Abstract
Red blood cell (RBC) deformability is severely decreased in patients with sickle cell anemia (SCA), which plays a role in the pathophysiology of the disease. However, investigation of RBC deformability from SCA patients demands careful methodological considerations. We assessed RBC deformability by ektacytometry (LORRCA MaxSis, Mechatronics, The Netherlands) in 6 healthy individuals and 49 SCA patients and tested the effects of different heights of the RBC diffraction patterns, obtained by altering the camera gain of the LORRCA, on the result of RBC deformability measurements, expressed as Elongation Index (EI). Results indicate that the pattern of RBCs from control subjects adopts an elliptical shape under shear stress, whereas the pattern of RBCs from individuals with SCA adopts a diamond shape arising from the superposition of elliptical and circular patterns. The latter represent rigid RBCs. While the EI measures did not change with the variations of the RBC diffraction pattern heights in the control subjects, we observed a decrease of EI when the RBC diffraction pattern height is increased in the SCA group. The differences in SCA EI values measured at 5 Pa between the different diffraction pattern heights correlated with the percent of hemoglobin S and the percent of sickled RBC observed by microscopy. Our study confirms that the camera gain or aperture of the ektacytometer should be used to standardize the size of the RBC diffraction pattern height when measuring RBC deformability in sickle cell patients and underscores the potential clinical utility of this technique.
Keywords: ektacytometry, sickle cell disease, red blood cell deformability, standardization, clinical relevance
Introduction
Sickle cell anemia (SCA) is a severe monogenic hemoglobinopathy characterized by the presence of abnormal hemoglobin (HbS). SCA patients are marked by chronic hemolysis, anemia, vasculopathy, diminished red blood cell (RBC) deformability, decreased RBC aggregation but increased RBC aggregates robustness and increased RBC adhesiveness to the vascular wall, and the recurrence of painful vaso-occlusive crises [14, 17, 20, 22]. Upon deoxygenation, HbS polymerizes, distorting the normal shape of the RBC and interfering with its natural deformability [6]. Variability in the degree of defective RBC deformability may predict the occurrence of certain complications, such as frequent vaso-occlusive crises [2, 12], leg ulcers [7] and glomerulopathy [11].
Although RBC deformability can be measured by multiple techniques, ektacytometry is currently one of the most widely employed because of its precision and ease of use [5]. Ektacytometry relies on laser diffraction analysis and, theoretically, allows for a direct and accurate measure of alterations in the deformability of sickle RBCs [21]. However, as Rabai et al [16] have recently reported, ektacytometric analysis of blood containing large intra-sample variation in cellular deformability can be problematic due to the fact that rigid cells do not properly align with deformable RBCs and/or do not produce elliptical diffraction patterns in response to increasing shear stress [16]. Detailed mathematical analyses have previously been employed to analyse diffraction pattern distortions [19] but Rabai et al [16] recently showed that the fraction of poorly deformable RBCs in a given sample could be more easily determined than with these mathematical analyses. They altered the height of the diffraction pattern by changing the size of the opening on the camera aperture or changing the gray level on the fitting software on an older version of the commercially available ektacytometer: the Laser-assisted Rotational Cell Analyzer (LORRCA, Mechatronics, The Netherlands). These alterations strongly affected the elongation index (EI; an index reflecting RBC deformability) values obtained at a given shear stress in blood from patients with SCA but not in blood from control subjects. The differences in image heights between diffraction patterns correlated well with the percentage of irreversibly sickled cells [16].
The insights provided by this study not only suggested the need for methodological standardization when investigating the deformability of erythrocytes from patients with SCA but also indicated potential clinical utility. Unfortunately, the camera aperture is not readily accessible on the latest generation of the LORRCA, the LORRCA MaxSis. We thus initiated a multi-center study to determine whether similar results could be obtained by changing the height of the diffraction pattern on the computer monitor using the camera gain of the LORRCA MaxSis.
