Abstract
Background
Circadian system contributes to the regulation of inflammatory processes, but the role of circadian misalignment as a risk factor for contracting Covid-19 has up to now been poorly studied. The aim of this study was to explore the relationship between circadian misalignment (chronic disturbance of the circadian system) and the risk of Covid-19 infection in a population of subjects suspected of contact or infection with SARS-CoV-2.
Methods
Cross-sectional single-center study conducted during a period without lockdown in winter 2021. Recruitment took place in a Covid-19 outpatient testing center. Subjects between 18 and 45 years old were included whether they were symptomatic or not, healthcare workers or not, in contact with a Covid-19 case or not. To determine social jetlag, a proxy of circadian misalignment, they were asked about their usual sleep-wake behaviors. Usual sleep duration and sleep-wake timing were explored on workdays and free days. Social jetlag was defined as at least 2 h shift of circadian alignment (defined as the difference between mid-sleep on workdays and mid-sleep on free days, mid-sleep as the median between bedtime and rise time).
Results
One thousand fourteen subjects were included (sampling rate: 10.8%, 39% men, mean age 28 ± 8) with 56 subjects positive for Covid-19 (positivity rate: 5.5%). Usual mean sleep duration was equivalent in both groups (7h47 versus 7h49, p = 0.733). Social jetlag greater than 2 h comprised 33.3% of subjects in the Covid-19 group versus 20.6% in the control group (p = 0.026). After adjustment on age, gender, BMI and work schedules, subjects presenting with social jetlag greater than 2 h had a 2.07-fold higher likelihood to test positive than subjects who had identical sleep-wake timing on workdays and free days (OR = 2.07, 95%CI = [1.12–3.80], p = 0.024).
Conclusion
Circadian misalignment not only is present in subjects infected by Covid-19 but could also be responsible for a higher likelihood of being infected. The chronobiological impact on the immune system or a higher likelihood of being exposed to social contacts during nocturnal activities could explain our findings, which need to be confirmed in a future large cohort study. Regular sleep-wake timing could ultimately become a target for preventing Covid-19 infection.
Keywords: Sleep medicine specialty, COVID-19, Immunology, Epidemiology
Abbreviations: BMI, Body Mass Index; OR, Odds-Ratio; CI, Confidence Interval; RT-PCR, Reverse Transcription-Polymerase Chain Reaction
1. Background
The Covid-19 epidemic has massively disrupted sleep-wake behaviors in western society [1]. Social distancing has disturbed daily life routines, increasing worry, isolation, stress, and screen time, which are known contributors to impaired sleep [2,3]. However, circadian clocks contribute to the regulation of inflammatory processes (control the function of our immune system, virus replication and the severity of infections) which are particularly important in this context of a pandemic [4]. Indeed, a recent study found that the expression of 30% of 332 human proteins that interact with SARS-CoV-2 proteins shows circadian oscillation [5], and that most potential drug targets exhibit robust 24-h oscillation in at least one organ or tissue in mammalian systems [6]. Moreover, people with circadian misalignment/disruption (a chronic disturbance of the circadian system) due to internal (age, blindness) or external (shift work, social jetlag) have a weaker immune system and could be more susceptible to such viral respiratory diseases [6]. Social jetlag is a chronic misalignment between internal circadian clocks and external time (social time). Taken together, these results support the hypothesis that circadian misalignment could promote SARS-CoV-2 infection [[6], [7], [8]]. Nevertheless, usual circadian misalignment as a risk factor for contracting Covid-19 has up to now been poorly studied. Only a 2021 study showed that night work, which involves circadian disruption, was associated with an 1.85-fold increased risk of Covid-19 infection [9]. Therefore, the aim of this study was to explore the relationship between usual circadian misalignment (described by social jetlag) and the risk of Covid-19 infection in the general population.
