Abstract
Background
Cognitive-behavioral therapy (CBT) is commonly adopted in pain management programs for patients with chronic low back pain (CLBP). However, the benefits of CBT are still unclear.
Objectives
This review investigated the effectiveness of CBT on pain, disability, fear avoidance, and self-efficacy in patients with CLBP.
Methods
Databases including PubMed, EMBASE, Web of Science, Cochrane Library, and PsycINFO were searched. RCTs examining the effects of CBT in adults with CLBP were included. The data about the outcome of pain, disability, fear avoidance, and self-efficacy were retained. Subgroup analysis about the effects of CBT on posttreatment was conducted according to CBT versus control groups (waiting list/usual care, active therapy) and concurrent CBT versus CBT alone. A random-effects model was used, and statistical heterogeneity was explored.
Results
22 articles were included. The results indicated that CBT was superior to other therapies in improving disability (SMD −0.44, 95% CI −0.71 to −0.17, P < 0.05), pain (SMD −0.32, 95% CI −0.57 to −0.06, P < 0.05), fear avoidance (SMD −1.24, 95% CI −2.25 to −0.23, P < 0.05), and self-efficacy (SMD 0.27, 95% CI 0.15 to 0.40, P < 0.05) after intervention. No different effect was observed between CBT and other therapies in all the follow-up terms. Subgroup analysis suggested that CBT in conjunction with other interventions was in favor of other interventions alone to reduce pain and disability (P < 0.05).
Conclusion
CBT is beneficial in patients with CLBP for improving pain, disability, fear avoidance, and self-efficacy in CLBP patients. Further study is recommended to investigate the long-term benefits of CBT. This meta-analysis is registered with Prospero (registration number CRD42021224837).
1. Introduction
Low back pain (LBP) is a public health concern that contributes to years lived with disability globally [1]. Globally, the estimated age-standardized point prevalence of LBP was 7.50% in 2017 [2] and ranked ninth for the cause of years lived with disability and health burden [3]. One study from the UK in 2018 claimed that about 10 to 15% of LBP cases go on to develop chronic low back pain (CLBP) which is defined as pain lasting over 12 weeks [4]. A review published in 2015 based on cross-sectional population and cohort studies reported that the CLBP prevalence was 19.6% in those aged between 20 and 59 [5]. CLBP is considered to have multifaceted pathophysiology that is influenced by somatic pathology and psychological and social factors [6]. Psychological indicators such as depression, anxiety, fear avoidance, and low self-efficacy are associated with an increased risk of developing pain and disability in patients with CLBP [7–10]. The management of CLBP includes medication and therapeutic exercise. While these interventions demonstrated modest improvement, pain recurrence remains [11]. One guideline in 2017 recommended a combined psychological and physical approach if previous treatment was ineffective or in cases where medium to high risk of chronicity was identified [12].
Cognitive-behavioral therapy (CBT) is a set of interventions that involve 4 broad components: the patient's knowledge and understanding about pain and their pain perception, the learning of active coping strategies, maintaining the coping strategies, and problem-solving plans to deal with pain and challenging situation. Although there are several systemic reviews of CBT intervention for pain alleviation in CLBP patients, most reviews have limitations. One is the restriction of a single comparison group [13]. Two reviews did not evaluate the long-term effects of CBT on CLBP patients [13, 14]. Two systemic reviews investigated the long-term effects but were published in the years 2007 and 2015 where their findings may be out-of-date [15, 16]. Therefore, the clinical benefits of CBT to reduce pain and disability immediately after treatment and during follow-up periods remain unclear. An updated pooled estimation of quantitative analysis with a larger sample size than previous studies would provide adequate power to evaluate the posttreatment and long-term effects of CBT in CLBP patients.
Psychological factors such as depression, anxiety, fear avoidance, and low self-efficacy are related to increased risk in patients with CLBP [7–10]. However, there has been already one review that investigated the effects of CBT on depression reduction in patients with CLBP [13]. In addition, there are not enough articles evaluating anxiety to complete a review. Therefore, the psychological outcomes in the present study were fear avoidance and self-efficacy instead of depression and anxiety. Touche et al. made a cross-sectional study to investigate the relationship between psychological variables, lumbar spine range of motion, and pain intensity in patients with CLBP. The results indicated that patients with low self-efficacy tend to increase pain intensity during lifting tasks [17]. Fear avoidance is characterized by escape and avoidance behaviors. The immediate consequences are reduced participation in daily activities due to the expectation of pain exacerbation [18]. For patients with CLBP, longstanding avoidance and physical inactivity have a detrimental impact on the musculoskeletal and cardiovascular systems, leading to disuse syndrome that further worsens the pain problem [19]. Therefore, specific intervention strategies should be implemented to improve self-efficacy and fear avoidance in patients with CLBP to achieve a positive clinical outcome. Wenzel et al. claimed that CBT may modify maladaptive behaviors and overcome avoidance behavior to improve self-care [20]. However, there is still a lack of review about the effects of CBT on improving self-efficacy and fear avoidance for CLBP patients. Therefore, it is imperative to conduct a systemic review to assess (1) the benefits of CBT on pain and disability relief at posttreatment and during different follow-up periods and (2) the effectiveness of CBT on improving fear avoidance and self-efficacy in patients with CLBP.
2. Materials and Methods
This review followed the guidelines for Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) (see Supplementary Material 1 for detailed information of PRISMA Checklist). The protocol was registered with Prospero (registration number CRD42021224837).
2.1. Criteria for Considering Studies for This Review
Studies were included in the review if they were randomized controlled trials (RCTs) that evaluated the effects of CBT on patients diagnosed with CLBP.
The inclusion criteria were as follows: (1) patients (>18 years old) diagnosed with chronic low back pain (pain duration >3 months); (2) with or without leg pain; (3) studies adopted CBT alone or CBT combined with other therapies as an intervention arm; (4) CBT delivered face-to-face, web-based, or telephone-based in one to one or group-based setting; (5) the comparison arm that may include waiting list (WL), usual care (UC), or any other active therapies (AT): exercise, physical therapy, or drug therapy; and (6) acceptance and commitment therapy.
Exclusion criteria were as follows: (1) patient population having other specific pathology, including spinal stenosis, lumbar instability, postsurgical pain, pregnancy-related LBP, spinal fractures, cauda equina, or spinal tumors; (2) other chronic pain caused by other pathologies such as rheumatoid arthritis, polymyalgia rheumatic, and fibromyalgia; and (3) lack of documentation of the CBT content.
2.2. Primary and Secondary Outcomes
Pain intensity and disability level were the primary outcomes. Fear of avoidance and/or self-efficacy were the secondary outcomes. Pain intensity was evaluated by the Visual Analog Scale (VAS) or Numeric Rating Scale (NRS). Disability was measured by the Roland Morris Disability Questionnaire (RMDQ) or the Oswestry Disability Index (ODI) and Activities of Daily Living (ADL). Fear of avoidance was evaluated by the Fear-Avoidance Beliefs Questionnaire (FABQ). Self-efficacy was assessed by the Pain Self-efficacy Questionnaire (PSEQ).
2.3. Search Methods for Identification of Studies and Data Extracted
Articles were searched in the following five electronic databases: (1) PubMed, (2) EMBASE, (3) Web of Science, (4) Cochrane Library, and (5) PsycINFO. All literature studies published in English between 1st January 1980 and 20th November 2021 were searched without any restriction of countries. Reference lists of all selected articles were independently screened to identify additional studies that were not identified in the initial search. Two reviewers (J. Y., F. Z) screened for eligible studies from titles and abstracts. Potentially relevant studies were obtained in full text and independently assessed for inclusion. Two reviewers (J. Y., F. Z) extracted data and assessed the quality of the evidence independently. Two reviewers (J. Y., X. C) assessed the risk of bias independently. Any disagreement was discussed to reach a consensus during moderation meetings.
The following data were extracted using a standardized format: author's name, year of publication, the country where the study was conducted, study period, follow-up time, the total number of people included in the study, blinding types, randomization procedure, and sociodemographic characteristics. The outcome of pain intensity, disability, fear of avoidance, and self-efficacy were recorded before and after the intervention and at the follow-up time points of 3, 6, and 12 months. Outcome values were extracted or converted into mean and standard deviations. If there were missing values, the authors attempted to contact the authors to acquire the missed values.
