Abstract
INTRODUCTION:
Blood-based biomarkers of amyloid pathology and neurodegeneration are entering clinical use. It is critical to understand what factors affect the levels of these markers.
METHODS:
Plasma markers (Aβ42, Aβ40, NfL, T-tau, Aβ42/40) were measured on the Quanterix Simoa® HD-1 analyzer for 996 Mayo Clinic Study of Aging (MCSA) participants, aged 51 to 95 years. All other data were collected during in-person MCSA visits or abstracted from the medical record.
RESULTS:
Among cognitively unimpaired (CU), all plasma markers correlated with age. Linear regression models revealed multiple relationships. For example, higher Charlson comorbidity index and chronic kidney disease were associated with higher levels of all biomarkers. Some relationships differed among MCI and dementia participants.
DISCUSSION:
Multiple variables affect plasma biomarkers of amyloid pathology and neurodegeneration among CU in the general population. Incorporating this information is critical for accurate interpretation of the biomarker levels and for the development of reference ranges.
Keywords: amyloid, neurofilament light chain, total tau, demographics, comorbid conditions, cognition
1. INTRODUCTION
The 2018 National Institute on Aging and Alzheimer’s Association (NIA-AA) Research Framework AT(N) proposed a biological definition of AD based on the underlying pathological process of amyloid (A), phosphorylated tau (T), and neurodegeneration (N) [1]. In that proposal, specific cerebrospinal fluid (CSF) and neuroimaging biomarkers of AT(N) were specified, but the flexibility to incorporate new biomarkers as they emerged was emphasized. Given the invasiveness of obtaining CSF and the time and cost associated with neuroimaging, identifying more easily obtainable biomarkers is an important goal. Blood-based biomarkers of AD and other dementias are ideally suited for use at the population level for screening or diagnosis and for serial assessments to track disease progression [2]. As blood-based biomarkers of amyloid pathology and neurodegeneration enter clinical use, it is essential to understand what factors influence the levels of these blood biomarkers for interpretation of the results [3]. This is particularly important for the establishment of reference ranges. For example, it is important to understand whether levels differ with age and by sex, or whether conditions such as chronic kidney disease (CKD), which can affect the clearance of proteins, should be considered.
In the present study, five plasma markers were examined among 996 participants enrolled in the population-based Mayo Clinic Study of Aging (MCSA): amyloid-β42 (Aβ42), amyloidβ-40 (Aβ40), the Aβ42/40 ratio, neurofilament light chain (NfL), and total tau (T-tau) protein. We considered several demographic, medical, and other factors as predictors of these plasma biomarker levels. We also determined whether the factors associated with the plasma biomarkers differed by clinical diagnosis.
2. METHODS
2.1. Mayo Clinic Study of Aging methods
The MCSA is a population-based study examining the epidemiology of cognitive decline among Olmsted County, Minnesota residents [4]. Age, sex, and ethnic characteristics of Olmsted County are similar to those of the state of Minnesota and the Upper Midwest [5]. However, compared to the entire US population, Olmsted County is less ethnically diverse (90% vs 75% white), more highly educated (91% vs 80% high school graduates), and wealthier ($51,316 vs $41,994 for median household income).
In 2004, Olmsted County residents between the ages of 70 and 89 were enumerated using the Rochester Epidemiology Project medical records-linkage system in an age- and sex-stratified random sampling design [6]. The study was extended to include those aged 50 and older in 2012. MCSA participants were prioritized for the blood biomarker assays of interest if they had concurrent or future imaging, including MRI, amyloid PET, and tau PET. Compared to MCSA participants not included in the present analysis, those included had slightly more years of education (mean: 14.6 vs 14.3 years) and were more frequently male (56.1% vs 49.7%). There were no differences in age, proportion with an APOE ε4 allele, or comorbidities.
MCSA visits included an interview by a study coordinator, physician examination, cognitive testing, and a blood draw completed on the same day [4]. Neuropsychometric testing includes nine tests covering four domains: memory [Auditory Verbal Learning Test Delayed Recall Trial [7], Wechsler Memory Scale-Revised Logical Memory-II & Visual Reproduction-II [8]], language [Boston Naming Test [9], category fluency [10]], visuospatial skills [WAIS-R Picture Completion and Block Design subtests [11]], and attention[Trailmaking Test B [10,12], WAIS-R Digit Symbol subtest [8]]. Using the mean and standard deviation (SD), test scores were converted to z-scores and tests within each domain were averaged and z-scored for domain-specific scores. Global cognition was calculated by z-scoring the average of the four cognitive domain z-scores.
2.2. MCI and dementia diagnostic determination
Clinical diagnoses were determined by a consensus committee comprised of a physician (medical history review, neurological examination, Short Test of Mental Status), study coordinator (Blessed Memory Test, Clinical Dementia Rating Scale, Functional Activities Questionnaire) and neuropsychologist who compared cognitive performance to normative data acquired on a separate sample, Mayo’s Older American Normative Studies [13]. Strict cognitive cutoffs were not used. A final decision was made after considering education, occupation, visual or hearing deficits, and reviewing all other participant information. Published criteria were used for the diagnosis of mild cognitive impairment (MCI) [14] and dementia [15]. Participants who performed in the normal range and did not meet criteria for MCI or dementia were deemed cognitively unimpaired (CU).
2.3. Protocol approvals standard, registrations and patient consents
The study was approved by Mayo Clinic and Olmsted Medical Center Institutional Review Boards. Written informed consent was obtained from all participants.
2.4. Assessment of Covariates
Participant demographics (age, sex, and years of education) were ascertained at the in-clinic examination. Participants’ height (cm) and weight (kg) were measured during the in-clinic exam and used to calculate body mass index (BMI) (kg/m2). Medical conditions (hypertension, dyslipidemia, diabetes, stroke, myocardial infarction, atrial fibrillation, cancer, and chemotherapy) were determined by medical record abstraction. All diagnoses via medical record abstraction were updated periodically, with the majority being up-to-date to the current visit. The Charlson Comorbidity Index [16] was obtained electronically using the REP medical records-linkage system [6]. CKD was determined using an algorithm that searches the medical record. Head trauma was reported by the participant. Depressive symptoms were assessed using the Beck Depression Inventory-II (BDI-II) [17] and anxiety symptoms with the Beck Anxiety Inventory (BAI) [18]. Apolipoprotein E (APOE) genotyping was performed from a blood sample.
2.5. Amyloid PET imaging
Amyloid Pittsburgh compound B (PiB)–PET images were acquired using a PET/CT scanner (DRX, GE Healthcare) operating in 3-dimensional mode within 8 weeks of the clinic visit [19]. Four, 5-minute dynamic frames were acquired from 40 to 60 minutes after injection [20,21]. Quantitative image analysis for PiB was done using our in-house fully automated image processing pipeline [22]. A global cortical PiB-PET retention ratio was computed by calculating the median uptake over voxels in the prefrontal, orbitofrontal, parietal, temporal, anterior cingulate, and posterior cingulate/precuneus regions of interest for each participant and dividing this by the median uptake over voxels in the cerebellar crus. No partial volume correction was used. The atlas and image recognition steps were based on a 3D T1-weighted volume MRI sequence. Participants were considered A+ based on a cutoff in standard uptake value ratio (SUVR) of 1.48 using the reliable worsening method [23], and updated to the SPM12 processing pipeline.
