Abstract
Growth differentiation factor 15 or macrophage inhibitory cytokine-1 (GDF15/MIC-1) is a divergent member of the transforming growth factor β superfamily and has a diverse pathophysiological role in cancers, cardiometabolic disorder, and other diseases. GDF15 can control hematopoietic growth, energy homeostasis, adipose tissue metabolism, body growth, bone remodeling, and response to stress signals. The role of GDF15 in cancer development and progression is complicated and depends on the specific cancer type, stage, and tumor microenvironment. Recently, research on GDF15 and GDF15-associated signaling has accelerated due to the identification of the GDF15 receptor: glial cell line-derived neurotrophic factor (GDNF) family receptor α-like (GFRAL). Therapeutic interventions to target GDF15 and/or GFRAL revealed the mechanisms that drive its activity and might improve overall outcomes of patients with metabolic disorders or cancer. The present review highlights the structure and functions of GDF15 and its receptor, emphasizing the pleiotropic role of GDF15 in obesity, tumorigenesis, metastasis, immunomodulation, and cachexia.
Keywords: Cancer, Obesity, Cachexia, GDF15, GFRAL
Graphical Abstract

1. Introduction
Growth differentiation factor 15 (GDF15), also known as macrophage inhibitory cytokine 1 (MIC-1), is a divergent member of the transforming growth factor β (TGF-β) superfamily. The GDF15 is secreted as a 40-kDa pro-peptide, cleaved to release a 25-kDa active circulating dimeric protein [1]. The placenta is the primary tissue that expresses high levels of GDF15 under physiological conditions for prenatal development. In addition to the placenta, expression of GDF15 has been observed in the prostate, heart, colon, pancreas, liver, and kidney [2, 3]. GDF15 is a stress response cytokine, and its expression levels can surge in response to various cellular stress signals, such as inflammation, hypoxia, tissue injuries, myocardial ischemia, and different malignancies. Elevated levels of circulating GDF15 have been reported in several cancers, including prostate, pancreatic, endometrial, and colorectum [4-7]. Within the tumor microenvironment, GDF15 is expressed by cancer-associated fibroblasts (CAF) and tumor-associated macrophages (TAM) [8]. However, due to the late discovery of GDNF receptor α–like (GFRAL) as a GDF15 receptor, the role of GDF15 in cancer is controversial (protumorigenic vs. antitumorigenic) and largely unidentified [9-13]. Herein, we discuss the molecular mechanisms regulating GDF15 expression, secretion, and its pleiotropic role in cellular functions. Further, we highlight the deregulation of GDF15-mediated signaling in different malignancies and its potential for the development of cancer therapies.
2. Structure and function of GDF15 and its receptors
The GDF15 encoding gene is located on chromosome 19 at the 19p13.11 locus. The entire gene, including untranslated regions, nearly consists of 7,007 bp with 2,746 bp of the coding region, comprising two exons separated by a 1,820 bp long intronic region. A conserved TATA box motif (TATAAA) is present near the start codon [14-16]. Similar to other peptide hormones, GDF15 is synthesized as a precursor protein known as pre-pro-GDF15 (308 amino acids), which is a biologically inactive form composed of an N-terminal (1-29 amino acid long) signal peptide for secretion and trafficking (Figure 1a). The removal of the signal peptide generates a ~40kDa pro-peptide-GDF15 (165 amino acids) that further undergoes proteolytic cleavage by subtilisin/kexin-type (PCSK) proteases, yielding a mature GDF15 (114 amino acids) of ~13kDa in size [17, 18]. The secreted mature GDF15 exists as a homodimer joined through disulfide linkages [10, 18]. GDF15 contains the hallmark cysteine knot (conserved 7 cysteine residues), but unlike the other TGF-β superfamily members, the mature protein consists of two additional cysteine residues and can be folded adequately without the pro-peptide. The monomeric precursor is prone to proteasomal degradation, and the pro-peptide modulates the proteasomal targeting, ensuring the dimeric precursor's existence in the endoplasmic reticulum [17, 18].
Figure 1: Different domains and structural organization of GDF15:
(A) The GDF15 gene is located on chromosome 19p13.11 and contains two exons and one intronic region. The primary immature linear sequence known as pre-pro-peptide GDF15 consists of a signal peptide (amino acid residues 1-29), following by a pro-peptide sequence (amino acids 30-194), and GDF15. The cleavage of the pre-pro-peptide liberates mature GDF15, which exists as a homodimer. (B) Representative three-dimensional model of GDF15 (cyan color) in complex with its receptor GFRAL (hot pink) as deduced by crystallographic studies; the interacting residues are shown in a ball and stick model. (C) Three-dimensional representation of the GDF15 and GFRAL complex. Dimeric GDF15 binds with two GFRAL molecules, providing multiple binding sites to target with small-molecule inhibitors. (D) Enlarged view of hydrogen bond-forming interfacial residues of the GDF15-GFRAL complex as shown in a ball and stick model. These interactions help in drug design and discovery. (E) Three-dimensional view of two molecules of monoclonal antibodies interacting with GFRAL, forming an antibody-protein complex. The interacting interfaces of the antibody heavy and light chains with the GFRAL complex are shown in an enlarged view (Site-I and Site-II). This three-dimensional structure of the antibody-GFRAL complex highlights two different binding sites (site-I and site-II) of GFRAL that could be exploited to develop specific inhibitors of GDF15-GFRAL interactions. The 3D structural model was generated using PyMOL, and structure coordinates were taken from Protein Data Bank (PDB ID: 5VZ3 & 6WMW).
Recently, multiple independent studies have reported that GFRAL acts as a receptor for GDF15. The reported three-dimensional structure suggested that GDF15 has a high affinity for GFRAL and that dimeric GDF15 interacted with GFRAL to perform its biological functions, such as body weight regulation and metabolism [9, 11, 13, 19]. Crystal structure studies have shown that GFRAL comprises 394 amino acid residues with a molecular mass of 44,518 Da. The binding site of GFRAL where GDF15 interacts comprises Leu132, Ala135, Glu136, Val139, Gly140, Val142, Asn145, Ala149, Leu152, Lys153, Ile196, Pro197, Gln200, Ser201, and Ala204 (Figure 1b). Glu136, Asn145, and Gln200 are important residues that participate in hydrogen bonding with GDF15. The structural elucidation of the GFRAL:GDF15 complex provides valuable insight into the mechanism of action, residues responsible for binding, potential binding interfaces, and architecture of active/binding sites, which in turn will be used to design and develop highly selective and potent competitive inhibitors against the GDF15/GFRAL axis [9, 11, 13, 19]. Additionally, following the binding of GDF15 to GFRAL, this complex was reported to further interact with the RET proto-oncogene (RET) and facilitate oncogenic signaling [9, 11, 13, 19]. The interacting network of GDF15: GFRAL and RET has expanded for the design and development of small-molecule inhibitors. For this, multiple drug-designing approaches with discrete targeting strategies can be used. The first approach includes designing the molecules targeting the binding interface between GDF15 and GFRAL, and the second approach is to target the GDF15: GFRAL binding interface with RET (Figure 1c). Suriben et al. recently reported that the antibody-mediated targeting of GFRAL decreases cancer-associated cachexia [20]. Interestingly, the outcomes of this study demonstrated that the antibody 3P10 shares the residues of GFRAL that bind with GDF15 and RET (Arg294, Thr295, Thr297, Gln298, Ser299), and suggested that antibody binding decreases the affinity of GFRAL with GDF15 and RET and blocked the activity (Figure 1e) [20]. This indicates that targeting RET or the GDF15 binding interface with GFRAL could provide a therapeutic approach for the design and development of small molecule inhibitors for therapy development.
3. GDF15 in Obesity and Diabetes:
Obesity and its associated disorders have become a major problem in Western countries [21]. The metabolic diseases can be modulated by lifestyle changes, physical exercise, and several pharmacological interventions, but due to inadequate treatment options or undesirable side effects, people are more prone to obesity. For example, Roux-en-Y gastric bypass surgery, which removes the half portion of the stomach to reduce the absorption of nutrients, is complex and invasive with various side effects [22]. Alternative therapies or pharmaceutical interventions targeting specific biological pathways are urgently needed. One such targetable molecule for obesity and diabetes is GDF15. In a genetically engineered mouse model, the absence of GDF15 resulted in an increase in body weight [23], while its overexpression resulted in lower body weight gain and fat mass [24, 25]. The underlying mechanism is that GDF15 acts through receptor GFRAL and co-receptor RET, extensively present in the hindbrain area called postrema, sending the signals via the vagus nerve to promote anorexia in mice [9-13] (Figure 2). Similarly, other studies suggest that GDF15 plays a vital role in the gut-brain axis because its receptors are present throughout the gut. In contrast, in the brain, receptor expression is restricted or observed in clusters. More studies are needed to understand the importance of GDF15 and its receptors in the intestinal tract [26].
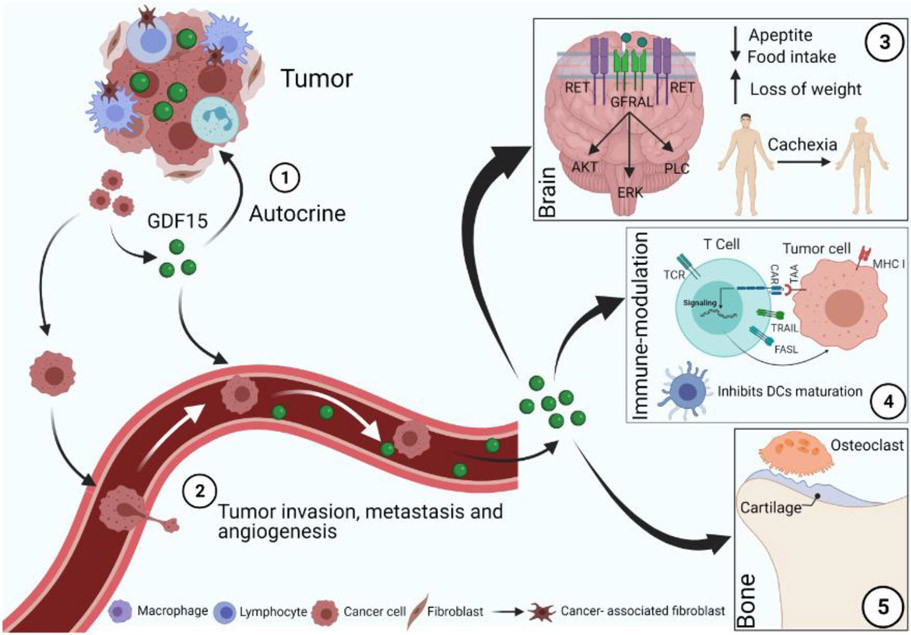
Figure 2: Pleiotropic effects of GDF15 in malignancies:
GDF15 regulates tumor metastasis, cachexia, and immune infiltration. 1) Tumor-secreted GDF15 controls tumorigenesis via an autocrine mode. 2) High GDF15 levels cause tumor invasion, angiogenesis, and distant organ metastasis. 3) Tumor-secreted GDF15 increases cancer-mediated weight loss or cachexia. GFRAL receptor is present in the brainstem area postrema and solitary tract nucleus. After binding to GFRAL, GDF15 induces activation and phosphorylation of the RET co-receptor. The ligand-receptor complex (GDF15-GFRAL-RET) activates the PI3K/AKT pathway and controls food intake, which ultimately leads to the loss of body weight or cachexia. 4) Tumor-derived GDF15 suppresses the maturation and function of DCs and inhibits DC-mediated T cell stimulation and cytotoxic T lymphocyte activation, suggesting an immunomodulatory role of GDF15. 5) GDF15 modulates the differentiation and function of the bone cells within the bone microenvironment during bone metastasis.
In diabetes, hyperglycemia is a chronic symptom that results in the formation of reactive oxygen species involved in cell injury and death [27, 28]. High glucose induces cell death through the PI3K/AKT/eNOS/NO signaling pathway, which can be blocked by increased GDF15, suggesting that the presence of high glucose up-regulates GDF15 expression, and its secretion modulates apoptosis as a negative feedback mechanism in HUVEC endothelial cells [29, 30]. During obesity, p53 is activated in the adipose tissue to promote pro-inflammatory cytokine secretion, insulin resistance, and diabetes. p53 also mediates expression of GDF15 in adipose tissue [31], which is further upregulated by high glucose supplementation in HUVEC cells in a p53-dependent manner [30]. In type 2 diabetic GDF15 knockout mice, increased glucose levels in the urine and decreased glucose transporters were observed (GLUT1, GLUT2, SGLT1, and SGLT2) [32]. Furthermore, type 2 diabetic women had two-fold higher serum GDF15 concentrations compared to control subjects [33], while in another study, plasma GDF15 levels were elevated in preeclampsia (serious blood pressure condition) and superimposed preeclampsia of diabetic patients [34]. The discrepancies associated with GDF15 expression levels in diabetes might be due to the anti-inflammatory response in early diabetes [35]. These studies strongly suggest that GDF15 could be used as a potential biomarker for obesity and the onset of diabetes.
In metabolic disorders such as obesity, GDF15 is a very promising target for pharmacological interventions.
