Abstract
Objective.
Determine and compare the efficacy of drugs to treat Mycobacterium kansasii (Mkn) pulmonary diseases by performing minimum inhibitory concentration(MIC) and time-kill studies.
Methods.
We used 13 drugs to determine the MIC (against the standard laboratory strain ATCC#12478 as well as 20 clinical isolates) and performed time-kill studies with 18 drugs from different classes using the standard laboratory strain of Mkn. The β-lactams antibiotics were tested with or without the combination of a β-lactamase inhibitor, avibactam. Inhibitory Sigmoid Emax model was used to describe the relationship between the drug concentrations and the bacterial burden.
Results.
Among the 13 tested drugs in the MIC experiments, the lowest MIC was recorded for bedaquiline. Among the 18 drugs used in the time-kill studies, the maximal kill with cefdinir, tebipenem, clarithromycin, azithromycin, moxifloxacin, levofloxacin, tedizolid, bedaquiline, pretomanid, and telacebac was greater than some of the drugs (isoniazid, rifampin, and ethambutol) in the standard combination therapy.
Conclusion.
We report preclinical data on the efficacy and potency of drugs that can potentially be repurposed to create safe, effective and likely shorter duration regimen for the treatment of Mkn pulmonary disease.
Keywords: nontuberculous mycobacteria, repurposed drugs, efficacy
INTRODUCTION
The incidence of pulmonary disease caused by nontuberculous mycobacteria (NTM) is on the rise.[1] Mycobacterium kansasii (Mkn) is an NTM with a clinical presentation very similar to the disease caused by Mycobacterium tuberculosis (Mtb).[2, 3] The current chemotherapy [2] to treat Mkn pulmonary disease is a regimen consist of isoniazid or a macrolide in combination of rifampin, and ethambutol; however requires 12 months therapy duration. Thus, there is an unmet need to develop more effective, safe, and shorter-course treatment regimens for Mkn pulmonary disease. Due to the lack of randomized controlled trials as well as pharmacokinetics/pharmacodynamics (PK/PD) informed studies to determine the optimal drug dose, regimen composition and even the susceptibility breakpoint for critical drugs within a multidrug regimen, the therapeutic approach has been largely empirical and remains extrapolated from Mtb. Recently a four drug regimen of current and repurposed drugs demonstrated the ability to shorten Mtb treatment to four months from the conventional six months of therapy [4], However, quest for such a shorter duration oral regimen for Mkn pulmonary diseases continues.
In contrast to most bacterial infections, there is a general belief that the drug minimum inhibitory concentration (MIC) and clinical response do not correlate for most drugs used to treat NTM diseases.[5] However, in our opinion, MIC is a good starting point to make an informed decision on antibiotics to treat a given bacterial infection. Studies on the role of MIC in NTM disease have been complicated by inconsistent methodologies including grouping of species and subspecies into a single analysis, the considerable challenge of predicting intracellular concentrations of drug for mycobacterial infections that exist in various local anatomic and immunological environments, as well as a lack of consideration of individual host PK variability. These challenges notwithstanding, MIC is still an important factor to determine the PK/PD optimized drug exposure, the clinical dose to achieve the optimal drug exposure target, and the susceptibility breakpoint above which the drug at optimal dose will fail to kill the bacteria, in this case Mkn. The present study summarizes the MIC and dose-response (time-kill) studies with several drugs from different classes to determine their efficacy and potency against Mkn. Efficacy of the drug can be described as the maximum effect (Emax) that a drug can produce regardless of the dose. Once Emax is achieved, increasing the drug dose will not produce an increased effect. Whereas, potency can be described as the amount of the drug required to produce a given effect. For example, EC50 is the concentration of the drug that can produce 50% of the maximum effect (bacterial kill). The findings presented here can help repurpose drugs for the treatment of Mkn pulmonary disease.
MATERIALS & METHODS
Drug, bacteria, and supplies
We used the following 18 drugs - isoniazid, rifampin, ethambutol, cefpodoxime, cefdinir, tebipenem, linezolid, tedizolid, clarithromycin, azithromycin, moxifloxacin, levofloxacin, minocycline, omadacycline, bedaquiline, pretomanid, sulfamethoxazole, and telacebac. The β-lactams antibiotics were tested with or without the combination of a β-lactamase inhibitor, avibactam,[6, 7] at a concentration of 15 mg/L. Drugs were purchased either from University of Texas Health Science Center at Tyler pharmacy or synthesized by the BOC Sciences (Sheryl, NY, USA). We used the standard laboratory strain (ATCC#12478) and a collection of 20 clinical isolates, listed in Table 1, to determine the MIC of the drugs. As the intent of the current study was to screen antibiotics for efficacy against Mkn, no patient demographic or clinical data (including the drugs in the combination regimen used to treat patients from which these clinical isolates were collected) was recorded. The concentration-response studies were performed only with the standard laboratory strain.
Table 1.
