Abstract
Background
This study aimed to compare the effectiveness of endometrial receptivity analysis (ERA)-guided personalized embryo transfer (pET) with conventional frozen embryo transfer (FET) in 281 Chinese women with recurrent implantation failure (RIF).
Material/Methods
A total of 281 eligible patients with RIF were recruited and assigned to ERA (ERA followed by pET) and FET groups. The clinical pregnancy outcomes were compared between the 2 groups.
Results
There were no significant differences between the ERA and FET groups in terms of endometrial thickness on the day of embryo transfer, mean attempts of assisted reproductive technology (ART) treatment, anti-Mullerian hormone, follicle-stimulating hormone, or antral follicle count in the fresh cycle (P>0.05). The ERA test identified 35% of samples as receptive and 65% as nonreceptive, and comparable pregnancy outcomes were observed between receptive and nonreceptive patients (P>0.05). Higher pregnancy and implantation rates were found in the ERA group than in the FET group (P<0.01), while no significant differences were detected between the 2 groups in terms of miscarriage rates (P>0.05).
Conclusions
In this study of Chinese women with RIF undergoing in vitro fertilization and embryo transfer, ERA-guided pET resulted in a significant improvement in pregnancy and implantation rates when compared with FET.
Keywords: Embryo Implantation, Embryo Transfer, Infertility
Background
Infertility, which is defined as a failure to conceive despite 12 months of regular, unprotected sexual intercourse, is a worldwide issue [1]. As a global public health concern, this disorder of the reproductive system is estimated to occur in 15% of all couples of reproductive age worldwide [2]. Results from the 2017 Global Burden of Disease Study showed that there were 123 085 individuals living with infertility across the globe, which was responsible for 957 000 years lived with disability [3]; more importantly, both the prevalence and the disease burden of infertility showed an upward trend from 1990 through 2017 [4]. In China, the prevalence of infertility is estimated to be 25% among couples of reproductive age, which poses heavy social, economic, family, and disease burdens in the country [5].
Although the exact cause remains unclear, multiple factors have been identified to be responsible for infertility [6], among which recurrent implantation failure (RIF) is accepted as a major cause of infertility [7–9]. Previous studies have identified many factors that contribute to the pathogenesis of RIF, and endometrial receptivity, which is crucial for embryo implantation, is widely considered as a primary cause for implantation failure [10–12]. A receptive endometrium is a prerequisite for successful embryo implantation and subsequent pregnancy [13].
The window of implantation (WOI), which is necessary for the implantation of the blastocyst in the uterus, usually starts between days 19 and 20 of the menstrual cycle, and generally lasts between 24 and 36 hours [14]. During the WOI, the endometrium expresses a sophisticated repertoire of proteins that allow it to become receptive to implantation by the embryo [15]. Precise prediction of the WOI is therefore of great significance to improve the implantation rate and the likelihood of pregnancy [16].
Endometrial receptivity analysis (ERA) was developed to identify the receptive state of the endometrium and personalize the time of embryo transfer through the profiling of the expression of 248 genes at different stages of the endometrial cycle. This assay has shown higher accuracy than histological dating in the identification of endometrial dating and receptivity [17]. More importantly, ERA results remain reproducible in the same individual across multiple menstrual cycles (from 29 through 40 months) as long as the body mass index (BMI), endometrial thickness, and treatment regimens are not changed during this period [17]. Previous studies have shown the effectiveness of ERA for the prediction of the WOI, and the ERA test was identified as an effective and reproducible approach to guiding pET [18–22]; however, the efficacy of such a tool for pET in Chinese women requires further investigation [23]. Therefore, this study aimed to compare the effectiveness of ERA-guided pET with conventional frozen embryo transfer (FET) in 281 Chinese women with RIF.
Material and Methods
Ethics Statement
The protocol of this study has been reviewed and approved by the Ethics Review Committee of Chengdu Xi’nan Gynecology Hospital (approval no. 2020-018). Signed informed consent was obtained from all participants.