2.6. Blood collection and plasma assays
Participants’ blood was collected in-clinic after an overnight fast, centrifuged, aliquoted, and stored at −80°C. Plasma Aβ40, Aβ42, T-tau and NfL were measured on the Quanterix HD-1 analyzer using the Simoa® Neurology 3-Plex A (N3PA) (tau, Aβ42, Aβ40) (catalog #101995) or Simoa® NF-light (catalog #103186) Advantage kits. Samples were diluted 1:4 using the instrument’s onboard dilution protocol and run in singlet. 8-point calibration curves and sample measurements were determined on the Simoa® HD-1 Analyzer software using a weighting factor 1/Y2 and a 4-parameter logistic curve fitting algorithm. Two levels of quality control material were included, flanking the samples at the front and end of each batch. In the N3PA kits, plasma Aβx-40 and Aβx-42 were quantified using a common detection antibody (6E10), which binds to the amyloid-β peptide at an RHD sequence at residues 5–7. Unique C-terminal antibodies were used for Aβ40 (2G3) and Aβ42 (H31L21) [24,25]. Plasma T-tau was quantified using a capture antibody that binds to the proline-rich P2 region in the mid-domain of the tau protein. The detection antibody binds at the N-terminal of the tau protein in which tyrosine 18 is not phosphorylated. The tau calibration curve was generated using a recombinant human tau 381 isoform with a single N-terminal insert and three microtubule binding domain repeats (3R/1N). In the NF-light kits, both the capture and detector antibodies (Uman Diagnostics article #27016–100, #27017–100) bind to the conserved rod domain of the NfL protein. Internal studies of imprecision for Aβ40, Aβ42, T-tau, and NfL (approximate concentrations: 15.0 and 220 pg/mL, 5.00 and 100 pg/mL, 2.00 and 75.0 pg/mL, 5.00 and 150 pg/mL), respectively, are as follows: Intra-assay imprecision was 7.8% and 3.8%, 8.3% and 3.6%, 5.9% and 3.3%, 18.2% and 4.2%. Inter-assay imprecision was 6.9% and 5.3%, 9.6% and 5.5%, 7.0% and 6.5%, 17.0% and 5.6%. The samples were run in two batches. Based on internal analysis of lot-to-lot variability in N3PA kits, it was found that multiplicative correction factors of 0.96, 0.78, and 0.99 needed to be applied to Aβ40, Aβ42, and T-tau, respectively, for the second round of samples run. Significant lot-to-lot variability was not observed for NfL.
2.7. Statistical analyses
Wilcoxon rank-sum tests were used to compare groups for continuous variables and chi-square tests for categorical variables. Spearman correlations were computed to examine associations between the plasma biomarkers and between each biomarker and age. In linear regression models, independent variables of interest were used to predict the plasma markers. The plasma markers were z-scored relative to the entire sample to compare coefficients. Both univariate and age (by decade) and sex-adjusted models were computed. Models with the cognitive z-scores were additionally adjusted for education. Models were run separately by clinical diagnosis (CU and MCI/dementia). An alpha level of 0.05 was employed. All analyses were completed using SAS version 9.4 (SAS Institute, Cary, NC) and R version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria).
3. RESULTS
3.1. Participant characteristics
The characteristics of the 996 participants, by clinical diagnosis, are shown in Table 1. The median age of the participants was 76.4 years, 56.1% were male, and 27.4% had an APOE ε4 allele. There were 859 CU, 133 MCI, and 4 dementia. Compared to CU, participants with MCI or dementia were older, had less years of education, worse global and domain-specific cognition, and more comorbidities. Plasma Aβ40, NfL, and T-tau were higher in those with MCI or dementia, while the Aβ42/40 ratio was lower (Table 1 and Figure 1).
TABLE 1.
Participant characteristics by clinical diagnosis
| All (N=996) | CU (N=859) | MCI/dementia (N=137) | ||
|---|---|---|---|---|
| Characteristics | Median (Q1, Q3)/N(%) | Median (Q1, Q3)/N(%) | Median (Q1, Q3)/N(%) | P value |
| Age (years) | 76.39 (68.26, 81.88) | 75.63 (66.97, 80.95) | 81.44 (75.60, 85.09) | <.001 |
| Male | 559 (56.1%) | 475 (55.3%) | 84 (61.3%) | .187 |
| Education (years) | 14 (12, 16) | 15 (12, 16) | 13 (12, 15) | <.001 |
| APOE ε4 Allele | 273 (27.4%) | 225 (26.2%) | 48 (35.0%) | .031 |
| Plasma Aβ42 (pg/mL) | 8.89 (7.17, 10.91) | 8.81 (7.15, 10.84) | 9.28 (7.65, 11.00) | .124 |
| Plasma Aβ40 (pg/mL) | 265.92 (224.64, 321.70) | 263.04 (222.72, 314.88) | 295.68 (243.84, 346.00) | <.001 |
| Plasma Aβ42/40 | 0.034 (0.030, 0.038) | 0.034 (0.030, 0.038) | 0.032 (0.028, 0.036) | .008 |
| Plasma NfL (pg/mL) | 17.90 (12.40, 25.23) | 17.0 (11.85, 24.05) | 23.50 (17.30, 32.50) | <.001 |
| Plasma T-tau (pg/mL) | 2.58 (1.86, 3.34) | 2.54 (1.84, 3.29) | 2.87 (2.00, 3.59) | .019 |
| BMI (kg/m2) | 27.43 (24.79, 30.91) | 27.47 (24.89, 31.02) | 26.93 (23.56, 29.70) | .020 |
| <18.5 | 8 (0.8%) | 6 (0.7%) | 2 (1.5%) | .119 |
| 18.5–24.9 | 264 (26.6%) | 218 (25.5%) | 46 (33.8%) | |
| 25–29.9 | 428 (43.2%) | 371 (43.4%) | 57 (41.9%) | |
| 30–34.9 | 214 (21.6%) | 188 (22.0%) | 26 (19.1%) | |
| 35–35.9 | 52 (5.2%) | 50 (5.8%) | 2 (1.5%) | |
| 40+ | 25 (2.5%) | 22 (2.6%) | 3 (2.2%) | |
| BDI depression | 72 (7.2%) | 56 (6.5%) | 16 (11.8%) | .029 |
| BAI total | 1 (0, 4) | 1 (0, 3) | 3 (0, 6) | <.001 |
| Current/former smoker | 445 (44.7%) | 379 (44.1%) | 66 (48.2%) | .375 |
| Charlson Comorbidity Index | 2 (1, 4) | 2 (1, 4) | 4 (2, 6) | <.001 |
| Hypertension | 672 (67.5%) | 568 (66.1%) | 104 (75.9%) | .023 |
| Chronic kidney disease | 87 (8.7%) | 69 (8.0%) | 18 (13.1%) | .049 |
| Dyslipidemia | 805 (80.8%) | 692 (80.6%) | 113 (82.5%) | .595 |
| Diabetes | 180 (18.1%) | 152 (17.7%) | 28 (20.4%) | .438 |
| Stroke | 31 (3.1%) | 20 (2.3%) | 11 (8.0%) | <.001 |
| Myocardial infarction | 125 (12.6%) | 95 (11.1%) | 30 (21.9%) | <.001 |
| Atrial fibrillation | 118 (11.8%) | 96 (11.2%) | 22 (16.1%) | .101 |
| Head trauma | 150 (16.2%) | 132 (16.5%) | 18 (14.1%) | .487 |
| Cancer | 238 (23.9%) | 200 (23.3%) | 38 (27.7%) | .256 |
| Chemotherapy | 40 (16.8%) | 35 (17.5%) | 5 (13.2%) | .512 |
| A+ | 172 (33.6%) | 131 (29.1%) | 41 (66.1%) | <.001 |
| Global z-score | 0.11 (−0.65, 0.75) | 0.25 (−0.31, 0.83) | −1.31 (−1.94, −0.99) | <.001 |
| Memory z-score | 0.08 (−0.68, 0.75) | 0.26 (−0.38, 0.83) | −1.45 (−1.88, −0.81) | <.001 |
| Language z-score | 0.10 (−0.58, 0.67) | 0.25 (−0.37, 0.76) | −1.05 (−1.67, −0.39) | <.001 |
| Attention z-score | 0.11 (−0.53, 0.69) | 0.23 (−0.33, 0.76) | −1.15 (−2.09, −0.43) | <.001 |
| Visual spatial z-score | 0.06 (−0.60, 0.69) | 0.18 (−0.44, 0.80) | −0.80 (−1.57, −0.09) | <.001 |
Abbreviations: Aβ42, Amyloid-beta 42; Aβ40, Amyloid-beta 40; Aβ42/40, Ratio of Amyloid-beta 42 to Amyloid-beta 40; APOE, Apolipoprotein E; BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory-II; BMI, Body Mass Index; CU, Cognitively Unimpaired; MCI, Mild Cognitive Impairment; NfL, Neurofilament Light Chain; Q1, First Quartile; Q3, Third Quartile; T-tau, Total Tau Protein; A+, PiB-PET SUVR ≥ 1.48. P values for continuous variables are from Wilcoxon rank-sum tests and for categorical variables they are from Chi-square tests. Missing Values: BMI, 5; BDI, 2; BAI, 1; Head Trauma, 68; Chemotherapy, 758; A+, 484; zGlobal, 47; zMemory, 9; zLanguage, 25; zAttention, 29; zVisual Spatial, 30.
