Abstract
Objective:
To evaluate whether the transition of a face-to-face behavioral intervention to videoconferencing-based telehealth delivery during the COVID-19 pandemic resulted in significantly smaller weight losses than those typically observed in gold standard, face-to-face programs.
Methods:
Participants were 160 adults with obesity (mean±SD age = 49.2±11.9 years, BMI = 36.1±4.2 kg/m2) enrolled in two cohorts of a 16-week comprehensive weight management program. Cohort 1 began in-person and transitioned to telehealth (Zoom) delivery during week 11 of intervention due to COVID-19; Cohort 2 was conducted completely remotely. A non-inferiority approach (using a clinically-relevant non-inferiority margin of 2.5%) was used to assess whether the weight losses observed were inferior to the 8% losses from baseline typically produced by gold-standard, face-to-face lifestyle interventions.
Results:
From baseline to post-intervention, participants lost an average of 7.37±4.88 kg, representing a reduction of 7.20±4.59%. This magnitude of weight change was significantly greater than 5.5%, t(159)=4.67, p<.001, and thus was within the proposed non-inferiority margin.
Conclusions:
These findings demonstrate that the results of behavioral weight-management interventions are robust, whether delivered in person or remotely, and that individuals can achieve clinically-meaningful benefits from behavioral treatment even during a global pandemic. Pragmatic “lessons learned”, including modified trial recruitment techniques, are discussed.
Keywords: Obesity, Obesity Treatment, Behavior Therapy, Clinical Care, Clinical Practice
Introduction
Disruptions in healthcare and health-related research resulting from the COVID-19 pandemic were partially offset by a rapid transition to telemedicine,1 with many face-to-face behavioral health interventions transitioned to remote delivery via modern videoconferencing platforms (e.g., Zoom).2 These transitions were implemented to prevent disruptions in ongoing clinical care; however, it is unclear what impact this shift may have had on intervention outcomes.
Research conducted prior to the COVID-19 pandemic suggested that the modality of remote delivery for behavioral interventions may affect program efficacy. In the weight management literature, research has demonstrated that comprehensive behavioral weight loss interventions delivered face-to-face typically produce weight losses of 8kg (or 8% from initial weight) in adults with obesity.3 Several large clinical trials have shown similar outcomes for programs using group telephone calls versus face-to-face groups,4,5 and one fully-powered equivalency trial demonstrated no difference in outcomes between these modalities.6 To date, only smaller pilot studies have implemented group-based interventions via newer videoconferencing technology;7-9 however, results have demonstrated weight losses smaller in magnitude (e.g., 4-5% from baseline weight) than traditional in-person interventions.
Beyond the shift to telemedicine, there is also evidence that the COVID-19 pandemic broadly impacted individuals’ health behaviors, resulting in decreases in physical activity,10,11 decreases in dietary quality (e.g., greater consumption of “unhealthy” foods), and increases in snacking between meals.10 Moreover, over two-thirds of behavioral intervention participants surveyed in two studies reported that the pandemic had negatively impacted their ability to adhere to program goals.2,12
Given the potential impact of transition to remote delivery and of other pandemic-related changes in health behaviors, it is vital to examine whether implementation of behavioral interventions via telehealth during the COVID19 pandemic adversely impacted program outcomes. Thus, the current study used a non-inferiority approach to assess whether program outcomes of a behavioral weight-loss intervention that transitioned from face-to-face to remote videoconference delivery during the COVID-19 pandemic were significantly worse than those typically observed for gold-standard, face-to-face programs. In addition, we have compiled and discussed pragmatic “lessons learned” regarding the implementation of behavioral intervention trials via telehealth.
