Abstract
Hepatocellular carcinoma (HCC) is one of the deadliest cancers in the world with a five-year survival rate of less than 20%. Nonetheless, selecting an appropriate therapeutic agent to inhibit the development of hepatoma cells is still a challenge. Bufalin, a component of the traditional Chinese medicine Chansu, has been shown to inhibit the proliferation, invasion and metastasis of HCC through various signaling pathways. In addition, bufalin and sorafenib demonstrate a synergistic effect in cancer therapeutics. This review highlighted on several focal signaling pathways involved in the inhibitory effects of bufalin on HCC and its synergistic mechanisms with sorafenib. The immunotherapy effect of bufalin has also been discussed as a novel property.
Keywords: bufalin, cancer, therapy, signaling pathways, immunotherapy effect
Introduction
Liver cancer is the sixth most commonly diagnosed cancer, and its mortality rate is the third most common among all cancers.1 Most liver diseases occur in developing countries and most patients with liver cancer are aged between 35 and 65 years, with the number of male patients being higher than that of female patients. These statistics show that hepatocellular carcinoma (HCC) is a life-threatening health problem. The prognosis of liver cancer is very poor such that approximately 5% to 15% of patients are eligible for surgical removal, a procedure suitable only for patients in the early stage of the disease because of diminished hepatic regenerative capacity, typically without cirrhosis. In terms of surgical complexity, right hepatectomy carries a higher risk for postoperative complications than left hepatectomy.2,3 Consequently, novel therapeutic strategies are essential for improving the clinical management of patients with HCC.
Bufalin, a component of the traditional Chinese medicine Chansu, is extracted from the skin and parotid venom glands of Bufo Bufo gargarizans cantor and has been shown to have antitumor activity.4 Many studies have reported that bufalin can inhibit proliferation, invasion and metastasis through a variety of mechanisms.
Effect and Mechanism of Action of Bufalin in Inhibiting Hepatocellular Carcinomas
AKT Signaling Pathway
AKT is the key protein of the AKT/GSK3β/β-catenin/E-cadherin signaling pathway in HCC, and its phosphorylation regulates 10 other main regulatory proteins of various signaling pathways. AKT belongs to the serine/threonine protein kinase family,5 and exists in three isoforms translated from three distinct genes: AKT1 (PKB alpha), AKT2 (PKB beta), and AKT 3 (PKB gamma).6 AKT is a crucial regulator that plays a key role in the PI3K/AKT pathway, which regulates several cellular processes including cell survival, cell growth/size, proliferation, transcription, glucose metabolism, synthesis, genome stability, and neovascularization; thus, any disturbance in this pathway has a notable impact on cellular homeostasis.7 With regard to AKT1, Xu et al8 showed that AKT1-mediated phosphorylation of mTORC2 is vital for triggering hepatocarcinogenesis in humans and mice, resulting from cellular growth through c-Myc activation. In addition, the increase in the expression of AKT1 was shown to result in an increase in the proliferation and migration of HepG2 cells (HCC cell line).9 Furthermore, the high expression of AKT2 was related to portal invasion, histopathological differentiation, and the number of tumor nodules.10 Recent studies on AKT3 have mainly focused on its expression in the testes and brain; however, a few reports have also presented its therapeutic implication in managing HCC.10
In the AKT/GSK3β/β-catenin/E-cadherin signaling pathway, AKT promotes the phosphorylation and expression of GSK3β and the nuclear translocation of β-catenin. GSK3β, a multifunctional serine/threonine kinase, regulates various cellular functions including cell survival and cell-fate specification, and participates in numerous signaling pathways, while β-catenin is an essential regulator of cell motility, invasion, and adhesion. The effect on β-catenin inhibits its downstream molecule, E-cadherin, which negatively regulates the expression of MMP-2 and MMP-9. Bufalin was shown to suppress the protein levels of FAK and Rho A, VEGF, MEKK3, MKK7, and uPA, as well as preventing the translocation of nuclear factor kappa B (NF-κB). These processes (signaling pathway and regulation of proteins) are closely related to the proliferation, migration, invasion, and adhesion of hepatoma cells.11 Qiu et al4 showed that bufalin could inhibit the AKT signaling pathway by negatively regulating the expression of AKT which is related to reduced levels of NF-κB,12 to prevent the activities of hepatoma cells.
