Abstract
Background
On 31st December 2019 in Wuhan, China, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), was acknowledged. This virus spread quickly throughout the world causing a global pandemic. The World Health Organization declared COVID-19 a pandemic disease on 11th March 2020. Since then, the whole world has come together and have developed several vaccines against this deadly virus. Similarly, several alternative searches for pandemic disease therapeutics are still ongoing. One of them has been identified as nanotechnology. It has demonstrated significant promise for detecting and inhibiting a variety of viruses, including coronaviruses. Several nanoparticles, including gold nanoparticles, silver nanoparticles, quantum dots, carbon dots, graphene oxide nanoparticles, and zinc oxide nanoparticles, have previously demonstrated remarkable antiviral activity against a diverse array of viruses.
Objective
This review aims to provide a basic and comprehensive overview of COVID-19's initial global outbreak and its mechanism of infiltration into human host cells, as well as the detailed mechanism and inhibitory effects of various nanoparticles against this virus. In addition to nanoparticles, this review focuses on the role of several antiviral drugs used against COVID-19 to date.
Conclusion
COVID-19 has severely disrupted the social and economic lives of people all over the world. Due to a lack of adequate medical facilities, countries have struggled to maintain control of the situation. Neither a drug nor a vaccine has a 100% efficacy rate. As a result, nanotechnology may be a better therapeutic alternative for this pandemic disease.
Keywords: Antiviral activity, COVID-19, Metal nanoparticles, Nanoparticles, SARS-CoV-2
Abbreviations: COVID-19:, Coronavirus disease 19; ACE-2:, Angiotensin-converting enzyme 2; CNT:, Carbon NanoTubes; PEG-NP:, Poly (ethylene glycol)-Nanoparticles; PEDV:, Porcine epidemic diarrhea virus; TGEV:, Transmissible gastroenteritis coronavirus; HBV:, Hepatitis B Virus; IFN-γ:, Interferon-gamma; DSS:, Dextran sodium sulfate; HSV-I:, Herpes simplex virus-I; IFN-α:, Interferon-alpha; TNF:, Tumor necrosis factor; VALs:, Viral attachment ligands; SENPs:, Single elemental nanoparticles; BBB:, Blood-brain barrier; SNC:, Silica nano-capsules
Graphical Abstract
1. Introduction
Viruses are sub-microscopic communicable agents that replicate only inside the living cells of an organism. Viruses can infect all life forms, from animals and plants to microorganisms. They can cause viral diseases and also potential death in humans and other mammals [1], [2]. As per recent reports, it has been estimated that viruses cause approximately 2,000,000 human deaths every year globally [3]. To date, vaccination has been the most effective approach to prevent viral infections. But unfortunately, the number of efficacious vaccines against viruses is relatively low. Presently, various medications have been developed for the cure of some viral diseases. For example, acyclovir is regarded as a potential anti-hepatitis B and anti-hepatitis C drug [4]. Even though these pharmaceuticals are regarded as a treatment of some viral diseases, side effects can occur in patients, particularly in children [5]. Additionally, the wide use of these chemical drugs does not necessarily take place in routine medical treatments, signifying the crucial need to fabricate new effective and safe antiviral medications. Among the numerous potential antiviral medications, nanotechnology has demonstrated its potential in this field. Nanotechnology is the field of research that deals with structures that are approximately 1–100 nm in at least one dimension Nanotechnology offers the potential for new and active therapeutics. Nanoparticles exhibit a totally new set of improved properties such as shape, size, crystalline structure and morphology. The potential of nanotechnology is rapidly evolving, and this field keeps on emerging with new discoveries every day, making a significant contribution to humankind. [6]. The wide use of nanotechnology-based probes for the detection of viruses, led to the manufacture of numerous bioelectronics and biosensors [7], [8], [9]. Various other nanomaterials have also been manufactured including the use of several virions and virus-like particles as prototypes, increasing the demand for research in the fields of biosynthesis and biocompatibility [10], [11]. Rigorous activity has also been dedicated to making fluorescent nanoprobes and research into finding suitable applications in the molecular mechanism of virus-infected cells [12], [13], [14]. Recently, a huge number of functionalized nanoparticles have been reported as potential candidates for viral propagation inhibition [15], [16].
Nanotechnology has also been broadly used in numerous other fields such as agriculture, gene delivery, imaging, artificial implants, and much more [17], [18], [19], [20], [21], [22], [23]. Nanoparticles also play a significant role in cancer treatment and the manufacture of different potential drugs against bacterial, fungal, and viral infections [24]. The basis of using nanotechnology in medicine is because of its minute size, i.e., 1–100 nm, which enables them to enter living cells and specifically into human cells. Additionally, nanomaterials avoid the encased drug or anti-infection agent from degenerating due to its shielding characteristics [25], [26]. Due to the rapid dispersion and capability to change by genetic mutations, viral infections are a potential threat to human beings. Suitable approaches are essential to reduce the spread of infectious diseases, mostly dependent on the seriousness of the disease and mode of transmission. In the past few years, a lot of new viruses such as Ebola, Nipah, Zika, and many Coronaviruses have emerged and caused a significant number of deaths, among which Coronaviruses are likely one of the most dangerous including MERS-CoV, SARS-
CoV, and SARS-CoV-2. According to global reports, as of January 2020, a total number of 2519 confirmed cases of MERS-CoV, with 866 deaths, having a case-fatality rate of 34.3% were reported [27]. The epidemic of SARS-CoV affected 26 countries and caused over 8000 confirmed cases in 2003 [28]. SARSCoV-2 is the phylogenetic successor to the previously known SARS-CoV.
Coronaviruses are a group of RNA viruses and are positioned in the family Coronaviridae in the Nidovirales order. SARS-CoV-2 was named the causal agent of COVID-19, and later it was declared a pandemic disease by the World Health Organization on 11th March 2020. The spread of COVID-19 has led to increased concerns for the development of vaccines against MERS-CoV, SARS-CoV, and SARS-CoV2 [29], [30]. Coronavirus disease 19, has proven to be a highly transmissible disease that can spread from one human to another by contact or by the release of aerosol droplets. A few of the symptoms observed are cough, cold, fever, headache, sore throat, body pain, loss of appetite. COVID-19 was initially reported during the middle of January 2020 in Wuhan, Hubei province in China. Coronaviruses which include SARS-CoV, SARS-CoV-2, hCoV-NL63 have been confirmed to be existing in tears through RTPCR techniques. Conjunctivitis is considered as another symptom of COVID-19, specifically after considering the high number of COVID-19 cases infected with conjunctivitis [31]. Oral diseases like mouth ulcers, necrotizing gingivitis, blisters, salivary gland alterations, gustatory dysfunction were primarily observed in the clinical reports of many COVID-19 patients. The lesions were observed to be associated with loss of taste and smell. SAR-CoV-2 showed tropism for endothelial cells and COVID-19 facilitated endotheliitis was observed to be capable of stimulating inflammation of oral tissues, enabling the spread of the virus. The critical symptoms include tissue homeostasis and deferred disease recovery, caused due to elevated levels of proinflammatory mediators in the COVID-19 infected patients [32].
Globally, as of 5th November 2021, there have been 248,467,363 confirmed cases of COVID-19, including 5,027,183 deaths. As of 4th November 2021, a total of 7,027,377,238 vaccine doses have been administered [33].
Since the breakout, various medications against COVID-19 were applied. A list of antiviral compounds was made a part of the guidelines from the National Health Commission of the People's Republic of China, including interferon, ribavirin, arbidol, chloroquine phosphate, lopinavir and ritonavir. Losartan, an Angiotensin receptor blocker was also recommended for the treatment of COVID-19. The COVID-19 treatment guidelines varied between countries. The guidelines presented by World Health Organization were very universal, suggesting administration of symptoms, and handling pediatric patients, pregnant women and patients with underlying comorbidities with great consciousness [34].
The World Health Organization has evaluated that the following vaccines against COVID-19 have met the necessary criteria for safety and efficacy which include AstraZeneca/Oxford vaccine, Johnson and Johnson, Moderna, Pfizer/Biontech, Sinopharm, Sinovac. There are numerous safe and effective vaccines that prevent people from becoming severely ill or dying from COVID-19. In addition to the key preventive measures of remaining at least 1 m away from each other, frequently cleaning your hands, covering a cough or sneeze in your elbow, wearing a mask and dodging poorly ventilated rooms or opening a window [35].
2. Mechanism of infiltration of SARS-CoV-2
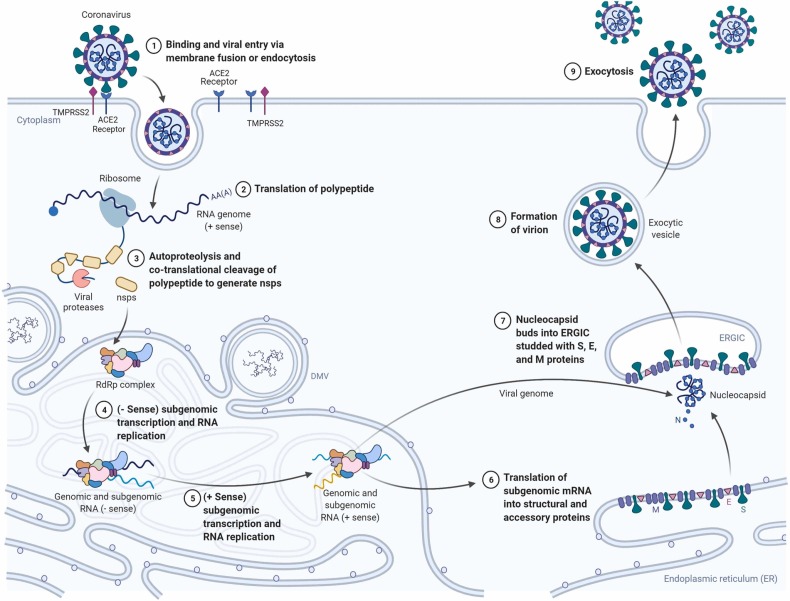
Viral infections can be considered as complex interactions between the host body and the virus. For protein synthesis the virus depends on the host cell, to firstly attach and then the viral genome penetrates the host cell and transcribes in the cytoplasm or the nucleus. Almost immediately, the replication process of the viral genome initiates and the viral mRNA and proteins are then synthesized and with the aid of the viral structural proteins the reassembly of progeny virions takes place and liberated from the host cell [36].
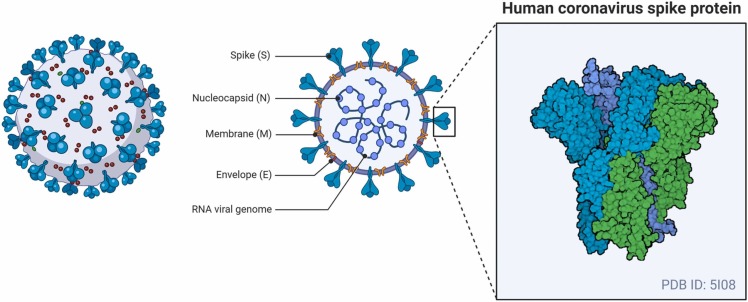
In the case of COVID-19, the chief factor for the commencement of the host-pathogen contact is the inhalation or physical contact with the SARS-CoV-2 virus. SARS-CoV-2, the human RNA virus is the solitary causal agent of the viral disease COVID-19. It has glycoprotein spikes on its outer surface which aid in the attachment and admission of the virus inside the host cell as shown in Fig. 1 [37]. Through numerous research reports it has been proven that SARS-CoV-2 is spread into the environment, primarily in the form of aerosols or droplets that are chiefly the respiratory secretions from an infected person who can either be a vulnerable host or a carrier. The virus can essentially survive in this media for a very long time on lifeless objects, in turn increasing the spread. The aerosol media helps the virus stay in a steady state for over a couple of hours on a steel surface, while the stability of the virus is approximately about 3 days [38]. The virus undergoes numerous genetic alterations to develop mutated characteristics for the initiation of the infection and ability to survive inside the host body [39]. The entry of the virus into the host cell is reliant on cellular proteases which split the spike proteins, Angiotensin-converting enzyme 2, which has been shown to be the most substantial receptor of SARS-CoV-2. The ACE-2 receptors has a very crucial lysine 31 residue, which recognizes the 394 glutamine residue of the SARS-CoV-2 in the receptor-binding region. The binding of the spike protein to the ACE-2 marks the initiation of the life cycle of SARS-CoV-2 [29], [40], [41]. After binding with ACE-2, the viral envelope fuses with the cell membrane via the endosomal pathway and SARS-CoV-2 then discharges the RNA into the host cell. The infective RNA translates to form viral replicase polyproteins pp1a and 1ab and in turn, it is broken down by viral proteinases into even smaller by products. Irregular transcription of the polymerase produces a sequence of sub-genomic mRNAs which are then translated into suitable viral proteins. Golgi body and endoplasmic reticulum play vital roles in the accumulation of the genome RNA and the virion’s viral proteins. Finally, it is carried out of the cell through vesicles [29]. Targeting the viral infection in its infancy stage, although potentially difficult, is of great significance as the site of action of the inhibitor might be extracellular and accessible [42]. The infiltration and budding mechanism of SARS-CoV-2 in and out of the human host cell are shown in Fig. 2.
Fig. 1.
Morphological structure and spike protein visualization of SARS-CoV-2 (Created with BioRender.com).
Fig. 2.
Infiltration mechanism of SARS-CoV-2 inside host cell (Created with BioRender.com).
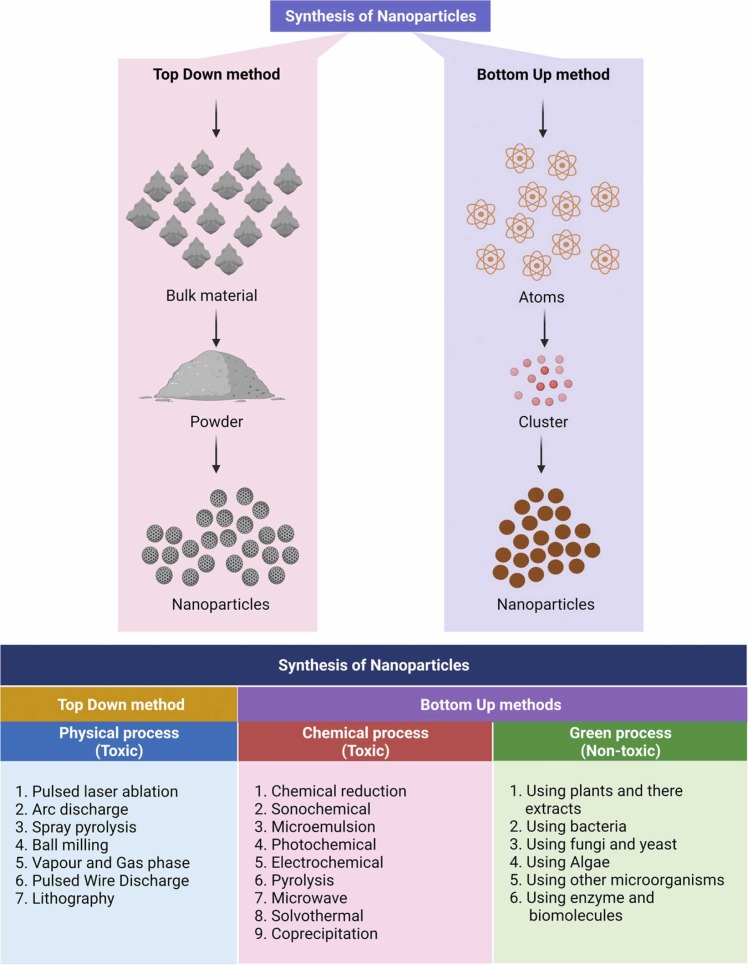
3. Synthesis of nanoparticles
The synthesis of nanoparticles can be classified into two groups ( Fig. 3):
-
(i)
Top-down method and
-
(ii)
bottom-up method. The top-down strategy emphasizes the physical path to nanoparticle creation, whereas the bottom-up method emphasizes the chemical and biological routes. Green nanoparticle synthesis can also be classified into the following categories (Fig. 3):
-
a.
Phyto-pathways, such as the use of plants and plant extracts.
-
b.
Microbial routes, such as using fungi, yeasts (eukaryotes), bacteria, and actinomycetes as templates.
-
c.
Bio-template routes, such as using membranes, viruses, and diatoms as templates.
Fig. 3.
Numerous ways for the creation of nanoparticles (Created with BioRender.com).
