Abstract
Background:
Bile acids are central to enterohepatic signaling pathways activated through natural receptors, farnesoid X receptor [FXR mediates synthesis of fibroblast growth factor-19 (FGF-19)] and G protein-coupled bile acid receptor 1 (GPBAR1, also known as TGR5). Although bile acid diarrhea (BAD) is more commonly encountered in ileal resection or disease, there is evidence documenting “idiopathic” BAD in about 20% of adolescents and 30% of adults presenting with chronic, non-bloody diarrhea often attributed to irritable bowel syndrome. Mechanism(s) leading to increased hepatic synthesis and colonic bile acid levels in “idiopathic” BAD include reduced synthesis of FGF-19 by the ileal mucosa, or genetic variation in hepatocyte proteins klotho β and FGF receptor 4 (FGFR4) that mediate negative feedback of bile acid synthesis.
Purpose:
The objective of this review is to summarize the diagnosis of BAD in adults and adolescents. In addition to 75SeHCAT retention for diagnosis of BAD, studies have validated fasting serum 7αC4 and FGF-19, respectively by-product and inhibitor of hepatic bile acid synthesis, as well as fecal bile acid measurements. These assays are widely available through reference labs and they are being simplified (e.g., measurement of primary fecal bile acids in a random stool sample). BAD has also been identified as a co-factor contributing to persistent diarrhea in other diseases in remission including inflammatory bowel disease, microscopic colitis, celiac disease, and neuroendocrine tumors. In summary, advances in diagnosis of BAD provide opportunities for generalists and pediatric and adult gastroenterologists to provide targeted treatment for BAD presenting as chronic non-bloody diarrhea.
Graphical Abstract
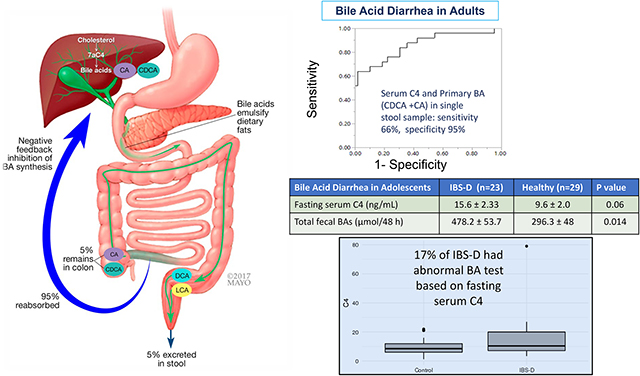
Introduction: Synthesis, Secretion and Circulation of Bile Acids
The objective of this review is to summarize the diagnosis of bile acid diarrhea (BAD) in adults and adolescents. Primary bile acids (BAs) are synthesized from cholesterol in the liver with the rate-limiting enzyme being 7α-hydroxylase (cytochrome P450 7A1, CYP7A1). These BAs are cholic acid (CA) and chenodeoxycholic acid (CDCA), which are conjugated with taurine and glycine, increasing aqueous solubility, and excreted in the bile. After storage in the gallbladder, BAs are delivered to the duodenum with ingestion of meals, emulsify fats and aid in their absorption.1
In the ileum, BAs are efficiently (~95%) absorbed via apical sodium bile acid transporter (ASBT), which is an active, energy-requiring process. In the ileal enterocytes, BAs stimulate the nuclear farnesoid X receptor (FXR) to produce fibroblast growth factor 19 (FGF-19). The latter is an enteroendocrine hormone that is transported to the liver and is actively transported into hepatocytes through FGF-receptor 4 (FGF-R4) interacting with a surface protein, klotho β. Within the hepatocyte, this leads to a small heterodimer protein (SHP) decreasing hepatic BA synthesis by CYP7A1. A by-product of this reaction is 7α-hydroxy-4-cholesten-3-one (C4), which is an indirect marker of the rate of BA synthesis.1
About 5–10% of the primary BAs unabsorbed in the ileum reach the colon, are deconjugated by bacterial bile salt hydrolases and dehydroxylated by bacterial 7α-dehydroxylase to form secondary bile acids, predominantly deoxycholic acid (DCA), lithocholic acid (LCA), and ursodeoxycholic acid (UDCA). In the colon, CDCA and DCA stimulate fluid secretion,2 increase mucosal permeability, and induce high amplitude propagated contractions,3,4 at least in part by stimulating TGR5 receptors located on enterocytes, smooth muscle cells, neural cells, immune cells including dendritic cells and macrophages, and enteroendocrine cells that produce glucagon-like peptide 1 (GLP-1).5 Although the human colon reabsorbs, by diffusion, at least 50% of the mass of bile acids reaching it,6 the BAs are excreted in stool are predominantly secondary BAs in health, with increased primary BAs excreted in some diarrheal diseases, perhaps due to more rapid transit through the colon and less time for dehydroxylation.
