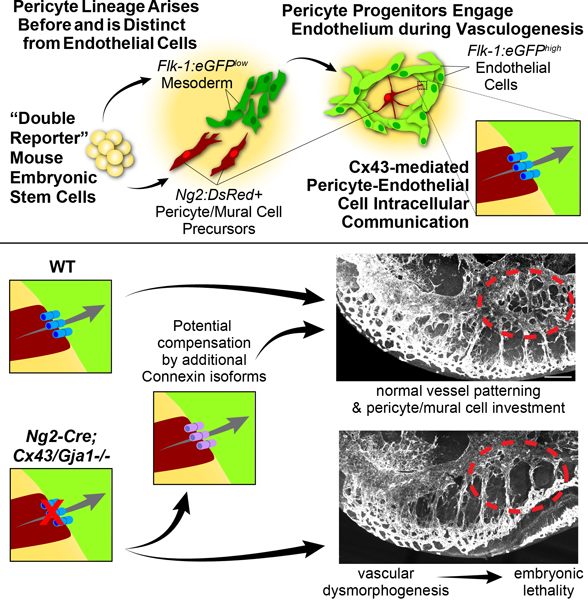
SUMMARY
Background:
Vascular pericytes (PCs) stabilize blood vessels and contribute to their maturation, while playing other key roles in microvascular function. Nevertheless, relatively little is known about involvement of their precursors in the earliest stages of vascular development, specifically during vasculogenesis.
Methods:
We combined high-power, time-lapse imaging with transcriptional profiling of emerging PCs and endothelial cells (ECs) in reporter mouse and cell lines. We also analyzed conditional transgenic animals deficient in Cx43/Gja1 expression within Ng2+ cells.
Results:
A subset of Ng2-DsRed+ cells, likely PC/mural cell precursors, arose alongside EC differentiation and organization, and physically engaged vasculogenic endothelium in vivo and in vitro. We found no overlap between this population of differentiating PC/mural progenitors and other lineages including hemangiogenic and neuronal/glial cell types. We also observed cell-cell coupling and identified Connexin43 (Cx43)-based gap junctions contributing to PC-EC precursor communication during vascular assembly. Genetic loss of Cx43/Gja1 in Ng2+ PC progenitors compromised embryonic blood vessel formation in a subset of animals, while surviving mutants displayed little to no vessel abnormalities, suggesting a resilience to Cx43/Gja1 loss in Ng2+ cells or potential compensation by additional connexin isoforms.
Conclusions:
Together, our data suggest that a distinct PC lineage emerges alongside vasculogenesis and directly communicates with the nascent endothelium via Cx43 during early vessel formation. Cx43/Gja1 loss in PC/mural cell progenitors can induce embryonic vessel dysmorphogenesis, but alternate connexin isoforms may be able to compensate. These data provide insight that may reshape the current framework of vascular development and may also inform tissue re-/vascularization strategies.
Keywords: pericytes, endothelial cells, vasculogenesis, Connexin43, gap junctions, embryonic stem cells, vascular development
Graphical Abstract
INTRODUCTION
Pericytes (PCs) are specialized vascular cells that extend along capillary networks and perform a range of established and emerging roles in cardiovascular function1. Current paradigms of vascular development suggest initial PC and mural cell engagement with the endothelium occurs during vessel maturation2–12. In addition, establishing and maintaining the PC lineage appears dependent on direct contact with terminally differentiated ECs13–15, and may even arise from hemangiogenic lineages16. As such, the timing of when PCs and mural cells initiate differentiation and first engage developing vessel networks is relatively unexplored, particularly during vasculogenesis.
Pericyte origins are largely ascribed to the embryonic mesenchyme and ectoderm-derived neural crest17,18. Overlap with other cell lineages has also been proposed, including cardiac endothelium16, neuronal/glial populations19,20, and tissue-resident stem cells21, though with some controversy22,23. Lineage-tracing studies indicate a close relationship between PCs and vascular smooth muscle cell (vSMC) differentiation1,17,24–26, reinforcing the notion of PCs as vascular-specific mural cells. Further, PCs embed within the specialized extracellular matrix (ECM) composing the vBM6,27–29 and form gap junctions with each other30 and with the endothelium13,31,32. However, it remains unclear when PC-EC intercellular channels first aggregate and become functional, and how this may influence embryonic vascular assembly and PC identity. Thus, a deeper understanding of PC differentiation with respect to early blood vessel organization is critical to advancing both our current understanding of vascular formation and the development of more comprehensive tissue re-/vascularization strategies including those utilizing induced-pluripotent stem cells (iPSCs).
In the current study, we applied high-power imaging modalities, time-lapse imaging, and detailed transcriptional profiling to reporter mouse and cell lines. With little to no overlap between PC/mural cell differentiation and other tissue compartments analyzed, we found a subset of Ng2-DsRed+ PC progenitors emerging alongside endothelial differentiation and the vasculogenic formation of developing blood vessels in our in vivo and in vitro models. In addition, we observed direct cell-cell communication during vasculogenesis with Connexin43 (Cx43)-based gap junctions contributing to intercellular communication between PC and EC precursors during early vessel formation.
METHODS (see Supplemental Materials for Additional Details)
The data that support the findings of this study are available from the corresponding author upon reasonable request. Additionally, Please see the Major Resources Table in the Supplemental Materials.
In Vivo Animal Models.
All animal work was conducted in accordance with a protocol that was approved by the Virginia Tech Institutional Animal Care and Use Committee (IACUC), which maintains compliance under the Animal Welfare Assurance number A-3208–01 (expiration 07–31-2025).
Cell Lines.
Wild-type Embryonic Stem Cells (WT-ESCs):
Wild-type Embryonic Stem Cells (WT-ESCs) were a gift from V.L. Bautch (University of North Carolina at Chapel Hill).
Double-Reporter Embryonic Stem Cells (DR-ESCs):
[EC/PC-DR-mESC (RRID:CVCL_XX13)]. DR-ESCs were obtained via blastocyst extraction, and all cultures were maintained in 5% CO2 at 37°C. DR-ESCs were amplified on irradiated mouse embryonic fibroblasts (iMEFs) in T75-flasks to 80% confluence and cryopreserved in 10% DMSO at passages 8–9.
ESC Maintenance and Differentiation:
DR-ESCs were maintained in an undifferentiated state in 2i System Medium. Cultures were passaged every 3–5 days, using a 1:3 dilution of 0.25%Trypsin/EDTA in DPBS. WT-ESCs were maintained in an undifferentiated state by using supplementation with medium conditioned by the 5637 human bladder cancer cell line (ATCC #HTB9) that produces LIF, thus eliminating the need for recombinant LIF and the dual-kinase inhibition. Differentiation of double reporter and WT ESCs was conducted as described previously33.
Blastocyst Extraction and Stem Cell Isolation.
A male flk-1:eGFP; ng2:DsRed mouse (flk-1:eGFP, The Jackson Laboratory #017006 and ng2:DsRed, The Jackson Laboratory #008241) was bred to a WT female for the purpose of blastocyst harvest and expansion in vitro. A single female was harvested and blastocyst clones were differentiated and screened for flk-1:eGFP; ng2:DsRed double-reporter expression. Blastocysts were collected at 3.5 days post coitus as previously described34,35.
Karyotyping Analysis.
Undifferentiated DR-ESCs were analyzed via karyotyping for chromosomal stability. Twenty-five chromosomal clusters were analyzed from 4 distinct preparations. Undifferentiated DR-ESCs were arrested in metaphase. Cultures were collected, washed, put into single-cell suspension, and treated with 75mM KCl for 20 minutes. Cells were fixed, spun down and re-suspended in fresh fixative twice. DNA of burst cells was labeled with Hoechst 34580 (ThermoFisher). Chromosomes were imaged on a Zeiss LSM880 confocal microscope and counted from 25 clusters.
Alkaline Phophatase Staining.
DR-ESCs were analyzed for pluripotency via alkaline phosphatase staining. DR-ESCs were fixed and stained for alkaline phosphatase36. Brightfield images of stained samples were acquired on a Zeiss Axio Observer microscope mounted with a Hamamatsu ORCA-Flash 4.0 V2 Digital CMOS camera.
Immunocytochemistry.
DR-ESCs were assessed for protein markers using immunocytochemistry and confocal microscopy. Cultures were fixed for 15 minutes at room temperature in 4% PFA, and immunostained as previously described37,38. Primary antibodies (Abs) used were: rat anti-mouse/human Oct3/4 at 1:200, rat anti-mouse platelet-endothelial cell adhesion molecule-1 (PECAM-1)/CD31 (BD Biosciences) at 1:1000, rabbit anti-mouse NG2 (Millipore) at 1:200, and rabbit polyclonal anti-phospho-Histone H3 (PH3, ser10, Millipore) at 1:500, rat anti-mouse PDGFRβ at 1:400, rabbit anti-mouse Desmin at 1:500, mouse anti-mouse A2B5 at 1:100. Secondary antibodies used were donkey anti-rat AlexaFluor647 (IgG; H+L) at ½ the primary Ab dilution factor (Invitrogen), and donkey anti-rabbit AlexaFluor647 (IgG; H+L) at ½ the primary Ab dilution. Incubation with DAPI for 30 min followed all staining. ESC cultures were imaged on a Zeiss LSM880 confocal microscope.
RNA isolation, Reverse Transcription and qRT-PCR.
DR-ESCs were collected to assess the gene expression levels of endothelial cell and pericyte markers. Real-time quantitative PCR was conducted in triplicate for each sample, using TaqMan Gene Expression Master Mix (Applied Biosystems) and the Applied Biosystems QuantStudio6. All targets were normalized to TATA-binding protein (tbp). Primer/probe sets were obtained from Applied Bio-Systems, with the exception of the dsred.T1 primer/probe set that was custom designed through Applied Biosystems using a custom DsRed Express (DsRed.T1) sequence. Applied Biosystems assay mixes for the following gene targets: Oct4, Fgf4, Sox2, Nanog and c-kit/CD117, Dab2, Gata6, Actc1 and Otx2, Pecam1/CD31, VE-Cadherin/Cdh5/CD44, Flk-1/Kdr/Vegfr2, Icam2/CD102, NG2/Cspg4, Pdgfrβ/CD140b, Desmin, and N-cadherin/Cdh2, Tgfβ1.
Fluorescence activated Cell Sorting (FACS).
DR-ESCs were processed and sorted via FACS using endogenous Ng2:DsRed (pericyte) and Flk-1:eGFP (endothelial cell) reporter expression for the purposes of transcriptional analysis via RNA-sequencing. FACS was performed on a Sony SH800 Flow cytometer.
RNA-Sequencing.
