Abstract
Despite treatment advancements and improved survival, approximately 1800 children in the United Stateswill die of cancer annually. Survival may depend on nonclinical factors, such as economic stability, neighborhood and built environment, health and health care, social and community context, and education, otherwise known as social determinants of health (SDoH). Extant literature reviews have linked socioeconomic status (SES) and race to disparate outcomes; however, these are not inclusive of all SDoH. Thus, we conducted a systematic review on associations between SDoH and survival in pediatric cancer patients. Of the 854 identified studies, 25 were included in this review. In addition to SES, poverty and insurance coverage were associated with survival. More studies that include other SDoH, such as social and community factors, utilize prospective designs, and conduct analyses with more precise SDoH measures are needed.
Keywords: adolescents, cancer health disparities, childhood cancer, pediatrics, social determinants of health, survival
1 |. INTRODUCTION
In the United States, approximately 16,000 cases of cancer are diagnosed in individuals ages 0–19 years, and an estimated 1800 children and adolescents will die of cancer each year.1,2 Malignant neoplasms are the third leading cause of deaths among children and adolescents after motor vehicle crashes and firearm injuries, accounting for 9% of all deaths in 2016.3 The most common cancer diagnoses for this population are leukemias, central nervous system (CNS) tumors, and lymphomas.2 Due to rapid advancements in diagnosis and treatment, 84% of pediatric cancer patients will survive 5 years or longer; however, survival may depend on nonclinical factors, such as social determinants of health (SDoH).4
Healthy People 2030 defines SDoH as “conditions in the environment in which people are born, live, learn, work, play, worship, and age that affect a wide range of health, functioning, and quality-of-life outcomes and risks.”5 SDoH can be categorized into five main domains: (a) economic stability, (b) educational access and quality, (c) healthcare access and quality, (d) neighborhood and built environment, and (e) social and community context. Within each domain are measurable underlying factors. Economic stability encompasses stable housing, food security, stable employment, and poverty. The neighborhood and built environment domain takes into account access to healthy foods, crime and violence, environment conditions, and housing quality. Health and healthcare consider whether individuals have access to healthcare and primary care and health literacy. Social and community contexts examine civic participation, discrimination, social cohesion, and incarceration. Education includes early childhood education, high school graduation, literacy, and higher education enrollment.
The relationship between SDoH and pediatric cancer outcomes and impact on families has been explored by numerous researchers. Treatment and care for pediatric cancer patients is resource intensive and can strain families physically, emotionally, and financially. SDoH, such as extent of economic stability or instability can vary across time. Bilodeau et al.’s study provided evidence of the dynamism of SDoH. In their cohort of 99 pediatric cancer families, 15% reported household material hardship (HMH) initially, but HMH increased to 33% after 6 months of chemotherapy.6 Similarly, another study by Bona et al. found that over the course of treatment, the proportion of families unable to meet basic needs increases and families of children undergoing chemotherapy could lose over 40% of their household income.7 Lack of social support (social and community context) and adverse economic situations, as demonstrated by Santacroce’s and Kneipp’s survey, are associated with severe distress and stress-related symptoms due to pediatric cancer treatment-induced financial burden.8 In addition to inducing financial and material hardship, nonclinical factors can also contribute to medication or treatment adherence among pediatric cancer patients. Hoppmann et al. have tested and validated risk prediction models for mercaptopurine nonadherence that includes race/ethnicity, annual household income, maternal and paternal education, and whether mothers serve as full-time caregivers.9 All of these studies point to the potential of SDoH as important factors that can be used to predict prognosis, health outcomes (e.g., survival), and health service utilization by pediatric cancer patients.
Yet, there is limited understanding of the extent to which SDoH impacts survival because pediatric cancer tends to be rare. Evidence demonstrating a relationship between SDoH and survival may also be impacted by an absence of standardized SDoH measurements, leaving researchers to rely on imprecise estimates from secondary data sources. Previous systematic reviews and studies have examined racial or ethnic disparities in survival. Bhatia’s review, for example, found that White children and adolescents had higher survival rates for acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), Hodgkin lymphoma (HL), rhabdomyosarcoma, and neuroblastoma than Black, Hispanic, and Asian children and adolescents.10 Kahn et al.’s secondary analysis of the Surveillance, Epidemiology, and End Results (SEER) database demonstrated mixed findings, with some racial disparities improving, some persisting, and others worsening.11 Another systematic review demonstrated that low socioeconomic status (SES) is associated with inferior pediatric cancer survival; however, SES alone does not encompass all SDoH.12 Studies and reviews that examine the relationship between SDoH and cancer survival have also primarily focused on cancers affecting adults.13–20 Thus, the purpose of this review is to summarize extant literature that examines the relationship between SDoH and pediatric cancer survival, and to assess how and which SDoH are captured in such studies.
2 |. METHODS
2.1 |. Information sources, eligibility criteria, and search strategy
This systematic review was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. An academic librarian with expertise in health sciences helped develop search strings. A strategy involving keyword searching, medical subject heading (MeSH) terms, filters, and manual reference reviews was used to identify studies investigating relationships between SDoH on survival outcomes in pediatric patients with cancer (Table 1). All studies published up until January 31, 2021 were included. The authors used Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia) for title, abstract, and full-text screening.
TABLE 1.