Methods
Data were collected across two cohorts of an existing, randomized-controlled trial evaluating the effect of an adaptive, smartphone-based intervention on long-term weight-loss maintenance. Prior to randomization in the parent maintenance trial, adults (age 18-70 years) with obesity (BMI 30-45 kg/m2) who owned a smartphone and had no medical conditions that would contraindicate weight loss completed a 16-week weight-loss program based on the Diabetes Prevention Program (DPP) lifestyle intervention13 (see Online Supporting Material for full inclusion/exclusion criteria and intervention protocol). Sessions were conducted weekly, for 60 minutes, in groups of 9-15 participants with two trained interventionists. Participants were encouraged to self-monitor weight, dietary intake, and physical activity daily using study-provided tools, and to turn in a summary of their previous week’s self-monitoring before each session. Participants were provided with caloric intake goals (1,200-1,800 kcal/day, based on initial weight) at the first session, and with physical activity goals (to gradually increase engagement in moderate-intensity physical activity, e.g. brisk walking, up to 300 min/week) in session 5. Cohort 1 began face-to-face in January 2020 and transitioned to videoconferencing (Zoom) after session 10, with no gap in the original session schedule; Cohort 2 began in October 2020 and was conducted entirely remotely via videoconferencing. The study was approved by the University of Florida IRB, and all participants provided informed consent.
Measures
Body weight was assessed at baseline and post-intervention using study-provided e-scales (BodyTrace, Inc) that have high concordance with weights measured in-person using calibrated clinic scales.14 Using previously-established protocols,15 participants were asked to weigh themselves first thing in the morning, after voiding but prior to having anything to eat or drink. Height and demographic characteristics were assessed via self-report. Days that participants self-monitored caloric intake and met caloric intake goals, and weeks that participants met physical activity goals were collected from the records returned to interventionists before each session.
Statistical Analyses
Under an intent-to-treat approach, multiple imputation was used for missing weight data; missing self-monitoring data were assumed to represent days that self-monitoring was not completed and goals were not met. Differences between cohorts in demographics, baseline characteristics, and attendance/adherence outcomes were examined using independent-sample t-tests for continuous variables, and chi-square and Fisher’s exact tests for categorical variables.
A non-inferiority approach was used to assess whether weight losses observed were inferior to the 8% losses from baseline typically produced by gold-standard, face-to-face interventions.3 Although various cut points (e.g., ≥5% and ≥10%) have been used to define clinically-significant weight loss, losses as small as ≥2.5% have been demonstrated to produce clinically-meaningful reductions in risk of type 2 diabetes.3 Thus, 2.5% was selected as a clinically-relevant non-inferiority margin, and a one-sample t-test was used to assess whether observed weight losses were significantly greater than 5.5%.
Results
A total of 160 participants were enrolled in the initial weight-loss program, with 147 (91.9%) completing post-intervention assessments (see Table S2 in the Online Supporting Material for demographics and attendance of completers versus non-completers). There were no differences in baseline or demographic characteristics by cohort (Table 1). Participants attended an average (mean±SD) of 12.52±3.43 (78.24±21.43%) of 16 group sessions, with no differences by cohort, p=.102. Participants self-monitored caloric intake on 75.8±31.3 days (72.2±29.8% of a possible 105 days, reflecting 15 sessions for which self-monitoring records were returned prior to session start), met their calorie goal on 55.8±29.7 days (53.1±28.3% of possible days), and met their activity goal on 4.7±3.1 weeks (42.4±28.2% of a possible 11 weeks, reflecting 11 sessions for which self-monitoring records were returned after physical activity goals were introduced), with no differences between cohorts (ps > .05). Figure 1 displays weekly averages for weight loss and program adherence, by cohort.
Table 1.
Participant Demographic and Baseline Characteristics, Overall and by Cohort.