It has been suggested that, after early childhood, the ability to synthetize BAs and the patterns of BAs in children and adolescents are similar to those found in adults.7,8
Definition and Categories of Bile Acid Malabsorption and Diarrhea
In bile acid malabsorption (BAM), excess BAs reach the colon, increasing colonic motility and secretion, and causing chronic diarrhea, urgency, and abdominal cramping.9 There are four categories of BAD. Type I includes any terminal ileal disease that would prevent the reabsorption of BAs (e.g. Crohn’s disease, ileal resection, and radiation ileitis)10 and chronic colitis and short bowel syndrome in children and adolescents;11,12 type II or “idiopathic” with no underlying disease and typically presents as chronic diarrhea or diarrhea-predominant irritable bowel syndrome (IBS-D);10 type III results from malabsorption or biliopancreatic diseases including cholecystectomy10 or cystic fibrosis with or without pancreatic insufficiency in children;13 a separate category of type III (sometimes called type IV) results from excessive BA synthesis without a clear source of impaired bile acid re-absorption within the gastrointestinal tract, as seen in patients with hypertriglyceridemia or those on metformin treatment.14 Despite normal ileal BA absorption, 60% of patients with lymphocytic colitis and 27% of those with microscopic colitis were diagnosed with BAD,15 possibly a result of ileal atrophy.16 Similarly, increased fecal BA levels have been described in children and adolescents with colonic inflammation from inflammatory bowel disease independent of ileal abnormalities.11 Although the term BAD is sometimes used interchangeably with BAM, a recent editorial pointed out that BAM and BAD are not equivalent. BAM is a necessary antecedent for BAD, but demonstrating BAM is not necessarily proof that BAD is present since the latter is best reserved for patients with evidence of BAM and diarrhea that is responsive to bile acid sequestrant.17 Others have argued that the fundamental mechanism is not necessarily malabsorption of bile acids, but rather increased hepatic synthesis that exceeds the reabsorptive capacity of the active transport mechanisms in the ileum, and that most such patients (shown predominantly in those with <10% 75SeHCAT retention)18 have partial or complete response to bile acid sequestrants.19 To keep the terminology simple, we elect to use the abbreviation BAD.
Potential Mechanisms Underpinning Idiopathic Bile Acid Diarrhea
The strongest evidence regarding the mechanism of idiopathic BAD is the reduced production of FGF-19 by ileal enterocytes, leading to increased hepatic synthesis of BAs,20 which is accompanied by increased serum 7α-C4. The low FGF-19 may result from reduced FGF-19 and ASBT mRNA expression in ileal biopsies from patients with BAD,21 and this has also been confirmed by studies of FGF-19 protein expression based on immunohistochemistry.22,23 A second mechanism that probably accounts for <1% of idiopathic BAD results from a mutation in the gene (SLC10A2) for the ileal BA transporter protein, ASBT.24 A third potential mechanism is based on associations of genetic variants in GPBAR1 rs11554825, klotho β rs17618244, and FGFR4 rs351855, with acceleration of colonic transit (as a surrogate of diarrhea) in patients presenting with IBS-D or functional diarrhea.9,25,26
Symptoms and Signs of Bile Acid Malabsorption
In an online survey of 100 patients with BAD, 85% reported urgency, 54% abdominal pain, 88% occasional incontinence, and 52% felt the need to be close to the bathroom. After treatment with bile acid sequestrants, gastrointestinal and systemic symptoms improved or resolved by at least 50%, and there was a significant improvement in work absences and altered work hours.27 Patients with unexplained diarrhea or irritable bowel syndrome-diarrhea (IBS-D) who have increased fecal bile acid excretion typically have higher BMI, increased stool weight and fat, and accelerated colonic transit compared to patients without increased fecal bile acid excretion.28
Diagnosis of Bile Acid Malabsorption
Diagnosis is based on documentation of impaired ileal BA absorption, decreased hepatic feedback inhibition, and increased hepatic BA synthesis.29
The 75selenium homotaurocholic acid test (75SeHCAT) measures the retention of radiolabeled BAs in the abdomen 7 days after oral ingestion: cut-offs for mild, moderate, and severe BAM are <15%, <10%, and <5% retention, respectively. Responses to a BA binder, cholestyramine, are respectively 70%, 80% and 96%.18 This test is regarded as the gold standard diagnostic method, but it is not widely available, requires a gamma camera, and involves exposure to radiation. This test is not currently performed in the USA.
A 48-hour fecal bile acid excretion test involves consumption of a diet containing100 g fat/day for 4 days and collecting stool during the final 48 hours. Total and primary fecal BA levels have significant positive association with 75SeHCAT retention.30,31 Total and primary fecal bile acids were significant predictors of increased stool weight, frequency, and consistency,32–34 fecal weight >400 g/48h and acceleration of colonic transit.35 Three criteria are diagnostic of BAD: total fecal BAs ≥2,337 μmol/48h,6 primary BAs (CDCA and CA) >10%, or total fecal BAs ≥1,000 μmol/48h plus primary BAs >4%.35,36 In an analysis of 986 patients who underwent 48-hour fecal bile acid measurement for clinical assessment of chronic diarrhea, 26% of patients had elevated total fecal BAs, whereas 46% of patients had fecal primary BAs >10%, indicating that measuring total fecal BAs alone will miss a subgroup of patients who have features of BAD.35 This test is offered by a reference laboratory in the USA.
Fasting serum 7α-hydroxy-4-cholesten-3-one (7αC4) collected before 9:00 a.m. because of diurnal variation) is measured by C18 liquid chromatography, tandem mass spectrometry.37 Serum 7αC4 is an indirect measurement of BA synthesis and has been validated in comparison with 75SeHCAT (cut-off value of 7αC4 >48.4 ng/mL: sensitivity of 90% and specificity of 79%)38 and with 48-hour fecal bile acid (cut-off value of 7αC4 >52.5 ng/mL in functional diarrhea39 and >48.3 ng/mL in patients with Crohn’s disease with diarrhea likely attributable to BAD).40 This test is offered by reference laboratories in the USA.
Fasting serum fibroblast growth factor-19 (FGF-19) is inversely correlated with serum 7αC4,36 and the diagnostic cut-off is ≤61.7 pg/mL. Fasting FGF-19 is not sensitive (29%) or specific (78%) enough relative to fecal BA excretion39 to screen for BAD. CDCA-stimulated serum FGF-19 may be a more sensitive diagnostic “stress test” for BAD.41 This is a research test in the USA.
Relative to 75SeHCAT <10% (moderate BAD), fasting serum 7αC4 >48.4 ng/mL had a sensitivity of 90% and specificity of 79%,38 and fasting serum FGF-19 <145 pg/mL had a sensitivity of 58% and a specificity of 84%.42 Relative to total 48-hour fecal bile acids >2,337 μmol/48 hours, fasting 7αC4 >52.5 ng/mL had sensitivity of 25% and specificity of 90%,39 and fasting FGF-19 <61.7 pg/mL had sensitivity of 32% and specificity of 78%.39 These data suggest that the serum tests alone had insufficient sensitivity as potential diagnostic tests.
