Abstract
Background:
Posttraumatic stress disorder (PTSD) is associated with autonomic dysfunction as indicated by deficits in the sympathetic and parasympathetic nervous systems. These abnormalities are expressed as elevated heart rate and reduced heart rate variability (HRV), respectively. Intermittent theta-burst stimulation (iTBS), a form of transcranial magnetic stimulation, has demonstrated effectiveness in PTSD. Nevertheless, it remains unclear whether HRV may be an iTBS biomarker for PTSD and whether iTBS impacts autonomic activity.
Materials and Methods:
Fifty Veterans with PTSD participated in a randomized controlled trial, receiving 10 daily sessions of sham-controlled iTBS (right dorsolateral prefrontal cortex, 1,800 pulses/day, 80% active motor threshold, 9.5min). With a usable dataset of (n=47), HRV parameters were assessed as predictors of clinical response immediately after stimulation. iTBS effects on autonomic response (mean RR interval, RMSSD, total power, and LF/HF ratio) were evaluated using an ultra-short approach.
Results:
Total power and RMSSD were significant predictors of acute clinical response to iTBS. Individuals with higher total power had better response to iTBS with improved symptoms on the Clinician Administered PTSD Scale (rs=−0.58,p=0.004), and higher functionality on the Social and Occupational Function Scale (rs=0.43,p=0.04). Similarly, higher RMSSD was associated with superior outcomes (rs=−0.44,p=0.04). No other significant changes in HRV metrics were observed (p≥.05).
Conclusions:
Our findings indicate that autonomic activity is a potential low-cost and technically simple predictive biomarker of iTBS response in PTSD. Less autonomic dysfunction was associated with superior clinical improvements with iTBS. Future studies might consider HRV acquisition during iTBS, as well as prospective testing of these findings in patients with elevated hyperarousal.
Keywords: Posttraumatic Stress Disorder, Autonomic Nervous System, Theta Burst Stimulation, Heart Rate Variability, Biomarker
Introduction
Post-traumatic Stress Disorder (PTSD) is a chronic psychiatric illness experienced by approximately 8% of individuals exposed to trauma 1. PTSD is characterized by avoidance of trauma-related stimuli, intrusive symptoms, negative changes in mood and cognition, and hyperarousal, which result in remarkable distress and functional impairment 2–4. Notably, individuals with PTSD demonstrate exaggerated fear responses (e.g., heightened startle) and impaired fear inhibition (e.g., hypervigilance), reflecting disruption of the sympathetic and parasympathetic nervous systems, respectively. These autonomic nervous system (ANS) abnormalities are generally expressed as elevated heart rate (HR) or blood pressure (sympathetic) and reduced heart rate variability (HRV; parasympathetic). Given that dropout rates remain high for PTSD treatments 5, there is a critical need to establish biomarkers of treatment response, and ANS indices are strong candidates given their established role in the disorder.
HRV is a neurocardiac parameter based on time intervals of successive heartbeats, more specifically R waves, which is indicative of the ANS response to physical and/or psychological stressors6–9. There are several indices of HRV, including time domain measures such as the root mean square of successive differences between normal-to-normal R intervals (RMSSD) and the standard deviation of normal-to-normal R intervals (SDNN), and frequency measures such as high-frequency (0.15–0.40 Hz; HF), low-frequency (0.01–0.15 Hz; LF), and the low/high frequency ratio (LF/HF). High frequency or HF-HRV is thought to reflect parasympathetic nervous system activity, or specifically cardiac vagal control over HR. HRV at rest is a common indicator of emotion regulation capacity and overall psychological health, with higher levels of resting HF-HRV being associated with better outcomes10. Numerous studies have demonstrated that individuals with PTSD exhibit lower HF-HRV at rest and in response to stressful stimuli compared to healthy and trauma-exposed controls11–14. Similar findings were observed in a recent systematic review and meta-analysis that included 19 studies assessing HRV parameters in individuals with PTSD compared to healthy controls15; this study found that PTSD was associated with reduced HF-HRV, as well as RMSSD (a time domain measure of overall ANS activity). A study by Hopper and colleagues (2006) indicated that HR was only elevated among individuals with PTSD and low HF-HRV, suggesting that HF-HRV may be a more specific marker of ANS dysfunction than HR alone12.
The prefrontal cortex exerts top-down control over brain regions involved in the regulation of the ANS, such as the amygdala 16. Stimulation of prefrontal cortical areas might therefore result in modulation of ANS, as shown by prior trials 17–19 20. Theta-burst stimulation (TBS), a newer form of repetitive transcranial magnetic stimulation (rTMS) 21,22, is a promising tool to modulate the ANS 17–19. In particular, intermittent TBS (iTBS) has been linked to enhanced cortical excitability by facilitating synaptic connections presumably due to long-term potentiation-like (LTP) effects21. Among the available neuromodulation modalities, iTBS stands out for its efficacy and safety profile, as well as the advantage of being able to be delivered in a short period of time. In this context, iTBS has been investigated as a potential therapeutic alternative to traditional psychological and pharmacological therapies. In a double-blind, sham-controlled clinical trial, Iseger et al. evaluated the effects of iTBS on ANS parameters, including multiple HRV measures, upon stimulation of the dorsolateral prefrontal cortex (DLPFC) in 15 patients with Major Depressive Disorder (MDD). The authors detected significant improvements in multiple HRV indices during active iTBS application (LF-HRV, HF-HRV, SDNN, and RMSSD), suggesting that it may have enhanced parasympathetic activity by transsynaptic activation. 17 Prefrontal iTBS in a PTSD population has been shown to have therapeutic benefits, with stimulation enhancing social/occupational function and improving depressive and PTSD symptoms 23. However, it is not yet clear whether these benefits are accompanied by HRV changes, and if so, whether HRV features might work as potential biomarkers to predict response to iTBS in PTSD.
Given that impaired baseline/resting HRV measures have frequently been implicated in PTSD14, 15, the current study used a sub-sample from Philip et al. to examine HRV in the context of iTBS for PTSD 23. The goals of this study were to: (a) describe baseline HRV frequency and time-domain parameters in Veterans with PTSD; (b) evaluate HRV parameters as potential predictors of clinical response; and (c) assess the effects of active iTBS, compared to sham, on ANS response (ultra-short-term HRV features) in PTSD.