Minimum inhibitory concentration of 13 drugs against M. kansasii.
| DRUG | INH | RIF | EMB | SMX | CLA | AZI | TZD | LZD | PTM | MINO | BDQ | CFD | TBP+AVI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range (mg/L) | 0.25 to 32 | 0.03 to 4 | 1 to 64 | 2 to 128 | 0.06 to 8 | 0.25 to 32 | 0.06 to 8 | 0.12 to 8 | 1 to 64 | 0.12 to 16 | 0.03 to 2 | 0.25 to 32 | 0.12 to 128 |
| ATCC | 1 | 1 | 8 | 128 | 1 | 32 | 1 | 4 | 64 | 8 | 0.03 | 32 | 0.5 |
| UVA_1 | 2 | 1 | 16 | 128 | 0.5 | 32 | 0.5 | 2 | 64 | 16 | 0.03 | 32 | ND |
| UVA_2 | 32 | 2 | 32 | 128 | 0.5 | 32 | 1 | 4 | 64 | 16 | 0.03 | 32 | ND |
| TY_1 | 2 | 0.5 | 8 | 128 | 8 | 32 | 2 | 4 | 64 | 2 | 2 | 32 | 0.25 |
| TY_2 | 1 | 1 | 4 | 128 | 0.5 | 8 | 1 | 2 | 8 | 4 | 0.03 | 16 | 0.25 |
| TY_3 | 0.5 | 0.25 | 2 | 128 | 0.12 | 8 | 0.5 | 2 | 1 | 2 | 0.03 | 32 | 0.25 |
| TY_4 | 0.5 | 0.5 | 2 | 28 | 0.5 | 16 | 0.5 | 2 | 4 | 2 | 0.03 | 16 | 0.25 |
| TY_5 | 1 | 0.5 | 2 | 128 | 0.5 | 16 | 0.5 | 2 | 4 | 1 | 0.03 | 8 | 0.25 |
| TY_6 | 0.5 | 0.5 | 4 | 128 | 0.5 | 8 | 0.5 | 2 | 4 | 2 | 0.03 | 32 | 0.25 |
| TY_7 | 0.5 | 0.5 | 4 | 128 | 0.5 | 16 | 0.5 | 2 | 4 | 2 | 0.03 | 16 | 0.25 |
| TY_8 | 1 | 0.5 | 4 | 128 | 0.5 | 16 | 0.5 | 2 | 2 | 2 | 0.03 | 16 | 1 |
| TY_9 | 32 | 4 | 64 | 128 | 8 | 32 | 8 | 8 | 64 | 16 | 0.03 | 32 | 0.25 |
| TY_10 | 1 | 2 | 4 | 128 | 2 | 32 | 1 | 4 | 8 | 4 | 0.03 | 32 | 0.5 |
| TY_11 | 32 | 4 | 64 | 128 | 8 | 32 | 8 | 8 | 64 | 16 | 2 | 32 | 0.25 |
| TY_12 | 0.5 | 0.5 | 4 | 128 | 0.5 | 32 | 1 | 4 | 16 | 4 | 0.03 | 0.5 | 0.25 |
| TY_13 | 0.5 | 0.5 | 2 | 128 | 0.25 | 8 | 0.5 | 2 | 8 | 2 | 0.03 | 16 | 1 |
| TY_14 | 1 | 0.5 | 4 | 128 | 1 | 32 | 1 | 4 | 8 | 4 | 0.03 | 32 | 0.5 |
| TY_15 | 8 | 4 | 64 | 128 | 2 | 32 | 1 | 8 | 64 | 16 | 0.03 | 32 | 0.5 |
| TY_16 | 1 | 0.5 | 4 | 128 | 1 | 16 | 0.5 | 2 | 8 | 4 | 0.03 | 16 | 0.5 |
| TY_17 | 0.5 | 1 | 4 | 128 | 1 | 32 | 0.5 | 2 | 8 | 4 | 0.03 | 32 | 1 |
| TY_18 | 32 | 4 | 64 | 128 | 2 | 10 | 8 | 8 | 64 | 16 | 0.03 | 32 | 2 |
| MIC 50 | 1 | 0.5 | 4 | 128 | 0.5 | 32 | 1 | 2 | 8 | 4 | 0.03 | 32 | 0.25 |
| MIC 90 | 32 | 4 | 64 | 128 | 8 | 32 | 8 | 8 | 64 | 16 | 1.6 | 32 | 1 |
INH, isoniazid; RIF, rifampin; EMB, ethambutol; SMX, sulfamethoxazole; CLA, clarithromycin; AZI, azithromycin; LZD, linezolid; TZD, tedizolid; PTM, pretomanid; MINO, minocycline; BDQ, bedaquiline; CFD, cefdinir; TBP, tebipenem; AVI, avibactam; ND, not done
MIC and concentration-response studies
The MIC experiments were performed using the broth micro-dilution method.[8] Before each experiment, first, bacteria were grown to logarithmic phase growth followed by preparation of McFarland 0.5 turbidity-adjusted inoculum preparation using the Middlebrook 7H9 broth supplemented with 10% oleic acid, albumin, dextrose, and catalase (OADC).[8–12] Second, the turbidity-adjusted inoculum was 100-fold diluted to achieve the initial bacterial burden of ~105 log10 CFU/mL. In the third step, 180 μL of the inoculum was added to each of the 96-well of a tissue culture plate prefilled with 20 μL of each drug concentration (10x). The plates were sealed in a Ziplock bag to prevent evaporation and cultures were incubated at 370C. After 7-days of incubation, plates were read using an inverted mirror, and the drug concentration completely inhibiting the bacterial growth (absence of bacterial pellet) was recorded as the MIC. The experiments were performed twice with three-replicates per drug concentration.
For the static concentration-response studies, the drug concentration range was like those used in the MIC experiments. The experiment was performed in 15 mL screw-capped tubes with a total volume of 5 mL. The inoculum was prepared as described above and bacteria were co-incubated with drugs for 7-days at 370C. On study day 7, the cultures were washed twice with normal saline to remove the carry-over drug, 10-fold serially diluted in normal saline, and spread on Middlebrook 7H10 agar supplemented with 10% OADC. The agar cultures were incubated at 370C, sealed in a Ziplock bag, and colony-forming units were recorded after 10 days of incubation. In addition to the monotherapy experiments, we also performed experiments with isoniazid, rifampin, and ethambutol as 2- and 3-drug combinations to benchmark the efficacy of drugs in the standard regimen [13] used to treat Mkn pulmonary disease. We used EC80 concentration of INH, RIF, and EMB in the combination studies.
