Abstract
Historically, non-small-cell lung cancer (NSCLC) has been regarded as a nonimmunogenic tumor; however, recent studies have shown that NSCLCs are among the most responsive cancers to monoclonal antibody immune checkpoint inhibitors (ICIs). ICIs have dramatically improved clinical outcomes for a subset of patients (∼20%) with locally advanced and metastatic NSCLC, and they have also demonstrated promise as neoadjuvant therapy for early-stage resectable disease. Nevertheless, the majority of patients with NSCLC are refractory to ICIs for reasons that are poorly understood. Thus, major questions are: how do we initially identify the patients most likely to derive significant clinical benefit from these therapies; how can we increase the number of patients benefiting; what are the mechanisms of primary and acquired resistance to immune-based therapies; are there additional immune checkpoints besides PD-1/PD-L1 and CTLA-4 that can be targeted to provide greater clinical benefit to patients; and how do we best combine ICI therapy with surgery, radiotherapy, chemotherapy, and targeted therapy? To answer these questions, we need to deploy the latest technologies to study tumors and their microenvironment and how they interact with components of the innate and adaptive immune systems. There is also a need for new preclinical model systems to investigate the molecular mechanisms of resistance to treatment and identify novel therapeutic targets. Recent advances in technology are beginning to shed new light on the immune landscape of NSCLC that may uncover biomarkers of response and maximize the clinical benefit of immune-based therapies. Identification of the mechanisms of resistance should lead to the identification of novel targets and the generation of new therapeutic strategies that improve outcomes for a greater number of patients. In the sections below, we discuss the results of studies examining the immune microenvironment in NSCLC, summarize the clinical experience with immunotherapy for NSCLC, and review candidate biomarkers of response to these agents in NSCLC.
Lung cancer is the most common cause of cancer-related mortality worldwide and accounts for more than 1.7 million deaths each year (Bray et al. 2018). Approximately 85% of lung cancers are characterized as non-small-cell lung cancer (NSCLC), which includes three major subtypes: adenocarcinoma (40%), squamous cell carcinoma (30%), and large-cell carcinoma (10%) (Herbst et al. 2018). For many years, the major pillars of NSCLC care consisted of surgery, chemotherapy (CT), and radiation therapy (RT). Recently, immunotherapy was added as a fourth pillar of the cancer treatment plan and provided the first opportunity to treat patients for a potential cure. Now, treatment is guided by molecular testing, and some patients whose tumors harbor actionable molecular aberrations receive personalized therapy that includes small molecule inhibitors (Chen et al. 2014; Garinet et al. 2018). Whereas these agents have been shown to provide a survival benefit for some patients with NSCLC (Kris et al. 2014), not all lung cancers harbor actionable genomic targets.
Immune checkpoint inhibitors (ICIs) may be the best treatment option for a subset of patients with NSCLC. Current clinical guidelines recommend that all newly diagnosed NSCLC patients have their tumors submitted for molecular analyses to complement standard histologic evaluation. After complete staging, decisions are made about whether the patient should undergo a curative surgical resection, with or without neo-adjuvant (pre and/or postoperative) platinum-based CT, potentially followed by adjuvant osimertinib if tumors harbor an epidermal growth factor receptor (EGFR) mutation (mut) (Wu et al. 2020), or if an approach with chemoradiotherapy (CRT) with consolidation immunotherapy (Gray et al. 2020) should be considered. If a patient has metastatic disease and their tumor has an actionable genomic alteration (e.g., EGFR mutations, ALK [anaplastic lymphoma kinase] rearrangements), the patient will receive a targeted therapy directed against that aberration. If not, the patient will receive first-line therapy with ICI with or without CT, and with or without another biologic agent (e.g., bevacizumab) (Chiang and Herbst 2020). The molecular analyses also provide information on the tumor mutation burden (TMB), mutations in key oncogenes/tumor suppressor genes, and standard tumor immunohistochemistry (IHC) information on the expression of PD-L1 (programmed death-ligand 1), which may be used to estimate the likelihood of response to the ICI therapy. ICIs are generally better tolerated than CT and have the potential to provide durable responses by imparting immunologic memory (Brahmer 2013). However, the results generated from recent clinical studies suggest that the majority of unselected patients with lung cancer are refractory to ICIs for reasons that remain unclear. Consequently, there in an urgent need to better understand the cellular and molecular mechanisms that govern the antitumor immune response to identify novel therapeutic targets and develop biomarkers predictive of response to immune-based therapies.
The mechanisms that mediate tumor resistance to ICIs are not completely understood and are the subject of much investigative effort. Tumor genomic aberrations recently shown to be associated with lack of responses to ICIs in NSCLC include STK11 mutations (Skoulidis et al. 2018), EGFR and ALK alterations (Gainor et al. 2016; Gavralidis and Gainor 2020). While some of these genetic alterations are useful biomarkers routinely obtained in patients newly diagnosed with NSCLC to guide therapy, we need to better understand how they contribute to ICI resistance. The examination of the tumor immune contexture, which refers to the spatial orientation, density, and functional role of the different immune cell populations in tumors (Fridman et al. 2012), can yield invaluable information regarding patient prognosis and therapeutic response (Fridman et al. 2017). Analyses of the immune microenvironment have been aided by a variety of techniques, including NanoString technology, RNA-sequencing, cytometry by time-of-flight, intracellular staining flow cytometry, barcoding antibody-based protein arrays (Lyons et al. 2017), and single-cell RNA sequencing (Shalek et al. 2013; Sinjab et al. 2021). In the next sections, we review characteristics of the immune microenvironment in NSCLC and the mechanisms responsible for immune editing of tumors. We also discuss the results from select studies that have led to the use of ICIs for the treatment of NSCLC and the role of biomarkers of therapeutic response.
TUMOR IMMUNE MICROENVIRONMENT IN NSCLC
In health, the innate and adaptive immune systems (Fig. 1) engage in a cooperative series of interactions to protect the lung from pathogens and particulate matter that may enter the respiratory tract (Chen and Kolls 2013). Recent studies suggest that the ability of the immune system to ensure pulmonary homeostasis may be regulated, in part, by the composition of bacteria residing in the gut and lung (Budden et al. 2017). It has also become increasingly clear that the pulmonary immune system may undergo dramatic changes in response to aging (Sharma et al. 2009; Lloyd and Marsland 2017) and lung disease (Sharma et al. 2009). How these changes in immune function affect the development and immunogenicity of NSCLC is not fully understood.
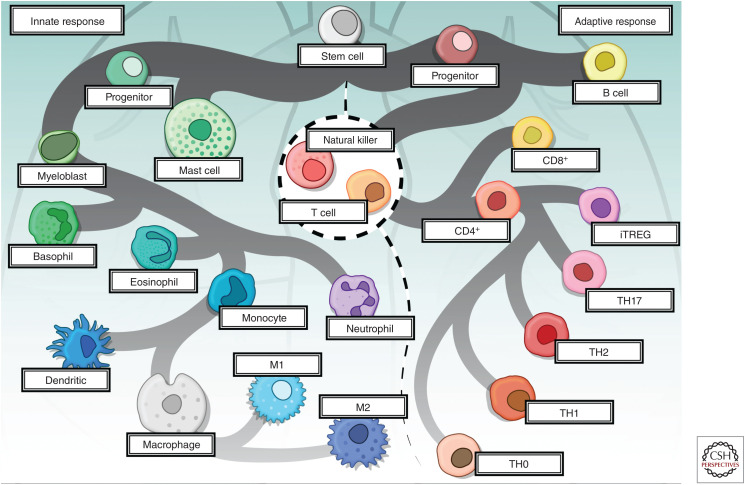
Figure 1.
The cellular mediators of the innate and adaptive immune response. Hematopoietic stem cells differentiate into lymphoid and myeloid progenitors that further branch out into specific cell types mediating innate and adaptive immune response. Cells of the innate immune system provide initial response against non-self antigens, while adaptive immune response is highly specific and is mediated by the activation of lymphocytes. The cellular components of innate immunity include mast cells, basophils, eosinophils, and phagocytic cells, including dendritic cells, macrophages, and neutrophils. Soluble molecules, including complement, acute phase proteins, chemokines, and cytokines make up the humoral component of innate immunity. The cellular components of adaptive immunity include T lymphocytes (CD8+ and CD4+ [TH1, TH2, TH17, and iTREG]), B lymphocytes, and natural killer (NK) cells while immunoglobulins, chemokines, and cytokines make up the humoral component of the adaptive immune system. NK cells, neutrophils, and iTREG cell subsets overlap between both arms of immune responses. (CD) Cluster of differentiation, (TH) T helper cells, (iTREG) induced T regulatory cells.
Reports comparing the immune content of the uninvolved lung with the cancerous lung suggest that the two tissues exhibit marked differences in their immune infiltrates. Studies examining the spatial distribution of tumor-infiltrating immune cells indicate that the cells have a tendency to localize in the stromal compartment and invasive margin of early-stage NSCLC (Lavin et al. 2017). Neutrophils are among the most prevalent immune cells found in NSCLC (Kargl et al. 2017; Stankovic et al. 2019). When compared with blood neutrophils, tumor-associated neutrophils (TANs) exhibit an activated phenotype (CD62LloCD54hi) and express a distinct pattern of chemokine receptors, including up-regulation of C-C motif chemokine receptor (CCR) CCR5, CCR7 and C-X-C motif chemokine receptor CXCR3, and CXCR4 (Eruslanov et al. 2014). TANs isolated from early-stage NSCLCs were found to produce proinflammatory cytokines (MCP-1 [monocyte chemoattractant protein-1], IL-8, MIP-1α [macrophage inflammatory protein-1α], IL-6) and stimulate T-cell proliferation and IFN-γ (interferon γ) secretion (Eruslanov et al. 2014), and to cross-present antigens and amplify effector T-cell responses (Singhal et al. 2016). Whereas the results of these studies advocate a cytotoxic role for TANs in early-stage NSCLC, examinations of experimental lung cancer models suggest that TANs may acquire protumorigenic properties as the disease progresses (Mishalian et al. 2013).
Monocytes traffic to tumors in response to a variety of chemotactic factors in the tumor microenvironment, including MCP-1, TGF-β (transforming growth factor β), and VEGF (vascular endothelial growth factor) (Mantovani et al. 1986; Wahl et al. 1987; Barleon et al. 1996). Tissue-resident alveolar macrophages may contribute to the pool of tumor-associated macrophages (TAMs), but reports indicate that the majority of TAMs are derived from monocyte recruitment from the periphery (Yang and Zhang 2017). TAMs comprise only 5% of the immune infiltrate in NSCLC (Stankovic et al. 2019), and yet are one of the key effectors in mediating cancer-related inflammation (Sica 2010). Macrophage content in early-stage NSCLC is comparable to that in uninvolved lung, but TAMs express higher levels of PD-L1 (Lavin et al. 2017; Liu et al. 2020), a protein that suppresses the adaptive arm of the immune system. In general, TAMs are associated with an M2 phenotype, oriented toward promoting tumor growth, tissue remodeling, and suppressing adaptive immunity (Mantovani et al. 2008). TAMs may also modulate tumor progression through their promotion of tumor angiogenesis by producing potent endothelial cell mitogens, including VEGF and fibroblast growth factor 2 (FGF-2) (Baird et al. 1985). VEGF may also interfere with immune cell recruitment by reducing lymphocyte adhesion to activated endothelial cells (Bouzin et al. 2007).
