Abstract
The pharmacokinetics of cefepime following administration of a single 2-g dose were evaluated for 12 adult patients with thermal burn injury and suspected or documented infection. Serial blood and urine samples for cefepime concentration determination were obtained for 24 h following drug administration. Serum and urine cefepime concentrations were determined by high-performance liquid chromatography and serum concentrations were fit to a two-compartment pharmacokinetic model. Mean (standard deviation [SD]) age, actual body weight (ABW), percent total body surface area burned, and days postburn at the time of study were 41 (13) years, 84 (22) kg, 36 (17)%, and 9 (3) days, respectively. Mean (SD) measured creatinine clearance (CLCR), total clearance (CLT), renal clearance (CLR), alpha phase half-life, beta phase half-life, and volume of distribution at steady state (VSS) were 135 (31) ml/min, 8.8 (2.4) liters/h, 8.1 (2.0) liters/h, 0.33 (0.14) h, 2.8 (0.6) h, and 0.43 (0.10) liters/kg ABW, respectively. Cefepime CLT and CLR in burn patients were similar to previously reported values for healthy volunteers when normalized by CLCR. Stepwise multiple regression was used to associate CLT with CLCR and days postburn (r2 = 0.861), CLR with CLCR and days postburn (r2 = 0.773), nonrenal clearance with percent third-degree (% 3°) burn and albumin concentration (r2 = 0.550), and VSS only with % 3° burn (r2 = 0.624). Simulated steady-state serum concentrations obtained by using the patients’ pharmacokinetic parameters exceeded the susceptibility interpretive standard (breakpoint) of cefepime for at least 60% of the dosing interval with dosing regimens of 1 g every 8 h (q8h), 2 g q8h, and 2 g q12h. Despite differences in pharmacokinetic parameters between our patients and healthy volunteers, it appears that these dosing regimens may be adequate in similar burn patients.
Numerous pathophysiological changes occur as a result of burn injury, and these changes may alter the pharmacokinetic characteristics and effectiveness of antimicrobial agents administered to burn patients (9, 21, 26). Several reports have demonstrated the necessity of larger and/or more frequently administered doses for antimicrobials which are primarily renally eliminated (2, 10, 11, 16, 17, 23, 28, 35, 37). Major reasons for this include increased renal elimination of drugs resulting from burn-induced increased glomerular filtration rate, alterations in fluid balance (affecting apparent distribution volume), increased metabolic rate, and altered protein binding (21, 24). Therefore, it is important to rigorously evaluate antimicrobials to determine what effect burn injury may have on the pharmacokinetic disposition and resultant dosing requirements in these patients.
Cefepime is a newer cephalosporin antibiotic possessing a wider spectrum of antibacterial activity and greater potency than most earlier cephalosporins (29). Its spectrum of activity includes pathogens such as Pseudomonas aeruginosa and Staphylococcus aureus, making it a potential candidate for treatment of bacterial infections in this population. Because of known alterations of drug disposition with other β-lactams and its potential usage in this patient population, the objective of this study was to characterize the pharmacokinetics of cefepime following a single dose in burn patients requiring antibiotics for suspected or documented infection.
(This study was presented in part at the American College of Clinical Pharmacy Spring Practice and Research Forum, Palm Springs, Calif., April 1998.)
MATERIALS AND METHODS
Patients.
All patients aged 18 to 80 years admitted to the Medical University of South Carolina Burn Unit with thermal burn injury having a percent total body surface area burned (% TBSAB) ≥15% (excluding first-degree burns) and requiring antimicrobial therapy (either suspected or documented infection) were eligible for study enrollment. The study was approved by the institutional review board, and written informed consent was obtained for all patients. Patients excluded from the study were the following: patients with a history of allergy to cefepime or cephalosporins or penicillins, patients with reduced renal function (estimated prestudy creatinine clearance [CLCR] < 30 ml/min calculated by the method of Cockroft and Gault [12]), patients with hepatic impairment (serum bilirubin and alanine aminotransferase levels above the normal upper limit by factors of ≥2 and ≥4, respectively), or those undergoing any type of dialysis. All patients were studied following completion of fluid resuscitation and monitored in accordance with standard burn patient care at our institution.
Patient data.
Information collected for all patients included age, sex, weight on day of study, height, date of burn, date of study, % TBSAB, percent second-degree burn (% 2° burn), percent third-degree burn (% 3° burn), review of systems and physical findings pertinent to the evaluation of liver and renal function, pre- and poststudy urinalysis, blood chemistry (SMA-7, SMA-25), and complete blood count with differential, dosage, schedule, and route of administration of concurrent medications, and fluid intake and output.
Drug administration and sample collection.
All patients received 2 g of cefepime (lot D6V89A; expiration date January 1999; Bristol-Myers Squibb Company, Princeton, N.J.). Cefepime was reconstituted as directed by the manufacturer and added to a 100-ml 0.9% saline minibag. A sample was frozen at −70°C within 1 h of preparation for subsequent cefepime concentration determination. The contents of the cefepime minibag were infused intravenously over 30 min with a programmable pump, and the actual volume delivered was recorded. Immediately following the end of the infusion, the administration line was flushed with 0.9% saline. The calculation of the actual amount of drug administered was based on the assayed concentration of cefepime in the minibag and the volume delivered. The actual amount of drug administered was used for all subsequent pharmacokinetic calculations. Blood samples were collected at the following times: predose, and 30, 40, 50, and 60 min and 2, 3, 4, 6, 8, 10, 12, 18, and 24 h after the start of the infusion. Each sample was collected with an additive-free VACUTAINER tube (Becton Dickinson, Franklin Lakes, N.J.) either through a central or peripheral line. At each sample point, 5 ml of blood was drawn through the line and placed in a separate container prior to collecting the sample. Samples were obtained by peripheral venipuncture only when an intravenous catheter was not available. Following collection, all blood samples were immediately placed on ice until they could be centrifuged at 2,000 × g (Centra-8R; IEC, Needham Heights, Mass.) for 6 min. The serum was removed and placed into polypropylene containers and frozen at −70°C. Serum samples were assayed within 7 months.
