarditis in rabbits that were systemically infected with 106 CFU of fungus. These animals all survived the 21-day observation period. In these subjects, the fungal densities in the vitreous humor, choroid-retina, and kidney did not change (Table 4). The fungal burden progressively increased in cardiac vegetations (Table 4). Thus, infection in this sublethal model was not self-clearing; the fungus persisted in each site for up to 21 days.
Effect of therapy on survival.
All untreated animals that were infected with 2 × 107 CFU of C. albicans died within 6 days. Those that received 5FC monotherapy died or were humanely euthanized within 18 days of infection (mean ± SD: 8.3 ± 2.7 days).
Approximately 93% of animals that received Flu, AmB, Flu plus 5FC, and Flu plus AmB survived until they were sacrificed. There were 1 to 2 deaths in each of these treatment groups. None of these animals died within 24 h of their scheduled sacrifice times; thus all of these animals were excluded from further analysis.
WBC counts were obtained from eight 5FC recipients immediately before they were euthanized. The WBC counts ranged between 18,000 and 25,000 (mean ± SD was 21,400 ± 1,843 cells/ml) cells/ml of blood (normal WBC count is <10,000 cells/ml). At least 94% of the WBCs in each animal were polymorphonuclear leukocytes. The serum ALT, AST, alkaline phosphatase, and total bilirubin levels in recipients of 5FC monotherapy were similar to those of other treatment groups (data not shown). Furthermore, in noninfected rabbits the results of WBC and liver studies of animals after 0, 5, 14, and 21 days of 5FC therapy were similar. Thus, death was not due to myelotoxicity or 5FC-induced hepatic necrosis (23). Animals died of their infections.
Comparative efficacies of the treatment groups in C. albicans endophthalmitis.
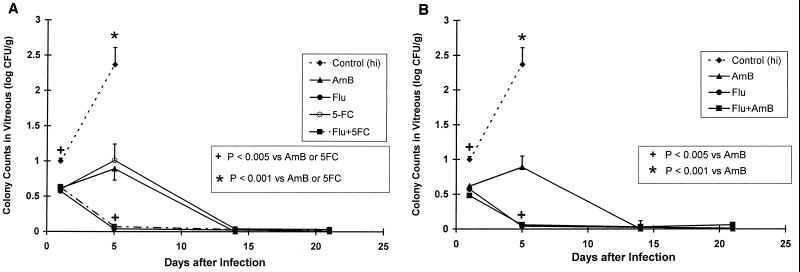
On day 5, Flu alone and in combination with 5FC or AmB sterilized the vitreous humor more rapidly than AmB or 5FC monotherapies (P < 0.005; Fig. 2). However, by day 14 all therapies demonstrated equivalent efficacies.
FIG. 2.
Relative efficacies of AmB, Flu, 5FC, and the combinations of Flu plus 5FC (A) and Flu plus AmB (B) in the treatment of C. albicans vitritis. The antifungal drug regimens were AmB at 1 mg/kg/day, Flu at 85.2 mg/kg (given in two divided doses each day), 5FC at 50 mg/kg/day, and the combination of the Flu and 5FC regimens. The drugs were given for 1, 5, 14, or 21 days. Twenty-four hours after the last dose, animals were sacrificed and quantitative cultures (to measure log10 [CFU] ± 1 standard error of the mean) were conducted. As shown, all antifungal therapies were superior to no therapy. All controls that were infected with 2 × 107 CFU of C. albicans died within 6 days of infection. Infected rabbits that received 5FC monotherapy died within 18 days. On day 5 of therapy, Flu alone and Flu in combination with 5FC or AmB were statistically superior to AmB or 5FC monotherapies (A; P < 0.005) for each comparison; AmB-plus-Flu therapy was similar to Flu alone but more active than AmB (B; P < 0.005). However, the AmB regimen was similar to these regimens by treatment day 14.
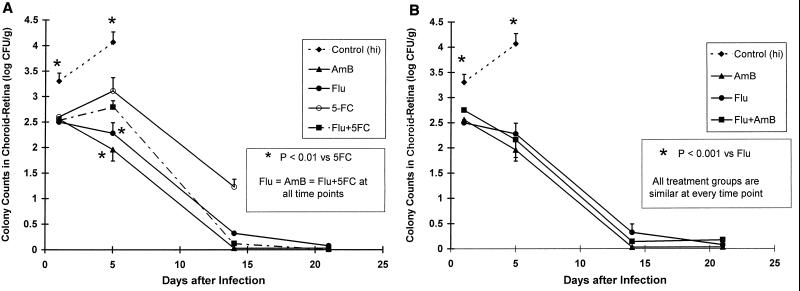
In the choroid-retina, all therapies were superior to 5FC monotherapy (P < 0.01) on day 5 of therapy (Fig. 3A). We could not assess their relative activities versus that of 5FC monotherapy on day 14 because only two rabbits receiving 5FC monotherapy survived to this time point. The efficacies of Flu, AmB, and Flu plus 5FC were equivalent. Of note, AmB and Flu monotherapies and the combination of AmB and Flu demonstrated similar activities in the choroid-retina at all time points (Fig. 3B).