Methods
Design of the study
Protocols were approved by the appropriate Institutional Review Board for each institution and subjects provided written informed consent. Whole blood was collected into K2 EDTA vacutainer tubes (BD Vacutainer) from 6 healthy volunteers and 49 patients with SCA at steady-state (no vaso-occlusive crises and no acute chest syndrome in the previous two months), whom 6 were chronically transfused. Blood was used for the quantification of HbS, blood smears and ektacytometry measurements.
Ektacytometry, blood smears and HbS quantification
25 μL of pre-oxygenated blood [4] was added to 5 mL of an iso-osmolar polyvinylpyrrolidone solution with a mean viscosity of 30 mPa.s (Mechatronics, The Netherlands). After the application of one mL of the thoroughly mixed RBC suspension, the LORRCA MaxSis was briefly operated at maximum shear stress (50 Pa) and the camera gain was adjusted to obtain a diffraction pattern on the computer monitor with heights of 3.8 cm, 4.5 cm and 5.4 cm, respectively, and measured directly from the screen with a ruler. The Elongation Index (EI) calculated from the diffraction pattern collected by the camera of the LORRCA Maxsis reflected RBC deformability [4]. EI data were then obtained in triplicate for each image height at 9 pre-selected shear stresses (i.e., 0.5, 0.89, 1.58, 2.81, 5.0, 8.89, 15.8, 28.1 and 50 Pa) corresponding to those used by Rabai et al [16]. At each shear stress, we calculated the differences (expressed in percentage) of EI-value between the different diffraction pattern heights (i.e. Δ3.8/4.5, Δ3.8/5.4 and Δ4.5/5.4). The percentage of sickled cells was obtained microscopically (100x magnification) by examining about 2000 red blood cells on a blood smear stained with May-Grunwald-Giemsa [1]. The HbS fraction was quantified by high-performance liquid chromatography [15] using the Variant II™ system and the ‘Short Beta Thal Program’ (Bio-Rad, Hercules, CA, USA)
Statistical analysis
A Mann-Whitney test or a student’ t test was used to compare the EI-values at each stress and diffraction pattern heights between SCA patients and healthy controls. A one-way Kruskal-Wallis ANOVA or parametric ANOVA was performed for each group to compare EI-values at each stress between the three diffraction pattern heights. Pearson correlations were performed between the percentages of HbS or of sickled cells and 1) RBC deformability (EI) measured at 5 Pa and 2) the differences of RBC deformability (EI) values between the different diffraction pattern heights at 5 Pa. The value of 5 Pa represents the shear stress that can be found in capillaries [10] as well as in arteries [18]. The significance level was defined as p < 0.05. Data are displayed as means ± SD. Analyses were conducted using SPSS software (version 20, IBM SPSS Statistics, Chicago, IL, USA).
Results
Diffraction patterns
At high shear stress, RBCs from control patients exhibited an elliptical diffraction pattern whereas RBCs from patients with sickle cell disease generated a diamond shaped diffraction pattern. The diamond shape of the diffraction pattern was more marked at 5.4 cm than at 4.5 or 3.8 cm. Figure 1 shows an example of diffraction pattern for a SCA patient obtained at 3.8 and 5.4 cm.
Figure 1.

Examples of a red blood cell diffraction pattern obtained at different heights (left = 3.8 cm, right = 5.4 cm) after camera gain adjustment in one patient with sickle cell anemia (SCA).
Effects of the diffraction pattern heights on RBC deformability measures in controls and SCA patients
As shown in the Figure 2 (2A, 2B, 2C), RBC deformability was lower in SCA than in controls at any shear stress and at any diffraction pattern height (p < 0.001). Changing the diffraction pattern heights by adjusting with the camera gain of the LORRCA MaxSis did not affect the measured EI-values in the control group (Figure 3A). In contrast, EI values were affected by the diffraction pattern heights in the SCA group (Figure 3B): the three curves obtained were significantly different from each other (p < 0.01 at the two lowest shear stresses and p < 0.001 for the other shear stresses).