Structure of searches and search strategies employed in PubMed database
| Search | PubMed search string | Results found |
|---|---|---|
| 1 | “social determinants” and “pediatric” and “child*” and (“neoplasms”[MESH] or cancer) | 11 |
| Filters applied: English, Child: birth-18 years | ||
| 2 | (“neoplasms”[MESH] or cancer) and “survival” and (“social determinants of health”[majr]) and (child*) | 14 |
| Filters applied: English, Child: birth-18 years | ||
| 3 | (“cancer” or “neoplasms”[MESH]) and (“disparities”) and (“social determinants of health”[majr]) and (“child*”) | 11 |
| 4 | (“Neoplasms”[Mesh] or cancer) and (Child* or adolescent* or pediatric*) and (survival or remission or outcome*) and “Healthcare Disparities”[Majr] | 222 |
| 5 | (“Neoplasms”[Mesh] or cancer) and (Child* or adolescent* or pediatric*) and (survival or remission or outcome*) and (“Social Determinants of Health”[Majr] or “Socioeconomic Factors”[Majr] or “Social Conditions”[Majr] or “Healthcare Disparities”[Majr] or “Health Status Disparities”[Majr] or “health disparities” or “health disparity” or “healthcare disparity” or “Healthcare disparities” or “social factors” or “economic status” or “determinants of health”) not ((Africa[mh] or asia[mh] or europe[mh] or islands[mh] or oceania[mh] or canada[mh] or mexico[mh] or South America[mh] or Central America[mh]) not ((Africa[mh] or asia[mh] or europe[mh] or islands[mh] or oceania[mh] or canada[mh] or mexico[mh] or South America[mh] or Central America[mh]) and (United States[mh] or African americans[mh] or Indians, North American[mh] or Asian americans[mh] or Hispanic americans[mh] or “America” or “united states” or “refugee” or refugees”))) | 846 |
| Filters applied: English, Child: birth-18 years, End Date: December 31, 2020 |
The following inclusion criteria were used to determine eligible studies: (a) published within the last two decades (January 1, 2000 to January 31, 2021); (b) published in English language; (c) conducted with US-based patient data; (d) examined children, ages 0 through 19 years; (e) study population diagnosed with any type of cancer; (f) included results of at least one social determinant; (g) assessed survival as a primary or secondary outcome measure (e.g., 1-, 5-, 10-year survival, etc.); and (h) completed study. We used Healthy People 2030′s framework to determine whether predictor variables or covariates fit the definition for social determinants.
2.2 |. Study selection and data collection
YHT reviewed titles and abstracts to ensure that the study met the criteria for pediatric cancer patient. YHT reviewed all full articles to determine which studies met inclusion criteria. Coauthors applied inclusion and exclusion criteria to manually search for relevant articles and assisted with full-text review. Authors erred on the side of inclusion whenever disputes arose.
The authors extracted the following information from each study: (a) author name, (b) year published, (c) sample size, (d) social determinant(s) collected, (e) survival outcome measured, (f) effect size type (e.g., Cox proportional hazard ratios [HR] or odds ratios), (g) effect size estimate, (h) statistical significance when provided, (k) type of cancer, (l) time range, and (m) study design. We considered findings significant at the level α = .05. Authors primarily focused on assessing effect sizes of multivariable analyses.
2.3 |. Quality assessment
All studies included in the review were observational, so the Newcastle–Ottawa Scale (NOS) tool for retrospective cohort studies was used to assess quality. For cohort studies, NOS scores study quality based on representativeness of exposed and nonexposed cohorts, ascertainment of exposure, comparability of cohorts, assessment of outcome, adequate follow-up period, and adequate follow-up of cohorts.
3 |. RESULTS