2. Methods
We conducted a cross-sectional single-center study during a period between February and April 2021 without lockdown but with very stringent socio-economic restrictions including curfew from 6 p.m. to 6 a.m. Recruitment took place at the entering of the Covid-19 outpatient testing center at Bordeaux University Hospital (southwest of France). Strict compliance with barrier gestures from investigators and participants was required. Patients between 18 and 45 years old who accepted to participate were included whether they were symptomatic or not, healthcare workers or not, in contact with a Covid-19 case or not. Following their test, participants answered an internet-based questionnaire on their sociodemographic data (age, gender, body mass index (BMI), profession and work schedules), their current symptoms and their medical background. Participants were asked about their usual sleep-wake timing. They were asked what time they usually go to bed (bedtime) and get up (rise time) on workdays and on free days, and how many hours of actual sleep they get (sleep duration) [10]. Mean sleep duration was defined as the average sleep duration over a full week, including workdays and free days. We then used these answers to estimate a proxy of their circadian misalignment: social jetlag (defined as the difference between mid-sleep on workdays and mid-sleep on free days, mid-sleep as the median between bedtime and rise time [11]). Short mean sleep duration was defined as fewer than the 7 h per night recommended by the National Sleep Foundation [12] and social jetlag as at least 2 h shift [13].
2.1. Statistical analysis
The primary endpoint was the SARS-CoV-2 detection using RT-PCR on nasopharyngeal samples (positive/negative) performed in a hospital-accredited center following the recommendations of the French Ministry of Health. Socio-demographical data and sleep-wake behaviors were compared between the Covid-19 group and the control group using a X2 test for qualitative variables or the t-test for quantitative variables. Adjusted odds-ratio (OR), 95% confidence interval (CI) and p-value were then assessed using a logistic regression with age, gender, BMI, and work schedules as covariates. The threshold of 0.05 was used to determine significance.
3. Results
3.1. Population
Out of 9419 patients (41% of men, mean age of 28 ± 8) tested in the center during the study period and meeting the inclusion criteria, 1014 participants were included since they were tested and interviewed during the work shifts of the student nurses assigned to the project (sampling rate: 10.8%). The population interviewed was representative of the whole sample in terms of age and sex (39% men, mean age 28 ± 8). Out of the 1014 subjects recruited, 56 participants tested positive for Covid-19 (5.5%). This positivity rate was similar to the department's data over the period (from 4.3% on March 12 to 7.5% on April 23). Table 1 describes socio-demographic and clinical patterns of the Covid-19 group and the control group. A total of 20.6% of participants were overweight, including 6% obese, 27.5% had shifted schedules including 18.3% with consecutive hours of more than 12 h, 29.1% were healthcare workers and 39.5% were students. Cough, fatigue, fever, headache, sore throat, aches, loss of taste and/or smell, shortness of breath and chest pain were more frequent in the Covid-19 group than in the control group. Presence of at least one chronic disease was equivalent in both groups (10.7% versus 10.2%).
Table 1.
Characteristics of study population.
| Variables | Covid-19 Positive (n = 56) |
Covid-19 Negative (n = 958) |
|---|---|---|
| Age | 28.9 ± 8.4 | 28.0 ± 8.3 |
| Gender = Female (%) | 36 (64.3%) | 587 (61.3%) |
| BMI | ||
| <18 | 4 (7.1%) | 22 (2.3%) |
| [18–25] | 40 (71.4%) | 741 (77.4%) |
| [25–30] | 6 (10.7%) | 142 (14.8%) |
| >30 | 6 (10.7%) | 53 (5.5%) |
| Work schedules | ||
| Fixed day work | 48 (85.7%) | 664 (69.3%) |
| Fixed night work | 1 (1.8%) | 21 (2.2%) |
| Shifted without consecutive 12 h | 2 (3.6%) | 92 (9.6%) |
| Shifted with consecutive 12 h | 5 (8.9%) | 181 (18.9%) |
| Healthcare workers = Yes (%) | 12 (21.4%) | 282 (29.3%) |
| Students = Yes (%) | 20 (35.7%) | 381 (39.8%) |
| Symptoms | ||
| Cough | 29 (51.2%) | 150 (15.7%) |
| Fatigue | 40 (71.4%) | 251 (26.2%) |
| Fever | 19 (33.9%) | 64 (6.7%) |
| Headache | 34 (60.7%) | 195 (20.3%) |
| Sore throat | 25 (44.6%) | 166 (17.3%) |
| Aches | 23 (41.1%) | 94 (9.8%) |
| Loss of taste and/or smell | 14 (25.0%) | 15 (1.6%) |
| Shortness of breath | 8 (14.3%) | 35 (3.7%) |
| Chest pain | 3 (5.4%) | 18 (1.9%) |
| Medical background | ||
| Any | 6 (10.7%) | 98 (10.2%) |
| Endocrine disease (diabetes, dysthyroid) | 0 | 12 (1.3%) |
| Chronic inflammatory bowel disease | 0 | 8 (0.8%) |
| Mood disorder (depression, bipolarity) | 0 | 7 (0.7%) |
| Sleep apnea | 1 (1.8%) | 5 (0.5%) |
| Chronic obstructive pulmonary disease | 2 (3.6%) | 2 (0.2%) |
| Multiple sclerosis | 0 | 2 (0.2%) |
| Chronic renal failure | 1 (1.8%) | 0 |
| Others | 3 (5.4%) | 54 (5.6%) |
| Mean sleep durationa | 07h47 ± 50′ | 07h49 ± 56′ |
| Social jetlagb (≥2 h) | 18 (33.3%) | 193 (20.6%) |
11 missing data (n = 1003).
