Abstract
Objective:
To examine the factors that underlie physical impairments and function is needed to develop targeted rehabilitation interventions. Thus, the purpose of this systematic review was to examine physical impairments and physical function in children and adolescents with sickle cell disease (SCD).
Data Sources:
PubMed, Embase (embase.com), CINAHL (EBSCO), the Cochrane Central Register of Controlled Trials (Wiley), and Dissertations and Theses (ProQuest) were searched from January 1, 1990 to September 25, 2020. References retrieved were required to include a term for sickle cell disease and a term for physical impairments or physical function. Results were limited to articles with children and adolescents and in the English language.
Study Selection:
A total of 3,054 non-duplicate articles were independently screened by two reviewers, resulting in 240 articles for full-text review. The full-text review, performed by two independent reviewers, resulted in 67 articles.
Data Extraction:
Data was extracted from each full text to a custom Excel document by a single reviewer and was verified by a secondary reviewer.
Data Synthesis:
The studies identified in this systematic review offer evidence that children and adolescents with SCD demonstrate physical impairments and physical function limitations compared to controls as noted by varying percentages in deficits up to 19–58% in muscle and bone composition/symptoms, muscle strength, cardiopulmonary function, motor performance, physical activity, and physical function domains of quality of life questionnaires.
Conclusions:
Children and adolescents with SCD present with physical impairments and physical function limitations. Scientists and clinicians should consider developing collaborative standards to define and objectively measure physical impairment and function in this population to comprehensively examine the underlying factors that contribute to physical impairments and function.
Keywords: sickle cell disease, physical impairment, physical function, pediatric, child, adolescent
Sickle cell disease (SCD) is the most common serious genetically-inherited condition identified by newborn screening in the United States with a rate of one in every 350 African American newborns, an estimated 2,000 children per year.1,2 Over the first six months to 12 months of life, fetal hemoglobin transitions to adult hemoglobin S or C in infants with SCD leading to polymerization of abnormal hemoglobins and abnormally shaped red blood cells. These changes result in the conditions of hemoglobin SS disease (HbSS) or hemoglobin SC disease (HbSC) SCD, or sickle cell beta null (HbSβ0) or beta plus (HbSβ+) thalassemia. The sickled-form of hemoglobin causes improper blood flow and transportation of oxygen, known as sickle cell anemia. The sickle-shaped cells lack the flexibility needed to transverse circulation, are fragile, have a shortened life span, and have increased adhesiveness to vascular endothelium. This cascade causes vaso-occlusion in small blood vessels and local ischemia resulting in painful episodes, known as vaso-occlusive crises. Vaso-occlusive crises and anemia can lead to chronic damage to organs and tissues and an inflammatory cascade, causing further tissue damage of the bones (avascular necrosis and osteomyelitis), muscle (myonecrosis), brain (cerebral infarction), and lungs (acute chest syndrome, pulmonary hypertension, and chronic lung disease).2–4 In combination, these factors can affect physical function such as walking, running, and jumping, thus limiting participation in school, play, and sports activities.5–10
SCD affects many body systems including physical impairments of the neuromuscular (pain, muscle tone, balance), musculoskeletal (strength, range of motion), and cardiopulmonary (endurance, energy expenditure) systems. Physical impairments can lead to deficits in physical function. Physical function is defined as, “the ability to perform the basic actions that are essential for maintaining independence and carrying out more complex activities.”11 In children and adolescents with SCD, an increase in episodes and intensity of pain is associated with decreased physical function.12 Decreased physical function is associated with an increased number of missed days of school and parental missed days of work.13,14 Therefore, it is important to understand how SCD affects physical impairments and changes in physical function.
Evidence supports underlying factors contribute to physical impairments and function. Muscle extensibility, muscle size, and neuromuscular activation contribute to muscle strength and physical function in children and adolescents with differing health conditions. In children and adolescents with lower-extremity bone cancer, hemophilia, and cerebral palsy, impairments in muscle extensibility and muscle size were correlated with muscle strength and physical function as measured by the Timed Up and Go, Timed Up and Down Stairs, 9-minute run-walk tests, Gross Motor Function Measure and gait characteristics.15–18 In children without health conditions, neuromuscular activation influences early stages of strength development, which is important for physical function such as running and jumping.19–21
It is important to understand how SCD affects physical impairments and changes in physical function and to understand the factors that underlie physical impairments and function. Thus, the purpose of this systematic review was to identify literature published between 1990 and 2020 to examine physical impairments and physical function in children and adolescents with SCD to provide a basis for the development of targeted rehabilitation interventions for children and adolescents with SCD.
2. Methods
The systematic review procedures were guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and are registered in the PROSPERO (CRD42020220176). This review is exploratory; therefore, studies were not excluded based on quality.
2.1. Search Methods
Searches were run and constructed by a medical librarian (AGS) in PubMed, Embase (embase.com), CINAHL (EBSCO), the Cochrane Central Register of Controlled Trials (Wiley), and Dissertations and Theses (ProQuest). Each search was customized for the database and contained both keywords and controlled vocabulary. References retrieved were required to include a term for sickle cell disease and a term for physical impairments or physical function. The complete search algorithm is provided in Appendix 1. Search strategies included limits to studies with children and adolescents as subjects, in the English language, and published since 1990. This date range was selected due to the paucity of published articles prior to 1990. A total of 3,054 references were retrieved on September 25, 2020.
2.2. Inclusion criteria and study characteristics
Articles were included if the studies measured physical impairment, physical function, and/or quality of life measurements that include measurements of physical impairment and/or physical function. Studies had to be in the English language and published since 1990. The study population was children and adolescents with sickle cell disease between birth and 21 years of age. Studies with the following research design were included: (1) observational studies, (2) cross-sectional studies, (3) randomized experimental studies, (4) quasi-experimental studies, (5) case reports, (6) mix-methods, and (7) retrospective case studies.
2.3. Exclusion criteria
Studies were excluded if the study participants had underlying neurological disorders not related to the sickle cell disease diagnosis and if the participants had undergone a stem cell transplant. Studies reporting only pain and no other measures of physical impairment or limitation were excluded. Manuscripts with the following study design were excluded: (1) meta-analyses, (2) umbrella reviews, (3) systematic reviews, (4) narrative reviews, (5) position papers, (6) scoping reviews, (7) white paper/editorials, and (8) qualitative studies.
2.4. Article selection and data extraction
Titles and abstracts screening and full-text reviews were performed by two independent reviewers using Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia). If the two independent reviews did not agree, a third independent reviewer served as a tiebreaker. Data were extracted from each full text to a custom Microsoft Excel spreadsheet (Microsoft Corporation, Redmond, WA, USA) by a single reviewer and were verified by a secondary reviewer. Extraction data points included article information (author, title, year), study design, study objective, participant diagnoses, inclusion/exclusion criteria, number of participants, participant age range, outcome measures and data, and the comparison group. Clear protocol and instructions were provided for all steps of screening, selection, and extraction.
3. Results
The search strategy resulted in 3,054 non-duplicate articles; 67 studies were included in the synthesis. The PRISMA flow diagram is outlined in Figure 1. Of the excluded studies, the most common study designs were narrative reviews and position papers. The resultant 67 studies included 50 cross-sectional observational studies, 12 longitudinal studies, three case studies, one mixed-methods study, and one quasi-experimental study related to physical impairment and physical function outcomes.
Figure 1.
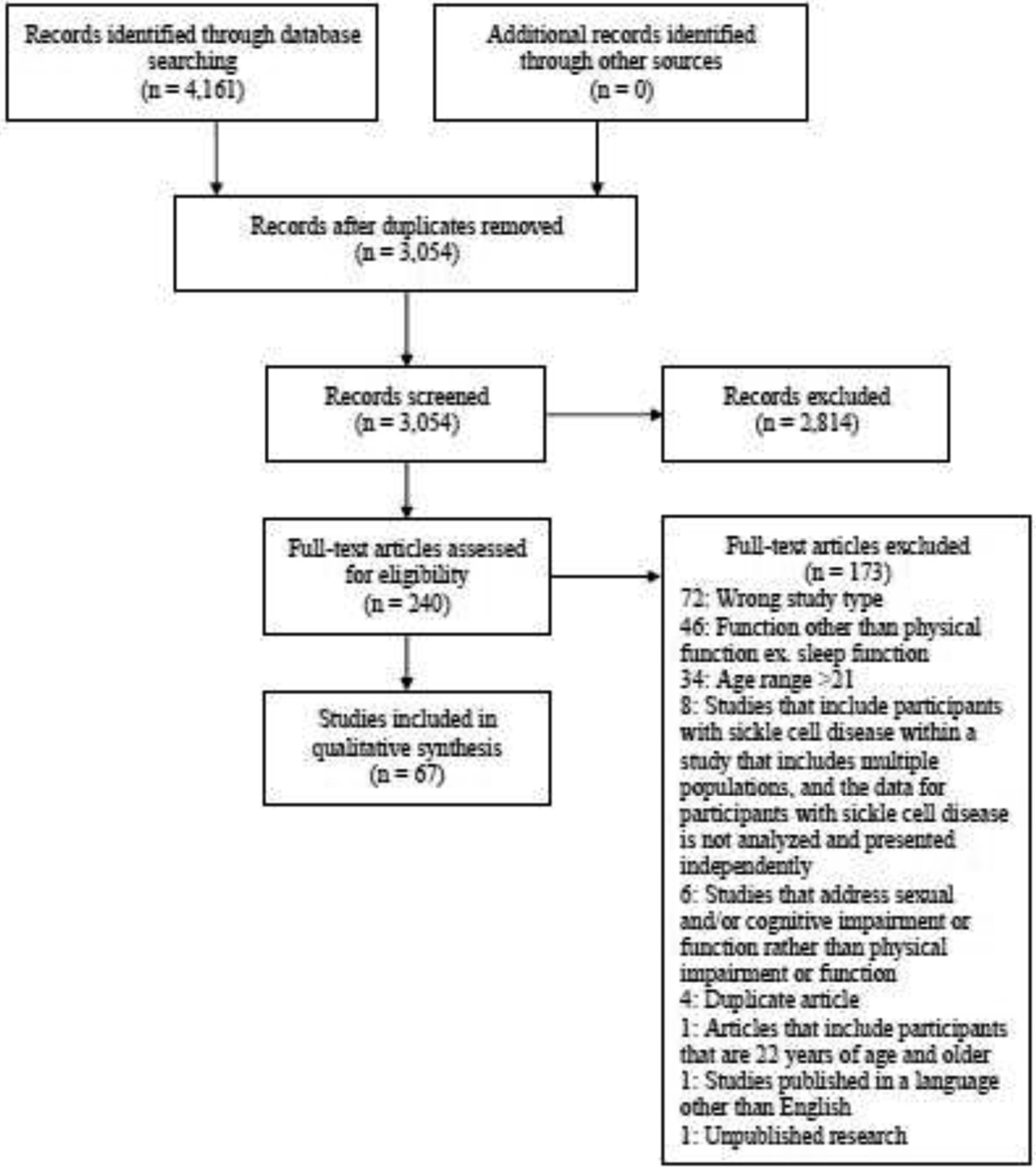
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flow Chart
Of the three case studies, two explored the range of motion of the hip before and after casting and surgical management for a dislocated hip replacement and a slipped capital femoral epiphysis, respectively.22,23 The third case study described a differential diagnosis of autoimmune rheumatic disorders in SCD.24 These case studies represent unique diagnoses and therefore were not discussed in this review. Seven studies included health-related quality of life surveys but did not report results related to specific physical impairments or limitations.14,25–30 Therefore, these seven studies will not be discussed any further.