Urine samples were collected during the study period at the following intervals: immediately prior to drug administration and from 0 to 4, 4 to 8, 8 to 12, and 12 to 24 h after the start of the infusion. At each interval, the urine was thoroughly mixed and the volume was recorded. A 3-ml sample was removed for cefepime analysis and immediately placed on ice prior to pooling the remaining urine for a 24-h urine collection and CLCR measurement. The 3-ml sample was mixed with 6 ml of 0.2 M sodium acetate buffer (pH 4.25) and immediately frozen at −70°C until the time of cefepime assay. Urine samples were assayed within 10 months of collection. Preliminary studies indicated that cefepime in serum and urine samples was stable (retention of ≥91% of the original concentration) during the period of frozen storage (unpublished data).
Cefepime serum assay.
Cefepime serum and urine samples were analyzed by a modification of the method described by Barbhaiya et al. (3). High-performance liquid chromatography (HPLC) equipment used for the cefepime serum and urine assays consisted of a pump (model 510; Waters, Milford, Mass.), sample injector (715 Ultra WISP; Waters), UV light absorbance detector (Lambda-Max model 481; Waters), and an integrator (Chromatopac C-R3A; Shimadzu, Kyoto, Japan). Chromatographic separation was performed with a reverse-phase HPLC column (Nova-Pak C-18; 3.9 by 150 mm; Waters). The mobile phase consisted of an 86% 0.0023 M 1-octanesulfonic acid sodium salt in HPLC-grade water and 14% (vol/vol) acetonitrile adjusted to pH 2.3 with 85% phosphoric acid. Dissolved gases were removed from the final product by filtration through a 0.45-μm-pore-size nylon membrane filter (Whatman, Maldstone, England) while the sample was stirred under vacuum. The mobile-phase flow rate was 1 ml/min, the setting for full-scale absorbance units was 0.05, and the detection wavelength was 280 nm.
Patient serum samples were allowed to thaw at room temperature prior to analysis. When necessary, samples were diluted with pooled human serum into the range of the assay. Protein precipitation was accomplished by adding an equal volume of 5% trichloroacetic acid to all patient samples as well as standards, vortexing for 20 s, and centrifuging at 3,000 × g (IEC Centra-8R) for 10 min. Samples were injected in duplicate with an injection volume of 75 μl. The retention time of cefepime was approximately 10 min, and no interfering peaks were observed.
Serum standards were prepared from laboratory grade cefepime (batch CCB4V0189, lot 189; Bristol-Myers Squibb Company, Syracuse, N.Y.). Standards were prepared with pooled human serum (Abbott Laboratories, North Chicago, Ill.) to produce concentrations of 0.5, 1, 5, 8, 10, 25, 35, 50, and 75 μg/ml. Quality control samples had concentrations ranging from 0.5 to 75 μg/ml. Two standard curves were used to accommodate the wide range of anticipated concentrations while assuring linearity. They ranged from 0.5 to 10 μg/ml (r2 ≥ 0.998) and from 10 to 75 μg/ml (r2 ≥ 0.999). The intraday coefficients of variation were ≤7 and ≤1% for the low- and high-concentration standard-curve quality control samples, respectively; the corresponding interday coefficients of variation were ≤8 and ≤5%, respectively. A standard curve was considered acceptable if the quality control samples were within 15% of the nominal concentration.
Cefepime urine assay.
The HPLC equipment used for the cefepime urine assay was the same as that listed above. Chromatographic separation was performed with a reverse-phase HPLC column (Partisil 5 ODS-3 C18; 4.6 by 100 mm; Whatman). The mobile phase consisted of 49.7% HPLC grade methyl alcohol, 40.4% of a 0.01 M sodium dodecyl sulfate solution (pH 3; adjusted with glacial acetic acid), 5.3% tetrahydrofuran, 3.9% of a 5% trichloroacetic acid solution, and 0.7% of a 2.49 M (vol/vol) phosphoric acid solution. The mobile phase was filtered through a 0.45-μm-pore-size nylon membrane filter (Whatman) while the sample was stirred under vacuum to remove dissolved gases. The mobile-phase flow rate was 2.8 ml/min, the detection wavelength was 280 nm, and the settings for full-scale absorbance units were 0.02 and 0.05 for the low-concentration and high-concentration standard curves, respectively.
Patient urine samples were allowed to thaw at room temperature prior to analysis. They were diluted with an equal volume of 0.2 M sodium acetate buffer into the range of the assay when necessary. Samples were injected in duplicate, and the injection volume was 10 μl. The retention time of cefepime was approximately 10 min.