T lymphocytes are enriched in early-stage NSCLCs when compared to matched uninvolved lung specimens (Ganesan et al. 2013; Lavin et al. 2017; Stankovic et al. 2019). In an examination of resected tumors from 68 patients with localized NSCLC, the most common T cells in tumors were CD4+ cells (26%) followed by CD8+ cells (22%) (Stankovic et al. 2019). Results from single-cell analysis of 32 resected NSCLCs and matched uninvolved lung tissues indicate that tumors contain higher frequencies of nonfunctional T cells and Forkhead box P3 (Foxp3)-expressing CD4+ T regulatory (TREG) cells than the uninvolved lung (Lavin et al. 2017). Regulatory CD4+CD25+ T cells that were isolated from stage I–II NSCLCs inhibited the proliferation of autologous peripheral blood T cells (Woo et al. 2002), suggesting that immune suppression may be an early event in the disease process. Increased TREG counts are associated with poor outcomes in NSCLC (Shimizu et al. 2010; Tao et al. 2012), whereas high densities of CD8+ T cells are an independent positive prognostic predictor for resectable NSCLC (Al-Shibli et al. 2008). Predominant infiltration of CD8+ T cells and macrophages into the cancer compartment as opposed to the stromal compartment has also been shown to be an independent predictor of survival in patients with advanced NSCLC (Kawai et al. 2008). Studies have shown that natural killer (NK) cells are reduced in NSCLC compared to noncancerous lung tissue, and that tumor-infiltrating NK cells have diminished cytotoxic function (Platonova et al. 2011; Lavin et al. 2017; Stankovic et al. 2019).
B lymphocytes are one of the most prevalent immune cells in NSCLC (Del Mar Valenzuela-Membrives et al. 2016; Stankovic et al. 2019), and are commonly found in tertiary lymphoid structures (TLS) located at the interface between tumor and stroma. The organization of these structures resembles that of secondary lymphoid organs. TLS contain a T-cell zone with lysosome-associated membrane glycoprotein 3-positive mature dendritic cells (DCs) and a segregated follicular zone where CD20+ B cells actively proliferate and differentiate into plasma cells (Germain et al. 2014; Fridman et al. 2017). A high density of follicular B cells correlates with long-term survival in patients with NSCLC (Germain et al. 2014). Immunohistochemical studies of resected NSCLCs suggest that the molecular mechanisms that mediate T-cell recruitment to TLS are similar to those that govern T-cell trafficking to lymph nodes and involve adhesive interactions between CD62L (L selectin) expressed on T cells and its corresponding counter-receptors located on the surface of high endothelial venules (de Chaisemartin et al. 2011). In addition to CD20+ B cells, the follicular zone also contains CD3+ T cells, a network of CD21+ DCs, CD68+ macrophages, and shows evidence of an ongoing humoral response (Germain et al. 2014). B cells play an important role in regulating NSCLC progression. Several studies have demonstrated an antitumor role for B cells in NSCLC by virtue of their ability to produce tumor-specific antibodies (Germain et al. 2014) and enhance effector T-cell responses (Wang et al. 2019a). Tumor-infiltrating B cells also present antigen to CD4+ T cells and can modulate the CD4+ T-cell phenotype (Bruno et al. 2017). Alternatively, B regulatory cells have the potential to impair an antitumor immune response by producing immunosuppressive cytokines, such as interleukin (IL)-10 and TGF-β (Wang et al. 2019a). This dual ability of the immune system to both restrain and promote tumor growth forms the basis for the cancer immunoediting hypothesis, which is discussed below.
IMMUNOEDITING AND MECHANISMS OF IMMUNOSUPPRESSION IN NSCLC
The cancer immunoediting hypothesis proposes that the immune system provides protection against tumor formation and shapes tumor immunogenicity through a series of three related processes: elimination, equilibrium, and escape (Schreiber et al. 2011). The hypothesis provides a framework for studying the complex cellular and molecular interactions between tumor and immune cells. During the elimination phase, the innate and adaptive immune system cooperate to recognize transformed cells that have escaped intrinsic tumor suppression and eliminate the cells before a tumor becomes clinically detectable (O'Donnell et al. 2019). If the cancer is completely cleared during the elimination phase, the process is complete. However, cancer cells that are able to survive the elimination phase may pass into the equilibrium phase. During the equilibrium phase, net growth is restricted and cellular immunogenicity is edited by the adaptive immune system (Koebel et al. 2007; O'Donnell et al. 2019). Tumor variants possessing either dampened immunity or the capacity to attenuate immune responses may enter the escape phase and give rise to clinically apparent cancers (Koebel et al. 2007).
T cells play a critical role during the equilibrium process by virtue of their ability to recognize tumor antigens and restrict tumor growth. Antigens capable of eliciting a T-cell response are typically placed into three categories: viral antigens, antigens that are the product of a gene mutation or rearrangement, and antigens that are encoded by cancer-germline genes (Coulie et al. 2014). Smoking-related NSCLCs possess one of the highest TMBs among all cancers (Alexandrov et al. 2013). However, studies in melanoma, which have a TMB comparable to that of NSCLC (>10 somatic mutations [mut] per megabase [mb] of coding DNA [deoxyribonucleic acid]), suggest that only a small fraction of nonsynonymous mutations actually results in neoantigens capable of eliciting tumor-infiltrating lymphocyte (TIL) reactivity (Linnemann et al. 2015; Schumacher and Schreiber 2015). The diminished number of immunogenic neoantigens in NSCLC may result from deficiencies in the antigen-presenting machinery (Korkolopoulou et al. 1996). Promoter hypermethylation has also been identified as a mechanism of transcriptomic neoantigen depletion (Rosenthal et al. 2019).
Studies have also shown that soluble factors in the tumor immune microenvironment may contribute to tumor escape. DCs play a critical role in mediating an antitumor immune response by acquiring, processing, and presenting tumor-associated antigens on major histocompatibility complex (MHC) molecules and providing the costimulatory and soluble factors that shape the T-cell response (Wculek et al. 2020). Cell-based studies suggest that cross talk between NSCLC cells and DCs may contribute to an impaired immune response via secretion of immunosuppressive cytokines, such as TGF-β1 from DCs (Dumitriu et al. 2009).
Tumor metabolism may also play a critical role in cancer development and therapeutic resistance (Chang et al. 2015; Altman et al. 2016; Nakazawa et al. 2016), and is now regarded as a hallmark of cancer (Hanahan and Weinberg 2011). Cancer cells rewire their metabolism to promote their growth and ensure their survival through increased glucose metabolism even in the presence of functioning mitochondria (i.e., the Warburg effect) (Liberti and Locasale 2016). The up-regulation of cancer cell glycolysis leads to accumulation of lactate in the tumor microenvironment, which, in turn, leads to the polarization of TAMs to an M2 phenotype (Colegio et al. 2014). Lactate also suppresses the activity of T cells and NK cells (Renner et al. 2017). Increased tumor glycolysis limits T-cell infiltration of NSCLCs and melanomas (Cascone et al. 2018). Melanoma-derived lactic acid impaired effector T-cell function in vitro, and inhibition of lactic acid rendered patient-derived melanoma cells susceptible to autologous TIL-mediated killing (Cascone et al. 2018). In a retrospective analysis of tumor preoperative positron emission tomography (PET) 18F-FDG uptake in 59 resected NSCLCs, we found that each 18F-FDG PET parameter was positively correlated with tumor bulk expression of glycolysis-related genes and increased FDG (fluorodeoxyglucose) uptake was associated with an immunosuppressive and poorly immune infiltrated tumor microenvironment (Mitchell et al. 2020). Other molecules, such as ectoenzymes, are up-regulated on tumor and tumor-infiltrating cells and lead to immunosuppressive conditions. A good example that has been identified as a mediator of tumor immunosuppression contributing to ICI-resistance in NSCLC is CD38, which is induced by all-trans retinoic acid and IFN-β in the tumor microenvironment. CD38 drives adenosine production and modulates the intermediates of the adenosine pathway, producing inhibition of CD8+ T-cell function (Chen et al. 2018; Konen et al. 2020).
IMMUNE CHECKPOINT PROTEINS
Another way in which tumors limit an immune response is by exploiting immune checkpoint proteins (Fig. 2). The programmed cell death protein 1 (PD-1) immune checkpoint is expressed on the surface of T cells, B cells, NK cells, and some myeloid cells (Agata et al. 1996). PD-1 has two known ligands, PD-L1 and PD-L2 (programmed death-ligand 2), and engagement of PD-1 with its ligands inhibits T-cell receptor (TCR)-mediated lymphocyte proliferation and cytokine secretion (Freeman et al. 2000). PD-L1 is constitutively expressed on several immune cell types (Ishida et al. 2002), and expression is inducible on cancer cells in response to IFN-γ (Dong et al. 2002). EGFR mutations may also up-regulate PD-L1 on NSCLC cells (Akbay et al. 2013). PD-L2 has a more restricted expression pattern and is inducible on DCs and macrophages in response to IFN-γ, granulocyte-macrophage colony-stimulating factor (GM-CSF), and IL-4 (Yamazaki et al. 2002). In NSCLC, PD-L1 expression is particularly abundant in macrophages in both the tumor and stromal compartments (Liu et al. 2020). PD-L1-expressing tumor cells and antigen-presenting cells can suppress tumor immunity through multiple mechanisms, including induction of T-cell apoptosis, anergy, functional exhaustion, and IL-10 production (Dong et al. 1999, 2002; Selenko-Gebauer et al. 2003; Chen and Han 2015).
Figure 2.
Immune checkpoint receptors expressed on immune cells and their respective ligands on antigen-presenting cells (APCs) and/or tumor cells highlighting select targetable receptors. (PD-1) Programmed cell death protein 1, (PD-L1) programmed cell death-ligand 1, (CTLA4) cytotoxic T-lymphocyte-associated protein 4, (LAG3) lymphocyte activation gene 3, (MHC-II) major histocompatibility complex-class II, (TIM3) T-cell immunoglobulin and mucin-domain containing 3.
The cytotoxic T lymphocyte-associated protein 4 (CTLA-4) immune checkpoint protein functions to down-regulate immune responses. CTLA-4 attenuates TCR signaling through competition with the costimulatory molecule CD28 for the B7 ligands CD80 and CD86 (Wei et al. 2018). CTLA-4 also suppresses T-cell responses by blocking IL-2 production, IL-2 receptor expression, and cell-cycle progression of activated T cells (Walunas et al. 1996). The physiologic function of CTLA-4 is to suppress T-cell responses to self-antigens by controlling TREG activity (Chen and Han 2015). Consequently, blocking CTLA-4 signaling can result in autoimmune damage to different organ systems (Tarhini 2013). Studies in melanoma indicate that autoimmunity correlates with tumor regression in patients treated with anti-CTLA-4 inhibitors (Attia et al. 2005).
The lymphocyte activation gene-3 (LAG-3) (CD223) immune checkpoint protein is ∼20% identical to CD4 (Triebel et al. 1990). LAG-3 is expressed on activated CD4+ and CD8+ T cells, TREGs, a subpopulation of NK cells, B cells, and plasmacytoid DCs (Qin et al. 2019). MHC-II is a canonical LAG-3 ligand and galectin-3, LSECtin, a-synuclein and fibrinogen-like protein 1 (FGL1) also interact with LAG-3 (Qin et al. 2019). LAG-3 signaling plays a negative regulatory role in T helper 1 (TH1) cell activation, proliferation, and cytokine secretion (Workman et al. 2002). Dual blockade of LAG-3 and PD-1 has been shown to enhance antitumor immunity and suppress tumor growth by reinvigorating CD8+ TILs and decreasing TREGs in the tumor microenvironment (Huang et al. 2015a).
T-cell immunoglobulin and mucin-domain containing-3 (TIM-3) is an immune checkpoint protein expressed on CD4+ TH1 and CD8+ T cytotoxic cells and on other immune cells, including B cells, TREGs, NK cells, DCs, monocytes, and macrophages (Anderson 2012). Four distinct ligands have been reported to bind to the IgV domain of TIM-3, including galectin-9, high-mobility group protein B1 (HMGB1), carcinoembryonic antigen cell adhesion molecule 1 (Ceacam-1), and phosphatidyl serine (PtdSer) (Qin et al. 2019). The binding of TIM-3 to its ligands induces intracellular calcium flux of TH1 cells, leading to cell death and negative regulation of T-cell responses (Huang et al. 2015b).
In the sections below, we summarize some of the key findings from completed and ongoing clinical studies of cellular therapy and ICIs for the treatment of patients with NSCLC.