The remaining 57 studies had components related to physical impairments and physical function limitations. Of the 57 studies, one study included interventions specifically targeting physical impairments and physical function limitations.31 Only baseline data is reported for longitudinal studies.
3.1. Body Structure and Function Impairments
3.1.1. Muscle and Bone Composition/Symptoms
Changes in muscle and bone composition in children with SCD were described in two studies32,33 and musculoskeletal symptoms were reported in three studies.10,34 Children with SCD, compared to children without SCD, presented with a reduction of lean muscle mass (5 to 18%), bone density (3 to 6%), bone mineral content (7%), and bone area (6%).32,33,35 Musculoskeletal symptoms of joint stiffness (16% to 19%), decreased flexibility, and muscle tenderness (8%) were reported by children and adolescents with SCD (Table 1).10,34–36
Table 1.
Muscle and Bone Composition/Symptoms
| Study | N | Age range/mean | Diagnoses | Non-SCD Control | Relevant Outcome Measures | Findings Related to Physical Impairments and Function |
|---|---|---|---|---|---|---|
| Buison et al. 200532 | 90 | 4–19 years/10.7 ± 4.2 years | HbSS | Yes |
|
|
| Jacob et al. 201334 | 76 | 10–17 years/13.0 ± 1.9 years | HbSS HbSC |
No |
|
|
| Moheeb et al. 200710 | 141 | 9–12 years/10.1 ± 0.076 years* | HbSS | Yes |
|
|
| Rhodes et al. 200933 | 64 | 10–13 years/11.29 ± 11.2 (male) 11.70 ± 1.62 (female) | SCA | Yes |
|
|
| Wali & Moheeb 201135 | 93 | 10.4–14.6 years/12.81 ± 0.136 years* | SCA | Yes |
|
Abbreviations: N = number of participants with sickle cell disease; SCA = Sickle Cell Anemia; SCD = Sickle Cell Disease; DEXA= dual energy X-ray absorptiometry. Results are reported as mean ± standard deviation, except where noted otherwise.
Standard Error.
3.1.2. Muscle Strength
Children and adolescents with SCD present with impaired muscle strength. Muscle strength was an outcome measure in eight studies (Table 2).10,35,37–42 Compared to controls, children and adolescents with SCD presented with a significant reduction in maximal handgrip strength (4 to 43%), vertical jump peak power (24 to 29%), jump height (10 to 23%), ankle plantarflexion torque (17 to 37%), and back strength (7 to 25%).10,35,38–40 Generalized weakness was reported in 14 to 88% of children and adolescents with SCD.41,42
Table 2.
Muscle Strength
| Study | N | Age range/mean | Diagnoses | Non-SCD Control | Relevant Outcome Measures | Findings Related to Physical Impairments and Function |
|---|---|---|---|---|---|---|
| Brownell et al. 202037 | 22 | 9–19 years/13.8 ± 3.3 years | HbSS | No |
|
|
| Dougherty et al. 201140 | 35 | 5–13 years/9 ± 2.2 years | HbSS | Yes |
|
|
| Dougherty et al. 201838 | 21 | 5–17 years/11 ± 4 years | HbSS | Yes |
|
|
| Dougherty et al. 202039 | 21 | 5–20 years/11 ± 4 years | HbSS | Yes |
|
|
| Jacob et al. 201241 | 31 | 10–17 yers | HbSS, HbSC | No |
|
|
| Moheeb et al. 200710 | 50 | 9–12 years/10.1 ± 0.076* years | HbSS | Yes |
|
|
| Patra et al. 201342 | 330 | 9 months-14 years/7.60 (males) 9.22 (females) | SCA | Yes |
|
|
| Wali & Moheeb 201135 | 93 | 10.4–14.6 years/12.81 ± 0.136* years | SCA | Yes |
|
|
Abbreviations: N = number of participants with sickle cell disease; NS = not significant; SCA = Sickle Cell Anemia; SCD = Sickle Cell Disease. Results are reported as mean ± standard deviation, except where noted otherwise.
Standard error.
Unknown units of measure.
3.1.3. Cardiopulmonary Function
Ten studies included outcomes of cardiopulmonary function (Table 3).8,9,31,33,43–48 Children and adolescents with SCD presented with impaired cardiopulmonary function at rest, during maximal and submaximal cardiopulmonary testing, and these impairments may be amenable to exercise intervention. Compared to healthy controls, children and adolescents with SCD presented with impaired resting cardiopulmonary function. The cardiopulmonary function was measured during rest and/or exercise through vital signs (heart rate, respiratory rate, blood pressure, pulse oximetry), and blood and gas properties. Children and adolescents with SCD presented with higher resting heart rate and metabolic rate, and impaired adjusted resting energy expenditure and activity-related energy expenditure.33,43,44,48 Pulmonary blood flow and stroke volume were consistently greater in children and adolescents with SCD compared to controls at rest, during exercise, and during recovery.45 Arteriovenous oxygen was consistently one-third lower in SCD compared to controls.45 However, no ventilatory differences were detected.45
Table 3.
Cardiopulmonary Function
| Study | N | Age range/mean | Diagnoses | Non-SCD Control | Relevant Outcome Measures | Findings Related to Physical Impairments and Function |
|---|---|---|---|---|---|---|
| Barden et al. 200043 | 36 | 5–18 years/11.3 ± 3.8 years | HbSS | Yes |
|
|
| Buchowski et al. 200244 | 28 | 14–18 years/15.6 ± 1.5 years | SCA | Yes |
|
|
| Chaudry et al. 201345 | 50 | 10–18 years/14.1 ± 2.4 years (male) 14.2 ± 2.5 years (female) |
HbSS HbSC |
Yes |
|
|
| Hostyn, et al. 20138 | 46 | 6–18 years/9.15 ± 3.06 years | HbSS HbSC HbSβ0 HbSβ+ |
No |
|
|
| Liem et al. 201546,47 | 60 | 8–21 years/15.1 ± 3.4 years | HbSS HbSβ0 |
Yes |
|
|
| Liem et al. 201731 | 10 | 13–21 years/14.4 ± 2.5 years | HbSS HbSβ0 |
No |
|
|
| Melo et al. 20179 | 57 | 6–18 years/11.9 ± 3.5 years | HbSS | Yes |
|
|
| Rhodes et al. 200933 | 64 | 10–13 years/11.29 ± 11.2 years (male) 11.70 ± 1.62 years (female) | SCA | Yes |
|
|
| Singhal et al. 199748 | 16 | 16.1–20.1 years/18.0 ± 1.1 years | SCD | Yes |
|
|
Abbreviations: N = number of participants with sickle cell disease; SCA = Sickle Cell Anemia; SCD = Sickle Cell Disease; bpm = beats per minute; rpm = respirations per minute. Results are reported as mean ± standard deviation, except where noted otherwise.
During maximal and submaximal cardiopulmonary testing, children and adolescents with SCD performed poorer than controls. During maximal cardiopulmonary exercise testing, using cycle ergometer, children and adolescents with SCD demonstrated reduced total exercise time (28%), peak work rate (29%), weight-adjusted peak VO2 max (27%), and ventilatory threshold (23%) and efficiency (9%) compared to controls.46,47 Impaired gas exchange and impaired oxygen uptake and delivery compared to controls were also observed.46,47 After the 6-minute walk test (6MWT), children with SCD reported higher rates of perceived exertion (4.1 out of 10) as compared to controls (1.2).9 Children with HbSS/HbSβ0 presented with decreased pulse oximetry and increased respiratory rate compared to children with HbSC/HbSβ+.8
A single intervention study by Liem et al. (2017) explored a 12-week individualized cardiovascular exercise training program for adolescents with SCD. The adolescents were prescribed three exercise sessions per week for 12 weeks on a stationary bicycle at the participant’s home.31 The training protocol was progressed throughout the duration of the intervention by increasing goals for target heart rate and duration of exercise.31 Participants demonstrated an increased mean peak rate of oxygen consumption (VO2) and peak workload after the first seven weeks of training.31 However, there was no significant improvement in exercise parameters, including maximal VO2, peak workload, and ventilatory threshold, at the end of the training program.31 In this study, 77% of the subjects completed 89% of prescribed sessions without exercise-related adverse events.31
3.2. Physical Function
3.2.1. Motor Performance
Gross motor and fine motor performance were measured in 17 studies (Table 4).5,6,51–57,7–10,37,39,49,50 The results of these studies suggest children and adolescents with SCD may present with deficits in motor performance.
Table 4.
Motor Performance
| Study | N | Age range/mean | Diagnoses | Non-SCD Control | Relevant Outcome Measures | Findings Related to Physical Impairments and Function |
|---|---|---|---|---|---|---|
| Armstrong et al. 201356 | 193 | 7–18 months/12.7 ± 2.7mo | HbSS HbSβ0 |
No |
|
|
| Brousse et al. 20205 | 51 | 5–17 years/11.9 ± 3.8 years | HbSS HbSβ0 |
No |
|
|
| Brownell et al. 202037 | 22 | 9–12 years/13.8 ± 3.3 years | HbSS | No |
|
|
| Burkhardt et al. 201749 | 32 | 7–17.25 years/11.14 ± 4.48 years | HbSS HbSC HbSβ0 |
Yes |
|
|
| Dedeken et al. 20146 | 46 | 6.1–19.7 years/12 years | HbSS HbSC HbSβ0 HbSβ+ |
No |
|
|
| Dougherty et al. 202039 | 21 | 5–20 years/11 ± 4 years |
HbSS | Yes |
|
|
| Drazen et al. 201550 | 43 | 1–34 months/8.8 ± 7.5 months | HbSS HbSC HbSβ0 HbSβ+ SPFH |
No |
|
|
| Glass et al. 201357 | 80 | Mean: 20.5 months | HbSS HbSC |
No |
|
|
| Hostyn et al. 20138 | 46 | 6–18 years/9.15 ± 3.06 years | HbSS HbSC HbSβ0 HbSβ+ |
No |
|
|
| Melo et al. 20179 | 57 | 6–18 years/11.9 ± 3.5 years | HbSS | Yes |
|
|
| Millis et al. 199451† | 30 | Only 10-year olds | HbSS | Yes |
|
|
| Möckesch et al. 20177 | 46 | 10–16 years/15 ± 2.4 years (HbSS) 15.1 ± 2.6 years (HbSC) | HbSS, HbSC |
No |
|
|
| Moheeb et al. 200710 | 50 | 9–12 years/10.1 ± 0.076 years | HbSS | Yes |
|
|
| Newby et al. 201852 | 67 | 5–18 years/11.36 ± 3.51 years | HbSS HbSC HbSβ0 HbSβ+ |
Yes |
|
|
| Schatz & Roberts 200753 | 61 | 12–18 & 32–40 months/14.8 ± 2.6 & 37.3 ± 3.4 months (HbSS/HbSβ0); 15.9 ± 2.1 & 36.1 ± 2.5 months (HbSC/HbSβ+) | HbSS HbSC HbSβ0 HbSβ+ |
No |
|
|
| Zempsky et al. 201354 | 25 | 7–21 years/16.6 ± 2.4 years | HbSS HbSC HbSβ+ |
No |
|
|
| Zempsky et al. 201455 | 159 | 7.26–21.82 years/15.73 ± 3.63 years | HbSS HbSC HbSβ0 HbSβ+ |
No |
|
|
Abbreviations: N = number of participants with sickle cell disease; NS = not significant; SCD = Sickle Cell Disease; SPFH = S persistent fetal hemoglobin. Results are reported as mean ± standard deviation, except where noted otherwise.
