Abstract
In tandem with the ever-increasing ageing population in low and middle-income countries, the burden of dementia is rising on the African continent. Dementia prevalence varies from 2.3 to 20.0% and incidence rates are 13.3 per 1000 person years with increasing mortality in parts of rapidly transforming Africa. Differences in nutrition, cardiovascular factors, co-morbidities, infections, mortality and detection likely contribute to lower incidence. Alzheimer’s disease, vascular dementia and HIV/AIDS associated neurocognitive disorders are the most common dementia subtypes. Comprehensive longitudinal studies with robust methodology and regional coverage would provide more reliable information. The apolipoprotein E (APOE) ε4 allele is most studied but has shown differential effects within African ancestry compared to Caucasian. More candidate gene and genome-wide association studies are needed to relate to dementia phenotypes. Validated culture-sensitive cognitive tools not influenced by education and language differences are critically needed for implementation across multidisciplinary groupings such as the proposed African dementia consortium.
Keywords: Africa, Dementia, Alzheimer’s disease, Vascular dementia, Epidemiology, Genetics, Neuropathology. Biomarkers, Precision Medicine, Consortium
1. INTRODUCTION
Globally, Alzheimer’s disease and other dementias constitute a major public health priority with substantial negative individual, social, and economic impacts.1,2 The current estimates from the World Health Organization (WHO) indicate that by 2050, 150 million persons, representing a 204% increase from 2017, will be living with dementia.3,4 Indications are that the majority of these increases will be found in low- and middle-income countries (LMIC) including within Africa.3–5 Worldwide, dementia is the 5th leading cause of death and the second leading contributor to death from neurological diseases.6 Recent estimates suggest that over 818 billion USD is spent annually on dementia related care worldwide and by 2028 the worldwide cost of dementia care is estimated to be >2 trillion USD.7 These include direct medical and other formal and informal health and social care costs.
The projection that over 68% of persons with dementia will reside in LMICs by 20505 is largely due to the demographic transition and population growth in the LIMCs including certain African countries, which are amongst the world’s most populous. The burden of dementia is shared by the person, their immediate family, caregivers, and the health, social, legal, and financial systems of the community at large. In Africa, as in many other underserved populations, additional strains on dementia care exist, attributable to globalisation, rapid socio-economic transitions, and the gradual erosion of key informal care systems such as multigenerational family structures which are the bedrock of dementia care.8 As such, the continent needs to devise robust alternate plans for the care of persons with dementia within the formal healthcare sector, taking advantage of global advancements in preventive, therapeutic and rehabilitative care of the condition.
Currently, there is a dearth of information on the basic and translational science of dementia in Africa. There is paucity of neuroimaging and fluid biomarker studies, and very few neuropathological and genomic studies limited to candidate gene reports in pockets of cohorts. Basic and clinical research in Alzheimer’s disease and other dementias are also constrained in countries with greater public awareness and affluence. There is therefore a limitation in the capacity for rigorous endophenotyping and the delivery of evidence-based personalized/precision approaches to dementia care, especially in the context of the unique diversity of African genomes and their interactions with the local environment. This review aims to summarize the current epidemiological evidence on dementia in Africa, highlight challenges, identify knowledge gaps, and suggest future research directions and goals.
2. CURRENT EVIDENCE
2.1. Epidemiology
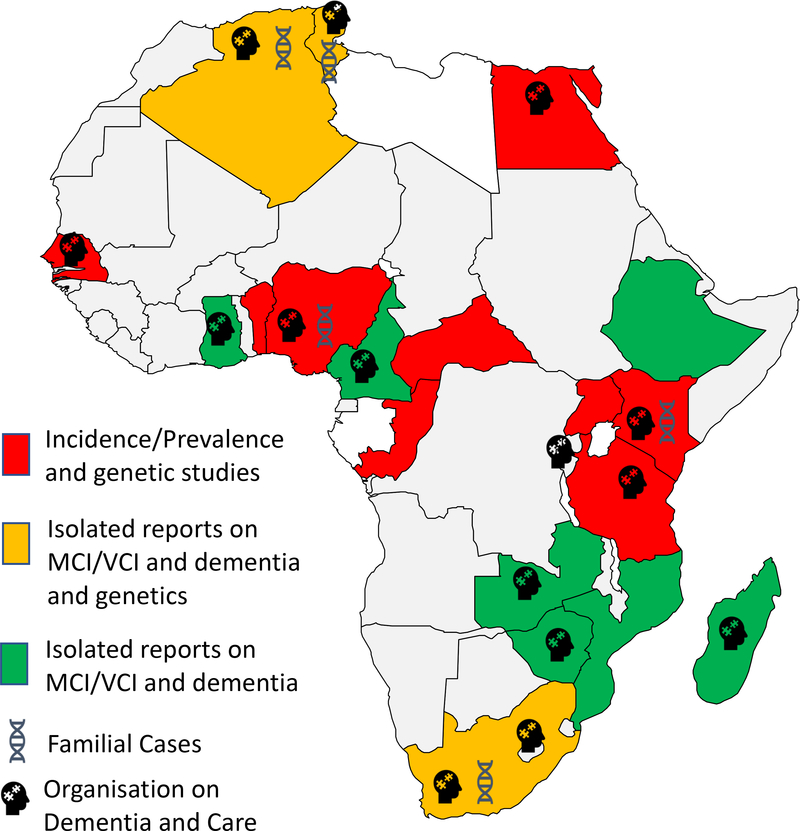
More than two-thirds of the world’s population of older people ( ≥ 65 years old) reside in less developed countries, many of whom are in Africa.9 The prevalence and incidence of dementia increase with age.10 However, despite the projected large increases in the number of persons living with dementia, current estimates of prevalence and incidence of dementia from multiple studies in Africa are among the lowest in the world. While this may be due to numerous factors including low life expectancy in many African nations, it is however to be noted that dementia data derived from observational studies using similar methodological approaches and designed to reflect the diversity of Africa are still relatively few even though growing. The paucity of data is a reflection of the challenges of conducting quality research in many resource poor African countries. (Figure 1)
Figure 1: Dementia in Africa and Dementia Care and Support Organisations.
Map of Africa showing limited number of countries where incidence and prevalence studies have been conducted over the past 30 years. Some countries have reported isolated reports on dementia cases in particular Alzheimer’s disease or vascular dementia. Limited number of countries in which candidate gene investigations have been carried out. In several African countries dementia care and support organisations exist. Most of these are member organisations of ADI (https://www.alzint.org). Further reports of cases and deaths due to dementia in African countries are known (www.afro.who.int) but are not published in peer-reviewed sources.
2.2. Incidence
Information on the incidence of dementia in Africa is currently sparse (Figure 1). There are four recent reports on dementia incidence from Western Africa, and one each from Central Africa and Northern Africa respectively. Notably, most of the data on incidence are from one country, Nigeria. Current incidence estimates from Sub - Saharan Africa (SSA) are similar to that for other low- and middle- income countries at 13.26/1 000 person years implying 367 698 new cases each year.11 The Alzheimer’s Disease International (ADI) meta-analysis shows that incidence doubles for every 7.7 year increase in age in sub- Saharan Africa (SSA).11 For Northern Africa, a recent review on the epidemiology of dementia in the Middle East and North Africa (MENA) estimated a crude incidence of 27/1,000 over a 20-year period for Egypt.12 Similar to prevalence, the reported annual incidence rates of dementia in Africa are generally lower than rates reported among populations of older persons living in Europe and North America.13 Differences in diet and burden of cardiovascular risk factors, medical co-morbidities, access to quality health care and mortality have been suggested as possible reasons for the lower incidence of dementia in Africa compared to higher income regions of the world.14 In one study comparing the incidence of dementia and Alzheimer’s disease in two comparative cohorts of African Americans and Yoruba Nigerians aged 70 years or over and evaluated a decade apart in 1992 and 2001 respectively, the standardised annual incidence rates of dementia and AD were relatively stable in the Yoruba African cohort (dementia: 1.7% vs 1.4%; AD: 1.5% vs 1.0%) whereas there was a significant decline among the African Americans (dementia: 3.6% vs 1.4%; AD: 2.5% vs 1.3%).15
2.3. Prevalence
Studies in Africa have generally reported varied but generally lower prevalence of dementia compared with findings in Europe and America.16 Limitations with many African studies include low quality of methods used, types of study settings (i.e. in-patients, outpatients, nursing homes, autopsy), and limited coverage of the different African regions.17(Table 1). The pattern of the findings is such that hospital-based studies report the lowest prevalence estimates of dementia in Africa.17 However, 48% of a sample of nursing home residents in Nigeria met the clinical diagnostic criteria for dementia.18
Table 1:
Summary of community-based epidemiological studies of dementia in Africa
| Author/Site, Country | Year | Criteria | Sample (n) | Age (yrs) | Prevalence | Identified Risk Factors | Limitations | ||
|---|---|---|---|---|---|---|---|---|---|
| Dementia | AD | VaD | |||||||
| West Africa | |||||||||
| Hendrie et al22/ Ibadan, Nigeria | 1995 | DSM III-R ICD 10 |
2494 | ≥ 65 | 2.3% | - | No consideration for educational status. Cultural bias in diagnosis. High rate in Indianapolis cohort. Age was determined using historical landmarks. | ||
| Ogunniyi et al16/Ibadan, Nigeria | 1997 | DSM III-R ICD 10 |
2494 | ≥ 65 | 64.3% | 28.6% | - | Age wasdetermined using historical landmarks. High illiteracy rates. No radiological confirmation | |
| Hall et al63/Ibadan, Nigeria | 2006 | DSM III-R ICD 10 |
1075 | ≥ 70 | Dyslipidemia. | Small number of AD cases. Survivor bias as most of the attrition from earlier cohort were due to mortality. Criteria for control group uncertain. Cross-sectional design not appropriate to determine association | |||
| Ochayi and Thatcher78/Jos, Nigeria | 2006 | CSID | 280 | ≥ 65 | 6.4% | Age, Female sex, BMI, NSAIDs | Possible over-estimation of dementia rate due to one stage process used. Wide confidence intervals for estimates. Estimated ages. Cross-sectional design. | ||
| Gureje et al20/Southwest, Nigeria | 2006 | 10-WDRT DSM IV |
2152 | ≥ 65 | 10.1 % | Age, Female sex, Lifetime history of alcohol use | Cross-sectional design. Incomplete information about disabilities. | ||
| Guerchet et al24 /Djidja, Benin | 2009 | DSM-IV | 514 | ≥ 65 | 2.6% | 53.8% | 7.7% | Age | Self-reported education. Informal age confirmation. Cross-sectional design. Low proportion of subject schooled. No radiological confirmation |
| Yusuf et al26/Zaria, Nigeria | 2011 | DSM IV ICD 10 |
322 | ≥ 65 | 2.8% | 66.7% | 33.3% | Age | One stage selection. No radiological confirmation |
| Paraiso et al25/Cotonou, Benin | 2011 | DSM-IV | 1162 | ≥ 65 | 3.7% | Age, Female sex | Sub-section of CSI-D used. Neuropsychology test do not have adjusted normative values for illiterate population. Relatives not involved to confirm details | ||
| Gureje et al37/Southwest, Nigeria | 2011 | 10-WDRT CHIF |
1225 | ≥ 65 | Age, Gender, Poor economic status, Rural Living, Social Isolation | Preponderance of persons with little or no education. Use of 10-WDRT | |||
| Akinyemi et al41/ Ibadan and Abeokuta, Nigeria | 2014 | DSM-IV ASA/AHA |
143 (Stroke survivors) | ≥.45 | 8.4% | Age, Low Education, Medial temporal lobe atrophy, Pre-stroke cognition | Modest sample size. Incomplete neuroimaging. | ||
| Ogunniyi et al42/Lalupon, Nigeria | 2016 | DSM-IV | 613 | ≥ 65 | 2.9% | 58.8% | 11.7% | Age | Lack of neuroimaging. Identification of treatable conditions |
| Ojagbemi et al35/Southwest, Nigeria | 2016 | 10-WDRT CHIF |
2149 | ≥ 65 | Age, Gender, Socioeconomic status, Pre-existing cognitive decline, Occupational complexity. | Inaccurate survival data. Attrition. Small size of dementia mortality sample | |||
| Sarfo et al40/Kumasi, Ghana | 2017 | DSM-IV | 147 (Stroke survivors) | Age, Education, Functional ability | Modest sample size. Cross-sectional study. Neuroimaging not available for review. Lack of pre-stroke cognitive status. | ||||
| Adoukonou et al/ Parakou, Benin | 2020 | DSM-IV-TR | 440 | ≥ 50 | 3.2% | 64.3% | 21.4% | Age, Living alone, Low vegetable intake | Not generalizable. Verbal report of vascular factors. Participants may have benefitted from having a better socioeconomic status and better access to health care than the overall older population. Sample size was small. High level of refusals. Use of the brief version of the CSI-D also carries some limitations. |