Urine standards were prepared from laboratory grade cefepime powder (batch CCB4V0189, lot 189; Bristol-Myers Squibb Company). Standards were prepared in 0.2 M sodium acetate buffer (pH 4.25) to produce concentrations of 1.6, 3.1, 6.3, 12.5, 25, 50, 100, 200, 400, 600, and 800 μg/ml. Quality control samples had concentrations ranging from 1.6 to 800 μg/ml. Two standard curves were used for the urine assay to assure linearity over the wide range of concentrations to be measured. The low-concentration standard curve ranged from 1.6 to 12.5 μg/ml (r2 ≥ 0.999), and the high-concentration standard curve ranged from 12.5 to 800 μg/ml (r2 ≥ 0.999). The intraday coefficients of variation were ≤2 and ≤3% for the low- and high-concentration standard-curve quality control samples, respectively; the corresponding interday coefficients of variation were ≤4 and ≤8%, respectively. A standard curve was considered acceptable if the quality control samples were within 15% of the nominal concentration.
Pharmacokinetic analysis.
The serum concentration-time profile for each patient was fit to a two-compartment model with a weighting selection of 1/y2 (where y is the observed concentration) by using RSTRIP (15). Determination of the optimal compartmental model was based on visual inspection of the concentration-time curves, minimization of the residual sum of squares, and the model selection criterion obtained from RSTRIP, which is an adaptation of the Akaike information criterion (34). Pharmacokinetic parameters included the area under the concentration-time curve (AUC) from 0 h to the last measured serum concentration (AUC0–t), AUC from 0 h to infinity (AUC0–∞), alpha phase rate constant (α), and beta phase rate constant (β). β was also calculated as the negative slope of the terminal elimination phase by linear least-squares regression of at least four points. The alpha phase half-life was calculated as t1/2α = 0.693/α, and the beta phase half-life was calculated as t1/2β = 0.693/β. The AUC was calculated by the linear trapezoidal method and extrapolated to infinity as follows: AUC0–∞ = AUC0–t + Clast/β where Clast is the last measured serum concentration. In addition, a noncompartmental analysis was performed. The area under the first moment of the concentration-time curve and the volume of distribution at steady state (VSS) were calculated with standard pharmacokinetic equations (18). Total clearance (CLT), renal clearance (CLR), and nonrenal clearance (CLNR) were calculated as follows: CLT = (actual dose administered)/AUC0–∞, CLR = (amount of cefepime recovered in urine during 0 to 8 h)/AUC from 0 to 8 h, and CLNR = CLT − CLR. CLT, CLR, CLNR, CLCR, and VSS were divided by actual body weight (ABW), lean body weight (LBW), a formula for corrected body weight that accounts for obesity (LBW + 0.4 [ABW − LBW]), and body surface area to normalize these parameters for differences in body weight among the patients studied. By least-squares analysis, the normalization factor that provided the strongest relationship between a pharmacokinetic parameter and the patient demographic factors was identified, and then this factor was used in subsequent analyses. Urinary excretion of cefepime as a percent of the dose recovered in 24 h was calculated as (amount of cefepime recovered in the urine/actual dose administered) × 100.
Pharmacodynamic analysis.
We assessed the time the serum concentration exceeded the MIC (T > MIC). The steady-state serum concentration-time profile for each patient was simulated assuming a one-compartment open model with a 0.5-h infusion time by using patient-specific t1/2β and VSS. Theoretical regimens simulated consisted of 1 g every 8 h (q8h), 2 g q8h, 1 g q12h, and 2 g q12h. The percentage of a dosing interval that the serum concentrations remained above a given MIC (%T > MIC) was calculated as [(T > MIC) × 100]/dosing interval. MICs utilized in this analysis were 8 (National Committee for Clinical Laboratory Standards susceptibility interpretive standard for cefepime), 4, 2, and 1 μg/ml (25). All calculations were verified by visual inspection of the serum concentration-time profiles.
Statistical analysis.
Descriptive statistics were used to summarize the pharmacokinetic parameters. Simple and stepwise multiple linear regression, by the method of least squares, was used to describe the relationships between pharmacokinetic parameters and demographic characteristics of interest (i.e., measured CLCR, % 2° burn, % 3° burn, age, days post-burn-injury, and albumin concentration [in grams per deciliter]). These relationships were analyzed with the StatView statistical software package, version 4.51 (Abacus Concepts, Inc., Berkeley, Calif.). A P value ≤0.05 was considered to be statistically significant.
RESULTS
Thirteen adult patients (10 male and 3 female) were enrolled. One patient was excluded from the study when venous access was lost. The analysis is based on the remaining twelve patients. Patient demographics are displayed in Table 1. All patients were studied following completion of fluid resuscitation and within 14 days of burn injury. The severity of the burn injury varied widely among patients with % 2° burn ranging from 0 to 50% and % 3° burn ranging from 0 to 70%. The mean (standard deviation [SD]) dose of cefepime administered was 1,844 (55) mg.
TABLE 1.
