Abstract
Objective:
Irritability is a common characteristic in ADHD. We examined whether dysfunction in neural connections supporting threat and reward processing was related to irritability in adolescents and young adults with ADHD.
Method:
We used resting-state fMRI to assess connectivity of amygdala and nucleus accumbens seeds in those with ADHD (n = 34) and an age- and gender-matched typically-developing comparison group (n = 34).
Results:
In those with ADHD, irritability was associated with atypical functional connectivity of both seed regions. Amygdala seeds showed greater connectivity with right inferior frontal gyrus and caudate/putamen, and less connectivity with precuneus. Nucleus accumbens seeds showed altered connectivity with middle temporal gyrus and precuneus.
Conclusion:
The irritability-ADHD presentation is associated with atypical functional connectivity of reward and threat processing regions with cognitive control and emotion processing regions. These patterns provide novel evidence for irritability-associated neural underpinnings in adolescents and young adults with ADHD. The findings suggest cognitive and behavioral treatments that address response to reward, including omission of an expected reward and irritability, may be beneficial for ADHD.
Keywords: functional connectivity, irritability, adolescence, ADHD, amygdala, nucleus accumbens
Introduction
Irritability—proneness to anger and agitation—is common in individuals with ADHD, affecting between 25% and 70% of youth with the disorder (Geller et al., 2002; Shaw et al., 2014). In adolescents with ADHD, irritability is associated with increased sleep problems, social difficulties, parental stress, and days missed from school (Mulraney et al., 2017) and alarmingly, related to a marked increase in risk for suicide completion (James et al., 2004), engaging in self-harming behaviors (Swanson et al., 2014), and substance abuse (Harty et al., 2017). However, despite the clinical importance of irritability combined with ADHD, relatively little is known about the underlying pathophysiology.
Alterations in threat- and reward-based processing neural circuits have been proposed to account for irritability. Specifically, altered function of a circuit comprising the prefrontal cortex (PFC), anterior cingulate cortex (ACC), striatum, and amygdala may give rise to irritability in response to a frustrative non-reward, that is, a result of not attaining a goal (Brotman et al., 2017). In contrast, dysfunction in a circuit involving the PFC, amygdala, hypothalamus, and periaqueductal gray may propel irritability in response to a threat such as, for example, aggressive acts or criticism (Brotman et al., 2017). Previous studies suggest that emotional lability—a construct related to irritability—is associated with altered functional connectivity of the amygdala in those with ADHD (Hulvershorn et al., 2013; Yu et al., 2020). Neuroimaging of resting-state functional connectivity—that is, the temporal correlation of neural activity between brain regions during rest—is a common approach to assess altered communication among spatially remote brain regions in clinical populations (Fox & Greicius, 2010). Compared to previous research reporting atypical amygdala functional connectivity, less is known about connectivity in reward processing regions and their association with irritability despite ample evidence that alterations in striatal-reward regions are common in those with ADHD (Konrad & Eickhoff, 2010; Rubia, 2018; Samea et al., 2019). To address this gap in the literature, we examined both amygdala and nucleus accumbens (NAcc) connectivity to determine whether irritability in adolescents and young adults with ADHD is associated with atypical connectivity of these two regions that form part of these two networks, with other brain regions. Identifying such connections may provide new clues about what behaviors to intervene with depending on the regions with which the amygdala and NAcc demonstrate coordinated communication in relation to irritability.
We focused on the amygdala as a central region within the threat-based circuit and on the NAcc as a central region within the reward processing circuit. In children with ADHD, previous resting-state investigations suggest an association between emotional lability and increased functional connectivity between the amygdala and the rostral ACC as well as decreased functional connectivity with posterior insula/superior temporal gyrus (Hulvershorn et al., 2013). Longitudinally, among children with ADHD, an irritable subtype—characterized by increased negative emotionality and greater risk for subsequent comorbidities—exhibited reduced amygdala–insula functional connectivity (Karalunas et al., 2014). A more recent study also employing a seed-based approach reported an association between higher emotional lability and lower functional connectivity of a subregion of the right amygdala with the right dorsolateral PFC and bilateral inferior parietal lobes (Yu et al., 2020). The limited overlap in findings among these studies suggests that more attention is needed to understand the relation between irritability in ADHD and underlying threat circuitry function.
Given these preliminary findings, irritability in ADHD is likely to be associated with dysfunctional amygdala functional connectivity. However, common abnormalities in reward processing suggest that functional connectivity of striatal regions may also play a role in irritability in youth with ADHD, possibly contributing to aberrant behavioral response to frustrative non-rewards (Brotman et al., 2017). Compared to the general population, adolescents and adults with ADHD show striatal hyporesponsiveness during reward anticipation (Plichta & Scheres, 2013). Additionally, resting-state functional connectivity between the left NAcc and the left orbitofrontal cortex correlated with symptoms of emotional lability in 7- to 12-year-old children diagnosed with ADHD (Posner et al., 2013). Behaviorally, children with ADHD prefer immediate, small rewards over larger, delayed rewards (Marco et al., 2009; Marx et al., 2021; Schweitzer & Sulzer-Azaroff, 1995), which has been linked to both NAcc-PFC functional connectivity and amygdala hyperactivity (Costa Dias et al., 2013; Plichta et al., 2009). Taken together, these studies suggest that aberrant functional connectivity in the reward network, and involving the NAcc specifically, may contribute to irritability symptoms in ADHD.
We examined functional connectivity of the amygdala and NAcc seeds and their relations to increased irritability symptoms in those with ADHD, compared to age- and gender-matched typically developing (TD) adolescents and young adults. Informed by prior research using resting-state functional connectivity to examine the neural basis of irritability and emotional lability in children and adults with ADHD (Hulvershorn et al., 2013; Karalunas et al., 2014; Yu et al., 2020), we hypothesized that higher irritability symptoms would be associated with altered resting-state connectivity of the amygdala and NAcc in adolescents and young adults with ADHD, compared to their TD peers. We predicted that functional connectivity with regions of the PFC would be particularly sensitive to differing levels of irritability but refrained from making specific predictions about the direction of connectivity.