Data analysis
The individual MIC against 20 clinical isolates was used to calculate MIC50 and MIC90 of each drug. We used the 4-parameter inhibitory Sigmoid Emax model to describe the relationship between the drug concentrations and the bacterial burden. The 4-parameters in the model were Econ or growth in the non-treated controls, Emax or maximal bacterial kill compared to the non-treated controls on study day 7, EC50 or effective concentration mediating 50% of the Emax, and the steep portion of the slope (H) as Hill coefficient. GraphPad Prism (v9) was used for plotting the data.
RESULTS.
Table 1 lists the concentration range of each drug used in the MIC experiments as well as show the individual strains MIC, and the MIC50 and the MIC90 of the drugs against 20 clinical isolates. The results of the experiments with isoniazid, rifampin, and ethambutol are shown in Figure 1A–C and Table 2. In the monotherapy concentration-response studies, the Mkn kill below stasis (or bacterial burden in the inoculum on day 0) with isoniazid was 1.88 log10 CFU/mL (Figure 1A), with rifampin was 2.28 log10 CFU/mL (Figure 1B), and with ethambutol was 1.66 log10 CFU/mL (Figure 1C). The Emax (compared to nontreated control on study day 7) and EC50 of isoniazid, rifampin, and ethambutol, based on the inhibitory Sigmoid model, were 1.88 log10 CFU/mL and 0.88 mg/L, 2.28 log10 CFU/mL and 0.09 mg/L, and 1.66 log10 CFU/mL and 1.39 mg/L, respectively. Table 2 summarizes the results of the combination studies where each drug was used at EC80 concentration; isoniazid 2.5 mg/L, rifampin 0.2 mg/L, and ethambutol 4 mg/L. The bacterial burden in the inoculum (stasis) was 5.75 log10 CFU/mL, which grew to 8.12 log10 CFU/mL in 7-days. As shown in Table 2, the Mkn kill with the 2- and 3-drug combinations was higher than each drug alone. Moreover, the Mkn kill with the isoniazid-rifampin 2-drug combination was not significantly different from the three-drug combination, suggesting ethambutol could be replaced with another potent drug in the standard regimen.
Figure 1. Efficacy of drugs in the standard regimen and three β-lactam antibiotics against M. kansasii.
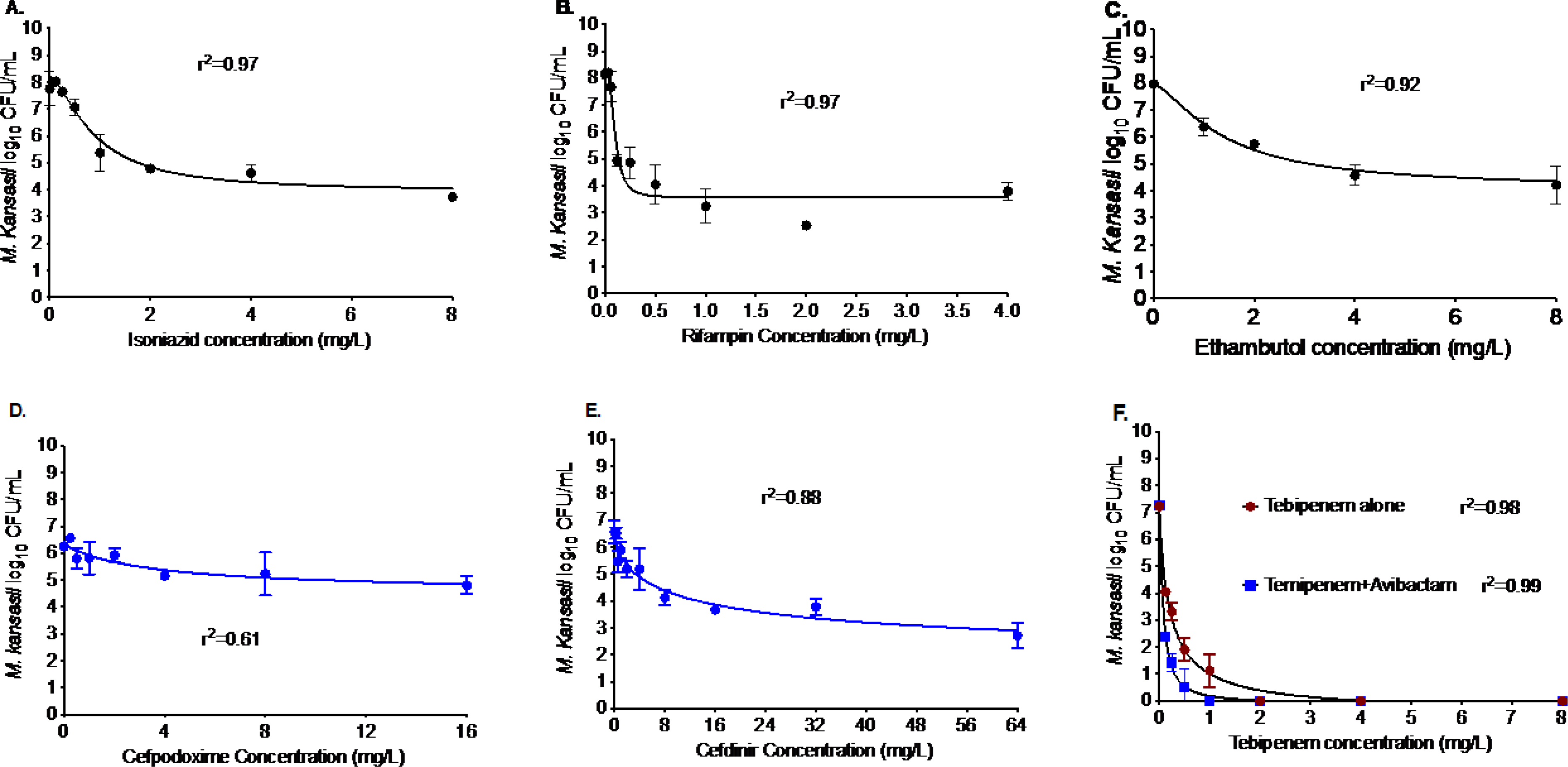
Relationship between the bacterial burden and (A) isoniazid, (B) rifampin, (C) ethambutol concentration on day 7 and r2 to show good model fit. The Mkn kill below stasis with isoniazid was 1.88 log10 CFU/mL, with rifampin was 2.28 log10 CFU/mL, and with ethambutol was 1.66 log10 CFU/mL. Among the three-beta-lactamase antibiotics, (D) and (E) show the results for cefpodoxime and cefdinir in combination with avibactam. There was no kill below stasis with cefpodoxime, whereas cefdinir showed a 1.50 log10 CFU/mL Mkn kill below stasis. In comparison, the Mkn kill with (F) tebipenem was independent of the β-lactamase inhibitor, avibactam. The kill below stasis with tebipenem was 4.48 log10 CFU/mL.