FACS-sorted populations were input for RNA-sequencing to determine cell-specific gene expression. Two biological replicates were obtained and processed. Whole cells were sent to Genewiz (NJ) for ultra-low input RNA-sequencing via Illumina HiSeq 2×150 bp at approximately 350M paired reads per lane. Raw data was delivered in FASTQ format and aligned to the mus musculus genome assembly, GRCm38.p6, and analyzed via Kallisto39. A cutoff of 5 transcripts per million (TPM) was used. Cell-specific (Flk-1:eGFP endothelial cells Ng2:DsRed pericyte) transcript expression was assessed via RNA-sequencing, as transcripts per million (TPM).
Short-Acquisition Time-Lapse Imaging.
DR-ESCs were imaged using epi-fluorescence to assess Flk-1:eGFP vessel structures and Ng2:DsRed pericytes relative to contracting cardiomyocytes. Short-acquisition time-lapse imaging (less than 5 mins) was used to image live cultures with a 20x objective on a Zeiss Axio Observer microscope mounted with a Hamamatsu ORCA-Flash 4.0 V2 Digital CMOS camera.
Long-Acquisition Live Confocal Imaging.
Ultra-long time-course confocal microscopy was used to obtain real-time videos of differentiating DR-ESCs as vessel structures arise de novo. A compressed z-stack of 6–10 images was obtained for each scan with 3–4μm between focal planes for each time point. Representative time-lapse sequences shown are from non-consecutive images. Twenty-one confocal live-imaging videos were obtained, spanning days 6–12 of differentiating DR-ESCs.
ImageJ/FIJI Vessel Analysis.
Vessel structures during early development were characterized in differentiating DR-ESCs (from day 6–12) via analysis of 21 real-time videos obtained from ultra-long time-course confocal microscopy (described above). Branch points per mm of vessel length, branch length (mm), percent of total vessel area, timing of emergent Flk-1:eGFPlow mesoderm, Flk-1:eGFPhigh endothelial cell and Ng2:DsRed pericyte fluorescent signals, and percent of Ng2:DsRed pericytes and Flk-1:eGFPhigh endothelial cells that arose from Flk-1:eGFPlow mesoderm precursors, were quantified. In each case, the analysis was blinded and utilized ImageJ software. Lastly, movies were analyzed for the subpopulation of Ng2-DsRed+ cells that engaged with Flk-1-eGFP+ cells. The totals were tallied across all movies to obtain an overall percentage.
Microinjection Dye Transfer.
Direct cell-to-cell communication during early vessel development was assessed via single-cell microinjection of Cascade Blue dye (596 MW) into Ng2:DsRed+ pericytes in differentiating DR-ESCs. We obtained transmission, fluorescence images of individual fluorophore from the selected field of view before and after microinjection of dye, using Axiocam MRm camera attached to the microscope.
Magnet-Assisted Cell Sorting (MACS).
Endothelial cells (PECAM-1+) and pericytes (An2+) were sorted from WT-ESCs via MACs for the purpose of qRT-PCR and immuno-blot analysis of connexin-43 expression. Four to eight biological replicates were collected for qRT-PCR analysis. Pericytes and endothelial cells were enriched from WT ESCs cultures at day 10 differentiation and sorted via MACS (Miltenyi Biotec) as previously described38,40.
SDS-PAGE and Western Blot.
Protein lysates were separated by SDS-PAGE and immuno-blotted using anti-phospho-Cx43 to determine overall protein level and phosphorylation states of Cx43. Connexin43 expression was normalized to GAPDH. Membranes was exposed (in TBST) to primary antibodies rabbit anti-mouse phospho-Connexin43 (Cell Signaling, Cat #3511) at 1:1000 and goat anti-GAPDH (Abcam, Cat ab9485) at 1:1000 overnight at 4°C and to secondary antibodies donkey anti-rabbit AlexaFluor 488 (Jackson ImmunoResearch Cat #711–545-152) and donkey anti-goat AlexaFluor 647 at 1:10,000 (Jackson ImmunoResearch Cat #705–605-147) for 2h at room temperature. Protein bands were imaged and quantified on a ChemiDoc system (Bio-Rad) using Bio-Rad Image Lab 5.1 software.
Immunohistochemistry.
Tissues were collected from mice at embryonic day 9 (Ng2Cre/+; Cx43+/+ and Ng2Cre/+; Cx43lox/lox), embryonic day 9.5 (WT C57BL/6J), 14.5 (Flk-1:eGFP; Ng2:DsRed skin) and post-natal days 4 (Ng2:DsRed brain) and 7 (Ng2:DsRed retina) and examined for Connexin43 (Cx43) expression or vessel patterns via immunolabeling. Tissues were blocked, permeabilized, and incubated 2 hours at room temperature or overnight at 4°C in PBST containing 1:500 (or 1:200 for embryos) rabbit anti-mouse Cx43 and/or 1:500 (or 1:200 for embryos) goat anti-mouse PECAM-1, and/or 1:200 rat anti-mouse PDGFRβ. The tissues were washed in PBS and incubated for 2h at RT in TBST with anti-goat AlexaFluor 647, anti-rabbit AlexaFluor 488, and/or anti-rat DyLight 550, at 2x dilution of the respective primary. Tissues were washed in PBS. DAPI was added at 1:1000 to the second to last wash and tissues were slide mounted for confocal or SoRa imaging.
Quantification and Statistical Analysis.
Statistical comparisons were made using GraphPad Prism 6.0, and P-values less than 0.05 were considered significant. Quantitative qRT-PCR RQ values were averaged, and the standard error of the mean (SEM) was found for each group. Three biological replicates were each generated from the average of two experimental replicates. Thus, each group was compared by one-way ANOVA followed by multiple comparisons via pair-wise Tukey t-test for each gene evaluated. For these groups, we analyzed our data for normality and equal variance as a pre-condition for applying a one-way ANOVA with Tukey post-hoc test. RNA-seq data was analyzed using the non-parametric Kruskal-Wallis test with Dunn’s post-hoc test. Vessel morphology data was analyzed by a two-tailed, unpaired Mann-Whitney test to compare ranks with a confidence level of 95%.
RESULTS
A Subset of Pericyte Precursors Emerge Adjacent to Developing Cardiac Tissue
Pericyte origins are often attributed to the neural crest and developing mesenchyme17,18, with several other embryonic sources described16,19–21. Additionally, differentiating PCs within the mural cell lineage have been associated with cardiac vasculature24 including the heart outflow tract26. To determine the spatio-temporal emergence of PC progenitors relative to the formation of cardiovascular structures, we incorporated “double-reporter” (DR) constructs into a mouse model of embryonic tissue development, in which an EC lineage contained a Flk-1-eGFP construct41, and a subset of PC precursors expressed the DsRed fluorescent protein under control of the neural glial antigen-2 (NG2)/Cspg4 promoter (Ng2-DsRed)42. We focused in vivo analysis on embryonic days 8.5 (E8.5) and 9.5 (Figure 1), as these stages provided observations of a critical time window for cardiovascular development43. These time-points are also prior to the emergence of NG2+ oligodendrocyte precursors (OPCs) and Schwann cells associated with neural tissue around E13–1544,45. In E8.5 DR embryos we found a population of Ng2-DsRed+ cells in close proximity to Flk-1-eGFP+ cells within the embryo proper, near the neural fold and within the cephalic mesenchyme46 (Figure 1A). At E9.5, we found these Ng2-DsRed+ cells prominently along the heart outflow tract and adjacent regions of the aortic root and ascending/dorsal aorta (Figure 1B and Online Video S1) – structures that form in part via vasculogenic processes5. Additionally, we found PDGFRβ+ mural cells closely associated with vessels within the cranial vasculature and along yolk sac vessels (Figure 1C). These data demonstrate that non-glial and non-Schwann cell NG2+ perivascular cells are present during formation of early cardiovascular structures.
Figure 1. Perivascular Cells Emerge Adjacent to Developing Cardiac Tissue.

(A): Single plane confocal images of embryonic day 8.5 (E8.5) whole mouse embryos (embryo 1 – i-iii and iv-vi, embryo 2 – vii – ix), with endogenous reporters Flk-1-eGFP (green), and Ng2-DsRed (red). me, mesenchyme; nf, neural fold; cm, cephalic mesenchyme. (B): Heart outflow tract of an E9.5 Ng2-DsRed+ embryo. (i) Epi-fluorescent image of an E9.5 heart from a Flk-1-eGFP-negative; Ng2-DsRed-positive mouse embryo, demonstrating Ng2-DsRed+ cells along the heart outflow tract (red) with gross animal morphology (dim green); (ii): Higher magnification of the Ng2-DsRed+ cells along the heart outflow tract. See Online Video S1. (C): Representative confocal images of E9.5 WT embryo cranial vasculature (i-iii) and yolk sac vessel (iv-vi) labeled for PECAM-1 and PDGFRβ. Arrowheads denote PC/mural cells along nascent vasculature.
To extend these findings, we isolated and validated a DR embryonic stem cell (DR-ESC) line (Figures S1-S3)34,36,47,48, derived from our DR in vivo model. We confirmed expression of commonly accepted PC and EC markers1,5 throughout differentiation (Figure 2A & B). With the exception of Pecam1/CD31 expression (which was consistent with previous studies49,50), each marker steadily increased throughout differentiation from a baseline of little-to-no transcript expression in undifferentiated cells. Levels of Tgfb1 (Figure 2B), a mesodermal germ layer marker and a key stimulus for propagating PC/vSMC lineages14,51,52, were also up-regulated. RNA Sequencing (RNA-Seq) analysis of distinct populations enriched by fluorescence-activated cell sorting (FACS; Figure S3) further revealed increased expression of expected markers for each cell type throughout differentiation (Figure 2C).
Figure 2. DR-ESC Endogenous Reporters Coincide Vascular EC and PC Lineage Markers.

(A): EC marker expression from unsorted undifferentiated, days 5 and 12 differentiated DR-ESCs. (B): Relative PC marker expression and Tgfβ1 (mural cell differentiation cue) from unsorted undifferentiated, days 5 and 12 differentiated DR-ESCs. Specific P-values are shown. Error bars, SEM (n=3). See Figures S1-S3 for additional DR-ESC validation. (C): Cell-specific expression of vascular markers by RNA-Seq for FACS-sorted Flk-1-eGFP+ and Ng2-DsRed+ cells in undifferentiated, days 7 and 10 differentiated DR-ESCs. See Figure S3 for sorting details. TPM, Transcripts per million. Medians with confidence limits. (n=2). (D): Days 6–8 differentiated DR-ESC-derived Ng2-DsRed+ cells (ii, vi, x; iv, viii and xii) labeled for PDGFRβ (i; iv), Desmin (v; viii), and NG2 (ix; xii). Flk-1-eGFP+ cells (xi; green in xii). Arrowheads indicate co-labeled cells (ix-xii). (E): DR-ESC-derived Ng2-DsRed+ cells (ii, vi, x; red in iv, viii, xii) not labeled by the OPC marker A2B5 (i, v, ix; blue in iv, viii, xii) in days 6 (i-iv), 8 (v-viii), 10 (ix-xii) differentiated DR-ESCs. Flk-1-eGFP+ mesoderm (iii) and differentiating ECs (vii, xi; green in iv, viii, xii). Dotted ovals (v-viii) and arrowheads (ix-xii) note A2B5 signal adjacent to, but not overlapping with, Flk-1-eGFP+ ECs. See Figure S4.
