Abstract
A cardinal feature common to embryonic development and tissue reorganization, as well as to wound healing and cancer cell invasion, is collective cellular migration. During collective migratory events the phenomena of cell jamming and unjamming are increasingly recognized, and underlying mechanical, genomic, transcriptional, and signaling events are increasingly coming to light. In this brief perspective I propose a synthesis that brings together in a new way two key concepts. On the one hand, it has been suggested that the unjammed phase of the cellular collective evolved under a selective pressure favoring fluid-like migratory dynamics as would be required so as to accommodate episodes of tissue evolution, development, plasticity, and repair. Being dynamic, such an unjammed migratory phase is expected to be energetically expensive compared with the jammed non-migratory phase, which is presumed to have evolved under a selective pressure favoring a solid-like homeostatic regime that, by comparison, is energetically economical and mechanically stable. On the other hand, well before the discovery of cell jamming and unjamming Kauffman proposed the general biological principle that living systems exist in a solid regime near the edge of chaos, and that natural selection achieves and sustains such a poised state. Here I propose that, in certain systems at least, this poised solid-like state as predicted in the abstract by Kauffman is realized in the particular by the jammed regime just at the brink of unjamming.
Keywords: Chaos, jamming, unjamming, epithelium, collective, migration, plasticity, development, invasion, repair, criticality
Two sentence summary:
In his groundbreaking book The Origins of Order, the natural philosopher Stuart A. Kauffman proposed the general biological principle that living systems exist in a solid regime near the edge of chaos, and that natural selection achieves and sustains such a poised state. In this brief perspective I propose that, in certain systems at least, this poised state as predicted in the abstract by Kaufman is realized in the particular by the jammed regime just at the brink of unjamming.
INTRODUCTION:
Inert disordered collective systems are commonplace. Examples include sand in a pile or grain in a hopper as well as powders, pastes, colloids, slurries, suspensions, and foams. Living disordered collective systems are also familiar, with perhaps the best example being the cellular assembly comprising any confluent multicellular tissue such as the epithelial layer that covers all body surfaces and lines the lumen of every hollow organ. Whether inert or living, it is thought that disordered collective systems such as these attain a collective solid-like phase when they jam and attain a fluid-like phase when they unjam. This notion was first suggested by Cates et al.1, popularized by Liu and Nagel2, later elaborated by others3, 4 for the case of inert granular materials, and by still others for the case of living multicellular tissues.5–8 Here I will use a particular model system ––tumor cell invasion–– as an example by which to generalize these findings and set them into a broad biological context.
What’s so special about jammed systems?
For at least three reasons jammed systems have garnered special interest in soft matter science and physical biology.
The first reason that jammed systems warrant special attention is that each constituent particle ––whether a sand grain in sand pile or a cell in a tissue–– can become trapped, i.e., caged, by surrounding nearest neighbors and thereby undergo kinetic arrest. Being a granular system of discrete particles at the microscale, rather than a continuum, when a small force is applied that force then becomes transmitted in chains from particle-to-particle, or cell-to-cell, and these force chains assemble into a network that can support an applied shear stress if not indefinitely, then for a very long time.1, 9 By this criterion taken at the level of coarse graining, the material collective thus comprises an elastic solid. Examples of such solid-like jammed phases include bubbles comprising a stable shaving foam, sand grains comprising a stable sand pile, or cells comprising a stable living tissue. When an applied shear stress exceeds the yield stress, however, then such a collective system can unjam and thus transition from a solid-like phase that is shape-stable to a fluid-like phase that readily flows. The second reason for special attention is that in the solid-like jammed phase the system becomes locked in its micro-scale configuration, ergodicity is therefore broken, and the system becomes trapped away from thermodynamic equilibrium. System energy is not minimized, therefore. As such, results derived from principles of energy minimization, the associated concept of thermodynamic equilibrium, or the assumption of small departures from thermodynamic equilibrium, all fail to explain the material properties of the solid-like phase or the fluid-like phase or the transition between them. The shortcoming of these approaches in this context is attributable to the fact that thermal fluctuations in such systems are far too small to drive microstructural rearrangements of trapped constituent particles over constraining energy barriers that define a rugged energy landscape, thereby trapping the system away from its minimum-energy equilibrium configuration. That is to say, these jammed systems can be regarded as being effectively athermal. Finally, unlike equilibrium thermal systems such as water, which attains a solid phase upon cooling by virtue of a structural transition from disorder to order ––i.e., crystalline–– these athermal systems attain a solid-like phase by virtue of caging, and resulting kinetic arrest, and thus remain disordered ––i.e., amorphous–– in the solid-like and fluid-like phases alike. For these three reasons, taken together, jammed systems are of substantial interest but remain poorly understood.3