Patient Enrollment
Patients undergoing FET at the blastocyst stage on days 5 or 6 during the period of November 2019 through March 2021 were recruited for this study. The inclusion criteria were (1) ages of 18 through 37 years; (2) BMI between 18.5 and 30 kg/m2; (3) couples experiencing multiple embryo implantation failures (at least 2 cycles of embryo transfer, or transfer of at least 3 good-quality blastocysts with a Gardner’s score of 4BB or higher) [24]; and (4) at least 1 remaining blastocyst with a Gardner’s score of 4BB or higher. Patients with genetic disorders, anatomical abnormality of the genital tract, infections, endocrine diseases, immune disorders, or severe asthenospermia were excluded from the study. Finally, a total of 281 eligible patients were included in the study and assigned to the ERA group (n=140) or the FET group (n=141). We collected participants’ demographic features from the medical records.
Endometrial Sampling and Processing
Patients in the ERA group underwent pET, while patients in the FET group underwent conventional frozen embryo transfer. Patients in the ERA group underwent the ERA test under either a hormonal replacement therapy (HRT) cycle or a natural cycle [19]. In the HRT cycle, 50 to 70 mg of endometrial biopsy samples were collected from the uterine fundus using a sterile suction tube (Shanghai Jiaobao Medical Health Care Technology Co., Ltd.; Shanghai, China) 120 ± 3 h after the start of administration with utrogestan vaginal 300 mg capsules (CYNDEA PHARMA SL; Olvega, Spain) every 12 h and 20 mg dydrogesterone (Abbott Biologicals B.V.; Amstelveen, The Netherlands) twice a day on day 5 from the start of menstruation (P+5). In the natural cycle, endometrial specimens were sampled 7 days after the luteinizing hormone surge (LH+7) or 7 days after the administration of human chorionic gonadotropin (hCG+7). All endometrial specimens were transferred to cryotubes (Biosigma S.p.A.; Cona, Italy) containing 1.5 mL RNA later solution (Qiagen GmbH; Hilden, Germany) and were shaken vigorously to allow for the stabilization of the genetic material present in the tissue. Endometrial specimens were stored at 4°C for at least 4 h or stored at −20°C and then shipped at room temperature for the ERA test. Patients in the FET group did not receive ERA and underwent blastocyst transfer between 120 and 126 h after corpus luteum transformation.
ERA and WOI Prediction
All endometrial specimens were sent to Hangzhou Veritas Genetics Medical Institute Co., Ltd. (Hangzhou, China) for ERA. Total RNA was extracted from endometrial specimens using a QIAGEN QIA cube robotic workstation and QIAGEN spin-column kits (Qiagen; Hilden, Germany), and good-quality RNA samples (RNA integrity number R7) were employed for subsequent ERA as described previously [20].
The transcriptomic sequencing data were processed using RNASeq, and the endometrial receptivity status was assessed by the ERA computational predictor as described previously [17]. The endometrium was classified as receptive or nonreceptive according to the ERA assessment, and pET was done at the time determined by the ERA in the ERA group.
