Abstract
Heme-containing peroxidases catalyze the oxidation of a variety of substrates by consuming hydrogen peroxide (H2O2), and play diversified roles in physiology and pathology including innate immunity, the synthesis of thyroid hormone and the extracellular matrix, as well as the pathogenesis of several inflammatory diseases. Peroxidasin (PXDN), also known as Vascular Peroxidase-1 (VPO1), is a newly identified peroxidase and expresses in multiple cells and tissues including cardiovascular system and the lung. Recent studies imply its roles in the innate immunity, cardiovascular physiology and diseases, and extracellular matrix formation. Studies on the role of PXDN in human diseases are entering a new and exciting stage, and this review provides the insights into this emerging field of PXDN.
Graphical Abstract
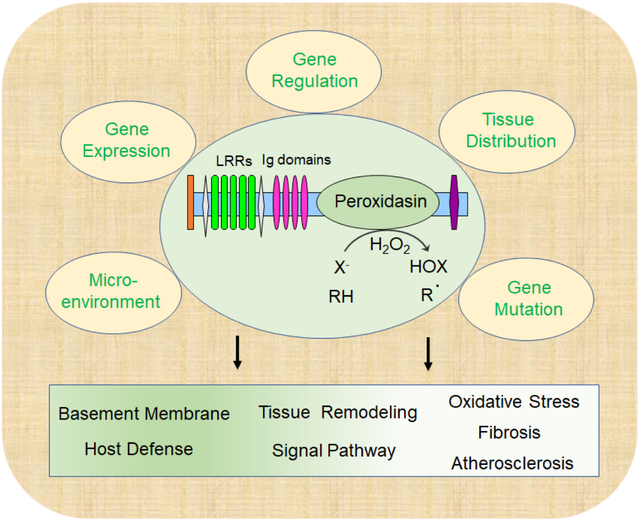
The animal heme-containing peroxidases (hPxs) are found in organisms ranging from Nematode, Drosophila to mammals. This enzyme family in humans includes eight numbers while murine has seven, in which PXDNL is missing. hPxs play diversified roles in host defenses, thyroid hormone synthesis and inflammatory responses. In pathology, hPxs are involving in infections, atherosclerosis, allergy and other inflammatory responsive diseases. The classic hPxs, including myeloperoxidase (MPO), eosinophil peroxidase (EPO), lactoperoxidase (LPO), thyroid peroxidase (TPO), exhibit highly restricted tissue distribution. For example, MPO expresses in neutrophils and monocytes [1]; EPO expresses in eosinophils [2]; TPO expresses in thyroid [3]. LPO expresses in some glands and secrets into milk, saliva and mucus of airways [4]. Peroxidasin (PXDN), also formerly known as vascular peroxidase-1 (VPO1), is the most recently identified mammalian hPx. It was first described in Drosophila in 1994 [5]. During recent years, its physiological and pathological functions have been progressively discovered. The publications related to PXDN are increased yearly. This review summarizes the important discoveries of mammalian PXDN in physiology and pathology.
1. Biology and biochemistry of PXDN
1.1. Identification of mammalian PXDN
Two new mammalian homologs highly expressed in cardiovascular system were completely identified in 2008. Based on their expression features and following the traditional nomenclature of hPxs, they were named VPO1 and VPO2 [6]. VPO1 and VPO2 were initially identified using BLAST searching of the NCBI expression sequence tag (EST) and genomic databases using the peroxidase domain of human Duox1 as the probe. Analysis of human genome databases reveals that VPO1 gene is located tail-to-tail with TPO on chromosome 2p25. VPO1 gene is approximately 110 kb containing 25 exons and 22 introns. VPO2 is located at 8q11 with 491 kb containing 26 exons. Protein sequence analysis reveals that VPO1 and VPO2 are approximately 63% identical, and they are closely related in sequence and domain structure to the previously described insect peroxidase (peroxidasin) [6]. Since the orthologue of VPO1 in Drosophila was found and named peroxidasin in 1994 [5], the Human Gene Nomenclature Committee (HUGO) approved the gene name and symbol as peroxidasin and PXDN, respectively. Following this, VPO2 is named peroxidasin-like and its symbol is PXDNL. In this review, the official name and symbol are used thereafter instead of the alias of VPO1 and VPO2.
Mammalian PXDN was certainly found through years and a variety of approaches. Mitchell et al first identified the 3′ region of PXDN gene, about 8.1-kb, through subtractive hybridization and the EST assembly in melanoma. This gene contains a homolog of interleukin-1 receptor antagonist and was named melanoma gene-50 (MG50) [7]. A short sequence at the 5’ region is different with the report by Cheng et al [6]. Cheng group has established a PXDN stably expressing cell line. PXDN is successfully over-expressed and purified [6]. Thus, it has not completely identified until the work from Cheng group. The discovery and exploration of PXDN in the past decade have yielded a definitively physiological purpose as a multi-functional peroxidase with many important roles.
1.2. Protein structure of PXDN
Alignment of human and mouse PXDN reveals a 91% identity at the amino acid level, which is similar as human and mouse MPO with 86% identity [6]. Fig. 1 shows the eight members and the domain structure of human hPx family [6]. PXDN and PXDNL have additional N-terminal domain. Molecular weights of PXDN and PXDNL are predicted as 165 kDa, which are much larger than MPO (89 kDa, precursor form) and other classic peroxidases. The N-terminus of PXDN and PXDNL is composed of multiple domains: 1) five leucine-rich repeats (LRRs); 2) LRR N-terminal or C-terminal domain, which are often located at N- or C-terminal of LRRs; and 3) four immunoglobulin (Ig) C-2 type domains. PXDN and PXDNL also contain a von Willebrand factor type C domain (VWC) at the extreme C-terminus following the peroxidase domain. These structural domains (except for the peroxidase domain) are predicted to mediate protein-protein interaction or protein-ligand interaction [6]. PXDN and PXDNL possess structural features that are not present in other hPxs, which may contribute to its capacity to localize to specific tissue/cellular sites and support the targeted biological modifications. It is worthy to note although PXDNL contains the peroxidase domain, it apparently lacks the critical residues for heme binding and is not active [8]. Recent study indicated that the N-terminus of PXDN interacts with LPS and facilitates the bactericide of Gram-negative bacteria [9]. Ig domains of PXDN are required to form sulfilimine cross-links suggesting they promote PXDN colocalization with collagen IV [10]. LRRs of PXDN can bind to laminin in basement membranes [11].
Figure 1.
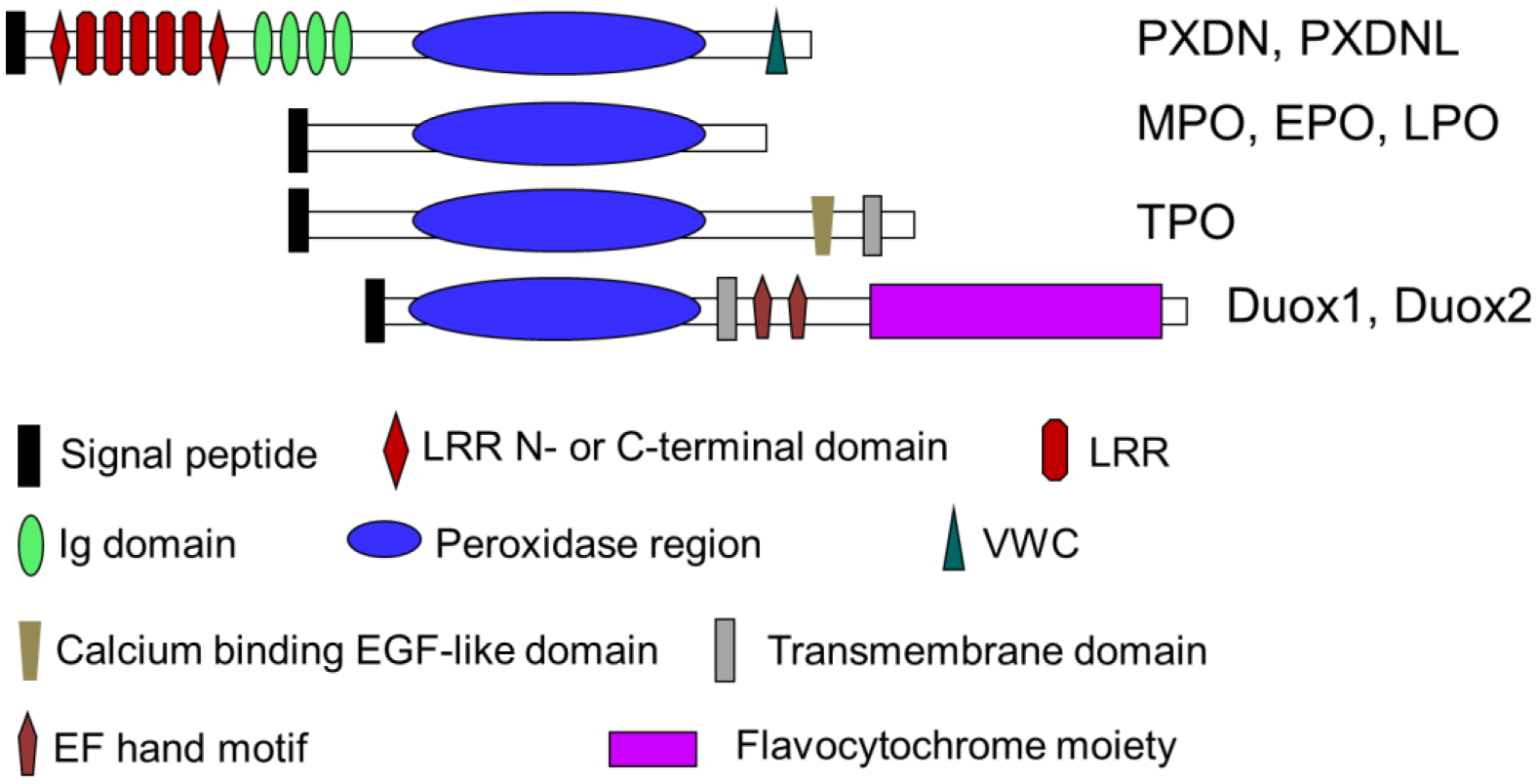
Members of human hPxs and the domain structure.