Jamming in confluent tissues:
In the case of confluent tissues, cell jamming and unjamming are now increasingly recognized in the context of collective epithelial cellular migration, which in the healthy tissue is a cardinal feature of tissue development, plasticity, and repair. To cite just a few prominent examples, cell jamming and unjamming have been identified in embryonic development and tissue reorganization in the healthy tissue10–13, as well as in wound healing7, cancer cell invasion14, 15, asthma5, and idiopathic pulmonary fibrosis16. Underlying mechanical, genomic, transcriptional, and signaling events are increasingly coming to light.15, 17, 18
In the case of confluent tissues, energy barriers that serve to impede structural rearrangements, and thus can cause the system to jam, are thought to vanish altogether in certain defined circumstances, in which case the system spontaneously unjams.19–21 Alternatively, internal propulsive forces or externally imposed shear can agitate the collective and thereby inject into the system the energy required to overcome non-vanishing energy barriers and thus drive microstructural rearrangements.8, 21 As addressed below, energy barriers that typify the jammed system ––which by definition is solid-like, elastic, and static–– are not to be confused with frictional energy dissipation, and associated metabolic demands as would be required to propel a migrating unjammed system.22, 23
A fundamental question:
The notion of fluidization of a solid-like multicellular collective is not at all new.24–30 More recently, it has become well-established that certain cases of fluidization of a multicellular collective can be cast into the interpretive context of a jammed-to-unjammed transition.8, 12–14, 21, 25, 31, 32 Moreover, to describe the wide range of material phases that a multicellular collective might find to be attainable while remaining consistent within the laws of physics, various jamming phase diagrams have been proposed including the one addressed in greater detail below.33 6 14 34
Across the jamming phase space, therefore, every locus is physically attainable in principle. The jamming phase space thus describes the full range of possibilities. But which among these physically attainable possibilities are the most biologically plausible? This is a new question. And, I argue, a fundamental one. The novel proposition addressed here is that under the influence of certain selective pressures the living collective system evolved so as to adapt toward certain preferred regions within the jamming phase space.
What this paper is not about:
First, I do not deal here with system-wide coordination provided by means other than jamming, such as by planar cell polarity, chemical signaling, morphogen signaling or electrical signaling.
Second, I do not address here the important but subtle distinctions between the jamming transition, the glass transition3 35 36, 37, and the flocking transition.38, 39 In that connection, the terms jamming and unjamming have been used somewhat loosely in the literature to describe these similar but different phenomenon. As such, it is often not clear whether in a given tissue the solid-like to fluid-like transition is due to a classical jamming mechanism in the narrow sense1 2 3, 4 or a somewhat different microscale mechanism.20 40 This issue is non-trivial but has been addressed suitably elsewhere.3 In brief, collections of particles with appreciable activity, such as cells in a tissue, pedestrians in a crowd, or automobiles in traffic, technically speaking cannot jam simply because, as a matter of its strict definition, jamming is a zero-activity limit. More properly, it should be said that collections of cells become solid-like as they approach a glass transition.41 42 Nevertheless, the jamming nomenclature as used in this communication has come to be widely used in biology.35
Finally, the focus of this communication is not the physics underlying jamming and unjamming. The physics community continues to make substantial strides in that direction. Rather, this paper is designed to shift the spotlight slightly away from the physics of cell jamming per se and more toward the manner in which this new physics brings a fresh perspective to certain issues in physiology, pathophysiology, and perhaps even evolution.
A particular model system:
The arguments developed below can be illustrated with any sufficiently rich jamming phase diagram. As such, the arguments below are not restricted to this particular case, and instead are rather general. But to fix ideas we focus on one model. In brief, the model begins with a confluent cluster of cells in a 2D matrix. Using an agent-based approach, each cell expresses capacities for mutual volume exclusion, cell-cell adhesion, cortical tension, elasticity, viscosity, propulsion, and polarization. From an initial state, the system is then allowed to evolve as cells migrate into a surrounding matrix comprising fixed post-like obstructions at a prescribed spatial density, which are taken to represent connective tissue fibers (Fig. 1, insets A,B,C). This minimal model is sufficiently complex to express a remarkably rich variety of phenomenologies, and thus becomes a useful tool to fix certain ideas as generalized below. If such a model were elaborated further, such as by extension to 3-dimensions or by incorporation of cell-based proteolytic digestion of connective tissue, for example, the resulting jamming phase diagram might differ in its details, both large and small. But the central argument put forward below is insensitive to those details. Of course this minimal computational model has numerous controllable variables. But for illustrative purposes we restrict attention to only two key variables that are systematically varied: collagen density and cellular propulsive force (also referred to as cell motility). For each combination the locus of every cell was determined as a function of time.
Fig. 1.
