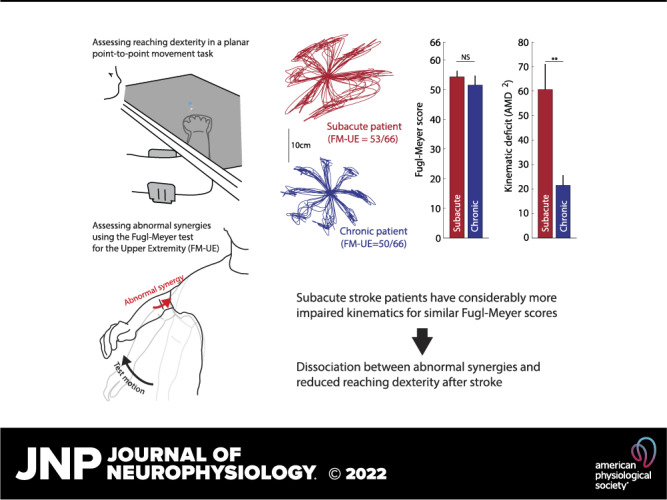
Keywords: dexterity, hemiparesis, motor control, stroke, synergy
Abstract
Most patients with stroke experience motor deficits, usually referred to collectively as hemiparesis. Although hemiparesis is one of the most common and clinically recognizable motor abnormalities, it remains undercharacterized in terms of its behavioral subcomponents and their interactions. Hemiparesis comprises both negative and positive motor signs. Negative signs consist of weakness and loss of motor control (dexterity), whereas positive signs consist of spasticity, abnormal resting posture, and intrusive movement synergies (abnormal muscle co-activations during voluntary movement). How positive and negative signs interact, and whether a common mechanism generates them, remains poorly understood. Here, we used a planar, arm-supported reaching task to assess poststroke arm dexterity loss, which we compared with the Fugl-Meyer stroke scale; a measure primarily reflecting abnormal synergies. We examined 53 patients with hemiparesis after a first-time ischemic stroke. Reaching kinematics were markedly more impaired in patients with subacute (<3 mo) compared to chronic (>6 mo) stroke even for similar Fugl-Meyer scores. This suggests a dissociation between abnormal synergies (reflected in the Fugl-Meyer scale) and loss of dexterity, which in turn suggests different underlying mechanisms. Moreover, dynamometry suggested that Fugl-Meyer scores capture weakness as well as abnormal synergies, in line with these two deficits sharing a neural substrate. These findings have two important implications: First, clinical studies that test for efficacy of rehabilitation interventions should specify which component of hemiparesis they are targeting and how they propose to measure it. Metrics used widely for this purpose may not always be chosen appropriately. For example, as we show here, the Fugl-Meyer score may capture some hemiparesis components (abnormal synergies and weakness) but not others (loss of dexterity). Second, there may be an opportunity to design rehabilitation interventions to address specific subcomponents of hemiparesis.
NEW & NOTEWORTHY Motor impairment is common after stroke and comprises reduced dexterity, weakness, and abnormal muscle synergies. Here we report that, when matched on an established synergy and weakness scale (Fugl-Meyer), patients with subacute stroke have worse reaching dexterity than chronic ones. This result suggests that the components of hemiparesis are dissociable and have separable mechanisms and, thus, may require distinct assessments and treatments.
INTRODUCTION
Stroke is one of the leading causes of disability globally, with an estimated 13.7 million individuals suffering a stroke each year (1). A large fraction of strokes (up to 70%–80%) results in some degree of motor impairment (2–4), which makes activities of daily living harder, compromising quality of life (5, 6). Hemiparesis (or upper motor neuron syndrome) is clinically quite recognizable but remains undercharacterized in terms of its behavioral components. It has been known since the late 19th century that hemiparesis comprises loss of ability (negative signs) and an intrusive movement disorder (positive signs) (7, 8). Negative signs consist of weakness and loss of dexterity or fractionated motor control, whereas positive signs consist of spasticity, abnormal resting postures, and abnormal synergies whereby multiple muscles or joints become co-activated during voluntary movement. How positive and negative signs relate to each other remains poorly understood. Bridging this knowledge gap will be essential for the treatment of hemiparesis, as it will allow us to 1) differentiate components of hemiparesis, allowing clinicians to more reliably track motor recovery after stroke; 2) better isolate and target individual components to make rehabilitation more effective; and 3) better assess the efficacy of rehabilitation interventions.
Among positive signs of stroke, abnormal synergies have been the focus of particular attention and widely recognized as a crucial characteristic of motor impairment after stroke. Twitchell’s (9) classic work describes the time course of recovery of voluntary movement after stroke, from plegia to flexor then extensor synergies to out-of-synergy movements. Given that recovery can get stuck at any point along this sequence, Brunnstrom (10) suggested therapeutic procedures to increase the chance of a patient progressing through it. A scale was subsequently developed to measure and track recovery from synergies—the Fugl-Meyer scale (11).
The negative signs of stroke are weakness and loss of dexterity or motor control. Dexterity generally refers to the ability to flexibly and independently control muscles and joints to generate the movement repertoire required by a given task. Importantly, the term should not be considered synonymous with or reserved for finger movements. Dexterity might require practice, and after stroke, it can recover independently of weakness (12, 13). Notably, the intrusion of abnormal synergies might mask dexterity. A previous study showed that when patients with chronic stroke made three-dimensional (3-D) reaching movements within synergy, trajectories appeared comparable with those made by healthy controls (14). In contrast, when these same patients attempted an out-of-synergy reach that required elbow extension, the trajectory was degraded by an intrusive flexor synergy. This intrusion is thought to be invoked in part by the patient needing to lift their arm against gravity, as several studies have shown that external support of the weight of the arm can reduce the effect of abnormal synergies and increase the arm’s available workspace (15–17). Thus, here we examined the relationship between abnormal synergies and loss of arm dexterity after stroke. Although these two are both signs of motor stroke, it is not given that they depend on each other: strokes can result in heterogeneous lesions, with differential weightings on cortical areas and descending pathways that could result in varying degrees of expression of each sign. We hypothesized that, if abnormal synergies and loss of arm dexterity shared a common neural substrate, they would be closely tied even when assessed when the contaminating effect of weakness is minimized through arm support. To obtain a measure of arm dexterity loss, we quantified kinematics in a planar reaching task. The apparatus provided full support of the weight of the arm, allowing us to assess arm dexterity while minimizing weakness and the intrusion of synergies; moreover, the apparatus constrained the trunk, minimizing the use of compensatory strategies. To measure the extent of abnormal synergies, we used the Fugl-Meyer scale for the upper extremity (FM-UE), which was specifically designed to quantify abnormal synergies poststroke (9–11). Because recovery of reaching dexterity and abnormal synergies may have different time courses, we compared two separate groups of patients, each at a different time stage poststroke (18): one during the early subacute stage, during which most of spontaneous recovery occurs (up to 3 mo poststroke), and another during the chronic stage (at least 6 mo poststroke). Finally, because FM-UE may also reflect weakness (12, 19), we used dynamometry to directly test strength in a subset of the patients to investigate the relative contribution of weakness to our FM-UE versus reaching dexterity comparisons.
MATERIALS AND METHODS
Participants and Ethics Statement
Participants were recruited as part of a multiple-task study of the motor learning, control, and physiology of patients with stroke (PaLaS study, Physiology and Learning after Stroke). The study compared these modalities between two stages in recovery: subacute (<3 mo poststroke) and chronic (> 6 mo poststroke). Table 1 shows details for each of the 53 patients with stroke (27 subacute and 26 chronic) included in this paper, whereas Table 2 shows summary demographics and assessment metrics for the two patient groups and 17 healthy, age-range-matched controls (age comparisons between controls and either patient group or between patient groups: all P > 0.26). Recruitment and data collection took place in Johns Hopkins University and the Kennedy Krieger Institute in Baltimore, MD, from December 2015 through February 2020; participant flow through the study is shown in Fig. 1. Patients were recruited from the stroke and rehabilitation units at Johns Hopkins, previous study participants, respondents to advertisement (flyers posted within the hospital), and stroke support groups to which the study was advertised. Healthy controls were recruited through advertisement and among previous study participants. All participants received monetary compensation for their time ($20/h). Participants provided informed consent in accordance with the Declaration of Helsinki, whereas procedures were approved by the Johns Hopkins Institutional Review Board.
Table 1.