Method
Participants
In the total ADHD sample, participants included 56 adolescents and young adults (aged 12–23 years) with a diagnosis of ADHD-Combined Presentation. Following exclusion criteria and sample-matching procedures detailed below, the final sample in the current study included 34 youth in the ADHD group and 34 youth in the TD group. We refer to the participants as “adolescents,” acknowledging that the age span includes young adults as well. Two licensed psychologists with extensive experience diagnosing ADHD (JFD, JBS) evaluated initial phone screening data to determine eligibility for the study. Participants meeting the phone screen criteria were invited to proceed to the next phase of the study, which included an in-depth, in-person psychological evaluation. Participants were evaluated according to the Diagnostic and Statistical Manual of Mental Disorders-IV-TR or 5th Edition (DSM 5, which was used upon its publication) criteria for ADHD and all other major psychiatric disorders (e.g., mood disorders, anxiety, obsessive-compulsive, trauma, psychosis, addiction, oppositional defiant, and conduct disorders). Diagnostic interviews included the participant and one of their parents/caregivers for adolescents, while adult participants could choose to have their parents, partner, or spouse rate their current behavior, depending upon who was most familiar with their current behavior (Diagnostic Interview Schedule for Child and Adolescents and Young Adult version; Shaffer et al., 2000). Parents also reported on symptoms using the Conners-3 Parent Rating Scale (CPRS-3) or the Conners’ Adult ADHD Rating Scale (CAARS-O), Observer Form—Long Version (with parent ratings on the young adults) (Conners, 2008). Parents of young adults also completed the Barkley Adult ADHD Rating Scale—Retrospective Scale (Barkley, 2011) to establish the presence of significant ADHD behavior before the age of 12 years.
A licensed psychologist (JFD) reviewed all of the diagnostic information to determine final ADHD diagnosis and presence of other disorders based on the diagnostic interview and DSM Predominantly Inattentive and Predominantly Hyperactive/Impulsive Presentation Scales from the CPRS or CAARS. Conners-3 Teacher Rating Scales (CTRS) further informed diagnosis if there were contradictions between the interview and parent rating scale data. In complex cases (i.e., disagreement between CPRS, CTRS, and the clinical interview), further follow-up interviews were conducted by JFD, and JFD and JBS both reviewed all diagnostic information to make a final expert diagnosis determination.
Inclusion criteria included IQ ≥ 80 and age between 12 and 23 years, with additional inclusion criteria for the ADHD group of meeting DSM-IV-TR or DSM-5 criteria for ADHD, Combined Presentation or Hyperactive/Impulsive Presentation. However, all participants in this study met criteria for the Combined Presentation and none for the Hyperactive/Impulsive Presentation. Exclusion criteria included: IQ score < 80; presence of a math or reading learning disability; a self- or parent-reported history of head trauma, neurological disorder, or major medical problem; prescribed psychoactive medication beyond ADHD medications (i.e., other than stimulants or atomoxetine); presence of any other DSM-IV-TR or DSM-5 Axis I diagnosis besides ADHD, oppositional defiant disorder, or conduct disorder; a positive drug screen on the day of the imaging session; and MRI contra-indications. Of the 56 clinical participants, 22 (39%) were excluded due to excessive head motion (mean frame-wise displacement > 0.35) resulting in a final clinical sample of 34 adolescents with ADHD (22 male, 12 female). The 22 excluded participants did not differ significantly from the remaining 34 patients in their average IQ, inattentive symptoms, and irritability ratings or, in the distribution of gender, race, ethnicity, household income, and maternal education. The excluded participants were however on average younger (t(54) = 3.134, p = .003) and with higher hyperactive-impulsive symptoms (t(53) = −2.657, p = .010). Twenty-three of the ADHD participants were on prescription medication (see Table 1 for medication information) but withheld medication for 48 to 96 hours (i.e., at least five half-lives) prior to the MRI scan with their physician’s approval. Thirty-four TD adolescents were matched to the ADHD group on sex, age, head motion (see Table 2). The same exclusion criteria applied to this control group with the addition of a score <60 on the CPRS- 3 or CAARS ADHD Total Scale. Table 2 shows average symptom scores and demographic information for each group. Informed written parental consent and child assent were obtained from all participants. The University of California, Davis Institutional Review Board approved the study.
Table 1.
Medication Information for the ADHD group.
| N | |
|---|---|
| Number of subjects unmedicated | 11 |
| Number of subjects medicated | 23 |
| Atomoxetine | 1 |
| Methylphenidate hydrochloride extended release (Concerta) | 5 |
| Dextro-amphetamine (Adderall) | 2 |
| Dextro-amphetamine extended release (Adderall XR) | 7 |
| Lisdexamfetamine dimesylate (Vyvanse) | 5 |
| Methylphenidate | 2 |
| Dexmethylphenidate HCL extended release (Focalin XR) | 1 |
Table 2.
Demographic, Clinical, and MRI Head Motion Information for the ADHD and Control Groups.
| ADHD (n = 34) | TD (n = 34) | Statistic | |
|---|---|---|---|
| Gender: Male/female | 22/12 | 22/12 | |
| Mean (SD) Age (years) | 16.29 (2.68) | 15.99 (2.85) | t(66) = 0.46, p = n.s. |
| Race: White/Black or African American/Asian/Multiracial/other or unknown | 25/1/0/7/1 (73.5%/2.9%/0%/20.6%/2.9%) | 18/2/2/10/2 (52.9%/5.9%/5.9%/29.4%/5.9%) | χ2 (4, N = 68) = 4.34, p = n.s. |
| Ethnicity: Not Hispanic/Hispanic/unknown | 22/8/4 (64.7%/23.5%/11.8%) | 24/10/0 (70.6%/29.4%/0%) | χ2 (2, N = 34) = 4.31, p = n.s. |
| Maternal education: bachelor’s degree or higher | 28 (82.4%) | 27 (79.4%) | χ2 (1, N = 68) = .10, p = n.s. |
| Household income: above $ 100k | 19 (55.9%) | 12 (35.3%) | χ2 (1, N = 68) = 2.62, p = n.s. |
| Mean (SD) IQ | 106.82 (11.56) | 114.15 (10.11) | t(66) = −2.78, p = .007 |
| Mean (SD) Conners’ DSM inattention* | 80.91 (10.37) | 42.56 (5.10) | t(48.08) = 19.35, p < .001 |
| Mean (SD) Conners’ DSM hyperactive/impulsive* | 78.21 (12.19) | 43.35 (4.71) | t(42.64) = 15.55, p < .001 |
| Mean (SD) irritability | 6.32 (3.90) | 1.21 (1.68) | t(44.9) = 7.03, p < .001 |
| Mean (SD) framewise displacement | 0.19 (0.09) | 0.17 (0.06) | t(66) = 0.98, p = n.s. |
| Mean (SD) relative motion (mm) | 0.09 (0.04) | 0.09 (0.03) | t(66) = 0.94, p = n.s. |
| Mean (SD) absolute motion (mm) | 0.33 (0.23) | 0.25 (0.17) | t(66) = 1.60, p = n.s. |
Mean score based on the t-scores for the CPRS’ Parent Scores (participants 17 years and younger) and CAARS-O (participants 18 years and older).
Measures.