| Characteristic | Overall (n = 160) |
Cohort 1 (n = 78) |
Cohort 2 (n = 82) |
p |
|---|---|---|---|---|
| Age, mean (SD) years | 49.2 (11.9) | 49.7 (12.3) | 48.8 (11.6) | .626 |
| Weight, mean (SD) kg | 102.5 (15.9) | 102.9 (15.7) | 102.1 (16.1) | .753 |
| BMI, mean (kg/m2) | 36.1 (4.2) | 36.2 (3.8) | 36.1 (4.5) | .836 |
| Sex | .224 | |||
| Male, n (%) | 30 (18.8) | 18 (23.1) | 12 (14.6) | |
| Female, n (%) | 130 (81.3) | 60 (76.9) | 70 (85.4) | |
| Race | .510 | |||
| American Indian or Alaskan Native, n (%) | 2 (1.3) | 2 (2.6) | 0 (0.0) | |
| Asian, n (%) | 4 (2.5) | 3 (3.8) | 1 (1.2) | |
| Black or African American, n (%) | 29 (18.1) | 15 (19.2) | 14 (17.1) | |
| White, n (%) | 122 (76.3) | 57 (73.1) | 65 (79.3) | |
| Other, n (%) | 4 (1.9) | 1 (1.3) | 2 (2.4) | |
| Ethnicity | .916 | |||
| Hispanic or Latino, n (%) | 16 (10.0) | 8 (10.3) | 8 (9.8) | |
| Not Hispanic or Latino, n (%) | 144 (90.0) | 70 (89.7) | 74 (90.2) | |
| Education | .086 | |||
| High School, n (%) | 5 (3.1) | 2 (2.6) | 3 (3.7) | |
| Vocational Training or Some College, n (%) | 24 (15.0) | 13 (16.7) | 11 (13.4) | |
| Associate Degree, n (%) | 21 (13.1) | 15 (19.2) | 6 (7.3) | |
| College/University Degree, n (%) | 51 (31.9) | 26 (33.3) | 25 (30.5) | |
| Graduate or Professional Education, n (%) | 59 (36.9) | 22 (28.2) | 37 (45.1) | |
| Marital Status | .386 | |||
| Married, n (%) | 93 (58.1) | 49 (62.8) | 44 (53.7) | |
| Separated/Divorced/Widowed, n (%) | 33 (20.6) | 14 (17.9) | 19 (23.2) | |
| Never Married, n (%) | 30 (18.8) | 12 (15.4) | 18 (22.0) | |
| Other, n (%) | 4 (2.5) | 3 (3.8) | 1 (1.2) | |
| Income | .158 | |||
| 0-25,000, n (%) | 5 (3.1) | 4 (5.1) | 1 (1.2) | |
| 25,001-50,000, n (%) | 35 (21.9) | 17 (21.8) | 18 (22.0) | |
| 50,001-75,000, n (%) | 39 (24.4) | 14 (17.9) | 25 (30.5) | |
| 75,001-100,000, n (%) | 28 (17.5) | 17 (21.8) | 11 (13.4) | |
| 100,001-125,000, n (%) | 24 (15.0) | 14 (17.9) | 10 (12.2) | |
| 125,000+, n (%) | 26 (16.3) | 10 (12.8) | 16 (19.5) | |
| No Response, n (%) | 3 (1.9) | 2 (2.6) | 1 (1.2) |
NOTE: p values are provided for differences between Cohort 1 and Cohort 2.
Figure 1.
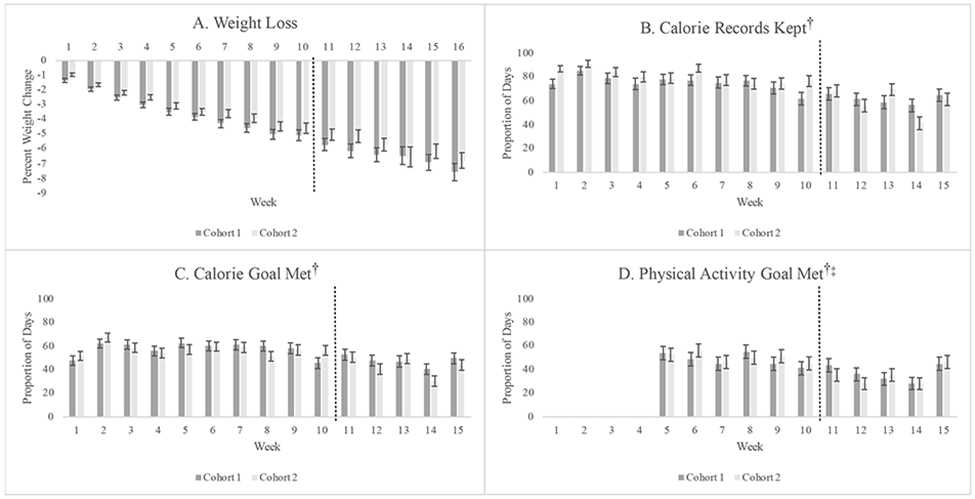
Average weight change (a) and proportion of days calorie records kept (b), calorie goals met (c), and physical activity goals met (d) each week during the 16-week intervention, by cohort.