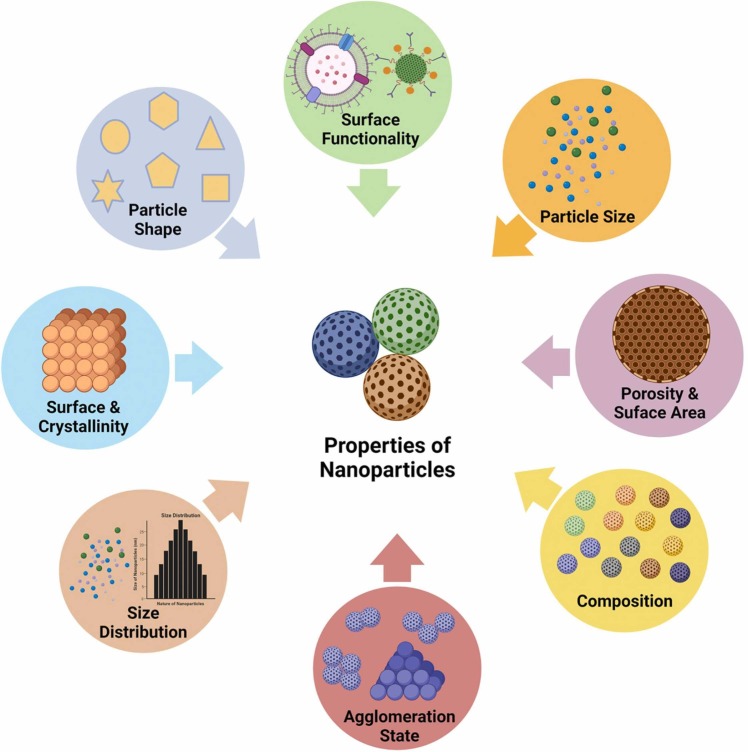
4. Properties of nanoparticles
Over the course of time Nanotechnology has proven to be of great significance, and one of the basic constituents of which is nanoparticles. Nanoparticles range from 1 to 100 nanometers in size and primarily constitute carbon-based, metal-based, metal-oxides based components and organic matter. At the nanoscale, nanoparticles display a variety of exclusive physical, chemical and biological properties, in comparison to their corresponding elements at higher scales [43] ( Fig. 4). This phenomenon takes place owing to a much larger surface area to volume ratio, amplified reactivity in a chemical process, and improved mechanical strength, etc [44].
Fig. 4.
Physicochemical properties of nanoparticles [43] (Created with BioRender.com).
A nanoparticle can be either zero-dimensional, or one dimensional, or two dimensional, or even three dimensional. A zero-dimensional nanoparticle has fixed length, breadth, and height at a single point, nanodots. A one-dimensional nanoparticle can possess only one parameter, for example, graphene. A two-dimensional nanoparticle has its length and breadth, for example, carbon nanotubes. A three-dimensional nanoparticle has all the parameters like its length, breadth and height, for example, gold nanoparticles [45].
The nanoparticles can vary in shape, size and structure. They can be spherical, tubular, cylindrical, spiral, flat, conical, etc. or even irregular and differ from 1 nm to 100 nm in size. Few of the nanoparticles are crystalline or amorphous with single or multi-crystal solids either agglomerated or detached [46].
The physical properties comprise observable characters such as the color of the nanoparticle, its absorption, light penetration and reflective characters, and reflective characters in a solution or when coated onto a surface. The mechanical characters such as elasticity, tensile strengths, ductility and flexibility that play an important role are also included.
The chemical properties of nanoparticles include the responsiveness of the nanoparticles to the target, along with stability and sensitivity to factors including moisture, heat, light and atmosphere. The antiviral, antibacterial, antifungal, disinfectant properties of a few nanoparticles are exemplary for biomedical and therapeutic applications [47] Table 1 lists the features of many nanoparticles in detail [47], [48], [49], [50], [51], [52], [53], [54], [55], [56].
Table 1.
| Sl. No. | Nanoparticles | Properties | Ref. |
|---|---|---|---|
| Carbon-based nanoparticles | |||
| 1. | Graphene | Extreme strength, thermal, electrical conductivity, light absorption. | [48] |
| 2. | Carbon Nano Tubes |
High electrical and thermal conductivity, tensile strength, flexible and elastic. |
[49] |
| Metal-based nanoparticles | |||
| 1. | Iron | Reactive and unstable, sensitive to air (oxygen) and water. | [50] |
| 2. | Silver | Absorbs and scatters light, stable, anti-bacterial, disinfectant. | [51] |
| 3. | Gold | Interactive with visible light, reactive. | [52] |
| 4. | Copper | Ductile, very high thermal and electrical conductivity, highly flammable solids. | [53] |
| 5. | Zinc | Antibacterial, anti-corrosive, antifungal, UV filtering. | [54] |
| Metal-oxide based nanoparticles | |||
| 1. | Titanium oxide | High surface area, magnetic, inhibits bacterial growth. | [55] |
| 2. | Iron oxide | Reactive and unstable. | [56] |
| 3. | Zinc oxide | Antibacterial, anti-corrosive, antifungal and UV filtering. | [54] |
5. Biodegradability of the nanoparticle formulas in various disease treatment
To improve intracellular medication and gene delivery to numerous tissues, biodegradable nanoparticles are used as carriers for pharmaceuticals [57], [58], [59], [60], [61]. By enhancing bioavailability, dissolvability, and retention duration, biodegradable nanoparticles could be used to improve the theoretical value of H2O and/or insoluble solvent and bioactive particles [61]. Until recently, biodegradable nanoparticles were employed to treat a number of diseases. Table 2 summarizes the full list [61], [62], [63], [64], [65], [66], [67], [68].
Table 2.
Types of biodegradable nanoparticles and their effectivity [61], [62], [63], [64], [65], [66], [67], [68].
| Sl. No. | Types of Biodegradable Nanoparticles | Effective Against | Ref. |
|---|---|---|---|
| 1 | Poly (ethylene glycol)- Nanoparticles (PEG-NP) |
Cancer Therapy | [62], [63] |
| 2 | Polymeric Nanoparticles | Peptide Delivery | [64] |
| 3 | Nano-and micro-particles containing captured or adsorbed antigen |
Gene Delivery | [64], [65] |
| 4 | PEG-coated Nanoparticles | Long-acting Delivery | [66] |
| 5 | Indinavir Nanosuspensions | Anti-HIV Treatment | [61], [68] |
6. Antiviral activity of nanoparticles against coronavirus infections
Since the outbreak of the COVID-19 numerous drugs have been examined to find suitable antiviral treatments. Following the rules of the National Health Commission, various antiviral drugs were tested, which included chloroquine phosphate, interferon, arbidol, ribavirin, lopinavir, and ritonavir. Losartan, an angiotensin receptor blocker was also examined [69]. These antiviral drugs which have undergone extensive examination aimed to fulfill two different requirements: first, inhibiting the viral replication cycle and second, to control the symptoms of the disease [70]. Among these drugs, chloroquine and hydroxychloroquine are polymerase inhibitors and obstructed glycosylation of host receptors, proteolytic processing and prevented the development of viral proteins by suppressing endosomal acidification [70]. Lopinavir and Ritonavir, a United States Food and Drug Administration authorized oral drug, showed significant effects on various coronaviruses as reported from in vitro studies by inhibiting 3-chymotrypsinlike protease [70]. Another significant drug examined was camostat mesylate, a serine protease inhibitor, which efficiently suppressed transmembrane protease serine 2 and was successful in inhibiting SARS-CoV-2 infection in human lung cells. Tocilizumab and Sarilumab were reported to bind with interleukin-6 (IL6) receptors and hindered the activation and signaling of interleukin-6 (IL-6). Ivermectin is another antiparasitic drug reported to incapacitate cell transport proteins which in turn obstructed their entry into the nucleus [70], [71]. Remdesivir (GS-5734), a monophosphate prodrug of parent adenosine analog, when metabolized, produces an active nucleoside triphosphate. In the case of COVID-19, remdesivir inhibits RNA dependent RNA polymerase. Remdesivir is a broad-spectrum antiviral drug with confirmed effects against RNA viruses, including Coronaviridae, Flaviviridae and Filoviridae [71], [72]. Though these drugs showed some positive effects against SARS-CoV-2, none was able to eradicate the virus. These drugs with their mechanism of action against SARS-CoV-2 are listed in Table 3 [70], [71], [72], [73], [74], [75].
Table 3.
| SL. No. | Name | Action |
|---|---|---|
| 1. | Chloroquine and Hydroxychloroquine | Obstructs glycosylation of host receptors and proteolytic processing. Inhibits endosomal acidification by ceasing the production of SARSCoV-2 viral proteins. |
| 2. | Lopinavir/ritonavir | Repression of 3-chymotrypsin-like protease in the SARS-CoV-2. |
| 3. | Nafamostat and Camostat | Inhibits transmembrane protease serine 2. Prevents host cell entry. |
| 4. | Famotidine | A papain-like protease which is encoded by the genome of SARS-CoV-2 is essential for the entry of SARS-CoV-2 into the hostcell, which is restricted by famotidine. |
| 5. | Ivermectin | Incapacitates cell transport proteins which in turn obstruct their entry into the nucleus. |
| 6. | Tocilizumab and Sarilumab | Binds with IL-6 receptor. Prevents IL-6 receptor activation. Inhibits IL-6signaling. |
| 7. | Remdesivir | Gives rise to an active nucleoside triphosphate. Inhibits RNA dependent RNA polymerase. |
Nanomaterials have been significantly used as antiviral agents or in the case of delivery platforms for antiviral compounds for many years [76], [77], [78]. Cho et al., invented a concoction consisting of titanium dioxide (TiO2) nanoparticles and silver colloids, which acted as a dispersion stabilizer and also exhibited potential antifungal, antibacterial, and antiviral actions [79], [80]. Reports on the antiviral tests showed that there was a 100-fold dilution at a rate of 99.99% or higher in the composition concentration on antiviral action against porcine epidemic diarrhea virus and transmissible gastroenteritis coronavirus. Ciejka et al., established a biopolymeric substance to tackle the NL63 human coronavirus, the substance would form nanospheres that had the potential to adsorb coronaviruses [81], [82]. Against a range of coronaviruses, it has been shown that nanomaterials can have antiviral actions, although a lot more emphasis must be placed on exploring antiviral nanomedicines against SARS-CoV, MERS-CoV, and SARS-CoV-2 [83]. Antiviral actions of the nanoparticles should potentially aim for the attachment, penetration, replication, and budding of viruses. The probable mechanisms by which nanoparticles act are deactivation of the virus, obstructing the attachment of viruses to the host cells, and thereby inhibiting viral replication. The working action of nanoparticles also depends on the type and form of nanoparticles used [84]. Nanoparticles can modify the structure of capsid proteins and in turn reduce virulence, which can be accredited to both chemical and physical means of reducing the active viral load [85]. A summary of the antiviral actions portrayed by various nanoparticles is listed in Table 4 [16].
Table 4.
Antiviral actions portrayed by various nanoparticles [16].
| SL. No. | Name of the Nanoparticle | Virus | Effects on Viruses |
|---|---|---|---|
| 1. | Angiotensin-Converting Enzyme-2 coated Quantum Dots |
SARS-CoV-2 | Halts SARS-CoV-2 entry into cells by inhibiting the attachment of the virus to the ACE2 protein, while also inhibiting activation of the accessory serine protease TMPRSS2. |
| 2. | Silver Sulfide (Ag2S) nanoclusters | SARS-CoV-2 | Blocks viral RNA synthesis and budding in Coronavirus |
| 3. | TGEV-conjugated colloidal gold | Transmissible gastroenteritis coronavirus | Found to elicit higher concentrations of IFN-γ and superior titers of neutralizing antibody in vaccinated animals |
| 4. | Gold nanoparticles | SARS-CoV | Induction of IgG response of SARS-CoV in BALB/c mice |
| 5. | Silver nanoparticles and silver nanowires | Transmissible gastroenteritis coronavirus | Capable of reducing the number of apoptotic cells elicited by TGEV |
| 6. | Zinc oxide nanoparticles | H1N1 Influenza Virus | Inhibit virus only after viral entry into host cells in case of H1N1 influenza virus |
| 7. | Graphene oxide nanoparticles | Herpes Simplex Virus | Attachment inhibition in case of Herpesvirus |
| 8. | Gold nanoparticles | Herpes Simplex Virus | Prevent viral attachment and penetration in case of Herpesvirus |
| 9. | Graphene oxide nanoparticles | Respiratory Syncytial Virus | Directly inactivate the virus and inhibit attachment in case of respiratory syncytial virus |
| 10. | Silver nanoparticles | Hepatitis B Virus | Interaction with double-stranded DNA/ binding with viral particles |
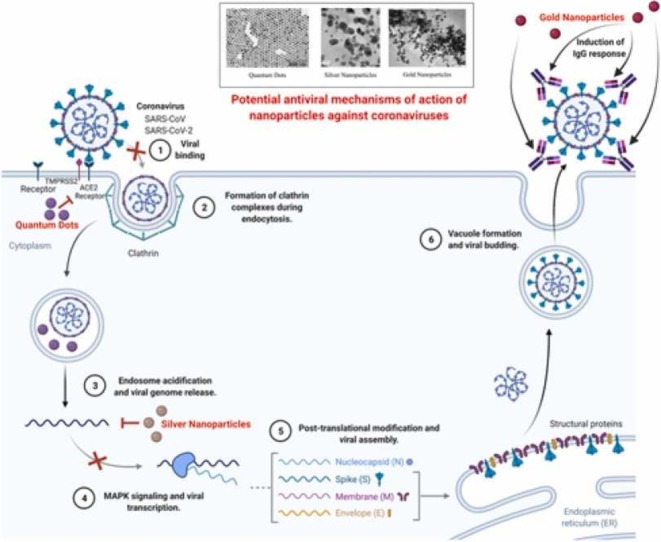
It is essential to discover safe and compatible antiviral agents to help stop the spread of viral infections because viral diseases pose a major threat to human health and the economy. Nanoparticles such as silver nanoparticles (AgNPs), gold nanoparticles (AuNPs), quantum dots (QDs), carbon dots (CDs), graphene oxide (GO), zinc oxide (ZnO) all possess extraordinary antimicrobial and antiviral actions [86]. Fig. 5 shows electron microscopic images of a couple of the nanoparticles with antibacterial and antiviral properties.
Fig. 5.
Electron microscopic images of nanoparticles: zinc oxide nanoparticles, gold nanoparticles, silver nanoparticles, selenium nanoparticles, graphene oxide nanoparticles, quantum dots, copper nanoparticles, iron oxide nanoparticles respectively.
6.1. Gold nanoparticles
Gold nanoparticles (AuNPs) serve an important role in the production of virus inhibitors and functional gold nanoparticles have been shown to be effective against the influenza virus, simplex herpes virus, and human immunodeficiency virus [87]. Bowman and his colleagues, found that multivalent gold nanoparticles can prevent the fusion of the human immunodeficiency virus by multiple molecular interactions. In addition, they also demonstrated that gold nanoparticles cannot prevent the fusion of human immunodeficiency virus alone, but when bound to other covalent molecules, gold nanoparticles can rapidly increase the antiviral effects by undergoing multivalent interactions [88]. In another study, Javier et al. produced an array of dendronized anionic gold nanoparticles and demonstrated that Human Immunodeficiency Virus could be suppressed more efficiently than dendrons alone. The results of this study further proved that the accumulation of gold nanoparticles can be inhibited by the presence of excess sulfur atoms within the dendrons, along with the coordination of the S(CH2)3SO3 moiety, whereas modified gold nanoparticles are considered to be more effective against HIV [89]. According to Papp et al. gold nanoparticles that have been surface coated with sialic acid, can suppress influenza virus infections [90]. Moreover, gold nanoparticles, surface coated with sialic acid, inhibit the attachment of the influenza virus to the host cells, and in turn, lower the probability for the virus to develop drug resistance. The size of gold nanoparticles plays an important role in antiviral activities [91]. Papp et al. established that surface area standardized polysulfated gold nanoparticles of diameters equal to or larger than that of the virus could suppress the attachment of the virus to the host cell more effectively compared to the smaller particles [84], [90]. AuNPs also help in the detection of SARS and are mostly utilized in developing specific and rapid molecular detection methods, primarily through two kinds of assays: 1) pp1ab gene detection based colorimetric assays which include the capability of AuNPs to specially adsorb single-stranded DNA rather than double-stranded DNA and recognize the existence of target DNA. 2) Nucleocapsid protein gene detection based electrochemical assays [92]. Certain short single-stranded DNA fragments attach themselves to the surface of AuNPs, which in turn increases the particle colloidal stability and also the capability of the AuNPs to endure elevated salt concentrations in marginal levels without substantial cluster formation or color change.
After attachment of the short single-stranded DNA fragments, the target DNA precisely produces double-stranded DNA, which can then be effortlessly isolated, leaving the AuNPs to accumulate due to the presence of salt. The accumulation of the particles results in a change of color from red to blue, signifying the presence of the target SARS nucleic acids. This color change can easily be observed by the naked eye or accurately measured by ultraviolet-visible spectroscopy in association with the target DNA concentration [93], [94].
The greatest effective medical intervention is most likely the discovery of vaccines, which are used against various deadly infectious diseases by boosting one’s immunity [95]. Since nanoparticles have been observed to have various immunostimulatory potency, they have been used in the development of various types of vaccines including vaccines against various coronaviruses [96]. In 2011, it was observed by Staroverov et al. that gold nanoparticles stimulated a protective immune response against a type of coronavirus called TGEV in inoculated mice and rabbits [97].
TGEV related colloidal gold was observed to trigger higher concentrations of Interferon-gamma and improve concentrations of neutralizing antibodies in immunized animals. It was also observed that antigen-colloidal gold complex-based immunization amplified proliferation of T cells ten times higher than that observed in free antigen, and thus resulted in greater protective immunity against TGEV. In a different study, animals inoculated with similar gold particle-transmissible gastroenteritis virus antigen complexes showed elevated levels of IFN-γ, Interleukin 1 beta (IL-1β), and Interleukin 6 (IL-6) than those animals directly inoculated with antigen only [98]. Hence, gold nanoparticles associated with any kind of virus could be recognized as possible antiviral candidates for the development of vaccines.
