Abstract
Objective:
To determine the longitudinal relationships between abnormal glucose metabolism and physical function in persons with HIV (PWH) and without HIV.
Design:
Prospective cohort study of men with or at risk for HIV in four US cities between 2006 and 2018.
Methods:
Men with or at risk for HIV from the Multicenter AIDS Cohort Study (MACS) had semi-annual assessments of glycemic status, grip strength, and gait speed. We used linear mixed models with random intercept to assess associations between glycemic status and physical function. Glycemic status was categorized as normal, impaired fasting glucose (IFG), controlled diabetes mellitus (DM) (hemoglobin A1C [HbA1C] <7.5%), or uncontrolled DM (HbA1C ≥7.5%).
Results:
Of 2,240 men, 52% were PWH. DM was similar among PWH (7.7 %) vs persons without HIV (6.7%, p=0.36) at baseline. PWH had slower gait speed (1.17 m/s vs 1.20 m/s, p<0.01) but similar grip strength (40.1 kg vs 39.8, p=0.76) compared to persons without HIV at baseline. In multivariate models, gait speed decline was greater with controlled DM (−0.018 m/s [−0.032, −0.005], p=0.01) and grip strength decline was greater with controlled (−0.560 kg [−1.096, −0.024, p=0.04) and uncontrolled DM (−0.937 kg [−1.684, −0.190], p=0.01), regardless of HIV serostatus compared to normoglycemic individuals.
Discussion:
Abnormal glucose metabolism was associated with declines in gait speed and grip strength regardless of HIV serostatus. These data suggest that improvement in glucose control should be investigated as an intervenable target to prevent progression of physical function limitations among PWH.
Keywords: diabetes mellitus, glycemic status, physical function, grip strength, gait speed, HIV-1
Introduction
While the routine use of antiretroviral therapy (ART) has transformed HIV into a chronic disease, people with HIV (PWH) experience both earlier onset and increased rates of chronic non-infectious aging-related comorbidities compared to the general population[1, 2]. Older PWH experience more rapid declines in physical function compared to persons without HIV [3, 4]. Impaired physical function has been associated with greater risk of adverse health outcomes in PWH including disability, falls, reduced quality of life, and mortality [5-9]. Therefore, insight into mechanisms underlying physical function declines and interventions to prevent functional impairment in this vulnerable population are urgently needed.
Abnormal glucose metabolism has been associated with physical function impairment in the general population[10-14]. Decreased muscle quality and mitochondrial dysfunction have been identified as potential causes of this relationship[15, 16]. Since the incidence and prevalence of diabetes mellitus (DM) have been higher among PWH compared to the general population, these relationships may be particularly relevant in driving physical function impairment among PWH [17, 18]. A cross-sectional study from the Multicenter AIDS Cohort Study (MACS) showed that worse insulin resistance was associated with frailty, a complex phenotype in which gait speed and grip strength are key components, in men with HIV. In this study, insulin resistance was also significantly worse among non-frail men with HIV compared to non-frail men without HIV [19]. A cross-sectional analysis in the Hawaii Aging with HIV-Cardiovascular Disease Study also showed that frailty was significantly associated with increased insulin resistance in PWH[20]. However, no longitudinal studies have compared the relationship between abnormal glucose metabolism and physical function over time in persons with and without HIV.
The purpose of this study was to determine the longitudinal relationship between glycemic status and physical function in men with and without HIV in the MACS. We aimed to better understand the relative contributions of dysglycemia and HIV to observed physical function declines.
Methods
Study participants
The MACS is one of the largest ongoing prospective observational cohort studies of PWH that includes men with HIV and demographically similar men without HIV. Since 1984, 6,972 participants have been recruited from four sites (Baltimore/Washington DC, Chicago, Los Angeles, and Pittsburgh). MACS eligibility criteria and follow-up assessment protocols have been previously described[21, 22]. In brief, MACS participants complete semiannual study visits which include detailed interviews, physical examination, and collection of blood for laboratory testing and storage.
In 2006, semi-annual assessments of physical function (i.e., gait speed and grip strength) were added to the MACS protocol. The present study included all participants who underwent at least two measurements of gait speed and/or grip strength between 2006 and 2018. The MACS protocol has been approved by the institutional review board at each study site. Informed consent has been obtained from all study participants.
Glycemic status assessment
Fasting serum glucose levels have been measured at each MACS visit since 1999. For this study, participants were categorized as one of four glycemic statuses: normal, impaired fasting glucose (IFG), controlled diabetes mellitus (DM), or uncontrolled DM. Men were categorized as having IFG if they had a fasting blood glucose level between 100-125 mg/dl. DM was defined as self-reported DM as a medical condition, use of anti-diabetic medications, or a fasting blood glucose level of 126 mg/dl or greater for at least two consecutive visits. DM was further dichotomized as controlled or uncontrolled based on hemoglobin A1C (HbA1C) level: HbA1C less than 7.5% was considered controlled and 7.5% or greater on two consecutive visits was considered uncontrolled DM. Diagnosis of DM was treated as an absorbent state, but glycemic status was reassessed for each participant at each MACS follow-up visit.
Physical function assessments
Gait speed was assessed as the faster of two timed 4-meter walk assessments at usual pace[3]. Grip strength was assessed using a hand-held dynamometer[4]. Participants were asked to squeeze the dynamometer “as hard as you can” three times using their dominant hand. The average of these three measures was used for the current analysis.
Other covariates and risk factors
Demographic factors included in the analysis are listed in Table 1. They included age, race, education level, study site, and enrollment year, body mass index (BMI), and use of tobacco, marijuana, alcohol, or intravenous drugs. Liver disease and arthritis were defined as prior or current self-reported diagnosis. Hypertension was defined as systolic pressure ≥140 mm Hg, diastolic pressure ≥90 mm Hg or self-reported diagnosis of hypertension with use of antihypertensive medications. Kidney disease was defined as estimated glomerular filtration rate < 60 ml/min/1.73 m2 body surface areas calculated by the CKD-EPI Creatinine Equation. Hepatitis C virus (HCV) infection was defined by a detectable serum HCV antibody or HCV RNA. Hepatitis B virus (HBV) infection was defined as detectable serum hepatitis B surface antigen. Supplemental testosterone use was assessed by self-report. Depressive symptoms were defined as a score of 16 or greater on the Center for Epidemiologic Studies Depression Scale (CESD). For PWH, additional covariates considered were number of years on ART; cumulative exposure (in years) to zidovudine, didanosine, stavudine, efavirenz, or any protease inhibitor; nadir CD4+ T-lymphocyte cell count/mm3 (CD4); and plasma HIV-1 RNA viral load. Covariates were updated at each visit.
Table 1.