PXDN contains covalently bound heme, which is consistent with the molecular model showing conservation of two of the three residues that form a covalent bond with heme in MPO [6]. The extinction coefficient for the ferric Soret band (412 nm) of native PXDN is approximately 112 ± 10 mM−1cm−1. This extinction coefficient is similar as LPO. Using heme concentration obtained from this extinction coefficient, the α band of the reduced pyridine hemochrome of PXDN is calculated to exhibit an extinction coefficient of approximately 22 mM−1cm−1, which is also similar to that of the corresponding band for LPO [12]. PXDN is high N-glycosylated protein with four highly glycosylated sites and six partially glycosylated sites [13]. Secreted PXDN reveals homotrimeric form [13]. The oligomerization of PXDN occurs intracellularly [14].
1.3. Sources of PXDN
PXDN mRNA is detected in a variety of tissues and cells by RT-PCR and Southern blots while the protein is found in multiple adult and embryonic tissues including heart, liver, lung, pancreas, placenta, brain and skeletal muscle with relatively high in the cardiovascular system and the lung [6, 9]. PXDN expresses and secrets in kidney and myofibroblasts [15]. PXDN is high expression in eyes [16, 17]. Immunohistochemistry shows PXDN expression in prostate cancer [18] and oral squamous cell carcinoma [19]. Inflammatory factors such as tumor necrosis factor-α (TNF-α) and lipopolysaccharide (LPS) induce PXDN expression and secretion in vitro and in vivo [9, 20–22]. Interestingly, oxidized low density lipoprotein (ox-LDL) is able to induce PXDN expression in aorta [21].
PXDN is also found in multiple cells and cell lines. Using RT-PCR, immunoblot assay and immunohistochemistry/immunofluorescence, PXDN has been detected in, vascular endothelial cells (VECs); vascular smooth muscle cells (VSMCs), alveolar type II epithelial cells [9], myofibroblasts [15], lens epithelium [16, 17], corneal epithelium [16, 17], mesenchymal cells in the vitreous and inner neuroblastic layer of fetal eyes [17], H9c2 [6], HUVECs [9] and glioblastoma cells [23]. Interestingly, PXDN secrets into blood and bronchoalveolar lavage fluid (BALF) [9, 20]. The concentration of PXDN in serum/plasma is much higher than MPO [20]. PXDN concentration in serum/plasma was measured by immunoblot assay using the anti-PXDN multiclonal antibody. Interestingly, the PXDN concentration in mouse serum is higher than that in human plasma (average 2.6 vs. 1.1 μM) [20]. Additionally, PXDN is separated from MPO in plasma. The fractions of PXDN and MPO are used for 3,3’,5,5’-Tetramethylbenzidine (TMB) oxidation assay, and the absorbance at 280 nm and 412 nm. Both fractions of PXDN and MPO have chloride-dependent bactericidal activities, which are inhabitable by 4-Aminobenzoic acid hydrazide (ABAH) [24]. These confirm PXDN expression in blood. However, the concentration of PXDN in blood is the only report until current review. Thus, PXDN is a wide-spread hPx.
1.4. Catalytic mechanisms and in vivo substrates
PXDN, like other numbers of the mammalian family of peroxidases, reveals the typical peroxidase activity [6, 25, 26]. Fig. 2 shows the catalytic cycles of PXDN. PXDN-dependent 3,3’,5,5’-tetramethylbenzidine (TMB) oxidation is effectively reconstituted in PXDN-transfected cells [6]. Cellular PXDN peroxidase activity is H2O2 dose-dependent, and the activity is inhibited in a dose-dependent manner by a specific inhibitor of hPxs, 4-Aminobenzoic acid hydrazide (ABAH) [6]. The Km value of PXDN for H2O2 is 1.5 mM and the peroxidase activity of PXDN is much lower than MPO and LPO (4 – 5 %) [6]. In co-transfection experiments, PXDN is capable for using H2O2 generated from NADPH oxidases (Noxs) to catalyze peroxidative reactions [6]. PXDN can also directly oxidize tyrosine by using H2O2 to form di-tyrosine [20]. Sevcnikar et al measured the kinetics of PXDN compound I and compound II reacting with the endogenous one-electron donors, nitrite, ascorbate, urate, tyrosine and serotonin [26]. In addition, PXDN compound I can react with halides to form hypohalous acids through halogenation cycle. Obinger group has elucidated the kinetics of compound I reduction by thiocyanate (1.8 × 107 M−1·s−1), iodide (1.7 × 107 M−1 ·s−1) and bromide (5.6 × 106 M−1 ·s −1) [25]. The rate constants for the other one-electron donors are calculated to be (7.7 ± 0.1) × 103 M−1·s−1 (serotonin), (4.0 ± 0.1) × 103 M−1·s−1 (nitrite) [26]. Hypohalous acids are known for its ability to modify diverse molecules via chlorination or bromination. These data suggest that PXDN can directly or indirectly oxidize numerous organic and inorganic substrates, such as aromatic amino acids, indole derivatives and a variety of other species [27].
Figure 2.
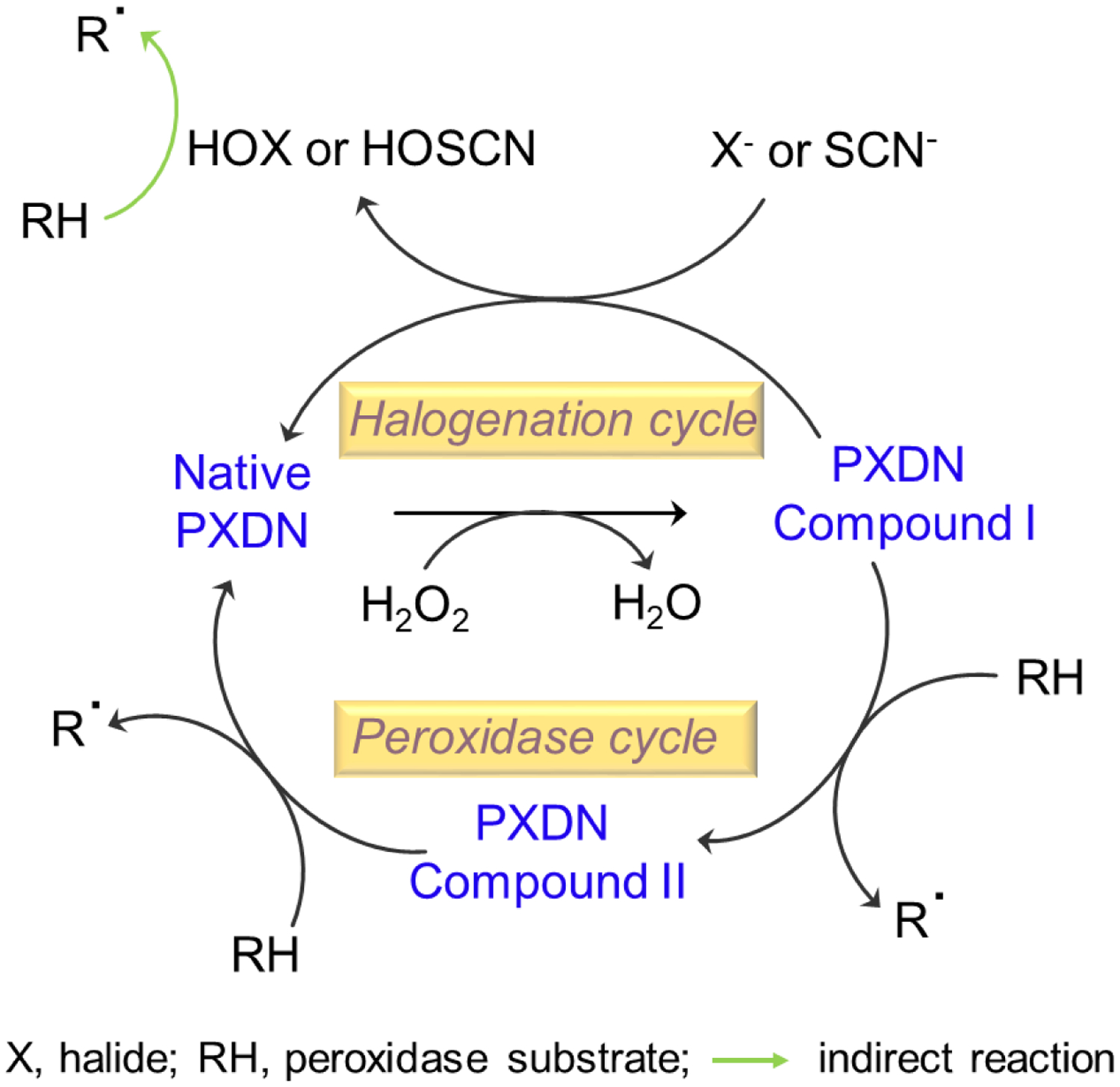
Two-electron redox reaction (halogenation cycle) and two one-electron redox reactions (peroxidase cycle) of PXDN.