CLINICAL EXPERIENCE WITH ADOPTIVE CELL THERAPY WITH TILs IN METASTATIC NSCLC
Adoptive T-cell transfer therapy in metastatic melanoma patients has produced durable complete response rates of 10%–15% (Rosenberg and Restifo 2015). A phase 1 trial recently reported the initial results of TIL transfer as a therapeutic approach in patients with metastatic NSCLC (Creelan et al. 2020). Autologous TILs cultured and expanded from a metastatic lesion were administered as a single infusion in patients who progressed after four doses of nivolumab, followed by IL-2 intermediate treatment, and nivolumab maintenance treatment for 1 year. TIL therapy led to a best objective response rate (ORR) of 25% with an overall manageable toxicity profile and most patients exhibited low or no tumor PD-L1 expression as well as low TMB. Interestingly, infused TILs were reactive to autologous tumor and, in ∼10% of cases, to predicted neoepitopes. Analyses of the TCR clonotype and phenotype indicated good persistence of the transferred TILs, which, in some cases, correlated with clinical benefit (Creelan et al. 2020). These results are encouraging and lend support for continuing to test and optimize TIL therapy for patients with NSCLC. An ongoing phase 2 trial is evaluating a single dose of patient's tumor-derived TILs followed by either a high or low dose of aldesleukin (a recombinant form of human IL-2) in subjects with metastatic NSCLC (NCT02133196).
CLINICAL TRIALS OF IMMUNE CHECKPOINT INHIBITORS FOR ADVANCED/METASTATIC NSCLC
First-Line Therapy
First-line therapy with nivolumab, a fully humanized IgG4 (immunoglobulin G4) antibody directed against PD-1, or atezolizumab, a fully humanized IgG1 monoclonal antibody that targets PD-L1, in patients with PD-L1 positive metastatic NSCLC did not produce survival benefit compared with CT in either the CheckMate 026 (Carbone et al. 2017) or IMpower110 (Spigel et al. 2019) trials, respectively. A significant survival improvement was only noted with atezolizumab in tumors with high PD-L1 expression. In the phase 3 KEYNOTE-024 trial comparing pembrolizumab, a fully humanized IgG4 antibody targeting PD-1, with CT in patients with advanced NSCLC, patients whose tumors expressed PD-L1 ≥ 50% and were treated with pembrolizumab, had longer progression-free survival (PFS) and overall survival (OS) than patients treated with CT (Reck et al. 2016). In the phase 3 KEYNOTE-042 trial comparing pembrolizumab with CT for patients with metastatic NSCLC, the OS benefit with pembrolizumab was largely due to patients with high PD-L1 expression (Mok et al. 2019; Morgensztern 2019). A recent retrospective analysis revealed that survival outcomes with first-line pembrolizumab treatment are improved in patients with high PD-L1 expression, especially for those whose tumors have a PD-L1 ≥ 90% (Aguilar et al. 2019).
The CheckMate 227 study is a multipart phase 3 trial that compared first-line nivolumab plus ipilimumab (CTLA-4 inhibitor) with CT in patients with advanced/metastatic NSCLC (Hellmann et al. 2018). PFS was significantly longer with nivolumab plus ipilimumab than with CT (7.2 vs. 5.5 mo) in patients with a high TMB (≥10 mut/mb), irrespective of PD-L1 expression. There was no PFS difference noted in patients with low TMB (<10 mut/mb), and no differences in OS benefit between both tumor TMB cutoffs assessed. In the portion of the study that reported the coprimary end point OS in patients with PD-L1 ≥ 1%, nivolumab plus ipilimumab was associated with a significant OS improvement compared to CT (17.2 vs. 12.2 mo). This benefit occurred for populations with PD-L1 ≥ 1% (HR 0.79) and PD-L1 < 1% (HR 0.62) (Hellmann et al. 2019). These results led to Food and Drug Administration (FDA) approval of the combination as first-line treatment for patients with metastatic NSCLC with no EGFR and ALK genomic aberrations and whose tumors express PD-L1 (≥1%), as determined by an FDA-approved test. In the phase 3 MYSTIC trial, durvalumab (PD-L1 inhibitor) alone or in combination with tremelimumab (CTLA-4 inhibitor) did not induce significant survival improvements in patients with PD-L1 > 25% when compared to CT (Rizvi et al. 2020).
CT can potentially synergize with ICIs through several different mechanisms, including killing tumor cells and improving the T cell-to-tumor ratio, reducing immunosuppressive molecules, and by releasing antigens for presentation, thereby broadening the antitumor response (Herbst and Sznol 2016). CT has also been shown to increase PD-L1 expression in NSCLC cells (Parra et al. 2018). In the phase 3 trial KEYNOTE-189 comparing first-line pembrolizumab plus CT versus CT alone in metastatic nonsquamous NSCLC without EGFR or ALK aberrations, the combination significantly increased both 12-mo OS (69.2% vs. 49.4%) and median PFS (8.8 vs. 4.9 mo) compared with CT (Gandhi et al. 2018). In the phase 3 KEYNOTE-407 trial that investigated the addition of pembrolizumab to first-line CT in patients with squamous NSCLC, pembrolizumab plus CT produced a significant increase in OS compared with CT (15.9 vs. 11.3 mo) regardless of PD-L1 expression (Paz-Ares et al. 2018). Other phase 3 trials tested atezolizumab combined with CT (IMpower132 and IMpower130) (Barlesi et al. 2018; West et al. 2019), and atezolizumab with carboplatin, paclitaxel, and bevacizumab (ABCP) in the three-arm phase 3 trial IMpower150 (Socinski et al. 2018). In this study, treatment with ABCP improved the PFS and OS compared with BCP (bevacizumab + carboplatin + paclitaxel), but ACP (atezolizumab + carboplatin + paclitaxel) did not produce a significant survival benefit compared with BCP. In patients with squamous histology, the addition of atezolizumab to CT did not improve OS in the IMpower131 trial, with the exception of patients with high tumor PD-L1 expression (Jotte et al. 2019). The phase 3 CheckMate 9LA trial testing the addition of nivolumab and ipilimumab to two cycles of CT compared to up to four cycles of CT alone in the first-line setting in patients with metastatic NSCLC showed that OS, PFS, and ORR were improved with combination chemoimmunotherapy compared with CT alone, irrespective of PD-L1 expression (Reck et al. 2020). At the 2021 American Society for Clinical Oncology (ASCO) Annual Meeting, updated data demonstrated continued benefit from the combination strategy: at a minimum follow-up of 2 yr, the median OS was 15.8 mo with chemoimmunotherapy versus 11.0 mo with CT alone and the 2-yr OS rates were 38% versus 26%, respectively (Reck et al. 2021). These studies provide evidence that the chemoimmunotherapy combination is superior to CT alone as the first-line treatment for patients with advanced NSCLC, regardless of tumor PD-L1 expression status. However, at this time, there is no clear evidence that the efficacy of CT plus ICI is superior to that of ICIs alone in patients with high tumor PD-L1 expression (Remon et al. 2020).
Second-Line Therapy
The phase 3 CheckMate 017 trial compared the safety and efficacy of nivolumab with docetaxel in patients with advanced squamous NSCLC who had progressed on first-line CT (Brahmer et al. 2015). The median OS was 9.2 and 6.0 mo for nivolumab and docetaxel, respectively. Similar results were obtained in the CheckMate 057 trial comparing second-line nivolumab with docetaxel in patients with nonsquamous NSCLC (Borghaei et al. 2015), with a median OS of 12.2 and 9.4 mo with nivolumab and docetaxel, respectively. Consequently, nivolumab was approved by the FDA for treatment of patients with metastatic squamous and nonsquamous NSCLC with progression on or after failure with CT.
In the phase 2/3 KEYNOTE-10 study comparing 2 mg/kg pembrolizumab, 10 mg/kg pembrolizumab, and docetaxel in patients with previously treated, PD-L1-positive, advanced NSCLC, the median OS was 10.4 mo, 12.7 mo, and 8.5 mo, respectively (Herbst et al. 2016). Among patients with tumor PD-L1 ≥ 50%, OS was significantly longer with pembrolizumab 2 mg/kg and 10 mg/kg versus docetaxel. Updated results of KEYNOTE-010 demonstrated that pembrolizumab treatment improved OS over docetaxel in the PD-L1 expression ≥ 50% and ≥ 1% groups after a median follow up time of 42.6 mo (Herbst et al. 2020). In the phase 2 POPLAR trial (Fehrenbacher et al. 2016), OS was 12.6 mo for atezolizumab versus 9.7 mo for docetaxel in patients with NSCLC who progressed on CT. Improvement correlated with PD-L1 expression on tumor and tumor-infiltrating immune cells, suggesting that PD-L1 expression is predictive for atezolizumab benefit. Similar results were reported in the OAK randomized phase 3 study (Rittmeyer et al. 2017).
ICI Therapy in Patients with Actionable Tumor Molecular Aberrations
Recent evidence suggests that the clinical activity and efficacy of ICIs are limited in patients with NSCLC harboring aberrations in EGFR and ALK. In a retrospective study evaluating the activity of PD-1 and PD-L1 inhibitors in 58 patients with NSCLC, responses were noted in 3.6% (1/28) of tumors harboring EGFR mutations or ALK rearrangements compared with 23.3% (7/30) of tumors characterized as EGFR wild-type and ALK-negative/unknown (Gainor et al. 2016). In a meta-analysis of more than 3000 patients with advanced NSCLC treated with a PD-1 or PD-L1 inhibitor or docetaxel, ICI therapy prolonged OS versus docetaxel in the EGFR wild-type subgroup, but not in the EGFR-mutant subgroup (Lee et al. 2018).
Recent studies have investigated the use of ICIs in combination with additional therapies for patients whose tumors harbor EGFR and ALK genomic alterations. The phase 3 IMpower150 study allowed enrollment of patients with EGFR and ALK alterations and demonstrated that ABCP treatment prolonged PFS in the overall population and in this subgroup of patients. Ongoing phase 3 trials are testing the clinical efficacy of ICI-CT combination in patients with EGFR-mutant NSCLC after progression on EGFR tyrosine kinase inhibitors (TKIs) (NCT03515837). Initial reports suggest that the combination of ICIs and TKIs induces higher frequency of toxicities compared to single agents without increased clinical activity (Antonia et al. 2014; Ma et al. 2016; Liang et al. 2018; Creelan et al. 2021). A retrospective analysis of patients harboring EGFR-mutant NSCLC demonstrated that sequential treatment with ICI therapy followed by osimertinib treatment was associated with onset of severe immune-related toxicities, particularly in patients who received osimertinib within 3 mo of last ICI dose (Schoenfeld et al. 2019). These results show the limited applicability of ICIs in patients with NSCLC harboring EGFR and ALK alterations and emphasize the importance of carefully considering the administration of these agents to these subgroups of patients.
CLINICAL TRIALS OF ICIs IN LOCALLY ADVANCED/UNRESECTABLE NSCLC
Tumor RT promotes immune effects by inducing proinflammatory signals that facilitate the recruitment of immune cells to the tumor microenvironment and by increasing neoantigen exposure and availability (Barker et al. 2015). Consequently, there is considerable interest in examining therapeutic effects of combined immunotherapy and RT. The randomized phase 3 PACIFIC study compared the anti-PD-L1 antibody durvalumab as consolidation therapy with placebo in patients with stage III NSCLC who did not have disease progression after two or more cycles of platinum-based CRT. Consolidation therapy with durvalumab produced significant improvements compared with placebo in both PFS (17.3 vs. 5.6 mo) and OS (not reached vs. 28.7 mo) (Antonia et al. 2018). These results established the PACIFIC regimen as the standard of care in this population. Subgroup analyses indicated that PFS and OS were superior if durvalumab was delivered fewer than 14 d after the completion of RT-CT. The 3-yr OS rates were 57% with durvalumab versus 43.5% with placebo (Gray et al. 2020). Updated results from the PACIFIC study were presented at the ASCO Annual Meeting 2021 (Spigel et al. 2021) and demonstrated that, at a median follow-up duration of 34.2 mo in all patients, OS (median 47.5 vs. 29.1 mo) and PFS (median 16.9 vs. 5.6 mo) remained consistent with the results from the primary analyses. The 60-mo OS rates were 42.9% and 33.4% with durvalumab and placebo, respectively, and 60-mo PFS rates were 33.1% and 19.0%, respectively (Spigel et al. 2021).