Demographic characteristics of study population by HIV serostatus at baseline
| Characteristic | Men without HIV N=1070 |
Men with HIV N=1170 |
p-value |
|---|---|---|---|
| Age (years), mean (SD) | 49.2 (11.7) | 44.8 (10.2) | <0.001 |
| BMI (kg/m2), mean (SD) | 27.1 (5.2) | 25.8 (4.6) | <0.001 |
| Race, N (%) | |||
| White | 786 (73.5%) | 674 (57.6%) | |
| Black | 229 (21.4%) | 379 (32.4%) | |
| Other | 55 (5.1%) | 117 (10.0%) | <0.001 |
| College education, N (%) | 636 (63.8%) | 501 (46.0%) | <0.001 |
| Center, N (%) | |||
| Baltimore | 278 (26.0%) | 282 (24.1%) | |
| Chicago | 159 (14.9%) | 276 (23.6%) | |
| Pittsburgh | 314 (29.4%) | 283 (24.2%) | |
| Los Angeles | 319 (29.8%) | 329 (28.1%) | <0.001 |
| Cohort, N (%) | |||
| 1984 | 633 (59.2%) | 364 (31.1%) | |
| 1987 | 43 (4.0%) | 90 (7.7%) | |
| 2001 | 346 (32.3%) | 451 (38.5%) | |
| 2010 | 48 (4.5%) | 265 (22.7%) | <0.001 |
| Smoking status, N (%) | |||
| Never | 340 (32.1%) | 359 (31.2%) | |
| Former | 463 (43.6%) | 409 (35.5%) | |
| Current | 258 (24.3%) | 384 (33.3%) | <0.001 |
| History of cocaine use, N (%) | 472 (44.4%) | 572 (49.1%) | 0.027 |
| History of marijuana use, N (%) | 800 (75.3%) | 815 (70.0%) | 0.005 |
| Alcohol use, N (%) | |||
| None | 158 (15.0%) | 206 (18.0%) | |
| Low-moderate | 621 (58.8%) | 629 (54.9%) | |
| Moderate-binge | 277 (26.2%) | 310 (27.1%) | 0.098 |
| Kidney disease, N (%) | 58 (6.9%) | 171 (17.6%) | <0.001 |
| Hypertension, N (%) | 396 (37.9%) | 387 (33.9%) | 0.051 |
| Arthritis, N (%) | 40 (3.9%) | 45 (3.9%) | 0.942 |
| Depression (CESD≥16) , N (%) | 237 (23.0%) | 321 (29.1%) | 0.001 |
| Hepatitis B infection, N (%) | 10 (0.9%) | 42 (3.6%) | <0.001 |
| Hepatitis C infection, N (%) | 46 (4.3%) | 82 (7.0%) | 0.006 |
| Glycemic status, N (%) | |||
| Normal | 498 (60.4%) | 554 (63.5%) | |
| IFG | 272 (33.0%) | 252 (28.9%) | |
| Controlled DM | 33 (4.0%) | 57 (6.5%) | |
| Uncontrolled DM | 22 (2.7%) | 10 (1.2%) | 0.004 |
| Grip strength (kg), mean (SD) | 39.8 (8.8%) | 40.1 (9.4) | 0.760 |
| Gait speed (m/s), mean (SD) | 1.20 (0.22) | 1.17 (0.22) | 0.001 |
| Testosterone use, N (%) | 61 (5.7%) | 195 (16.7%) | <0.001 |
| Years of ART, mean (SD) | - | 5.1 (4.7) | - |
| Cumulative ART exposure (years), mean (SD) | |||
| AZT | - | 2.6 (3.6) | - |
| DDI | - | 0.8 (1.9) | - |
| d4T | - | 1.6 (2.5) | - |
| EFV | - | 1.3 (2.2) | - |
| Any PI | - | 2.9 (3.4) | - |
| CD4 nadir (cells/uL), N (%) | - | 334.7 (212.1) | - |
| <200 | - | 300 (25.8%) | |
| 200-500 | - | 652 (56.1%) | |
| >500 | - | 210 (18.1%) | - |
| Undetectable HIV viral load, N (%) | - | 805 (69.6%) | - |
ART, antiretroviral therapy; AZT, zidovudine; BMI, body mass index; d4T, stavudine; DDI, didanosine; DM, diabetes mellitus; EFV, efavirenz; IFG, impaired fasting glucose; PI, protease inhibitor; SD, standard deviation
Statistical analysis
Comparison of baseline characteristics were performed using Student t tests for normally distributed continuous variables, Wilcoxon rank-sum tests for variables with skewed distributions, and Chi-square test for categorical data.
We used linear mixed models with random intercept to assess the association of glycemic status and physical function. This approach examined within-individual changes in physical function (i.e., grip strength and gait speed) over the time-period of study follow-up, accounting for within-individual correlations. The present analysis included all participants with a minimum of two study visits and had complete data for all covariates of interest. Due to the relatively large portion of missingness (>20%) in testosterone use, last observation carried forward method was used. For the two initial models, physical function (either grip strength or gait speed) was a function of follow-up time, glycemic status, HIV serostatus, and the clinical characteristics and other confounding factors as listed above. To examine whether there was an association between glycemic status and changes in physical function over time, a three-way interaction term of follow-up time*glycemic status*HIV serostatus was then added to the initial model. Subgroup analyses were also performed among PWH using the same linear mixed model format with addition of HIV specific risk factors, including nadir CD4, current CD4, undetectable HIV-1 RNA (suppressed on assay available at that time) and time on ART (in years). All the analyses were performed using Stata 14.0 SE (StataCorp, College Station, TX).
Results
Demographic characteristics
Baseline characteristics for the 1,170 PWH and 1,070 persons without HIV included in this study are shown in Table 1. The mean follow-up time was 8.7 years. Compared to persons without HIV, PWH tended to be younger and were more likely to be non-White, current smokers, have a lower BMI, have a history of cocaine or injection drug use, use supplemental testosterone, or to have liver disease, kidney disease, depression, and HBV or HCV. PWH were less likely to have a college education, a history of marijuana use, or hypertension. At baseline, persons without HIV were more likely to have IFG or uncontrolled DM, but PWH were more likely to have controlled DM. Baseline mean HbA1cfor each glycemic category by HIV serostatus is shown in Table 2. No statistically significant difference in grip strength by HIV serostatus was noted at baseline. Gait speed was significantly lower in PWH at baseline (p=0.001).
Table 2.