Patient characteristics at T1
| Time Since Stroke | Lesion Side | Age, 5-Yr Range | Sex | MoCa | FM-UE | ARAT |
|---|---|---|---|---|---|---|
| Subacute patients (days) | ||||||
| 29 | R | 31-35 | M | 23 | 59 | 55.5 |
| 8 | L | 61-65 | F | 22 | 53 | 39 |
| 17 | R | 66-70 | F | 23 | 54.5 | 57 |
| 15 | R | 66-70 | F | 23 | 56 | 45 |
| 12 | R | 66-70 | M | 20 | 59 | 45 |
| 30 | R | 21-25 | F | 27 | 64 | 57 |
| 57 | L | 56-60 | M | 25 | 33.5 | 36 |
| 17 | L | 41-45 | F | 20 | 53 | 34.5 |
| 8 | R | 61-65 | M | 21 | 52.5 | 41 |
| 57 | L | 26-30 | M | 28 | 61.5 | 57 |
| 19 | L | 36-40 | F | 26 | 58 | 54 |
| 47 | R | 51-55 | M | 24 | 57 | 56 |
| 9 | L | 61-65 | M | 19 | 56 | 48.5 |
| 12 | R | 81-85 | F | 22 | 38 | 37.5 |
| 43 | R | 71-75 | F | 27 | 21 | 9 |
| 47 | R | 61-65 | F | 30 | 63.5 | 57 |
| 6 | L | 61-65 | M | 20 | 65 | 57 |
| 55 | R | 51-55 | M | 24 | 44 | 37 |
| 21 | L | 51-55 | F | 22 | 64.5 | 57 |
| 19 | R | 91-95 | M | 27 | 26 | n.d. |
| 70 | R | 66-70 | F | 28 | 59.5 | 56 |
| 47 | R | 71-75 | F | 23 | 45 | 54 |
| 14 | L | 61-65 | F | 25 | 61 | 55.5 |
| 17 | R | 46-50 | M | 30 | 62.5 | 57 |
| 12 | L | 71-75 | F | 25 | 57.5 | 56.5 |
| 58 | L | 46-50 | M | 29 | 15 | 11 |
| 51 | R | 56-60 | M | 27 | 11.5 | 3 |
| Chronic patients (months) | ||||||
| 27 | R | 26-30 | F | 30 | 58 | 44.5 |
| 54 | R | 41-45 | M | 30 | 13 | 3 |
| 42 | R | 66-70 | M | 27 | 60 | 57 |
| 27 | R | 66-70 | M | 25 | 13 | 6 |
| 27 | L | 56-60 | M | 25 | 58 | 54.5 |
| 39 | R | 56-60 | M | 25 | 47.5 | 44 |
| 24 | R | 46-50 | M | 29 | 12 | 3 |
| 76 | R | 61-65 | M | 28 | 10 | 7 |
| 11 | R | 51-55 | F | 23 | 62 | 57 |
| 88 | R | 61-65 | F | 25 | 17 | 3 |
| 24 | R | 56-60 | M | 20 | 29.5 | 5.5 |
| 8 | R | 76-80 | M | 20 | 50 | 43 |
| 59 | R | 46-50 | M | 22 | 24 | 42 |
| 20 | R | 56-60 | M | 20 | 17.5 | 3 |
| 72 | L | 36-40 | M | 27 | 60.5 | 55 |
| 58 | L | 51-55 | F | 21 | 9 | 2 |
| 35 | R | 51-55 | M | 26 | 64.5 | 55.5 |
| 7 | L | 66-70 | M | 28 | 64.5 | 57 |
| 7 | R | 46-50 | M | 29 | 27 | 14 |
| 8 | R | 51-55 | F | 28 | 36 | 14.5 |
| 17 | R | 56-60 | F | 26 | 13.5 | n.d. |
| 9 | R | 41-45 | F | 28 | 33 | 25.5 |
| 7 | L | 36-40 | M | 26 | 61 | 57 |
| 59 | R | 56-60 | F | 23 | 18.5 | 3 |
| 95 | L | 66-70 | F | 27 | 64 | 57 |
| 103 | L | 36-40 | F | 27 | 48.5 | 52.5 |
Showing time since stroke, lesion side, age (nonoverlapping 5-yr range), sex, Fugl-Meyer assessment for the upper extremity (FM-UE, max. 66), Montreal Cognitive Assessment (MoCA, max. 30), and Action Research Arm Test (ARAT, max. 57). F, female; L, left; M, male; n.d., not done; R, right.
Table 2.
Demographics and clinical characteristics of participants
| Subacute Stroke Patients | Chronic Stroke Patients | Controls | |
|---|---|---|---|
| n | 27 | 26 | 17 |
| Age | 58.8 ± 16.3 | 54.6 ± 11.5 | 57.6 ± 12.4 |
| Sex | 13M/14F | 16M/10F | 8M/9F |
| Affected side | 11R/16L | 7R/19L | n/a |
| Handedness | 24R/1L/2A | 24R/2L | 15R/1L/1A |
| FM-UE | 50.0 ± 15.6 | 37.4 ± 21.1 | 66 ± 0 |
| ARAT | 45.1 ± 15.9 | 30.6 ± 23.6 | 57 ± 0 |
| MoCA | 24.4 ± 3.2 | 25.6 ± 3.1 | 27.9 ± 1.6 |
| Time since stroke | 29.5 ± 19.8 days | 38.6 ± 29.6 mo | n/a |
±Indicates standard deviation across participants. A, ambidextrous; ARAT, Action Research Arm Test; FM-UE, Fugl-Meyer assessment for the upper extremity; F, female; L, left; M, male; MoCA, Montreal Cognitive Assessment; n/a, not applicable; R, right. Handedness was assessed using the Edinburgh inventory (56), asking patients to report based on their preferences before the stroke.
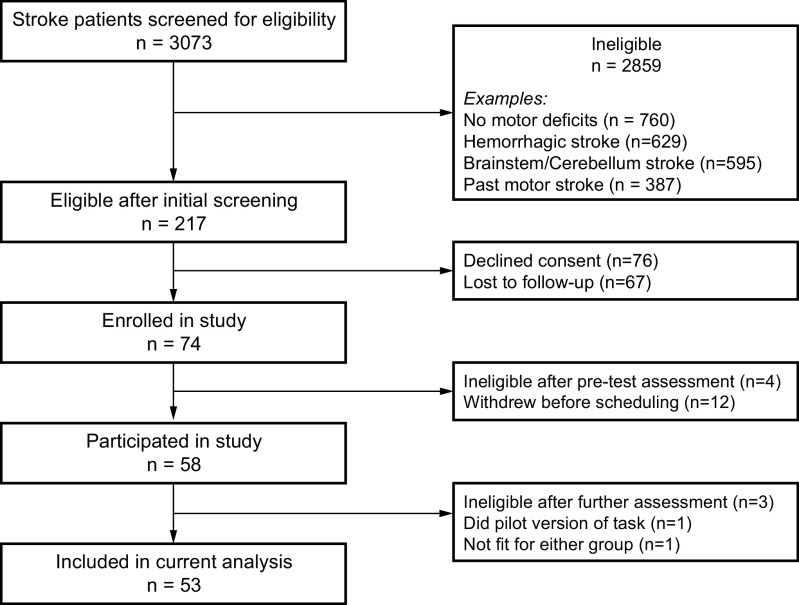
Figure 1.
Patient flow through the study (n indicates number of patients in each step). Note that the same patient might fulfill more than one ineligibility criteria.
Eligibility Criteria
Patients recruited had to be over 21 yr old, have had their first ischemic supratentorial stroke with motor deficits, exhibit residual movement with the affected arm, and be able to provide informed consent and understand the tasks involved. Exclusion criteria relevant to the tasks described in this paper included hemorrhagic transformation or associated intracranial hemorrhage; severe congestive heart failure; unstable angina; uncontrolled hypertension; dementia [assessed based on the Montreal Cognitive Assessment, MoCA (20)]; severe aphasia or ideomotor apraxia, neglect, or hemianopia; and orthopedic or pain issues.
Sessions
Participants underwent two sessions: the main session (T1) and a 1-mo follow-up (T2). For the subacute group, T1 took place 29.5 ± 19.8 days poststroke (average ± standard deviation) and T2 took place 39.5 ± 8.5 days later. For the chronic group, T1 took place 38.6 ± 29.6 mo poststroke and T2 took place 39.2 ± 7.8 days later. Thirteen patients in the main session (10 in the subacute and 3 in the chronic group) were subsequently lost to follow-up and thus did not complete the 1-mo follow-up session.
Impairment Assessment Using the Fugl-Meyer Scale for the Upper Extremity
Assessments were separately scored by at least two different raters: Keller—a trained physical therapist with extensive clinical experience administering the scale for over 10 years; Branscheidt—a clinical-research neurologist trained to administer the scale routinely in patients; and Hadjiosif, who was trained to administer and rate the scale for the purposes of the study. To obtain the final value, scores were averaged between reviewers (hence some having decimal values). Assessments were captured on video to allow for review in cases of substantial score differences. In cases of substantial score differences (3 points or more), assessment videos were again reviewed together by raters to determine a consensus score. For the analysis comparing changes in arm dexterity and FM-UE from T1 to T2 (Fig. 6), a participant was excluded from the chronic group due to missing FM-UE score. We used the entirety of the score (0–66) for our main analysis. At certain points, as mentioned in our results, we additionally performed comparisons based on subcomponents of FM-UE scores focusing on 1) items referring to movement of the proximal arm [items 3–17 and 31–33—i.e., everything apart from parts I/VI (reflexes), VII (wrist), and VIII (hand)], 2) items related to within-synergy movements (part II of FM-UE, “Flexor Synergy” items 3–8), or 3) items related to out-of-synergy movements (parts IV and V of the FM-UE: “Movement combining synergies” and “Movement out of synergy” containing a total of 6 items). The FM-UE scoring form we used is included in the Supplemental Materials.
Lesion Size
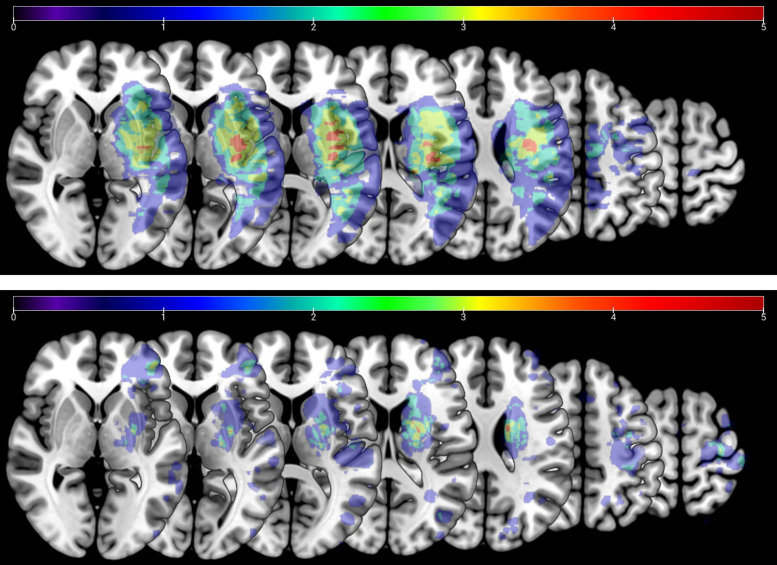
A large fraction of our patients (34 out of 53) had clinical MRI images available through their records (imaging was not performed specifically for this study). This enabled us to compare lesion size between subacute and chronic populations. Lesion boundaries were manually delineated on each axial slice of a patient’s T2-weighted FLAIR or DWI image by a trained neurologist using MRICron software (https://www.nitrc.org/projects/mricron; see Fig. 2 for averaged lesion distribution map). We normalized the obtained volume of interest (VOI) to the Montreal Neurological Institute (MNI) template using the clinical toolbox (https://www.nitrc.org/projects/clinicaltbx) with SPM12 (https://fil.ion.ucl.ac.uk/spm). The T2 image was co-registered to the T1 image, and these parameters were used to reslice the lesion into the native T1 space.