1.5. Regulation of PXDN
Like other members in this family, incorporation of heme into the PXDN apoprotein is critical. Inadequate incorporation of heme will cause the lack of activity. Incubation of hematin increases PXDN activity, but has no effect on PXDN protein expression [6]. Sodium butyrate (NaBu) does not increase PXDN activity, but induces PXDN protein expression approximately five-folds in recombinant HEK293 cells [6]. PXDN activity increased significantly in the presence of both hematin and NaBu, which implies that NaBu induces significant PXDN apoprotein expression, but exogenous heme is needed to optimally reconstitute the active enzyme [6, 13].
PXDN is induced by a wide variety of activators, including proinflammatory stimulators and metabolic factors. Horikoshi et al reported that PXDN is highly expressed when human colon cancer EB1 cells undergo p53-dependent apoptosis upon induction of p53 by exposure to ZnCl2 [28]. They considered PXDN as p53 response gene. Treatment with two common proinflammatory stimuli, LPS and TNF-α, increases the constitutive secretion of PXDN in HUVECs as well as in C57BL/6 mice [20]. PXDN expression is up-regulated with the development of hypertension [29], which indicates a regulatory role of mechanical force on PXDN expression. Angiotensin II up-regulates PXDN mRNA and protein expression in a concentration- and time-dependent manner in VSMCs [30]. ox-LDL, an atherogenic factor, also induces PXDN expression in VECs [31] and in vivo [22]. Zalan et al find increased PXDN protein expression during transforming growth factor-1 (TGF-1)-induced myofibroblast differentiation [15]. Moreover, PXDN expression is up-regulated significantly both in a rat model of ischemia-reperfusion (IR) and H9c2 cell model of hypoxia/reoxygenation [32]. These data illustrate a potential role for PXDN in cardiovascular diseases, such as vascular remodeling in hypertension, atherosclerosis, pulmonary hypertension, abdominal aortic aneurysm, endothelial dysfunction, cardiac hypertrophy and cardiac fibrosis. Interestingly, folic acid downregulates PXDN expression by altering DNA methylation at PXDN promoter [33]. Exogenous H2O2, tert-butylhydroquinone or sulforaphane can induce PXDN expression in Hela and HEK293 cells through Nrf2 pathway [34]. Increased expression of PXDN is detected in a variety of cancers including melanoma [7, 35], ovarian cancer [36], prostate cancer [18], and oral squamous cell carcinoma [19]. Interestingly, Tauber et al. reported that the PXDN expression is strongly correlated with heme oxygenase-1 (HO-1) in fourteen common cancer types [37].
2. PXDN-dependent oxidation products
2.1. Generation of hypohalous acids
hPxs participate in host defense, thyroid hormone biosynthesis, and pathological conditions via the generation of hypohalous acids [3, 38–40]. It is widely accepted that the generation of hypohalous acids by hPxs occurs via a two-electron oxidation of halides (Fig. 2) [41, 42]. Surprisingly, PXDN, like MPO, can oxidize Cl−, Br−, or SCN− to generate hypochlorous acid (HOCl), hypobromous acid (HOBr) and hypothiocyanous acid (HOSCN), respectively, in the presence of H2O2 [43]. Under the concentrations of halides (100 μM KBr, 100 μM KSCN, and 100 mM NaCl) at pH 7.4, PXDN utilizes approximately ~45% of H2O2 for the generation of HOBr, ~35% for HOSCN, and ~18% for HOCl [43]. The enzymatic properties and substrate specificity of PXDN are similar as MPO. PXDN is second hPx to generate HOCl. However, the significantly lower catalytic rate constants of PXDN relative to MPO suggest other physiological roles for this novel heme-containing peroxidase [43]. The activity of PXDN in the generation of HOCl and HOSCN is greater with a combination of halides and the “pseudohalide” thiocyanate than either of these agents alone [43]. Therefore, one should not underestimate the synergistic effects of these substrates when considering the physiological and pathological roles of PXDN. Under certain pathological conditions, such as excessive salt intake, cigarette smoking, and Cl− accumulation in cells (such as cystic fibrosis [44]), increases in halides and thiocyanate may enhance oxidant generation and perpetuate tissue inflammation.
HOCl generation by PXDN is demonstrated by multiple approaches. By means of mass-spectrometry, chlorotyrosine and chlorotaurine are detected in the reaction of PXDN/H2O2/NaCl/tyrosine and PXDN/H2O2/NaCl/taurine, respectively [24]. In the control experiments, MPO generates chlorotaurine whereas LPO does not as expected. In addition, using a Free Radical Analyzer for real-time measurement of H2O2, H2O2 is decomposed in the reaction of PXDN/H2O2/NaCl, not in LPO/ H2O2/NaCl. Interestingly, adding Cl− to PXDN/H2O2/Br−, PXDN/H2O2/SCN−, or PXDN/H2O2/Br/SCN− increases the H2O2 decomposition [43]. The partially purified PXDN from plasma, like MPO in the same preparation, reveals bactericidal activities which are dependent on chloride and inhabitable by 4-Aminobenzoic acid hydrazide (ABAH) [24]. In multiple biochemical assays, PXDN-mediated HOCl generation is detected. These include 1) detection of Cl-taurine generation by monitoring the absorbance at 252 nm; 2) taurine chlorination-TMB oxidation assay; 3) PXDN and chloride dose-dependent experiments of HOCl generation; 4) inhibition of PXDN-mediated HOCl generation by inhibitory reagents such as AHAH, NaN3, catalase and methionine; 5) PXDN-mediated monochlorodimedone (MCD) chlorination assay; 6) PXDN-mediated 2-nitro-5-thiobenzoic acid (TNB) oxidation [24]. In these assays, MPO is the positive control while LPO is negative control of HOCl generation. However, contradictory data are reported. Obinger group reported that oxidation of chloride (140 mM) by PXDN at pH 7.4 is negligible since the redox potential is too low and PXDN is not able to form the electron-withdrawing sulfonium ion heme to protein linkage [13]. Additionally, the same group reported the kinetic data using stopped-flow study on a truncated PXDN containing only Ig domain and peroxidase region. The data unravel that the truncated PXDN can follow the halogenation cycle. But chloride cannot act as a two-electron donor of compound I, whereas thiocyanate, iodide, and bromide efficiently restore the ferric resting state [25]. One possible explanation for the contradictory data is that the reduction potential of PXDN was measured at pH 7.4 [25]. In the pH, negligible amount of HOCl may not be detectable by stopped-flow. Indeed, PXDN-mediated HOCl generation is pH-dependent [24]. At pH 7.4, PXDN only generates very small amount of HOCl, which is much less than that at pH 5.5. In addition, the stopped-flow assay only detected HOCl formation in milliseconds [25] while HOCl formation was measured either in real-time or in reactions for 30 minutes in mass spectrometry and biochemical assays [24]. Thus, the contradictory data warrant further stopped-flow study by lowering pH and/or increasing PXDN concentration.