Baseline hemoglobin A1C [mean (SD)] by HIV serostatus and glycemic status
| Men without HIV N=1070 |
Men with HIV N=1170 |
|
|---|---|---|
| Glycemic status | ||
| Normal | 5.39 (0.42) | 5.29 (0.49) |
| IFG | 5.64 (0.55) | 5.39 (0.54) |
| Controlled DM | 6.39 (0.67) | 6.15 (0.77) |
| Uncontrolled DM | 9.32 (1.70) | 9.32 (2.40) |
Grip strength
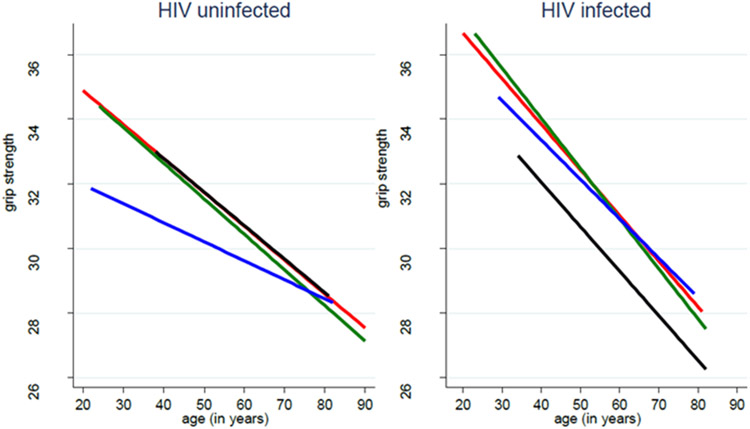
Compared to persons with normal glycemic status, men with controlled (β −0.560 kg, p=0.040) or uncontrolled DM (β −0.937kg, p=0.014) had significant declines in grip strength (Table 3). IFG and HIV serostatus were not significantly associated with grip strength. Other characteristics significantly associated with change in grip strength included current smoking status, older age, higher BMI, depression and HCV infection. No grip strength decline over time was observed by glycemic status in both PWH or persons without HIV (Figure 1 and Supplemental Table 1).
Table 3.
Association between glycemic status and physical function: results from multivariate linear mixed models
| Grip Strength | Gait speed | |||
|---|---|---|---|---|
| Variables | Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value |
| Age, years | -0.129 (−0.166, −0.093) | <0.001 | -0.003 (−0.003, −0.002) | <0.001 |
| Glycemic status | ||||
| Normal | Ref | Ref | ||
| IFG | −0.144 (−0.429, 0.140) | 0.321 | −0.004 (−0.012, 0.003) | 0.230 |
| Controlled DM | −0.560 (−1.096, −0.024) | 0.040 | −0.018 (−0.032, −0.005) | 0.007 |
| Uncontrolled DM | −0.937 (−1.684, −0.190) | 0.014 | −0.007 (−0.026, 0.011) | 0.437 |
| HIV infection | 0.483 (−0.269, 1.235) | 0.208 | −0.002 (−0.016, 0.012) | 0.773 |
| BMI (class) | ||||
| Underweight/normal | ref | ref | ||
| Overweight | 0.720 (0.334, 1.105) | <0.001 | −0.008 (−0.017, 0.001) | 0.085 |
| Obese | 0.944 (0.406, 1.483) | 0.001 | −0.026 (−0.038, −0.013) | <0.001 |
| Race | ||||
| White | ref | ref | ||
| Black | −0.975 (−2.003, 0.053) | 0.063 | −0.047 (−0.067, −0.028) | <0.001 |
| Other | −2.935 (−4.484, −1.386) | <0.001 | −0.006 (−0.035, 0.023) | 0.684 |
| College education | 0.243 (−0.347, 0.832) | 0.419 | 0.024 (0.011, 0.036) | <0.001 |
| Center | ||||
| Baltimore | ref | ref | ||
| Chicago | 0.331 (−0.752, 1.414) | 0.549 | −0.053 (−0.073, −0.033) | <0.001 |
| Pittsburgh | 0.925 (−0.081, 1.932) | 0.072 | −0.055 (−0.073, −0.036) | <0.001 |
| Los Angeles | −0.932 (−1.968, 0.104) | 0.078 | −0.063 (−0.082, −0.044) | <0.001 |
| Cohort | ||||
| 1984 | ref | ref | ||
| 1987 | 1.154 (−0.582, 2.890) | 0.193 | −0.005 (−0.037, 0.027) | 0.775 |
| 2001 | 2.019 (0.916, 3.122) | <0.001 | 0.017 (−0.003, 0.038) | 0.101 |
| 2010 | 1.370 (−0.219, 2.958) | 0.091 | −0.010 (−0.041, 0.020) | 0.505 |
| Smoking status | ||||
| Never | ref | ref | ||
| Former | −0.768 (−1.578, 0.042) | 0.063 | −0.025 (−0.040, −0.010) | 0.001 |
| Current | −1.050 (−1.930, −0.169) | 0.019 | −0.041 (−0.058, −0.024) | <0.001 |
| History of cocaine use | 0.421 (−0.297, 1.139) | 0.251 | 0.009 (−0.005, 0.024) | 0.194 |
| History of marijuana use | 0.528 (−0.231, 1.286) | 0.173 | 0.014 (−0.001, 0.030) | 0.074 |
| Alcohol use status | ||||
| None | ref | ref | ||
| Low-moderate | 0.121 (−0.318, 0.560) | 0.589 | 0.012 (0.001, 0.022) | 0.033 |
| Moderate-binge | 0.074 (−0.454, 0.602) | 0.784 | 0.009 (−0.004, 0.022) | 0.161 |
| Liver disease | −0.648 (−2.217, 0.921) | 0.418 | −0.028 (−0.062, 0.006) | 0.104 |
| Kidney disease | −0.345 (−0.721, 0.034) | 0.075 | −0.012 (−0.022, −0.003) | 0.010 |
| Hypertension | 0.177 (−0.134, 0.489) | 0.265 | −0.001 (−0.009, 0.007) | 0.813 |
| Arthritis | −0.702 (−1.372, −0.032) | 0.040 | −0.003 (−0.020, 0.014) | 0.745 |
| Depression (CESD⩾16) | −0.388 (−0.705, −0.072) | 0.016 | −0.020 (−0.027, −0.012) | <0.001 |
| Testosterone use | 0.379 (−0.235, 0.994) | 0.226 | −0.005 (−0.019, 0.010) | 0.520 |
| Hepatitis B infection | 0.782 (−1.573, 3.137) | 0.515 | −0.034 (−0.079, 0.010) | 0.129 |
| Hepatitis C infection | −2.297 (−3.773, −0.820) | 0.002 | −0.028 (−0.057, 0.002) | 0.063 |
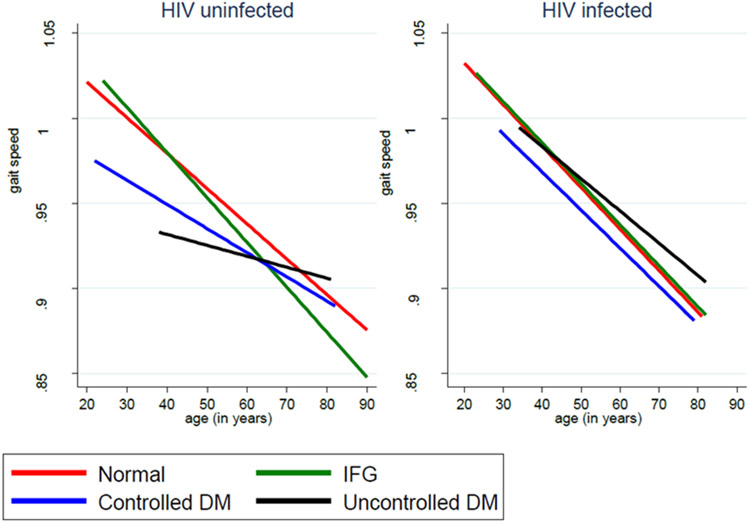
Figure 1. Physical function over time by glycemic status and HIV serostatus.