Table 2.
Extent of M. kansasii kill with isoniazid, rifampin, and ethambutol alone or in combination at static concentration.
| H | R | E | HR | HE | RE | HRE | |
|---|---|---|---|---|---|---|---|
| Kill below stasis (log10 CFU/mL) | 1.69 | 1.39 | 1.44 | 3.66 | 2.41 | 2.51 | 3.46 |
| Emax (on day 7; log10 CFU/mL) | 3.76 | 3.76 | 3.82 | 6.03 | 4.79 | 4.88 | 5.83 |
H, isoniazid; R, rifampin; E, ethambutol.
β-lactams are the largest class of antibiotics, used to treat several bacterial infections. However, their potential to treat Mkn pulmonary disease has not been explored systematically. The results of the experiments with cefpodoxime, cefdinir, and tebipenem are shown in Figure 1D–F. The experiments with tebipenem were performed with and without avibactam combination, whereas cefpodoxime and cefdinir were tested only in combination with avibactam, at a concentration of 15 mg/L. As shown in Figure 1D, cefpodoxime in combination with avibactam killed 1.84 log10 CFU/mL Mkn in 7-days, where EC50 was calculated as 3.53 mg/L. The results of the cefdinir and avibactam combination are shown in Figure 1E, showing 5.13 log10 CFU/mL Mkn kill in 7-days at static concentration with an EC50 of 12.09 mg/L. Figure 1F shows that tebipenem alone or in combination with avibactam killed 7.25 log10 CFU/mL Mkn in 7-days. However, the EC50 was 3-fold lower in combination with avibactam (0.21 mg/L versus 0.07 mg/L).
The British Thoracic Society (BTS) recommends combining macrolides with ethambutol and rifampin to treat Mkn pulmonary diseases.[14] In Figure 2 we show the results for two macrolides - clarithromycin, and azithromycin. The maximal kill (Emax) with clarithromycin (Figure 2A), after 7-days of co-incubation at static concentrations, was 3.48 log10 CFU/mL compared to the non-treated controls. The clarithromycin EC50 was calculated as 2.21 mg/L. In comparison, azithromycin (Figure 2B) showed an Emax of 6.61 log10 CFU/mL in 7-days with an EC50 of 3.75 mg/L. Thus, both macrolides showed good efficacy against Mkn in the test-tube experiments.
Figure 2. Efficacy of macrolide, fluoroquinolone, and tetracycline class of antibiotics against M. kansasii.
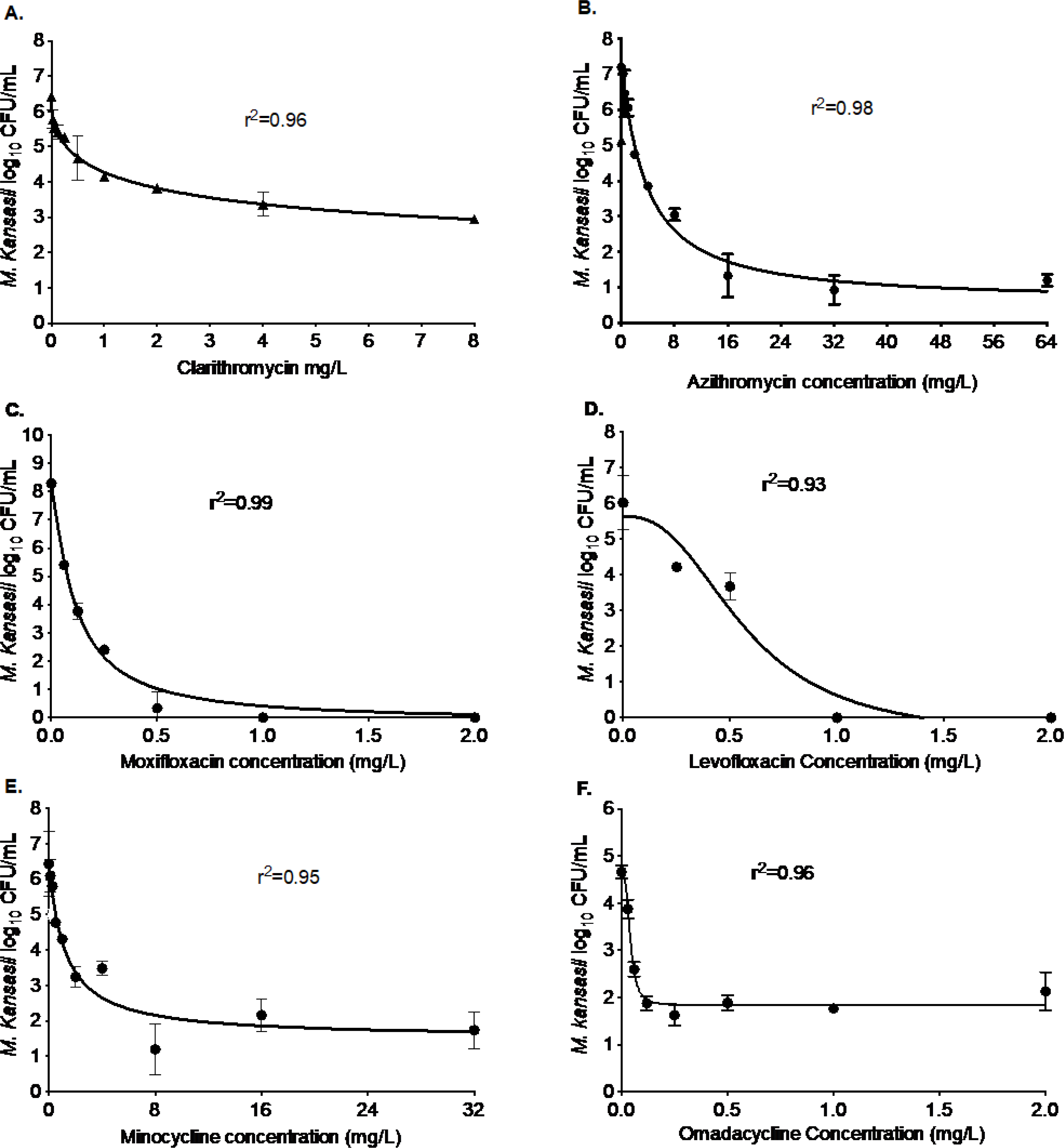