FIGURE 1.
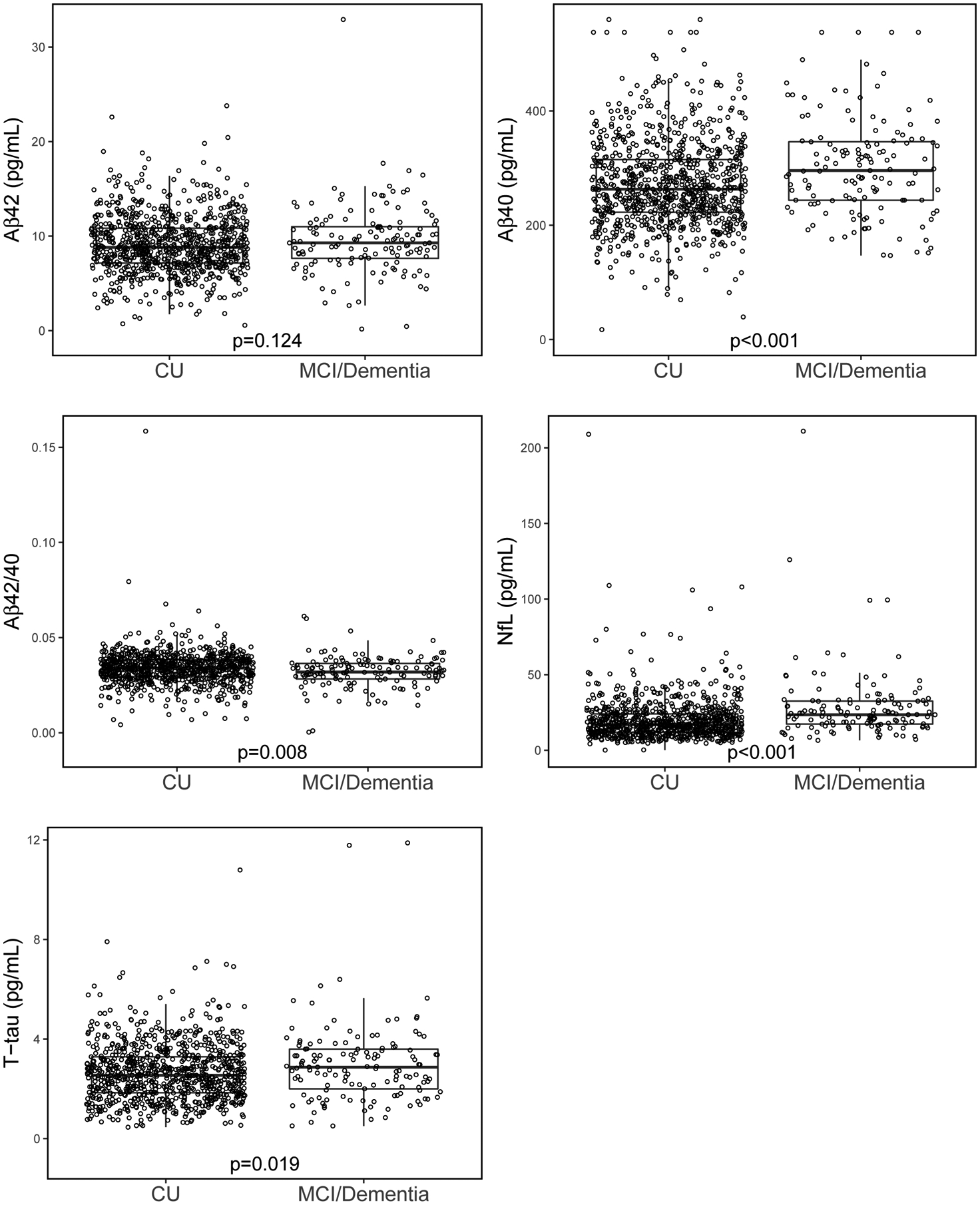
Boxplots of plasma markers by cognitive diagnosis
Abbreviations: Aβ40, Amyloid-beta 40; Aβ42, Amyloid-beta 42; Aβ42/40, Ratio of Amyloid-beta 42 to Amyloid-beta 40; CU, Cognitively Unimpaired; MCI, Mild Cognitive Impairment; NfL, Neurofilament Light Chain; T-tau, Total Tau Protein. P value is from Wilcoxon rank-sum test.
Correlations between the plasma markers, by clinical diagnosis, are shown in Figure 2. Among CU, the weakest correlation was T-tau with NfL (Spearman’s rho=0.202, P< .001) and the strongest was Aβ40 with Aβ42 (rho=0.730, P< .001). Similar correlations were observed for those with MCI or dementia. All plasma markers, except the Aβ42/40 ratio amongst those with MCI or dementia, correlated with age (Figure 3). Plasma Aβ40, Aβ42, NfL, and T-tau positively correlated with age. The Aβ42/40 ratio negatively correlated with age. Among CU, women had higher levels of T-tau and a lower Aβ42/40 ratio. None of the plasma markers differed by sex among the participants with MCI or dementia (Figure S1).
FIGURE 2.