2.2. Tyrosine crosslink and bromo-tyrosine.
The di-tyrosine bond is an oxidative covalent cross-link between two tyrosine molecules. Di-tyrosine cross-linking is increasingly identified as a marker of oxidative stress, aging and diseases, and it is detected in diverse pathological conditions [45]. hPxs catalyze the single-electron oxidation of tyrosine to form tyrosyl radicals, which can cross-link to form di-tyrosine [45]. The capacity of PXDN to promote di-tyrosine formation is compared with MPO and LPO. MPO and LPO are equally potent in catalyzing di-tyrosine formation from tyrosine ethyl ester whereas the specific activity of PXDN is ~12.5-fold lower [43]. PXDN most readily oxidizes tyrosine ethyl ester while less oxidizes D- and L-tyrosine. Interestingly, when reacting with the mixture of D- and L-tyrosine, PXDN only yield ~65% of di-tyrosine compared to reaction with D- or L-isomer of tyrosine alone. This suggests that tyrosine with the same polar side group (either D-isomer or L-isomer) facilitating di-tyrosine formation [20]. PXDN-mediated di-tyrosine formation is optimal under basic conditions, which is similar as other hPxs. PXDN produced approximately 1.5 folds di-tyrosine at pH 8.5 compared to pH 7.5, and this effect is dose-dependent at tyrosine concentrations from 50 to 250 μM [20]. Therefore, PXDN may catalyze di-tyrosine formation and consequently promote protein cross-linking under a variety of physiological and pathophysiological conditions. In addition to directly oxidizing tyrosine to form di-tyrosine, PXDX indirectly forms bromo-tyrosine through PXDN-generated HOBr [46]. It is reported that PXDN can brominate the bystander tyrosine (Tyr-1485 of COL4A2) of collagen IV [47]. Since PXDN can generate HOCl, it is predicted that PXDN may form chloro-tyrosine.
2.3. Lipid oxidation
Evidence shows that mammalian hPxs play an important role in the oxidation of lipids and proteins [48]. HOCl generated by MPO oxidizes LDL and high-density lipoprotein (HDL) [48, 49]. ApoE in VLDL and recombinant ApoE are readily oxidized by PXDN/H2O2/Cl−, including intra- and inter-molecular crosslinks and degradation [21]. PXDN-oxidized ApoE forms a weaker bond to lipid emulsion particles and has a reduced capability of lipid efflux from foam cells [21]. These data suggest that PXDN is a new regulator of lipid homeostasis, which implies a role for PXDN in the genesis and development of atherosclerosis. Recently, it was found that PXDN performs LDL oxidation in the presence of H2O2 and chloride [22, 50]. With higher concentration in plasma than MPO, PXDN is responsible for LDL oxidation in vivo and its expression is up-regulated by angiotensin II treatment in VSMCs and endothelial cells [29].
2.4. Sulfilimine formation
Collagen IV scaffold is essential for the structure of cells and tissues, and it is dysfunctional in several diseases [51]. Recent studies of bovine and Drosophila tissues reveal that the scaffold is stabilized by sulfilimine chemical bonds (S=N) that covalently cross-link methionine and hydroxylysine residues at the interface of adjoining triple helical protomers [52, 53]. PXDN, which is embedded in the basement membrane, produces hypohalous acid intermediates that oxidize methionine to form the sulfilimine cross-link [54]. The bond is a covalent cross-link between methionine-93 and lysine-211 or hydroxylysine-211 (Lys211/Hyl211) residues of non-collagenous (NC1) domains of collagen IV, and stabilizes the collagen NC1 domains by adjoining triple-helical protomers [54]. Bromide, not chloride, is considered as essential halide for the crosslink formation of NC1 domains [52]. PXDN-mediated sulfilimine cross-link in collagen IV is considered as the unique feature of PXDN. Growing reports and interests have been seen in recently years. The sulfilimine cross-link mediated by PXDN is important for extracellular matrix assembly and critical for cell survival and growth [55]. The result provides signaling cues for PXDN in the regulation of cell behavior and function in tissue genesis and homeostasis. Additionally, post-translational proteolytic processing of PXDN by proprotein-convertases facilitates PXDN integration into the extracellular matrix [56]. This process may be important for the PXDN-mediated collagen IV-crosslinking activity. Further study reveals that PXDN mediates bromine enrichment in basement membranes, implying that PXDN activity is largely restricted to basement membranes in mammalian tissues [47]. Interestingly, PXDN can also brominate the bystander tyrosine (Tyr-1485 of COL4A2) of collagen IV [47]. This is consistent with the report from Winterbourn group, that PXDN mediates bromination of tyrosine residues in the extracellular matrix [46]. Thus, bromotyrosine may be as a specific biomarker of PXDN-mediated bromination. As PXDN plays an important role in sulfilimine cross-links of collagen IV and bromine enrichment in basement membranes, it further demonstrates that Ig domains of PXDN are required to form sulfilimine cross-links [10]. These data suggest that Ig domains of PXDN promote PXDN colocalization with collagen IV. LRRs of PXDN can bind to laminin in basement membranes [11]. If PXDN mediates another extracellular matrix (ECM) protein such as laminin merits the further study. The growing interests have been seen in the PXDN-mediated sulfilimine cross-link in collagen IV and its role in the basement membrane integrity.
3. Physiological and pathological implications of PXDN
3.1. Host defense
PXDN plays dual function in host defense. Its N-terminus containing five leucine-rich repeats and four immunoglobulin domains allows it for interaction with LPS, a membrane component of gram-negative bacteria, while its C-terminal peroxidase domain mediates bactericidal activity of PXDN by generation of hypohalous acids [9]. Physiological concentration of PXDN completely kills E.coli and P. aeruginosa. This effect is dependent on H2O2 and Cl−, and is inhibited by peroxidase inhibitors as well as H2O2 scavenger catalases [24]. However, PXDN cannot kill Gram-positive bacteria [9]. Deficiency of PXDN results in a failure to eradicate P. aeruginosa and increased mortality in a murine model of lung infections [9]. PXDN is abundantly expressed in pulmonary epithelial cells and secreted into lung epithelial lining fluid [9]. The PXDN-expressing cells show bactericide to P. aeruginosa [9]. These observations indicate that PXDN plays a role in innate immunity. If PXDN compensates MPO-deficiency in host defense remains to be determined.
3.2. Atherosclerosis
Evidence accumulated during the last decade suggests a role for MPO in inflammation related to atherogenesis [39, 57]. Like MPO, PXDN might also contribute to the development of atherosclerosis based on expression profile and oxidization capacity of lipoproteins and other biomacromolecules. PXDN is located in atherosclerotic plaques in LDLR-deficient mice, and immunofluorescence assays show that it is close to ApoE [21]. PXDN-mediated oxidation of apoproteins and lipids in LDL is verified using a variety of approaches. Exposure of PXDN-oxidized LDL to human macrophages leads to an accumulation of ox-LDL and foam cell formation [21, 22]. PXDN is highly expressed in human and mouse atherosclerotic samples, but it did not co-localize with a marker of microphages, CD68. Administration of the inflammatory factor LPS or TNF-α via the mouse tail vein increases PXDN expression in the aorta and plasma secretions [22]. PXDN is able to bind to LDL, and PXDN-mediated oxidation of apoproteins and lipids in LDL causes accumulation of LDL in monocyte-like cells and promoted formation of foam cells [22]. TNF-α also promotes formation and retention of PXDN-oxidized LDL in aortic walls [22]. In addition, VSMCs exposing to ox-LDL increases PXDN expression and calcification, mediated by PXDN/HOCl/PI3K/AKT, ERK1/2, and P38 MAPK/Runx2 signaling pathways [50]. Interestingly, PXDN gene among other nine vascular homeostasis related genes is hypermethylated in atherosclerotic human aorta samples comparing with a donor-matched healthy [58], suggesting a role of PXDN in atherosclerosis. Another report verifies the result [59]. Thus, PXDN gene belongs to atherosclerosis-linked differentially methylated regions. The biological significance and molecular mechanism of elevated methylation of PXDN gene in atherosclerotic aorta are currently unclear. As PXDN locates in atherosclerotic plaque and mediates lipoprotein oxidation, it is assumed that methylation of PXDN gene in atherosclerosis is a protective mechanism to suppress PXDN expression. Collectively, these data support an important role of PXDN in the genesis and development of atherosclerosis.
3.3. Heart and vessel remodeling
Numerous studies indicate that NOX-dependent signaling is involved in the development of cardiomyocyte hypertrophy, interstitial fibrosis and post-myocardial infarction remodeling [60, 61]. PXDN can utilize H2O2 generated in cells expressing NOX1, NOX2, NOX3, NOX4, and NOX5 [6]. Several lines of evidence suggest that the NOX/PXDN pathway-mediated oxidative stress plays an important role in myocardial IR injury, endothelial cell apoptosis and/or smooth muscle cell proliferation [62]. Zhang group reported a novel pathway of NOX2/PXDN in myocardium in which PXDN coordinates with NOX2 and amplifies the role of NOX-derived ROS in oxidative injury following ischemia-reperfusion. In addition, they found that increased PXDN activity contributes to ischemia-reperfusion -induced cardiac dysfunction, and the inhibition of PXDN activity has potential clinical value in protecting the myocardium against ischemia-reperfusion injury [32].