Figure 2.
Lesion distribution overlay for chronic (top, n = 10) and subacute (bottom, n = 19) patients with moderate/mild Fugl-Meyer scale for the upper extremity (FM-UE) impairment (FM-UE ≥ 26). Averaged lesion distribution mapped to Johns Hopkins University-Montreal Neurological Institute (MNI) space, with lesion flipped to one hemisphere. Color bar indicates patient count.
Reaching Task Details
Participants sat on a robotic chair (Kinarm exoskeleton, BKIN Technologies), which provided arm support while allowing for planar motion (Fig. 3). The chair was positioned against a screen that occluded vision of the arm but allowed projection of targets, and a cursor indicating hand position, at arm level. Participants made 10-cm reaching point-to-point movements to eight targets arranged at 45° intervals about a start position as shown in Fig. 3B. Targets and start position were 1 cm in radius, where the cursor was 0.5 cm in radius. The start position was defined relative to each participant’s shoulder midpoint and was typically (in 47 out of 53 patients, and all 17 control participants) 45 cm from it but could range from 45 to 50 cm to accommodate different sizes and positioning of participants. Upon positioning the cursor on the start position, a cyan target would appear in one of the eight different target positions. Participants were instructed to initiate a movement to the target soon after it appeared. A movement timer would begin as soon as the participant had moved outside the start position.
Figure 3.
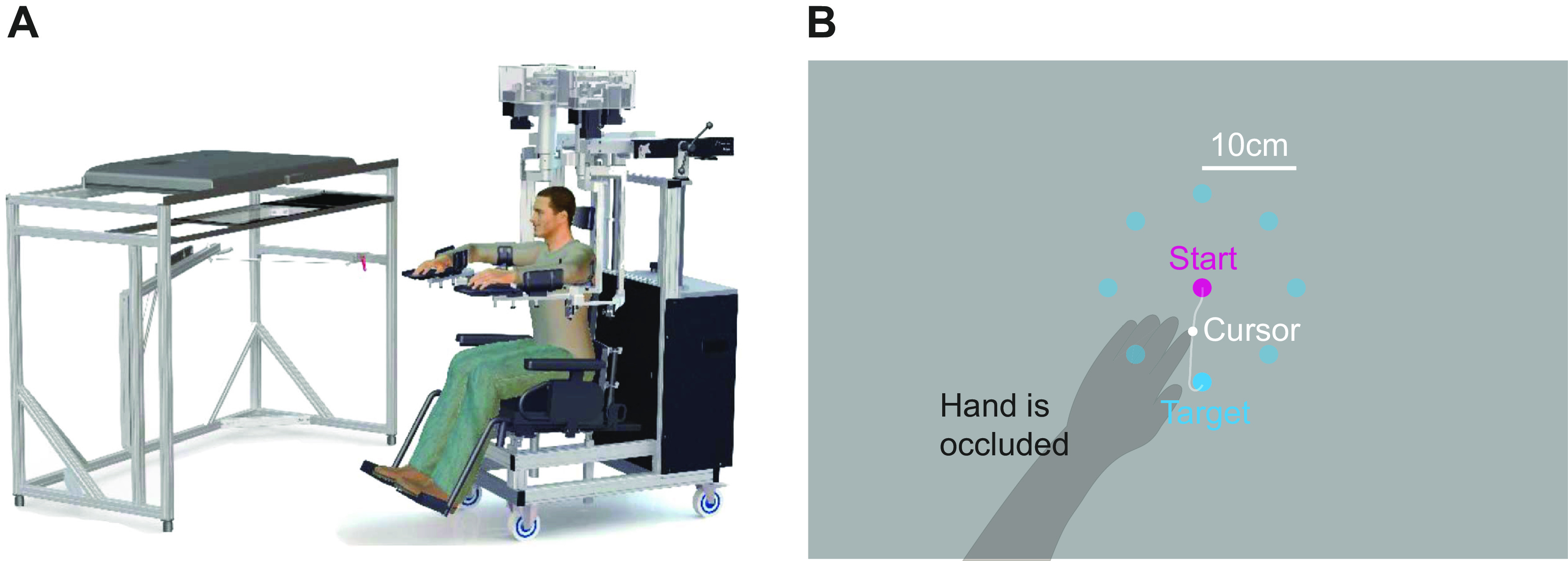
Task design and example trajectories. A: illustration of the experimental apparatus (Kinarm, BKIN Technologies, Kingston, ON, Canada, figure adapted from Ref. 55). B: the task: participants had to make point-to-point outward reaching movements to eight different targets (cyan) beginning from a starting position (pink). Vision of the hand was occluded; instead, during movement, a cursor was displayed on screen on the plane of the arm.
The movement would end either when the participant reached the target or an 800-ms timer ran out. Upon movement end, the cursor would freeze momentarily and the target would change color based on the participant’s speed. Specifically, it would change to orange if the movement terminated on the target but too quickly (movement time < 200 ms) or change to red if the movement failed to reach the target before the 800-ms timer ran out. If the cursor reached the target within the desired 200 ms to 800 ms from onset, and was held inside the target for an additional 500 ms, the target would turn green. After an additional 250 ms wait time, Kinarm would actively return the arm back to the starting position.
Patients completed two blocks of 88 movements each (11 to each of the eight different targets) with their paretic arm. Breaks were given between the blocks as necessary. Healthy controls completed four blocks (two with each arm), to obtain reference trajectories for both the left and the right arm for comparison with trajectories of left-paretic and right-paretic patients, respectively.
Dynamometry
We measured strength by dynamometry in 25 of our patients (11 in the subacute and 14 in the chronic stage; not all of them, as dynamometry was only added later in the study). Strength was assessed in 11 subacute and 6 chronic patients recruited as part of the main session, plus an additional eight of our earlier subacute patients (who were enrolled in a different study and were at the chronic stage at the time of the measurement). Participants sat on a chair, with their trunk straight and their forearm supported on a table in pronation with the elbow at 90° and the shoulder in an open packed position (∼60° of abduction and 30° of horizontal adduction) (21). Using a handheld button dynamometer (MicroFet 2, Hoggan Scientific), held by the experimenter against the participant, we measured the maximum effort of four muscle groups: elbow flexors, elbow extensors, shoulder (horizontal) adductors, and shoulder (horizontal) abductors. The average of two trials was taken for each condition. Both the paretic and nonparetic arms were tested, and strength was expressed as a % of the force exerted with the nonparetic arm.
Data Analysis
Analysis was performed using MATLAB (MathWorks, Natick, MA). Position data were smoothed using an 8th-order, 8-Hz low-pass Butterworth filter and differentiated to obtain velocity. For the purpose of analysis, we estimated movement onset using a method similar to that described previously in Ref. 12: we identified the time of peak speed (first zero-crossing of acceleration that was >8 cm/s; if no zero-crossing corresponded to a velocity >8 m/s, we used the moment of peak velocity across the entire velocity profile) and, then, going backward, identified movement onset as the time speed surpassed 2 cm/s. We identified movement end by going forward from the time of peak speed and finding the moment when speed remained <2 cm/s for more than 0.1 s. To avoid familiarization effects (see Supplemental Fig. S1; all supplemental material is available at https://doi.org/10.6084/m9.figshare.17345102), we focused our analysis on the second block (88 movements).
Data exclusion criteria.
On occasion, after setting up a participant on the robotic chair, reaching some of the targets was impossible because of mechanical constraints. We thus excluded these targets from our analysis. This case was rare: in the main session, targets were excluded in only two out of 53 patients. From the remaining trajectories, we excluded movements in which 1) movement direction at peak speed was ≥90° away from target direction, 2) the participant had not moved beyond 30% of the target distance (the criteria used in Ref. 12 and previously Ref. 22), or 3) the movement onset analysis described in the previous paragraph failed to provide an estimate of movement onset (as in when the peak velocity was lower than the 2 cm/s onset criterion; these movements were clearly abortive). For the second training block that we focus on in this paper, these criteria excluded 3.72% of patients’ movements with the paretic arm; for healthy controls, the corresponding value was 0.07% for the left and right arms together (these are the percentages calculated after a small number of targets were excluded altogether due to mechanical constraints). In the interest of completeness and transparency, we repeated our analyses without the exclusion criteria 1) and 2), showing qualitatively identical findings (see Supplemental Fig. S3).
Functional principal component analysis.
To assess the quality of kinematics, we used functional principal component analysis (fPCA), a method that applies principal component analysis to functional data (23). In summary, this data-driven analysis allows trajectories to be evaluated without prior assumptions about which trajectory features ought to be emphasized. Here, we used fPCA to estimate the Mahalanobis distance (MD) of patients’ trajectories from the reference population of healthy, age-range-matched controls. Here, MD can be understood as a generalization of the z-score; it is large when the movement is impaired (i.e., it is dissimilar to the healthy controls used as a reference) and small when the movement is similar to healthy controls. For each patient, we averaged these distances across trials and target directions into the average squared Mahalanobis distance (AMD2) separately for session T1 and for session T2. Additional details are provided in Supplemental Materials, adapted from a previous report (12).
Estimation of baseline value for AMD2 in controls.
The functional principal component analysis described earlier compares patients’ trajectories with the population of corresponding trajectories from healthy controls. Because of variability within the reference population itself, AMD2 scores would be nonzero for controls themselves. To estimate an AMD2 value for this baseline, we calculated AMD2 between the trajectories of each control participant compared and the trajectories of all other controls.