2.4. Assessment of Risk of Bias in Included Studies
The risk of bias for each study was independently assessed by two reviewers according to the 13 criteria recommended by the Cochrane Back and Neck Review Group [21]. It is a tool that is the same as the recommended Cochrane Collaboration but has additional items relevant to the assessment of nondrug trials. It also contains 6 domains of selection bias, performance bias, detection bias, attritions bias, reporting bias, and other bias. For each study, each criterion was scored as “low,” “high,” or “unclear risk.” A consensus method was adopted to conclude the risk of bias of the included studies. However, if an agreement was not achieved at any stage, a third review author was consulted. The GRADE (Grading of Recommendations Assessment, Development, and Evaluation) guideline was applied to assess the confidence of the effect estimates based on the following criteria: risk of bias, inconsistency, imprecision, indirectness, and publication bias.
2.5. Measures of Treatment Effects
Data analysis was conducted in Review Manager Software 5.4.1. All of the outcomes were performed as the change values of mean difference and standard deviation (SD) before treatment and after treatment. As these outcomes were continuous data from different scales, the effect sizes were calculated by standardized mean difference (SMD) and 95% confidence intervals. A two-sided P value of less than 0.05 was considered statistically significant. A random-effects model was used to analyze the data. A negative effect size indicated that CBT was more beneficial than the comparison therapies. When there were several comparison groups in the same study, we halved the number of participants in the shared intervention group, which corrected the error introduced by double-counting [22].
2.6. Heterogeneity and Sensitivity Analysis
Statistical heterogeneity was examined graphically by forest plots, standardized Chi-squared (χ2) test, and I2 statistic. I2 statistics were interpreted as follows: statistical significance was considered at a P value of <0.05 and an I2 of >50%. An I2 of ≥50% might be considered as substantial heterogeneity. Sensitivity analysis was performed to test the influence of each study by visual evaluation of the funnel plot and exclusion sensitivity plot, searching for any asymmetry. Subgroup analyses were conducted according to different control groups: WL/UC, AT, and CBT + Control vs. Control group.
3. Results
3.1. Searched Studies and Characteristics
The literature search identified 1752 articles from 5 electronic databases. After excluding irrelevant studies, 22 eligible articles, covering 20 separate RCTs, met the inclusion criteria [23–44] (Figure 1). Turner et al. [42] and Cherkin et al. 2016 belong to the same RCT and Harris et al. [30] was part of a larger randomized controlled multicenter trial conducted by Reme et al. [37]. All of the included 20 RCTs were published in English and conducted from nine different countries (6 in the United States, 3 in Germany, 3 in the United Kingdom, 2 in Norway, 2 in Australia, one each in the Netherlands, Sweden, Italy, and Pakistan). In total, 3003 patients were examined. Study sample sizes ranged from 44 to 363 (mean = 152). The intervention types in 15 studies were face-to-face, three studies were Internet-based, and 4 studies were based on telephone, text, audiotape, and mixed methods (face-to-face and telephone). The mean intervention duration of CBT was 10 weeks, ranging from 3 to 54 weeks. Five studies had three months follow-up, 6 studies had six months follow-up, and five included one-year follow-up. One article had nine months of follow-up. The comparison group included WL/UC (n = 12), AT (n = 7). Other detailed descriptions of the characteristics of the included studies are shown in Table 1. The description of CBT and the comparison groups intervention type are shown in Table 2.
Figure 1.
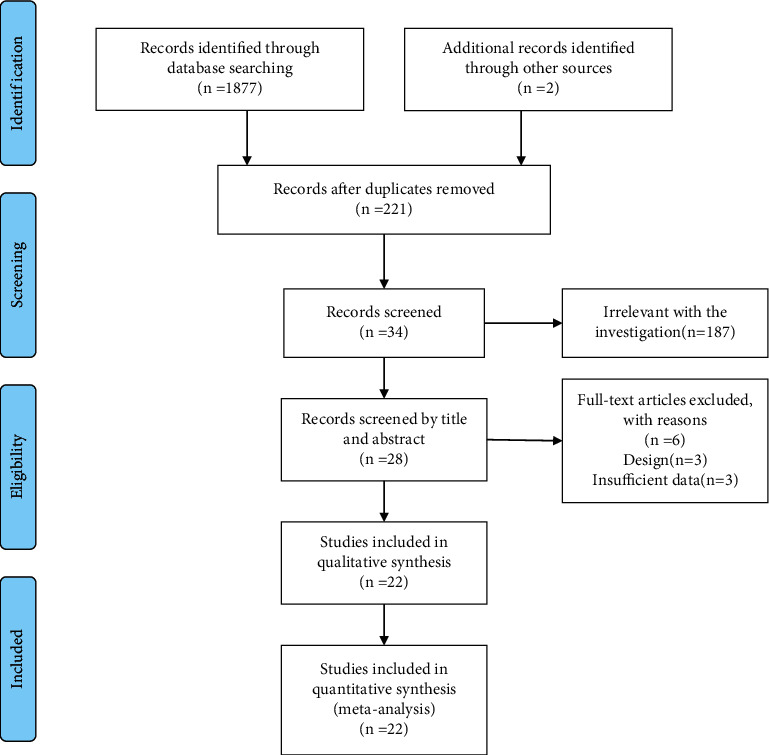
An illustration of the flow at each stage of the study.
Table 1.
Basic characteristics of the included studies.
| Author year | Country | Total sample size (male/female) | Educational attainment (primary/high) | Marital status (married/unmarried) | Outcomes | Follow-up time (month) |
|---|---|---|---|---|---|---|
| Balser 1997 | German | 76 (18/58) | NA | NA | Pain intensity, disability1 | 3 |
| Beth 2021 | USA | 263 (130/131) | 6/257 | 160/103 | VAS, PESQ | 3 |
| Buhrman 2004 | Sweden | 51 (19/32) | 24/32 | NA | Pain diary | 3 |
| Carpenter 2012 | USA | 131 (22/109) | 60/71 | NA | Pain intensity, RMD, FABQ, self-efficacy scale | |
| Cherkin 2016 | USA | 341 (224/117) | 26/3155 | 249/92 | Characteristic pain intensity, RDQ (modified) | 6, 12 |
| Christiansen 2010 | German | 60 (23/37) | 48/12 | 36/24 | ADL, NRS | 3 |
| Godfrey 2020 | UK | 219 (89/130) | 111/134 | 127/92 | NRS, RMD, PESQ | 12 |
| Gould 2020 | USA | 67 (60/7) | NA | NA | Pain intensity2, RMDQ | |
| Harris 2017 | Norway | 147 (73/74) | 87/60 | 102/45 | ODI, FABQ | |
| Ivar brox 2003 | Norway | 61 (25/36) | NA | 54/7 | ODI, FABQ, VAS | |
| Johnson 2007 | UK | 234 (94/140) | NA | 171/63 | VAS, RMDQ | 3, 9, 15 |
| Khan 2014 | Pakistan | 54 (25/29) | NA | NA | VAS, RMDQ | |
| Monticone 2013 | Italy | 90 (38/52) | 82/8 | 60/30 | NRS, RMD, TSK | |
| Newton 1995 | Australia | 44 (NA) | NA | NA | Pain diary, PDI | |
| Petrozzi 2019 | Australia | 106 (52/54) | NA | NA | PSEQ, RMDQ, NRS | 6, 12 |
| Pincus 2015 | UK | 99 (35/64) | NA | NA | RMDQ, NRS | 6 |
| Reme 2016 | Norway | 308 (140/168) | 190/88 | 215/93 | ODI | 6, 12 |
| Rutledge 2018a | US | 61 (55/6) | 25/36 | 40/21 | RMDQ, NRS | |
| Rutledge 2018b | US | 66 (41/25) | 10/56 | 40/26 | RMDQ, NRS | |
| Schweikert 2006 | Germany | 363 (339/24) | NA | NA | Pain, Disability3 | 6 |
| Smeets 2006 | Netherlands | 162 (80/82) | 104/58 | NA | VAS, RDQ | |
| Turner 2016 | USA | 341 (224/171) | 26/315 | 249/92 | PESQ | 6, 12 |
NA: not answer; DDS: Du¨sseldorf disability scale; HCS: Heidelberg coping scale; 1pain diary, 2descriptor differential scale (higher scores indicating higher pain intensity); 3pain (German school grades). Disability: Hannover functional questionnaire. Primary educational attainment, ≤12 years education. High educational attainment, college or higher educational experience.
Table 2.