Materials and Methods
Trial design
The parent modified parallel-group double-blind sham-controlled trial was performed at the Veterans Affairs Medical Center (VAMC) in Providence, Rhode Island, from May 2016 to December 2017. Methods were previously reported by Philip et al., please refer to the original study for a more detailed description of the methods (ClinicalTrials.gov NCT02769312) 23.
Participants
Fifty individuals with PTSD participated in the parent trial based on the following inclusion criteria: (a) diagnosis of chronic PTSD based on DSM-5 criteria; (b) between ages 18 and 70 years; (c) failure of at least one evidence-based treatment for PTSD (defined as failure to achieve clinically significant reduction in symptoms with adequate trial(s) of pharmacotherapy and/or psychotherapy); (d) remained clinically symptomatic despite ongoing treatment for at least 6 weeks prior to the study procedures; and (e) being capable of understanding and providing informed consent. Participants who had any primary psychotic disorder, bipolar I disorder, ongoing substance use disorder (moderate/severe), or active suicidality were excluded from the study. Other exclusions criteria were non-MRI safe cardiac pacemaker, implanted device or metallic implant at the upper thoracic spine or higher, in addition to TMS-specific exclusions such as pregnancy risk, history of moderate or severe traumatic brain injury, active unstable medical conditions, severe neurological disorders/impairment, CNS tumors, seizures, or cerebrovascular disease.
Setting
Recruitment began in May 2016 utilizing a combined approach including a broad-based strategy, by placing advertisements to Veterans in the community, and a targeted plan by contacting professionals from the Providence VAMC Mental Health/PTSD services, and VA community-based outpatient clinics. Furthermore, the VA Computerized Patient Record System (CPRS) was also reviewed as an additional recruitment tool. One hundred sixteen potential participants were prescreened by phone interview, and 56 eligible subjects were screened during an on-site visit at the laboratory. In the end, a naturalistic sample of 50 Veterans with chronic PTSD was included in the parent trial. For this secondary analysis, individuals who concluded the double-blind iTBS period (10 sessions) and had at least one ECG recording were included.
Randomization and Blinding
Subjects were randomly allocated to undergo active iTBS vs. sham stimulation on a 1:1 basis, stratified by sex and PTSD symptom severity. An external investigator, who had no knowledge of other aspects of the trial, performed the randomization procedure.
For reliable blinding, neither subjects nor raters had any information as to whether active or sham stimulation had been applied. Given that iTBS requires use of different coils for active and sham interventions, a research assistant was invited exclusively for designating the coils. To assess the blinding effectiveness, at the end of the 10th session, subjects were asked to guess whether they had been assigned to the active or sham group.
Ethics Statement
The Providence VA Institutional Review Board approved the study protocol. In accordance with the ethical principles for medical research involving human beings of the Declaration of Helsinki, all participants were provided with detailed verbal and written information about the study and signed written consent 24.
Interventions
Participants received daily session of sham-controlled iTBS for 2 consecutive weeks (10 business days), delivered to the right dorsolateral prefrontal cortex (DLPFC), 1,800 pulses/day at an intensity of 80% of the active motor threshold (AMT), for 9.5 minutes, using a Magstim Rapid 2+1 system (Magstim, Whitland, U.K.). Right DLPFC was adopted as the stimulation target given prior evidence of successful clinical outcomes in PTSD studies 25,26, in addition to decreased amygdala activation to trauma-related stimuli 16, Utilizing scalp measures, the coil was placed over the F4 electrode location, based on the 10–20 EEG International System, as it corresponds to the right DLPFC area. The targeted location was rechecked in each session to secure reliable and precise placements. At the end of the double-blind period, all subjects were offered the possibility of undergoing 10 unblinded active iTBS sessions, aiming to assess the cumulative effects of a greater number of iTBS sessions. The parent study flow diagram based on CONSORT may be found at Philip et al. 23, and a schematic diagram outlining the study procedures that are relevant for this secondary analysis is illustrated in Figure 1.
Figure 1.
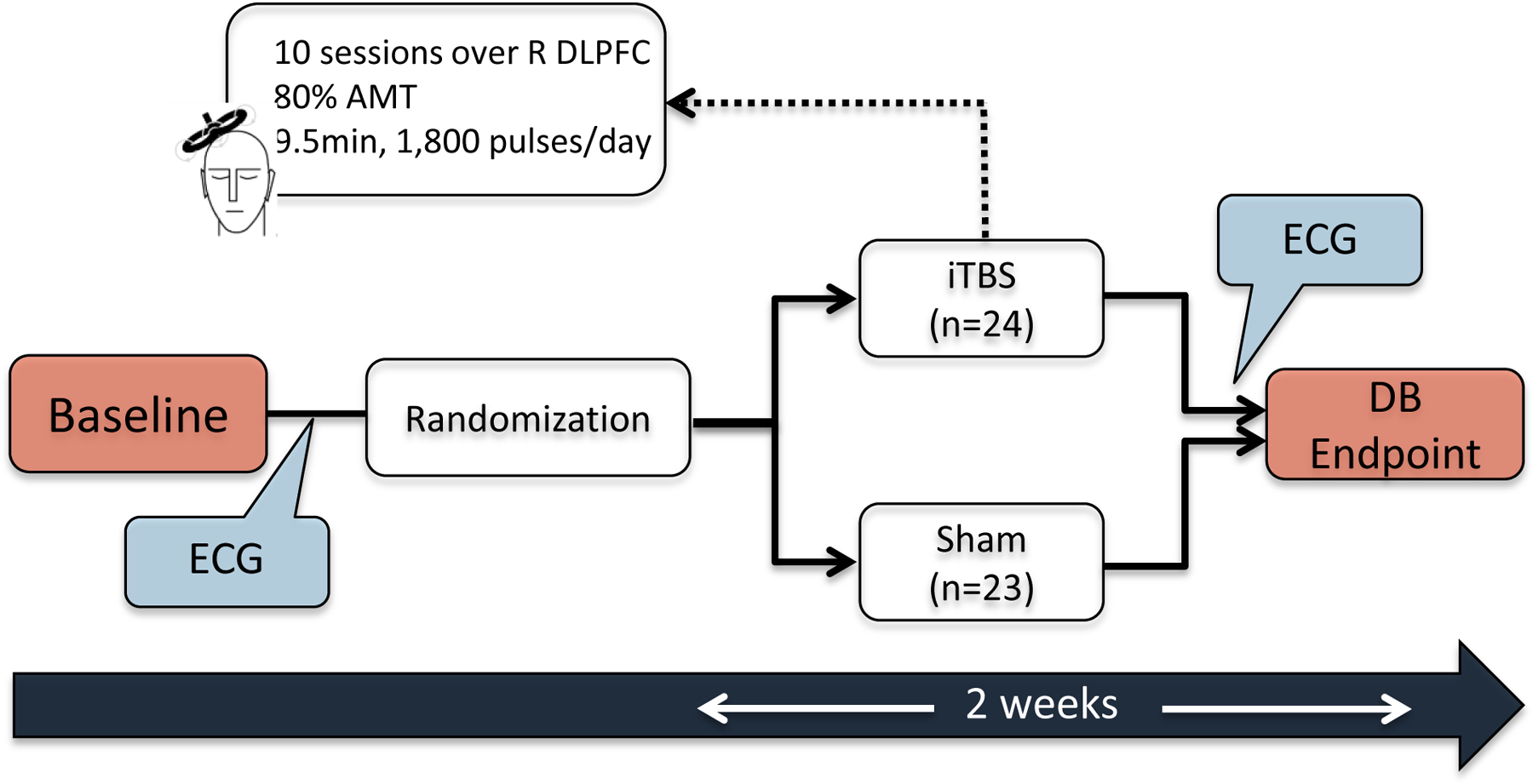
Study procedures diagram
Safety
Safety assessment was performed at the end of each stimulation session by documenting spontaneous reported adverse events, in addition to the active query of possible iTBS side effects such as seizure, headache, and dizziness.