NOTE: Dashed lines represent the point at which Cohort 1 transitioned from in-person to telehealth intervention delivered via Zoom.
†Self-monitoring records were not returned after the final session on week 16; thus, there are only 15 weeks of self-monitoring data available.
‡Physical activity goals were not set until week 5.
From baseline to post-intervention, participants lost an average of 7.37±4.88 kg, representing a reduction of 7.20±4.59%. This magnitude of weight change was significantly greater than 5.5%, t(159)=4.67, p<.001, and thus was within the proposed non-inferiority margin. Overall, 112 participants (70.0%) lost ≥5% of their baseline weight, and 42 (26.3%) lost ≥10% of their baseline weight. There were no significant differences in weight-loss outcomes between Cohort 1 and Cohort 2 (Table 2), and observed weight losses were non-inferior to traditional face-to-face programs for both Cohort 1, t(77)=4.20, p<.001, and Cohort 2, t(81)=2.47, p=.008.
Table 2.
Weight Change Outcomes from Baseline to Post-Intervention, by Cohort.
| Outcome | Cohort 1 (n = 78) |
Cohort 2 (n = 82) |
p |
|---|---|---|---|
| Weight Change, mean (SD) kg | 7.9 (5.0) | 6.8 (4.7) | .073 |
| Weight Change, mean (SD) % | 7.6 (4.5) | 6.8 (4.7) | .118 |
| Participants losing ≥ 5% from baseline, n (%) | 56 (71.8) | 56 (68.3) | .378 |
| Participants losing ≥ 10% from baseline, n (%) | 22 (28.2) | 20 (24.4) | .356 |
Discussion
Adults with obesity who completed a DPP-based behavioral weight-loss program that transitioned from face-to-face to videoconference delivery due to the COVID-19 pandemic lost an average of 7% of their initial body weight. These outcomes were not significantly inferior to the 8% losses typically observed in gold-standard, face-to-face programs,3 and were similar to the 7% losses observed for the initial DPP lifestyle program.16
Taken together with results from previous trials of telephone-based interventions,4-6 results of the current study demonstrate that behavioral weight management interventions are robust to modifications in delivery modality. These findings have particular relevance given emerging research suggesting that many individuals prefer videoconferencing for delivery of healthcare interventions.2,17 Furthermore, Pew18 estimates that over 97% of U.S. adults own a mobile phone (with 85% owning a smartphone), with no differences by race/ethnicity, suggesting that interventions delivered via telephone and videoconferencing have potential for high reach. Although the parent trial did not formally collect data on participant experiences with telehealth, we observed few challenges from participants or interventionists during the transition from in-person sessions to Zoom. Similar to other studies,2,17 some participants noted preference for telehealth (e.g., as it reduced barriers to attendance such as traffic, parking, or childcare). Even fewer technical challenges were reported during Cohort 2; many participants noted during enrollment that they had used Zoom or similar videoconferencing programs for work, social events, or medical appointments.
Results also demonstrate that behavioral interventions can produce clinically-meaningful outcomes even during a global pandemic that otherwise demonstrated adverse impacts on health behavior.2,10-12 Based on our clinical observations, the nature of challenges experienced by participants varied widely but were similar to those reported previously.2,10-12 Although some participants (e.g., those employed in healthcare) noted increased stress and time limitations due to in-person work, others transitioned to home-based telework. Unique challenges related to working from home included lack of daily structure, increased access to food, and increased sedentary time; conversely, some individuals found that working from home provided greater control over food choices and ability to engage in physical activity throughout the day. Many parents experienced challenges related to childcare and virtual learning. Overall, the intervention protocol offered flexibility (e.g., through weekly discussion of barriers, targeted problem solving, and individual goal setting) that likely helped individuals navigate unique challenges related to COVID-19.