Figure 2.
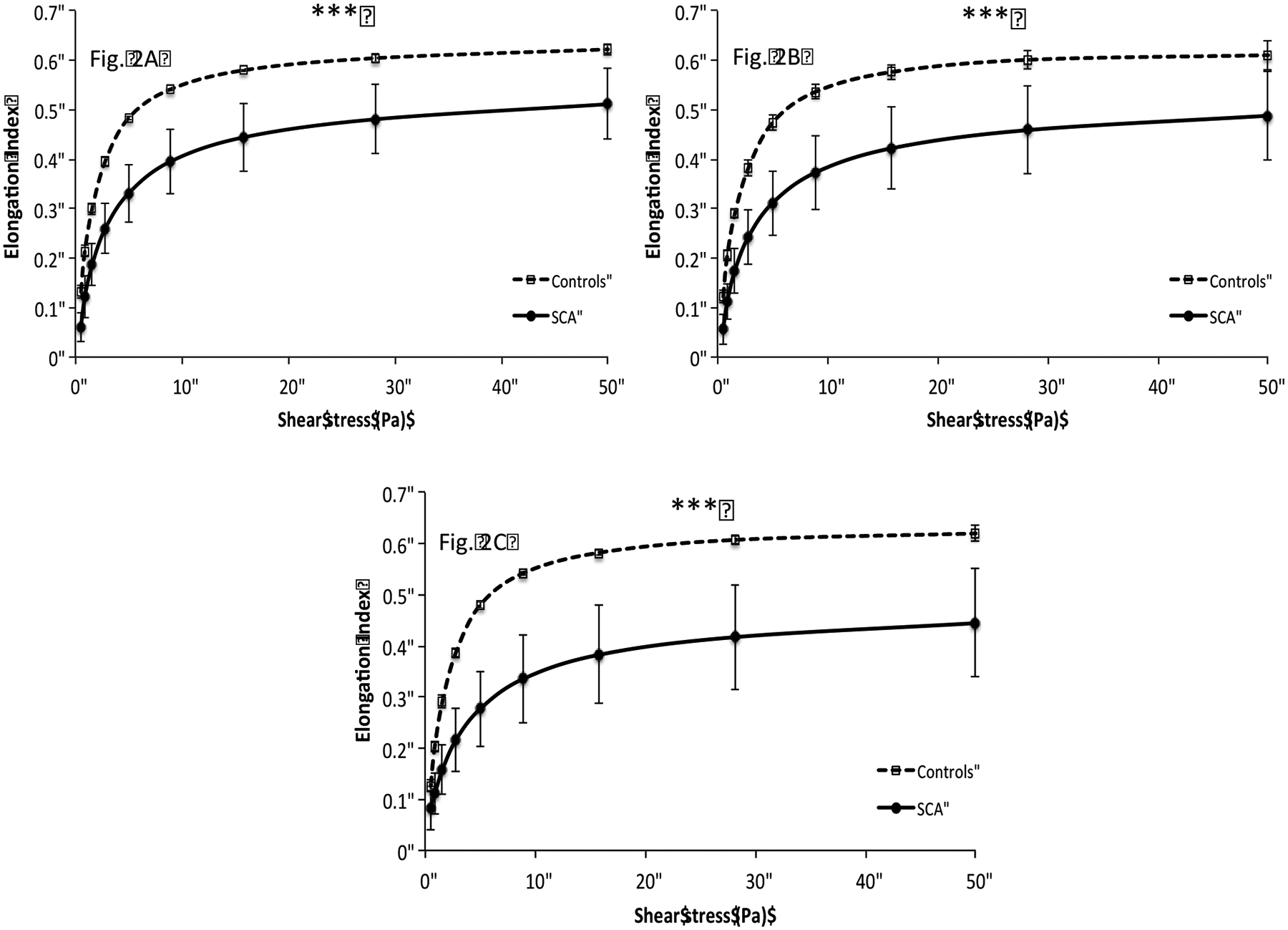
Comparison of red blood cell deformation curves between controls and SCA individuals at different diffraction pattern heights (Fig. 2A = 3.8 cm, Fig. 2B = 4.5 cm, Fig. 2C = 5.4 cm). Elongation index values were decreased in SCA patients at all shear stresses (***p < 0.001).