CBT and comparison group information.
| Author year | Groups | Type of CBT | CBT sessions | CBT duration (W) | Comparison type |
|---|---|---|---|---|---|
| Balser 1997 | CBT + UC vs. UC | Face-to-face | 12 sessions ∗ 120 min | 12 | UC: various forms of medical treatment such as pain medication, nerve blocks, transcutaneous electrical stimulation, and physical therapy, but not surgery. |
|
| |||||
| Beth 2021 | CBT vs. PE CBT vs. ER | Face-to-face | 8 sessions ∗ 120 min | 8 | PE: pain education, matched to empowered relief on 4 key factors: duration, structure, format, and site. ER: empowered relief consists of a single-session, 2-hour pain class that includes pain neuroscience education, mindfulness principles, and CBT skills. |
|
| |||||
| Buhrman 2004 | CBT vs. WL | Internet-based | Webpages | 8 | WL: nonspecific treatment control had been used instead of a waiting list. |
|
| |||||
| Carpenter 2012 | CBT vs. WL | Internet-based | Webpages | 3 | WL: nonspecific treatment control had been used instead of a waiting list. |
|
| |||||
| Cherkin 2016 | CBT vs. UC, CBT vs. MBSR | Face-to-face | 2 sessions ∗ 120 min ∗ 8 W | 8 | UC: free to seek whatever treatment but no MBSR training or CBT. MBSR: mindfulness-based stress reduction, a program does not focus specifically on a particular condition such as pain. |
|
| |||||
| Christiansen 2010 | CBT + UC vs. UC | Face-to-face | 2 sessions ∗ 30 min | 3 | UC: standard outpatient back pain program. |
|
| |||||
| Godfrey 2020 | ACT + PT vs. PT | Face-to-face and telephone call | 3 sessions ∗ (60 min ∗ 2 + 20 min) | 6 | PT: usual physical therapy including manual therapy techniques. |
|
| |||||
| Gould 2020 | CBT + placebo vs. placebo | Face-to-face | 6 sessions (1 ∗ 90 min + 5 ∗ 60 min) | 8 | Placebo: a dose of benztropine mesylate 0.125 mg daily was chosen. |
|
| |||||
| Harris 2017 | BI vs. BI + CBT vs. BI + PE | Face-to-face | 7 sessions ∗ 90 min | 12 | BI: brief intervention, a brief cognitive, clinical examination program addressing pain and fear avoidance. PE: Group physical exercise consisted of strength and endurance training and relaxation. |
|
| |||||
| Ivar brox 2003 | Surgery vs. CBT + PT | Face-to-face | NA | 12 | Surgery: posterolateral fusion with transpedicular screws of the L4–L5 segment and/or the L5–S1 segment. PT: customarily prescribed physiotherapy, including exercises. |
|
| |||||
| Johnson 2007 | CBT + UC vs. UC | Face-to-face and leaflet | 8 sessions ∗ 120 min | 6 | UC: received no further intervention and continued to be treated as usual. |
|
| |||||
| Khan 2014 | CBT + exercise vs. exercise | Face-to-face | 3 sessions ∗ 12 ∗ ? min | 12 | Exercise: general exercise protocol under the supervision of a physical therapist. |
|
| |||||
| Monticone 2013 | CBT + exercise vs. exercise | Face-to-face | 5 ∗ 60 min + 12 ∗ 60 min | 54 | Exercise: general exercise protocol under the supervision of a physical therapist. |
|
| |||||
| Newton 1995 | CBT vs. EMGBF vs. WL | Face-to-face | 5 ∗ 90 min | 4 | EMGBF: electromyographic biofeedback, consisted firstly of a psychoeducational session, then introduced to the pain-tension-pain cycle. WL: nonspecific treatment control had been used instead of a waiting list. |
|
| |||||
| Petrozzi 2019 | CBT + PT vs. PT | Internet-based | 5 modules, online-based | 8 | PT: included manual therapy in combination with other modalities such as advice, education, and exercise. |
|
| |||||
| Pincus 2015 | CBT vs. PT | Face-to-face | 8 sessions ∗ 50 min | 12 | PT: physiotherapy was delivered as usual within services, with the stipulation that it included at least 60% exercise. |
|
| |||||
| Reme 2016 | BI + CBT vs. BI | Audiotaped | 7 sessions ∗ ? min | 8 | BI: brief intervention, a brief cognitive, clinical examination program based on a noninjury model addressing pain and fear avoidance, where return to normal activity and work is the main goal. |
|
| |||||
| Rutledge 2018a | CBT vs. UC | Text-based | 12 sessions (1 ∗ 120 min + 11 ∗ 30 min) | 8 | UC: controlled for nonspecific benefits of therapy. |
|
| |||||
| Rutledge 2018b | CBT vs. UC | Telephone-based | 12 sessions (1 ∗ 120 min + 11 ∗ 30 min) | 8 | UC: controlled for nonspecific benefits of therapy. |
|
| |||||
| Schweikert 2006 | CBT + UC vs. UC | Face-to-face | 6 sessions ∗ 90 min | 3 | UC: standardized conventional 3-week inpatient rehabilitation program consisting of daily physiotherapy, massage of the spinal region, electrotherapeutical measures, 1-hour seminar regarding back training, twice-daily exercise program. |
|
| |||||
| Smeets 2006 | CBT vs. PT, CBT vs. WL | Face-to-face | 18 sessions, total: 11.5 ∗ 60 min | 10 | PT: aerobic training on a bicycle and strength and endurance training. WL: not allowed to participate in diagnostic or therapeutic procedures because of their CLBP. |
|
| |||||
| Turner 2016 | CBT vs. UC, CBT vs. MBSR | Face-to-face | 2 sessions ∗ 120 min ∗ 8 W | 8 | UC: free to seek whatever treatment but no MBSR training or CBT. MBSR: mindfulness-based stress reduction: a program does not focus specifically on a particular condition such as pain. |
UC: usual care; PE: pain education; ER: empowered relief; WL: waiting-list; PT: physical therapy; BI: brief intervention; MBSR: mindfulness-based stress reduction; EMGBF: electromyographic biofeedback.
3.2. Risk of Bias in Included Studies
The risk of bias was assessed based on the Cochrane Back and Neck Review Group (Figure 2). All of the 22 studies randomly assigned patients into groups. Random sequence generation was based on computer (n = 15) [23, 25, 30–33, 36–43], random number table [24, 27] (n = 2), toss dice [26] (n = 1), third office [28, 29, 44] (n = 3), and unclear randomization method [34] (n = 1). Due to the content of CBT intervention, the participants and CBT providers were not blinded in most studies, whereas the outcome accessors were blinded in most studies.
Figure 2.
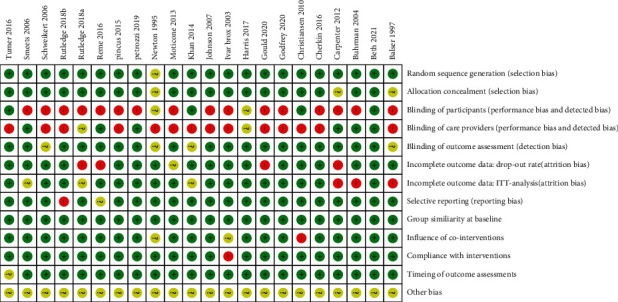
Risk of bias of the included studies. Most studies were low risk in the selection bias, while the performance and detected bias were high risks.
3.3. Quality of the Evidence and Effects of Intervention
The quality of the evidence ranged between high and very low due to the performance bias and high heterogeneity. Fifteen studies that investigated pain intensity and 18 studies that investigated disability were included. Five studies investigated the effect of CBT on self-efficacy and five studies assessed the outcome of fear avoidance. The quality of the evidence and the effect estimates of all the outcomes are shown in Table 3.
Table 3.