FIG. 3.
Relative efficacies (log10 [CFU] ± 1 standard error of the mean) of AmB, Flu, 5FC, and the combinations Flu plus 5FC (A) and Flu plus AmB (B) in the treatment of C. albicans choroid-retinitis. The dosages of antifungal drugs are described in the legend for Fig. 2. AmB was statistically superior to 5FC (P < 0.01) on day 5 of treatment (A) but was similar to Flu alone and Flu plus AmB (B). After day 5 all treatment groups were similar. The addition of 5FC or AmB to Flu did not improve the outcome.
C. albicans pyelonephritis.
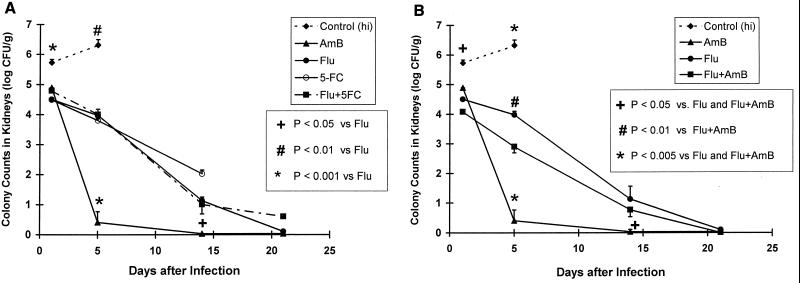
As shown in Fig. 4, AmB monotherapy reduced the fungal densities in kidneys more rapidly than the other treatment regimens (P < 0.001 and P ≤ 0.038 on days 5 and 14, respectively). However, by day 21 all treatment groups were similar. 5FC monotherapy was not compared with the other regimens on day 14 because there were too few animals in this group for analysis. The addition of 5FC to Flu was no more efficacious than Flu alone (Fig. 4A). Although fungal counts in the kidneys of AmB-plus-Flu recipients tended to be lower than those in rabbits that received Flu monotherapy, the differences did not reach statistical significance (Fig. 4B). However, AmB monotherapy was more active than AmB plus Flu therapy on days 5 and 14 of therapy (P = 0.001 and P = 0.038, respectively). Thus, this combination was antagonistic.
FIG. 4.
AmB was the most active agent in reducing the fungal densities (log10 [CFU] ± 1 standard error of the mean) in the kidney on days 5 and 14. However, by day 21 the AmB regimen was similar to all the other regimens. There was no difference between Flu, Flu plus 5FC, and Flu plus AmB. Since Flu-plus-AmB therapy was statistically less active than AmB monotherapy on days 5 and 14 (P < 0.001 and P = 0.038, respectively), antagonism was observed with this drug combination (B). In this case, antagonism was manifested as a slower rate of clearance of the fungus from the kidney.
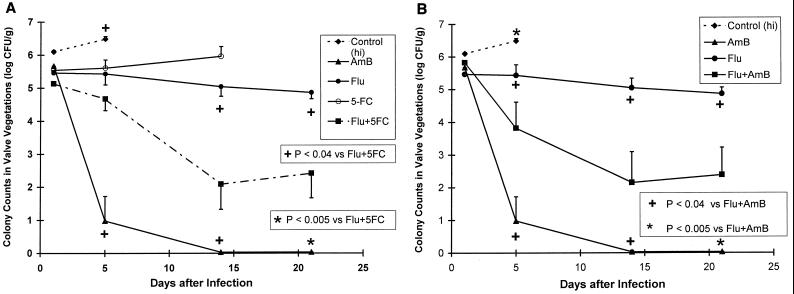
C. albicans endocarditis.
AmB was more effective than Flu and 5FC monotherapies in reducing fungal counts in cardiac vegetations after day 1 (Fig. 5A). The activity of Flu plus 5FC was similar to that of Flu monotherapy on day 5 but greater than that of Flu monotherapy on days 14 and 21 (P < 0.02 at each time point). At these time points, this drug combination decreased the fungal density by at least 2.5 log10 CFU versus Flu monotherapy. Nevertheless, AmB was the most active drug.
FIG. 5.
AmB was more effective than 5FC and Flu monotherapies in reducing fungal densities in cardiac vegetations (log10 [CFU] ± 1 standard error of the mean) beginning on day 5. Also, on day 5 the activity of Flu plus 5FC was similar to that of Flu monotherapy (A). However, on days 14 and 21 Flu-plus-5FC therapy was more effective than Flu alone (P < 0.03 at each time point). Flu-plus-AmB therapy was significantly more active than Flu monotherapy (B). However, Flu-plus-AmB therapy was significantly less active than AmB monotherapy (B; P < 0.04, P < 0.036, and P < 0.008 on days 5, 14, and 21, respectively); thus, the combination of AmB plus Flu was antagonistic.