PC differentiation into neuronal/glial cell populations has been described19,20, although controversial22,23. Therefore, we immunolabeled differentiating DR-ESCs for accepted PC markers1 – platelet-derived growth factor receptor-β (PDGFRβ), desmin, and NG2, all of which co-labeled a subset of Ng2-DsRed+ cells, likely PCs and/or their progenitors (Figure 2D and Figure S4). These observations were largely consistent with other ESC-based systems used to explore the earliest stages of mural cell differentiation53. Additionally, immunolabeling for the OPC marker A2B5 yielded signals adjacent to, but not coinciding with, Flk-1-eGFP+ vessels with no apparent co-labeling of Ng2-DsRed+ cells (Figure 2E and Figure S4). Transcript levels of oligodendrocyte markers Mbp, Olig1, Olig2, and Plp1 from RNA-seq data of Ng2-DsRed+ sorted DR-ESCs, remained below a cutoff of transcripts per million (TPM) throughout differentiation (data not shown), further distinguishing Ng2-DsRed+ cells from glial cell types. Taken together, our data provide evidence to support the idea that a distinct subset of Ng2-DsRed+ cells in our models differentiates into a population displaying numerous hallmarks of the PC/mural cell lineage.
Similarly, we identified distinct populations of Flk-1+ cells, described previously41. Consistent with these reports, we found distinguishable Flk-1-eGFPhigh cells, which expressed the EC marker Platelet-Endothelial Cell Adhesion Molecule-1 (PECAM-1/CD31), arising from PECAM-1-negative Flk-1-eGFPlow cells of the primitive mesoderm (Figure 3A and Online Video S2). To further define the emergence of ECs from the primitive mesoderm throughout differentiation, we labeled DR-ESCs with PECAM-1 at days 6, 8, and 12 (Figure 3B and Figure S4), finding robust and consistent co-expression with Flk-1-eGFPhigh cells. Further, we detected an increase in the Flk-1 receptor on the surface of coalescing Flk-1-eGFPhigh cells (Figure 3C and Figure S4), verifying active Flk-1 protein synthesis. Together, these data support a high degree of EC and PC lineage fidelity for the Flk-1-eGFP and Ng2-DsRed reporters in DR-ESCs.
Figure 3. Differentiating ECs Organize into Primitive Vascular Networks in Proximity to Contracting Cardiomyocytes.

. (A): Day 6 differentiated DR-ESCs (i-iv, v-viii) labeled for PECAM-1 (ii, vi; blue in iv, viii). Arrows denote Flk-1-eGFPhigh cells (i, v; greenhigh – iv, viii). Arrowheads denote precursor Flk-1-eGFPlow mesoderm (i, v; greenlow – iv, viii). Nuclei, DAPI (iii, vii; white in iv, viii). See Online Video S2. (B): DR-ESC-derived cells (Flk-1-eGFP+; i, iv, vii; green in iii, vi, ix) labeled for PECAM-1 (ii, v, viii; blue in iii, vi,, ix) from days 6 (i-iii), 8 (iv-vi), 12 (vii-ix) of differentiation. Nuclei, DAPI (white in iii, vi, ix). (C): DR-ESC-derived cells (Flk-1-eGFP+; i, iv, vii; green in iii, vi, ix) labeled for Flk-1 receptor (blue) from days 6 (i-iii), 8 (iv-vi), 10 (vii-ix) of differentiation. Nuclei, DAPI (white in iii, vi, ix). See Figure S4. (D): Flk-1-eGFP+ cells (i; green in iii, v) and Ng2-DsRed+ cells (ii; red in iii, v) in vessels near contracting cardiomyocytes (white dashed line) and neighboring areas. See Online Video S3.
Validation of the emerging EC and PC identities within DR-ESCs facilitated insight into PC differentiation dynamics during embryonic tissue formation. Consistent with observing a population of Ng2-DsRed+ PC precursors along the outflow tract of a contracting embryonic heart (Figure 1B), we found DR-ESC-derived Ng2-DsRed+ PC/mural cell progenitors closely associated with primitive vascular structures within and adjacent to cardiomyocytes contracting in synchrony (Figure 3D and Online Video S3). Vessels were also apparent at distal and spatially heterogeneous locations (Figure 3D), suggestive of primitive capillary networks that arise in other embryonic and extra-embryonic tissues (e.g. yolk sac) during development. Together, these data suggest that a subpopulation of NG2+ mural precursors arise proximally to early-stage cardiac cells.
A Pericyte Progenitor Population Emerges Prior to Vasculogenesis Within In Vitro and In Vivo Models
Observations of PC progenitors along vasculature at these early stages inspired the hypothesis that a population within the PC/mural cell lineage may differentiate earlier than previously described2–5. Therefore, we conducted live imaging of cellular differentiation and vessel organization in differentiating DR-ESCs over several days, an approach difficult to achieve in vivo. Ultra-long time-lapse imaging (up to 120–140 hrs) facilitated real-time detection of each progenitor cell type/reporter and the earliest phases of vasculogenesis and angiogenic remodeling. Our analysis revealed distinct Ng2-DsRed reporter signals at the earliest captured time point during live imaging (day 6, Figure 4A), which consistently appeared prior to Flk-1-eGFPhigh signals produced by differentiating ECs (Figure 4A and B, and Online Video S4)41. We quantified mRNA transcripts of DsRed and eGFP via qRT-PCR in undifferentiated cultures (day 1) through day 9 of differentiation (Figure 4C). Transcript levels of DsRed increased on day 3, whereas eGFP transcripts appeared on day 5 that reflect the Flk-1-eGFPlow population within the emerging mesoderm, prior to observed EC differentiation and coalescence (Flk-1-eGFPhigh). These data suggest that a subset of Ng2-DsRed+ PC precursors may arise prior to EC differentiation, consistent with their early appearance during in vivo and in vitro embryonic cardiovascular development. This earlier emergence of PC progenitors in vivo was recently supported by single cell RNA-Sequencing (scRNA-seq) of mouse embryogenesis (E9.5-E13.5)54 in which a population of cells (Sub-cluster 21.8) expressed numerous established and emerging PC markers55–57, aligning with the RNA-Seq expression profile of Ng2:DsRed+ cells sorted from day 7 differentiated DR-ESCs (Figures S5 and S6). These data coincide with the proposed timing for the differentiation of a PC progenitor subpopulation to occur alongside, and perhaps before, endothelial emergence and vasculogenic organization.
Figure 4. Ng2-DsRed+ Cells Emerge Prior to Vasculogenesis and Are Distinct from Hemangiogenic Lineages.
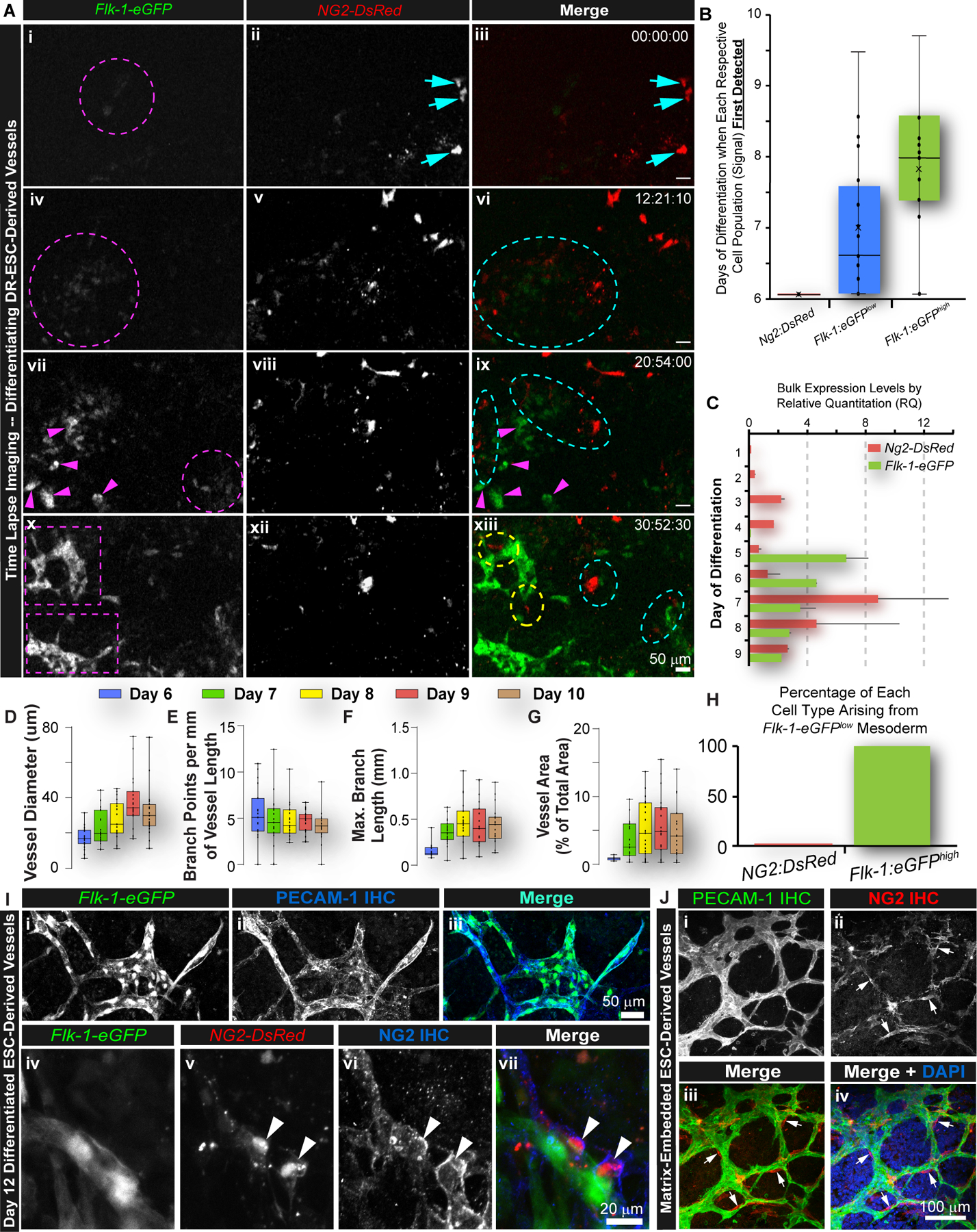
(A): Time-lapse images of DR-ESC differentiation over ~30 hours. Magenta arrowheads (vii, ix) denote emergence of ECs (x; xii). Dashed magenta circles are precursor Flk-1-eGFPlow mesoderm (i, iv, vii; green in iii, vi, ix). Dashed purple boxes indicated EC coalescence into primitive vessels (x). Cyan arrows are Ng2-DsRed+ cells (ii, v, viii, xii; red in iii, vi, ix, xiii) arising before Flk-1-eGFP+ signals. Dashed cyan ovals note Ng2-DsRed+ cell and Flk-1-eGFP+ mesoderm interactions (vi, ix, xii). Dashed yellow ovals note Ng2-DsRed+ cells interacting with ECs undergoing organization (xii). Time (hh:mm:sec). See Online Video S4. (B): Initial day of detection of Ng2-DsRed+ cells, Flk-1-eGFP+ mesodermal precursor cells (Flk-1-eGFPlow), and Flk-1-eGFP+ ECs (Flk-1-eGFPhigh) in days 6 to 11 differentiating DR-ESC (n=21, blinded). (C): Relative quantitation (RQ) of DsRed (Ng2) and eGFP (Flk-1) transcripts from unsorted DR-ESCs at day 1 (minimally differentiated) through day 9 of differentiation. Error bars, SD, (n=2). (D-G): Morphological quantifications of DR-ESC-derived vessels at days 6–10 of differentiation - vessel diameter (D), branch points per mm of vessel length (E), branch length (mm) (F), and vessel area as a percent of total area (G). (H): Percentages of Ng2-DsRed+ and Flk-1-eGFPhigh cells arising from Flk-1-eGFPlow mesoderm (n=21, blinded). (I): Day 12 differentiated DR-ESC-derived cells (Flk-1-eGFP+; i; green in iii) labeled for PECAM-1 (ii, blue in iii). Scale bar, 50 μm. Ng2-DsRed+ cells (v; red in vii) along Flk-1-eGFP+ cells (iv; green vii) labeled for cell surface NG2 protein (vi; blue in vii). Scale bar, 20 μm. (J): Matrix-embedded ESC-derived vessels labeled for PECAM-1 (i, green in iii and iv) and NG2 protein (ii, red in iii and iv). Scale bar, 100 μm. Arrows (ii, iii, iv) denote NG2+ cells at branch points within the PECAM-1+ EC network of primitive vessels.