As shown in the figure, (A) clarithromycin, (B) azithromycin, (C) moxifloxacin, (D) levofloxacin, (E) minocycline, and (F) omadacycline were able to kill Mkn in the test-tube experiments. The extent of the kill varied between the antibiotics of the same class. The highest concentration of moxifloxacin and levofloxacin used in the experiments were 32 and 64 mg/L. However, for clarity of presentation, we truncated the x-axis. The kill below stasis with clarithromycin was 1.64 log10 CFU/mL, with azithromycin was 3.95 log10 CFU/mL, with moxifloxacin was 4.13 log10 CFU/mL, with levofloxacin was 4.53 log10 CFU/mL, with minocycline was 2.87 log10 CFU/mL, and with omadacycline was 2.64 log10 CFU/mL.
Fluoroquinolones are another class of antibiotics with the potential to be used for the treatment of Mkn pulmonary disease. In Figure 2C–D, we show the results of the two fluoroquinolones. After 7-days of co-incubation, the Emax of moxifloxacin was 8.38 log10 CFU/mL compared to the non-treated controls with an EC50 0.11 mg/L (Figure 2C). In comparison, levofloxacin (Figure 2D) showed an Emax of 6.21 log10 CFU/mL on day 7 of the study with an EC50 of 0.57 mg/L. Thus, both moxifloxacin and levofloxacin should further be explored for the treatment of Mkn pulmonary disease.
Figure 2E–F shows the results of the two tetracycline antibiotics tested for efficacy against Mkn. On day 7 of the study, compared to the non-treated controls, the Emax of minocycline was recorded as 4.96 log10 CFU/mL and the EC50 was calculated as 1.21 mg/L (Figure 2E). The second tetracycline, omadacycline (Figure 2F) showed an Emax of 2.81 log10 CFU/mL on day 7 of the study with an EC50 of 0.04 mg/L (the lowest EC50 of any single drug tested excepting bedaquiline, as shown below). Thus, while efficacy (Emax) of minocycline was higher, omadacycline showed better potency as EC50 was 30-fold lower.
Oxazolidinone is another class of antibiotics that could be potentially repurposed for the treatment of Mkn pulmonary disease. Figure 3 shows the results of the two oxazolidinones, we tested for efficacy against Mkn. In the 7-days static concentration experiment, the Emax of linezolid was 3.96 log10 CFU/mL compared to the non-treated controls with an EC50 2.18 mg/L (Figure 3A). In comparison, tedizolid (Figure 3B) showed a higher Emax of 7.32 log10 CFU/mL, for the same study duration, with an EC50 of 0.43 mg/L. Thus, in terms of both efficacy (Emax) and potency (EC50), tedizolid could be a better choice for the treatment of Mkn pulmonary disease.
Figure 3. Efficacy of drugs with potential to repurpose them for the treatment of M. kansasii pulmonary disease.