Statistical comparisons.
To compare AMD2 between subacute and chronic patients, or changes in AMD2 between T1 and T2, we used a bootstrap procedure (24) using 10,000 permutations within each subgroup. This provided estimates of the two-tailed P values we report. We opted to use bootstrap over more typical t tests, as the latter require assumptions of normality that may not hold for our data, especially for the distribution of AMD2 across participants. For simplicity, we used a similar bootstrap method for all other comparisons in the paper (other than the analysis used for Fig. 7) and also to calculate all SEMs reported. To examine the relationship between weakness and FM-UE (Fig. 7), we used Spearman’s rank correlation to avoid assumptions about linearity.
Figure 7.
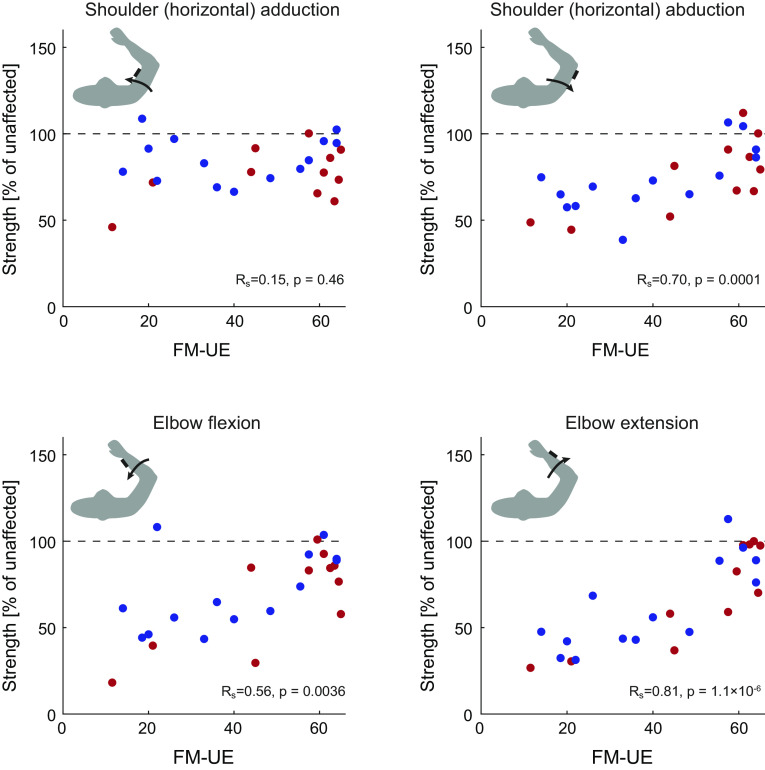
Relationship of weakness and Fugl-Meyer scale for the upper extremity (FM-UE). Comparing strength (expressed as a % of nonparetic strength) with each patients’ FM-UE scores revealed strong correlations between FM-UE and shoulder abduction strength, elbow flexion strength, and elbow extension strength, but not shoulder adduction strength (which was relatively high across the whole patient population). For each panel, each dot represents a patient [red: subacute (n = 11); blue: chronic, at the time of examination (n = 14)].
RESULTS
Patients with Subacute Stroke Had Worse Reaching Dexterity Compared to Patients with Chronic Stroke despite Similar Fugl-Meyer Scores
We first assessed the quality of reaching movements using our two-dimensional (2-D) reaching task. Examples of participants’ trajectories with the paretic arm are shown in Fig. 4A. We made two primary observations. First, subacute patients had markedly worse trajectories compared with chronic patients for similar FM-UE scores. Second, there was convergence onto the shape of trajectories of the control population as patients’ FM-UE scores improved.
Figure 4.
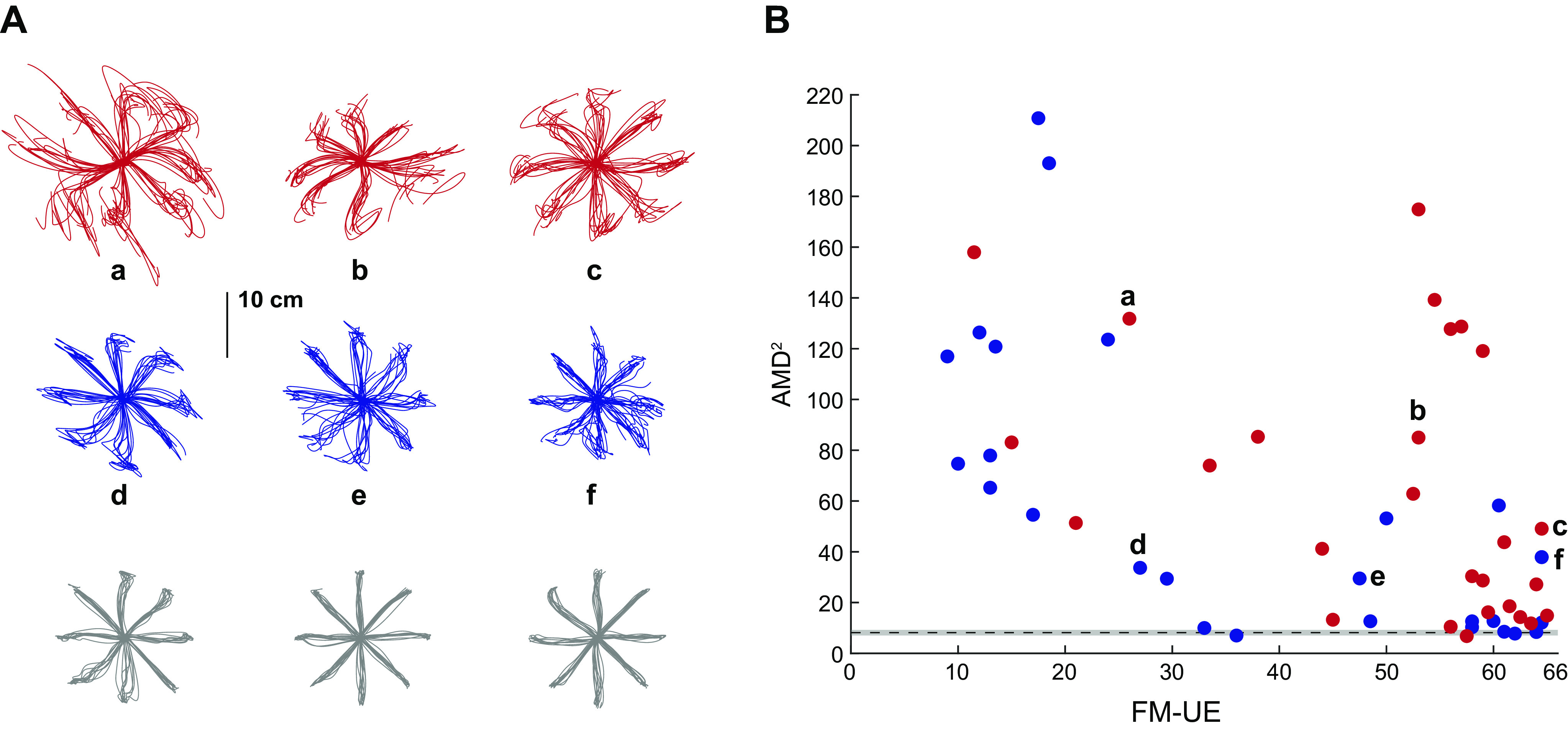
Subacute patients had worse kinematics compared with chronic patients for similar Fugl-Meyer scores. A: exemplar movement trajectories, using the paretic arm, for three subacute patients (red), three chronic patients (blue), and three controls (gray). B: scatter plot of kinematic abnormality (average squared Mahalanobis distance, AMD2) vs. Fugl-Meyer scale for the upper extremity (FM-UE) for the subacute (red, n = 27) and chronic (blue, n = 26) groups. Note the higher AMD2 for subacute patients, especially the ones with moderately and mildly impaired FM-UE (≥26). The lowercase letters (a–f) point to the corresponding trajectories on A. The black dashed line indicates baseline calculated based on control data (n = 17); the gray band indicates the corresponding 95% confidence interval estimated using bootstrap.
To formally assess reaching trajectories in patients with stroke and generate a scalar comparison metric, we used the AMD2 metric obtained through functional principal component analysis (fPCA, see materials and methods). Consistent with the trajectory shapes shown in Fig. 4A, AMD2 scores indicated impaired trajectory kinematics for patients with stroke compared with controls (average ± SEM AMD2 for all patients: 61.4 ± 7.5; for all controls: 8.13 ± 0.56). Interestingly, however, subacute patients tended to show markedly worse kinematics than chronic patients despite similar or higher FM-UE scores, as illustrated in Fig. 4B. Because very few participants in the subacute stage had low FM-UE scores, resulting in a higher average FM-UE for the subacute group compared with the chronic group (on average, FM-UE of 50.0 ± 3.0 for the subacute vs. 37.4 ± 4.0 for the chronic group, see Fig. 4B), we performed additional analysis on patients with moderate and mild impairment [FM-UE ≥ 26, the cut-off is based on previous work (19, 25)]. For these patients (24 subacute and 16 chronic), despite similar FM-UE (54.3 ± 2.0 for the subacute vs. 51.5 ± 3.2, means ± SE for the chronic patients, respectively, P = 0.47), trajectory abnormalities were substantially greater in the subacute group (AMD2 of 60.5 ± 10.2 vs. 21.5 ± 4.1, P < 10−4, Fig. 5). These two groups were significantly different (P = 0.0002) even when the subacute participant with the highest AMD2 (174.9, Fig. 5B) was excluded as a potential outlier. We also considered whether this AMD2 difference between subacute and chronic patients could be due to FM-UE capturing different types of abnormality in each group. In other words, despite similar overall FM-UE scores, could there be systematic differences in subcomponents of the FM-UE between subacute and chronic patients? We thus isolated and compared 1) the part of the FM-UE focusing on movement of the proximal arm (see materials and methods) and 2) the part of the FM-UE focusing on out-of-synergy movement between these two subgroups (subacute vs. chronic mild/moderate patients). We found no significant differences (proximal part: 30.8 ± 1.0 vs. 29.7 ± 1.6 for subacute vs. chronic, P = 0.56; out-of-synergy part: 10.2 ± 0.5 vs. 9.7 ± 0.8 for subacute vs. chronic, P = 0.59), ruling out that AMD2 differences could be explained by differences specific to the synergy subcategory of the FM-UE that are not apparent when taking the whole score into account.