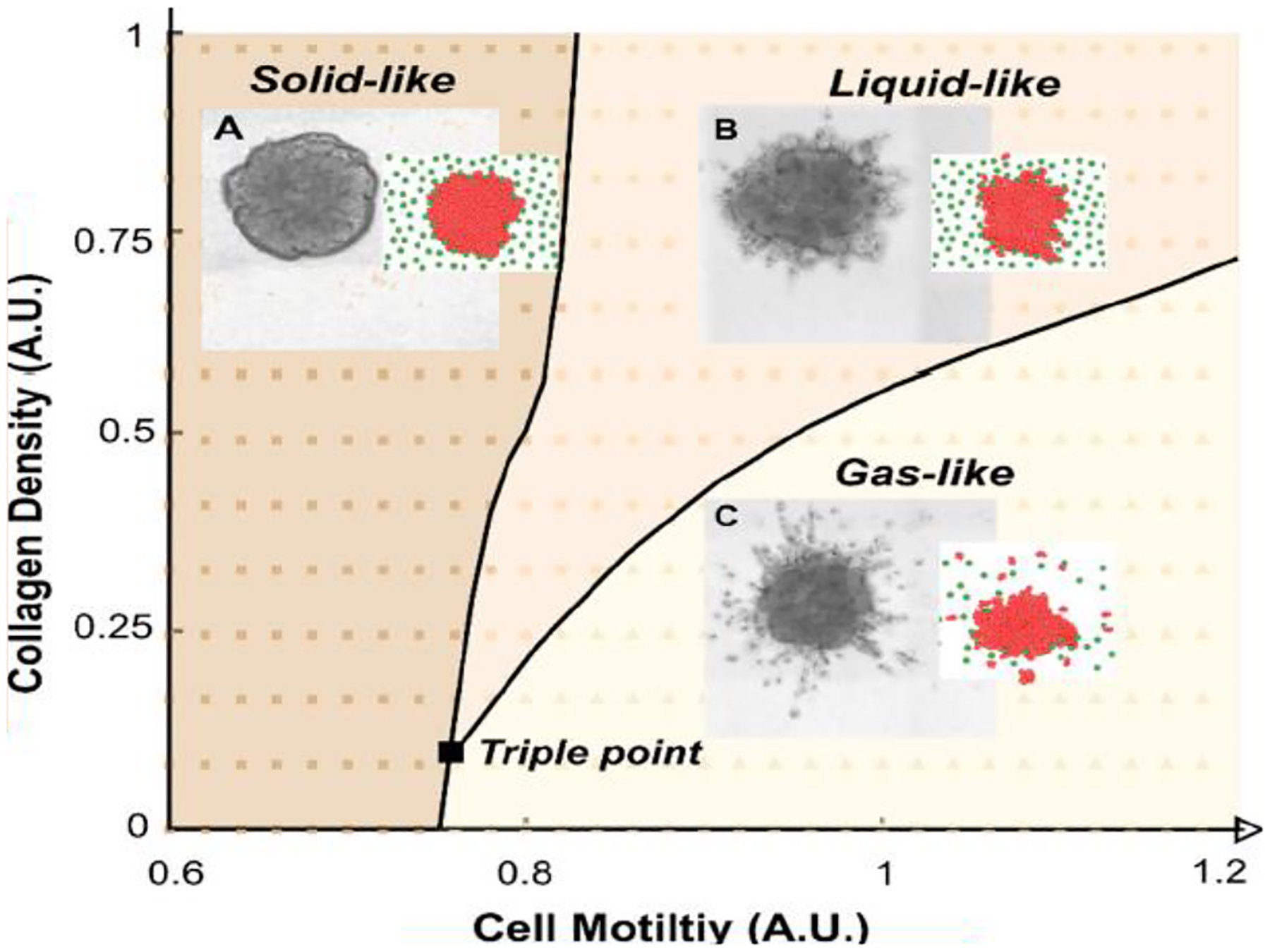
By one computational model, solid-like, liquid-like, and gas-like modes of cell invasion into matrix are observed, as are co-existence lines at which two phases can co-exist, and a triple point at which all three phase can co-exist. Adapted from Kang, Ferruzzi et al.,45
Three migratory phenotypes:
Three migratory phenotypes are observed. First, when cell motility is sufficiently small or collagen density is sufficiently large, only minimal invasion is observed (Fig. 1 inset A). Each cell remains confluent with its immediate neighbors and restricted to a locus close to its original position. This computational simulation reasonably approximates the phenotypic behavior observed experimentally when a spheroid comprising 1000 to 5000 non-tumorigenic epithelial cells (MCF10A) is embedded in collagen at a density of 4 mg/ml. Experimental data indicate that cell shapes are cobblestone in appearance with elongation aspect ratios consistent with cells close to the jamming transition.40, 43, 44 Kang, Ferruzzi et al. referred to this situation as the solid-like jammed regime.45
Second, when cell motility is made sufficiently larger, or collagen density is made sufficiently smaller, there arise continuous invasive tongues that penetrate into the matrix (Fig. 1 inset B). This computational simulation reasonably approximates the phenotypic behavior observed experimentally when a spheroid of MCF10A cells is embedded in collagen at a density of 2 mg/ml, as well as the behavior that is observed when a cluster of aggressive MDA-MB-231 cells is embedded in collagen at a density of 4 mg/ml; MDA-MB-231 cells are post-metastatic and express mesenchymal markers including high Vimentin and low E-Cadherin. Experimental data indicate that cell shapes at the base of the invasive tongue are systematically elongated and migrate faster than cells in the MCF10A spheroid. Kang, Ferruzzi et al. referred to this as the fluid-like unjammed regime.45 They noted, however, that with increasing penetration into the matrix cells comprising the invasive tongue tend to rejam. For the invading cellular collective, these observations support the interpretation that high density collagen promotes steric hindrance and an associated confinement-induced re-jamming.46, 47
Third, when cell motility is sufficiently large but collagen density is sufficiently small, individual cells and small cell clusters are seen to separate from the primary spheroid, scatter, and invade into the matrix. This computational simulation reasonably approximates the phenotypic behavior observed experimentally when a spheroid of the aggressive MDA-MB-231 cells is embedded in collagen at a density of 2 mg/ml. Experimental data show that, depending upon experimental parameters, cell shapes and velocities within the spheroid can indicate either a fluid-like or a solid-like regime, whereas cells scattering into matrix comprise a gas-like regime.45
Gradual perturbations beget abrupt changes:
How does the transition between these different modes of invasion occur as a function of the degree of matrix confinement? Using graded concentrations of collagen (1 to 4 mg/ml), Kang, Ferruzzi et al. tracked over time the number of single MDA-MB-231 cells that had detached from the continuous primary spheroid, escaped that spheroid, and invaded in a gas-like fashion into the matrix (Fig. 2). The number of such single invading cells or cell clusters was found to be dependent not only upon time but, more importantly, dependent upon collagen concentration (Figure 2). On day 0 no cell escape is evident at any collagen concentration; immediately after embedding in collagen, all cells remain within the spheroid. On day 1 a modest level of cell escape becomes evident at lower collagen concentrations (1 and 2 mg/ml) but not at higher concentrations. On day 2 the number of detached invading cells becomes much larger and highly sensitive to collagen concentration. By day 3, remarkably, the number of detached invading cells stabilizes into a striking, almost biphasic, dependence on collagen concentration. The collagen concentration demarking this step-like transition for MDA-MB-231 spheroids falls between 2 and 3 mg/ml. Overall, these findings support the existence of a critical collagen density at which MDAMB-231 cells at the spheroid periphery transition in an almost switch-like fashion between distinct modes of invasion, namely, liquid-like versus gas-like. These events likely depend upon active remodeling of matrix by metalloproteases, cell generated traction forces, and the manner in which these tractions act to align or compress collagen fibers.48–50