Gold nanoparticles have distinctive surface plasmons, recognized in wavelength-selective absorption. In a previous colorimetric assay, gold nanoparticles coated with a self-congregated double-layered DNA strand supplemented the open reading frame 1a on the MERS-CoV E protein [99]. In an akin assay, it was observed that gold nanoparticles were coated with SARS-CoV E protein with the help of a mediator goldbinding polypeptide [100]. Gold nanoparticles when associated with a green fluorescent protein, exhibited differences in color and absorbance after interacting with a corresponding antibody, resulting in quantitative detection of SARS-CoV [101]. In a biosensor, gold nanoparticles having 20 nm diameter were used for the recognition of SARS-CoV depending upon the variations in the fluorescence of a fluorophorelabelled with anti-SARS-CoV N protein secondary antibodies [102], [103].
Sulfonate ligands are mainly used to functionalize metallic nanoparticles because of their heparan sulfate proteoglycans site mimicking ability on the surface of the cell. Correspondingly, the binding of the virus to the surface of nanoparticles takes place and thereby inhibiting the infection in cells. According to a current study, AuNPs with extended links of sulfonate mercaptoethanesulfonate (MES) and undecanesulfonic acid (MUS) gave rise to irrevocable distortion in numerous virus types, including in vitro and in vivo studies of respiratory syncytial virus. After investigating the mechanism behind this, it was known that AuNPs functionalized with undecanesulfonic acid (MUS) leads to a multivalent binding to the virus, which in turn deforms the capsid structure. Thus, this multivalent binding could be a fascinating approach in inhibiting COVID-19 [104].
6.2. Silver nanoparticles
Silver nanoparticles are considered a significant chemical drug because of their exceptional physicochemical and chemical characteristics along with other biological characteristics, namely antiinflammatory, antiplatelet, antifungal, angiogenesis, anti-cancer, and antibacterial actions [105], [106], [107], [108]. Due to their high synthesis mechanism, silver nanoparticles receive a lot of attention in the medical as well as biological fields. Despite having reported therapeutic actions in wound dressing, antibacterial lotions, and long-term burn care, silver nanoparticles are still in the preliminary stages of development, having only a few reports on their viricidal actions [109], [110]. Sreekanth et al. established a basic green ultrasonication synthesis method for the formulation of pseudo spherical silver nanoparticles developed from aqueous extracts of Panax ginseng roots, and in turn, the resulting silver nanoparticles exhibited viricidal actions against influenza A virus [111].
Silver interacts with thiol groups, which are functional sites in the respiratory enzymes of bacterial cells. The attachment of silver on the cell wall and cell membrane takes place, and in turn inhibition of respiration occurs [112]. According to current observations the anti-bacterial actions of silver ions were mainly caused within the cell by its interaction within the cytoplasm. The silver ions penetrate via ion channels and no damage is caused to the cell membranes [113]. Denaturation of ribosomes occur, and it inhibits the expression of ATP-producing enzymes and proteins. The cells being unable to maintain membrane structures results in cell death due to these mechanisms. EFTEM, 2DE, and MALDITOF MS were used to determine the bactericidal properties of a silver ion solution on Escherichia coli. It was observed by EFTEM that the silver ion quickly infiltrates the interior of Escherichia coli rather than remaining in the cell membrane area. Furthermore, analysis using 2-DE and MALDI-TOF MS showed that silver ions impacted a ribosomal subunit protein, as well as certain enzymes and proteins. These findings suggest that one of the silver ion's key bactericidal mechanisms is due to its interaction with the ribosome, which results in reduced expression of enzymes and proteins required for ATP synthesis [113].
The release of carbohydrate compounds like succinate, mannitol, and amino acids like glutamine, proline, and phosphate from Escherichia coli cells along with the absorption of phosphate is inhibited in the presence of silver [113]. Silver nanoparticles have a huge surface area, so their antimicrobial efficiency is much higher, which allows them better interaction with the microorganisms. Adsorption of nanoparticles onto the cellular membrane takes place and these nanoparticles penetrate the membrane and enter the bacterial cell. The interaction between sulfur-containing proteins of the cell membrane along with the phosphorous comprising components such as DNA tends to interact with the silver nanoparticles. Silver nanoparticles on entry inside a bacterial cell, form a low molecular complex which in turn causes the bacteria to fuse. Thus, the DNA is shielded by the silver ions and inhibits replication. The respiratory chain is also affected by the silver nanoparticles resulting in cell death. Once the silver ions enter into the bacterial cell, their bactericidal actions take effect [114], [115], [116], [117].
Silver nanoparticles are one of the chief nanoparticles used in antiviral activity, they are highly active against viruses like HIV-1, Hepatitis B virus, Monkey Pox virus, Tacaribe virus, Influenza virus, Herpes Simplex virus, and Respiratory Syncytial virus [118], [119], [120], [121], [122], [123], [124]. Nanoparticles in the 1–100 nm size range are mainly used for the suppression of viruses [42]. The size of the nanoparticle plays an important role in the interaction between the viruses and the nanoparticles. The greater the size of the nanoparticles, the more the interaction and suppression of the viral genome takes place. The size-dependent phenomenon of nanoparticles is of great significance when they enter the cell and apply their antiviral activity against the viral genome [42].
Minute nanoparticles first enter inside the host cell, then enter inside the viral genome where the obstruction of replication of viral vectors takes place. In another instance, these minute nanoparticles get attached to the viral genome confirming that polymerase action does not take place and in turn obstructing the formation of further virion progenies. Capping agents like polymers, polysaccharides, and surfactants escalate the effectiveness of nanoparticles; capped silver nanoparticles are highly efficient when compared to bare nanoparticles [125].
More severe lung fibrosis and inflammation are the typical effects of COVID-19 on a patient. Cytokine storm causes the initiation of pulmonary as well as systemic inflammation by controlling the proinflammatory signaling which is also involved in lung fibrosis. Silver nanoparticles in this regard exhibit promising therapeutic effects, having potential anti-inflammatory and anti-fibrotic properties by virtue of their capability of abrogate the inflammatory cytokines by controlling their transcriptional action. A recent experiment exhibited that AgNPs showed anti-fibrotic properties in a dextran sodium sulfate induced colitis model. AgNPs reduced fibrosis and collagen accumulation by reducing the expression of profibrotic genes like Col 1a1 and Col 1a2 as evident from histological discoveries and confirmed by mRNA expression studies. Therefore, AgNPs may terminate the higher levels of cytokines and cytokine facilitated inflammation and fibrosis in COVID-19 [126].
6.3. Graphene oxide nanoparticles
Graphene oxide is known for its applications in biomedical fields and currently various studies are being conducted to demonstrate its antiviral properties. Song et al., observed that graphene oxide had an inhibitory effect on hand-foot-and-mouth disease (EV71) and endemic gastrointestinal avian influenza (H9N2) and can be nominated as a potential candidate for virus inhibition [127]. Both these viruses could also be inhibited by controlled temperature conditions. At a room temperature of 25 °C, graphene oxide showed a lesser inhibiting effect but as the temperature increased to 37 °C or much higher temperature like 56 °C, the inhibiting effect kept on increasing. It was also observed in this study that graphene oxide alone could destroy the virus without any kind of customization. The results illustrated that the virus fitted into the edge and basal plane of graphene oxide which suggested the mechanism of virus destruction depended on the physicochemical interactions of graphene oxide with envelope viruses and capsid. The resulting data also demonstrated that in the absence of graphene oxide, degeneration of EV71 was not observed, but treating the virus for 5 mins with graphene oxide destroyed the virus. This virus inhibition mechanism can further be applied to various antiviral fields without worrying about environmental interferences and the efficiency of antiviral activity of graphene oxide can be increased by combining it with other antiviral compounds producing a synergistic virus obstruction agent against the violent invasion of viruses [128].
Graphene oxide coated with curcumin has proven to have remarkable inhibitory actions against respiratory syncytial virus infection and greater biocompatibility within host cells. Experimental data further proved that curcumin coated graphene oxide could inhibit the respiratory syncytial virus infecting the host cells by direct inactivation of the virus or by preventing the virus and the host cells attachment and thereby disrupting the virus replication. This outstanding synergistic anti-viral agent was developed due to the combined effect of curcumin and graphene oxide, and it is a potential nanomedicine used for the therapy of respiratory syncytial virus infection. These versatile, and functional, graphene oxide antiviral agents bring a new perception in production of nanomedicines. Sametband et al., developed an effective graphene oxide nanomaterial to inhibit attachment of herpes simplex virus-I to the host cells [129]. They formulated a graphene oxide and partly reduced sulfonated composite (rGO-SO3), for inhibiting herpes simplex virus-I infections by an aggressive suppressing strategy. Additional experimental studies proved that nanocomposites are much more effective in lowering viral infection rates, obstructing the attachment of viruses to the host cells and most importantly the nanomaterials do not affect intercellular diffusion at all. Thus, these internal abilities of graphene oxide and its composites will help in further production of safer and much needed antiviral nanomedicines with less aftereffects [84], [130].
Ye et al. applied graphene oxide on PEDV which is an RNA virus, to investigate the antiviral effect of graphene oxide. Graphene oxide added prior to virus adsorption lowered the level of action of PEDV viral protein and the ability to form plaques as shown by plaque-forming assays and immunofluorescence assays. Graphene oxide shows antiviral effects against various ranges of RNA and DNA viruses. Moreover, the antiviral effects observed in this study were time and concentration-dependent, with the antiviral activity increasing with the increase in the incubation period and graphene oxide concentration. Even at a lower concentration of 1.5 μg/mL graphene oxide, substantial antiviral actions were demonstrated. Another experiment performed by Ye et al. revealed that only the pre-incubation of graphene oxide showed crucial inhibiting actions. However, in cell culture, graphene oxide was not able to obstruct virus replication spreading to surrounding cells. According to this report, graphene oxide disabled the virus before entering the cell [131].
Chen et al. revealed the antiviral action of nanocomposites of graphene oxide and silver (GOAg) against enveloped and non-enveloped viruses. Solutions with various dilution levels were incubated with respective diluted solutions of feline coronavirus to demonstrate the antiviral action of graphene oxide and silver nanocomposite. The composite particles were removed, and the supernatant was examined with a virus inhibition assay. The results showed that 0.1 mg/mL of graphene oxide and silver nanocomposite inhibited 24.8% infection of feline coronavirus and the efficiency of GO-Ag was much higher than that of graphene oxide alone. While preventing infection of viruses, negatively charged graphene oxide sheets interacted with positively charged lipid membranes and silver nanoparticles became attached to the sulfur groups of viral proteins. Therefore, it was shown that graphene oxide was able to suppress enveloped viruses at non-cytotoxic concentrations, whereas graphene oxide sheets having silver nanoparticles were able to disrupt the infection triggered by both enveloped and non-enveloped viruses [131].
Against human coronavirus NL63, Ciejka et al. produced a bio-polymeric material to develop nanospheres or microspheres having potential to inhibit coronaviruses [82], [83]. The antiviral properties of graphene combined with antibacterial actions of other silver and titanium oxide nanoparticles can be utilized to create graphene composites comprising nanoparticles with antiviral properties to inhibit and exterminate the SARS-CoV-2 viruses [132]. In another study, with the help of graphene the detection of SARS-CoV-2 in clinical samples was trialed by creating a sensor where graphene sheets of a field-effect transistor were coated with other specific antibodies against the spike protein of SARS-CoV-2. In this study it was possible to identify SARS-CoV-2 spike protein at a concentration of 1 fg/mL in phosphate-buffered saline and 100 fg/mL clinical transport medium [133].
6.4. Zinc oxide nanoparticles
Zinc is one of the most crucial metals in biological systems due to its utility as a coenzyme, fundamental element and signaling molecule [134]. One of the most vital roles of zinc is boosting the body’s immunity. Zinc synchronizes the development, separation, multiplication, and functioning of lymphocytes and leukocytes [135]. Signaling involved in the regulation of inflammatory responses is also performed by zinc [136]. It also provides nutritional immunity [137]. Similarly, changes in zinc concentration can significantly affect the immune response in the body, resulting in vulnerability to infectious and inflammatory diseases, including pneumonia, malaria, tuberculosis, acquired immune deficiency syndrome [138]. As zinc supplementation has been successfully used against diseases such as diabetes and cardiovascular diseases, due to its immunological properties, it has been proposed that it can also be used as a treatment for COVID-19 by increasing antiviral resistance [139], [140]. Reportedly, zinc was also used in the case of H1N1 influenza (Swine Flu) as a possible agent to boost immunity against the virus [141].
During the COVID-19 pandemic, zinc has been considered as a potential supportive treatment therapy for COVID-19 infection due to its antiviral effect as well as immunity enhancing effect [142]. Specifically, a potential decrease in the replication process was seen in the case of SARS-Coronavirus RNA polymerase (RNA-dependent RNA polymerase) by the Zn2+ cations particularly in combination with Zinc ionophore pyrithione [143]. Past reports suggested that chloroquine is a zinc ionophore that increased the Zn2+ flow into the cell [144]. It was also theorized that an increase in the intracellular Zn2+ concentrations by chloroquine can result in an increased antiviral effect against SARS-CoV-2. Since chloroquine treatment has shown adverse side effects in the past, the use of zinc without chloroquine might have an equally positive effect [145]. Theoretically, zinc ionophores like epigallocatechin-gallate and quercetin, with substantially lower toxicity levels, can also be used as an alternative, though clinical trials supported by in vitro studies must be performed to support this theory [146]. In the case of COVID-19, Zn2+ ions can be specifically targeted in the configuration of the viral proteins. For example, papain-like protease in MERS-CoV and SARS-CoV are responsible for the discharge of disulfiram-instigated Zn2+ in the cell resulting in protein deterioration [147]. Zn-releasing drugs like disulfiram can be considered as potential antiviral agents, and thus can also be used in the treatment for SARS-CoV-2. For entering into the target cell, SARS-CoV-2 requires ACE-2 just like SARS-CoV, regulation of the ACE-2 receptor is considered as a potential therapeutic treatment for COVID-19 [148]. Speth et al. validated that exposure to zinc (100 µM) showed decreased action of recombinant human ACE-2 in rat lungs. Commonly, zinc is considered to play a vital role in the treatment of respiratory infections due to its antiinflammatory and antioxidant action [149], [150].
Through adjustment in viral particle entry, replication, fusion, viral protein translation and secretion for a lot of viruses, including those involved in respiratory system pathology, zinc has shown a significant impact on viral infections [151], [152]. The use of zinc ionophores such as pyrithione and hinokitiol to increase the intracellular Zn concentrations can considerably change the replication rates of picornavirus, which is considered to be the leading cause of common cold [153]. Zinc was previously used against rhinovirus for stopping its replication process in the early 1970 s [154]. Moreover, treatment with zinc has shown the increased expression of interferon α by leukocytes and in turn, making it a potential antiviral agent against rhinovirus-infected cells [155], [156], [157].
Zinc oxide nanoparticles are considered to be one of the most essential metal nanoparticles as they exhibit substantial anti-microbial actions against numerous kinds of microorganisms, including viruses. The antiviral action exhibited by zinc oxide micro-nano structures in virus-infected corneal tissues were assessed, and it was observed that negatively charged zinc oxide micro-nano structures proficiently trap the virions through a novel virostatic medium restricting their entry into the human corneal fibroblasts. Zinc oxide nanoparticles that have been surface-modified could alter the infection potential of herpes simplex virus-I by neutralizing the virus compared to electrostatic interference of hydrophobic zinc nanoparticles with the virus [158]. Ghaffari et al. established the antiviral action of zinc oxide nanoparticles and PEGylated zinc oxide nanoparticles against the H1N1 influenza virus [159]. The results exhibited that postexposure of influenza virus with PEGylated zinc oxide nanoparticles and zinc oxide nanoparticles alone, at the maximum non-toxic concentrations, could lead to 2.8 and 1.2 log10 TCID50 decreases in virus titer when compared to unPEGylated zinc oxide nanoparticles. Zn2+ ions significantly suppressed replication of Nidovirus, and increased concentrations of intracellular Zn2+ ions could effectively disrupt the replication of a range of RNA viruses [85], [160].