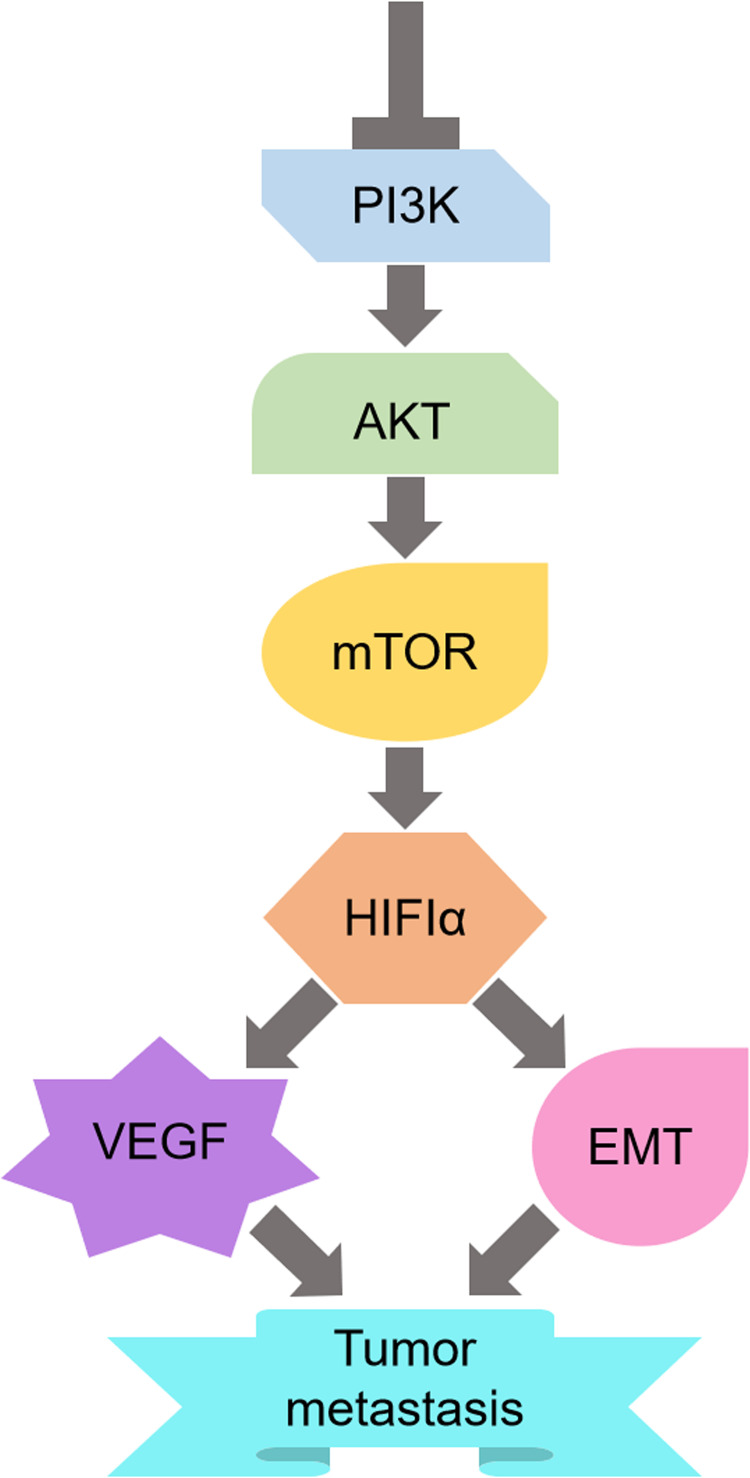
In addition, Wang et al13 reported that bufalin inhibited HCC metastasis by negatively regulating the PI3/AKT/mTOR pathway. Through this pathway, HIF-1α expression which can mediate EMT and VEGF involved in the antimetastatic process, is downregulated (Figure 1). Nonetheless, there are many other upstream pathways of HIF-1α besides PI3K/AKT/mTOR; therefore, the suppression of one mere pathway would not be enough to reduce the expression of HIF-1α.