As shown in Fig. 5B, the combination of AmB and Flu resulted in a significant decrease in fungal counts versus Flu monotherapy beginning on day 14 of therapy (P < 0.02). However, AmB monotherapy was more active than AmB plus Flu at all time points (P < 0.02, P = 0.036, and P < 0.008 at days 5, 14, and 21, respectively). Thus, the combination of Flu and AmB demonstrated antagonism compared with AmB monotherapy.
Proportion of sites that were culture negative.
The percentages of vitreous humor, choroid-retina, kidney, and valvular vegetation samples that were culture negative in each treatment group are shown in Table 5. On day 5 of therapy, Flu alone and in combination with AmB or 5FC was more efficacious than AmB monotherapy in sterilizing the vitreous humor (P ≤ 0.003 for each drug relative to AmB). However the treatments were similar by day 14. The efficacies with which the various treatments sterilized the choroid-retina were similar at all time points.
TABLE 5.
Numbers of samples from the various sites that were culture negative after 5, 14, or 21 days of therapy
| Site and drug(s) | No. of culture-negative samples/total no. of samples after indicated days of therapyd
|
||
|---|---|---|---|
| 5 | 14 | 21 | |
| Vitreous humor | |||
| Control | 0/22 (0)a | ||
| AmB | 3/12 (25)a | 16/16 (100) | 16/16 (100) |
| Flu | 12/12 (100)c | 16/16 (100) | 16/16 (100) |
| 5FC | 1/12 (17) | 4/4 (100) | No data |
| Flu + 5FC | 12/12 (100)b | 18/18 (100) | 16/16 (100) |
| Flu + AmB | 12/12 (100)b | 16/18 (89) | 13/16 (81) |
| Choroid-retina | |||
| Control | 0/22 (0) | ||
| AmB | 1/12 (8) | 16/16 (100) | 16/16 (100) |
| Flu | 1/12 (8) | 12/16 (75) | 16/16 (100) |
| 5FC | 0/12 (0) | 2/4 (50) | No data |
| Flu + 5FC | 0/12 (0) | 17/18 (94) | 16/16 (100) |
| Flu + AmB | 6/12 (50) | 15/18 (83) | 14/16 (88) |
| Kidney | |||
| Control | 0/11 (0) | ||
| AmB | 4/6 (67) | 8/8 (100) | 8/8 (100) |
| Flu | 0/0 (0) | 4/8 (50) | 8/8 (100) |
| 5FC | 0/6 (0) | 0/2 (0) | No data |
| Flu + 5FC | 0/6 (0) | 6/9 (67) | 6/8 (75) |
| Flu + AmB | 0/6 (0) | 5/9 (56) | 6/8 (88) |
| Valve vegetations | |||
| Control | 0/10 (0) | ||
| AmB | 4/6 (67) | 8/8 (100)a | 8/8 (100)a |
| Flu | 0/6 (0) | 0/8 (0)c | 0/8 (0)c |
| 5FC | 0/6 (0) | 0/2 (0) | No data |
| Flu + 5FC | 0/6 (0) | 5/9 (55) | 3/8 (38) |
| Flu + AmB | 1/6 (17) | 5/9 (55) | 4/8 (50) |
P < 0.0003 versus Flu.
P < 0.003 versus AmB.
P < 0.0003 versus AmB.
The left and right vitreous humor and choroid-retina results were similar. Thus the results were combined. No information was available for the 5FC group on day 21 since all animals in this group died by day 18. Differences between groups were determined by Fisher’s exact test with Bonferroni’s correction. On day 5 seven pair-wise comparisons were made, and a difference of P < 0.007 was considered statistically significant. On days 14 and 21, when a control group did not exist, the 5FC monotherapy group was excluded from analysis because of the small number of subjects. Thus, four pair-wise comparisons were made at these time points and P < 0.01 was considered significant.
AmB monotherapy tended to sterilize a greater proportion of kidneys than Flu and AmB plus Flu on days 5 and 14 of treatment. However, the differences were not significant.
AmB was superior to Flu in sterilizing valvular vegetations on days 14 and 21 of therapy (P < 0.0003 at each time point). AmB therapy tended to sterilize more cardiac vegetations than AmB-plus-Flu therapy at each time point (not significant).
Time to sterilization of the kidney and valvular vegetation.
AmB monotherapy sterilized the kidney more rapidly than the combination of AmB and Flu (P was significant at 0.04 by Kaplan-Meier and Wilcoxon analyses). The rates of sterilization for AmB plus Flu and Flu monotherapy were similar (P = 0.88). Infected cardiac vegetations also were sterilized more rapidly with AmB than with AmB plus Flu (P was significant at 0.026). Thus, in the treatment of C. albicans pyelonephritis and endocarditis, AmB plus Flu demonstrated antagonism relative to AmB monotherapy.
Also, the combination of Flu and 5FC sterilized cardiac vegetations significantly faster than Flu alone (P = 0.03). In the kidney, the activities of Flu and Flu plus 5FC were similar (P = 0.50).