21 missing data (n = 993).
3.2. Circadian misalignment
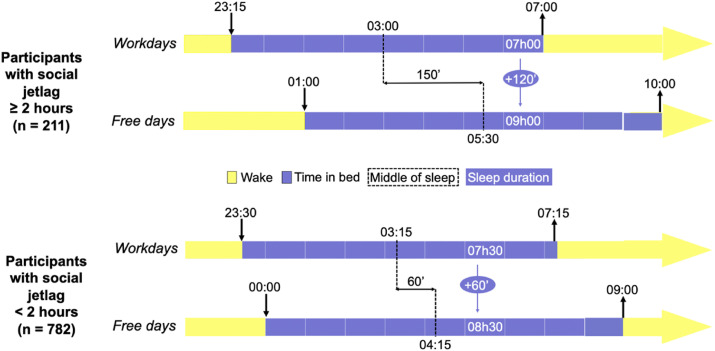
Two-hundred eleven participants (20.8%) had social jetlag. Fig. 1 describes the sleep-wake timing of participants according to their social jetlag. Usual bedtime and rise time were 11:15 p.m. and 7:00 a.m. on workdays and 1:00 a.m. and 10:00 a.m. on free days in participants with social jetlag, versus 11:30 p.m. and 7:15 a.m. on workdays and 12.00 p.m. and 9:00 a.m. on free days for others (respectively, p = 0.450, p = 10−9, p = 0.089 and p = 10−16). Social jetlag was present in 33.3% of participants in the Covid-19 group versus 20.6% of the control group (p = 0.026). In other words, the positivity rate was 8.5% (18/211) in subjects with social jetlag while the positivity rate was 4.6% (36/782) in subjects with regular sleep-wake timing on workdays and free days.
Fig. 1.
Sleep-wake timing of participants according to their social jetlag.
3.3. Sleep duration
Usual sleep duration before workdays and free days were equivalent in both groups: 7h18 and 9h00 in the Covid-19 group and 7h27 and 8h52 in the control group (respectively, p = 0.476 and p = 0.204) as usual mean sleep duration (7h47 in the Covid-19 group versus 7h49 in the control group, p = 0.733.
3.4. Association between usual social jetlag, sleep duration and Covid-19 status
Social jetlag was significantly associated with Covid-19 infection after adjustment for age, gender, BMI, and usual work schedules (OR = 2.07, 95%CI = [1.12–3.80], p = 0.024). Mean sleep duration was not associated with Covid-19 infection after adjusting for age, gender, BMI and work schedules (OR = 0.66, 95%CI = [0.27–1.59], p = 0.331).