Figure 1 shows the process of identifying articles for inclusion. Our search identified 847 unique manuscripts, and 25 articles were included in the final analysis. All articles were published between 2009 and 2021. All included studies ranged from moderate quality to high quality. Almost all studies relied on one registry, except for Acharya et al., who utilized the FCDS and TCR.21 The three most common source of data were SEER (eight out of 25 or 32%), TCR (five out of 25 or 20%), and CCR (four out of 25 or 16%). Other data sources used were the Dana Farber Cancer Institute (DFCI)/ALL Consortium, the Centers for Disease Control and Prevention (CDC)’s National Program of Cancer Registries (NPCR), the National Cancer Database (NCDB), the Children’s Oncology Group, the Pediatric Health Information System, the Center for International Blood and Bone Marrow Transplant Research, and University of California San Francisco’s Cancer Registry. Most studies focused on one type of cancer, with the most common being leukemias (ALL and AML), followed by CNS tumors. Additional study characteristics can be found in Table 2.
FIGURE 1.
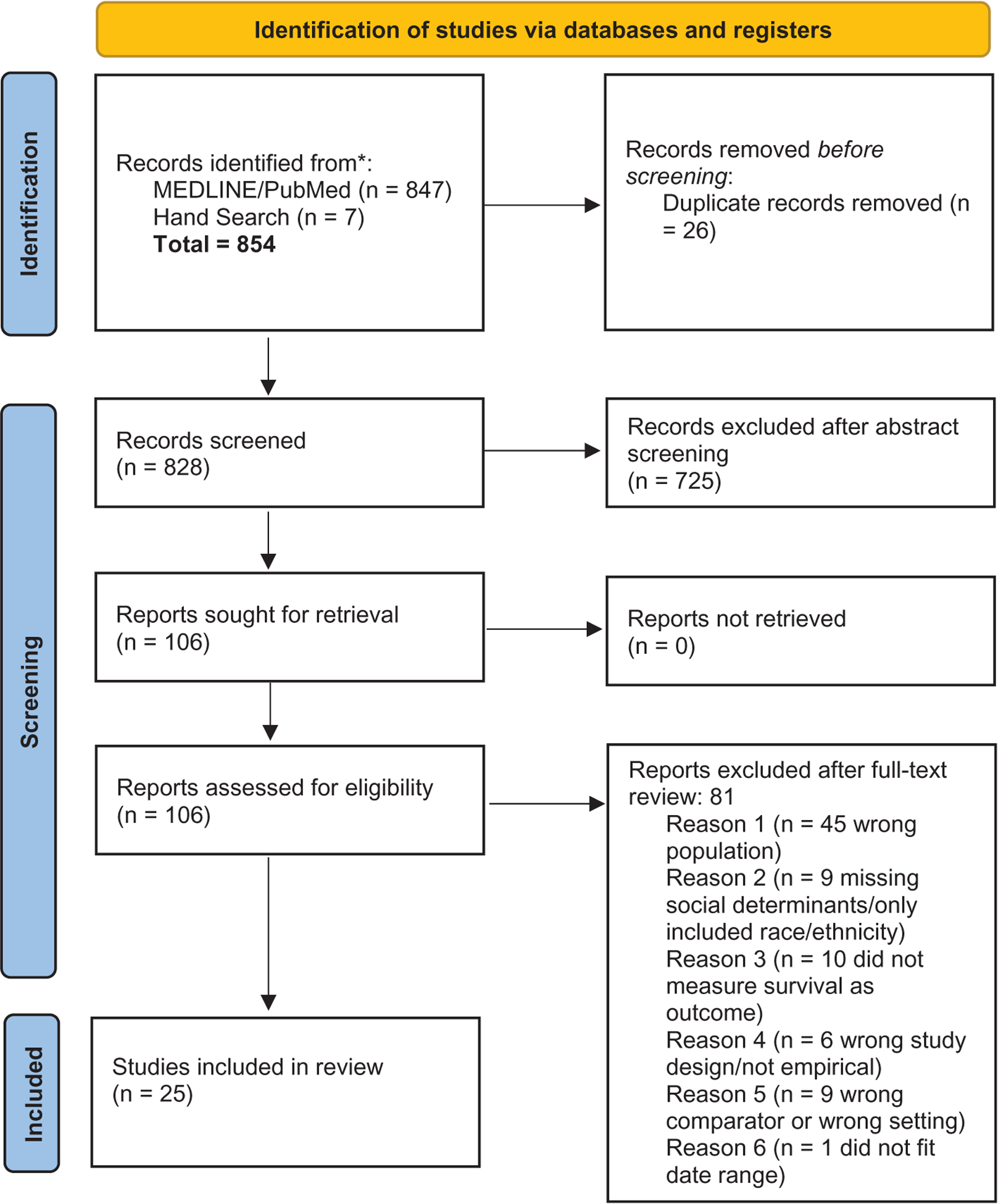
Process for eligible article inclusion
TABLE 2.
Information from the studies
| Reference | Population age (years) | Time range | Data source | Cancer type |
|---|---|---|---|---|
| Abrahão et al., 201522 | <1–19 | 1988–2011 | California Cancer Registry | APL |
| Abrahão et al., 201523 | Only analyzed 0–19 data in review | 1988–2011 | California Cancer Registry | ALL |
| Acharya et al., 201621 | 1–18 | 1995–2008 | Florida Cancer Data System, Texas Cancer Registry | ALL |
| Austin et al., 201524 | ≤18 | 1995–2009 | Texas Cancer Registry | Non-CNS solid tumor malignancy |
| Austin et al., 201625 | ≤18 | 1995–2009 | Texas Cancer Registry | CNS |
| Bona et al., 20167,31 | 1–18 | 2000–2010 | Dana Farber Cancer Institute | ALL |
| Bona et al., 202041 | ≤18 | 2005–2014 | Children’s Oncology Group, | High-risk neuroblastoma |
| Pediatric Health Information System | ||||
| Bona et al., 202136 | ≤18 | 2006–2015 | Center for International Blood and Bone Marrow Transplant Research | Generally mentioned “malignant disease” |
| Byrne et al., 201135 | Only included <10, 10–19 data in review | 1998–2002 | Florida Cancer Data System | AML |
| Colton et al., 201943 | Only analyzed 15–19 in review | 2007–2014 | SEER | Lymphoid leukemia, AML, HL, NH: (except Burkitt), astrocytomas, gliomas, hepatic carcinomas, malignant gonadal germ cell tumors, other and unspecified carcinomas |
| Cooney et al., 201826 | 0–19 | 1988–2012 | California Cancer Registry | High-grade glioma, medulloblastoma |
| Doganis et al., 201842 | 0–14 | 1990–2012 | SEER | Wilms tumor |
| Dressler et al., 201737 | 0–19 | 1998–2011 | NCDB | Medulloblastoma |
| Garner et al., 201739 | ≤21 | 1998–2012 | NCDB | WDTC |
| Hamilton et al., 201627 | ≤18 | 1995–2009 | Texas Cancer Registry | Melanoma |
| Kehm et al., 201828 | 0–19 | 2000–2012 | SEER | ALL, AML neuroblastoma, NHL, HL, astrocytoma, non-astrocytoma CNS tumors, non-rhabdomyosarcoma soft tissue sarcomas, rhabdomyosarcoma, Wilms tumor, osteosarcoma, germ cell tumors |
| Kent et al., 200929 | 0–14 | 1996–2005 | California Cancer Registry | Leukemia (ALL, AML, CLL, CML) |
| Khullar et al., 202038 | ≤21 | 2004–2015 | NCDB | HL |
| Knoble et al., 201632 | 0–19 | 1973–2012 | SEER | AML |
| Lee et al., 201745 | <15 | 2007–2009 | SEER | Leukemias, lymphomas, CNS neoplasms, neuroblastomas, PNS tumors, retinoblastomas, renal tumors, hepatic tumors malignant tumors, sarcomas, germ cell tumors, malignant epithelial neoplasms |
| Mitchell et al., 202030 | 0–19 | 2000–2015 | SEER | CNS |
| Penumarthy et al., 202044 | Only analyzed <15 in review | 2000–2015 | UC San Francisco Cancer Registry | Bone and soft tissue sarcomas |
| Ribeiro et al., 201533 | 0–19 | 2000–2009 | SEER | Langerhans cell histiocytosis |
| Schraw et al., 2020 | <20 | 1995–2011 | Texas Cancer Registry | ALL |
| Siegel et al., 201940 | <20 | 2001–2008 | CDC NPCR | CNS |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; CDC, Centers for Disease Control and Prevention; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; CNS, central nervous system; HL, Hodgkin lymphoma; NCDB, National Cancer Database; NHL, non-Hodgkin lymphoma; NPCR, National Program of Cancer Registries; PNS, peripheral nervous system; SEER, Surveillance, Epidemiology, and End Results; WDTC, well-differentiated thyroid cancer.