Figure 3.
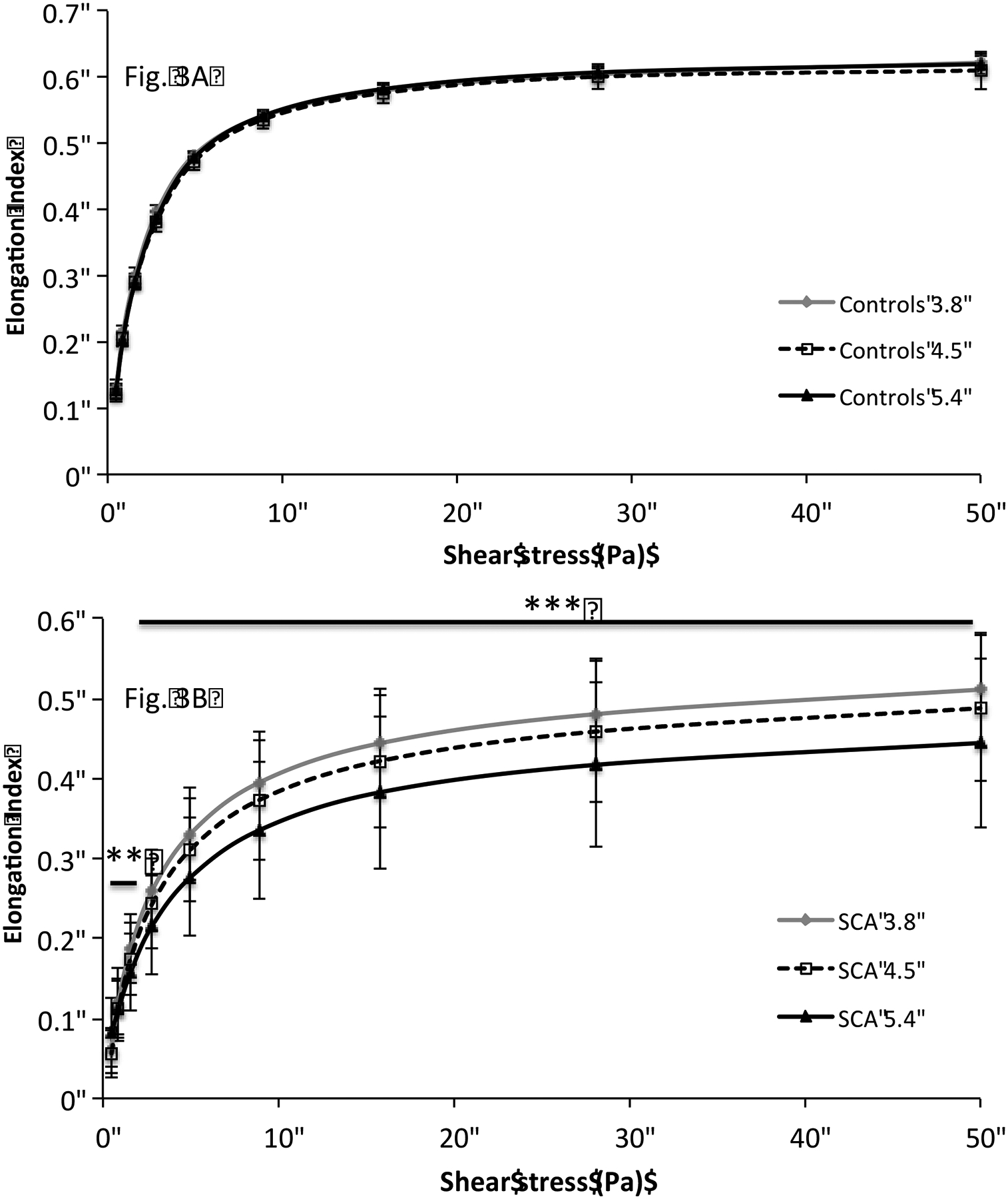
Comparison of red blood cell deformation curves obtained at different diffraction pattern heights in controls (Fig. 3A) and SCA individuals (Fig. 3B). RBC deformation curve was significantly affected by the changes in the red blood cell diffraction pattern heights in SCA group (**p < 0.01; ***p < 0.001).