Several studies are investigating concurrent administration of ICIs and RT for unresectable stage III NSCLC. The ETOP NICOLAS trial is evaluating the safety and efficacy of nivolumab concurrent with CT-RT in 82 patients. In the first 21 patients, no grade 3 or higher pneumonitis was observed by the end of the 3-mo post-RT follow-up period and no unexpected or increased toxicities were reported (Peters et al. 2019). The DETERRED trial investigated concurrent atezolizumab with CT-RT followed by CT-atezolizumab and maintenance atezolizumab and reported that concurrent atezolizumab and CT-RT is safe without increased toxicities (Lin et al. 2019). The KEYNOTE-799 phase 2 nonrandomized 2-cohort study investigated pembrolizumab plus concurrent CRT (cCRT) in 112 NSCLC patients with locally advanced and untreated NSCLC and demonstrated promising antitumor activity of pembrolizumab plus cCRT and an overall manageable safety and toxicity profile (Jabbour et al. 2021).
In addition to promoting antitumor activity at the primary tumor site, RT can also induce systemic effects outside of the irradiation field (i.e., abscopal effects) (Demaria and Formenti 2020). Whereas the precise biological mechanisms underlying an abscopal response remain unclear, the effect has been connected to mechanisms involving the immune system and there is growing interest in combining immunotherapy with RT as a therapeutic strategy to enhance abscopal response rates (Ngwa et al. 2018). There is some evidence to support this supposition. A recent study found that combined RT and CTLA-4 blockade induced systemic antitumor T cells in CT-refractory NSCLC, whereas anti-CTLA-4 antibodies had failed to demonstrate efficacy alone or in combination with CT (Formenti et al. 2018). The investigators reported an ORR of 18% with two complete and five partial responses. These findings demonstrate that RT can synergize with ICIs to produce control of both primary NSCLC and distal metastases.
CLINICAL TRIALS OF ICIs FOR EARLY-STAGE NSCLC
Adjuvant Immunotherapy Trials
Surgery is the preferred treatment approach for patients with operable early-stage NSCLC (Howington et al. 2013). However, more than 50% of patients experience tumor recurrence after surgery alone (Martin et al. 2002). The use of perioperative (neoadjuvant or adjuvant) CT as a means to prevent disease recurrence after surgery provides only a modest (∼5%) improvement in 5-yr OS and is also a source of considerable toxicity (Pignon et al. 2008; NSCLC Meta-Analysis Collaborative Group 2014). ICIs are being evaluated in the adjuvant setting with the goal to eradicate micrometastatic disease and reduce the risk of relapse, based on the rationale that the postsurgical stress response is characterized by physiological cellular mediators that may impair innate and acquired immune responses by generating an immunosuppressive state that favors tumor progression (Bakos et al. 2018). Several phase 3 trials are investigating adjuvant ICIs for resectable stage IB–IIIA NSCLC. The randomized phase 3 open-label IMpower010 trial evaluated adjuvant atezolizumab compared to best supportive care (BSC) after adjuvant platinum-based CT in patients with early-stage resected NSCLC (Wakelee et al. 2021). The primary end point of disease-free survival (DFS) was analyzed in three subgroups: patients with stage II–IIIA tumors and PD-L1 ≥ 1%; all randomly assigned patients with stage II–IIIA NSCLC; and intention-to-treat (ITT) population with stage IB–IIIA NSCLC. In patients with stage II–IIIA resected NSCLC and PD-L1 tumor composite score ≥ 1%, adjuvant atezolizumab reduced the risk of disease progression or death by 34% versus BSC. Median DFS was not reached in the atezolizumab-treated group versus 35.3 mo for patients who received BSC and no new safety concerns were noted. These results are promising to implement an ICI agent in the adjuvant setting for some patients with resected NSCLC; however, several challenges remain in this disease setting, including the lack of established predictive biomarkers of therapeutic benefit, the unclear ideal length of adjuvant treatment and the role of DFS used as a primary end point as compared to the use of OS.
Neoadjuvant Immunotherapy
The results from the clinical trials of ICIs in patients with metastatic NSCLC provided the impetus for testing checkpoint inhibitors in resectable, early-stage NSCLC. Administering ICIs in the neoadjuvant setting provides an opportunity to prime the antitumor immune response and eradicate micrometastases at an early time point when tumor-specific antigens may possess less heterogeneity and induce greater antitumor control. Neoadjuvant treatment also allows rapid assessment of tumor sensitivity or resistance to therapy, particularly when there are evaluable surrogate end points at the time of surgery that can correlate with hard end points of clinical efficacy. Clinical trials using OS as a primary end point require a decade or longer to complete (Hellmann et al. 2014). Major pathologic response (MPR), defined as ≤10% viable tumor cells in resected tumor specimens, provides an objective criterion of response to neoadjuvant CT and correlates with long-term outcomes (Pataer et al. 2012). MPR has been incorporated as a candidate surrogate of survival benefit in neoadjuvant trials testing new therapeutic strategies for patients with early-stage NSCLC.
In the first feasibility study, neoadjuvant nivolumab had a tolerable toxicity profile, did not delay surgery, and resulted in an MPR rate of 45% (Forde et al. 2018). TMB, but not pretreatment PD-L1 expression, was associated with MPR. In the Lung Cancer Mutation Consortium 3 (LCMC3) study, neoadjuvant atezolizumab produced a 21% MPR rate, tumor PD-L1 ≥ 50% expression was associated with MPR to therapy, and a weak correlation was noted between MPR and TMB with a trend toward less pathological regression in STK11-mutated tumors (Carbone et al. 2021). In the phase 2 randomized NEOSTAR trial, which tested neoadjuvant nivolumab as monotherapy or in combination with ipilimumab in 44 patients with resectable NSCLC (Cascone et al. 2021), nivolumab produced a 22% MPR rate, whereas the combination therapy produced a 38% MPR rate in the ITT population (Cascone et al. 2021). Tumor PD-L1 expression at baseline was associated with radiographic responses and greater tumor regression; however, responses were also observed in some patients lacking tumor PD-L1 expression pretherapy (Cascone et al. 2021). In a phase 1b study, the neoadjuvant PD-1 inhibitor sintilimab produced a 40.5% MPR rate in 37 Chinese patients with resected NSCLC (Gao et al. 2020). The NADIM phase 2 trial tested neoadjuvant nivolumab plus CT followed by surgical resection and adjuvant nivolumab treatment for 1 yr in 46 patients with resectable stage IIIA N2 NSCLC. The MPR response was 83% (34/41) in the resected patients or 73% (34/46) in the ITT patients and 24 mo OS was 89.9% (Provencio et al. 2020). Neoadjuvant atezolizumab plus CT induced MPR rates of 57% in treated patients with resectable NSCLC, and no significant associations between MPR and PD-L1 expression were observed (Shu et al. 2020). The initial results of the phase 3 randomized study CheckMate 816 investigating neoadjuvant platinum doublet CT plus nivolumab versus CT in patients with operable NSCLC without EGFR or ALK genomic aberrations and using pathological complete response (pCR) and event-free survival (EFS) as primary end points were recently reported (Forde et al. 2021). The pCR rate was 24% with chemoimmunotherapy as compared to 2.2% with CT alone in the ITT population. The MPR rates were 36.9% with combination therapy versus 8.9% with CT alone. This study is the first phase 3 trial in the neoadjuvant immunotherapy setting to show the benefit of an ICI plus platinum-doublet CT over CT alone. Several ongoing phase 3 trials are evaluating perioperative combinations of ICIs with CT, including IMpower030 (atezolizumab), KEYNOTE-671 (pembrolizumab), AEGEAN (durvalumab), and CheckMate 77T (nivolumab) trials. Much investigative effort is directed toward the identification of several emerging tissue-, blood-, and host-based biomarkers that could be used to maximize the clinical benefit of ICIs (Fig. 3). In the next section, we discuss the evidence supporting the role for some of these biomarkers, including tumor PD-L1, TMB, circulating tumor DNA (ctDNA), and gut microbiome.
Figure 3.
Emerging tissue-, blood-, and host-based biomarkers of potential clinical benefit or therapeutic resistance to immune checkpoint inhibitors (ICIs) under evaluation in NSCLC. (PD-L1) Programmed death-ligand 1, (TILs) tumor-infiltrating lymphocytes, (TLS) tertiary lymphoid structures, (TMB) tumor mutation burden, (TCR) T-cell receptor, (ITH) intratumoral heterogeneity, (NKs) natural killer cells, (PD-1) programmed cell death protein 1, (NLR) neutrophil-to-lymphocyte ratio, (M:L) myeloid-to-lymphoid ratio, (PLR) platelet-to-lymphocyte ratio, (ctDNA) circulating tumor DNA, (bTMB) blood tumor mutation burden.
PD-L1 EXPRESSION AS A BIOMARKER FOR ICIs IN NSCLC
PD-L1 expression has been evaluated as a prospective selection marker for ICI therapy in both first-line and second-line settings in patients with NSCLC. PD-L1 expression is determined by standard immunohistochemical staining of tissue sections that are then read by pathologists as the tumor proportion score (TPS), which represents the best estimated percentage (0%–100%) of tumor cells showing partial or complete membranous PD-L1 staining (Lantuejoul et al. 2020) and percentage of immune cells with similar expression (immune cell proportion score [ICPS]). Despite the fact that only pembrolizumab and nivolumab plus ipilimumab requires a PD-L1 IHC assay as a companion diagnostic to determine patient eligibility for therapy, PD-L1 IHC has also been established as a complementary diagnostic for other PD-L1 inhibitors to determine patient eligibility (Büttner et al. 2017). Recently, two large studies demonstrated that three of the five IHC assays for assessing PD-L1 expression were closely aligned on tumor cell staining (22C3, 28-8, and SP263 assays), whereas the SP142 assay exhibited fewer stained tumor cells overall, and the 73–10 assay had higher sensitivity (Rimm et al. 2017; Tsao et al. 2018). These studies also showed that there is reliability among pathologists in tumor cell PD-L1 scoring with all assays, but poor reliability in immune cell PD-L1 scoring (Tsao et al. 2018).
The results of the clinical studies thus far indicate that there is considerable variability in PD-L1 as a biomarker of response to ICIs. While patients whose tumors express elevated PD-L1 levels may exhibit improved responses to treatment, patients whose tumors lack PD-L1 expression may also receive benefit. PD-L1 expression remains an imperfect marker of response for several reasons, including tumor heterogeneity, the fact that it is assessed as a continuous variable, and different conditions and therapies may regulate its expression (Lantuejoul et al. 2020). Therefore, the use of PD-L1 IHC expression, at this time, should be dedicated to select patients with advanced NSCLC eligible to treatment with ICIs.