Preclinical studies GDF15
Owing to the implications of GDF15 in metabolic disorders, multiple preclinical studies were performed or ongoing to develop potential therapeutic molecules. Administration of recombinant GDF15 (rhGDF15) in C57BL6 mice resulted in a decrease in food intake in a dose-dependent manner [36], while, in another study, treatment of GDF15 also resulted in a higher intake of water, decreased sugar consumption, and lower body weight in mice, rats, and obese cynomolgus monkeys [26]. Similar results were also observed upon administration of mouse or human recombinant GDF15 in ob/ob mice and db/db diabetic mice [26]. For instance, rhGDF15 treatment (10 mmol/kg) decreased food intake, body weight, glucose, and insulin levels [26]. Further, studies suggested that either daily administration or a long-acting dose (weekly) of rhGDF15 reduces body weight, food intake, and plasma triglycerides in obese cynomolgus monkeys [11, 26]. Thus, the overall effects of GDF15 in obesity prevention are due to a decrease in high caloric intake [11].
Antibodies against GFD15 have been used to study the effect of GDF15 on different metabolic parameters [21]. A short treatment of mice with GDF15 monoclonal antibodies after feeding a high-fat diet (HFD) led to higher body weight with higher rates of obesity compared to control animals [37]. Moreover, blocking of GDF15 via mAb26, mAb1, and mAb2 antibodies and silencing of GFRAL, increased body weight in HFD fed mice [26, 37]. Similarly, 3P10 antibody prevents GDF15 from interacting with its receptors (GFRAL/RET axis), and its administration led to a reversal of lipid oxidation in mice with tumors. These studies suggest that certain genes are involved in the regulation of lipid metabolism in adipose tissue through the GFRAL/RET axis and that they contribute to decreased fat and muscle mass in cachectic mice [20].
Since GDF15 has a short half-life (~3 hr) in circulation, research has been focused on developing stable GDF15 analogs for the treatment of associated diseases [26]. One such long-lasting GDF15 analog is human serum albumin (HSA) GDF15 used for treating obese and cachexia patients. These analogs are engineered by fusing GDF15 with the Fc domain of immunoglobulin or HSA to prevent its clearance from circulation [26]. Administration Fc-GDF15 caused weight reduction in both obese mice and cynomolgus monkey models [26]. Similarly, HSA-GDF15 treatment for 4 weeks led to a significant decrease in the body weight of obese cynomolgus monkeys [11].
Clinical studies of GDF15
In clinical practice, the role of GDF15 as a biomarker is limited because we do not yet understand its mechanism of action. The relationships between GDF15, insulin resistance, and obesity are well established. However, its regulation in response to dietary intake needs to be investigated in detail [30, 38, 39]. Studies using oral administration of a carbohydrate- or fat-rich meal showed that both glucose and insulin independently mediate GDF15 expression at the transcript level and increase its levels in the circulation of lean and obese (pre-and post-bariatric surgery) individuals [40]. To understand the relationship between GDF15 and β-cell function, an oral glucose tolerance test (OGTT, 75 g) was performed in obese subjects, and based on OGTT, the cohort was divided into pre-diabetic and diabetic groups [41]. Both groups showed higher GDF15 levels, which correlated with glycosylated hemoglobin, glucose, and insulin levels, suggesting the involvement of GDF15 in the β-cell function of patients with severe obesity [41].
Next, a case-controlled study showed that GDF15 expression in serum and adipose tissue could be used to differentiate between pre-diabetic (n=30) and diabetic (n=73) patients. Interestingly, diabetic individuals had a significantly higher GDF15 level in the serum compared to pre-diabetic and healthy groups. In addition, GDF15 levels in visceral adipose tissue were also elevated in diabetic (n=17) subjects compared to healthy individuals (n=29), suggesting a large cohort is required to validate GDF15 as a potential candidate marker for diabetes [42]. Furthermore, patients with lower extremity atherosclerotic disease (LEAD) had significantly higher serum GDF15 levels than those without LEAD, and serum GDF15 levels were strongly correlated with an increased risk of LEAD in the diabetic group [43].
The most prescribed anti-diabetic drug, metformin, decreases glucose and insulin levels and, most importantly, body weight [44-46]. However, the molecular mechanism of metformin in decreasing body weight is not known, while its insulin-sensitizing action is well established [47]. A randomized controlled study showed that metformin administration increased circulatory GDF15 levels and reduced food intake and body weight [48, 49]. The proposed mechanism is that metformin induces the expression of integrated stress response regulators such as activating transcription factor 4 (ATF4) and C/EBP homologous protein (CHOP) to stimulate GDF15 secretion from the peripheral tissue. The secreted GDF15 binds to GFRAL/RET present in the brainstem, leading to decreased food intake and body weight [49, 50].
4. GDF15 and malignancies
The role of GDF15 in cancer biology is contradictory in different malignancies, as both pro-tumorigenic and anti-tumorigenic roles have been described [1, 5, 51-54]. However, in several malignancies (including melanoma, prostate, breast, gastrointestinal, pancreatic, and colorectal cancers), levels of circulating serum GDF15 can be nearly 200-fold higher and are associated with poor survival, suggesting GDF15 is a marker for cancer progression [51, 55-57].
GDF15 in prostate cancer
Prostate cancer (PCa) is the most common malignancy among men in the United States. PCa patients exhibit elevated levels of serum GDF15, which serve as a potential biomarker for PCa prognosis [4]. In addition, serum GDF15 is a potential marker to discriminate between benign hyperplasia and PCa [58]. Higher serum GDF15 levels were detected in PCa patients after docetaxel treatment and were associated with shorter survival [59]. Further, in combination with serum prostate-specific antigens (PSA), GDF15 provides a more specific diagnostic tool than existing markers for PCa patients [60].
Due to the lack of appropriate models, the role of GDF15 in PCa has been unclear. Several in vitro and in vivo experiments with PCa cells have revealed conflicting results. One of the earliest findings revealed that GDF15 is high in the prostate tissues [61]. Further, immunohistochemistry and FISH analyses revealed that GDF15 is highly expressed in prostate luminal cells. Additionally, GDF15 was responsive to endogenous prostate ligand androgens [61]. Similarly, one study showed that androgen treatment could induce GDF15 expression and secretion in LNCaP PCa cells [62]. Furthermore, we observed that GDF15 overexpression increased the proliferative and clonogenic ability of PCa cells [63, 64].
In PCa cells (PC3), the introduction of wild-type p53 resulted in overexpression of GDF15 and inhibition of both cancer cells’ migratory and invasion propensity. It was shown that either recombinant GDF15 treatment or suppressing wild-type p53 using siRNA diminished p53/GDF15-mediated cell migration and invasion [65]. Moreover, higher expression of GDF15 was also observed in prostatic adenocarcinoma compared to benign prostatic glandular tissue, supporting the pro-tumorigenic role of GDF15 in PCa [62, 64]. In line with this study, downregulation of GDF15 was shown to enhance the efficacy of taxel-based chemotherapies [66]. Overexpression of GDF15 enhanced the invasion and metastasis potential of PCa cells through the activation of the FAK-RhoA signaling pathway and actin cytoskeleton rearrangement [63]. The data together imply that GDF15 has a positive effect on tumor growth.
On the other hand, GDF15 overexpression in DU145 PCa cells reduced cell adhesion and induced apoptosis [67]. Another recent study suggested that overexpression of GDF15 limits the progression of spontaneous PCa in transgenic adenocarcinoma of mouse prostate (TRAMP) mice via modulation of CD8 T cell activity and subsequent stimulation of tumor immunity [68]. However, aging TRAMP mice overexpressing GDF15 demonstrated enhanced metastases, suggesting that like TGF-β, GDF15 also has a dual role in PCa [69]. The authors suggest that GDF15 may play an inhibitory role in early tumor development while promoting metastasis at a later stage, possibly through differential regulation.
GDF15 regulates several inflammatory pathways, demonstrating both tumor-suppressing and tumor-promoting functions in PCa [70]. A recent report showed that GDF15 promotes early prostate carcinogenesis via regulating NF-κB and macrophage signaling; however, GDF15? expression appears to exert opposite effects on the risk of prostate tumor development [71]. In another case, crossing TRAMP mice with GDF15 transgenic mice reduced primary tumor growth and increased survival while inducing metastasis. It is interesting to note that TRAMP mice express a specific T-antigen, which is known to inhibit P53 expression, and hence the observed GDF15 expression may be the result of P53-mediated regulation [72]. Interestingly, a GDF15 gene polymorphism (H6D C-to-G) was associated with a decreased risk of PCa occurrence and an augmented rate of PCa-specific death due to increased aggressiveness of the tumor [73]. All these studies have revealed that GDF15 may play a cell type-specific role in promoting or inhibiting cell growth and apoptosis [74]. However, as multiple transcription factors are involved in PCa progression and many of these TFs are deregulated in the process, the differential regulation of GDF15 should be studied in a context-dependent manner.
In addition to cancer cells, GDF15 was also secreted from other cells present within the tumor microenvironment or metastatic sites such as cancer-associated fibroblasts (CAF) and osteocytes. CAF are abundantly found in the tumor stroma, and secretion of GDF15 from CAF regulates PCa progression in vivo and anchorage-independent growth in vitro [75].
Bone is the most common site of PCa metastasis, and GDF15 plays a vital role in PCa bone metastasis. Increased serum GDF15 has been reported in PCa bone metastasis and may be assessed to diagnose bone metastases in PCa patients [76]. Overexpression of GDF15 increased the potential of PCa cell lines to metastasize to the bone. Intratibial injections of GDF15-overexpressing DU-145 cell lines increased osteoclast activation and cachexia development [77]. A recent report suggested that GFRAL is expressed on PCa cell lines, and osteocyte-derived GDF15 promotes PCa growth in bone via upregulation of EGR1 expression [78]. Altogether, GDF15 is overexpressed in PCa, and high serum levels of GDF15 are strongly associated with the severity and metastasis ability of PCa and related cachexia.
Conclusively, the serum concentration of GDF15 is a novel and potential biomarker capable of predicting PCa prognosis, particularly in discriminating between benign hyperplasia and PCa. An elevated level of GDF15 in serum is associated with aggressiveness, metastasis, and shortened survival of PCa patients. In PCa bone metastasis, the overexpression of GDF15 potentiates bone resorption through osteoclastogenesis and cachexia.
GDF15 in breast cancer
Breast cancer is one of the leading causes of mortality in women. GDF15 expression correlates with ER-negative and HER2-positive status in patients with breast cancer. GDF15 overexpression decreases E-cadherin and upregulates mesenchymal markers and transcriptional factors to induce epithelial to mesenchymal transition (EMT) in breast cancer cells [79]. However, an in vitro study by Gkretsi et al. suggested that GDF15 suppresses breast cancer cell invasion through modulation of focal adhesion gene expression [80]. It has also been suggested that silencing of Ras suppressor-1 (a suppressor of Ras transformation) leads to the downregulation of GDF15 and modulates actin rearrangement and metastasis [81]. Ferroportin (iron efflux pump) hepcidin (a peptide hormone that binds to ferroportin and triggers its degradation) regulates breast cancer progression [82]. The 3D culture system of MCF-7 cells exhibited higher GDF15 expression than the monolayer culture, and GDF15 secreted by breast epithelial cells induced hepcidin expression in breast cancer spheroids via activation of the SMAD1-5-8 pathway [83]. The mechanism of action and signaling pathways underlying this effect are not known. Kim et al. suggested that GDF15 induced phosphorylation of AKT and ERK-1/2 and the transactivation of ErbB2 in SK-BR3 breast cancer cells; thus, GDF15 may participate in cell growth of certain ErbB2 over-expressing tumors [84]. GDF15 expression is increased in trastuzumab-resistant HER2-overexpressing breast cancer cells via enhanced p38 phosphorylation, suggesting a possible role of GDF15 in therapy resistance [85]. Another study by Sasahara et al. showed the significance of GDF15 in breast cancer tumor sphere formation and maintenance of cancer stem cells via activation of GDF15-ERK1/2-GDF15 circuits [86]. The transcriptional co-activator yes-associated protein (YAP), which functions as an oncogene, is a major downstream effector of the Hippo pathway frequently amplified in various cancers [87]. One study reported that YAP promotes metastasis of breast cancer cell lines (ER+ and PR−) through epigenetic repression of GDF15, suggesting the significance of YAP-GDF15 signaling in breast cancer metastasis [88].
Breast cancer cells likely maintain GDF15 production by the autocrine/paracrine system and act as cancer stem cells. Targeting GDF15, either by attenuation with anti-GDF15 antibodies or blocking with pharmacological inhibitors, would be beneficial to eradicate GDF15high cancer stem-like cells.