PXDN also plays an important role in vascular smooth muscle cell proliferation via NOX-H2O2-PXDN-HOCl-ERK1/2 pathways, which may contribute to vascular remodeling in hypertension [63]. PXDN is significantly increased in human and mouse abdominal aortic aneurysm tissues. Moreover, PXDN is found to regulate VSMC phenotypic switch through the PXDN/ H2O2/HOCl/ERK1/2 signaling pathway and plays a key role in the development of abdominal aortic aneurysm [64]. NOX/PXDN pathway-mediated oxidative stress and the inflammatory reaction also play a role in pulmonary vascular remodeling and right ventricle hypertrophy in a hypoxia-induced rat model of pulmonary artery hypertension [65]. Additionally, PXDN promotes hypoxia-induced proliferation, apoptosis resistance, and migration in pulmonary artery smooth muscle cells (PASMCs) via the NOX4/PXDN/HOCl/NF-κB signaling pathway [66]. NOX/PXDN-dependent regulation of cardiac and vessel remodeling may lead to new therapeutic targets for related diseases. However, Sirokmany et al. reported that PXDN-mediated crosslinking of collagen IV is independent of Noxs [67].
An elevated expression of PXDNL is detected in failing myocardium in a recent study. The increased PXDNL levels in the failing heart may contribute to ECM dysregulation because of its antagonism of PXDN function [8]. This discovery opens exciting research on the role of PXDN and PXDNL in the highly adaptive nature of the ECM.
3.4. Endothelial dysfunction
Proper endothelial function is essential for the regulation of vascular tone, reduction in platelet aggregation, inhibition of endothelial inflammatory responses, and the control of intimal smooth muscle cell proliferation. Systemic endothelial dysfunction and local coronary endothelial dysfunction are associated with increased risk for cardiovascular disease [68]. PXDN expresses in the endothelial cells and secretes into blood. PXDN exhibits with much higher concentration in plasma than MPO [20]. Therefore, it is reasonable to speculate that PXDN also plays an important role in vascular tone under physiological and pathological conditions. Indeed, PXDN is found to contribute to endothelial dysfunction in hypertension [29]. PXDN expression in aorta is up-regulated, and the aortic relaxation to acetylcholine is deteriorated in Spontaneously Hypertensive Rats [29]. In addition, evidence indicates that PXDN plays a regulatory role in asymmetric dimethylarginine (ADMA) formation and metabolism in endothelial cells. ADMA is the endogenous inhibitor of nitric oxide synthases and contributes to endothelial dysfunction and cardiovascular diseases [69]. The intracellular level of ADMA is regulated by the activity of dimethylarginine dimethylaminohydrolase [70]. Dimethylarginine dimethylaminohydrolase-2 is the major enzyme responsible for ADMA metabolism in endothelial cells. PXDN/H2O2/HOCl system increases ADMA production through inhibiting dimethylarginine dimethylaminohydrolase-2 activity whereas treatment with siRNA of PXDN reduces ADMA production [71]. These data indicate that the inhibitory role of PXDN on dimethylarginine dimethylaminohydrolase-2 activity might occur through the generation of HOCl. Additionally, folic acid supplementation may prevent oxidative stress-induced apoptosis and suppresses reactive oxygen species (ROS) levels through downregulating PXDN, resulting in changes in DNA methylation, which may contribute to beneficial effects on endothelial function [33]. Endothelial cell apoptosis and programmed necrosis are very important in endothelial dysfunction during pathological conditions. PXDN-induced oxidative stress plays a role in ox-LDL induced endothelial cell apoptosis [31]. In addition, PXDN promotes endothelial programmed necrosis under hyperlipidemic conditions through activation of β-catenin signaling [72]. Recent study indicates that PXDN is associated with glycation end-products mediated diabetic vascular endothelial dysfunction in diabetes mellitus. Glycation end-products significantly increase the expression of PXDN and 3-Cl-tyrosine in HUVECs. In diabetic mouse model (db/db mice), knockdown of PXDN by silencing PXDN with siRNAs in vivo through tail vein injection restores the impaired endothelium-dependent relaxation function with accompanying with up-regulation of Ser1177 phosphorylation of eNOS and NO production, causing restoration of diabetic vascular endothelial function [73].
3.5. Fibrosis
ECM deposition and abnormal cross-linking of components, such as collagen, are critical to pathogenesis of fibrosis [74]. Peroxidases in plants and lower animal species frequently participate in ECM formation. In the presence of H2O2, peroxidases enzymatically cross-link extracellular proteins through tyrosine residues [75, 76]. ECM stabilization by di-tyrosine bridges is well-documented during sea urchin fertilization, where secreted ovoperoxidase is responsible for the formation of cross-links [77]. Di-tyrosine formation is also involved in the stabilization of C. elegans cuticle, where dual oxidases, carrying NADPH oxidase and peroxidase-like domains, provide H2O2 for the crosslinking reaction [78]. The domain organization of PXDN suggests that it is ideally suited for the stabilization of the ECM through protein-protein interactions. PXDN has a C- terminal vWF C-type domain. vWF C-type domain is found in a variety of plasma proteins, integrins, collagens, mucins and other extracellular proteins participating in oligomerization, protein complex formation, homeostasis and signal transduction [79, 80]. In addition, the presence of leucine-rich repeats and immunoglobulin domains in PXDN suggests that PXDN readily associates with other ECM proteins [5, 6]. It has been demonstrated that myofibroblasts secrete PXDN into the extracellular space where it organizes into a fibril-like network and co-localizes with fibronectin, which helps form the extracellular matrix [15]. PXDN expression is increased in a murine model of kidney fibrosis, in that PXDN localizes in the peritubular space in fibrotic kidneys [15]. In human ischemic cardiomyopathy and murine model of cardiac fibrosis after myocardial infarction, PXDN expression is significantly increased and plays a role in regulating cardiac fibroblasts differentiation, collagen I synthesis, and proliferation through HOCl/Smad2/3 and /ERK1/2 signaling pathways [81]. During TGF-β1-induced epithelial-mesenchymal transition, PXDN expression decreases by up to 47% in two cervical-carcinoma cell lines, with concomitant increases in Snai1 and vimentin, and decrease in E-cadherin. TGF-β1 induces Snai1 binding to the PXDN promoter and significantly represses luciferase reporter gene expression [82]. Zalan Peterfi et al reported that PXDNL plays a role in heart fibrosis [8]. They found that PXDN is produced by cardiomyocytes and localizes at cell-cell junctions, and PXDNL forms a complex with PXDN and antagonizes its peroxidase activity. PXDNL expression increases in the failing myocardium, in which fibrosis is a major characteristic. As described above, PXDN plays a unique role in formation of sulfilimine bonds in the NC1 domain of collagen IV [52, 53]. If PXDN mutation causes defects of collagen IV and fibrosis needs further study. These data indicate that PXDN represents a previously unknown pathway in extracellular matrix formation with a potentially important role in fibrogenesis. The discovery of this novel matrix-associated protein with its multifunctional domains opens an exciting avenue for fibrosis research.
4. Beyond of oxidation - Gene mutation
In 2011, Khan et al first reported homozygous mutations of PXDN in three consanguineous families with congenital cataract-microcornea with mild-to-moderate corneal opacity and a consanguineous Cambodian family with developmental glaucoma and severe corneal opacification [16]. Thereafter, Choi A et al reported that four new mutations of PXDN in two families [83]. The PXDN mouse mutation induced by N-ethyl-N-nitrosourea leads to a recessive phenotype [17]. This mutation causes severe anterior segment dysgenesis and microphthalmia that resemble the manifestations in patients with PXDN mutation. A microarray study using embryonic samples of PXDN-deficient mice identified 121 (75 upregulated and 46 downregulated) differentially expressed genes between PXDN mutation and normal tissues [84]. Though the functional enrichment analysis and protein-protein interaction network analysis, the crucial genes including Cdkn1b, Acta1 and Tnnt3 were screened. The study provides a comprehensive elucidation of the regulatory mechanisms in PXDN mutation-induced eye disorder and the novel biomarkers which may be used for the prognosis and prevention of the disorder [84]. Moreover, PXDN mutants exhibited an early onset glaucoma and progressive retinal dysgenesis. Transcriptome profiling revealed that PXDN affected the transcription of developmental and eye disease-related genes during early eye development [17]. Defective PXDN impairs sulfilimine bond formation in collagen IV, which is a constituent of the basement membrane. This effect suggests that the eye defects result in the loss of basement membrane integrity in the developing eye [16]. These findings support that PXDN is necessary for cell proliferation, differentiation and basement membrane consolidation during eye development. The PXDN gene mutation is involved in eye-related disease and development, but the consequences of the mutations on heart, blood vessels and other cells/tissues remain to be elucidated. Thus, further works are needed to determine the possible role of PXDN deficiency in these tissues and organisms, as well as the mechanism by which the mutations cause diverse phenotypic effects.