Standard error.
The unusually fast reported times for 20- and 40-yard swim for control sample were unable to be validated due to unsuccessful attempts to contact the corresponding author.
In three studies, infants’ and toddlers’ gross motor performance was measured by the Bayley Scale of Infant Development.49,50,52 Drazen et al. (2016) reported, as compared to a normative population, infants and toddlers with SCD present with below-average performance in fine motor (31.7%) and gross motor (32.6%) performance. However, Armstrong et al. (2013) and Glass et al. (2013) reported on average, infants and toddlers with SCD did not present with motor delay. One study in children with SCD using the Vineland Adaptative Behavior Scale (VABS) motor scale, reported lower age-adjusted motor scores at 32–40 months of age compared to 12–18 months of age.55
Two studies reported motor performance using the Bruininks-Oseretsky Test of Motor Proficiency (BOT) Short Form to quantify motor proficiency with baseline scores in children and adolescents with SCD.37,39 The mean BOT score in children and adolescents with SCD was reported as 62 to 67 out of 88 compared to 62 in controls.37,39 Burkhardt (2017) and Newby et al. (2018) explored fine motor performance in children with SCD. Burkhardt et al. (2017) found children and adolescents with SCD presented with impaired fine motor function, whereas Newby et al. (2018) reported children with SCD performed average for fine motor dexterity.51,54
Motor performance was reported to be impaired in children and adolescents with SCD performing tasks required for participation in school, play, and sports activities. While performing the 6MWT, children and adolescents with SCD performed below norm-referenced distances (72 to 83% of predicted distance).5–9 Children with SCD had poorer performance on the 20-yard swim, 40-yard swim, 100-yard “potato race”, and jump height compared to age- and sex-matched controls.10,53 Zempky et al. (2013, 2014) measured physical function via Functional Independence Measure (FIM) with baseline motor scores of 54 to 57 out of 91 in children and adolescents with SCD during vaso-occlusive pain episodes. During hospitalizations, children and adolescents with SCD demonstrated improvements in physical function from initial FIM scores at admission to before discharge.56
3.2.2. Physical Activity
In four studies, physical activity was used as an outcome measure (Table 5).44,48,58,59 Physical activity has been defined as bodily movements produced by skeletal muscles that require energy expenditure.60 Physical activity was measured using accelerometers, heart rate monitors, metabolic parameters, or surveys. Children and adolescents with SCD present with decreased amount and level of physical activity by 24 to 58% and spend more time at lower physical activity intensities with mean physical activity at light intensity.44,48,58,59
Table 5.
Physical Activity
| Study | N | Age range/mean | Diagnoses | Non-SCD Control | Relevant Outcome Measures | Related to Physical Impairments and Function |
|---|---|---|---|---|---|---|
| Buchowski et al. 200244 | 28 | 14–18 years/15.6 ± 1.5 years | SCA | Yes |
|
|
| Karlson et al. 201758 | 30 | 8–18 years/13.9 ± 2.9 years | HbSS HbSC HbSβ0 HbSβ+ |
No |
|
|
| Melo et al. 201859 | 50 | 6–18 years/12.02 ± 3.63 years | HbSS | Yes |
|
|
| Singhal et al. 199748 | 16 | 16.1–20.1 years/18.0 ± 1.1 years | SCD | Yes |
|
|
Abbreviations: N = number of participants with sickle cell disease; SCA = Sickle Cell Anemia; SCD = Sickle Cell Disease; MET = metabolic equivalent of task. Results are reported as mean ± standard deviation, except where noted otherwise.
3.3. Health-related Quality of Life: Physical Function
Of the resultant studies, 28 analyzed reported health-related quality of life questionnaires with domains of physical function or mobility (Table 6).7,9,64–73,12,74–81,13,39,57,59,61–63 These questionnaires included: the Patient-Reported Outcome Measurement Information System (PROMIS), Pediatric Quality of Life Inventory (PedsQL), Functional Disability Inventory (FDI), Child Health Questionnaire (CHQ), Physical Activity Questionnaire for Older Children and Adolescents (PAQ-C), Barthel Index for Activities of Daily Living (Barthel Index), 36-Item Short Form Health Survey (SF-36), Child Activities Limitations Interview (CALI), EUROQOL, Youth Acute Pain Functional Ability Questionnaire (YAPFAQ), National Health and Nutrition Survey (NHANES), International Physical Activity Questionnaire (IPAQ), Child Physical Activity Questionnaire, and a self-designed questionnaire. Physical function using questionnaires were used to assess differences between children and adolescents with SCD compared to controls (healthy or with the sickle-cell trait), to assess differences between SCD groups with medical or symptomatic conditions, and to provide objective measurements at baseline for SCD samples.
Table 6.
Health-related Quality of Life: Physical Function
| Study | N | Age range/mean | Diagnoses | Non-SCD Control | Relevant Outcome Measures | Findings Related to Physical Impairments and Function |
|---|---|---|---|---|---|---|
| Adeyemo et al. 201579 | 80 | 15–18 years/16 ± 1.5 years | HbSS HbSC |
Yes |
|
|
| Barakat et al. 200880 | 42 | 12–18 years/15 ± 1.82 years | HbSS | No |
|
|
| Dampier et al. 201013 | 1,772 | 2–18 years/9.6 ± 4.7 years | HbSS HbSC HbSβ+ HbSβ0 |
No |
|
|
| Dampier et al. 201612 | 121 | 8–17 years/12.5 ± 3.1 years |
HbSS HbSC |
Yes |
|
|
| Dampier et al. 201676 | 235 | 8–17 years/12.5 ± 2.8 years | HbSS HbSC HbSβ+ HbSβ0 |
Yes |
|
|
| Dougherty et al. 202039 | 21 | 5–20 years/11 ± 4 years | HbSS | Yes |
|
|
| Hoff et al. 200681 | 56 | 8–17 years/12.14 ± 2.46 years | HbSS HbSC HbSβ+ |
No |
|
|
| Hussein et al. 201961 | 100 | 6–12 years/8.90 ± 2.06 | SCA | No |
|
|
| Kambasu et al. 201962 | 140 | 8–17 years/14.25 years | HbSS | NO |
|
|
| Karlson et al. 202075 | 206 | 8–17 years/11.72 ± 4.42 years | HbSS HbSC |
No |
|
|
| Malheiros et al. 201563 | 71 | 8–21 years/12.19 ± 3.2 years | SCA with and without hip dysfunction | No |
|
|
| Matos et al. 201864 | 24 | 8–18 years/11.1 ± 3.9 years |
SCA | Yes |
|
|
| Melo et al. 20179 | 57 | 6–18 years/11.9 ± 3.5 years | HbSS | Yes |
|
|
| Melo et al. 201859 | 50 | 6–18 years/12.02 ± 3.63 years | HbSS | Yes |
|
|
| Menezes et al. 200865 | 100 | 5–18 years | HbSS HbSC HbSβ+ |
Yes |
|
|
| Möckesch et al. 20177 | 46 | 10–16 years/15 ± 2.4 (HbSS) 15.1 ± 2.6 (HbSC) |
HbSS HbSC |
Yes |
|
|
| Omwanghe et al. 201777 | 100 | 6–12th graders | SCD | Yes |
|
|
| Oliver-Carpenter et al. 201166 | 47 | 6–18 years/11.93 ± 3.8 years | HbSS HbSC HbSβ+ HbSβ0 |
Yes |
|
|
| Palermo et al. 200267 | 58 | 5–18 years/10.97 ± 3.41 | HbSS HbSC HbSβ+ |
Yes |
|
|
| Palermo et al. 200578 | 42 | 9–17 years/13.36 ± 1.34 years | HbSS HbSC HbSβ+ |
No |
|
|
| Panepinto et al. 200568 | 99 | 5–8 years/10.67 ± 3.71 years | HbSS HbSC HbSβ+ HbSβ0 HbS tacoma |
No |
|
|
| Patel & Pathan 200569 | 25 | 8–18 years/11.2 ± 1.7 years | SCA | Yes |
|
|
| Sil et al. 201670 | 100 | 8–18 years/13.54 ± 2.7 years | HbSS HbSC HbSβ+ HbSβ0 |
No |
|
|
| Sil et al. 202071 | 42 | 8–18 years/14.95 years | HbSS HbSC HbSβ+ HbSβ0 |
No |
|
|
| Singh et al. 202072 | 67 | 8–18 years/10.7 ± 3.6 years | HbSS HbSC HbSβ+ HbSβ0 Other |
No |
|
|
| Singh et al. 201973 | 164 | 5–17 years/10 ± 4 years | HbSS HbSC HbSβ0 Other |
No |
|
|
| Thornburg et al. 201174 | 191 | 2–18 years/10.4 ± 4.7 years | HbSS HbSC HbSβ+ HbSβ0 HbSOA rab HbSGP hil-adelphia |
No |
|
|
| Zempsky et al. 201455 | 159 | 7.2621.82 years/15.73 ± 3.63 years | HbSS HbSC HbSβ+ HbSβ0 |
No |
|
|
Abbreviations: N = number of participants with sickle cell disease; NS = not significant; SCA = Sickle Cell Anemia; SCD = Sickle Cell Disease; Barthel Index = The Barthel Index for Activities of Daily Living; CALI = Child Activities Limitations Interview; CHQ = Child Health Questionnaire; FDI = Functional Disability Index; FIM = Functional Independence Measure; IPAQ = International physical activity questionnaire; NHANES = National Health and Nutrition Examination Survey; PAQ-C = Physical Activity Questionnaire for Older Children; PedsQL = Pediatric Quality of Life Inventory; PROMIS = Patient-Reported Outcomes Measurement Information System; SF-36 = 36-Item Short Form Health Survey; YAPFAQ = Youth Acute Pain Functional Ability Questionnaire. Results are reported as mean ± standard deviation, except where noted otherwise.