In terms of pragmatic “lessons learned,” the transition to remote procedures required considerable additional staff time. As one example, we were able to maintain similar sample demographics across cohorts by transitioning community-based recruitment approaches to digital formats (e.g., rather than attending/presenting at community events and church services, we connected with community leaders and pastors via email, telephone, and videoconferencing; see Online Supporting Material); however, this required more extensive outreach to a greater number of organizations. To prevent technical challenges from interfering with orientation and consenting processes, Cohort 2 used one-on-one Zoom sessions, vastly increasing the number of sessions conducted (as Cohort 1 conducted these in groups of 18-20 potential participants). Given that minimal technical challenges were experienced with Zoom, our team has since successfully piloted a group Zoom orientation/consenting process to reduce staff burden in future cohorts.
The current study had several important strengths, including the use of a manualized protocol based on materials from a gold-standard behavioral intervention, and integration of e-scales, allowing for objective weight measurement following transition to remote assessment procedures. Despite these strengths, the study also had important limitations. Interpretation of outcomes is limited by the lack of a randomized control condition; however, the vast literature on behavioral weight management programs has demonstrated consistent outcomes for initial weight loss,3 providing a meaningful population mean to which current outcomes could be compared. Generalizability of results to the broader population of adults with obesity may also be limited by sample demographics, as the study sample was primarily female, non-Hispanic White, and highly-educated. This may be especially important as the COVID-19 pandemic disproportionally affected individuals with lower socio-economic status and those from racial/ethnic minority backgrounds.19,20 Future studies should investigate whether individuals from historically-marginalized groups similarly benefit from remotely-delivered weight management interventions.
Conclusion
Overall, results of the current study demonstrate that behavioral weight-management interventions are robust to transitions in delivery modality and that they can produce clinically-meaningful outcomes even during a global pandemic. In alignment with the preference of many individuals for remote delivery of healthcare interventions,2,6 findings support the clinical utility of delivering behavioral interventions via telehealth.
Supplementary Material
What is already known about this subject?
Many behavioral weight management interventions were moved to remote, video-conferencing delivery during the COVID-19 pandemic.
Gold-standard, face-to-face behavioral weight loss interventions consistently produce weight losses around 8 kg (8% of initial body weight) in adults with obesity, and telephone-based interventions have demonstrated similar outcomes. Only small pilot studies have investigated videoconferencing delivery; results have demonstrated weight losses of 4-5%.
What does this study add?
Participants in two cohorts of a 16-week behavioral weight management program that was either transitioned to videoconferencing (Cohort 1) or delivered entirely remotely (Cohort 2) lost an average of 7 kg (7% from baseline weight).
The average weight loss experienced by participants was not significantly inferior to the 8% losses typically observed in gold-standard, face-to-face programs.
Pragmatic “lessons learned” are provided to support future remote intervention trials.
How might your results change the direction of research or the focus of clinical practice?
Given other research demonstrating that many individuals may prefer videoconferencing as a mode of intervention delivery, the current results support continued delivery of comprehensive behavioral weight loss programs via videoconferencing services.
The built-in adaptability of existing intervention protocols (which focus on addressing individual barriers to change) may support their ability to help participants navigate unique challenges related to the COVID-19 pandemic.
Acknowledgements
We would like to acknowledge and thank all of the individuals who have participated in the Project STAR trial, along with the students and staff of the University of Florida Health Promotion lab who delivered the intervention and collected data for this study, and Dr. David Janicke, who served as the Safety Officer for the STAR trial.
Funding:
This study was funded by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, under award R01DK119244.