6.5. Quantum dots
Traditional quantum dots possessing antiviral properties are still unidentified, so various groups have performed several types of research on this topic [84]. It has been shown that glutathione capped cadmium telluride quantum dots have antiviral effects on the pseudorabies virus [161]. Cadmium telluride quantum dots were seen to change the viral surface protein structure and prevent the pseudorabies virus from getting into the host cells. Cd2+ released from cadmium telluride quantum dots also reduced the number of viruses infecting the host cells. Important anti-pseudorabies virus effects were found for the surface charge and size of quantum dots where the positively charged quantum dots had higher inhibitory efficacy than negatively charged quantum dots [84]. With a different mechanism from other functional nanoparticles, the antiviral effects of less toxic quantum dots such as silver sulfide nanocrystals have potentially excellent viral inhibitory ability [162]. It was found that carbon dots have significant viricidal potential which are closely linked to their carbon precursors and synthesis conditions, which suggest the need for systematic investigation of various antiviral functions of carbon dots. Some experiments have shown that carbon dots are capable of inhibition of pseudorabies virus and porcine reproductive and respiratory syndrome virus multiplication. Blue and cyan, fluorescent carbon dots were shown to be capable of inhibiting pseudorabies virus and its antiviral mechanisms were explained through RNA exaction and real-time polymerase chain reaction assays. Carbon dots have a major effect on the intrinsic immune response of the host cells by inducing them to produce more interferons, which play an important role in protection against viruses [84]. Carbon dots can also improve the expression of several interferon-stimulated genes which are an important part of the antiviral mechanism based on carbon dots [163]. Benzoxazine monomer-derived carbon dots were created which are capable of blocking infections like flaviviruses and non-enveloped viruses, like porcine parvovirus and adenoviruses [164]. The surface-functionalized carbon nanodots (C-dots) with boronic acid or amine can be used to combat herpes simplex virus type 1 by hindering the entry of the virus into the host cells [165]. A one-step method was discovered for preparing uniform and stable cationic carbon dots (CCM-CDs) with antiviral properties by using curcumin [166]. By using the porcine epidemic diarrhea virus as a coronavirus model, the inhibitory efficiency of CCM-CDs on viral replication was examined and it was found that CCM-CDs can inhibit the proliferation of porcine epidemic diarrhea virus with greater efficacy than carbon dots modified by non-curcumin. Glycyrrhizic acid which is an essential ingredient in Chinese herbal medicine, helped to develop a new kind of highly bio-compatible quantum dot, glycyrrhizic acid-based carbon dots, which can prevent porcine reproductive and respiratory syndrome virus proliferation viral with titerss of up to 5 orders of magnitude [167]. Through several other investigations, it was found that glycyrrhizic-acid based carbon dots are capable of inhibiting invasion and replication of porcine reproductive and respiratory syndrome virus, inducing antiviral innate immune responses and suppressing intracellular reactive oxygen species accumulation caused by the infection of porcine reproductive and respiratory syndrome virus. High antiviral capacity against pseudorabies virus and porcine epidemic diarrhea virus was observed in glycyrrhizic-acid based carbon dots. All these findings led to the use of carbon dots in modified Chinese medicines for high bacterial activity [84]. Viral infectious diseases like the human immunodeficiency virus, Ebola virus, and SARS-CoV can be extended to one of the functions of nanomaterials [168]. Quantum dots with a size range between 60 and 140 nm can be manufactured to the required size (1–10 nm) and shape that can efficiently penetrate SARS-CoV-2 [169]. The S protein of SARS-CoV-2 could be sequestered by using the positive surface charge of carbon-based quantum dots [170]. In addition, cationic surface charges exhibited by quantum dots interfere with the virus' negative RNA strand, leading to the development of reactive oxygen species within SARS-CoV-2 [84], [171]. Activation of interferon-stimulated genes can be induced by carbon dots that inhibit viral replication, especially interferon-alpha development [172]. Also, the inclusion of preferred functional quantum dots helps in interacting effectively with the SARS-CoV-2 entry receptors and the genomic replication can be affected [168]. It was found that the functional group of carbon dots interact with human coronavirus-229E's S protein, thus prohibiting the virus from interaction with the host cell membrane and prevents the virus from entering into the host cells. More detailed experiments and optimization of quantum dots for approaching SARS-CoV-2, through new functional molecules, will lead to the discovery of nanostructures for COVID-19 therapeutics in the near future for defeating this deadly disease [173].
Currently, for the treatment of human coronavirus HCoV-229E infection, various carbon dots were examined. These nanoscale materials are derived from modified boronic acid ligands and ethylenediamine or citric acid. Carbon nanoparticles were successful in inhibiting and deactivating HCoV-229E entry in a concentration-dependent manner. The interaction of the functional groups of these carbon quantum dots and HCoV-229E entry receptors led to the inhibition of replication of the virus [174].
6.6. Other nanoparticles
Titanium, zinc, and copper, as well as metal oxide nanoparticles such as iron oxide, zinc oxide, and titanium dioxide have also exhibited distinct antiviral effects [175]. Recently, Cagno et al. established the antiviral activities of broad-spectrum, non-toxic nanoparticles against Human papilloma virus, Respiratory syncytial virus, Dengue and Lenti virus. The authors showed that a sequence of antiviral nanoparticles with extended and flexible linkers, imitating heparin sulfate proteoglycans, greatly preserved target of viral attachment ligands and could accomplish effective viral inhibition [176]. Nanoparticles consisting of a simple spherical core particle, known as core-shell nanoparticle is entirely covered by a shell of a different material. This material can be monometallic or bimetallic in nature. Many of these core-shell nanoparticles have exhibited biomedical applications [175]. Some of these nanoparticles and limitations of nanoparticles for nanotechnology-based therapeutics have been described in the following sections.
6.6.1. Copper nanoparticles
In response to a viral infection, Cu can trigger autophagy. The cells can withstand external stress of the virus through this mechanism. It has been determined that the presence of Cu in the host's body causes induction of this mechanism and forms autophagic vacuoles, as well as apoptosis. For therapeutic use, the toxicity of copper to humans was observed to be very low as a consequence of homeostatic mechanisms which inhibited excessive Cu intake. However, Cu supplements when taken in excessive quantities within a short amount of time (more than 10 mg/ day) can cause terrible side effects [177]. A diet of less fiber, ascorbic acid, divalent metals, iron, fructose, zinc and cysteine with high protein intake and polybasic amino acids has been advised for optimum Cu absorption. Thus Cu, which previously showed specific results against viruses, can be considered as a major potential agent acting against SARS-CoV-2. Cu taken as dietary supplements can provide stronger immunity towards COVID-19 [178].
Cu is thus an important emerging contender and a possible therapeutic agent as a result of these findings [179]. Compared to other regularly used materials such as stainless steel and plastic, Cu has been recognized as a material that inactivates the virus in a shorter period of time [38]. A previous analysis looking into the CoV-229E strain backed up this study. Brass with a minimum of 70% Cu was found to be more effective against CoV-229E in this analysis, and the efficacy rose as the percentage of Cu increased [180], [181].
6.6.2. Iron oxide nanoparticles
The magnetic features that iron oxide nanoparticles possess are connected to small nanoparticles having superparamagnetic characteristics which can be seen as a reaction to any other foreign magnetic field. This effect is dependent on modifications to the surface with other molecules or sulfonates. Bromberg et al. performed a study where they synthesized core-shell silica magnetic nanoparticles functionalized with poly hexamethylene biguanide. They concluded that these nanoparticles showed prominent virucidal effects on various strains of viruses, mainly because of amino alkylation of their genomic materials and thereby blocking genome replication and bringing about the inactivation of the virus. Due to iron homeostasis dysregulation in COVID-19, various studies are ongoing using iron oxide nanoparticles [182], [183], [184].
6.6.3. Selenium nanoparticles
Ebselen is an organic selenium species which by binding covalently to the COVID-19 virion Mpro via cell membranes has been seen to be able to inhibit COVID-19 [185]. Ebselen has been proven to be safe in humans and is also less cytotoxic in nature. The most suitable concentrations for Ebselen to inhibit COVID19 infected Vero cells is 10 μM. However, serious liver damage was also recorded in cases of COVID-19. Ebselen on the contrary was found to be able to prevent liver damage caused by a wide range of substances, immune systems, and microorganisms [186]. The toxicity of Selenium at high doses is a key problem when utilizing it to fight various viruses. Nano selenium has been suggested as a treatment for several diseases like Cancer and Huntington's disease as nano selenium has been discovered to be less toxic in nature than other selenium compounds [187], [188], [189].
6.6.4. Synergistic effects of nanoparticles
In his study, Bankier et al., analysed the effects of single elemental nanoparticles - silver, copper and tungsten carbide on Gram-positive (Staphylococcus aureus) and Gram-negative (Pseudomonas aeruginosa) bacteria. For this study silver and copper nanoparticles were selected, since both nanoparticles are regular antibacterial agents. Even though the antiviral activities of tungsten carbide were reported earlier, it was also selected for this study because its antibacterial properties were unknown [190] . Along with this, to identify the strongest antibacterial effect, the combination of nanoparticles of same elements in varying ratios were also examined on the same bacterial species at different concentrations such as 0.05, 0.10 and 0.25 w/v %. The action of single elemental nanoparticles and combination of nanoparticles on these species were judged by determining the quantity of live and dead cells after 24 h interaction of nanoparticles with bacteria. With the help of flow cytometry, quantification of bacterial cells was conducted and the synergistic effects of a combination of nanoparticles were compared to single elemental nanoparticles. Accordingly, potent antibacterial effects on Pseudomonas aeruginosa were observed with no antibacterial effects on Staphylococcus aureus [191].
Tungsten carbide nanoparticles did not exhibit any effect on Pseudomonas aeruginosa and Staphylococcus aureus. However, copper single elemental nanoparticles had antimicrobial effects on Pseudomonas aeruginosa and minor toxicity against Staphylococcus aureus. Antimicrobial effects of Silver (at all concentrations) and Copper (at 0.05 and 0.10 w/v%) single element nanoparticles were less prominent against Staphylococcus aureus. Robust antibacterial action was demonstrated by the Silver single element nanoparticles utilized in this experiment. This research exhibited that when a minimum dose of 0.05 w/v% was utilized against Pseudomonas aeruginosa, extermination of 99% of the bacterial population occurred [192].
6.6.5. Toxicity
Decrease in the toxicity of the incorporated drug may be observed if nanoparticles are used as drug carriers [193], although the source of the toxicity caused cannot be differentiated between the nanoparticles or the drug. Gold nanoparticles have a unique structure and set of properties which make them beneficial in a wide range of biological applications. However, at higher concentrations, using these systems have exhibited toxicity. Goodman et al. established that for 2 nm gold particles, the cationic particles were discreetly toxic, while the anionic particles were comparatively non-toxic [194].
For thiol derivatized polyethylene glycol (PEG) - colloidal gold nanoparticles with tumor necrosis factor (TNF) an improved anti-tumor action was observed when compared to free TNF [195]. For the uptake of nanoparticles, phagocytosis by macrophages does not appear to be essential. While dealing with nanoparticles, the immune system is not completely inactive. For 100 nm polystyrene particles, an IgE adjuvant action was detected in an animal model of ovalbumin allergy [196], [197]. After the intraperitoneal injection of C60 conjugated with serum proteins, antibodies against fullerenes could be induced [198].
7. Nano-systems against new strains of SARS-CoV-2
The latest variants of the SARS-CoV-2 give rise to a major threat to the speedy international recovery from COVID-19 infection. The newer strains of SARS-CoV-2 is capable of reproduction, mutation and can spread much rapidly, and thereby suggesting these are going to be present always. The latest variants of SARS-CoV-2 from Brazil, the United Kingdom and South Africa, along with the double variants found in India were observed to escalate rapidly and in certain regions even be much life-threatening compared to the primary strains [199].
Performance and appearance of nanosystems are considered to be vital components, these characteristics comprise anticipated functionality to interact with bioactives, the surface of preferred porosity, antiviral and antimicrobial action, and tunable optical, magnetic, electrical, and molecular characters [200], [201], [202], [203], [204].
Since improved and effective surface sterilization has been validated using nanomaterials with tunable antiviral characters, it is apparent that the application of nanotechnology to combat newer SARS-CoV-2 variants may rise. The COVID-19 infection primarily transmits through aerosol molecules but have been noted to be present on various surfaces for long hours still being able to infect individuals. Considering airborne transmission as a vital concern, the trapping and neutralization of viral droplets or particulates and aerosol in indoor air using effective technology become very essential. Air purifiers are emerging as an alternative technological solution. For example, the air purifier of Molekule Inc TM sanitizes indoor air based on a photochemical oxidation procedure where the function of a photosensitive nanoparticle is immensely pivotal. FDA has approved this technology which effectively eliminates viruses, bacteria, allergens, volatile organic compounds, and particulates from indoor air [202].
New methods of surface sanitization have been developed as a potential method to eliminate SARSCoV-2. Among nanosystems such as copper, silver, and a few others, have potential uses as antiviral surface coatings which in turn can be used as antivirals [202], [205]. Titanium dioxide nanoparticle-based photocatalysts are able to eliminate the virus. The photocatalytic features of TiO2 have been detected to achieve improved results in antiviral/antimicrobial settings with a larger surface area [206].
For the inhibition and treatment of viral diseases, personalized nanomedicine is being established and is greatly emerging as the future therapy. Nanomedicine has exhibited a high percentage of acceptability and performance, and thus a targeted viral infection can be treated and managed proficiently with it. The site-specific delivery of an antiviral cargo of high efficacy improving health and enabling real-time evaluation is the main reason why nanomedicines are emerging so well [207], [208], [209], [210].
A multi-drug loaded, time-release nanotechnology method to identify the various biomarkers of SARS-CoV-2 and the suitable resources to deliver as an antiviral treatment to the affected area can be employed to treat the symptoms associated with COVID-19. This multifaceted method has shown feasibility in the treatment of HIV and in penetrating the blood-brain barrier without adverse effects [207], [208]. With various lethal variants of the SARS-CoV-2 emerging frequently, the research and development of this technology to treat this virus and its long-term effects is the most promising method in antiviral care [211].
8. Controlled nanoparticle composite subjects
Xu et al. developed an advanced electrostatically mustered silica nano-capsules containing layer-by-layer film, where a distinct structure with decent stability, high loading capability and prolonged drug release profile at physiological circumstances were observed. The SNC/CS films further demonstrated sturdy and reversible swelling characters with the alteration in pH levels caused by protonation and de-protonation. The films were capable in encapsulating huge amount of fulvestrant, a homophobic anti-tumor drug found within oil cores of the mustered SNCs and assisted on-demand or fluctuated pH-controlled drug release profiles. These capabilities of layer-by-layer films provides evidence of the application of these films for future efficient and sustained-release drug delivery arrangements [212] (DOI: 10.1021/acsabm.9b00381).
Xu et al. used BCM and HA biopolymers to develop hydrogen-bonded layer-by-layer films with controlled assembly, high constancy and decent drug loading capability. BCM/HA films established repeatable swelling/de-swelling behaviors in response to the alteration in environmental temperature, which was brought about by the protonation and de-protonation of PNIPAM component in the films. The films could efficiently load anti-tumor drug Osimertinib in the hydrophobic cores of the BCMs in the films. The layer-by-layer films could be possibly used for programmable release of therapeutic compounds in medical devices in upcoming times [213].
In another study, the first proof-of-concept platform of temperature-responsive SNC-incorporated layer by layer films with a distinct inner structure and a continuous release profile for on-demand in vitro drug delivery was established by Xu et al. Using surface-initiated atom-transfer radical polymerization, temperature-responsive block polymers were functionalized on the exterior of silica nanocapsules (SNCs) by a “grafting-from” method. Favipiravir, a potent drug candidate for the treatment of COVID-19, was condensed in polymer-coated SNCs and later combined into distinct films by layer-bylayer self-assembly. SNCs with coronae of higher steric interference caused in a greater layering distance during film development. Moreover, the variance in the continued release rates of the drug specified their varied diffusion coefficients and intermolecular interactions within the multilayer films, due to the presence of a methyl spacer at the amino group of nano-capsule coronae and frailer ionic pairing between SNC coronae and PMAA homopolymers. The profile of drug release from the films was reliant on the temperature value of the neighboring environment. At 37 °C and 40 °C, the films were able to efficiently entrap favipiravir, with as low as 50% released in 80 days, while a quicker favipiravir release was activated by exposure to a lower temperature value at 25 °C [214].
9. Limitations of nanoparticles for nanotechnology-based therapeutics
Mammals, particularly humans are exposed to nanoparticles which are present in different products including medications. They can penetrate into the body by various ways including inhalation, oral and transcutaneous routes. The size of nanoparticles allows its uptake and transport into the cells by both endocytosis and transcytosis [215]. Cu supplements when taken in excessive quantities within a short amount of time (more than 10 mg/ day) can cause dramatic side effects [181]. Previous reports have suggested that relations between SARS-CoV-2 and hemoglobin occur in the erythrocyte precursors, resulting in hemoglobin denaturation and iron breakdown dysregulation. These iron level variations can decrease the use of these nanoparticles in clinical applications as they might cause side effects [183]. High concentrations of gold nanoparticles decrease body weight, hematocrit, red blood cells and spleen index [216].
Although nanomedicines show a broad spectrum of applications and have drawn interest from various scientific communities around the world, a barrier still exists between the excellent technological developments and the effective commercialization of nanotechnology-based treatments. Until recently, commercialization of nanotechnology-based treatments was run by start-up and small to medium scale companies. Large pharmaceutical companies still show less interest in investing in the emerging nanotechnology-based treatments. Small companies undergo difficulties to find investors to support the development of these new medicines. Furthermore, enterprises manufacturing nanomedical products are prone to considerably higher per unit costs [217].
9.1. Cost–effectiveness of nanoparticle based medicine
The use of specific decision-making frameworks can significantly increase the success rate of bringing high-priced nanotherapeutics to patients, and thus market uptake. The usage of profound, standard cost-effective evaluations results in the creation of value for money in healthcare market as it takes the focus away towards reducing healthcare costs while preserving quality of care. Through integrating and repaying cost-effective interventions, or therapies or pharmaceuticals with the lowest cost per health effect the society gains value for money [218].