HRV data acquisition and analysis
Preceding the first stimulation session and following the last intervention of the double-blind phase, subjects underwent resting electrocardiogram (ECG) recording, for at least 5 minutes, while sitting in a comfortable chair (the position was standardized for all participants). Two electrodes were applied on the patient’s right upper and left lower chest, using Biopac ECG100C amplifier with MP150 data acquisition and AcqKnowledge 4.1 software (Biopac Systems Inc., Goleta, CA, USA). HRV analysis was conducted separately utilizing Kubios software (version 3.4.1, HRV Premium, Kuopio, Finland)27. An automatic QRS detection (manually reviewed by two staff members), and automated algorithms for RR interval artifacts correction, addressing missing, extra and/or ectopic beats, were performed.
An ultra-short-term HRV approach was used, carrying out the analysis on ECG excerpts of less than 5 minutes (periods of 30s, 45s, 60s, and 195s). Ultra-short-term HRV features have been previously shown to be reliable surrogates of short HRV features (5 min recordings)28,29, including in a study addressing HRV parameters on psychological stress 29.
For this secondary analysis, our HRV outcomes were RMSSD, very-low-frequency (VLF - 0.0033–0.04 Hz), low-frequency (LF - 0.04–0.15 Hz), and high-frequency (HF - 0.15–0.4 Hz) bands (all expressed in absolute signal and presented in ms2), as well as the total power (TP), low frequency/high frequency power ratio (LF/HF), and parasympathetic nervous system (PNS) and sympathetic nervous system (SNS) indexes 6,30. These PNS and SNS indexes were generated by Kubios software through algorithms based on: the mean RR interval, RMSSD, and Poincaré plot index SD1 (in normalized units); and mean RR, Baevsky’s stress index, and Poincaré plot index SD2 (in normalized units), respectively. Although the longer recording epoch (195s) better represents autonomic activity, measures of both HRV domains were calculated for all epochs based in prior findings of more significant heart rate changes at the beginning of the ECG recording17. The mean RR interval, mean HR, as well as their respective standard deviations, were determined for each ultra-short-term period.
Statistical analysis
The Shapiro–Wilk test was applied to assess the normality of data. Continuous variables were described as means and standard deviations (SD), while categorical data were summarized as a percentage. A non-parametric two-sample Wilcoxon rank-sum (Mann–Whitney) test was used to compare HRV outcomes between the active iTBS and sham groups, while the Wilcoxon matched-pairs signed rank test was applied for within-group comparisons. Additionally, Spearman’s correlation was performed to assess the relationship between baseline HRV parameters and clinical measures following iTBS. The clinical outcomes included: (a) changes in PTSD symptoms measured by the Clinician Administered PTSD Scale for DSM-5 (CAPS)31, in addition to (b) the Social and Occupational Function Scale (SOFAS)32, and (c) the Inventory of Depressive Symptomatology Self-Report (IDSSR)33, all considering outcomes obtained immediately after the last iTBS. No imputation method was utilized for missing HRV data. Regarding clinical outcomes, missing data was handled by applying multiple imputations (n=20 imputations; for further information, please refer to Philip et al.) 23. Statistical analysis was performed using Stata statistical software program, version 15.0 (StataCorp LP, College Station, TX, USA). Statistical significance was determined at 5% and all p-values were two-sided.
Results
Baseline demographic and clinical data are shown in Table 1; no statistically significant differences were found between the active iTBS and sham groups. From the 50 individuals included in the original trial, three were not able to be included in this secondary analysis as the ECG recordings had significant artifact that precluded HRV analysis, leaving 47 participants. All patients had ongoing treatment (pharmacotherapy and/or psychotherapy); and were allowed to continue without changes.
Table 1.