4. Discussion
Our study shows that usual social jetlag, a proxy of circadian misalignment, is frequent (20.8%) and significantly associated with Covid-19 infection in the general population, and it suggests that chronic circadian disruption could weaken the immune system and promote infection [6,7]. This result is in line with the conclusions of a 2022 review which conclude that alterations of circadian rhythms have been identified as possible new risk factors for alteration of physiologic metabolism, including immunity [14]. Although the study period included a curfew from 6 p.m. to 6 a.m., one cannot exclude social gatherings in apartments and homes, which could explain the higher rate of contamination in subjects with a delayed sleep onset [15,16]. On the other hand, usual sleep duration was not associated with Covid-19 infection. Many studies have found a link between sleep deprivation and immunity, and particularly Covid-19 infection [[17], [18], [19], [20]]. The lack of association in this sample may be due to the low prevalence of significant deprivation (sleep duration less than 6 h per night on workdays: 5.2% versus 35.9% in a 2019 French study conducted before the outbreak [21]). Indeed, sleep duration has increased significantly since the beginning of the Covid-19 epidemic in western society [22].
The study has some limitations. First, the subjects were included on a voluntary basis, although their characteristics were quite similar to the source population, except for a high proportion of students. However, supplementary analyses showed that the association between social jetlag and Covid-19 infection was consistent across both groups (students: 31.6% of social jetlag in the Covid-19 group versus 21.2% in the control group and non-students: 34.3% versus 20.1%). Second, the design was cross-sectional, although we focused on usual sleep-wake timing, which limited reverse causality. A large cohort study is now required to confirm our findings. Finally, the formula used to calculate social jetlag does not take into account sleep latency as described by Roenneberg et al. and could lead to an erroneous estimate [11].
In conclusion, circadian misalignment, ie, a chronic disturbance of the circadian system observed frequently in shift workers and evening subjects with sleep-wake timing constrained by work, could prove to be another risk factor for Covid-19 infection in the general population due to continuing social constraints. Defining the most appropriate schedules for (tele)-working or staying at home to maintain a healthy body and circadian clock could help to reduce the risk of infection and rapid transmission and manage the dynamics of the current pandemic and future ones. Thus, ‘temporal’ social interventions need to be considered along with ‘spatial’ social distancing to help curb the spread of SARS-CoV-2.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Acknowledgments
We would like to thank the entire administrative and nursing team of the outpatient testing center who allowed us to carry out this study. Also, we would like to thank Patrick Cassai and Luc Durand for their support in writing the project and submitting it to the ethics committee, to Marie-France Koltes for welcoming and supervising the students and conducting the patient survey, Aurélien Boiseau for his involvement in data management, follow-up of participants and for having conducted the patient survey and finally Cécile Klochendler for the design and supervision of this research. Finally, we thank the students who help us by informing patients about the subject of our research.
Footnotes
None.
The ICMJE Uniform Disclosure Form for Potential Conflicts of Interest associated with this article can be viewed by clicking on the following link: https://doi.org/10.1016/j.sleep.2022.03.015.
Conflict of interest
The following is the Supplementary data to this article:
References
- 1.Jahrami H., BaHammam A.S., Bragazzi N.L., et al. Sleep problems during the COVID-19 pandemic by population: a systematic review and meta-analysis. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. 1 févr 2021;17(2):299–313. doi: 10.5664/jcsm.8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott H., Biello S.M., Woods H.C. Social media use and adolescent sleep patterns: cross-sectional findings from the UK millennium cohort study. BMJ Open. 1 sept 2019;9(9) doi: 10.1136/bmjopen-2019-031161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford M.B. Social distancing during the COVID-19 pandemic as a predictor of daily psychological, social, and health-related outcomes. J Gen Psychol. juill 2021;148(3):249–271. doi: 10.1080/00221309.2020.1860890. [DOI] [PubMed] [Google Scholar]