Grade of evidence and effect estimates.
| Analyses | No of studies and participants | Effect estimates (95% CI) | I 2 (%) | Grade |
|---|---|---|---|---|
| Primary outcomes | ||||
| Pain | ||||
| After intervention | 2169 (15 studies) | −0.32 (−0.57 to –0.06) | 87 | Low1,2 |
| 3 months follow-up | 524 (4 studies) | 0.17 (−0.53 to 0.19) | 71 | Low1,2 |
| 6 months follow-up | 757 (3 studies) | −0.1 (−0.25 to 0.05) | 0 | High3 |
| 12 months follow-up | 1037 (5 studies) | −0.19 (−0.38 to 0.01) | 54 | Low1,2 |
| Disability | ||||
| After intervention | 2237 (16 studies) | −0.44 (−0.71 to −0.17) | 89 | Low1,2 |
| 3 months follow-up | 294 (2 studies) | −0.19 (−0.42 to 0.04) | 80 | Very low1,2,3 |
| 6 months follow-up | 757 (3 studies) | −0.11 (−0.31 to 0.09) | 43 | High |
| 12 months follow-up | 1184 (6 studies) | −0.52 (−1.38 to 0.34) | 11 | Moderate1 |
| Secondary outcomes | ||||
| Fear avoidance | 505 (5 studies) | −1.24 (−2.25 to −0.23) | 96 | Low1,2 |
| Self-efficacy | 1060 (5 studies) | 0.27 (0.15 to 0.40) | 74 | Moderate1 |
| Subgroups analyses | ||||
| Pain | ||||
| CBT vs. WL/UC | 367 (5 studies) | −0.05 (−0.35 to 0.26) | 45 | Low1,3 |
| CBT vs. AT | 667 (4 studies) | −0.03 (−0.51 to 0.46) | 88 | Moderate2 |
| Concurrent CBT | 1035 (8 studies) | −0.67 (−1.21 to −0.13) | 94 | low1,2 |
| Disability | ||||
| CBT vs. WL/UC | 564 (6 studies) | −0.34 (−0.56 to −0.12) | 37 | Moderate1 |
| CBT vs. AT | 400 (4 studies) | −0.03 (−0.23 to 0.18) | 1 | Moderate1 |
| Concurrent CBT | 1243 (9 studies) (9 studies) | −0.81 (−1.35 to −0.27) | 95 | Moderate2 |
GRADE interpretation: 1>50% of subjects came from studies with a performance bias; 2the heterogeneity was large (I2 >50%, representing potentially substantial heterogeneity); 3the total population size is less than 400 or there is only one study.
3.4. Primary Outcomes
Pain Intensity. Fifteen studies (n = 2169 participants) were involved in comparing the effects of CBT with other therapies before and after the interventions. We found low-quality evidence that there was a better effect of CBT on reducing pain compared with other therapies. The effect was statistically significant (SMD −0.32, 95% CI −0.57 to −0.06, I2 = 87%, P=0.01). Two articles (Monticone et al. 2013 [33], Khan et al. 2014 [32]) with extreme outliers and accounted for a large percentage of the statistical heterogeneity were excluded. However, the overall effect changed to a negative result (SMD −0.11, 95% CI −0.23 to −0.01, I2 = 37%, P=0.07). There was no statistical significance at the follow-up 3, 6, and 12 months (Figure 3).
Figure 3.
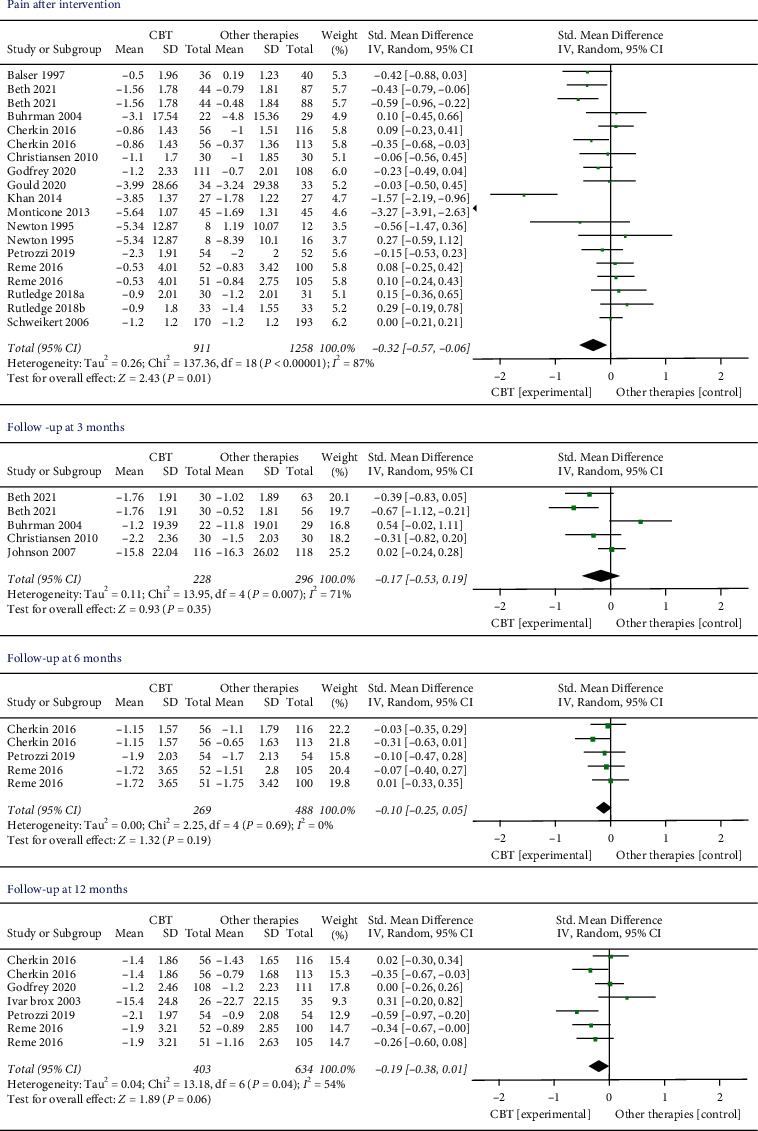
Pain intensity immediately after intervention and during the follow-up period. Compared with other therapies, the overall effect of CBT on pain outcome immediately after intervention was significant (P < 0.05). All the follow-up periods failed to show statistical significance.
Disability. There were 16 trials (n = 2237 participants) that compared the effects of CBT to other therapies before and after the interventions. The results showed that CBT provided a significant disability improvement compared with other therapies (SMD −0.44, 95% CI −0.71 to −0.17, I2 = 89%, P=0.001). Two articles [32, 33] with extreme outliers and accounted for a large percentage of the statistical heterogeneity were excluded, while the overall effect remained unchanged (SMD −0.16, 95% CI −0.25 to −0.06, I2 = 11%, P=0.001). Two, 3, and 5 studies measured the pain at 3, 6, and 12 months later, respectively. However, there was no significant difference in all the follow-up times (Figure 4).
Figure 4.
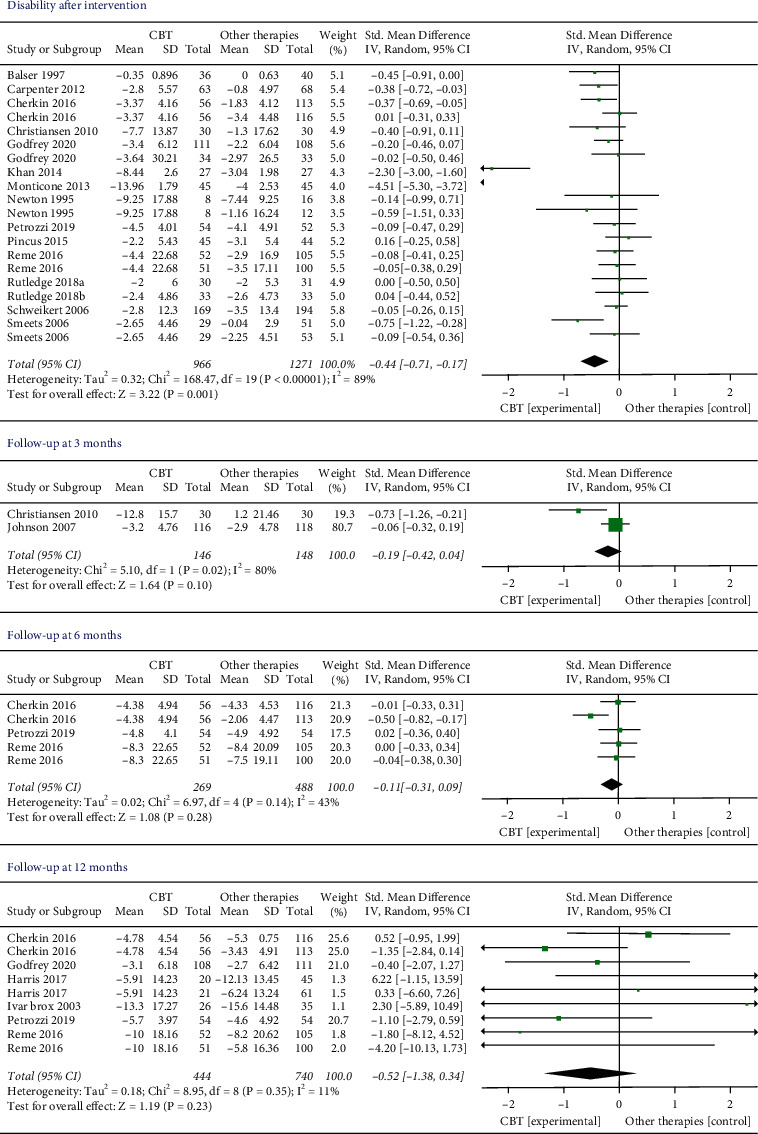
Disability levels immediately after intervention and during the follow-up period. Compared with other therapies, the overall effect of CBT on disability outcome immediately after intervention was significant (P < 0.05). All the follow-up periods failed to show statistical significance.