Clinical Follow-up and Outcomes
All patients were followed up until May 15, 2021, and the following were calculated and compared between the ERA and the FET groups: biochemical pregnancy rate, clinical pregnancy rate, implantation rate, clinical miscarriage rate, biochemical miscarriage rate, ectopic pregnancy rate, cumulative biochemical pregnancy rate, cumulative clinical pregnancy rate, cumulative implantation rate, cumulative clinical miscarriage rate, cumulative biochemical miscarriage rate, and cumulative ectopic pregnancy rate. The biochemical pregnancy rate was defined as the proportion of women who were positive for serum β-human chorionic gonadotropin (β-hCG) (>25 mIU/mL), without confirmation by vaginal ultrasound; clinical pregnancy rate was defined as the proportion of women who were positive for β-hCG, and a gestational sac was visualized by vaginal ultrasound at the fifth week of pregnancy; implantation rate was defined as the number of gestational sacs detected by vaginal ultrasound at the fifth gestational week divided by the number of embryos transferred; clinical miscarriage rate was defined as the proportion of women with spontaneous pregnancy losses in whom a gestational sac was previously observed; biochemical miscarriage rate was defined as the proportion of women with pregnancy losses in whom only the detection of β-hCG was positive, without a gestational sac visualized by vaginal ultrasound at the fifth week of pregnancy; ectopic pregnancy rate was defined as the proportion of women with pregnancies outside of the uterine cavity diagnosed by ultrasound, surgical visualization, or histopathology; cumulative biochemical pregnancy rate, cumulative clinical pregnancy rate, cumulative implantation rate, cumulative clinical miscarriage rate, cumulative biochemical miscarriage rate, and cumulative ectopic pregnancy rate were the appellate counterpart indicators (biochemical pregnancy rate, the clinical pregnancy rate, implantation rate, clinical miscarriage rate, biochemical miscarriage rate, ectopic pregnancy rate) following the same type of transfer arm into which the patient was randomized to up to a 12-month follow-up period, respectively. In addition, the blastocyst quality was assessed using Gardner’s scoring system, and a good-quality blastocyst was defined by a Gardner’s score of 4BB or higher [24].
Statistical Analysis
All statistical analyses were performed using the statistical software SPSS version 21.0 (IBM Corp., Armonk, NY, USA). All measurement data were expressed as mean±standard deviations (SD), and all categorical data were described as proportions. Comparison of measurement data was done with the t test, and differences between proportions were tested for statistical significance with the chi-squared test or Fisher exact test. A P value of <0.05 was considered statistically significant.
Results
Patients Characteristics
In the ERA group, patient age ranged from 24 to 37 years, BMI was between 18.55 and 29.78 kg/m2, and endometrial thickness ranged from 0.5 to 1.5 cm on the day of embryo transfer. In the FET group, patient age ranged from 23 to 37 years, BMI was between 18.73 and 29.38 kg/m2, and endometrial thickness ranged from 0.5 to 1.7 cm on the day of embryo transfer. Patients in the ERA group experienced 5.79±0.97 attempts with assisted reproductive technology (ART) treatment and patients in the FET group underwent 6.10±0.83 attempts. There were no significant differences between the ERA and FET groups in terms of age, BMI, endometrial thickness on the day of embryo transfer, mean attempts of ART treatment, or anti-Mullerian hormone (AMH), follicle-stimulating hormone (FSH), or antral follicle count (AFC) in the fresh cycle (P>0.05) (Table 1).
Table 1.
Comparison of the baseline patient characteristics between the endometrial receptivity analysis (ERA) and conventional frozen embryo transfer (FET) groups.
| Characteristic | ERA group (n=140) | FET group (n=141) | P value |
|---|---|---|---|
| Age (years) | 32.01±2.99 | 31.87±3.21 | >0.05 |
| BMI (kg/m2) | 21.21±0.8 | 21.17±0.79 | >0.05 |
| AMH in the fresh cycle (ng/mL) | 4.26±0.71 | 5.12±1.98 | >0.05 |
| FSH in the fresh cycle (IU) | 7.40±1.69 | 7.00±1.67 | >0.05 |
| AFC in the fresh cycle | 16.35±8.19 | 18.25±9.29 | >0.05 |
| Endometrial thickness on the day of embryo transfer (cm) | 0.97±0.11 | 0.95±0.23 | >0.05 |
| Number of attempts with ART | 5.79±0.97 | 6.10±0.83 | >0.05 |
BMI – body mass index; AMH – anti-Mullerian hormone; FSH – follicle-stimulating hormone; AFC – antral follicle count; ART – assisted reproductive technology.
In addition, there were 60 patients who transferred day 6 embryos and 80 who transferred day 5 embryos in the ERA group, while 56 patients transferred day 6 embryos and 85 transferred day 5 embryos in the FET group. There were no significant differences detected in the clinical outcomes between patients who transferred day 5 embryos and patients who transferred day 6 embryos in the ERA group or in the FET group, except that a significantly higher biochemical miscarriage rate was observed in patients who transferred day 5 embryos than in those who transferred day 6 embryos in the FET group (Table 2).