Figure 1.
Schematic of the mechanism by which bufalin inhibits HCC invasion and metastasis.
Wnt/β-Catenin Signaling Pathway
The Wnt/β-catenin signaling pathway plays a major role in the occurrence and development of various cancers. It consists of Wnt signaling ligands, viz. Fz receptor proteins and co-receptor LRP5/6, as well as disheveled, axin, β-catenin, APC, casein kinase 1a, and GSK-3β. Among these molecules, β-catenin is an important protein whose accumulation is a sign of the activated state of the Wnt/β-catenin signaling pathway.14
In normal mature cells, β-catenin is maintained at a low level because of the lack of Wnt. During embryonic development and tissue regeneration, β-catenin accumulates and passes through the nuclear membrane to conjugate with factors in the nucleus, thereby increasing the downstream target proteins that are related to cell proliferation, apoptosis, matrix dissolution, and angiogenesis, such as cyclin D1, MMP-7, COX-2, c-myc, surviving, and VEGF.15 Among them, cyclin D1 promotes cell transformation from the G1 phase to the S phase resulting in cell proliferation.16 MMP-7 not only participates in various extracellular matrix lysis, but also damages diverse tumor suppressor compositions on the cell surface.17 Consequently, the overexpression of cyclin D1, MMP-7, and COX-2 plays an essential role in the proliferation, invasion, and metastasis of hepatoma cells.18
Low E-cadherin expression leads to a significant increase in free β-catenin. In addition, the increased phosphorylation at the GSK-3β ser9 site and reduced expression of E-cadherin are regarded as the main causes of activation of the Wnt/β-catenin pathway in HCC cells.19
In a previous study,14 bufalin decreased the phosphorylation of the GSK-3β Ser9 site and the translocation of β-catenin in HCC cells, then regulated the extranuclear signal transduction and endonuclear target molecules, which subsequently led to lower expression levels of cyclin D1, MMP-7, and COX-2 in HCC cells. In this way, bufalin inhibited the proliferation, invasion, and metastasis of HCC cells.
Hedgehog Signaling Pathway
Hedgehog is a highly conserved intercellular signal transduction system that contains Hedgehog (Hh), Patched-Smoothened, Glioma (Gli), Costal-2, Fuse (Fu), Suppressor of Fuse, and protein kinase A. Among them, Hh, Smo, Gli, and Fu are positive regulatory molecules in the Hh signaling pathway, while the remainder are negative regulators.20
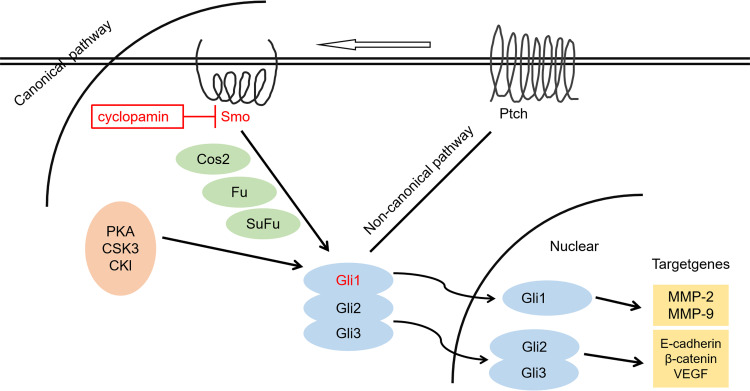
Epithelial mesenchymal transition (EMT) is a biological process in which epithelial cells lose polarity through a specific program and obtain the characteristics of mesenchymal cells. Extracellular matrix (ECM) degradation is an important contributor to tumor invasion and metastasis. Bufalin inhibits EMT, ECM degradation, and angiogenesis of hepatoma cells by regulating the expressions of Ptch1, Gli1, and Gli3 proteins in the Hedgehog signaling pathway. In addition, bufalin inhibits the downstream target molecules of MMP-2 and MMP-9 in hepatoma cells, which leads to the degradation of a variety of protein components in the ECM. Similarly, bufalin treatment increases E-cadherin expression and decreases β-catenin and VEGF expression in hepatoma cells by influencing Gli3 protein expression (Figure 2).21
Figure 2.