| Central Africa | |||||||||
| Guerchet et al27/ Bangui, CAR | 2010 | DSM-IV | 496 | ≥ 65 | 8.1% | 82.5% | 17.5% | DSM-IV underestimate. No radiological confirmation | |
| Guerchet et al27 / Brazzaville, Congo | 2010 | DSM-IV | 520 | ≥ 65 | 6.7% | 68.6% | 31.4% | ||
| Guerchet et al65/ Bangui CAR and Brazzaville, Congo | 2012 | DSM-IV | 977 | ≥ 65 | 7.6 % | Age, Female sex, Hypertension, Peripheral artery disease, Low BMI, Depression, Lack of education | High rate of missing data. Cross-sectional design. Absence of APOE genotyping. No radiological confirmation | ||
| East Africa | |||||||||
| Longdon et al29/Kilimanjaro, Tanzania Paddick et al63/Kilimanjaro, Tanzania |
2013 2014 |
DSM-IV | 1198 | ≥ 70 | 6.4% | 48.7% | 41.0% | Diabetes | No radiological confirmation. Incomplete radiological and laboratory investigations. Too little number for subtypes. Attrition. Non-medically trained census enumerators. |
| Mubangizi et al30/Rural Southwest, Uganda | 2020 | Brief CSID | 400 | ≥ 60 | 20.0% | Age. But having some education, exercise and ventilated kitchen were protective | No structured clinical interviews. Brief CSID used. Early and midlife exposure variables were measured by self-reporting. | ||
| Yoseph et al162/Kilimanjaro, Tanzania | 2021 | DSM- V | 3011 | ≥ 70 | 8.9% | - | |||
| South Africa | |||||||||
| Ramlall et al/Nursing Homes, South Africa | 2013 | DSM-IV-TR | 140 | ≥ 60 | 7.9% | 40.0% | Blackouts, Hypertension, Exercise, Visual and Hearing impairment. | Poor sampling – small size, low number of black participants, low number of dementia cases. Inter-rater reliability not quantified. | |
| De Jager et al13/ Amatole District, South Africa | 2017 | CSID | 1394 | ≥ 65 | 11.0% | Older age, dépressive symptoms | No clinician resources to provide a DSM-IV diagnosis of dementia. Targeted sample size not achieved. Sampling involved only low-income rural community | ||
| North Africa | |||||||||
| Farrag et al34/ Assiut Governorate, Egypt | 1998 | DSM-III R | 2000 | ≥ 60 | 4.5% | 53.0% | 22.9% | - | NS |
| El Tallawy et al23/ Al Kharga District, Egypt | 2012 | DSM-IV-TR | 8173 | ≥ 50 | 2.3% | 51.2% | 28.7% | - | NS |
| El Tallawy et al32/ Al-Quseir city, Egypt | 2014 | DSM-IV | 4329 | ≥ 50 | 3.8% | 48.3% | 36.8% | - | NS |
| Khedr et al33/ Qena Governorate, Egypt | 2015 | DSM-IV | 619 | ≥ 60 | 5.1% | 34.3% | 25.7% | - | NS |
Abbreviations: DSM- IIIR – Diagnostic & Statistical Manual of Mental Disorders–3rd Edition Revised; ICD- 10 – International Classification of Diseases 10th Revision; WDRT - 10-Word Delayed Recall Test; CHIF - Clinician Home-based Interview; DSM – IV - Diagnostic & Statistical Manual of Mental Disorders– 4th Edition; DSM – IV-TR- Diagnostic & Statistical Manual of Mental Disorders– 4th Edition Text Revision; CSID – Community Screening Instrument for Dementia; CAR – Central Africa Republic; NS - not stated
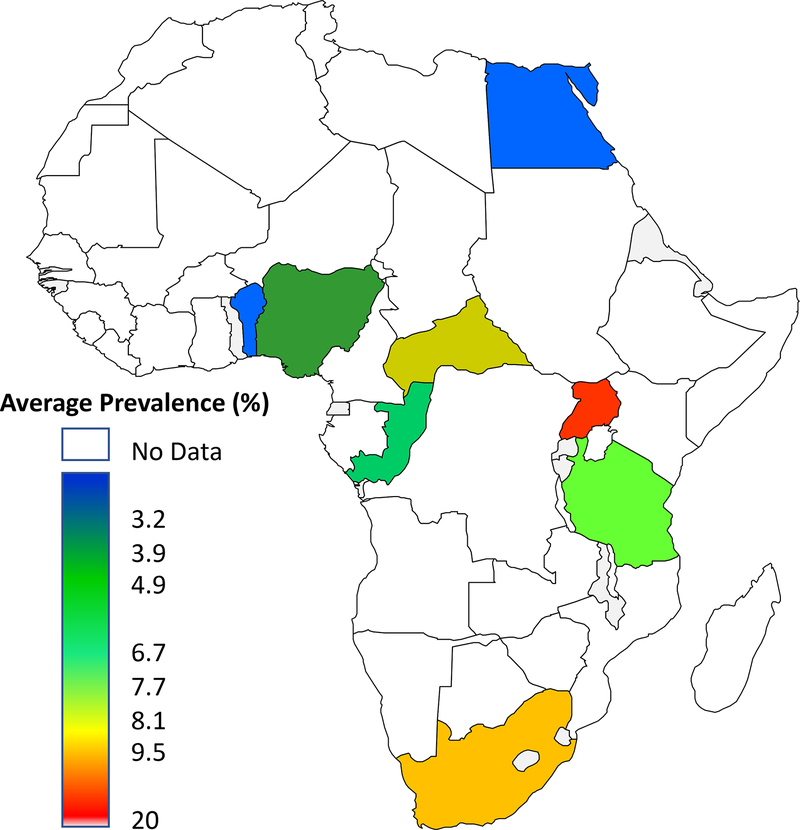
As highlighted in a systematic review by Mavrodaris et al, variation in dementia prevalence depends on the criteria used and methodology employed.19 Overall, higher prevalence estimates of up to 20.0% have been reported in community-based studies using different approaches and multiple rating scales for defining dementia.20,21,30 There are also important geographical variations in the prevalence estimates of dementia. The lowest prevalence rate of 2.3% has been reported from Ibadan, Nigeria22 and Al Kharga in Egypt.23 The reported prevalence of dementia appears low in Western Africa with most studies reporting prevalence around 3%24–26 and much lower than figures above 6% in Central, Eastern, and Southern Africa.21,27–30 Figures from Northern Africa tend to be intermediate and range from 2.3% to 5.1%.23,31–34 ( Figure 2; Table 1)
Figure 2: Prevalence of Dementia in Africa.
Heat map showing wide range of dementia prevalence in African countries determined over the past 25 years. Dementia prevalence studies have also been conducted in Senegal and Kenya but the data are not published yet.
2.4. Mortality
The recent report on dementia in SSA published by ADI included results from four African studies that have estimated dementia mortality risk. The result showed an increased mortality risk with an hazard ratio ranging from HR=1.5 (95%CI: 1.2–1.8)35 to HR=6.3 (95%CI: 3.2–12.6)36 and an estimate from meta-analysis of HR=2.3 (95%CI: 1.0–3.5).11 Contrary to expectations of a higher risk of dying from dementia in the developing compared to developed countries, some studies in Africa reported a lower risk of mortality from dementia than has been reported in several middle- and high-income countries.35 Urban dwelling and anthropometric evidence of under-nutrition were independent predictors of dementia mortality in the Ibadan Study on Ageing cohort.35
2.5. Economic cost
Data on the direct costs of dementia in Africa are largely non-existent. However, it has been estimated that the cost of dementia in 2015 represented 6.2 billion US dollars for SSA, of which 70% is attributable to the cost of informal care most often provided by relatives and families of people living with dementia (Guerchet et al., 2017).11 A limit of this estimate is that it is based on imputations using the countries’ Gross Domestic Product and the medical/social/informal cost distribution from a multicentric study in LMICs from the 10/66 Dementia Research Group. There is a lack of original data regarding health service utilization and cost of services in African countries, which could inform a better and more precise estimation of the cost of dementia in the region.37
2.6. Risk factors
A number of known risk factors for dementia have been evaluated by various studies in Africa. While the results do not significantly differ from those reported in other regions, the strength of the evidence is limited by the fact that most of these studies were cross-sectional in design. New risk factors for dementia such as air and environmental pollution need further study in Africa. Hence, more longitudinal studies that can inform context-specific interventions are needed from multiple African regions.
2.6.1. Non-modifiable Risks
2.6.1.1. Age and sex
Age is the most consistent non-modifiable risk factor for cognitive impairment and dementia. Various studies have corroborated the known association with both prevalent and incident dementia in the African context.11,12,21,22,38–41 Regarding association with sex, many studies have also reported that dementia and AD are either more prevalent in the female sex,22,24–26,29 or the male sex42 or have found no association21. The association with the female sex may be linked to a higher life expectancy and often poorer education.
2.6.1.2. Genetic factors
Using mainly candidate gene approaches, some genetic loci/alleles have been associated with dementia phenotypes in Africa, with the apolipoprotein E (APOE) gene being the most studied (Table 2). Whereas the APOE ε2 allele is protective,43 the APOE ε4 allele increases the risk of AD in Caucasians44 but not conclusively in indigenous African populations (particularly those in SSA). Older population-based studies showed weak or no association between APOE ε4 and cognitive decline24 or AD24,45–47 whereas more recent data reported a significant association between APOE ε4 homozygosity48 and incident AD among Yoruba Nigerians.49 It is particularly intriguing that whereas APOE ε4 allele has been clearly associated with AD in Northern Africa, the association has been non–existent or rather weak in SSA.50 While gene – environment interactions might influence the link, there is also the plausibility of the existence of novel genetic variants that have stronger genetic contribution to AD biology in SSA populations.8,51 Further studies are therefore needed to explore the geographical disparities in the relative association of the APOE alleles and AD in different African regions, although ancestry-specific genetic factors near APOE have been implicated.52 The APOE ε4 allele has also been linked with mortality53 and white matter integrity in adults with HIVinfection.54 Studies from Northern Africa have reported other genetic associations of AD with mutations in the Amyloid Precursor Protein (APP), Presenilin 1 (PSEN1) and Presenilin 2 (PSEN2) genes.55,56 In South Africa, PSEN1 mutation has been reported in a family with early onset AD57 and the CHMP2B polymorphisms in a South African family with frontotemporal dementia (FTD).58 Newer studies have also reported associations of ACEI and PON1-L55M T alleles, GTICC haplotype and increased dementia risk, whereas AADTT reduced dementia risk by 80%.48 Landoulsi et al. reported no major association of TREM2 gene with late-onset AD in a Tunisian cohort59 but plasminogen activator inhibitor 1 (PAI-1) 4G/5G polymorphism demonstrated increased risk of dementia.60 Considering the great genetic diversity on the African continent, it is probable that unique genetic variants at previously identified loci and novel loci associated with both the risk of and protection from AD and related dementias remain to be discovered.
Table 2:
Candidate gene studies for dementia phenotypes in Africa
| Authors (year) | Gene Name | Study Population | Salient Findings |
|---|---|---|---|
| Osuntokun et al (1995) 44 | APOE Ɛ4 | Yoruba Nigerian | No association was reported between APOE Ɛ4 alleles and AD |
| Lane et al (2003) 52 | APOE Ɛ4 | African American and Yoruba Nigerian | No association was observed between APOE Ɛ4 alleles, age and mortality risk among the Yoruba Nigerians. |
| Heckmann et al (2004) 56 | PSEN1 | Southern Africa | PSEN1 mutations associated with early onset AD but no effect of APOE Ɛ4 |
| Momeni et al (2006) 57 | CHMP2B | South African | Mutation in CHMP2B gene associated with frontotemporal dementia |
| Gureje et al (2006) 45 | APOE Ɛ4 | Nigerian | Lack of association between APOE Ɛ4 and AD |
| Chen et al (2008) 46 | APOE Ɛ4 | Kenyan | Lack of association between APOE Ɛ4 and AD |
| Guerchet et al (2009)23 | APOE Ɛ4 | Beninoise | No association between APOE Ɛ4 and cognitive decline |
| Hoare et al (2013) 53 | APOE Ɛ4 | South African | APOE Ɛ4 is associated with memory impairment and white matter integrity in HIV positive individuals |
| El Kadmiri et al (2014) 54 | APP | Moroccan | 7 novel mutations (frameshift mutations) in the APP gene on exons 16 and 17 had genetic contributions to AD |
| El Kadmiri et al (2014) 55 | PSEN1, PSEN2 | Moroccan | Mutations in both genes have a genetic effect on early onset AD |
| Hendrie et al (2014) 48 | APOE Ɛ4 | African Americans and Yoruba Nigerians |
One or two copies of APOE Ɛ4 allele is/are significant risk for both AD and cognitive decline in African Americans. Only homozygous carriers of the APOE Ɛ4 among Yoruba Nigerians had a significant risk factor for AD but not cognitive decline |
| Fekih-Mrissa et al (2017) 59 | PAI 1 | Tunisian | Variants of PAI-1h ad significantly increased risk for AD. Homozygotes are at higher risk while female gender was also at increased risk. |
| Landoulsi et al (2018) 58 | TREM2 | Tunisian | TREM2 has no association with late onset AD which has significant risk in Caucasian populations |
| Haithem et al (2018) 47 | APOE, ACE1, PON1 | Tunisian | All studied genes had polymorphisms associated with dementia risk individually and collectively with a cumulative and synergistic effect |
Abbreviations: APOE – Apolipoprotein E; PSEN1- Presenilin 1; PSEN2 – Presenilin 2; APP – Amyloid Precursor Protein; CHMP2B – charged multivesicular body protein 2B; PAI 1- Plasminogen activator inhibitor-1; TREM2 - Triggering receptor expressed on myeloid cells 2; ACE1- Angiotensin-converting enzyme; PON1- Serum paraoxonase and arylesterase 1.