GDF15 in pancreatic cancer
Pancreatic cancer (PC) is one of the leading causes of cancer-related deaths worldwide [89]. One of the major obstacles in PC early detection is the lack of biomarkers. Similar to other cancers, PC patients exhibit elevated serum GDF15 compared with healthy controls and benign pancreatic neoplasms [3, 90]. Specifically, the study by Kaur et al. reported that GDF15 along with neutrophil gelatinase-associated lipocalin (NGAL) and well-established PC biomarker CA19-9 were significantly elevated in PC compared to healthy control (HC) patients. Most importantly, GDF15 was found to have more specificity (94%), while NGAL demonstrated less specificity (92%) to distinguish early-stage PC patients from HCs. Notably, the combination of biomarkers such as carbohydrate antigen (CA) 19-9 (74%) and GDF15 (78%) were found to have more diagnostic precision in differentiating chronic pancreatitis (CP) versus resectable PC than either marker alone? [90]. Later, a meta-analysis evaluating 2826 PC patients from 14 studies demonstrated that the diagnostic accuracy of GDF15 is comparable with CA19-9 in terms of specificity and sensitivity. The authors concluded that both GDF15 and CA19-9 are promising biomarker candidates in PC, and a combined panel using both GDF15 and CA19-9 will have more diagnostic value [91]. On the other hand, GDF15 showed greater specificity in differentiating PC from CP than CA19-9. The early diagnostic accuracy of CA19-9 is hampered due to low sensitivity [92]. Recently, a pilot study through the Australian Pancreatic Cancer Screening Program identified an increase in the median baseline of serum GDF15 as a predictor of pancreatic neoplasm in asymptomatic patients compared to individuals diagnosed with benign disease [93]. Thus, this study advocates that the GDF15 detection strategy should be recommended to pre-malignant detection programs in asymptomatic populations.
GDF15 was shown to modulate TAM activity by impeding NF-κB signaling, suggesting that GDF15 suppresses macrophage surveillance during the early stage of PC progression [94]. The upregulation of GDF15 expression via p38 MAPK signaling occurred by Twist (helix-loop-helix transcription factor family member) to promote PC progression and drug resistance [95]. Similarly, NR5A2 (a nuclear receptor subfamily 5 group A member 2) transcription factor was reported to drive PC progression by upregulating GDF15, which suggests that it could act as a therapeutic target for PC [96]. Studies using biomechanical compression of PC cells showed that solid stress could enhance the transcriptional regulation and secretion of GDF15 by activating the AKT/CREB1 pathway, upstream of GDF15. The findings established the signaling mechanism by which solid stress activates pancreatic fibroblasts to secrete GDF15, which further acts on PC cells through an autocrine signal to promote invasion and migration properties [97]. Recently it has been demonstrated that PC cells secrete GDF15 and promote growth and metastasis via GFRAL-mediated autocrine signaling [98].
Similar to many tumor markers of PC, GDF15 has only a modest ability to distinguish between PC and pancreatitis. Elevated GDF15 diminishes the antitumor immune response by interfering with NF-κB signaling and macrophage infiltration at the tumor site, and it facilitates cancer progression and drug resistance via NR5A2 and p38 MAPK signaling. The GDF15-GFRAL axis mediated by autocrine signaling is important to promote growth and metastasis. Additional studies are required to establish GDF15 as a PC biomarker, and its diagnostic utility could be improved by correlating with other serum markers like hepatocarcinoma-intestine-pancreas/pancreatitin-associated protein I and tissue inhibitor of metalloproteinase 1.
GDF15 in gastric cancer:
Gastric cancer (GC) is the 3rd leading cause of cancer-related death worldwide. The role of GDF15 in the pathogenesis of GC remains unclear since the available literature supports both the pro-tumorigenic and tumor suppressor activity of GDF15. In GC, GDF15 activates AKT and ERK signaling and enhances metastasis through the transactivation of ErbB2 tyrosine kinase [84]. Conversely, nuclear protein CXXC4 has been shown to inhibit the growth of GC cells through upregulation of GDF15 [99]. Further, GDF15 contributes to the progression and invasion of GC through up-regulation of the urokinase-type plasminogen activator (uPA) system via the ERK1/2-dependent pathway [100]. In addition, overexpression of GDF15 was reported to induce cancer stem cell-like properties in GC cell lines [101]. Recently, Buchholz et al. analyzed the immunohistochemical expression of GDF15, GFRAL, and RET in GC patient samples and suggested that all three molecules are significantly and jointly elevated in GC tissues, providing clinical implications for the prognosis of GC patients [102].
GDF15 regulates HIF-1α expression via transactivation of ErbB2, and modulating GDF15 expression in GC could reduce the acquisition of stem cell-like properties [103]. GDF15 could be a promising target for the clinical treatment of GC with altered tumor suppressor CXXC4. However, the GDF15-GFRAL-RET axis requires further mechanistic investigation in GC.
GDF15 in colorectal cancer
Colorectal cancer (CRC) is the 2nd cause of cancer-related death in the United States. Physiological GDF15 serum levels are in the range of 150 to 1,150 pg/ml [104], and its elevated expression is linked to various cancers, including CRC, suggesting it could be a new potential biomarker for cancer diagnostics. However, its functional significance in CRC progression is unknown [104, 105]. Clinically, the expression of GDF15 is significantly higher in CRC tissues and serum, which results in poor survival [106]. Specifically, a retrospective blinded evaluation of CRC patient serum and adenomatous polyps revealed that GDF15 sensitivity for early detection of CRC was significantly higher than for carcinoembryonic antigen (CEA) by ELISA or immunoassay [106]. In another study, serum levels of GDF15 were found to be increased in hepatic metastatic CRC patients compared to healthy subjects, and patients with higher GDF-15 levels had worse outcomes [107]. To determine a marker for premalignant colonic polyps, serum GDF15 levels were evaluated in a polyp prevention trial in which a nonsteroidal anti-inflammatory drug was used [108]. Increased expression of GDF15 was associated with a greater number of adenomas, suggesting that GDF15 could be a serum biomarker for the early detection of adenomatous colonic polyposis [108]. Additionally, moderate to high GDF15 staining intensity was observed in CRC patients who had a higher tumor relapse rate compared with other CRC patients using microarray analysis; additionally, higher plasma GDF15 levels correlated with a high incidence of CRC recurrence and poor survival [109]. Two additional studies of CRC patients reported similar results, wherein detection of high plasma GDF15 levels before the diagnosis of CRC correlated with greater mortality, especially for those with tumors positive for prostaglandin-endoperoxide synthase 2 [110].
Mechanistic studies using in vitro and in vivo models show that GDF15 plays an important role in CRC progression and metastasis [53]. EMT modulation was a known factor associated with cancer metastasis. The EMT process was enhanced in CRC cells by GDF15 via binding to TGF-β receptor, which activated Smad 2 & 3 pathways [53]. Further, overexpression of GDF15 resulted in a significant increase in cell viability, invasion, and migratory potential of the CRC cell lines [111]. Like the previous study, expression of EMT markers, i.e., N-cadherin, vimentin, and Twist1, were increased upon GDF15 overexpression in CRC LoVo cells [111]. Intravenous administration of HT29 CRC cells overexpressing luciferase-labeled GDF15 in NOD/SCID mice led to an increase in lung metastasis [112]. Conversely, Yamaguchi et al. reported an anti-tumorigenic effect of GDF15 in a CRC cell line [113]. This function was found to be mediated by PI3K signaling, whereby AKT negatively regulated GSK-3 to induce GDF15 expression. Targeting PI3K and GSK3 by LY294002 and IL-6-hydroxymethyl-chiro inositol 2(R)-2-O-methyl-3-O-octadecylcarbonate, respectively, induced GDF15 expression, which led to increased cell apoptosis and cell cycle and growth arrest in HCT-116 cells [113]. As in numerous other cancers, GDF15 expression in CRC is strongly associated with metastasis, poor prognosis, high incidence of recurrence, and poor survival of the patients. Since the sensitivity of GDF15 is significantly higher than CEA, GDF15 could be used as a biomarker for the early diagnosis of CRC. Overexpressed GDF15 promotes EMT (N-cadherin, vimentin, and Twist1) and binds to TGF-β, activating Smad2 &3, which are the major pathways involved in CRC. Overall, the role of GDF15 in CRC progression suggests that GDF15 could be a potential marker in the clinical setting for the management of CRC patients.
Mechanisms of action of GDF15 in cancer cells
GDF15 exhibits a broad spectrum of functions across tissues, and the mode of action of GDF15, its interacting receptors, and their downstream signaling pathways are still unclear. Even though for decades, the literature suggested that GDF15 has diverse functions in several tissues, its interacting receptors, and their exact downstream signaling pathways were discovered only recently. Several earlier studies suggested that GDF15 binds with TGFβRII and activates downstream SMAD2/3 pathways [114, 115]. Notably, several authors have reported that commercially available GDF15 preparations are contaminated with TGF-β1, which are the possible cause of artifacts showing the effects of GDF15 through SMAD2/3 or via TGFβRII [116, 117] (Figure 3). However, in recent studies, GDF15 failed to show any binding with TGF-β receptor, and its metabolic effects were attributed to binding with GFRAL receptor. Following GDF15 binding, GFRAL interacts with its co-receptor RET and signals via ERK and AKT pathways [9-11, 13, 118, 119]. Since the presence of GFRAL is highly restricted to the brain tissue, the effect of GDF15 on cancer cells might be mediated by pathways other than GFRAL. In support of this, GDF15 was reported to promote osteosarcoma cell invasion and migration through activation of the SMAD pathway [120]. Similarly, in cervical cancer, GDF15 activates AKT and ERK signaling through phosphorylation of a tyrosine-protein kinase (ErbB2), suggesting GDF15 signals independently of GFRAL in cancer cells [121] (Figure 3 & 4).
Figure 3: GDF15 signaling:
GDF15 signaling in cancer (Left): Secreted GDF15 from the cancer cells binds to TGF-beta to promote metastasis through SMAD2/3 signaling. Further, GDF15 forms a complex with ErbB2 and activates PI3K/AKT and MAPK pathways to promote cancer cell proliferation and migration. The latter effect involves phosphorylation of the CREB1 transcription factor, which binds on the GDF15 promoter, activating an autocrine circuit of GDF15 secretion. GDF15/GFRAL/RET signaling axis in obesity and cachexia (Right): After GFRAL binds to GDF15, it recruits and phosphorylates the co-receptor RET which subsequently mediates downstream activation of AKT, ERK, and PLC molecules. Activation of these targets results in regulation of body weight, energy intake and leads to decreased incidence of diabetes mellitus. In cancer cachexia, GDF15 signaling additionally induces muscle proteolysis.
Figure 4: Role of GDF15 in various cancer types and involvement of key signaling molecules.
1) GDF15 is elevated in tumor tissues and plasma, and its expression correlates with aggressiveness and recurrence of the tumor. High serum GDF15 levels have been used as a biomarker in malignancies such as prostate, pancreatic, hepatocellular, osteosarcoma, and colorectal cancer and are associated with decreased survival. 2) Overexpression of GDF15 results in distant metastasis of prostate, colorectal, and gall bladder cancers. 3) In vitro studies suggested that GDF15 increases the phosphorylation of key cell signaling molecules such as ERK, AKT, Smad2&3, ErbB2, and FAK-RhoA kinases. 4) Radioresistance is a major cause of treatment failure. GDF15 contributes to radioresistance and cancer stemness in head and neck cancer by regulating ROS levels and activating the SMAD pathway, suggesting GDF15 could be a predictive marker of radioresistance.
5. Immunomodulatory aspects of GDF15 in the tumor microenvironment
The immune contexture of a tumor has a substantial role in patient survival. Several growth factors modulate the crosstalk between the tumor and immune cells, establishing an immunosuppressive microenvironment. Macrophages play a distinct role in tumor progression and are modulated by tumor suppressive and secretory factors from the tumor microenvironment. Tumor-secreted GDF15 impedes the antitumor activity of the activated macrophages through the inhibition of TNF-α secretion [38]. The potent inflammatory properties of GDF15 suggest that it has an immunomodulatory role in the tumor microenvironment. Kleinertz et al. have shown that circulating GDF15 is associated with the growing dysfunction of natural killer (NK) cells during systemic inflammation [122]. In CRC, high serum GDF15 levels are correlated with a reduction in lymphocyte infiltration [104]. A retrospective study showed that high expression of GDF15 is associated with an increase in NK cells; however, these metastatic CRC patients also showed a reduction in CD8+ T cells and PD-L1 positivity in the TME, which correlated with worse outcomes and resistance to anti-PDL1 therapy [123]. Downregulation of GDF15 increased T cell infiltration and prolonged survival in glioblastoma [124]. In PC, tumor-secreted GDF15 evades macrophage-mediated immune surveillance via TGF-beta-activated kinase (TAK1)-mediated inhibition of NF-κB signaling during the early stages of tumorigenesis [94]. Similarly, overexpression of GDF15 in the TRAMP transgenic mice model of PCa delayed tumorigenesis, but increased distant organ metastasis, suggesting a role for GDF15 in immunosurveillance [69]. Dendritic cells (DCs) are antigen-presenting cells and play a critical role in antigen-specific immune responses. GDF15 is one of the tumor-derived factors that can suppress the maturation and function of DCs and inhibit the ability of DCs to stimulate T cells and cytotoxic T lymphocyte, suggesting GDF15 might be a novel target in immunotherapy [125]. Similarly, overexpression of GDF15 in DCs induces immune-inhibitory molecules, enhancing T cell exhaustion and promoting the generation of regulatory T cells via modulation of TGF-β receptors I and II, suggesting a GFRAL-independent role for GDF15 in DCs. [126]. Overall, the available evidence suggests that GDF15 plays an immunoinhibitory role. Since GFRAL expression is confined to brainstem neurons, the immunomodulatory effects of GDF15 in immune cells are likely to be mediated via GFRAL-independent signaling pathways, which have yet to be investigated.