A combination test that has been proposed using fasting serum 7αC4 >52.5 ng/mL together with >10% primary BAs in a single, random stool sample43 performed well (63% sensitivity at 90% specificity) relative to BAD based on the three diagnostic criteria of the 48-hour fecal bile acid measurement. There is significant overall correlation between fasting serum C4 and % fecal primary bile acids and total fecal BA concentration.43 This combination test is not yet offered as a clinical diagnostic test in the USA.
Epidemiology of Idiopathic BAD and Associations with other Intestinal Diseases
Prevalence of idiopathic BAD in adults and adolescents without other underlying medical conditions
Adults:
Prevalence of idiopathic BAD in adults is based on two systematic reviews conducted in patients with functional diarrhea. One involved 15 prospective studies involving mostly 75SeHCAT retention and showed severe BAD (<5% retention) in 10%, moderate BAD (<10% retention) in 32%, and mild BAD (<15% retention) in 26% of patients.18 A second systematic review of 36 studies involving 5,028 patients included other diagnostic methods and the average prevalence was 30%.44
In 1,071 consecutive patients with chronic diarrhea undergoing SeHCAT scanning at Leeds teaching hospitals in the UK, 61% of the patients had no known risk factors other than chronic diarrhea, and 35.7% of those individuals had BAD and about 3/5 had moderate or severe BA malabsorption.45
Given that it is estimated that 5% of the population in developed countries has chronic diarrhea of >4 weeks’ duration at any point in time46 and that BAD affects at least 25% of patients with chronic diarrhea,18 it was proposed that ~1% of adults in Western countries have BAD.47
Adolescents:
In a cross-sectional study of adolescents7 which included 26 patients with IBS-D and 56 healthy controls recruited in two pediatric tertiary care centers, 20% of pediatric patients had abnormal fasting serum C4. Moreover, a preliminary report48 measured total fecal 48-hour bile acids and percent of primary fecal BAs which were significantly higher in 53 adolescents with IBS-D compared to 41 healthy adolescent controls. Total fecal BAs >688 μmol/48h had 87% specificity and 90% sensitivity to identify fecal weight of >377 g/48h (significant diarrhea); 33% of the adolescents with IBS-D had total fecal BA >688 μmol/48h.
BAD in association with other gastrointestinal diseases
a. In two studies of Crohn’s disease without ileal resection totalling 134 patients,49,50 BAD was reported in 11% based on serum markers and in 32% based on 75SeHCAT retention. A study of 31 pediatric patients with chronic colitis assessed by colonoscopy (including 15 patients with Crohn’s and 16 with ulcerative colitis with moderate or severe inflammatory activity in the ascending colon) showed patients had a significantly higher fecal excretion of 14C-cholic acid after i.v. administration (24.1%) than patients with no or mild inflammatory activity (6.6%).11
b. Post-cholecystectomy: A systematic review of 25 studies that included 3,388 patients showed only 9.1% of patients developed diarrhea after cholecystectomy, with two-thirds associated with evidence of BAD.51 Similarly, in 125 consecutive patients who underwent laparoscopic cholecystectomy, 25.2% developed diarrhea at one week after surgery and 5.7% had diarrhea at 3 months.52
c. Microscopic colitis: In one study, 43% of patients with microscopic colitis had BAD [lymphocytic (60%); collagenous (27%) colitis], and 86% of those with BAD responded to cholestyramine.15 Findings in other studies of BAD in microscopic colitis have been conflicting.53–55 The cause of BAD in microscopic colitis may be related to the villous atrophy, inflammation, and collagen deposition in the ileum.56,57
d. Diverse intestinal inflammatory diseases in remission: A study of 47% of 51patients with celiac disease in remission, 53% of 34 patients with collagenous colitis in remission, and 50% of 32 with lymphocytic colitis in remission showed patients had increased fecal BA excretion.58
e. Neuroendocrine tumors: In 22 patients with neuroendocrine tumors and no ileal resection, 13/15 tested (87%) had BAD based on a 48h fecal BA measurement.59
Management of Patients Without Other Underlying Medical Conditions
Diagnostic test or empiric trial of BA sequestrants?
When diagnostic tests are not available, empiric treatment with bile acid sequestrants is advocated for patients with suspected BAD. However, compliance with a therapeutic trial may be suboptimal,60 compromising interpretation of a negative response. Severity of BAD can predict response to treatment, and this supports the rationale to objectively measure the indices consistent with BAD rather than resorting to empiric treatment.18,61 British and Canadian gastroenterology organizations have published guidelines supporting diagnosis with empiric trial with bile acid sequestrants for suspected BAD in patients presenting with chronic diarrhea.62,63 A positive test for the diagnosis of BAD was associated with reduced healthcare utilization in referral centers in the UK64 and USA.65 In the latter retrospective study of almost 1,000 patients being evaluated for chronic unexplained diarrhea, there was high healthcare utilization in referred patients (average 1.2 transaxial imaging, 2.6 endoscopic procedures, and 1.6 miscellaneous tests/person) before positive BAD diagnosis.65
Treatment of BAD
Dietary modifications:
A diet with <20% of total daily caloric intake as fat complements efficacy of BA sequestrants in relief of abdominal discomfort, distension, urgency, and stool consistency and frequency.66
Bile acid sequestrants:
Three BA sequestrants are available in either powder or tablet formulations: cholestyramine, colestipol, and colesevelam. These medications are ingested with meals to bind free BAs and reduce delivery to the colon. With all BA sequestrants, it is advisable to avoid ingestion of medications or supplements within 2 hours of the sequestrant because of interference with absorption and pharmacokinetics. With long-term treatment, periodic monitoring of Vitamins A and D levels and prothrombin time are advised,67,68 though this recommendation was not supported by the Canadian guideline.62 The optimal timing of dosing bile acid sequestrants for BAD has not been well studied. Although dosing recommendations in the label suggest that the sequestrants should be administered with meals in hypercholesterolemia, the optimal timing for BAD may be a few hours after the final meal of the day so that the sequestrant is in the colon, ready to bind the bile acids that “leak” from the small intestine after meals.