2.6.2. Modifiable Risks
2.6.2.1. Cardiovascular risk factors
Several studies have demonstrated associations between traditional cardiovascular risk factors – hypertension,61,62 type 2 diabetes,26,63 dyslipidaemia,64 and peripheral arterial disease65,66 and cognitive impairment - and dementia phenotypes.67 While peripheral arterial disease and systemic hypertension were linked to prevalent dementia in Central and West Africa, respectively17, high total cholesterol and low-density lipoprotein were predictors of incident dementia in Nigeria.64 Mild cognitive impairment (MCI) is a precursor of dementia. In one community–based study from Lalupon, southern Nigeria, MCI was associated with mean arterial pressure (MAP) and pulse pressure68 while a Ugandan community–based study found an association between cognitive impairment and carotid artery plaques.69 Hypertension and type 2 diabetes were also found to be associated co-morbidities in Nigerian hospital–based dementia cohorts.70–72 Other less established risk factors have also been shown to increase the risk of dementia in Africa include homocysteine,73 and folate.74
2.6.2.2. Literacy and education
As shown in the extant dementia literature particularly from the West, studies from Africa have also demonstrated an association between low educational attainment and increased dementia risk.41,65,75 Based on the cognitive reserve hypothesis,76–78 it is suggested that education might interfere with the phenotypic expression of dementia. However, other studies have reported a lack of association between education and dementia on the continent22,29,79,80. Nonetheless, it is noted that the majority of older Africans included in these studies had no formal education lasting 7 years and only a minority had a few years of formal education. It is likely that this low level of formal education may not be an appropriate signature of cognitive reserve. Indeed, many older Africans even centenarians despite having no formal Western education play key social roles and have communal responsibilities that better reflect their cognitive ability and likely plays a role in maintaining cognitive reserve. Clearly, traditional systems of learning which can also improve cognitive abilities need to be considered. As such, one has to consider the limitations and appropriateness of directly translated cognitive scales that have been used in the past in interpreting the association. Fortunately, newer context–sensitive tools are being developed for better cognitive evaluation in the region to address these observations.81–83
2.6.2.3. Lifestyle factors and social isolation
A review of the literature from Africa demonstrates limited evidence on the relationship between lifestyle factors such as diet, physical activity, smoking and alcohol and cognitive impairment and dementia. Anthropometric markers of malnutrition such as reduced body mass index,65,84 low arm muscular circumference, low mid-upper arm circumference84–86 and lower consumption of oily foods have been associated with dementia.85 Undernutrition, especially with low consumption of oleaginous diet was associated with prevalent dementia in a study involving two countries from Central Africa17,85 while a history of smoking, current smoking, and weight loss were linked to incident dementia in Nigeria.84 Pre-stroke daily fish intake was found to be protective against cognitive impairment among stroke survivors.41. It has also been suggested that the lower risk of dementia among Yoruba Nigerians is related to low levels of saturated fat and high fiber content in their traditional diet which consists of yam tubers (Dioscorea rotundata), grains, vegetables, and fish.64 Fiber has many beneficial effects, including alteration of the gut microbiota and consequent implication for the gut-brain axis and related brain disorders. Based on data from the WHO’s Study on Global AGEing and Adult Health (SAGE), a cross-sectional, community-based study conducted in South Africa demonstrated an association between food insecurity and cognitive impairment.87 However, findings from the two studies on the link between alcohol and dementia were contradictory.85,88 The importance of lifestyle and environmental factors and their interaction with genetic factors has been elucidated in comparative cross-national studies.89 The care structure for older persons in traditional African societies provides a rich social support network with older persons often living in multigenerational settings, although this is now been eroded by migration, urbanization and globalization.90 The effect of these changes in care structure on the trends of cognitive impairment and dementia in Africa deserve further research focus. Available data, however, showed that living with others was protective against dementia in the Ibadan cohort of the Ibadan – Indianapolis Dementia Project91 while low social network92 and poor social engagement38 were risk factors for prevalent and incident dementia in a Senegalese and another Nigerian study respectively. The role of sleep, hearing loss, head trauma, air pollution and environmental toxins such as lead have not been studied as potential risk factors for dementia in the African context.
2.6.2.4. In utero and early life factors
The role of in – utero and early life exposure in dementia occurrence has been the focus of several studies in high income countries,93,94 but these have not been widely explored in Africa. Well-designed longitudinal studies to explore the role of early life factors will require considerable funding and expertise. Pilleron et al, however, reported a significant association between the death of one parent during childhood and dementia in late life from studies conducted in the Central African region.95
2.6.2.5. HIV and Other Infections
Substantial evidence abounds demonstrating HIV as a cause of neurocognitive disorders with about two-thirds of people living with HIV diagnosed with probable HIV associated neurocognitive disorder (HAND).96–102 With over 11.3 million HIV/AIDS patients affected by HAND98, it has been suggested that the burden of HAND in Africa is likely to rank among the highest of any region in the world. There are several reasons for the high burden of HAND on the continent. First, Africa has the highest HIV/AIDS burden in the world. Secondly, in many parts of Africa late presentation of HIV is rife with relatively advanced infection characterized by severe immunosuppression, which directly predisposes to HAND.101,103 HIV also increases the risk of atherosclerotic strokes104 and of tuberculosis including tuberculous meningitis and tuberculomas that indirectly increase risk of cognitive decline and dementia. Furthermore, the development of new genetic recombinant forms of HIV1 as well as its genetic variation in patients with HAND as reported from Cameroon,105,106 pose additional challenges to reduction of the HAND burden and vaccination research. When feasible, including HIV screening in the future generations of population-based studies of dementia could fill some knowledge gap.
The role of toxoplasmosis infection in dementia emphasizes the fact that parasitic infectious agents might be particularly important contributors to cognitive impairment and dementia in Africa.107 A recent case-control study reported a higher prevalence of cognitive impairment in people with epilepsy, particularly decreased executive function and verbal fluency, than in people without epilepsy in an onchocerciasis-endemic area of Cameroon.108 Evidence from children with retinopathy-positive cerebral malaria suggests some cognitive impairment109,110 Longitudinal studies are necessary to delineate the role of parasitic diseases in cognitive function and dementia in Africa.
2.7. Dementia subtypes in Africa
Alzheimer disease (AD) and vascular cognitive impairment and dementia (VCID) remain the most commonly reported dementia phenotypes (Table 1).37 A report from the Ibadan-Indianapolis dementia study suggests that in a densely populated urban community in Ibadan, south-Western Nigeria, only 12% of all new cases of dementia between 1995 and 2001 received a diagnosis of vascular dementia based on DSM III-R and ICD 10 criteria.13 In a recent systematic review, the proportion of VCID in multiple African studies ranged between 17 and 41% for all phenotypes of dementia depending on the type of study sample.111 Other dementia phenotypes reported in Africa include frontotemporal dementia,112 dementia with Lewy bodies,113, Parkinson’s disease dementia,71 and cognitive impairment or dementia associated with Creutzfeldt-Jakob disease,114 Huntington disease115,116 and sickle cell disease (SCD).117–121 However, confirmation of dementia subtypes is only definitive following post-mortem neuropathologic examination and this level of diagnostic certainty has not been achieved in existing studies from Africa122,123 with the exception of the first reported case of dementia with Lewy bodies113. SCD is well known to predispose to vascular brain injury, particularly silent cerebral infarction (SCI), which are often associated with cognitive impairment.121 Studies in Cameroon and Nigeria have revealed that executive function in particular, attention and working memory are severely affected in SCD children with high cerebral blood flow velocities.118–120 A recent comparative magnetic resonance imaging (MRI) study in Tanzania showed that SCI, vasculopathy, and hemoglobin are independent risk factors for diffuse white matter injury in children with SCD.117
2. 8. Living with dementia in Africa
A common problem in Africa and probably other LMICs for persons living with dementia is grappling with stigmatization. Limited studies have reported that stigmatization is rooted in belief systems, commonly cultural124 or supernatural,125 where persons with dementia are thought to be witches. Even though the role of traditional healers, community leaders and faith healers in health promotion cannot be overlooked, there is nevertheless evidence that these community opinion leaders commonly do not view dementia as a specific disease but rather a feature of normal ageing.126–128 Occasionally, carers and even healthcare workers hold similar belief, an observation that suggests a need for education of both the general population and healthcare workers.125,129 There is evidence in support of higher educational attainment being associated with less stigmatising attitudes.125 The need for education is particularly key as caregivers are unlikely to seek health interventions without adequate information. In Nigeria about a third of people feel that even individuals living with dementia would prefer not to know or let others know their disease status.130
To relieve caregiver burden, formal home care is a viable option in high-income countries but not in Africa. Negative attitudes to formal care exist among some family members and in some society irrespective of economic status, and religious beliefs contributing to the perspective.131 A study in South Africa showed that less than a third of respondents were willing to pay for formal home caregivers, with higher education, female sex and older age associated with willingness to pay.132 The challenges of formal care setting include the risk of delirium associated with dementia due to infective causes.133,134 However, there are also limitations with formal care, among which is sub-optimal clinical practices among healthcare workers. A qualitative study carried out in Southwestern Uganda noted that healthcare staff did not have adequate specific mental health training for assessment and diagnosis of dementia. Healthcare workers with some specialized training in mental health were more likely to use neuropsychological tests and brain imaging in the diagnosis of dementia.135
2.9. Treatment options
2.9.1. Pharmacological:
While there are no approved disease-modifying medications for most of the primary dementias, a few strategies such as immunotherapy directed against amyloid and/or tau, and inhibition of amyloid synthesis are currently in advanced stages of development.136–138 There are reports on the potential use of medicinal plants.139,140 However, the current treatment approaches are directed at symptom relief, with options including adjustment of neurotransmitters (acetylcholine, norepinephrine, and serotonin), behavioural modification, and treatment of medical complications. Access to and affordability of pharmacological agents for symptomatic management remains a significant challenge in Africa. It is also necessary to enlist common relevant medications on the WHO essential drug list. Traditional approaches to the treatment of dementia in Africa are often influenced by the belief about the origin of the ailment or the trajectory including trial of medicinal products and hospital care. African family and/or caregivers of individuals with dementia often consider the use of herbal or folk remedies and reports on the potential efficacy of some local African medicinal products are emerging139,140 However, when anticipated improvement in memory, functioning or quality of life is not achieved, alternative and informal care approaches are often resorted to.