No significant three-way interactions between physical function, HIV serostatus, and glycemic status over time were noted.
Because the finding that HIV serostatus was not associated with grip strength differed from prior analysis in the MACS[4], we performed several sensitivity analyses. In analyses restricted to participants over the age of 40 or 45 years old, the effect of HIV serostatus on grip strength remained non-significant. When glycemic status was removed from the model, HIV did have a significant effect on grip strength decline (β - 0.719 kg, 95% CI: −0.011, −1.428, p=0.046, data not shown).
Gait speed
In our multivariate linear mixed model, men with controlled DM had significant declines in gait speed compared to men with normoglycemia (β −0.018 m/s, p=0.007). Men who had uncontrolled DM or IFG also demonstrated gait speed declines compared to those with normoglycemia, but these associations did not reach statistical significance. HIV serostatus was not significantly associated with gait speed. Other characteristics significantly associated with changes in gait speed included obesity, black race, smoking, kidney disease and depression. No gait speed decline over time was observed by glycemic status in both PWH or persons without HIV (Figure 1 and Supplemental Table 1).
Since HIV serostatus had previously been associated with gait speed decline in the MACS[3], we performed additional sensitivity analyses. In analyses restricted to participants over the age of 40 or 45 years old or when glycemic status was removed from the model, the effect of HIV serostatus on gait speed remained non-significant (data not shown).
Analyses restricted to PWH
In a subgroup analysis restricted to PWH, uncontrolled DM (β −1.874 kg, p<0.001) but not IFG or controlled DM was found to be associated with grip strength decline, as shown in Table 4. Longer time on ART was associated with improvement in grip strength (β 0.183 kg per year of ART, p=0.001), but greater cumulative exposure to didanosine (ddI) was associated with decline in grip strength (β −0.227 kg per year of ddI, p=0.041). No significant associations between glycemic status and gait speed were noted among PWH. Greater cumulative exposure to zidovudine (AZT) was significantly associated with gait speed decline (β −0.005 m/s per year of AZT, p<0.001).
Table 4.
Association between glycemic status and physical function in men with HIV: results from multivariate linear mixed models
| Grip strength | Gait speed | |||
|---|---|---|---|---|
| Variables | Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value |
| Age, years | −0.206 (−0.269, −0.143) | <0.001 | −0.003 (−0.004, −0.002) | <0.001 |
| Glycemic status | ||||
| Normal | Ref | Ref | ||
| IFG | −0.014 (−0.416, 0.389) | 0.946 | 0.004 (−0.006, 0.015) | 0.413 |
| Controlled DM | −0.193 (−0.919, 0.533) | 0.602 | −0.012 (−0.031, 0.006) | 0.186 |
| Uncontrolled DM | −1.874 (−2.924, −0.824) | <0.001 | 0.006 (−0.020, 0.032) | 0.646 |
| Years of ART | 0.183 (0.073, 0.293) | 0.001 | 0.002 (−0.000, 0.004) | 0.078 |
| HIV medications, cumulative years | ||||
| AZT | −0.097 (−0.220, 0.026) | 0.124 | −0.005 (−0.007, −0.002) | <0.001 |
| DDI | −0.227 (−0.445, −0.009) | 0.041 | −0.002 (−0.006, 0.002) | 0.396 |
| d4T | −0.020 (−0.233, 0.193) | 0.853 | −0.003 (−0.007, 0.001) | 0.156 |
| EFV | −0.026 (−0.143, 0.092) | 0.669 | 0.001 (−0.001, 0.003) | 0.353 |
| Any PI | 0.011 (−0.099, 0.122) | 0.839 | 0.002 (−0.000, 0.004) | 0.055 |
| CD4 nadir | ||||
| <200 | Ref | Ref | ||
| 200-500 | 0.341 (−0.874, 1.557) | 0.582 | 0.016 (−0.006, 0.038) | 0.160 |
| >500 | 1.440 (−0.179, 3.060) | 0.081 | 0.016 (−0.014, 0.045) | 0.308 |
| Suppressed HIV viral load | 0.068 (−0.602, 0.737) | 0.843 | −0.006 (−0.023, 0.011) | 0.490 |
Models were adjusted for all variables listed in table 3.
Discussion
Our data support the hypothesis that abnormal glucose metabolism is associated with similar declines in physical function among men with and without HIV. In the current study, IFG was not significantly associated with change in physical function, but DM was associated with declines in both gait speed and grip strength compared to participants with normoglycemia.
We found an effect of glycemic control on the relationship between DM and grip strength, with uncontrolled DM having a greater effect on grip strength decline than controlled DM (−0.937 kg vs −0.560 kg). In these models, the effect of controlled and uncontrolled DM on grip strength decline were equivalent to 4.34 and 7.26 years of aging, respectively. We similarly found that the effect of controlled DM on gait speed decline was equivalent to 6 years of aging. These findings suggest that DM is a more significant risk factor than advancing age for declines in physical function among men with and without HIV.
These findings are consistent with findings of prior studies conducted in the general population identifying DM as a risk factor for sarcopenia that can accelerate age-related declines in skeletal muscle mass and function[23-25]. Few studies have considered glycemic status beyond DM as a risk factor for physical function declines. A recent study of community-dwelling older adults without HIV found that uncontrolled DM (HbA1C ≥7%) was associated with increased risk of weak grip and that glycemic control modified this relationship[26]. DM and its associated complications can have myriad effects on physical function. Decreased insulin signaling in persons with DM can cause dysregulated protein turnover which can ultimately lead to decreases in muscle mass[27]. Moreover, insulin resistance is associated with mitochondrial dysfunction[28], increased oxidative stress[29], and chronic inflammation[30, 31] that can contribute to reduced skeletal muscle mass, strength, and function[32, 33]. Chronic hyperglycemia can also increase advanced glycation end products that accumulate in skeletal muscle thereby increasing muscle stiffness and leading to reduced physical function[34, 35]. In addition, diabetic neuropathy leads to wasting and weakness of distal skeletal muscles[36, 37] and increased intermuscular adipose tissue, which is associated with decreased muscle strength and function[38].
Compared to persons without HIV, many studies have found a higher prevalence of DM among PWH [17, 18]. In addition to traditional risk factors, HIV-related factors increase risk for development of type 2 DM. PWH experience systemic inflammation related to chronic HIV infection that has been associated with incident DM[39]. Various antiretroviral agents can also cause mitochondrial toxicity, body composition changes (e.g., decreased subcutaneous adipose tissue and increased visceral adipose tissue), and excess hyperglycemia that contribute to insulin resistance and development of DM[40].