Regulation and control of bufalin on invasive and metastatic molecules of HCC cells via the Hedgehog signaling pathway.
Fas- and Mitochondrial-Mediated Apoptotic Pathway
In the intrinsic pathway, Bax and Bcl-2 which belong to the Bcl-2 family of proteins, have crucial effects on initiating the mitochondrial death cascade. Bax, a pro-apoptotic protein, is upregulated by bufalin. Bufalin also inhibits the expression of the anti-apoptotic protein Bcl-2. These changes directly lead to the disruption of ΔΨm and the release of cytochrome c, which activates a caspase cascade consisting of caspase-9 and caspase-3.22
In the extrinsic pathway, the Fas pathway has been shown to be highly efficient in inhibiting tumor cells.22 Bufalin also upregulates the expression of Fas and activates caspase-8 and caspase-10. The major regions of caspase-8 and caspase-10 activate caspase-3, while the minor portions cleave Bid to tBid, which also leads to the release of cytochrome c.
Caspase-3 in these caspase cascades induces the cleavage of PRAP together during apoptosis in both intrinsic and extrinsic pathways.23
Synergistic Effect of Bufalin and Sorafenib
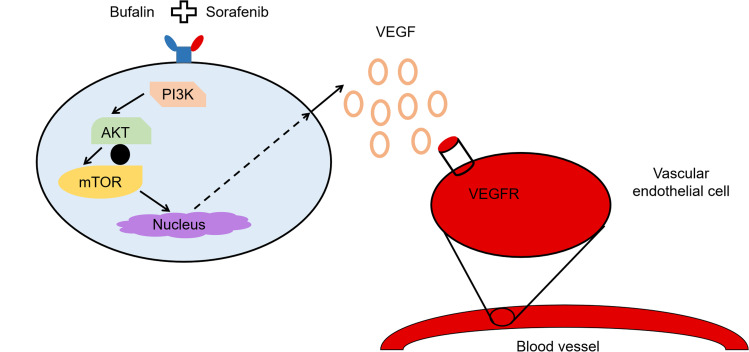
Wang et al24 found that the synergistic effect of bufalin and sorafenib is related to the regulation of cells, viz. tumor cells and endothelial cells. The synergistic treatment effects of these molecules regulated HCC cells via the mTOR/VEGF pathway, resulting in the downregulation of VEGF secretion. Consequently, the mTOR/VEGF pathway may be considered a target for strengthening the synergism (Figure 3).
Figure 3.
Schematic illustration of the mechanism by which bufalin augments sorafenib-induced inhibition of angiogenesis through the mTOR/VEGF signaling pathway in HCC.
Gao et al25 demonstrated that bufalin enhanced the anti-cancer effect of sorafenib in PLC/PRF/5 and Hep G-2 cells. The mechanism was postulated to involve sorafenib-mediated inhibition of cell growth by the decreased phosphorylation of ERK, which was positively regulated by bufalin-induced increased phosphorylation of AKT. This proposed effect on AKT by bufalin is contrary to the findings of Zhai et al.26
Zhai et al26 reported that bufalin reversed both inherent and acquired resistance to sorafenib in HCC cells by inhibiting sorafenib-mediated AKT activation. This effect was said to result from bufalin-induced ER stress, which predominantly reduced p-AKT, inhibited cell growth, and promoted apoptosis via the IRE1 pathway. Although the latent mechanism by which bufalin reverses resistance to sorafenib has not been verified, the ER stress pathway participates in crosstalk with various pathways related to cell growth and apoptosis.26
From the aforementioned studies, some conclusions seem to be on the contrary, which may result from different perspectives and the advancing technology over the years. However, numerous studies have confirmed that the expression of AKT can be reduced by bufalin, which was consistent with the findings of this review. As previously alluded, bufalin enhances the anti-cancer effect of sorafenib by downregulating the expression of AKT, and AKT is a key protein whose phosphorylation regulates more than 10 signaling proteins to participate in processes such as cell proliferation, apoptosis, migration, and invasion. Molecular signal transduction of cells is a complex process that has multiple paths simultaneously. Different perspectives and experimental conditions may draw different conclusions, which may be associated with simultaneous interferences from other factors. For instance, Gao et al25 reported an increased synergistic effect of sorafenib from dual administration with bufalin; however, these findings require further study and discussion prior to potential application into clinical settings. Overall, bufalin has been shown to have an inhibitory effect on the occurrence and development of tumors.