Fig. 2.

A. Invasion of a cancer spheroid comprising MDA-MB-231 cells into collagen matrix at graded degrees of collagen density. B. Cells confluent with the primary spheroid are depicted in blue, and those that have scattered from the spheroid are depicted in red. C. The number of detached single cells or small cell clusters as a function of days of maturation and collagen density. By day 3, remarkably, the number of detached and invading cells stabilizes into a striking, almost biphasic, dependence on collagen concentration. Adapted from Kang, Ferruzzi et al.,45
Mapping a hypothetical jamming phase diagram:
Computational simulations performed over a range of cell motilities and collagen densities reveal a jamming phase diagram that depicts solid-like, liquid-like, and gas-like regimes (Fig. 1). The boundaries between these regimes define coexistence lines between phases, i.e., conditions for which two phases can co-exist. The boundaries between phases also depict a triple point at which all three phases can co-exist. The transition from solid-like to liquid-like behavior in this non-equilibrium system is reminiscent of transitions that occur in familiar equilibrium systems: the transition from solid-like to liquid-like regimes is reminiscent of melting; the transition from liquid-like to gas-like regimes is reminiscent of evaporation; and the transition from solid-like to gas-like regimes is reminiscent of sublimation. The existence of each of these non-equilibrium transitions and associated coexistence of these non-equilibrium phases has been confirmed experimentally.45
Where does physiology happen?
If this jamming phase diagram describes the full range of physically attainable possibilities, where in this space does physiology play out? This, I suggest, is a fundamental question with non-trivial implications. I argue here that certain regions within this jamming phase space are more conducive to essential multicellular physiological functions whereas others are less so.
Consider the following gedankenexperiment, beginning with the idea that all living tissue is to some degree heterogeneous in space. Therefore, when the system as a whole is deep in a solid-like regime we might expect to find a few small islands of multiple cells, with each such island being effectively liquid-like, melted, and therefore capable of complex behaviors such as mutual cellular arrangements or collective cellular migration. But these small liquid-like islands are embedded in sea of cells that are solid-like and therefore effectively frozen. As a result, complex behaviors in any one liquid-like melted island are shielded in the sense that they cannot propagate into the surrounding solid-like space, and therefore these islands cannot influence one another. Large perturbations in the local liquid-like island can therefore exert at best only small effects globally, and the system as a whole remains solid-like and frozen. It is perhaps for this reason, as well as others, that the jammed phase has been referred to as being tumor suppressive.51
By contrast, when the system as a whole is deep in a liquid-like regime we might expect to find a few small islands of multiple cells that are effectively solid-like and frozen, as it were. Being solid-like, any complex behavior within such an island, such as mutual cellular rearrangements or migration, would have to be highly cooperative. But these small solid-like islands are embedded in a sea of cells that are liquid-like, melted, and, by virtue of viscosity, mechanically dissipative. As such, cooperative behaviors within the small solid-like island are shielded in the sense that they become dissipated and diffused. The behavior in these islands is thus attenuated in the surrounding liquid-like sea, and these islands therefore cannot influence one another. Large perturbations in local solid-like islands can therefore exert at best small effects globally, and the system as a whole remains liquid-like and melted.