5. Conclusions
This manuscript reviews the emerging mammalian PXDN from gene identification, characterization of biochemical and enzymatic properties, gene expression and regulation, gene mutation, and physiological and pathological functions. PXDN-generated reactive oxidants are important components for host defense, collagen IV synthesis in basement membrane development and tissue genesis, signaling pathways and homeostasis under physiological conditions. In diseases characterized by increased oxidant stress, PXDN as a new mediator is involved in the impairment of endothelial functions, pathogenesis of vascular diseases such as atherosclerosis, and vascular remodeling, and tissue fibrosis. In next few years, the studies should fill the large gaps in several aspects. First, what is the precisely regulatory mechanism of PXDN expression? Second, PXDN mutation is the cause of severe deficits in eye development. So far, other symptoms and signs except lung host defense are not well determined. If under challenging, will additional symptoms, signs and/or molecular changes be observed and discovered? Third, providing the importance of sulfilimine cross-links in collagen IV and in the structural integrity of basement membranes, what is the specific function of PXDN-mediated formation of sulfilimine bond in collagen IV? Particularly, what is the functional impact of PXDN-mutation mediated lack of NC1 hexamers in collagen IV? Fourth, PXDN catalyzes the decomposition of H2O2. How do the mutation and aberrant expression of PXDN modulate the homeostasis of H2O2? And what is the consequence? Finally, PXDN has a unique and longer N-terminus with five LRRs and four Ig domains. What are the roles of the individual LRR and Ig domain? Given our increasing knowledge of PXDN in physiology and pathology, studies on the roles of PXDN are entering a new and exciting era.
Highlights.
PXDN is the newly-identified heme-containing peroxidase. It is extensively reviewed in the manuscript.
The review manuscript includes gene identification, characterization of biochemical and enzymatic properties, gene expression and regulation, gene mutation, and physiological and pathological functions pf PXDN.
The updated references are provided.
The future studies on PXDN field are prospected.
Acknowledgments
This work was supported by the National Heart, Blood, and Lung Institute, and National Institute of Allergy and Infectious Disease, the National Institutes of Health under Award Numbers R01HL086836, R21AI101642, R01AI141724 and R21AI139900, and American Heart Association grant 12GRNT12040409 (to G. Cheng). R. Shi was supported by grants from the National Program on Key Basic Research Project of China (No. 2019YFF0216304), National Nature Science Foundation of China (No. 82170292) and Outstanding Youth Foundation Project of Hunan Nature Science Foundation (No. 2019JJ20036).
Abbreviations:
- H2O2
hydrogen peroxide
- hPxs
heme-containing peroxidases
- VPO1
vascular peroxidase-1
- PXDN
peroxidasin
- VPO2
Vascular peroxidase-2
- PXDNL
peroxidasin-like
- MPO
myeloperoxidase
- EPO
eosinophil peroxidase
- LPO
lactoperoxidse
- TPO
thyroid peroxidase
- HOCl
hypochlorous acid
- HOBr
hypobromous acid
- HOSCN
hypothiocyanous acid
- Noxs
NADPH oxidases
- NC1
C-terminal non-collagenous domain
- ADMA
asymmetric dimethylarginine
- eNOS
endothelial nitric oxide synthase
- ECM
extracellular matrix
- ROS
reactive oxygen species
- DDAH2
dimethylarginine dimethylaminohydrolase-2
- LPS
lipopolysaccharide
- TNF-α
tumor necrosis factor-α
- ox-LDL
oxidized low-density lipoprotein
- HDL
high-density lipoprotein
- NCBI
the National Center for Biotechnology Information
- EST
expression sequence tag
- VECs
vascular endothelial cells
- VSMCs
vascular smooth muscle cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Klebanoff SJ Myeloperoxidase: friend and foe. J Leukoc Biol 77:598–625; 2005. [DOI] [PubMed] [Google Scholar]
- [2].Weiss SJ; Test ST; Eckmann CM; Roos D; Regiani S Brominating oxidants generated by human eosinophils. Science 234:200–203; 1986. [DOI] [PubMed] [Google Scholar]
- [3].Surks MI; Schwartz HL; Oppenheimer JH Tissue iodoprotein formation during the peripheral metabolism of the thyroid hormones. J Clin Invest 48:2168–2175; 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wolfson LM; Sumner SS Antibacterial Activity of the Lactoperoxidase System: A Review. J Food Prot 56:887–892; 1993. [DOI] [PubMed] [Google Scholar]
- [5].Nelson RE; Fessler LI; Takagi Y; Blumberg B; Keene DR; Olson PF; Parker CG; Fessler JH Peroxidasin: a novel enzyme-matrix protein of Drosophila development. EMBO J 13:3438–3447; 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cheng G; Salerno JC; Cao Z; Pagano PJ; Lambeth JD Identification and characterization of VPO1, a new animal heme-containing peroxidase. Free Radic Biol Med 45:1682–1694; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mitchell MS; Kan-Mitchell J; Minev B; Edman C; Deans RJ A novel melanoma gene (MG50) encoding the interleukin 1 receptor antagonist and six epitopes recognized by human cytolytic T lymphocytes. Cancer Res 60:6448–6456; 2000. [PubMed] [Google Scholar]
- [8].Peterfi Z; Toth ZE; Kovacs HA; Lazar E; Sum A; Donko A; Sirokmany G; Shah AM; Geiszt M Peroxidasin-like protein: a novel peroxidase homologue in the human heart. Cardiovasc Res 101:393–399; 2014. [DOI] [PubMed] [Google Scholar]
- [9].Shi R; Cao Z; Li H; Graw J; Zhang G; Thannickal VJ; Cheng G Peroxidasin contributes to lung host defense by direct binding and killing of gram-negative bacteria. PLoS Pathog 14:e1007026; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ero-Tolliver IA; Hudson BG; Bhave G The Ancient Immunoglobulin Domains of Peroxidasin Are Required to Form Sulfilimine Cross-links in Collagen IV. J Biol Chem 290:21741–21748; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sevcnikar B; Schaffner I; Chuang CY; Gamon L; Paumann-Page M; Hofbauer S; Davies MJ; Furtmuller PG; Obinger C The leucine-rich repeat domain of human peroxidasin 1 promotes binding to laminin in basement membranes. Arch Biochem Biophys 689:108443; 2020. [DOI] [PubMed] [Google Scholar]
- [12].Morrison M; Bayse GS Catalysis of iodination by lactoperoxidase. Biochemistry 9:2995–3000; 1970. [DOI] [PubMed] [Google Scholar]
- [13].Soudi M; Paumann-Page M; Delporte C; Pirker KF; Bellei M; Edenhofer E; Stadlmayr G; Battistuzzi G; Boudjeltia KZ; Furtmuller PG; Van Antwerpen P; Obinger C Multidomain human peroxidasin 1 is a highly glycosylated and stable homotrimeric high spin ferric peroxidase. J Biol Chem 290:10876–10890; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Paumann-Page M; Tscheliessnig R; Sevcnikar B; Katz RS; Schwartz I; Hofbauer S; Pfanzagl V; Furtmuller PG; Obinger C Monomeric and homotrimeric solution structures of truncated human peroxidasin 1 variants. Biochim Biophys Acta Proteins Proteom 1868:140249; 2020. [DOI] [PubMed] [Google Scholar]
- [15].Peterfi Z; Donko A; Orient A; Sum A; Prokai A; Molnar B; Vereb Z; Rajnavolgyi E; Kovacs KJ; Muller V; Szabo AJ; Geiszt M Peroxidasin is secreted and incorporated into the extracellular matrix of myofibroblasts and fibrotic kidney. Am J Pathol 175:725–735; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Khan K; Rudkin A; Parry DA; Burdon KP; McKibbin M; Logan CV; Abdelhamed ZI; Muecke JS; Fernandez-Fuentes N; Laurie KJ; Shires M; Fogarty R; Carr IM; Poulter JA; Morgan JE; Mohamed MD; Jafri H; Raashid Y; Meng N; Piseth H; Toomes C; Casson RJ; Taylor GR; Hammerton M; Sheridan E; Johnson CA; Inglehearn CF; Craig JE; Ali M Homozygous mutations in PXDN cause congenital cataract, corneal opacity, and developmental glaucoma. American journal of human genetics 89:464–473; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yan X; Sabrautzki S; Horsch M; Fuchs H; Gailus-Durner V; Beckers J; Hrabe de Angelis M; Graw J Peroxidasin is essential for eye development in the mouse. Human molecular genetics 23:5597–5614; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dougan J; Hawsawi O; Burton LJ; Edwards G; Jones K; Zou J; Nagappan P; Wang G; Zhang Q; Danaher A; Bowen N; Hinton C; Odero-Marah VA Proteomics-Metabolomics