Autophagy
Autophagy has received increasing attention in cancer research as it plays a key role in cancer cell survival and death. It is a physiological process that leads to the segregation and degradation of cytoplasmic content through a lysosomal mechanism. Furthermore, it allows cellular components to recycle and supply cellular energy under nutrient starvation, infection, and other stress conditions.27,28 Miao et al found that bufalin can cause HepG2 autophagy.29 Their findings concluded that autophagy plays a two-sided role in cancer, viz. in prosurvival and proapoptotic processes, with the detailed mechanisms in cancer cells being dependent on the type of cancer, stage, and microenvironment. In addition, their results showed that autophagy induced by bufalin acted as a proapoptotic mechanism, and bufalin-induced cell death in HCC cancer cells was dependent, at least in part, on the induction of autophagy.
AMP-activated protein kinase (AMPK) is a principal energy-preserving intracellular enzyme that is activated under stress conditions and can induce autophagy through inhibition of the serine/threonine kinase mammalian target of rapamycin (mTOR), a major repressor of autophagy.30–32 Bufalin induced autophagy in HepG2 cells, which was followed by the inhibition of phosphorylation of both mTOR and its substrate p70S6K. In contrast, bufalin dose-dependently increased AMPK activation. Therefore, the induction of autophagy by bufalin was related to the activation of AMPK and inhibition of the mTOR signaling pathway. Similarly, Tsai et al33 demonstrated that the AKT/mTOR signaling pathway promotes autophagy in bufalin-treated SK-HEP-1 cells. In addition, Hsu et al34 have found that hepatoma cells could be effectively killed by bufalin via cell cycle arrest at the G2/M phase due to autophagy and not apoptosis. Sheng et al35 indicated that bufalin induced autophagy in liver cancer cells by upregulating the protein expression of LC3-II and Beclin-1, and by downregulating that of P62. However, these authors also reported that the therapeutic efficacy of bufalin in HCC can be improved by targeting autophagy-related proteins.
Meanwhile, in another study,36 the inhibition of autophagy could increase the cytotoxicity of bufalin in HCC cells, resulting from the efficient prevention of apoptosis and cell death mediated by the ER stress response, thus maintaining cell homeostasis and survival.
Immunotherapy Properties of Bufalin
To date, immunotherapy has attracted widespread concern and research. In particular, bufalin was shown to enhance the immune responses in vivo in a leukemia-induced mouse model (BALB/c mice).37 Shih et al37 proved that bufalin may increase immune responses not only by promoting the monocyte (CD11b) population as well as T-cell and B-cell proliferation, but also by improving macrophage phagocytosis in mice with leukemia in vivo.
However, not many studies that demonstrate the effect of bufalin in modulating immune responses in HCC in vitro have been reported in literature. A study by Yang et al38 reported that bufalin inhibited APOBEC3F, CCR9, CCR10, CXCR4, and pIgR proteins in the intestinal immune network for IgA production. Because the APOBEC3F-induced intestinal immune network of the IgA production signaling pathway plays a key role in tumor progression in HCC, the authors suggested that downregulation of APOBEC3F and inhibiting the activation of the intestinal immune network of the IgA production signaling pathway may be a novel mechanism of the antitumor effect of bufalin. Nevertheless, their experiments were all conducted in vitro, which led to many limitations of the study. Unfortunately, reports on the targeting of other myeloid and lymphoid immune cells for immunotherapy, which proved to be a promising therapeutic strategy for liver cancer influenced by bufalin, were not available in literature.
Toll-like receptor 3 (TLR3) agonists which are polyriboinosinic–polyribocytidylic acid [poly (I:C)] have been reported as potential immunotherapy adjuvants for cancer, with low concentrations having being said to improve the metastatic capacity of TLR3C HCC.39 In line with this, bufalin has been shown to inhibit the risk of metastasis of poly (I:C).39 Feng et al39 showed the potential immunotherapy effect of bufalin. However, further research is needed to explore the mechanism of action of the molecule and the detailed immunotherapy effect in HCC.