3.5. Secondary Outcomes
Self-Efficacy. Five studies included 1060 participants that assessed the results of self-efficacy. We found low-quality evidence that CBT was significantly more effective than other interventions (SMD 0.27, 95% CI 0.15 to 0.40, I2 = 74%, P=0.0008).
Fear Avoidance. Five studies containing 505 participants measured the outcome of fear avoidance. There was low-quality evidence that CBT may produce better effects compared to other interventions. The effect was statistically significant (SMD −1.24, 95% CI −2.25 to −0.23, I2 = 96%, P=0.002) (Figure 5).
Figure 5.
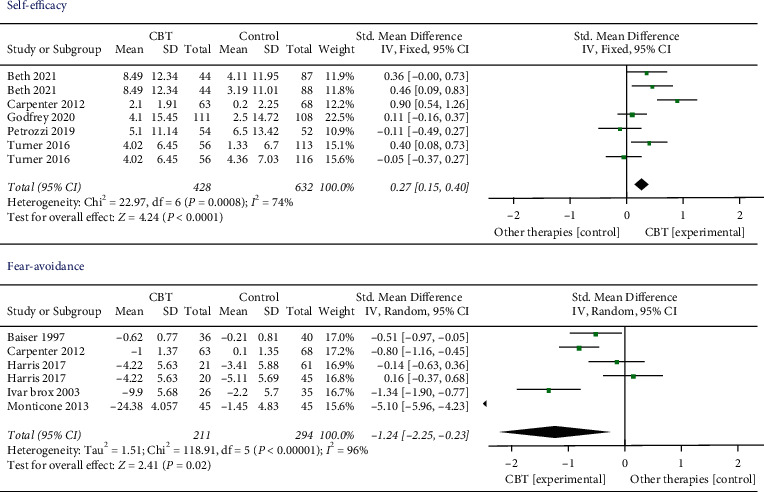
Self-efficacy and fear avoidance after intervention. The overall effect of CBT on self-efficacy and fear avoidance after intervention was in favor of other therapies (P < 0.05).
3.6. Subgroup Analysis
We evaluated the subgroup of CBT on posttreatment according to CBT versus control groups (waiting list/usual care, active therapy) and concurrent CBT versus CBT alone about the outcome of pain and disability (Figures 6 and 7). CBT vs. WL/UC. Five studies and six studies compared the effects of CBT with WL/UC on the results of pain and disability, respectively. For the result on pain, low-quality evidence suggested that CBT did not result in a statistically better effect than the sham WL/UC group (SMD −0.05, 95% CI −0.35 to 0.26; participants = 367; I2 = 45%). For disability, moderate-quality evidence suggested that CBT had a significantly better effect than WL/UC (SMD −0.34, 95% CI −0.56 to −0.12; participants = 564; I2 = 37%, P=0.003).
Figure 6.
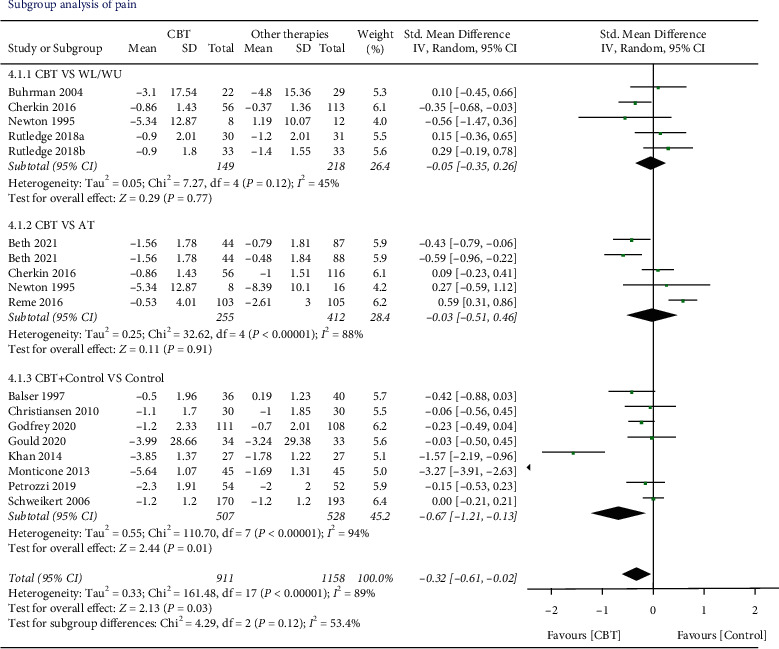
The outcome of the pain of different control subgroups. Combined CBT with other therapies showed a greater overall effect than other therapies alone (P < 0.05).
Figure 7.
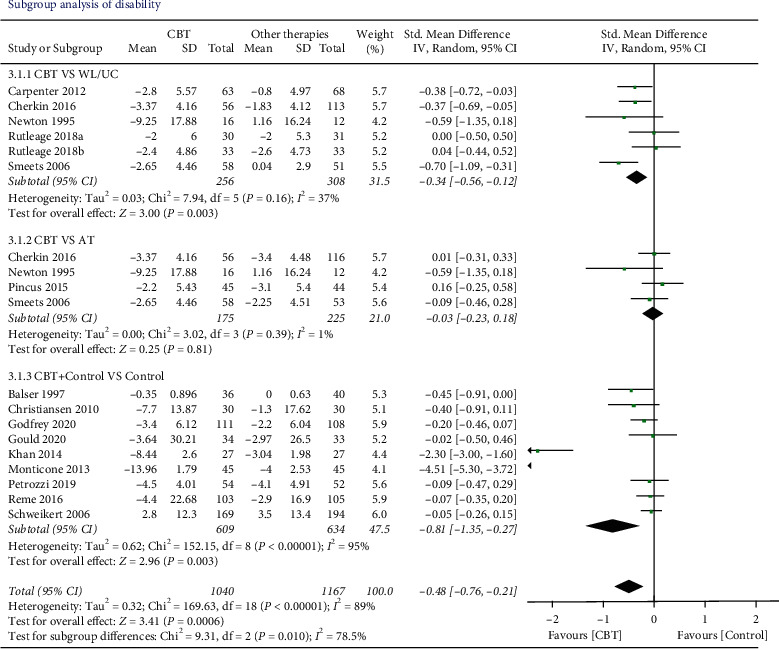
The outcome of disability of different control subgroups. Compared with the waiting list or usual care, the overall effect of CBT on improving disability showed statistical significance (P < 0.05). Combining CBT with other therapies showed a greater overall effect than other therapies alone (P < 0.05).
CBT versus AT. Four studies examined the effects of CBT versus AT on the result of pain and disability, respectively. It was found that there was no better treatment effect comparing CBT to AT on either pain (SMD −0.03, 95% CI −0.51 to 0.46; participants = 667; studies = 4; I2 = 88%, P=0.91) or disability (SMD 0.03, 95% CI −0.23 to 0.18; participants = 400; studies = 4; I2 = 1%, P=0.81).
CBT plus Control vs. Control. For the outcome of pain, eight studies were included in comparing the effects of combined CBT and control with the control alone. The results showed CBT plus control provided a better effect on pain-relieving than control alone (SMD −0.67, 95% CI −1.21 to −0.13; participants = 1035; studies = 8; I2 = 94%, P=0.01), and 9 studies included the outcome of disability within the design of CBT plus control versus control. Statistically significant better effects were found (SMD −0.81, 95% CI −1.35 to −0.27; participants = 1243; I2 = 95%; P=0.003). However, when two articles [32, 33] with extreme outliers were excluded, the overall effect of pain changed to a negative result (SMD −0.12, 95% CI −0.25 to 0.02, I2 = 0%, P=0.09) and the effect of disability remained unchanged (SMD −0.13, 95% CI −0.25 to −0.01, I2 = 0%, P=0.03).