DISCUSSION
In the present study we evaluated the relative efficacies of AmB, Flu, 5FC, and the combinations of Flu and 5FC and AmB and Flu for the treatment of C. albicans endocarditis, endophthalmitis, and pyelonephritis. We used a dose of Flu in rabbits that resulted in a steady-state AUC0–24 that was equivalent to the AUC measured in humans who received Flu at 1,600 mg/day (30). We chose this dosage because 1,600 mg of Flu per day is the maximum dosage in humans that is not associated with central nervous system side effects (4). Furthermore, Louie et al. (31) demonstrated that it is the AUC/MIC ratio and not the peak serum concentration or time that this azole remains above the MIC for C. albicans that best predicts outcome. The use of high-dose Flu in the present study provided us with the opportunity to compare the effects of regimens that incorporated the maximum clinically relevant dose of Flu with the effects of those that incorporated AmB. The pharmacodynamic parameter that predicts the outcome for AmB is unknown. Thus, we used a dose of AmB that is commonly used in rabbits by other investigators to permit comparisons between studies (16, 43, 50). Furthermore, the AmB dose of 1 mg/kg/day in rabbits resulted in t1/2β, trough, and AUC0–24 values similar to those seen in the serum of humans who receive 1 mg of AmB-desoxycholate/kg/day (6, 12, 22). We also examined the efficacy of 5FC at 50 mg/kg/day, alone and in combination with Flu. This dosage of 5FC results in steady-state serum concentrations of 40 to 60 μg/ml in rabbits (48), which are the levels that are measured in humans given clinically prescribed doses. Higher dosages of 5FC were not evaluated because dosages of ≥75 mg/kg/day cause fatal fulminant hepatic necrosis in healthy rabbits (23).
In our infection model we found that all the treatment regimens examined were better than no therapy in improving survival. However, 5FC monotherapy could prolong survival for a maximum of 18 days, perhaps due to the emergence of 5FC resistance by C. albicans during therapy (9, 49). All the other treatment regimens resulted in approximately a 93% survival rate, which enabled us to evaluate the relative efficacies of AmB, Flu, Flu plus 5FC, and AmB plus Flu in the vitreous humor, choroid-retina, kidney, and cardiac vegetations.
In our study, high-dose Flu, alone and in combination with 5FC or AmB, sterilized the vitreous humor more rapidly than either AmB or 5FC as monotherapy. In the choroid-retina, AmB and Flu monotherapies demonstrated equal efficacies, but AmB was more active than Flu monotherapy in both the kidney and cardiac vegetations. Flu-plus-5FC therapy was superior to Flu monotherapy in the treatment of C. albicans endocarditis, but in ocular sites and the kidney this combination was no better than Flu alone. AmB-plus-Flu therapy was antagonistic relative to AmB monotherapy in both the kidney and cardiac vegetations.
For antifungal drug monotherapies, in vitro studies and animal models of systemic candidiasis and endocarditis show that AmB is more active than Flu (7, 25, 28, 29, 43, 50). However, in the present study the relative efficacies of AmB and Flu monotherapies in the vitreous humor, choroid-retina, kidney, and cardiac vegetation were dependent on the site of infection. This finding may be due, in part, to differences with which these drugs penetrate into these sites. The superior efficacy of Flu monotherapy over AmB in the treatment of C. albicans vitritis may be due to greater penetration of this azole into the vitreous humor despite the presence of a “blood-ocular barrier” between the blood vessels of the eye and the vitreous humor (3). Savani et al. (44) found that vitreous humor Flu concentrations were 21 and 27% of serum Flu concentrations in the absence and presence, respectively, of inflammation. Previously (35) and in the present study, we found the AUC of Flu in the vitreous humor to be 74% of the AUC in serum. In contrast, the concentration of AmB in this site was ≤0.1 μg/ml (references 17 and 20 and this study). Thus, the superior activity of Flu over AmB monotherapy in the treatment of Candida vitritis may be due to the greater penetration of Flu into the vitreous humor.
In contrast, in the choroid-retina the efficacies of AmB and Flu monotherapies were equivalent. This finding may be due to the fact that the blood-ocular barrier separates only a portion of the choroid-retina from the systemic circulation (3). Thus, the quantitative culture results for this site may represent the average of the superior effect of AmB in portions of the choroid-retina where there is no barrier to AmB penetration and the inferior activity of this drug in portions of the choroid-retina where a barrier does exist. Both AmB and Flu readily penetrate into renal parenchyma (references 10, 19, and 26 and this study). Thus, similar to findings based on the outcomes in systemic models of candidiasis, AmB is the more effective agent at this site.
For the treatment of Candida endocarditis, we found AmB monotherapy to be more active than Flu. These results are consistent with those reported by others (13, 43, 50). The superior efficacy of AmB monotherapy may be due to a combination of factors. First, AmB is fungicidal against C. albicans while Flu is fungistatic (19, 25). Second, the platelet and fibrin in the vegetations that are deposited around the organism (5, 11) form a barrier to Flu and AmB penetration. While neutrophils are abundantly seen in infected kidney parenchyma (27), fungal vegetations are often devoid of neutrophils (5, 11). Without the added effect of the host’s immune defenses within this site, the fungicidal activity of AmB as monotherapy would have a theoretical advantage.