We now consider in particular a special domain, one that is neither deep in the liquid-like regime nor deep in the solid-like regime, but rather is poised in the solid-like regime but just at the brink of unjamming (Fig. 3). For three reasons this regime is singular. First, being poised at the brink of unjamming, this regime allows for collective cellular behaviors that are not only dynamic and complex but also cooperative. Second, this regime is also capable of percolating across space so as to span the physical boundaries of the system. For example, being poised at the brink of unjamming allows for system-wide cooperative dynamical events such as tissue remodeling, wound repair, development, branching, and growth. By contrast, deep within the solid-like regime, as well as deep within the fluid-like regime, for the reasons described above events such as these become increasingly implausible. Finally, being poised at the brink of unjamming allows for rather potent and sensitive mechanical modulation of these processes. By shifting only slightly more deeply into the solid-like regime, for example, dynamic events would become progressively and dramatically slowed or, in the limit, stabilized altogether. Conversely, by shifting only slightly away from the solid-like regime and toward the liquid-like regime dynamic events would become increasing possible and accelerated. Moreover, being thus poised at the edge of unjamming, a very small but well-chosen modulation in certain state variables may alter system-wide behavior rather dramatically, thereby affording a sensitive mechanism for tuning and control of system-wide behavior.
Fig. 3. In any jamming phase diagram, where does physiology play out?

In the heterogeneous multicellular system, one might expect to find deep in the solid-like regime small islands comprising isolated melted domains. Similarly, one would also expect to find deep in the liquid-like regime small islands of isolated solid-like domains. But in neither situation can the complex and cooperative behaviors that define physiology percolate across the system. Such physiological behaviors include but are not limited to development, remodeling, plasticity, invasion, and wound repair. As such, certain regions within this phase space are more conducive to essential multicellular physiological functions whereas others are far less so. In particular, being poised in the solid-like regime but just at the brink of unjamming allows for the existence ––as well as the fine control–– of collective cellular behaviors that are not only dynamic and complex but also cooperative and system-spanning. Dysregulation of that control has been implicated in pathologies including cancer cell invasion14, 15, asthma5, and idiopathic pulmonary fibrosis16.
For these reasons, taken together, I propose that in a variety of systems and circumstances, well beyond those illustrated here, physiology happens, and can only happen, in the solid-like regime just at the brink of unjamming. Of course, if this special regime were to become dysregulated it would allow for pathological events such as asthmatic remodeling of the airway wall, idiopathic pulmonary fibrosis, and cancer cell invasion and scattering.16, 52 45
Jamming or unjamming first?
In evolution of early multicellular organisms, or in embryonic tissue development, which comes first, jamming or unjamming? Here we turn again to Kauffman, who in 2002 introduced the concept he called the adjacent possible.53 His theory of the adjacent possible proposes that “biological systems are able to morph into more complex systems only by making incremental, relatively less energy consuming changes in their makeup”. That is to say, evolution advances mainly by cobbling together available resources to new uses. Based upon this idea, I reason as follows. Being highly dynamic and requiring tissue plasticity and remodeling, the earliest multicellular organisms, as well the developing embryonic tissue, are reasoned to be unjammed but energetically expensive. Little data is available about energy metabolism associated with unjamming. We do know, however, that at the leading unjammed edge of an advancing epithelial layer the cytoplasmic redox ratio becomes progressively smaller, the NADH lifetime becomes progressively shorter, and the mitochondrial membrane potential and glucose uptake become progressively larger.22 While short of being definitive, these observations are suggestive that the unjammed migratory phase is energetically expensive compared with the jammed phase. To the extent that this might be so, the jammed phase, logically, would represent an obvious adjacent possible with respect to the unjammed phase. In tissue development as in evolution, the unjammed phase, which is dynamic but energetically expensive, is reasoned to precede the jammed phase which follows, which is homeostatic but energetically economical.
What about the EMT?
Mature tissues are characterized by loss of cell motility, which in turn stabilizes the tissue mechanically and decreases energy metabolism. These features, taken together, suggest that the jamming transition and emergence of the associated solid-like phase may be among the final stages of tissue maturation. But in order for any pathology that dedifferentiates or deregulates epithelial cells, including carcinomas, to become a systemic disease ––as opposed to a localized aberration–– a fluid-like phase allowing for epithelial motility must be restored. In that connection, the unjamming transition is distinct from and not to be confused with the epithelial-to-mesenchymal transition (EMT).54 Either provides a gateway to epithelial motility, fluidity, and malleability. We know, moreover, that the unjamming transition can occur in the absence of EMT, whereas the converse remains unclear.54 It remains unclear, as well, how unjamming and EMT might work independently, sequentially, or cooperatively to effect morphogenesis, wound repair, and tissue remodeling, as well as fibrosis, cancer invasion, and metastasis.