We assessed the formation and remodeling of early vasculature to strengthen in vivo comparisons. We found primitive vessels forming during DR-ESC differentiation with additional remodeling occurring throughout the imaging time-course (Figure 4D-G). Interestingly, dynamic changes in vessel structure and organization stabilized over time, reaching a plateau across all metrics quantified. In all cases, an Ng2-DsRed+ PC progenitor subset was observed prior to vasculogenic formation of primordial networks that were refined further by additional angiogenic remodeling at later time points. Mirroring PC localization within an in vivo capillary plexus, NG2+ cells were also found frequently at branch points of primitive vascular networks formed by differentiating WT ESCs embedded in a fibrin matrix (Figure 4J). Collectively, these observations suggest the potential repositioning of PC/mural cell precursor engagement with the endothelium during the time-course of vascular development, placing them earlier in the vasculogenic phases of vessel formation.
Early Pericyte Differentiation Appears Distinct from Hemangiogenic Lineages
In addition to the established PC origins described above17,18, alternative PC origins have also been suggested58, including hemangiogenic lineages such as the cardiac endothelium16. Therefore, we sought to detect occurrences of Ng2-DsRed+ cells (which likely include cells within the PC lineage) emerging from the hemangiogenic mesoderm (Flk-1-eGFPlow) by tracking reporter cell populations from live imaging analysis of DR-ESC differentiation. We found no evidence of Ng2-DsRed+ cells arising from Flk-1-eGFPlow cells (Figure 4H), while most, if not all, Flk-1-eGFPhigh cells emerged from this compartment in our DR-ESC model. These observations were consistent with in vivo DR embryonic tissues, which showed no overlap in the DsRed and eGFP (low or high) signals (Figure 1), and ex vivo DR tissues59, supporting the notion that PC and EC progenitors likely emerge from discrete lineages. Distinctions between these populations were further verified by overlapping reporter signals with respective cell surface markers – PECAM-1 for ECs and NG2 surface protein for PCs (Figure 4I). RNA-seq and qRT-PCR transcriptional profiles, alongside live imaging and immunolabeling data, were consistent with the idea that Ng2-DsRed+ cells, which include a subset of PC progenitors in our DR-ESC model and in DR embryos, do not emerge from hemangiogenic lineages that are known to give rise to the endothelial compartment.
A Subset of Ng2-DsRed+ Pericyte Precursors Physically Engage Endothelial Cells During Vasculogenesis
Pericyte and mural cell progenitor association with the earliest stages of vascular development, specifically with vasculogenesis, remains relatively unexplored. Our observations suggested that mural cell and PC precursors may emerge concurrently with, if not prior to, EC organization into primitive vascular structures. This raised the question of whether a subset of Ng2-DsRed+ PC progenitors might engage the endothelium during vasculogenic formation of early blood vessels in our DR-ESC model. To begin addressing this question, we conducted ultra-long time-lapse imaging of differentiation and vascular development in DR-ESCs. At time points coinciding with vasculogenesis (i.e. days 5–7 of ESC differentiation60–63), we observed a population of Ng2-DsRed+ cells, likely including PC precursors, rapidly migrating within regions of Flk-1-eGFP+ mesoderm and differentiating ECs. This was followed by a slowing of translocation concurrent with multi-directional filopodia extensions and direct contact with both Flk-1-eGFP+ and Ng2-DsRed+ cells (Figure 5A, Online Video S5, Figure S7, and Online Video S6). We captured high-resolution confocal images of these interactions (Figure 5B), applying orthogonal and multi-dimensional analysis of each signal to provide additional support for physical engagement of these potential PC progenitors with vasculogenic ECs (Figure 5C-D and S8). These cell-cell interactions occurred for approximately 68.8% of the Ng2-DsRed+ cells observed (Figure S7), and they persisted into the angiogenic phase of vascular development. Live imaging over days 12–13 of DR-ESC differentiation provided numerous examples of Ng2-DsRed+ cells engaged with sprouting ECs and vessels undergoing lumenization (Figure 5E, Online Videos S7 and S8). Therefore, while it is established that in mature vasculature PCs interact with the endothelium64,65 and with other PCs30, physical association between PC and EC lineages has only been implied during early vascular development13,14. Here, we demonstrate direct interactions between populations of emerging PCs and ECs during the earliest stages of vasculogenesis in our models.
Figure 5. A Subset of Ng2-DsRed+ Cells Physically Engage Emerging Endothelial Cells During Vessel Formation.

(A): Time-lapse images of Flk-1-eGFP+ mesoderm (Flk-1-eGFPlow: i-iv & greenlow in ix-xii) and coalescing ECs (Flk-1-eGFPhigh: iii-iv, greenhigh: xi-xii) over day 5–6 of DR-ESC differentiation. Ng2-DsRed+ cells (v-viii & red in ix-xii) engage with multiple ECs (white arrows – vii & xi). Time (hh:mm). See Online Video S5, Figure S7, and Online Video S6. (B): Confocal image of Ng2-DsRed+ cells (i, red in iii) adjacent to Flk-1-eGFP+ cells (ii, green in iii), prior to vascular formation. Three distinct interactions are indicated by solid and dashed cyan arrows and a cyan arrowhead (iii). Scale bar, 50 μm. (C): Orthogonal, single-plane analysis of each interaction (cyan arrowhead in i, cyan arrow in ii, and dashed cyan arrow in iii). Dashed white circles/ovals indicate the interactions targeted by the white crosshair lines of the orthogonal single-plane analysis. (D): Three-dimensional renderings of z-stacks, shown compressed in ii (and in panel (C)), as viewed from the bottom right angle (i) and bottom left angle (iii). The intensities of the Ng2-DsRed (red) and Flk-1-eGFP (green) signals are represented three-dimensionally in an X-Y intensity plot (iv). See Figure S8. (E): Time-lapse images of a sprouting Flk-1-eGFP+ cells (i-v & xi-xv) interacting with Ng2-DsRed+ cells (vi-x & red in xi-xv; white arrows – i-ii, vi-vii, & xi-xii) over days 12–13 of ESC differentiation. Dividing Ng2-DsRed+ cell (cyan, magenta arrows – viii & xiii) yields a daughter cell (cyan arrows – ix-x & xiv-xv) that engages an EC sprout and migrates to a nearby branch point (cyan arrows – x & xv). Time (h:mm). See Online Video S7.
Connexin43 Contributes to Cell-Cell Communication between PC and EC Lineages during Early Vessel Formation
Physical engagement of Ng2-DsRed+ cells, likely a subset of PC/mural cell precursors, with vasculogenic ECs suggested the potential of intercellular communication among these cells (Figures 5, S7, and Online Videos 5-7). In fully formed microvascular networks, PCs form connexin-based gap junctions with neighboring PCs30 and ECs13,31,32, but relatively little is known about the formation of these intercellular channels during vascular development. To determine if Ng2-DsRed+ cells in our DR model were functionally coupled to neighboring vascular cells, we used a microinjection dye transfer assay at day 8 of differentiation (Figure 6A). We filled Ng2-DsRed+ cells, a subset likely to include PC precursors, with the intracellular dye Cascade Blue (Figure 6B), which emits primarily in the ultraviolet (UV) range (with modest green/eGFP crossover). We addressed potential signal overlap by image acquisition before and after microinjection (Figure 6A and C). During injection, adjacent Ng2-DsRed+ cells began to fluoresce in the UV range, indicating cell-cell coupling (Figure 6B), extending observations from mature vessels30,66. Following 30 minutes of dye transfer, we assessed Cascade Blue fluorescence by confocal microscopy (Figure 6C). Successful dye exchange was strongly detected from the microinjected Ng2-DsRed+ cell to two adjacent Flk-1-eGFP+ cells, consistent with intercellular coupling of a subset of these cells to nascent ECs. We observed dye signal in two additional cells (Figure 6C) – one in close proximity to a Flk-1-eGFP+/Cascade Blue+ cell, undergoing direct or indirect intercellular transfer. Overall, these data demonstrate direct coupling of Ng2-DsRed+ cells with differentiating ECs during vascular cell differentiation and the formation of primitive vasculature in our ESC model.
Figure 6. Ng2-DsRed+ Cells Establish Intercellular Communication with Vasculogenic Endothelium.