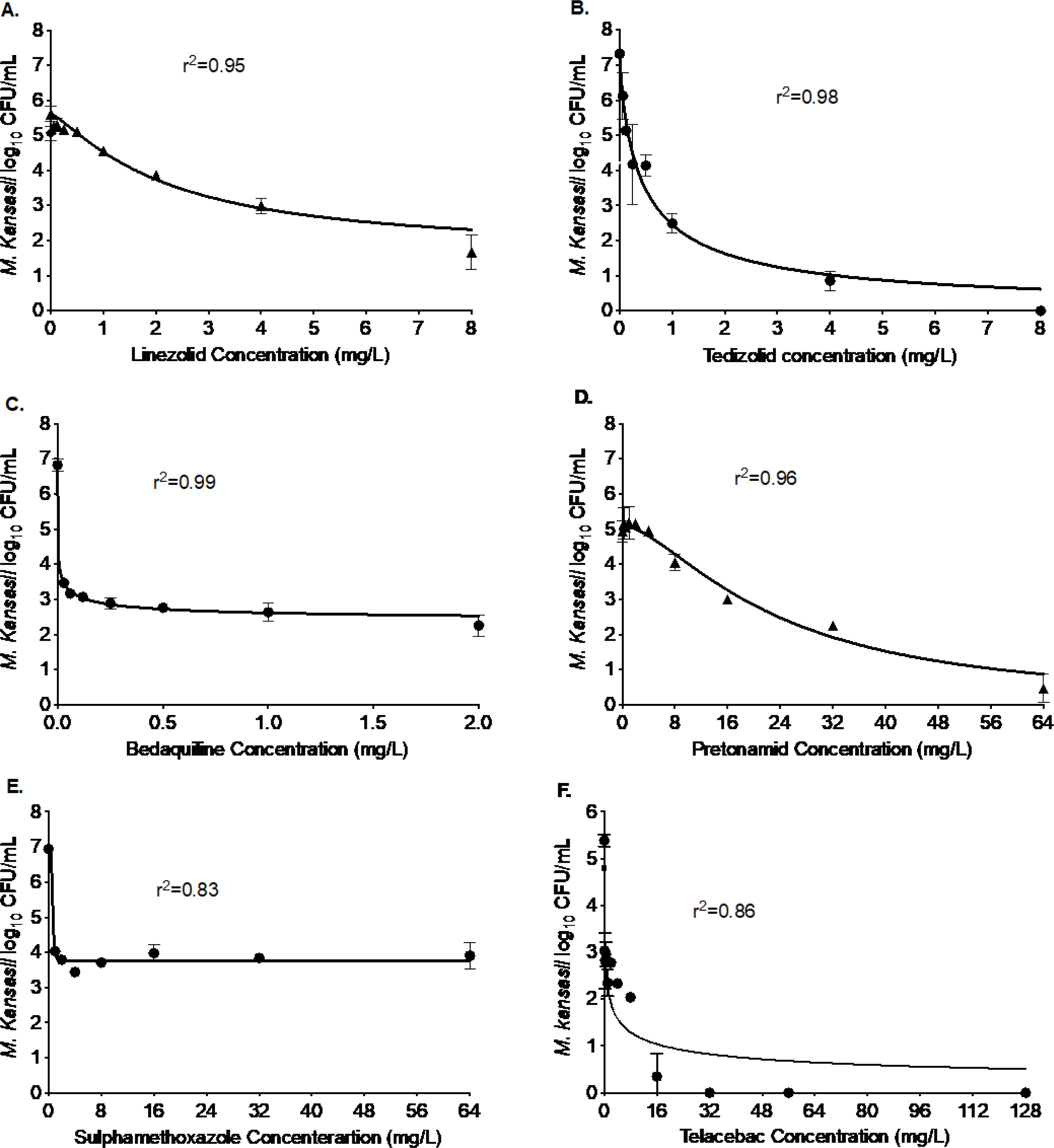
The figure below shows excellent bacterial kill compared to the nontreated controls on day 7 with each of the six drugs, namely, (A) linezolid, (B) tedizolid, (C) bedaquiline, (D) pretomanid, (D) sulfamethoxazole, and (E) telacebac. The kill below stasis with linezolid was 3.4 log10 CFU/mL, with tedizolid was 4.22 log10 CFU/mL, with bedaquiline was 3.10 log10 CFU/mL, with pretomanid was 4.73 log10 CFU/mL, with sulfamethoxazole was 1.82 log10 CFU/mL, and with telacebac was 4.80 log10 CFU/mL.
Bedaquiline, pretomanid, telacebac, and sulfamethoxazole are among the antimicrobials that are either specifically designed to treat M. tuberculosis or Gram-positive and Gram-negative bacterial infections.[15–19] In Figure 3C–F, we show the results of these four drugs with the intent to repurpose them for treatment of Mkn pulmonary disease. Figure 3C shows the results of the bedaquiline studies, that killed 4.57 log10 CFU/mL in 7-days with an EC50 of 0.002 mg/L. Figure 3D shows the results for pretomanid concentration-response studies. The Emax of pretomanid was 5.12 log10 CFU/mL and EC50 was calculated as 23.05 mg/L. Next, in Figure 3E we show the results of the sulfamethoxazole that killed 3.18 log10 CFU/mL in 7-days static concentration experiments, and the EC50 of the sulfamethoxazole was calculated as 0.63 mg/L. Finally, Figure 3F shows the results of telacebac, where the maximal Mkn kill was recorded as 5.89 log10 CFU/mL with an EC50 of 0.39 mg/L. Thus, bedaquiline, pretomanid, sulfamethoxazole, and telacebac have the potential to treat Mkn pulmonary disease, particularly in combination with other drugs that differentially maximize either efficacy or potency.
In addition to the results described above, the Supplementary Table 1 summarizes the model parameters for each of the drugs including Econ, Emax, EC50, and Hill constant that could be used to calculate the drug exposure for the combination studies.
DISCUSSION
In the United States, Mkn is the second most common NTM after Mycobacterium avium complex.[20] Mkn is considered to be as one of the most virulent of the NTM.[21, 22] However, the data on Mkn disease incidence are scant in part because it has not been commonly considered a transmissible public health threat, and it is not a reportable pathogen in many municipalities. The 2020 multi-society NTM treatment guideline recommends daily or intermittent therapy when a macrolide-based regimen is used and daily therapy when an INH based-regimen is used. [2] The 2020 guidelines also recommend that the Mkn could be treated for a fixed duration of 12 months instead of 12 months beyond culture conversion.[2] The current ATS guidelines acknowledge that the level of evidence for the currently recommended regimen for NTM infections as having the lowest evidence categorization by GRADE (Grades of Recommendation Assessment, Development and Evaluation) criteria, [3, 23]. Our findings suggest significant opportunity for the development of new treatment regimens by repurposing the drugs.