3.1 |. Socioeconomic status
The most common factor assessed was SES (52.2%), a measure that encompasses more than one SDoH (see Table 3). No study used individual-level SES measures, as the data were not available in datasets. In studies that included SES in analyses, researchers measured SES at the neighborhood, county, or census tract level. Some studies derived SES from seven block-level census variables, which is a method validated by the Agency for Healthcare Research and Quality (AHRQ).22–30 Acharya et al. used census tract-level poverty rate, measured as the percentage of households within a census tract living under the poverty threshold, as a measure of SES.21 Bona et al. measured community-level SES using the median household income and percentage of families in poverty by zip code data from the US Census Bureau and partitioned patients into low-poverty and high-poverty categories depending on whether at least 20% of residents within a zip code live at or below the poverty level.31 Knoble et al. conducted factor analysis of 23 SES variables to derive a four-factor solution that accounted for co-occurrence of social risk factors.32 Ribeiro et al. used Census 2000 data to determine median values for crowding, rural/urban status, educational attainment, and poverty levels, which they then used as cutoff values.33 Schraw et al. used the area deprivation index (ADI), developed and validated by Singh.34 The ADI uses census tract data to create a composite index that includes 21 indicators covering education, employment, median family income, income disparity, median home value, median gross rent, median monthly mortgage, home ownership rate, population below poverty threshold, single-parent households, lack of transportation (motor vehicle), lack of telephone, housing with incomplete plumbing, and crowding.34
TABLE 3.
Key findings from the studies
| Reference | Cohort size | Measures | Key findings | |
|---|---|---|---|---|
| Domain 1: Economic stability | ||||
| Acharya et al., 201621 | 4719 | HR and 95% CI | 5%–20% FPL: 1.29 (1.03–1.61) 20%–100% FPL: 1.80 (1.41–2.30) |
|
| Bona et al., 20167,31 | 575 | OS probability percentage and 95% CI | Low poverty: 85% (89%–94%) High poverty: 92% (74%–92%) |
|
| Bona et al., 202041 | 371 | HR and 95% CI | Neighborhood poverty: NS Household poverty: 2.79 (1.63–4.79) Neighborhood and household poverty: 3.70 (2.08–6.59) |
|
| Bona et al., 202136 | 2037 | HR and 95% CI | Neighborhood poverty all-cause mortality: NS | |
| Byrne et al., 201135 | 186 | HR and 95% CI, median survival time (months) | Community-level Poverty 10.1%–15% Residents: 1.11 (1.00–1.22) ≥15% Residents: 1.15 (1.04–1.27) |
|
| Dressler et al., 201737 | 3647 | HR and 95% CI | Median household income <$30,000: 1.39 (1.10–1.75) $35,000–$45,999: 1.28 (1.05–1.55) |
|
| Garner et al., 201739 | 9585 | Kaplan–Meier OS | NS | |
| Khullar et al., 202038 | 9285 | HR and 95% CI for OS | NS | |
| Ribeiro et al., 201533 | 145 | 5-Year relative survival (%) and 95% CI | NS | |
| Domain 2: Education access and quality | ||||
| Garner et al., 201739 | 9585 | Kaplan–Meier OS | NS | |
| Khullar et al., 202038 | 9285 | HR and 95% CI | NS | |
| Ribeiro et al., 201533 | 145 | 5-Year relative survival (%) and 95% CI | NS | |
| Domain 3: Healthcare access and quality | ||||
| Abrahão et al., 2015 | 9295 | HR and 95% CI | Public insurance: 1.15 (1.01–1.32) Unknown insurance: 1.77 (1.38–2.26) |
|
| Abrahão et al., 2015 | 784 | OR and 95% CI | No insurance: 2.67 (1.10–6.52) Unknown insurance: 0.22 (0.06–0.79) |
|
| Bona et al., 202136 | 2037 | HR and 95% CI | Medicaid: 1.23 (1.07–1.41) | |
| Byrne et al., 201135 | 186 | HR and 95% CI, median survival time (months) | Medicaid: 1.25 (1.06–1.47) | |
| Colton et al., 201943 | 4539 | HR and 95% CI | Public/no insurance Lymphoid leukemia: 1.80 (1.21–2.68) AML: 2.21 (1.49–3.27) HL: 2.39 (1.13–5.02) |
|
| Garner et al., 201739 | 9585 | Kaplan–Meier OS | NS | |
| Kent et al., 200929 | 3409 | HR and 95% CI | No/unknown insurance: 1.56 (1.26–1.94) | |
| Lee et al., 201745 | 8219 | HR and 95% CI | NS | |
| Mitchell et al., 202030 | 9577 | HR and 95% CI | Medicaid: 1.18 (1.04–1.34) | |
| Penumarthy et al., 202044 | 1106 | HR and 95% CI | NSa | |
| Domain 4: Neighborhood and built environment | ||||
| Austin et al., 201524 | 4603 | HR and 95% | NS | |
| Austin et al., 201625 | 2421 | HR and 95% | Travel distance: NS | |
| Doganis et al., 201842 | 2243 | HR and 95% CI | Rural: NS | |
| Hamilton et al., 201627 | 235 | HR and 95% CI | Travel distance: NS | |
| Khullar et al., 202038 | 9285 | HR and 95% CI | Travel distance: NS | |
| Ribeiro et al., 201533 | 145 | 5-Year relative survival (%) and 95% CI | Crowding: NS | |
| Domain 5: Social and community context | ||||
| No studies retrieved | ||||
| Other | ||||
| Abrahão et al., 2015 | 9295 | HR and 95% CI | Lowest 20% SES: 1.30 (1.04–2.27) | |
| Abrahão et al., 2015 | 784 | OR and 95% CI | Neighborhood SES quintiles Quintile 1 (lowest 20%): 1.03 (0.44–2.44) Quintile 2: 1.08 (0.46–2.53) Quintile 3: 0.93 (0.39–2.23) Quintile 4: 0.81 (0.32–2.02) |
|
| Austin et al., 201524 | 4603 | HR and 95% CI | NS | |
| Austin et al., 201625 | 2421 | HR and 95% CI | NS | |
| Cooney et al., 201826 | 1200 | HR and 95% CI, median survival time (months) and 95% CI | b | |
| Hamilton et al., 201627 | 235 | HR and 95% CI | SES ≤25%: 4.3 (1.4–13.9) | |
| Kehm et al., 201828 | 31 866 | HR and 95% CI | SES is a significant mediator, but did not report HR and 95% CI for SES | |
| Kent et al., 200929 | 3409 | HR and 95% CI | NS | |
| Knoble et al., 201632 | 3651 | HR and 95% CI | Factor 1: 1.07 (1.02–1.12) | |
| Mitchell et al., 202030 | 9577 | HR and 95% CI | 3rd Most deprived: (1.03–1.51) 2nd Most deprived: 1.31 (1.08–1.58) Most deprived: 1.45 (1.20–1.74) |
|
| Schraw et al., 2020 | 4104 | HR and 95% CI | Most disadvantaged: 1.57 (1.23–2.00) | |
Abbreviations: ADI, area deprivation index; ALL, acute lymphoblastic leukemia; AML, acute myelocytic leukemia; CI, confidence interval; FPL, Federal Poverty Line; HL, Hodgkin lymphoma, HR, hazard ratio; NS, not statistically significant in multivariable analyses; OS, overall survival; SDoH, social determinants of health; SES, socioeconomic status.