Perspective
Traditional Chinese medicines, which have been researched for potential anti-cancer properties for many years have demonstrated low toxicity in normal cancer cells in numerous cases. In particular, bufalin has been recorded as one of the most efficient anti-cancer medicines that has been studied in clinical trials for treatment of various cancers, such as HCC, non-small cell lung cancer, and pancreatic cancer.40 Fortunately, its effects on proliferation, invasion, and metastasis of HCC cells have been confirmed, and have been summarized in this review. Thus, bufalin has the potential to become a treatment for HCC in clinical settings.
However, the mechanism of its effect on autophagy has not yet attained consensus in the Oncology community. Therefore, autophagy of HCC cells requires further research, and subsequent molecular targeted drug therapy discoveries for autophagy-related proteins may promote the development of bufalin. Importantly, the immunotherapy effect of bufalin has been discovered; however, other myeloid and lymphoid immune cell applications as immunotherapy agents, which are promising therapeutic strategies for liver cancer, were not available in reviewed literature. Therefore, we expect further detailed research on this effect in the coming years.
Acknowledgments
This was supported by Ying Liu, the host of the Dalian Medical Science Research Program (No. 2011043), and Han-yu Jiang, the host of the Provincial College Student Innovation and Entrepreneurship Training Program (NO.S202111258024). We would like to thank Editage (www.editage.cn) for English language editing.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Hong L, Hui L, Dan Z, Wen P. Effect of anlotinib combined with systemic immunotherapy in the treatment of advanced liver cancer. J Guangdong Med Univ. 2021;39:2. [Google Scholar]
- 3.Anwanwan D, Singh SK, Singh S, Saikam V, Singh R. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer. 2020;1873(1):188314. doi: 10.1016/j.bbcan.2019.188314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiu DZ, Zhang ZJ, Wu WZ, Yang YK. Bufalin, a component in Chansu, inhibits proliferation and invasion of hepatocellular carcinoma cells. BMC Complement Altern Med. 2013;13(1):185. doi: 10.1186/1472-6882-13-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellacosa A, Testa JR, Staal SP, Tsichlis PN. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991;254(5029):274–277. doi: 10.1126/science.254.5029.274 [DOI] [PubMed] [Google Scholar]
- 6.Mroweh M, Roth G, Decaens T, Marche PN, Lerat H, Macek Jílková Z. Targeting Akt in hepatocellular carcinoma and its tumor microenvironment. Int J Mol Sci. 2021;22(4):1794. doi: 10.3390/ijms22041794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martelli AM, Tabellini G, Bressanin D, et al. The emerging multiple roles of nuclear Akt. Biochim Biophys Acta. 2012;1823(12):2168–2178. doi: 10.1016/j.bbamcr.2012.08.017 [DOI] [PubMed] [Google Scholar]
- 8.Xu Z, Xu M, Liu P, et al. The mTORC2-Akt1 cascade is crucial for c-Myc to promote hepatocarcinogenesis in mice and humans. Hepatology. 2019;70(5):1600–1613. doi: 10.1002/hep.30697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dai F, Yu W, Song J, Li Q, Wang C, Xie S. Extracellular polyamines-induced proliferation and migration of cancer cells by ODC, SSAT, and Akt1-mediated pathway. Anticancer Drugs. 2017;28(4):457–464. doi: 10.1097/CAD.0000000000000465 [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Huang W, Ran Y, et al. miR-582-5p inhibits proliferation of hepatocellular carcinoma by targeting CDK1 and AKT3. Tumour Biol. 2015;36(11):8309–8316. doi: 10.1007/s13277-015-3582-0 [DOI] [PubMed] [Google Scholar]