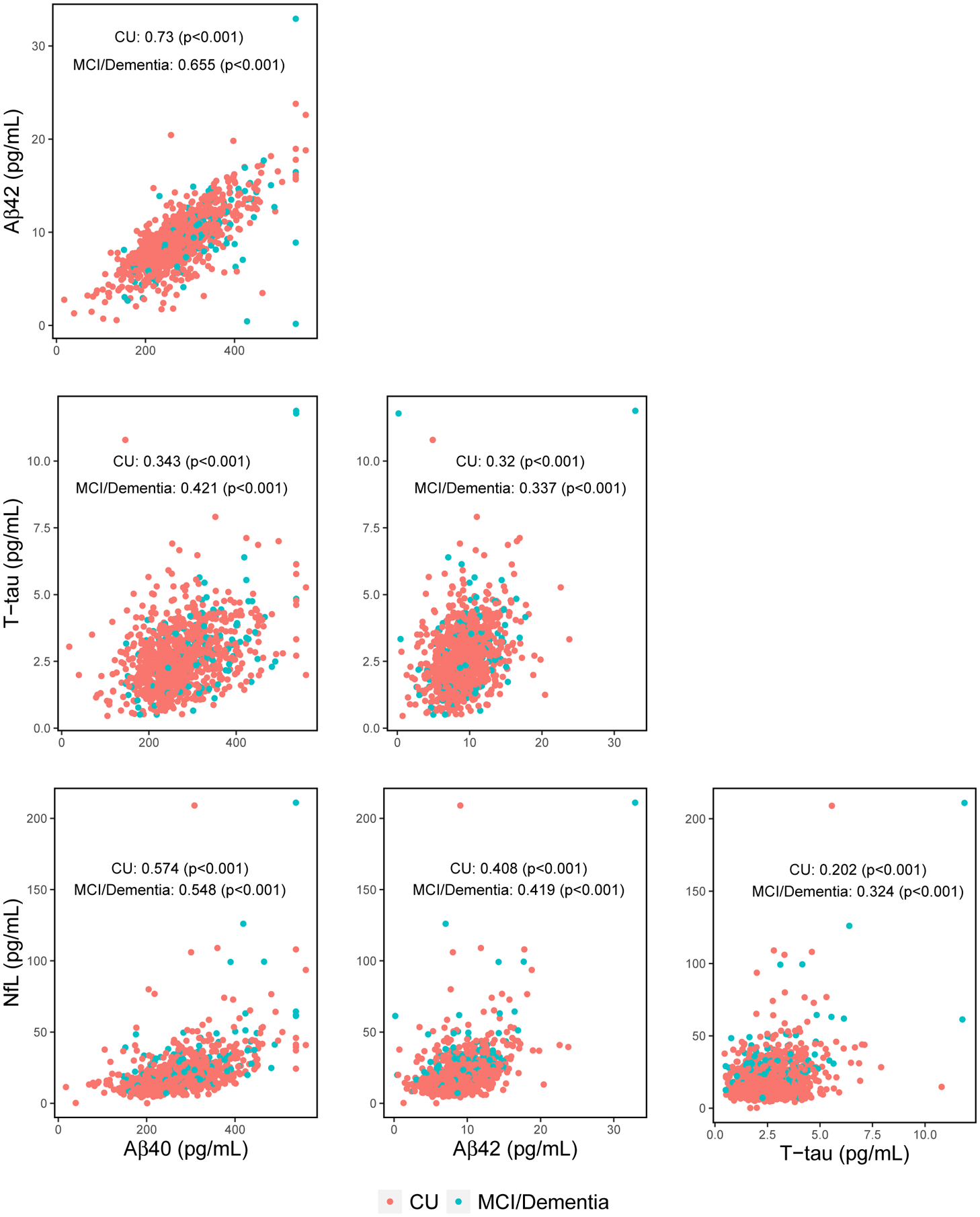
Spearman correlation matrix of plasma markers
Abbreviations: Aβ40, Amyloid-beta 40; Aβ42, Amyloid-beta 42; Aβ42/40, Ratio of Amyloid-beta 42 to Amyloid-beta 40; APOE, Apolipoprotein E; CU, Cognitively Unimpaired; MCI, Mild Cognitive Impairment; NfL, Neurofilament Light Chain; T-tau, Total Tau Protein.
FIGURE 3.
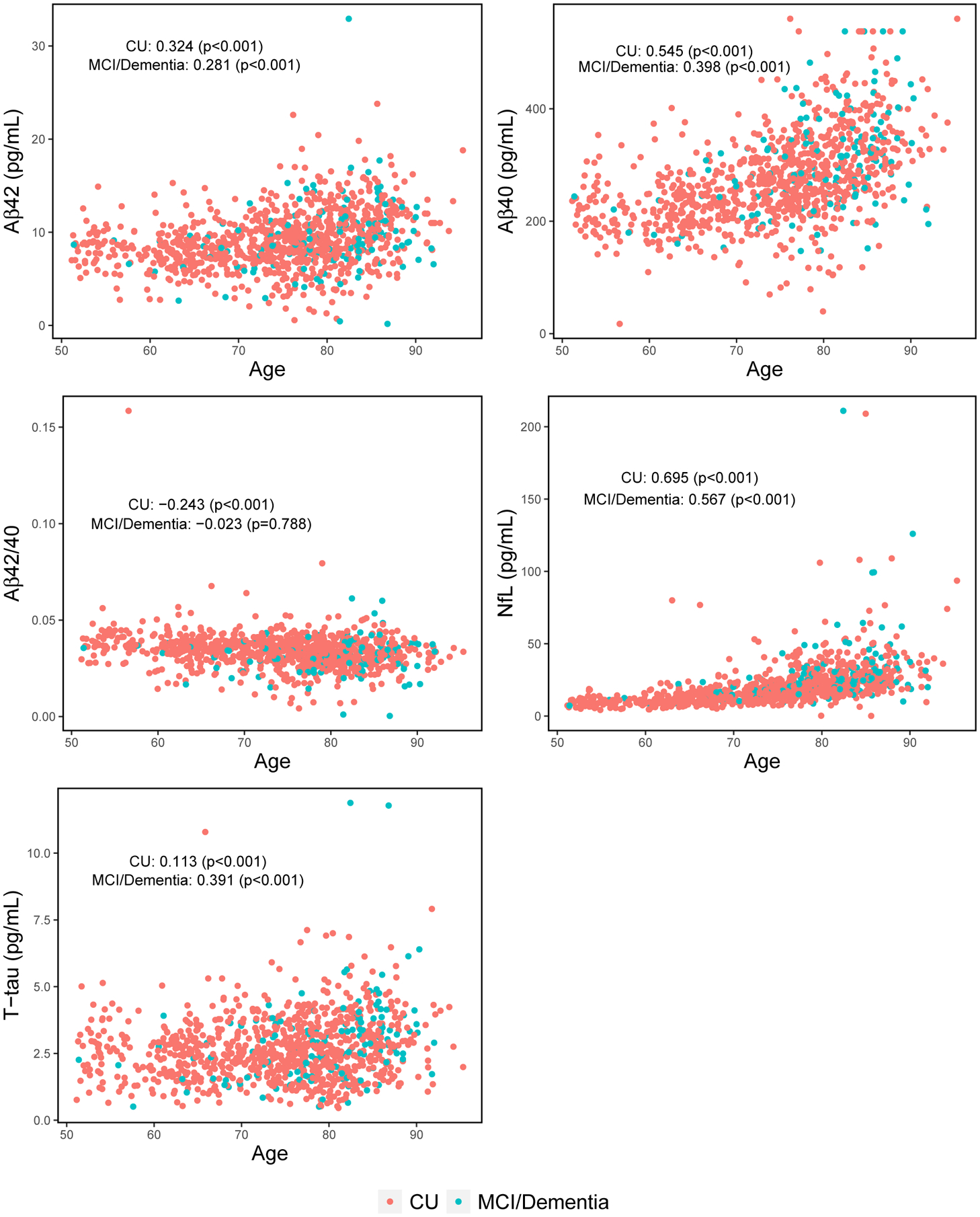
Spearman correlations of plasma markers with age
Abbreviations: Aβ40, Amyloid-beta 40; Aβ42, Amyloid-beta 42; Aβ42/40, Ratio of Amyloid-beta 42 to Amyloid-beta 40; CU, Cognitively Unimpaired; MCI, Mild Cognitive Impairment; NfL, Neurofilament Light Chain; T-tau, Total Tau Protein.