The only randomized trial of cholestyramine efficacy in BAD showed response rates of 40% and 53.8% respectively in patients with 75SeHCAT retention <10% (moderate to severe BAD) or 20% (mild to borderline abnormal).69
In an open-label trial in patients with 75SeHCAT retention <20%, colestipol reduced stool frequency and IBS severity score.70 In a comparison of cholestyramine and hydroxypropyl cellulose in patients with chronic water diarrhea (some with 75SeHCAT <15%), there was no difference in the primary endpoint of proportion with mean ≤3 liquid bowel movements per week.69 However, hydroxypropyl cellulose also binds BAs without affecting hepatic bile acid synthesis.
In another open-label study in patients with BAD based on high 48h fecal BA excretion, colesevelam, 1875 mg twice daily for 10 days, decreased stool consistency and increased stool excretion of sequestered bile acids, with compensatory increase in hepatic synthesis of BAs shown by increased serum C4.71 Colesevelam also slowed emptying of the ascending colon compared with placebo in IBS-D; the treatment effect was associated with baseline serum C4, which reflects the hepatic bile acid synthesis rate.72 A small placebo-controlled trial of colesevelam did not reveal clinical efficacy, but colesevelam increased delivery of total and secondary BAs to stool, hepatic BA synthesis, and colonic mucosal expression of genes that regulate BAs, farnesoid X, and GPBAR1 receptors.73 The latter may be compensatory mechanisms following the intraluminal sequestration of BAs. Further controlled trials are necessary to assess the effects of BA sequestrants for BAD, especially as long-term therapy is usually required.
FXR agonists:
FXR agonists were initially developed for cholestatic liver diseases; they are attractive for treating BAD because they target the underlying pathophysiology of idiopathic BAD. Efficacy of FXR agonists in BAM has been shown in in vitro and in two in vivo studies. FXR agonists attenuated calcium and cyclic adenosine monophosphate (cAMP) dependent chloride secretion on colonic epithelium.74 A 2-week trial of obeticholic acid (which is chemically 6-ethyl CDCA), 25mg daily, in patients with primary and secondary BAD showed improvements in stool frequency and form, and total diarrhea index, with corresponding increase in FGF-19 and decrease in serum 7αC4 and fecal BAs.75 However, obeticholic acid is associated with pruritus76 (3.93-fold with 25 mg and 1.65-fold with 10 mg dose) in patients with non-cholestatic non-alcoholic steatohepatitis.
In a separate multicenter, placebo-controlled clinical trial, the non-bile acid molecule, tropifexor, retarded ascending colon emptying in patients with BAD, although the clinical endpoints were not significantly altered in that small clinical trial.77
Conclusions
The bile acid field has expanded in relevance, with documented prevalence in about one-third of adolescents and adults presenting with suspected IBS-diarrhea. Advances in diagnosis have been facilitated by the validation of screening serum tests, fecal bile acid excretion, and the 75SeHCAT retention test for BAD. Importantly, primary fecal BA measurements identify additional patients with BAD, and early validation studies have shown that the combination of fasting serum 7αC4 and primary BAs in a random stool sample has high diagnostic accuracy. These diagnostic tests are relevant not only in unexplained chronic diarrhea or presumed IBS-diarrhea, but also in microscopic colitis, celiac disease in remission, short gut syndrome, Crohn’s disease in apparent remission and without ileal resection, and neuroendocrine tumors. These tests help identify patients with BAD and have potential to individualize care of unexplained diarrhea. Agonists of FXR receptors provide novel approaches for treatment of BAD and are promising as complementary or alternative treatments to BA sequestrants.
Figure 1. Synthesis, secretion, and enterohepatic circulation of BAs in humans.

(1) Primary BAs are synthesized in hepatocytes from cholesterol. (2) BAs are conjugated to glycine and taurine and are stored in the gallbladder at high concentrations. (3) After feeding, conjugated BAs are secreted in the intestine where they emulsify dietary fats and form mixed micelles that facilitate digestion and absorption of the products of triglyceride digestion. (4) Conjugated BAs are actively absorbed by the apical sodium BA co-transporter (IBAT) at the apical membrane of enterocytes of the terminal ileum. (5) In the colon, bacteria deconjugate and dehydroxylate primary BAs to form secondary BAs, which are passively absorbed. (6) Conjugated and unconjugated BAs enter the portal vein and recirculate to the liver for reuse. BA, bile acid; CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; IBAT, ileal bile acid transporter; LCA, lithocholic acid; Na, sodium; UDCA, ursodeoxycholic acid. Reproduced with permission from ref. 1, Bunnett NW. Neuro-humoral signalling by bile acis and the TGR5 receptor in the gastrointestinal tract. J Physiol 2014;592:2943–2950. All permission requests for this image should be made to the copyright holder.
Table 1. Current and future bile acid diarrhea (BAD) diagnostic tests (BA=bile acids; primary BAs are cholic acid and chenodeoxycholic acid). Information in pediatric patients is limited.