Conners’ Parent Rating Scale—3 (Conners, 2008):
The CPRS-3 contains 108-items that are rated on how frequently certain behaviors occur from 0 (never, seldom) to 3 (very often). The questionnaire has good internal reliability (Cronbach’s α ranging from .75 to .94 for all scales), high test-retest reliability, and effective discriminatory power (Conners et al., 1998).
Conners’ Adult ADHD Rating Scales (CAARS-O) (observer ratings):
For participants aged 18 and over, ADHD symptoms were assessed by the CAARS-O. Internal consistency of the CAARS-O subscales ranges from 0.81 (Hyperactivity/Restlessness) to 0.89 (Problems With Self-Concept) (Conners et al., 1999).
For the purpose of the present study, a separate score for irritability was derived by summing scores on items 14, 48, 73, 81, 100 of the CPRS-3 and items 8, 19, 23, 61 of the CAARS-O. Internal consistency of the CPRS-3 and CAARS irritability items were 0.92 and 0.84 respectively. The items chosen from each questionnaire correspond to items on the Affective Reactivity Index (ARI) (Stringaris et al., 2012). To validate our irritability measure we correlated CPRS-3 and CAARS irritability scores with ARI scores in a sub-sample of participants that completed both questionnaires. Correlations were high: CPRS-3 (n = 21): r = .96, p< .0001; CAARS (n = 12): r = .71, p = .01.
Resting state MRI data acquisition:
Imaging data were acquired on a Siemens 3.0 T TIM Trio MRI scanner. T2-weighted functional images were acquired with the following parameters: TR = 2,000 ms; TE = 25 ms; flip angle = 80°, 36 slices, matrix = 64 × 64; FOV = 220 mm; voxel size = 3.4 × 3.4 × 3.4 mm. The duration of the resting state scan was 6 minutes 4 seconds. The T1-weighted high-resolution anatomical image was acquired with the following parameters: TR = 1,900 ms; TE = 3.06 ms; flip angle = 8°; 160 slices; FOV = 256 mm; acquisition voxel size = 1.0 × 1.0 × 1.0 mm. Participants were instructed to look at a fixation point during the scan acquisition.
Functional connectivity analysis.
All analyses including image preprocessing were carried out using the CONN toolbox v. 17.0 (http://www.nitrc.org/projects/conn/) (Whitfield-Gabrieli & Nieto-Castanon, 2012). T1 structural scans were segmented into gray matter, white matter, and cerebrospinal fluid and then normalized to the Montreal Neurological Institute (MNI) template. Preprocessing of functional images included slice timing correction, spatial coregistration of functional data to each participant’s structural scan, spatial normalization to MNI space, and spatial smoothing using a 6 mm full-width-at-half-maximum Gaussian kernel. Prior to smoothing, we identified outlier volumes across subjects in acquisitions with a framewise displacement above 0.9 mm or global BOLD signal changes above 5 SD using artifact removal toolbox (ART) (https://www.nitrc.org/projects/artifact_detect/). For each subject, outlier volumes, head-motion (six-parameters of translation and rotation), and other spurious sources of noise (e.g., signal from white matter and cerebrospinal fluid) were regressed out using the aCompcor method (Behzadi et al., 2007). Then, temporal high-pass filtering (0.008–0.09 Hz) was applied to the residual BOLD time course to exclude remaining physiological noise (e.g., respiratory effects).
Seed regions were defined using the default ROIs within CONN based on the Harvard-Oxford Subcortical Atlas, left and right amygdala as well as left and right NAcc. First-level correlation maps were produced by extracting the denoised BOLD time course from each seed and computing Pearson’s correlation coefficients between that time course and the time courses of all other voxels in the brain. Correlation coefficients were Fisher-transformed into Z scores, which increases normality and allows for improved second-level General Linear Model analyses. We adjusted the cluster significant threshold for the four seed regions to an FDR cluster-corrected threshold of p < .0125. Unpaired t-tests were performed to assess between-group differences in seed-to-voxel functional connectivity. As the groups were matched on sex, age, and head motion, we did not include these variables as covariates in our initial analysis. To ensure effects were due to irritability symptoms above and beyond hyperactive-impulsive symptoms as suggested previously (Hulvershorn et al., 2013) in a follow-up analysis we controlled for hyperactive-impulsive symptoms. We also assessed separately if results would hold if we controlled for differences in full-scale IQ.
Results
Demographic and clinical information for the sample is provided in Table 2. The groups were closely matched on age and sex and no significant differences were observed for maternal education level, household income, race, and ethnicity. As expected, the ADHD group scored significantly lower on full-scale IQ (though still in the normal range), and significantly higher on core ADHD symptom measures of inattention and impulsivity/hyperactivity and on irritability (see Table 2).
Amygdala seeds:
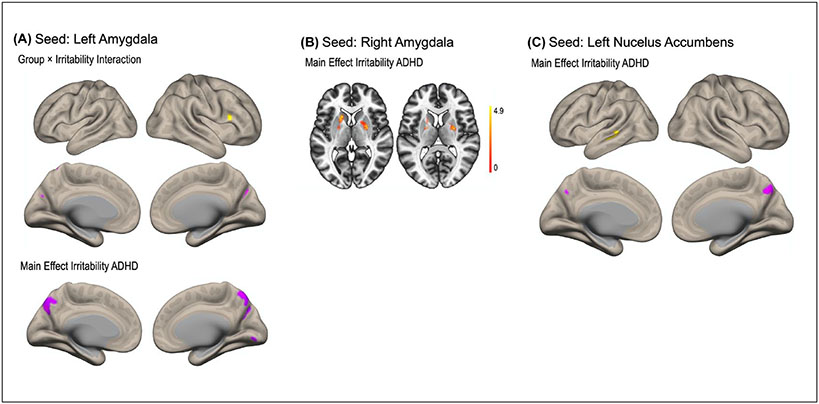
A group × irritability interaction effect was found for functional connectivity between the left amygdala and right inferior frontal gyrus and between the left amygdala and bilateral precuneus/cuneus (Figure 1, Table 3). Irritability symptoms were more positively associated with functional connectivity between the left amygdala and the right inferior frontal gyrus in the ADHD group compared to the TD group. For functional connectivity between the left amygdala and the bilateral precuneus/cuneus, irritability was more negatively associated in the ADHD compared to the TD group. This interaction was not observed for the right amygdala. Simple main effects of irritability in the ADHD group showed that higher irritability was associated with higher functional connectivity between the left amygdala and bilateral putamen/caudate and lower functional connectivity of the left amygdala with the precuneus and lingual gyrus. This simple main effect of irritability and functional connectivity with the putamen/caudate was also observed for the right amygdala. No simple main effect of irritability was observed in the TD group. Both the interaction and the simple main effects of irritability remained significant when controlling for hyperactive-impulsive symptoms. The group × irritability interaction for the left amygdala also remained significant when covarying for IQ differences, suggesting that neither IQ nor hyperactive-impulsive symptoms were related to the observed associations with irritability.