Differences in the penetration of AmB and Flu into the vitreous humor, choroid-retina, kidney, and cardiac vegetations may also explain the different interactions between AmB and Flu at these sites that were observed when these drugs were used in combination. Since Flu readily penetrates into the vitreous humor (35, 44) while AmB concentrations are seldom detectable (references 17 and 20 and this study), it is not surprising that the efficacy of AmB plus Flu was similar to that of Flu monotherapy for the treatment of C. albicans vitritis. In contrast, both drugs readily penetrate into renal tissue (references 10, 19, and 26 and this study); thus, the antagonistic interaction between AmB and Flu is readily appreciated. The blood-ocular barrier may limit AmB penetration to only a portion of the choroid-retina (3). Thus, the observed additive effect between AmB and Flu at this site may reflect the average of the superior outcome of Flu in portions of the choroid-retina in which AmB penetration is limited by the blood-ocular barrier and the antagonistic effect between AmB and Flu in areas where the blood-ocular barrier is absent. Although information on the penetration of AmB and Flu into cardiac vegetations is incomplete (13), the pharmacokinetic profile of these drugs in vegetations is sufficient to result in antagonism.
The combination of Flu and 5FC was more effective than Flu monotherapy in the treatment of Candida endocarditis. The fungal density in vegetations was at least 2.5 log10 CFU/g lower with this drug combination by day 14 of therapy than with either drug alone (the differences were statistically significant, with P < 0.03 at 14 and 21 days). Others attempted to define the interaction between Flu and 5FC by using models of exogenous Candida endophthalmitis (38) and endocarditis (13). Their studies were inconclusive because the doses of 5FC used in the 5FC and Flu-plus-5FC groups were toxic to rabbits, resulting in excessive mortalities.
The antagonistic interaction between AmB and Flu observed in the present study confirms what we and others observed in vitro (7, 34, 39) and in our murine model of systemic candidiasis (29). In our mouse study, the combination of AmB and Flu resulted in higher densities of fungi in kidneys and a worsening of survival versus AmB monotherapy. A stepwise reduction in the antagonistic effect was seen when the same doses of these drugs were given to mice infected with C. albicans strains for which the Flu MICs were higher (0.5 to 128 μg/ml). Antagonism between AmB and Flu was not seen in mice infected with a highly Flu-resistant strain of C. albicans (Flu MIC of 512 μg/ml).
In contrast to our results, Sugar et al. (45) reported that the interaction between AmB and Flu was additive in their murine model of systemic candidiasis. However, in their model the 90 to 100% survival observed in animals that received AmB monotherapy placed the survivorship on the top of the dose-response curve. This makes it very difficult to observe an antagonistic interaction between AmB and Flu unless Flu completely abolishes the activity of AmB. Also, the results of the quantitative cultures of kidneys were below the sensitivity of their assay for all the treatment groups examined. Thus, it is difficult to make any conclusions about the interaction between AmB and Flu from those studies.
Using a neutropenic mouse model of systemic candidiasis, Sanati et al. (43) reported survival rates for AmB, Flu, and Flu-plus-AmB recipients of approximately 70, 58, and 43%, respectively. The difference in outcome between combination therapy and AmB monotherapy was not statistically significant; however, the possibility of a type II error could not be excluded. In contrast to our results, Sanati et al. (43) observed an “indifferent” interaction between Flu and AmB in their rabbit model of C. albicans endocarditis. Importantly, we reported the same rank order in fungal densities as Sanati et al. for Flu, AmB-plus-Flu, and AmB therapies. However, our model resulted in a 5-log10 CFU/g difference between Flu and AmB monotherapies versus a 2-log10 CFU/g difference in the model of Sanati et al. Because of the larger difference between groups, our experimental model was more sensitive in identifying antagonism between AmB and Flu.
The clinical significance of the negative interaction between AmB and Flu observed in both our rabbit and mouse (29) models of C. albicans infection is uncertain. The delay in clearance of C. albicans from the kidney suggests that this combination should not be used in individuals with rapidly fatal infections caused by fungal species that are usually susceptible to both AmB and Flu. However, in stable persons, outcomes with AmB, Flu, and AmB plus Flu may be similar after completion of a prescribed antifungal drug course. Of note, all in vivo studies report that the activity of AmB plus Flu is never less than that of Flu monotherapy. Thus, for infections in which clinical studies have demonstrated Flu and AmB monotherapies to have equivalent efficacies, such as catheter-related fungemia due to C. albicans (40), outcomes associated with AmB plus Flu, Flu, and AmB should be similar. Finally, our endocarditis treatment results demonstrate that the antagonism between AmB and Flu persists throughout therapy in a site that is devoid of neutrophils and, perhaps, other host immune mechanisms. This finding suggests that in neutropenic and other immunocompromised individuals, AmB plus Flu should be used with caution as an empiric therapy or as treatment of an infection due to fungal species that are usually susceptible to both antifungal agents.