We hypothesized that excess DM risk among PWH may contribute to and exacerbate accelerated physical declines previously described in this population. In our model restricted to PWH, uncontrolled DM was associated with a significant decline in grip strength. Although we did not find a significant HIV interaction in our models, we did find that the effect of uncontrolled DM on grip strength decline was 2 times greater in our model restricted to PWH than in our analysis of all MACS participants (−1.87 kg vs −0.937 kg); these findings may reflect poorer diabetes control among some PWH, or could suggest that the adverse effects of uncontrolled DM on physical function, particularly grip strength, may be heightened among PWH.
In our study, HIV serostatus was not an independent risk factor for declines in grip strength or gait speed among MACS participants; this finding contrasts with prior analyses from the MACS that indicated HIV was associated with faster declines in both grip strength and gait speed[3, 4]. The study populations analyzed in these prior studies were slightly different: analyses of gait speed and grip strength trajectories were restricted to men aged 40 and older for gait and 50 and older for grip. In addition, follow-up time for previous analyses of gait and grip continued through 2013 and 2014, respectively, compared to through 2018 in the current study. However, in our sensitivity analyses restricted to men over the ages of 40 or 45 years old at baseline, the effect of HIV on physical function remained non-significant. Of note, these prior models of gait speed and grip strength did not include DM or glycemic status. When glycemic status was removed from our current models, HIV serostatus did have a significant effect on grip strength but not gait speed. These findings suggest that glycemic status is an important mediator of grip strength decline among PWH.
Our findings indicate that interventions to prevent and improve control of DM may help to attenuate physical function declines in PWH and persons without HIV. Optimized glucose control and use of certain diabetes medications have been associated with better functional outcomes among elderly individuals without HIV[46]. Previous studies demonstrated that use of insulin sensitizers, such as metformin or thiazolidinediones, attenuated muscle loss and declines in physical function among older men and women with DM[47, 48]. Dipeptidyl peptidase 4 (DPP4) inhibitors, which increase insulin secretion, have the potential to restore insulin signaling in skeletal muscle[57]; cross-sectional and observational studies have noted an association between their use and greater muscle mass and strength[58, 59]. However, the risks and benefits of such medications and intensive glucose management must be evaluated further, particularly in older PWH who are at increased risk for geriatric syndromes such as polypharmacy and falls [60-62]. Non-pharmacologic therapies, such as diet and exercise-based interventions, can prevent development of DM[63], improve glucose control in individuals with known DM[64, 65], and can increase physical function in older adults with and without DM[66-69]. Exercise-based interventions have resulted in significant improvement in physical function among PWH[70] and warrant further evaluation among persons living with both HIV and diabetes.
While lifestyle interventions to reduce obesity may be beneficial to prevent DM and its negative effects on physical function, the current study found that elevated BMI had differential effects on gait speed and grip strength. While obesity was associated with significant declines in gait speed, higher BMI class (both overweight and obese) was associated with improved grip strength. Obesity has been linked to gait speed impairment among both PWH and the general population[71, 72]. Individuals with obesity may experience more balance impairment and physical fatigue leading to slower gait. In addition, excess adipose tissue can reduce the ratio of lean to non-lean muscle mass and thereby decrease skeletal muscle quality and function[73]. Some studies in the general population have suggested that obesity is associated with lower hand grip strength[74, 75], while others have noted that higher BMI is associated with higher grip strength in men[76].[76]. In men with greater amounts of lean muscle mass, BMI can overestimate obesity[77], which may explain the positive association between BMI and grip strength in our current study. Additional analyses are needed to define more clearly the relationship between body composition and physical function in PWH compared to the general population.
Depression was associated with declines in both gait speed and grip strength. Mood disorders and reduced physical function are known to be highly overlapping and mutually reinforcing syndromes[29, 78].
Among PWH in the current study, longer duration of didanosine and zidovudine use were associated with grip strength and gait speed declines, respectively, while longer duration of ART was associated with grip strength improvement. Both didanosine and zidovudine have been shown to cause mitochondrial toxicity[79] that can contribute to physical function declines[80, 81]. However, neither didanosine nor zidovudine are routinely used in newer ART regimens. Therefore, PWH without didanosine and zidovudine exposure may face less physical function impairment than PWH without such exposures.
Several limitations of this study must be acknowledged. First, MACS participants are all male and a majority are White. Additional studies are needed to determine if similar associations are observed in other demographic groups. In addition, the overall number of men with either controlled or uncontrolled DM was low, which limited the power of our findings. While our statistical models accounted for many of the factors that affect the relationship between glycemic status and physical function, complete data for some confounders (e.g., steroid use) was not available. Furthermore, there was significant missingness in testosterone use data. Also, survivorship bias may exist in this cohort; some MACS participants have been involved in the study for over 20 years, but those with more severe complications of HIV or aging may have been more likely drop out. Moreover, the generalizability of our results to other cohorts of PWH with less exposure to older more toxic ART regimens and shorter duration of HIV is not clear.
As the population of older PWH continues to grow, it is imperative to develop targeted interventions aimed at preserving physical function and independence and promoting resiliency in this population. This study represents the largest longitudinal evaluation to date of the relationship between glycemic status and physical function among men with and without HIV. These data suggest that glycemic control may be an important modifiable risk factor to preserve physical function, regardless of HIV serostatus. Accordingly, efforts to prevent, screen for, and optimally manage pre-diabetes and DM in aging PWH should constitute a priority for clinicians and researchers.
Supplementary Material
Acknowledgements
The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). MWCCS (Principal Investigators): Atlanta CRS (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-HL146241; Baltimore CRS (Todd Brown and Joseph Margolick), U01-HL146201; Bronx CRS (Kathryn Anastos and Anjali Sharma), U01-HL146204; Brooklyn CRS (Deborah Gustafson and Tracey Wilson), U01-HL146202; Data Analysis and Coordination Center (Gypsyamber D’Souza, Stephen Gange and Elizabeth Golub), U01-HL146193; Chicago-Cook County CRS (Mardge Cohen and Audrey French), U01-HL146245; Chicago-Northwestern CRS (Steven Wolinsky), U01-HL146240; Northern California CRS (Bradley Aouizerat, Jennifer Price, and Phyllis Tien), U01-HL146242; Los Angeles CRS (Roger Detels and Matthew Mimiaga), U01-HL146333; Metropolitan Washington CRS (Seble Kassaye and Daniel Merenstein), U01-HL146205; Miami CRS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-HL146203; Pittsburgh CRS (Jeremy Martinson and Charles Rinaldo), U01-HL146208; UAB-MS CRS (Mirjam-Colette Kempf, Jodie Dionne-Odom, and Deborah Konkle-Parker), U01-HL146192; UNC CRS (Adaora Adimora), U01-HL146194. The MWCCS is funded primarily by the National Heart, Lung, and Blood Institute (NHLBI), with additional co-funding from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Institute On Aging (NIA), National Institute Of Dental & Craniofacial Research (NIDCR), National Institute Of Allergy And Infectious Diseases (NIAID), National Institute Of Neurological Disorders And Stroke (NINDS), National Institute Of Mental Health (NIMH), National Institute On Drug Abuse (NIDA), National Institute Of Nursing Research (NINR), National Cancer Institute (NCI), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute on Minority Health and Health Disparities (NIMHD), and in coordination and alignment with the research priorities of the National Institutes of Health, Office of AIDS Research (OAR). MWCCS data collection is also supported by UL1-TR000004 (UCSF CTSA), UL1-TR003098 (JHU ICTR), UL1-TR001881 (UCLA CTSI), P30-AI-050409 (Atlanta CFAR), P30-AI-073961 (Miami CFAR), P30-AI-050410 (UNC CFAR), P30-AI-027767 (UAB CFAR), and P30-MH-116867 (Miami CHARM).