Figure 1.
Resting-state functional connectivity maps with seeds in the left (A) and right (B) amygdala and left nucleus accumbens (C). (A) Irritability in the ADHD group was positively associated (yellow) with functional connections between the left amygdala and right inferior frontal gyrus and negatively (purple) with connectivity between the left amygdala and bilateral precuneus compared to the typically developing control group. (B) Irritability levels in the ADHD group were positively associated with functional connectivity between the right amygdala and bilateral putamen/caudate. (C) A main effect of irritability in the ADHD group revealed stronger connectivity between the left NAcc and a region in the left posterior middle temporal gyrus and weaker connectivity between the left NAcc and the precuneus.
Table 3.
The Four Seed Regions (Left and Right Amygdala, Left and Right Nucleus Accumbens) and Significant Target Regions Associated with Irritability.
| Coordinates |
|||||||
|---|---|---|---|---|---|---|---|
| x | y | z | Cluster size (k) | Beta | T | p | |
| Seed: left amygdala | |||||||
| Group × Irritability interaction | |||||||
| (+) right IFG | +54 | +16 | +24 | 137 | .08 | 4.85 | .005 |
| (−) precuneus/cuneal cortex | +22 | −70 | +32 | 136 | −.08 | −4.18 | .005 |
| (−) precuneus | −4 | −60 | +62 | 102 | −.07 | −4.23 | .012 |
| Main effect irritability: ADHD | |||||||
| (−) precuneus | −14 | −68 | +38 | 1,310 | −.03 | −6.10 | <.001 |
| (−) lingual gyrus | +04 | −82 | −08 | 120 | −.03 | −4.02 | .009 |
| (+) left caudate/putamen | −18 | +10 | +04 | 144 | .03 | 4.49 | .005 |
| Main effect irritability: TD | n.s. | ||||||
| Seed: right amygdala | |||||||
| Group × Irritability interaction | n.s. | ||||||
| Main effect irritability: ADHD | |||||||
| (+) bilateral putamen/caudate | −16 | +16 | +02 | 293 | .03 | 4.68 | .002 |
| (+) right putamen/caudate | +26 | −04 | +10 | 262 | .03 | 4.46 | .003 |
| Main effect irritability: TD | n.s. | ||||||
| Seed: left nucleus accumbens | |||||||
| Group × Irritability interaction | n.s. | ||||||
| Main effect irritability: ADHD | |||||||
| (−) precuneus | +10 | −64 | +40 | 163 | −.03 | −4.53 | .002 |
| (+) left pMTG | −52 | −38 | −04 | 126 | .03 | 4.52 | .004 |
| Main effect irritability: TD | n.s. | ||||||
| Seed: right nucleus accumbens | |||||||
| Group × Irritability interaction | n.s. | ||||||
| Main effect irritability: ADHD | n.s. | ||||||
| (+) left pMTG | −52 | −40 | −06 | 119 | .02 | 4.42 | .025 |
| Main effect irritability: TD | n.s. | ||||||
Note. All results are p < .0125 (FDR-corrected). (+) = indicates a positive association; (−) = indicates a negative association; IFG = inferior frontal gyrus; pMTG = posterior middle temporal gyrus.
Nucleus Accumbens seeds:
No significant group × irritability interaction effect for either the left or the right NAcc was found. A main effect of irritability in the ADHD group, however, showed that higher irritability was associated with greater functional connectivity between the left NAcc and a region in the left posterior middle temporal gyrus, and lower functional connectivity between the left NAcc and a cluster in the precuneus (Figure 1, Table 3). A trend for greater functional connectivity between the right NAcc and the left posterior middle temporal gyrus with higher levels of irritability was also observed but did not survive correction for multiple comparisons (p = .025). When statistically adjusting for symptoms of hyperactivity/impulsivity, the simple main effects of irritability observed for the left NAcc no longer survived the threshold for multiple comparisons (p = .028).
Discussion
Given poor outcomes for individuals with ADHD and high levels of irritability, we aimed to identify neural markers of irritability in adolescents with ADHD and contrast these to those within typically developing adolescents. We focused on two regions, the amygdala and the nucleus accumbens, to examine circuits that support reward and threat behaviors. Crucially, we observed an association between irritability symptoms and functional connectivity between the amygdala and inferior frontal and posterior midline regions with varying irritability symptoms in the ADHD but not the TD group. In addition to the group differences, irritability symptoms in the ADHD group were also associated with greater functional connectivity between the amygdala and the caudate/putamen as well as between the left NAcc and a posterior temporal region. The results support altered functional connectivity of both threat and reward circuits, when ADHD is accompanied by irritability, extending to other key regions involved in cognitive control and emotion processing.
The observed association between irritability and functional connectivity between the left amygdala and the right IFG was significantly more positive in the ADHD group compared to the TD group suggesting a diminished ability for cognitive control, specifically inhibition and emotion regulation typically attributed to the IFG (Aron & Poldrack, 2005). Right IFG and amygdala show strong positive functional connectivity during task-based emotion paradigms (Kerestes et al., 2017) and become negatively connected during successful regulation of negative emotions (Ochsner et al., 2012). As the observed positive association between irritability and IFG-amygdala functional connectivity remained even after controlling for hyperactive-impulsive symptoms, the results confirm previously reported associations with less efficient emotion regulation mechanisms in irritable youth (Leibenluft, 2017).
Compared to the TD group, irritability was negatively associated with functional connectivity between the left amygdala and posterior midline regions within the precuneus in the ADHD group. Amygdala-precuneus connections support successful emotion regulation by directing attention away from affective information (Ferri et al., 2016; Roy et al., 2009; Zhang & Li, 2012). Reduced functional connectivity therefore may increase irritability by preventing an attentional shift away from emotional stimuli (Ferri et al., 2016). Such altered or reduced connectivity between these two regions has been reported in several other psychopathologies known for high levels of irritability including children diagnosed with bipolar disorder (Rich et al., 2008; Stoddard et al., 2015), adolescents with depression (Cullen et al., 2014), adults with childhood maltreatment (van der Werff et al., 2013), post-traumatic stress disorder (Nicholson et al., 2015), and schizophrenia (Mukherjee et al., 2012).
Group by irritability interactions were only observed for the left but not right amygdala and with connectivity to right but not left IFG. The left amygdala has been shown to respond more consistently to negative emotions during task fMRI studies using emotion paradigms (Wager et al., 2008) and the right IFG plays a more dominant role in regulating emotions and during inhibitory control compared to its left counterpart. Even though we did not formally test laterality differences, atypical asymmetry in brain structure and function is a common observation in ADHD (Langleben et al., 2001; Shaw et al., 2009; Silk et al., 2016) and has also been implicated in the neurobiology of irritability (Althoff et al., 2017; Chaarani et al., 2020); as such it is likely, that left and right fronto-limbic and fronto-striatal connections differentially contribute to the pathophysiology of irritability in ADHD.