The analysis did not stratify results by pediatric patients.
The study did not report results for SES, but mentioned that racial disparities were mitigated by accounting for SES.
Except for Garner et al., Abrahão et al.’s acute promyelocytic leukemia (APL) study, and Austin et al.’s paper on solid tumor malignancy, all other studies that included SES in their models as the main predictor or covariate, found significant associations between SES and survival. Kehm et al. tested the mediating effect of SES and reported that SES was a significant mediator of race/ethnicity and survival.28 Abrahão et al.’s ALL study, Acharya et al., Byrne et al., Hamilton et al., Kent et al., Ribeiro et al., and Mitchell et al. found that patients in the lowest SES, in the highest poverty level, most disadvantaged, or most economically deprived were more likely to experience higher risk of death.
3.2 |. SDoH domain 1: Economic stability
Similar to how SES was addressed, investigators who included a poverty variable in their analyses used community level data rather than individual data. Byrne et al., Garner et al., Dressler et al., Khullar et al., and Siegel et al. included poverty variables in their analyses as measures of economic stability. In Byrne et al.’s paper, community poverty level was measured as the percentage of households in a census block whose income was below the poverty line and categorized poverty level into four categories.35 Byrne et al.’s sample included patients less than 10 years up to age 59 years and did not do subset analyses for patients under 18 years; however, they did find that residing in an area with the lowest poverty level was an independent predictor of worse survival among AML patients.35 Similarly to Byrne et al., Bona et al.’s study of hematopoietic cell transplant recipients measured neighborhood poverty as the proportion of persons living below 100% of the FPL. Among malignant patients, neighborhood poverty did not contribute to significant differences in all-cause mortality, but was associated with transplant-related mortality.36 Dressler et al., Khullar et al., and Garner et al. used median household income by zip code.37–39 In Dressler’s study of children with medulloblastoma, a median income of less than $30,000 or between $35,000 and $45,999 was associated with lower survival.37 Khullar et al.’s study demonstrated a significant association between worse survival and median income below $63,000. On the other hand, Garner et al. found no difference in overall survival (OS) when adjusting for poverty. Siegel et al. included county-level economic status data from the CDC’s NPCR, which applies the Appalachian Regional Commission’s index-based county economic classification system. Their analyses demonstrated that those in the top 25% and transitional (25%–75%) economic groups had lower risk of death than those with unknown or lower economic status.40 Only one study from 2020 by Bona et al. measured household poverty in addition to neighborhood poverty and found that the former was associated with worse OS (3.08, 95% confidence interval [CI]: 1.76–5.39), but the latter measure of poverty was not significantly associated with difference in OS.41 Moreover, this study linked dual poverty exposure (both neighborhood and household poverty) to worse OS.
3.3 |. SDoH domain 2: Neighborhood and built environment
Only three studies specifically examined the influence of geography. No studies reported significant relationships between rurality or crowding and survival.33,39,42 Two studies, Hamilton et al. and Khullar et al., included driving distance to the treatment center in their analyses and also did not find statistically significant relationships.27,38
3.4 |. SDoH domain 3: Health and healthcare
We considered insurance status as a measure of health and health-care. Cancer databases such as SEER or the CCR did not reliably collect insurance data until 1996. Unlike SES, poverty, or education, insurance coverage was reported at the individual level. In our cohort of studies, 43.5% included insurance coverage as a predictor variable or covariate. There were mixed findings regarding the potential impacts of insurance on cancer survival, and findings appeared to differ by cancer type. Abrahão et al.’s ALL study demonstrated that having no insurance, public insurance, or unknown insurance was associated with lower OS compared to private insurance.23 However, in APL patients, Abrahão et al. only found a significantly higher risk of death among uninsured patients. In AML patients, being insured by Medicaid alone was associated with lower overall median survival times, whereas other types of insurance had no impact on median survival time. Public or no insurance was significantly associated with death for adolescent patients (ages 15–19 years) with lymphoid leukemia, AML, HL, and unspecified carcinomas; however, there was no significant relationship between public or no insurance and death in patients with non-Hodgkin lymphomas, astrocytomas, gliomas, hepatic carcinomas, fibrosarcomas, and gonadal germ cell tumors. Kent et al. found that no or unknown insurance was associated with worse survival rates than having private insurance in leukemia patients among all race/ethnic groups except Asian and Pacific Islanders. In HL patients, those uninsured, covered by Medicaid, or have other nonprivate insurance had worse survival outcomes compared to patients with private insurance. In patients with bone and soft tissue sarcomas, low-income public insurance was also associated with worse survival when accounting for all other covariates. For patients with unspecified malignant disease who received hematopoietic cell transplant treatment, those on public insurance (Medicaid) had higher probability of all-cause mortality.36
Some studies found no association between insurance and survival. Bona et al. found a significant difference in mortality for Medicaid patients; however, unknown insurance status was not associated with a difference in mortality.36 Lee et al. found that mean survival times after 5 years did not significantly differ by insurance type, even though there was an increased hazard of cancer death for uninsured patients compared to public or private, public, or any insurance. When adjusted for socioeconomic factors and cancer type, Lee et al. did not find any difference in insurance status and mortality. Additionally, Garner et al. did not report any quantitative findings but noted that there was no difference in OS by insurance type. Mitchell et al.’s study of patients with primary CNS tumors reported no difference in OS by insurance type when adjusting for sex, age, year of diagnosis, tumor category, race/ethnicity, and SES. When only adjusting for sex, age, year of diagnosis, and tumor category, patients with public insurance (Medicaid) appeared to have worse survival rates.