- 4.Irwin M.R., Opp M.R. Sleep health: reciprocal regulation of sleep and innate immunity. Neuropsychopharmacology. janv 2017;42(1):129–155. doi: 10.1038/npp.2016.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ray S., Valekunja U.K., Stangherlin A., et al. Circadian rhythms in the absence of the clock gene Bmal1. Science. 14 févr 2020;367(6479):800–806. doi: 10.1126/science.aaw7365. [DOI] [PubMed] [Google Scholar]
- 6.Ray S., Reddy A.B. COVID-19 management in light of the circadian clock. Nat Rev Mol Cell Biol. 22 juill 2020:1–2. doi: 10.1038/s41580-020-0275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Z L., S T., X Z. COVID-19, circadian rhythms and sleep: from virology to chronobiology. Interface Focus. 10 déc 2021;11(6) doi: 10.1098/rsfs.2021.0043. https://pubmed.ncbi.nlm.nih.gov/34956600/ [Internet] Disponible sur: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salehinejad M.A., Azarkolah A., Ghanavati E., et al. Circadian disturbances, sleep difficulties and the COVID-19 pandemic. Sleep Med. 14 juill 2021;S1389-S9457(21):393–402. doi: 10.1016/j.sleep.2021.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fatima Y., Bucks R.S., Mamun A.A., et al. Shift work is associated with increased risk of COVID-19: findings from the UK Biobank cohort. J Sleep Res. 8 mars 2021:e13326. doi: 10.1111/jsr.13326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roenneberg T., Wirz-Justice A., Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms. févr 2003;18(1):80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- 11.Roenneberg T., Pilz L.K., Zerbini G., et al. Chronotype and social jetlag: a (self-) critical review. Biology. 12 juill 2019;8(3):E54. doi: 10.3390/biology8030054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirshkowitz M., Whiton K., Albert S.M., et al. National Sleep Foundation's updated sleep duration recommendations: final report. Sleep Health. déc 2015;1(4):233–243. doi: 10.1016/j.sleh.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Beauvalet J.C., Quiles C.L., Oliveira MAB de, et al. Social jetlag in health and behavioral research: a systematic review. ChronoPhysiol Ther. 8 mai 2017;7:19–31. [Google Scholar]
- 14.Ragnoli B., Pochetti P., Pignatti P., et al. Sleep deprivation, immune suppression and SARS-CoV-2 infection. Int J Environ Res Public Health. janv 2022;19(2):904. doi: 10.3390/ijerph19020904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whaley C.M., Cantor J., Pera M., et al. Assessing the association between social gatherings and COVID-19 risk using birthdays. JAMA Intern Med. 1 août 2021;181(8):1090–1099. doi: 10.1001/jamainternmed.2021.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grant R., Charmet T., Schaeffer L., et al. Impact of SARS-CoV-2 Delta variant on incubation, transmission settings and vaccine effectiveness: results from a nationwide case-control study in France. Lancet Reg Health – Eur. 25 nov 2021 doi: 10.1016/j.lanepe.2021.100278. https://www.thelancet.com/journals/lanepe/article/PIIS2666-7762(21)00264-7/fulltext [Internet] 0(0). Disponible sur: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pataka A., Kotoulas S., Sakka E., et al. Sleep dysfunction in COVID-19 patients: prevalence, risk factors, mechanisms, and management. J Personalized Med. 14 nov 2021;11(11):1203. doi: 10.3390/jpm11111203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishikura I.A., Rosa D.S., Hachul H., et al. Sleep deficit in COVID-19 health-care workers may increase the infection risk. Scand J Publ Health. nov 2021;49(7):697–699. doi: 10.1177/14034948211007679. [DOI] [PubMed] [Google Scholar]
- 19.Mello M.T.D., Silva A., Guerreiro R. de C., et al. Sleep and COVID-19: considerations about immunity, pathophysiology, and treatment. Sleep Sci Sao Paulo Braz. sept. 2020;13(3):199–209. doi: 10.5935/1984-0063.20200062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.H K., S H., C L., et al. COVID-19 illness in relation to sleep and burnout. BMJ Nutr Prev Health. 22 mars 2021;4(1) doi: 10.1136/bmjnph-2021-000228. https://pubmed.ncbi.nlm.nih.gov/34308120/ [Internet] Disponible sur: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Léger D. Le temps de sommeil en France/Sleep Time in France. 30.
- 22.Trakada A., Nikolaidis P.T., Andrade M.D.S., et al. Sleep during « lockdown » in the COVID-19 pandemic. Int J Environ Res Publ Health. 5 déc 2020;17(23):E9094. doi: 10.3390/ijerph17239094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.