The results presented below are the age- and sex-adjusted linear model results (Figures 4 and 5 and Tables S1 and S2). Results from the univariate linear models are shown in Figures S2 and S3 and Tables S3 and S4. To better understand the effect sizes of the examined factors on the blood biomarkers, we also show differences in the biomarkers by amyloid PET status for those with available data. Of the 859 CU participants, 450 (52%) had a concurrent amyloid PET scan. Those with an amyloid PET scan were younger (median: 73.5 vs 77.1, P<.001), included a larger proportion of men (59.6% vs 50.6%, P=.009), had lower plasma levels of Aβ40 (250.6 vs 275.5, P<.001) and NfL (15.9 vs 18.5, P<.001), and a higher Aβ42/40 ratio (0.035 vs 0.033, P=.007) compared to those without.
FIGURE 4.
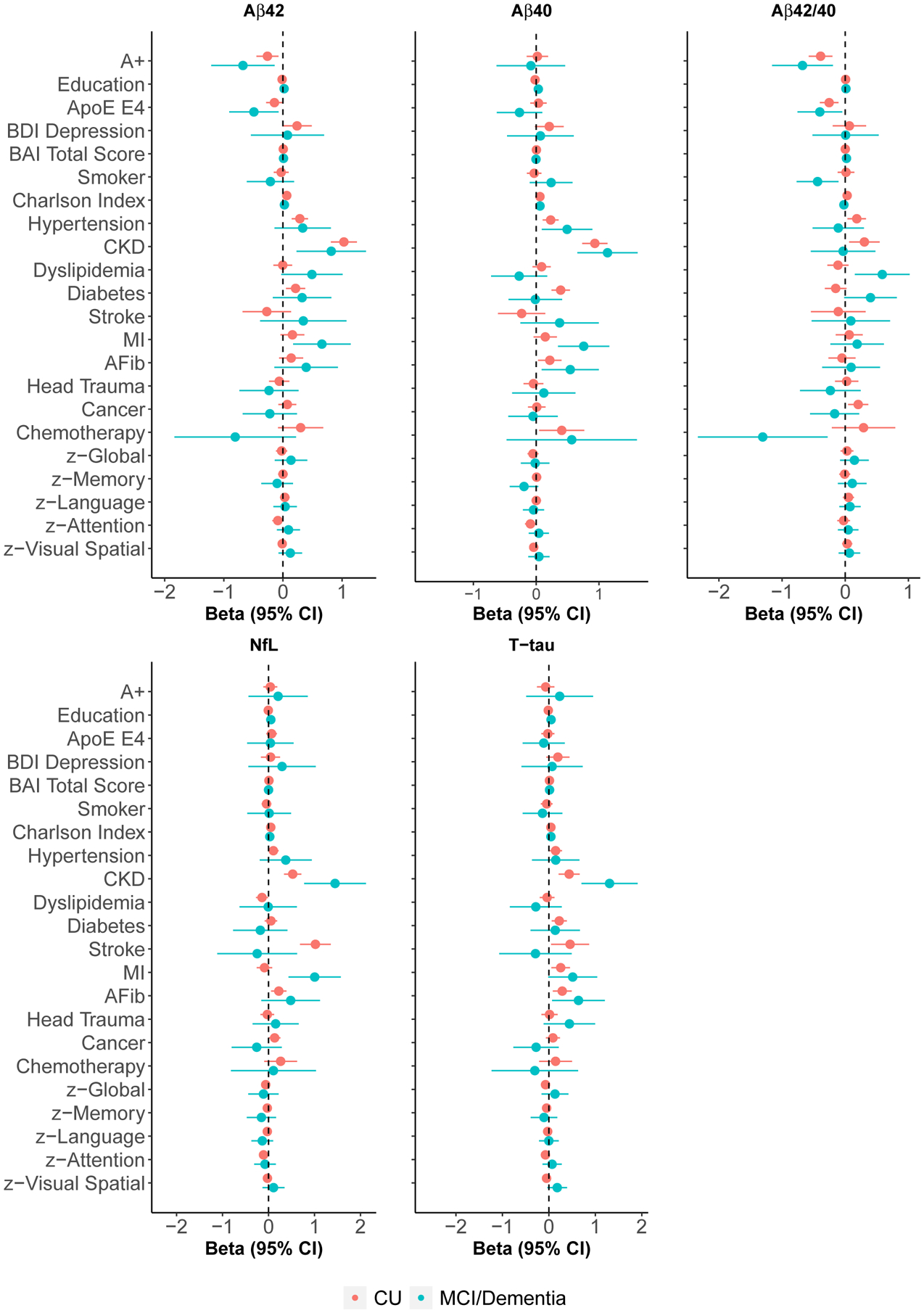
Forest plots of linear model results adjusted for age and sex
Abbreviations: Aβ40, Amyloid-beta 40; Aβ42, Amyloid-beta 42; Aβ42/40, Ratio of Amyloid-beta 42 to Amyloid-beta 40; A+, PiB-PET SUVR ≥ 1.48; AFib, Atrial Fibrillation; APOE, Apolipoprotein E; BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory-II; Beta, Linear Regression Model Coefficient; CI, Confidence Interval; CKD, Chronic Kidney Disease; CU, Cognitively Unimpaired; MCI, Mild Cognitive Impairment; MI, Myocardial Infarction; NfL, Neurofilament Light Chain; T-tau, Total Tau Protein. Models corresponding to the global and domain cognitive z-scores additionally adjust for education.
FIGURE 5.