Standard error.
Median (Interquartile range [IQR]).
Compared to controls, children and adolescents with SCD present with a significant deficit of physical function (17%; SF-36), physical activity (49–51%; PAQ-C, NHANES, IPAQ), physical function (39 to 55%; PedsQL), and physical functioning (17%) and physical role impact (19%; CHQ).7,9,59,61,67,68,70,80 Children and adolescents with SCD presented with poorer upper extremity function by 9% and lower extremity function by 8% as measured by the PROMIS compared to controls.39 Children and adolescents without health conditions reported “no problems” with mobility, however, 24% of children and adolescents with SCD reported “problems on some days” with mobility as measured by the EUROQOL.39,72 As compared to normative samples, children and adolescents with SCD presented within the normative range for the CHQ and PROMIS measures of mobility and upper extremity dexterity.12,62,79 Children and adolescents with SCD reported lower levels of physical activity on the IPAQ and NHANES compared to controls.7,80 Although most studies did not explore relationships between age and physical function, PedsQL data from Menenez et al. (2008)68 suggest that younger children (5–7 years of age) report less percentage of physical function deficits compared to controls (39%) than older children (8–12 years of age; 49%) and adolescents (13–18 years of age; 55%).
Seven studies assessed differences in self-reported physical function between SCD groups with medical or symptomatic conditions including migraine/headaches, hip dysfunction, pain patterns, and those receiving and not receiving hydroxyurea. Palermo et al. (2005) studied children and adolescents with SCD who presented migraine ± aura, tension headache, or no headache. This study reported a significant difference in functional disability, as measured by the FDI, between migraine and tension headache, and migraine and no headache groups.81 Malheiros et al. (2015) compared children and adolescents with and without hip dysfunction using the Barthel Index and PedsQL. Those with hip dysfunction reported significantly lower physical activity compared to those without hip dysfunction, but no differences were noted in activities of daily living.66 Sil et al. (2016, 2020) compared children and adolescents with SCD with varying frequencies of pain. This study identified significantly lower functional disability scores on the FDI in those with no pain compared to episodic pain, and no pain compared to chronic pain.73,74 Using the PROMIS measure, Singh et al. (2019, 2020) assessed physical activity and strength impact in children and adolescents with SCD who received or did not receive pain medication in the prior week, and varying levels of pain. Pain medication status and pain level did not affect physical activity, but those who received pain medicine in the past week and those with higher pain scores reported lower strength impact scores.75,76 Thornburg et al. (2011) reported significantly higher physical function as measured by the PedsQL in children and adolescents with SCD on hydroxyurea compared to those not on hydroxyurea.
The remaining 10 articles including health-related quality of life questionnaires related to physical function reported raw baseline values for the FDI, YAPFAQ, PedsQL, CALI, CHQ, Child Physical Activity Questionnaire, and a self-made questionnaire.13,57,62–65,69,71,77,78
4. Discussion
The studies identified in this systematic review offer emerging evidence that children and adolescents with SCD demonstrate physical impairments and physical function limitations related to muscle and bone composition/symptoms, muscle strength, cardiopulmonary function, motor performance, physical activity, and physical function domains of quality of life questionnaires. The manuscripts included utilized a variety of outcomes and procedures to measure physical impairment and physical function. Objective measurements of physical impairments and function were used in 47% of the studies. Subjective child and/or parent proxy reports were used in 47% of the studies. A combination of both objective and subjective measurements of physical impairment and physical function were used in 5.3% of the studies.
The body structure and function impairments identified in this review begin in childhood and progress well into adulthood including muscle and bone composition and cardiopulmonary function. Young adult women (mean age of 31 years) with SCD demonstrate a high mean percentage of fat, low fat-free mass, and low bone mineral density despite average weight and bone mass indexes.82 Similarly, low bone mineral density is prevalent in 80% of young adults with SCD (mean age of 36 years), where the lumbar spine is the most common site.83 This high incidence of low bone mineral density may be attributed to the failure of children and adolescents with SCD to obtain optimal peak bone mass during growth.83,84 Factors including female sex, older age, higher body mass index, and lower hemoglobin levels demonstrated relationships with lower fitness levels as measured by maximal treadmill exercise testing in a large sample of adults (mean age of 43 years) with SCD from the Cooperative Study of Sickle Cell Disease.85 Therefore, the early identification of physical impairments and initiation of targeted interventions in individuals with SCD during childhood and adolescence may have the potential to influence the physical impairment and function into adulthood.
Few published studies focus on factors that influence physical function besides pain in children and adolescents with SCD.21,86,87 Although pain impacts physical function,56 other factors such as changes in muscle force production and muscle power can affect physical function in children.21,87 In SCD, muscle hypoxia affects muscle remodeling, which could lead to impaired force production.4 Muscle performance capacity can be quantified through measurements of peak muscle force or joint torque.88 Factors contributing to muscle force production include not only muscle size (muscle thickness, cross-sectional area, volume), but also neuromuscular activation (amplitude of activation, rate of activation, rate force development) as measured by electromyography and dynamometry. Muscle size is associated with the ability to produce maximal strength.89 Measurements of neuromuscular activation, such as the rate of muscle activation, can be measured through electromyography and may be critical in not only the ability to produce maximal strength but also the quick and coordinated movements required in many physical function activities.20,21 In populations other than SCD, relationships between underlying factors and physical function are well-studied. For example, in children and adolescents with childhood cancer undergoing treatment for acute lymphoblastic leukemia, strength was significantly associated with physical function.90,91 The Timed Up and Go test was correlated with knee extension strength, and the Timed Up and Down Stairs and the 9-Minute Run-Walk tests were correlated with ankle dorsiflexion strength.90,91 Interventions focusing on muscle force production in healthy children and adolescents improve performance in physical function activities, such as running, jumping, and throwing.92,93 Thus, further exploration of strength and the underlying factors are warranted in children and adolescents of SCD.
This systematic review did not identify any published articles related to balance function in children and adolescents with SCD, even though balance deficits can occur due to impaired perfusion to the brain and downstream dysfunction in the neuromuscular system.94 Silva et al. (2018) identified deficits in balance in adults with SCD without known neurological involvement compared with healthy controls.94 Deficits in balance in children and adolescents are measured through static, dynamic, transfer, and mobility testing.95 Balance assessment can be performed through timing tasks (single leg stance, timed up and go), performing standardized testing (BOT-2 balance subscale, Berg Balance Scale), or using specialized force plates to measure a child or adolescent’s center of pressure during tasks (limits of stability, Modified Clinical Test of Sensory Interaction in Balance).95,96 In children and adolescents, deficits in balance have been associated with impaired physical function.97 Therefore, the underlying factors that contribute to balance need to be thoroughly examined in children and adolescents with SCD to assist in the development of targeted intervention programs aimed to improve physical function.
There are very few rehabilitation intervention studies in children and adolescents with SCD. One intervention study was identified through this systematic review. Besides the one pediatric study included in our review,31 we identified a manuscript published outside of this systematic review’s date range. Alcorn et al. (1984)98 explored the range of motion, gait, and length of hospitalization in children and adolescents with SCD hospitalized for painful vaso-occlusive crises. The intervention included heat fluidotherapy twice daily, initiated on hospital day two. Once ambulatory, the children and adolescents participated in moderate strength and endurance exercise in 10-to-30 minutes durations that included recreational gymnastics, stationary bike riding, and games.98 The authors concluded exercise and heat therapy may contribute to a reduction in the length of hospitalization in children and adolescents with SCD and painful vaso-occlusive crises.98 Both the Liem et al. (2017) and Alcorn et al. (1984) studies demonstrate children and adolescents can improve with a focused intervention program. There is a significant need for further study for rehabilitation interventions targeting physical impairment and physical function in children and adolescents with SCD.
Many of the studies identified in this systematic review included questionnaires as the main measure of physical function. Questionnaires offer a reliable and valid method for obtaining subjective information regarding physical function. However, children and adolescents often report higher physical function compared to their caregiver’s reports.13,62,65,69,71 Although questionnaires have practical and clinical utility, using questionnaires only without an objective assessment measure of physical impairment and function may not provide the comprehensive picture required for measuring change over time in response to a targeted intervention. Objective measures provide specific information about a participant’s body structure function and physical function that can be directly compared to controls and used to measure change over time. Discrepancies between subjective and objective measurements favor the use of objective measurements or a combination of these two types of measurement methods for children and adolescents.99,100 Therefore, the results from this systematic review support the utility of both methods of measurement when examining physical impairments and physical function in children and adolescents with SCD.
Disease-modifying medical management, such as hydroxyurea, shows promise in the reduction of physical symptoms and serious medical complications of SCD,101 thus may also demonstrate a role in the reduction of physical impairments and improvements in physical function. Hydroxyurea is an antimetabolite medication, which has the capacity to increase fetal hemoglobin production thus decreasing the likelihood of red blood cell sickling.101 Wali & Moheeb (2011)35 report that hydroxyurea not only increased hemoglobin concentrations in adolescents with SCD but also improved physical fitness, as measured by mean heart rate and non-fatigued time on a treadmill test. The physical fitness of these children and adolescents with SCD after taking hydroxyurea for a minimum of two years was more similar to controls compared to their non-medicated baseline.35 Thornburg et al. (2011)77 studied the health-related quality of life in children and adolescents with SCD receiving hydroxyurea compared to those not receiving hydroxyurea. After adjusted for age, sex, and SCD genotype and disease severity, children and adolescents receiving hydroxyurea reported greater PedsQL total and physical functioning scores compared to their non-medicated peers.77 The use of hydroxyurea also benefits other areas of health-related quality of life including social function, pain recall, general health perception.102 According to Badawy et al. (2017), higher levels of adherence in taking hydroxyurea suggest a better health-related quality of life in adolescents and young adults with SCD.103 Further studies are needed to explore the potential effects of hydroxyurea therapy on physical impairments and function in children and adolescents with SCD.
The findings of this systematic review, with a detailed search strategy across multiple search engines, highlight the breadth of physical impairments and physical function limitations experienced by children and adolescents with SCD. This review identified that many previous studies have relied heavily on health-related quality of life questionnaires and identified gaps in evidence including skeletal muscle properties and performance, gross motor performance, and balance. Additionally, the relationships between physical impairments and gross motor performance have not been explored. These findings can help to provide a basis for clinical practice, and future research exploring objective measurement of physical impairments and function in this unique population. With an increased understanding of how SCD and its management affects physical impairments and function, clinicians and rehabilitation scientists can explore the effects of targeted interventions to mitigate these adverse effects. This review has also identified the need for rehabilitation scientists and clinicians to develop collaborative standards to define and objectively measure changes in physical impairment and function to allow for data analysis of larger samples of children and adolescents with SCD.