TMB AS A BIOMARKER FOR ICIs IN NSCLC
TMB refers to the number of somatic mutations within the coding region of a tumor. It is reasonable to assume that a tumor with a high TMB would be more likely to express a greater number of neoantigens as compared to a tumor with a low TMB and thus be more responsive to ICIs. The relationship between TMB and clinical efficacy of ICIs has been investigated. The CheckMate 026 study demonstrated that patients with PD-L1 positive tumors and high TMB treated with first-line nivolumab had higher response rates (47% vs. 28%) and improved PFS (9.7 vs. 5.8 mo) versus CT (Carbone et al. 2017). However, there were no differences in OS between the nivolumab and CT group. In the CheckMate 227 phase 3 multipart trial, PFS was found significantly longer with first-line nivolumab plus ipilimumab therapy than with chemotherapy among patients with metastatic NSCLC and with a high TMB (≥10 mut/mb), irrespective of PD-L1 expression level (Hellmann et al. 2018). However, in a subsequent report, a similar OS benefit was noted in NSCLC patients treated with nivolumab plus ipilimumab, regardless of high or low TMB (≥10 vs. <10 mut/mb, respectively) (Hellmann et al. 2019). In a retrospective analysis of two large randomized trials of previously treated, metastatic NSCLC patients receiving ICIs, and in a separate nontrial cohort, blood TMB (bTMB) identified patients with improved response rates and PFS from ICI therapy (Gandara et al. 2018; Wang et al. 2019b). In the phase 3 MYSTIC trial (Rizvi et al. 2020), exploratory analyses showed that a bTMB threshold of ≥20 mut/mb was associated with improved OS in patients treated with durvalumab plus tremelimumab versus CT. Nevertheless, the role of TMB as a predictive biomarker remains unclear in the early-stage and metastatic disease setting and challenging to implement in practice due to lack of a standard cutoff for high TMB, variabilities in the assays used to determine TMB, and absence of prospective validation for survival benefit.
ctDNA AS A BIOMARKER FOR ICIs IN NSCLC
Results from recent studies suggest that analyzing the fragments of tumor cell DNA from tumor cells that are released in the circulation may represent a valuable approach for monitoring disease burden in NSCLC patients who have undergone surgery or been treated with ICIs. DNA fragments have been shown to gain access to the circulation and other bodily fluid compartments through processes of apoptosis, necrosis, or active secretion (Heitzer et al. 2020). A recent report in patients with stage I–III NSCLC has shown that ctDNA can serve as a biomarker to detect molecular residual disease (MRD) following surgery and for defining the clonality of relapsing disease (Abbosh et al. 2020). The investigators reported that of the 45 patients that had recurrence of their primary NSCLC, ctDNA was detected at or before clinical relapse in 37 of these patients (Abbosh et al. 2020). In a study of 40 patients with stage I–III lung cancer who were treated with curative-intent therapies, ctDNA was detected in the first blood sample collected posttherapy in 94% of patients who experienced disease recurrence (Chaudhuri et al. 2017). ctDNA analysis also demonstrated the potential to inform consolidation ICI therapy after CRT in patients with locally advanced, unresectable NSCLC (Moding et al. 2020). Patients with MRD after CRT who received consolidation ICI experienced improved outcomes compared to those who did not receive consolidation ICI, suggesting the utility of ctDNA for the personalization of ICI therapy while avoiding unnecessary toxicities (Moding et al. 2020). In an analysis of 87 patients enrolled in the CheckMate 816 trial, ctDNA was more likely to clear when nivolumab was administered in combination with neoadjuvant CT versus CT alone (56% vs. 34%, respectively), and pCR was observed in 46% of patients with ctDNA clearance as compared to 13% of those without (Forde et al. 2021).
GUT MICROBIOME AS A BIOMARKER FOR ICI THERAPY IN NSCLC
Recent evidence suggests that the composition of bacteria residing in the gut may play a key role in determining tumor responses to ICIs and that microbial-derived metabolites may be a central regulator of systemic immune function (Rooks and Garrett 2016). Melanoma patients who responded to anti-PD-1 therapy could be distinguished from nonresponders by the diversity and composition of their gut microbiome (Gopalakrishnan et al. 2018). This effect was mediated by elevated antigen presentation and improved effector T-cell function (Gopalakrishnan et al. 2018). In patients with advanced NSCLC treated with ICIs, concomitant antibiotic administration altered gut microbiome and led to reduced tumor response to ICIs when compared to patients without antibiotic treatment (Routy et al. 2018). In a study involving a cohort of Chinese patients with advanced NSCLC treated with anti-PD-1 therapy, 16S rRNA gene sequencing revealed higher diversity of gut microbiome at baseline followed by stable microbiome population during treatment in responders to ICIs (Jin et al. 2019). In the phase 2 randomized NEOSTAR trial, we found that administration of neoadjuvant ICIs had no significant impact on the diversity, structure, and composition of microbiomes as assessed by 16S sequencing (Cascone et al. 2021). Our exploratory analyses also demonstrated that a relative higher abundance of Ruminococcus and Akkermansia spp. in pretherapy samples was associated with MPR to neoadjuvant nivolumab plus ipilimumab therapy (Cascone et al. 2021). Studies in melanoma also reported that higher diversity and relative abundance of fecal Ruminococcaceae are associated with response to ICIs (Gopalakrishnan et al. 2018), and metagenomics of baseline stool samples in patients with advanced NSCLC and kidney cancer that were refractory to ICIs revealed low levels of Akkermansia muciniphila (Routy et al. 2018). The ability of distinct commensal bacteria to impact tumor immunogenicity and increase the efficacy of ICIs provides an extraordinary opportunity to improve clinical outcomes for patients with resectable NSCLC. However, while encouraging, these results were exploratory in nature and will require validation in larger cohorts.
CONCLUSIONS
ICIs have rapidly changed the treatment landscape for patients with locally advanced/unresectable and metastatic NSCLC. New ICI combinations or regimens with ICI therapy combined with CT or RT are being explored in metastatic disease and the results from those studies are informing new trials for patients with early-stage disease in the perioperative setting. Additional understanding of the immune biology of lung tumors is critical for defining the role of the immune system in controlling tumor formation, progression, and metastasis. Technological advances are providing a deeper understanding of the immune biology of NSCLC that will likely be translated into new therapeutic strategies to improve clinical outcomes. These include tumor molecular profiling (sequencing, expression, epigenome, proteomic, and metabolomic); multiparameter IHC profiling and single-cell RNA sequencing analyses of the tumor and immune cells; identification of tumor neoantigens and individual patient T- and B-cell repertories before and after ICI therapy; and, finally, correlation of these findings with clinicopathological characteristics and therapeutic outcome. This understanding will also yield biomarkers that optimize patient selection and identify those patients most likely to benefit from treatment, thereby maximizing the clinical effectiveness of ICIs.
COMPETING INTEREST STATEMENT
T.C. reports speaker's fees from the Society for the Immunotherapy of Cancer, Bristol Myers Squibb and Roche; advisory/consulting fees from MedImmune, AstraZeneca, Bristol Myers Squibb, EMD Serono, Arrowhead Pharmaceuticals, Genentech and Merck & Co.; clinical research funding to The University of Texas MD Anderson Cancer Center from Boehringer Ingelheim, MedImmune, AstraZeneca, Bristol Myers Squibb and EMD Serono. D.L.G. reports honoraria for scientific advisory boards from AstraZeneca, Sanofi, Alethia Biotherapeutics and Lilly, research support from Janssen, Takeda, Ribon Therapeutics, Astellas, NGM Biopharmaceuticals and AstraZeneca.
ACKNOWLEDGMENTS
T.C and D.L.G are partially supported by the Lung SPORE Grant 5 P50 CA070907, the NIH/NCI P30 CA016672 Cancer Center Support Grant, and the generous philanthropic contributions to the University of Texas MD Anderson Cancer Center Lung Cancer Moon Shot Program. Work in the Cascone laboratory is partially supported by the Conquer Cancer Foundation of the American Society of Clinical Oncology Career Development Award 2018, the University of Texas MD Anderson Cancer Center Khalifa Scholars Program (from Khalifa Bin Zayed Al Nahyan Foundation) and the Physician Scientist Program; the Rexanna's Foundation for Fighting Lung Cancer, and the Bob Mayberry Foundation. Work in the Gibbons laboratory is supported by NIH R37CA214609 and CPRIT RP200235.
Footnotes
Editors: Christine M. Lovly, David P. Carbone, and John D. Minna
Additional Perspectives on Lung Cancer: Disease Biology and Its Potential for Clinical Translation available at www.perspectivesinmedicine.org
REFERENCES
- Abbosh C, Frankell A, Garnett A, Harrison T, Weichert M, Licon A, et al. 2020. Abstract CT023: phylogenetic tracking and minimal residual disease detection using ctDNA in early-stage NSCLC: A lung TRACERx study. Cancer Res 80: CT023-CT. [Google Scholar]
- Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubat T, Yagita H, Honjo T. 1996. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol 8: 765–772. 10.1093/intimm/8.5.765 [DOI] [PubMed] [Google Scholar]
- Aguilar EJ, Ricciuti B, Gainor JF, Kehl KL, Kravets S, Dahlberg S, Nishino M, Sholl LM, Adeni A, Subegdjo S, et al. 2019. Outcomes to first-line pembrolizumab in patients with non-small-cell lung cancer and very high PD-L1 expression. Ann Oncol 30: 1653–1659. 10.1093/annonc/mdz288 [DOI] [PubMed] [Google Scholar]
- Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, Mikse OR, Cherniack AD, Beauchamp EM, Pugh TJ, et al. 2013. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 3: 1355–1363. 10.1158/2159-8290.CD-13-0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Børresen-Dale AL, et al. 2013. Signatures of mutational processes in human cancer. Nature 500: 415–421. 10.1038/nature12477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund LT. 2008. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res 14: 5220–5227. 10.1158/1078-0432.CCR-08-0133 [DOI] [PubMed] [Google Scholar]
- Altman BJ, Stine ZE, Dang CV. 2016. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer 16: 619–634. 10.1038/nrc.2016.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AC. 2012. Tim-3, a negative regulator of anti-tumor immunity. Curr Opin Immunol 24: 213–216. 10.1016/j.coi.2011.12.005 [DOI] [PubMed] [Google Scholar]
- Antonia SJ, Brahmer JR, Gettinger S, Chow LQ, Juergens R, Shepherd FA, Laurie SA, Gerber DE, Goldman J, Shen Y, et al. 2014. Nivolumab (ANTI-PD-1; BMS-936558, ONO-4538) in combination with platinum-based doublet chemotherapy (Pt-Dc) or erlotinib in advanced non-small cell lung cancer (NSCLC). Int J Radiat Oncol 90: S153. [Google Scholar]
- Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, et al. 2018. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 379: 2342–2350. 10.1056/NEJMoa1809697 [DOI] [PubMed] [Google Scholar]
- Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, et al. 2005. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol 23: 6043–6053. 10.1200/JCO.2005.06.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird A, Mormède P, Bőhlen P. 1985. Immunoreactive fibroblast growth factor in cells of peritoneal exudate suggests its identity with macrophage-derived growth factor. Biochem Biophys Res Commun 126: 358–364. 10.1016/0006-291X(85)90614-X [DOI] [PubMed] [Google Scholar]