Patient demographics
| Patient | Sex | Age (yr) | ABW (kg) | % TBSAB | % 2° Burn | % 3° Burn | Days postburn |
|---|---|---|---|---|---|---|---|
| 1 | Male | 21 | 107 | 30 | 25 | 5 | 2 |
| 2 | Male | 60 | 88 | 30 | 17 | 13 | 7 |
| 3 | Male | 38 | 84 | 18 | 18 | 0 | 10 |
| 4 | Female | 42 | 122 | 30 | 10 | 20 | 10 |
| 5 | Male | 30 | 61 | 27 | 9 | 18 | 7 |
| 6 | Male | 31 | 71 | 40 | 35 | 5 | 10 |
| 7 | Male | 53 | 116 | 40 | 0 | 40 | 8 |
| 8 | Male | 44 | 71 | 65 | 50 | 15 | 9 |
| 9 | Male | 50 | 56 | 18 | 10 | 8 | 13 |
| 10 | Male | 61 | 78 | 22 | 22 | 0 | 14 |
| 11 | Female | 26 | 96 | 45 | 5 | 40 | 8 |
| 12 | Male | 32 | 67 | 70 | 0 | 70 | 7 |
| Mean (SD) | 41 (13) | 84 (22) | 36 (17) | 17 (15) | 20 (21) | 9 (3) |
The pharmacokinetic parameters for all patients are shown in Table 2. Serum concentration-time data were best fit to a two-compartment model with a weighting factor of 1/y2 except for two patients (patients 4 and 9). In these patients, the β phase was better described by a weighting factor of 1/y. For patient 9, we were unable to characterize the α phase due to incomplete sample collection immediately following cefepime administration. We were unable to measure CLCR and to calculate CLR for one patient due to incomplete urine collection. In the remaining patients, the 24-h mean (SD) percent urinary excretion was 90 (12)%.
TABLE 2.
Pharmacokinetic parameters
| Patient | CLCR (ml/min) | AUC0–∞ (μg · h/ml) | VSS (liters/ kg ABW) | t1/2α (h) | t1/2β (h) | CLT (liters/h) | CLR (liters/h) | 24-h urinary excretion (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 182 | 135 | 0.37 | 0.39 | 2.8 | 14.0 | 12.5 | 90 |
| 2 | 122 | 196 | 0.36 | 0.19 | 2.2 | 10.0 | 9.4 | 95 |
| 3 | 189 | 193 | 0.33 | 0.37 | 1.9 | 9.2 | 8.2 | 96 |
| 4 | 191 | 163 | 0.48 | 0.45 | 3.3 | 11.1 | 9.4 | 82 |
| 5 | 125 | 258 | 0.38 | 0.10 | 1.9 | 6.9 | 6.7 | 99 |
| 6 | 139 | 251 | 0.31 | 0.63 | 2.2 | 7.2 | 7.8 | 107 |
| 7 | 164 | 223 | 0.43 | 0.29 | 4.0 | 8.4 | 8.4 | 98 |
| 8 | 104 | 294 | 0.43 | 0.35 | 3.0 | 6.3 | 5.8 | 90 |
| 9 | 150 | 266 | 0.55 | NCa | 2.5 | 7.0 | 6.3 | 88 |
| 10 | NAb | 333 | 0.34 | 0.28 | 2.8 | 5.6 | NA | NA |
| 11 | 184 | 157 | 0.50 | 0.31 | 3.5 | 11.3 | 9.3 | 82 |
| 12 | 130 | 215 | 0.66 | 0.27 | 3.1 | 8.8 | 5.7 | 63 |
| Mean (SD) | 135 (31) | 224 (59) | 0.43 (0.10) | 0.33 (0.14) | 2.8 (0.6) | 8.8 (2.4) | 8.1 (2.0) | 90 (12) |
NC, not calculated for this patient.
NA, urine data not available for this patient.
In the analysis of the relationships between pharmacokinetic parameters and patient demographics, CLT, CLR, CLNR, and CLCR were normalized by LBW whereas VSS was normalized by ABW. CLT was significantly associated with CLCR by simple linear regression (r2 = 0.575; P = 0.0068). This is depicted in Fig. 1. With stepwise multiple regression, the inclusion of days postburn in addition to CLCR enhanced the explanation of variability in CLT (r2 = 0.861; P = 0.0004). CLR was significantly associated with CLCR (r2 = 0.519; P = 0.0124). With stepwise multiple regression, inclusion of days postburn in addition to CLCR helped explain the variability in CLR (r2 = 0.773; P = 0.0026). CLNR was significantly associated with % 3° burn (r2 = 0.376; P = 0.0448) by simple linear regression. With stepwise multiple regression, the inclusion of albumin concentration in addition to % 3° burn better explained the variability in CLNR (r2 = 0.550; P = 0.0411). By simple linear regression and stepwise multiple regression, only % 3° burn was significantly associated with VSS (r2 = 0.624; P = 0.0022). Patient demographics included in each stepwise multiple-regression relationship were individually statistically significant with the exception of albumin concentration, which was included in the CLNR relationship because it contributed considerably to the explanation of variability for this parameter.
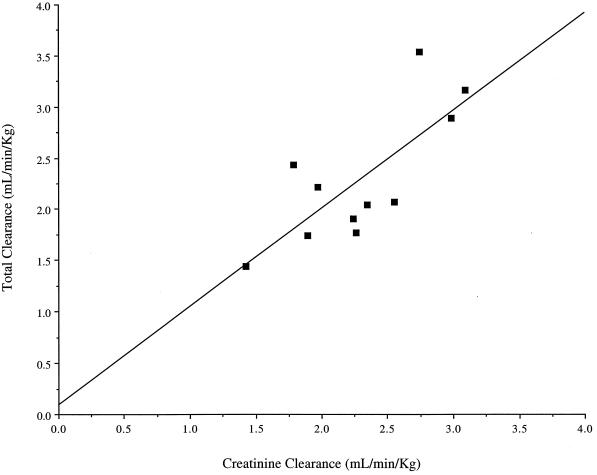
FIG. 1.