6. GDF15 in tumor-induced anorexia and cachexia
Severe weight loss and loss of appetite are common symptoms reported in the advanced stages of cancer patients. Cachexia is a multifactorial syndrome characterized by a significant reduction in body weight, predominately from loss of skeletal muscle and adipose tissue [127]. Anorexia, in combination with cachexia, deteriorates the cancer patient's condition [128]. Cachexia compromises the patient’s response to and dose of chemo and radiotherapy and contributes to morbidity and mortality. Transgenic mice with ubiquitously overexpressed human GDF15 showed a reduction in body weight [24]. Similarly, Johnen et al. reported that tumor-derived GDF15 induced fat and lean tissue mass loss, and its elevation was associated with weight loss and cachexia [129]. Subcutaneous implantation of PCa cell lines (DU145) overexpressing GDF15 induced weight loss in mice due to reduced food intake. Moreover, treatment with GDF15 antibodies reversed the observed weight loss via activating hypothalamic neurons, suggesting the association of GDF15 with cachexia and anorexia [129]. GDF15 and GFRAL have been extensively studied for possible regulation of appetite and body weight, after the identification of brainstem-restricted expression of GFRAL [9, 11, 13, 119]. Serum analysis of a cohort of metastatic lung cancer patients suggested an association between high serum GDF15 and weight loss in different cancers [130]. Recently, it has been reported that muscle secretes a high amount of GDF15 only during the daytime, which suggests that serum GDF15 concentration varies according to circadian rhythm; since muscle-secreted GDF15 promotes anorexia during muscle mitochondria dysfunction, it may have a pathological role in the regulation of systemic energy metabolism via modulating the muscle-brain axis [131]. Neutralization of GDF15 with a potent monoclonal antibody (mAB2) acutely reverses the anorexia and weight loss induced by recombinant GDF15, but not the anorexia and weight loss induced by low-dose of lipopolysaccharide [132]. Still, detailed pharmacokinetics of mAB2 and a more extended study duration are required to better understand this therapeutic effect [132]. These studies suggest that GDF15 has the potential to serve as a therapeutic target to treat cancer-associated anorexia and metabolic dysfunctions.
7. Conclusion and future prospective
Elevated serum GDF15 levels have been observed in several malignancies and are considered a potential tumor biomarker. Most of the in vivo preclinical and clinical studies have suggested that GDF15 promotes metastasis. Further studies are warranted to establish GDF15 as a metastasis biomarker in specific cancers for the early diagnosis of metastatic disease. Studies support both a tumor-supportive and tumor-suppressive role of GDF15; therefore, GDF15 effects seem to depend on context and cancer type, similar to TGF- β. Notably, in the case of cancer, GDF15 plays a differential role in a stage-specific manner. In the early stages of cancer, it controls the cancer cell growth and increases the aggressiveness of tumor cells in the late stages. It would be interesting to validate these in vitro findings in an in vivo setting using conditionally floxed GDF15 mice.
The recent discovery of the GFRAL receptor escalated the research paradigm of GDF15. Currently, several clinical trials are focused on the therapeutic potential of GDF15/GFRAL as druggable targets (Table 1). The implications of the GDF15-GFRAL axis in anorexia and cachexia established the pathophysiological role of this axis. However, this effect is not observed during pregnancy, where high serum GDF15 levels are observed. This raises a future research question to see the role of high serum GDF15 during pregnancy. In addition, it remains elusive whether the effects of increased GDF15 expression in pathological conditions such as metabolic diseases, tissue injury, inflammation, and cancer are GFRAL dependent or independent.
Table 1:
GDF15 oriented therapeutics and clinical trials
| Drug/drug combination | Affected Pathway/Outcomes |
Clinical Trial/References |
|---|---|---|
| GDF-15 neutralizing antibody (CTL-002) -monotherapy and/or in combination with an Anti-PD-1 checkpoint inhibitor Metformin |
-GDF-15 inhibits LFA-1 activation on CD8+ T cells. -interfering effector T cell recruitment. -Changes in circulating GDF-15 -changes in body weight and visceral fat |
Phase-I, NCT04725474 (GDFATHER) [133] [134] |
| GDF15 with Liraglutide treatment | -Functional Impact of GLP-1 for Heart Failure Treatment’ (FIGHT) | [135] |
| NGM120 (GFRAL antagonist monoclonal antibody blocking GDF15 signaling) | -GFRAL-GDF15 pathway inhibitor -for pancreatic cancer and cancer-related cachexia - metastatic pancreatic cancer -metastatic castration-resistant prostate cancer -bladder cancer -melanoma -non-small cell lung cancer -colorectal cancer -gastric cancer -esophageal cancer -ovarian cancer -head neck squamous cell carcinoma |
Phase-I, -II NCT04068896 |
| GDF15 based TPF induction chemotherapy | -for oral squamous cell carcinoma patient -improve overall survival of patients |
Phase-II, NCT02285530 |
| Effect of exercise on GDF-15 in prediabetic patients | - application of guideline-based exercise programs on GDF-15 levels in individuals with prediabetes. | NCT04799938 |
Given that GDF15 acts on cell types without detectable GFRAL expression, the GFRAL-independent effects of GDF15, especially immunomodulation, remain unclear. Experimental designs using both GDF15 and GFRAL knockout mice will be required to elucidate the GFRAL independent effects of GDF15 if any. Apart from the mechanistic studies, the structural insight of GDF15-GFRAL-RET complex provides an interesting and diverse platform for designing and developing small-molecule inhibitors for future cancer therapies.
Highlights.
Growth differentiation factor 15 (GDF15) is a member of the TGF-β superfamily.
GDF15 has a role in cancers, metabolic, and its associated diseases.
Pro-tumorigenic and anti-tumorigenic role of GDF15 is controversial.
GFRAL is the receptor of GDF15 present in the brain and regulates anorexia and cachexia.
Immunomodulatory effects of GDF15 are mediated via GFRAL-independent manner.
Acknowledgement
Figures were created with BioRender.com. We also would like to thank Dr. Jessica Mercer for editing this manuscript.
Funding Sources
The authors are, in part, supported by grants from the National Institutes of Health (NIH) U01 CA185148, DOD W81XWH-18-1-0308 (SKB), and R01 CA218545, R01 CA241752 (MWN), and DOD W81XWH-21-1-0640 (JAS).
Abbreviations
- BC
Breast cancer
- CAF
Cancer-associated fibroblasts
- CEA
Carcinoembryonic antigen
- CRC
Colorectal cancer
- DCs
Dendritic cells
- GC
Gastric cancer
- GDF15
Growth differentiation factor:15
- GDNF
Glial-cell-derived neurotrophic factors
- GFRAL
Glial cell line-derived neurotrophic factor family receptor α-like
- HAS
Human serum albumin
- HFD
High fat diet
- LEAD
Lower extremity atherosclerotic disease
- mAB
Monoclonal antibody
- MIC1
Macrophage inhibitory cytokines
- NK Cells
Natural killer cells
- OGTT
Oral glucose tolerance test
- PC
Pancreatic cancer
- PCa
Prostate cancer
- RET
Rearranged during transfection, tyrosine kinase receptor
- TAK1
TGF-beta-activated kinase
- TAM
Tumor-associated macrophages
- TGF- β
Transforming growth factor-β
- TRAMP
Transgenic adenocarcinoma of mouse prostate
- uPA
Urokinase-type plasminogen activator
Biography

Jawed Akhtar Siddiqui, Ph.D.
Jawed Siddiqui is an Assistant Professor at the University of Nebraska Medical Center, Omaha, NE, USA. Dr. Siddiqui obtained his Ph.D. degree in 2012 in Endocrinology from the Central Drug Research Institute, India. After completing Ph.D. research studies, Dr. Siddiqui joined the University of California, San Diego, for his postdoctoral studies. Dr. Siddiqui worked on research projects to explore the role of Chromogranin A and - Catestatin (CST-KO) conditional knock-out mice models to study their involvement in metabolic disorders such as diabetes and hypertension. In 2013, Dr. Siddiqui moved to New York University for another postdoc research. Dr. Siddiqui has made major scientific contributions by investigating the role of Monocyte Chemoattractant Protein-1(MCP-1/CCL2) in the anabolic and catabolic action of Parathyroid Hormone in bone. The main objective of Dr. Siddiqui current research work is to identify the molecular mechanism of prostate cancer pathogenesis and bone metastasis and identification and development of potential drug candidates and novel therapy. Dr. Siddiqui’s research work on Growth differentiation factor (GDF15) and bone metastasis is supported by the Department of Defence (DOD). Dr. Siddiqui is at the forefront of new research technologies to diagnose and treat endocrine and metabolic diseases and cancers through the field of Endocrinology and Bone research.

Ramesh Pothuraju, Ph.D.
Dr. Pothuraju currently working as Instructor in Biochemistry and Molecular Biology at University of Nebraska Medical Center (UNMC), Omaha, USA. His research is focused on clinically approved labelling (IRDye800) method for mucin antibodies to identify the colorectal cancer (CRC) progression and working in mucins and drug resistance. During initial years of postdoctoral training at UNMC, he studied differential expression of mucins in pancreatic as well as CRC progression and developed genetically engineered mouse models for CRC. Before joining at UNMC, he obtained his M.Sc. (2009) and Ph.D. (2015) degrees from the National Dairy Research Institute, Karnal, India. During his Ph.D. program he studied the anti-obesity effects of probiotics (Lactobacilli) and herbal ingredients in diet induced obesity and insulin resistance mouse model. He gained extensive research experience more than 10 years in functional foods, nanotechnology, obesity, diabetes, and their related metabolic disorders along with pancreatic and CRC. He has published several research and review articles (>35 articles) in peer reviewed scientific journals and cited >670 with H-index: 15 and i10-index: 22. In addition, he has served as an Associate Editor, Editorial Board Member as well as reviewer for several international and national journals.

Parvez Khan, PhD. Parvez is a Postdoctoral Fellow at Department of Biochemistry & Molecular Biology, University of Nebraska Medical Center (UNMC), USA. He has been focused on small cell lung cancer, microRNAs, tumor microenvironment, and tumor targeting through small molecule inhibitors and nanoparticles. His research interest is to understand the molecular mechanism of small cell lung cancer metastasis and the identification of novel therapeutic targets and therapeutics. Parvez completed his PhD in Molecular Biophysics/Biological Sciences in 2016, Centre for Interdisciplinary Research in Basic Sciences, Jamia Millia Islamia, New Delhi, India. He Worked on protein folding problems and the identification of small molecule inhibitors for protein kinases especially for cancer associated kinases such as MAPK, FASTK, and CDKs. He did a MSc in Biotechnology in 2011 by Hamdard University, New Delhi, India. He published more than 50 articles in peer-reviewed journals receiving high citations and performed multiple oral communications in national and international platforms.

Gunjan Sharma, Ph.D. Dr. Sharma a Postdoc fellow in the Department of Biochemistry and Molecular Biology, University of Nebraska Medical center (UNMC), USA, currently carrying out his research on the mechanism of GDF15 mediated bone metastasis in cancer. During his first Postdoctoral study in the Department of Immunology and Cancer Research, Hebrew University-Hadassah Medical Center, Jerusalem, Israel, he worked on radio-resistance and DNA repair mechanisms of cancer cells. Dr. Sharma received his Ph.D. degree in Biotechnology on the topic of “Studies on anticancer activity of novel transition metal-arene complexes in vitro and in vivo” at the Department of Zoology, Banaras Hindu University Varanasi in the year of 2018. He has published 29 publications in highly reputed journals. His dedication and growth in the field of Cancer Biology is continued till date.

Sakthivel Muniyan, Ph.D.
Dr. Muniyan is an Assistant professor at the University of Nebraska medical center, Omaha, NE. He is a well-known researcher in the field of prostate cancer and mucin biology. Currently, he is working in the area of MUC16 and chemoresistance. He is serving as a reviewer and board member of several journals.

Parthasarathy Seshacharyulu, Ph.D. Parthasarathy Seshacharyulu is a junior faculty with 16 years of research experience in cancer biology. Since he joined UNMC as a post-doctoral researcher in 2010, he started developing mice models on prostate and pancreatic cancer. His current interests are (i) to investigate the role of PP2A subunits in prostate cancer advancement and (ii) to test USFDA approved drugs as radio/chemo-sensitizers to enhance therapeutic outcomes of lethal prostate and pancreatic cancer. He has obtained several awards for best poster presentation, oral presentation, and research activities at the national and international levels. In addition, he has published 35 peer-reviewed publications. His long-term goal is to identify stromal contributors of prostate and pancreatic cancer as potential targets and test its targeting approach using the mice models and their derived tools (syngeneic cell lines and tumoroids), which he developed over the years to enhance conventional therapies.

Maneesh Jain, Ph.D.