- Bakos O, Lawson C, Rouleau S, Tai LH. 2018. Combining surgery and immunotherapy: turning an immunosuppressive effect into a therapeutic opportunity. J Immunother Cancer 6: 86. 10.1186/s40425-018-0398-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker HE, Paget JT, Khan AA, Harrington KJ. 2015. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer 15: 409–425. 10.1038/nrc3958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marme D. 1996. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood 87: 3336–3343. 10.1182/blood.V87.8.3336.bloodjournal8783336 [DOI] [PubMed] [Google Scholar]
- Barlesi F, Nishio M, Cobo M, Steele N, Paramonov V, Parente B, Dear R, Berard H, Peled N, Seneviratne L, et al. 2018. IMpower132: efficacy of atezolizumab (atezo) + carboplatin (carbo)/cisplatin (cis) + pemetrexed (pem) as 1L treatment in key subgroups with stage IV non-squamous non-small cell lung cancer (NSCLC). Ann Oncol 29: viii743– viii744. 10.1093/annonc/mdy424.066 [DOI] [Google Scholar]
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al. 2015. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373: 1627–1639. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzin C, Brouet A, De Vriese J, Dewever J, Feron O. 2007. Effects of vascular endothelial growth factor on the lymphocyte-endothelium interactions: identification of caveolin-1 and nitric oxide as control points of endothelial cell anergy. J Immunol 178: 1505–1511. 10.4049/jimmunol.178.3.1505 [DOI] [PubMed] [Google Scholar]
- Brahmer JR. 2013. Harnessing the immune system for the treatment of non-small-cell lung cancer. J Clin Oncol 31: 1021–1028. 10.1200/JCO.2012.45.8703 [DOI] [PubMed] [Google Scholar]
- Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al. 2015. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373: 123–135. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. 2018. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68: 394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- Bruno TC, Ebner PJ, Moore BL, Squalls OG, Waugh KA, Eruslanov EB, Singhal S, Mitchell JD, Franklin WA, Merrick DT, et al. 2017. Antigen-presenting intratumoral B cells affect CD4+ TIL phenotypes in non-small cell lung cancer patients. Cancer Immunol Res 5: 898–907. 10.1158/2326-6066.CIR-17-0075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budden KF, Gellatly SL, Wood DL, Cooper MA, Morrison M, Hugenholtz P, Hansbro PM. 2017. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol 15: 55–63. 10.1038/nrmicro.2016.142 [DOI] [PubMed] [Google Scholar]
- Büttner R, Gosney JR, Skov BG, Adam J, Motoi N, Bloom KJ, Dietel M, Longshore JW, López-Ríos F, Penault-Llorca F, et al. 2017. Programmed death-ligand 1 immunohistochemistry testing: a review of analytical assays and clinical implementation in non-small-cell lung cancer. J Clin Oncol 35: 3867–3876. 10.1200/JCO.2017.74.7642 [DOI] [PubMed] [Google Scholar]
- Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F, et al. 2017. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med 376: 2415–2426. 10.1056/NEJMoa1613493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone D, Lee J, Kris M, Wistuba I, Kwiatkowski D, Owen D, Bunn P, Johnson B, Oezkan F, Tang Y, et al. 2021. OA06.06 clinical/biomarker data for neoadjuvant atezolizumab in resectable stage IB-IIIB NSCLC: primary analysis in the LCMC3 study. J Thorac Oncol 16: S115–S116. 10.1016/j.jtho.2021.01.294 [DOI] [Google Scholar]
- Cascone T, McKenzie JA, Mbofung RM, Punt S, Wang Z, Xu C, Williams LJ, Wang Z, Bristow CA, Carugo A, et al. 2018. Increased tumor glycolysis characterizes immune resistance to adoptive T cell therapy. Cell Metab 27: 977–987.e4. 10.1016/j.cmet.2018.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascone T, William WN, Weissferdt A, Leung CH, Lin HY, Pataer A, Godoy MCB, Carter BW, Federico L, Reuben A, et al. 2021. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med 27: 504–514. 10.1038/s41591-020-01224-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Qiu J, O'Sullivan D, Buck MD, Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJ, et al. 2015. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162: 1229–1241. 10.1016/j.cell.2015.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri AA, Chabon JJ, Lovejoy AF, Newman AM, Stehr H, Azad TD, Khodadoust MS, Esfahani MS, Liu CL, Zhou L, et al. 2017. Early detection of molecular residual disease in localized lung cancer by circulating tumor DNA profiling. Cancer Discov 7: 1394–1403. 10.1158/2159-8290.CD-17-0716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Han X. 2015. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest 125: 3384–3391. 10.1172/JCI80011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Kolls JK. 2013. T cell-mediated host immune defenses in the lung. Annu Rev Immunol 31: 605–633. 10.1146/annurev-immunol-032712-100019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. 2014. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer 14: 535–546. 10.1038/nrc3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Diao L, Yang Y, Yi X, Rodriguez BL, Li Y, Villalobos PA, Cascone T, Liu X, Tan L, et al. 2018. CD38-mediated immunosuppression as a mechanism of tumor cell escape from PD-1/PD-L1 blockade. Cancer Discov 8: 1156–1175. 10.1158/2159-8290.CD-17-1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang AC, Herbst RS. 2020. Frontline immunotherapy for NSCLC—the tale of the tail. Nat Rev Clin Oncol 17: 73–74. 10.1038/s41571-019-0317-y [DOI] [PubMed] [Google Scholar]
- Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, et al. 2014. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513: 559–563. 10.1038/nature13490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulie PG, Van den Eynde BJ, van der Bruggen P, Boon T. 2014. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer 14: 135–146. 10.1038/nrc3670 [DOI] [PubMed] [Google Scholar]
- Creelan B, Wang C, Teer J, Toloza E, Mullinax J, Yao J, Koomen J, Kim S, Chiappori A, Saller J, et al. 2020. Durable complete responses to adoptive cell transfer using tumor infiltrating lymphocytes (TIL) in non-small cell lung cancer (NSCLC): a phase I trial. Cancer Res 80. 10.1158/1538-7445.am2020-ct056 [DOI] [Google Scholar]
- Creelan BC, Yeh TC, Kim SW, Nogami N, Kim DW, Chow LQM, Kanda S, Taylor R, Tang W, Tang M, et al. 2021. A phase 1 study of gefitinib combined with durvalumab in EGFR TKI-naive patients with EGFR mutation-positive locally advanced/metastatic non-small-cell lung cancer. Br J Cancer 124: 383–390. 10.1038/s41416-020-01099-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chaisemartin L, Goc J, Damotte D, Validire P, Magdeleinat P, Alifano M, Cremer I, Firdman WH, Sautès-Fridman C, Dieu-Nosjean MC. 2011. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res 71: 6391–6399. 10.1158/0008-5472.CAN-11-0952 [DOI] [PubMed] [Google Scholar]
- Del Mar Valenzuela-Membrives M, Perea-García F, Sanchez-Palencia A, Ruiz-Cabello F, Gómez-Morales M, Miranda-León MT, Galindo Angel I, Fárez-Vidal ME. 2016. Progressive changes in composition of lymphocytes in lung tissues from patients with non-small-cell lung cancer. Oncotarget 7: 71608–71619. 10.18632/oncotarget.12264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria S, Formenti SC. 2020. The abscopal effect 67 years later: from a side story to center stage. Br J Radiol 93: 20200042. 10.1259/bjr.20200042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Zhu G, Tamada K, Chen L. 1999. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 5: 1365–1369. 10.1038/70932 [DOI] [PubMed] [Google Scholar]
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. 2002. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8: 793–800. 10.1038/nm730 [DOI] [PubMed] [Google Scholar]
- Dumitriu IE, Dunbar DR, Howie SE, Sethi T, Gregory CD. 2009. Human dendritic cells produce TGF-β1 under the influence of lung carcinoma cells and prime the differentiation of CD4+CD25+Foxp3+ regulatory T cells. J Immunol 182: 2795–2807. 10.4049/jimmunol.0712671 [DOI] [PubMed] [Google Scholar]
- Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, Stephen TL, Ranganathan A, Deshpande C, Akimova T, Vachani A, Litzky L, Hancock WW, et al. 2014. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Invest 124: 5466–5480. 10.1172/JCI77053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, et al. 2016. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 387: 1837–1846. 10.1016/S0140-6736(16)00587-0 [DOI] [PubMed] [Google Scholar]
- Forde PM, Chaft JE, Pardoll DM. 2018. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med 379: e14. 10.1056/NEJMc1808251 [DOI] [PubMed] [Google Scholar]
- Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, Felip E, Broderick S, Brahmer J, Swanson SJ, et al. 2021. Nivolumab NIVO) + platinum-doublet chemotherapy (chemo) vs chemo as neoadjuvant treatment (tx) for resectable (IB-IIIA) non-small cell lung cancer (NSCLC) in the phase 3 CheckMate 816 trial. Cancer Res 10.1158/1538-7445.AM2021-CT003 [DOI] [Google Scholar]
- Formenti SC, Rudqvist NP, Golden E, Cooper B, Wennerberg E, Lhuillier C, Vanpouille-Box C, Friedman K, Ferrari de Andrade L, Wucherpfennig KW, et al. 2018. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med 24: 1845–1851. 10.1038/s41591-018-0232-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. 2000. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 192: 1027–1034. 10.1084/jem.192.7.1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman WH, Pagès F, Sautès-Fridman C, Galon J. 2012. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 12: 298–306. 10.1038/nrc3245 [DOI] [PubMed] [Google Scholar]
- Fridman WH, Zitvogel L, Sautès-Fridman C, Kroemer G. 2017. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol 14: 717–734. 10.1038/nrclinonc.2017.101 [DOI] [PubMed] [Google Scholar]
- Gainor JF, Shaw AT, Sequist LV, Fu X, Azzoli CG, Piotrowska Z, Huynh TG, Zhao L, Fulton L, Schultz KR, et al. 2016. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res 22: 4585–4593. 10.1158/1078-0432.CCR-15-3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandara DR, Paul SM, Kowanetz M, Schleifman E, Zou W, Li Y, Rittmeyer A, Fehrenbacher L, Otto G, Malboeuf C, et al. 2018. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med 24: 1441–1448. 10.1038/s41591-018-0134-3 [DOI] [PubMed] [Google Scholar]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, et al. 2018. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378: 2078–2092. 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- Ganesan AP, Johansson M, Ruffell B, Yagui-Beltran A, Lau J, Jablons DM, Coussens LM. 2013. Tumor-infiltrating regulatory T cells inhibit endogenous cytotoxic T cell responses to lung adenocarcinoma. J Immunol 191: 2009–2017. 10.4049/jimmunol.1301317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao SG, Li N, Gao SY, Xue Q, Ying JM, Wang SH, Tao X, Zhao J, Mao Y, Wang B, et al. 2020. Neoadjuvant PD-1 inhibitor (sintilimab) in NSCLC. J Thorac Oncol 15: 816–826. 10.1016/j.jtho.2020.01.017 [DOI] [PubMed] [Google Scholar]
- Garinet S, Laurent-Puig P, Blons H, Oudart JB. 2018. Current and future molecular testing in NSCLC, what can we expect from new sequencing technologies? J Clin Med 7: 144. 10.3390/jcm7060144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavralidis A, Gainor JF. 2020. Immunotherapy in EGFR-mutant and ALK-positive lung cancer: implications for oncogene-driven lung cancer. Cancer J 26: 517–524. 10.1097/PPO.0000000000000491 [DOI] [PubMed] [Google Scholar]
- Germain C, Gnjatic S, Tamzalit F, Knockaert S, Remark R, Goc J, Lepelley A, Becht E, Katsahian S, Bizouard G, et al. 2014. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med 189: 832–844. 10.1164/rccm.201309-1611OC [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, et al. 2018. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359: 97–103. 10.1126/science.aan4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JE, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, Cho BC, et al. 2020. Three-year overall survival with durvalumab after chemoradiotherapy in stage III NSCLC-update from PACIFIC. J Thorac Oncol 15: 288–293. 10.1016/j.jtho.2019.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: the next generation. Cell 144: 646–674. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- Heitzer E, Auinger L, Speicher MR. 2020. Cell-free DNA and apoptosis: how dead cells inform about the living. Trends Mol Med 26: 519–528. 10.1016/j.molmed.2020.01.012 [DOI] [PubMed] [Google Scholar]