In the ADHD group, heightened irritability was associated with increased amygdala-striatal functional connectivity, suggesting associations between irritability and both altered reward processing and arousal (Beauchaine & Tackett, 2020; Brotman et al., 2017). Similarly, the left NAcc showed greater functional connectivity with a region in the left posterior middle temporal gyrus with increasing irritability in the patient group. The stronger functional connectivity between the two regions may thus hint at more rigid reward expectancies (Badre & Wagner, 2007) that may result in pathological temper outbursts or reactive aggression when reward expectancies are not met (Brotman et al., 2017). Given that there were no significant group differences for these associations these main effects should be interpreted cautiously. It is possible that the low variability of the irritability ratings in the TD group contributed to the lack of significant group differences. It would be valuable to compare the ADHD group to another clinical group to determine whether the main effects observed here are specific to ADHD. Additionally, a dimensional symptom-based approach independent of diagnostic category as proposed by the RDoC framework (Cuthbert & Insel, 2013) may provide complementary information on the neural underpinnings of irritability.
Our results differ from those reported by Hulvershorn et al. (2013), who found a positive association between emotional lability and left amygdala-medial PFC functional connectivity. Age differences across samples or measurement of irritability as compared to emotional lability may be relevant. Our findings also differ to some extent from those reported by Yu et al. (2020) who found that emotional lability was correlated positively with connectivity of the right (superficial) amygdala and dlPFC as well as inferior parietal regions. These authors, however, restricted their analysis to regions that initially showed significant between-group differences during a task. Significantly, comparable to our results, they found that the ADHD group exhibited weaker connectivity between the amygdala and the precuneus compared to controls.
Our findings should be interpreted in light of several limitations. First, although we used a well-validated clinical questionnaire, the instrument was not specifically developed to measure irritability and may have missed important aspects of the irritability phenotype (Vidal-Ribas et al., 2016). Second, our study is limited to a cross-sectional examination of irritability and functional connectivity. Given the long-term clinical implications of heightened irritability, it is important to examine neural markers of improvement or worsening of irritability-related impairments longitudinally. Third, our final sample size was diminished considerably after losing 39% of the clinical sample due to excessive head motion. Even though heightened head motion is common in participants with ADHD (Kong et al., 2014; Satterthwaite et al., 2012) the reduced sample size limits the generalizability of our results especially to younger ADHD participants and those with higher hyperactive-impulsive symptoms. Fourth, parental education and income, along with participant IQ, were relatively high, potentially also reducing generalizability. Thus, follow-up studies will need to recruit participants with more varied incomes and educational levels that reflect the general population. Lastly, differences in developmental stage or brain maturation may account for some of the results observed here given the wide age-range of our participants. Future studies may separately utilize irritability ratings, age, and individual brain maturation indices (Cao et al., 2015; Dosenbach et al., 2010; Truelove-Hill et al., 2020) to examine developmental and maturational effects on irritability in those diagnosed with ADHD and typically developing youth.
Our sample was carefully phenotyped clinically, and the results present important information on the neural correlates of irritability in adolescents, who are most representative of adolescents with the ADHD, Combined Presentation. We recruited participants with evidence of significant impulsivity and therefore, meet criteria for the Combined Presentation, and excluded volunteers with the Inattentive ADHD Presentation, because of a need in the field to characterize the relation between behavioral symptoms of impulsivity and associated neural functioning. Furthermore, there is evidence of functional connectivity differences between the Inattentive and Combined Presentations of ADHD (Fair et al., 2012) and thus recruiting from Inattentive ADHD populations may have obscured some potential findings.
Taken together, we revealed alterations in functional connectivity of the amygdala and the NAcc in association with heightened irritability in ADHD. Anomalies with connections to frontal, temporal, and posterior midline regions may give rise to not only increased impulsivity and difficulties in attention but also to heightened irritability. These observed associations between irritability and functional connectivity of NAcc and amygdala suggest treatments that address response to reward, primarily omission of an expected reward and irritability may be beneficial for those with ADHD. This may include training to increase inhibitory and cognitive control or reduction in the effect of stimuli eliciting irritability, including cognitive or behavioral training such as exposure therapy (Kircanski et al., 2019; Linke et al., 2020), perhaps meditation techniques, or pharmacological approaches that affect how one responds to cues signaling reward loss, absence of an expected reward, and other situations associated with irritability.
Acknowledgments
We would like the acknowledge the kind support of all our research participants, as well as research staff Arthur Tadeus Hartanto, Maria B.E. Menor, Jessica Nguyen, Shannon Hoffman and Drs. Erin Calfee, Lauren Boyle, Marcia Unger.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Institute of Mental Health grants R01MH091068 (Schweitzer) and U54 HD079125 (Abbeduto).
Biography
Prerona Mukherjee, Ph.D., is an Assistant Professional Researcher in the Department of Psychiatry and Behavioral Sciences and the MIND Institute. She completed her Ph.D. at the University of Edinburgh and did her postdoctoral training at Stony Brook University and University of California, Davis.
Veronika Vilgis, Ph.D., completed her Ph.D. at the University of Melbourne, Australia and was a postdoctoral fellow at the Center for Mind and Brain at the University of California, Davis. She was the recipient of an Early Investigator Grant from the Brain and Behavior Research Foundation.
Shawn Rhoads, M.A. is a doctoral candidate in Psychology at Georgetown University. After he graduated from the University of Southern California with a B.A. in both physics and psychology, he worked as a lab manager at the Center for Mind and Brain, University of California, Davis before beginning his postgraduate studies.
Rajpreet Chahal, Ph.D., completed her Ph.D. in Human Development at the University of California, Davis, and received an NIH TL1 (T32), fellowship in clinical and translational science from the NIH-funded Clinical and Translational Science Center (CTSC) at UC Davis and is currently a postdoctoral fellow in the Department of Psychology at Stanford University.
Catherine Fassbender, Ph.D., is an Assistant Professor of School of Psychology at Dublin City University. She completed her Ph.D. at Department of Psychology and Institute of Neuroscience at Trinity College Dublin, postdoctoral fellowships at the University of Maryland and faculty member at the University of California, Davis, Department of Psychiatry and Behavioral Sciences and relocated to Dublin City University in 2019.
Ellen Leibenluft, M.D., is Senior Investigator (tenured), Chief of the Section on Mood Dysregulation and Neuroscience and Co-Branch Chief of the Emotion and Development Branch, Intramural Research Program, National Institute of Mental Health. Dr. Leibenluft is a member of the American College of Neuropsychopharmacology, served on the American Psychiatric Association Work Group on Childhood Disorders for DSM-5 and several editorial boards.