Figure 5.
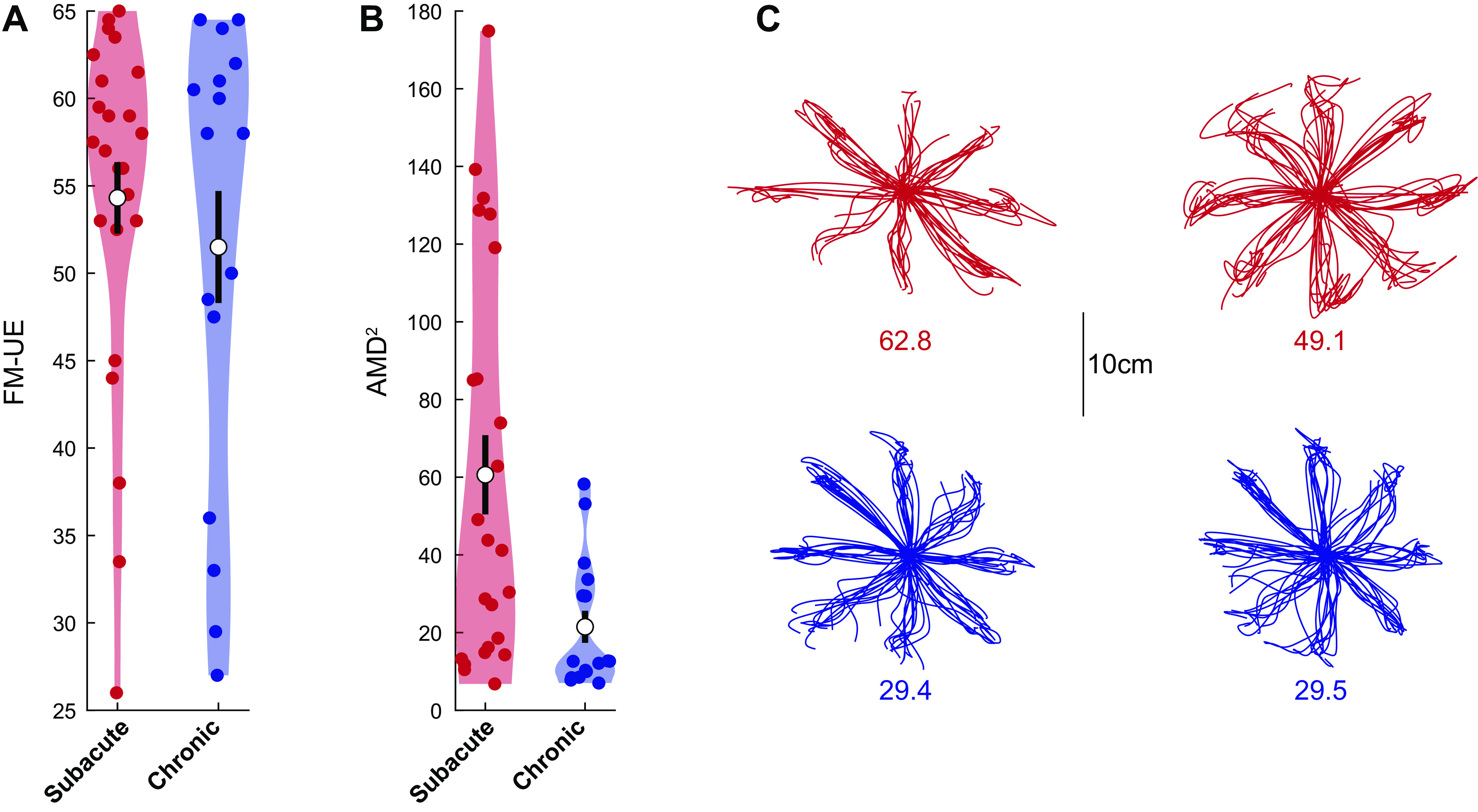
Subacute patients had worse kinematics compared with chronic patients despite similar Fugl-Meyer scores. Violin plots of Fugl-Meyer scale for the upper extremity (FM-UE) (A) and average squared Mahalanobis distance (AMD2) (B) for chronic (n = 16) and subacute (n = 24) patients with moderate/mild (FM-UE ≥ 26) impairment. Note how, for this FM range, where both subacute and chronic patients were adequately represented, we found much worse motor control (higher AMD2) for subacute patients compared with chronic patients, despite similar FM-UE. White circles indicate mean values and thick black lines indicate means ± SE. C: reaches for two patients closest to the mean AMD2 for each subgroup. Numbers indicate specific AMD2 scores.
The difference in arm dexterity despite similar FM-UE that we observed suggests that impaired arm dexterity (a negative sign) is dissociable from abnormal synergies (a positive sign on which FM-UE is based)—one is not causing the other. This failure on part of the FM-UE to capture differences in the quality of motor control during the subacute recovery stage further suggests that additional assessments may be needed to capture the full spectrum of the poststroke motor control phenotype, and/or recovery of motor control within the subacute stage may lag behind corresponding improvements in FM-UE.
Finally, we examined whether our finding could be explained by larger lesion volume in the subacute group. For the part of mild/moderately impaired patients who also had imaging available, we still observed similar FM-UE scores (53.4 ± 2.4 for the 19 subacute vs. 46.3 ± 4.1 for the 10 chronic patients, P = 0.14) and significantly larger AMD2 in the subacute group (65.1 ± 12.0 vs. 16.4 ± 3.1, P < 10−4). Yet, lesion size was larger in the chronic subgroup [61,029 ± 25,540 voxels for chronic (n = 10) vs. 9,269 ± 3,206 voxels for subacute (n = 19), P = 0.0138], suggesting that reduced dexterity in the subacute group is very unlikely to be explained by lesion volume.
As Patients Progressed through the Subacute Stage, the Relationship between Abnormal Synergies and Reaching Dexterity Increasingly Resembled That of the Chronic Group
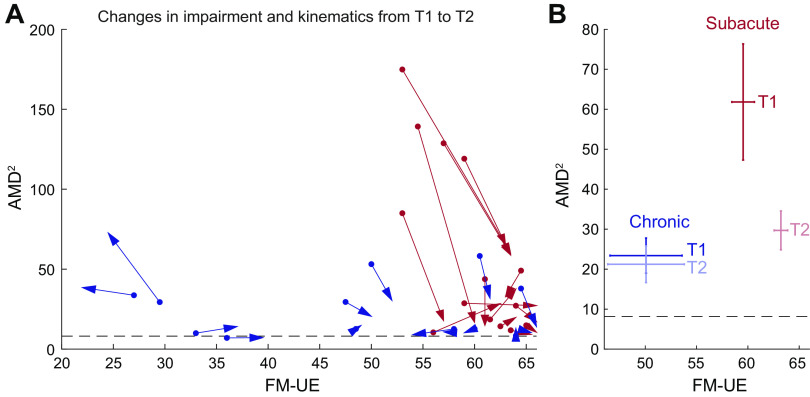
If the differences in kinematics between subacute and chronic patients with similar FM-UE were indeed due to time poststroke, we would expect that, given time, these differences would become smaller as recovery of the subacute group progressed. Conversely, if there was no improvement in kinematics for the subacute group, this could suggest that time poststroke was not the driving factor and that the subacute group had impaired kinematics for other reasons. To examine this, we tested for changes in both FM-UE and AMD2 in the subset of patients who completed the 1-mo follow-up (T2) session and had been classified as moderate/high FM-UE (≥26) during the main (T1) session—14 patients in each group. We saw that, indeed, the relationship between AMD2 and FM-UE in the subacute group in the 1-mo follow-up (T2 session) tended to approach the relationship observed for chronic patients, with the most kinematically impaired subacute patients drastically reducing their AMD2 (illustrated by the long downward-facing red arrows in Fig. 6A). On average, subacute patients improved both their FM-UE (59.5 ± 1.1 vs. 63.2 ± 0.7, P < 10−4 estimated using bootstrap) and their kinematics (AMD2 of 61.8 ± 14.5 vs. 29.7 ± 4.9, P = 0.0016). In contrast, chronic patients improved neither FM-UE nor kinematics (50.1 ± 3.5 vs. 50.1 ± 3.7, P = 0.96 for FM-UE and 23.4 ± 4.4 vs. 21.2 ± 4.6, P = 0.59 for AMD2), as illustrated in Fig. 6B. We note that the lack of improvement in kinematics for the chronic group suggests no effect of savings or additional practice in the point-to-point reaching task (aside from a familiarization effect, illustrated in Supplemental Fig. S1), meaning that the changes in AMD2 we see in the subacute group represent improvements in reaching dexterity rather than motor learning.
Figure 6.
Changes in kinematics and Fugl-Meyer scale for the upper extremity (FM-UE) as recovery progressed. A: individual changes in kinematic abnormalities (average squared Mahalanobis distance, AMD2) and FM-UE between the main session (T1, dots) and the 1-mo follow-up (T2, tip of arrowpoints). B: subject averages for these two groups. Error bars indicate SEM. In both A and B, patients were only included if 1) they completed both T1 and T2 sessions and 2) were classified in the moderate/mild group during T1. Red, subacute patients; blue, chronic patients. Included are n = 14 patients from each group.