The authors gratefully acknowledge the contributions of the study participants and dedication of the staff at the MWCCS sites.
This study was also supported by NIA AG054366 to KME, NIDDK T32 DK007169 and NIA 5R03AG067980-02 to MCM, NIAID K24 120834 to TTB.
Footnotes
Conflicts of Interest: TTB has served as a consultant to Gilead Sciences, Merck, ViiV Healthcare, and Theratechnologies. Jingyan Yang is currently employed by Pfizer Inc. and holds Pfizer stock.
References
- 1.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med 2011; 62:141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasse B, Ledergerber B, Furrer H, Battegay M, Hirschel B, Cavassini M, et al. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis 2011; 53(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 3.Schrack JA, Althoff KN, Jacobson LP, Erlandson KM, Jamieson BD, Koletar SL, et al. Accelerated Longitudinal Gait Speed Decline in HIV-Infected Older Men. J Acquir Immune Defic Syndr 2015; 70(4):370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schrack JA, Jacobson LP, Althoff KN, Erlandson KM, Jamieson BD, Koletar SL, et al. Effect of HIV-infection and cumulative viral load on age-related decline in grip strength. AIDS 2016; 30(17):2645–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johs NA, Wu K, Tassiopoulos K, Koletar SL, Kalayjian RC, Ellis RJ, et al. Disability Among Middle-Aged and Older Persons With Human Immunodeficiency Virus Infection. Clin Infect Dis 2017; 65(1):83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma A, Hoover DR, Shi Q, Holman S, Plankey MW, Tien PC, et al. Longitudinal study of falls among HIV-infected and uninfected women: the role of cognition. Antivir Ther 2018; 23(2):179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tassiopoulos K, Abdo M, Wu K, Koletar SL, Palella FJ Jr., Kalayjian R, et al. Frailty is strongly associated with increased risk of recurrent falls among older HIV-infected adults. AIDS 2017; 31(16):2287–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erlandson KM, Allshouse AA, Jankowski CM, Mawhinney S, Kohrt WM, Campbell TB. Relationship of physical function and quality of life among persons aging with HIV infection. AIDS 2014; 28(13):1939–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erlandson KM, Perez J, Abdo M, Robertson K, Ellis RJ, Koletar SL, et al. Frailty, Neurocognitive Impairment, or Both in Predicting Poor Health Outcomes Among Adults Living With Human Immunodeficiency Virus. Clin Infect Dis 2019; 68(1):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Rekeneire N, Resnick HE, Schwartz AV, Shorr RI, Kuller LH, Simonsick EM, et al. Diabetes is associated with subclinical functional limitation in nondisabled older individuals: the Health, Aging, and Body Composition study. Diabetes Care 2003; 26(12):3257–3263. [DOI] [PubMed] [Google Scholar]
- 11.Gregg EW, Beckles GL, Williamson DF, Leveille SG, Langlois JA, Engelgau MM, et al. Diabetes and physical disability among older U.S. adults. Diabetes Care 2000; 23(9):1272–1277. [DOI] [PubMed] [Google Scholar]
- 12.Kalyani RR, Tra Y, Yeh HC, Egan JM, Ferrucci L, Brancati FL. Quadriceps strength, quadriceps power, and gait speed in older U.S. adults with diabetes mellitus: results from the National Health and Nutrition Examination Survey, 1999-2002. J Am Geriatr Soc 2013; 61(5):769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuo CK, Lin LY, Yu YH, Wu KH, Kuo HK. Inverse association between insulin resistance and gait speed in nondiabetic older men: results from the U.S. National Health and Nutrition Examination Survey (NHANES) 1999-2002. BMC Geriatr 2009; 9:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okoro CA, Zhong Y, Ford ES, Balluz LS, Strine TW, Mokdad AH. Association between the metabolic syndrome and its components and gait speed among U.S. adults aged 50 years and older: a cross-sectional analysis. BMC Public Health 2006; 6:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park SW, Goodpaster BH, Strotmeyer ES, de Rekeneire N, Harris TB, Schwartz AV, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes 2006; 55(6):1813–1818. [DOI] [PubMed] [Google Scholar]
- 16.Phielix E, Mensink M. Type 2 diabetes mellitus and skeletal muscle metabolic function. Physiol Behav 2008; 94(2):252–258. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez-Romieu AC, Garg S, Rosenberg ES, Thompson-Paul AM, Skarbinski J. Is diabetes prevalence higher among HIV-infected individuals compared with the general population? Evidence from MMP and NHANES 2009-2010. BMJ Open Diabetes Res Care 2017; 5(1):e000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med 2005; 165(10):1179–1184. [DOI] [PubMed] [Google Scholar]
- 19.Erlandson KM, Ng DK, Jacobson LP, Margolick JB, Dobs AS, Palella FJ Jr., et al. Inflammation, Immune Activation, Immunosenescence, and Hormonal Biomarkers in the Frailty-Related Phenotype of Men With or at Risk for HIV Infection. J Infect Dis 2017; 215(2):228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chow DC, Bernas MA, Gangcuangco LM, Huynh J, Kohorn LB, Kallianpur KJ, et al. Frailty Is Associated With Insulin Resistance in Chronic Human Immunodeficiency Virus Infection. Clin Infect Dis 2020; 71(4):1127–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR Jr. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol 1987; 126(2):310–318. [DOI] [PubMed] [Google Scholar]
- 22.Dudley J, Jin S, Hoover D, Metz S, Thackeray R, Chmiel J. The Multicenter AIDS Cohort Study: retention after 9 1/2 years. Am J Epidemiol 1995; 142(3):323–330. [DOI] [PubMed] [Google Scholar]
- 23.Park SW, Goodpaster BH, Strotmeyer ES, Kuller LH, Broudeau R, Kammerer C, et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care 2007; 30(6):1507–1512. [DOI] [PubMed] [Google Scholar]
- 24.Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol 2014; 2(10):819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mesinovic J, Zengin A, De Courten B, Ebeling PR, Scott D. Sarcopenia and type 2 diabetes mellitus: a bidirectional relationship. Diabetes Metab Syndr Obes 2019; 12:1057–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nebuloni CC, Maximo RO, de Oliveira C, Alexandre TDS. Uncontrolled Diabetes as an Associated Factor with Dynapenia in Adults Aged 50 Years or Older: Sex Differences. J Gerontol A Biol Sci Med Sci 2020; 75(6):1191–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aleman-Mateo H, Lopez Teros MT, Ramirez FA, Astiazaran-Garcia H. Association between insulin resistance and low relative appendicular skeletal muscle mass: evidence from a cohort study in community-dwelling older men and women participants. J Gerontol A Biol Sci Med Sci 2014; 69(7):871–877. [DOI] [PubMed] [Google Scholar]