- 11.Zhang ZJ, Yang YK, Wu WZ. Bufalin attenuates the stage and metastatic potential of hepatocellular carcinoma in nude mice. J Transl Med. 2014;12(1):57. doi: 10.1186/1479-5876-12-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen YY, Lu HF, Hsu SC, et al. Bufalin inhibits migration and invasion in human hepatocellular carcinoma SK-Hep1 cells through the inhibitions of NF-kB and matrix metalloproteinase-2/-9-signaling pathways. Environ Toxicol. 2015;30(1):74–82. doi: 10.1002/tox.21896 [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Zhang C, Xu L, et al. Bufalin suppresses hepatocellular carcinoma invasion and metastasis by targeting HIF-1α via the PI3K/AKT/mTOR pathway. Oncotarget. 2016;7(15):20193–20208. doi: 10.18632/oncotarget.7935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gai JQ, Sheng X, Qin JM, Sun K, Zhao W, Ni L. The effect and mechanism of bufalin on regulating hepatocellular carcinoma cell invasion and metastasis via Wnt/β-catenin signaling pathway. Int J Oncol. 2016;48(1):338–348. doi: 10.3892/ijo.2015.3250 [DOI] [PubMed] [Google Scholar]
- 15.Saito-Diaz K, Chen TW, Wang X, et al. The way Wnt works: components and mechanism. Growth Factors. 2013;31(1):1–31. doi: 10.3109/08977194.2012.752737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu H, Liu J, Chakraborty C. Analysing the effect of mutation on protein function and discovering potential inhibitors of CDK4: molecular modelling and dynamics studies. PLoS One. 2015;10(8):e0133969. doi: 10.1371/journal.pone.0133969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu J, Guan X, Zhang K, Li YT, Bai P, Wu J. A/G polymorphism of matrix metalloproteinase 7 gene promoter region and cancer risk: a meta-analysis. Biomed Rep. 2013;1(5):792–796. doi: 10.3892/br.2013.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee MJ, Xu DY, Li H, et al. Pro-oncogenic potential of NM23-H2 in hepatocellular carcinoma. Exp Mol Med. 2012;44(3):214–224. doi: 10.3858/emm.2012.44.3.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolfe A, Thomas A, Edwards G, Jaseja R, Guo GL, Apte U. Increased activation of the Wnt/β-catenin pathway in spontaneous hepatocellular carcinoma observed in farnesoid X receptor knockout mice. J Pharmacol Exp Ther. 2011;338(1):12–21. doi: 10.1124/jpet.111.179390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hui CC, Angers S. Gli proteins in development and disease. Annu Rev Cell Dev Biol. 2011;27(1):513–537. doi: 10.1146/annurev-cellbio-092910-154048 [DOI] [PubMed] [Google Scholar]
- 21.Sheng X, Sun X, Sun K, Sui H, Qin J, Li Q. Inhibitory effect of bufalin combined with Hedgehog signaling pathway inhibitors on proliferation and invasion and metastasis of liver cancer cells. Int J Oncol. 2016;49(4):1513–1524. doi: 10.3892/ijo.2016.3667 [DOI] [PubMed] [Google Scholar]
- 22.Goto M. Elevation of soluble Fas (APO-1, CD95) ligand in natural aging and Werner syndrome. Biosci Trends. 2008;2(3):124–127. [PubMed] [Google Scholar]
- 23.Qi F, Inagaki Y, Gao B, et al. Bufalin and cinobufagin induce apoptosis of human hepatocellular carcinoma cells via Fas- and mitochondria-mediated pathways. Cancer Sci. 2011;102(5):951–958. doi: 10.1111/j.1349-7006.2011.01900.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, Zhang C, Chi H, Meng Z. Synergistic anti-hepatoma effect of bufalin combined with sorafenib via mediating the tumor vascular microenvironment by targeting mTOR/VEGF signaling. Int J Oncol. 2018;52(6):2051–2060. doi: 10.3892/ijo.2018.4351 [DOI] [PubMed] [Google Scholar]
- 25.Gao Y, Li HX, Xu LT, et al. Bufalin enhances the anti-proliferative effect of sorafenib on human hepatocellular carcinoma cells through downregulation of ERK. Mol Biol Rep. 2012;39(2):1683–1689. doi: 10.1007/s11033-011-0908-x [DOI] [PubMed] [Google Scholar]