Combined Approach Identifies Peroxidasin as a Protector against Metabolic and Oxidative Stress in Prostate Cancer. Int J Mol Sci 20; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kurihara-Shimomura M; Sasahira T; Shimomura H; Kirita T Peroxidan Plays a Tumor-Promoting Role in Oral Squamous Cell Carcinoma. Int J Mol Sci 21; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cheng G; Li H; Cao Z; Qiu X; McCormick S; Thannickal VJ; Nauseef WM Vascular peroxidase-1 is rapidly secreted, circulates in plasma, and supports dityrosine cross-linking reactions. Free Radic Biol Med 51:1445–1453; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yang Y; Cao Z; Tian L; Garvey W; Cheng G VPO1 Mediates ApoE Oxidation and Impairs the Clearance of Plasma Lipids. PLoS ONE 8:e57571; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yang Y; Shi R; Cao Z; Zhang G; Cheng G VPO1 mediates oxidation of LDL and formation of foam cells. Oncotarget; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kadioglu O; Saeed MEM; Mahmoud N; Azawi S; Mrasek K; Liehr T; Efferth T Identification of novel drug resistance mechanisms by genomic and transcriptomic profiling of glioblastoma cells with mutation-activated EGFR. Life Sci:119601; 2021. [DOI] [PubMed] [Google Scholar]
- [24].Li H; Cao Z; Moore DR; Jackson PL; Barnes S; Lambeth JD; Thannickal VJ; Cheng G Microbicidal activity of vascular peroxidase 1 in human plasma via generation of hypochlorous acid. Infect Immun 80:2528–2537; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Paumann-Page M; Katz RS; Bellei M; Schwartz I; Edenhofer E; Sevcnikar B; Soudi M; Hofbauer S; Battistuzzi G; Furtmuller PG; Obinger C Pre-steady-state Kinetics Reveal the Substrate Specificity and Mechanism of Halide Oxidation of Truncated Human Peroxidasin 1. J Biol Chem 292:4583–4592; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sevcnikar B; Paumann-Page M; Hofbauer S; Pfanzagl V; Furtmuller PG; Obinger C Reaction of human peroxidasin 1 compound I and compound II with one-electron donors. Arch Biochem Biophys 681:108267; 2020. [DOI] [PubMed] [Google Scholar]
- [27].Panasenko OM; Gorudko IV; Sokolov AV Hypochlorous acid as a precursor of free radicals in living systems. Biochemistry (Mosc) 78:1466–1489; 2013. [DOI] [PubMed] [Google Scholar]
- [28].Horikoshi N; Cong J; Kley N; Shenk T Isolation of Differentially Expressed cDNAs from p53-Dependent Apoptotic Cells: Activation of the Human Homologue of the Drosophila Peroxidasin Gene. Biochemical and Biophysical Research Communications 261:864–869; 1999. [DOI] [PubMed] [Google Scholar]
- [29].Yang L; Bai Y; Li N; Hu C; Peng J; Cheng G; Zhang G; Shi R Vascular VPO1 expression is related to the endothelial dysfunction in spontaneously hypertensive rats. Biochem Biophys Res Commun 439:511–516; 2013. [DOI] [PubMed] [Google Scholar]
- [30].Shi R; Hu C; Yuan Q; Yang T; Peng J; Li Y; Bai Y; Cao Z; Cheng G; Zhang G Involvement of vascular peroxidase 1 in angiotensin II-induced vascular smooth muscle cell proliferation. Cardiovasc Res 91:27–36; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bai YP; Hu CP; Yuan Q; Peng J; Shi RZ; Yang TL; Cao ZH; Li YJ; Cheng G; Zhang GG Role of VPO1, a newly identified heme-containing peroxidase, in ox-LDL induced endothelial cell apoptosis. Free Radic Biol Med 51:1492–1500; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhang YS; He L; Liu B; Li NS; Luo XJ; Hu CP; Ma QL; Zhang GG; Li YJ; Peng J A novel pathway of NADPH oxidase/vascular peroxidase 1 in mediating oxidative injury following ischemia-reperfusion. Basic Res Cardiol 107:266; 2012. [DOI] [PubMed] [Google Scholar]
- [33].Cui S; Lv X; Li W; Li Z; Liu H; Gao Y; Huang G Folic acid modulates VPO1 DNA methylation levels and alleviates oxidative stress-induced apoptosis in vivo and in vitro. Redox Biol 19:81–91; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hanmer KL; Mavri-Damelin D Peroxidasin is a novel target of the redox-sensitive transcription factor Nrf2. Gene 674:104–114; 2018. [DOI] [PubMed] [Google Scholar]
- [35].Paumann-Page M; Kienzl NF; Motwani J; Bathish B; Paton LN; Magon NJ; Sevcnikar B; Furtmuller PG; Traxlmayr MW; Obinger C; Eccles MR; Winterbourn CC Peroxidasin protein expression and enzymatic activity in metastatic melanoma cell lines are associated with invasive potential. Redox Biol 46:102090; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zheng YZ; Liang L High expression of PXDN is associated with poor prognosis and promotes proliferation, invasion as well as migration in ovarian cancer. Ann Diagn Pathol 34:161–165; 2018. [DOI] [PubMed] [Google Scholar]
- [37].Tauber S; Jais A; Jeitler M; Haider S; Husa J; Lindroos J; Knofler M; Mayerhofer M; Pehamberger H; Wagner O; Bilban M Transcriptome analysis of human cancer reveals a functional role of heme oxygenase-1 in tumor cell adhesion. Mol Cancer 9:200; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Nauseef WM Myeloperoxidase in human neutrophil host defence. Cell Microbiol 16:1146–1155; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Heinecke JW Mechanisms of oxidative damage by myeloperoxidase in atherosclerosis and other inflammatory disorders. J Lab Clin Med 133:321–325; 1999. [DOI] [PubMed] [Google Scholar]
- [40].Nicholls SJ; Hazen SL Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol 25:1102–1111; 2005. [DOI] [PubMed] [Google Scholar]
- [41].Furtmuller PG; Burner U; Obinger C Reaction of myeloperoxidase compound I with chloride, bromide, iodide, and thiocyanate. Biochemistry 37:17923–17930; 1998. [DOI] [PubMed] [Google Scholar]
- [42].Battistuzzi G; Bellei M; Bortolotti CA; Sola M Redox properties of heme peroxidases. Arch Biochem Biophys 500:21–36; 2010. [DOI] [PubMed] [Google Scholar]
- [43].Li H; Cao Z; Zhang G; Thannickal VJ; Cheng G Vascular peroxidase 1 catalyzes the formation of hypohalous acids: characterization of its substrate specificity and enzymatic properties. Free Radic Biol Med 53:1954–1959; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Grandjean Lapierre S; Phelippeau M; Hakimi C; Didier Q; Reynaud-Gaubert M; Dubus JC; Drancourt M Cystic fibrosis respiratory tract salt concentration: An Exploratory Cohort Study. Medicine (Baltimore) 96:e8423; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].DiMarco T; Giulivi C Current analytical methods for the detection of dityrosine, a biomarker of oxidative stress, in biological samples. Mass Spectrom Rev 26:108–120; 2007. [DOI] [PubMed] [Google Scholar]
- [46].Bathish B; Paumann-Page M; Paton LN; Kettle AJ; Winterbourn CC Peroxidasin mediates bromination of tyrosine residues in the extracellular matrix. J Biol Chem 295:12697–12705; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].He C; Song W; Weston TA; Tran C; Kurtz I; Zuckerman JE; Guagliardo P; Miner JH; Ivanov SV; Bougoure J; Hudson BG; Colon S; Voziyan PA; Bhave G; Fong LG; Young SG; Jiang H Peroxidasin-mediated bromine enrichment of basement membranes. Proc Natl Acad Sci U S A 117:15827–15836; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Boudjeltia KZ; Delporte C; Van Antwerpen P; Franck T; Serteyn D; Moguilevsky N; Raes M; Vanhamme L; Vanhaeverbeek M; Van Meerhaeghe A; Roumeguere T Myeloperoxidase-dependent LDL modifications in bloodstream are mainly predicted by angiotensin II, adiponectin, and myeloperoxidase activity: a cross-sectional study in men. Mediators Inflamm 2013:750742; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bergt C; Pennathur S; Fu X; Byun J; O’Brien K; McDonald TO; Singh P; Anantharamaiah GM; Chait A; Brunzell J; Geary RL; Oram JF; Heinecke JW The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc Natl Acad Sci U S A 101:13032–13037; 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Tang Y; Xu Q; Peng H; Liu Z; Yang T; Yu Z; Cheng G; Li X; Zhang G; Shi R The role of vascular peroxidase 1 in ox-LDL-induced vascular smooth muscle cell calcification. Atherosclerosis 243:357–363; 2015. [DOI] [PubMed] [Google Scholar]