Amount of primary bile acids reflects direct or indirect secretory potential (CDCA and CA respectively, as CA undergoes 7α hydroxylation in the colon to form the secretory, deoxycholic acid). Modified with permission from ref. 29, Vijayvargiya P, Camilleri M. Commentary: Current practice in the diagnosis of bile acid diarrhea. Gastroenterology 2019;156:1233–1238.
| Diagnostic Test | 75SeHCAT | Fasting serum C4 | Fasting serum FGF19 | Total fecal BAs | Primary BAs >4% + total fecal BAs | Fecal primary BAs >10% | Combined tests of fecal primary BAs + fasting serum C4 |
|---|---|---|---|---|---|---|---|
| What does it measure? | Ileal capacity to reabsorb radio-labeled bile acid retention (%) on day 7 | Hepatic bile acid synthesis | Amount of feedback inhibition to hepatic bile acid synthesis | Total fecal bile acid excreted from the colon | Amount of primary bile acids reflects direct or indirect secretory potential (CDCA and CA respectively) with total fecal BA excretion | Amount of bile acids that are directly synthesized from the liver with secretory potential | Combining serum and stool biomarkers to simplify diagnosis of bile acid diarrhea |
| Diagnostic cutoffs | <5% (severe) <10%+ (moderate) <15% (mild) |
≥52.5 ng/mL | ≤61.7 pg/mL | ≥2,337 μmol/48h | Primary BA >4% + total fecal BA >1,000 μmol/48h | >10% primary BA | Fecal sample primary BA >10% + serum C4 ≥52.5 ng/mL |
| Sensitivity relative to fecal wt >400g/48h | 15% | 28% | 59% | 46% | 49% | ||
| Specificity relative to fecal wt >400g/48h | 86% | 75% | 92% | 97% | 91% | ||
| Diet, radiation and equipment required for measurement | γ camera + radiation exposure; 7-day test | HPLC; Measure before 9am |
ELISA; Measure before 9am |
HPLC Requires 100-gram high fat diet × 4 days and 2-day stool collection |
HPLC + HPLC single, random stool sample + C4 measured before 9am |
||
| Comment or pitfalls of testing | ? best for type I BAD | ? diagnostic accuracy | ? diagnostic accuracy | Analysis of fecal BAs reflects BA in colon | Identify additional patients | Identify additional patients | Identifying BAD based on 48h fecal BA: 63% Sensitivity with 90% specificity |
Acknowledgement:
The authors thank Mrs. Cindy Stanislav for excellent secretarial assistance.
Funding:
Dr. Camilleri’s research on bile acid diarrhea is supported by NIH grant R01-DK115950.
Footnotes
Disclosures: The authors have no conflicts of interest.
Data Sharing and Data Accessibility
Data sharing is not applicable in this review article as no new data were created or analyzed.
References
- 1.Bunnett NW. Neuro-humoral signalling by bile acids and the TGR5 receptor in the gastrointestinal tract. J Physiol 2014;592:2943–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wingate DL, Krag E, Mekhjian HS, Phillips SF. Relationships between ion and water movement in the human jejunum, ileum and colon during perfusion with bile acids. Clin Sci Mol Med 1973;45:593–606. [DOI] [PubMed] [Google Scholar]
- 3.Kirwan WO, Smith AN, Mitchell WD, Falconer JD, Eastwood MA. Bile acids and colonic motility in the rabbit and the human. Gut 1975;16:894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bampton PA, Dinning PG, Kennedy ML, Lubowski DZ, Cook IJ. The proximal colonic motor response to rectal mechanical and chemical stimulation. Am J Physiol Gastrointest Liver Physiol 2002;282:G443–G449. [DOI] [PubMed] [Google Scholar]
- 5.Brighton CA, Rievaj J, Kuhre RE, Glass LL, Schoonjans K, Holst JJ, Gribble FM, Reimann F. Bile acids trigger GLP-1 release predominantly by accessing basolaterally located G protein–coupled bile acid receptors. Endocrinology 2015;156:3961–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mekhjian HS, Phillips SF, Hofmann AF. Colonic absorption of unconjugated bile acids: perfusion studies in man. Dig Dis Sci 1979;24:545–550. [DOI] [PubMed] [Google Scholar]
- 7.Beinvogl BC, Manini ML, Camilleri M, Donato LJ, Harmsen WS, Absah I, Burch E, Schechter NL, Nurko S. Markers of bile acid metabolism in pediatric diarrhea predominant irritable bowel syndrome and healthy controls. J Pediatr Gastroenterol Nutr 2021;72:859–865. [DOI] [PubMed] [Google Scholar]
- 8.Xiong JJ, Hu HW, Xu CZ, Yin J-W, Liu M, Zhang L-Z, Duan Y, Huang Y-K. Developmental patterns of fecal bile acids in healthy neonates and children. Med Sci Monit 2021;27:e928214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camilleri M Physiological underpinnings of irritable bowel syndrome: neurohormonal mechanisms. J Physiol 2014;592:2967–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fromm H, Malavolti M. Bile acid-induced diarrhoea. Clin Gastroenterol 1986;15:567–582. [PubMed] [Google Scholar]
- 11.Holmquist L, Andersson H, Rudic N, Ahrén C, Fällström SP. Bile acid malabsorption in children and adolescents with chronic colitis. Scand J Gastroenterol 1986;21:87–92. [DOI] [PubMed] [Google Scholar]
- 12.Ohkohchi N, Andoh T, Izumi U, Igarashi Y, Ohi R. Disorder of bile acid metabolism in children with short bowel syndrome. J Gastroenterol 1997;32:472–479. [DOI] [PubMed] [Google Scholar]
- 13.Colombo C, Roda A, Roda E, Piceni Sereni L, Brega A, Fugazza R, Giunta A. Bile acid malabsorption in cystic fibrosis with and without pancreatic insufficiency. J Pediatr Gastroenterol Nutr 1984;3:556–562. [DOI] [PubMed] [Google Scholar]