Demographic and clinical features at baseline
| iTBS (n=24) | Sham (n=23) | |||
|---|---|---|---|---|
| Age (years) † | 49.04 (12.84) | 52.13 (11.53) | ||
|
| ||||
| n | % | n | % | |
|
| ||||
| Female sex (%) | 5 | 20.83 | 3 | 13.04 |
| Race (%) ‡ | ||||
| African American | 0 | 0 | 1 | 4.35 |
| American Indian/Alaska Native | 1 | 4.17 | 0 | 0 |
| Multiracial | 2 | 8.33 | 1 | 4.35 |
| White | 21 | 87.50 | 19 | 82.61 |
| Ethnicity ‡ | ||||
| Not of Hispanic origin | 22 | 91.67 | 21 | 91.3 |
| Hispanic origin | 0 | 0 | 2 | 8.70 |
| Education ‡ | ||||
| Less than high school | 1 | 4.17 | 1 | 4.35 |
| High school or equivalent | 2 | 8.33 | 4 | 17.39 |
| Some college | 10 | 41.67 | 7 | 30.43 |
| Trade or vocational degree | 3 | 12.50 | 0 | 0 |
| Bachelor’s degree | 2 | 8.33 | 7 | 30.43 |
| Advanced degree and/or education beyond college | 2 | 8.33 | 3 | 13.05 |
| Employment Status ‡ | ||||
| Full time | 6 | 25.00 | 4 | 17.39 |
| Part time | 0 | 0 | 2 | 8.70 |
| Unemployed | 10 | 41.67 | 9 | 39.13 |
| Retired | 6 | 25.00 | 7 | 30.43 |
| Service connected disability (mental health) | 14 | 58.34 | 15 | 65.21 |
| Military History Branch ‡ |
||||
| Army | 7 | 29.17 | 7 | 30.43 |
| Navy | 8 | 33.33 | 5 | 21.74 |
| Marines | 2 | 8.33 | 1 | 4.35 |
| Air Force | 0 | 0 | 3 | 13.04 |
|
| ||||
| Clinical Variables § | ||||
|
| ||||
| PTSD Symptom Severity | ||||
| CAPS-5 Score | 48.34 (9.97) | 46.96 (10.89) | ||
| PCL-5 Score | 49.46 (9.55) | 49.22 (11.28) | ||
| Social/Occupational Function & Quality of Life | ||||
| SOFAS Score | 44.50 (13.39) | 44.74 (15.57) | ||
| Depressive Symptom Severity | ||||
| IDSSR Score | 42.58 (12.10) | 38.83 (11.95) | ||
Age presented as mean ± standard deviation (SD);
Totals do not sum up to 100% due to participants non-response;
Clinical variables described as mean ± SD; CAPS-5, Clinician Administered PTSD Scale for DSM5; IDSSR, Inventory of Depressive Symptomatology, Self-Report; iTBS, Intermittent Theta-burst Stimulation; PCL-5, PTSD Checklist for DSM-5; PTSD, Posttraumatic Stress Disorder; SD, Standard deviation; SOFAS, Social and Occupational Function Scale.
Baseline HRV parameters of the Veterans with PTSD included in this analysis are shown in Table 2. These values correspond to parasympathetic nervous system (PNS) and sympathetic nervous system (SNS) indexes, in addition to frequency and time-domain features, all obtained for the longest epoch available (195s), as longer ECG segments generate more reliable parameters of autonomic response 6,17.
Table 2.
HRV outcomes at baseline and at the end of double-blind period, comparing within and between active iTBS vs. sham in PTSD (195 seconds epoch)
| HRV Variables | Baseline† (mean/SD) | Pre (mean/SD) | Post (mean/SD) | Within-groups (p) ‡ | Between-groups § (p) | |||
|---|---|---|---|---|---|---|---|---|
| iTBS | Sham | iTBS | Sham | iTBS | Sham | |||
| PNS index | −.96 (1.16) | −.94 (1.36) | −.99 (.90) | −.98 (1.00) | −.92 (.89) | 0.43 | 0.26 | 0.60 |
| SNS index | 2.47 (2.41) | 2.70 (1.92) | 2.20 (2.93) | 2.12 (1.95) | 2.18 (2.86) | 0.08 | 0.88 | 0.82 |
| Time-domain | ||||||||
| Mean RR (ms) | 827.37 (122.99) | 812.13 (109.93) | 845.83 (137.94) | 830.09(132.13) | 868.28 (136.56) | 0.25 | 0.15 | 0.30 |
| Mean HR (bpm) | 74.18 (11.71) | 75.16 (10.02) | 72.99 (13.67) | 74.01 (11.52) | 71.00 (13.14) | 0.17 | 0.22 | 0.30 |
| RMSSD (ms) | 22.52 (28.41) | 25.04 (37.28) | 19.46 (11.05) | 21.30 (16.80) | 17.70 (11.45) | 0.50 | 0.57 | 0.64 |
| Stress Index | 21.62 (11.48) | 22.90 (10.27) | 20.07 (12.91) | 19.58 (9.26) | 20.88 (12.71) | 0.07 | 0.57 | 0.52 |
| Frequency-domain | ||||||||
| VLF (ms2) | 38.90 (48.49) | 21.12 (18.16) | 60.42 (63.72) | 35.27 (33.69) | 35.09 (27.98) | 0.04* | 0.16 | 0.99 |
| LF (ms2) | 378.17 (997.19) | 478.20 (1342.56) | 257.08 (183.58) | 403.33 (879.97) | 319.99 (478.06) | 0.85 | 0.54 | 0.82 |
| HF (ms2) | 312.38 (933.09) | 422.66 (1252.52) | 178.88 (177.10) | 186.34 (194.10) | 134.90 (155.21) | 0.82 | 0.64 | 0.66 |
| Total Power (ms2) | 731.91 (1469.38) | 926.39 (1962.93) | 496.48 (318.92) | 625.08 (1015.02) | 490.01 (588.17) | 0.77 | 0.47 | 0.69 |
| LF/HF | 2.82 (3.13) | 2.43 (2.83) | 3.28 (3.48) | 3.30 (4.33) | 3.76 (5.58) | 0.43 | 0.09 | 0.92 |
| EDR (Hz) | .25 (.05) | .26 (.05) | .24 (.06) | .26 (.06) | .23 (.05) | 0.60 | 0.88 | 0.24 |
Baseline HRV parameters of all veterans included in the analysis.
P-values correspond to Wilcoxon matched-pairs signed rank test.
P-values correspond to Mann-Whitney U test.
Statistical significance (p< .05). HF, High frequency; HR, Heart rate; HRV, Heart rate variability; LF, Low frequency; LF/HF, Low frequency/high frequency ratio; PNS, Parasympathetic nervous system; PTSD, posttraumatic stress disorder; RMSSD, Root mean square of the successive differences; RR, RR interval; SD, Standard deviation; SNS, Sympathetic nervous system; TP, total power; VLF, Very low frequency.
The PNS index (mean ± SD= −0.96 ± 1.16; generated in Kubios) was negative, suggesting parasympathetic activity below the normative average value for a healthy population, while the SNS index (mean ± SD= 2.47 ± 2.41) indicated a sympathetic response above the standard values for a healthy population. When assessed by group (active vs. sham), no differences were observed for the HRV variables at baseline.