4.1. Study Limitations
This systematic review explored manuscripts related to physical impairments and physical function in children and adolescents with SCD published since 1990. Although the search strategy was comprehensive, we were able to identify an intervention study outside the date range for this systematic review search. Therefore, a larger search range may have improved capturing the full breadth of literature for this specific population. The systematic search strategy of this review excluded grey literature and manuscripts not published in the English language. This review was exploratory, and due to the heterogeneity of study designs, participant characteristics, and outcome measurements across studies, a meta-analysis was not performed.
5. Conclusions
This systematic review identified 57 articles supporting that children and adolescents with SCD present with physical impairments and physical function limitations. Further research is needed to comprehensively examine the underlying factors that contribute to physical impairments and function in children and adolescents with SCD. Further studies exploring the factors contributing to physical impairments and physical function in children and adolescents with SCD will support the development of targeted intervention programs that aim to improve physical performance. Additionally, rehabilitation scientists and clinicians should consider developing collaborative standards to define and objectively measure changes in physical impairment and function experienced by children and adolescents with SCD.
6. Acknowledgments
This work was supported by the Dr. Gladys E. Wadsworth Physical Therapy Research Fund from the Department of Physical Therapy and Rehabilitation Science within the University of Maryland School of Medicine. This publication was made possible by a NIAMS-funded predoctoral fellowship to K.R. (T32AR007592).
List of abbreviations:
- 6MWT
6-minute walk test
- Barthel Index
The Barthel Index for Activities of Daily Living
- BOT
Bruininks-Oseretsky Test of Motor Proficiency
- CALI
Child Activities Limitations Interview
- CHQ
Child Health Questionnaire
- FDI
Functional Disability Inventory
- FIM
Functional Independence Measure
- HbSC
hemoglobin SC disease
- HbSS
hemoglobin SS disease
- HbSβ+
sickle cell beta plus thalassemia
- HbSβ0
sickle cell beta null thalassemia
- IPAQ
International physical activity questionnaire
- NHANES
National Health and Nutrition Examination Survey
- PAQ-C
Physical Activity Questionnaire for Older Children and Adolescents
- PedsQL
Pediatric Quality of Life Inventory
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROMIS
Patient-Reported Outcome Measurement Information System
- SCD
Sickle cell disease
- SF-36
36-Item Short Form Health Survey
- VO2
Rate of oxygen consumption
- YAPFAQ
Youth Acute Pain Functional Ability Questionnaire
Appendix 1. Search Strategies
[Limits: publication date: 1990-present, English language]
PubMed
(“anemia, sickle cell”[mesh] OR sickle cell[tiab] OR sickle anemia[tiab] OR sickle anaemia[tiab] OR hemoglobin S disease[tiab] OR haemoglobin S disease[tiab] OR hemoglobin SS[tiab] OR haemoglobin SS[tiab] OR hemoglobin SC[tiab] OR haemoglobin SC[tiab] OR hemoglobin SD[tiab] OR haemoglobin SD[tiab] OR SCA[tiab] OR sicklemia[tiab] OR sicklaemia[tiab] OR sickling[tiab] OR drepanocyt*[tiab])
AND
(muscle tone[tiab] OR spasticity[tiab] OR hemiparesis[tiab] OR haemiparesis[tiab] OR ataxi*[tiab] OR proprioception[tiab] OR kinesthesi*[tiab] OR kinaesthesi*[tiab]OR static balance[tiab] OR dynamic balance[tiab] OR sitting balance[tiab] OR standing balance[tiab] OR range of motion[tiab] OR flexibility[tiab] OR extensibility[tiab] OR contracture[tiab] OR gait[tiab] OR muscle strength[tiab] OR weakness[tiab] OR muscle force[tiab] OR torque[tiab] OR musc* power[tiab] OR functional strength[tiab] OR muscle activation[tiab] OR neuromuscular activation[tiab] OR electromyography[tiab] OR muscle size[tiab] OR muscle architecture[tiab] OR posture[tiab] OR scoliosis[tiab] OR lordosis[tiab] OR kyphosis[tiab] OR physical function*[tiab] OR motor function*[tiab] OR muscle function*[tiab] OR gross motor[tiab] OR fine motor[tiab] OR motor performance[tiab] OR physical performance[tiab] OR sports[tiab] OR physical activity[tiab] OR endurance[tiab] OR fatigue[tiab] OR fitness[tiab] OR exercise capacity[tiab] OR functional capacity[tiab] OR exertion[tiab] OR developmental delay*[tiab] OR neurodevelopmental delay*[tiab] OR functional status[tiab] OR functional disability[tiab] OR motor deficit*[tiab] OR physical deficit*[tiab] OR exercise tolerance[tiab] OR “exercise tolerance”[mesh] OR “exercise test”[mesh] OR “motor activity”[mesh] OR “musculoskeletal physiological phenomena”[mesh] OR “muscle spasticity”[mesh] OR “paresis”[mesh] OR “proprioception”[mesh] OR “muscle weakness”[mesh] OR “scoliosis”[mesh] OR “lordosis”[mesh] OR “kyphosis”[mesh] OR “motor skills”[mesh])
NOT
(adult[mesh] NOT (adolescent[mesh] OR child[mesh] OR infant[mesh]))
Embase (embase.com)
(‘sickle cell anemia’/exp OR ‘sickle cell’:ab,ti OR ‘sickle anemia’:ab,ti OR ‘sickle anaemia’:ab,ti OR ‘hemoglobin S disease’:ab,ti OR ‘haemoglobin S disease’:ab,ti OR ‘hemoglobin SS’:ab,ti OR ‘haemoglobin SS’:ab,ti OR ‘hemoglobin SC’:ab,ti OR ‘haemoglobin SC’:ab,ti OR ‘hemoglobin SD’:ab,ti OR ‘haemoglobin SD’:ab,ti OR SCA:ab,ti OR sicklemia:ab,ti OR sicklaemia:ab,ti OR sickling:ab,ti OR drepanocyt*:ab,ti)
AND
(‘muscle tone’:ab,ti OR spasticity:ab,ti OR hemiparesis:ab,ti OR haemiparesis:ab,ti OR ataxi*:ab,ti OR proprioception:ab,ti OR kinesthesi*:ab,ti OR kinaesthesi*:ab,ti OR ‘static balance’:ab,ti OR ‘dynamic balance’:ab,ti OR ‘sitting balance’:ab,ti OR ‘standing balance’:ab,ti OR ‘range of motion’:ab,ti OR flexibility:ab,ti OR extensibility:ab,ti OR contracture:ab,ti OR gait:ab,ti OR ‘muscle strength’:ab,ti OR weakness:ab,ti OR ‘muscle force’:ab,ti OR torque:ab,ti OR ‘musc* power’:ab,ti OR ‘functional strength’:ab,ti OR ‘muscle activation’:ab,ti OR ‘neuromuscular activation’:ab,ti OR electromyography:ab,ti OR ‘muscle size’:ab,ti OR ‘muscle architecture’:ab,ti OR posture:ab,ti OR scoliosis:ab,ti OR lordosis:ab,ti OR kyphosis:ab,ti OR ‘physical function*’:ab,ti OR ‘motor function*’:ab,ti OR ‘muscle function*’:ab,ti OR ‘gross motor’:ab,ti OR ‘fine motor’:ab,ti OR ‘motor performance’:ab,ti OR ‘physical performance’:ab,ti OR sports:ab,ti OR ‘physical activity’:ab,ti OR endurance:ab,ti OR fatigue:ab,ti OR fitness:ab,ti OR ‘exercise capacity’:ab,ti OR ‘functional capacity’:ab,ti OR exertion:ab,ti OR ‘developmental delay*’:ab,ti OR ‘neurodevelopmental delay*’:ab,ti OR ‘functional status’:ab,ti OR ‘functional disability’:ab,ti OR ‘motor deficit*’:ab,ti OR ‘physical deficit*’:ab,ti OR ‘exercise tolerance’:ab,ti OR ‘muscle characteristics and function’/exp OR spasticity/de OR hemiparesis/de OR ‘motor dysfunction’/exp OR proprioception/de OR kinesthesia/de OR ‘body equilibrium’/de OR ‘range of motion’/de OR ‘muscle strength’/de OR scoliosis/de OR lordosis/de OR kyphosis/exp OR ‘physical activity’/exp OR ‘physical capacity’/exp OR ‘physical performance’/exp OR endurance/de OR fitness/de OR exercise/exp OR ‘functional status’/de OR ‘exercise test’/exp OR ‘motor activity’/exp)
NOT
(([adult]/lim OR [aged]/lim) NOT ([newborn]/lim OR [infant]/lim OR [child]/lim OR [adolescent]/lim))
NOT
[conference abstract]/lim
CINAHL (EBSCO)
(TI (“sickle cell” OR “sickle anemia” OR “sickle anaemia” OR “hemoglobin S disease” OR “haemoglobin S disease” OR “hemoglobin SS” OR “haemoglobin SS” OR “hemoglobin SC” OR “haemoglobin SC” OR “hemoglobin SD” OR “haemoglobin SD” OR SCA OR sicklemia OR sicklaemia OR sickling OR drepanocyt*) OR AB (“sickle cell” OR “sickle anemia” OR “sickle anaemia” OR “hemoglobin S disease” OR “haemoglobin S disease” OR “hemoglobin SS” OR “haemoglobin SS” OR “hemoglobin SC” OR “haemoglobin SC” OR “hemoglobin SD” OR “haemoglobin SD” OR SCA OR sicklemia OR sicklaemia OR sickling OR drepanocyt*) OR (MH “anemia, sickle cell+”))
AND
(TI (“muscle tone” OR spasticity OR hemiparesis OR haemiparesis OR ataxi* OR proprioception OR kinesthesi* OR kinaesthesi* OR “static balance” OR “dynamic balance” OR “sitting balance” OR “standing balance” OR “range of motion” OR flexibility OR extensibility OR contracture OR gait OR “muscle strength” OR weakness OR “muscle force” OR torque OR “musc* power” OR “functional strength” OR “muscle activation” OR “neuromuscular activation” OR electromyography OR “muscle size” OR “muscle architecture” OR posture OR scoliosis OR lordosis OR kyphosis OR “physical function*” OR “motor function*” OR “muscle function*” OR “gross motor” OR “fine motor” OR “motor performance” OR “physical performance” OR sports OR “physical activity” OR endurance OR fatigue OR fitness OR “exercise capacity” OR “functional capacity” OR exertion OR “developmental delay*” OR “neurodevelopmental delay*” OR “functional status” OR “functional disability” OR “motor deficit*” OR “physical deficit*” OR “exercise tolerance”)) OR (AB (“muscle tone” OR spasticity OR hemiparesis OR haemiparesis OR ataxi* OR proprioception OR kinesthesi* OR kinaesthesi* OR “static balance” OR “dynamic balance” OR “sitting balance” OR “standing balance” OR “range of motion” OR flexibility OR extensibility OR contracture OR gait OR “muscle strength” OR weakness OR “muscle force” OR torque OR “musc* power” OR “functional strength” OR “muscle activation” OR “neuromuscular activation” OR electromyography OR “muscle size” OR “muscle architecture” OR posture OR scoliosis OR lordosis OR kyphosis OR “physical function*” OR “motor function*” OR “muscle function*” OR “gross motor” OR “fine motor” OR “motor performance” OR “physical performance” OR sports OR “physical activity” OR endurance OR fatigue OR fitness OR “exercise capacity” OR “functional capacity” OR exertion OR “developmental delay*” OR “neurodevelopmental delay*” OR “functional status” OR “functional disability” OR “motor deficit*” OR “physical deficit*” OR “exercise tolerance”))
NOT
((MH “adult+”) NOT (MH “adolescence+” OR MH “child+”))
Cochrane Central Register of Controlled Trials (Wiley)
(“sickle cell” OR “sickle anemia” OR “sickle anaemia” OR “hemoglobin S disease” OR “haemoglobin S disease” OR “hemoglobin SS” OR “haemoglobin SS” OR “hemoglobin SC” OR “haemoglobin SC” OR “hemoglobin SD” OR “haemoglobin SD” OR SCA OR sicklemia OR sicklaemia OR sickling OR drepanocyt*):ti,ab,kw
AND