J. Faye Dixon, Ph.D., Professor of Psychiatry and Behavioral Sciences and the MIND Institute, University of California, Davis. She has a long history of work in child psychopathology, specifically the areas of depression, anxiety, PTSD, ADHD and learning differences in children. Currently, Dr. Dixon is the director of clinical management and community outreach for the AIR (Attention, Impulsivity & Regulation) Lab. She is currently serving on the APSARD Board of Directors.
Murat Pakyurek, M.D., is Professor, Medical Director for Behavioral Health Center, Child and Adolescent Behavioral Health Center, and the MIND Institute ADHD Clinic and Chief, Child and Adolescent Psychiatry Division. His clinical and research interests include autism spectrum disorders, ADHD, anxiety disorders in children, catatonia, and tele-psychiatry.
Wouter van den Bos, Ph.D., is Associate Professor at the Developmental Psychology, Department of the University of Amsterdam & Director of the Connected Minds Lab, Adjunct Research Scientist at the Max Planck Institute for Human Development Center for Adaptive Rationality, and Faculty at the Max Planck UCL Centre for Computational Psychiatry and Ageing Research. His research is focused on how changes in brain function and structure relate to typical and atypical development of learning and decision-making.
Stephen P. Hinshaw, Ph.D. is Professor in the Department of Psychology, University of California, Berkeley, and the Vice-Chair for Child and Adolescent Psychology, Department of Psychiatry and Behavioral Sciences, University of California, San Francisco. He has authored groundbreaking research articles in ADHD, research in girls with ADHD and stigma and mental illness. He has been awarded several awards, including the Sarnat International Prize in Mental Health, from the National Academy of Medicine.
Amanda E. Guyer, Ph.D. is Professor in the Human Development and Family Studies Program of the Department of Human Ecology, Chair of the Human Development Graduate Group, and Associate Director of the Center for Mind and Brain, University of California, Davis. She is the Director of the Teen Experiences, Emotions & Neurodevelopment (TEEN) Lab and is a UC Davis Chancellor’s Fellow and Fellow of the Association for Psychological Science. Her research focuses on behavioral and neural underpinnings of social-emotional functioning and mental health in adolescence.
Julie B. Schweitzer, Ph.D., is Professor of Psychiatry and Behavioral Sciences and MIND Institute, University of California Davis. She directs the Attention, Impulsivity and Regulation (AIR) Laboratory, Mentoring Academy for Research Excellence (MARE) and the UC Davis Mentored Clinical Research Training Program. Her research interests include the identification and treatment of impulsivity, inattention and dysregulation of cognition and emotions in ADHD and the development on nonpharmacologic neurotherapeutics.
Footnotes
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Hinshaw receives book royalties from Oxford University Press and St. Martin’s Press. Mr. Rhoads and Drs. Mukherjee, Vilgis, Chahal, Fassbender, van den Bos, Guyer, Pakyurek, and Schweitzer report no competing interests.
References
- Althoff R, Chaarani B, Kan K-J, Mackey S, Spechler P, Orr C, Hudson K, Stringaris A, & Garavan H (2017). 40. Neural correlates of adolescent irritability and its comorbidity. Biological Psychiatry, 81(10), S17. 10.1016/j.biopsych.2017.02.051 [DOI] [Google Scholar]
- Aron AR, & Poldrack RA (2005). The cognitive neuroscience of response inhibition: Relevance for genetic research in attention-deficit/hyperactivity disorder. Biological Psychiatry, 57(11), 1285–1292. 10.1016/j.biopsych.2004.10.026 [DOI] [PubMed] [Google Scholar]
- Badre D, & Wagner AD (2007). Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia, 45(13), 2883–2901. 10.1016/j.neuropsychologia.2007.06.015 [DOI] [PubMed] [Google Scholar]
- Barkley RA (2011). Barkley Adult ADHD Rating Scale-IV (BAARS-IV). Guilford Press. [Google Scholar]
- Beauchaine TP, & Tackett JL (2020). Irritability as a trans-diagnostic vulnerability trait: current issues and future directions. Behavior Therapy, 51(2), 350–364. 10.1016/j.beth.2019.10.009 [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, & Liu TT (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage, 37(1), 90–101. 10.1016/j.neuroimage.2007.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman MA, Kircanski K, Stringaris A, Pine DS, & Leibenluft E (2017). Irritability in youths: A translational model. American Journal of Psychiatry, 174(6), 520–532. 10.1176/appi.ajp.2016.16070839 [DOI] [PubMed] [Google Scholar]
- Cao B, Mwangi B, Hasan KM, Selvaraj S, Zeni CP, Zunta-Soares GB, & Soares JC (2015). Development and validation of a brain maturation index using longitudinal neuroanatomical scans. NeuroImage, 117, 311–318. 10.1016/j.neuroimage.2015.05.071 [DOI] [PubMed] [Google Scholar]
- Chaarani B, Kan K-J, Mackey S, Spechler PA, Potter A, Banaschewski T, Millenet S, Bokde ALW, Bromberg U, Büchel C, Cattrell A, Conrod PJ, Desrivières S, Flor H, Frouin V, Gallinat J, Gowland P, Heinz A, Ittermann B, & Stedman A (2020). Neural correlates of adolescent irritability and its comorbidity with psychiatric disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 59(12), 1371–1379. 10.1016/j.jaac.2019.11.028 [DOI] [PubMed] [Google Scholar]
- Conners CK (2008). Conners 3rd edition manual. Multi-Health Systems. [Google Scholar]
- Conners CK, Erhardt D, Epstein JN, Parker JD, Sitarenios G, & Sparrow E (1999). Self-ratings of ADHD symptoms in adults I: Factor structure and normative data. Journal of Attention Disorders, 3(3), 141–151. 10.1177/108705479900300303 [DOI] [Google Scholar]
- Conners CK, Sitarenios G, Parker JDA, & Epstein JN (1998). The revised Conners’ Parent Rating Scale (CPRS-R): Factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology, 26(4), 257–268. [DOI] [PubMed] [Google Scholar]