2.9.2. Non-pharmacological:
Non-pharmacological approaches are important in the management of dementia worldwide and probably more so in Africa. Generally healthcare staff and home care workers prefer interpersonal approaches above medication to manage distressing behavioural disorders, except in certain situations.141 Non-pharmacological interventions aimed at reducing disabilities consist of patient education, cognitive interventions, and lifestyle modifications.142 Cognitive stimulation therapy has been studied in Nigeria and Tanzania with reported clinical benefits including substantial improvements in cognition, anxiety and other behavioural symptoms.143–145
3. GAPS AND FUTURE DIRECTIONS
3.1. Cognitive evaluation in Africa
Robust cognitive tools that are culture–sensitive with excellent psychometric properties and resistant to the differences due to effects of education and languages are needed for deployment across multiple regions of Africa. The need to utilize cognitive and functional assessment instruments that are culturally appropriate and adopt common approaches to clinical evaluation of dementia across African countries cannot be over-emphasized. This is because comparison of dementia rates from different studies may be fraught with difficulties due to variations in assessment tools and approaches. Various investigators have tried to improve on neuropsychological tests used on the continent with a view to adjusting for the peculiarities of the environment, ease of administration and educational status. The Community Screening Instrument for Dementia (CSI-D) is a screening tool that was developed for settings with low education.146,147 It has been deployed in several dementia epidemiological studies in sub-Saharan Africa and the 10/66 dementia research group has developed a shorter version of it with excellent psychometric properties.148 The Rowland Universal Dementia Assessment Scale (RUDAS) has been validated for dementia screening in Arabic-speaking population.149 The Intervention for Dementia in Elderly Africans (IDEA) Cognitive Screen83 is a six-item instrument that was more recently developed with components derived from the CSI-D (items 1–4). Item 5 was taken from the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) 10-word recall test, while the sixth item was designed to measure praxis and consists of a matchstick design test originally developed by Baiyewu et al.150 The IDEA Cognitive Screen therefore includes measures of delayed recall, orientation, two measures of executive function, verbal fluency and abstract reasoning, praxis and long-term memory. No items were included requiring reading, writing, drawing or calculation in order to reduce possible educational bias.81,82 It has been validated in Nigeria (Yoruba language) and Tanzania (Swahili language) with excellent psychometric properties including the Area Under the Receiver Operating Characteristic Curve (AUROC) of 0.99 in Nigeria and 0.91 in Tanzania.81,83 Cut off score is <7 for possible dementia. The IDEA cognitive screen was used to conduct a dementia prevalence study in Lalupon near Ibadan, Nigeria and obtained rates that were largely similar to previous rates obtained (using the CSI-D) in the Ibadan -Indianapolis Dementia Project.42 The International HIV Dementia Scale which was developed in Uganda has been used as a screening tool for HAND in several African countries.151,152
The next important step for promoting reliable cognitive evaluation in Africa is the development of normative data across the lifespan, based on the validated culturally - sensitive neuropsychological test batteries and screening instruments. Examples of normative data have been reported from Cameroon in the paediatric and adult populations.99,153
For functional assessment, the Clinician Home-based Interview to assess Function (CHIF) was developed and validated by the Indianapolis-Ibadan dementia research group.154 The IDEA study Instrumental Activities of Daily Living (IDEA – IADL) was also recently developed and validated among rural dwelling Tanzanians. It had an AUROC of 0.896 for DSM -IV dementia and 0.937 when used in conjunction with the IDEA Cognitive Screen, with no bias for age, sex or education.155 A shorter version, the 3-item IDEA-IADL questionnaire has also been validated for evaluation of instrumental activities of daily living.156 The Alzheimer’s Disease Assessment Scale - Cognitive (ADAS-Cog) has been adapted for low literacy and has good psychometric properties.157 The IDEA - IADL App-Based Cognitive Screening instrument mobile application has now been developed and validated among non-specialist rural community workers. The AUROC was 0.78 with good sensitivity and fair specificity.158 In Central Africa, the Central African Daily Interference Scale (CA-DFI), a 10-item scale, has also been developed with psychometric properties supporting the reliability and internal validity of the CA-DFI scale as a promising tool for functional assessment in the elderly for the diagnosis of dementia in Central Africa.159
3.2. Tracking epidemiological trends
African populations are aging rapidly such that by the year 2050, 212 million older persons 60 years of age and older o will be residing on the continent in keeping with trends in other LMICs.160 Concomitant with this is a projected increase in the prevalence of aging – associated disorders of the brain such as Alzheimer’s and Parkinson’s diseases. Hence, more epidemiological studies are needed in Africa that will accurately track the secular trends of cognitive impairment and dementia and dementia subtypes in Africa. Community-based studies in Tanzania have reported worsening cognitive decline over 2 years of follow-up161 and increased prevalence of dementia from 6.4% to 8.9% in a rural cohort of older persons > 70 years over a 9-year interval and using the same methods of cognitive assessment.162 This is in contrast to declining trends of dementia reported in high-income countries. Over the years, there has been improvement in dementia epidemiological studies and results suggest an increasing prevalence.11 Increasing prevalence may also suggest greater awareness of dementia in Africa. Other potential, but less studied sources of variation in prevalence estimates of dementia in Africa include genetic predisposition, lifestyle factors, urban versus rural distribution of study participants, differences in literacy levels and changes in the age structure of the studied population. Tracking epidemiologic trends provides relevant data needed to inform identification of specific modifiable risk factors for cognitive impairment and dementia that can inform the development of culturally appropriate interventions and the formulation of elderly–friendly policies in African countries.11,161
3.3. Insights from neuropathologic evaluation
Accurate phenotyping of dementia subtypes depends on neuropathologic techniques as the gold standard. Although Alzheimer’s disease is typically characterised by the presence of amyloid plaques and neurofibrillary tangles, concomitant vascular and/or neurodegenerative pathologies are often detected and produce mixed phenotypes. For example, in a community-based autopsy cohort, approximately 60% of patients with clinical diagnoses of Alzheimer’s-type disease were in fact affected by vascular disease pathology, TDP - 43, or Lewy body pathology rather than plaques and tangles.163 Clinicopathological studies are therefore critical in shaping our understanding of the aetiology, natural history and mechanisms of disease and to help in formulating the frameworks necessary for the discovery of new therapeutic and preventative interventions.123
In Africa, a post-mortem study of brain tissue of neurologically normal Nigerian Africans showed incidental Lewy body pathology burden similar to figures that were then reported among individuals of European ancestry from the UK and USA.164 The significance of this finding was the suggestion that the correspondence of the frequency of Parkinsonian pre-symptomatic neuropathology (and indirectly the risk of Parkinson’s disease) in Nigerian Africans and Caucasians in the UK and USA might indicate similarity in the predisposition to Parkinson’s disease, while the disparity in prevalence (lower in Nigerian Africans) might be attributable to lower life expectancy in the latter.165 This also implies that as African populations age, the prevalence of PD and PD dementia might rise in parallel. However, in an autopsy survey of 198 brains of Nigerians aged 40 years and above (including 45 individuals (23%) who were above 65 years of age) to determine the occurrence of pathological hallmarks of AD, findings showed mild cortical neuronal loss and absence of neurofibrillary tangles, senile plaques and amyloid angiopathy – characteristic pathological features hallmarks of AD.166 Clinically at that time, dementia was rather rare.167 A similar post-mortem study on non-demented elderly East Africans reported significant neocortical amyloid beta deposits and tau protein reactive neurofibrillary tangles evident in the hippocampus in 15.2% and 12.5% of the subjects respectively similar to findings in age-matched elderly Caucasian control subjects from Cleveland, USA.168
Other studies involving multiracial populations of North America have reported racial disparities in the epidemiology and neuropathology of cognitive impairment and dementia. In a report from the Rush Study, African American subjects were less likely to have Alzheimer pathology as a single dementia pathology compared to Caucasian subjects (19.5% vs 42.0%), but were more likely to have Alzheimer pathology mixed with an additional pathology (70.7% vs 50.6%) particularly Alzheimer pathology and Lewy bodies, and Alzheimer pathology, Lewy bodies, and infarcts. Furthermore, black subjects also had more severe arteriolar sclerosis and atherosclerosis.169 Similarly, a recent multiracial Brazilian neuropathological study showed a comparable reduction in AD pathology but higher vascular pathology in the brains of subjects of African ancestry.170
3.4. Unravelling the genetic architecture
The observations reported above beg the question of whether there is a protective gene at play that mitigates the amyloid depositing effect of APOE ε4 in African ancestry populations. This lack of clarity is due to the fact that little is yet known about the genetic architecture of AD among indigenous Africans and there is inconsistency in the reported association of AD with APOE-ε4 allele among Africans.8,46,49,50 Even though Africa is the origin of modern humans and harbors the greatest genetic diversity in global populations only a fraction of the genetic diversity among Africans has been surveyed with < 2% of genome wide association studies (GWAS) comprising African data.171 In a recent high-depth study of African genomes aimed at further exploration of African genomic diversity, whole-genome sequencing analyses were performed on 426 individuals from 50 ethnolinguistic groups under the aegis of the Human Health and Heredity in Africa (H3Africa) Consortium. The study found more than 3 million previously undescribed genetic variants.172 The implications of these observations are enormous for understanding the genetic basis of cognitive impairment and dementia in African ancestry and global populations. Thus, greater representation of indigenous Africans in dementia genomic research including GWAS and whole exome or whole genome sequencing approaches will enhance diversity and inclusiveness and enable novel insights into the biology of brain ageing, cognition, AD and other phenotypes. In addition, fine mapping of loci and variants already described will be enhanced to pinpoint precise causal genetic variants.173 Furthermore, such studies will facilitate translational genomics, development of Afrocentric polygenic risk scores, unravel new pathways, biomarkers and drug targets for the enhancement of precision/ personalized dementia care. It will also improve our understanding of disparities in dementia phenotypes, risk factors and outcomes.123,174,175 Furthermore, such efforts will be in consonance with the US National Dementia Plan to prevent and effectively treat AD and AD - related dementias (ADRDs) by 20251 and the National Academy of Medicine recommendation to utilize global health research to benefit Americans and global populations.176
3.5. Fluid, neuroimaging and other biomarkers
Of all the causes of primary dementia worldwide, AD and VCID account for 70 – 80%, the other major causes being frontotemporal dementia, Parkinson’s disease dementia and Lewy body dementia.142 All primary dementias except VCID are due to neurodegenerative proteinopathies– misfolded protein form inclusion bodies that are toxic to the neurones and implicated in neuroinflammation, glial reaction and neurodegeneration. Amyloid beta (AD), tau protein (AD, fronto-temporal lobar degeneration, cortico-basal degeneration, and progressive supra-nuclear palsy), TAR DNA-binding protein 43 (TDP-43) (fronto-temporal lobar degeneration(FTLD), RNA- binding Fused in Sarcoma (FUS) (FTLD), and alpha-synuclein (Parkinson’s disease dementia, Lewy body dementia) are the main proteins elucidated and the neuropathological process initiation often precedes the recognizable clinical expression of disease by several years.142
Recent studies have highlighted the role of biomarkers – to predict the likelihood of progression to dementia from MCI (cerebral amyloid angiopathy, mesial temporal lobe atrophy on MRI), to confirm clinical diagnosis, and standardize clinical research as clearly captured in the recent National Institute of Aging – Alzheimer’s Association (NIA-AA) AD Diagnostic Criteria.174 There are major advances in imaging and fluid biomarkers particularly in AD neurobiology. Cerebrospinal fluid (CSF) biomarkers including amyloid beta 1–42, phosphorylated (p-tau)181 and total (t-tau) tau are of diagnostic significance. CSF Aβ1–42 and positron emission tomography (PET) amyloid imaging with Pittsburgh Compound B are markers of brain beta amyloid deposition, whereas increased levels of CSF total (t-tau) and phosphorylated (p-tau) tau, hypometabolism on fluoro - deoxyglucose PET scan and atrophy on structural MRI scan are markers of neurodegeneration.142,175 And more recent data have revealed the utility of blood p – tau181 as a potential diagnostic marker.177,178
A recent meta-analysis found that cerebrospinal fluid t-tau and p-tau 181 were consistently lower in African American than Caucasian individuals, in samples with normal cognition or with mild cognitive impairment/dementia.179 This suggests that racial differences should be taken into consideration in interpreting differences in biomarker levels in the dementia phenotypes among individuals of different ancestries. For the other dementia phenotypes, there are also useful neuroimaging modalities including MRI and FDG-PET, tau-PET for FTLD, dopamine transport scan for LBD/PDD, and diffusion tensor imaging for VaD.142 SPECT studies are desirable for differentiating dementia subtypes particularly where PET may not be available and use of Pittsburgh Compound B for in-vivo imaging of amyloid may be difficult.180
In Africa, there are limited datasets on imaging and fluid biomarkers. Africa lags behind in these advances due to lack of investments in relevant research infrastructure and expertise and the high cost of these advanced modalities. Nevertheless, there is a ray of hope in the horizon as efforts are beginning to yield fruits. Global brain atrophy and medial temporal lobe atrophy on MRI were significantly associated with cognitive impairment in a cohort of Nigerian stroke survivors181 while MRI - determined thinning of the corpus callosum was associated with central nervous system disease severity and reduced immunity in a cohort of South African children living with HIV.182 Other studies have evaluated the role of CSF-based biomarkers,183 and peripheral blood cell biomarkers in HIV-associated neurocognitive disorders.97 Muscle strength measured using handgrip strength has been considered as a biomarker of mild cognitive impairment in LMICs.184
3.6. Integrative transomics and precision medicine for dementia
Emerging insights from progress in multi - omics research suggest that dysregulation of molecular pathways at multiple levels including the genome, epigenome, transcriptome, proteome and the metabolome in response to numerous risk factors and triggers are involved in the neuropathological cascades that ultimately result in cognitive impairment and dementia.185,186 Thus, an integrative approach involving the exploration of sociodemographic, lifestyles, clinical, imaging, genetic, epigenetic, fluid biomarkers and pathologic substrates of cognitive decline and dementia in diverse populations including indigenous Africans will provide useful insights into new pathways, processes and networks, potential identification and characterization of novel biomarkers for prevention, risk profiling, early detection, diagnosis, prognosis and treatment of cognitive disorders in a precision medicine framework.187,188
4. COVID- 19 AND DEMENTIA IN AFRICA
The corona virus disease 2019 (COVID - 19) caused by the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) is a pandemic of current global proportions and importance.189 It is generally known that individuals living with cognitive impairment and dementia are particularly susceptible to the viral infection because of their age, co-existing morbidities, immune senescence and reduced ability to adhere to the preventive protocols.190,191 In Africa, there have been >2.7 million COVID-19 cases and long term consequences are of particular concern (www.afro.who.int). Mortality rates are also very high in this population even though African data are sparse.192,193 The peculiar challenges of COVID - 19 and the elderly in Africa are premised on further erosion of the social support structure by social distancing, weak and fragile healthcare systems, worsening poverty and poor healthcare financing.191–193 The situation is further aggravated by the dependence on carers and caregivers for the performance of activities of daily living. This is fraught with the risk of infection with COVID −19 of both the person living with dementia as well as the caregiver. Social distancing also predisposed to exacerbations of neuropsychiatric symptoms like anxiety, agitation and depression.194 To mitigate these challenges in the African context, social connection and interactions needs to be maintained with older adults, persons living with dementia and their carers inspite of ‘spatial distancing’. Family members and healthcare providers can keep in touch through various digital technologies including video and audio phone calls, WhatsApp etc.192 In the wake of the 2020 lockdown, unique telemedicine -based care models of care have been developed to meet the care needs of elderly Africans. In Nigeria for instance, a “Care in Place” policy was implemented in a pioneering geriatric centre. This involved the provision of home based care for ambulatory geriatric patients in order to prevent avoidable hospital visits and with the attendant risk of infection with the virus.195 Other recommendations from the Lancet Commission194 on dementia prevention, treatment and care and dementia experts190 are generally applicable within the African context.