(“muscle tone” OR spasticity OR hemiparesis OR haemiparesis OR ataxi* OR proprioception OR kinesthesi* OR kinaesthesi* OR “static balance” OR “dynamic balance” OR “sitting balance” OR “standing balance” OR “range of motion” OR flexibility OR extensibility OR contracture OR gait OR “muscle strength” OR weakness OR “muscle force” OR torque OR “musc* power” OR “functional strength” OR “muscle activation” OR “neuromuscular activation” OR electromyography OR “muscle size” OR “muscle architecture” OR posture OR scoliosis OR lordosis OR kyphosis OR “physical function*” OR “motor function*” OR “muscle function*” OR “gross motor” OR “fine motor” OR “motor performance” OR “physical performance” OR sports OR “physical activity” OR endurance OR fatigue OR fitness OR “exercise capacity” OR “functional capacity” OR exertion OR “developmental delay*” OR “neurodevelopmental delay*” OR “functional status” OR “functional disability” OR “motor deficit*” OR “physical deficit*” OR “exercise tolerance”):ti,ab,kw
Dissertations and Theses (ProQuest)
AB,TI(“sickle cell” OR “sickle anemia” OR “sickle anaemia” OR “hemoglobin S disease” OR “haemoglobin S disease” OR “hemoglobin SS” OR “haemoglobin SS” OR “hemoglobin SC” OR “haemoglobin SC” OR “hemoglobin SD” OR “haemoglobin SD” OR SCA OR sicklemia OR sicklaemia OR sickling OR drepanocyt*)
AND
AB,TI(“muscle tone” OR spasticity OR hemiparesis OR haemiparesis OR ataxi* OR proprioception OR kinesthesi* OR kinaesthesi* OR “static balance” OR “dynamic balance” OR “sitting balance” OR “standing balance” OR “range of motion” OR flexibility OR extensibility OR contracture OR gait OR “muscle strength” OR weakness OR “muscle force” OR torque OR “musc* power” OR “functional strength” OR “muscle activation” OR “neuromuscular activation” OR electromyography OR “muscle size” OR “muscle architecture” OR posture OR scoliosis OR lordosis OR kyphosis OR “physical function*” OR “motor function*” OR “muscle function*” OR “gross motor” OR “fine motor” OR “motor performance” OR “physical performance” OR sports OR “physical activity” OR endurance OR fatigue OR fitness OR “exercise capacity” OR “functional capacity” OR exertion OR “developmental delay*” OR “neurodevelopmental delay*” OR “functional status” OR “functional disability” OR “motor deficit*” OR “physical deficit*” OR “exercise tolerance”)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interests
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: Disclaimers: The views expressed in the submitted article are our own and not an official position of our institutions. This work is original, has not been previously published, nor is under review by any other journal.
References
- 1.Buchanan G, Vichinsky E, Krishnamurti L, Shenoy S. Severe Sickle Cell Disease-Pathophysiology and Therapy. Biol. Blood Marrow Transplant [Internet]. 2010;16:S64–7. Available from: 10.1016/j.bbmt.2009.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang WC. The pathophysiology, prevention, and treatment of stroke in sickle cell disease. Curr. Opin. Hematol 2007;14:191–7. [DOI] [PubMed] [Google Scholar]
- 3.Yaster M, Kost-Byerly S, Maxwell LG. The management of pain in sickle cell disease. Pediatr. Clin. North Am [Internet]. 2000;47:699–710. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10835998 [DOI] [PubMed] [Google Scholar]
- 4.Merlet AN, Chatel B, Hourdé C, Ravelojaona M, Bendahan D, Féasson L, et al. How Sickle Cell Disease Impairs Skeletal Muscle Function: Implications in Daily Life. Med. Sci. Sports Exerc [Internet]. 2019;51:4–11. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30095751 [DOI] [PubMed] [Google Scholar]
- 5.Brousse V, Pondarre C, Arnaud C, Kamden A, de Montalembert M, Boutonnat-Faucher B, et al. One-Fifth of Children with Sickle Cell Anemia Show Exercise-Induced Hemoglobin Desaturation: Rate of Perceived Exertion and Role of Blood Rheology. J. Clin. Med [Internet]. 2020;9:133. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31947773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dedeken L, Chapusette R, Lê PQ, Heijmans C, Devalck C, Huybrechts S, et al. Reduction of the six-minute walk distance in children with sickle cell disease is correlated with silent infarct: results from a cross-sectional evaluation in a single center in Belgium. PLoS One [Internet]. 2014;9:e108922. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25275451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Möckesch B, Charlot K, Jumet S, Romana M, Divialle-Doumdo L, Hardy-Dessources M-DD, et al. Micro- and macrovascular function in children with sickle cell anaemia and sickle cell haemoglobin C disease. Blood Cells, Mol. Dis [Internet]. 2017;64:23–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28340403 [DOI] [PubMed] [Google Scholar]
- 8.Hostyn SV, Carvalho WB de, Johnston C, Braga JAP. Evaluation of functional capacity for exercise in children and adolescents with sickle-cell disease through the six-minute walk test. J. Pediatr. (Rio. J) [Internet]. 2013;89:588–94. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24055098 [DOI] [PubMed] [Google Scholar]
- 9.Melo HN, Stoots SJ-MM, Pool MA, Carvalho VO, Almeida LOC, Aragão MLDC, et al. Physical activity level and performance in the six-minute walk test of children and adolescents with sickle cell anemia. Rev. Bras. Hematol. Hemoter [Internet]. 2017;39:133–9. Available from: 10.1016/j.bjhh.2017.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moheeb H, Wali YA, El-Sayed MS. Physical fitness indices and anthropometrics profiles in schoolchildren with sickle cell trait/disease. Am. J. Hematol [Internet]. 2007;82:91–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16986131 [DOI] [PubMed] [Google Scholar]
- 11.Painter P, Stewart AL, Carey S. Physical functioning: definitions, measurement, and expectations. Adv. Ren. Replace. Ther [Internet]. 1999;6:110–23. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10230878 [DOI] [PubMed] [Google Scholar]
- 12.Dampier C, Jaeger B, Gross HE, Barry V, Edwards L, Lui Y, et al. Responsiveness of PROMIS® Pediatric Measures to Hospitalizations for Sickle Pain and Subsequent Recovery. Pediatr. Blood Cancer [Internet]. 2016;63:1038–45. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17849473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dampier C, Lieff S, LeBeau P, Rhee S, McMurray M, Rogers Z, et al. Health-related quality of life in children with sickle cell disease: A report from the comprehensive sickle cell centers clinical trial consortium. Pediatr. Blood Cancer 2010;55:485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maikler VE, Broome ME, Bailey P, Lea G. Childrens’ and adolescents’ use of diaries for sickle cell pain. J. Soc. Pediatr. Nurs [Internet]. 2001;6:161–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11777329 [DOI] [PubMed] [Google Scholar]
- 15.Stephensen D, Drechsler WI, Scott OM. Influence of ankle plantar flexor muscle architecture and strength on gait in boys with haemophilia in comparison to typically developing children. Haemophilia. 2014;20:413–20. [DOI] [PubMed] [Google Scholar]
- 16.Moreau NG, Simpson KN, Teefey SA, Damiano DL. Muscle Architecture Predicts Maximum Strength and Is Related to Activity Levels in Cerebral Palsy. Phys. Ther 2010;90:1619–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ko I-H, Kim J-H, Lee B-H. Relationships between lower limb muscle architecture and activities and participation of children with cerebral palsy. J. Exerc. Rehabil 2013;9:368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchese VG, Spearing E, Callaway L, Rai SN, Zhang L, Hinds PS, et al. Relationships among range of motion, functional mobility, and quality of life in children and adolescents after limb-sparing surgery for lower-extremity sarcoma. Pediatr. Phys. Ther 2006;18:238–44. [DOI] [PubMed] [Google Scholar]
- 19.Waugh CM, Korff T, Fath F, Blazevich AJ. Rapid force production in children and adults: mechanical and neural contributions. Med. Sci. Sports Exerc [Internet]. 2013;45:762–71. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23190586 [DOI] [PubMed] [Google Scholar]
- 20.Dotan R, Mitchell C, Cohen R, Klentrou P, Gabriel D, Falk B. Child-adult differences in muscle activation--a review. Pediatr. Exerc. Sci [Internet]. 2012;24:2–21. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22433260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milliken LA, Faigenbaum AD, Loud RL, Westcott WL. Correlates of upper and lower body muscular strength in children. J. strength Cond. Res [Internet]. 2008;22:1339–46. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18545169 [DOI] [PubMed] [Google Scholar]
- 22.Meehan JP, Jamali AA, Ryan JA. Pantaloon hip spica cast and constrained liner for the treatment of early total hip dislocation in a young patient with sickle cell disease. Am. J. Orthop (Belle Mead. NJ: ). [Internet]. 2009;38:184–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20145795 [PubMed] [Google Scholar]
- 23.Segal LS, Wallach DM. Slipped capital femoral epiphysis in a child with sickle cell disease. Clin. Orthop. Relat. Res [Internet]. 2004;198–201. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15021154 [DOI] [PubMed] [Google Scholar]
- 24.Bali S, D’Cruz D, Lazaro M, Inusa BPD. Juvenile polymyositis with unremitting pain and progressive loss of motor and bulbar function on a background of sickle cell disease. BMJ Case Rep. 2015;2015:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chua-Lim C, Moore RB, McCleary G, Shah A, Mankad VN. Deficiencies in school readiness skills of children with sickle cell anemia: a preliminary report. South. Med. J [Internet]. 1993;86:397–402. Available from: https://journals.lww.com/jpho-online/Fulltext/2018/07000/Muscle_Strength,_Power,_and_Torque_Deficits_in.3.aspx [DOI] [PubMed] [Google Scholar]
- 26.Anderson LM, Allen TM, Thornburg CD, Bonner MJ. Fatigue in children with sickle cell disease: Association with neurocognitive and social-emotional functioning and quality of life. J. Pediatr. Hematol. Oncol 2015;37:584–9. [DOI] [PubMed] [Google Scholar]
- 27.Gentry B, Hall L, Dancer J. A parental survey of speech, language, and physical development of infants and toddlers with sickle cell disease. Percept. Mot. Skills [Internet]. 1997;85:1105–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9399326 [DOI] [PubMed] [Google Scholar]
- 28.Iyer SR, Iyer RR, Oza GD, Rane RM, Khandwala RM, Desai PK, et al. Sickle cell syndromes in and around Bardoli. J. Assoc. Physicians India [Internet]. 1994;42:885–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7868492 [PubMed] [Google Scholar]