- Costa Dias TG, Wilson VB, Bathula DR, Iyer SP, Mills KL, Thurlow BL, Stevens CA, Musser ED, Carpenter SD, Grayson DS, Mitchell SH, Nigg JT, & Fair DA (2013). Reward circuit connectivity relates to delay discounting in children with attention-deficit/hyperactivity disorder. European Neuropsychopharmacology, 23(1), 33–45. 10.1016/j.euroneuro.2012.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KR, Westlund MK, Klimes-Dougan B, Mueller BA, Houri A, Eberly LE, & Lim KO (2014). Abnormal amygdala resting-state functional connectivity in adolescent depression. JAMA Psychiatry, 71(10), 1138–1147. 10.1001/jamapsychiatry.2014.1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, & Insel TR (2013). Toward the future of psychiatric diagnosis: The seven pillars of RDoC. BMC Medicine, 11(1), 126. 10.1186/1741-7015-11-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Nelson SM, Wig GS, Vogel AC, Lessov-Schlaggar CN, Barnes KA, Dubis JW, Feczko E, Coalson RS, Pruett JR, Barch DM, Petersen SE, & Schlaggar BL (2010). Prediction of individual brain maturity using fMRI. Science, 329(5997), 1358–1361. 10.1126/science.1194144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Nigg JT, Iyer S, Bathula D, Mills KL, Dosenbach NU, Schlaggar BL, Mennes M, Gutman D, Bangaru S, Buitelaar JK, Dickstein DP, Di Martino A, Kennedy DN, Kelly C, Luna B, Schweitzer JB, Velanova K, Wang Y-F, …, Milham MP (2012). Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Frontiers in Systems Neuroscience, 6, 80. 10.3389/fnsys.2012.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri J, Schmidt J, Hajcak G, & Canli T (2016). Emotion regulation and amygdala-precuneus connectivity: Focusing on attentional deployment. Cognitive Affective & Behavioral Neuroscience, 16(6), 991–1002. 10.3758/s13415-016-0447-y [DOI] [PubMed] [Google Scholar]
- Fox MD, & Greicius M (2010). Clinical applications of resting state functional connectivity. Frontiers in Systems Neuroscience, 4, 19. 10.3389/fnsys.2010.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller B, Zimerman B, Williams M, Del Bello MP, Bolhofner K, Craney JL, Frazier J, Beringer L, & Nickelsburg MJ (2002). DSM-IV mania symptoms in a prepubertal and early adolescent bipolar disorder phenotype compared to attention-deficit hyperactive and normal controls. Journal of Child and Adolescent Psychopharmacology, 12(1), 11–25. 10.1089/10445460252943533 [DOI] [PubMed] [Google Scholar]
- Harty SC, Gnagy EM, Pelham WE Jr., & Molina BSG (2017). Anger-irritability as a mediator of attention deficit hyperactivity disorder risk for adolescent alcohol use and the contribution of coping skills. Journal of Child Psychology and Psychiatry, 58(5), 555–563. 10.1111/jcpp.12668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulvershorn LA, Mennes M, Castellanos FX, Di Martino A, Milham MP, Hummer TA, & Roy AK (2013). Abnormal amygdala functional connectivity associated with emotional lability in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 53(3), 351–361.e1. 10.1016/j.jaac.2013.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James A, Lai FH, & Dahl C (2004). Attention deficit hyperactivity disorder and suicide: A review of possible associations. Acta Psychiatrica Scandinavica, 110(6), 408–415. 10.1111/j.1600-0447.2004.00384.x [DOI] [PubMed] [Google Scholar]
- Karalunas SL, Fair D, Musser ED, Aykes K, Iyer SP, & Nigg JT (2014). Subtyping attention-deficit/hyperactivity disorder using temperament dimensions: Toward biologically based nosologic criteria. JAMA Psychiatry, 71(9), 1015–1024. 10.1001/jamapsychiatry.2014.763 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kerestes R, Chase HW, Phillips ML, Ladouceur CD, & Eickhoff SB (2017). Multimodal evaluation of the amygdala’s functional connectivity. NeuroImage, 148, 219–229. 10.1016/j.neuroimage.2016.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircanski K, Craske MG, Averbeck BB, Pine DS, Leibenluft E, & Brotman MA (2019). Exposure therapy for pediatric irritability: Theory and potential mechanisms. Behaviour Research and Therapy, 118, 141–149. 10.1016/j.brat.2019.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X, Zhen Z, Li X, Lu H, Wang R, Liu L, He Y, Zang Y, & Liu J (2014). Individual differences in impulsivity predict head motion during magnetic resonance imaging. PLoS ONE, 9(8), e104989. 10.1371/journal.pone.0104989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad K, & Eickhoff SB (2010). Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Human Brain Mapping, 31(6), 904–916. 10.1002/hbm.21058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langleben DD, Austin G, Krikorian G, Ridlehuber HW, Goris ML, & Strauss HW (2001). Interhemispheric asymmetry of regional cerebral blood flow in prepubescent boys with attention deficit hyperactivity disorder. Nuclear Medicine Communications, 22(12), 1333–1340. 10.1097/00006231-200112000-00009 [DOI] [PubMed] [Google Scholar]
- Leibenluft E (2017). Pediatric irritability: A systems neuroscience approach. Trends in Cognitive Sciences, 21(4), 277–289. 10.1016/j.tics.2017.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke J, Kircanski K, Brooks J, Perhamus G, Gold AL, & Brotman MA (2020). Exposure-based cognitive-behavioral therapy for disruptive mood dysregulation disorder: An evidence-based case study. Behavior Therapy, 51(2), 320–333. 10.1016/j.beth.2019.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco R, Miranda A, Schlotz W, Melia A, Mulligan A, Muller U, Andreou P, Butler L, Christiansen H, Gabriels I, Medad S, Albrecht B, Uebel H, Asherson P, Banaschewski T, Gill M, Kuntsi J, Mulas F, Oades R, …, Sonuga-Barke EJ (2009). Delay and reward choice in ADHD: An experimental test of the role of delay aversion. Neuropsychology, 23(3), 367–380. 10.1037/a0014914 [DOI] [PubMed] [Google Scholar]
- Marx I, Hacker T, Yu X, Cortese S, & Sonuga-Barke E (2021). ADHD and the choice of small immediate over larger delayed rewards: A comparative meta-analysis of performance on simple choice-delay and temporal discounting paradigms. Journal of Attention Disorders, 25(2), 171–187. 10.1177/1087054718772138 [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Whalley HC, McKirdy JW, McIntosh AM, Johnstone EC, Lawrie SM, & Hall J (2012). Lower effective connectivity between amygdala and parietal regions in response to fearful faces in schizophrenia. Schizophrenia Research, 134(2–3), 118–124. 10.1016/j.schres.2011.09.033 [DOI] [PubMed] [Google Scholar]
- Mulraney M, Zendarski N, Mensah F, Hiscock H, & Sciberras E (2017). Do early internalizing and externalizing problems predict later irritability in adolescents with attention-deficit/hyperactivity disorder? Australian and New Zealand Journal of Psychiatry, 51(4), 393–402. 10.1177/0004867416659365 [DOI] [PubMed] [Google Scholar]