5. THE AFRICAN DEMENTIA CONSORTIUM
The broad aim of the African Dementia Consortium (AfDC) is to bring together African dementia researchers in a multidisciplinary framework and generate clinical, cognitive, socioeconomic, neuroimaging, genomic and biomarker data to improve the phenotypic characterization of dementia in Africans. The network will also identify novel biomarkers and interventions for prevention and treatment. The AfDC will further foster the translation of evidence to policy and practice and contribute to efforts to reduce the burden of dementia among Africans, African ancestry populations in Diaspora and ultimately contribute to the reduction of the global burden of dementia.
In order of priority, AfDC will focus on research areas including: (1) epidemiological studies to define trends in prevalence, incidence, and risk factors for dementia in Africa; (2) genetic studies to unmask novel variants that predispose to Alzheimer’s disease and related dementias (ADRD) in African populations and also increase African participation in global genomic studies including trans - ancestry meta analyses in dementia; (3) detection of unique biomarkers for dementia; (4) conduct of dementia clinical trials involving African populations; (5) capacity building and networking among dementia researchers living or working in Africa particularly early career investigators; (6) facilitation of translational dementia research (7) promotion of implementation science for translation of research evidence to practice and policy in Africa and (8) training and education of the next generation of research leaders. The consortium will build effective synergies through collaborative research networks with researchers within Africa and with partners from North America, Latin America, Europe, Asia and other regions of the world.
6. CONCLUSIONS
It is imperative that we invest resources to better reduce the current vast gaps in knowledge regarding Alzheimer’s and other dementias in the African continent. The ageing of the population makes this an economic and social, as well as a moral and ethical imperative. Furthermore, undertaking further genetic, biomarker and pathological studies in this genetically and environmentally diverse region promises to lead to an improved understanding of the biology of AD that will benefit individuals and populations all over the world and further promote effective prevention, treatment and care as recently outlined by the Lancet Commission on Dementia.194 Equity in access to dementia diagnosis, treatment and access to care as well as dementia prevention strategies should remain core to the future efforts engaged in dementia science and care in Africa.
Supplementary Material
Supplementary File 1: Organisations on Dementia Care and Support in Africa:
ACKNOWLEDGEMENTS
ROA is supported by the UK Royal Society/African Academy of Sciences FLAIR Grants FLR/R1/191813 and FCG/R1/201034, and GCRF Networking Grant from the UK Academy of Medical Sciences and Global Brain Health Institute/Alzheimer’s Association/Alzheimer’s Society UK Grant GBHI ALZ UK-21-724204. ROA, MG L M-M, KR and KB are Senior Atlantic Fellows of the Global Brain Health Institute. ROA, MOO, AA and FSS are also supported by Grants U54HG007479 and U01HG010273 from the National Institutes of Health (NIH), USA as part of the H3Africa Consortium. MOO, ROA FSS and AA are further supported by NIH grant R01NS107900. SS is supported by NIH grants U01 AG052409 and R01 AG054076 RNK’s research on elderly post-stroke dementia has been supported by the Medical Research Council, RCUK Newcastle Centre for Brain Ageing and Vitality (MRC G0500247), Medical Research Council (UK), Alzheimer’s Research UK, the Dunhill Medical Trust, UK and the Newcastle National Institute for Health Research Biomedical Research Centre in Ageing and Age- Related Diseases, Newcastle upon Tyne Hospitals National Health Service Foundation Trust.
Footnotes
CONFLICTS OF INTEREST
All authors have no conflict of interest related to this article.
REFERENCES
- 1.Corriveau RA et al. Alzheimer’s Disease-Related Dementias Summit 2016: National research priorities. Neurology 89, 2381–2391, doi: 10.1212/WNL.0000000000004717 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corriveau RA et al. The Science of Vascular Contributions to Cognitive Impairment and Dementia (VCID): A Framework for Advancing Research Priorities in the Cerebrovascular Biology of Cognitive Decline. Cellular and molecular neurobiology 36, 281–288, doi: 10.1007/s10571-016-0334-7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prince M, Guerchet M & Prina M The Global Impact of Dementia: 2013–2050. (2013).
- 4.WHO. Global action plan on the public health response to dementia 2017 – 2025. (2017). [Google Scholar]
- 5.Prince M et al. The Global Impact of Dementia - An analysis of prevalence, incidence, cost and trends. (London, 2015). [Google Scholar]
- 6.Collaborators, G. B. D. N. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18, 459–480, doi: 10.1016/S1474-4422(18)30499-X (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wimo A et al. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement 13, 1–7, doi: 10.1016/j.jalz.2016.07.150 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogunniyi A & Akinyemi RO Epidemiology and Genetics of Alzheimer’s disease with Special Reference to Africans. Nigerian Journal of Health Sciences 3, 1–6 ( 2003). [Google Scholar]
- 9.Nations, U. World Population Ageing 2019. (2020). [Google Scholar]
- 10.Alzheimer’s Disease International. The Global Impact of Dementia An analysis of prevalence, incid ence, cost & trends, (Alzheimer’s Disease International, London, 2015). [Google Scholar]
- 11.Guerchet M et al. Dementia in sub-Saharan Africa: Challenges and opportunities. (2017).
- 12.Bhalla D et al. Incidence and Risk Profile of Dementia in the Regions of Middle East and North Africa. NED 50, 144–152, doi: 10.1159/000487761 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Hendrie HC et al. Incidence of dementia and Alzheimer disease in 2 communities: Yoruba residing in Ibadan, Nigeria, and African Americans residing in Indianapolis, Indiana. JAMA 285, 739–747, doi: 10.1001/jama.285.6.739 (2001). [DOI] [PubMed] [Google Scholar]
- 14.Ogunniyi A et al. Hypertension and incident dementia in community-dwelling elderly Yoruba Nigerians. Acta Neurol. Scand. 124, 396–402, doi: 10.1111/j.1600-0404.2011.01491.x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao S et al. Dementia incidence declined in African-Americans but not in Yoruba. Alzheimers Dement 12, 244–251, doi: 10.1016/j.jalz.2015.06.1894 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogunniyi A et al. Profile of dementia in a Nigerian community--types, pattern of impairment, and severity rating. J Natl Med Assoc 89, 392–396 (1997). [PMC free article] [PubMed] [Google Scholar]
- 17.Ojagbemi A & Bello T The Low Prevalence of Dementia in Sub-Saharan Africa- A systematic review and meta-analysis of geographical variations and associations. Afr J Med Med Sci 49, 9–21 (2020). [Google Scholar]
- 18.Baiyewu O, Adeyemi JD & Ogunniyi A Psychiatric disorders in Nigerian nursing home residents. Int J Geriatr Psychiatry 12, 1146–1150, doi: (1997). [DOI] [PubMed] [Google Scholar]
- 19.Mavrodaris A, Powell J & Thorogood M Prevalences of dementia and cognitive impairment among older people in sub-Saharan Africa: a systematic review. Bull. World Health Organ. 91, 773–783, doi: 10.2471/BLT.13.118422 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gureje O, Ogunniyi A, Kola L & Afolabi E Functional disability in elderly Nigerians: Results from the Ibadan Study of Aging. J Am Geriatr Soc 54, 1784–1789, doi: 10.1111/j.1532-5415.2006.00944.x (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Jager CA, Msemburi W, Pepper K & Combrinck MI Dementia Prevalence in a Rural Region of South Africa: A Cross-Sectional Community Study. J. Alzheimers Dis. 60, 1087–1096, doi: 10.3233/JAD-170325 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hendrie HC et al. Prevalence of Alzheimer’s disease and dementia in two communities: Nigerian Africans and African Americans. Am J Psychiatry 152, 1485–1492, doi: 10.1176/ajp.152.10.1485 (1995). [DOI] [PubMed] [Google Scholar]
- 23.El Tallawy HN et al. Prevalence of dementia in Al Kharga District, New Valley Governorate, Egypt. NED 38, 130–137, doi: 10.1159/000335655 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Guerchet M et al. Cognitive impairment and dementia in elderly people living in rural Benin, west Africa. Dement Geriatr Cogn Disord 27, 34–41, doi: 10.1159/000188661 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Paraiso MN et al. Prevalence of dementia among elderly people living in Cotonou, an urban area of Benin (West Africa). NED 36, 245–251, doi: 10.1159/000328255 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Yusuf AJ, Baiyewu O, Sheikh TL & Shehu AU Prevalence of dementia and dementia subtypes among community-dwelling elderly people in northern Nigeria. International Psychogeriatrics 23, 379–386, doi: 10.1017/S1041610210001158 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Guerchet M et al. Prevalence of dementia in elderly living in two cities of Central Africa: the EDAC survey. Dement Geriatr Cogn Disord 30, 261–268, doi: 10.1159/000320247 (2010). [DOI] [PubMed] [Google Scholar]
- 28.Guerchet M et al. Epidemiology of dementia in Central Africa (EPIDEMCA): protocol for a multicentre population-based study in rural and urban areas of the Central African Republic and the Republic of Congo. SpringerPlus 3, 1044, doi: 10.1186/2193-1801-3-338 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Longdon AR et al. The prevalence of dementia in rural Tanzania: a cross-sectional community-based study. Int J Geriatr Psychiatry 28, 728–737, doi: 10.1002/gps.3880 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Mubangizi V, Maling S, Obua C & Tsai AC Prevalence and correlates of Alzheimer’s disease and related dementias in rural Uganda: cross-sectional, population-based study. BMC Geriatrics 20, 48, doi: 10.1186/s12877-020-1461-z (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tallawy HNAE et al. Epidemiology of Major Neurological Disorders Project in Al Kharga District, New Valley, Egypt. NED 35, 291–297, doi: 10.1159/000320240 (2010). [DOI] [PubMed] [Google Scholar]
- 32.El Tallawy HN et al. Prevalence of dementia in Al-Quseir city, Red Sea Governorate, Egypt. Clin Interv Aging 9, 9–14, doi: 10.2147/CIA.S48325 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khedr E et al. Prevalence of mild cognitive impairment and dementia among the elderly population of Qena Governorate, Upper Egypt: a community-based study. J. Alzheimers Dis. 45, 117–126, doi: 10.3233/JAD-142655 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Farrag A, Farwiz HM, Khedr EH, Mahfouz RM & Omran SM Prevalence of Alzheimer’s disease and other dementing disorders: Assiut-Upper Egypt study. Dement Geriatr Cogn Disord 9, 323–328, doi: 10.1159/000017084 (1998). [DOI] [PubMed] [Google Scholar]