- 29.Sibinga EMS, Shindell DL, Casella JF, Duggan AK, Wilson MH. Pediatric patients with sickle cell disease: use of complementary and alternative therapies. J. Altern. Complement. Med [Internet]. 2006;12:291–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16646728 [DOI] [PubMed] [Google Scholar]
- 30.Anie KA, Telfair J. Multi-site study of transition in adolescents with sickle cell disease in the United Kingdom and the United States. Int. J. Adolesc. Med. Health 2005;17:169–78. [DOI] [PubMed] [Google Scholar]
- 31.Liem RI, Akinosun M, Muntz DS, Thompson AA. Feasibility and safety of home exercise training in children with sickle cell anemia. Pediatr. Blood Cancer [Internet]. 2017;64:1–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28598539 [DOI] [PubMed] [Google Scholar]
- 32.Buison AM, Kawchak DA, Schall JI, Ohene-Frempong K, Stallings VA, Leonard MB, et al. Bone area and bone mineral content deficits in children with sickle cell disease. Pediatrics [Internet]. 2005;116:943–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16199706 [DOI] [PubMed] [Google Scholar]
- 33.Rhodes M, Akohoue SA, Shankar SM, Fleming I, Qi An A, Yu C, et al. Growth patterns in children with sickle cell anemia during puberty. Pediatr. Blood Cancer [Internet]. 2009;53:635–41. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19544390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacob E, Duran J, Stinson J, Lewis MA, Zeltzer L. Remote monitoring of pain and symptoms using wireless technology in children and adolescents with sickle cell disease. J. Am. Assoc. Nurse Pract [Internet]. 2013;25:42–54. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3624763/pdf/nihms412728.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wali YA, Moheeb H. Effect of hydroxyurea on physical fitness indices in children with sickle cell anemia. Pediatr. Hematol. Oncol [Internet]. 2011;28:43–50. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21083357 [DOI] [PubMed] [Google Scholar]
- 36.Koppelmans V, Scott JM, Downs ME, Cassady KE, Yuan P, Pasternak O, et al. Exercise effects on bed rest-induced brain changes. PLoS One 2018;13:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brownell JN, Schall JI, Mcanlis CR, Smith-Whitley K, Norris CF, Stallings VA. Effect of High-dose Vitamin A Supplementation in Children With Sickle Cell Disease: A Randomized, Double-blind, Dose-finding Pilot Study. J. Pediatr. Hematol. Oncol [Internet]. 2020;42:83–91. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31764511 [DOI] [PubMed] [Google Scholar]
- 38.Dougherty KA, Bertolaso C, Schall JI, Smith-Whitley K, Stallings VA. Muscle Strength, Power, and Torque Deficits in Children With Type SS Sickle Cell Disease. J. Pediatr. Hematol. Oncol [Internet]. 2018;40:348–54. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29621064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dougherty KA, Schall JI, Bertolaso C, Smith-Whitley K, Stallings VA. Vitamin D Supplementation Improves Health-Related Quality of Life and Physical Performance in Children with Sickle Cell Disease and in Healthy Children. J. Pediatr. Health Care [Internet]. 2020;34:424–34. Available from: 10.1016/j.pedhc.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dougherty KA, Schall JI, Rovner AJ, Stallings VA, Zemel BS. Attenuated Maximal Muscle Strength and Peak Power in Children With Sickle Cell Disease. J. Pediatr. Hematol. Oncol [Internet]. 2011;33. Available from: https://journals.lww.com/jpho-online/Fulltext/2011/03000/Attenuated_Maximal_Muscle_Strength_and_Peak_Power.4.aspx [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacob E, Stinson J, Duran J, Gupta A, Gerla M, Ann Lewis M, et al. Usability testing of a smartphone for accessing a web-based e-diary for self-monitoring of pain and symptoms in sickle cell disease. J. Pediatr. Hematol. Oncol [Internet]. 2012;34:326–35. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22627570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patra PK, Panigrahi SK, Banerjee G. Epidemeological profile of sickle cell disease prevalent in Chhattisgarh, Central India. Int. J. Pharma Bio Sci 2013;4:513–8. [Google Scholar]
- 43.Barden EM, Zemel BS, Kawchak DA, Goran MI, Ohene-Frempong K, Stallings VA. Total and resting energy expenditure in children with sickle cell disease. J. Pediatr [Internet]. 2000;136:73–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10636978 [DOI] [PubMed] [Google Scholar]
- 44.Buchowski MS, Townsend KM, Williams R, Chen KY. Patterns and energy expenditure of free-living physical activity in adolescents with sickle cell anemia. J. Pediatr [Internet]. 2002;140:86–92. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11815769 [DOI] [PubMed] [Google Scholar]
- 45.Chaudry RA, Bush A, Rosenthal M, Crowley S. The impact of sickle cell disease on exercise capacity in children. Chest [Internet]. 2013;143:478–84. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22922408 [DOI] [PubMed] [Google Scholar]
- 46.Liem RI, Onyejekwe K, Olszewski M, Nchekwube C, Zaldivar FP, Radom-Aizik S, et al. The acute phase inflammatory response to maximal exercise testing in children and young adults with sickle cell anaemia. Br. J. Haematol 2015;171:854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liem RI, Reddy M, Pelligra SA, Savant AP, Fernhall B, Rodeghier M, et al. Reduced fitness and abnormal cardiopulmonary responses to maximal exercise testing in children and young adults with sickle cell anemia. Physiol. Rep 2015;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singhal A, Davies P, Wierenga KJJ, Thomas P, Serjeant G. Is there an energy deficiency in homozygous sickle cell disease? Am. J. Clin. Nutr [Internet]. 1997;66:386–90. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9250118 [DOI] [PubMed] [Google Scholar]
- 49.Armstrong FD, Elkin TD, Brown RC, Glass P, Rana S, Casella JF, et al. Developmental function in toddlers with sickle cell anemia. Pediatrics [Internet]. 2013;131:e406–14. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23296434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glass P, Brennan T, Wang J, Luchtman-Jones L, Hsu L, Bass CM, et al. Neurodevelopmental deficits among infants and toddlers with sickle cell disease. J. Dev. Behav. Pediatr [Internet]. 2013;34:399–405. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23838585 [DOI] [PubMed] [Google Scholar]
- 51.Burkhardt L, Lobitz S, Koustenis E, Rueckriegel SM, Hernáiz Driever P. Cognitive and fine motor deficits in a pediatric sickle cell disease cohort of mixed ethnic origin. Ann. Hematol [Internet]. 2017;96:199–213. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27796476 [DOI] [PubMed] [Google Scholar]
- 52.Drazen CH, Abel R, Gabir M, Farmer G, King AA. Prevalence of Developmental Delay and Contributing Factors Among Children With Sickle Cell Disease. Pediatr. Blood Cancer [Internet]. 2016;63:504–10. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26575319 [DOI] [PubMed] [Google Scholar]
- 53.Millis RM, Baker FW, Ertugrul L, Douglas RM, Sexcius L. Physical performance decrements in children with sickle cell anemia. J. Natl. Med. Assoc 1994;86:113–6. [PMC free article] [PubMed] [Google Scholar]
- 54.Newby RF, Epping A, Geiger JA, Miller MS, Scott JP. Visual Motor Integration in Children With Sickle Cell Disease. J. Pediatr. Hematol. Oncol [Internet]. 2018;40:495–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30044354 [DOI] [PubMed] [Google Scholar]
- 55.Schatz J, Roberts CW. Neurobehavioral impact of sickle cell disease in early childhood. J. Int. Neuropsychol. Soc [Internet]. 2007;13:933–43. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17942011 [DOI] [PubMed] [Google Scholar]
- 56.Zempsky WT, Palermo TM, Corsi JM, Lewandowski AS, Zhou C, Casella JF. Daily changes in pain, mood and physical function in children hospitalized for sickle cell disease pain. Pain Res. Manag [Internet]. 2013;18:33–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23457684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zempsky WT, O’Hara EA, Santanelli JP, New T, Smith-Whitley K, Casella J, et al. Development and validation of the Youth Acute Pain Functional Ability Questionnaire (YAPFAQ). J. Pain [Internet]. 2014;15:1319–27. Available from: 10.1016/j.jpain.2014.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karlson CW, Baker AM, Bromberg MH, David Elkin T, Majumdar S, Palermo TM. Daily Pain, Physical Activity, and Home Fluid Intake in Pediatric Sickle Cell Disease. J. Pediatr. Psychol [Internet]. 2017;42:335–44. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27370016 [DOI] [PubMed] [Google Scholar]
- 59.Melo HN, Stoots SJ-MM, Pool MA, Carvalho VO, Aragão MLDC, Gurgel RQ, et al. Objectively measured physical activity levels and sedentary time in children and adolescents with sickle cell anemia. PLoS One [Internet]. 2018;13:1–10. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30521638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep [Internet]. 1985;100:126–31. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3920711 [PMC free article] [PubMed] [Google Scholar]
- 61.Adeyemo TA, Ojewunmi OO, Diaku-Akinwumi IN, Ayinde OC, Akanmu AS. Health related quality of life and perception of stigmatisation in adolescents living with sickle cell disease in Nigeria: A cross sectional study. Pediatr. Blood Cancer [Internet]. 2015;62:1245–51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25810358 [DOI] [PubMed] [Google Scholar]
- 62.Barakat LP, Patterson CA, Daniel LC, Dampier C. Quality of life among adolescents with sickle cell disease: mediation of pain by internalizing symptoms and parenting stress. Health Qual. Life Outcomes [Internet]. 2008;6:60. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18691422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoff AL, Palermo TM, Schluchter M, Zebracki K, Drotar D. Longitudinal relationships of depressive symptoms to pain intensity and functional disability among children with disease-related pain. J. Pediatr. Psychol [Internet]. 2006;31:1046–56. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16150876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hussein AA, Hassan MB, Sachit AA. Determination of Daily Living Activities of School Age Children with Sickle Cell Anemia in Al Nasiriya City. Indian J. Public Heal. Res. Dev [Internet]. 2019;10:1077. Available from: http://www.indianjournals.com/ijor.aspx?target=ijor:ijphrd&volume=10&issue=6&article=203 [Google Scholar]