- 28.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 2002; 51(10):2944–2950. [DOI] [PubMed] [Google Scholar]
- 29.Russo A, Cesari M, Onder G, Zamboni V, Barillaro C, Pahor M, et al. Depression and physical function: results from the aging and longevity study in the Sirente geographic area (ilSIRENTE Study). J Geriatr Psychiatry Neurol 2007; 20(3):131–137. [DOI] [PubMed] [Google Scholar]
- 30.Banerjee M, Saxena M. Interleukin-1 (IL-1) family of cytokines: role in type 2 diabetes. Clin Chim Acta 2012; 413(15-16):1163–1170. [DOI] [PubMed] [Google Scholar]
- 31.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 2006; 116(7):1793–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meng SJ, Yu LJ. Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci 2010; 11(4):1509–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu CK, Lyass A, Larson MG, Massaro JM, Wang N, D'Agostino RB Sr., et al. Biomarkers of oxidative stress are associated with frailty: the Framingham Offspring Study. Age (Dordr) 2016; 38(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dalal M, Ferrucci L, Sun K, Beck J, Fried LP, Semba RD. Elevated serum advanced glycation end products and poor grip strength in older community-dwelling women. J Gerontol A Biol Sci Med Sci 2009; 64(1):132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Semba RD, Bandinelli S, Sun K, Guralnik JM, Ferrucci L. Relationship of an advanced glycation end product, plasma carboxymethyl-lysine, with slow walking speed in older adults: the InCHIANTI study. Eur J Appl Physiol 2010; 108(1):191–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allen MD, Choi IH, Kimpinski K, Doherty TJ, Rice CL. Motor unit loss and weakness in association with diabetic neuropathy in humans. Muscle Nerve 2013; 48(2):298–300. [DOI] [PubMed] [Google Scholar]
- 37.Andreassen CS, Jakobsen J, Ringgaard S, Ejskjaer N, Andersen H. Accelerated atrophy of lower leg and foot muscles--a follow-up study of long-term diabetic polyneuropathy using magnetic resonance imaging (MRI). Diabetologia 2009; 52(6):1182–1191. [DOI] [PubMed] [Google Scholar]
- 38.Tuttle LJ, Sinacore DR, Cade WT, Mueller MJ. Lower physical activity is associated with higher intermuscular adipose tissue in people with type 2 diabetes and peripheral neuropathy. Phys Ther 2011; 91(6):923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown TT, Tassiopoulos K, Bosch RJ, Shikuma C, McComsey GA. Association between systemic inflammation and incident diabetes in HIV-infected patients after initiation of antiretroviral therapy. Diabetes Care 2010; 33(10):2244–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feeney ER, Mallon PW. Insulin resistance in treated HIV infection. Best Pract Res Clin Endocrinol Metab 2011; 25(3):443–458. [DOI] [PubMed] [Google Scholar]
- 41.Sax PE, Erlandson KM, Lake JE, McComsey GA, Orkin C, Esser S, et al. Weight Gain Following Initiation of Antiretroviral Therapy: Risk Factors in Randomized Comparative Clinical Trials. Clin Infect Dis 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bourgi K, Rebeiro PF, Turner M, Castilho JL, Hulgan T, Raffanti SP, et al. Greater Weight Gain in Treatment Naive Persons Starting Dolutegravir-Based Antiretroviral Therapy. Clin Infect Dis 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kerchberger AM, Sheth AN, Angert CD, Mehta CC, Summers NA, Ofotokun I, et al. Weight Gain Associated with Integrase Stand Transfer Inhibitor Use in Women. Clin Infect Dis 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Norwood J, Turner M, Bofill C, Rebeiro P, Shepherd B, Bebawy S, et al. Brief Report: Weight Gain in Persons With HIV Switched From Efavirenz-Based to Integrase Strand Transfer Inhibitor-Based Regimens. J Acquir Immune Defic Syndr 2017; 76(5):527–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lake JE, Wu K, Bares SH, Debroy P, Godfrey C, Koethe JR, et al. Risk Factors for Weight Gain Following Switch to Integrase Inhibitor-Based Antiretroviral Therapy. Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang CP, Hazuda HP. Better glycemic control is associated with maintenance of lower-extremity function over time in Mexican American and European American older adults with diabetes. Diabetes Care 2011; 34(2):268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee CG, Boyko EJ, Barrett-Connor E, Miljkovic I, Hoffman AR, Everson-Rose SA, et al. Insulin sensitizers may attenuate lean mass loss in older men with diabetes. Diabetes Care 2011; 34(11):2381–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee CG, Schwartz AV, Yaffe K, Hillier TA, LeBlanc ES, Cawthon PM, et al. Changes in physical performance in older women according to presence and treatment of diabetes mellitus. J Am Geriatr Soc 2013; 61(11):1872–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saisho Y. Metformin and Inflammation: Its Potential Beyond Glucose-lowering Effect. Endocr Metab Immune Disord Drug Targets 2015; 15(3):196–205. [DOI] [PubMed] [Google Scholar]
- 50.Horiuchi T, Sakata N, Narumi Y, Kimura T, Hayashi T, Nagano K, et al. Metformin directly binds the alarmin HMGB1 and inhibits its proinflammatory activity. J Biol Chem 2017; 292(20):8436–8446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fang J, Yang J, Wu X, Zhang G, Li T, Wang X, et al. Metformin alleviates human cellular aging by upregulating the endoplasmic reticulum glutathione peroxidase 7. Aging Cell 2018:e12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moiseeva O, Deschenes-Simard X, St-Germain E, Igelmann S, Huot G, Cadar AE, et al. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-kappaB activation. Aging Cell 2013; 12(3):489–498. [DOI] [PubMed] [Google Scholar]