The Contribution of Strength to the Fugl-Meyer Score Did Not Explain Its Dissociation with Reaching Dexterity
Our findings suggest a dissociation between abnormal synergies, assessed through the FM-UE scale, and arm dexterity, assessed through kinematics. However, although FM-UE is a synergy-based measure, it may also reflect weakness—for example, we have previously found a strong correspondence between FM-UE improvement and recovery of strength (12). Could the FM-UE versus dexterity dissociation in our data instead reflect a strength/dexterity dissociation, as has been shown in earlier work (26)?
To answer this question, we measured weakness at the joints involved in the reaching task, to compare weakness with each patient’s FM-UE score. We thus used dynamometry to measure patients’ horizontal shoulder adduction/abduction and elbow flexion/extension strength. As dynamometry was only later added to the study assessments, it was only performed in 25 patients (11 subacute and 14 chronic, see materials and methods). With the exception of horizontal shoulder adduction, strength correlated with FM-UE (see Fig. 7). In particular, not only elbow extension strongly correlated with FM-UE (Spearman’s rank correlation Rs = 0.81, P = 1.1 × 10−6) but elbow flexion also did (Rs = 0.56, P = 0.0036). The latter relationship was present even when we examined only the FM-UE items strictly related to out-of-synergy movements (out-of-synergy FM-UE vs. elbow flexor strength: Rs = 0.58, P = 0.0022). As elbow flexor strength correlated with the part of FM-UE that evaluates movements out of flexor synergy, we find it unlikely that strength increases at the elbow are the cause of the ability to move out of synergy; rather, the correlations suggest a recovery process that is common for strength and synergies. We also found that elbow extensor strength correlated—even better than elbow flexor strength did—to the part of FM-UE that evaluates movements within flexor synergy (Rs = 0.74, P = 0.00003). Similarly, we find it unlikely that increased extension strength is the cause of the ability to move within flexor synergy, instead suggesting a common recovery process.
DISCUSSION
Significance of Our Findings for Assessing Poststroke Motor Impairment
Here, we sought to dissect the hemiparesis phenotype into its constituent components. Given the prevalence and impact of hemiparesis, it is important to assess specific poststroke motor impairments with quantitative metrics to enable clinicians to reliably characterize the initial deficit and to predict and track recovery. This knowledge may help optimize rehabilitation (27–29) and also enable researchers to compare the effectiveness of different experimental treatments and interventions to enhance recovery. The FM is widely used in tracking poststroke motor recovery, in fact it is the de facto impairment measure from the International Classification of Functioning, Disability, and Health used for studies and trials (12, 27, 30–40). Here, however, we have shown that FM-UE might miss one of the components of hemiparesis—reaching dexterity loss—even though it might well capture weakness and abnormal synergies. Our findings, showing a poor correspondence between these two types of impairment, suggest the need for more careful matching between the rehabilitation intervention being tested in clinical studies and the chosen primary outcome measure: hemiparesis—or upper motor neuron syndrome—is too vague a term, as it lumps weakness, synergies, and dexterity loss.
Why would FM-UE not be that suitable for assessing reaching dexterity deficits? The primary reason is that is not what it was designed for—instead, it was created to assess synergies outside the context of reaching or any other kind of functional movement (11). Recent work has shown that reaching dexterity in patients with stroke is improved with weight support; conversely, without weight support, dexterity might be masked by weakness and abnormal synergies. Beer et al. (16) found that external arm support allowed for significantly greater peak torques when moving to distal targets requiring elbow extension and/or shoulder flexion, i.e., arm support facilitated movements that required breaking out of flexor synergy. At the same time, there was little, if any, effect of external arm support for movements to proximal targets that involved elbow flexion and shoulder extension. A subsequent study by the same group also found that, while providing arm support allows for greater range and speed of elbow extension (15), this improvement was independent of reduced shoulder strength or elbow flexor/extensor strength imbalance. This suggested that abnormal synergies—and not merely weakness per se—were the sign alleviated by arm support. This finding mirrored earlier results for 3-D movements (14), which showed a critical effect of synergy intrusion in the absence of weight support. Thus, tasks that require the patient to make multijoint movements in 3-D without support, like most of the test components of the FM-UE, will primarily reflect weakness and synergies, masking residual dexterity.
Potential Mechanisms behind Differences in Motor Control between Subacute and Chronic Patients
Here, our data showed a clear dissociation between the FM-UE score and the quality of planar reaches. It still needs to be explained, however, why the dissociation took the specific form it did: it is not merely that FM-UE scores are poor predictors of the quality of reaching, but there was a clear bias whereby planar reaches were substantially worse in the subacute as compared with the chronic group in patients with similar FM-UE scores. A potential explanation for this discrepancy may be that the residual corticospinal tract needs time and practice to reach its maximal level of potential performance. Thus, improvements in negative signs might lag improvements in positive ones. This explanation, however, appears to contradict our previous work showing that recovery of planar kinematics occurs over the first 5 wk poststroke and then plateaus (12). We considered the potential explanation that at least some of the patients in the subacute group were still within this 5-wk window and therefore had not yet reached their full recovery. We reasoned that this could still be the case despite the fact that, overall, this group would be less impaired than the chronic group with respect to the FM-UE because they have similar scores and yet could only be expected to improve further. Indeed, the subacute patients with the highest AMD2 scores on T1 tended to improve drastically on T2, as illustrated in Fig. 6A.
The Fugl-Meyer Assessment, Abnormal Synergies, and Weakness
In this study, we relied on FM-UE as a measure of abnormalities in muscle synergy. As we mentioned in the introduction, FM-UE was designed to capture the stages of poststroke recovery described by Twitchell (9) and Brunnstrom (10), a prominent feature of which is the intrusion of abnormal synergies. In turn, this made the scale a strong indicator of synergy abnormality. In line with this idea, the degree of synergy abnormality assessed with EMG was found to strongly (negatively) correlate with FM-UE scores across patients (41, 42). It should be noted that there is currently no gold standard for synergy assessment; even with EMG, typical muscle synergy analysis methods miss kinematic dimensions relevant to the task (43). Another method that has been used is to instruct the patient to produce movements isolated about one joint and compare the excursion about that joint with the resulting unwanted excursions about the noninstructed joints (14). This is, however, just a more kinematically granular quantification of synergy based on exactly the same principle of joint isolation as the FM-UE. Thus, future studies could obtain joint kinematics in this way, but at the current time, there is no better validated measure than the FM-UE. This is not the case for reaching dexterity, for which there is no existing clinically validated measure that controls for weakness, synergies, and compensation, and, arguably, therefore almost by definition needs a novel kinematic approach.
We also found that FM-UE scores also correlate with weakness, in line with our previous work suggesting that FM-UE recovery mirrors strength recovery (12). This relationship was present for not only elbow extension strength but also elbow flexion strength (Fig. 7); it would be unlikely to have a causal relationship between increased flexion strength and ability to move out of flexor synergy. Our findings thus indicate that there is indeed a dissociation between synergy and arm dexterity rather than only a dissociation between strength and arm dexterity. This observation is consistent with previous findings in which increased extensor reach was observed when arm support was given: externally provided arm support is orthogonal to extensor (or flexor) strength (17). Instead, ours and previous findings suggest that the FM-UE/weakness correlation might indicate a shared substrate between abnormal synergies and weakness.
The shared substrate hypothesis would fit with observations that abnormal synergies are mitigated when weakness is itself mitigated through arm support (15–17). A potential explanation is that damage to the (contralateral) corticospinal tract (CST) after stroke may lead to increased reliance on the (ipsilateral) reticulospinal tract (RST), providing some strength at the expense of abnormal synergies (44). In support of this theory, it was shown that corticospinal lesions in macaques led to increased responsivity in reticulospinal pathways that innervated forearm flexor muscles (45); moreover, it was recently shown that strength training in monkeys involves adaptations in the reticulospinal but not the corticospinal tract (46). This previous work, together with our findings, thus suggests an anatomical and physiological dissociation (corticospinal vs. reticulospinal) that may map onto the behavioral dissociation (positive vs. negative signs).
This theory, however, raises an apparent paradox: if upregulation of the RST increases both strength and abnormal synergies, then how do patients proceed to recover from synergies without concomitant loss of strength? There are a few possible nonmutually exclusive answers to this question that relate to recovery of CST function. First, as the CST becomes able to provide some strength, reliance on the RST may be reduced. Second, the RST might be unable to provide strength for some muscles, which instead may rely on CST recovery: for example, CST integrity may be necessary for strength in the first dorsal interosseous, a distal muscle, but not the biceps (47). Studies in monkeys using spike-triggered averaging have found that ipsilateral RST projections led to facilitation in the biceps but suppression in the triceps (48, 49); thus, the (contralateral) CST—which can facilitate either flexors or extensors (50)—may be necessary for control of extensor muscles, which is needed to break out of the flexor synergy. Third, given that both CST and RST converge upon spinal interneurons (51, 52), the CST might directly regulate RST (53). Thus, CST recovery may restore its regulatory effect upon the RST, reducing abnormal synergies but maintaining strength.
Limitations
Our comparison of kinematics between subacute and chronic groups focused on patients with moderate and mild impairment based on their FM-UE score. This was due to the very low number of subacute participants with low FM-UE scores, which prevented reliable comparisons with the corresponding part of the chronic group: we were able to recruit only three subacute patients with a FM-UE of <26 compared with 10 chronic patients in the same subgroup. We interpret this difference as the result of the difficulty of recruiting patients in the subacute stage after stroke. Both subacute and chronic patients were recruited irrespective of their FM-UE score (within the ~10–64 range). However, a subacute patient with high impairment will be likely to spend more time in the hospital and in rehabilitation, thus less likely to have time on research participation before the subacute time window expires. Moreover, increased impairment itself might make them less likely to be interested in joining in the first place, given that they may need more time to adjust. Hence, our observation in this study and others is that subacute patients who are able and eager to participate will tend to be less impaired in the first place.