Dr. Jain is a professor at the University of Nebraska Medical Center, Omaha, USA. Dr. Jain obtained his Ph.D. in 2002 from Institute of Microbial Technology Chandigarh, (Jawaharlal Nehru University), India. Dr. Jain’s research is focused early detection and targeted therapies for solid tumors with a special focus on pancreatic cancer. Dr. Jain’s lab has developed several reagents and tools to exploit mucins as disease biomarkers and therapeutic targets. His laboratory is developing antibody-based targeted therapy for pancreatic cancer. He is also leading efforts to develop mucin based immunotherapeutic approaches pancreatic cancer. His laboratory is actively engaged in characterizing the role of tumor microenvironment in therapy resistance and disease progression. Jain Lab is leading efforts in developing therapeutic strategies to selectively modulate the tumor microenvironment for improving the efficacy of chemotherapy, radiation therapy and immunotherapy. To this end, Dr. Jain’s laboratory is focused on studying the role of the endothelin axis in pancreatic tumor microenvironment, particularly in tumor stroma and tumor-associated macrophage recruitment using a combination of genetically engineered models of pancreatic cancer and conditional endothelin ligand and receptor knockout mice. Over the last 13 years, Dr Jain’s lab has been continuously funded by several research awards from NIH including R01, U01 and P01 grants.

Mohd (Wasim) Nasser, Ph.D.
Mohd Wasim Nasser, Ph.D. MW Nasser graduated in 2002 in the field of Biotechnology from Hamdard University, New Delhi. He worked as a postdoctoral fellow in the field of chemokines, inflammation and cancer biology at the Meharry Medical College, Nashville, TN; followed by JLC-BBRI North Carolina Center University, Durham NC. He started his senior postdoctoral training and followed by Junior Faculty training at the Ohio State University to discover the novel role of microRNAs in cancer progression and identified players involved in tumor microenvironment that led to metastasis. MW Nasser joined University of Nebraska Medical Center (UNMC) as an Assistant professor and currently, he is an Associate Professor level in the department of Molecular Biology and Biochemistry at UNMC. The current focus of his group is to understand the tumor microenvironment of lung and breast cancers that leads to develop brain metastasis. He has published so far 51 research and review articles in well reputed high impact journals in the field of cancer and inflammation.

Prof. Surinder K. Batra
Dr. S.K. Batra, Ph.D. is Professor and Chair of Biochemistry and Molecular Biology, University of Nebraska Medical Center, USA. Dr. Batra leads several program projects, such as the Clinical Validation Center (CVC) grant of EDRN and the NCI program project grant (PPG) on pancreatic cancer metastasis. In 2013, Dr. Batra was named as UNMC's seventh Scientist Laureate by the chancellor, and in 2016, he received the Outstanding Research and Creative Activity Award.
Dr. Batra is a pioneer in the field of MUCIN biology. His lab has cloned and characterized several mucins (MUC) differentially expressed and glycosylated in pancreatic cancer and other GI malignancies. His lab has developed several genetically engineered models, various cell lines to investigate the role of mucin and other oncogenes on the development and the progression of pancreatic cancer and evaluate the efficacy of different therapeutics. Dr. Batra's lab has received NIH funding to study the role of Growth differentiation factors 15 (GDF15) in pancreatic and prostate cancer and published several research papers on GDF15 in different malignancies.
Currently, his research is focused on numerous aspects, including therapeutic targeting (EGFR, mucins), targeted radiation therapy, development of radio-sensitizers (FDPS), understanding the role of cytokines and chemokines in tumor microenvironment and metastasis.
Footnotes
Declaration of Competing Interest
SKB is a co-founder of Sanguine Diagnostics and Therapeutics, Inc. The other authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Eling TE, Baek SJ, Shim M, Lee CH, NSAID activated gene (NAG-1), a modulator of tumorigenesis, J Biochem Mol Biol 39(6) (2006) 649–55. [DOI] [PubMed] [Google Scholar]
- [2].Kempf T, Horn-Wichmann R, Brabant G, Peter T, Allhoff T, Klein G, Drexler H, Johnston N, Wallentin L, Wollert KC, Circulating concentrations of growth-differentiation factor 15 in apparently healthy elderly individuals and patients with chronic heart failure as assessed by a new immunoradiometric sandwich assay, Clin Chem 53(2) (2007) 284–91. [DOI] [PubMed] [Google Scholar]
- [3].Koopmann J, Buckhaults P, Brown DA, Zahurak ML, Sato N, Fukushima N, Sokoll LJ, Chan DW, Yeo CJ, Hruban RH, Breit SN, Kinzler KW, Vogelstein B, Goggins M, Serum macrophage inhibitory cytokine 1 as a marker of pancreatic and other periampullary cancers, Clin Cancer Res 10(7) (2004) 2386–92. [DOI] [PubMed] [Google Scholar]
- [4].Brown DA, Lindmark F, Stattin P, Balter K, Adami HO, Zheng SL, Xu J, Isaacs WB, Gronberg H, Breit SN, Wiklund FE, Macrophage inhibitory cytokine 1: a new prognostic marker in prostate cancer, Clin Cancer Res 15(21) (2009) 6658–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mimeault M, Batra SK, Divergent molecular mechanisms underlying the pleiotropic functions of macrophage inhibitory cytokine-1 in cancer, J Cell Physiol 224(3) (2010) 626–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Staff AC, Trovik J, Eriksson AG, Wik E, Wollert KC, Kempf T, Salvesen HB, Elevated plasma growth differentiation factor-15 correlates with lymph node metastases and poor survival in endometrial cancer, Clin Cancer Res 17(14) (2011) 4825–33. [DOI] [PubMed] [Google Scholar]
- [7].Wang X, Li Y, Tian H, Qi J, Li M, Fu C, Wu F, Wang Y, Cheng D, Zhao W, Zhang C, Wang T, Rao J, Zhang W, Macrophage inhibitory cytokine 1 (MIC-1/GDF15) as a novel diagnostic serum biomarker in pancreatic ductal adenocarcinoma, BMC Cancer 14 (2014) 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fairlie WD, Moore AG, Bauskin AR, Russell PK, Zhang HP, Breit SN, MIC-1 is a novel TGF-beta superfamily cytokine associated with macrophage activation, J Leukoc Biol 65(1)(1999) 2–5. [DOI] [PubMed] [Google Scholar]
- [9].Emmerson PJ, Wang F, Du Y, Liu Q, Pickard RT, Gonciarz MD, Coskun T, Hamang MJ, Sindelar DK, Ballman KK, Foltz LA, Muppidi A, Alsina-Fernandez J, Barnard GC, Tang JX, Liu X, Mao X, Siegel R, Sloan JH, Mitchell PJ, Zhang BB, Gimeno RE, Shan B, Wu X, The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL, Nat Med 23(10) (2017) 1215–1219. [DOI] [PubMed] [Google Scholar]
- [10].Hsu JY, Crawley S, Chen M, Ayupova DA, Lindhout DA, Higbee J, Kutach A, Joo W, Gao Z, Fu D, To C, Mondal K, Li B, Kekatpure A, Wang M, Laird T, Horner G, Chan J, McEntee M, Lopez M, Lakshminarasimhan D, White A, Wang SP, Yao J, Yie J, Matern H, Solloway M, Haldankar R, Parsons T, Tang J, Shen WD, Alice Chen Y, Tian H, Allan BB, Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15, Nature 550(7675) (2017) 255–259. [DOI] [PubMed] [Google Scholar]
- [11].Mullican SE, Lin-Schmidt X, Chin CN, Chavez JA, Furman JL, Armstrong AA, Beck SC, South VJ, Dinh TQ, Cash-Mason TD, Cavanaugh CR, Nelson S, Huang C, Hunter MJ, Rangwala SM, GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates, Nat Med 23(10) (2017) 1150–1157. [DOI] [PubMed] [Google Scholar]
- [12].Tsai VW, Manandhar R, Jorgensen SB, Lee-Ng KK, Zhang HP, Marquis CP, Jiang L, Husaini Y, Lin S, Sainsbury A, Sawchenko PE, Brown DA, Breit SN, The anorectic actions of the TGFbeta cytokine MIC-1/GDF15 require an intact brainstem area postrema and nucleus of the solitary tract, PLoS One 9(6) (2014) e100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yang L, Chang CC, Sun Z, Madsen D, Zhu H, Padkjaer SB, Wu X, Huang T, Hultman K, Paulsen SJ, Wang J, Bugge A, Frantzen JB, Norgaard P, Jeppesen JF, Yang Z, Secher A, Chen H, Li X, John LM, Shan B, He Z, Gao X, Su J, Hansen KT, Yang W, Jorgensen SB, GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand, Nat Med 23(10) (2017) 1158–1166. [DOI] [PubMed] [Google Scholar]
- [14].International C Human Genome Sequencing, Finishing the euchromatic sequence of the human genome, Nature 431(7011) (2004) 931–45. [DOI] [PubMed] [Google Scholar]
- [15].Grimwood J, Gordon LA, Olsen A, Terry A, Schmutz J, Lamerdin J, Hellsten U, Goodstein D, Couronne O, Tran-Gyamfi M, Aerts A, Altherr M, Ashworth L, Bajorek E, Black S, Branscomb E, Caenepeel S, Carrano A, Caoile C, Chan YM, Christensen M, Cleland CA, Copeland A, Dalin E, Dehal P, Denys M, Detter JC, Escobar J, Flowers D, Fotopulos D, Garcia C, Georgescu AM, Glavina T, Gomez M, Gonzales E, Groza M, Hammon N, Hawkins T, Haydu L, Ho I, Huang W, Israni S, Jett J, Kadner K, Kimball H, Kobayashi A, Larionov V, Leem SH, Lopez F, Lou Y, Lowry S, Malfatti S, Martinez D, McCready P, Medina C, Morgan J, Nelson K, Nolan M, Ovcharenko I, Pitluck S, Pollard M, Popkie AP, Predki P, Quan G, Ramirez L, Rash S, Retterer J, Rodriguez A, Rogers S, Salamov A, Salazar A, She X, Smith D, Slezak T, Solovyev V, Thayer N, Tice H, Tsai M, Ustaszewska A, Vo N, Wagner M, Wheeler J, Wu K, Xie G, Yang J, Dubchak I, Furey TS, DeJong P, Dickson M, Gordon D, Eichler EE, Pennacchio LA, Richardson P, Stubbs L, Rokhsar DS, Myers RM, Rubin EM, Lucas SM, The DNA sequence and biology of human chromosome 19, Nature 428(6982) (2004) 529–35. [DOI] [PubMed] [Google Scholar]
- [16].Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C, Stange-Thomann Y, Stojanovic N, Subramanian A, Wyman D, Rogers J, Sulston J, Ainscough R, Beck S, Bentley D, Burton J, Clee C, Carter N, Coulson A, Deadman R, Deloukas P, Dunham A, Dunham I, Durbin R, French L, Grafham D, Gregory S, Hubbard T, Humphray S, Hunt A, Jones M, Lloyd C, McMurray A, Matthews L, Mercer S, Milne S, Mullikin JC, Mungall A, Plumb R, Ross M, Shownkeen R, Sims S, Waterston RH, Wilson RK, Hillier LW, McPherson JD, Marra MA, Mardis ER, Fulton LA, Chinwalla AT, Pepin KH, Gish WR, Chissoe SL, Wendl MC, Delehaunty KD, Miner TL, Delehaunty A, Kramer JB, Cook LL, Fulton RS, Johnson DL, Minx PJ, Clifton SW, Hawkins T, Branscomb E, Predki P, Richardson P, Wenning S, Slezak T, Doggett N, Cheng JF, Olsen A, Lucas S, Elkin C, Uberbacher E, Frazier M, Gibbs RA, Muzny DM, Scherer SE, Bouck JB, Sodergren EJ, Worley KC, Rives CM, Gorrell JH, Metzker ML, Naylor SL, Kucherlapati RS, Nelson DL, Weinstock GM, Sakaki Y, Fujiyama A, Hattori M, Yada T, Toyoda A, Itoh T, Kawagoe C, Watanabe H, Totoki Y, Taylor T, Weissenbach J, Heilig R, Saurin W, Artiguenave F, Brottier P, Bruls T, Pelletier E, Robert C, Wincker P, Smith DR, Doucette-Stamm L, Rubenfield M, Weinstock K, Lee HM, Dubois J, Rosenthal A, Platzer M, Nyakatura G, Taudien S, Rump A, Yang H, Yu J, Wang J, Huang G, Gu J, Hood L, Rowen L, Madan A, Qin S, Davis RW, Federspiel NA, Abola AP, Proctor MJ, Myers RM, Schmutz J, Dickson M, Grimwood J, Cox DR, Olson MV, Kaul R, Raymond C, Shimizu N, Kawasaki K, Minoshima S, Evans GA, Athanasiou M, Schultz R, Roe BA, Chen F, Pan H, Ramser J, Lehrach H, Reinhardt R, McCombie WR, de la Bastide M, Dedhia N, Blocker H, Hornischer K, Nordsiek G, Agarwala R, Aravind L, Bailey JA, Bateman A, Batzoglou S, Birney E, Bork P, Brown DG, Burge CB, Cerutti L, Chen HC, Church D, Clamp M, Copley RR, Doerks T, Eddy SR, Eichler EE, Furey TS, Galagan J, Gilbert JG, Harmon C, Hayashizaki Y, Haussler D, Hermjakob H, Hokamp K, Jang W, Johnson LS, Jones TA, Kasif S, Kaspryzk A, Kennedy S, Kent WJ, Kitts P, Koonin EV, Korf I, Kulp D, Lancet D, Lowe TM, McLysaght A, Mikkelsen T, Moran JV, Mulder N, Pollara VJ, Ponting CP, Schuler G, Schultz J, Slater G, Smit AF, Stupka E, Szustakowki J, Thierry-Mieg D, Thierry-Mieg J, Wagner L, Wallis J, Wheeler R, Williams A, Wolf YI, Wolfe KH, Yang SP, Yeh RF, Collins F, Guyer MS, Peterson J, Felsenfeld A, Wetterstrand KA, Patrinos A, Morgan MJ, de Jong P, Catanese JJ, Osoegawa K, Shizuya H, Choi S, Chen YJ, Szustakowki J, C. International Human Genome Sequencing, Initial sequencing and analysis of the human genome, Nature 409(6822) (2001) 860–921. [DOI] [PubMed] [Google Scholar]