Table 2.
Comparison of the clinical outcomes between transfer of day 5 and day 6 embryos in the endometrial receptivity analysis (ERA) and conventional frozen embryo transfer (FET) groups.
| Clinical outcome | ERA group (n=140) | FET group (n=141) | ||||
|---|---|---|---|---|---|---|
| Transfer of day-5 embryos | Transfer of day-6 embryos | P value | Transfer of day-5 embryos | Transfer of day-6 embryos | P value | |
| Biochemical pregnancy rate, n (%) | 56/89 (62.92%) | 32/51 (62.75%) | >0.05 | 36/88 (40.91%) | 19/53 (35.85%) | >0.05 |
| Clinical pregnancy rate, n (%) | 47/89 (52.81%) | 23/51 (45.1) | >0.05 | 19/88 (21.59%) | 16/53 (30.19%) | >0.05 |
| Implantation rate, n (%) | 64/141 (45.39% | 27/77 (35.06% | >0.05 | 23/139 (16.55%) | 19/84 (22.62) | >0.05 |
| Clinical miscarriage rate, n (%) | 4/56 (7.14%) | 5/32 (15.63%) | >0.05 | 6/36 (16.67%) | 3/19 (15.79%) | >0.05 |
| Biochemical miscarriage rate, n (%) | 9/56 (16.07%) | 9/32 (28.13%) | >0.05 | 16/36 (44.44%) | 2/19 (10.53%) | <0.05 |
| Ectopic pregnancy rate, n (%) | 0/56 (0) | 0/32 (0) | >0.05 | 0/36 (0) | 0/19 (0) | >0.05 |
| Embryos transferred per patient, N | 1.58±0.32 | 1.51±0.45 | >0.05 | 1.58±0.54 | 1.58±0.47 | >0.05 |
| High-quality embryos transferred per patient, N | 0.72±0.43 | 0.78±0.27 | >0.05 | 1.07±0.35 | 0.92±0.22 | >0.05 |
| Cumulative biochemical pregnancy rate, n (%) | 62/88 (70.45%) | 37/52 (71.15%) | >0.05 | 40/79 (50.63%) | 28/62 (45.16) | >0.05 |
| Cumulative clinical pregnancy rate, n (%) | 50/88 (56.82%) | 30/52 (57.69%) | >0.05 | 31/79 (39.24%) | 26/62 (41.94%) | >0.05 |
| Cumulative implantation rate, n (%) | 67/141 (47.52%) | 35/76 (46.05%) | >0.05 | 39/129 (30.23%) | 29/99 (29.29%) | >0.05 |
| Cumulative clinical miscarriage rate, n (%) | 4/62 (6.45%) | 6/37 (16.22%) | >0.05 | 4/40 (10%) | 4/28 (14.29%) | >0.05 |
| Cumulative biochemical miscarriage rate, n (%) | 11/62 (17.74%) | 7/37 (18.92%) | >0.05 | 8/40 (20%) | 1/28 (3.57%) | >0.05 |
| Cumulative ectopic pregnancy rate, n (%) | 0/62 (0) | 0/37 (0) | >0.05 | 0/40 (0) | 0/28 (0) | >0.05 |
| Cumulative embryos transferred per patient, N | 1.6±0.39 | 1.46±0.41 | >0.05 | 1.53±0.41 | 1.58±0.39 | >0.05 |
| Cumulative high-quality embryos transferred per patient, N | 0.77±0.29 | 0.71±0.3 | >0.05 | 0.940.11± | 0.89±0.21 | >0.05 |
ERA Results and Follow-up Outcomes
The ERA assessment presented a receptive result in 49 of the 140 patients tested (35%), and most of the nonreceptive results were pre-receptive (Figure 1). At least 1 high-quality embryo was transferred in the patients with a receptive endometrium, and the clinical follow-up showed a 48.98% clinical pregnancy rate and a 44.16% implantation rate in those patients. Among the 91 patients with a nonreceptive endometrium, the clinical follow-up showed a 50.55% clinical pregnancy rate and a 40.43% implantation rate. No ectopic pregnancies were found in patients undergoing ERA. There were no significant differences between receptive and nonreceptive patients in terms of pregnancy, implantation, or miscarriage rates (P>0.05) (Table 3).