ACKNOWLEDGMENTS
We thank personnel in the Animal Resources Facility for their care of the animals.
This project was supported by Pfizer Inc. and grant RO1EY089977-01 from the National Eye Institute (M.H.M.).
REFERENCES
- 1.Akaike H. A new look at the statistical model identification. IEEE Trans Automated Control. 1974;19:716–723. [Google Scholar]
- 2.Akler M E, Vellend H, McNeely D M, Walmsley S L, Gold W L. Use of fluconazole in the treatment of candidal endophthalmitis. Clin Infect Dis. 1995;20:657–664. doi: 10.1093/clinids/20.3.657. [DOI] [PubMed] [Google Scholar]
- 3.Alm A. Ocular circulation. In: Hart W M Jr, editor. Adler’s physiology of the eye: clinical applications. 9th ed. Boston, Mass: Mosby Year Book; 1992. pp. 198–227. [Google Scholar]
- 4.Anaissie E J, Kontoyiannis D M, Huls C, Vartivarian S E, Karl C, Prince R A, Bosso J, Bodey G P. Safety, plasma concentrations, and efficacy of high-dose fluconazole in invasive mold infections. J Infect Dis. 1995;172:599–602. doi: 10.1093/infdis/172.2.599. [DOI] [PubMed] [Google Scholar]
- 5.Andriole V T, Kravetz H M, Roberts W C, Utz J P. Candida endocarditis. Clinical and pathologic studies. Am J Med. 1962;32:251–285. doi: 10.1016/0002-9343(62)90294-2. [DOI] [PubMed] [Google Scholar]
- 6.Ayestaran A, Lopez R M, Montoro J B, Estibalez A, Pou L, Julia A, Lopez A, Pascual B. Pharmacokinetics of conventional formulation versus fat emulsion formulation of amphotericin B in a group of patients with neutropenia. Antimicrob Agents Chemother. 1996;40:609–612. doi: 10.1128/aac.40.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banerjee P, Liu Q-F, Louie A, Shayegani M, Taber H, Drusano G, Miller M. Abstracts of the 97th General Meeting of the American Society for Microbiology. Washington, D.C.: American Society for Microbiology; 1997. Comparison of checkerboard isobolograms and computer generated 3D-plots for evaluation of the in-vitro interactions between antifungal drugs, abstr. C-252a; p. 164. [Google Scholar]
- 8.Bannatyne R M, Cheung R. Discrepant results of amphotericin B assays on fresh versus frozen serum samples. Antimicrob Agents Chemother. 1997;12:550. doi: 10.1128/aac.12.4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett J E. Flucytosine. Ann Intern Med. 1977;86:319–321. doi: 10.7326/0003-4819-86-3-319. [DOI] [PubMed] [Google Scholar]
- 10.Brammer K W, Farrow P R, Faulkner J K. Pharmacokinetics and tissue penetration of fluconazole in humans. Rev Infect Dis. 1990;12(Suppl. 3):S318–S326. doi: 10.1093/clinids/12.supplement_3.s318. [DOI] [PubMed] [Google Scholar]
- 11.Calderone R A, Rotondo M F, Sande M A. Candida albicans endocarditis: ultrastructural studies of vegetation formation. Infect Immun. 1978;20:279–289. doi: 10.1128/iai.20.1.279-289.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chavanet P Y, Garry I, Charlier N, Caillot C, Kisterman J P, D’Athis M, Portier H. Trial of glucose versus fat emulsion preparation of amphotericin for use in HIV infected patients with candidiasis. Br Med J. 1992;305:921–925. doi: 10.1136/bmj.305.6859.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chemlal K, Saint-Julien L, Joly V, Farinotti R, Seta N, Yeni P, Carbon C. Comparison of fluconazole and amphotericin B for treatment of experimental Candida albicans endocarditis in rabbits. Antimicrob Agents Chemother. 1996;40:263–266. doi: 10.1128/aac.40.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Como J A, Dismukes W E. Oral azole drugs as systemic antifungal therapy. N Engl J Med. 1994;330:263–272. doi: 10.1056/NEJM199401273300407. [DOI] [PubMed] [Google Scholar]
- 15.Durack D T, Beeson P B, Petersdorf R G. Experimental bacterial endocarditis. III. Production and progress of the disease in rabbits. Br J Exp Pathol. 1973;54:142–151. [PMC free article] [PubMed] [Google Scholar]
- 16.Filler S G, Crislip M A, Mayer C L, Edwards J E., Jr Comparison of fluconazole and amphotericin B for treatment of disseminated candidiasis and endophthalmitis in rabbits. Antimicrob Agents Chemother. 1991;35:288–292. doi: 10.1128/aac.35.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher J F. Penetration of amphotericin B into the human eye. J Infect Dis. 1983;147:164. doi: 10.1093/infdis/147.1.164. [DOI] [PubMed] [Google Scholar]