However, we would not expect that the difference in kinematics between the subacute and chronic groups seen for moderate- and mild-impairment patients would also manifest in highly impaired patients. First, patients with high impairment would show difficulty with kinematics regardless of their recovery stage: lower and lower FM-UE scores would amount to impairment closer and closer to complete lack of movement. For example, a plegic patient would have a FM-UE score very close to zero, and being unable to move at all, they would have a complete deficit in their kinematics in our task, regardless of time after stroke. Second, the very fact that the FM-UE score failed to capture a good part of motor control deficits, specifically during the subacute stage, paired with the assumption that the subacute patients who participated may have had less overall impairment, suggests that subacute patients with low FM-UE may tend to fare better in components of impairment not well-captured by the FM-UE (one of which is motor control). Finally, there seems to be a difference how severe versus moderate/mild patients recover: in contrast to moderate/mild patients, a fraction of severe patients may not recover much at all and, thus, show less improvement as they advance to the chronic stage (2, 19, 54). Although this observation was derived by comparing more general impairment scales (and FM-UE itself), it might also hold for movement kinematics as well.
We also note two more limitations. First, we did not formally examine patients’ sensory deficits, which could be a component of further study. Second, our comparison of lesion size versus kinematic impairment in subacute versus chronic patients, other than the fact that not all patients had clinical imaging available, has the limitation that it does not provide an estimate of the amount of CST damage in each patient. That said, it would be very unlikely that the groups, each with clinically diagnosed hemiparesis, would differ systematically in the degree of CST involvement at the time of their strokes such that the larger stroke had less CST involvement. Third, in our dynamometry versus Fugl-Meyer analysis (Fig. 7), the number of patients in each subgroup is too small to allow us to evaluate this relationship separately for subacute versus chronic patients, which raises the possibility that the correlations we find may be in part driven by differences between the subacute and chronic groups. We find this unlikely, however, given previous work showing that FM-UE changes as patients progress from the acute to the chronic stage, paralleling changes in weakness (12). Finally, here we examined kinematics in 2-D with full arm support. Fully evaluating poststroke motor control, however, would need to examine kinematics in all three dimensions. 3-D movement control may contain features not prominent in horizontal planar movement, such as the engagement of more muscles, a wider range of joint configurations, and dealing with the effects of weight bearing. For the latter part, especially, it may be worthwhile to systematically examine the relationship between FM-UE and kinematics under intermediate amounts of arm support rather than either full or none to properly map the interplay between synergies, strength, and kinematics.
Conclusion
Here, we show that there is a dissociation between loss of reaching dexterity and presence of abnormal muscle synergies in the contralesional arm after stroke; two prominent and characteristic signs of hemiparesis. To dissect these two signs, we designed a reaching task in which we isolated arm dexterity from synergy by providing weight support, and we separately assessed abnormal synergies using the Fugl-Meyer score for the upper extremity (FM-UE). We found a large difference in dexterity deficits between subacute and chronic patients with similar levels of abnormal synergy: patients in the chronic stage had more normal planar reaching trajectories even with worse FM-UE scores. These dissociations suggest that abnormal synergies and dexterity deficits reflect distinct components of hemiparesis, perhaps attributable to damage to separable systems. Finally, we found that FM-UE scores correlated with arm weakness. This suggests that both synergies and weakness are independent of arm dexterity loss. In short, our findings suggest that recovery from hemiparesis does not proceed uniformly across its components. Stroke rehabilitation should be tailored for each patient based on their specific component deficits; a form of behavioral precision medicine.
SUPPLEMENTAL DATA
Supplemental Figs. S1–S3 (including Supplemental Fig. S2 which contains a summary of nonparetic reaching data collected following paretic testing) and extended Methods: https://doi.org/10.6084/m9.figshare.17345102.
GRANTS
This work has been supported by NIH Grant 5R01HD053793 and the Sheikh Khalifa Stroke Institute.
DISCLOSURES
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare in the past 36 mo, J.W.K. has received payments or honoraria for talks about stroke recovery, participated on a Data Safety Monitoring Board/Advisory Board for an aphasia trial, served at the Scientific Advisory Board of Burke Rehabilitation Institute, and had equity in Mindmaze, a company that sells stroke rehabilitation technology; A.J.B. served as Councilor for the Society for Neuroscience. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
A.M.H., M.B., A.J.B., P.A.C., and J.W.K. conceived and designed research; A.M.H., M.B., M.A.A., K.D.R., and J.K. performed experiments; A.M.H. analyzed data; A.M.H., M.B., P.A.C., and J.W.K. interpreted results of experiments; A.M.H. prepared figures; A.M.H. drafted manuscript; A.M.H., M.B., M.A.A., K.D.R., J.K., A.J.B., P.A.C., and J.W.K. edited and revised manuscript; A.M.H., M.B., M.A.A., K.D.R., J.K., A.J.B., P.A.C., and J.W.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jeff Goldsmith for help in implementing functional principal components analysis; Jing Xu, Adrian Haith, Juan Camilo Cortes, and Martin Lindquist for helpful discussions; Amanda Therrien for help with experiment software; as well as Kahori Kita and Kendra Cherry-Allen for help with assessments.
REFERENCES
- 1.GBD 2016 Stroke Collaborators . Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18: 439–458, 2019. doi: 10.1016/S1474-4422(19)30034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakayama H, Jørgensen HS, Raaschou HO, Olsen TS. Recovery of upper extremity function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil 75: 394–398, 1994. doi: 10.1016/0003-9993(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 3.Rathore SS, Hinn AR, Cooper LS, Tyroler HA, Rosamond WD. Characterization of incident stroke signs and symptoms: findings from the atherosclerosis risk in communities study. Stroke 33: 2718–2721, 2002. doi: 10.1161/01.str.0000035286.87503.31. [DOI] [PubMed] [Google Scholar]
- 4.Parker V, Wade D, Hewer RL. Loss of arm function after stroke: measurement, frequency, and recovery. Int Rehabil Med 8: 69–73, 1986. doi: 10.3109/03790798609166178. [DOI] [PubMed] [Google Scholar]
- 5.Niemi ML, Laaksonen R, Kotila M, Waltimo O. Quality of life 4 years after stroke. Stroke 19: 1101–1107, 1988. doi: 10.1161/01.STR.19.9.1101. [DOI] [PubMed] [Google Scholar]
- 6.Viitanen M, Fugl-Meyer K, Bernspång B, Fugl-Meyer AR. Life satisfaction in long-term survivors after stroke. Scand J Rehabil Med 20: 17–24, 1988. [PubMed] [Google Scholar]
- 7.Hughlings Jackson J. Evolution and dissolution of the nervous system. Selected Writings John Hughlings-Jackson 2: 45–75, 1884. [Google Scholar]
- 8.Pearce JMS. Positive and negative cerebral symptoms: the roles of Russell Reynolds and Hughlings Jackson. J Neurol Neurosurg Psychiatry 75: 1148, 2004. doi: 10.1136/jnnp.2004.038422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Twitchell TE. The restoration of motor function following hemiplegia in man. Brain 74: 443–480, 1951. doi: 10.1093/brain/74.4.443. [DOI] [PubMed] [Google Scholar]
- 10.Brunnstrom S. Motor testing procedures in hemiplegia: based on sequential recovery stages. Phys Ther 46: 357–375, 1966. doi: 10.1093/ptj/46.4.357. [DOI] [PubMed] [Google Scholar]
- 11.Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med 7: 13–31, 1975. [PubMed] [Google Scholar]
- 12.Cortes JC, Goldsmith J, Harran MD, Xu J, Kim N, Schambra HM, Luft AR, Celnik P, Krakauer JW, Kitago T. A short and distinct time window for recovery of arm motor control early after stroke revealed with a global measure of trajectory kinematics. Neurorehabil Neural Repair 31: 552–560, 2017. doi: 10.1177/1545968317697034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu J, Ejaz N, Hertler B, Branscheidt M, Widmer M, Faria AV, Harran MD, Cortes JC, Kim N, Celnik PA. Separable systems for recovery of finger strength and control after stroke. J Neurophysiol 118: 1151–1163, 2017. doi: 10.1152/jn.00123.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zackowski KM, Dromerick A, Sahrmann S, Thach W, Bastian AJ. How do strength, sensation, spasticity and joint individuation relate to the reaching deficits of people with chronic hemiparesis? Brain 127: 1035–1046, 2004. doi: 10.1093/brain/awh116. [DOI] [PubMed] [Google Scholar]
- 15.Beer RF, Ellis MD, Holubar BG, Dewald JP. Impact of gravity loading on post‐stroke reaching and its relationship to weakness. Muscle Nerve 36: 242–250, 2007. doi: 10.1002/mus.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beer RF, Dewald JP, Dawson ML, Rymer WZ. Target-dependent differences between free and constrained arm movements in chronic hemiparesis. Exp Brain Res 156: 458–470, 2004. doi: 10.1007/s00221-003-1807-8. [DOI] [PubMed] [Google Scholar]