- Hellmann MD, Chaft JE, William WN, Rusch V, Pisters KM, Kalhor N, Pataer A, Travis WD, Swisher SG, Kris MG, et al. 2014. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol 15: e42–e50. 10.1016/S1470-2045(13)70334-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers S, Salman P, et al. 2018. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 378: 2093–2104. 10.1056/NEJMoa1801946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, Park K, Alexandru A, Lupinacci L, de la Mora Jimenez E, et al. 2019. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med 381: 2020–2031. 10.1056/NEJMoa1910231 [DOI] [PubMed] [Google Scholar]
- Herbst RS, Sznol M. 2016. Diminished but not dead: chemotherapy for the treatment of NSCLC. Lancet Oncol 17: 1464–1465. 10.1016/S1470-2045(16)30524-1 [DOI] [PubMed] [Google Scholar]
- Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al. 2016. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387: 1540–1550. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- Herbst RS, Morgensztern D, Boshoff C. 2018. The biology and management of non-small cell lung cancer. Nature 553: 446–454. 10.1038/nature25183 [DOI] [PubMed] [Google Scholar]
- Herbst RS, Garon EB, Kim DW, Cho BC, Perez-Gracia JL, Han JY, Dubos Arvis C, Majem M, Forster MD, Monnet I, et al. 2020. Long-term outcomes and retreatment among patients with previously treated, programmed death-ligand 1positive, advanced non-small-cell lung cancer in the KEYNOTE-010 study. J Clin Oncol 38: 1580–1590. 10.1200/JCO.19.02446 [DOI] [PubMed] [Google Scholar]
- Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. 2013. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 143: e278S–e313S. 10.1378/chest.12-2359 [DOI] [PubMed] [Google Scholar]
- Huang RY, Eppolito C, Lele S, Shrikant P, Matsuzaki J, Odunsi K. 2015a. LAG3 and PD1 co-inhibitory molecules collaborate to limit CD8+ T cell signaling and dampen antitumor immunity in a murine ovarian cancer model. Oncotarget 6: 27359–27377. 10.18632/oncotarget.4751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, Dougan SK, Petersen B-S, Melum E, Pertel T, et al. 2015b. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature 517: 386–390. 10.1038/nature13848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida M, Iwai Y, Tanaka Y, Okazaki T, Freeman GJ, Minato N, Honjo T. 2002. Differential expression of PD-L1 and PD-L2, ligands for an inhibitory receptor PD-1, in the cells of lymphohematopoietic tissues. Immunol Lett 84: 57–62. 10.1016/S0165-2478(02)00142-6 [DOI] [PubMed] [Google Scholar]
- Jabbour SK, Lee KH, Frost N, Breder V, Kowalski DM, Pollock T, Levchenko E, Reguart N, Martinez-Marti A, Houghton B, et al. 2021. Pembrolizumab plus concurrent chemoradiation therapy in patients with unresectable, locally advanced, stage III non-small cell lung cancer: the phase 2 KEYNOTE-799 nonrandomized trial. JAMA Oncol 10.1001/jamaoncol.2021.2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Dong H, Xia L, Yang Y, Zhu Y, Shen Y, Zheng H, Yao C, Wang Y, Lu S. 2019. The diversity of gut microbiome is associated with favorable responses to anti-programmed death 1 immunotherapy in Chinese patients with NSCLC. J Thorac Oncol 14: 1378–1389. 10.1016/j.jtho.2019.04.007 [DOI] [PubMed] [Google Scholar]
- Jotte R, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Abreu DR, Hussein M, Soo R, Conter H, Kozuki T, Huang K, et al. 2019. OA14.02 IMpower131: final OS results of carboplatin + nab-paclitaxel ± atezolizumab in advanced squamous NSCLC. J Thorac Oncol 14: S243–S244. 10.1016/j.jtho.2019.08.484 [DOI] [PubMed] [Google Scholar]
- Kargl J, Busch SE, Yang GH, Kim KH, Hanke ML, Metz HE, Hubbard JJ, Lee SM, Madtes DK, McIntosh MW, et al. 2017. Neutrophils dominate the immune cell composition in non-small cell lung cancer. Nat Commun 8: 14381. 10.1038/ncomms14381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai O, Ishii G, Kubota K, Murata Y, Naito Y, Mizuno T, Aokage K, Saijo N, Nishiwaki Y, Gemma A, et al. 2008. Predominant infiltration of macrophages and CD8+ T cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer 113: 1387–1395. 10.1002/cncr.23712 [DOI] [PubMed] [Google Scholar]
- Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. 2007. Adaptive immunity maintains occult cancer in an equilibrium state. Nature 450: 903–907. 10.1038/nature06309 [DOI] [PubMed] [Google Scholar]
- Konen JM, Fradette JJ, Gibbons DL. 2020. The good, the bad and the unknown of CD38 in the metabolic microenvironment and immune cell functionality of solid tumors. Cells 9: 52. 10.3390/cells9010052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkolopoulou P, Kaklamanis L, Pezzella F, Harris AL, Gatter KC. 1996. Loss of antigen-presenting molecules (MHC class I and TAP-1) in lung cancer. Br J Cancer 73: 148–153. 10.1038/bjc.1996.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kris MG, Johnson BE, Berry LD, Kwiatkowski DJ, Iafrate AJ, Wistuba II, Varella-Garcia M, Franklin WA, Aronson SL, Su PF, et al. 2014. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 311: 1998–2006. 10.1001/jama.2014.3741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantuejoul S, Sound-Tsao M, Cooper WA, Girard N, Hirsch FR, Roden AC, Lopez-Rios F, Jain D, Chou TY, Motoi N, et al. 2020. PD-L1 testing for lung cancer in 2019: perspective from the IASLC pathology committee. J Thorac Oncol 15: 499–519. 10.1016/j.jtho.2019.12.107 [DOI] [PubMed] [Google Scholar]
- Lavin Y, Kobayashi S, Leader A, Amir ED, Elefant N, Bigenwald C, Remark R, Sweeney R, Becker CD, Levine JH, et al. 2017. Cell 169: 750–765.e17. 10.1016/j.cell.2017.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CK, Man J, Lord S, Cooper W, Links M, Gebski V, Herbst RS, Gralla RJ, Mok T, Yang JCH. 2018. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non-small cell lung carcinoma: a systematic review and meta-analysis. JAMA Oncol 4: 210–216. 10.1001/jamaoncol.2017.4427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Liu X, Wang M. 2018. Immunotherapy combined with epidermal growth factor receptor-tyrosine kinase inhibitors in non-small-cell lung cancer treatment. Onco Targets Ther 11: 6189–6196. 10.2147/OTT.S178497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberti MV, Locasale JW. 2016. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci 41: 211–218. 10.1016/j.tibs.2015.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SH, Lin Y, Mok I, Young JA, Phan S, Sandler A, Papadimitraopoulou V, Heymach J, Tsap AS. 2019. Phase II trial combining atezolizumab concurrently with chemoradiation therapy in locally advanced non-small cell lung cancer. J Clin Oncol 37: 8512. 10.1200/JCO.2019.37.15_suppl.8512 [DOI] [Google Scholar]
- Linnemann C, van Buuren MM, Bies L, Verdegaal EM, Schotte R, Calis JJ, Behjati S, Velds A, Hilkmann H, El Atmioui D, et al. 2015. High-throughput epitope discovery reveals frequent recognition of neo-antigens by CD4+ T cells in human melanoma. Nat Med 21: 81–85. 10.1038/nm.3773 [DOI] [PubMed] [Google Scholar]
- Liu Y, Zugazagoitia J, Ahmed FS, Henick BS, Gettinger S, Herbst RS, Schalper KA, Rimm DL. 2020. Immune cell PD-L1 co-localizes with macrophages and is associated with outcome in PD-1 pathway blockade therapy. Clin Cancer Res 26: 970–977. 10.1158/1078-0432.CCR-19-1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd CM, Marsland BJ. 2017. Lung homeostasis: influence of age, microbes, and the immune system. Immunity 46: 549–561. 10.1016/j.immuni.2017.04.005 [DOI] [PubMed] [Google Scholar]
- Lyons YA, Wu SY, Overwijk WW, Baggerly KA, Sood AK. 2017. Immune cell profiling in cancer: molecular approaches to cell-specific identification. NPJ Precis Oncol 1: 26. 10.1038/s41698-017-0031-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma BBY, Rudin CM, Cervantes A, Dowlati A, Costa D, Schmid P, Heist R, Villaflor VM, Sarkar I, Huseni MA, et al. 2016. Preliminary safety and clinical activity of erlotinib plus atezolizumab from a phase Ib study in advanced NSCLC. Ann Oncol 27: ix139–ix156. 10.1093/annonc/mdw594 [DOI] [Google Scholar]
- Mantovani A, Ming WJ, Balotta C, Abdeljalil B, Bottazzi B. 1986. Origin and regulation of tumor-associated macrophages: the role of tumor-derived chemotactic factor. Biochim Biophys Acta 865: 59–67. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. 2008. Cancer-related inflammation. Nature 454: 436–444. 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- Martin J, Ginsberg RJ, Venkatraman ES, Bains MS, Downey RJ, Korst RJ, Kris MG, Rusch VW. 2002. Long-term results of combined-modality therapy in resectable non-small-cell lung cancer. J Clin Oncol 20: 1989–1995. 10.1200/JCO.2002.08.092 [DOI] [PubMed] [Google Scholar]
- Mishalian I, Bayuh R, Levy L, Zolotarov L, Michaeli J, Fridlender ZG. 2013. Tumor-associated neutrophils (TAN) develop pro-tumorigenic properties during tumor progression. Cancer Immunol Immunother 62: 1745–1756. 10.1007/s00262-013-1476-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KG, Amini B, Wang Y, Carter BW, Godoy MCB, Parra ER, Behrens C, Villalobos P, Reuben A, Lee JJ, et al. 2020. 18F-fluorodeoxyglucose positron emission tomography correlates with tumor immunometabolic phenotypes in resected lung cancer. Cancer Immunol Immunother 69: 1519–1534. 10.1007/s00262-020-02560-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moding EJ, Liu Y, Nabet BY, Chabon JJ, Chaudhuri AA, Hui AB, Bonilla RF, Ko RB, Yoo CH, Gojenola L, et al. 2020. Circulating tumor DNA dynamics predict benefit from consolidation immunotherapy in locally advanced non-small-cell lung cancer. Nat Cancer 1: 176–183. 10.1038/s43018-019-0011-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G, Srimuninnimit V, Laktionov KK, Bondarenko I, et al. 2019. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 393: 1819–1830. 10.1016/S0140-6736(18)32409-7 [DOI] [PubMed] [Google Scholar]
- Morgensztern D. 2019. KEYNOTE-042 and the role for single agent pembrolizumab in patients with PD-L1 tumor proportion score 1–49%. J Thorac Dis 11: S1963–S1965. 10.21037/jtd.2019.07.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa MS, Keith B, Simon MC. 2016. Oxygen availability and metabolic adaptations. Nat Rev Cancer 16: 663–673. 10.1038/nrc.2016.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC. 2018. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer 18: 313–322. 10.1038/nrc.2018.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NSCLC Meta-Analysis Collaborative Group. 2014. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet 383: 1561–1571. 10.1016/S0140-6736(13)62159-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell JS, Teng MWL, Smyth MJ. 2019. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol 16: 151–167. 10.1038/s41571-018-0142-8 [DOI] [PubMed] [Google Scholar]
- Parra ER, Villalobos P, Behrens C, Jiang M, Pataer A, Swisher SG, Williams WN, Zhang J, Lee J, Cascone T, et al. 2018. Effect of neoadjuvant chemotherapy on the immune microenvironment in non-small cell lung carcinomas as determined by multiplex immunofluorescence and image analysis approaches. J Immunother Cancer 6: 48. 10.1186/s40425-018-0368-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pataer A, Kalhor N, Correa AM, Raso MG, Erasmus JJ, Kim ES, Behrens C, Lee JJ, Roth JA, Stewart DJ, et al. 2012. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 7: 825–832. 10.1097/JTO.0b013e318247504a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A, et al. 2018. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379: 2040–2051. 10.1056/NEJMoa1810865 [DOI] [PubMed] [Google Scholar]
- Peters S, Felip E, Dafni U, Belka C, Guckenberger M, Irigoyen A, Nadal E, Becker A, Vees H, Pless M, et al. 2019. Safety evaluation of nivolumab added concurrently to radiotherapy in a standard first line chemo-radiotherapy regimen in stage III non-small cell lung cancer—the ETOP NICOLAS trial. Lung Cancer 133: 83–87. 10.1016/j.lungcan.2019.05.001 [DOI] [PubMed] [Google Scholar]
- Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, Dunant A, Torri V, Rasell R, Seymour L, et al. 2008. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE collaborative group. J Clin Oncol 26: 3552–3559. 10.1200/JCO.2007.13.9030 [DOI] [PubMed] [Google Scholar]
- Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P, Andre P, Dieu-Nosjean MC, ALifano M, Regnard JF, et al. 2011. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res 71: 5412–5422. 10.1158/0008-5472.CAN-10-4179 [DOI] [PubMed] [Google Scholar]
- Provencio M, Nadal E, Insa A, García-Campelo MR, Casal-Rubio J, Dómine M, Marjem M, Rodríguez-Abreu D, Martínez-Martí A, De Castro Carpeño J, et al. 2020. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 21: 1413–1422. 10.1016/S1470-2045(20)30453-8 [DOI] [PubMed] [Google Scholar]
- Qin S, Xu L, Yi M, Yu S, Wu K, Luo S. 2019. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol Cancer 18: 155. 10.1186/s12943-019-1091-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. 2016. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375: 1823–1833. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- Reck M, Ciuleanu TE, Dols MC, Schenker M, Zurawski B, Menezes J, Richardet E, Bennouna J, Felip E, Juan-Vidal O, et al. 2020. Nivolumab (NIVO) + ipilimumab (IPI) + 2 cycles of platinum-doublet chemotherapy (chemo) vs 4 cycles chemo as first-line (1L) treatment (tx) for stage IV/recurrent non-small cell lung cancer (NSCLC): CheckMate 9LA. J Clin Oncol 38: 9501. 10.1200/JCO.2020.38.15_suppl.9501 [DOI] [Google Scholar]
- Reck M, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, de Menezes JJ, Richardet E, Bennouna J, Felip E, Juan-Vidal O, et al. 2021. First-line nivolumab (NIVO) plus ipilimumab (IPI) plus two cycles of chemotherapy (chemo) versus chemo alone (4 cycles) in patients with advanced non-small cell lung cancer (NSCLC): two-year update from CheckMate 9LA. J Clin Oncol 39: 9000. 10.1200/JCO.2021.39.15_suppl.9000 [DOI] [Google Scholar]
- Remon J, Passiglia F, Ahn MJ, Barlesi F, Forde PM, Garon EB, Gettinger S, Goldberg SB, Herbst RS, Horn L, et al. 2020. Immune checkpoint inhibitors in thoracic malignancies: review of the existing evidence by an IASLC expert panel and recommendations. J Thorac Oncol 15: 914–947. 10.1016/j.jtho.2020.03.006 [DOI] [PubMed] [Google Scholar]
- Renner K, Singer K, Koehl GE, Geissler EK, Peter K, Siska PJ, Kreutz M. 2017. Metabolic hallmarks of tumor and immune cells in the tumor microenvironment. Front Immunol 8: 248. 10.3389/fimmu.2017.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimm DL, Han G, Taube JM, Yi ES, Bridge JA, Flieder DB, Homer R, West WW, Wu H, Roden AC, et al. 2017. A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA Oncol 3: 1051–1058. 10.1001/jamaoncol.2017.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Cobo Dols M, et al. 2017. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389: 255–265. 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi NA, Cho BC, Reinmuth N, Lee KH, Luft A, Ahn MJ, van den Heuvel MM, Cobo M, Vicente D, Smolin A, et al. 2020. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer: the MYSTIC phase 3 randomized clinical trial. JAMA Oncol 6: 661–674. 10.1001/jamaoncol.2020.0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooks MG, Garrett WS. 2016. Gut microbiota, metabolites and host immunity. Nat Rev Immunol 16: 341–352. 10.1038/nri.2016.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Restifo NP. 2015. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 348: 62–68. 10.1126/science.aaa4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R, Cadieux EL, Salgado R, Bakir MA, Moore DA, Hiley CT, Lund T, Tanić M, Reading JL, Joshi K, et al. 2019. Neoantigen-directed immune escape in lung cancer evolution. Nature 567: 479–485. 10.1038/s41586-019-1032-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, et al. 2018. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359: 91–97. 10.1126/science.aan3706 [DOI] [PubMed] [Google Scholar]
- Schoenfeld AJ, Arbour KC, Rizvi H, Iqbal AN, Gadgeel SM, Girshman J, Kris MG, Riely GJ, Yu HA, Hellmann MD. 2019. Severe immune-related adverse events are common with sequential PD-(L)1 blockade and osimertinib. Ann Oncol 30: 839–844. 10.1093/annonc/mdz077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber RD, Old LJ, Smyth MJ. 2011. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 331: 1565–1570. 10.1126/science.1203486 [DOI] [PubMed] [Google Scholar]
- Schumacher TN, Schreiber RD. 2015. Neoantigens in cancer immunotherapy. Science 348: 69–74. 10.1126/science.aaa4971 [DOI] [PubMed] [Google Scholar]
- Selenko-Gebauer N, Majdic O, Szekeres A, Höfler G, Guthann E, Korthäuer U, Zlabinger G, Steinberger P, Pickl WF, Stockinger H, et al. 2003. B7-H1 (programmed death-1 ligand) on dendritic cells is involved in the induction and maintenance of T cell anergy. J Immunol 170: 3637–3644. 10.4049/jimmunol.170.7.3637 [DOI] [PubMed] [Google Scholar]
- Shalek AK, Satija R, Adiconis X, Gertner RS, Gaublomme JT, Raychowdhury R, Schwartz S, Yosef N, Malboeuf C, Lu D, et al. 2013. Single-cell transcriptomics reveals bimodality in expression and splicing in immune cells. Nature 498: 236–240. 10.1038/nature12172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G, Hanania NA, Shim YM. 2009. The aging immune system and its relationship to the development of chronic obstructive pulmonary disease. Proc Am Thorac Soc 6: 573–580. 10.1513/pats.200904-022RM [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K, Nakata M, Hirami Y, Yukawa T, Maeda A, Tanemoto K. 2010. Tumor-infiltrating Foxp3+ regulatory T cells are correlated with cyclooxygenase-2 expression and are associated with recurrence in resected non-small cell lung cancer. J Thorac Oncol 5: 585–590. 10.1097/JTO.0b013e3181d60fd7 [DOI] [PubMed] [Google Scholar]
- Shu CA, Gainor JF, Awad MM, Chiuzan C, Grigg CM, Pabani A, Garofano RF, Stoopler MB, Cheng SK, White A, et al. 2020. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 21: 786–795. 10.1016/S1470-2045(20)30140-6 [DOI] [PubMed] [Google Scholar]
- Sica A. 2010. Role of tumour-associated macrophages in cancer-related inflammation. Exp Oncol 32: 153–158. [PubMed] [Google Scholar]
- Singhal S, Bhojnagarwala PS, O'Brien S, Moon EK, Garfall AL, Rao AS, Quatromoni JG, Stephen TL, Litzky L, Deshpande C, et al. 2016. Origin and role of a subset of tumor-associated neutrophils with antigen-presenting cell features in early-stage human lung cancer. Cancer Cell 30: 120–135. 10.1016/j.ccell.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinjab A, Han G, Treekitkarnmongkol W, Hara K, Brennan PM, Dang M, Hao D, Wang R, Dai E, Dejima H, et al. 2021. Resolving the spatial and cellular architecture of lung adenocarcinoma by multiregion single-cell sequencing. Cancer Discov 10.1158/2159-8290.CD-20-1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoulidis F, Goldberg ME, Greenawalt DM, Hellmann MD, Awad MM, Gainor JF, Schrock AB, Hartmaier RJ, Travucco SE, Gay L, et al. 2018. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov 8: 822–835. 10.1158/2159-8290.CD-18-0099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, et al. 2018. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378: 2288–2301. 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- Spigel D, de Marinis F, Giaccone G, Reinmuth N, Vergnenegre A, Barrios CH, Morise M, Felip E, Andric ZG, Geater S, et al. 2019. IMpower110: interim overall survival (OS) analysis of a phase III study of atezolizumab (atezo) vs platinum-based chemotherapy (chemo) as first-line (1L) treatment (tx) in PD-L1-selected NSCLC. Ann Oncol 30: v915. 10.1093/annonc/mdz293 [DOI] [Google Scholar]
- Spigel DR, Faivre-Finn C, Gray JE, Vicente D, Planchard D, Paz-Ares LG, Vansteenkiste JF, Garassino MC, Hui R, Quantin X, et al. 2021. Five-year survival outcomes with durvalumab after chemoradiotherapy in unresectable stage III NSCLC: an update from the PACIFIC trial. J Clin Oncol 39: 8511. 10.1200/JCO.2021.39.15_suppl.8511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankovic B, Bjørhovde HAK, Skarshaug R, Aamodt H, Frafjord A, Müller E, Hammarström C, Beraki K, Bækkevold ES, Woldbæk PR, et al. 2019. Immune cell composition in human non-small cell lung cancer. Front Immunol 9: 3101. 10.3389/fimmu.2018.03101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao H, Mimura Y, Aoe K, Kobayashi S, Yamamoto H, Matsuda E, Okabe K, Matsumoto T, Sugi K, Ueoka H. 2012. Prognostic potential of FOXP3 expression in non-small cell lung cancer cells combined with tumor-infiltrating regulatory T cells. Lung Cancer 75: 95–101. 10.1016/j.lungcan.2011.06.002 [DOI] [PubMed] [Google Scholar]
- Tarhini A. 2013. Immune-mediated adverse events associated with ipilimumab ctla-4 blockade therapy: the underlying mechanisms and clinical management. Scientifica (Cairo) 2013: 857519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, Hercend T. 1990. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med 171: 1393–1405. 10.1084/jem.171.5.1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao MS, Kerr KM, Kockx M, Beasley MB, Borczuk AC, Botling J, Bubendorf L, Chirieac L, Chen G, CHou TY, et al. 2018. PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of blueprint phase 2 project. J Thorac Oncol 13: 1302–1311. 10.1016/j.jtho.2018.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl SM, Hunt DA, Wakefield LM, McCartney-Francis N, Wahl LM, Roberts AB, Sporn MB. 1987. Transforming growth factor type β induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci 84: 5788–5792. 10.1073/pnas.84.16.5788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakelee HA, Altorki NK, Zhou C, Csőszi T, Vynnychenko IO, Goloborodko O, Luft A, Akopov A, Martinez-Marati A, Kenmotsu H, et al. 2021. IMpower010: primary results of a phase III global study of atezolizumab versus best supportive care after adjuvant chemotherapy in resected stage IB-IIIA non-small cell lung cancer (NSCLC). J Clin Oncol 39: 8500. 10.1200/JCO.2021.39.15_suppl.8500 [DOI] [Google Scholar]
- Walunas TL, Bakker CY, Bluestone JA. 1996. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med 183: 2541–2550. 10.1084/jem.183.6.2541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SS, Liu W, Ly D, Xu H, Qu L, Zhang L. 2019a. Tumor-infiltrating B cells: their role and application in anti-tumor immunity in lung cancer. Cell Mol Immunol 16: 6–18. 10.1038/s41423-018-0027-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Duan J, Cai S, Han M, Dong H, Zhao J, Zhu B, Wang S, Zhou M, Sung J, et al. 2019b. Assessment of blood tumor mutational burden as a potential biomarker for immunotherapy in patients with non-small cell lung cancer with use of a next-generation sequencing cancer gene panel. JAMA Oncol 5: 696–702. 10.1001/jamaoncol.2018.7098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. 2020. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol 20: 7–24. 10.1038/s41577-019-0210-z [DOI] [PubMed] [Google Scholar]
- Wei SC, Duffy CR, Allison JP. 2018. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 8: 1069–1086. 10.1158/2159-8290.CD-18-0367 [DOI] [PubMed] [Google Scholar]
- West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, Kopp HG, Daniel D, McCune S, Mekhail T, et al. 2019. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 20: 924–937. 10.1016/S1470-2045(19)30167-6 [DOI] [PubMed] [Google Scholar]
- Woo EY, Yeh H, Chu CS, Schlienger K, Carroll RG, Riley JL, Kaiser LR, June CH. 2002. Cutting edge: regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol 168: 4272–4276. 10.4049/jimmunol.168.9.4272 [DOI] [PubMed] [Google Scholar]
- Workman CJ, Dugger KJ, Vignali DA. 2002. Cutting edge: molecular analysis of the negative regulatory function of lymphocyte activation gene-3. J Immunol 169: 5392–5395. 10.4049/jimmunol.169.10.5392 [DOI] [PubMed] [Google Scholar]
- Wu YL, Tsuboi M, He J, John T, Grohe C, Majem M, Goldman JW, Laktionov K, Kim SW, Kato T, et al. 2020. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med 383: 1711–1723. 10.1056/NEJMoa2027071 [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, et al. 2002. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol 169: 5538–5545. 10.4049/jimmunol.169.10.5538 [DOI] [PubMed] [Google Scholar]
- Yang L, Zhang Y. 2017. Tumor-associated macrophages: from basic research to clinical application. J Hematol Oncol 10: 58. 10.1186/s13045-017-0430-2 [DOI] [PMC free article] [PubMed] [Google Scholar]