- 14.Scarpello JH, Hodgson E, Howlett HC. Effect of metformin on bile salt circulation and intestinal motility in type 2 diabetes mellitus. Diabetic Med 1998;15:651–656. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez-Banares F, Esteve M, Salas A, Forné TM, Espinos JC, Martín-Comin J, Viver JM. Bile acid malabsorption in microscopic colitis and in previously unexplained functional chronic diarrhea. Dig Dis Sci 2001;46:2231–2238. [DOI] [PubMed] [Google Scholar]
- 16.Pardi DS. Diagnosis and management of microscopic colitis. Am J Gastroenterol 2017;112:78–85. [DOI] [PubMed] [Google Scholar]
- 17.Schiller LR. BAM=/=BAD. Dig Dis Sci. 2021. Aug 7. doi: 10.1007/s10620-021-07196-8 [DOI] [Google Scholar]
- 18.Wedlake L, A’Hern R, Russell D, Thomas K, Walters JRF, Andreyev HJN. Systematic review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2009;30:707–717. [DOI] [PubMed] [Google Scholar]
- 19.Walters JRF. Making the diagnosis of bile acid diarrhea. Am J Gastroenterol 2020;115:1974–1975. [DOI] [PubMed] [Google Scholar]
- 20.Walters JRF, Tasleem AM, Omer OS, Brydon WG, Dew T, le Roux CW. A new mechanism for bile acid diarrhea: defective feedback inhibition of bile acid biosynthesis. Clin Gastroenterol Hepatol 2009;7:1189–1194. [DOI] [PubMed] [Google Scholar]
- 21.Johnston IM, Nolan JD, Pattni SS, Appleby RN, Zhang JH, Kennie SL, Madhan GK, Jameie-Oskooei S, Pathmasrirengam S, Lin J, Hong A, Dixon PH, Williamson C, Walters JRF. Characterizing factors associated with differences in FGF19 blood levels and synthesis in patients with primary bile acid diarrhea. Am J Gastroenterol 2016;111:423–432. [DOI] [PubMed] [Google Scholar]
- 22.Chang C, Jiang J, Sun R, Wang S, Chen H. Downregulation of serum and distal ileum fibroblast growth factor19 in bile acid diarrhoea patients. Dig Dis Sci 2021. May 26. doi: 10.1007/s10620-021-07042-x. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Camilleri M BAD on the runs: improved diagnosis of idiopathic bile acid diarrhea. Dig Dis Sci 2021. May 26. doi: 10.1007/s10620-021-07044-9. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Montagnani M, Love MW, Rossel P, Dawson PA, Qvist P. Absence of dysfunctional ileal sodium-bile acid cotransporter gene mutations in patients with adult-onset idiopathic bile acid malabsorption. Scand J Gastroenterol 2001;36:1077–1080. [DOI] [PubMed] [Google Scholar]
- 25.Camilleri M, Shin A, Busciglio I, Carlson P, Acosta A, Bharucha AE, Burton D, Lamsam J, Lueke A, Donato LJ, Zinsmeister AR. Genetic variation in GPBAR1 predisposes to quantitative changes in colonic transit and bile acid excretion. Am J Physiol 2014;307:G508–G516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camilleri M, Klee EW, Shin A, Carlson P, Li Y, Grover M, Zinsmeister AR. Irritable bowel syndrome-diarrhea: characterization of genotype by exome sequencing, and phenotypes of bile acid synthesis and colonic transit. Am J Physiol 2014;306:G13–G26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bannaga A, Kelman L, O’Connor M, Pitchford C, Walters JRF, Arasaradnam RP. How bad is bile acid diarrhoea: an online survey of patient-reported symptoms and outcomes. BMJ Open Gastroenterol 2017;4(1):e000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camilleri M, Busciglio I, Acosta A, Shin A, Carlson P, Burton D, Ryks M, Rhoten D, Lamsam J, Lueke A, Donato LJ, Zinsmeister AR. Effect of increased bile acid synthesis or fecal excretion in irritable bowel syndrome-diarrhea. Am J Gastroenterol 2014;109:1621–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vijayvargiya P, Camilleri M. Commentary: Current practice in the diagnosis of bile acid diarrhea. Gastroenterology 2019;156:1233–1238. [DOI] [PubMed] [Google Scholar]
- 30.Scheurlen C, Kruis W, Bull U, Stellaard F, Lang P, Paumgartner G. Comparison of 75SeHCAT retention half-life and fecal content of individual bile acids in patients with chronic diarrheal disorders. Digestion 1986;35:102–108. [DOI] [PubMed] [Google Scholar]
- 31.Sciarretta G, Fagioli G, Furno A, Vicini G, Cecchetti L, Grigolo B, Verri A, Malaguti P. 75Se HCAT test in the detection of bile acid malabsorption in functional diarrhoea and its correlation with small bowel transit. Gut 1987;28:970–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin A, Camilleri M, Vijayvargiya P, Busciglio I, Burton D, Ryks M, Rhoten D, Lueke A, Saenger A, Girtman A, Zinsmeister AR. Bowel functions, fecal unconjugated primary and secondary bile acids, and colonic transit in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 2013;11:1270–1275.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong BS, Camilleri M, Carlson P, McKinzie S, Busciglio I, Bondar O, Dyer RB, Lamsam J, Zinsmeister AR. Increased bile acid biosynthesis is associated with irritable bowel syndrome with diarrhea. Clin Gastroenterol Hepatol 2012;10:1009–1015.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vijayvargiya P, Camilleri M, Burton D, Busciglio I, Lueke A, Donato LJ. Bile and fat excretion are biomarkers of clinically significant diarrhoea and constipation in irritable bowel syndrome. Aliment Pharmacol Ther 2019;49:744–758. [DOI] [PubMed] [Google Scholar]