The origins of order:
It has been suggested that the unjammed phase of the cellular collective evolved under a selective pressure favoring fluid-like migratory dynamics as would be required so as to accommodate dynamics associated with episodes of tissue development, plasticity, and repair. This unjammed phase, being dynamic, is reasoned to be energetically expensive compared with the jammed phase, which evolved under a selective pressure favoring a solid-like homeostatic regime that, by comparison, is non-migratory but energetically economical and mechanically stable.22
But well before the discovery of cell jamming Kauffman proposed the general biological principle that living systems exist in a solid regime near the edge of chaos, and that natural selection achieves and sustains such a poised state.55 Kauffman formulated this principle based upon abstract considerations rooted in non-linear Boolean networks and self-organized criticality55 53, and these ideas were later generalized still further by others.56 In his book, Investigations, Kaufman goes on to emphasize that by the terms ‘order’ and ‘chaos’ what he is referring to is different from ––and not to be confused with–– classical concepts of entropy as framed by Boltzmann and Gibbs in the context of statistical thermodynamics of systems at thermodynamic equilibrium.53 Instead, he deals with what he calls the active autonomous agent, which comprises an open dissipative system far from thermodynamic equilibrium, such as the living cell. For example, he argues that as the cell ‘swims, scrambles, pokes, twists, and pounces’, it co-evolves to an ‘edge of chaos’, which he defines as that region lying just between ‘overrigid and overfluid’ behavior. Further, he connects this co-evolution to the adjacent possible, wherein these autonomous agents ‘push their way into novelty’, be that novelty molecular, morphological, behavioral, or organizational.
Kauffman’s framework is so general as to be innately abstract, vague and qualitative. By contrast, our knowledge of cell jamming and unjamming, while still incomplete, is concrete, specific and quantitative. For example, we now know that as epithelial cells progressively unjam, and thus transition from a solid-like to a fluid-like collective phase, their cell stiffnesses and migration velocities tend to increase, their cell shapes become more elongated and more variable, and their propulsive forces exerted on the substrate, and junctional forces exerted on immediate neighbors, tend to increase.31, 50, 52, 57 In addition, the size of cooperative swirls tends to decrease.41, 52
So the question arises, Do the solid-like and fluid-like phases associated with jamming and unjamming fit as a subset within Kauffman’s generalized concept of ‘overrigid and overfluid’ behavior? Or should his framework be regarded merely as analogy or metaphor? Certainly, it can address none of the experimental findings associated with cell jamming/unjamming in concrete or specific terms and, as he points out, can make no specific predictions. The best that it can do, he says, is to tell stories describing events as they unfold.53 Based upon available evidence, perhaps the strongest statement that can be made at this time is that the jammed solid-like phase of the multicellular collective ––poised just at the brink of unjamming–– is strongly reminiscent of Kauffman’s generalized solid-like regime poised at the edge of chaos.55 A similar situation may be recapitulated at a subcellular scale of organization.58 59 60
Acknowledgments:
The author thanks Josef Käs and James P. Butler for their helpful comments. This work was supported by NIH grant number 1R01HL148152
References:
- 1.Cates ME, Wittmer JP, Bouchaud JP and Claudin P, Physical Review Letters, 1998, 81, 1841–1844. [DOI] [PubMed] [Google Scholar]
- 2.Liu AJ and Nagel SR, Nature, 1998, 396, 21–22. [Google Scholar]
- 3.Berthier L, Flenner E and Szamel G, The Journal of Chemical Physics, 2019, 150, 200901. [DOI] [PubMed] [Google Scholar]
- 4.Trappe V, Prasad V, Cipelletti L, Segre PN and Weitz DA, Nature, 2001, 411, 772–775. [DOI] [PubMed] [Google Scholar]
- 5.Park JA, Kim JH, Bi D, Mitchel JA, Qazvini NT, Tantisira K, Park CY, McGill M, Kim SH, Gweon B, Notbohm J, Steward R Jr., Burger S, Randell SH, Kho AT, Tambe DT, Hardin C, Shore SA, Israel E, Weitz DA, Tschumperlin DJ, Henske EP, Weiss ST, Manning ML, Butler JP, Drazen JM and Fredberg JJ, Nat Mater, 2015, 14, 1040–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadati M, Taheri Qazvini N, Krishnan R, Park CY and Fredberg JJ, Differentiation, 2013, 86, 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trepat X, Wasserman M, Angelini T, Millet E, Weitz D, Butler J and Fredberg J, Nature Physics, 2009, 5, 426–430. [Google Scholar]