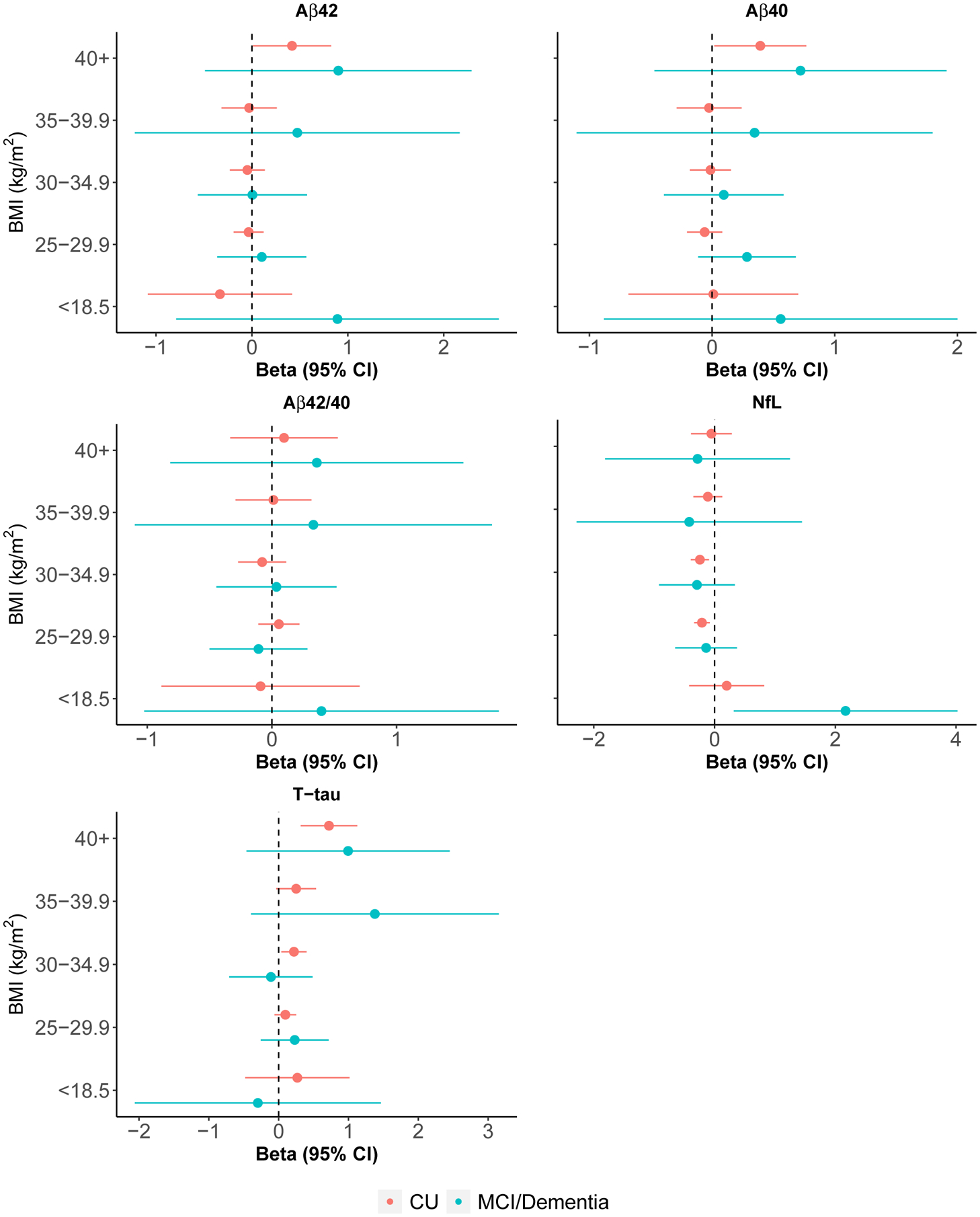
Forest plots of BMI linear model results adjusted for age and sex compared to BMI of 18.5 to 24.9
Abbreviations: Aβ40, Amyloid-beta 40; Aβ42, Amyloid-beta 42; Aβ42/40, Ratio of Amyloid-beta 42 to Amyloid-beta 40; APOE, Apolipoprotein E; Beta, Linear Regression Model Coefficient; BMI, Body Mass Index; CI, Confidence Interval; CU, Cognitively Unimpaired; MCI, Mild Cognitive Impairment; NfL, Neurofilament Light Chain; T-tau, Total Tau Protein.
3.2. Aβ42
In age- and sex-adjusted linear regression models, CU participants with hypertension, diabetes, or CKD had higher Aβ42 levels, on average, than those without each condition (Figure 4 and Table S1). Most notably, those with CKD, versus without, had Aβ42 levels about one SD higher. For reference, A+ participants had Aβ42 levels about 0.26 SD lower than A-. Each increase in comorbid conditions, as measured by the Charlson comorbidity index, was associated with higher Aβ42 levels. Furthermore, those with BMI≥40 had higher Aβ42 when compared to the reference BMI group of 18.5–24.9 (Figure 5 and Table S1). Participants with an APOE ε4 allele, on the other hand, had lower Aβ42 levels. Amongst those with MCI or dementia, CKD and a history of myocardial infarction were associated with higher Aβ42 levels whereas the presence of an APOE ε4 allele was associated with lower levels (Figure 4 and Table S2).
3.3. Aβ40
Hypertension, diabetes, atrial fibrillation, chemotherapy (amongst those with cancer), and CKD were associated with higher Aβ40 levels amongst CU (Figure 4 and Table S1). As with Aβ42, those with CKD had about one SD higher Aβ40 levels that those without. Aβ40 levels did not differ by amyloid PET status. Lower z-scores in the attention domain, a larger number of comorbidities, and BMI≥40 were associated with higher Aβ40 levels (Figure 5 and Table S1). Among MCI and dementia participants, Charlson comorbidity index, hypertension, atrial fibrillation, myocardial infarction, and CKD were associated with higher Aβ40 levels (Figure 4 and Table S2).
3.4. Aβ42/40
Hypertension, previous cancer diagnosis, CKD, and the Charlson comorbidity index were associated with a higher Aβ42/40 ratio in age- and sex-adjusted linear models among CU participants (Figure 4 and Table S1). The relationship of CKD with Aβ42/40 was not as strong (about 0.3 SD higher) as it was for Aβ40 or Aβ42 alone but was still significant. Being A+ (as compared to A-) had a similar effect but in the opposite direction (about 0.3 SD lower). Those with an APOE ε4 allele had a lower Aβ42/40 ratio (Figure 5 and Table S1). Among those with MCI or dementia, presence of an APOE ε4 allele was also associated with a lower Aβ42/40 ratio (Figure 4 and Table S2). In addition, three variables not associated with the ratio among CU were associated with the ratio among those who had MCI or dementia: smoking and chemotherapy were associated with a lower Aβ42/40 while dyslipidemia was associated with a higher ratio.
3.5. NfL
Amongst CU, a history of stroke, atrial fibrillation, history of cancer, and CKD were associated with higher NfL levels, on average, when compared to those without those conditions in age- and sex-adjusted linear models (Figure 4 and Table S1). Those with CKD had NfL levels about a half SD higher than those without; those with history of stroke were about one SD higher. NfL levels did not differ by amyloid PET status. In addition, an increasing number of comorbidities and a lower attention domain z-score were associated with higher levels of NfL whereas dyslipidemia was associated with lower levels. CU participants with a BMI of 25–29.9 (−0.21[−0.34, −0.08]) and 30–34.9 (−0.24[−0.39, −0.09]) had lower levels of NfL, on average, compared to those with a BMI between 18.5 and 24.9 (Figure 5 and Table S1).
Among participants with a diagnosis of MCI or dementia, CKD was associated with even higher NfL levels than among CU (Figure 4 and Table S2). Furthermore, those with BMI<18.5, versus BMI 18.5–24.9, had NfL levels about 2 SD higher (Figure 5 and Table S2). Myocardial infarction was also associated with higher NfL.
3.6. Total tau
Among CU, those with hypertension, diabetes, a history of stroke, myocardial infarction, atrial fibrillation, CKD, and an increasing Charlson comorbidity index had higher levels of T-tau than those without in age- and sex-adjusted analyses (Figure 4 and Table S1). T-tau levels did not differ by amyloid PET status. A higher BMI was also associated with higher T-tau levels (Figure 5 and Table S1). Among those with MCI or dementia, only atrial fibrillation and CKD were associated with higher T-tau levels (Figure 4 and Table S2). The effect of CKD on T-tau levels was much stronger for MCI or dementia participants compared to CU.
4. DISCUSSION
As blood-based biomarkers of amyloid pathology and neurodegeneration near clinical use, it is essential to understand what factors may influence the levels of these markers to best interpret the results. This information is especially important for the establishment of reference ranges. In the present study, we examined whether several factors were associated with plasma Aβ42, Aβ40, Aβ42/40, NfL, and T-tau among 996 participants enrolled in the population-based MCSA. Multiple associations were found that should be considered in the clinical interpretation of these plasma biomarker results.
The CU participants are most relevant for developing reference ranges, so we stratified results by cognitive status. Amongst CU participants, age was positively correlated with Aβ42, Aβ40, NfL, and T-tau levels and negatively correlated with the Aβ42/40 ratio. Notably, T-tau correlated the weakest with age. These results are in agreement with previous studies [26–32], and further suggest that age should be considered when interpreting these biomarkers.