- [17].Li JJ, Liu J, Lupino K, Liu X, Zhang L, Pei L, Growth Differentiation Factor 15 Maturation Requires Proteolytic Cleavage by PCSK3, −5, and −6, Mol Cell Biol 38(21) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Assadi A, Zahabi A, Hart RA, GDF15, an update of the physiological and pathological roles it plays: a review, Pflugers Arch 472(11) (2020) 1535–1546. [DOI] [PubMed] [Google Scholar]
- [19].Hsu J-Y, Crawley S, Chen M, Ayupova DA, Lindhout DA, Higbee J, Kutach A, Joo W, Gao Z, Fu D, Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15, Nature 550(7675) (2017) 255–259. [DOI] [PubMed] [Google Scholar]
- [20].Suriben R, Chen M, Higbee J, Oeffinger J, Ventura R, Li B, Mondal K, Gao Z, Ayupova D, Taskar P, Li D, Starck SR, Chen HH, McEntee M, Katewa SD, Phung V, Wang M, Kekatpure A, Lakshminarasimhan D, White A, Olland A, Haldankar R, Solloway MJ, Hsu JY, Wang Y, Tang J, Lindhout DA, Allan BB, Antibody-mediated inhibition of GDF15-GFRAL activity reverses cancer cachexia in mice, Nat Med 26(8) (2020) 1264–1270. [DOI] [PubMed] [Google Scholar]
- [21].Hale C, Veniant MM, Growth differentiation factor 15 as a potential therapeutic for treating obesity, Mol Metab 46 (2021) 101117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nielsen JB, Pedersen AM, Gribsholt SB, Svensson E, Richelsen B, Prevalence, severity, and predictors of symptoms of dumping and hypoglycemia after Roux-en-Y gastric bypass, Surg Obes Relat Dis 12(8) (2016) 1562–1568. [DOI] [PubMed] [Google Scholar]
- [23].Tsai VW, Macia L, Johnen H, Kuffner T, Manadhar R, Jorgensen SB, Lee-Ng KK, Zhang HP, Wu L, Marquis CP, Jiang L, Husaini Y, Lin S, Herzog H, Brown DA, Sainsbury A, Breit SN, TGF-b superfamily cytokine MIC-1/GDF15 is a physiological appetite and body weight regulator, PLoS One 8(2) (2013) e55174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Baek SJ, Okazaki R, Lee SH, Martinez J, Kim JS, Yamaguchi K, Mishina Y, Martin DW, Shoieb A, McEntee MF, Eling TE, Nonsteroidal anti-inflammatory drug-activated gene-1 over expression in transgenic mice suppresses intestinal neoplasia, Gastroenterology 131(5)(2006) 1553–60. [DOI] [PubMed] [Google Scholar]
- [25].Macia L, Tsai VW, Nguyen AD, Johnen H, Kuffner T, Shi YC, Lin S, Herzog H, Brown DA, Breit SN, Sainsbury A, Macrophage inhibitory cytokine 1 (MIC-1/GDF15) decreases food intake, body weight and improves glucose tolerance in mice on normal & obesogenic diets, PLoS One 7(4) (2012) e34868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Xiong Y, Walker K, Min X, Hale C, Tran T, Komorowski R, Yang J, Davda J, Nuanmanee N, Kemp D, Wang X, Liu H, Miller S, Lee KJ, Wang Z, Veniant MM, Long-acting MIC-1/GDF15 molecules to treat obesity: Evidence from mice to monkeys, Sci Transl Med 9(412) (2017). [DOI] [PubMed] [Google Scholar]
- [27].Afanas'ev I, Signaling of reactive oxygen and nitrogen species in Diabetes mellitus, Oxid Med Cell Longev 3(6) (2010) 361–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chrysovergis K, Wang X, Kosak J, Lee SH, Kim JS, Foley JF, Travlos G, Singh S, Baek SJ, Eling TE, NAG-1/GDF-15 prevents obesity by increasing thermogenesis, lipolysis and oxidative metabolism, Int J Obes (Lond) 38(12) (2014) 1555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ho FM, Lin WW, Chen BC, Chao CM, Yang CR, Lin LY, Lai CC, Liu SH, Liau CS, High glucose-induced apoptosis in human vascular endothelial cells is mediated through NF-kappaB and c-Jun NH2-terminal kinase pathway and prevented by PI3K/Akt/eNOS pathway, Cell Signal 18(3) (2006) 391–9. [DOI] [PubMed] [Google Scholar]
- [30].Li J, Yang L, Qin W, Zhang G, Yuan J, Wang F, Adaptive induction of growth differentiation factor 15 attenuates endothelial cell apoptosis in response to high glucose stimulus, PLoS One 8(6) (2013) e65549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kelly JA, Lucia MS, Lambert JR, p53 controls prostate-derived factor/macrophage inhibitory cytokine/NSAID-activated gene expression in response to cell density, DNA damage and hypoxia through diverse mechanisms, Cancer Lett 277(1) (2009) 38–47. [DOI] [PubMed] [Google Scholar]
- [32].Mazagova M, Buikema H, van Buiten A, Duin M, Goris M, Sandovici M, Henning RH, Deelman LE, Genetic deletion of growth differentiation factor 15 augments renal damage in both type 1 and type 2 models of diabetes, Am J Physiol Renal Physiol 305(9) (2013) F1249–64. [DOI] [PubMed] [Google Scholar]
- [33].Dostalova I, Roubicek T, Bartlova M, Mraz M, Lacinova Z, Haluzikova D, Kavalkova P, Matoulek M, Kasalicky M, Haluzik M, Increased serum concentrations of macrophage inhibitory cytokine-1 in patients with obesity and type 2 diabetes mellitus: the influence of very low calorie diet, Eur J Endocrinol 161(3) (2009) 397–404. [DOI] [PubMed] [Google Scholar]
- [34].Sugulle M, Dechend R, Herse F, Weedon-Fekjaer MS, Johnsen GM, Brosnihan KB, Anton L, Luft FC, Wollert KC, Kempf T, Staff AC, Circulating and placental growth-differentiation factor 15 in preeclampsia and in pregnancy complicated by diabetes mellitus, Hypertension 54(1) (2009) 106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Carstensen M, Herder C, Brunner EJ, Strassburger K, Tabak AG, Roden M, Witte DR, Macrophage inhibitory cytokine-1 is increased in individuals before type 2 diabetes diagnosis but is not an independent predictor of type 2 diabetes: the Whitehall II study, Eur J Endocrinol 162(5) (2010) 913–7. [DOI] [PubMed] [Google Scholar]
- [36].Patel S, Alvarez-Guaita A, Melvin A, Rimmington D, Dattilo A, Miedzybrodzka EL, Cimino I, Maurin AC, Roberts GP, Meek CL, Virtue S, Sparks LM, Parsons SA, Redman LM, Bray GA, Liou AP, Woods RM, Parry SA, Jeppesen PB, Kolnes AJ, Harding HP, Ron D, Vidal-Puig A, Reimann F, Gribble FM, Hulston CJ, Farooqi IS, Fafournoux P, Smith SR, Jensen J, Breen D, Wu Z, Zhang BB, Coll AP, Savage DB, O'Rahilly S, GDF15 Provides an Endocrine Signal of Nutritional Stress in Mice and Humans, Cell Metab 29(3) (2019) 707–718 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tsai VW, Lin S, Brown DA, Salis A, Breit SN, Anorexia-cachexia and obesity treatment may be two sides of the same coin: role of the TGF-b superfamily cytokine MIC-1/GDF15, Int J Obes (Lond) 40(2) (2016) 193–7. [DOI] [PubMed] [Google Scholar]
- [38].Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, Zhang HP, Donnellan M, Mahler S, Pryor K, Walsh BJ, Nicholson RC, Fairlie WD, Por SB, Robbins JM, Breit SN, MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily, Proc Natl Acad Sci U S A 94(21) (1997) 11514–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Karczewska-Kupczewska M, Kowalska I, Nikolajuk A, Adamska A, Otziomek E, Gorska M, Straczkowski M, Hyperinsulinemia acutely increases serum macrophage inhibitory cytokine-1 concentration in anorexia nervosa and obesity, Clin Endocrinol (Oxf) 76(1) (2012) 46–50. [DOI] [PubMed] [Google Scholar]
- [40].Schernthaner-Reiter MH, Kasses D, Tugendsam C, Riedl M, Peric S, Prager G, Krebs M, Promintzer-Schifferl M, Clodi M, Luger A, Vila G, Growth differentiation factor 15 increases following oral glucose ingestion: effect of meal composition and obesity, Eur J Endocrinol 175(6) (2016) 623–631. [DOI] [PubMed] [Google Scholar]
- [41].Schernthaner-Reiter MH, Itariu BK, Krebs M, Promintzer-Schifferl M, Stulnig TM, Tura A, Anderwald CH, Clodi M, Ludvik B, Pacini G, Luger A, Vila G, GDF15 reflects beta cell function in obese patients independently of the grade of impairment of glucose metabolism, Nutr Metab Cardiovasc Dis 29(4) (2019) 334–342. [DOI] [PubMed] [Google Scholar]
- [42].Roy D, Purohit P, Modi A, Khokhar M, Shukla RKG, Chaudhary R, Sankanagoudar S, Sharma P, Growth Differentiation Factor-15 as a Biomarker of Obese Pre-diabetes and Type 2 Diabetes Mellitus in Indian Subjects: A Case-control Study, Curr Diabetes Rev (2021). [DOI] [PubMed] [Google Scholar]
- [43].He X, Su J, Ma X, Lu W, Zhu W, Wang Y, Bao Y, Zhou J, The association between serum growth differentiation factor 15 levels and lower extremity atherosclerotic disease is independent of body mass index in type 2 diabetes, Cardiovasc Diabetol 19(1) (2020) 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM, Diabetes G Prevention Program Research, Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin, N Engl J Med 346(6) (2002) 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V, Indian Diabetes Prevention P, The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1), Diabetologia 49(2) (2006) 289–97. [DOI] [PubMed] [Google Scholar]
- [46].Lachin JM, Christophi CA, Edelstein SL, Ehrmann DA, Hamman RF, Kahn SE, Knowler WC, Nathan DM, Group DDKR, Factors associated with diabetes onset during metformin versus placebo therapy in the diabetes prevention program, Diabetes 56(4) (2007) 1153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rena G, Hardie DG, Pearson ER, The mechanisms of action of metformin, Diabetologia 60(9) (2017) 1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gerstein HC, Pare G, Hess S, Ford RJ, Sjaarda J, Raman K, McQueen M, Lee S, Haenel H, Steinberg GR, Investigators O, Growth Differentiation Factor 15 as a Novel Biomarker for Metformin, Diabetes Care 40(2) (2017) 280–283. [DOI] [PubMed] [Google Scholar]
- [49].Coll AP, Chen M, Taskar P, Rimmington D, Patel S, Tadross JA, Cimino I, Yang M, Welsh P, Virtue S, Goldspink DA, Miedzybrodzka EL, Konopka AR, Esponda RR, Huang JT, Tung YCL, Rodriguez-Cuenca S, Tomaz RA, Harding HP, Melvin A, Yeo GSH, Preiss D, Vidal-Puig A, Vallier L, Nair KS, Wareham NJ, Ron D, Gribble FM, Reimann F, Sattar N, Savage DB, Allan BB, O'Rahilly S, GDF15 mediates the effects of metformin on body weight and energy balance, Nature 578(7795) (2020) 444–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ouyang J, Isnard S, Lin J, Fombuena B, Peng X, Chen Y, Routy JP, GDF-15 as a Weight Watcher for Diabetic and Non-Diabetic People Treated With Metformin, Front Endocrinol (Lausanne) 11 (2020) 581839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Bauskin AR, Brown DA, Kuffner T, Johnen H, Luo XW, Hunter M, Breit SN, Role of macrophage inhibitory cytokine-1 in tumorigenesis and diagnosis of cancer, Cancer Res 66(10) (2006) 4983–6. [DOI] [PubMed] [Google Scholar]
- [52].Corre J, Hebraud B, Bourin P, Concise review: growth differentiation factor 15 in pathology: a clinical role?, Stem Cells Transl Med 2(12) (2013) 946–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Li C, Wang J, Kong J, Tang J, Wu Y, Xu E, Zhang H, Lai M, GDF15 promotes EMT and metastasis in colorectal cancer, Oncotarget 7(1) (2016) 860–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Unsicker K, Spittau B, Krieglstein K, The multiple facets of the TGF-beta family cytokine growth/differentiation factor-15/macrophage inhibitory cytokine-1, Cytokine Growth Factor Rev 24(4) (2013) 373–84. [DOI] [PubMed] [Google Scholar]