(A-C): Cyan arrows note the 1st (solid arrow) and 2nd (dashed arrow) Ng2-DsRed+ cells sequentially microinjected. The 2nd microinjected Ng2-DsRed+ cell (dashed arrow) did not survive the procedure (white asterisk). Cyan and magenta arrowheads respectively note an Ng2-DsRed+ cell and Flk-1-eGFP+ cells that received dye transfer. (A): Day 8 differentiated DR-ESCs before dye microinjection visualizing (40x) the ultraviolet (UV) spectrum (minimal signal in i, iv), Ng2-DsRed+ cells (ii; red in iv), and Flk-1-eGFP+ mesoderm (iii; greenlow - iv) and ECs (iii; greenhigh - iv). (B): Cascade Blue dye UV emission (i), NG2-DsRed+ cells (ii), and Flk-1-eGFP+ ECs and mesoderm (iii) during the 1st PC microinjection. Dye-filled needle (dashed black lines) emits signal in both UV and green spectra. (C): Cascade Blue dye (i; blue in iv) following transfer. Two additional cells with dye that appear to lack DsRed or eGFP signals (yellow arrows).
Connexin-based gap junctions facilitate intercellular communication in quiescent, functional vasculature. EC coupling to neighboring ECs and vSMCs allows exchange of second messengers and ions67. Here, we took an unbiased approach to determine which connexin isoforms may be involved during Ng2-DsRed+ cell-EC coupling. We assessed RNA-Seq transcriptional profiles of FACS-enriched Ng2-DsRed+ and Flk-1-eGFP+ cells from undifferentiated, day 7 and day 10 differentiating DR-ESCs (Figure 7A). During differentiation, both cell populations up-regulated Cx43/Gja1, by far the highest expression relative to all other mapped connexins. We corroborated these findings in day 10 differentiated ESCs focusing on: (i) cell-specific mRNA expression in EC and PC/mural progenitors isolated by magnetic bead-assisted cell separation (MACS) (Figure 7B), (ii) positive Cx43 labeling of gap junction plaques at the cell-cell interface of presumptive PCs and ECs (Figure 7C), and (iii) total protein via Western Blot (Figure 7D-F and Figure S10). In addition, we identified cell-specific phosphorylation states in Cx43 immuno-blots (Figure 7D), where phosphorylated Cx43 species correspond to differential electrophoretic isoforms68. Relative quantification of the Cx43 phosphorylation sites in MACS-sorted NG2+ cells (i.e. P0-P3 bands) revealed a distinct increase in the P1 band (Figure 7F) compared to ECs. This migration pattern corresponds to augmented phosphorylation at Serine365 (S365), suggesting localization of Cx43 at the plasma membrane and protection against gap junction down-regulation69. Thus, our data support the idea that the direct PC-EC coupling observed in the ESC model is facilitated, at least in part, by Cx43 heterocellular gap junctions, though other connexin isoforms such as Cx4513 cannot be fully excluded from potential involvement.
Figure 7. During Vessel Formation, Cells within a PC Lineage Preferentially Express and Localize Connexin43 at their Endothelial Interface.

(A): Transcript levels of cell-specific Connexin (Cx) isoforms from FACS-sorted (Flk-1-eGFP+ and Ng2-DsRed+) subpopulations of undifferentiated, days 7 and 10 differentiated DR-ESCs. TPM, Transcripts per million. Medians with confidence limits. (n=2). (B): Fold difference in Gja1 (Cx43) in MACS-sorted NG2+ cells vs. CD31/PECAM-1+ cells from WT-ESCs via qRT-PCR. (C): Day 10 differentiated WT ESCs labeled for PECAM-1 (i; green in iv, v), NG2 (ii; red in iv, v), and Cx43 (iii; blue in iv, v). Nuclei, DAPI (white in v). (D): Westerm Blot for Cx43 and GAPDH (housekeeping) from CD31+ cells and NG2+ cells isolated from day 10 WT-ESC-derived vessels by MACS. Raw immunoblot provided in Figure S10. (E): Fold difference in total NG2+ cell Cx43 protein vs. CD31/PECAM-1+ cell Cx43 levels, summing intensities for all Cx43 bands (panel D). (F): Fold difference in each Cx43 phospho-isoform from NG2+ cells vs. CD31+ cells isolated by MACS. (G): Embryonic day 9.5 brain microvessel labeled for EC PECAM-1 (i; blue in v), PC PDGFRβ (ii; red in v), and Cx43 (iii; green in v). Nuclei, DAPI (iv; white in v). Scale bar, 10 μm. Cx43 GJ plaques at the cell-cell interface (dashed white ovals). (H): Embryonic day 14.5 (E14.5) skin vessels from a Flk-1-eGFP; Ng2-DsRed embryo, visualizing ECs (i; green in v) and PCs (ii; red in v), and labeled Cx43 (iii; blue in v), within GJ plaques at the cell-cell interface (dashed white oval). Nuclei, DAPI (iv, white in v). Scale bar, 5 μm. See Figure S9. (I): P7 mouse retina microvessels labeled for EC PECAM-1 (i; blue in v), Ng2-DsRed+ cells (ii; red in v), and Cx43 (iii; green in v). Nuclei, DAPI (iv; white in v). Scale bar, 10 μm. Cx43 GJ plaques at cell-cell interface (yellow arrowheads).
To verify that these observations aligned with in vivo Cx43 localization at the PC-EC interface during embryonic vascular development, we analyzed Cx43 distribution in nascent vessels of E9.5 (brain) and E14.5 (skin) mice using high-power confocal microscopy (Figure 7G-H and Figure S9). We observed Cx43-enriched gap junction plaques appearing at the border between PDGFRβ+ or Ng2-DsRed+ PCs and PECAM-1+ or Flk-1-eGFP+ ECs, including cells bridging across two early-stage vessels. Because PCs are abundant in a variety of organ systems, such as the retina18, we addressed PC engagement with the remodeling retinal endothelium via Cx43-based gap junctions. We labeled PECAM-1 and Cx43 in developing retina capillary beds of a P7 Ng2-DsRed+ mouse (Figure 7I). At this developmental time-point, ECs sprout from a single vessel layer, facilitating a more tractable volumetric analysis70,71. We consistently observed Cx43-enriched plaques seemingly localized at the PC-EC interface, which coincided with data collected from embryonic stages.
Observing this preference for Cx43 within EC and PC lineages during vessel formation, we generated animals in which: (i) Cre-recombinase was expressed under the control of the Ng2/Cspg4 promoter (Ng2Cre/+), and (ii) exon 2 of the Cx43/Gja1 gene was flanked by loxP sites (Cx43lox/lox)72. In addition to containing control littermates, resultant litters included mice lacking substantial, if any, Cx43/Gja1 expression in Ng2+ cells including PCs. We observed vascular defects, specifically reduced branching morphogenesis, in a subset of E9 Ng2Cre/+; Cx43lox/lox embryos as compared to littermate controls (Figure 8A). These abnormalities contributed to a certain degree of embryonic lethality, as we found a reduction in the number of surviving Ng2Cre/+; Cx43lox/lox mutants, though Mendelian ratios of each genotype were overall relatively comparable. Interestingly, the Ng2Cre/+; Cx43lox/lox animals that survived to adulthood did not exhibit overt vascular dysmorphogenesis or disruption in PC differentiation in peripheral tissues at 10 weeks of age (Figure 8B-F). Therefore, while we did find vessel defects present in Ng2Cre/+; Cx43lox/lox animals at embryonic stages, mutant animals may be able to recover as they mature into adulthood. These animals may suffer from other cardiovascular complications such as aberrant blood pressure regulation seen with conditional deletion of Cx43/Gja1 in ECs72. Identifying these potential complications was beyond the scope of the current study, however, and will need to be addressed in future work.
Figure 8. Conditional Deletion of Cx43/Gja1 in Ng2+ Pericytes Induces Variable Vascular Dysmorphogenesis and Pericyte Investment.

(A): Embryonic day 9 of Ng2Cre/+; Cx43+/+ and Ng2Cre/+; Cx43lox/lox mouse major vessels (location denoted within whole embryo, yellow box inside insert) labeled for EC PECAM-1. Scale bar, 200 μm. Abnormal vessel morphology (dashed red ovals). (B): Graph of genotype distribution from Ng2Cre/+; Cx43lox/+ x Cx43lox/lox animal crosses as a percent of total animals generated. Inset number is total number of animals for each genotype. (C): Postnatal 10-week mouse retina microvessels from a Ng2Cre/+; Cx43lox/+ littermate (i-iii) and an Ng2Cre/+; Cx43lox/lox animal (iv-vi) labeled for PECAM-1/CD31 (i, iv; blue in iii, vi) and PDGFRβ (ii, v; red in iii, vi). Scale bars, 100 μm. (D): Branch points per mm of vessel length for Ng2Cre/+; Cx43lox/+ and Ng2Cre/+; Cx43lox/lox animals (n=3 for each genotype). (E): PDGFRβ+ pericytes per mm of vessel length for Ng2Cre/+; Cx43lox/+ and Ng2Cre/+; Cx43lox/lox animals (n=3 for each genotype). (F): PDGFRβ+ pericytes per vessel branch point for Ng2Cre/+; Cx43lox/+ and Ng2Cre/+; Cx43lox/lox animals (n=3 for each genotype). (G): Gene search analysis of Cx45/Gjc1 from the Mouse Organogenesis Cell Atlas database54. (H): Cell type and T-SNE cluster analysis of Cx45/Gjc1 expression from a cluster of cells within a presumptive PC lineage (Cluster 21.8). (I): Postnatal 10-week mouse retina microvessels from a Ng2Cre/+; Cx43lox/+ littermate (i-v) and an Ng2Cre/+; Cx43lox/lox animal (vi-x) labeled and analyzed by line scans for PECAM-1/CD31 (i and vi; blue in iv, ix), PDGFRβ (ii, vii; red in iv, ix), and Cx45 (iii, viii; green in iv, ix). Nuclei, DAPI (white in iv, ix). Scale bars, 5 μm. Line scan analysis (v, x) of each signal along the dotted white lines, “s” denotes scan start location, and “e” denotes scan end location in iv and ix, corresponding to v and x.
Nevertheless, these data suggested an additional hypothesis, specifically that another connexin isoform may be able to compensate for the loss of Cx43 in Ng2+ cells, which includes PCs and mural cells. Previous studies13 and the single cell transcriptional profiling databases discussed above54–57 point to Cx45 as a potential connexin isoform able to compensate for the genetic loss of Cx43/Gja1 (Figure 8G-H). Indeed Cx45 was found to be highly localized to the PC-EC interface in 10-week postnatal retina vessels of both Ng2Cre/+; Cx43lox/lox animals and their littermate controls (Figure 8I). Considering these data alongside previous studies suggests that, while Cx43 may be a preferred connexin isoform for facilitating PC communication with ECs in certain scenarios14,15,73,74, other connexin isoforms such as Cx45 can compensate for Cx43 loss and may in fact become the more dominant connexin isoform in mature vessels. These compensation mechanisms and Connexin isoform preference appear to be tissue- and perhaps time-dependent, as vascular mural cells appear to maintain a relatively high expression of Cx43 expression in certain regions such as the aortic root58. In contrast, Cx43 seems to be expressed at much lower levels in PCs/mural cells within more peripheral vascular beds58. Additional studies will be necessary to elucidate the potential functional roles of Connexin isoforms in modulating PC differentiation/identity and sustaining vessel formation and function including blood pressure regulation.