3.7. Heterogeneity Inspection and Sensitivity Analysis
By visual inspection, outliers were removed to assess their influence on the overall effect. Two articles [32, 33] included in the analysis showed extreme outliers and raised the heterogeneity to very high values. For Monticone et al. [33], it had no overlap with any other study in the analysis. High efficacy estimate was suspected as the long intervention period (1 year) compared with other studies (mean = 7.95 weeks). However, the author did not offer any explanation in the article. For Khan et al. [32], the author did not provide an explanation for the presence of extreme outliers.
4. Discussion
This is the first meta-analysis to evaluate the effect of CBT beyond the intervention period by not restricting CBT intervention types or CBT providers. This is also the first review to investigate the effect of CBT on improving self-efficacy. Twenty-one studies were included in this meta-analysis. Most of the studies were low- to moderate-quality evidence and two studies were found to be of high-quality evidence.
4.1. Pain and Disability during Different Periods
For pain intensity immediately after the intervention, 15 studies of low-quality evidence supported the small or very small benefits of CBT over other therapies for reducing pain. However, this finding must be interpreted with caution as there were two outlier articles with high heterogeneity that result in contradicting results upon removal of those two studies. Sixteen studies of low quality of evidence reported CBT was superior to other therapies immediately after intervention to reduce disability. The quality of evidence was hampered by the high heterogeneity and performance bias. Thus, further well-designed RCTs are required to provide high-quality evidence.
No significant difference was observed between CBT and other therapies for relieving pain or improving disability during all follow-up time points. These results seem to suggest that the effects of CBT do not go beyond the intervention period. It is unclear as to the exact reason for the lack of benefit during the follow-up period. It has long been documented that relatively little is known about the specific biobehavioral mechanisms of CBT that lead to chronic pain and disability improvement [45, 46]. Further study is required to establish the optimal strategy to maintain the medium-long-term efficacy of CBT. A systematic review on CBT also indicated that even the short-term effect of CBT is limited and an adequate reinforcement phase is essential to maintain the benefit of CBT acquired during the intervention period [47]. Despite the finding of the present study, further investigation is required to confirm the finding on the benefit of CBT of pain and disability beyond the intervention period as the studies included in this analysis had inadequate follow-up periods.
4.2. Secondary Outcomes and Subgroup Analysis
Subgroup analysis indicated significant improvement in pain and disability when CBT is provided in conjunction with other therapies. Compared to alternative active treatments, the intervention regime that incorporated CBT as an adjunct produced significant improvements in the domains of the pain experience, cognitive coping, appraisal (positive coping measures), and reduced behavioral expression of pain [48, 49]. This is among the first review to measure the effect of CBT on improving self-efficacy. Moderate evidence for the improvement of self-efficacy suggested CBT had better effects compared with other therapies. Three out of the five included studies showed significant self-efficacy improvements associated with CBT [27, 42, 43], but the high heterogeneity of the studies prevented quantitative comparisons. Self-efficacy has been highlighted to influence the improvement of pain intensity and functioning. Pre- to posttreatment changes in self-efficacy for managing pain mediated the effects of CBT on pain [50]. However, the effect of CBT on self-efficacy has not been studied adequately and further studies are recommended.
Five studies analyzed the fear avoidance outcome in this review and the pooled effects suggested that CBT could reduce fear avoidance. In patients with CLBP, regression analysis showed that fear avoidance beliefs about work accounted for 23% of the variance of disability in activities of daily living and 26% of the variance of work loss, even after allowing for the severity of pain [51]. A similar review showed that a decrease in avoidance values during treatment was associated with less pain and disability [52]. Therefore, early CBT treatment may promote the recovery of pain and disability.
Considering the potential influence of sociodemographic characteristics, we also investigated the pooled effects from the aspects of educational background, gender, and marital status (see Supplementary materials 2. Subgroup analysis of pain and disability from sociodemographic characteristics: sFig. 1, sFig. 2). The results showed that educational attainment may play a role in CBT intervention outcomes. Participants with higher educational background (college grade or higher) may benefit from the CBT intervention in pain relief (P=0.04). One published responder analysis in 2021 also showed some predictors for the treatment effects of yoga, physical therapy, and self-care book, including having higher school education, income, employment, few work-related fear avoidance beliefs, and high pain self-efficacy [53]. One article analyzed the factors that might negatively affect the outcome of CBT in patients with low back pain. It was reported that CLBP patients with anxiety, strong focus on pain, and high medical dependency may be categorized to a nonadaptation group for CBT [54].
4.3. Agreements and Disagreements with Other Studies or Reviews
The findings of the primary outcomes for CBT were in line with previous systematic reviews [14, 15] which indicated a greater effect on reducing pain disability. Another review included 10 RCTs that showed CBT + PT was advantageous for reducing pain and disability and enhancing functional capacity in CLBP patients [13], which was consistent with our subgroup conclusion that CBT combined with other therapies was superior to other therapies alone in improving pain and disability. For the outcome of fear avoidance, a study conducted by Baez et al. [55] reported similar findings to the present study. The authors concluded that there was inconsistent, patient-oriented evidence (grade B) to support the use of CBT and psychoeducation to treat fear avoidance beliefs in patients with acute, subacute, and chronic low back pain. However, this review did not give a specific analysis of each type of back. The findings of this study about the follow-up effects of CBT on pain and disability were inconsistent with the findings reported in another meta-analysis conducted by Richmond et al. [16]. The study reported a moderate to large significant effect in favor of CBT compared with active treatment at short- (6–12 weeks) and long-term (26–52 weeks) follow-up. This inconsistency may be explained by different inclusion criteria between the two studies. We included patients diagnosed with chronic low back pain (pain duration >3 months), whereas Richmond et al. included LBP patients at both chronic and subacute (< 6 weeks) phases. Secondly, we analyzed pain and disability outcomes at different follow-up time points which compared the CBT with any control therapies. Richmond et al. [16] divided the comparison group into WL/UC and guideline-based active treatments. Therefore, the included RCTs to analyze were different. The most recent review investigated the effects of preoperative CBT on patients who were scheduled to undergo spine surgery for a degenerative disorder of the lumbar spine. The results showed that there were no additional effects of CBT interventions on outcomes in patients scheduled for lumbar surgery compared to usual care. This may be caused by the included patients at high risk for poor postoperative outcomes [56].
4.4. Clinical Significance
The minimal clinical significance will be considered if the pain severity was reported to be a 30% reduction from baseline [57]. Ten of the included studies indicated CBT induced a clinically significant reduction in pain intensity or disability level [23, 24, 28, 29, 32–34, 39, 43, 44], whereas 7 studies reported limited or no clinical effects [25, 30, 31, 35, 38, 40, 41]. Five studies did not report the clinical significance of CBT [26, 27, 36, 37, 42]. Despite the pooled significant effects on reduced disability, improved fear avoidance, and self-efficacy, the results must be interpreted with caution since the effect was estimated by the pooled SMD. There was also a lack of consistency in the implementation of CBT regimes and control therapies. Therefore, no firm conclusion could be drawn on the clinical significance of the observed effect.
4.5. Limitations
One of the limitations is the variations and differences between the deliveries of CBT intervention protocol of the included studies which might influence the outcome. CBT is a tailored intervention and the exact protocol, such as intervention delivery format, duration time, and the professional qualification of the providers, may vary between studies. The inherent inability to blind participants to the treatment received was a source of potential performance bias favoring CBT intervention. These substantial differences in the CBT program are particularly problematic for the direct comparison between different studies. Another limitation is that the present review only included research studies published in English which may cause language bias.
5. Conclusion
CBT intervention may be beneficial in reducing pain and disability in people with chronic low back pain. CBT as an adjunct to other types of therapy may be more effective than CBT or other therapies alone in reducing pain and disability. CBT may be effective in improving fear avoidance and self-efficacy. Further study is recommended to investigate the long-term benefit of CBT to enable the development of an appropriate strategy to maintain the benefits.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (grant number: 82002375).
Data Availability
The data of primary and secondary results supporting this meta-analysis are from previously reported studies and datasets, which have been cited.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Authors' Contributions
Jiajia Yang and Wai Leung Ambrose Lo contributed equally to this work.