- [55].Joshi JP, Brown NE, Griner SE, Nahta R, Growth differentiation factor 15 (GDF15)-mediated HER2 phosphorylation reduces trastuzumab sensitivity of HER2-overexpressing breast cancer cells, Biochem Pharmacol 82(9) (2011) 1090–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Welsh JB, Sapinoso LM, Kern SG, Brown DA, Liu T, Bauskin AR, Ward RL, Hawkins NJ, Quinn DI, Russell PJ, Sutherland RL, Breit SN, Moskaluk CA, Frierson HF Jr., Hampton GM, Large-scale delineation of secreted protein biomarkers overexpressed in cancer tissue and serum, Proc Natl Acad Sci U S A 100(6) (2003) 3410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Yan Y, Yue X, Yang S, Zhao Z, Wu P, Liu H, The expression of growth differentiation factor 15 in gallbladder carcinoma, J BUON 26(1) (2021) 218–228. [PubMed] [Google Scholar]
- [58].Bansal N, Kumar D, Gupta A, Chandra D, Sankhwar SN, Mandhani A, Relevance of MIC-1 in the Era of PSA as a Serum Based Predictor of Prostate Cancer: A Critical Evaluation, Sci Rep 7(1) (2017) 16824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zhao L, Lee BY, Brown DA, Molloy MP, Marx GM, Pavlakis N, Boyer MJ, Stockler MR, Kaplan W, Breit SN, Sutherland RL, Henshall SM, Horvath LG, Identification of candidate biomarkers of therapeutic response to docetaxel by proteomic profiling, Cancer Res 69(19) (2009) 7696–703. [DOI] [PubMed] [Google Scholar]
- [60].Li J, Veltri RW, Yuan Z, Christudass CS, Mandecki W, Macrophage inhibitory cytokine 1 biomarker serum immunoassay in combination with PSA is a more specific diagnostic tool for detection of prostate cancer, PLoS One 10(4) (2015) e0122249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Paralkar VM, Vail AL, Grasser WA, Brown TA, Xu H, Vukicevic S, Ke HZ, Qi H, Owen TA, Thompson DD, Cloning and characterization of a novel member of the transforming growth factor-beta/bone morphogenetic protein family, J Biol Chem 273(22) (1998) 13760–7. [DOI] [PubMed] [Google Scholar]
- [62].Karan D, Chen SJ, Johansson SL, Singh AP, Paralkar VM, Lin MF, Batra SK, Dysregulated expression of MIC-1/PDF in human prostate tumor cells, Biochem Biophys Res Commun 305(3) (2003) 598–604. [DOI] [PubMed] [Google Scholar]
- [63].Senapati S, Rachagani S, Chaudhary K, Johansson SL, Singh RK, Batra SK, Overexpression of macrophage inhibitory cytokine-1 induces metastasis of human prostate cancer cells through the FAK-RhoA signaling pathway, Oncogene 29(9) (2010) 1293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Chen SJ, Karan D, Johansson SL, Lin FF, Zeckser J, Singh AP, Batra SK, Lin MF, Prostate-derived factor as a paracrine and autocrine factor for the proliferation of androgen receptor-positive human prostate cancer cells, Prostate 67(5) (2007) 557–71. [DOI] [PubMed] [Google Scholar]
- [65].Cheng JC, Chang HM, Leung PC, Wild-type p53 attenuates cancer cell motility by inducing growth differentiation factor-15 expression, Endocrinology 152(8) (2011) 2987–95. [DOI] [PubMed] [Google Scholar]
- [66].Mimeault M, Johansson SL, Batra SK, Marked improvement of cytotoxic effects induced by docetaxel on highly metastatic and androgen-independent prostate cancer cells by downregulating macrophage inhibitory cytokine-1, Br J Cancer 108(5) (2013) 1079–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Liu T, Bauskin AR, Zaunders J, Brown DA, Pankhurst S, Russell PJ, Breit SN, Macrophage inhibitory cytokine 1 reduces cell adhesion and induces apoptosis in prostate cancer cells, Cancer Res 63(16) (2003) 5034–40. [PubMed] [Google Scholar]
- [68].Husaini Y, Tsai VW, Manandhar R, Zhang HP, Lee-Ng KKM, Lebhar H, Marquis CP, Brown DA, Breit SN, Growth differentiation factor-15 slows the growth of murine prostate cancer by stimulating tumor immunity, PLoS One 15(6) (2020) e0233846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Husaini Y, Qiu MR, Lockwood GP, Luo XW, Shang P, Kuffner T, Tsai VW, Jiang L, Russell PJ, Brown DA, Breit SN, Macrophage inhibitory cytokine-1 (MIC-1/GDF15) slows cancer development but increases metastases in TRAMP prostate cancer prone mice, PLoS One 7(8) (2012) e43833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wang X, Baek SJ, Eling TE, The diverse roles of nonsteroidal anti-inflammatory drug activated gene (NAG-1/GDF15) in cancer, Biochem Pharmacol 85(5) (2013) 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Rybicki BA, Sadasivan SM, Chen Y, Kravtsov O, Palangmonthip W, Arora K, Gupta NS, Williamson S, Bobbitt K, Chitale DA, Tang D, Rundle AG, Iczkowski KA, Growth and differentiation factor 15 and NF-kappaB expression in benign prostatic biopsies and risk of subsequent prostate cancer detection, Cancer Med (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Kannan K, Amariglio N, Rechavi G, Givol D, Profile of gene expression regulated by induced p53: connection to the TGF-beta family, FEBS Lett 470(1) (2000) 77–82. [DOI] [PubMed] [Google Scholar]
- [73].Hayes VM, Severi G, Southey MC, Padilla EJ, English DR, Hopper JL, Giles GG, Sutherland RL, Macrophage inhibitory cytokine-1 H6D polymorphism, prostate cancer risk, and survival, Cancer Epidemiol Biomarkers Prev 15(6) (2006) 1223–5. [DOI] [PubMed] [Google Scholar]
- [74].Wollmann W, Goodman ML, Bhat-Nakshatri P, Kishimoto H, Goulet RJ Jr., Mehrotra S, Morimiya A, Badve S, Nakshatri H, The macrophage inhibitory cytokine integrates AKT/PKB and MAP kinase signaling pathways in breast cancer cells, Carcinogenesis 26(5) (2005) 900–7. [DOI] [PubMed] [Google Scholar]
- [75].Bruzzese F, Hagglof C, Leone A, Sjoberg E, Roca MS, Kiflemariam S, Sjoblom T, Hammarsten P, Egevad L, Bergh A, Ostman A, Budillon A, Augsten M, Local and systemic protumorigenic effects of cancer-associated fibroblast-derived GDF15, Cancer Res 74(13) (2014) 3408–17. [DOI] [PubMed] [Google Scholar]
- [76].Selander KS, Brown DA, Sequeiros GB, Hunter M, Desmond R, Parpala T, Risteli J, Breit SN, Jukkola-Vuorinen A, Serum macrophage inhibitory cytokine-1 concentrations correlate with the presence of prostate cancer bone metastases, Cancer Epidemiol Biomarkers Prev 16(3) (2007) 532–7. [DOI] [PubMed] [Google Scholar]
- [77].Wakchoure S, Swain TM, Hentunen TA, Bauskin AR, Brown DA, Breit SN, Vuopala KS, Harris KW, Selander KS, Expression of macrophage inhibitory cytokine-1 in prostate cancer bone metastases induces osteoclast activation and weight loss, Prostate 69(6) (2009) 652–61. [DOI] [PubMed] [Google Scholar]
- [78].Wang W, Yang X, Dai J, Lu Y, Zhang J, Keller ET, Prostate cancer promotes a vicious cycle of bone metastasis progression through inducing osteocytes to secrete GDF15 that stimulates prostate cancer growth and invasion, Oncogene 38(23) (2019) 4540–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Peake BF, Eze SM, Yang L, Castellino RC, Nahta R, Growth differentiation factor 15 mediates epithelial mesenchymal transition and invasion of breast cancers through IGF-1R-FoxM1 signaling, Oncotarget 8(55) (2017) 94393–94406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Gkretsi V, Stylianou A, Kalli M, Louca M, Voutouri C, Zaravinos A, Stylianopoulos T, Silencing of Growth Differentiation Factor-15 Promotes Breast Cancer Cell Invasion by Down-regulating Focal Adhesion Genes, Anticancer Res 40(3) (2020) 1375–1385. [DOI] [PubMed] [Google Scholar]
- [81].Gkretsi V, Louca M, Stylianou A, Minadakis G, Spyrou GM, Stylianopoulos T, Inhibition of Breast Cancer Cell Invasion by Ras Suppressor-1 (RSU-1) Silencing Is Reversed by Growth Differentiation Factor-15 (GDF-15), Int J Mol Sci 20(1) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Pinnix ZK, Miller LD, Wang W, D'Agostino R Jr., Kute T, Willingham MC, Hatcher H, Tesfay L, Sui G, Di X, Torti SV, Torti FM, Ferroportin and iron regulation in breast cancer progression and prognosis, Sci Transl Med 2(43) (2010) 43ra56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Blanchette-Farra N, Kita D, Konstorum A, Tesfay L, Lemler D, Hegde P, Claffey KP, Torti FM, Torti SV, Contribution of three-dimensional architecture and tumor-associated fibroblasts to hepcidin regulation in breast cancer, Oncogene 37(29) (2018) 4013–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Kim KK, Lee JJ, Yang Y, You KH, Lee JH, Macrophage inhibitory cytokine-1 activates AKT and ERK-1/2 via the transactivation of ErbB2 in human breast and gastric cancer cells, Carcinogenesis 29(4) (2008) 704–12. [DOI] [PubMed] [Google Scholar]
- [85].Donnelly SM, Paplomata E, Peake BM, Sanabria E, Chen Z, Nahta R, P38 MAPK contributes to resistance and invasiveness of HER2- overexpressing breast cancer, Curr Med Chem 21(4) (2014) 501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Sasahara A, Tominaga K, Nishimura T, Yano M, Kiyokawa E, Noguchi M, Noguchi M, Kanauchi H, Ogawa T, Minato H, Tada K, Seto Y, Tojo A, Gotoh N, An autocrine/paracrine circuit of growth differentiation factor (GDF) 15 has a role for maintenance of breast cancer stem-like cells, Oncotarget 8(15) (2017) 24869–24881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Huang J, Wu S, Barrera J, Matthews K, Pan D, The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP, Cell 122(3) (2005) 421–34. [DOI] [PubMed] [Google Scholar]
- [88].Wang T, Mao B, Cheng C, Zou Z, Gao J, Yang Y, Lei T, Qi X, Yuan Z, Xu W, Lu Z, YAP promotes breast cancer metastasis by repressing growth differentiation factor-15, Biochim Biophys Acta Mol Basis Dis 1864(5 Pt A) (2018) 1744–1753. [DOI] [PubMed] [Google Scholar]
- [89].Siegel RL, Miller KD, Fuchs HE, Jemal A, Cancer Statistics, 2021, CA Cancer J Clin 71(1) (2021) 7–33. [DOI] [PubMed] [Google Scholar]
- [90].Kaur S, Chakraborty S, Baine MJ, Mallya K, Smith LM, Sasson A, Brand R, Guha S, Jain M, Wittel U, Singh SK, Batra SK, Potentials of plasma NGAL and MIC-1 as biomarker(s) in the diagnosis of lethal pancreatic cancer, PLoS One 8(2) (2013) e55171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Yang Y, Yan S, Tian H, Bao Y, Macrophage inhibitory cytokine-1 versus carbohydrate antigen 19-9 as a biomarker for diagnosis of pancreatic cancer: A PRISMA-compliant meta-analysis of diagnostic accuracy studies, Medicine (Baltimore) 97(9) (2018) e9994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Hogendorf P, Durczynski A, Skulimowski A, Kumor A, Poznanska G, Strzelczyk J, Growth differentiation factor (GDF-15) concentration combined with Ca125 levels in serum is superior to commonly used cancer biomarkers in differentiation of pancreatic mass, Cancer Biomark 21(3) (2018) 505–511. [DOI] [PubMed] [Google Scholar]
- [93].O'Neill RS, Emmanuel S, Williams D, Stoita A, Macrophage inhibitory cytokine-1/growth differentiation factor-15 in premalignant and neoplastic tumours in a high-risk pancreatic cancer cohort, World J Gastroenterol 26(14) (2020) 1660–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Ratnam NM, Peterson JM, Talbert EE, Ladner KJ, Rajasekera PV, Schmidt CR, Dillhoff ME, Swanson BJ, Haverick E, Kladney RD, Williams TM, Leone GW, Wang DJ, Guttridge DC, NF-kappaB regulates GDF-15 to suppress macrophage surveillance during early tumor development, J Clin Invest 127(10) (2017) 3796–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Ji H, Lu HW, Li YM, Lu L, Wang JL, Zhang YF, Shang H, Twist promotes invasion and cisplatin resistance in pancreatic cancer cells through growth differentiation factor 15, Mol Med Rep 12(3) (2015) 3841–3848. [DOI] [PubMed] [Google Scholar]