DISCUSSION
Pericytes make essential contributions to vessel maturation and stabilization, yet relatively little is known about the involvement of their progenitors during the earliest stages of vascular development. In the current study, we show that a distinct PC precursor lineage emerges prior to vasculogenic formation of nascent blood vessels during mouse embryogenesis and in a complementary ESC model. We found a subpopulation of Ng2-DsRed+ cells, likely mural cell and PC progenitors, in both scenarios localizing within and around primitive cardiac tissue, notably alongside the heart outflow tract in vivo, which gives rise to the aortic root/ascending aorta and pulmonary trunk. ESC-derived Ng2-DsRed+ cells, including presumptive PC precursors, did not overlap with hemangiogenic lineages, nor did they appear to give rise to neuronal/glial cell types. Interestingly, we found that this population of Ng2-DsRed+ cells physically engaged the endothelium during vasculogenesis, undergoing direct cell-cell coupling comparable to intercellular communication observed in functional vascular structure and networks. Crosstalk between subsets of potential PC and EC progenitors was supported by localization of Cx43-based gap junctions at the cell-cell interface, which were more prominent during ESC-derived vasculogenesis and were also found in remodeling blood vessels in vivo. Conditional deletion of Cx43/Gja1 in Ng2+ cells, including vascular PCs, led to embryonic blood vessel defects in a subset of animals; however, not all mutants succumbed embryonically nor did all mutants display overt vascular dysmorphogenesis or aberrant PC differentiation in peripheral tissues, suggesting compensation by or a shift towards other connexin isoforms such as Cx45. Taken together, these observations suggest that a discrete mural cell/PC lineage arises alongside, and perhaps before, EC differentiation, physically engaging the endothelium during vasculogenesis and directly communicating with emerging ECs via Cx43-mediated coupling.
Pericyte involvement within the vascular development timeline is most often described in vessel maturation processes3–5. Specifically, as the vasculature moves toward quiescence, PCs are implicated in vBM deposition6,7,75 and reinforcing EC junctions9–12, among other functions. During angiogenesis, PCs facilitate remodeling and vessel stabilization75, and are thought to interact with ECs primarily after early tube and plexus formation occurs, allowing for greater vascular plasticity2. However, live imaging of early stage DR-ESC differentiation presented herein revealed Ng2-DsRed+ cells, likely including PC precursors and mural cell intermediates24,76, directly contacting Flk-1-eGFP+ cells before and during vasculogenic organization of ECs. Moreover, a subset of Ng2-DsRed+ PC progenitors appeared to dynamically engage with surrounding Flk-1-eGFPhigh ECs, as well as Flk-1-eGFPlow mesoderm, in synchrony with EC activity, aligning with our previous observations12,59 and in vivo scRNA-Seq profiling of developing mouse embryos54. Though not a specific focus within the current study, PC precursor contact with emerging and differentiated ECs likely promotes lineage commitment for both cell types13–15. Here, we also found that PC-EC contact via Cx43 gap junctions may play a role in vessel morphogenesis in a tissue-specific and time-dependent manner. Together, these findings suggest that PC/mural cell progenitor differentiation and crosstalk with blood vessel formation likely occurs earlier in vascular development than previously appreciated.
Vascular PC origins are most often attributed to the neural crest and embryonic mesenchyme17,18, although several studies suggest the existence of a common ESC-derived Flk-1+ precursor16,77. In the models and time points explored in our current study, we found no evidence of Ng2-DsRed+ cells arising from Flk-1-eGFP+ cells, and no instances of convergence between Ng2-DsRed and Flk-1-eGFP signals by fluorescent reporter or transcriptional profiling after cell-specific sorting, consistent with previous observations12,21,40,78. Furthermore, PC trans-differentiation into neuronal/glial cell types has also been reported19,20, although debated22,23. We did not detect any overlap in the OPC marker A2B5 with Ng2-DsRed+ cells, which was corroborated by RNA-Seq analysis. Interestingly, the A2B5 signal was observed nearby (but not coinciding with) Flk-1-eGFP+ vessels derived from DR-ESCs. These observations suggest that OPCs may arise within differentiating DR-ESCs, highlighting the potential utility of this cell line in studying neurovascular development79 and in advancing stem cell-based models80,81 and therapeutic approaches. Therefore, while blood vessel formation involves coordination among cell types of various lineages, cells within the PC/mural lineage do not appear to share common origins with hemangiogenic/EC or glial/neuronal populations within the in vitro and in vivo models presented herein.
Connexin-based gap junctions facilitate intercellular communication between numerous cell types within the cardiovascular system. Cx43 in particular is involved in epicardial development82,83, and global Cx43 knockout mice develop heart outflow tract defects84–86, among other abnormalities. Cx43 has been shown to contribute to PC differentiation14 and vessel maturation73. Pericytes within global Cx43 null mice may therefore remain immature and fail to properly stabilize the vasculature, specifically along the heart outflow tract, which ultimately forms the aortic root/ascending aorta and pulmonary trunk. Interestingly, scRNA-Seq approaches have identified mural cell populations along the pulmonary trunk and aortic root as highly expressive of Cx43/Gja158, mirroring our observation of Ng2-DsRed+ cells enriched along the heart outflow tract. Collectively, these observations also suggest the potential for PC/mural cell diversity to reflect EC heterogeneity during development and beyond, perhaps utilizing distinct molecules and mechanisms for their interactions depending on the stage and/or tissue region. Cx45 for example may become the dominant connexin in PCs, particularly in peripheral and/or non-cardiac vascular beds54–56,58, and may be able to compensate for Cx43 loss or down-regulation, as suggested in the current study and by others13. Both isoforms appear to modulate TGFβ signaling such that disruption of these connexins leads to vascular defects including aberrant mural cell investment14,87. In the present study, we found several lines of evidence supporting the production and localization of Cx43 at the interface of cells within the EC and PC lineages comprising newly formed vasculature. Dye transfer experiments further supported direct cell-cell communication between a subset of Ng2-DsRed+ cells and the developing endothelium via the presence of Cx43-based gap junctions. Thus, the coupling of PCs and their progenitors to neighboring vascular cells within developing blood vessel networks may originate well before the maturation phase.
While PC roles in blood vessel function are being resolved in greater detail, contributions of their precursors to the earliest stages of vascular development remain relatively underexplored. In this study, we found that a subset of cells within the PC/mural cell lineage likely emerges before and alongside vasculogenesis in vitro and in vivo, and engages differentiating ECs during primitive vessel formation. Our observations further suggest that PCs and their progenitors may interact with vascular development beyond mechanisms underlying vessel stabilization and maturation. Greater understanding of PC precursor crosstalk with the earliest and most basic assembly of vascular structures is critical for expanding their therapeutic utility. For instance, a focus on PCs and mechanisms governing their differentiation and potential contributions to vascular morphogenesis will likely bolster vascularization strategies in tissue-engineered constructs and organoids88,89, and iPSC-based models80,81 and therapeutics90. These approaches may be further enhanced by promoting connexin-mediated PC communication with neighboring PCs and the endothelium, as our study and others13,14,30–32 demonstrate that gap junctions support blood vessel formation and function. Thus, insights presented herein of the PC/mural cell lineage engaging with the earliest stages of vessel development are vital to the advancement of novel therapeutic strategies to treat vascular-related pathologies.
Supplementary Material
HIGHLIGHTS.
A pericyte/mural cell precursor lineage emerged prior to vasculogenic formation of nascent blood vessels in embryo and stem cell models.
Pericyte and mural cell progenitors physically engaged the endothelium during vasculogenesis, undergoing direct cell-cell coupling.
Conditional deletion of Cx43/Gja1 in pericytes/mural cells led to embryonic vascular defects in a subset of mutants, while surviving mutants displayed little to no vessel abnormalities, suggesting compensatory mechanisms.
ACKNOWLEDGMENTS
The authors thank the Chappell Lab members for stimulating discussion during manuscript preparation.
SOURCES OF FUNDING
This work was supported by the American Heart Association grant 19POST34380560 (to LBP) and 19TPA34910121 (to JCC), by the NIH / NHLBI grant R00-HL105779 (to JCC), R56-HL133826 (to JCC), and R01-HL146596 (to JCC).
Abbreviations:
- Cx43
Connexin43
- DR-ESCs
double-reporter embryonic stem cells
- EC
endothelial cell
- PC
pericyte
- vBM
vascular basement membrane
- iPSCs
induced-pluripotent stem cells
- vSMC
vascular smooth muscle cell
Footnotes
DISCLOSURES
The authors declare no competing interests.