In nanomedicine research, one major flaw of current cost-effectiveness is that almost all studies continue to account just for direct treatment costs, completely ignoring secondly costs. A complete cost taxonomy is critically needed in the nanomedicine sector to demonstrate that 'expensive' nanodrugs of upcoming generations are also cost effective or even saving costs for society. Furthermore, in the field of nanomedicine the hospital standpoint continues to shape cost assessment. Indirect costs are never evaluated, as a result of this framework, lower production and transportation costs from less treatment-related adverse outcomes and long-term complications can result in significant savings thus condemning more effective nanodrugs. Because survival can be improved, more impactful nanomedicines can result in relatively reduced foregone interests due to early death. Furthermore, because patients may spend significantly fewer days in the hospital, indirect patient visiting costs for family and friends may be significantly reduced. As a result of fewer treatment-related adverse outcomes and long-term complications of the disease direct costs for added therapies and drugs may also be reduced. The indirect, undefinable cost in pain and suffering, or the 'human loss,' could be significantly reduced (though this cost should preferably be included in estimates of quality of life). The wide range of cost definitions makes cross-study comparisons difficult which is the final source of concern. An updated cost model with costs ideally judged from a societal perspective, combining direct and indirect costs irrespective of who bears them is urgently needed. Even with the significantly increased number of studies on cost-effectiveness of nanomedicine, the cost-effectiveness research of nanomedicines is still in its initial stages. Homogenous, comprehensive cost-effectiveness is one of the major missing links between the advancement of exceptional new drugs and the well-organized provision of scarce monetary resources. Once accepted by major investors, the administrative model could be used for policy decision-making [218].
Nanomedicine may perhaps contribute drastically to affordable care, but the first step being assessment by efficient cost–effectiveness studies. Only then will ‘unattractive’ nanodrugs (those with significantly higher acquisition costs) disclose their potential dominance as cost-effective for the society. In particular, large direct and indirect investments can be realized by the administration of operative nanodrugs. Without this, ‘expensive’ nanomedicines which have the accurate set of characteristics to seek reimbursement will have only minute possibility of reaching the patient, downgraded instead to a valley of ‘forgotten’ capable therapeutics.
10. Future perspectives
Mechanism of infiltration by coronaviruses can be helpful information used to design nanoparticles and decipher the route of administration of the nanoparticle to effectively target coronavirus infections in humans.
11. Conclusion
Every year, infectious diseases lead to a huge number of deaths. Evolution has led to the development of various new diseases, some bacterial and many viral. Respiratory infection caused by numerous viruses is one of the major reasons for the increased worldwide mortality rate. Recently, the emergence of COVID-19 caused by the SARS-CoV-2 has resulted in over 4 million deaths as of 13th July 2021. COVID-19 has crippled the normal lives of people all around the world, both socially and economically. Due to the lack of proper medical facilities in developing countries, it has been very hard for nations to bring the situation under control. Nanotechnology has proven to be of great significance and has exhibited great potential in the fields of antiviral activity and therapeutic treatments. The field of nanotechnology is one of the most active areas of research in contemporary materials science. Nanoparticles have completely new or improved properties that are based on specific characteristics like size, shape, crystalline structure, and morphology. Nanoparticles in crystalline form have found marvelous applications in the field of high sensitivity bio-molecular detection, disease and chemical diagnostics, antimicrobial, and therapeutic compounds. Nanotechnology has exhibited prophylactic and therapeutic activity against a range of viruses. Nanoparticles might truly change the way people think and operate; a nanoparticle vaccine could change the world for the better. In response to orthodox antigen-based vaccines, nanoparticle-based vaccines have a significantly superior potential to induce advanced protective immunity responses. Additionally, reports have exhibited that nano-assays play a significant role in furnishing higher sensitivity and specificity, compared to present methods when used for rapid detection of viral infection at initial stages. Along with various other vaccines, namely Pfizer BioNTech, AstraZeneca-University of Oxford, Moderna and Janssen Sputnik vaccine, which are being administered worldwide, many studies are still ongoing for the development of a working nanoparticle-based vaccine directed at SARS-CoV-2.
CRediT authorship contribution statement
Joy Sarkar: Conceptualization, Writing – original draft, Writing – review & editing, Supervision. Sunandana Das: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft. Sahasrabdi Aich: Conceptualization, Formal analysis, Investigation, Writing – original draft. Prithu Bhattacharyya: Conceptualization, Methodology, Writing – original draft. Krishnendu Acharya: Writing – review & editing, Supervision.
Conflict of interest
On behalf of all listed authors, the corresponding author declares that there is not any sort of financial and non-financial conflict of interest in the subject materials mentioned in this manuscript.
Acknowledgments
We do not have any funding support from any organizational or institutional level. The authors acknowledge the invaluable assistance provided by the scholars whose articles are cited and listed in the manuscript's references. The authors are also grateful to the authors/editors/publishers of all the articles, journals, and books that were used to review and discuss the literature for this article.
References
- 1.Gerba C.P., Betancourt W.Q. Viral aggregation: impact on virus behavior in the environment. Environ. Sci. Technol. 2017;51:7318–7325. doi: 10.1021/acs.est.6b05835. [DOI] [PubMed] [Google Scholar]
- 2.Rivera A., Messaoudi I. Pathophysiology of ebola virus infection: current challenges and future hopes. ACS Infect. Dis. 2015;1:186–197. doi: 10.1021/id5000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colpitts C.C., Verrier E.R., Baumert T.F. Targeting viral entry for treatment of hepatitis B and C virus infections. ACS Infect. Dis. 2015;1:420–427. doi: 10.1021/acsinfecdis.5b00039. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhry R.M., Nelson K.L., Drewes J.E. Mechanisms of pathogenic virus removal in a full-scale membrane bioreactor. Environ. Sci. Technol. 2015;49:2815–2822. doi: 10.1021/es505332n. [DOI] [PubMed] [Google Scholar]
- 5.Nitsche C., Holloway S., Schirmeister T., Klein C.D. Biochemistry and medicinal chemistry of the dengue virus protease. Chem. Rev. 2014;114:11348–11381. doi: 10.1021/cr500233q. [DOI] [PubMed] [Google Scholar]
- 6.Jahn W. Review: chemical aspects of the use of gold clusters in structural biology. J. Struct. Biol. 1999;127:106–112. doi: 10.1006/jsbi.1999.4123. [DOI] [PubMed] [Google Scholar]
- 7.Caygill R.L., Blair G.E., Millner P.A. A review on viral biosensors to detect human pathogens. Anal. Chim. Acta. 2010;681:8–15. doi: 10.1016/j.aca.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 8.Chen L., Zhang X., Zhou G., Xiang X., Ji X., Zheng Z., He Z., Wang H. Simultaneous determination of human enterovirus 71 and coxsackievirus B3 by dual-color quantum dots and homogeneous immunoassay. Anal. Chem. 2012;84:3200–3207. doi: 10.1021/ac203172x. [DOI] [PubMed] [Google Scholar]
- 9.Daaboul G.G., Yurt A., Zhang X., Hwang G.M., Goldberg B.B., Ünlü M.S. Highthroughput detection and sizing of individual low-index nanoparticles and viruses for pathogen identification. Nano Lett. 2010;10:4727–4731. doi: 10.1021/nl103210p. [DOI] [PubMed] [Google Scholar]
- 10.Luo K., Jung S., Park K.-H., Kim Y.-R. Microbial biosynthesis of silver nanoparticles in different culture media. J. Agric. Food Chem. 2018;66:957–962. doi: 10.1021/acs.jafc.7b05092. [DOI] [PubMed] [Google Scholar]
- 11.Lee S.Y., Krishnamurthy S., Cho C.-W., Yun Y.-S. Biosynthesis of gold nanoparticles using ocimum sanctum extracts by solvents with different polarity. ACS Sustain. Chem. Eng. 2016;4:2651–2659. doi: 10.1021/acssuschemeng.6b00161. [DOI] [Google Scholar]
- 12.Zhang Y., Ke X., Zheng Z., Zhang C., Zhang Z., Zhang F., Hu Q., He Z., Wang H. Encapsulating quantum dots into enveloped virus in living cells for tracking virus infection. ACS Nano. 2013;7:3896–3904. doi: 10.1021/nn305189n. [DOI] [PubMed] [Google Scholar]
- 13.Pan H., Zhang P., Gao D., Zhang Y., Li P., Liu L., Wang C., Wang H., Ma Y., Cai L. Noninvasive visualization of respiratory viral infection using bioorthogonal conjugated near-infrared-emitting quantum dots. ACS Nano. 2014;8:5468–5477. doi: 10.1021/nn501028b. [DOI] [PubMed] [Google Scholar]
- 14.Luo R., Fang L., Jin H., Wang D., An K., Xu N., Chen H., Xiao S. Label-free quantitative phosphoproteomic analysis reveals differentially regulated proteins and pathway in PRRSV-infected pulmonary alveolar macrophages. J. Proteome Res. 2014;13:1270–1280. doi: 10.1021/pr400852d. [DOI] [PubMed] [Google Scholar]
- 15.White K.M., Abreu P., Wang H., De Jesus P.D., Manicassamy B., García-Sastre A., Chanda S.K., DeVita R.J., Shaw M.L. Broad spectrum inhibitor of influenza A and B viruses targeting the viral nucleoprotein. ACS Infect. Dis. 2018;4:146–157. doi: 10.1021/acsinfecdis.7b00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L., Liang J. An overview of functional nanoparticles as novel emerging antiviral therapeutic agents. Mater. Sci. Eng. C. 2020;112 doi: 10.1016/j.msec.2020.110924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prasad R., Bhattacharyya A., Nguyen Q.D. Nanotechnology in sustainable agriculture: recent developments, challenges, and perspectives. Front. Microbiol. 2017;8:1–13. doi: 10.3389/fmicb.2017.01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curtis A., Wilkinson C. Nantotechniques and approaches in biotechnology. Trends Biotechnol. 2001;19:97–101. doi: 10.1016/S0167-7799(00)01536-5. [DOI] [PubMed] [Google Scholar]
- 19.Mansur H.S., Mansur A.A.P., Curti E., De Almeida M.V. Functionalizedchitosan/quantum dot nano-hybrids for nanomedicine applications: towards biolabeling and biosorbing phosphate metabolites. J. Mater. Chem. B. 2013;1:1696. doi: 10.1039/c3tb00498h. [DOI] [PubMed] [Google Scholar]
- 20.Gaikwad A., Joshi M., Patil K., Sathaye S., Rode C. Fluorescent carbon-dots thin film for fungal detection and bio-labeling applications. ACS Appl. Bio Mater. 2019;2:5829–5840. doi: 10.1021/acsabm.9b00795. [DOI] [PubMed] [Google Scholar]
- 21.Riley M., Vermerris W. Recent advances in nanomaterials for gene delivery—a review. Nanomaterials. 2017;7:94. doi: 10.3390/nano7050094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan W.C. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science. 1998;281:2016–2018. doi: 10.1126/science.281.5385.2016. [DOI] [PubMed] [Google Scholar]
- 23.Streicher R.M., Schmidt M., Fiorito S. Nanosurfaces and nanostructures for artificial orthopedic implants. Nanomedicine. 2007;2:861–874. doi: 10.2217/17435889.2.6.861. [DOI] [PubMed] [Google Scholar]
- 24.Misra R., Acharya S., Sahoo S.K. Cancer nanotechnology: application of nanotechnology in cancer therapy. Drug Discov. Today. 2010;15:842–850. doi: 10.1016/j.drudis.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Kim B.Y.S., Rutka J.T., Chan W.C.W. Nanomedicine. N. Engl. J. Med. 2010;363:2434–2443. doi: 10.1056/NEJMra0912273. [DOI] [PubMed] [Google Scholar]
- 26.Wang H., Zhu W., Feng L., Chen Q., Chao Y., Dong Z., Liu Z. Nanoscale covalent organic polymers as a biodegradable nanomedicine for chemotherapy-enhanced photodynamic therapy of cancer. Nano Res. 2018;11:3244–3257. doi: 10.1007/s12274-017-1858-y. [DOI] [Google Scholar]
- 27.World Health Organization, MERS Situation update, January 2020, World Health Organization, 2020, p. 1. 〈http://www.emro.who.int/health-topics/mers-cov/mersoutbreaks.html〉.
- 28.World Health Organization, SARS (Severe Acute Respiratory Syndrome), World Health Organization, (n.d.), p. 1. 〈https://www.who.int/ith/diseases/sars/en/〉.
- 29.Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020 doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. JAMA. 2020;323:1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 31.Panoutsopoulos .AA. Conjunctivitis as a sentinel of SARS-CoV-2 infection: a need of revision for mild symptoms. SN Compr. Clin. Med. 2020;2:859–864. doi: 10.1007/S42399-020-00360-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brandini D.A., Takamiya A.S., Thakkar P., Schaller S., Rahat R., Naqvi A.R. Covid-19 and oral diseases: crosstalk, synergy or association? Rev. Med. Virol. 2021 doi: 10.1002/RMV.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coronavirus Disease (COVID-19), (n.d.). 〈https://www.who.int/emergencies/diseases/novel-coronavirus-2019?gclid=Cj0KCQjw8p2MBhCiARIsADDUFVHFvJy5G9pjBtstjrJ3UQRXhtef7NOlXqvO2D7KpsbU_jjWiwolyrMaAoAiEALw_wcB〉, (Accessed 8 November 2021).
- 34.Tobaiqy M., Qashqary M., Al-Dahery S., Mujallad A., Hershan A.A., Kamal M.A., Helmi N. Therapeutic management of patients with COVID-19: a systematic review. Infect. Prev. Pract. 2020;2 doi: 10.1016/J.INFPIP.2020.100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coronavirus Disease (COVID-19): Vaccines, (n.d.). 〈https://www.who.int/news-room/q-adetail/coronavirus-disease-(covid-19)-vaccines?adgroupsurvey={adgroupsurvey}&gclid=Cj0KCQjw8p2MBhCiARIsADDUFVGFAiXLwkgUyHIu7JCrJtbF5iuJwVShEI9rh4X_5a1gUD_ZJodarWwaApjuEALw_wcB〉, (Accessed 8 November 2021).