- [96].Guo F, Zhou Y, Guo H, Ren D, Jin X, Wu H, NR5A2 transcriptional activation by BRD4 promotes pancreatic cancer progression by upregulating GDF15, Cell Death Discov 7(1) (2021) 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Kalli M, Minia A, Pliaka V, Fotis C, Alexopoulos LG, Stylianopoulos T, Solid stress-induced migration is mediated by GDF15 through Akt pathway activation in pancreatic cancer cells, Sci Rep 9(1) (2019) 978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Zhao Z, Zhang J, Yin L, Yang J, Zheng Y, Zhang M, Ni B, Wang H, Upregulated GDF-15 expression facilitates pancreatic ductal adenocarcinoma progression through orphan receptor GFRAL, Aging (Albany NY) 12(22) (2020) 22564–22581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Han M, Dai D, Yousafzai NA, Wang F, Wang H, Zhou Q, Lu H, Xu W, Feng L, Jin H, Wang X, CXXC4 activates apoptosis through up-regulating GDF15 in gastric cancer, Oncotarget 8(61) (2017) 103557–103567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Lee DH, Yang Y, Lee SJ, Kim KY, Koo TH, Shin SM, Song KS, Lee YH, Kim YJ, Lee JJ, Choi I, Lee JH, Macrophage inhibitory cytokine-1 induces the invasiveness of gastric cancer cells by up-regulating the urokinase-type plasminogen activator system, Cancer Res 63(15)(2003) 4648–55. [PubMed] [Google Scholar]
- [101].Guo J, Bian Y, Wang Y, Chen L, Yu A, Sun X, S100A4 influences cancer stem cell-like properties of MGC803 gastric cancer cells by regulating GDF15 expression, Int J Oncol 49(2) (2016) 559–68. [DOI] [PubMed] [Google Scholar]
- [102].Buchholz K, Antosik P, Grzanka D, Gagat M, Smolinska M, Grzanka A, Gzil A, Kasperska A, Klimaszewska-Wisniewska A, Expression of the Body-Weight Signaling Players: GDF15, GFRAL and RET and their clinical relevance in Gastric Cancer, J Cancer 12(15) (2021) 4698–4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Yu S, Li Q, Yu Y, Cui Y, Li W, Liu T, Liu F, Activated HIF1alpha of tumor cells promotes chemoresistance development via recruiting GDF15-producing tumor-associated macrophages in gastric cancer, Cancer Immunol Immunother 69(10) (2020) 1973–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Brown DA, Ward RL, Buckhaults P, Liu T, Romans KE, Hawkins NJ, Bauskin AR, Kinzler KW, Vogelstein B, Breit SN, MIC-1 serum level and genotype: associations with progress and prognosis of colorectal carcinoma, Clin Cancer Res 9(7) (2003) 2642–50. [PubMed] [Google Scholar]
- [105].Spanopoulou A, Gkretsi V, Growth differentiation factor 15 (GDF15) in cancer cell metastasis: from the cells to the patients, Clin Exp Metastasis 37(4) (2020) 451–464. [DOI] [PubMed] [Google Scholar]
- [106].Wang X, Yang Z, Tian H, Li Y, Li M, Zhao W, Zhang C, Wang T, Liu J, Zhang A, Shen D, Zheng C, Qi J, Zhao D, Shi J, Jin L, Rao J, Zhang W, Circulating MIC-1/GDF15 is a complementary screening biomarker with CEA and correlates with liver metastasis and poor survival in colorectal cancer, Oncotarget 8(15) (2017) 24892–24901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Vocka M, Langer D, Fryba V, Petrtyl J, Hanus T, Kalousova M, Zima T, Petruzelka L, Growth/differentiation factor 15 (GDF-15) as new potential serum marker in patients with metastatic colorectal cancer, Cancer Biomark 21(4) (2018) 869–874. [DOI] [PubMed] [Google Scholar]
- [108].Brown DA, Hance KW, Rogers CJ, Sansbury LB, Albert PS, Murphy G, Laiyemo AO, Wang Z, Cross AJ, Schatzkin A, Danta M, Srasuebkul P, Amin J, Law M, Breit SN, Lanza E, Serum macrophage inhibitory cytokine-1 (MIC-1/GDF15): a potential screening tool for the prevention of colon cancer?, Cancer Epidemiol Biomarkers Prev 21(2) (2012) 337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Wallin U, Glimelius B, Jirstrom K, Darmanis S, Nong RY, Ponten F, Johansson C, Pahlman L, Birgisson H, Growth differentiation factor 15: a prognostic marker for recurrence in colorectal cancer, Br J Cancer 104(10) (2011) 1619–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Mehta RS, Chong DQ, Song M, Meyerhardt JA, Ng K, Nishihara R, Qian Z, Morikawa T, Wu K, Giovannucci EL, Fuchs CS, Ogino S, Chan AT, Association Between Plasma Levels of Macrophage Inhibitory Cytokine-1 Before Diagnosis of Colorectal Cancer and Mortality, Gastroenterology 149(3) (2015) 614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Zhang Y, Wang X, Zhang M, Zhang Z, Jiang L, Li L, GDF15 promotes epithelial-to-mesenchymal transition in colorectal [corrected], Artif Cells Nanomed Biotechnol 46(sup2) (2018) 652–658. [DOI] [PubMed] [Google Scholar]
- [112].Li YL, Chang JT, Lee LY, Fan KH, Lu YC, Li YC, Chiang CH, You GR, Chen HY, Cheng AJ, GDF15 contributes to radioresistance and cancer stemness of head and neck cancer by regulating cellular reactive oxygen species via a SMAD-associated signaling pathway, Oncotarget 8(1) (2017) 1508–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Yamaguchi K, Lee SH, Eling TE, Baek SJ, Identification of nonsteroidal anti-inflammatory drug-activated gene (NAG-1) as a novel downstream target of phosphatidylinositol 3-kinase/AKT/GSK-3beta pathway, J Biol Chem 279(48) (2004) 49617–23. [DOI] [PubMed] [Google Scholar]
- [114].Artz A, Butz S, Vestweber D, GDF-15 inhibits integrin activation and mouse neutrophil recruitment through the ALK-5/TGF-betaRII heterodimer, Blood 128(4) (2016) 529–41. [DOI] [PubMed] [Google Scholar]
- [115].Xu J, Kimball TR, Lorenz JN, Brown DA, Bauskin AR, Klevitsky R, Hewett TE, Breit SN, Molkentin JD, GDF15/MIC-1 functions as a protective and antihypertrophic factor released from the myocardium in association with SMAD protein activation, Circ Res 98(3) (2006) 342–50. [DOI] [PubMed] [Google Scholar]
- [116].Olsen OE, Skjaervik A, Stordal BF, Sundan A, Holien T, TGF-beta contamination of purified recombinant GDF15, PLoS One 12(11) (2017) e0187349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Tsai VWW, Husaini Y, Sainsbury A, Brown DA, Breit SN, The MIC-1/GDF15-GFRAL Pathway in Energy Homeostasis: Implications for Obesity, Cachexia, and Other Associated Diseases, Cell Metab 28(3) (2018) 353–368. [DOI] [PubMed] [Google Scholar]
- [118].Cimino I, Coll AP, Yeo GSH, GDF15 and energy balance: homing in on a mechanism, Nat Med 23(10) (2017) 1119–1120. [DOI] [PubMed] [Google Scholar]
- [119].Saarma M, Goldman A, Obesity: Receptors identified for a weight regulator, Nature 550(7675) (2017) 195–197. [DOI] [PubMed] [Google Scholar]
- [120].Chen G, Wang M, Liu X, GDF15 promotes osteosarcoma cell migration and invasion by regulating the TGFbeta signaling pathway, Mol Med Rep 20(5) (2019) 4262–4270. [DOI] [PubMed] [Google Scholar]
- [121].Li S, Ma YM, Zheng PS, Zhang P, GDF15 promotes the proliferation of cervical cancer cells by phosphorylating AKT1 and Erk1/2 through the receptor ErbB2, J Exp Clin Cancer Res 37(1) (2018) 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Kleinertz H, Hepner-Schefczyk M, Ehnert S, Claus M, Halbgebauer R, Boiler L, Huber-Lang M, Cinelli P, Kirschning C, Flohe S, Sander A, Waydhas C, Vonderhagen S, Jager M, Dudda M, Watzl C, Flohe SB, Circulating growth/differentiation factor 15 is associated with human CD56(bright) natural killer cell dysfunction and nosocomial infection in severe systemic inflammation, EBioMedicine 43 (2019) 380–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Jayachandran P, Xiu J, Soni S, Goldberg RM, Weinberg BA, Lou E, Hall MJ, Khushman M.d.M., Sohal D, Battaglin F, Arai H, Zhang W, Wang J, Korn WM, Millstein J, Lenz H-J, GDF15 expression in metastatic colorectal cancer, Journal of Clinical Oncology 39(3_suppl) (2021) 116–116.33151787 [Google Scholar]
- [124].Roth P, Junker M, Tritschler I, Mittelbronn M, Dombrowski Y, Breit SN, Tabatabai G, Wick W, Weller M, Wischhusen J, GDF-15 contributes to proliferation and immune escape of malignant gliomas, Clin Cancer Res 16(15) (2010) 3851–9. [DOI] [PubMed] [Google Scholar]
- [125].Zhou Z, Li W, Song Y, Wang L, Zhang K, Yang J, Zhang W, Su H, Zhang Y, Growth differentiation factor-15 suppresses maturation and function of dendritic cells and inhibits tumor-specific immune response, PLoS One 8(11) (2013) e78618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Zhang Y, Zhang G, Liu Y, Chen R, Zhao D, McAlister V, Mele T, Liu K, Zheng X, GDF15 Regulates Malat-1 Circular RNA and Inactivates NFkappaB Signaling Leading to Immune Tolerogenic DCs for Preventing Alloimmune Rejection in Heart Transplantation, Front Immunol 9 (2018) 2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Siddiqui JA, Pothuraju R, Jain M, Batra SK, Nasser MW, Advances in cancer cachexia: Intersection between affected organs, mediators, and pharmacological interventions, Biochim Biophys Acta Rev Cancer 1873(2) (2020) 188359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Lerner L, Hayes TG, Tao N, Krieger B, Feng B, Wu Z, Nicoletti R, Chiu MI, Gyuris J, Garcia JM, Plasma growth differentiation factor 15 is associated with weight loss and mortality in cancer patients, J Cachexia Sarcopenia Muscle 6(4) (2015) 317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Johnen H, Lin S, Kuffner T, Brown DA, Tsai VW, Bauskin AR, Wu L, Pankhurst G, Jiang L, Junankar S, Hunter M, Fairlie WD, Lee NJ, Enriquez RF, Baldock PA, Corey E, Apple FS, Murakami MM, Lin EJ, Wang C, During MJ, Sainsbury A, Herzog H, Breit SN, Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1, Nat Med 13(11) (2007) 1333–40. [DOI] [PubMed] [Google Scholar]
- [130].Lerner L, Gyuris J, Nicoletti R, Gifford J, Krieger B, Jatoi A, Growth differentiating factor-15 (GDF-15): A potential biomarker and therapeutic target for cancer-associated weight loss, Oncol Lett 12(5) (2016) 4219–4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Ost M, Igual Gil C, Coleman V, Keipert S, Efstathiou S, Vidic V, Weyers M, Klaus S, Muscle-derived GDF15 drives diurnal anorexia and systemic metabolic remodeling during mitochondrial stress, EMBO Rep 21(3) (2020) e48804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Breen DM, Jagarlapudi S, Patel A, Zou C, Joaquim S, Li X, Kang L, Pang J, Hales K, Ziso-Qejvanaj E, Vera NB, Bennett D, He T, Lambert M, Kelleher K, Wu Z, Zhang BB, Lin L, Seeley RJ, Bezy O, Growth differentiation factor 15 neutralization does not impact anorexia or survival in lipopolysaccharide-induced inflammation, iScience 24(6) (2021) 102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Melero I, Calvo E, Dummer R, Garralda E, Schuler MH, Goebeler M-E, Bargou RC, Gromke T, Tabernero J, Ramelyte E, Miguel MD, Sanmamed MF, Rodriguez-Ruiz ME, Fettes P, Klar K, Ruediger M, Schuberth-Wagner C, Haake M, Wischhusen J, Leo E, A phase I, first-in-human clinical trial of the GDF-15 neutralizing antibody CTL-002 in subjects with advanced-stage solid tumors (ACRONYM: GDFATHER), Journal of Clinical Oncology 39(15_suppl) (2021) TPS2658–TPS2658. [Google Scholar]
- [134].Carreras-Badosa G, Gomez-Vilarrubla A, Mas-Pares B, Martinez-Calcerrada JM, Xargay-Torrent S, Prats-Puig A, Puerto-Carranza E, Diaz-Roldan F, de Zegher F, Ibanez L, Bassols J, Lopez-Bermejo A, A 24-month metformin treatment study of children with obesity: Changes in circulating GDF-15 and associations with changes in body weight and visceral fat, Pediatr Obes (2021) e12845. [DOI] [PubMed] [Google Scholar]
- [135].Sharma A, Greene S, Vaduganathan M, Fudim M, Ambrosy AP, Sun JL, McNulty SE, Hernandez AF, Borlaug BA, Velazquez EJ, Mentz RJ, DeVore AD, Alhanti B, Margulies K, Felker GM, Growth differentiation factor-15, treatment with liraglutide, and clinical outcomes among patients with heart failure, ESC Heart Fail 8(4) (2021) 2608–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]