- 35.Ojagbemi A, Bello T & Gureje O Cognitive Reserve, Incident Dementia, and Associated Mortality in the Ibadan Study of Ageing. Journal of the American Geriatrics Society 64, 590–595, doi: 10.1111/jgs.14015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paddick S-M et al. Mortality rates in community-dwelling Tanzanians with dementia and mild cognitive impairment: a 4-year follow-up study. Age and Ageing 44, 636–641, doi: 10.1093/ageing/afv048 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Kalaria RN et al. Alzheimer’s disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet neurology 7, 812–826, doi:S1474–4422(08)70169–8 [pii] 10.1016/S1474-4422(08)70169-8 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gureje O, Ogunniyi A, Kola L & Abiona T Incidence of and Risk Factors for Dementia in the Ibadan Study of Aging: INCIDENCE OF AND RISK FACTORS FOR DEMENTIA. Journal of the American Geriatrics Society 59, 869–874, doi: 10.1111/j.1532-5415.2011.03374.x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramlall S, Chipps J, Pillay BJ & Bhigjee AL Mild cognitive impairment and dementia in a heterogeneous elderly population: prevalence and risk profile. Afr J Psychiatry (Johannesbg) 16, doi: 10.4314/ajpsy.v16i6.58 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Sarfo FS, Akassi J, Adamu S, Obese V & Ovbiagele B Burden and Predictors of Post-Stroke Cognitive Impairment in a Sample of Ghanaian Stroke Survivors. J Stroke Cerebrovasc Dis 26, 2553–2562, doi: 10.1016/j.jstrokecerebrovasdis.2017.05.041 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akinyemi RO et al. Profile and determinants of vascular cognitive impairment in African stroke survivors: the CogFAST Nigeria Study. J Neurol Sci 346, 241–249, doi: 10.1016/j.jns.2014.08.042 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Ogunniyi A, Adebiyi AO, Adediran AB, Olakehinde OO & Siwoku AA Prevalence estimates of major neurocognitive disorders in a rural Nigerian community. Brain Behav 6, e00481, doi: 10.1002/brb3.481 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corder EH et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet 7, 180–184, doi: 10.1038/ng0694-180 (1994). [DOI] [PubMed] [Google Scholar]
- 44.Corder EH et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261, 921–923, doi: 10.1126/science.8346443 (1993). [DOI] [PubMed] [Google Scholar]
- 45.Osuntokun BO et al. Lack of an association between apolipoprotein E epsilon 4 and Alzheimer’s disease in elderly Nigerians. Ann Neurol. 38, 463–465, doi: 10.1002/ana.410380319 (1995). [DOI] [PubMed] [Google Scholar]
- 46.Gureje O et al. APOE ε4 is not associated with Alzheimer’s disease in elderly Nigerians. Ann Neurol. 59, 182–185, doi: 10.1002/ana.20694 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen C-H et al. A comparative study to screen dementia and APOE genotypes in an ageing East African population. Neurobiol. Aging 31, 732–740, doi: 10.1016/j.neurobiolaging.2008.06.014 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haithem H et al. Association between dementia and vascular disease-associated polymorphisms in a Tunisian population. Int. J. Neurosci. 128, 32–41, doi: 10.1080/00207454.2017.1348353 (2018). [DOI] [PubMed] [Google Scholar]
- 49.Hendrie HC et al. APOE ε4 and the risk for Alzheimer disease and cognitive decline in African Americans and Yoruba. International Psychogeriatrics 26, 977–985, doi: 10.1017/S1041610214000167 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akinyemi RO et al. Neurogenomics in Africa: Perspectives, progress, possibilities and priorities. Journal of the neurological sciences 366, 213–223, doi: 10.1016/j.jns.2016.05.006 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fluegge K Environmental factors influencing the link between APOE ε4 and Alzheimer’s disease (AD) risk. International Psychogeriatrics 31, 305–306, doi: 10.1017/S1041610218000984 (2019). [DOI] [PubMed] [Google Scholar]
- 52.Rajabli F et al. Ancestral origin of ApoE epsilon4 Alzheimer disease risk in Puerto Rican and African American populations. PLoS Genet 14, e1007791, doi: 10.1371/journal.pgen.1007791 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lane KA et al. Apolipoprotein E and mortality in African-Americans and Yoruba. J. Alzheimers Dis. 5, 383–390, doi: 10.3233/jad-2003-5505 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoare J et al. Relationship between apolipoprotein E4 genotype and white matter integrity in HIV-positive young adults in South Africa. Eur Arch Psychiatry Clin Neurosci 263, 189–195, doi: 10.1007/s00406-012-0341-8 (2013). [DOI] [PubMed] [Google Scholar]
- 55.El Kadmiri N et al. Novel mutations in the amyloid precursor protein gene within Moroccan patients with Alzheimer’s disease. J. Mol. Neurosci. 53, 189–195, doi: 10.1007/s12031-014-0278-7 (2014). [DOI] [PubMed] [Google Scholar]
- 56.El Kadmiri N et al. Novel presenilin mutations within Moroccan patients with Early-Onset Alzheimer’s Disease. Neuroscience 269, 215–222, doi: 10.1016/j.neuroscience.2014.03.052 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Heckmann JM et al. Novel presenilin 1 mutation with profound neurofibrillary pathology in an indigenous Southern African family with early-onset Alzheimer’s disease. Brain 127, 133–142, doi: 10.1093/brain/awh009 (2004). [DOI] [PubMed] [Google Scholar]
- 58.Momeni P et al. Genetic variability in CHMP2B and frontotemporal dementia. Neurodegener Dis 3, 129–133, doi: 10.1159/000094771 (2006). [DOI] [PubMed] [Google Scholar]
- 59.Landoulsi Z et al. Genetic Analysis of TREM2 Variants in Tunisian Patients with Alzheimer’s Disease. Med Princ Pract 27, 317–322, doi: 10.1159/000489779 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fekih-Mrissa N et al. The Plasminogen Activator Inhibitor 1 4G/5G Polymorphism and the Risk of Alzheimer’s Disease. Am J Alzheimers Dis Other Demen 32, 342–346, doi: 10.1177/1533317517705223 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ogunniyi A et al. Hypertension and incident dementia in community-dwelling elderly Yoruba Nigerians. Acta Neurol Scand 124, 396–402, doi: 10.1111/j.1600-0404.2011.01491.x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tianyi FL, Agbor VN, Njamnshi AK & Atashili J Factors Associated with the Prevalence of Cognitive Impairment in a Rural Elderly Cameroonian Population: A Community-Based Study in Sub-Saharan Africa. Dement Geriatr Cogn Disord 47, 104–113, doi: 10.1159/000496825 (2019). [DOI] [PubMed] [Google Scholar]
- 63.Paddick S-M et al. The prevalence of dementia subtypes in rural Tanzania. Am J Geriatr Psychiatry 22, 1613–1622, doi: 10.1016/j.jagp.2014.02.004 (2014). [DOI] [PubMed] [Google Scholar]
- 64.Hall K et al. Cholesterol, APOE genotype, and Alzheimer disease: an epidemiologic study of Nigerian Yoruba. Neurology 66, 223–227, doi: 10.1212/01.wnl.0000194507.39504.17 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guerchet M et al. Factors associated with dementia among elderly people living in two cities in Central Africa: the EDAC multicenter study. J Alzheimers Dis 29, 15–24, doi: 10.3233/JAD-2011-111364 (2012). [DOI] [PubMed] [Google Scholar]
- 66.Guerchet M et al. Association between a low ankle-brachial index and dementia in a general elderly population in Central Africa (Epidemiology of Dementia in Central Africa Study). Journal of the American Geriatrics Society 61, 1135–1140, doi: 10.1111/jgs.12310 (2013). [DOI] [PubMed] [Google Scholar]
- 67.Akinyemi RO, Mukaetova-Ladinska EB, Attems J, Ihara M & Kalaria RN Vascular risk factors and neurodegeneration in ageing related dementias: Alzheimer’s disease and vascular dementia. Current Alzheimer research 10, 642–653 (2013). [DOI] [PubMed] [Google Scholar]
- 68.Adebiyi AO, Ogunniyi A, Adediran BA, Olakehinde OO & Siwoku AA Cognitive Impairment Among the Aging Population in a Community in Southwest Nigeria. Health Educ Behav 43, 93S–99S, doi: 10.1177/1090198116635561 (2016). [DOI] [PubMed] [Google Scholar]
- 69.Mworozi K, Ameda F, Byanyima RK & Nakasujja N Carotid artery plaque detected on ultrasound is associated with impaired cognitive state in the elderly: A population-based study in Wakiso district, Uganda. J Clin Neurosci 68, 194–200, doi: 10.1016/j.jocn.2019.06.011 (2019). [DOI] [PubMed] [Google Scholar]
- 70.Ogunniyi A, Lekwauwa UG, Falope ZF & Osuntokun BO Clinically-diagnosed dementing illnesses in Ibadan: features, types and associated conditions. Afr J Med Med Sci 22, 61–64 (1993). [PubMed] [Google Scholar]
- 71.Amoo G et al. Profile of clinically-diagnosed dementias in a neuropsychiatric practice in Abeokuta, South-Western Nigeria. Afr J Psychiatry (Johannesbg) 14, 377–382, doi: 10.4314/ajpsy.v14i5.5 (2011). [DOI] [PubMed] [Google Scholar]
- 72.Yusuf AJ et al. Low education and lack of spousal relationship are associated with dementia in older adults with diabetes mellitus in Nigeria. Psychogeriatrics 18, 216–223, doi: 10.1111/psyg.12309 (2018). [DOI] [PubMed] [Google Scholar]
- 73.Hendrie HC et al. Homocysteine levels and dementia risk in Yoruba and African Americans. International Psychogeriatrics 25, 1859–1866, doi: 10.1017/S1041610213001294 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smach MA et al. Folate and Homocysteine in the Cerebrospinal Fluid of Patients with Alzheimer’s Disease or Dementia: A Case Control Study. Eur Neurol 65, 270–278, doi: 10.1159/000326301 (2011). [DOI] [PubMed] [Google Scholar]
- 75.Paddick S-M et al. The association between educational level and dementia in rural Tanzania. Dement Neuropsychol 8, 117–125, doi: 10.1590/S1980-57642014DN82000006 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stern Y What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc 8, 448–460 (2002). [PubMed] [Google Scholar]
- 77.Stern Y Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 11, 1006–1012, doi: 10.1016/S1474-4422(12)70191-6 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stern Y & Barulli D Cognitive reserve. Handb Clin Neurol 167, 181–190, doi: 10.1016/B978-0-12-804766-8.00011-X (2019). [DOI] [PubMed] [Google Scholar]
- 79.Ochayi B & Thacher TD Risk factors for dementia in central Nigeria. Aging Ment Health 10, 616–620, doi: 10.1080/13607860600736182 (2006). [DOI] [PubMed] [Google Scholar]
- 80.Ogunniyi A et al. Risk factors for incident Alzheimer’s disease in African Americans and Yoruba. Metab Brain Dis 21, 235–240, doi: 10.1007/s11011-006-9017-2 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gray WK et al. Community validation of the IDEA study cognitive screen in rural Tanzania. Int J Geriatr Psychiatry 31, 1199–1207, doi: 10.1002/gps.4415 (2016). [DOI] [PubMed] [Google Scholar]
- 82.Gray WK et al. Development and Validation of the Identification and Intervention for Dementia in Elderly Africans (IDEA) Study Dementia Screening Instrument. J Geriatr Psychiatry Neurol 27, 110–118, doi: 10.1177/0891988714522695 (2014). [DOI] [PubMed] [Google Scholar]
- 83.Paddick SM et al. Validation of the Identification and Intervention for Dementia in Elderly Africans (IDEA) cognitive screen in Nigeria and Tanzania. BMC Geriatr 15, 53, doi: 10.1186/s12877-015-0040-1 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ogunniyi A et al. Weight loss and incident dementia in elderly Yoruba Nigerians: a 10-year follow-up study. Int Psychogeriatr 23, 387–394, doi: 10.1017/S1041610210001390 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pilleron S et al. Diet, Alcohol Consumption and Cognitive Disorders in Central Africa: A Study from the EPIDEMCA Program. J Nutr Health Aging 19, 657–667, doi: 10.1007/s12603-015-0487-y (2015). [DOI] [PubMed] [Google Scholar]
- 86.Pilleron S et al. Association between mild cognitive impairment and dementia and undernutrition among elderly people in Central Africa: some results from the EPIDEMCA (Epidemiology of Dementia in Central Africa) programme. Br. J. Nutr. 114, 306–315, doi: 10.1017/S0007114515001749 (2015). [DOI] [PubMed] [Google Scholar]
- 87.Koyanagi A et al. Food Insecurity Is Associated with Mild Cognitive Impairment among Middle-Aged and Older Adults in South Africa: Findings from a Nationally Representative Survey. Nutrients 11, doi: 10.3390/nu11040749 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gureje O, Ogunniyi A & Kola L The profile and impact of probable dementia in a sub-Saharan African community: Results from the Ibadan Study of Aging. J Psychosom Res 61, 327–333, doi: 10.1016/j.jpsychores.2006.07.016 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hendrie HC Lessons learned from international comparative crosscultural studies on dementia. Am J Geriatr Psychiatry 14, 480–488, doi: 10.1097/01.JGP.0000192497.81296.fb (2006). [DOI] [PubMed] [Google Scholar]
- 90.Apt N Aging in Africa: Past Experiences and Strategic Directions. Ageing International 37, 93–103 (2012). [Google Scholar]
- 91.Ogunniyi A et al. Epidemiology of dementia in Nigeria: results from the Indianapolis-Ibadan study. Eur J Neurol 7, 485–490 (2000). [DOI] [PubMed] [Google Scholar]