- 53.Perez-Revuelta BI, Hettich MM, Ciociaro A, Rotermund C, Kahle PJ, Krauss S, et al. Metformin lowers Ser-129 phosphorylated alpha-synuclein levels via mTOR-dependent protein phosphatase 2A activation. Cell Death Dis 2014; 5:e1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cho K, Chung JY, Cho SK, Shin HW, Jang IJ, Park JW, et al. Antihyperglycemic mechanism of metformin occurs via the AMPK/LXRalpha/POMC pathway. Sci Rep 2015; 5:8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duca FA, Cote CD, Rasmussen BA, Zadeh-Tahmasebi M, Rutter GA, Filippi BM, et al. Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat Med 2015; 21(5):506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a Tool to Target Aging. Cell Metab 2016; 23(6):1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lamers D, Famulla S, Wronkowitz N, Hartwig S, Lehr S, Ouwens DM, et al. Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome. Diabetes 2011; 60(7):1917–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rizzo MR, Barbieri M, Fava I, Desiderio M, Coppola C, Marfella R, et al. Sarcopenia in Elderly Diabetic Patients: Role of Dipeptidyl Peptidase 4 Inhibitors. J Am Med Dir Assoc 2016; 17(10):896–901. [DOI] [PubMed] [Google Scholar]
- 59.Bouchi R, Fukuda T, Takeuchi T, Nakano Y, Murakami M, Minami I, et al. Dipeptidyl peptidase 4 inhibitors attenuates the decline of skeletal muscle mass in patients with type 2 diabetes. Diabetes Metab Res Rev 2018; 34(2). [DOI] [PubMed] [Google Scholar]
- 60.Guaraldi G, Malagoli A, Calcagno A, Mussi C, Celesia BM, Carli F, et al. The increasing burden and complexity of multi-morbidity and polypharmacy in geriatric HIV patients: a cross sectional study of people aged 65 - 74 years and more than 75 years. BMC Geriatr 2018; 18(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.American Geriatrics Society Expert Panel on Care of Older Adults with Diabetes M, Moreno G, Mangione CM, Kimbro L, Vaisberg E. Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 update. J Am Geriatr Soc 2013; 61(11):2020–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim TW, Walley AY, Ventura AS, Patts GJ, Heeren TC, Lerner GB, et al. Polypharmacy and risk of falls and fractures for patients with HIV infection and substance dependence. AIDS Care 2018; 30(2):150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duncan AD, Goff LM, Peters BS. Type 2 diabetes prevalence and its risk factors in HIV: A cross-sectional study. PLoS One 2018; 13(3):e0194199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yarasheski KE, Cade WT, Overton ET, Mondy KE, Hubert S, Laciny E, et al. Exercise training augments the peripheral insulin-sensitizing effects of pioglitazone in HIV-infected adults with insulin resistance and central adiposity. Am J Physiol Endocrinol Metab 2011; 300(1):E243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fitch KV, Anderson EJ, Hubbard JL, Carpenter SJ, Waddell WR, Caliendo AM, et al. Effects of a lifestyle modification program in HIV-infected patients with the metabolic syndrome. AIDS 2006; 20(14):1843–1850. [DOI] [PubMed] [Google Scholar]
- 66.Safeek RH, Hall KS, Lobelo F, Del Rio C, Khoury AL, Wong T, et al. Low Levels of Physical Activity Among Older Persons Living with HIV/AIDS Are Associated with Poor Physical Function. AIDS Res Hum Retroviruses 2018; 34(11):929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Byrne H, Caulfield B, De Vito G. Effects of Self-directed Exercise Programmes on Individuals with Type 2 Diabetes Mellitus: A Systematic Review Evaluating Their Effect on HbA1c and Other Metabolic Outcomes, Physical Characteristics, Cardiorespiratory Fitness and Functional Outcomes. Sports Med 2017; 47(4):717–733. [DOI] [PubMed] [Google Scholar]
- 68.Allet L, Armand S, de Bie RA, Golay A, Monnin D, Aminian K, et al. The gait and balance of patients with diabetes can be improved: a randomised controlled trial. Diabetologia 2010; 53(3):458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Montoya JL, Jankowski CM, O'Brien KK, Webel AR, Oursler KK, Henry BL, et al. Evidence-informed practical recommendations for increasing physical activity among persons living with HIV. AIDS 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Erlandson KM, MaWhinney S, Wilson M, Gross L, McCandless SA, Campbell TB, et al. Physical function improvements with moderate or high-intensity exercise among older adults with or without HIV infection. AIDS 2018; 32(16):2317–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bauer LO, Wu Z, Wolfson LI. An obese body mass increases the adverse effects of HIV/AIDS on balance and gait. Phys Ther 2011; 91(7):1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tabue-Teguo M, Peres K, Simo N, Le Goff M, Perez Zepeda MU, Feart C, et al. Gait speed and body mass index: Results from the AMI study. PLoS One 2020; 15(3):e0229979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol 2018; 14(9):513–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stenholm S, Sallinen J, Koster A, Rantanen T, Sainio P, Heliovaara M, et al. Association between obesity history and hand grip strength in older adults--exploring the roles of inflammation and insulin resistance as mediating factors. J Gerontol A Biol Sci Med Sci 2011; 66(3):341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim Y, White T, Wijndaele K, Sharp SJ, Wareham NJ, Brage S. Adiposity and grip strength as long-term predictors of objectively measured physical activity in 93 015 adults: the UK Biobank study. Int J Obes (Lond) 2017; 41(9):1361–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hardy R, Cooper R, Aihie Sayer A, Ben-Shlomo Y, Cooper C, Deary IJ, et al. Body mass index, muscle strength and physical performance in older adults from eight cohort studies: the HALCyon programme. PLoS One 2013; 8(2):e56483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rothman KJ. BMI-related errors in the measurement of obesity. Int J Obes (Lond) 2008; 32 Suppl 3:S56–59. [DOI] [PubMed] [Google Scholar]
- 78.Stegenga BT, Nazareth I, Torres-Gonzalez F, Xavier M, Svab I, Geerlings MI, et al. Depression, anxiety and physical function: exploring the strength of causality. J Epidemiol Community Health 2012; 66(7):e25. [DOI] [PubMed] [Google Scholar]
- 79.Benbrik E, Chariot P, Bonavaud S, Ammi-Said M, Frisdal E, Rey C, et al. Cellular and mitochondrial toxicity of zidovudine (AZT), didanosine (ddI) and zalcitabine (ddC) on cultured human muscle cells. J Neurol Sci 1997; 149(1):19–25. [DOI] [PubMed] [Google Scholar]
- 80.Bourdel-Marchasson I, Biran M, Dehail P, Traissac T, Muller F, Jenn J, et al. Muscle phosphocreatine post-exercise recovery rate is related to functional evaluation in hospitalized and community-living older people. J Nutr Health Aging 2007; 11(3):215–221. [PubMed] [Google Scholar]
- 81.Joseph AM, Adhihetty PJ, Buford TW, Wohlgemuth SE, Lees HA, Nguyen LM, et al. The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high- and low-functioning elderly individuals. Aging Cell 2012; 11(5):801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.