- 17.Sukal TM, Ellis MD, Dewald JP. Shoulder abduction-induced reductions in reaching work area following hemiparetic stroke: neuroscientific implications. Exp Brain Res 183: 215–223, 2007. doi: 10.1007/s00221-007-1029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernhardt J, Hayward KS, Kwakkel G, Ward NS, Wolf SL, Borschmann K, Krakauer JW, Boyd LA, Carmichael ST, Corbett D, Cramer SC. Agreed definitions and a shared vision for new standards in stroke recovery research: the stroke recovery and rehabilitation roundtable taskforce. Int J Stroke 12: 444–450, 2017. doi: 10.1177/1747493017711816. [DOI] [PubMed] [Google Scholar]
- 19.Krakauer JW, Carmichael ST. Broken Movement: The Neurobiology of Motor Recovery After Stroke. Cambridge, MA: MIT Press, 2017. [Google Scholar]
- 20.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53: 695–699, 2005. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 21.Bohannon RW. Shoulder position influences elbow extension force in healthy individuals. J Orthop Sports Phys Ther 12: 111–114, 1990. doi: 10.2519/jospt.1990.12.3.111. [DOI] [PubMed] [Google Scholar]
- 22.Kitago T, Goldsmith J, Harran M, Kane L, Berard J, Huang S, Ryan SL, Mazzoni P, Krakauer JW, Huang VS. Robotic therapy for chronic stroke: general recovery of impairment or improved task-specific skill? J Neurophysiol 114: 1885–1894, 2015. doi: 10.1152/jn.00336.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldsmith J, Kitago T. Assessing systematic effects of stroke on motorcontrol by using hierarchical function-on-scalar regression. J R Stat Soc Ser C Appl Stat 65: 215, 2016. doi: 10.1111/rssc.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. New York, NY: Chapman and Hall/CRC, 1994. [Google Scholar]
- 25.Duncan PW, Lai SM, Keighley J. Defining post-stroke recovery: implications for design and interpretation of drug trials. Neuropharmacology 39: 835–841, 2000. doi: 10.1016/s0028-3908(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 26.Ada L, O'Dwyer N, Green J, Yeo W, Neilson P. The nature of the loss of strength and dexterity in the upper limb following stroke. Hum Movement Sci 15: 671–687, 1996. doi: 10.1016/0167-9457(96)00015-2. [DOI] [Google Scholar]
- 27.Duncan PW, Goldstein LB, Matchar D, Divine GW, Feussner J. Measurement of motor recovery after stroke. Outcome assessment and sample size requirements. Stroke 23: 1084–1089, 1992. doi: 10.1161/01.str.23.8.1084. [DOI] [PubMed] [Google Scholar]
- 28.Stinear C. Prediction of recovery of motor function after stroke. Lancet Neurol 9: 1228–1232, 2010. doi: 10.1016/S1474-4422(10)70247-7. [DOI] [PubMed] [Google Scholar]
- 29.Stinear CM, Byblow WD, Ackerley SJ, Barber PA, Smith M-C. Predicting recovery potential for individual stroke patients increases rehabilitation efficiency. Stroke 48: 1011–1019, 2017. doi: 10.1161/STROKEAHA.116.015790. [DOI] [PubMed] [Google Scholar]
- 30.Rabadi MH, Rabadi FM. Comparison of the action research arm test and the fugl-meyer assessment as measures of upper-extremity motor weakness after stroke. Arch Phys Med Rehabil 87: 962–966, 2006. doi: 10.1016/j.apmr.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 31.Chollet F, Tardy J, Albucher J-F, Thalamas C, Berard E, Lamy C, Bejot Y, Deltour S, Jaillard A, Niclot P, Guillon B, Moulin T, Marque P, Pariente J, Arnaud C, Loubinoux I. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol 10: 123–130, 2011. doi: 10.1016/S1474-4422(10)70314-8. [DOI] [PubMed] [Google Scholar]
- 32.Crisostomo EA, Duncan PW, Propst M, Dawson DV, Davis JN. Evidence that amphetamine with physical therapy promotes recovery of motor function in stroke patients. Ann Neurol 23: 94–97, 1988. doi: 10.1002/ana.410230117. [DOI] [PubMed] [Google Scholar]
- 33.Francisco G, Chae J, Chawla H, Kirshblum S, Zorowitz R, Lewis G, Pang S. Electromyogram-triggered neuromuscular stimulation for improving the arm function of acute stroke survivors: a randomized pilot study. Arch Phys Med Rehabil 79: 570–575, 1998. doi: 10.1016/s0003-9993(98)90074-0. [DOI] [PubMed] [Google Scholar]
- 34.Kwakkel G, Winters C, van Wegen EEH, Nijland RHM, van Kuijk AAA, Visser-Meily A, de Groot J, de Vlugt E, Arendzen JH, Geurts ACH, Meskers CGM; EXPLICIT-Stroke Consortium. Effects of unilateral upper limb training in two distinct prognostic groups early after stroke: the EXPLICIT-stroke randomized clinical trial. Neurorehabil Neural Repair 30: 804–816, 2016. doi: 10.1177/1545968315624784. [DOI] [PubMed] [Google Scholar]
- 35.Van der Lee JH, Wagenaar RC, Lankhorst GJ, Vogelaar TW, Devillé WL, Bouter LM. Forced use of the upper extremity in chronic stroke patients: results from a single-blind randomized clinical trial. Stroke 30: 2369–2375, 1999. doi: 10.1161/01.str.30.11.2369. [DOI] [PubMed] [Google Scholar]
- 36.Fasoli SE, Krebs HI, Stein J, Frontera WR, Hogan N. Effects of robotic therapy on motor impairment and recovery in chronic stroke. Arch Phys Med Rehabil 84: 477–482, 2003. doi: 10.1053/apmr.2003.50110. [DOI] [PubMed] [Google Scholar]
- 37.Lo AC, Guarino PD, Richards LG, Haselkorn JK, Wittenberg GF, Federman DG, Ringer RJ, Wagner TH, Krebs HI, Volpe BT. Robot-assisted therapy for long-term upper-limb impairment after stroke. New Engl J Med 362: 1772–1783, 2010. doi: 10.1056/NEJMoa0911341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lum PS, Burgar CG, Shor PC, Majmundar M, Van der Loos M. Robot-assisted movement training compared with conventional therapy techniques for the rehabilitation of upper-limb motor function after stroke. Arch Phys Med Rehabil 83: 952–959, 2002. doi: 10.1053/apmr.2001.33101. [DOI] [PubMed] [Google Scholar]
- 39.Duncan P, Studenski S, Richards L, Gollub S, Lai SM, Reker D, Perera S, Yates J, Koch V, Rigler S, Johnson D. Randomized clinical trial of therapeutic exercise in subacute stroke. Stroke 34: 2173–2180, 2003. doi: 10.1161/01.STR.0000083699.95351.F2. [DOI] [PubMed] [Google Scholar]
- 40.Feys HM, De Weerdt WJ, Selz BE, Cox Steck GA, Spichiger R, Vereeck LE, Putman KD, Van Hoydonck GA. Effect of a therapeutic intervention for the hemiplegic upper limb in the acute phase after stroke. Stroke 29: 785–792, 1998. doi: 10.1161/01.STR.29.4.785. [DOI] [PubMed] [Google Scholar]
- 41.Bourbonnais D, Vanden Noven S, Carey KM, Rymer WZ. Abnormal spatial patterns of elbow muscle activation in hemiparetic human subjects. Brain 112: 85–102, 1989. doi: 10.1093/brain/112.1.85. [DOI] [PubMed] [Google Scholar]
- 42.Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain 118: 495–510, 1995. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- 43.Barradas VR, Kutch JJ, Kawase T, Koike Y, Schweighofer N. When 90% of the variance is not enough: residual EMG from muscle synergy extraction influences task performance. J Neurophysiol 123: 2180–2190, 2020. doi: 10.1152/jn.00472.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McPherson JG, Chen A, Ellis MD, Yao J, Heckman C, Dewald JP. Progressive recruitment of contralesional cortico‐reticulospinal pathways drives motor impairment post stroke. J Physiol 596: 1211–1225, 2018. doi: 10.1113/JP274968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zaaimi B, Edgley SA, Soteropoulos DS, Baker SN. Changes in descending motor pathway connectivity after corticospinal tract lesion in macaque monkey. Brain 135: 2277–2289, 2012. doi: 10.1093/brain/aws115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glover IS, Baker SN. Cortical, corticospinal, and reticulospinal contributions to strength training. J Neurosci 40: 5820–5832, 2020. doi: 10.1523/JNEUROSCI.1923-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schambra HM, Xu J, Branscheidt M, Lindquist M, Uddin J, Steiner L, Hertler B, Kim N, Berard J, Harran MD. Differential poststroke motor recovery in an arm versus hand muscle in the absence of motor evoked potentials. Neurorehabil Neural Repair 33: 568–580, 2019. doi: 10.1177/1545968319850138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davidson AG, Buford JA. Motor outputs from the primate reticular formation to shoulder muscles as revealed by stimulus-triggered averaging. J Neurophysiol 92: 83–95, 2004. doi: 10.1152/jn.00083.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davidson AG, Schieber MH, Buford JA. Bilateral spike-triggered average effects in arm and shoulder muscles from the monkey pontomedullary reticular formation. J Neurosci 27: 8053–8058, 2007. doi: 10.1523/JNEUROSCI.0040-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheney PD, Fetz EE, Mewes K. Neural mechanisms underlying corticospinal and rubrospinal control of limb movements. Prog Brain Res 87: 213–252, 1991. doi: 10.1016/s0079-6123(08)63054-x. [DOI] [PubMed] [Google Scholar]
- 51.Riddle CN, Edgley SA, Baker SN. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J Neurosci 29: 4993–4999, 2009. doi: 10.1523/JNEUROSCI.3720-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riddle CN, Baker SN. Convergence of pyramidal and medial brain stem descending pathways onto macaque cervical spinal interneurons. J Neurophysiol 103: 2821–2832, 2010. doi: 10.1152/jn.00491.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schepens B, Drew T. Descending signals from the pontomedullary reticular formation are bilateral, asymmetric, and gated during reaching movements in the cat. J Neurophysiol 96: 2229–2252, 2006. doi: 10.1152/jn.00342.2006. [DOI] [PubMed] [Google Scholar]
- 54.Jørgensen HS, Nakayama H, Raaschou HO, Vive-Larsen J, Støier M, Olsen TS. Outcome and time course of recovery in stroke. II. Time course of recovery. The Copenhagen Stroke Study. Arch Phys Med Rehabil 76: 406–412, 1995. doi: 10.1016/S0003-9993(95)80568-0. [DOI] [PubMed] [Google Scholar]
- 55.Tyryshkin K, Coderre AM, Glasgow JI, Herter TM, Bagg SD, Dukelow SP, Scott SH. A robotic object hitting task to quantify sensorimotor impairments in participants with stroke. J Neuroeng Rehabil 11: 47, 2014. doi: 10.1186/1743-0003-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figs. S1–S3 (including Supplemental Fig. S2 which contains a summary of nonparetic reaching data collected following paretic testing) and extended Methods: https://doi.org/10.6084/m9.figshare.17345102.