- 35.Vijayvargiya P, Camilleri M, Chedid V, Carlson P, Busciglio I, Burton D, Donato LJ. Analysis of fecal primary bile acids detects increased stool weight and colonic transit in patients with chronic functional diarrhea. Clin Gastroenterol Hepatol 2019;17:922–929.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peleman C, Camilleri M, Busciglio I, Burton D, Donato LJ, Zinsmeister AR. Colonic transit and bile acid synthesis or excretion in patients with irritable bowel syndrome–diarrhea without bile acid malabsorption. Clin Gastroenterol Hepatol 2017;15:720–727, e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donato LJ, Lueke A, Kenyon SM, Meeusen JW, Camilleri M. Description of analytical method and clinical utility of measuring serum 7-alpha-hydroxy-4-cholesten-3-one (7aC4) by mass spectrometry. Clin Biochem 2018;52:106–111. [DOI] [PubMed] [Google Scholar]
- 38.Sauter GH, Munzing W, von Ritter C, Paumgartner G. Bile acid malabsorption as a cause of chronic diarrhea diagnostic value of 7α-hydroxy-4-cholesten-3-one in serum. Dig Dis Sci 1999;44:14–19. [DOI] [PubMed] [Google Scholar]
- 39.Vijayvargiya P, Camilleri M, Carlson P, Lueke A, O’Neill J, Burton D, Busciglio I, Donato L. Performance characteristics of serum C4 and FGF19 measurements to exclude the diagnosis of bile acid diarrhoea in IBS‐diarrhoea and functional diarrhoea. Aliment Pharmacol Ther 2017;46:581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Battat R, Duijvestein M, Vande Casteele N, Singh S, Dulai PS, Valasek MA, Mimms L, McFarland J, Hester KD, Renshaw M, Jain A, Sandborn WJ, Boland BS. Serum concentrations of 7α-hydroxy-4-cholesten-3-one are associated with bile acid diarrhea in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2019;17:2722–2730.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borup C, Syversen C, Bouchelouche P, Damgaard M, Graff J, Rumessen JJ, Munck LK. Diagnosis of bile acid diarrhoea by fasting and postprandial measurements of fibroblast growth factor 19. Eur J Gastroenterol Hepatol 2015;27:1399–1402. [DOI] [PubMed] [Google Scholar]
- 42.Pattni SS, Brydon WG, Dew T, Johnston IM, Nolan JD, Srinivas M, Basumani P, Bardhan KD, Walters JRF. Fibroblast growth factor 19 in patients with bile acid diarrhoea: a prospective comparison of FGF19 serum assay and SeHCAT retention. Aliment Pharmacol Ther 2013;38:967–976. [DOI] [PubMed] [Google Scholar]
- 43.Vijayvargiya P, Camilleri M, Taylor A, Busciglio I, Loftus EV Jr, Donato LJ. Combined fasting serum C4 and primary bile acids from a single stool sample to diagnose bile acid diarrhea. Gastroenterology 2020;159:1952–1954.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valentin N, Camilleri M, Altayar O, Vijayvargiya P, Acosta A, Nelson AD, Murad MH. Biomarkers for bile acid diarrhoea in functional bowel disorder with diarrhoea: a systematic review and meta-analysis. Gut 2016;65:1951–1959. [DOI] [PubMed] [Google Scholar]
- 45.Lim SJ, Gracie DJ, Kane JS, Mumtaz S, Scarsbrook AF, Chowdhury FU, Ford AC, Black CJ. Prevalence of, and predictors of, bile acid diarrhea in outpatients with chronic diarrhea: A follow-up study. Neurogastroenterol Motil 2019;31:e13666. [DOI] [PubMed] [Google Scholar]
- 46.Fine KD, Schiller LR. AGA Technical Review on the Evaluation and Management of Chronic Diarrhea. Gastroenterology 1999;116:1464–1486. [DOI] [PubMed] [Google Scholar]
- 47.Walters JR, Pattni SS. Managing bile acid diarrhoea. Ther Adv Gastroenterol 2010;3:349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elsisy MF, Manini ML, Chedid VG, Vijayvargiya P, Ezaizi Y, Camilleri M. Diagnosing bile acid diarrhea in pediatric IBS-D patients using 48 hours fecal bile acids testing. Gastroenterology 2021;160:S609–S610. [Google Scholar]
- 49.Lenicek M, Duricova D, Komarek V, Gabrysova B, Lukas M, Smerhovsky Z, Vitek L. Bile acid malabsorption in inflammatory bowel disease: assessment by serum markers. Inflamm Bowel Dis 2011;17:1322–1327. [DOI] [PubMed] [Google Scholar]
- 50.Nyhlin H, Merrick MV, Eastwood MA. Bile acid malabsorption in Crohn’s disease and indications for its assessment using SeHCAT. Gut 1994;35:90–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farahmandfar MR, Chabok M, Alade M, Bouhelal A, Patel B. Post-cholecystectomy diarrhoea: a systematic review. Surgical Science 2012;3:332–338. [Google Scholar]
- 52.Yueh T-P, Chen F-Y, Lin T-E, Chuang M-T. Diarrhea after laparoscopic cholecystectomy: Associated factors and predictors. Asian J Surg 2014;37:171–177. [DOI] [PubMed] [Google Scholar]
- 53.Giardiello FM, Bayless TM, Jessurun J, Hamilton SR, Yardley JH. Collagenous colitis: Physiologic and histopathologic studies in seven patients. Ann Intern Med 1987;106:46–49. [DOI] [PubMed] [Google Scholar]
- 54.Kingham JG, Levison DA, Ball JA, Dawson AM. Microscopic colitis-a cause of chronic watery diarrhoea. Br Med J 1982;285:1601–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eusufzai S, Löfberg R, Veress B, Einarsson K, Angelin B. Studies on bile acid metabolism in colagenous colitis: no evidence of bile acid malabsorption as determined by the SeHCAT test. Eur J Gastroenterol Hepatol 1992;4:317–321. [Google Scholar]
- 56.Einarsson K, Eusufzai S, Johansson U, Lofberg R, Theodorsson E, Veress B. Villous atrophy of distal ileum and lymphocytic colitis in a woman with bile acid malabsorption. Eur J Gastroenterol Hepatol 1992;4:585–590. [Google Scholar]