EDTA-anticoagulated blood from 5 additional SCA patients were studied in a special LORRCA MaxSis adapted with a more easily accessible camera aperture in order to confirm that similar effects on image height by adjustments of either camera aperture or gain could be obtained (data not shown).
Correlation of differences in EI values, measured at 5 Pa, with the percentage of sickled cells and hemoglobin S
Consistent with a previous study performed in a limited number of sickle cell patients [16], the differences in EI measured at 5 Pa correlated with the percent of sickled cells (Δ3.8/4.5: r = 0.41; p < 0.01; Δ3.8/5.4: r = 0.49; p < 0.001; Δ4.5/5.4: r = 0.42; p < 0.01). EI values also correlated with the percent of sickled cells as well (3.8: r = −0.60; p < 0.001; 4.5: r = −0.59; p < 0.001; 5.5: r = −0.59; p < 0.001). We also found a correlation between the differences in EI measured at 5 Pa and the percent of HbS (Δ3.8/4.5: r = 0.37; p < 0.01; Δ3.8/5.4: r = 0.35; p < 0.05; Δ4.5/5.4: r = 0.30; p < 0.05). There was no significant correlation between percent of HbS and the EI values.
Discussion
Ektacytometry is the gold standard to measure RBC deformability in humans and in animals but its use requires precaution [4]. The diamond shape (Figure 1) generated by RBCs from patients with SCA in response to shear stress arises from the superposition of an elliptical pattern and a circular pattern. The elliptical shape arises from deformable erythrocytes and the circular pattern arises from rigid RBCs. The latter cells type tumbles in all directions, producing a circular diffraction pattern, rather than orienting properly along the flow vector. The higher the percentage of sickled (rigid) RBC in SCA blood is, the lower the mean RBC deformability.
Using the camera gain to change the diffraction pattern size produces results similar to those previously obtained by altering the camera aperture or gray level by Rabai et al [16]. While changing the diffraction pattern height did not change the EI values at a given shear stress in healthy controls, these values were highly affected in SCA patients. These results clearly indicate the need for standardization of diffraction pattern height to allow the comparison of results between different laboratories investigating the deformability profiles of RBCs in sickle cell patients and indicate that this can be easily accomplished using the readily accessible camera gain on the LORRCA MaxSis.
The correlation found between the differences in RBC deformability as a function of diffraction pattern height and both the percentage of sickled cells and HbS highlights the fact that ektacytometry has clinical relevance and utility in the context of SCA. Increased number of dense sickled cells has been shown to correlate with several complications of the hemolytic/endothelial dysfunction phenotype [9] in SCA, such as skin ulcers, priapism and renal dysfunction [3]. Decreased RBC deformability in SCA is highly suspected to contribute to several complications and to enhance the risk for hemolysis [7–8, 11]. Using the camera gain of the LORRCA MaxSis to measure RBC deformability (EI) at different diffraction pattern heights could be useful to quickly quantify the amount of circulating rigid and dense RBCs for a given SCA patient. Moreover, positive RBC rheological effects of hydroxurea therapy could be easily tracked by ektacytometry [13] and the determination of several diffraction pattern heights since this molecule increases the percent of fetal hemoglobin and decreases the percent of HbS and the number of dense sickled RBCs [3, 23].