- 92.Toure et al. Risk factors for dementia in a senegalese elderly population aged 65 years and over. Dement Geriatr Cogn Dis Extra 2, 160–168, doi: 10.1159/000332022 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Miller DB & O’Callaghan JP Do early-life insults contribute to the late-life development of Parkinson and Alzheimer diseases? Metabolism 57 Suppl 2, S44–49, doi: 10.1016/j.metabol.2008.07.011 (2008). [DOI] [PubMed] [Google Scholar]
- 94.Borenstein AR, Copenhaver CI & Mortimer JA Early-life risk factors for Alzheimer disease. Alzheimer Dis Assoc Disord 20, 63–72, doi: 10.1097/01.wad.0000201854.62116.d7 (2006). [DOI] [PubMed] [Google Scholar]
- 95.Pilleron S et al. Association between Stressful Life Events and Cognitive Disorders in Central Africa: Results from the EPIDEMCA Program. NED 44, 99–107, doi: 10.1159/000375462 (2015). [DOI] [PubMed] [Google Scholar]
- 96.Debalkie Animut M, Sorrie MB, Birhanu YW & Teshale MY High prevalence of neurocognitive disorders observed among adult people living with HIV/AIDS in Southern Ethiopia: A cross-sectional study. PLoS ONE 14, e0204636, doi: 10.1371/journal.pone.0204636 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jumare J et al. Peripheral blood lymphocyte HIV DNA levels correlate with HIV associated neurocognitive disorders in Nigeria. J. Neurovirol. 23, 474–482, doi: 10.1007/s13365-017-0520-5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nakku J, Kinyanda E & Hoskins S Prevalence and factors associated with probable HIV dementia in an African population: a cross-sectional study of an HIV/AIDS clinic population. BMC Psychiatry 13, 126, doi: 10.1186/1471-244X-13-126 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kanmogne GD et al. Effects of HIV infection, antiretroviral therapy, and immune status on the speed of information processing and complex motor functions in adult Cameroonians. Sci Rep 10, 14016, doi: 10.1038/s41598-020-70981-4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kanmogne GD et al. HIV-associated neurocognitive disorders in sub-Saharan Africa: a pilot study in Cameroon. BMC Neurol 10, 60, doi: 10.1186/1471-2377-10-60 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kelly CM et al. HIV associated neurocognitive disorders (HAND) in Malawian adults and effect on adherence to combination anti-retroviral therapy: a cross sectional study. PLoS ONE 9, e98962, doi: 10.1371/journal.pone.0098962 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Njamnshi AK et al. Risk factors for HIV-associated neurocognitive disorders (HAND) in sub-Saharan Africa: the case of Yaounde-Cameroon. J Neurol Sci 285, 149–153, doi: 10.1016/j.jns.2009.06.043 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eaton P et al. Risk factors for symptomatic HIV-associated neurocognitive disorder in adults aged 50 and over attending a HIV clinic in Tanzania. Int J Geriatr Psychiatry, doi: 10.1002/gps.5357 (2020). [DOI] [PubMed] [Google Scholar]
- 104.Benjamin LA Bryer AEHCKSSTCMD. Hiv infection and stroke: current perspectives and future directions. The Lancet Neurology 11, 878–890 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Teto G et al. Gag P2/NC and pol genetic diversity, polymorphism, and drug resistance mutations in HIV-1 CRF02_AG- and non-CRF02_AG-infected patients in Yaounde, Cameroon. Sci Rep 7, 14136, doi: 10.1038/s41598-017-14095-4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Acharya A, Fonsah JY, Mbanya D, Njamnshi AK & Kanmogne GD Near-Full-Length Genetic Characterization of a Novel HIV-1 Unique Recombinant with Similarities to A1, CRF01_AE, and CRFO2_AG Viruses in Yaounde, Cameroon. AIDS Res Hum Retroviruses 35, 762–768, doi: 10.1089/AID.2019.0042 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bouscaren N et al. Prevalence of toxoplasmosis and its association with dementia in older adults in Central Africa: a result from the EPIDEMCA programme. Trop. Med. Int. Health 23, 1304–1313, doi: 10.1111/tmi.13151 (2018). [DOI] [PubMed] [Google Scholar]
- 108.Njamnshi AK et al. Epilepsy-associated neurocognitive disorders (EAND) in an onchocerciasis-endemic rural community in Cameroon: A population-based case-control study. Epilepsy Behav 112, 107437, doi: 10.1016/j.yebeh.2020.107437 (2020). [DOI] [PubMed] [Google Scholar]
- 109.Boivin MJ et al. Early and middle childhood developmental, cognitive, and psychiatric outcomes of Malawian children affected by retinopathy positive cerebral malaria. Child Neuropsychol 25, 81–102, doi: 10.1080/09297049.2018.1451497 (2019). [DOI] [PubMed] [Google Scholar]
- 110.Brim R et al. Cognitive Outcomes and Psychiatric Symptoms of Retinopathy-Positive Cerebral Malaria: Cohort Description and Baseline Results. Am J Trop Med Hyg 97, 225–231, doi: 10.4269/ajtmh.17-0020 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Akinyemi RO et al. Stroke, cerebrovascular diseases and vascular cognitive impairment in Africa. Brain research bulletin, doi: 10.1016/j.brainresbull.2018.05.018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Akinyemi RO, Owolabi MO, Makanjuola VA, Ogunseyinde AO & Ogunniyi A Frontotemporal dementia in a Nigerian woman: case report and brief review of the literature. Afr J Med Med Sci 38, 71–75 (2009). [PubMed] [Google Scholar]
- 113.Ogunnyi A et al. Dementia With Lewy Bodies in a Nigerian: A Case Report. International psychogeriatrics / IPA 14, 211–218, doi: 10.1017/S1041610202008402 (2002). [DOI] [PubMed] [Google Scholar]
- 114.Adam AM & Akuku O Creutzfeldt-Jakob disease in Kenya. Trop. Med. Int. Health 10, 710–712, doi: 10.1111/j.1365-3156.2005.01435.x (2005). [DOI] [PubMed] [Google Scholar]
- 115.Bouhouche A et al. Clinical and genetic data of Huntington disease in Moroccan patients. Afr Health Sci 15, 1232–1238, doi: 10.4314/ahs.v15i4.23 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Baine FK, Krause A & Greenberg LJ The Frequency of Huntington Disease and Huntington Disease-Like 2 in the South African Population. NED 46, 198–202, doi: 10.1159/000444020 (2016). [DOI] [PubMed] [Google Scholar]
- 117.Jacob M et al. White Matter Integrity in Tanzanian Children With Sickle Cell Anemia: A Diffusion Tensor Imaging Study. Stroke 51, 1166–1173, doi: 10.1161/STROKEAHA.119.027097 (2020). [DOI] [PubMed] [Google Scholar]
- 118.Prussien KV et al. Associations of transcranial doppler velocity, age, and gender with cognitive function in children with sickle cell anemia in Nigeria. Child Neuropsychol 25, 705–720, doi: 10.1080/09297049.2018.1526272 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Oluwole OB, Noll RB, Winger DG, Akinyanju O & Novelli EM Cognitive functioning in children from Nigeria with sickle cell anemia. Pediatr Blood Cancer 63, 1990–1997, doi: 10.1002/pbc.26126 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ruffieux N et al. Association between biological markers of sickle cell disease and cognitive functioning amongst Cameroonian children. Child Neuropsychol 19, 143–160, doi: 10.1080/09297049.2011.640932 (2013). [DOI] [PubMed] [Google Scholar]
- 121.Rees DC, Williams TN & Gladwin MT Sickle-cell disease. Lancet 376, 2018–2031, doi: 10.1016/S0140-6736(10)61029-X (2010). [DOI] [PubMed] [Google Scholar]
- 122.Akinyemi RO et al. Brain banking in low and middle-income countries: Raison D’être for the Ibadan Brain Ageing, Dementia And Neurodegeneration (IBADAN) Brain Bank Project. Brain Res. Bull. 145, 136–141, doi: 10.1016/j.brainresbull.2018.08.014 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jellinger KA Neuropathological assessment of the Alzheimer spectrum. J Neural Transm (Vienna) 127, 1229–1256, doi: 10.1007/s00702-020-02232-9 (2020). [DOI] [PubMed] [Google Scholar]
- 124.Brooke J & Ojo O Contemporary views on dementia as witchcraft in sub-Saharan Africa: A systematic literature review. J Clin Nurs 29, 20–30, doi: 10.1111/jocn.15066 (2020). [DOI] [PubMed] [Google Scholar]
- 125.Spittel S, Maier A & Kraus E Awareness challenges of mental health disorder and dementia facing stigmatisation and discrimination: a systematic literature review from Sub-Sahara Africa. J Glob Health 9, 020419, doi: 10.7189/jogh.09.020419 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yusuf AJ & Baiyewu O Beliefs and attitudes towards dementia among community leaders in northern Nigeria. West Afr J Med 31, 8–13 (2012). [PubMed] [Google Scholar]
- 127.Hindley G et al. The role of traditional and faith healers in the treatment of dementia in Tanzania and the potential for collaboration with allopathic healthcare services. Age and Ageing 46, 130–137, doi: 10.1093/ageing/afw167 (2017). [DOI] [PubMed] [Google Scholar]
- 128.Agyeman N et al. “When someone becomes old then every part of the body too becomes old”: Experiences of living with dementia in Kintampo, rural Ghana. Transcult Psychiatry 56, 895–917, doi: 10.1177/1363461519847054 (2019). [DOI] [PubMed] [Google Scholar]
- 129.Mkhonto F & Hanssen I When people with dementia are perceived as witches. Consequences for patients and nurse education in South Africa. J Clin Nurs 27, e169–e176, doi: 10.1111/jocn.13909 (2018). [DOI] [PubMed] [Google Scholar]
- 130.Adebiyi AO, Fagbola MA, Olakehinde O & Ogunniyi A Enacted and implied stigma for dementia in a community in south-west Nigeria. Psychogeriatrics 16, 268–273, doi: 10.1111/psyg.12156 (2016). [DOI] [PubMed] [Google Scholar]
- 131.Ramaboa K & Fredericks I Muslims’ Affective and Cognitive Attitudes towards Formal Dementia Care in South Africa: Do They Vary according to Family Structure and the Experience of Familial Caregiving? Dement Geriatr Cogn Disord 48, 261–270, doi: 10.1159/000505833 (2019). [DOI] [PubMed] [Google Scholar]
- 132.Ramaboa K & Fredericks I Demographic Characteristics Associated with the Likelihood to Use Paid Home Care for People with Dementia among South African Muslims. Dement Geriatr Cogn Disord 48, 337–348, doi: 10.1159/000506511 (2019). [DOI] [PubMed] [Google Scholar]
- 133.Lewis EG et al. Risk Factors for Delirium in Older Medical Inpatients in Tanzania. Dement Geriatr Cogn Disord 44, 160–170, doi: 10.1159/000479058 (2017). [DOI] [PubMed] [Google Scholar]
- 134.Yaria JO et al. Delirium in Elderly Patients:Frequency and Precipitants in a Tertiary Hospital Setting. West Afr J Med 36, 183–188 (2019). [PubMed] [Google Scholar]
- 135.Kamoga R et al. Dementia assessment and diagnostic practices of healthcare workers in rural southwestern Uganda: a cross-sectional qualitative study. BMC Health Serv Res 19, 1005, doi: 10.1186/s12913-019-4850-2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kwon S, Iba M, Kim C & Masliah E Immunotherapies for Aging-Related Neurodegenerative Diseases-Emerging Perspectives and New Targets. Neurotherapeutics 17, 935–954, doi: 10.1007/s13311-020-00853-2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Plascencia-Villa G & Perry G Status and future directions of clinical trials in Alzheimer’s disease. Int Rev Neurobiol 154, 3–50, doi: 10.1016/bs.irn.2020.03.022 (2020). [DOI] [PubMed] [Google Scholar]
- 138.Weninger S et al. Active immunotherapy and alternative therapeutic modalities for Alzheimer’s disease. Alzheimers Dement (N Y) 6, e12090, doi: 10.1002/trc2.12090 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ali SK et al. In-vitro evaluation of selected Egyptian traditional herbal medicines for treatment of Alzheimer disease. BMC Complement Altern Med 13, 121, doi: 10.1186/1472-6882-13-121 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thakur A, Chun YS, October N, Yang HO & Maharaj V Potential of South African medicinal plants targeting the reduction of Abeta42 protein as a treatment of Alzheimer’s disease. J Ethnopharmacol 231, 363–373, doi: 10.1016/j.jep.2018.11.034 (2019). [DOI] [PubMed] [Google Scholar]
- 141.van Wyk A, Manthorpe J & Clark C The behaviours that dementia care home staff in South Africa find challenging: An exploratory study. Dementia (London) 16, 865–877, doi: 10.1177/1471301215622092 (2017). [DOI] [PubMed] [Google Scholar]
- 142.Salardini A An Overview of Primary Dementias as Clinicopathological Entities. Semin Neurol 39, 153–166, doi: 10.1055/s-0039-1683445 (2019). [DOI] [PubMed] [Google Scholar]