- 57.Marteau P, Lavergne-Slove A, Lemann M, Bouhnik Y, Bertheau P, Becheur H, Galian A, Rambaud JC. Primary ileal villous atrophy is often associated with microscopic colitis. Gut 1997;41:561–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vijayvargiya P, Gonzalez Izundegui D, Calderon G, Tawfic S, Batbold S, Saifuddin H, Duggan P, Melo V, Thomas T, Heeney M, Beyde A, Miller J Jr, Valles K, Oyemade K, Brant JF, Atieh J, Donato LJ, Camilleri M. Increased fecal bile acid excretion in a significant subset of patients with other inflammatory diarrheal diseases. Dig Dis Sci 2021. Apr 22. doi: 10.1007/s10620-021-06993-5. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khanna L, Halfdanarson TR, Sonbol MB, Eiring R, Prond T, Camilleri M. Bile acid malabsorption in patients with neuroendocrine tumors. Dig Dis Sci (2021, in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilcox C, Turner J, Green J. Systematic review: the management of chronic diarrhoea due to bile acid malabsorption. Aliment Pharmacol Ther 2014;39:923–939. [DOI] [PubMed] [Google Scholar]
- 61.Orekoya O, McLaughlin J, Leitao E, Johns W, Lal S, Paine P. Quantifying bile acid malabsorption helps predict response and tailor sequestrant therapy. Clin Med 2015;15:252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sadowski DC, Camilleri M, Chey WD, Leontiadis GI, Marshall JK, Shaffer EA, Tse F, Walters JRF. Canadian Association of Gastroenterology clinical practice guideline on the management of bile acid diarrhea. Clin Gastroenterol Hepatol 2020;18:24–41. [DOI] [PubMed] [Google Scholar]
- 63.Arasaradnam RP, Brown S, Forbes A, Fox MR, Hungin P, Kelman L, Major G, O’Connor M, Sanders DS, Sinha R, Smith SC, Thomas P, Walters JRF. Guidelines for the investigation of chronic diarrhoea in adults: British Society of Gastroenterology, 3rd edition. Gut 2018;67:1380–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Turner JM, Pattni SS, Appleby RN, Walters JFR. A positive SeHCAT test results in fewer subsequent investigations in patients with chronic diarrhoea. Frontline Gastroenterol 2017;8:279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vijayvargiya P, Gonzalez Izundegui D, Calderon G, Tawfic S, Batbold S, Camilleri M. Fecal bile acid testing in assessing patients with chronic unexplained diarrhea: implications for healthcare utilization. Am J Gastroenterol 2020;115:1094–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jackson A, Lalji A, Kabir M, Muls A, Gee C, Vyoral S, Shaw C, Andreyev HJN. PTU-128: the efficacy of using low-fat dietary interventions to manage bile acid malabsorption. Gut 2017;66(Suppl.2):A114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barkun AN, Love J, Gould M, Pluta H, Steinhart H. Bile acid malabsorption in chronic diarrhea: pathophysiology and treatment. Can J Gastroenterol 2013;27:653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Westergaard H. Bile acid malabsorption. Curr Treat Options Gastroenterol 2007;10:28–33. [DOI] [PubMed] [Google Scholar]
- 69.Fernandez-Banares F, Rosinach M, Piqueras M, Ruiz-Cerulla A, Modolell I, Zabana Y, Guardiola J, Esteve M. Randomised clinical trial: colestyramine vs. hydroxypropyl cellulose in patients with functional chronic watery diarrhoea. Aliment Pharmacol Ther 2015;41:1132–1140. [DOI] [PubMed] [Google Scholar]
- 70.Bajor A, Törnblom H, Rudling M, Ung K-A, Simrén M. Increased colonic bile acid exposure: a relevant factor for symptoms and treatment in IBS. Gut 2015;64:84–92. [DOI] [PubMed] [Google Scholar]
- 71.Camilleri M, Acosta A, Busciglio I, Boldingh A, Dyer RB, Zinsmeister AR, Lueke A, Gray A, Donato LJ. Effect of colesevelam on faecal bile acids and bowel functions in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2015;41:438–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Odunsi-Shiyanbade ST, Camilleri M, McKinzie S, Burton D, Carlson P, Busciglio IA, Lamsam J, Singh R, Zinsmeister AR. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin Gastroenterol and Hepatol 2010;8:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vijayvargiya P, Camilleri M, Carlson P, Nair A, Linker Nord S, Ryks M, Rhoten D, Burton D, Busciglio I, Lueke A, Harmsen WS, Donato LJ. Effects of colesevelam on bowel symptoms, biomarkers, and colonic mucosal gene expression in patients with bile acid diarrhea in a randomized trial. Clin Gastroenterol Hepatol 2020;18:2962–2970.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mroz MS, Keating N, Ward JB, Sarker R, Amu S, Aviello G, Donowitz M, Fallon PG, Keely SJ. Farnesoid X receptor agonists attenuate colonic epithelial secretory function and prevent experimental diarrhoea in vivo. Gut 2014;63:808–817. [DOI] [PubMed] [Google Scholar]
- 75.Walters J, Johnston I, Nolan J, Vassie C, Pruzanski ME, Shapiro DA. The response of patients with bile acid diarrhoea to the farnesoid X receptor agonist obeticholic acid. Aliment Pharmacol Ther 2015;41:54–64. [DOI] [PubMed] [Google Scholar]
- 76.Kulkarni AV, Tevethia HV, Arab JP, Candia R, Premkumar M, Kumar P, Sharma M, Nageshwar Reddy D, Rao Padaki N. Efficacy and safety of obeticholic acid in liver disease-A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol 2021;45:101675. [DOI] [PubMed] [Google Scholar]
- 77.Camilleri M, Linker Nord S, Burton D, Oduyebo I, Zhang Y, Chen J, Im K, Bhad P, Badman MK, Sanders DS, Walters JRF. Randomised clinical trial: Significant biochemical and colonic transit effects of the farnesoid X receptor agonist, tropifexor, in patients with primary bile acid diarrhoea. Aliment Pharmacol Ther 2020;52:808–820. [DOI] [PubMed] [Google Scholar]