In conclusion, while measurement of RBC deformability by ektacytometry does not require standardization of the diffraction pattern height in the general population, attention is required in sickle cell patients. We routinely use a standardized diffraction pattern height of 4.5 cm in our laboratories that allows comparisons between different SCA patients and follow-up of cohorts over time. In addition, the use of the camera gain of the LORRCA MaxSis to modulate the diffraction pattern heights may yield additional information that is clinically relevant to sickle cell patients.
References
- [1].Alvarez O, Montague NS, Marin M, O’Brien R and Rodriguez MM, Quantification of Sickle Cells in the Peripheral Smear as a Marker of Disease Severity. Fetal Pediatr Pathol (2014). [DOI] [PubMed] [Google Scholar]
- [2].Ballas SK and Smith ED, Red blood cell changes during the evolution of the sickle cell painful crisis. Blood 79 (1992), 2154–2163. [PubMed] [Google Scholar]
- [3].Bartolucci P, Brugnara C, Teixeira-Pinto A, Pissard S, Moradkhani K, Jouault H and Galacteros F, Erythrocyte density in sickle cell syndromes is associated with specific clinical manifestations and hemolysis. Blood 120 (2012), 3136–3141. [DOI] [PubMed] [Google Scholar]
- [4].Baskurt OK, Boynard M, Cokelet GC, Connes P, Cooke BM, Forconi S, Liao F, Hardeman MR, Jung F, Meiselman HJ, Nash G, Nemeth N, Neu B, Sandhagen B, Shin S, Thurston G and Wautier JL, New guidelines for hemorheological laboratory techniques. Clin Hemorheol Microcirc 42 (2009), 75–97. [DOI] [PubMed] [Google Scholar]
- [5].Baskurt OK, Hardeman MR, Uyuklu M, Ulker P, Cengiz M, Nemeth N, Shin S, Alexy T and Meiselman HJ, Comparison of three commercially available ektacytometers with different shearing geometries. Biorheology 46 (2009), 251–264. [DOI] [PubMed] [Google Scholar]
- [6].Bunn HF, Pathogenesis and treatment of sickle cell disease. N Engl J Med 337 (1997), 762–769. [DOI] [PubMed] [Google Scholar]
- [7].Connes P, Lamarre Y, Hardy-Dessources MD, Lemonne N, Waltz X, Mougenel D, Mukisi-Mukaza M, Lalanne-Mistrih ML, Tarer V, Tressieres B, Etienne-Julan M and Romana M, Decreased hematocrit-to-viscosity ratio and increased lactate dehydrogenase level in patients with sickle cell anemia and recurrent leg ulcers. PLoS One 8 (2013), e79680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Connes P, Lamarre Y, Waltz X, Ballas SK, Lemonne N, Etienne-Julan M, Hue O, Hardy-Dessources MD and Romana M, Haemolysis and abnormal haemorheology in sickle cell anaemia. Br J Haematol 165 (2014), 564–572. [DOI] [PubMed] [Google Scholar]
- [9].Kato GJ, Gladwin MT and Steinberg MH, Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev 21 (2007), 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Koutsiaris AG, Tachmitzi SV, Batis N, Kotoula MG, Karabatsas CH, Tsironi E and Chatzoulis DZ, Volume flow and wall shear stress quantification in the human conjunctival capillaries and post-capillary venules in vivo. Biorheology 44 (2007), 375–386. [PubMed] [Google Scholar]
- [11].Lamarre Y, Romana M, Lemonne N, Hardy-Dessources MD, Tarer V, Mougenel D, Waltz X, Tressieres B, Lalanne-Mistrih ML, Etienne-Julan M and Connes P, Alpha thalassemia protects sickle cell anemia patients from macro-albuminuria through its effects on red blood cell rheological properties. Clin Hemorheol Microcirc 57 (2014), 63–72. [DOI] [PubMed] [Google Scholar]