- Nicholson AA, Densmore M, Frewen PA, Théberge J, Neufeld RW, McKinnon MC, & Lanius RA (2015). The dissociative subtype of posttraumatic stress disorder: Unique resting-state functional connectivity of basolateral and centromedial amygdala complexes. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 40(10), 2317–2326. 10.1038/npp.2015.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, & Buhle JT (2012). Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251(1), E1–E24. 10.1111/j.1749-6632.2012.06751.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta MM, & Scheres A (2013). Ventral–striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: A meta-analytic review of the fMRI literature. Neuroscience and Biobehavioral Reviews, 38, 125–134. 10.1016/j.neubiorev.2013.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta MM, Vasic N, Wolf RC, Lesch K-P, Brummer D, Jacob C, Fallgatter AJ, & Grön G (2009). Neural hyporesponsiveness and hyperresponsiveness during immediate and delayed reward processing in adult attention-deficit/hyperactivity disorder. Biological Psychiatry, 65(1), 7–14. 10.1016/j.biopsych.2008.07.008 [DOI] [PubMed] [Google Scholar]
- Posner J, Rauh V, Gruber A, Gat I, Wang Z, & Peterson BS (2013). Dissociable attentional and affective circuits in medication-naïve children with attention-deficit/hyperactivity disorder. Psychiatry Research Neuroimaging, 213(1), 24–30. 10.1016/j.pscychresns.2013.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich BA, Fromm SJ, Berghorst LH, Dickstein DP, Brotman MA, Pine DS, & Leibenluft E (2008). Neural connectivity in children with bipolar disorder: Impairment in the face emotion processing circuit. Journal of Child Psychology and Psychiatry, 49(1), 88–96. 10.1111/j.1469-7610.2007.01819.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, & Milham MP (2009). Functional connectivity of the human amygdala using resting state fMRI. NeuroImage, 45(2), 614–626. 10.1016/j.neuroimage.2008.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K (2018). Cognitive neuroscience of Attention deficit hyperactivity disorder (ADHD) and its clinical translation. Frontiers in Human Neuroscience, 12, 100. 10.3389/fnhum.2018.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samea F, Soluki S, Nejati V, Zarei M, Cortese S, Eickhoff SB, Tahmasian M, & Eickhoff CR (2019). Brain alterations in children/adolescents with ADHD revisited: A neuroimaging meta-analysis of 96 structural and functional studies. Neuroscience and Biobehavioral Reviews, 100, 1–8. 10.1016/j.neubiorev.2019.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, Gur RC, & Gur RE (2012). Impact of in-scanner head motion on multiple measures of functional connectivity: Relevance for studies of neurodevelopment in youth. NeuroImage, 60(1), 623–632. 10.1016/j.neuroimage.2011.12.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer JB, & Sulzer-Azaroff B (1995). Self-control in boys with attention deficit hyperactivity disorder: Effects of added stimulation and time. Journal of Child Psychology and Psychiatry, 36(4), 671–686. 10.1111/j.1469-7610.1995.tb02321.x [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, & Schwab-Stone ME (2000). NIMH diagnostic interview schedule for children version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry, 39(1), 28–38. 10.1097/00004583-200001000-00014 [DOI] [PubMed] [Google Scholar]
- Shaw P, Lalonde F, Lepage C, Rabin C, Eckstrand K, Sharp W, Greenstein D, Evans A, Giedd JN, & Rapoport J (2009). Development of cortical asymmetry in typically developing children and its disruption in attention-deficit/ hyperactivity disorder. Archives of General Psychiatry, 66(8), 888–896. 10.1001/archgenpsychiatry.2009.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Stringaris A, Nigg J, & Leibenluft E (2014). Emotion dysregulation in attention deficit hyperactivity disorder. American Journal of Psychiatry, 171, 276–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk TJ, Vilgis V, Adamson C, Chen J, Smit L, Vance A, & Bellgrove MA (2016). Abnormal asymmetry in frontostriatal white matter in children with attention deficit hyperactivity disorder. Brain Imaging and Behavior, 10(4), 1080–1089. 10.1007/s11682-015-9470-9 [DOI] [PubMed] [Google Scholar]
- Stoddard J, Hsu D, Reynolds RC, Brotman MA, Ernst M, Pine DS, Leibenluft E, & Dickstein DP (2015). Aberrant amygdala intrinsic functional connectivity distinguishes youths with bipolar disorder from those with severe mood dysregulation. Psychiatry Research, 231(2), 120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris A, Goodman R, Ferdinando S, Razdan V, Muhrer E, Leibenluft E, & Brotman MA (2012). The affective reactivity index: A concise irritability scale for clinical and research settings. Journal of Child Psychology and Psychiatry, 53(11), 1109–1117. 10.1111/j.1469-7610.2012.02561.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson EN, Owens EB, & Hinshaw SP (2014). Pathways to self-harmful behaviors in young women with and without ADHD: A longitudinal examination of mediating factors. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 55(5), 505–515. 10.1111/jcpp.12193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truelove-Hill M, Erus G, Bashyam V, Varol E, Sako C, Gur RC, Gur RE, Koutsouleris N, Zhuo C, Fan Y, Wolf DH, Satterthwaite TD, & Davatzikos C (2020). A multidimensional neural maturation index reveals reproducible developmental patterns in children and adolescents. The Journal of Neuroscience, 40(6), 1265–1275. 10.1523/JNEUROSCI.2092-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werff SJ, Pannekoek JN, Veer IM, van Tol M-J, Aleman A, Veltman DJ, Zitman FG, Rombouts SA, Elzinga BM, & van der Wee NJ (2013). Resting-state functional connectivity in adults with childhood emotional maltreatment. Psychological Medicine, 43(9), 1825–1836. [DOI] [PubMed] [Google Scholar]
- Vidal-Ribas P, Brotman MA, Valdivieso I, Leibenluft E, & Stringaris A (2016). The status of irritability in psychiatry: A conceptual and quantitative review. Journal of the American Academy of Child and Adolescent Psychiatry, 55(7), 556–570. 10.1016/j.jaac.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, & Ochsner KN (2008). Prefrontal-Subcortical pathways mediating successful emotion regulation. Neuron, 59(6), 1037–1050. 10.1016/j.neuron.2008.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, & Nieto-Castanon A (2012). Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2(3), 125–141. 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- Yu X, Liu L, Chen W, Cao Q, Zepf FD, Ji G, Wu Z, An L, Wang P, Qian Q, Zang Y, Sun L, & Wang Y (2020). Integrity of Amygdala subregion-based functional networks and emotional lability in drug-naïve boys with ADHD. Journal of Attention Disorders, 24(12), 1661–1673. 10.1177/1087054716661419 [DOI] [PubMed] [Google Scholar]
- Zhang S, & Li CS (2012). Functional connectivity mapping of the human precuneus by resting state fMRI. NeuroImage, 59(4), 3548–3562. 10.1016/j.neuroimage.2011.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]