3.5 |. SDoH domain 4: Social and community context
No study included in this review examined social and community context at the patient level, zip code level, or geocode level. We searched for inclusion of community capacity, civic participation, reported discrimination, incarceration and crime rates, and measures of social cohesion or connectedness in statistical models. No study included such measures.
3.6 |. SDoH domain 5: Education
Several studies included education as separate variable in their analyses instead of including education within SES or some other composite index. Garner et al. used zip code level education, measured as the number of adults without a high school degree, and partitioned data into quartiles. Garner et al. did not find a statistically significant difference in survival by proportion of adults in a zip code attaining a high school degree and did not report quantitative results for this finding. Likewise, Khullar et al. did not find statistically significant association between education attainment and survival.38 Ribeiro et al. categorized low education attainment as greater than 16.6% of persons 25 years or older in a county with less than high school graduate, and high education attainment as less than or equal to 16.6% of persons 25 years or older with less than a high school degree.33 While 5-year relative survival rates for Langerhans cell histiocytosis was higher among patients residing in less educated counties, 97.0% (95% CI: 78%–99.6%) versus 87.8% (95% CI: 79.1%–93.0%), there was no statistically significant difference (p = .156)
3.7 |. Interaction effects: Race/ethnicity
All studies included in this review recorded patient race/ethnicity. However, few studies reported testing of interactions between race/ethnicity and social determinants. Cooney et al., Garner et al., and Penumarthy et al. did not find any influence of race/ethnicity on survival.26,39,44 All other studies that included race/ethnicity in their models demonstrated a significant association between race/ethnicity and survival in unadjusted, adjusted, or both models. In general, non-Hispanic Black, African American, or Hispanic were associated with worse survival outcomes compared to White patients, even when adjusting for SES, insurance, and other variables.
4 |. DISCUSSION
We conducted a systematic review that examines any association between social determinants and cancer survival among pediatric patients. Previous reviews have linked race and ethnicity as well as SES to cancer survival. As defined by Healthy People 2030, SDoH span multiple categories that race/ethnicity and SES alone do not address. Findings from this review generally support existing literature linking SES to poor survival outcomes. Additionally, this review examines several studies that test the relationship between poverty (or income), education, insurance coverage, geography (rural vs. urban and driving distance), and crowding. Only insurance coverage, particularly being uninsured or having low-income public insurance, was associated with poorer survival outcomes. Finally, this review identifies several social determinants that have not been extensively studied in the context of pediatric cancer survival: food security, stable employment (and not overall unemployment rates), health literacy, civic participation, social cohesion, and discrimination.
Inconsistent findings on associations between SDoH and pediatric cancer survival may be attributed to retrospective designs and secondary data sources. Cancer registries and census data report social determinants data at the county, zip code, or census tract level. Thus, estimated effect sizes may be biased or imprecise. These issues high-light opportunities for investigators to identify different data sources, such as electronic health records or health information exchanges or to collect primary data. Moreover, the absence of prospective studies presents opportunities for researchers to design prospective studies that test interventions, such as implementing universal SDoH screening similarly to the approach taken by Power-Hays et al.46 Other approaches, such as administering surveys to about basic resource needs and financial burden, have been demonstrated to be feasible in recent studies.6,8
Many of the articles included in this systematic review rely on the SEER database for analysis. SEER data comes from registries in the following states: Connecticut, Georgia, California, Hawaii, Idaho, Iowa, Kentucky, Louisiana, Massachusetts, New Mexico, New York, Washington, Utah, and Wisconsin.47,48 SEER data also includes the Alaska Native Tumor, Arizona Indians, and Cherokee Nation registries.47,48 Data from these registries, which encompass 26% of the US population, are then extrapolated to represent the national pediatric cancer data.49 Using the SEER database has several advantages, such as a large sample size and long follow-up periods. A caveat of using the SEER database is that participating registries may change over time. For example, population-based cancer registries from Detroit, Michigan, and New Jersey no longer participate in the SEER program.47,48 A second limitation of the SEER database is that there is a higher proportion of foreign-born and urban-dwelling individuals represented than in the actual US population.49 SEER data may also suffer from missing or inaccurate data due to underreporting of radiation therapy, radiation fields, doses, and intent; low coding reliability for rare histologies; patient migration; and selection bias.49
There are several limitations associated with this systematic review. First, only PubMed/MEDLINE’s database was searched, so this review may have missed key references indexed in other databases. Second, by narrowing the age range to only pediatric patients, we may have missed articles that combined child and adolescent with young adult and adult populations. Third, by using reference review as the only method of hand-searching additional references, we may have also missed white papers, gray literature, pre-print articles, articles with null findings, and published literature not indexed in PubMed. Fourth, we could not conduct meta-analyses, given the heterogeneity of the articles, and therefore could not approximate the extent of publication bias. Finally, NOS used for quality assessment is less time consuming than other quality assessment methods but has its limitations, which include low to moderate interrater reliability. Nonetheless, we believe that the articles included in this systematic review are representative of the body of literature and that this review contributes to understanding the role of SDoH in pediatric cancer outcomes.