In the active iTBS group, there was a statistically significant negative correlation between CAPS score and baseline total power (rs=−0.58,p=0.004) and RMSSD (rs=−0.44,p=0.04), respectively, in addition to a positive correlation between baseline total power and SOFAS score (rs=0.43,p=0.04) (Figures 2 and 3). No statistical significance was found for the correlation analyses in the sham group (all p ≥ 0.05). Similarly, no statistical significance was found between HRV features and IDSSR scores.
Figure 2.
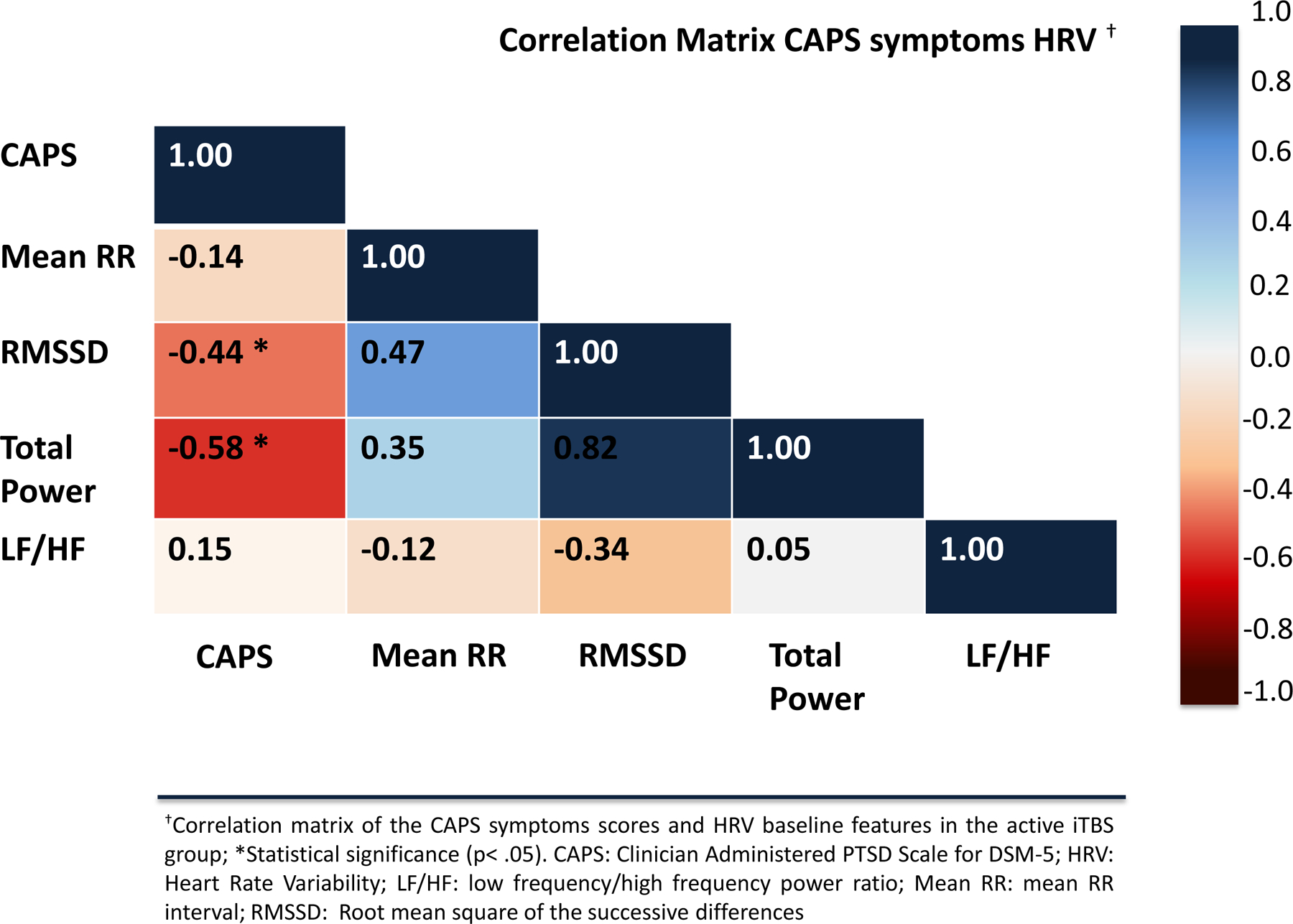
Correlation matrix of the CAPS symptoms scores and HRV features in the active iTBS group
Figure 3.
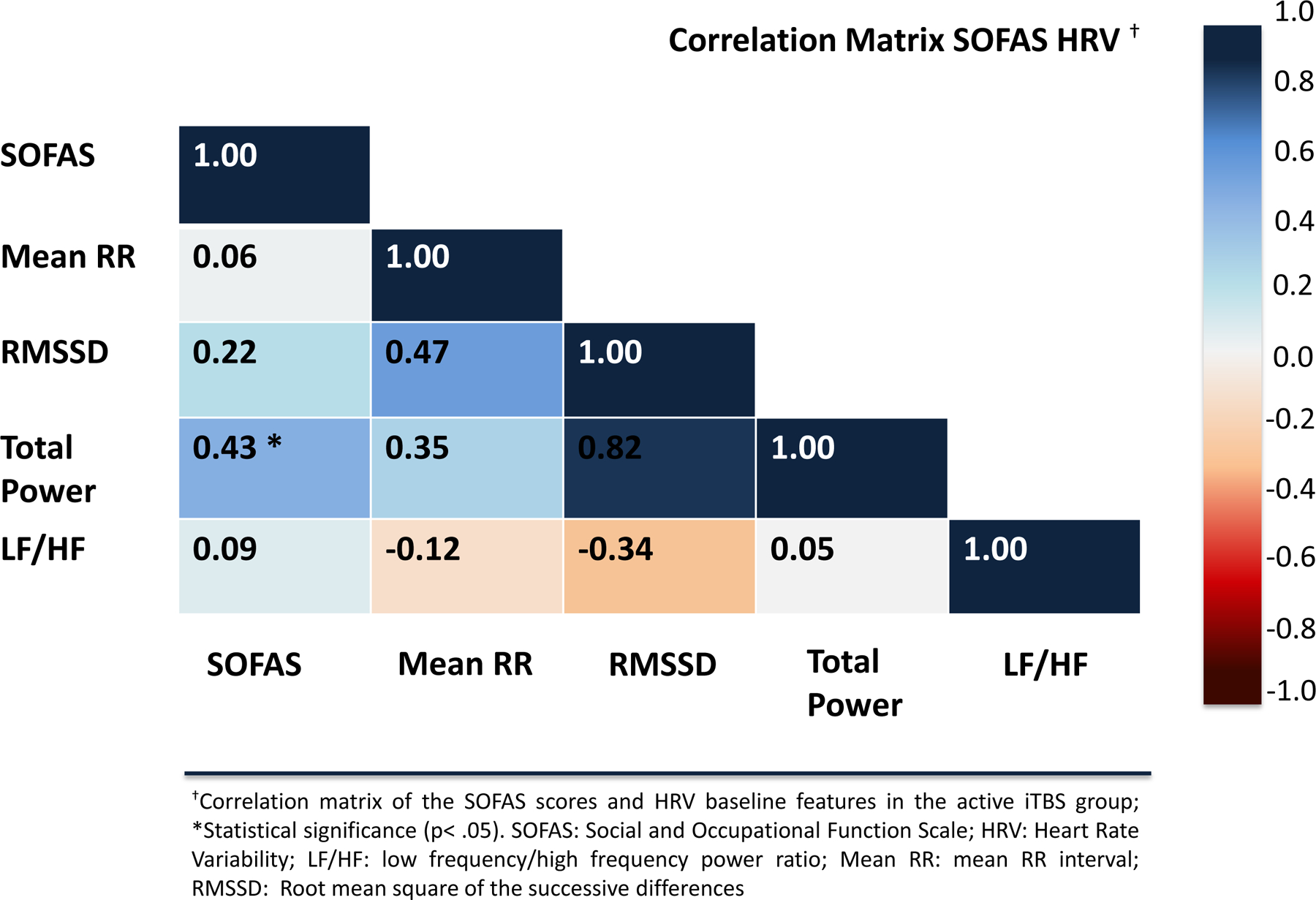
Correlation matrix of the SOFAS scores and HRV features in the active iTBS group
With regard to iTBS direct effects on HRV measures, no significant changes were observed in all excerpts (periods of 30s, 45s, 60s, and 195s) within or between-groups (all p ≥ 0.05), except for the VLF when comparing pre and post values in the active group (p= .04) (Table 2, and tables S1, S2, and S3 in the Supplementary Material).
Discussion
To the best of our knowledge, this is the first study to assess HRV measures as predictors of response to iTBS for PTSD, and to evaluate iTBS effects on ANS activity in PTSD. This secondary analysis examined the relationship between HRV parameters and clinical changes immediately after the last iTBS session. HRV features, total power and RMSSD obtained by applying an ultra-short-term approach, seem to be potential predictors of acute clinical response to iTBS for PTSD. These findings suggest that autonomic activity might serve as a low-cost predictive biomarker to identify PTSD patients most likely to respond to iTBS.
Our results indicate that PTSD patients that have higher baseline RMSSD 6,34, demonstrated better clinical outcomes immediately after the last iTBS session. Similarly, those with higher baseline total power, a sympathetic-driven parameter of autonomic response, had better acute responses to iTBS with improved symptoms on the CAPS, in addition to higher functionality on the SOFAS. Among the HRV features, RMSSD stands out for being an accurate and reliable indirect measure of vagal activity7, which has also shown high sensitivity and specificity to identify autonomic abnormalities in prior trials35,36. This is possibly a reason why RMSSD has been identified as a potential predictor of iTBS response in our study. Our findings are also consistent with prior studies demonstrating that PTSD is associated with abnormalities in ANS activity, such as lower RMSSD and total power values, reflecting reduced parasympathetic and increased sympathetic responding, respectively. Among our sample, despite overall reduced RMSSD and total power, individuals with higher baseline values were found to be more likely to respond to active stimulation. In other words, PTSD patients with less ANS dysfunction were more likely to respond to iTBS.
While iTBS and other TMS modalities have been widely applied in numerous neuropsychiatric disorders with promising results, there is a paucity of successful trials investigating potential biomarkers of treatment response. To date, the most optimistic outcomes have been shown by researchers utilizing sophisticated functional mapping techniques as biological markers, however, these methods are associated with high costs and increased time commitment 37. In this context, HRV appears as a potential biological marker with broad applicability, given its safe, feasible, low-cost, and technically simple profile.
No significant differences were observed for the HRV features at baseline or post intervention, when assessed between-groups. Overall, we did not observe direct effects of iTBS on HRV itself, except for the VLF when comparing within the active group, which it is not associated with any specific physiological or clinical meaning. This lack of response might be attributed to the study population profile, including individuals who have failed at least one evidence-based treatment for PTSD (pharmacotherapy and/or psychotherapy). These patients might have a more severe presentation likely marked by pronounced autonomic regulation impairment. Interestingly, our findings are inconsistent with the recent work from Iseger et al. 17. There are several potential explanations behind this discrepancy; these include pathophysiological differences between the populations under study (i.e., PTSD vs. MDD), some distinct protocol parameters and anatomical target. For instance, in the MDD study, subjects underwent thirty stimulation sessions with an intensity of 120% of the resting motor threshold, and the ECG data was acquired during the stimulation, which allowed them to obtain the effects of iTBS over HRV measures immediately, instead of after a short interval. Iseger et al. found a significantly larger HR deceleration during active iTBS that was limited to the first minute of stimulation. No significance was observed when analyzing the whole recording, which the authors attributed to the probability of HRV changes being more prominent at the beginning of the recording, possibly related to the fast nature of the parasympathetic responses modulated by iTBS. Therefore, based on their hypothesis, our lack of significant findings could be explained by the acquisition of ECG just before and just after the stimulation, when potential effects on HRV parameters might have faded.
Another potential reason for the divergent results may be due to the differing anatomical targets (right vs. left DLPFC). A meta-analysis assessing the efficacy of non-invasive brain stimulation techniques in the modulation of HRV parameters showed that the stimulation target was a significant moderator of response 20. In particular, trials stimulating the prefrontal cortex (PFC) revealed significant increases in HRV, with the majority having targeted the left PFC. As explained in the original trial by Philip et al., the decision to apply iTBS over the right DLPFC was based on prior findings suggesting PTSD symptom reduction (one of the primary outcomes for that analysis) following high-frequency modulation of this area, likely due to top-down inhibition of the amygdala resulting in decreased response to trauma-related stimuli16. Designed to assess the effects of iTBS in the modulation of cardiovascular parameters in MDD, Iseger et al. defined the left DLPFC as their target based on previous trials showing MDD symptom improvement as well as the interconnections between this area and the anterior cingulate cortex (ACC) 17. The ACC is a critical regulatory area of the anterior limbic circuit that composes the central autonomic network (CAN) 38,39 and has been associated with the modulation of the ANS and, therefore, its indirect stimulation might explain their significant HRV findings.
Our results describing the patient population are generally consistent with the existing literature. Increased sympathetic and attenuated parasympathetic activity are expected adaptive mechanisms in the context of threatening stimuli, preparing the individual for the environmental demand 40. Our descriptive analysis results are consistent with prior evidence that PTSD is associated with maladaptive ANS activity, as supported by parasympathetic activity below average values observed in healthy populations, as well as an increased sympathetic activity 40,41. Based on our HRV analysis indexes, enhancement of sympathetic activity was more pronounced than the reduction in parasympathetic activity. Similar findings were observed in prior studies, suggesting that ANS impairment in PTSD is the result of an overactive sympathetic branch and hypoactive parasympathetic response, with the former being predominant 11–14. The imbalance results in a sustained fight or flight response, which in turn is associated with poor quality of life and function in PTSD patients. This work underscores the need to develop novel therapeutic methods that might be able to restore ANS activity to a more normative state.
The current study had several limitations: (a) issues inherent to the secondary analysis of clinical trials, namely that recruitment did not focus on autonomic or related activity; (b) the sensitive nature of HRV features and how they fluctuate based on stress-induced physiological changes, resting status, and baseline comorbidities, including cardiovascular diseases, what might represent a challenge to its application as a biomarker in the clinical setting 42,43; (c) the naturalistic veterans patient population, predominantly composed by males, which may have introduced confounding factors related to ongoing treatment, in addition to cardiovascular and related clinical factors; (d) small sample size, precluding more complex analyses or conclusions; (e) the lack of correction for multiple comparisons, therefore requiring careful interpretation of our findings in the context of its exploratory nature; (f) the lack of recording during stimulation, which may have prevented us from detecting potential modulation of the autonomic response by iTBS given the fast nature of the parasympathetic response; and (g) absence of direct measures of frontolimbic activity (i.e., neuroimaging), making us unable to conclude whether iTBS modulated these networks. Furthermore, unlike RMSS 6,7,28,34,44, total power is a HRV parameter that has not been extensively studied 6, so its physiological meaning is not fully understood and, in addition, its reliability in ultra-short ECG recordings remains unclear.
Conclusion
In conclusion, our findings indicate that ultra-short-term HRV features might work as a low-cost and technically simple predictive biomarker of iTBS clinical response in PTSD. Individuals with less autonomic impairment were more prone to acutely respond to iTBS, with improvements in both PTSD symptoms and social & occupational function. In regard to the effects of iTBS in the modulation of HRV parameters, future trials might consider targeting the left DLPFC, simultaneous iTBS delivery and ECG acquisition, combining iTBS to evidence-based treatment for PTSD (e.g. pharmacotherapy and/or psychotherapy), as well as prospective testing of these findings in patients with elevated hyperarousal.
Supplementary Material
Sources of Financial Support
Effort on this manuscript was supported in part by VA grants I21 RX002032, I01 RX002450 & I01 HX002572, NIH grants R01 MH120126, R25 MH101076, P20 GM130452, and the VA RR&D Center for Neurorestoration and Neurotechnology at the Providence VA Medical Center.
Footnotes
Conflict of Interest Statement
The authors have no conflict of interests to report.
References
- 1.Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress. 2013;26(5):537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shalev A, Liberzon I, Marmar C. Post-Traumatic Stress Disorder. N Engl J Med. 2017;376(25):2459–2469. [DOI] [PubMed] [Google Scholar]
- 3.Yehuda R. Post-traumatic stress disorder. N Engl J Med. 2002;346(2):108–114. [DOI] [PubMed] [Google Scholar]
- 4.Association AP. Diagnostic and Statistical Manual of Mental Disorders Vol 5th ed.. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 5.Haagen JF, Smid GE, Knipscheer JW, Kleber RJ. The efficacy of recommended treatments for veterans with PTSD: A metaregression analysis. Clin Psychol Rev. 2015;40:184–194. [DOI] [PubMed] [Google Scholar]
- 6.Shaffer F, Ginsberg JP. An Overview of Heart Rate Variability Metrics and Norms. Front Public Health. 2017;5:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaffer F, McCraty R, Zerr CL. A healthy heart is not a metronome: an integrative review of the heart’s anatomy and heart rate variability. Front Psychol. 2014;5:1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malliani A. Heart rate variability: from bench to bedside. Eur J Intern Med. 2005;16(1):12–20. [DOI] [PubMed] [Google Scholar]
- 9.Berntson GG, Bigger JT Jr., Eckberg DL, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–648. [DOI] [PubMed] [Google Scholar]
- 10.Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Ann Behav Med. 2009;37(2):141–153. [DOI] [PubMed] [Google Scholar]
- 11.Hauschildt M, Peters MJ, Moritz S, Jelinek L. Heart rate variability in response to affective scenes in posttraumatic stress disorder. Biol Psychol. 2011;88(2–3):215–222. [DOI] [PubMed] [Google Scholar]