Relationship between CLCR and cefepime CLT. CLT = 0.08810 + 0.9568(CLCR); r2 = 0.575; P = 0.0068.
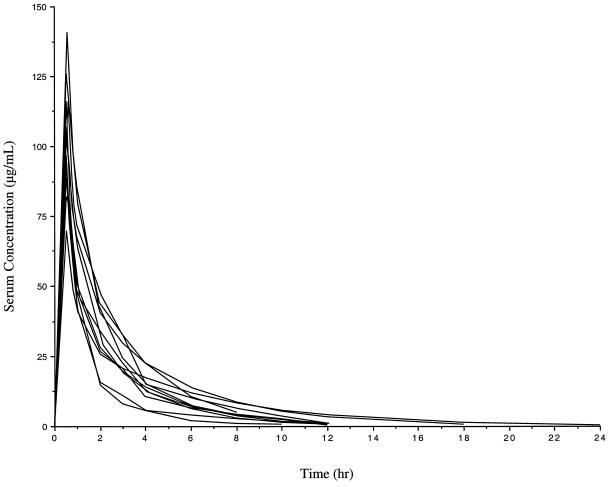
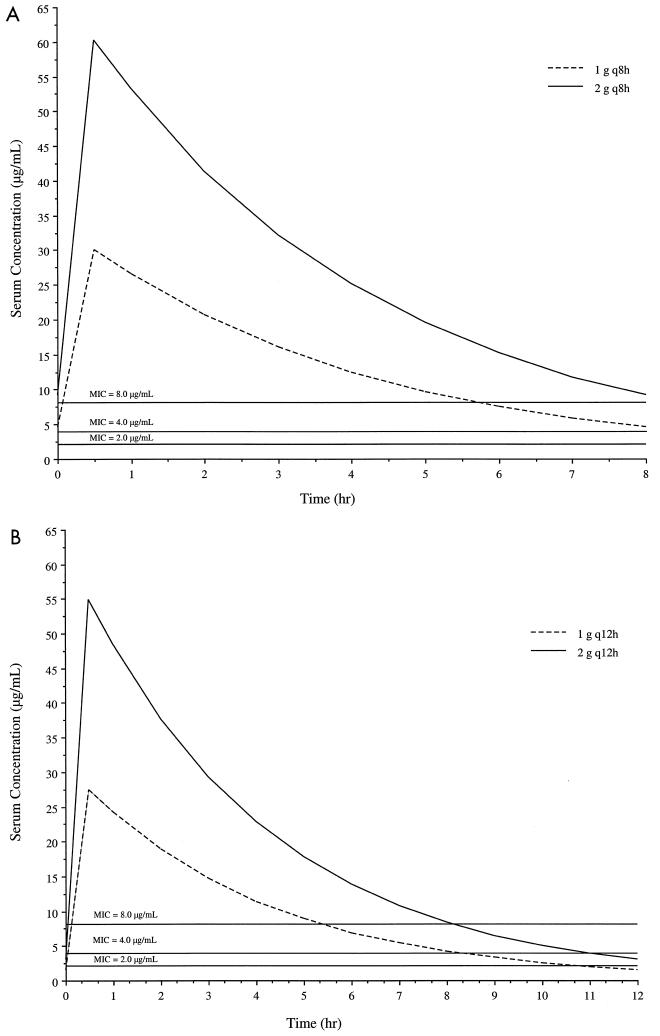
The fitted serum concentration-time profiles for the patients are shown in Fig. 2. The mean (SD) observed serum concentration at the end of the infusion was 110 (23) μg/ml, whereas the observed concentrations at 8 and 12 h after the start of the infusion (SD) were 5.5 (2.6) μg/ml and 2.3 (1.6) μg/ml, respectively. The estimated %T > MIC values for dosing regimens consisting of 1 g q8h, 2 g q8h, 1 g q12h, and 2 g q12h administered over 0.5 h, obtained by using patient-specific pharmacokinetic parameters, are shown in Table 3. Simulated steady-state serum concentration-time profiles based on mean pharmacokinetic parameters with the same dosing regimens are shown in Fig. 3. In each panel, the x axis represents the time after the start of the infusion. All dosing regimens except 1 g q12h maintained a %T > MIC of at least 60% throughout the dosing interval for all MICs at or below 8 μg/ml, a susceptibility interpretive guideline for cefepime (25).
FIG. 2.
Fitted serum concentration-time profile for each patient after a single 2-g dose of cefepime administered over 0.5 h (patient 4 was omitted due to a 1-h infusion).
TABLE 3.
Estimate of %T > MIC
| MIC (μg/ml) | Mean %T > MIC (range) for regimen:
|
|||
|---|---|---|---|---|
| 1 g q12h | 2 g q12h | 1 g q8h | 2 g q8h | |
| 8 | 45 (36–56) | 68 (52–85) | 73 (56–89) | 96 (79–100) |
| 4 | 68 (52–85) | 89 (67–100) | 96 (79–100) | 100 (100) |
| 2 | 89 (68–100) | 97 (83–100) | 100 (100) | 100 (100) |
| 1 | 97 (83–100) | 100 (98–100) | 100 (100) | 100 (100) |
FIG. 3.