- [51].Khoshnoodi J; Pedchenko V; Hudson BG Mammalian collagen IV. Microsc Res Tech 71:357–370; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].McCall AS; Cummings CF; Bhave G; Vanacore R; Page-McCaw A; Hudson BG Bromine is an essential trace element for assembly of collagen IV scaffolds in tissue development and architecture. Cell 157:1380–1392; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Fidler AL; Vanacore RM; Chetyrkin SV; Pedchenko VK; Bhave G; Yin VP; Stothers CL; Rose KL; McDonald WH; Clark TA; Borza DB; Steele RE; Ivy MT; Aspirnauts; Hudson JK; Hudson BG A unique covalent bond in basement membrane is a primordial innovation for tissue evolution. Proc Natl Acad Sci U S A 111:331–336; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bhave G; Cummings CF; Vanacore RM; Kumagai-Cresse C; Ero-Tolliver IA; Rafi M; Kang JS; Pedchenko V; Fessler LI; Fessler JH; Hudson BG Peroxidasin forms sulfilimine chemical bonds using hypohalous acids in tissue genesis. Nat Chem Biol 8:784–790; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lee SW; Kim HK; Naidansuren P; Ham KA; Choi HS; Ahn HY; Kim M; Kang DH; Kang SW; Joe YA Peroxidasin is essential for endothelial cell survival and growth signaling by sulfilimine crosslink-dependent matrix assembly. FASEB J 34:10228–10241; 2020. [DOI] [PubMed] [Google Scholar]
- [56].Kovacs HA; Lazar E; Varady G; Sirokmany G; Geiszt M Characterization of the Proprotein Convertase-Mediated Processing of Peroxidasin and Peroxidasin-like Protein. Antioxidants (Basel) 10; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lau D; Baldus S Myeloperoxidase and its contributory role in inflammatory vascular disease. Pharmacol Ther 111:16–26; 2006. [DOI] [PubMed] [Google Scholar]
- [58].Zaina S; Heyn H; Carmona FJ; Varol N; Sayols S; Condom E; Ramirez-Ruz J; Gomez A; Goncalves I; Moran S; Esteller M DNA methylation map of human atherosclerosis. Circ Cardiovasc Genet 7:692–700; 2014. [DOI] [PubMed] [Google Scholar]
- [59].Lacey M; Baribault C; Ehrlich KC; Ehrlich M Atherosclerosis-associated differentially methylated regions can reflect the disease phenotype and are often at enhancers. Atherosclerosis 280:183–191; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Bedard K; Krause KH The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87:245–313; 2007. [DOI] [PubMed] [Google Scholar]
- [61].Nabeebaccus A; Zhang M; Shah AM NADPH oxidases and cardiac remodelling. Heart Fail Rev 16:5–12; 2011. [DOI] [PubMed] [Google Scholar]
- [62].Ma QL; Zhang GG; Peng J Vascular peroxidase 1: a novel enzyme in promoting oxidative stress in cardiovascular system. Trends Cardiovasc Med 23:179–183; 2013. [DOI] [PubMed] [Google Scholar]
- [63].Yang W; Liu Z; Xu Q; Peng H; Chen L; Huang X; Yang T; Yu Z; Cheng G; Zhang G; Shi R Involvement of vascular peroxidase 1 in angiotensin II-induced hypertrophy of H9c2 cells. Journal of the American Society of Hypertension: JASH 11:519–529 e511; 2017. [DOI] [PubMed] [Google Scholar]
- [64].Peng H; Zhang K; Liu Z; Xu Q; You B; Li C; Cao J; Zhou H; Li X; Chen J; Cheng G; Shi R; Zhang G VPO1 Modulates Vascular Smooth Muscle Cell Phenotypic Switch by Activating Extracellular Signal-regulated Kinase 1/2 (ERK 1/2) in Abdominal Aortic Aneurysms. J Am Heart Assoc 7:e010069; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Liu B; Luo XJ; Yang ZB; Zhang JJ; Li TB; Zhang XJ; Ma QL; Zhang GG; Hu CP; Peng J Inhibition of NOX/VPO1 pathway and inflammatory reaction by trimethoxystilbene in prevention of cardiovascular remodeling in hypoxia-induced pulmonary hypertensive rats. J Cardiovasc Pharmacol 63:567–576; 2014. [DOI] [PubMed] [Google Scholar]
- [66].You B; Liu Y; Chen J; Huang X; Peng H; Liu Z; Tang Y; Zhang K; Xu Q; Li X; Cheng G; Shi R; Zhang G Vascular peroxidase 1 mediates hypoxia-induced pulmonary artery smooth muscle cell proliferation, apoptosis resistance and migration. Cardiovasc Res 114:188–199; 2018. [DOI] [PubMed] [Google Scholar]
- [67].Sirokmany G; Kovacs HA; Lazar E; Konya K; Donko A; Enyedi B; Grasberger H; Geiszt M Peroxidasin-mediated crosslinking of collagen IV is independent of NADPH oxidases. Redox Biol 16:314–321; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Targonski PV; Bonetti PO; Pumper GM; Higano ST; Holmes DR Jr.; Lerman A Coronary endothelial dysfunction is associated with an increased risk of cerebrovascular events. Circulation 107:2805–2809; 2003. [DOI] [PubMed] [Google Scholar]
- [69].Bouras G; Deftereos S; Tousoulis D; Giannopoulos G; Chatzis G; Tsounis D; Cleman MW; Stefanadis C Asymmetric Dimethylarginine (ADMA): a promising biomarker for cardiovascular disease? Curr Top Med Chem 13:180–200; 2013. [DOI] [PubMed] [Google Scholar]
- [70].Vallance P; Leiper J Cardiovascular biology of the asymmetric dimethylarginine:dimethylarginine dimethylaminohydrolase pathway. Arterioscler Thromb Vasc Biol 24:1023–1030; 2004. [DOI] [PubMed] [Google Scholar]
- [71].Peng H; Chen L; Huang X; Yang T; Yu Z; Cheng G; Zhang G; Shi R Vascular peroxidase 1 up regulation by angiotensin II attenuates nitric oxide production through increasing asymmetrical dimethylarginine in HUVECs. Journal of the American Society of Hypertension: JASH 10:741–751 e743; 2016. [DOI] [PubMed] [Google Scholar]
- [72].Zhang YZ; Wang L; Zhang JJ; Xiong XM; Zhang D; Tang XM; Luo XJ; Ma QL; Peng J Vascular peroxide 1 promotes ox-LDL-induced programmed necrosis in endothelial cells through a mechanism involving beta-catenin signaling. Atherosclerosis 274:128–138; 2018. [DOI] [PubMed] [Google Scholar]
- [73].Jing C; Zhang G; Liu Z; Xu Q; Li C; Cheng G; Shi R Peroxidasin promotes diabetic vascular endothelial dysfunction induced by advanced glycation end products via NOX2/HOCl/Akt/eNOS pathway. Redox Biol 45:102031; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Bonnans C; Chou J; Werb Z Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 15:786–801; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Heinecke JW; Li W; Daehnke HL 3rd; Goldstein JA Dityrosine, a specific marker of oxidation, is synthesized by the myeloperoxidase-hydrogen peroxide system of human neutrophils and macrophages. J Biol Chem 268:4069–4077; 1993. [PubMed] [Google Scholar]
- [76].Marquez LA; Dunford HB Kinetics of oxidation of tyrosine and dityrosine by myeloperoxidase compounds I and II. Implications for lipoprotein peroxidation studies. J Biol Chem 270:30434–30440; 1995. [DOI] [PubMed] [Google Scholar]
- [77].LaFleur GJ Jr.; Horiuchi Y; Wessel GM Sea urchin ovoperoxidase: oocyte-specific member of a heme-dependent peroxidase superfamily that functions in the block to polyspermy. Mech Dev 70:77–89; 1998. [DOI] [PubMed] [Google Scholar]
- [78].Edens WA; Sharling L; Cheng G; Shapira R; Kinkade JM; Lee T; Edens HA; Tang X; Sullards C; Flaherty DB; Benian GM; Lambeth JD Tyrosine cross-linking of extracellular matrix is catalyzed by Duox, a multidomain oxidase/peroxidase with homology to the phagocyte oxidase subunit gp91phox. J Cell Biol 154:879–891; 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Bork P Shuffled domains in extracellular proteins. FEBS Lett 286:47–54; 1991. [DOI] [PubMed] [Google Scholar]
- [80].Zhang JL; Huang Y; Qiu LY; Nickel J; Sebald W von Willebrand factor type C domain-containing proteins regulate bone morphogenetic protein signaling through different recognition mechanisms. J Biol Chem 282:20002–20014; 2007. [DOI] [PubMed] [Google Scholar]
- [81].Liu Z; Xu Q; Yang Q; Cao J; Wu C; Peng H; Zhang X; Chen J; Cheng G; Wu Y; Shi R; Zhang G Vascular peroxidase 1 is a novel regulator of cardiac fibrosis after myocardial infarction. Redox Biol 22:101151; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Sitole BN; Mavri-Damelin D Peroxidasin is regulated by the epithelial-mesenchymal transition master transcription factor Snai1. Gene 646:195–202; 2018. [DOI] [PubMed] [Google Scholar]
- [83].Choi A; Lao R; Ling-Fung Tang P; Wan E; Mayer W; Bardakjian T; Shaw GM; Kwok PY; Schneider A; Slavotinek A Novel mutations in PXDN cause microphthalmia and anterior segment dysgenesis. Eur J Hum Genet 23:337–341; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Yang Y; Xing Y; Liang C; Hu L; Xu F; Mei Q An examination of the regulatory mechanism of Pxdn mutation-induced eye disorders using microarray analysis. Int J Mol Med 37:1449–1456; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]