- 143.Paddick S-M et al. Cognitive stimulation therapy as a sustainable intervention for dementia in sub-Saharan Africa: feasibility and clinical efficacy using a stepped-wedge design. International Psychogeriatrics 29, 979–989, doi: 10.1017/S1041610217000163 (2017). [DOI] [PubMed] [Google Scholar]
- 144.Mkenda S et al. Cognitive stimulation therapy as a low-resource intervention for dementia in sub-Saharan Africa (CST-SSA): Adaptation for rural Tanzania and Nigeria. Dementia (London) 17, 515–530, doi: 10.1177/1471301216649272 (2018). [DOI] [PubMed] [Google Scholar]
- 145.Olakehinde O et al. Managing dementia in rural Nigeria: feasibility of cognitive stimulation therapy and exploration of clinical improvements. Aging Ment Health 23, 1377–1381, doi: 10.1080/13607863.2018.1484883 (2019). [DOI] [PubMed] [Google Scholar]
- 146.Hall KS et al. Community screening interview for dementia (CSI ‘D’); performance in five disparate study sites. International journal of geriatric psychiatry 15, 521–531 (2000). [DOI] [PubMed] [Google Scholar]
- 147.Hall KS, Hendrie HC & Brittain HM The development of a dementia screening interview in two distinct languages. International Journal of Methods in Psychiatric Research 3, 1–28 (1993). [Google Scholar]
- 148.Prince M et al. A brief dementia screener suitable for use by non-specialists in resource poor settings--the cross-cultural derivation and validation of the brief Community Screening Instrument for Dementia. Int J Geriatr Psychiatry 26, 899–907, doi: 10.1002/gps.2622 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chaaya M et al. Validation of the Arabic Rowland Universal Dementia Assessment Scale (A-RUDAS) in elderly with mild and moderate dementia. Aging Ment Health 20, 880–887, doi: 10.1080/13607863.2015.1043620 (2016). [DOI] [PubMed] [Google Scholar]
- 150.Baiyewu O et al. The Stick Design test: a new measure of visuoconstructional ability. J Int Neuropsychol Soc 11, 598–605, doi:S135561770505071X [pii] 10.1017/S135561770505071X (2005). [DOI] [PubMed] [Google Scholar]
- 151.Njamnshi AK et al. The International HIV Dementia Scale is a useful screening tool for HIV-associated dementia/cognitive impairment in HIV-infected adults in Yaounde-Cameroon. J Acquir Immune Defic Syndr 49, 393–397, doi: 10.1097/qai.0b013e318183a9df (2008). [DOI] [PubMed] [Google Scholar]
- 152.Joska JA et al. Validity of the International HIV Dementia Scale in South Africa. AIDS Patient Care STDS 25, 95–101, doi: 10.1089/apc.2010.0292 (2011). [DOI] [PubMed] [Google Scholar]
- 153.Ruffieux N et al. Neuropsychology in Cameroon: first normative data for cognitive tests among school-aged children. Child Neuropsychol 16, 1–19, doi: 10.1080/09297040902802932 (2010). [DOI] [PubMed] [Google Scholar]
- 154.Hendrie HC et al. The development of a semi-structured home interview (CHIF) to directly assess function in cognitively impaired elderly people in two cultures. International Psychogeriatrics 18, 653–666, doi: 10.1017/S104161020500308X (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Collingwood C et al. Development and community-based validation of the IDEA study Instrumental Activities of Daily Living (IDEA-IADL) questionnaire. Glob Health Action 7, 25988, doi: 10.3402/gha.v7.25988 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stone L et al. Screening for Instrumental Activities of Daily Living in Sub-Saharan Africa: A Balance Between Task Shifting, Simplicity, Brevity, and Training. J Geriatr Psychiatry Neurol 31, 248–255, doi: 10.1177/0891988718790400 (2018). [DOI] [PubMed] [Google Scholar]
- 157.Paddick SM et al. Adaptation and validation of the Alzheimer’s Disease Assessment Scale - Cognitive (ADAS-Cog) in a low-literacy setting in sub-Saharan Africa. Acta Neuropsychiatr 29, 244–251, doi: 10.1017/neu.2016.65 (2017). [DOI] [PubMed] [Google Scholar]
- 158.Paddick SM et al. Effectiveness of App-Based Cognitive Screening for Dementia by Lay Health Workers in Low Resource Settings. A Validation and Feasibility Study in Rural Tanzania. J Geriatr Psychiatry Neurol, 891988720957105, doi: 10.1177/0891988720957105 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Edjolo A et al. Development of the Central Africa Daily Functioning Interference Scale for Dementia Diagnosis in Older Adults: The EPIDEMCA Study. Dement Geriatr Cogn Disord 47, 29–41, doi: 10.1159/000492782 (2019). [DOI] [PubMed] [Google Scholar]
- 160.Dotchin CL, Akinyemi RO, Gray WK & Walker RW Geriatric medicine: services and training in Africa. Age and ageing 42, 124–128, doi: 10.1093/ageing/afs119 (2013). [DOI] [PubMed] [Google Scholar]
- 161.Heward J et al. A longitudinal study of cognitive decline in rural Tanzania: rates and potentially modifiable risk factors. Int Psychogeriatr 30, 1333–1343, doi: 10.1017/S1041610217002861 (2018). [DOI] [PubMed] [Google Scholar]
- 162.Yoseph M et al. Prevalence estimates of dementia in older adults in rural Kilimanjaro 2009–2010 and 2018–2019: Is there evidence of changing prevalence? Int J Geriatr Psychiatry, doi: 10.1002/gps.5498 (2021). [DOI] [PubMed] [Google Scholar]
- 163.Nelson PT et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain 142, 1503–1527, doi: 10.1093/brain/awz099 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jendroska K et al. Incidental Lewy body disease in black Africans. Lancet 344, 882–883, doi: 10.1016/s0140-6736(94)92854-1 (1994). [DOI] [PubMed] [Google Scholar]
- 165.Akinyemi RO Epidemiology of Parkinsonism and Parkinson’s disease in Sub-Saharan Africa: Nigerian profile. Journal of neurosciences in rural practice 3, 233–234, doi: 10.4103/0976-3147.102586 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Osuntokun BO, Ogunniyi A, Junaid TA & Lekwauwa UG Autopsy survey for Alzheimer’s disease in Nigerian Africans: a preliminary report. Afr J Med Med Sci 24, 75–79 (1995). [PubMed] [Google Scholar]
- 167.Ogunniyi AO, Osuntokun BO, Lekwauwa UB & Falope ZF Rarity of dementia (by DSM-III-R) in an urban community in Nigeria. East Afr Med J 69, 64–68 (1992). [PubMed] [Google Scholar]
- 168.Ogeng’o JA et al. Cerebral amyloid beta protein deposits and other Alzheimer lesions in non-demented elderly east Africans. Brain Pathol 6, 101–107, doi: 10.1111/j.1750-3639.1996.tb00790.x (1996). [DOI] [PubMed] [Google Scholar]
- 169.Barnes LL et al. Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology 85, 528–534, doi: 10.1212/WNL.0000000000001834 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schlesinger D et al. African ancestry protects against Alzheimer’s disease-related neuropathology. Mol Psychiatry 18, 79–85, doi: 10.1038/mp.2011.136 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sirugo G, Williams SM & Tishkoff SA The Missing Diversity in Human Genetic Studies. Cell 177, 1080, doi: 10.1016/j.cell.2019.04.032 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Choudhury A et al. High-depth African genomes inform human migration and health. Nature 586, 741–748, doi: 10.1038/s41586-020-2859-7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Andrews SJ, Fulton-Howard B & Goate A Interpretation of risk loci from genome-wide association studies of Alzheimer’s disease. Lancet Neurol 19, 326–335, doi: 10.1016/S1474-4422(19)30435-1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jack CR et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14, 535–562, doi: 10.1016/j.jalz.2018.02.018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jack CR et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 12, 207–216, doi: 10.1016/S1474-4422(12)70291-0 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dzau V, Fuster V, Frazer J & Snair M Investing in Global Health for Our Future. The New England journal of medicine 377, 1292–1296, doi: 10.1056/NEJMsr1707974 (2017). [DOI] [PubMed] [Google Scholar]
- 177.Karikari TK et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol 19, 422–433, doi: 10.1016/S1474-4422(20)30071-5 (2020). [DOI] [PubMed] [Google Scholar]
- 178.Suarez-Calvet M et al. Novel tau biomarkers phosphorylated at T181, T217 or T231 rise in the initial stages of the preclinical Alzheimer’s continuum when only subtle changes in Abeta pathology are detected. EMBO Mol Med 12, e12921, doi: 10.15252/emmm.202012921 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chaudhry A & Rizig M Comparing fluid biomarkers of Alzheimer’s disease between African American or Black African and white groups: A systematic review and meta-analysis. J Neurol Sci, 117270, doi: 10.1016/j.jns.2020.117270 (2020). [DOI] [PubMed] [Google Scholar]
- 180.Villemagne VL et al. Molecular Imaging Approaches in Dementia. Radiology, 200028, doi: 10.1148/radiol.2020200028 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Akinyemi RO et al. Medial temporal lobe atrophy, white matter hyperintensities and cognitive impairment among Nigerian African stroke survivors. BMC Res Notes 8, 625, doi: 10.1186/s13104-015-1552-7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Andronikou S et al. Correlating brain volume and callosal thickness with clinical and laboratory indicators of disease severity in children with HIV-related brain disease. Childs Nerv Syst 30, 1549–1557, doi: 10.1007/s00381-014-2434-3 (2014). [DOI] [PubMed] [Google Scholar]
- 183.Abassi M et al. Cerebrospinal fluid biomarkers and HIV-associated neurocognitive disorders in HIV-infected individuals in Rakai, Uganda. J. Neurovirol. 23, 369–375, doi: 10.1007/s13365-016-0505-9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Vancampfort D et al. Associations between handgrip strength and mild cognitive impairment in middle-aged and older adults in six low- and middle-income countries. Int J Geriatr Psychiatry 34, 609–616, doi: 10.1002/gps.5061 (2019). [DOI] [PubMed] [Google Scholar]
- 185.Badhwar A et al. A multiomics approach to heterogeneity in Alzheimer’s disease: focused review and roadmap. Brain 143, 1315–1331, doi: 10.1093/brain/awz384 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nativio R et al. An integrated multi-omics approach identifies epigenetic alterations associated with Alzheimer’s disease. Nat Genet 52, 1024–1035, doi: 10.1038/s41588-020-0696-0 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Collins FS & Varmus H A new initiative on precision medicine. N Engl J Med 372, 793–795, doi: 10.1056/NEJMp1500523 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Coote JH & Joyner MJ Is precision medicine the route to a healthy world? Lancet 385, 1617, doi: 10.1016/s0140-6736(15)60786-3 (2015). [DOI] [PubMed] [Google Scholar]
- 189.Norton A, Mphahlele J, Yazdanpanah Y, Piot P & Bayona MT Strengthening the global effort on COVID-19 research. Lancet 396, 375, doi: 10.1016/S0140-6736(20)31598-1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mok VCT et al. Tackling challenges in care of Alzheimer’s disease and other dementias amid the COVID-19 pandemic, now and in the future. Alzheimers Dement 16, 1571–1581, doi: 10.1002/alz.12143 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Baiyewu O, Elugbadebo O & Oshodi Y Burden of COVID-19 on mental health of older adults in a fragile healthcare system: the case of Nigeria: dealing with inequalities and inadequacies. Int Psychogeriatr 32, 1181–1185, doi: 10.1017/S1041610220001726 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gyasi RM COVID-19 and mental health of older Africans: an urgency for public health policy and response strategy. Int Psychogeriatr 32, 1187–1192, doi: 10.1017/S1041610220003312 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gyasi RM Fighting COVID-19: Fear and Internal Conflict among Older Adults in Ghana. J Gerontol Soc Work 63, 688–690, doi: 10.1080/01634372.2020.1766630 (2020). [DOI] [PubMed] [Google Scholar]
- 194.Livingston G et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446, doi: 10.1016/S0140-6736(20)30367-6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Adebusoye LA et al. Caring for older adults during the COVID pandemic and beyond: experience from a specialized tertiary facility for the care of older persons in a low resource setting. Pan Africa Medical Journal 35, 99 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary File 1: Organisations on Dementia Care and Support in Africa: