Abstract
Objective:
Patient-clinician goal concordance is associated with improved outcomes in certain chronic diseases, but not explored in rheumatoid arthritis (RA). We examined goal concordance, correlates of concordance, and the association of concordance with health outcomes.
Methods:
Adult RA patients seen ≥1 time in prior 12 months at one of two rheumatology clinics participated. Patients and their clinician independently ranked top three goals for RA treatment from eight options prior to a routine visit. Patients completed post-visit surveys on health, demographics, health literacy, and adherence. Goal concordance was defined as the patient’s #1 goal being among the clinician’s top three goals for that patient. Bivariable and multivariable logistic regression models were used to examine correlates of concordance.
Results:
Patients were 58% female, 16% Spanish-speaking, and 29% had limited health literacy. Among 204 patient-clinician dyads, 20% were goal-discordant. “Have less pain” was selected by both patient and clinician in 81% of dyads, followed by “have fewer problems doing daily activities” by 63%. Otherwise, clinicians prioritized avoiding side effects, while patients ranked improved sleep, fatigue, and mood. Longer disease duration was associated with discordance (median 13.3 years, IQR 5.2–20 among discordant vs. 7 years, IQR 4–14; p=0.039); higher depressive symptoms were associated with concordance (8.1% vs 24%, p=0.04). Goal concordance was associated with higher medication adherence (AOR 2.76, 95% CI 1.01–7.56).
Conclusion:
One in five patient-clinician dyads had discordant treatment goals. Goal concordance was associated with higher medication adherence. Studies to improve goal elicitation and communication of RA patients’ priorities are needed.
Current treatment paradigms for rheumatoid arthritis (RA), a chronic disabling condition with high societal and personal cost, include shared decision making (SDM) between patients and clinicians (1). A key element of effective SDM is understanding patients’ goals, which often reflect desired improvements in quality of life, and how they align with or differ from clinicians’ goals. For clinicians who care for persons with RA, goals are commonly driven by clinical metrics and guidelines, including the treat-to-target approach, with a target of low disease activity or remission. Patients’ goals are often different. In a systematic literature review of RA patient goals, we identified more than 400 goals which fell into four distinct areas: (a) the bodily experience of RA; (b) achieving normalcy and maintaining wellness; (c) social connectedness and support; and (d) interpersonal and healthcare system interactions (2). Persons with RA report challenges in sharing their treatment goals with their clinicians (3). Despite achievement of remission, patients continue to report lower quality of life compared with the general population, suggesting that even patients who reach the clinical goal of remission do not necessarily reach their own individual goals and continue to experience limitations, including fatigue and mood disturbances such as depression and anxiety (4).
Does it matter if clinicians and patients share common goals? Agreement around shared goals or “goal concordance” is associated with improved outcomes in other complex chronic conditions, such as diabetes (5), and during end of life care (6). Effective RA patient-clinician communication is central to accurate diagnosis, symptom assessment, treatment selection, and avoidance of unsafe use of costly therapies, yet communication around goals in RA is largely understudied. Patient preferences and values vary significantly (7) and thus alignment of goals is essential in RA and may serve as a model for other chronic conditions with complex decision making around medications, safety, and cost. Patient-clinician discordance around the assessment of disease activity has been consistently reported in RA (8–12) and underscores communication gaps between patients and clinicians. Persons with RA have expressed reluctance or fear of raising issues around pain with their clinicians (4). These barriers to alignment between clinicians and patients impede effective SDM and delivery of patient-centered care, where clinicians and patients work together toward the same set of goals, determined by patient preferences and priorities.
Additional barriers to effective communication and SDM in RA in majority English-language dominant health care settings include limited health literacy (LHL), limited English language proficiency (LEP), and depressed mood (8). In a prior study of more than 500 RA patients, we found suboptimal decision-making communication among those with LHL and LEP (13). These known barriers to communication have yet to be studied with regard to goal concordance. Therefore, in this study, we aimed to: 1) measure goal concordance between RA patients and their clinicians; 2) examine whether barriers to communication (LEP, LHL or depression) were independently associated with goal discordance; and 3) evaluate the association of goal concordance with outcomes of self-efficacy, trust in physician, medication adherence, and disease activity.
PATIENTS AND METHODS
Study design.
We conducted a cross-sectional study to measure goal concordance (or agreement) between persons with RA and their rheumatology clinicians and sought to: 1) identify correlates of goal concordance and 2) measure the association of goal concordance with patient outcomes.
Study population.
Patient participants.
Eligible patients were consecutively enrolled from two rheumatology clinical sites (university-based and Veterans Affairs outpatient-based rheumatology specialty clinics) beginning in October 2016. Patient participants included in this study must have been seen by a rheumatology clinician at least once over the previous 12 months, were ≥ 18 years of age, and met the 2010 American College of Rheumatology criteria for RA. Clinician participants included rheumatology attendings, fellows in training, or advance practice partners (nurse practitioner or physician’s assistant). The research protocol was approved by the joint Portland VA/OHSU Institutional Review Board (IRB # 15851); patient participants provided written informed consent and clinician participants were consented via information sheet.
Procedures.
Patient participants with upcoming clinic appointments were screened for study eligibility, and those meeting inclusion criteria were mailed a letter to introduce the study, which included a return letter where patients could opt out or request more information. Among those who did not opt out, patients were contacted prior to their appointment or approached in the clinic waiting room, and if interested, consented to enroll in the study. Enrolled patients completed a brief goals measure (Appendix 1a) prior to the clinic visit. The measure was developed by the study team based on a review of the literature on patient goals (2), focus groups (14), and multi-stakeholder input. Patient goals identified in the systematic review and focus groups were grouped into broad domains in the goals measure in order to create a brief, operable tool for clinical use. The goals measure asks patients to rank the top three most important things they want their RA treatment to do. Options were: have less pain, improve function, work outside the home, avoid side effects, improve sleep, feel less tired, improve mood, not affect fertility, or “other”, where patients can write-in a goal. Clinician participants were asked independently of the patient goals measure to rank their top three priorities for treatment for their patient from the same list of goals patients used prior to the clinic visit for each unique patient (Appendix 1b). After completion of the goals measure, patients would then proceed to their usual clinic visit as scheduled, and after the visit patients completed a second survey, which included items on demographics, health literacy, disease duration, medications, depressive symptoms, post-traumatic stress disorder (PTSD), self-efficacy, trust in physician, disease activity and medication adherence. Patient participants were compensated with a $25 gift card
Measures.
Goal concordance.
The measure of interest in all analyses was patient-clinician goal concordance. This was defined as the patient’s #1 goal being ranked among the top 3 listed by the clinician. This definition is guided by prior literature on goal concordance in diabetes {Heisler, 2003 #32;Zulman, 2010 #31}.
Primary correlates of concordance: Limited English language proficiency (LEP), limited health literacy, depressive symptoms.
Using methodology established by Karliner et al to ascertain LEP (15), participants responded to two questions to evaluate English language proficiency. Health literacy was measured using a single-item literacy screener, a self-reported and validated measure that has been used among RA populations in other studies (16, 17) . Depressive symptoms were measured using the Patient Health Questionnaire-8, an 8-item self-report measure, validated in multiple languages and used among patients with limited health literacy (18). Scores range from 0–24 with a score of ≥ 10 corresponding to moderate depressive symptoms or greater.
Patient outcomes.
In our study model, we hypothesized that goal concordance would be associated with downstream outcomes of increased self-efficacy, higher trust in physician, improved medication adherence and lower disease activity.
Self-efficacy.
Self-efficacy was measured using the English-language validated 6-item “Self-efficacy for Managing Chronic Disease” scale (19). Item scores range from 1 to 10 with higher scores reflecting greater self-efficacy; the summary score is the mean of the six items. For Spanish speakers, we used the “Spanish Arthritis Self-Efficacy” 8-item scale (20); item score range and scoring are the same as the English language scale.
Trust in physician.
Low trust in physician has been shown to be associated with suboptimal communication around shared decision making (13), as well as confidence in deciding to take a disease-modifying antirheumatic drug (DMARD) (21). We measured trust with the 11-item Trust in Physician scale (22) that has been validated in patients with RA (23). Responses are summed and the value is transformed to a 0–100 scale. A score below the median was considered suboptimal. After data collection had been completed, it was noted that the final 3 questions of the scale were inadvertently omitted from the survey. Despite this, the Cronbach’s alpha for the scale with 8-items was 0.799 and it felt reasonable to proceed with including it as an a priori selected patient outcome.
Adherence.
We measured self-report adherence with the validated 8-item Morisky’s Medication Adherence Scale (MMAS-8) (24). The MMAS-8 scoring system classifies patients as having low, moderate or high adherence; in prior studies patients who had low or moderate adherence were considered nonadherent (25). We dichotomized adherence at low/moderate compared to high.
Disease activity.
We captured disease activity using a composite measure, the Clinical Disease Activity Index (CDAI), which is a sum of 4 components: 28 tender joint count, 28 swollen joint count, patient global assessment and clinician global assessment of disease activity. CDAI scores range from 0–76. A minimal clinically important difference in CDAI is 6 (26).
Covariates.
Patient demographics of age, gender (male vs. female), race/ethnicity (white, Black, Latinx/Hispanic, vs. other), partner status (coupled, which included married or living with a partner, yes vs. no), education (high school or less vs. some college or more) and annual income (<$40,000 vs. ≥$40,000) were all captured by self-report on the survey. Given that we included a number of Veteran patient participants in the study and PTSD has been associated with incident RA (27) and poorer prognosis/more aggressive disease activity (28), participants completed a 4-item screener for PTSD (29) and if they scored 3 or higher on the screener, they were instructed to complete a full measure of PTSD (30).
Analysis.
Descriptive statistics were used to summarize patient characteristics according to goal concordance. Goals ranked by patient and clinician were described graphically and were enumerated as those selected by both patient and clinician, by patient only and clinician only.
We performed a series of bivariate analyses to test for differences in patient characteristics, primary correlates and patient outcomes according to goal concordance, for which we used chi-square tests for categorical variables and t-tests or Mann-Whitney U tests for continuous variables. To determine the association of our primary correlates with concordance, and of concordance with patient outcomes, we built a series of regression models, including both unadjusted (no covariates) and adjusted models. We used the following analysis approaches: logistic regression for all primary correlates of concordance, trust in physician and medication adherence, and linear regression for self-efficacy and disease activity. Our first step in creating an adjusted model was to retain covariates in the model if they changed the regression coefficient, for either our primary correlates of concordance or concordance itself, by approximately 20% or more, e.g. a change-in-estimate variable selection strategy (31). Next, we used a forward selection procedure, based on Bayesian Information Criteria (BIC), to incorporate covariates that improved model fit. Last, we considered site and gender in our adjusted models, as these would adjust for differences among patients at the clinic and system level. When building our primary correlates of concordance models, we were conservative in the number of covariates we added to each model in order to minimize the number of subjects per variable (SPV), and for this reason, did not include site and gender in the same model; these factors in combination greatly impacted the standard error due to the predominance of male patients at the VA site. Models presented include site, as this factor provided a better model fit. Additionally, we used Firth’s bias penalized likelihood logistic regressions to reduce potential bias generated from the small sample size in certain covariate profiles. As multiple patients were treated by a single clinician, we assessed if clustering by clinician should be taken into account; preliminary models incorporating clustering by clinician did not improve model fit, and for this reason, we do not present these models. Model assumptions were checked and no violations were noted. All analyses were performed in Stata/SE, version 15, and graphics created in R version 4.0.2 using package ggplot2 version 3.3.3.
RESULTS
A total of 208 patient-clinician dyads were enrolled (estimated patient participation rate 62%); goal-ranking instructions were not followed by one patient and two clinicians, and one patient lacked clinical information, leaving 204 dyads included in the analysis, 63% from the university and 37% from the VA (Table 1). Overall, patients were predominantly female (58%) with an average age of 57.2 years (14.2 standard deviation or SD), and median 8 years disease duration (interquartile range or IQR 4 – 16). Nearly one third had a high school education or less (31.6%), 15.1% had LEP, and 29% had limited health literacy. There were a total of 15 clinicians (8 female), which included 14 physicians (4 of whom were rheumatology fellows) and one advanced practice partner.
Table 1.
Patient characteristics, overall and by concordance
| Total | Discordant | Concordant | p-value | ||
|---|---|---|---|---|---|
| N=204 | N=40 | N=164 | |||
| Site, n (%) | OHSU | 122 (62.7%) | 26 (65.0%) | 96 (62.2%) | 0.86 |
| VA | 76 (37.3%) | 14 (35.0%) | 62 (37.8%) | ||
|
| |||||
| Gender, n (%) | Male | 85 (41.7%) | 18 (45.0%) | 67 (40.9%) | 0.72 |
| Female | 119 (58.3%) | 22 (55.0%) | 97 (59.1%) | ||
|
| |||||
| Age (years), mean (SD) | 57.2 (14.2) | 58.2 (15.1) | 56.9 (14.0) | 0.62 | |
|
| |||||
| Language, n (%) | English | 168 (84.4%) | 35 (92.1%) | 133 (82.6%) | 0.21 |
| Spanish | 31 (15.6%) | 3 (7.9%) | 28 (17.4%) | ||
|
| |||||
| Limited English language proficiency, n (%) | 30 (15.1%) | 3 (7.9%) | 27 (16.8%) | 0.17 | |
|
| |||||
| Coupled, n (%) | 110 (57.9%) | 22 (61.1%) | 88 (57.1%) | 0.71 | |
|
| |||||
| Education, n (%) | High school or less | 60 (31.6%) | 15 (40.5%) | 45 (29.4%) | 0.24 |
| Some college or more | 130 (68.4%) | 22 (59.5%) | 108 (70.6%) | ||
|
| |||||
| Income, >$40,000, n (%) | Less than $40,000 | 85 (45.5%) | 12 (33.3%) | 73 (48.3%) | 0.14 |
| Greater than $40,000 | 102 (54.5%) | 24 (66.7%) | 78 (51.7%) | ||
|
| |||||
| People in household, median (IQR) | 2 (2–4) | 2 (2–3) | 2 (2–4) | 0.92 | |
|
| |||||
| Race/Ethnicity, n (%) | White | 123 (68.3%) | 25 (78.1%) | 98 (66.2%) | 0.62 |
| Black | 6 (3.3%) | 0 (0.0%) | 6 (4.1%) | ||
| Latinx/Hispanic | 39 (21.7%) | 6 (18.8%) | 33 (22.3%) | ||
| Other | 12 (6.7%) | 1 (3.1%) | 11 (7.4%) | ||
|
| |||||
| Limited health literacy, n (%) | 55 (28.6%) | 13 (35.1%) | 42 (27.1%) | 0.42 | |
|
| |||||
| Disease duration (years), median (IQR) | 8 (4–16) | 13 (5–21) | 7 (4–15) | 0.039 | |
|
| |||||
| Number of medications, median (IQR) | 1 (1–2) | 1 (0–2) | 1 (1–2) | 0.10 | |
|
| |||||
| Depressive symptoms, n (%) | 38 (20.8%) | 3 (8.1%) | 35 (24.0%) | 0.040 | |
|
| |||||
| PTSD, n (%) | 13 (7.1%) | 2 (5.6%) | 11 (7.5%) | 1.00 | |
|
| |||||
| Self-efficacy score, mean (SD) | 6.3 (2.1) | 6.3 (2.1) | 6.3 (2.1) | 0.96 | |
|
| |||||
| Trust in Physician, n (%) | 106 (53.8%) | 19 (51.4%) | 87 (%) | 0.74 | |
|
| |||||
| Disease activity score (CDAI), mean (SD) | 12.8 (10.5) | 10.5 (9.7) | 13.2 (10.8) | 0.21 | |
|
| |||||
| Medication Adherence, n (%) | High | 63 (33.5%) | 7 (20.6%) | 56 (36.4%) | 0.11 |
| Low/Medium | 125 (66.5%) | 27 (79.4%) | 98 (63.6%) | ||
Abbreviations: IQR, interquartile range; PTSD, post-traumatic stress disorder; SD, standard deviation; OHSU, Oregon Health & Science University; VA, Veterans Affairs; CDAI, Clinical Disease Activity Index
Goal concordance.
A total of 164 dyads (80%) met the definition of concordance (patient’s top goal ranked among the clinician’s top 3). This agreement was largely driven by 124 dyads being concordant on “have less pain” as a goal, (132 patients selected it as their #1 goal, Figure 1). The next most frequently selected goal was “have fewer problems doing daily activities,” with 32 dyads concordant on this goal (61.8% selected by both patient and clinician, Table 2). “Improve sleep” was ranked by 20.1% of patients, with 0 dyads concordant on this goal. Similarly, “feel less tired” was selected by 27.5% of patients but only 6.4% of clinicians. “Improve mood” was a goal selected by 12.3% of patients but only 1 clinician. Interestingly, goal ranking by patients varied by disease duration. Patients with early RA (≤ 2 years duration) were more likely to select “work outside the home” and “improve mood” than those with longer disease duration, who more often chose “have fewer problems with daily activities” (supplementary table 1). : “Other” goals included ambulation, preventing joint damage or deformities, going back to work, gain knowledge about prognosis, and to live normally.
Figure 1.
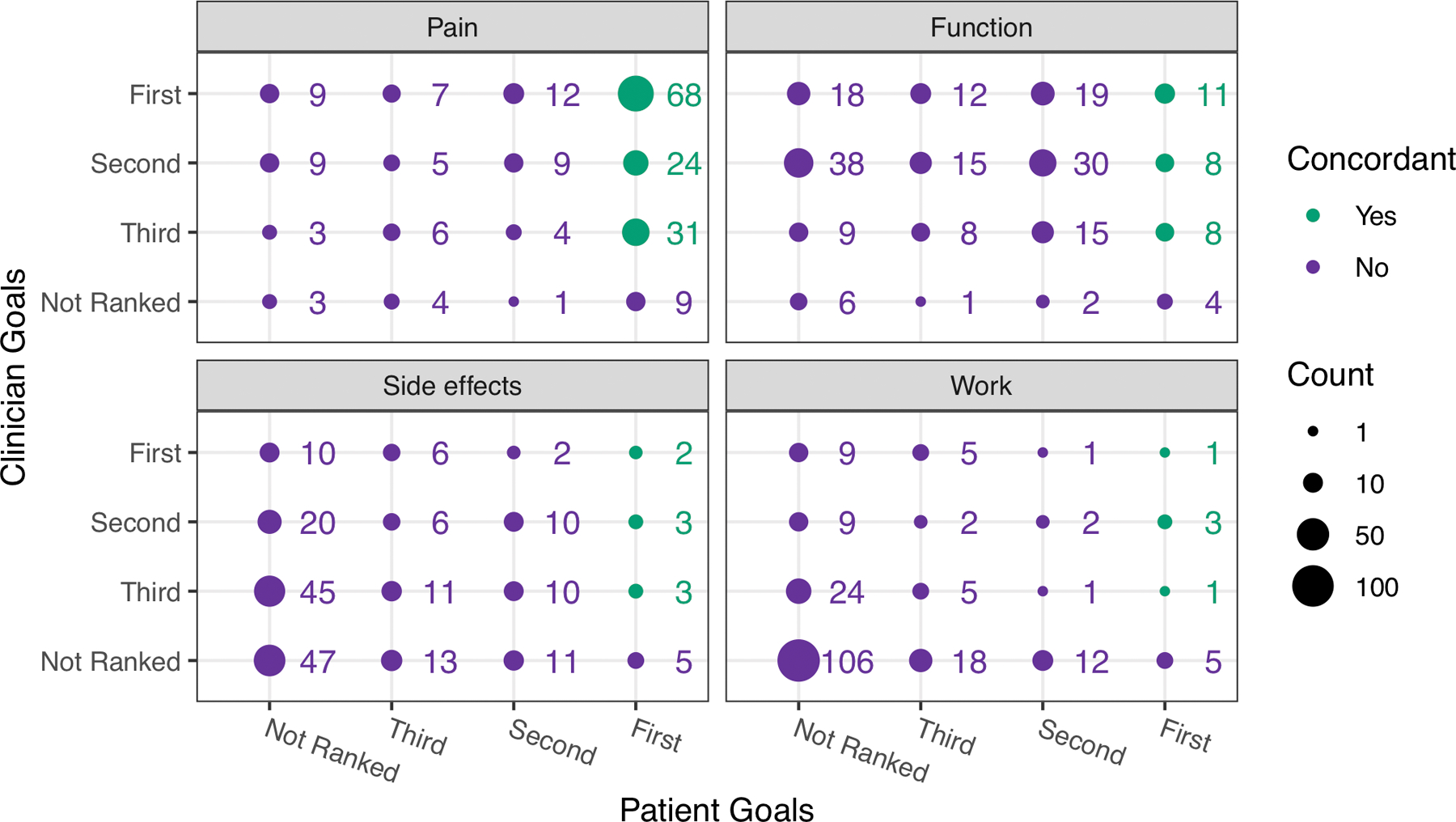
This plot displays the clinician-selected top goals for RA treatment (y-axis) and the patient-ranked goals (x-axis) as either first, second or third for each goal listed on the goals measure. Wording on the measure asked patients and clinicians to select the top three most important things for treatment to do for them from a list of 8 options. We display results from the most commonly selected goals here: 1) have less pain; 2) have fewer problems doing my daily activities (function); 3) be able to work outside the home; 4) avoid side effects from medicine. A green dot indicates that the clinician selected the patient’s number 1 goal (defined as “goal concordant”); a purple dot indicates the goal was selected by either or both patient or clinician but did not meet the definition of concordance. The size of the dot reflects the number of times it was selected.
Table 2.
Patient and clinician selection of RA treatment goals organized by: goals selected by both patient and clinician, selected by the patient only, and selected by the clinician only.
|
Goal (selected as one of top 3 goals) |
Selected by both
N (%) |
Selected by patient only
N (%) |
Selected by clinician only
N (%) |
|---|---|---|---|
| Have less pain | 166 ( 81.4) | 14 ( 6.9) | 21 ( 10.3) |
| Have fewer problems doing daily activities | 126 ( 61.8) | 7 ( 3.4) | 65 ( 31.9) |
| Be able to work outside the home | 21 ( 10.3) | 35 ( 17.2) | 42 ( 20.6) |
| Avoid side effects from medicine | 53 ( 26.0) | 29 ( 14.2) | 75 ( 36.8) |
| Improve sleep | 3 ( 1.5) | 38 ( 18.6) | 6 ( 2.9) |
| Feel less tired | 13 ( 6.4) | 56 ( 27.5) | 13 ( 6.4) |
| Improve mood | 1 ( 0.5) | 25 ( 12.3) | 1 ( 0.5) |
| Not affect ability to have children | 1 ( 0.5) | 8 ( 3.9) | 0 (−) |
| Other | 0 (−) | 11 ( 5.4) | 4 ( 2.0) |
Correlates of goal concordance.
In bivariate analyses, disease duration was shorter in goal concordant (median 7 years, IQR 4–15) compared with discordant (median 13 years, IQR 5–21) dyads, (p=0.04). Depressive symptoms by PHQ-8 (score ≥ 10) were more common among patients in concordant dyads (24.0% vs. 8.1%, p=0.04). Multivariable logistic models constructed for each primary predictor of interest are shown in Table 3. Limited health literacy was associated with a non-significant lower odds of goal concordance (adjusted odds ratio or AOR 0.57, 95% CI 0.26, 1.25), while depressive symptoms were associated with a non-significant higher odds of goal concordance (AOR 2.8, 95% CI 0.85, 9.25). LEP, when adjusted for disease duration and income, was not associated with goal concordance. In all three models, longer disease duration was associated with lower odds of goal concordance (AOR 0.96, 95% CI 0.93, 0.99 in the health literacy model).
Table 3.
Results of unadjusted and adjusted logistic regression models comparing the odds of RA patient-clinician goal concordance for three primary predictors of interest
| Main Predictor | Unadjusted | p | Adjusted* | p |
|---|---|---|---|---|
| Model 1 | ||||
| Limited Health Literacy | 0.680 (0.320, 1.442) | 0.314 | 0.569 (0.260, 1.247) | 0.159 |
| Disease duration (years) | 0.958 (0.925, 0.993) | 0.019 | ||
| Income (>$40,000) | 0.610 (0.282, 1.318) | 0.208 | ||
| VA site | 1.333 (0.602, 2.952) | 0.478 | ||
|
| ||||
| Model 2 | ||||
|
| ||||
| LEP | 2.074 (0.642, 6.696) | 0.223 | 1.507 (0.452, 5.025) | 0.505 |
| Disease duration (years) | 0.966 (0.934, 1.000) | 0.051 | ||
| Income (>$40,000) | 0.598 (0.278, 1.282) | 0.186 | ||
|
| ||||
| Model 3 | ||||
|
| ||||
| Depressive symptoms | 3.138 ( 0.981, 10.037) | 0.054 | 2.802 ( 0.848, 9.251) | 0.091 |
| Disease duration | 0.965 ( 0.931, 1.000) | 0.051 | ||
| Income (>$40,000) | 0.618 ( 0.278,1.375) | 0.238 | ||
| VA site | 1.378 ( 0.618, 3.070) | 0.433 | ||
For each model, the adjusted analyses included variables listed for each model respectively.
Abbreviations: LEP = Limited English language proficiency; p = p-value.
Goal concordance as predictor of health outcomes.
Using separate models for each outcome, in both unadjusted and adjusted analyses, we did not see an association of goal concordance with self-efficacy, trust in physician, or disease activity (Table 4). Goal concordance was associated with higher medication adherence (AOR 2.76, 95% CI 1.01 – 7.56) when controlling for gender, income, education, depression, disease duration and site. Education (> high school) was also associated with high medication adherence (AOR 2.80, 95% CI 1.19, 6.58).
Table 4.
Results of unadjusted and adjusted logistic and linear regression models assessing the association between patient outcomes and patient-clinician goal concordance as the main effect.
| Outcome | Unadjusted Estimate | p | Adjusted Estimate | p | |
|---|---|---|---|---|---|
| Self-efficacy score | Goal concordant | −0.019 (−0.822, 0.785) | 0.963 | 0.532 (−0.294, 1.357) | 0.205 |
| Female gender | NA | NA | −0.372 (−1.271, 0.526) | 0.414 | |
| Income >$40,000 | NA | NA | 0.412 (−0.324, 1.148) | 0.271 | |
| Some college or more | NA | NA | −0.305 (−1.103, 0.493) | 0.451 | |
| Coupled | NA | NA | −0.002 (−0.724, 0.720) | 0.996 | |
| Depressive symptoms | NA | NA | −2.027 (−2.814, −1.240) | 0.000 | |
| Disease duration (years) | NA | NA | −0.009 (−0.042, 0.023) | 0.566 | |
| VA site | NA | NA | −0.269 (−1.165, 0.628) | 0.554 | |
|
| |||||
| Trust in Physician | Goal concordant | 1.129 (0.552, 2.310) | 0.740 | 1.361 (0.610, 3.038) | 0.452 |
| Female gender | NA | NA | 0.899 (0.379, 2.130) | 0.809 | |
| Income >$40,000 | NA | NA | 2.430 (1.281, 4.610) | 0.007 | |
| Depressive symptoms | NA | NA | 0.695 (0.317, 1.522) | 0.362 | |
| Disease duration (years) | NA | NA | 1.010 (0.977, 1.045) | 0.546 | |
| VA site | NA | NA | 1.055 (0.434, 2.563) | 0.906 | |
|
| |||||
| Disease Activity score * | Goal concordant | 2.698 (−1.566, 6.962) | 0.213 | 1.609 (−2.838, 6.056) | 0.476 |
| Female gender | NA | NA | 5.021 (0.119, 9.923) | 0.045 | |
| Income >$40,000 | NA | NA | −0.412 (−3.928, 3.104) | 0.817 | |
| Some college or more | NA | NA | −1.053 (−4.938, 2.832) | 0.593 | |
| Health Lit | NA | NA | 0.075 (−3.760, 3.910) | 0.969 | |
| Depressive symptoms | NA | NA | 7.129 (2.992, 11.266) | 0.001 | |
| Disease duration (years) | NA | NA | 0.044 (−0.127, 0.215) | 0.612 | |
| VA site | NA | NA | 0.847 (−4.313, 6.007) | 0.746 | |
|
| |||||
| High Medication Adherence | Goal concordant | 2.204 (0.902, 5.388) | 0.083 | 2.760 (1.007, 7.564) | 0.048 |
| Female gender | NA | NA | 0.989 (0.386, 2.532) | 0.981 | |
| Income >$40,000 | NA | NA | 0.863 (0.421, 1.766) | 0.686 | |
| Some college or more | NA | NA | 2.799 (1.192, 6.575) | 0.018 | |
| Depressive symptoms | NA | NA | 0.528 (0.219, 1.272) | 0.155 | |
| Disease duration (years) | NA | NA | 1.016 (0.981, 1.052) | 0.372 | |
| VA site | NA | NA | 0.968 (0.372, 2.517) | 0.947 | |
Disease activity measured by the Clinical Disease Activity Index (CDAI)
Sensitivity analysis – examination of goal concordance excluding the ranking of “less pain.”
As described above, the majority of patient-clinician dyads (80%) were goal concordant, which was driven by the prioritization by both patients and clinicians of “have less pain.” RA patients are asked about pain levels at every visit, and pain is included in many disease activity measures for RA. Given the diverse and broad range of patient goals, we explored the proportion of concordant dyads when pain reduction was not included in the list of goals. If a patient ranked pain reduction as their #1 goal, we then considered their #2 goal as their first choice for this analysis. It should be noted that 126 patients (62%) ranked pain reduction as their #1 goal, and of these 126, 119 (94%) were concordant. In contrast, 116 of dyads (57%) were concordant when pain reduction was not considered as part of the goal measure. With respect to predictors of goal concordance, depressive symptoms were no longer associated with concordance, and the direction of the association shifted (AOR 0.77, 95% CI 0.37, 1.62). This suggests that the finding of the association of depressive symptoms and goal concordance in the main analysis was overwhelmingly driven by agreement around the goal of having less pain among patients with more depressive symptoms. In addition, LHL was associated with lower odds of goal concordance when adjusting for disease duration, income and site (AOR 0.50, 95% CI 0.26, 0.95) in this sensitivity analysis. Goal concordance was no longer associated with medication adherence when pain reduction was excluded from the goals list (AOR 1.11, 95% CI 0.56, 2.18).
Additional sensitivity analyses in which the definitions of goal concordance were altered to be more strict, revealed a lower frequency of concordance (Table 5): 1) patient’s #1 ranked goal among the clinician’s #1–2: 59% concordant; 2) patient’s #1 ranked goal among the clinician’s #1 goal: 41% concordant.
Table 5.
Results of sensitivity analysis: frequency of patient-clinician goal concordance across three definitions of concordance and without pain ranked as a goal
| Concordance definition | Concordance, n (%) |
|---|---|
| #1 ranked goal of patient among #1–3 of clinician | 164 (80%) |
| #1 ranked goal of patient among #1–2 of clinician | 121 (59%) |
| #1 ranked goal of patient among #1 of clinician | 83 (41%) |
| #1 ranked goal of patient among #1–3 of clinician (without pain) | 116 (57%) |
DISCUSSION
In this novel, cross-sectional study of more than 200 patients with RA and their rheumatology clinicians, one in five dyads lacked agreement around the most important goals. The current treatment paradigm of treat-to-target prioritizes the clinician-driven goal of low disease activity or remission whereas patient goals are typically more complex and often go unelicited or unaddressed (2, 14, 32). Goal concordance was driven overwhelmingly by the prioritization of having less pain. The majority of patients (64%) selected pain reduction as their #1 goal and, of these, 94% were goal concordant. Beyond pain reduction, RA patients more often ranked goals of improved sleep, less fatigue, and improved mood, while clinicians chose avoiding side effects of medicine. Clinicians may prioritize the avoidance of side effects in part due to a sense of responsibility for causing them through prescribing practices.
Depressive symptoms were associated with nearly three times the odds of being goal concordant, although this relationship did not reach statistical significance. Persons with RA have concomitant depression in up to 40% of cases; depression is a known barrier to communication, and has been identified as a predictor of patient-clinician discordance around the assessment of disease activity in RA (8, 9). Despite higher rates of goal concordance among those with depression in this study, the focus on pain may not allow space for discussion of goals around mood (selected by 12.3% of patients, 0.5% clinicians). Limited health literacy and LEP were not significantly associated with goal concordance in the main analysis, however the sensitivity analysis without the ranking of pain reduction showed lower odds of concordance among those with LHL (AOR 0.50, 95% CI 0.26, 0.95). Our prior work identified LHL as a significant predictor for suboptimal communication around shared decision making (13). Our goals measure prioritized the use of plain language to describe goals and included input from patients in its development. Communication of goals and the high priority for less pain, as it is discussed at every RA clinical visit, may not be impacted by literacy or LEP status. Given that LEP and LHL are known predictors of suboptimal communication (33), future studies of interventions to enhance goal concordance in RA and other chronic diseases should continue to measure and include persons with these barriers to communication in the development and testing of goal elicitation tools and strategies. Furthermore, the adoption of universal precautions for health literacy improves medication adherence in RA (34).
Goal concordance was associated with higher medication adherence. This finding reinforces the importance of communication and shared goals in selection of therapy and has potential impacts on longer-term control of disease activity (35), however longitudinal studies are needed. Medication adherence to DMARDs in RA has been reported to be as low as 25% (36). A recent study where patients were asked to rank the most important factors related to DMARD adherence identified improving symptoms, maintaining independence, and shared decision making as high priorities (37). In addition, we have identified beliefs in the necessity of medications to be associated with improved treatment adherence in RA (38).
While this study adds to our understanding of goal concordance in RA it is not without limitations. The cross-sectional study design does not allow for determination of causality. We used a cross-sectional measure of adherence as a proxy to examine the relationship between goal concordance and adherence; ideally, one would look at impact of goal concordance on adherence over time. Participants were located in a single geographic location which may limit generalizability. However, the inclusion of patients and clinicians from two separate health care systems, one with a large number of males with RA, is a strength, as this is an often understudied population due to the natural higher prevalence of RA among women. The proportion of patients with LEP was low and may not allow for identification of independent associations of LEP with goal concordance. Our inclusion of LHL and LEP patients in this study is a strength, given these are known predictors of poor communication and poorer outcomes in RA. Three items from the 11-item Trust in Physician scale were inadvertently omitted from the survey however, the Cronbach’s alpha for the 8-item scale was 0.799 and thus this was included as an a priori selected patient outcome. Despite an extensive systematic literature review and series of focus groups which informed the goals measure, the major themes of “bodily experience, achieving normalcy and wellness, social connectedness and support, and interpersonal interactions” may not be comprehensively represented in the tool. However, all participants had the option to select “other” on the measure and write in a goal of their choosing.” We did not collect data on patient-clinician relationship duration which may influence goal concordance, however all patients had been seen at least once in the prior twelve months as part of eligibility criteria. Our definition of goal concordance was generous and may have underestimated the degree of discordance in our population; the sensitivity analysis, which removed the #1 goal of having less pain, revealed more than a doubling of the rate of discordance (20% vs. 43%) when this rather expected goal is removed. Additionally, if we alter the definition of concordance to agreement of the patient’s #1 ranked goal among #1 or #1–2 goals listed by the clinician, the frequency of goal discordance increases as well (59% and 41%, respectively). Future studies may require refining the definition of goal concordance in RA and measuring this concept in multiple different settings and populations. We sought to measure concordance around goals for therapy, however equally important may be agreement around strategies to achieve those goals. A shared treatment approach is integral to shared decision making. Establishing shared goals can be considered the first step in the SDM journey.
CONCLUSION
In this novel study of more than 200 patients with RA and their rheumatology clinicians, one in five dyads lacked agreement around the most important goals. Goal concordance was independently associated with higher medication adherence. Systematic approaches to communication around goals and shared decision making may create an environment where sharing goals is normalized and working together to achieve them through both pharmacologic and non-pharmacologic means becomes standard of care. Areas of sleep, fatigue, and mood need more explicit elicitation on the part of clinicians, and broader consideration of non-pharmacologic therapy (e.g., more discussion of physical activity), and enhanced collaborative care with mental health and primary care clinicians should be studied.
Supplementary Material
Significance and Innovation bullets.
Goal concordance (or agreement) between patients and clinicians can lead to improved health outcomes in chronic disease but has not been examined in rheumatoid arthritis (RA).
One in five patient-clinician dyads were discordant around RA treatment goals.
Patients with longer RA disease duration were more likely to be discordant with their clinician. This may highlight shifting goals over time for patients (more focus on function and mood with longer duration).
Goal concordance was independently associated with higher adherence, which suggests that clearer communication around treatment goals may lead to improved adherence and outcomes.
Funding information:
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Department of Veterans Affairs. Dr. Barton’s work was supported by the NIH (grant K23-AR-064372).
References
- 1.Smolen JS. Treat-to-target as an approach in inflammatory arthritis. Curr Opin Rheumatol 2016;28:297–302. [DOI] [PubMed] [Google Scholar]
- 2.Hulen E, Ervin A, Schue A, Evans-Young G, Saha S, Yelin EH, et al. Patient goals in rheumatoid arthritis care: A systematic review and qualitative synthesis. Musculoskeletal Care 2017;15:295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strand V, Wright GC, Bergman MJ, Tambiah J, Taylor PC. Patient expectations and perceptions of goal-setting strategies for disease management in rheumatoid arthritis. J Rheumatol 2015;42:2046–54. [DOI] [PubMed] [Google Scholar]
- 4.Scott IC, Machin A, Mallen CD, Hider SL. The extra-articular impacts of rheumatoid arthritis: Moving towards holistic care. BMC Rheumatol 2018;2:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heisler M, Vijan S, Anderson RM, Ubel PA, Bernstein SJ, Hofer TP. When do patients and their physicians agree on diabetes treatment goals and strategies, and what difference does it make? J Gen Intern Med 2003;18:893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paladino J, Bernacki R, Neville BA, Kavanagh J, Miranda SP, Palmor M, et al. Evaluating an intervention to improve communication between oncology clinicians and patients with life-limiting cancer: A cluster randomized clinical trial of the serious illness care program. JAMA Oncol 2019;5:801–9. [DOI] [PubMed] [Google Scholar]
- 7.Fraenkel L, Nowell WB, Michel G, Wiedmeyer C. Preference phenotypes to facilitate shared decision-making in rheumatoid arthritis. Ann Rheum Dis 2018;77:678–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barton JL, Imboden J, Graf J, Glidden D, Yelin EH, Schillinger D. Patient-physician discordance in assessments of global disease severity in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2010;62:857–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desthieux C, Granger B, Balanescu AR, Balint P, Braun J, Canete JD, et al. Determinants of patient-physician discordance in global assessment in psoriatic arthritis: A multicenter european study. Arthritis Care Res (Hoboken) 2017;69:1606–11. [DOI] [PubMed] [Google Scholar]