REFERENCES
- 1.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 2011;21:193–215. doi: S1534–5807(11)00269–3 [pii] 10.1016/j.devcel.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 2.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro-oncology 2005;7:452–464. doi: 10.1215/S1152851705000232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marmé D, Fusenig NE. Tumor angiogenesis : basic mechanisms and cancer therapy New York: Springer; 2008. [Google Scholar]
- 4.Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development 1998;125:1591–1598. [DOI] [PubMed] [Google Scholar]
- 5.Potente M, Makinen T. Vascular heterogeneity and specialization in development and disease. Nat Rev Mol Cell Biol 2017;18:477–494. doi: 10.1038/nrm.2017.36 [DOI] [PubMed] [Google Scholar]
- 6.Sava P, Cook IO, Mahal RS, Gonzalez AL. Human microvascular pericyte basement membrane remodeling regulates neutrophil recruitment. Microcirculation 2015;22:54–67. doi: 10.1111/micc.12173 [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Klett F, Potas JR, Hilpert D, Blazej K, Radke J, Huck J, Engel O, Stenzel W, Genove G, Priller J. Early loss of pericytes and perivascular stromal cell-induced scar formation after stroke. J Cereb Blood Flow Metab 2013;33:428–439. doi: 10.1038/jcbfm.2012.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stratman AN, Davis GE. Endothelial cell-pericyte interactions stimulate basement membrane matrix assembly: influence on vascular tube remodeling, maturation, and stabilization. Microsc Microanal 2012;18:68–80. doi: 10.1017/S1431927611012402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winkler EA, Birk H, Burkhardt JK, et al. Reductions in brain pericytes are associated with arteriovenous malformation vascular instability. J Neurosurg 2018:1–11. doi: 10.3171/2017.6.JNS17860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res 2006;312:623–629. doi: 10.1016/j.yexcr.2005.10.019 [DOI] [PubMed] [Google Scholar]
- 11.Jo DH, Kim JH, Heo JI, Kim JH, Cho CH. Interaction between pericytes and endothelial cells leads to formation of tight junction in hyaloid vessels. Molecules and Cells 2013;36:465–471. doi: 10.1007/s10059-013-0228-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao H, Darden J, Chappell JC. Establishment and Characterization of an Embryonic Pericyte Cell Line. Microcirculation 2018:e12461. doi: 10.1111/micc.12461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang JS, Dai C, Kurjiaka DT, Burt JM, Hirschi KK. Connexin45 regulates endothelial-induced mesenchymal cell differentiation toward a mural cell phenotype. Arterioscler Thromb Vasc Biol 2013;33:362–368. doi: 10.1161/ATVBAHA.112.255950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirschi KK, Burt JM, Hirschi KD, Dai C. Gap junction communication mediates transforming growth factor-beta activation and endothelial-induced mural cell differentiation. Circ Res 2003;93:429–437. doi: 10.1161/01.RES.0000091259.84556.D5 [DOI] [PubMed] [Google Scholar]
- 15.Brandt MM, van Dijk CGM, Maringanti R, Chrifi I, Kramann R, Verhaar MC, Duncker DJ, Mokry M, Cheng C. Transcriptome analysis reveals microvascular endothelial cell-dependent pericyte differentiation. Sci Rep 2019;9:15586. doi: 10.1038/s41598-019-51838-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Q, Zhang H, Liu Y, Adams S, Eilken H, Stehling M, Corada M, Dejana E, Zhou B, Adams RH. Endothelial cells are progenitors of cardiac pericytes and vascular smooth muscle cells. Nature communications 2016;7:12422. doi: 10.1038/ncomms12422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Etchevers HC, Vincent C, Le Douarin NM, Couly GF. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development 2001;128:1059–1068. [DOI] [PubMed] [Google Scholar]
- 18.Trost A, Schroedl F, Lange S, Rivera FJ, Tempfer H, Korntner S, Stolt CC, Wegner M, Bogner B, Kaser-Eichberger A, Krefft K, Runge C, Aigner L, Reitsamer HA. Neural crest origin of retinal and choroidal pericytes. Invest Ophthalmol Vis Sci 2013;54:7910–7921. doi: 10.1167/iovs.13-12946 [DOI] [PubMed] [Google Scholar]
- 19.Karow M, Sanchez R, Schichor C, Masserdotti G, Ortega F, Heinrich C, Gascon S, Khan MA, Lie DC, Dellavalle A, Cossu G, Goldbrunner R, Gotz M, Berninger B. Reprogramming of pericyte-derived cells of the adult human brain into induced neuronal cells. Cell Stem Cell 2012;11:471–476. doi: 10.1016/j.stem.2012.07.007 [DOI] [PubMed] [Google Scholar]
- 20.Guelfi S, Duffau H, Bauchet L, Rothhut B, Hugnot JP. Vascular Transdifferentiation in the CNS: A Focus on Neural and Glioblastoma Stem-Like Cells. Stem Cells Int 2016;2016:2759403. doi: 10.1155/2016/2759403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003 [DOI] [PubMed] [Google Scholar]
- 22.Guimaraes-Camboa N, Cattaneo P, Sun Y, Moore-Morris T, Gu Y, Dalton ND, Rockenstein E, Masliah E, Peterson KL, Stallcup WB, Chen J, Evans SM. Pericytes of Multiple Organs Do Not Behave as Mesenchymal Stem Cells In Vivo. Cell Stem Cell 2017;20:345–359 e345. doi: 10.1016/j.stem.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cano E, Gebala V, Gerhardt H. Pericytes or Mesenchymal Stem Cells: Is That the Question? Cell Stem Cell 2017;20:296–297. doi: 10.1016/j.stem.2017.02.005 [DOI] [PubMed] [Google Scholar]
- 24.Volz KS, Jacobs AH, Chen HI, Poduri A, McKay AS, Riordan DP, Kofler N, Kitajewski J, Weissman I, Red-Horse K. Pericytes are progenitors for coronary artery smooth muscle. Elife 2015;4. doi: 10.7554/eLife.10036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pouget C, Pottin K, Jaffredo T. Sclerotomal origin of vascular smooth muscle cells and pericytes in the embryo. Dev Biol 2008;315:437–447. doi: 10.1016/j.ydbio.2007.12.045 [DOI] [PubMed] [Google Scholar]
- 26.Arima Y, Miyagawa-Tomita S, Maeda K, Asai R, Seya D, Minoux M, Rijli FM, Nishiyama K, Kim KS, Uchijima Y, Ogawa H, Kurihara Y, Kurihara H. Preotic neural crest cells contribute to coronary artery smooth muscle involving endothelin signalling. Nature communications 2012;3:1267. doi: 10.1038/ncomms2258 [DOI] [PubMed] [Google Scholar]
- 27.Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood 2009;114:5091–5101. doi: blood-2009–05-222364 [pii] 10.1182/blood-2009-05-222364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baluk P, Morikawa S, Haskell A, Mancuso M, McDonald DM. Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. Am J Pathol 2003;163:1801–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Courtoy PJ, Boyles J. Fibronectin in the microvasculature: localization in the pericyte-endothelial interstitium. J Ultrastruct Res 1983;83:258–273. [DOI] [PubMed] [Google Scholar]
- 30.Alarcon-Martinez L, Villafranca-Baughman D, Quintero H, Kacerovsky JB, Dotigny F, Murai KK, Prat A, Drapeau P, Di Polo A. Interpericyte tunnelling nanotubes regulate neurovascular coupling. Nature 2020. doi: 10.1038/s41586-020-2589-x [DOI] [PubMed] [Google Scholar]
- 31.Ivanova E, Kovacs-Oller T, Sagdullaev BT. Vascular Pericyte Impairment and Connexin43 Gap Junction Deficit Contribute to Vasomotor Decline in Diabetic Retinopathy. J Neurosci 2017;37:7580–7594. doi: 10.1523/JNEUROSCI.0187-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li AF, Sato T, Haimovici R, Okamoto T, Roy S. High glucose alters connexin 43 expression and gap junction intercellular communication activity in retinal pericytes. Invest Ophthalmol Vis Sci 2003;44:5376–5382. [DOI] [PubMed] [Google Scholar]
- 33.Chappell JC, Cluceru JG, Nesmith JE, Mouillesseaux KP, Bradley V, Hartland C, Hashambhoy-Ramsay YL, Walpole J, Peirce SM, Gabhann FM, Bautch VL. Flt-1 (VEGFR-1) Coordinates Discrete Stages of Blood Vessel Formation. Cardiovasc Res 2016;111:84–93. doi: 10.1093/cvr/cvw091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Czechanski A, Byers C, Greenstein I, Schrode N, Donahue LR, Hadjantonakis AK, Reinholdt LG. Derivation and characterization of mouse embryonic stem cells from permissive and nonpermissive strains. Nat Protoc 2014;9:559–574. doi: 10.1038/nprot.2014.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bryja V, Bonilla S, Arenas E. Derivation of mouse embryonic stem cells. Nat Protoc 2006;1:2082–2087. doi: 10.1038/nprot.2006.355 [DOI] [PubMed] [Google Scholar]
- 36.O’Connor MD, Kardel MD, Iosfina I, Youssef D, Lu M, Li MM, Vercauteren S, Nagy A, Eaves CJ. Alkaline phosphatase-positive colony formation is a sensitive, specific, and quantitative indicator of undifferentiated human embryonic stem cells. Stem Cells 2008;26:1109–1116. doi: 10.1634/stemcells.2007-0801 [DOI] [PubMed] [Google Scholar]
- 37.Kappas NC, Zeng G, Chappell JC, Kearney JB, Hazarika S, Kallianos KG, Patterson C, Annex BH, Bautch VL. The VEGF receptor Flt-1 spatially modulates Flk-1 signaling and blood vessel branching. J Cell Biol 2008;181:847–858. doi: jcb.200709114 [pii], or 10.1083/jcb.200709114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chappell JC, Mouillesseaux KP, Bautch VL. Flt-1 (Vascular Endothelial Growth Factor Receptor-1) Is Essential for the Vascular Endothelial Growth Factor-Notch Feedback Loop During Angiogenesis. Arterioscler Thromb Vasc Biol 2013;33:1952–1959. doi: ATVBAHA.113.301805 [pii], or 10.1161/ATVBAHA.113.301805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol 2016;34:525–527. doi: 10.1038/nbt.3519 [DOI] [PubMed] [Google Scholar]
- 40.Darden J, Payne LB, Zhao H, Chappell JC. Excess vascular endothelial growth factor-A disrupts pericyte recruitment during blood vessel formation. Angiogenesis 2018. doi: 10.1007/s10456-018-9648-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ema M, Takahashi S, Rossant J. Deletion of the selection cassette, but not cis-acting elements, in targeted Flk1-lacZ allele reveals Flk1 expression in multipotent mesodermal progenitors. Blood 2006;107:111–117. doi: 10.1182/blood-2005-05-1970 [DOI] [PubMed] [Google Scholar]
- 42.Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development 2008;135:145–157. doi: 10.1242/dev.004895 [DOI] [PubMed] [Google Scholar]
- 43.Walls JR, Coultas L, Rossant J, Henkelman RM. Three-dimensional analysis of vascular development in the mouse embryo. PLoS One 2008;3:e2853. doi: 10.1371/journal.pone.0002853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trotter J, Karram K, Nishiyama A. NG2 cells: Properties, progeny and origin. Brain Res Rev 2010;63:72–82. doi: 10.1016/j.brainresrev.2009.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castro R, Taetzsch T, Vaughan SK, Godbe K, Chappell J, Settlage RE, Valdez G. Specific labeling of synaptic schwann cells reveals unique cellular and molecular features. Elife 2020;9. doi: 10.7554/eLife.56935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morgani SM, Su J, Nichols J, Massague J, Hadjantonakis AK. The transcription factor Rreb1 regulates epithelial architecture, invasiveness, and vasculogenesis in early mouse embryos. Elife 2021;10. doi: 10.7554/eLife.64811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gaztelumendi N, Nogues C. Chromosome instability in mouse embryonic stem cells. Sci Rep 2014;4:5324. doi: 10.1038/srep05324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wray J, Kalkan T, Smith AG. The ground state of pluripotency. Biochem Soc Trans 2010;38:1027–1032. doi: 10.1042/bst0381027 [DOI] [PubMed] [Google Scholar]
- 49.Li ZJ, Wang ZZ, Zheng YZ, Xu B, Yang RC, Scadden DT, Han ZC. Kinetic expression of platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) during embryonic stem cell differentiation. J Cell Biochem 2005;95:559–570. doi: 10.1002/jcb.20436 [DOI] [PubMed] [Google Scholar]
- 50.Robson P, Stein P, Zhou B, Schultz RM, Baldwin HS. Inner cell mass-specific expression of a cell adhesion molecule (PECAM-1/CD31) in the mouse blastocyst. Dev Biol 2001;234:317–329. doi: 10.1006/dbio.2001.0274 [DOI] [PubMed] [Google Scholar]
- 51.Gaengel K, Genove G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol 2009;29:630–638. doi: ATVBAHA.107.161521[pii] 10.1161/ATVBAHA.107.161521 [DOI] [PubMed] [Google Scholar]
- 52.Sieczkiewicz GJ, Herman IM. TGF-beta 1 signaling controls retinal pericyte contractile protein expression. Microvasc Res 2003;66:190–196. [DOI] [PubMed] [Google Scholar]
- 53.Lindskog H, Athley E, Larsson E, Lundin S, Hellstrom M, Lindahl P. New insights to vascular smooth muscle cell and pericyte differentiation of mouse embryonic stem cells in vitro. Arterioscler Thromb Vasc Biol 2006;26:1457–1464. doi: 10.1161/01.ATV.0000222925.49817.17 [DOI] [PubMed] [Google Scholar]
- 54.Cao J, Spielmann M, Qiu X, Huang X, Ibrahim DM, Hill AJ, Zhang F, Mundlos S, Christiansen L, Steemers FJ, Trapnell C, Shendure J. The single-cell transcriptional landscape of mammalian organogenesis. Nature 2019;566:496–502. doi: 10.1038/s41586-019-0969-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vanlandewijck M, He L, Mae MA, et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature 2018;554:475–480. doi: 10.1038/nature25739 [DOI] [PubMed] [Google Scholar]
- 56.He L, Vanlandewijck M, Mae MA, et al. Single-cell RNA sequencing of mouse brain and lung vascular and vessel-associated cell types. Sci Data 2018;5:180160. doi: 10.1038/sdata.2018.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He L, Vanlandewijck M, Raschperger E, Andaloussi Mae M, Jung B, Lebouvier T, Ando K, Hofmann J, Keller A, Betsholtz C. Analysis of the brain mural cell transcriptome. Sci Rep 2016;6:35108. doi: 10.1038/srep35108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muhl L, Genové G, Leptidis S, et al. Single-cell analysis uncovers fibroblast heterogeneity and criteria for fibroblast and mural cell identification and discrimination. Nat Commun 2020;11:3953. doi: 10.1038/s41467-020-17740-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Payne LB, Darden J, Suarez-Martinez AD, Zhao H, Hendricks A, Hartland C, Chong D, Kushner EJ, Murfee WL, Chappell JC. Pericyte migration and proliferation are tightly synchronized to endothelial cell sprouting dynamics. Integr Biol (Camb) 2021;13:31–43. doi: 10.1093/intbio/zyaa027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jakobsson L, Domogatskaya A, Tryggvason K, Edgar D, Claesson-Welsh L. Laminin deposition is dispensable for vasculogenesis but regulates blood vessel diameter independent of flow. FASEB J 2007. [DOI] [PubMed] [Google Scholar]
- 61.Tillet E, Vittet D, Feraud O, Moore R, Kemler R, Huber P. N-cadherin deficiency impairs pericyte recruitment, and not endothelial differentiation or sprouting, in embryonic stem cell-derived angiogenesis. Exp Cell Res 2005;310:392–400. doi: S0014–4827(05)00394–0 [pii] 10.1016/j.yexcr.2005.08.021 [DOI] [PubMed] [Google Scholar]
- 62.Bautch VL, Redick SD, Scalia A, Harmaty M, Carmeliet P, Rapoport R. Characterization of the vasculogenic block in the absence of vascular endothelial growth factor-A. Blood 2000;95:1979–1987. [PubMed] [Google Scholar]
- 63.Risau W, Sariola H, Zerwes HG, Sasse J, Ekblom P, Kemler R, Doetschman T. Vasculogenesis and angiogenesis in embryonic-stem-cell-derived embryoid bodies. Development 1988;102:471–478. [DOI] [PubMed] [Google Scholar]
- 64.Caruso RA, Fedele F, Finocchiaro G, Pizzi G, Nunnari M, Gitto G, Fabiano V, Parisi A, Venuti A. Ultrastructural descriptions of pericyte/endothelium peg-socket interdigitations in the microvasculature of human gastric carcinomas. Anticancer Res 2009;29:449–453. [PubMed] [Google Scholar]
- 65.Diaz-Flores L, Gutierrez R, Varela H, Rancel N, Valladares F. Microvascular pericytes: a review of their morphological and functional characteristics. Histol Histopathol 1991;6:269–286. [PubMed] [Google Scholar]
- 66.Kovacs-Oller T, Ivanova E, Bianchimano P, Sagdullaev BT. The pericyte connectome: spatial precision of neurovascular coupling is driven by selective connectivity maps of pericytes and endothelial cells and is disrupted in diabetes. Cell Discov 2020;6:39. doi: 10.1038/s41421-020-0180-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bagher P, Segal SS. Regulation of blood flow in the microcirculation: role of conducted vasodilation. Acta Physiol (Oxf) 2011;202:271–284. doi: 10.1111/j.1748-1716.2010.02244.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Solan JL, Lampe PD. Spatio-temporal regulation of connexin43 phosphorylation and gap junction dynamics. Biochim Biophys Acta Biomembr 2018;1860:83–90. doi: 10.1016/j.bbamem.2017.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Solan JL, Marquez-Rosado L, Sorgen PL, Thornton PJ, Gafken PR, Lampe PD. Phosphorylation at S365 is a gatekeeper event that changes the structure of Cx43 and prevents down-regulation by PKC. J Cell Biol 2007;179:1301–1309. doi: 10.1083/jcb.200707060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chappell JC, Darden J, Payne LB, Fink K, Bautch VL. Blood Vessel Patterning on Retinal Astrocytes Requires Endothelial Flt-1 (VEGFR-1). J Dev Biol 2019;7. doi: 10.3390/jdb7030018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 2003;161:1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liao Y, Day KH, Damon DN, Duling BR. Endothelial cell-specific knockout of connexin 43 causes hypotension and bradycardia in mice. Proc Natl Acad Sci U S A 2001;98:9989–9994. doi: 10.1073/pnas.171305298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Durham JT, Dulmovits BM, Cronk SM, Sheets AR, Herman IM. Pericyte chemomechanics and the angiogenic switch: insights into the pathogenesis of proliferative diabetic retinopathy? Invest Ophthalmol Vis Sci 2015;56:3441–3459. doi: 10.1167/iovs.14-13945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tien T, Muto T, Barrette K, Challyandra L, Roy S. Downregulation of Connexin 43 promotes vascular cell loss and excess permeability associated with the development of vascular lesions in the diabetic retina. Molecular vision 2014;20:732–741. [PMC free article] [PubMed] [Google Scholar]
- 75.Stratman AN, Pezoa SA, Farrelly OM, Castranova D, Dye LE, 3rd, Butler MG, Sidik H, Talbot WS, Weinstein BM. Interactions between mural cells and endothelial cells stabilize the developing zebrafish dorsal aorta. Development 2017;144:115–127. doi: 10.1242/dev.143131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mikawa T, Gourdie RG. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev Biol 1996;174:221–232. doi: 10.1006/dbio.1996.0068 [DOI] [PubMed] [Google Scholar]
- 77.Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, Naito M, Nakao K, Nishikawa S. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature 2000;408:92–96. doi: 10.1038/35040568 [DOI] [PubMed] [Google Scholar]
- 78.Kim JM, Hong KS, Song WK, Bae D, Hwang IK, Kim JS, Chung HM. Perivascular Progenitor Cells Derived From Human Embryonic Stem Cells Exhibit Functional Characteristics of Pericytes and Improve the Retinal Vasculature in a Rodent Model of Diabetic Retinopathy. Stem Cells Transl Med 2016;5:1268–1276. doi: 10.5966/sctm.2015-0342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maki T Novel roles of oligodendrocyte precursor cells in the developing and damaged brain. Clinical and Experimental Neuroimmunology 2017;8:33–42. doi: 10.1111/cen3.12358 [DOI] [Google Scholar]
- 80.Stebbins MJ, Gastfriend BD, Canfield SG, Lee MS, Richards D, Faubion MG, Li WJ, Daneman R, Palecek SP, Shusta EV. Human pluripotent stem cell-derived brain pericyte-like cells induce blood-brain barrier properties. Sci Adv 2019;5:eaau7375. doi: 10.1126/sciadv.aau7375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jamieson JJ, Searson PC, Gerecht S. Engineering the human blood-brain barrier in vitro. J Biol Eng 2017;11:37. doi: 10.1186/s13036-017-0076-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rhee DY, Zhao XQ, Francis RJ, Huang GY, Mably JD, Lo CW. Connexin 43 regulates epicardial cell polarity and migration in coronary vascular development. Development 2009;136:3185–3193. doi: 10.1242/dev.032334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Francis R, Xu X, Park H, Wei C-J, Chang S, Chatterjee B, Lo C. Connexin43 modulates cell polarity and directional cell migration by regulating microtubule dynamics. PloS one 2011;6:e26379–e26379. doi: 10.1371/journal.pone.0026379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ya J, Erdtsieck-Ernste EB, de Boer PA, van Kempen MJ, Jongsma H, Gros D, Moorman AF, Lamers WH. Heart defects in connexin43-deficient mice. Circ Res 1998;82:360–366. doi: 10.1161/01.res.82.3.360 [DOI] [PubMed] [Google Scholar]
- 85.Liu S, Liu F, Schneider AE, St Amand T, Epstein JA, Gutstein DE. Distinct cardiac malformations caused by absence of connexin 43 in the neural crest and in the non-crest neural tube. Development 2006;133:2063–2073. doi: 10.1242/dev.02374 [DOI] [PubMed] [Google Scholar]
- 86.Huang GY, Xie LJ, Linask KL, et al. Evaluating the role of connexin43 in congenital heart disease: Screening for mutations in patients with outflow tract anomalies and the analysis of knock-in mouse models. J Cardiovasc Dis Res 2011;2:206–212. doi: 10.4103/0975-3583.89804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kruger O, Plum A, Kim JS, Winterhager E, Maxeiner S, Hallas G, Kirchhoff S, Traub O, Lamers WH, Willecke K. Defective vascular development in connexin 45-deficient mice. Development 2000;127:4179–4193. [DOI] [PubMed] [Google Scholar]
- 88.Tourovskaia A, Fauver M, Kramer G, Simonson S, Neumann T. Tissue-engineered microenvironment systems for modeling human vasculature. Exp Biol Med (Maywood) 2014;239:1264–1271. doi: 10.1177/1535370214539228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Campagnolo P, Gormley AJ, Chow LW, Guex AG, Parmar PA, Puetzer JL, Steele JA, Breant A, Madeddu P, Stevens MM. Pericyte Seeded Dual Peptide Scaffold with Improved Endothelialization for Vascular Graft Tissue Engineering. Adv Healthc Mater 2016;5:3046–3055. doi: 10.1002/adhm.201600699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kusuma S, Shen YI, Hanjaya-Putra D, Mali P, Cheng L, Gerecht S. Self-organized vascular networks from human pluripotent stem cells in a synthetic matrix. Proc Natl Acad Sci U S A 2013;110:12601–12606. doi: 10.1073/pnas.1306562110 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