- 36.Galdiero S., Falanga A., Vitiello M., Cantisani M., Marra V., Galdiero M. Silver nanoparticles as potential antiviral agents. Molecules. 2011;16:8894–8918. doi: 10.3390/molecules16108894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181 doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Das P., Choudhuri T. Decoding the global outbreak of COVID-19: the nature is behind the scene. VirusDisease. 2020;31:106–112. doi: 10.1007/s13337-020-00605y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bertram S., Glowacka I., Muller M.A., Lavender H., Gnirss K., Nehlmeier I., Niemeyer D., He Y., Simmons G., Drosten C., Soilleux E.J., Jahn O., Steffen I., Pohlmann S. Cleavage and activation of the severe acute respiratory syndrome coronavirus spike protein by human airway trypsin-like protease. J. Virol. 2011;85:13363–13372. doi: 10.1128/JVI.05300-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glowacka I., Bertram S., Muller M.A., Allen P., Soilleux E., Pfefferle S., Steffen I., Tsegaye T.S., He Y., Gnirss K., Niemeyer D., Schneider H., Drosten C., Pohlmann S. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 2011;85:4122–4134. doi: 10.1128/JVI.0223210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galdiero S., Falanga A., Vitiello M., Cantisani M., Marra V., Galdiero M. Silver nanoparticles as potential antiviral agents. Molecules. 2011;16:8894–8918. doi: 10.3390/molecules16108894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeyaraj M., Gurunathan S., Qasim M., Kang M.-H., Kim J.-H., Comprehensive A. Review on the synthesis, characterization, and biomedical application of platinum nanoparticles. Nanomaterials. 2019;9:1719. doi: 10.3390/NANO9121719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smita S., Gupta S.K., Bartonova A., Dusinska M., Gutleb A.C., Rahman Q. Nanoparticles in the environment: assessment using the causal diagram approach. Environ. Health. 2012;11(1):1–11. doi: 10.1186/1476-069X–11S1-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cho E.J., Holback H., Liu K.C., Abouelmagd S.A., Park J., Yeo Y. Nanoparticle characterization: state of the art, challenges, and emerging technologies. Mol. Pharm. 2013;10:2093–2110. doi: 10.1021/MP300697H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Machado S., Pacheco J.G., Nouws H.P.A., Albergaria J.T., Delerue-Matos C. Characterization of green zero-valent iron nanoparticles produced with tree leaf extracts. Sci. Total Environ. 2015;533:76–81. doi: 10.1016/J.SCITOTENV.2015.06.091. [DOI] [PubMed] [Google Scholar]
- 47.S.A.M. Ealia, M.P. Saravanakumar, A review on the classification, characterisation, synthesis of nanoparticles and their application, IOP Conf. Ser. Mater. Sci. Eng., 263, 2017, 032019. 〈 10.1088/1757899X/263/3/032019〉. [DOI]
- 48.Huang X., Boey F., Zhang H. A brief review on graphene-nanoparticle composites. Cosmos. 2010;06:159–166. doi: 10.1142/S0219607710000607. [DOI] [Google Scholar]
- 49.de Volder M.F.L., Tawfick S.H., Baughman R.H., Hart A.J. Carbon nanotubes: present and future commercial applications. Science. 2013;339:535–539. doi: 10.1126/SCIENCE.1222453. [DOI] [PubMed] [Google Scholar]
- 50.Harshiny M., Iswarya C.N., Matheswaran M. Biogenic synthesis of iron nanoparticles using Amaranthus dubius leaf extract as a reducing agent. Powder Technol. C. 2015:744–749. doi: 10.1016/J.POWTEC.2015.09.021. [DOI] [Google Scholar]
- 51.Hulteen John C., Treichel David A., Smith Matthew T., Duval Michelle L., Jensen Traci R., van Duyne R.P. Nanosphere lithography: size-tunable silver nanoparticle and surface cluster arrays. J. Phys. Chem. B. 1999;103:3854–3863. doi: 10.1021/JP9904771. [DOI] [Google Scholar]
- 52.Syed B., Prasad N.M.N., Satish S. Endogenic mediated synthesis of gold nanoparticles bearing bactericidal activity. J. Microsc. Ultrastruct. 2016;4:162. doi: 10.1016/J.JMAU.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ryu C.H., Joo S.J., Kim H.S. Two-step flash light sintering of copper nanoparticle ink to remove substrate warping. Appl. Surf. Sci. 2016;384:182–191. doi: 10.1016/J.APSUSC.2016.05.025. [DOI] [Google Scholar]
- 54.Bajpai S.K., Jadaun M., Tiwari S. Synthesis, characterization and antimicrobial applications of zinc oxide nanoparticles loaded gum acacia/poly(SA) hydrogels. Carbohydr. Polym. 2016;153:60–65. doi: 10.1016/J.CARBPOL.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 55.Laad M., Jatti V.K.S. Titanium oxide nanoparticles as additives in engine oil. J. King Saud. Univ. Eng. Sci. 2018;30:116–122. doi: 10.1016/J.JKSUES.2016.01.008. [DOI] [Google Scholar]
- 56.Ruales-Lonfat C., Barona J.F., Sienkiewicz A., Bensimon M., Vélez-Colmenares J., Benítez N., Pulgarín C. Iron oxides semiconductors are efficients for solar water disinfection: a comparison with photo-Fenton processes at neutral pH. Appl. Catal. B Environ. 2015;166–167:497–508. doi: 10.1016/J.APCATB.2014.12.007. [DOI] [Google Scholar]
- 57.Roy K., Mao H.Q., Huang S.K., Leong K.W. Oral gene delivery with chitosan--DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat. Med. 1999;5:387–391. doi: 10.1038/7385. [DOI] [PubMed] [Google Scholar]
- 58.Rhaese S., von Briesen H., Rübsamen-Waigmann H., Kreuter J., Langer K. Human serum albumin-polyethylenimine nanoparticles for gene delivery. J. Control. Release Off. J. Control. Release Soc. 2003;92:199–208. doi: 10.1016/S0168-3659(03)00302-X. [DOI] [PubMed] [Google Scholar]
- 59.Bender A.R., von Briesen H., Kreuter J., Duncan I.B., Rubsamen-Waigmann H. Efficiency of nanoparticles as a carrier system for antiviral agents in human immunodeficiency virus-infected human monocytes/macrophages in vitro. Antimicrob. Agents Chemother. 1996;40:1467–1471. doi: 10.1128/AAC.40.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brzoska M., Langer K., Coester C., Loitsch S., Wagner T.O.F., Mallinckrodt C.v. Incorporation of biodegradable nanoparticles into human airway epithelium cells-in vitro study of the suitability as a vehicle for drug or gene delivery in pulmonary diseases. Biochem. Biophys. Res. Commun. 2004;318:562–570. doi: 10.1016/J.BBRC.2004.04.067. [DOI] [PubMed] [Google Scholar]
- 61.Patil V., Patel A. Biodegradable nanoparticles: a recent approach and applications. Curr. Drug Targets. 2020;21:1722–1732. doi: 10.2174/1389450121666200916091659. [DOI] [PubMed] [Google Scholar]
- 62.Nobs L., Buchegger F., Gurny R., Allémann E. Biodegradable nanoparticles for direct or two-step tumor immunotargeting. Bioconjug. Chem. 2006;17:139–145. doi: 10.1021/BC050137K. [DOI] [PubMed] [Google Scholar]
- 63.Gültekin H.E., Değim Z. Biodegradable polymeric nanoparticles are effective systems for controlled drug delivery. J. Pharm. Sci. 2013;38:107–118. [Google Scholar]
- 64.Damgé C., Reis C.P., Maincent P. Nanoparticle strategies for the oral delivery of insulin. Expert Opin. Drug Deliv. 2008;5:45–68. doi: 10.1517/17425247.5.1.45. [DOI] [PubMed] [Google Scholar]
- 65.Panyam J., Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 2003;55:329–347. doi: 10.1016/S0169-409X(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 66.Mohanraj V.J., Chen Y. Nanoparticles - a review. Trop. J. Pharm. Res. 2007;5:561–573. doi: 10.4314/tjpr.v5i1.14634. [DOI] [Google Scholar]
- 67.Patil D.R. Synthesis and charactrisation of silver nanoparticles using fungi and its antimicrobial activity. Int. J. Res. Stud. Biosci. 2015;3:146–152. [Google Scholar]
- 68.Mamo T., Moseman E.A., Kolishetti N., Salvador-Morales C., Shi J., Kuritzkes D.R., Langer R., von Andrian U., Farokhzad O.C. Emerging nanotechnology approaches for HIV/AIDS treatment and prevention. Nanomedicine. 2010;5:269–285. doi: 10.2217/NNM.10.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tobaiqy M., Qashqary M., Al-Dahery S., Mujallad A., Hershan A.A., Kamal M.A., Helmi N. Therapeutic management of patients with COVID-19: a systematic review. Infect. Prev. Pract. 2020;2 doi: 10.1016/j.infpip.2020.100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shaffer L. 15 drugs being tested to treat COVID-19 and how they would work. Nat. Med. 2020 doi: 10.1038/d41591-020-00019-9. [DOI] [PubMed] [Google Scholar]
- 71.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19) JAMA. 2020;323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 72.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.-X., Nicastri E., Oda R., Yo K., Quiros-Roldan E., Studemeister A., Redinski J., Ahmed S., Bernett J., Chelliah D., Chen D., Chihara S., Cohen S.H., Cunningham J., D’Arminio Monforte A., Ismail S., Kato H., Lapadula G., L’Her E., Maeno T., Majumder S., Massari M., Mora-Rillo M., Mutoh Y., Nguyen D., Verweij E., Zoufaly A., Osinusi A.O., DeZure A., Zhao Y., Zhong L., Chokkalingam A., Elboudwarej E., Telep L., Timbs L., Henne I., Sellers S., Cao H., Tan S.K., Winterbourne L., Desai P., Mera R., Gaggar A., Myers R.P., Brainard D.M., Childs R., Flanigan T. Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rajter J.C., Sherman M.S., Fatteh N., Vogel F., Sacks J., Rajter J.J. Use of ivermectin is associated with lower mortality in hospitalized patients with coronavirus disease 2019: the ivermectin in COVID nineteen study. Chest. 2021 doi: 10.1016/j.chest.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vallejos J., Zoni R., Bangher M., Villamandos S., Bobadilla A., Plano F., Campias C., Chaparro Campias E., Achinelli F., Guglielmone H.A., Ojeda J., Medina F., Farizano Salazar D., Andino G., Ruiz Diaz N.E., Kawerin P., Meza E., Dellamea S., Aquino A., Flores V., Martemucci C.N., Vernengo M.M., Martinez S.M., Segovia J.E., Aguirre M.G. Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19): a structured summary of a study protocol for a randomized controlled trial. Trials. 2020 doi: 10.1186/s13063-020-04813-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Poon W.-L., Alenius H., Ndika J., Fortino V., Kolhinen V., Meščeriakovas A., Wang M., Greco D., Lähde A., Jokiniemi J., Lee J.C.-Y., El-Nezami H., Karisola P. Nano-sized zinc oxide and silver, but not titanium dioxide, induce innate and adaptive immunity and antiviral response in differentiated THP-1 cells. Nanotoxicology. 2017;11:936–951. doi: 10.1080/17435390.2017.1382600. [DOI] [PubMed] [Google Scholar]
- 77.Miyako E., Nagata H., Hirano K., Sakamoto K., Makita Y., Nakayama K., Hirotsu T. Photoinduced antiviral carbon nanohorns. Nanotechnology. 2008;19 doi: 10.1088/0957-4484/19/7/075106. [DOI] [PubMed] [Google Scholar]
- 78.Calderón L., Harris R., Cordoba-Diaz M., Elorza M., Elorza B., Lenoir J., Adriaens E., Remon J.P., Heras A., Cordoba-Diaz D. Nano and microparticulate chitosan-based systems for antiviral topical delivery. Eur. J. Pharm. Sci. 2013;48:216–222. doi: 10.1016/j.ejps.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 79.Nikaeen G., Abbaszadeh S., Yousefinejad S. Application of nanomaterials in treatment, anti-infection and detection of coronaviruses. Nanomedicine. 2020;15:1501–1512. doi: 10.2217/nnm-2020-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.I.H. Cho, G.D. Lee, Y.Y. Yang, Composition with Sterilizing Activity Against Bacteria, Fungus and Viruses, Application Thereof and Method for Preparation Thereof, US 8673331 B2, 2014.
- 81.Lv X., Wang P., Bai R., Cong Y., Suo S., Ren X., Chen C. Inhibitory effect of silver nanomaterials on transmissible virus-induced host cell infections. Biomaterials. 2014;35:4195–4203. doi: 10.1016/j.biomaterials.2014.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ciejka J., Wolski K., Nowakowska M., Pyrc K., Szczubiałka K. Biopolymeric nano/microspheres for selective and reversible adsorption of coronaviruses. Mater. Sci. Eng. C. 2017;76:735–742. doi: 10.1016/j.msec.2017.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nikaeen G., Abbaszadeh S., Yousefinejad S. Application of nanomaterials in treatment, anti-infection and detection of coronaviruses. Nanomedicine. 2020;15:1501–1512. doi: 10.2217/nnm-2020-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen L., Liang J. An overview of functional nanoparticles as novel emerging antiviral therapeutic agents. Mater. Sci. Eng. C. 2020;112 doi: 10.1016/j.msec.2020.110924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gurunathan S., Qasim M., Choi Y., Do J.T., Park C., Hong K., Kim J.-H., Song H. Antiviral potential of nanoparticles—can nanoparticles fight against coronaviruses? Nanomaterials. 2020;10:1645. doi: 10.3390/nano10091645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lozano R., Naghavi M., Foreman K., Lim S., Shibuya K., Aboyans V., Abraham J., Adair T., Aggarwal R., Ahn S.Y., AlMazroa M.A., Alvarado M., Anderson H.R., Anderson L.M., Andrews K.G., Atkinson C., Baddour L.M., Barker-Collo S., Bartels D.H., Bell M.L., Benjamin E.J., Bennett D., Bhalla K., Bikbov B., Bin Abdulhak A., Birbeck G., Blyth F., Bolliger I., Boufous S., Bucello C., Burch M., Burney P., Carapetis J., Chen H., Chou D., Chugh S.S., Coffeng L.E., Colan S.D., Colquhoun S., Colson K.E., Condon J., Connor M.D., Cooper L.T., Corriere M., Cortinovis M., de Vaccaro K.C., Couser W., Cowie B.C., Criqui M.H., Cross M., Dabhadkar K.C., Dahodwala N., De Leo D., Degenhardt L., Delossantos A., Denenberg J., Des Jarlais D.C., Dharmaratne S.D., Dorsey E.R., Driscoll T., Duber H., Ebel B., Erwin P.J., Espindola P., Ezzati M., Feigin V., Flaxman A.D., Forouzanfar M.H., Fowkes F.G.R., Franklin R., Fransen M., Freeman M.K., Gabriel S.E., Gakidou E., Gaspari F., Gillum R.F., Gonzalez-Medina D., Halasa Y.A., Haring D., Harrison J.E., Havmoeller R., Hay R.J., Hoen B., Hotez P.J., Hoy D., Jacobsen K.H., James S.L., Jasrasaria R., Jayaraman S., Johns N., Karthikeyan G., Kassebaum N., Keren A., Khoo J.-P., Knowlton L.M., Kobusingye O., Koranteng A., Krishnamurthi R., Lipnick M., Lipshultz S.E., Ohno S.L., Mabweijano J., MacIntyre M.F., Mallinger L., March L., Marks G.B., Marks R., Matsumori A., Matzopoulos R., Mayosi B.M., McAnulty J.H., McDermott M.M., McGrath J., Memish Z.A., Mensah G.A., Merriman T.R., Michaud C., Miller M., Miller T.R., Mock C., Mocumbi A.O., Mokdad A.A., Moran A., Mulholland K., Nair M.N., Naldi L., Narayan K.M.V., Nasseri K., Norman P., O’Donnell M., Omer S.B., Ortblad K., Osborne R., Ozgediz D., Pahari B., Pandian J.D., Rivero A.P., Padilla R.P., Perez-Ruiz F., Perico N., Phillips D., Pierce K., Pope C.A., Porrini E., Pourmalek F., Raju M., Ranganathan D., Rehm J.T., Rein D.B., Remuzzi G., Rivara F.P., Roberts T., De León F.R., Rosenfeld L.C., Rushton L., Sacco R.L., Salomon J.A., Sampson U., Sanman E., Schwebel D.C., Segui-Gomez M., Shepard D.S., Singh D., Singleton J., Sliwa K., Smith E., Steer A., Taylor J.A., Thomas B., Tleyjeh I.M., Towbin J.A., Truelsen T., Undurraga E.A., Venketasubramanian N., Vijayakumar L., Vos T., Wagner G.R., Wang M., Wang W., Watt K., Weinstock M.A., Weintraub R., Wilkinson J.D., Woolf A.D., Wulf S., Yeh P.-H., Yip P., Zabetian A., Zheng Z.-J., Lopez A.D., Murray C.J. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Andresen H., Mager M., Grießner M., Charchar P., Todorova N., Bell N., Theocharidis G., Bertazzo S., Yarovsky I., Stevens M.M. Single-step homogeneous immunoassays utilizing epitope-tagged gold nanoparticles: on the mechanism, feasibility, and limitations. Chem. Mater. 2014;26:4696–4704. doi: 10.1021/cm500535p. [DOI] [Google Scholar]
- 88.Bowman M.-C., Ballard T.E., Ackerson C.J., Feldheim D.L., Margolis D.M., Melander C. Inhibition of HIV fusion with multivalent gold nanoparticles. J. Am. Chem. Soc. 2008;130:6896–6897. doi: 10.1021/ja710321g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peña-González C.E., García-Broncano P., Ottaviani M.F., Cangiotti M., Fattori A., Hierro-Oliva M., González-Martín M.L., Pérez-Serrano J., Gómez R., Muñoz-Fernández M.Á., Sánchez-Nieves J., de la Mata F.J. Dendronized anionic gold nanoparticles: synthesis, characterization, and antiviral activity. Chem. Eur. J. 2016;22:2987–2999. doi: 10.1002/chem.201504262. [DOI] [PubMed] [Google Scholar]