- 12.Hopper JW, Spinazzola J, Simpson WB, van der Kolk BA. Preliminary evidence of parasympathetic influence on basal heart rate in posttraumatic stress disorder. J Psychosom Res. 2006;60(1):83–90. [DOI] [PubMed] [Google Scholar]
- 13.Keary TA, Hughes JW, Palmieri PA. Women with posttraumatic stress disorder have larger decreases in heart rate variability during stress tasks. Int J Psychophysiol. 2009;73(3):257–264. [DOI] [PubMed] [Google Scholar]
- 14.Minassian A, Geyer MA, Baker DG, et al. Heart rate variability characteristics in a large group of active-duty marines and relationship to posttraumatic stress. Psychosom Med. 2014;76(4):292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ge F, Yuan M, Li Y, Zhang W. Posttraumatic Stress Disorder and Alterations in Resting Heart Rate Variability: A Systematic Review and Meta-Analysis. Psychiatry Investig. 2020;17(1):9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baeken C, De Raedt R, Van Schuerbeek P, et al. Right prefrontal HF-rTMS attenuates right amygdala processing of negatively valenced emotional stimuli in healthy females. Behav Brain Res. 2010;214(2):450–455. [DOI] [PubMed] [Google Scholar]
- 17.Iseger TA, Arns M, Downar J, Blumberger DM, Daskalakis ZJ, Vila-Rodriguez F. Cardiovascular differences between sham and active iTBS related to treatment response in MDD. Brain Stimul. 2020;13(1):167–174. [DOI] [PubMed] [Google Scholar]
- 18.Demirtas-Tatlidede A, Freitas C, Pascual-Leone A, Schmahmann JD. Modulatory effects of theta burst stimulation on cerebellar nonsomatic functions. Cerebellum. 2011;10(3):495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poppa T, de Witte S, Vanderhasselt MA, Bechara A, Baeken C. Theta-burst stimulation and frontotemporal regulation of cardiovascular autonomic outputs: The role of state anxiety. Int J Psychophysiol. 2020;149:25–34. [DOI] [PubMed] [Google Scholar]
- 20.Makovac E, Thayer JF, Ottaviani C. A meta-analysis of non-invasive brain stimulation and autonomic functioning: Implications for brain-heart pathways to cardiovascular disease. Neurosci Biobehav Rev. 2017;74(Pt B):330–341. [DOI] [PubMed] [Google Scholar]
- 21.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201–206. [DOI] [PubMed] [Google Scholar]
- 22.Lowe CJ, Manocchio F, Safati AB, Hall PA. The effects of theta burst stimulation (TBS) targeting the prefrontal cortex on executive functioning: A systematic review and meta-analysis. Neuropsychologia. 2018;111:344–359. [DOI] [PubMed] [Google Scholar]
- 23.Philip NS, Barredo J, Aiken E, et al. Theta-Burst Transcranial Magnetic Stimulation for Posttraumatic Stress Disorder. Am J Psychiatry. 2019;176(11):939–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlson RV, Boyd KM, Webb DJ. The revision of the Declaration of Helsinki: past, present and future. Br J Clin Pharmacol. 2004;57(6):695–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berlim MT, Van Den Eynde F. Repetitive transcranial magnetic stimulation over the dorsolateral prefrontal cortex for treating posttraumatic stress disorder: an exploratory meta-analysis of randomized, double-blind and sham-controlled trials. Can J Psychiatry. 2014;59(9):487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karsen EF, Watts BV, Holtzheimer PE. Review of the effectiveness of transcranial magnetic stimulation for post-traumatic stress disorder. Brain Stimul. 2014;7(2):151–157. [DOI] [PubMed] [Google Scholar]
- 27.Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-Aho PO, Karjalainen PA. Kubios HRV--heart rate variability analysis software. Comput Methods Programs Biomed. 2014;113(1):210–220. [DOI] [PubMed] [Google Scholar]
- 28.Baek HJ, Cho CH, Cho J, Woo JM. Reliability of ultra-short-term analysis as a surrogate of standard 5-min analysis of heart rate variability. Telemed J E Health. 2015;21(5):404–414. [DOI] [PubMed] [Google Scholar]
- 29.Castaldo R, Montesinos L, Melillo P, James C, Pecchia L. Ultra-short term HRV features as surrogates of short term HRV: a case study on mental stress detection in real life. BMC Med Inform Decis Mak. 2019;19(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montano N, Porta A, Cogliati C, et al. Heart rate variability explored in the frequency domain: a tool to investigate the link between heart and behavior. Neurosci Biobehav Rev. 2009;33(2):71–80. [DOI] [PubMed] [Google Scholar]
- 31.Weathers FW, Bovin MJ, Lee DJ, et al. The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5): Development and initial psychometric evaluation in military veterans. Psychol Assess. 2018;30(3):383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rybarczyk B. Social and Occupational Functioning Assessment Scale (SOFAS). In: Encyclopedia of Clinical Neuropsychology. New York, NY: Springer; 2011. [Google Scholar]
- 33.Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26(3):477–486. [DOI] [PubMed] [Google Scholar]
- 34.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 35.Silva A, Christofaro DGD, Bernardo AFB, Vanderlei FM, Vanderlei LCM. Sensitivity, Specificity and Predictive Value of Heart Rate Variability Indices in Type 1 Diabetes Mellitus. Arq Bras Cardiol. 2017;108(3):255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pivatelli FC, Dos Santos MA, Fernandes GB, et al. Sensitivity, specificity and predictive values of linear and nonlinear indices of heart rate variability in stable angina patients. Int Arch Med. 2012;5(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crosson B, Ford A, McGregor KM, et al. Functional imaging and related techniques: an introduction for rehabilitation researchers. J Rehabil Res Dev. 2010;47(2):vii–xxxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beissner F, Meissner K, Bar KJ, Napadow V. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J Neurosci. 2013;33(25):10503–10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sklerov M, Dayan E, Browner N. Functional neuroimaging of the central autonomic network: recent developments and clinical implications. Clin Auton Res. 2019;29(6):555–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williamson JB, Porges EC, Lamb DG, Porges SW. Maladaptive autonomic regulation in PTSD accelerates physiological aging. Front Psychol. 2014;5:1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan G, Dao TK, Farmer L, Sutherland RJ, Gevirtz R. Heart rate variability (HRV) and posttraumatic stress disorder (PTSD): a pilot study. Appl Psychophysiol Biofeedback. 2011;36(1):27–35. [DOI] [PubMed] [Google Scholar]
- 42.Kim HG, Cheon EJ, Bai DS, Lee YH, Koo BH. Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature. Psychiatry Investig. 2018;15(3):235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kleiger RE, Stein PK, Bigger JT Jr. Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiol. 2005;10(1):88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Esco MR, Flatt AA. Ultra-short-term heart rate variability indexes at rest and post-exercise in athletes: evaluating the agreement with accepted recommendations. J Sports Sci Med. 2014;13(3):535–541. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