Women had higher plasma T-tau levels and a lower Aβ42/40 ratio than men but NfL, Aβ42, and Aβ40 levels did not differ by sex. Two studies also reported higher T-tau levels for women [33,34], but other studies have not observed a sex difference for T-tau [35,36] or Aβ42/40 [37,38]. Notably, we did not observe a sex difference in T-tau or Aβ42/40 levels for those with MCI or dementia. Therefore, a potential explanation for the discrepancy between studies could be the percentage of cognitively impaired individuals included in the sample. In line with our observations of a lack of sex differences in NfL measures, previous studies of blood NfL have also not reported sex differences [26,34]. Contrary to our results, one study found higher Aβ40 levels in men [38].
In age- and sex-adjusted linear regression models of CU participants, CKD was associated with significantly elevated levels of all biomarkers. Several other medical conditions were also associated with the plasma biomarker levels. For example, the Charlson comorbidity index was associated with higher levels of all markers, hypertension was associated with higher levels of all markers except NfL, and diabetes was associated with higher levels of Aβ42, Aβ40, and T-tau. Elevated Aβ42 and Aβ40 have previously been found in those with hypertension, diabetes, and ischemic heart disease [39]. NfL has likewise been found to be positively associated with the presence of multiple cardiovascular conditions [40]. The propensity for increased medical conditions and comorbidities with age could explain some of the age associations. It is important to consider that the effect of some of the variables examined on the blood biomarker levels may be because they are risk factors for amyloid pathology and/or neurodegeneration whereas the influence of others may be due to physiological processes. For example, the effect of APOE on the plasma markers, especially Aβ42 and Aβ40, is likely due to the increased risk of Alzheimer’s disease for those with an APOE ε4 allele. By comparison, CKD could be both a risk factor and have an impact on blood levels due to physiological processes. A low estimated glomerular filtration rate and clinical diagnosis of CKD, for instance, have been associated with risk of dementia [41,42]. In addition, CKD results in a reduced clearance of proteins in the blood, and thus higher circulating blood levels for the biomarkers of interest. Because a low Aβ42/Aβ40 ratio is associated with elevated brain amyloid, the elevated ratio due to CKD could result in missed diagnoses. In contrast, CKD could result in higher levels of NfL and T-tau, which would make it appear that a patient has more significant neurodegeneration. Understanding the impact of each factor on the blood biomarkers, through an increased risk of disease or physiological processes, will be important for the future interpretation of the blood biomarkers in the context of clinical screening, diagnosis, or prognosis at the population level.
A higher BMI was associated with lower NfL levels. One study of participants with multiple sclerosis and controls demonstrated a linear inverse relationship between plasma NfL and BMI [43]. It has been hypothesized that a higher blood volume in those with higher BMI could be the underlying reason for the association between a higher BMI and lower NfL [44]. In the present study, this inverse association was observed for those with BMI<35. Of note, the current study had a much higher median age (76 vs 40 years) and BMI (27 vs 24) than the previous study so additional research examining the association between BMI and blood NfL at older ages is needed. In contrast to NfL, a higher BMI was associated with higher T-tau levels among CU participants. Given the inverse association between BMI and NfL, it was somewhat surprising that T-tau levels were positively associated with BMI. Tau has many physiological functions and the role of tau in the periphery is not well understood. The potential association between peripheral tau and insulin signaling could contribute to the positive association between T-tau and BMI, but more research is needed [45].
Only some relationships observed among the CU participants persisted among those with MCI/dementia. Most notably, the effect of CKD on NfL and T-tau levels was even greater than that observed among CU participants. Some relationships not seen amongst the CU group were present in the MCI/dementia group. The most prominent was the association between myocardial infarction and higher levels of Aβ42, Aβ40, and NfL.
A strength of the study is the large sample of well-characterized individuals enrolled in a population-based study which did not exclude participants based on medical conditions such as cerebrovascular disease and cancer. However, one limitation of the current study is that our sample is almost entirely White (99%). To create reference ranges that apply to a diverse patient population, studies of varying races and ethnicities as well as socioeconomic and population density (rural/urban) contexts are needed. When conducting studies amongst different races and ethnicities it will be important to not over-interpret the results. In this study, both BMI and CKD affected the levels of these plasma biomarkers. The prevalence of obesity, after adjusting for age, is higher amongst Blacks and Hispanic people compared to White people, and higher in rural populations than in urban settings [46]. Similar racial and ethnic observations have been found for CKD [47]. Thus, when interpreting these blood biomarkers in varying population settings, it will be important to consider the sample characteristics and the implications of selection bias.
Supplementary Material
ACKNOWLEDGMENTS
Funding for this study was provided by grants from the National Institutes of Health (U01 AG006786, R37 AG011378, R01 NS097495, and P30 AG062677) and the GHR Foundation. This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01 AG034676.
Role of the Funder/Sponsor:
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study, and all authors had final responsibility for the decision to submit for publication.
CONFLICTS OF INTEREST
Dr. Mielke served as a consultant to Brain Protection Company and Biogen and receives research support from the National Institutes of Health and the Department of Defense. She is a Senior Associate Editor for Alzheimer’s and Dementia: The Journal of the Alzheimer’s Association. Dr. Knopman serves on a Data Safety Monitoring Board for Biogen (fee paid to institution), the DIAN-TU study (receives personal consulting fees), Agenbio (unpaid), and an endovascular carotid reconstruction study (unpaid). He is an investigator in clinical trials sponsored by Biogen, Lilly Pharmaceuticals, and the Alzheimer’s Disease Cooperative Study; and receives research support from the National Institutes of Health and philanthropic funds. Dr. Bu has received consulting and/or speaking fees from AbbView, E-Scape, SciNeuro, Merck, and Alnylam. He recieves research support from the National Institutes of Health and the Cure Alzheimer’s Fund, has a patent pending for application of Wnt modulators, and serves as a member of the Scientific Review Committee for the Bright Focus Foundation and Research Leadership Group for Cure Alzheimer’s Fund. Dr. Vemuri received speaking fees from Miller Medical Communications, LLC and receives research support from the National Institutes of Health. Dr Graff-Radford receives NIH funding and serves as Assistant Editor for Neurology. He has received payment for speaking at the American Academy of Neurology Annual meeting. Dr. Jack serves on an independent data monitoring board for Roche but he receives no personal compensation from any commercial entity. He receives research support from the National Institutes of Health, the GHR Foundation and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic. Dr. Petersen is a consultant for Roche, Inc., Merck, Inc., Biogen, Inc., and Eisai, Inc. He has received payment for serving on a Data Safety Monitoring Board for Genentech, receives royalties from Oxford University Press and UpToDate, and receives research support from the National Institutes of Health. Dr. Machulda receives research support from the National Institutes of Health. Ms. Campbell has served in unpaid leadership roles for The American Society for Clinical Laboratory Science, American Society for Clinical Pathology, and the Clinical Laboratory Standards Institute. Mr. Syrjanen and Dr. Algeciras-Schimnich have no conflicts to report.