- 18.Granich G G, Kobayashi G S, Krogstad D J. Sensitive high-pressure liquid chromatographic assay for amphotericin B which incorporates an internal standard. Antimicrob Agents Chemother. 1986;29:584–588. doi: 10.1128/aac.29.4.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grant S M, Clissold S P. Fluconazole. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in superficial and systemic mycoses. Drugs. 1990;39:877–916. doi: 10.2165/00003495-199039060-00006. [DOI] [PubMed] [Google Scholar]
- 20.Green W R, Bennett J E, Goos R D. Ocular penetration of amphotericin B: a report of laboratory studies and a case report of postsurgical cephalsporium endophthalmitis. Arch Ophthalmol. 1965;73:769–775. doi: 10.1001/archopht.1965.00970030771004. [DOI] [PubMed] [Google Scholar]
- 21.Hallum J L, Williams T W., Jr . Candida endocarditis. In: Bodey G P, editor. Candidiasis: pathogenesis, diagnosis, and treatment. 2nd ed. New York, N.Y: Raven Press; 1993. pp. 357–369. [Google Scholar]
- 22.Heinemann V, Bosse D, Jehn U, Kahny B, Wachholz K, Debus A, Scholz P, Kolb H-J, Wilmanns W. Pharmacokinetics of liposomal amphotericin B (AmBisome) in critically ill patients. Antimicrob Agents Chemother. 1997;41:1275–1280. doi: 10.1128/aac.41.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones D B, Green M T, Osato M S, Broberg P H, Gentry L O. Endogenous Candida albicans endophthalmitis in the rabbit. Chemotherapy for systemic effect. Arch Ophthalmol. 1981;99:2182–2187. doi: 10.1001/archopht.1981.03930021058014. [DOI] [PubMed] [Google Scholar]
- 24.Jorgensen J H, Alexander G A, Graybill J R, Drutz D J. Sensitive bioassay for ketoconazole in serum and cerebrospinal fluid. Antimicrob Agents Chemother. 1981;20:59–62. doi: 10.1128/aac.20.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klepser M E, Wolfe E J, Jones R N, Nightingale C H, Pfaller M A. Antifungal pharmacodynamic characteristics of fluconazole and amphotericin B tested in Candida albicans. Antimicrob Agents Chemother. 1997;41:1392–1395. doi: 10.1128/aac.41.6.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee J W, Amantea M A, Francis P A, Navarro E E, Bacher J, Pizzo P A, Walsh T J. Pharmacokinetics and safety of a unilamellar liposomal formulation of amphotericin B (AmBisome) in rabbits. Antimicrob Agents Chemother. 1994;38:713–718. doi: 10.1128/aac.38.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Louie A, Baltch A L, Smith R P, Franke M A, Ritz W J, Singh J K, Gordon M A. Tumor necrosis factor alpha has a protective role in a murine model of systemic candidiasis. Infect Immun. 1994;62:2761–2772. doi: 10.1128/iai.62.7.2761-2772.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Louie A, Baltch A L, Smith R P, Franke M A, Ritz W J, Singh J K, Gordon M A. Fluconazole and amphotericin B antifungal therapies do not negate the protective effect of endogenous tumor necrosis factor in a murine model of fatal disseminated candidiasis. J Infect Dis. 1995;171:406–415. doi: 10.1093/infdis/171.2.406. [DOI] [PubMed] [Google Scholar]
- 29.Louie A, Banerjee P, Drusano G L, Shayegani M, Miller M H. Interaction between fluconazole and amphotericin B in mice with systemic infection due to fluconazole-susceptible or -resistant strains of Candida albicans. Antimicrob Agents Chemother. 1999;43:2841–2847. doi: 10.1128/aac.43.12.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Louie A, Liu Q-F, Drusano G L, Liu W, Mayers M, Anaissie E, Miller M H. Pharmacokinetic studies of fluconazole in rabbits characterizing doses which achieve peak levels in serum and area under the concentration-time curve values which mimic those of high-dose fluconazole in humans. Antimicrob Agents Chemother. 1998;42:1512–1514. doi: 10.1128/aac.42.6.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Louie A, Drusano G L, Banerjee P, Liu Q-F, Liu W, Kaw P, Shayegani M, Taber H, Miller M H. Pharmacodynamics of fluconazole in a murine model of systemic candidiasis. Antimicrob Agents Chemother. 1998;42:1105–1109. doi: 10.1128/aac.42.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maddux M S, Barriere S L. A review of the complications of amphotericin B therapy; recommendations for prevention and management. Drug Intell Clin Pharm. 1980;14:177–181. [Google Scholar]