Toward the goal of development of new effective drug regimens for Mkn, we show the MIC distribution of 13 different drugs, including the drugs in the standard combination regimen as well as drugs that are specifically designed for Mtb (bedaquiline, pretomanid, and telacebac) and used for the treatment of drug-resistant tuberculosis including linezolid. Elsewhere, Mkn was reported to be susceptible to sulfamethoxazole,[24] however, we found that all 20 clinical strains had MIC >128 mg/L. Among the macrolides, clarithromycin showed lower MICs compared to the azithromycin; among oxazolidinones, the clinical strains had lower MIC for tedizolid compared to the linezolid; tebipenem, alone and in combination with avibactam, had MICs comparable to one of the most potent drugs in the standard regimen -rifampin, and bedaquiline showed the lowest MIC among all the 13 drugs used in the MIC experiments.
While drug susceptibility testing (MIC) could help in initial decision making, it is important to know how well a drug kills the infecting organism. Thus, we compared the kill below stasis with isoniazid, rifampin, and ethambutol (1.88, 2.28, and 1.66 log10 CFU/mL, respectively) to several drugs, namely tebipenem, azithromycin, moxifloxacin, levofloxacin, minocycline, omadacycline, linezolid, tedizolid, bedaquiline, pretomanid, and telacebac, and found those drugs to have either a better kill below stasis or were able to kill the entire bacterial burden in the inoculum in the 7-days static-concentration experiments. Therefore, the next step should be to examine these drugs at dynamic concentrations, such as in the hollow fiber model system as proposed by Alffenaar et al[25] and Rampacci et al[26], to determine if the effect persists at fluctuating concentrations and what would be the PK/PD optimized exposure target for kill and resistance suppression.
It is a common belief that the in vitro susceptibility testing of NTM, including Mkn, is of little help for managing the treatment of these infections. However, there is some evidence that indeed the in vitro susceptibility of Mkn, even if based on the interpretative criteria used with Mtb, could correlate with clinical outcome.[27, 28] Therefore, a more expansive understanding of drug susceptibility, as we have developed in this this study, can be used to predict clinical outcome in patients with Mkn pulmonary disease, and design drug regimens for clinical studies with the dose to achieve the PK/PD optimized drug exposure target. For example, we have previously published PK/PD studies with moxifloxacin[12] that determined moxifloxacin of 800 mg/day as the PK/PD optimized dose for the treatment of Mkn pulmonary disease. In the next step, moxifloxacin was added to the currently recommended regimen of isoniazid-rifampin-ethambutol or replaced isoniazid or ethambutol.[9] It was observed that the addition of moxifloxacin resulted in faster Mkn kill with the potential to shorten the therapy duration to possibly six months or less. In another example we compared the kill effect of the novel drug combination of rifapentine-tedizolid-minocycline with the ATS recommended standard regimen and BTS recommended regimen that included a macrolide.[10] Notably, the experimental regimen performed better than the standard of care regimens. Thus, if these drugs are combined at doses determined using formal PK/PD studies, may lead to safe, tolerable, and shorter duration regimens, a significant leap in the management of an otherwise neglected disease.
Despite these promising results for repurposing the drugs for the treatment of Mkn pulmonary disease, our study has limitations. One could argue that the experiments were performed at static concentrations that might overestimate the kill due to constant drug exposure. However, the PK/PD studies[10] with rifapentine, tedizolid, and minocycline provided the crucial evidence that the effect seen at static concentration will likely persist at dynamic concentration. Additional PK/PD studies are warranted to test and rank various drug combinations that could potentially be advanced in the clinics.
To summarize, the development of new drugs specific to Mkn has not been a priority for the pharmaceutical industry and the information on potential drugs and regimens to advance in clinical trials is lacking. Therefore, repurposing drugs as we report here, is a promising strategy to improve the management of Mkn pulmonary disease.
Supplementary Material
Funding.
This work was supported by funding from the Department of Pulmonary Immunology [423500/14000], University of Texas System STARS award [250439/39411], 1R21AI148096-01 from the National Institute of Allergy and Infectious Diseases [NIAID], and 1R01HD099756-02 grant from Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and American Thoracic Foundation ATS Foundation/Insmed Research Award in Non-Tuberculous Mycobacteria (NTM) Lung Disease grant to SS. SKH is supported via the NIAID R01 AI137080 and U01 AI150508 grants.
Footnotes
Conflict of Interest. Tawanda Gumbo founded and is the president and CEO of Praedicare Inc., a contract research organization. JGP is an employee of Praedicare Inc. All other authors have nothing to declare.
Ethical Approval. Not required.