- 65.Kambasu DM, Rujumba J, Lekuya HM, Munube D, Mupere E. Health-related quality of life of adolescents with sickle cell disease in sub-Saharan Africa: A cross-sectional study. BMC Hematol 2019;19:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Malheiros CD, Lisle L, Castelar M, Sá KN, Matos MA. Hip Dysfunction and Quality of Life in Patients With Sickle Cell Disease. Clin. Pediatr. (Phila) [Internet]. 2015;54:1354–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25971463 [DOI] [PubMed] [Google Scholar]
- 67.Matos MA, Silva LL dos S, Alves GB, Júnior WS de A, Veiga D. Necrosis of the femoral head and health-related quality of life of children and adolescents. Acta Ortop. Bras 2018;26:227–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Menezes AS de O da P, Len CA, Hilário MOE, Terreri MTRA, Braga JAP. Quality of life in patients with sickle cell disease. Rev. Paul. Pediatr [Internet]. 2008;31:24–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23703040 [DOI] [PubMed] [Google Scholar]
- 69.Oliver-Carpenter G, Barach I, Crosby LE, Valenzuela J, Mitchell MJ. Disease management, coping, and functional disability in pediatric sickle cell disease. J. Natl. Med. Assoc [Internet]. 2011;103:131–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21443065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Palermo TM, Schwartz L, Drotar D, McGowan K. Parental report of health-related quality of life in children with sickle cell disease. J. Behav. Med [Internet]. 2002;25:269–83. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12055777 [DOI] [PubMed] [Google Scholar]
- 71.Panepinto JA, O’Mahar KM, DeBaun MR, Loberiza FR, Scott JP. Health-related quality of life in children with sickle cell disease: Child and parent perception. Br. J. Haematol 2005;130:437–44. [DOI] [PubMed] [Google Scholar]
- 72.Patel AB, Pathan HG. Quality of life in children with sickle cell hemoglobinopathy. Indian J. Pediatr [Internet]. 2005;72:567–71. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16077239 [DOI] [PubMed] [Google Scholar]
- 73.Sil S, Cohen LL, Dampier C. Psychosocial and functional outcomes in youth with chronic sickle cell pain. Clin. J. Pain [Internet]. 2016;32:527–33. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26379074 [DOI] [PubMed] [Google Scholar]
- 74.Sil S, Cohen LL, Bakshi N, Watt A, Hathaway M, Abudulai F, et al. Changes in Pain and Psychosocial Functioning and Transition to Chronic Pain in Pediatric Sickle Cell Disease: A Cohort Follow-up Study. Clin. J. Pain [Internet]. 2020;36:463–71. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32287106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singh A, Dasgupta M, Simpson PM, Brousseau DC, Panepinto JA. Can PROMIS domains of pain and physical functioning detect changes in health over time for children with sickle cell disease? Pediatr. Blood Cancer 2020;67:1–7. [DOI] [PubMed] [Google Scholar]
- 76.Singh A, DasGupta M, Simpson PM, Panepinto JA. Use of the new pediatric PROMIS measures of pain and physical experiences for children with sickle cell disease. Pediatr. Blood Cancer [Internet]. 2019;66:e27633. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30688017 [DOI] [PubMed] [Google Scholar]
- 77.Thornburg CD, Calatroni A, Panepinto JA. Differences in health-related quality of life in children with sickle cell disease receiving hydroxyurea. J. Pediatr. Hematol. Oncol [Internet]. 2011;33:251–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21516020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Karlson CW, Delozier AM, Seals SR, Britt AB, Stone AL, Reneker JC, et al. Physical Activity and Pain in Youth With Sickle Cell Disease. Fam. Community Health [Internet]. 2020;43. Available from: https://journals.lww.com/familyandcommunityhealth/Fulltext/2020/01000/Physical_Activity_and_Pain_in_Youth_With_Sickle.1.aspx [DOI] [PubMed] [Google Scholar]
- 79.Dampier C, Barry V, Gross HE, Lui Y, Thornburg CD, Dewalt DA, et al. Initial Evaluation of the Pediatric PROMIS® Health Domains in Children and Adolescents With Sickle Cell Disease. Pediatr. Blood Cancer 2016;63:1031–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Omwanghe OA, Muntz DS, Kwon S, Montgomery S, Kemiki O, Hsu LL, et al. Self-reported physical activity and exercise patterns in children with sickle cell disease. Pediatr. Exerc. Sci 2017;29:388–95. [DOI] [PubMed] [Google Scholar]
- 81.Palermo TM, Platt-Houston C, Kiska RE, Berman B. Headache Symptoms in Pediatric Sickle Cell Patients. J. Pediatr. Hematol. Oncol [Internet]. 2005;27:420–4. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3624763/pdf/nihms412728.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Woods KF, Ramsey LT, Callahan LA, Mensah GA, Litaker MS, Kutlar A, et al. Body composition in women with sickle cell disease. Ethn. Dis [Internet]. 2001;11:30–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11289248 [PubMed] [Google Scholar]
- 83.Sarrai M, Duroseau H, D’Augustine J, Moktan S, Bellevue R. Bone mass density in adults with sickle cell disease. Br. J. Haematol 2007;136:666–72. [DOI] [PubMed] [Google Scholar]
- 84.Soliman AT, Bererhi H, Darwish A, Alzalabani MM, Wali Y, Ansari B. Decreased bone mineral density in prepubertal children with sickle cell disease: Correlation with growth parameters, degree of siderosis and secretion of growth factors. J. Trop. Pediatr 1998;44:194–8. [DOI] [PubMed] [Google Scholar]
- 85.Badawy SM, Payne AB, Rodeghier MJ, Liem RI. Exercise capacity and clinical outcomes in adults followed in the Cooperative Study of Sickle Cell Disease (CSSCD). Eur. J. Haematol [Internet]. 2018;101:532–41. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29999202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liao H, Hwang A. Relations of balance function and gross motor ability for children with cerebral palsy. Percept. Mot. Skills [Internet]. 2003;96:1173–84. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12929770 [DOI] [PubMed] [Google Scholar]
- 87.Muehlbauer T, Besemer C, Wehrle A, Gollhofer A, Granacher U. Relationship between strength, balance and mobility in children aged 7–10 years. Gait Posture [Internet]. 2013;37:108–12. Available from: 10.1016/j.gaitpost.2012.06.022 [DOI] [PubMed] [Google Scholar]
- 88.Bohannon RW. Considerations and practical options for measuring muscle strength: A narrative review. Biomed Res. Int 2019;2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Secomb JL, Nimphius S, Farley ORL, Lundgren LE, Tran TT, Sheppard JM. Relationships between lower-body muscle structure and, lower-body strength, explosiveness and eccentric leg stiffness in adolescent athletes. J. Sport. Sci. Med 2015;14:691–7. [PMC free article] [PubMed] [Google Scholar]
- 90.Marchese VG, Chiarello LA, Lange BJ. Strength and functional mobility in children with Acute Lymphoblastic Leukemia. Med. Pediatr. Oncol 2003;40:230–2. [DOI] [PubMed] [Google Scholar]
- 91.Marchese VG, Chiarello LA. Relationships Between Specific Measures of Body Function, Activity, and Participation in Children with Acute Lymphoblastic Leukemia. Rehabil. Oncol 2004;22:5–9. [Google Scholar]
- 92.Behringer M, Vom Heede A, Matthews M, Mester J. Effects of strength training on motor performance skills in children and adolescents: a meta-analysis. Pediatr. Exerc. Sci [Internet]. 2011;23:186–206. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21633132 [DOI] [PubMed] [Google Scholar]
- 93.Johnson BA, Salzberg CL, Stevenson DA. A systematic review: plyometric training programs for young children. J. strength Cond. Res [Internet]. 2011;25:2623–33. Available from: https://journals.lww.com/nsca-jscr/Fulltext/2011/09000/A_Systematic_Review__Plyometric_Training_Programs.35.aspx [DOI] [PubMed] [Google Scholar]
- 94.Silva PO, Ferreira AS, Lima CM de A, Guimarães FS, Lopes AJ. Balance control is impaired in adults with sickle cell anaemia. Somatosens. Mot. Res [Internet]. 2018;35:109–18. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30010483 [DOI] [PubMed] [Google Scholar]
- 95.Verbecque E, Lobo Da Costa PH, Vereeck L, Hallemans A. Psychometric properties of functional balance tests in children: A literature review. Dev. Med. Child Neurol 2015;57:521–9. [DOI] [PubMed] [Google Scholar]
- 96.Christy JB, Payne J, Azuero A, Formby C. Reliability and diagnostic accuracy of clinical tests of vestibular function for children. Pediatr. Phys. Ther 2014;26:180–9. [DOI] [PubMed] [Google Scholar]
- 97.Marchese V, Sanders O, York T, Creath R, Rogers M. Motion Analysis of a Jumping Task in Childhood Leukemia Survivors. Rehabil. Oncol [Internet]. 2017;35:9–14. Available from: 10.1097/01.reo.0000000000000043 [DOI] [Google Scholar]
- 98.Alcorn R, Bowser B, Henley EJ, Holloway V. Fluidotherapy and exercise in the management of sickle cell anemia. A clinical report. Phys. Ther [Internet]. 1984;64:1520–2. Available from: http://www.ncbi.nlm.nih.gov/pubmed/6483980 [DOI] [PubMed] [Google Scholar]
- 99.Janz KF, Medema-Johnson HC, Letuchy EM, Burns TL, Gilmore JME, Torner JC, et al. Subjective and objective measures of physical activity in relationship to bone mineral content during late childhood: the Iowa Bone Development Study. Br. J. Sports Med [Internet]. 2008;42:658–63. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18603581 [DOI] [PubMed] [Google Scholar]
- 100.Götte M, Seidel CC, Kesting SV, Rosenbaum D, Boos J. Objectively measured versus self-reported physical activity in children and adolescents with cancer. PLoS One 2017;12:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang WC, Ware RE, Miller ST, Iyer RV, Casella JF, Minniti CP, et al. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG). Lancet (London, England) [Internet]. 2011;377:1663–72. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21571150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ballas SK, Barton FB, Waclawiw MA, Swerdlow P, Eckman JR, Pegelow CH, et al. Hydroxyurea and sickle cell anemia: effect on quality of life. Health Qual. Life Outcomes [Internet]. 2006;4:59. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16942629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Badawy SM, Thompson AA, Lai J-S, Penedo FJ, Rychlik K, Liem RI. Health-related quality of life and adherence to hydroxyurea in adolescents and young adults with sickle cell disease. Pediatr. Blood Cancer [Internet]. 2017;64. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27896936 [DOI] [PubMed] [Google Scholar]