- 26.Zhai B, Hu F, Yan H, et al. Bufalin reverses resistance to sorafenib by inhibiting Akt activation in hepatocellular carcinoma: the role of endoplasmic reticulum stress. PLoS One. 2015;10(9):e0138485. doi: 10.1371/journal.pone.0138485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye MX, Zhao YL, Li Y, et al. Curcumin reverses cis-platin resistance and promotes human lung adenocarcinoma A549/DDP cell apoptosis through HIF-1α and caspase-3 mechanisms. Phytomedicine. 2012;19(8–9):779–787. doi: 10.1016/j.phymed.2012.03.005 [DOI] [PubMed] [Google Scholar]
- 28.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290(5497):1717–1721. doi: 10.1126/science.290.5497.1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miao Q, Bi LL, Li X, et al. Anticancer effects of bufalin on human hepatocellular carcinoma HepG2 cells: roles of apoptosis and autophagy. Int J Mol Sci. 2013;14(1):1370–1382. doi: 10.3390/ijms14011370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A. Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 2009;16(7):966–975. doi: 10.1038/cdd.2009.33 [DOI] [PubMed] [Google Scholar]
- 31.Meijer AJ, Codogno P. Regulation and role of autophagy in mammalian cells. Int J Biochem Cell Biol. 2004;36(12):2445–2462. doi: 10.1016/j.biocel.2004.02.002 [DOI] [PubMed] [Google Scholar]
- 32.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1(1):15–25. doi: 10.1016/j.cmet.2004.12.003 [DOI] [PubMed] [Google Scholar]
- 33.Tsai SC, Yang JS, Peng SF, et al. Bufalin increases sensitivity to AKT/mTOR-induced autophagic cell death in SK-HEP-1 human hepatocellular carcinoma cells. Int J Oncol. 2012;41(4):1431–1442. doi: 10.3892/ijo.2012.1579 [DOI] [PubMed] [Google Scholar]
- 34.Hsu CM, Tsai Y, Wan L, Tsai FJ. Bufalin induces G2/M phase arrest and triggers autophagy via the TNF, JNK, BECN-1 and ATG8 pathway in human hepatoma cells. Int J Oncol. 2013;43(1):338–348. doi: 10.3892/ijo.2013.1942 [DOI] [PubMed] [Google Scholar]
- 35.Sheng X, Zhu P, Qin J, Li Q. The biological role of autophagy in regulating and controlling the proliferation of liver cancer cells induced by bufalin. Oncol Rep. 2018;39(6):2931–2941. doi: 10.3892/or.2018.6365 [DOI] [PubMed] [Google Scholar]
- 36.Hu F, Han J, Zhai B, et al. Blocking autophagy enhances the apoptosis effect of bufalin on human hepatocellular carcinoma cells through endoplasmic reticulum stress and JNK activation. Apoptosis. 2014;19(1):210–223. doi: 10.1007/s10495-013-0914-7 [DOI] [PubMed] [Google Scholar]
- 37.Shih YL, Chou JS, Chen YL, et al. Bufalin enhances immune responses in leukemic mice through enhancing phagocytosis of macrophage in vivo. In Vivo (Brooklyn). 2018;32(5):1129–1136. doi: 10.21873/invivo.11355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Z, Tao Y, Xu X, Cai F, Yu Y, Ma L. Bufalin inhibits cell proliferation and migration of hepatocellular carcinoma cells via APOBEC3F induced intestinal immune network for IgA production signaling pathway. Biochem Biophys Res Commun. 2018;503(3):2124–2131. doi: 10.1016/j.bbrc.2018.07.169 [DOI] [PubMed] [Google Scholar]
- 39.Feng Y, Chen Y, Meng Y, et al. Bufalin suppresses migration and invasion of hepatocellular carcinoma cells elicited by poly (I:C) therapy. Oncoimmunology. 2018;7(5):e1426434. doi: 10.1080/2162402X.2018.1426434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meng Z, Yang P, Shen Y, et al. Pilot study of huachansu in patients with hepatocellular carcinoma, nonsmall-cell lung cancer, or pancreatic cancer. Cancer. 2009;115(22):5309–5318. doi: 10.1002/cncr.24602 [DOI] [PMC free article] [PubMed] [Google Scholar]