- 90.Papp I., Sieben C., Ludwig K., Roskamp M., Böttcher C., Schlecht S., Herrmann A., Haag R. Inhibition of influenza virus infection by multivalent sialic-acid-functionalized gold nanoparticles. Small. 2010;6:2900–2906. doi: 10.1002/smll.201001349. [DOI] [PubMed] [Google Scholar]
- 91.Vonnemann J., Sieben C., Wolff C., Ludwig K., Böttcher C., Herrmann A., Haag R. Virus inhibition induced by polyvalent nanoparticles of different sizes. Nanoscale. 2014;6:2353. doi: 10.1039/c3nr04449a. [DOI] [PubMed] [Google Scholar]
- 92.H. Li, L. Rothberg, Colorimetric detection of DNA sequences based on electrostatic interactions with unmodified gold nanoparticles, Proc. Natl. Acad. Sci., 101, 2004, pp. 14036–14039. 〈 10.1073/pnas.0406115101〉. [DOI] [PMC free article] [PubMed]
- 93.Martínez-Paredes G., González-García M.B., Costa-García A. Genosensor for SARS virus detection based on gold nanostructured screen-printed carbon electrodes. Electroanalysis. 2009;21:379–385. doi: 10.1002/elan.200804399. [DOI] [Google Scholar]
- 94.Draz M.S., Shafiee H. Applications of gold nanoparticles in virus detection. Theranostics. 2018;8:1985–2017. doi: 10.7150/thno.23856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Afrough B., Dowall S., Hewson R. Emerging viruses and current strategies for vaccine intervention. Clin. Exp. Immunol. 2019;196:157–166. doi: 10.1111/cei.13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zaman M., Good M.F., Toth I. Nanovaccines and their mode of action. Methods. 2013;60:226–231. doi: 10.1016/j.ymeth.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 97.Staroverov S.A., Vidyasheva I.V., Gabalov K.P., Vasilenko O.A., Laskavyi V.N., Dykman L.A. Immunostimulatory effect of gold nanoparticles conjugated with transmissible gastroenteritis virus. Bull. Exp. Biol. Med. 2011;151:436. doi: 10.1007/s10517-011-1350-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Staroverov S.A., Volkov A.A., Mezhenny P.V., Domnitsky I.Yu, Fomin A.S., Kozlov S.V., Dykman L.A., Guliy O.I. Prospects for the use of spherical gold nanoparticles in immunization. Appl. Microbiol. Biotechnol. 2019;103:437–447. doi: 10.1007/s00253-018-9476-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim H., Park M., Hwang J., Kim J.H., Chung D.-R., Lee K., Kang M. Development of label-free colorimetric assay for MERS-CoV using gold nanoparticles. ACS Sens. 2019;4:1306–1312. doi: 10.1021/acssensors.9b00175. [DOI] [PubMed] [Google Scholar]
- 100.Park T.J., Lee S.Y., Lee S.J., Park J.P., Yang K.S., Lee K.-B., Ko S., Park J.B., Kim T., Kim S.K., Shin Y.B., Chung B.H., Ku S.-J., Kim D.H., Choi I.S. Protein nanopatterns and biosensors using gold binding polypeptide as a fusion partner. Anal. Chem. 2006;78:7197–7205. doi: 10.1021/ac060976f. [DOI] [PubMed] [Google Scholar]
- 101.Bian H., Xu F., Jia Y., Wang L., Deng S., Jia A., Tang Y. A new immunochromatographic assay for on-site detection of porcine epidemic diarrhea virus based on monoclonal antibodies prepared by using cell surface fluorescence immunosorbent assay. BMC Vet. Res. 2019;15:32. doi: 10.1186/s12917-019-1773-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang J.C., Chang Y.-F., Chen K.-H., Su L.-C., Lee C.-W., Chen C.-C., Chen Y.-M.A., Chou C. Detection of severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in human serum using a localized surface plasmon coupled fluorescence fiberoptic biosensor. Biosens. Bioelectron. 2009;25:320–325. doi: 10.1016/j.bios.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Uskoković V. Why have nanotechnologies been underutilized in the global uprising against the coronavirus pandemic? Nanomedicine. 2020;15:1719–1734. doi: 10.2217/nnm-2020-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.de V.M., Cardoso O., Moreira B.J., Comparetti E.J., Sampaio I., Ferreira L.M.B., Lins P.M.P., Zucolotto V. Is nanotechnology helping in the fight against COVID-19? Front. Nanotechnol. 2020;2 doi: 10.3389/fnano.2020.588915. [DOI] [Google Scholar]
- 105.Lee G.H., Lee S.J., Jeong S.W., Kim H.-C., Park G.Y., Lee S.G., Choi J.H. Antioxidative and antiinflammatory activities of quercetin-loaded silica nanoparticles. Colloids Surf. B Biointerfaces. 2016;143:511–517. doi: 10.1016/j.colsurfb.2016.03.060. [DOI] [PubMed] [Google Scholar]
- 106.Lü S., Wu Y., Liu H. Silver nanoparticles synthesized using Eucommia ulmoides bark and their antibacterial efficacy. Mater. Lett. 2017;196:217–220. doi: 10.1016/j.matlet.2017.03.068. [DOI] [Google Scholar]
- 107.Aiad I., Marzouk M.I., Shaker S.A., Ebrahim N.E., Abd-Elaal A.A., Tawfik S.M. Antipyrine cationic surfactants capping silver nanoparticles as potent antimicrobial agents against pathogenic bacteria and fungi. J. Mol. Liq. 2017;243:572–583. doi: 10.1016/j.molliq.2017.08.072. [DOI] [Google Scholar]
- 108.Kuppusamy P., Ichwan S.J.A., Parine N.R., Yusoff M.M., Maniam G.P., Govindan N. Intracellular biosynthesis of Au and Ag nanoparticles using ethanolic extract of Brassica oleracea L. and studies on their physicochemical and biological properties. J. Environ. Sci. 2015;29:151–157. doi: 10.1016/j.jes.2014.06.050. [DOI] [PubMed] [Google Scholar]
- 109.Rosa R.M., Silva J.C., Sanches I.S., Henriques C. Simultaneous photo-induced crosslinking and silver nanoparticle formation in a PVP electrospun wound dressing. Mater. Lett. 2017;207:145–148. doi: 10.1016/j.matlet.2017.07.046. [DOI] [Google Scholar]
- 110.Akbarzadeh A., Kafshdooz L., Razban Z., Dastranj Tbrizi A., Rasoulpour S., Khalilov R., Kavetskyy T., Saghfi S., Nasibova A.N., Kaamyabi S., Kafshdooz T. An overview application of silver nanoparticles in inhibition of herpes simplex virus. Artif. Cells Nanomed. Biotechnol. 2018;46:263–267. doi: 10.1080/21691401.2017.1307208. [DOI] [PubMed] [Google Scholar]
- 111.Sreekanth T.V.M., Nagajyothi P.C., Muthuraman P., Enkhtaivan G., Vattikuti S.V.P., Tettey C.O., Kim D.H., Shim J., Yoo K. Ultra-sonication-assisted silver nanoparticles using Panax ginseng root extract and their anti-cancer and antiviral activities. J. Photochem. Photobiol. B Biol. 2018;188:6–11. doi: 10.1016/j.jphotobiol.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 112.Klasen H.J. Historical review of the use of silver in the treatment of burns. I. Early uses. Burns. 2000;26:117–130. doi: 10.1016/S0305-4179(99)00108-4. [DOI] [PubMed] [Google Scholar]
- 113.Yamanaka M., Hara K., Kudo J. Bactericidal actions of a silver ion solution on Escherichia coli, studied by energy-filtering transmission electron microscopy and proteomic analysis. Appl. Environ. Microbiol. 2005;71:7589–7593. doi: 10.1128/AEM.71.11.7589-7593.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Feng Q.L., Wu J., Chen G.Q., Cui F.Z., Kim T.N., Kim J.O. A mechanistic study of the antibacterial effect of silver ions onEscherichia coli andStaphylococcus aureus. J. Biomed. Mater. Res. 2000;52:662–668. doi: 10.1002/10974636(20001215)52:4<662::AID-JBM10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 115.Morones J.R., Elechiguerra J.L., Camacho A., Holt K., Kouri J.B., Ramírez J.T., Yacaman M.J. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–2353. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 116.Rai M., Yadav A., Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009;27:76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 117.Sondi I., Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004;275:177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 118.Lara H.H., Ayala-Nuñez N.V., Ixtepan-Turrent L., Rodriguez-Padilla C. Mode of antiviral action of silver nanoparticles against HIV-1. J. Nanobiotechnol. 2010;8:1. doi: 10.1186/1477-3155-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lara H.H., Garza-Treviño E.N., Ixtepan-Turrent L., Singh D.K. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J. Nanobiotechnol. 2011;9:30. doi: 10.1186/1477-3155-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sun R.W.-Y., Chen R., Chung N.P.-Y., Ho C.-M., Lin C.-L.S., Che C.-M. Silver nanoparticles fabricated in Hepes buffer exhibit cytoprotective activities toward HIV-1 infected cells. Chem. Commun. 2005:5059. doi: 10.1039/b510984a. [DOI] [PubMed] [Google Scholar]
- 121.Rogers J.V., Parkinson C.V., Choi Y.W., Speshock J.L., Hussain S.M. A preliminary assessment of silver nanoparticle inhibition of monkeypox virus plaque formation. Nanoscale Res. Lett. 2008;3:129–133. doi: 10.1007/s11671-0089128-2. [DOI] [Google Scholar]
- 122.Speshock J.L., Murdock R.C., Braydich-Stolle L.K., Schrand A.M., Hussain S.M. Interaction of silver nanoparticles with Tacaribe virus. J. Nanobiotechnol. 2010;8:19. doi: 10.1186/1477-3155-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Baram-Pinto D., Shukla S., Perkas N., Gedanken A., Sarid R. Inhibition of herpes simplex virus type 1 infection by silver nanoparticles capped with mercaptoethane sulfonate. Bioconjug. Chem. 2009;20:1497–1502. doi: 10.1021/bc900215b. [DOI] [PubMed] [Google Scholar]
- 124.Papp I., Sieben C., Ludwig K., Roskamp M., Böttcher C., Schlecht S., Herrmann A., Haag R. Inhibition of influenza virus infection by multivalent sialic-acid-functionalized gold nanoparticles. Small. 2010;6:2900–2906. doi: 10.1002/smll.201001349. [DOI] [PubMed] [Google Scholar]
- 125.Bryaskova R., Pencheva D., Nikolov S., Kantardjiev T. Synthesis and comparative study on the antimicrobial activity of hybrid materials based on silver nanoparticles (AgNps) stabilized by polyvinylpyrrolidone (PVP) J. Chem. Biol. 2011;4:185–191. doi: 10.1007/s12154-011-0063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Allawadhi P., Singh V., Khurana A., Khurana I., Allwadhi S., Kumar P., Banothu A.K., Thalugula S., Barani P.J., Naik R.R., Bharani K.K. Silver nanoparticle based multifunctional approach for combating COVID-19. Sens. Int. 2021;2 doi: 10.1016/j.sintl.2021.100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Song Z., Wang X., Zhu G., Nian Q., Zhou H., Yang D., Qin C., Tang R. Virus capture and destruction by label-free graphene oxide for detection and disinfection applications. Small. 2015;11:1171–1176. doi: 10.1002/smll.201401706. [DOI] [PubMed] [Google Scholar]
- 128.Yang X.X., Li C.M., Li Y.F., Wang J., Huang C.Z. Synergistic antiviral effect of curcumin functionalized graphene oxide against respiratory syncytial virus infection. Nanoscale. 2017;9:16086–16092. doi: 10.1039/C7NR06520E. [DOI] [PubMed] [Google Scholar]
- 129.Sametband M., Kalt I., Gedanken A., Sarid R. Herpes simplex virus type-1 attachment inhibition by functionalized graphene oxide. ACS Appl. Mater. Interfaces. 2014;6:1228–1235. doi: 10.1021/am405040z. [DOI] [PubMed] [Google Scholar]
- 130.Iannazzo D., Pistone A., Ferro S., De Luca L., Monforte A.M., Romeo R., Buemi M.R., Pannecouque C. Graphene quantum dots based systems as HIV inhibitors. Bioconjug. Chem. 2018;29:3084–3093. doi: 10.1021/acs.bioconjchem.8b00448. [DOI] [PubMed] [Google Scholar]
- 131.Ye S., Shao K., Li Z., Guo N., Zuo Y., Li Q., Lu Z., Chen L., He Q., Han H. Antiviral activity of graphene oxide: how sharp edged structure and charge matter. ACS Appl. Mater. Interfaces. 2015;7:21571–21579. doi: 10.1021/acsami.5b06876. [DOI] [PubMed] [Google Scholar]
- 132.Shirvanimoghaddam K., Akbari M.K., Yadav R., Al-Tamimi A.K., Naebe M. Fight against COVID-19: the case of antiviral surfaces. APL Mater. 2021;9 doi: 10.1063/5.0043009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang D. Application of nanotechnology in the COVID-19 pandemic. Int. J. Nanomed. 2021;16:623–649. doi: 10.2147/IJN.S296383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Prasad A.S. Molecular, Genetic, and Nutritional Aspects of Major and Trace Minerals. Elsevier; 2017. Discovery of zinc for human health and biomarkers of zinc deficiency; pp. 241–260. [DOI] [Google Scholar]
- 135.Wessels I., Maywald M., Rink L. Zinc as a gatekeeper of immune function. Nutrients. 2017;9:1286. doi: 10.3390/nu9121286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Maywald M., Wessels I., Rink L. Zinc signals and immunity. Int. J. Mol. Sci. 2017;18:2222. doi: 10.3390/ijms18102222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Haase H., Rink L. Multiple impacts of zinc on immune function. Metallomics. 2014;6:1175. doi: 10.1039/c3mt00353a. [DOI] [PubMed] [Google Scholar]
- 138.Gammoh N.Z., Rink L. Zinc in infection and inflammation. Nutrients. 2017;9:624. doi: 10.3390/nu9060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J. Med. Virol. 2020;92:479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Read S.A., Obeid S., Ahlenstiel C., Ahlenstiel G. The role of zinc in antiviral immunity. Adv. Nutr. 2019;10:696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sandstead H.H., Prasad A.S. Zinc intake and resistance to H1N1 influenza. Am. J. Public Health. 2010;100:970–971. doi: 10.2105/AJPH.2009.187773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J. Med. Virol. 2020;92:479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.te Velthuis A.J.W., van den Worm S.H.E., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010 doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xue J., Moyer A., Peng B., Wu J., Hannafon B.N., Ding W.-Q. Chloroquine is a zinc ionophore. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0109180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Guastalegname M., Vallone A. Could chloroquine /hydroxychloroquine be harmful in coronavirus disease 2019 (COVID-19) treatment? Clin. Infect. Dis. 2020;71:888–889. doi: 10.1093/cid/ciaa321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dabbagh-Bazarbachi H., Clergeaud G., Quesada I.M., Ortiz M., O’Sullivan C.K., Fernández-Larrea J.B. Zinc ionophore activity of quercetin and epigallocatechin-gallate: from hepa 1-6 cells to a liposome model. J. Agric. Food Chem. 2014;62:8085–8093. doi: 10.1021/jf5014633. [DOI] [PubMed] [Google Scholar]
- 147.Lin M.-H., Moses D.C., Hsieh C.-H., Cheng S.-C., Chen Y.-H., Sun C.-Y., Chou C.-Y. Disulfiram can inhibit MERS and SARS coronavirus papain-like proteases via different modes. Antivir. Res. 2018;150:155–163. doi: 10.1016/j.antiviral.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-02005985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Truong‐Tran A.Q., Carter J., Ruffin R., Zalewski P.D. New insights into the role of zinc in the respiratory epithelium. Immunol. Cell Biol. 2001;79:170–177. doi: 10.1046/j.1440-1711.2001.00986.x. [DOI] [PubMed] [Google Scholar]
- 150.Speth R., Carrera E., Jean‐Baptiste M., Joachim A., Linares A. Concentration‐dependent effects of zinc on angiotensin‐converting enzyme‐2 activity (1067.4) FASEB J. 2014;28 doi: 10.1096/fasebj.28.1_supplement.1067.4. [DOI] [Google Scholar]
- 151.Ishida T. Review on the role of Zn2+ ions in viral pathogenesis and the effect of Zn2+ ions for host cell-virus growth inhibition. Am. J. Biomed. Sci. Res. 2019;2:28–37. doi: 10.34297/AJBSR.2019.02.000566. [DOI] [Google Scholar]
- 152.Read S.A., Obeid S., Ahlenstiel C., Ahlenstiel G. The role of zinc in antiviral immunity. Adv. Nutr. 2019;10:696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Krenn B.M., Gaudernak E., Holzer B., Lanke K., Van Kuppeveld F.J.M., Seipelt J. Antiviral activity of the zinc ionophores pyrithione and hinokitiol against picornavirus infections. J. Virol. 2009;83:58–64. doi: 10.1128/JVI.01543-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Korant B.D., Kauer J.C., Butterworth B.E. Zinc ions inhibit replication of rhinoviruses. Nature. 1974;248:588–590. doi: 10.1038/248588a0. [DOI] [PubMed] [Google Scholar]
- 155.Cakman I., Kirchner H., Rink L. Zinc supplementation reconstitutes the production of interferon-α by leukocytes from elderly persons. J. Interferon Cytokine Res. 1997;17:469–472. doi: 10.1089/jir.1997.17.469. [DOI] [PubMed] [Google Scholar]