- 8.Henkes S, Fily Y and Marchetti M, PHYSICAL REVIEW E, 2011, 84, 040301(R). [DOI] [PubMed] [Google Scholar]
- 9.Keys A, Abate A, Glotzer SC and Durian DJ, Nature Physics, 2007, 3, 260–264. [Google Scholar]
- 10.Atia L, Bi D, Sharma Y, Mitchel JA, Gweon B, Koehler S, DeCamp SJ, Lan B, Kim JH, Hirsch R, Pegoraro AF, Lee KH, Starr JR, Weitz DA, Martin AC, Park JA, Butler JP and Fredberg JJ, Nat Phys, 2018, 14, 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mongera A, Rowghanian P, Gustafson HJ, Shelton E, Kealhofer DA, Carn EK, Serwane F, Lucio AA, Giammona J and Campas O, Nature, 2018, 561, 401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spurlin JW, Siedlik MJ, Nerger BA, Pang M-F, Jayaraman S, Zhang R and Nelson CM, Development, 2019, 146, dev175257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petridou NI, Corominas-Murtra B, Heisenberg C-P and Hannezo E, Cell, 2021, DOI: 10.1016/j.cell.2021.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ilina O, Gritsenko PG, Syga S, Lippoldt J, La Porta CAM, Chepizhko O, Grosser S, Vullings M, Bakker GJ, Starruss J, Bult P, Zapperi S, Kas JA, Deutsch A and Friedl P, Nat Cell Biol, 2020, DOI: 10.1038/s41556-020-0552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palamidessi A, Malinverno C, Frittoli E, Corallino S, Barbieri E, Sigismund S, Beznoussenko GV, Martini E, Garre M, Ferrara I, Tripodo C, Ascione F, Cavalcanti-Adam EA, Li Q, Di Fiore PP, Parazzoli D, Giavazzi F, Cerbino R and Scita G, Nature Materials, 2019, DOI: 10.1038/s41563-019-0425-1. [DOI] [PubMed] [Google Scholar]
- 16.Stancil IT, Michalski JE, Davis-Hall D, Chu HW, Park J-A, Magin CM, Yang IV, Smith BJ, Dobrinskikh E and Schwartz DA, Nature Communications, 2021, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilic A, Ameli A, Park JA, Kho AT, Tantisira K, Santolini M, Cheng F, Mitchel JA, McGill M, O’Sullivan MJ, De Marzio M, Sharma A, Randell SH, Drazen JM, Fredberg JJ and Weiss ST, Sci Rep, 2020, 10, 966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Marzio M, Kilic A, Maiorino E, Mitchel JA, Mwase C, O’Sullivan MJ, McGill M, Chase R, Fredberg JJ, Park JA, Glass K and Weiss ST, Sci Adv, 2021, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bi D, Lopez J, Schwarz J and Manning M, Soft Matter, 2014. [DOI] [PubMed] [Google Scholar]
- 20.Bi D, Lopez JH, Schwarz JM and Manning ML, Nat Phys, 2015, advance online publication. [Google Scholar]
- 21.Bi D, Yang X, Marchetti MC and Manning ML, Physical Review X, 2016, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeCamp SJ, Tsuda VMK, Ferruzzi J, Koehler SA, Giblin JT, Roblyer D, Zaman MH, Weiss ST, Kilic A, De Marzio M, Park CY, Ogassavara NC, Mitchel JA, Butler JP and Fredberg JJ, Sci Rep, 2020, 10, 18302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duclut C, Paijmans J, Mandar C and Julicher F, arXiv pre-print server, 2021, DOI: arxiv:2111.10327. [Google Scholar]
- 24.Farhadifar R, Roper JC, Aigouy B, Eaton S and Julicher F, Curr Biol, 2007, 17, 2095–2104. [DOI] [PubMed] [Google Scholar]
- 25.Garcia S, Hannezo E, Elgeti J, Joanny J-F, Silberzan P and Gov NS, Proceedings of the National Academy of Sciences, 2015, DOI: 10.1073/pnas.1510973112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hakim V and Silberzan P, Reports on Progress in Physics, 2017, 80, 076601. [DOI] [PubMed] [Google Scholar]
- 27.Marel A-KP, Nils , Zorn M, Rädler JO and Elgeti J, New Journal of Physics, 2014, 16, 115005. [Google Scholar]
- 28.Poujade M, Grasland-Mongrain E, Hertzog A, Jouanneau J, Chavrier P, Ladoux B, Buguin A and Silberzan P, Proc Natl Acad Sci U S A, 2007, 104, 15988–15993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ranft J, Basan M, Elgeti J, Joanny J-F, Prost J and Jülicher F, Proceedings of the National Academy of Sciences, 2010, 107, 20863–20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarkar D, Gompper G and Elgeti J, Communications Physics, 2021, 4. [Google Scholar]
- 31.Atia L, Bi D, Sharma Y, Mitchel JA, Gweon B, Koehler SA, DeCamp SJ, Lan B, Kim JH, Hirsch R, Pegoraro AF, Lee KH, Starr JR, Weitz DA, Martin AC, Park J-A, Butler JP and Fredberg JJ, Nature Physics, 2018, DOI: 10.1038/s41567-018-0089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haeger A, Alexander S, Vullings M, Kaiser FMP, Veelken C, Flucke U, Koehl GE, Hirschberg M, Flentje M, Hoffman RM, Geissler EK, Kissler S and Friedl P, The Journal of Experimental Medicine, 2019, DOI: 10.1084/jem.20181184, jem.20181184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bi D, Lopez JH, Schwarz JM and Manning ML, Nat Phys, 2015, 11, 1074–1079. [Google Scholar]