Simulated steady-state serum concentration-time profiles of cefepime at 1 and 2 g q8h (A) and q12h (B) administered over 0.5 h based on mean pharmacokinetic parameters. Overlaid lines representing possible MICs are for showing T > MIC.
DISCUSSION
Burn injury results in numerous pathological changes in the body that can affect the disposition of antimicrobials (9, 21, 26). Since infection is a common complication of burn injury and since drug disposition may be altered in these patients, it is important to examine newer agents used in this patient population. Most of the published information regarding drug disposition in burn patients has been limited to aminoglycosides (7, 19, 20, 23, 27, 30, 35–37) and vancomycin (2, 11, 17, 28). Few studies have focused on the drug disposition of β-lactams in burn patients (1, 8, 10, 16, 32, 33). Consistent throughout these reports is the fact that drug disposition is influenced in this patient population. Similarly, we found cefepime pharmacokinetics to be altered in our burn patients.
Overall, the two cefepime pharmacokinetic parameters that appear to be most affected in burn patients are clearance and volume of distribution. The CLT and CLR for the study patients were approximately 10 and 20 to 30% higher, respectively, than those previously reported for healthy volunteers (4, 6). The patients in our study population also demonstrated an above-normal CLCR with a mean (range) of 153 (104 to 191) ml/min. Similarly, Loirat et al. (23) demonstrated a significant increase in CLCR among patients between the 4th and 35th days following burn injury compared to that for healthy controls. However, when normalized by CLCR, the ratios of CLT/CLCR and CLR/CLCR in our study were found to be similar to ratios calculated from a study of healthy volunteers (5). Thus, it appears that the increased CLT we observed was likely due to an elevated glomerular filtration rate in our patient population. Not all burn patients will have a CLCR elevated above normal. Patients with preexisting renal impairment may experience an increase in the glomerular filtration rate over their baseline, although their increased renal function may still be below normal levels.
As is expected with a drug that is eliminated primarily by glomerular filtration, we found a statistically significant association between CLT or CLR and CLCR by simple linear regression. When analyzed by stepwise multiple regression, days post-burn-injury also helped explain the variability in CLT and CLR. An inverse relationship between CLT or CLR and days post-burn-injury was found. Perhaps wound healing results in decreased drug clearance. CLNR was positively associated with both % 3° burn and albumin concentration. Because the mechanisms of CLNR were not assessed, we are unable to physiologically explain this finding. Lastly, the association between VSS and % 3° burn that we found may be related to the physiological effects resulting from thermal burn injury, an effect directly related to the medical interventions, or some combination of the above.
We found a strong relationship between CLCR and CLT with cefepime in this population. Other authors have found strong relationships between CLCR and CLT with other β-lactam antibiotics in burn patients. Boucher et al. (8) observed a significant relationship between CLCR and CLT with imipenem (r2 = 0.60; P = 0.0001) and Friedrich et al. (16) noted a similar significant relationship with aztreonam in burn patients (r = 0.95; P = 0.0018). In a study of the pharmacokinetics of piperacillin-tazobactam in burn patients, Bourget et al. (10) found a significant relationship between the CLT of piperacillin-tazobactam and CLCR (r = 0.83, P = 0.03), and Shikuma et al. (32) found a relationship between the CLT of piperacillin and CLCR (r = 0.49). In contrast to the findings of the previous authors, Walstad et al. (33) failed to find a relationship between CLCR and CLT of ceftazidime (r = 0.13), although a significant correlation between CLCR and CLR was found (r = 0.96). The authors report that this finding may be explained by an elevated CLNR in patients with large burns. Therefore, the CLT of β-lactams is variable in burn patients but appears to be associated with CLCR. Although the CLCR range observed in the patients we studied was limited (104 to 191 ml/min), the ratio of CLT/CLCR we observed in our study patients was consistent with that reported for healthy volunteers (5).
Although the calculated CLR for patient 6 appears to exceed the CLT, this is unlikely to occur physiologically and may be explained by experimental error, especially in the case of a drug for which the CLR approaches the CLT. In contrast, the low CLR relative to the CLT we observed for patient 12 may be related to the % 3° burn, which was the highest among all patients. This, coupled with the needed debridement surgery during the study, may explain an increased CLNR due to an associated increase in insensible fluid loss.
Similar to other investigators who noted an increase in the VSS of β-lactams, we noted an increase in the VSS of cefepime in burn patients. The VSS in our burn patient population (0.43 liters/kg) was approximately twice that previously reported for volunteers (0.18 to 0.24 liters/kg) with or without renal impairment (5, 6, 14). Walstad et al. (33) observed an increase in the VSS of ceftazidime in burn patients compared to that in other patients. The increase was almost twice that seen with ceftazidime in healthy volunteers (22). Bourget et al. (10), Boucher et al. (8), Friedrich et al. (16), and Adam et al. (1) reported similar increases in VSS with piperacillin-tazobactam, imipenem, aztreonam, and ticarcillin-clavulanate, respectively, in burn patients. Shikuma et al. (32) described approximately a threefold increase in VSS of piperacillin in burn patients compared to that in healthy subjects. Thus, it appears that the VSS of cefepime is increased, as has been previously described for other β-lactams. The increase in the VSS of cefepime may also be partially attributed to alterations in protein binding; other studies have shown changes in plasma protein levels and drug binding following burn injury (24). Plasma albumin levels in our patients were sometimes low, ranging from 1.7 to 3.4 g/dl. However, because the protein binding of cefepime is less than 20% (3), it is unlikely to explain the observed increase in VSS.