- 156.Berg K., Bolt G., Andersen H., Owen T.C. Zinc potentiates the antiviral action of human IFN-α tenfold. J. Interferon Cytokine Res. 2001;21:471–474. doi: 10.1089/10799900152434330. [DOI] [PubMed] [Google Scholar]
- 157.Skalny A., Rink L., Ajsuvakova O., Aschner M., Gritsenko V., Alekseenko S., Svistunov A., Petrakis D., Spandidos D., Aaseth J., Tsatsakis A., Tinkov A. Zinc and respiratory tract infections: perspectives for COVID-19 (review) Int. J. Mol. Med. 2020;46:17–26. doi: 10.3892/ijmm.2020.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Faten F., Rania S. Ibrahim, comparing surface chemical modifications of zinc oxide nanoparticles for modulating their antiviral activity against herpes simplex virus type-1. Int. J. Nanopart. Nanotechnol. 2018;4:1–14. doi: 10.35840/2631-5084/5521. [DOI] [Google Scholar]
- 159.Ghaffari H., Tavakoli A., Moradi A., Tabarraei A., Bokharaei-Salim F., Zahmatkeshan M., Farahmand M., Javanmard D., Kiani S.J., Esghaei M., Pirhajati-Mahabadi V., Monavari S.H., Ataei-Pirkooh A. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: another emerging application of nanomedicine. J. Biomed. Sci. 2019;26:70. doi: 10.1186/s12929-019-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ishida T. Review on the role of Zn2+ ions in viral pathogenesis and the effect of Zn2+ ions for host cell-virus growth inhibition. Am. J. Biomed. Sci. Res. 2019;2:28–37. doi: 10.34297/AJBSR.2019.02.000566. [DOI] [Google Scholar]
- 161.Du T., Cai K., Han H., Fang L., Liang J., Xiao S. Probing the interactions of CdTe quantum dots with pseudorabies virus. Sci. Rep. 2015;5:16403. doi: 10.1038/srep16403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Du T., Liang J., Dong N., Lu J., Fu Y., Fang L., Xiao S., Han H. Glutathione-capped Ag2S nanoclusters inhibit coronavirus proliferation through blockage of viral RNA synthesis and budding. ACS Appl. Mater. Interfaces. 2018;10:4369–4378. doi: 10.1021/acsami.7b13811. [DOI] [PubMed] [Google Scholar]
- 163.Liu H., Bai Y., Zhou Y., Feng C., Liu L., Fang L., Liang J., Xiao S. Blue and cyan fluorescent carbon dots: one-pot synthesis, selective cell imaging and their antiviral activity. RSC Adv. 2017;7:28016–28023. doi: 10.1039/C7RA03167J. [DOI] [Google Scholar]
- 164.Huang S., Gu J., Ye J., Fang B., Wan S., Wang C., Ashraf U., Li Q., Wang X., Shao L., Song Y., Zheng X., Cao F., Cao S. Benzoxazine monomer derived carbon dots as a broadspectrum agent to block viral infectivity. J. Colloid Interface Sci. 2019;542:198–206. doi: 10.1016/j.jcis.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 165.Barras A., Pagneux Q., Sane F., Wang Q., Boukherroub R., Hober D., Szunerits S. High efficiency of functional carbon nanodots as entry inhibitors of herpes simplex virus type 1. ACS Appl. Mater. Interfaces. 2016;8:9004–9013. doi: 10.1021/acsami.6b01681. [DOI] [PubMed] [Google Scholar]
- 166.Ting D., Dong N., Fang L., Lu J., Bi J., Xiao S., Han H. Multisite inhibitors for enteric coronavirus: antiviral cationic carbon dots based on curcumin. ACS Appl. Nano Mater. 2018;1:5451–5459. doi: 10.1021/acsanm.8b00779. [DOI] [PubMed] [Google Scholar]
- 167.Tong T., Hu H., Zhou J., Deng S., Zhang X., Tang W., Fang L., Xiao S., Liang J. Glycyrrhizic‐acid‐based carbon dots with high antiviral activity by multisite inhibition mechanisms. Small. 2020;16 doi: 10.1002/smll.201906206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Iannazzo D., Pistone A., Ferro S., De Luca L., Monforte A.M., Romeo R., Buemi M.R., Pannecouque C. Graphene quantum dots based systems as HIV inhibitors. Bioconjug. Chem. 2018;29:3084–3093. doi: 10.1021/acs.bioconjchem.8b00448. [DOI] [PubMed] [Google Scholar]
- 169.Prajapat M., Sarma P., Shekhar N., Avti P., Sinha S., Kaur H., Kumar S., Bhattacharyya A., Kumar H., Bansal S., Medhi B. Drug for corona virus: a systematic review. Indian J. Pharmacol. 2020;52:56. doi: 10.4103/ijp.IJP_115_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ting D., Dong N., Fang L., Lu J., Bi J., Xiao S., Han H. Multisite inhibitors for enteric coronavirus: antiviral cationic carbon dots based on curcumin. ACS Appl. Nano Mater. 2018;1:5451–5459. doi: 10.1021/acsanm.8b00779. [DOI] [PubMed] [Google Scholar]
- 171.Dong X., Moyer M.M., Yang F., Sun Y.-P., Yang L. Carbon dots’ antiviral functions against noroviruses. Sci. Rep. 2017;7:519. doi: 10.1038/s41598017-00675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Manivannan S., Ponnuchamy K. Quantum dots as a promising agent to combat COVID19. Appl. Organomet. Chem. 2020;34:1–6. doi: 10.1002/aoc.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Manivannan S., Ponnuchamy K. Quantum dots as a promising agent to combat COVID19. Appl. Organomet. Chem. 2020;34:1–6. doi: 10.1002/aoc.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tavakol S., Zahmatkeshan M., Mohammadinejad R., Mehrzadi S., Joghataei M.T., Alavijeh M.S., Seifalian A. The role of nanotechnology in current COVID-19 outbreak. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e06841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Singh L., Kruger H.G., Maguire G.E.M., Govender T., Parboosing R. The role of nanotechnology in the treatment of viral infections. Ther. Adv. Infect. Dis. 2017;4:105–131. doi: 10.1177/2049936117713593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cagno V., Andreozzi P., D’Alicarnasso M., Jacob Silva P., Mueller M., Galloux M., Le Goffic R., Jones S.T., Vallino M., Hodek J., Weber J., Sen S., Janeček E.-R., Bekdemir A., Sanavio B., Martinelli C., Donalisio M., Rameix Welti M.-A., Eleouet J.-F., Han Y., Kaiser L., Vukovic L., Tapparel C., Král P., Krol S., Lembo D., Stellacci F. Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nat. Mater. 2018;17:195–203. doi: 10.1038/nmat5053. [DOI] [PubMed] [Google Scholar]
- 177.Lönnerdal B. Bioavailability of copper. Am. J. Clin. Nutr. 1996;63:821S–829S. doi: 10.1093/ajcn/63.5.821. [DOI] [PubMed] [Google Scholar]
- 178.Andreou A., Trantza S., Filippou D., Sipsas N., Tsiodras S. COVID-19: the potential role of copper and N-acetylcysteine (NAC) in a combination of candidate antiviral treatments against SARS-CoV-2. In Vivo. 2020;34:1567–1588. doi: 10.21873/invivo.11946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cherukuri S., Potla R., Sarkar J., Nurko S., Harris Z.L., Fox P.L. Unexpected role of ceruloplasmin in intestinal iron absorption. Cell Metab. 2005;2:309–319. doi: 10.1016/j.cmet.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 180.Warnes S.L., Little Z.R., Keevil C.W. Human coronavirus 229E remains infectious on common touch surface materials. mBio. 2015;6 doi: 10.1128/mBio.0169715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jagaran K., Singh M. Nanomedicine for COVID-19: potential of copper nanoparticles. Biointerface Res. Appl. Chem. 2020;11:10716–10728. doi: 10.33263/BRIAC113.1071610728. [DOI] [Google Scholar]
- 182.Bromberg L., Bromberg D.J., Hatton T.A., Bandín I., Concheiro A., Alvarez-Lorenzo C. Antiviral properties of polymeric aziridine- and biguanide-modified core–shell magnetic nanoparticles. Langmuir. 2012;28:4548–4558. doi: 10.1021/la205127x. [DOI] [PubMed] [Google Scholar]
- 183.Bisso S., Leroux J.-C. Nanopharmaceuticals: a focus on their clinical translatability. Int. J. Pharm. 2020;578 doi: 10.1016/j.ijpharm.2020.119098. [DOI] [PubMed] [Google Scholar]
- 184.de V.M., Cardoso O., Moreira B.J., Comparetti E.J., Sampaio I., Ferreira L.M.B., Lins P.M.P., Zucolotto V. Is nanotechnology helping in the fight against COVID-19? Front. Nanotechnol. 2020;2 doi: 10.3389/fnano.2020.588915. [DOI] [Google Scholar]
- 185.Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., Zhang B., Li X., Zhang L., Peng C., Duan Y., Yu J., Wang L., Yang K., Liu F., Jiang R., Yang X., You T., Liu X., Yang X., Bai F., Liu H., Liu X., Guddat L.W., Xu W., Xiao G., Qin C., Shi Z., Jiang H., Rao Z., Yang H. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582:289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 186.Kono H., Arteel G.E., Rusyn I., Sies H., Thurman R.G. Ebselen prevents early alcoholinduced liver injury in rats. Free Radic. Biol. Med. 2001;30:403–411. doi: 10.1016/S0891-5849(00)00490-1. [DOI] [PubMed] [Google Scholar]
- 187.Pi J., Jiang J., Cai H., Yang F., Jin H., Yang P., Cai J., Chen Z.W. GE11 peptide conjugated selenium nanoparticles for EGFR targeted oridonin delivery to achieve enhanced anticancer efficacy by inhibiting EGFR-mediated PI3K/AKT and Ras/Raf/MEK/ERK pathways. Drug Deliv. 2017;24:1549–1564. doi: 10.1080/10717544.2017.1386729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cong W., Bai R., Li Y.-F., Wang L., Chen C. Selenium nanoparticles as an efficient nanomedicine for the therapy of Huntington’s disease. ACS Appl. Mater. Interfaces. 2019;11:34725–34735. doi: 10.1021/acsami.9b12319. [DOI] [PubMed] [Google Scholar]
- 189.He L., Zhao J., Wang L., Liu Q., Fan Y., Li B., Yu Y.-L., Chen C., Li Y.-F. Using nanoselenium to combat coronavirus disease 2019 (COVID-19)? Nano Today. 2021;36 doi: 10.1016/j.nantod.2020.101037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.G. Ren, J.S. Oxford, P.W. Reip, R. Lambkin-Williams, A. Mann, Anti-viral Formulations Nanomaterals and Nanoparticles, US 2013/009 1611 A1, 2013.
- 191.Grass G., Rensing C., Solioz M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011;77:1541–1547. doi: 10.1128/AEM.02766-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bankier C., Matharu R.K., Cheong Y.K., Ren G.G., Cloutman-Green E., Ciric L. Synergistic antibacterial effects of metallic nanoparticle combinations. Sci. Rep. 2019;9:16074. doi: 10.1038/s41598-019-52473-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kim S.Y., Lee Y.M., Baik D.J., Kang J.S. Toxic characteristics of methoxy poly(ethylene glycol)/poly(epsilon-caprolactone) nanospheres; in vitro and in vivo studies in the normal mice. Biomaterials. 2003;24:55–63. doi: 10.1016/S0142-9612(02)00248-X. [DOI] [PubMed] [Google Scholar]
- 194.Goodman C.M., McCusker C.D., Yilmaz T., Rotello V.M. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug. Chem. 2004;15:897–900. doi: 10.1021/BC049951I. [DOI] [PubMed] [Google Scholar]
- 195.Paciotti G.F., Myer L., Weinreich D., Goia D., Pavel N., McLaughlin R.E., Tamarkin L. Colloidal gold: a novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. 2004;11:169–183. doi: 10.1080/10717540490433895. [DOI] [PubMed] [Google Scholar]
- 196.Granum B., Gaarder P.I., Lovik M. IgE adjuvant effect caused by particles - immediate and delayed effects. Toxicology. 2001;156:149–159. doi: 10.1016/S0300483X(00)00375-9. [DOI] [PubMed] [Google Scholar]
- 197.Nygaard U.C., Aase A., Løvik M. The allergy adjuvant effect of particles - genetic factors influence antibody and cytokine responses. BMC Immunol. 2005;6:1–10. doi: 10.1186/1471-2172-6-11/FIGURES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.What are Potential Harmful Effects of Nanoparticles? (n.d.). 〈https://ec.europa.eu/health/scientific_committees/opinions_layman/en/nanotechnologies/l-3/6-health-effects-nanoparticles.htm〉, (Accessed 19 January 2022).
- 199.S.S.A. Karim, T. de Oliveira, New SARS-CoV-2 Variants — Clinical, Public Health, and Vaccine Implications, 384, 2021, pp. 1866–1868. 〈 10.1056/NEJMC2100362〉. [DOI] [PMC free article] [PubMed]
- 200.Ahmadivand A., Gerislioglu B., Ramezani Z., Kaushik A., Manickam P., Ghoreishi S.A. Functionalized terahertz plasmonic metasensors: femtomolar-level detection of SARSCoV-2 spike proteins. Biosens. Bioelectron. 2021;177 doi: 10.1016/J.BIOS.2021.112971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Paliwal P., Sargolzaei S., Bhardwaj S.K., Bhardwaj V., Dixit C., Kaushik A. Grand challenges in bio-nanotechnology to manage the COVID-19 pandemic. Front. Nanotechnol. 2020;0:5. doi: 10.3389/FNANO.2020.571284. [DOI] [Google Scholar]
- 202.Kaushik A.K., Dhau J.S., Gohel H., Mishra Y.K., Kateb B., Kim N.-Y., Goswami D.Y. Electrochemical SARS-CoV-2 sensing at point-of-care and artificial intelligence for intelligent COVID-19 management. ACS Appl. Bio Mater. 2020 doi: 10.1021/ACSABM.0C01004. [DOI] [PubMed] [Google Scholar]
- 203.A. Kaushik, Manipulative Magnetic Nanomedicine: The Future of COVID-19 Pandemic/endemic Therapy,18, 2020, pp. 531–534. 〈 10.1080/17425247.2021.1860938〉. [DOI] [PubMed]
- 204.Yalcin H.C., Kaushik A. Support of intelligent emergent materials to combat COVID-19 pandemic. Emerg. Mater. 2021;4 doi: 10.1007/S42247-021-00189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Talebian S., Wallace G.G., Schroeder A., Stellacci F., Conde J. Nanotechnology-based disinfectants and sensors for SARS-CoV-2. Nat. Nanotechnol. 2020;15(8):618–621. doi: 10.1038/s41565-020-0751-0. 15. [DOI] [PubMed] [Google Scholar]
- 206.Sadique Mohd A., Yadav S., Ranjan P., Verma S., Salammal S.T., Khan Mohd A., Kaushik A., Khan R. High-performance antiviral nano-systems as a shield to inhibit viral infections: SARS-CoV-2 as a model case study. J. Mater. Chem. B. 2021;9:4620–4642. doi: 10.1039/D1TB00472G. [DOI] [PubMed] [Google Scholar]
- 207.Varahachalam S.P., Lahooti B., Chamaneh M., Bagchi S., Chhibber T., Morris K., Bolanos J.F., Kim N.Y., Kaushik A. Nanomedicine for the SARS-CoV2: state-of-the-art and future prospects. Int. J. Nanomed. 2021;16:539–560. doi: 10.2147/IJN.S283686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.A. Kaushik, Manipulative Magnetic Nanomedicine: The Future of COVID-19 Pandemic/endemic Therapy, 18, 2020, pp. 531–534. 〈 10.1080/17425247.2021.1860938〉. [DOI] [PubMed]
- 209.A. Kaushik, R.D. Jayant, M. Nair, Nanomedicine for NeuroHIV/AIDS Management, 13, 2018, pp. 669–673. 〈 10.2217/NNM-2018-0005〉. [DOI] [PubMed]
- 210.Kaushik A., Dev Jayant R., Bhardwaj V., Nair M. Personalized nanomedicine for CNS diseases. Drug Discov. Today. 2018;23:1007–1015. doi: 10.1016/J.DRUDIS.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gage A., Brunson K., Morris K., Wallen S.L., Dhau J., Gohel H., Kaushik A. Perspectives of manipulative and high-performance nanosystems to manage consequences of emerging new severe acute respiratory syndrome coronavirus 2 variants. Front. Nanotechnol. 2021;0:45. doi: 10.3389/FNANO.2021.700888. [DOI] [Google Scholar]
- 212.Xu L., Chu Z., Wang H., Cai L., Tu Z., Liu H., Zhu C., Shi H., Pan D., Pan J., Fei X. Electrostatically assembled multilayered films of biopolymer enhanced nanocapsules for on-demand drug release. ACS Appl. Bio Mater. 2019;2:3429–3438. doi: 10.1021/ACSABM.9B00381/SUPPL_FILE/MT9B00381_SI_001.PDF. [DOI] [PubMed] [Google Scholar]
- 213.Li Z., Yang P., Yan S., Fang Q., Xue M., Qiu S., Robust A. Zeolitic imidazolate framework membrane with high H2/CO2 separation performance under hydrothermal conditions. ACS Appl. Mater. Interfaces. 2019;11:15748–15755. doi: 10.1021/ACSAMI.9B01051/SUPPL_FILE/AM9B01051_SI_001.PDF. [DOI] [PubMed] [Google Scholar]
- 214.Xu L., Zhang X., Chu Z., Wang H., Li Y., Shen X., Cai L., Shi H., Zhu C., Pan J., Pan D. Temperature-responsive multilayer films based on block copolymer-coated silica nanoparticles for long-term release of favipiravir. ACS Appl. Nano Mater. 2021;4:14014–14025. doi: 10.1021/ACSANM.1C03334. [DOI] [Google Scholar]
- 215.Exbrayat J.-M., Moudilou E.N., Lapied E. Harmful effects of nanoparticles on animals. J. Nanotechnol. 2015;2015:1–10. doi: 10.1155/2015/861092. [DOI] [Google Scholar]
- 216.Zhang Toxicologic effects of gold nanoparticles in vivo by different administration routes. Int. J. Nanomed. 2010:771. doi: 10.2147/IJN.S8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bosetti R. Cost–effectiveness of nanomedicine: the path to a future successful and dominant market? Nanomedicine. 2015;10:1851–1853. doi: 10.2217/nnm.15.74. [DOI] [PubMed] [Google Scholar]
- 218.R. Bosetti, S.L. Jones, Cost–Effectiveness of Nanomedicine: Estimating the Real Size of Nano-Costs, 14, 2019, pp. 1367–1370. 〈 10.2217/NNM-2019-0130〉. [DOI] [PubMed]