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- [1].Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement 2018;14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].O’Bryant SE, Mielke MM, Rissman RA, Lista S, Vanderstichele H, Zetterberg H, et al. Blood-based biomarkers in Alzheimer disease: current state of the science and a novel collaborative paradigm for advancing from discovery to clinic. Alzheimers Dement 2017;13:45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mielke MM. Consideration of sex differences in the measurement and interpretation of Alzheimer disease-related biofluid-based biomarkers. J Appl Lab Med 2020;5:158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008;30:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].St. Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc 2012;87:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].St. Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Pankratz JJ, Brue SM, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol 2012;41:1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rey A. L’examen Clinique en Psychologie. Paris: Presses Universitaires de France; 1964. [Google Scholar]
- [8].Wechsler D. Manual for the Wechsler Memory Scale-Revised. San Antonio, TX: The Psychological Corporation: Harcourt Brace Jovanovich; 1987. [Google Scholar]
- [9].Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- [10].Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3rd ed. New York: Oxford University Press; 2006. [Google Scholar]
- [11].Wechsler D. Wechsler Adult Intelligence Scale-Revised [Manual]. San Antonio, TX: Psychological Corporation: Harcourt Brace jovanovich; 1981. [Google Scholar]
- [12].Reitan R. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills 1958;8:271–276. [Google Scholar]
- [13].Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC, Kokmen E, et al. Mayo’s Older Americans Normative Studies: updated AVLT norms for ages 56 to 97. Clinical Neuropsychologist 1992;6:83–104. [Google Scholar]
- [14].Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256:183–194. [DOI] [PubMed] [Google Scholar]
- [15].American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). 4th ed. Washington, D.C.: American Psychiatric Association; 1994. [Google Scholar]
- [16].Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- [17].Beck AT, Steer RA, Brown GK. BDI-II, Beck Depression Inventory: Manual. 2nd ed. San Antonio, TX; Boston, MA: Psychological Corp.; Harcourt Brace; 1996. [Google Scholar]
- [18].Beck AT, Steer RA. Manual for the Beck Anxiety Inventory. San Antonio, TX: Psychological Corporation; 1990. [Google Scholar]
- [19].Lowe VJ, Kemp BJ, Jack CR Jr., Senjem M, Weigand S, Shiung M, et al. Comparison of 18F-FDG and PiB PET in cognitive impairment. J Nucl Med 2009;50:878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab 2005;25:1528–1547. [DOI] [PubMed] [Google Scholar]
- [21].McNamee RL, Yee SH, Price JC, Klunk WE, Rosario B, Weissfeld L, et al. Consideration of optimal time window for Pittsburgh compound B PET summed uptake measurements. J Nucl Med 2009;50:348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jack CR Jr., Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain 2008;131:665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jack CR Jr., Wiste HJ, Weigand SD, Therneau TM, Lowe VJ, Knopman DS, et al. Defining imaging biomarker cut points for brain aging and Alzheimer’s disease. Alzheimers Dement 2017;13:205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].De Meyer S, Schaeverbeke JM, Verberk IMW, Gille B, De Schaepdryver M, Luckett ES, et al. Comparison of ELISA- and SIMOA-based quantification of plasma Abeta ratios for early detection of cerebral amyloidosis. Alzheimers Res Ther 2020;12:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Baghallab I, Reyes-Ruiz JM, Abulnaja K, Huwait E, Glabe C. Epitomic characterization of the specificity of the anti-amyloid Abeta monoclonal antibodies 6E10 and 4G8. J Alzheimers Dis 2018;66:1235–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Khalil M, Pirpamer L, Hofer E, Voortman MM, Barro C, Leppert D, et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat Commun 2020;11:812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Disanto G, Barro C, Benkert P, Naegelin Y, Schadelin S, Giardiello A, et al. Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol 2017;81:857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mattsson N, Andreasson U, Zetterberg H, Blennow K. Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol 2017;74:557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lewczuk P, Ermann N, Andreasson U, Schultheis C, Podhorna J, Spitzer P, et al. Plasma neurofilament light as a potential biomarker of neurodegeneration in Alzheimer’s disease. Alzheimers Res Ther 2018;10:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].de Wolf F, Ghanbari M, Licher S, McRae-McKee K, Gras L, Weverling GJ, et al. Plasma tau, neurofilament light chain and amyloid-beta levels and risk of dementia; a population-based cohort study. Brain 2020;143:1220–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mattsson-Carlgren N, Janelidze S, Palmqvist S, Cullen N, Svenningsson AL, Strandberg O, et al. Longitudinal plasma p-tau217 is increased in early stages of Alzheimer’s disease. Brain 2020;143:3234–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mielke MM, Syrjanen JA, Blennow K, Zetterberg H, Vemuri P, Skoog I, et al. Plasma and CSF neurofilament light: relation to longitudinal neuroimaging and cognitive measures. Neurology 2019;93:e252–e260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pase MP, Beiser AS, Himali JJ, Satizabal CL, Aparicio HJ, DeCarli C, et al. Assessment of plasma total tau level as a predictive biomarker for dementia and related endophenotypes. JAMA Neurol 2019;76:598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Baldacci F, Lista S, Manca ML, Chiesa PA, Cavedo E, Lemercier P, et al. Age and sex impact plasma NFL and t-Tau trajectories in individuals with subjective memory complaints: a 3-year follow-up study. Alzheimers Res Ther 2020;12:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mattsson N, Zetterberg H, Janelidze S, Insel PS, Andreasson U, Stomrud E, et al. Plasma tau in Alzheimer disease. Neurology 2016;87:1827–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dage JL, Wennberg AM, Airey DC, Hagen CE, Knopman DS, Machulda MM, et al. Levels of tau protein in plasma are associated with neurodegeneration and cognitive function in a population-based elderly cohort. Alzheimers Dement 2016;12:1226–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Vergallo A, Megret L, Lista S, Cavedo E, Zetterberg H, Blennow K, et al. Plasma amyloid beta 40/42 ratio predicts cerebral amyloidosis in cognitively normal individuals at risk for Alzheimer’s disease. Alzheimers Dement 2019;15:764–775. [DOI] [PubMed] [Google Scholar]
- [38].Keshavan A, Pannee J, Karikari TK, Rodriguez JL, Ashton NJ, Nicholas JM, et al. Population-based blood screening for preclinical Alzheimer’s disease in a British birth cohort at age 70. Brain 2021;144:434–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Janelidze S, Stomrud E, Palmqvist S, Zetterberg H, van Westen D, Jeromin A, et al. Plasma beta-amyloid in Alzheimer’s disease and vascular disease. Sci Rep 2016;6:26801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Polymeris AA, Coslovksy M, Aeschbacher S, Sinnecker T, Benkert P, Kobza R, et al. Serum neurofilament light in atrial fibrillation: clinical, neuroimaging and cognitive correlates. Brain Commun 2020;2:fcaa166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Xu H, Garcia-Ptacek S, Trevisan M, Evans M, Lindholm B, Eriksdotter M, et al. Kidney function, kidney function decline, and the risk of dementia in older adults: a registry-based study. Neurology 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Burns CM, Knopman DS, Tupper DE, Davey CS, Slinin YM, Lakshminarayan K, et al. Prevalence and risk of severe cognitive impairment in advanced chronic kidney disease. J Gerontol A Biol Sci Med Sci 2018;73:393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Manouchehrinia A, Piehl F, Hillert J, Kuhle J, Alfredsson L, Olsson T, et al. Confounding effect of blood volume and body mass index on blood neurofilament light chain levels. Ann Clin Transl Neurol 2020;7:139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Feldschuh J, Enson Y. Prediction of the normal blood volume. Relation of blood volume to body habitus. Circulation 1977;56:605–612. [DOI] [PubMed] [Google Scholar]
- [45].Goncalves RA, Wijesekara N, Fraser PE, De Felice FG. The link between tau and insulin signaling: implications for Alzheimer’s disease and other tauopathies. Front Cell Neurosci 2019;13:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hales CM, Fryar CD, Carroll MD, Freedman DS, Aoki Y, Ogden CL. Differences in obesity prevalence by demographic characteristics and urbanization level among adults in the United States, 2013–2016. JAMA 2018;319:2419–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Peralta CA, Katz R, DeBoer I, Ix J, Sarnak M, Kramer H, et al. Racial and ethnic differences in kidney function decline among persons without chronic kidney disease. J Am Soc Nephrol 2011;22:1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