- 33.Madu A, Cioffe C, Mian U, Burroughs M, Tuomanen E, Mayers M, Schwartz E, Miller M. Pharmacokinetics of fluconazole in cerebrospinal fluid and serum of rabbits: validation of an animal model used to measure drug concentrations in cerebrospinal fluid. Antimicrob Agents Chemother. 1994;38:2111–2115. doi: 10.1128/aac.38.9.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin E, Maier F, Bhakdi S. Antagonistic effects of fluconazole and 5-fluorocytosine on candidacidal action of amphotericin B in human serum. Antimicrob Agents Chemother. 1994;38:1331–1338. doi: 10.1128/aac.38.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mian U K, Mayers M, Garg Y, Liu Q-F, Newcomer G, Madu C, Liu W, Louie A, Miller M. Comparison of fluconazole pharmacokinetics in serum, aqueous humor, vitreous humor, and cerebrospinal fluid following a single dose and at steady state. J Ocul Pharmacol Ther. 1998;14:459–471. doi: 10.1089/jop.1998.14.459. [DOI] [PubMed] [Google Scholar]
- 36.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing for yeast. Tentative standard M27-T. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1995. [Google Scholar]
- 37.Odds F C. Candida endocarditis, myocarditis, and other cardiovascular Candida infections. In: Odds F C, editor. Candida and candidosis. A review and bibliography. 2nd ed. London, United Kingdom: Bailliere, Tindall; 1988. pp. 175–180. [Google Scholar]
- 38.Park S S, D’Amico D J, Paton B, Baker A S. Treatment of exogenous Candida endophthalmitis in rabbits with oral fluconazole. Antimicrob Agents Chemother. 1995;39:958–963. doi: 10.1128/aac.39.4.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrou M A, Rogers T R. Interactions in vitro between polyenes and imidazoles against yeast. J Antimicrob Chemother. 1991;27:491–506. doi: 10.1093/jac/27.4.491. [DOI] [PubMed] [Google Scholar]
- 40.Rex J H, Bennett J E, Sugar A M, Pappas P G, van der Horst C M, Edwards J E, Washburn R G, Scheld W M, Karchmer A W, Dine A P, et al. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients with neutropenia. Candidemia Study Group and the National Institute. N Engl J Med. 1994;331:1325–1330. doi: 10.1056/NEJM199411173312001. [DOI] [PubMed] [Google Scholar]
- 41.Rubinstein E, Noreiga E R, Simberkoff M S, Holzman R, Rahal J J., Jr Fungal endocarditis: analysis of 24 cases and review of the literature. Medicine. 1975;54:331–344. [PubMed] [Google Scholar]
- 42.Sabra R, Branch R A. Amphotericin B nephrotoxicity. Drug Saf. 1990;5:94–108. doi: 10.2165/00002018-199005020-00003. [DOI] [PubMed] [Google Scholar]
- 43.Sanati H, Ramos C F, Bayer A S, Ghannoum M A. Combination therapy with amphotericin B and fluconazole against invasive candidiasis in neutropenic-mouse and infective-endocarditis rabbit models. Antimicrob Agents Chemother. 1997;41:1345–1348. doi: 10.1128/aac.41.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Savani D V, Perfect J R, Cobo L M, Durack D T. Penetration of new azole compounds into the eye and efficacy in experimental Candida endophthalmitis. Antimicrob Agents Chemother. 1987;31:6–10. doi: 10.1128/aac.31.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugar A M, Hitchcock C A, Troke P F, Picard M. Combination therapy of murine invasive candidiasis with fluconazole and amphotericin B. Antimicrob Agents Chemother. 1995;39:598–601. doi: 10.1128/AAC.39.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Venditti M, De Bernardis F, Micozzi A, Pontieri E, Chirletti P, Cassone A, Martino P. Fluconazole treatment of catheter-related right-sided endocarditis caused by Candida albicans and associated with endophthalmitis and folliculitis. Clin Infect Dis. 1992;14:422–426. doi: 10.1093/clinids/14.2.422. [DOI] [PubMed] [Google Scholar]
- 47.Walsh T J, Bacher J, Pizzo P A. Chronic silastic central venous catheterization for induction, maintenance and support of persistent granulocytopenia in rabbits. Lab Anim Sci. 1988;38:467–471. [PubMed] [Google Scholar]
- 48.Walsh T J, Lee J, Aoki S, Mechinaud F, Bacher J, Lecciones J, Thomas V, Rubin M, Pizzo P A. Experimental basis for use of fluconazole for preventive or early treatment of disseminated candidiasis in granulocytopenic hosts. Rev Infect Dis. 1990;12(Suppl. 3):S307–S317. doi: 10.1093/clinids/12.supplement_3.s307. [DOI] [PubMed] [Google Scholar]
- 49.Wise G J, Kozinn P J, Goldberg P. Flucytosine in the management of genitourinary candidiasis: 5 years of experience. J Urol. 1980;124:70–72. doi: 10.1016/s0022-5347(17)55301-x. [DOI] [PubMed] [Google Scholar]
- 50.Witt M D, Bayer A S. Comparison of fluconazole and amphotericin B for prevention and treatment of experimental Candida endocarditis. Antimicrob Agents Chemother. 1991;35:2481–2485. doi: 10.1128/aac.35.12.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]