Supplementary Materials
Supplementary Material 1: PRISMA Checklist. Supplementary Material 2: subgroup analysis of pain and disability from sociodemographic characteristics: sFig.1, sFig.2.
References
- 1.Buchbinder R., van Tulder M., Öberg B., et al. Low back pain: a call for action. Lancet . 2018;391(10137):2384–2388. doi: 10.1016/s0140-6736(18)30488-4. [DOI] [PubMed] [Google Scholar]
- 2.Wu A., March L., Zheng X., et al. Global low back pain prevalence and years lived with disability from 1990 to 2017: estimates from the Global Burden of Disease Study 2017. Annals of Translational Medicine . 2020;8:p. 299. doi: 10.21037/atm.2020.02.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diseases G. B. D., Injuries C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet . 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y., Campbell P., Strauss V. Y., Foster N. E., Jordan K. P., Dunn K. M. Trajectories and predictors of the long-term course of low back pain: cohort study with 5-year follow-up. Pain . 2018;159:252–260. doi: 10.1097/j.pain.0000000000001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meucci R. D., Fassa A. G., Faria N. M. Prevalence of chronic low back pain: systematic review. Revista de Saúde Pública . 2015;49 doi: 10.1590/S0034-8910.2015049005874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urits I., Hubble A., Peterson E., et al. An update on cognitive therapy for the management of chronic pain: a comprehensive review. Current Pain and Headache Reports . 2019;23(8):p. 57. doi: 10.1007/s11916-019-0794-9. [DOI] [PubMed] [Google Scholar]
- 7.Hayward R., Stynes S. Self-efficacy as a prognostic factor and treatment moderator in chronic musculoskeletal pain patients attending pain management programmes: a systematic review. Musculoskeletal Care . 2020;19 doi: 10.1002/msc.1533. [DOI] [PubMed] [Google Scholar]
- 8.Ibrahim M. E., Weber K., Courvoisier D. S., Genevay S. Big five personality traits and disabling chronic low back pain: association with fear-avoidance, anxious and depressive moods. Journal of Pain Research . 2020;13:745–754. doi: 10.2147/jpr.s237522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trinderup J. S., Fisker A., Juhl C. B., Petersen T. Fear avoidance beliefs as a predictor for long-term sick leave, disability and pain in patients with chronic low back pain. BMC Musculoskeletal Disorders . 2018;19:p. 431. doi: 10.1186/s12891-018-2351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Moraes V. E. B., de Goes S. M., Damiani L. P., de Mattos Pimenta C. A. Self-efficacy and fear avoidance beliefs in chronic low back pain patients: coexistence and associated factors. Pain Management Nursing . 2014;15:593–602. doi: 10.1016/j.pmn.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Artus M., van der Windt D. A., Jordan K. P., Hay E. M. Low back pain symptoms show a similar pattern of improvement following a wide range of primary care treatments: a systematic review of randomized clinical trials. Rheumatology . 2010;49(12):2346–2356. doi: 10.1093/rheumatology/keq245. [DOI] [PubMed] [Google Scholar]
- 12.de Campos T. F. Low back pain and sciatica in over 16s: assessment and management NICE guideline [NG59] Journal of Physiotherapy . 2017;63:p. 120. doi: 10.1016/j.jphys.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Hajihasani A., Rouhani M., Salavati M., Hedayati R., Kahlaee A. H. The influence of cognitive behavioral therapy on pain, quality of life, and depression in patients receiving physical therapy for chronic low back pain: a systematic review. PM&R . 2019;11(2):167–176. doi: 10.1016/j.pmrj.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 14.Hall A., Richmond H., Copsey B., et al. Physiotherapist-delivered cognitive-behavioural interventions are effective for low back pain, but can they be replicated in clinical practice? A systematic review. Disability and Rehabilitation . 2018;40:1–9. doi: 10.1080/09638288.2016.1236155. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman B. M., Papas R. K., Chatkoff D. K., Kerns R. D. Meta-analysis of psychological interventions for chronic low back pain. Health Psychology . 2007;26:1–9. doi: 10.1037/0278-6133.26.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Richmond H., Hall A. M., Copsey B., et al. The effectiveness of cognitive behavioural treatment for non-specific low back pain: a systematic review and meta-analysis. PLoS One . 2015;10(8) doi: 10.1371/journal.pone.0134192.e0134192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Touche R. L., Grande-Alonso M., Arnes-Prieto P., Paris-Alemany A. How does self-efficacy influence pain perception, postural stability and range of motion in individuals with chronic low back pain? Pain Physician . 2019;22(1):E1–E13. doi: 10.36076/ppj/2019.22.e1. [DOI] [PubMed] [Google Scholar]
- 18.Heuts P. H. T. G., Vlaeyen J. W. S., Roelofs J., et al. Pain-related fear and daily functioning in patients with osteoarthritis. Pain . 2004;110(1):228–235. doi: 10.1016/j.pain.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 19.Vlaeyen J. W. S., Linton S. J. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain . 2000;85(3):317–332. doi: 10.1016/s0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- 20.Wenzel A. Basic strategies of cognitive behavioral therapy. Psychiatric Clinics of North America . 2017;40(4):597–609. doi: 10.1016/j.psc.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Rubinstein S. M., de Zoete A., van Middelkoop M., Assendelft W. J. J., de Boer M. R., van Tulder M. W. Benefits and harms of spinal manipulative therapy for the treatment of chronic low back pain: systematic review and meta-analysis of randomised controlled trials. BMJ . 2019;364:p. l689. doi: 10.1136/bmj.l689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cumpston M., Li T., Page M. J., et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database of Systematic Reviews . 2019;10 doi: 10.1002/14651858.ED000142.ED000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cherkin D. C., Sherman K. J., Balderson B. H., et al. Effect of mindfulness-based stress reduction vs. cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA . 2016;315(12):1240–1249. doi: 10.1001/jama.2016.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basler H.-D., Jäkle C., Kröner-Herwig B. Incorporation of cognitive-behavioral treatment into the medical care of chronic low back patients: a controlled randomized study in German pain treatment centers. Patient Education and Counseling . 1997;31(2):113–124. doi: 10.1016/s0738-3991(97)00996-8. [DOI] [PubMed] [Google Scholar]
- 25.Brox J. I., Sorensen R., Friis A., et al. Randomized clinical trial of lumbar instrumented fusion and cognitive intervention and exercises in patients with chronic low back pain and disc degeneration. Spine (Phila Pa 1976) . 2003;28:1913–1921. doi: 10.1097/01.BRS.0000083234.62751.7A. [DOI] [PubMed] [Google Scholar]
- 26.Buhrman M., Fältenhag S., Ström L., Andersson G. Controlled trial of Internet-based treatment with telephone support for chronic back pain. Pain . 2004;111(3):368–377. doi: 10.1016/j.pain.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 27.Carpenter K. M., Stoner S. A., Mundt J. M. An online self-help CBT intervention for chronic lower back pain. The Clinical Journal of Pain . 2012;28:14–22. doi: 10.1097/AJP.0b013e31822363db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christiansen S., Oettingen G., Dahme B. A short goal-pursuit intervention to improve physical capacity: a randomized clinical trial in chronic back pain patients. Pain . 2010;149:444–452. doi: 10.1016/j.pain.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Gould H. M., Atkinson J. H., Chircop-Rollick T., et al. A randomized placebo-controlled trial of desipramine, cognitive behavioral therapy, and active placebo therapy for low back pain. Pain . 2020;161:1341–1349. doi: 10.1097/j.pain.0000000000001834. [DOI] [PubMed] [Google Scholar]
- 30.Harris A., Moe T. F., Eriksen H. R., et al. Brief intervention, physical exercise and cognitive behavioural group therapy for patients with chronic low back pain (the CINS trial) European Journal of Pain . 2017;21:1397–1407. doi: 10.1002/ejp.1041. [DOI] [PubMed] [Google Scholar]
- 31.Johnson R. E., Jones G. T., Wiles N. J., et al. Active exercise, education, and cognitive behavioral therapy for persistent disabling low back pain: a randomized controlled trial. Spine (Phila Pa 1976) . 2007;32:1578–1585. doi: 10.1097/BRS.0b013e318074f890. [DOI] [PubMed] [Google Scholar]