Footnotes
Data Sharing Plan: De-identified participant data will be publicly available via a data repository following completion of the parent clinical trial.
Clinical Trial Registration: Parent trial Clinicaltrials.gov identifier NCT04116853.
Disclosure: The authors declare no conflicts of interest.
References
- 1.Baum A, Kaboli PJ, Schwartz MD. Reduced in-person and increased telehealth outpatient visits during the COVID-19 pandemic. Ann Intern Med. January 2021;74:129–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardel MI, Manasse SM, Krukowski RA, et al. Covid-19 impacts mental health outcomes and ability/desire to participate in research among current research participants. Obesity. 2020;28(12):2272–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25 Suppl 2):S102–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Befort CA, VanWormer JJ, Desouza C, et al. Effect of behavioral therapy with in-clinic or telephone group visits vs in-clinic individual visits on weight loss among patients with obesity in rural clinical practice: a randomized clinical trial. JAMA. 2021;325(4):363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donnelly JE, Smith BK, Dunn L, et al. Comparison of a phone vs clinic approach to achieve 10% weight loss. Int J Obes. 2007;31:1270–1276. [DOI] [PubMed] [Google Scholar]
- 6.Donnelly JE, Goetz J, Gibson C, et al. Equivalent weight loss for weight management programs delivered by phone and clinic. Obesity. 2013;21(10):1951–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azar KMJ, Aurora M, Wang EJ, Muzaffar A, Pressman A, Palaniappan LP. Virtual small groups for weight management: an innovative delivery mechanism for evidence-based lifestyle interventions among obese men. Transl Behav Med. 2015;5(1):37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taetzsch A, Gilhooly CH, Bukhari A, et al. Development of a videoconference-adapted version of the community Diabetes Prevention Program, and comparison of weight loss with in-person program delivery. Mil Med. 2019;184(11-12):647–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.West DS, Stansbury M, Krukowski RA, Harvey J. Enhancing group-based internet obesity treatment: A pilot RCT comparing video and text-based chat. Obes Sci Pract. 2019;5(6):513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ammar A, Brach M, Trabelsi K, et al. Effects of COVID-19 home confinement on eating behaviour and physical activity: Results of the ECLB-COVID19 international online survey. Nutrients. 2020;12(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunton GF, Wang SD, Do B, Courtney J. Early effects of the COVID-19 pandemic on physical activity locations and behaviors in adults living in the United States. Prev Med Rep. 2020;20:101241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pellegrini CA, Webster J, Hahn KR, Leblond TL, Unick JL. Relationship between stress and weight management behaviors during the COVID-19 pandemic among those enrolled in an internet program. Obes Sci Pract. 2021;7(1):129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): Description of lifestyle intervention. Dia Care. 2002;25(12):2165–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross KM, Wing RR. Concordance of in-home “smart” scale measurement with body weight measured in-person. Obes Sci Pract. 2016;2(2):224–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krukowski RA, Ross KM. Measuring weight with electronic scales in clinical and research settings during the coronavirus disease 2019 pandemic. Obesity. 2020;28(7):1182–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wing RR, Hamman RF, Bray GA, et al. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res. 2004;12(9):1426–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ross KM, Hong YR, Krukowski RA, Miller DR, Lemas DJ, Cardel MI. Acceptability of research and health care visits during the COVID-19 pandemic: Cross-sectional survey study. JMIR Formative Research. 2021;5(6):e27185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pew Research Center. Demographics of mobile device ownership and adoption in the United States. Pew Research Center: Internet, Science & Tech. Published 2021. Accessed September 14, 2021. https://www.pewresearch.org/internet/fact-sheet/mobile/ [Google Scholar]
- 19.Rossen LM, Branum AM, Ahmad FB, Sutton P, Anderson RN. Excess deaths associated with COVID-19, by age and race and ethnicity — United States, January 26–October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(42):1522–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perry BL, Aronson B, Pescosolido BA. Pandemic precarity: COVID-19 is exposing and exacerbating inequalities in the American heartland. PNAS. 2021;118(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