- 34.Kang W, Ferruzzi J, Spatarelu C-P, Han YL, Sharma Y, Koehler SA, Mitchel JA, Khan A, Butler JP, Roblyer D, Zaman MH, Park J-A, Guo M, Chen Z, Pegoraro AF and Fredberg JJ, iScience, 2021, 24, 103252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atia L, Fredberg JJ, Gov NS and Pegoraro AF, Cells & Development, 2021, DOI: 10.1016/j.cdev.2021.203727, 203727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen D, Aw WY, Devenport D and Torquato S, Biophys J, 2016, 111, 2534–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atkinson S, Stillinger FH and Torquato S, Proc Natl Acad Sci U S A, 2014, 111, 18436–18441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giavazzi F, Paoluzzi M, Macchi M, Bi D, Scita G, Manning ML, Cerbino R and Marchetti MC, Soft Matter, 2018, 14, 3471–3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giavazzi F, Malinverno C, Corallino S, Ginelli F, Scita G and Cerbino R, arXive, 2017, arXiv:1707.03060 [cond-mat.soft]. [Google Scholar]
- 40.Merkel M and Manning ML, New Journal of Physics, 2018, 20, 022002. [Google Scholar]
- 41.Angelini TE, Hannezo E, Trepat X, Marquez M, Fredberg JJ and Weitz DA, Proc Natl Acad Sci U S A, 2011, 108, 4714–4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Angell CA, Science, 1995, 267, 1924–1935. [DOI] [PubMed] [Google Scholar]
- 43.Grosser S, Lippoldt J, Oswald L, Merkel M, Sussman DM, Renner F, Gottheil P, Morawetz EW, Fuhs T, Xie X, Pawlizak S, Fritsch AW, Wolf B, Horn L-C, Briest S, Aktas B, Manning ML and Käs JA, Physical Review X, 2021, 11, 011033. [Google Scholar]
- 44.Grosser S, Lippoldt J, Oswald L, Merkel M, Sussman DM, Renner F, Morawetz E, Pawlizak S, Fritsch A, Horn L, Aktas B, Manning M and Kaes J, Physical Review X, 2019, In press. [Google Scholar]
- 45.Kang W, Ferruzzi J, Spatarelu CP, Han YL, Sharma Y, Koehler SA, Mitchel JA, Khan A, Butler JP, Roblyer D, Zaman MH, Park JA, Guo M, Chen Z, Pegoraro AF and Fredberg JJ, iScience, 2021, 24, 103252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolf K and Friedl P, Trends Cell Biol, 2011, 21, 736–744. [DOI] [PubMed] [Google Scholar]
- 47.Haeger A, Krause M, Wolf K and Friedl P, Biochimica et biophysica acta, 2014, DOI: 10.1016/j.bbagen.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 48.Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG and Keely PJ, BMC Medicine, 2006, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steinwachs J, Metzner C, Skodzek K, Lang N, Thievessen I, Mark C, Munster S, Aifantis KE and Fabry B, Nat Methods, 2016, 13, 171–176. [DOI] [PubMed] [Google Scholar]
- 50.Kim JH, Pegoraro AF, Das A, Koehler SA, Ujwary SA, Lan B, Mitchel JA, Atia L, He S, Wang K, Bi D, Zaman MH, Park J-A, Butler JP, Lee KH, Starr JR and Fredberg JJ, Biochemical and Biophysical Research Communications, 2020, 521, 706–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frittoli E, Palamidessi A, Iannelli F, Zanardi F, Villa S, Barzaghi L, Ando H, Cancila V, Beznuskenko G, Chiara GD, Pagani M, Malinverno C, Bhattacharya D, Pisati F, Yu W, Galimberti V, Bonizzi G, Martini E, Mironov A, Gioia U, Fagagna FDAD, Rossi C, Bertalot G, Lucioni M, Tancredi R, Pedrazzoli P, Vecchione A, Perini C, Ferrari F, Lanzuolo C, Nader G, Foiani M, Piel M, Cerbino R, Giavazzi F, Tripodo C and Scita G, unpublished work.
- 52.Park J-A, Kim JH, Bi D, Mitchel JA, Qazvini NT, Tantisira K, Park CY, McGill M, Kim S-H, Gweon B, Notbohm J, Steward R Jr, Burger S, Randell SH, Kho AT, Tambe DT, Hardin C, Shore SA, Israel E, Weitz DA, Tschumperlin DJ, Henske EP, Weiss ST, Manning ML, Butler JP, Drazen JM and Fredberg JJ, Nat Mater, 2015, advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kauffman SA, Investigations, Oxford University Press, New York, 2000. [Google Scholar]
- 54.Mitchel JA, Das A, O’Sullivan MJ, Stancil IT, DeCamp SJ, Koehler S, Ocana OH, Butler JP, Fredberg JJ, Nieto MA, Bi D and Park JA, Nat Commun, 2020, 11, 5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kauffman SA, The Origins of Order, Oxford University Press, 1993. [Google Scholar]
- 56.Muñoz MA, Reviews of Modern Physics, 2018, 90. [Google Scholar]
- 57.Han YL, Pegoraro AF, Li H, Li K, Yuan Y, Xu G, Gu Z, Sun J, Hao Y, Gupta SK, Li Y, Tang W, Tang X, Teng L, Fredberg JJ and Guo M, Nat Phys, 2020, 16, 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fredberg JJ, Physiology (Bethesda), 2014, 29, 218–219. [DOI] [PubMed] [Google Scholar]
- 59.Fabry B, Maksym GN, Butler JP, Glogauer M, Navajas D and Fredberg JJ, Phys Rev Lett, 2001, 87, 148102. [DOI] [PubMed] [Google Scholar]
- 60.Schrödinger E, What is life?, Cambridge University Press, Cambridge, UK, 1944. [Google Scholar]