ACKNOWLEDGMENTS
The authors thank Rachel J. Hinrichs, MS, MSLS for her assistance with database searching. Yvette H. Tran received funding from the National Library of Medicine under Grant T15LM012502. The National Library of Medicine had no role in the study design, data collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit this manuscript for publication.
Funding information
U.S. National Library of Medicine, Grant/Award Number: 5T15LM012502-04
Abbreviations:
- ADI
area deprivation index
- ALL
acute lymphoblastic leukemia
- AML
acute myeloid leukemia
- APL
acute promyelocytic leukemia
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- CNS
central nervous system
- HL
Hodgkin lymphoma
- HMH
household material hardship
- NOS
Newcastle–Ottawa Scale
- NPCR
National Program of Cancer Registries
- OS
overall survival
- SDoH
social determinants of health
- SEER
Surveillance, Epidemiology, and End Results
- SES
socioeconomic status
Footnotes
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
REFERENCES
- 1.ACCO. US Childhood Cancer Statistics. ACCO. Accessed February 23, 2021. https://www.acco.org/us-childhood-cancer-statistics/ [Google Scholar]
- 2.National Cancer Institute. Cancer in Children and Adolescents. National Cancer Institute; September 1, 2017. Accessed February 23, 2021. https://www.cancer.gov/types/childhood-cancers/child-adolescent-cancers-fact-sheet [Google Scholar]
- 3.Cunningham RM, Walton MA, Carter PM. The major causes of death in children and adolescents in the United States. N Engl J Med. 2018;379(25):2468–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Key statistics for childhood cancers. American Cancer Society; 2021. Accessed February 23, 2021. https://www.cancer.org/cancer/cancer-in-children/key-statistics.html
- 5.Victorino CC, Gauthier AH. The social determinants of child health: variations across health outcomes - a population-based cross-sectional analysis. BMC Pediatr. 2009;9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bilodeau M, Ma C, Al-Sayegh H, Wolfe J, Bona K. Household material hardship in families of children post-chemotherapy. Pediatr Blood Cancer. 2018;65(1):e26743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bona K, London WB, Guo D, Frank DA, Wolfe J. Trajectory of material hardship and income poverty in families of children undergoing chemotherapy: a prospective cohort study. Pediatr Blood Cancer. 2016;63(1):105–111. [DOI] [PubMed] [Google Scholar]
- 8.Santacroce SJ, Kneipp SM. Influence of pediatric cancer–related financial burden on parent distress and other stress-related symptoms. Pediatr Blood Cancer. 2020;67(3):e28093. [DOI] [PubMed] [Google Scholar]
- 9.Hoppmann AL, Chen Y, Landier W, et al. Individual prediction of nonadherence to oral mercaptopurine (6MP) in children with acute lymphoblastic leukemia (ALL): results from COG AALL03N1 study. J Clin Oncol. 2020;38(15_suppl):10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatia S. Disparities in cancer outcomes: lessons learned from children with cancer. Pediatr Blood Cancer. 2011;56(6):994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahn JM, Keegan THM, Tao L, Abrahao R, Bleyer A, Viny AD. Racial disparities in the survival of American children, adolescents, and young adults with acute lymphoblastic leukemia, acute myelogenous leukemia, and Hodgkin lymphoma. Cancer. 2016;122(17):2723–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta S, Wilejto M, Pole JD, Guttmann A, Sung L. Low socioeconomic status is associated with worse survival in children with cancer: a systematic review. PLoS One. 2014;9(2):e89482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDaniel JT, Nuhu K, Ruiz J, Alorbi G. Social determinants of cancer incidence and mortality around the world: an ecological study. Glob Health Promot. 2019;26(1):41–49. [DOI] [PubMed] [Google Scholar]
- 14.Coughlin SS. Social determinants of breast cancer risk, stage, and survival. Breast Cancer Res Treat. 2019;177(3):537–548. [DOI] [PubMed] [Google Scholar]
- 15.Coughlin SS. Social determinants of colorectal cancer risk, stage, and survival: a systematic review. Int J Colorectal Dis. 2020;35(6):985–995. [DOI] [PubMed] [Google Scholar]
- 16.Coughlin SS. A review of social determinants of prostate cancer risk, stage, and survival. Prostate Int. 2020;8(2):49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerend MA, Pai M. Social determinants of black-white disparities in breast cancer mortality: A review. Cancer Epidemiol Biomarkers Prev. 2008;17(11):2913–2923. 10.1158/1055-9965.epi-07-0633 [DOI] [PubMed] [Google Scholar]
- 18.Merletti F, Galassi C, Spadea T. The socioeconomic determinants of cancer. Environ Health. 2011;10(1):S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown CR, Hambleton IR, Hercules SM, et al. Social determinants of breast cancer in the Caribbean: a systematic review. Int J Equity Health. 2017;16(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein J, von dem Knesebeck O. Socioeconomic inequalities in prostate cancer survival: a review of the evidence and explanatory factors. Soc Sci Med. 2015;142:9–18. [DOI] [PubMed] [Google Scholar]
- 21.Acharya S, Hsieh S, Shinohara ET, DeWees T, Frangoul H, Perkins SM. Effects of race/ethnicity and socioeconomic status on outcome in childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2016;38(5):350–354. [DOI] [PubMed] [Google Scholar]
- 22.Abrahão R, Ribeiro RC, Medeiros BC, Keogh RH, Keegan THM. Disparities in early death and survival in children, adolescents, and young adults with acute promyelocytic leukemia in California. Cancer. 2015;121(22):3990–3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abrahão R, Lichtensztajn DY, Ribeiro RC, et al. Racial/ethnic and socioeconomic disparities in survival among children with acute lymphoblastic leukemia in California, 1988–2011: A population-based observational study. Pediatric Blood & Cancer. 2015;62(10):1819–1825. 10.1002/pbc.25544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Austin MT, Nguyen H, Eberth JM, et al. Health disparities are important determinants of outcome for children with solid tumor malignancies. J Pediatr Surg. 2015;50(1):161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin MT, Hamilton E, Zebda D, et al. Health disparities and impact on outcomes in children with primary central nervous system solid tumors. J Neurosurg Pediatr. 2016;18(5):585–593. [DOI] [PubMed] [Google Scholar]