- 10.Desthieux C, Hermet A, Granger B, Fautrel B, Gossec L. Patient-physician discordance in global assessment in rheumatoid arthritis: A systematic literature review with meta-analysis. Arthritis Care Res (Hoboken) 2016;68:1767–73. [DOI] [PubMed] [Google Scholar]
- 11.Desthieux C, Molto A, Granger B, Saraux A, Fautrel B, Gossec L. Patient-physician discordance in global assessment in early spondyloarthritis and its change over time: The desir cohort. Ann Rheum Dis 2016;75:1661–6. [DOI] [PubMed] [Google Scholar]
- 12.Khan NA, Spencer HJ, Abda E, Aggarwal A, Alten R, Ancuta C, et al. Determinants of discordance in patients’ and physicians’ rating of rheumatoid arthritis disease activity. Arthritis Care Res (Hoboken) 2012;64:206–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barton JL, Trupin L, Tonner C, Imboden J, Katz P, Schillinger D, et al. English language proficiency, health literacy, and trust in physician are associated with shared decision making in rheumatoid arthritis. J Rheumatol 2014;41:1290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barton JL, Hulen E, Schue A, Yelin EH, Ono SS, Tuepker A, et al. Experience and context shape patient and clinician goals for treatment of rheumatoid arthritis: A qualitative study. Arthritis Care Res (Hoboken) 2018;70:1614–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karliner LS, Napoles-Springer AM, Schillinger D, Bibbins-Domingo K, Perez-Stable EJ. Identification of limited english proficient patients in clinical care. J Gen Intern Med 2008;23:1555–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barton JL, Koenig CJ, Evans-Young G, Trupin L, Anderson J, Ragouzeos D, et al. The design of a low literacy decision aid about rheumatoid arthritis medications developed in three languages for use during the clinical encounter. BMC Med Inform Decis Mak 2014;14:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris NS, MacLean CD, Chew LD, Littenberg B. The single item literacy screener: Evaluation of a brief instrument to identify limited reading ability. BMC Fam Pract 2006;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The phq-8 as a measure of current depression in the general population. J Affect Disord 2009;114:163–73. [DOI] [PubMed] [Google Scholar]
- 19.Lorig KR, Sobel DS, Ritter PL, Laurent D, Hobbs M. Effect of a self-management program on patients with chronic disease. Eff Clin Pract 2001;4:256–62. [PubMed] [Google Scholar]
- 20.Gonzalez VM, Stewart A, Ritter PL, Lorig K. Translation and validation of arthritis outcome measures into spanish. Arthritis Rheum 1995;38:1429–46. [DOI] [PubMed] [Google Scholar]
- 21.Martin RW, Head AJ, Rene J, Swartz TJ, Fiechtner JJ, McIntosh BA, et al. Patient decision-making related to antirheumatic drugs in rheumatoid arthritis: The importance of patient trust of physician. J Rheumatol 2008;35:618–24. [PubMed] [Google Scholar]
- 22.Thom DH, Ribisl KM, Stewart AL, Luke DA. Further validation and reliability testing of the trust in physician scale. The stanford trust study physicians. Med Care 1999;37:510–7. [DOI] [PubMed] [Google Scholar]
- 23.Freburger JK, Callahan LF, Currey SS, Anderson LA. Use of the trust in physician scale in patients with rheumatic disease: Psychometric properties and correlates of trust in the rheumatologist. Arthritis Rheum 2003;49:51–8. [DOI] [PubMed] [Google Scholar]
- 24.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 1986;24:67–74. [DOI] [PubMed] [Google Scholar]
- 25.Gadallah MA, Boulos DN, Gebrel A, Dewedar S, Morisky DE. Assessment of rheumatoid arthritis patients’ adherence to treatment. Am J Med Sci 2015;349:151–6. [DOI] [PubMed] [Google Scholar]
- 26.Curtis JR, Yang S, Chen L, Pope JE, Keystone EC, Haraoui B, et al. Determining the minimally important difference in the clinical disease activity index for improvement and worsening in early rheumatoid arthritis patients. Arthritis Care Res (Hoboken) 2015;67:1345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee YC, Agnew-Blais J, Malspeis S, Keyes K, Costenbader K, Kubzansky LD, et al. Post-traumatic stress disorder and risk for incident rheumatoid arthritis. Arthritis Care Res (Hoboken) 2016;68:292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boscarino JA, Forsberg CW, Goldberg J. A twin study of the association between ptsd symptoms and rheumatoid arthritis. Psychosom Med 2010;72:481–6. [DOI] [PubMed] [Google Scholar]
- 29.Freedy JR, Steenkamp MM, Magruder KM, Yeager DE, Zoller JS, Hueston WJ, et al. Post-traumatic stress disorder screening test performance in civilian primary care. Fam Pract 2010;27:615–24. [DOI] [PubMed] [Google Scholar]
- 30.Wilkins KC, Lang AJ, Norman SB. Synthesis of the psychometric properties of the ptsd checklist (pcl) military, civilian, and specific versions. Depress Anxiety 2011;28:596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heinze G, Wallisch C, Dunkler D. Variable selection - a review and recommendations for the practicing statistician. Biom J 2018;60:431–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barton JL, Markwardt S, Schue A, Saha S, Yelin EH. . Goal concordance in rheumatoid arthritis: Beyond pain reduction, is there agreement? Arthritis Rheum 2018;70. [Google Scholar]
- 33.Ladin K, Buttafarro K, Hahn E, Koch-Weser S, Weiner DE. “End-of-life care? I’m not going to worry about that yet.” Health literacy gaps and end-of-life planning among elderly dialysis patients. Gerontologist 2018;58:290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirsh J, Wood P, Keniston A, Boyle D, Quinzanos I, Caplan L, et al. Universal health literacy precautions are associated with a significant increase in medication adherence in vulnerable rheumatology patients. ACR Open Rheumatol 2020;2:110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li L, Cui Y, Yin R, Chen S, Zhao Q, Chen H, et al. Medication adherence has an impact on disease activity in rheumatoid arthritis: A systematic review and meta-analysis. Patient Prefer Adherence 2017;11:1343–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Curtis JR, Bykerk VP, Aassi M, Schiff M. Adherence and persistence with methotrexate in rheumatoid arthritis: A systematic review. J Rheumatol 2016;43:1997–2009. [DOI] [PubMed] [Google Scholar]
- 37.Voshaar MJH, Vriezekolk JE, van Dulmen AM, van den Bemt BJF, van de Laar M. Ranking facilitators and barriers of medication adherence by patients with inflammatory arthritis: A maximum difference scaling exercise. BMC Musculoskelet Disord 2021;22:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCulley C, Katz P, Trupin L, Yelin EH, Barton JL. Association of medication beliefs, self-efficacy, and adherence in a diverse cohort of adults with rheumatoid arthritis. J Rheumatol 2018;45:1636–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