Randomized control Trial. Not applicable
REFERENCE
- [1].Winthrop KL, Marras TK, Adjemian J, Zhang H, Wang P, Zhang Q. Incidence and Prevalence of Nontuberculous Mycobacterial Lung Disease in a Large U.S. Managed Care Health Plan, 2008–2015. Ann Am Thorac Soc. 2020;17:178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ, Andrejak C, et al. Treatment of nontuberculous mycobacterial pulmonary Disease: AnoOfficial ATS/ERS/ESCMID/IDSA clinical practice guideline: executive summary. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. [DOI] [PubMed] [Google Scholar]
- [4].Dorman SE, Nahid P, Kurbatova EV, Phillips PPJ, Bryant K, Dooley KE, et al. Four-Month Rifapentine Regimens with or without Moxifloxacin for Tuberculosis. N Engl J Med. 2021;384:1705–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].van Ingen J, Boeree MJ, van Soolingen D, Mouton JW. Resistance mechanisms and drug susceptibility testing of nontuberculous mycobacteria. Drug Resist Updat. 2012;15:149–61. [DOI] [PubMed] [Google Scholar]
- [6].Gordon EM, Duncton MAJ, Gallop MA. Orally Absorbed Derivatives of the beta-Lactamase Inhibitor Avibactam. Design of Novel Prodrugs of Sulfate Containing Drugs. J Med Chem. 2018;61:10340–4. [DOI] [PubMed] [Google Scholar]
- [7].Soroka D, Ourghanlian C, Compain F, Fichini M, Dubee V, Mainardi JL, et al. Inhibition of beta-lactamases of mycobacteria by avibactam and clavulanate. J Antimicrob Chemother. 2016. [DOI] [PubMed] [Google Scholar]
- [8].CLSI. Susceptibility testing of mycobacteria, nocardia spp., and other aerobic actinomycetes. 3rd ed. CLSI Standard M24. Wayne, PA: Clinical and Laboratory Standards Institute; 2018. [PubMed] [Google Scholar]
- [9].Srivastava S, Wang JY, Magombedze G, Chapagain M, Huang HL, Deshpande D, et al. Nouveau short-course therapy and morphism mapping for clinical pulmonary Mycobacterium kansasii. Antimicrob Agents Chemother. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chapagain M, Gumbo T, Heysell SK, Srivastava S. Comparison of a novel regimen of rifapentine, tedizolid, and minocycline with standard regimens for treatment of pulmonary Mycobacterium kansasii. Antimicrob Agents Chemother. 2020;64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Srivastava S, Gumbo T. Clofazimine for the treatment of Mycobacterium kansasii. Antimicrob Agents Chemother. 2018;62: pii: e00248–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Srivastava S, Pasipanodya J, Sherman CM, Meek C, Leff R, Gumbo T. Rapid drug tolerance and dramatic sterilizing effect of moxifloxacin monotherapy in a novel hollow-fiber model of intracellular Mycobacterium kansasii disease. Antimicrob Agents Chemother. 2015;59:2273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ, Andrejak C, et al. Treatment of Nontuberculous Mycobacterial Pulmonary Disease: An Official ATS/ERS/ESCMID/IDSA Clinical Practice Guideline. Clin Infect Dis. 2020;71:905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Haworth CS, Banks J, Capstick T, Fisher AJ, Gorsuch T, Laurenson IF, et al. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax. 2017;72:ii1–ii64. [DOI] [PubMed] [Google Scholar]
- [15].Davies Forsman L, Schon T, Simonsson US, Bruchfeld J, Larsson M, Jureen P, et al. Intra- and extracellular activities of trimethoprim-sulfamethoxazole against susceptible and multidrug-resistant Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2014;58:7557–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pethe K, Bifani P, Jang J, Kang S, Park S, Ahn S, et al. Discovery of Q203, a potent clinical candidate for the treatment of tuberculosis. NatMed. 2013;19:1157–60. [DOI] [PubMed] [Google Scholar]
- [17].Rustomjee R, Diacon AH, Allen J, Venter A, Reddy C, Patientia RF, et al. Early bactericidal activity and pharmacokinetics of the diarylquinoline TMC207 in treatment of pulmonary tuberculosis. Antimicrob Agents Chemother. 2008;52:2831–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Diacon AH, Dawson R, Hanekom M, Narunsky K, Maritz SJ, Venter A, et al. Early bactericidal activity and pharmacokinetics of PA-824 in smear-positive tuberculosis patients. Antimicrob Agents Chemother. 2010;54:3402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tyagi S, Nuermberger E, Yoshimatsu T, Williams K, Rosenthal I, Lounis N, et al. Bactericidal activity of the nitroimidazopyran PA-824 in a murine model of tuberculosis. Antimicrobial Agents and Chemotherapy. 2005;49:2289–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Toor A, De Freitas G, Torras J. Necrotizing pneumonia in a patient with untreated Mycobacterium kansasii infection. Respir Med Case Rep. 2019;27:100849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bloch KC, Zwerling L, Pletcher MJ, Hahn JA, Gerberding JL, Ostroff SM, et al. Incidence and clinical implications of isolation of Mycobacterium kansasii: results of a 5-year, population-based study. Ann Intern Med. 1998;129:698–704. [DOI] [PubMed] [Google Scholar]
- [22].Wolinsky E When is an infection disease? Rev Infect Dis. 1981;3:1025–7. [DOI] [PubMed] [Google Scholar]
- [23].Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schunemann HJ. What is “quality of evidence” and why is it important to clinicians? BMJ. 2008;336:995–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ahn CH, Wallace RJ Jr., Steele LC, Murphy DT. Sulfonamide-containing regimens for disease caused by rifampin-resistant Mycobacterium kansasii. Am Rev Respir Dis. 1987;135:10–6. [DOI] [PubMed] [Google Scholar]
- [25].Alffenaar JW, Martson AG, Heysell SK, Cho JG, Patanwala A, Burch G, et al. Therapeutic Drug Monitoring in Non-Tuberculosis Mycobacteria Infections. Clin Pharmacokinet. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rampacci E, Stefanetti V, Passamonti F, Henao-Tamayo M. Preclinical Models of Nontuberculous Mycobacteria Infection for Early Drug Discovery and Vaccine Research. Pathogens. 2020;9:641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Inderlied CB, Nash KA. Antimycobacterial agents: in vitro susceptibility testing, spectra of activity, mechanisms of action and resistance, and assays for activity in biologic fluids. 4 ed. Baltimore, Md.: The Williams & Wilkins Co.; 1996. [Google Scholar]
- [28].Inderlied CB, Pfyffer GE. Susceptibility test methods: mycobacteria. 8 ed. Washington, D.C.: ASM Press; 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