- [12].Lamarre Y, Romana M, Waltz X, Lalanne-Mistrih ML, Tressieres B, Divialle-Doumdo L, Hardy-Dessources MD, Vent-Schmidt J, Petras M, Broquere C, Maillard F, Tarer V, Etienne-Julan M and Connes P, Hemorheological risk factors of acute chest syndrome and painful vaso-occlusive crisis in children with sickle cell disease. Haematologica 97 (2012), 1641–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lemonne N, Charlot K, Waltz X, Ballas SK, Lamarre Y, Lee K, Hierso R, Connes C, Etienne-Julan M, Romana M and Connes P, Hydroxyurea treatment does not increase blood viscosity and improves red blood cell rheology. Haematologica (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mohandas N and Evans E, Rheological and adherence properties of sickle cells. Potential contribution to hematologic manifestations of the disease. Ann N Y Acad Sci 565 (1989), 327–337. [DOI] [PubMed] [Google Scholar]
- [15].Nebor D, Broquere C, Brudey K, Mougenel D, Tarer V, Connes P, Elion J and Romana M, Alpha-thalassemia is associated with a decreased occurrence and a delayed age-at-onset of albuminuria in sickle cell anemia patients. Blood Cells Mol Dis 45 (2010), 154–158. [DOI] [PubMed] [Google Scholar]
- [16].Rabai M, Detterich JA, Wenby RB, Hernandez TM, Toth K, Meiselman HJ and Wood JC, Deformability analysis of sickle blood using ektacytometry. Biorheology 51 (2014), 159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rees DC, Williams TN and Gladwin MT, Sickle-cell disease. Lancet 376 (2010), 2018–2031. [DOI] [PubMed] [Google Scholar]
- [18].Sheikh S, Rainger GE, Gale Z, Rahman M and Nash GB, Exposure to fluid shear stress modulates the ability of endothelial cells to recruit neutrophils in response to tumor necrosis factor-alpha: a basis for local variations in vascular sensitivity to inflammation. Blood 102 (2003), 2828–2834. [DOI] [PubMed] [Google Scholar]
- [19].Streekstra GJ, Dobbe JG and Hoekstra AG, Quantification of the fraction poorly deformable red blood cells using ektacytometry. Opt Express 18 (2010), 14173–14182. [DOI] [PubMed] [Google Scholar]
- [20].Tripette J, Alexy T, Hardy-Dessources MD, Mougenel D, Beltan E, Chalabi T, Chout R, Etienne-Julan M, Hue O, Meiselman HJ and Connes P, Red blood cell aggregation, aggregate strength and oxygen transport potential of blood are abnormal in both homozygous sickle cell anemia and sickle-hemoglobin C disease. Haematologica 94 (2009), 1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Vent-Schmidt J, Waltz X, Romana M, Hardy-Dessources MD, Lemonne N, Billaud M, Etienne-Julan M and Connes P, Blood Thixotropy in Patients with Sickle Cell Anaemia: Role of Haematocrit and Red Blood Cell Rheological Properties. PLoS One 9 (2014), e114412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Verger E, Schoevaert D, Carrivain P, Victor JM, Lapoumeroulie C and Elion J, Prior exposure of endothelial cells to hydroxycarbamide alters the flow dynamics and adhesion of sickle red blood cells. Clin Hemorheol Microcirc 57 (2014), 9–22. [DOI] [PubMed] [Google Scholar]
- [23].Ware RE, How I use hydroxyurea to treat young patients with sickle cell anemia. Blood 115 (2010), 5300–5311. [DOI] [PMC free article] [PubMed] [Google Scholar]