The %T > MIC for β-lactams has been associated with outcome in both animal infection models (13) and humans (31). Using a neutropenic-mouse thigh infection model, researchers have previously demonstrated, with cephalosporins against gram-negative organisms, that maintaining drug concentrations above the MIC for 60 to 70% of the dosing interval may be necessary to maximize bactericidal activity (13). Therefore, it is reasonable to design cephalosporin dosing regimens for humans based on this pharmacodynamic parameter. According to our pharmacokinetic simulations with MICs ≤8 μg/ml, a %T > MIC of at least 60% was accomplished with all assessed dosing regimens except 1 g q12h. It should also be noted that the 1-g-q8h regimen produced a %T > MIC similar to that produced by the 2-g-q12h regimen and represents a reduction in the total daily dose.
In conclusion, the CLT and VSS of cefepime were increased in our study population compared to those for healthy volunteers. The CLT may be explained by an increase in the glomerular filtration rate, whereas VSS is associated with the burn severity. Nonetheless, it appears that the dose does not need to be adjusted in similarly affected burn patients and that 1 g q8h and 2 g q12h provide similar %T > MIC, while the 1-g-q8h regimen allows a reduction in the daily dose.
ACKNOWLEDGMENTS
This work was supported, in part, by a grant from Bristol Myers Squibb Company, Princeton, N.J.
We acknowledge Linda B. Mihm for her contribution to the study and Archie J. Taylor for his expertise and assistance with HPLC. In addition, we are indebted to the entire Burn Unit staff, without whom this project would not have been possible.
REFERENCES
- 1.Adam D, Zellner P R, Koeppe P, Wesch R. Pharmacokinetics of ticarcillin/clavulanate in severely burned patients. J Antimicrob Chemother. 1989;24(Suppl. B):121–130. doi: 10.1093/jac/24.suppl_b.121. [DOI] [PubMed] [Google Scholar]
- 2.Bailie G R, Ackerman B H, Fischer J, Solem L D, Rotschafer J C. Increased vancomycin dosage requirements in young burn patients. J Burn Care Rehabil. 1984;5:376–378. [Google Scholar]
- 3.Barbhaiya R H, Forgue S T, Shyu W C, Papp E A, Pittman K A. High-pressure liquid chromatographic analysis of BMY-28142 in plasma and urine. Antimicrob Agents Chemother. 1987;31:55–59. doi: 10.1128/aac.31.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbhaiya R H, Forgue S T, Gleason C R, Knupp C A, Pittman K A, Weidler D J, Martin R R. Safety, tolerance, and pharmacokinetic evaluation of cefepime after administration of single intravenous doses. Antimicrob Agents Chemother. 1990;34:1118–1122. doi: 10.1128/aac.34.6.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbhaiya R H, Knupp C A, Pittman K A. Effects of age and gender on pharmacokinetics of cefepime. Antimicrob Agents Chemother. 1992;36:1181–1185. doi: 10.1128/aac.36.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbhaiya R H, Forgue S T, Gleason C R, Knupp C A, Pittman K A, Weidler D J, Movahhed H, Tenney J, Martin R R. Pharmacokinetics of cefepime after single and multiple intravenous administrations in healthy subjects. Antimicrob Agents Chemother. 1992;36:552–557. doi: 10.1128/aac.36.3.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bootman J L, Wertheimer A I, Zaske D. Individualizing gentamicin dosage regimens in burn patients with gram-negative septicemia: a cost-benefit analysis. J Pharm Sci. 1979;68:267–272. doi: 10.1002/jps.2600680304. [DOI] [PubMed] [Google Scholar]
- 8.Boucher B A, Hickerson W L, Kuhl D A, Bombassaro A M, Jaresko G S. Imipenem pharmacokinetics in patients with burns. Clin Pharmacol Ther. 1990;48:130–137. doi: 10.1038/clpt.1990.127. [DOI] [PubMed] [Google Scholar]
- 9.Boucher B A, Kuhl D A, Hickerson W L. Pharmacokinetics of systemically administered antibiotics in patients with thermal injury. Clin Infect Dis. 1992;14:458–463. doi: 10.1093/clinids/14.2.458. [DOI] [PubMed] [Google Scholar]
- 10.Bourget P, Lesne-Hulin A, Le Reveillé R, Le Bever H, Carsin H. Clinical pharmacokinetics of piperacillin-tazobactam combination in patients with major burns and signs of infection. Antimicrob Agents Chemother. 1996;40:139–145. doi: 10.1128/aac.40.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brater D C, Bawdon R E, Anderson S A, Purdue G F, Hunt J L. Vancomycin elimination in patients with burn injury. Clin Pharmacol Ther. 1986;39:631–634. doi: 10.1038/clpt.1986.111. [DOI] [PubMed] [Google Scholar]
- 12.Cockroft D W, Gault M H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 13.Craig W A. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1997;26:1–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 14.Cronqvist J, Nilsson-Ehle I, Öqvist B, Norrby S R. Pharmacokinetics of cefepime dihydrochloride arginine in subjects with renal impairment. Antimicrob Agents Chemother. 1992;36:2676–2680. doi: 10.1128/aac.36.12.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox J L, Lamson M L. RSTRIP user handbook, version 3.0. Salt Lake City, Utah: Micromath Inc.; 1987. [Google Scholar]