- 32.Khan M., Akhter S., Soomro R. R., Ali S. S. The effectiveness of cognitive behavioral therapy (CBT) with general exercises versus general exercises alone in the management of chronic low back pain. Pakistan Journal of Pharmaceutical Sciences . 2014;27:1113–1116. [PubMed] [Google Scholar]
- 33.Monticone M., Ferrante S., Rocca B., Baiardi P., Dal Farra F., Foti C. Effect of a long-lasting multidisciplinary program on disability and fear-avoidance behaviors in patients with chronic low back pain: results of a randomized controlled trial. The Clinical Journal of Pain . 2013;29:929–938. doi: 10.1097/AJP.0b013e31827fef7e. [DOI] [PubMed] [Google Scholar]
- 34.Newton-John T. R., Spence S. H., Schotte D. Cognitive-behavioural therapy versus EMG biofeedback in the treatment of chronic low back pain. Behaviour Research and Therapy . 1995;33:691–697. doi: 10.1016/0005-7967(95)00008-l. [DOI] [PubMed] [Google Scholar]
- 35.Petrozzi M. J., Leaver A., Ferreira P. H., Rubinstein S. M., Jones M. K., Mackey M. G. Addition of MoodGYM to physical treatments for chronic low back pain: a randomized controlled trial. Chiropractic & Manual Therapies . 2019;27:p. 54. doi: 10.1186/s12998-019-0277-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pincus T., Anwar S., McCracken L. M., et al. Delivering an optimised behavioural intervention (OBI) to people with low back pain with high psychological risk; results and lessons learnt from a feasibility randomised controlled trial of contextual cognitive behavioural therapy (CCBT) vs. physiotherapy. BMC Musculoskeletal Disorders . 2015;16:p. 147. doi: 10.1186/s12891-015-0594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reme S. E., Tveito T. H., Harris A., et al. Cognitive interventions and nutritional supplements (the CINS trial): a randomized controlled, multicenter trial comparing a brief intervention with additional cognitive behavioral therapy, seal oil, and soy oil for sick-listed low back pain patients. Spine (Phila Pa 1976) . 2016;41:1557–1564. doi: 10.1097/BRS.0000000000001596. [DOI] [PubMed] [Google Scholar]
- 38.Rutledge T, Atkinson J. H., Holloway R., et al. Randomized controlled trial of nurse-delivered cognitive-behavioral therapy versus supportive psychotherapy telehealth interventions for chronic back pain. The Journal of Pain . 2018;19:1033–1039. doi: 10.1016/j.jpain.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 39.Rutledge T., Atkinson J. H., Chircop-Rollick T., et al. Randomized controlled trial of telephone-delivered cognitive behavioral therapy versus supportive care for chronic back pain. The Clinical Journal of Pain . 2018;34:322–327. doi: 10.1097/AJP.0000000000000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schweikert B., Jacobi E., Seitz R., et al. Effectiveness and cost-effectiveness of adding a cognitive behavioral treatment to the rehabilitation of chronic low back pain. Journal of Rheumatology . 2006;33:2519–2526. [PubMed] [Google Scholar]
- 41.Smeets R. J., Vlaeyen J. W., Hidding A., et al. Active rehabilitation for chronic low back pain: cognitive-behavioral, physical, or both? First direct post-treatment results from a randomized controlled trial [ISRCTN22714229] BMC Musculoskeletal Disorders . 2006;7:p. 5. doi: 10.1186/1471-2474-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turner J. A., Anderson M. L., Balderson B. H., Cook A. J., Sherman K. J., Cherkin D. C. Mindfulness-based stress reduction and cognitive behavioral therapy for chronic low back pain: similar effects on mindfulness, catastrophizing, self-efficacy, and acceptance in a randomized controlled trial. Pain . 2016;157(11):2434–2444. doi: 10.1097/j.pain.0000000000000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Darnall B. D., Roy A., Chen A. L., et al. Comparison of a single-session pain management skills intervention with a single-session health education intervention and 8 sessions of cognitive behavioral therapy in adults with chronic low back pain. JAMA Network Open . 2021;4(8) doi: 10.1001/jamanetworkopen.2021.13401.e2113401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Godfrey E., Wileman V., Galea Holmes M., et al. Physical therapy informed by acceptance and commitment therapy (pact) versus usual care physical therapy for adults with chronic low back pain: a randomized controlled trial. The Journal of Pain . 2020;21(1-2):71–81. doi: 10.1016/j.jpain.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 45.Mansell G., Storheim K., Lochting I., Werner E. L., Grotle M. Identification of indirect effects in a cognitive patient education (COPE) intervention for low back pain. Physical Therapy . 2017;97:1138–1146. doi: 10.1093/ptj/pzx091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smeets R. J., Beelen S., Goossens M. E., Schouten E. G., Knottnerus J. A, Vlaeyen J. W. Treatment expectancy and credibility are associated with the outcome of both physical and cognitive-behavioral treatment in chronic low back pain. The Clinical Journal of Pain . 2008;24:305–315. doi: 10.1097/AJP.0b013e318164aa75. [DOI] [PubMed] [Google Scholar]
- 47.Monticone M., Cedraschi C., Ambrosini E., et al. Cognitive-behavioural treatment for subacute and chronic neck pain. The Cochrane Database of Systematic Reviews . 2015 doi: 10.1002/14651858.CD010664.pub2.CD010664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaapa E. H., Frantsi K., Sarna S., Malmivaara A. Multidisciplinary group rehabilitation versus individual physiotherapy for chronic nonspecific low back pain: a randomized trial. Spine (Phila Pa 1976) . 2006;31:371–376. doi: 10.1097/01.brs.0000200104.90759.8c. [DOI] [PubMed] [Google Scholar]
- 49.Morley S., Eccleston C., Williams A. Systematic review and meta-analysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain . 1999;80(1):1–13. doi: 10.1016/s0304-3959(98)00255-3. [DOI] [PubMed] [Google Scholar]
- 50.Turner J. A., Holtzman S., Mancl L. Mediators, moderators, and predictors of therapeutic change in cognitive-behavioral therapy for chronic pain. Pain . 2007;127(3):276–286. doi: 10.1016/j.pain.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 51.Waddell G., Newton M., Henderson I., Somerville D., Main C. J. A fear-avoidance beliefs questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain . 1993;52(2):157–168. doi: 10.1016/0304-3959(93)90127-b. [DOI] [PubMed] [Google Scholar]
- 52.Roseen E. J., Gerlovin H., Felson D. T., Delitto A., Sherman K. J., Saper R. B. Which chronic low back pain patients respond favorably to yoga, physical therapy, and a self-care book? Responder analyses from a randomized controlled trial. Pain Medicine . 2021;22:165–180. doi: 10.1093/pm/pnaa153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shimizu K., Inage K., Orita S., et al. Background factors for chronic low back pain resistant to cognitive behavioral therapy. Scientific Reports . 2021;11:p. 8227. doi: 10.1038/s41598-021-87239-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wertli M. M., Rasmussen-Barr E., Held U., Weiser S., Bachmann L. M., Brunner F. Fear-avoidance beliefs-a moderator of treatment efficacy in patients with low back pain: a systematic review. The Spine Journal . 2014;14(11):2658–2678. doi: 10.1016/j.spinee.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 55.Baez S., Hoch M. C., Hoch J. M. Evaluation of cognitive behavioral interventions and psychoeducation implemented by rehabilitation specialists to treat fear-avoidance beliefs in patients with low back pain: a systematic review. Archives of Physical Medicine and Rehabilitation . 2018;99:2287–2298. doi: 10.1016/j.apmr.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 56.Janssen E. R. C., Punt I. M., Clemens M. J., Staal J. B., Hoogeboom T. J., Willems P. C. Current prehabilitation programs do not improve the postoperative outcomes of patients scheduled for lumbar spine surgery: a systematic review with meta-analysis. Journal of Orthopaedic & Sports Physical Therapy . 2021;51:103–114. doi: 10.2519/jospt.2021.9748. [DOI] [PubMed] [Google Scholar]
- 57.Ostelo R. W., Deyo R. A., Stratford P., et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine (Phila Pa 1976) . 2008;33:90–94. doi: 10.1097/BRS.0b013e31815e3a10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: PRISMA Checklist. Supplementary Material 2: subgroup analysis of pain and disability from sociodemographic characteristics: sFig.1, sFig.2.
Data Availability Statement
The data of primary and secondary results supporting this meta-analysis are from previously reported studies and datasets, which have been cited.