- 26.Cooney T, Fisher PG, Tao L, Clarke CA, Partap S. Pediatric neuro-oncology survival disparities in California. J Neurooncol. 2018;138(1):83–97. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton EC, Nguyen HT, Chang YC, et al. Health disparities influence childhood melanoma stage at diagnosis and outcome. J Pediatr. 2016;175:182–187. [DOI] [PubMed] [Google Scholar]
- 28.Kehm RD, Spector LG, Poynter JN, Vock DM, Altekruse SF, Osy-puk TL. Does socioeconomic status account for racial and ethnic disparities in childhood cancer survival? Cancer. 2018;124(20):4090–4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kent EE, Sender LS, Largent JA, Anton-Culver H. Leukemia survival in children, adolescents, and young adults: influence of socioeconomic status and other demographic factors. Cancer Causes Control. 2009;20(8):1409–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell HK, Morris M, Ellis L, Abrahão R, Bonaventure A. Racial/ethnic and socioeconomic survival disparities for children and adolescents with central nervous system tumours in the United States, 2000–2015. Cancer Epidemiol. 2020;64:101644. [DOI] [PubMed] [Google Scholar]
- 31.Bona K, Blonquist TM, Neuberg DS, Silverman LB, Wolfe J. Impact of Socioeconomic Status on Timing of Relapse and Overall Survival for Children Treated on Dana-Farber Cancer Institute ALL Consortium Protocols (2000–2010). Pediatric Blood & Cancer. 2016;63(6):1012–1018. 10.1002/pbc.25928 [DOI] [PubMed] [Google Scholar]
- 32.Knoble NB, Alderfer MA, Hossain MJ. Socioeconomic status (SES) and childhood acute myeloid leukemia (AML) mortality risk: analysis of SEER data. Cancer Epidemiol. 2016;44:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ribeiro KB, Degar B, Antoneli CBG, Rollins B, Rodriguez-Galindo C. Ethnicity, race, and socioeconomic status influence incidence of Langerhans cell histiocytosis. Pediatr Blood Cancer. 2015;62(6):982–987. [DOI] [PubMed] [Google Scholar]
- 34.Singh GK. Area deprivation and widening inequalities in US mortality, 1969–1998. Am J Public Health. 2003;93(7):1137–1143. 10.2105/ajph.93.7.1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Byrne MM, Halman LJ, Koniaris LG, Cassileth PA, Rosenblatt JD, Cheung MC. Effects of poverty and race on outcomes in acute myeloid leukemia. Am J Clin Oncol. 2011;34(3):297–304. [DOI] [PubMed] [Google Scholar]
- 36.Bona K, Brazauskas R, He N, et al. Neighborhood poverty and pediatric allogeneic hematopoietic cell transplantation outcomes: a CIBMTR analysis. Blood. 2021;137(4):556–568. 10.1182/blood.2020006252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dressler EV, Dolecek TA, Liu M, Villano JL. Demographics, patterns of care, and survival in pediatric medulloblastoma. J Neurooncol. 2017;132(3):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khullar K, Rivera-Núñez Z, Jhawar SR, et al. Pediatric Hodgkin lymphoma: disparities in survival by race. Leuk Lymphoma. 2020;61(3):546–556. [DOI] [PubMed] [Google Scholar]
- 39.Garner EF, Maizlin II, Dellinger MB, et al. Effects of socioeconomic status on children with well-differentiated thyroid cancer. Surgery. 2017;162(3):662–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siegel DA, Li J, Ding H, Singh SD, King JB, Pollack LA. Racial and ethnic differences in survival of pediatric patients with brain and central nervous system cancer in the United States. Pediatr Blood Cancer. 2019;66(2):e27501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bona K, Li Y, Winestone LE, et al. Poverty and Targeted Immunotherapy: Survival in Children’s Oncology Group Clinical Trials for High-Risk Neuroblastoma. J Natl Cancer Inst. 2021;113(3):282–291. 10.1093/jnci/djaa107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doganis D, Panagopoulou P, Tragiannidis A, et al. Survival and mortality rates of Wilms tumour in Southern and Eastern European countries: socioeconomic differentials compared with the United States of America. Eur J Cancer. 2018;101:38–46. [DOI] [PubMed] [Google Scholar]
- 43.Colton MD, Goulding D, Beltrami A, et al. A U.S. population-based study of insurance disparities in cancer survival among adolescents and young adults. Cancer Med. 2019;8(10):4867–4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Penumarthy NL, Goldsby RE, Shiboski SC, Wustrack R, Murphy P, Winestone LE. Insurance impacts survival for children, adolescents, and young adults with bone and soft tissue sarcomas. Cancer Med. 2020;9(3):951–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee JM, Wang X, Ojha RP, Johnson KJ. The effect of health insurance on childhood cancer survival in the United States. Cancer. 2017;123(24):4878–4885. [DOI] [PubMed] [Google Scholar]
- 46.Power-Hays A, Li S, Mensah A, Sobota A. Universal screening for social determinants of health in pediatric sickle cell disease: a quality-improvement initiative. Pediatr Blood Cancer. 2020;67(1):e28006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.SEER. SEER Registries - About SEER. SEER. Accessed May 13, 2021. https://seer.cancer.gov/registries/index.html [Google Scholar]
- 48.SEER. List of SEER Registries - About SEER. SEER. Accessed May 13, 2021. https://seer.cancer.gov/registries/list.html [Google Scholar]
- 49.NCI SEER public-use data: applications and limitations in oncology research. Cancer Network. Accessed May 13, 2021. https://www.cancernetwork.com/view/nci-seer-public-use-data-applications-and-limitations-oncology-research [PubMed] [Google Scholar]