- 16.Friedrich L V, White R L, Kays M B, Brundage D M, Yarbrough D., III Aztreonam pharmacokinetics in burn patients. Antimicrob Agents Chemother. 1991;35:57–61. doi: 10.1128/aac.35.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garrelts J C, Peterie J D. Altered vancomycin dose vs. serum concentration relationship in burn patients. Clin Pharmacol Ther. 1988;44:9–13. doi: 10.1038/clpt.1988.105. [DOI] [PubMed] [Google Scholar]
- 18.Gibaldi M, Perrier D. Pharmacokinetics. New York, N.Y: Marcel Dekker, Inc.; 1982. [Google Scholar]
- 19.Glew R H, Moellering R C, Burke J F. Gentamicin dosage in children with extensive burns. J Trauma. 1976;16:819–823. doi: 10.1097/00005373-197610000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Hoey L L, Tschida S J, Rotschafer J C, Guay D R, Vance-Bryan K. Wide variation in single, daily-dose aminoglycoside pharmacokinetics in patients with burn injuries. J Burn Care Rehabil. 1997;18:116–124. doi: 10.1097/00004630-199703000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Jaehde U, Sörgel F. Clinical pharmacokinetics in patients with burns. Clin Pharmacokinet. 1995;29:15–28. doi: 10.2165/00003088-199529010-00003. [DOI] [PubMed] [Google Scholar]
- 22.Leeder J S, Spino M, Isles A F, Tesoro A M, Gold R, MacLeod S M. Ceftazidime disposition in acute and stable cystic fibrosis. Clin Pharmacol Ther. 1984;36:355–362. doi: 10.1038/clpt.1984.187. [DOI] [PubMed] [Google Scholar]
- 23.Loirat P, Rohan J, Baillet A, Beaufils F, David R, Chapman A. Increased glomerular filtration rate in patients with major burns and its effect on the pharmacokinetics of tobramycin. N Engl J Med. 1978;299:915–919. doi: 10.1056/NEJM197810262991703. [DOI] [PubMed] [Google Scholar]
- 24.Martyn J A J, Abernethy D R, Greenblatt D J. Plasma protein binding of drugs after severe burn injury. Clin Pharmacol Ther. 1984;35:535–539. doi: 10.1038/clpt.1984.73. [DOI] [PubMed] [Google Scholar]
- 25.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing. NCCLS document M100-S9. 19, no. 1. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1999. [Google Scholar]
- 26.Peck M D, Ward C G. Burn injury. In: Civetta J M, Taylor R W, Kirby R R, editors. Critical care. Philadelphia, Pa: Lippincott-Raven Publishers; 1997. pp. 1265–1275. [Google Scholar]
- 27.Polk R E, Mayhall C G, Smith J, Hall G, Kline B J, Swensson E, Haynes B W. Gentamicin and tobramycin penetration into burn eschar. Arch Surg. 1983;118:295–302. doi: 10.1001/archsurg.1983.01390030027005. [DOI] [PubMed] [Google Scholar]
- 28.Rybak M J, Albrecht L M, Berman J R, Warbasse L H, Svensson C K. Vancomycin pharmacokinetics in burn patients and intravenous drug abusers. Antimicrob Agents Chemother. 1990;34:792–795. doi: 10.1128/aac.34.5.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanders C C. Cefepime: the next generation? Clin Infect Dis. 1993;17:369–379. [PubMed] [Google Scholar]
- 30.Sawchuk R J, Zaske D E. Pharmacokinetics of dosing regimens which utilize multiple intravenous infusions: gentamicin in burn patients. J Pharmacokinet Biopharm. 1976;4:183–195. doi: 10.1007/BF01086153. [DOI] [PubMed] [Google Scholar]
- 31.Schentag J J. Pharmacokinetics and pharmacodynamics of beta-lactam antibiotics. Infect Med. 1992;9(Suppl. B):10–12. [Google Scholar]
- 32.Shikuma L R, Ackerman B H, Weaver R H, Solem L D, Strate R G, Cerra F B, Zaske D W. Thermal injury effects on drug disposition: a prospective study with piperacillin. J Clin Pharmacol. 1990;30:632–637. doi: 10.1002/j.1552-4604.1990.tb01866.x. [DOI] [PubMed] [Google Scholar]
- 33.Walstad R A, Aanderud L, Thurmann-Nielsen E. Pharmacokinetics and tissue concentrations of ceftazidime in burn patients. Eur J Clin Pharmacol. 1988;35:543–549. doi: 10.1007/BF00558251. [DOI] [PubMed] [Google Scholar]
- 34.Yamaoko K, Nakagawa T, Uno T. Application of Akaike’s information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. 1978;6:165–175. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]
- 35.Zaske D E, Sawchuk R J, Gerding D N, Strate R G. Increased dosage requirements of gentamicin in burn patients. J Trauma. 1976;16:824–828. doi: 10.1097/00005373-197610000-00014. [DOI] [PubMed] [Google Scholar]
- 36.Zaske D E, Sawchuk R J, Strate R G. The necessity of increased doses of amikacin in burn patients. Surgery. 1978;84:603–608. [PubMed] [Google Scholar]
- 37.Zaske D E, Bootman J L, Solem L D, Strate R G. Increased burn patient survival with individualized dosages of gentamicin. Surgery. 1982;91:142–149. [PubMed] [Google Scholar]