Figure 1.
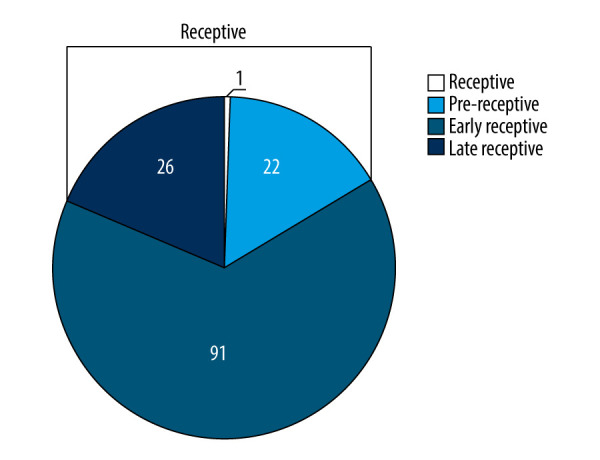
Endometrial receptivity analysis (ERA) assessment of endometrial receptivity in 140 Chinese women with recurrent implantation failure. Pre-receptive profile, P+6; early receptive, P+5.5; late receptive profile, P+4.5; P – progesterone. The figure was created using Microsoft Excel 2010.
Table 3.
Comparison of the clinical outcomes between the receptive and nonreceptive patients.
| Clinical outcome | Receptive subjects (n=49) | Non-receptive subjects (n=91) | P value |
|---|---|---|---|
| Biochemical pregnancy rate, n (%) | 29/49 (59.18%) | 59/91 (64.84%) | >0.05 |
| Clinical pregnancy rate, n (%) | 24/49 (48.98%) | 46/91 (50.55%) | >0.05 |
| Implantation rate, n (%) | 34/77 (44.16%) | 57/141 (40.43%) | >0.05 |
| Clinical miscarriage rate, n (%) | 3/29 (10.34%) | 6/59 (10.19%) | >0.05 |
| Biochemical miscarriage rate, n (%) | 5/29 (17.24%) | 13/59 (22.03%) | >0.05 |
| Ectopic pregnancy rate, n (%) | 0/29 (0.00%) | 0/59 (0.00%) | – |
| Cumulative biochemical pregnancy rate, n (%) | 35/49 (71.43%) | 64/91 (70.33%) | >0.05 |
| Cumulative clinical pregnancy rate, n (%) | 30/49 (61.22%) | 50/91 (54.95%) | >0.05 |
| Cumulative implantation rate, n (%) | 42/93 (45.16%) | 61/150 (40.67%) | >0.05 |
| Cumulative clinical miscarriage rate, n (%) | 2/35 (5.71%) | 8/64 (12.50%) | >0.05 |
| Cumulative biochemical miscarriage rate, n (%) | 4/35 (11.43%) | 14/64 (21.88%) | >0.05 |
| Cumulative ectopic pregnancy rate, n (%) | 0/35 (0.00%) | 0/64 (0.00%) | – |
There were no significant differences between the ERA and FET groups in terms of the number of embryos or high-quality embryos transferred per patient (P>0.05). We observed a higher pregnancy and implantation rate in the ERA group than in the FET group (P<0.01); however, no significant differences were found between the 2 groups in terms of miscarriage rate (P>0.05). In addition, no ectopic pregnancies occurred in either group (Table 4).
Table 4.
Comparison of the clinical outcomes between the endometrial receptivity analysis (ERA) and conventional frozen embryo transfer (FET) groups.
| Clinical outcome | ERA group (n=140) | FET group (n=141) | P value |
|---|---|---|---|
| Embryos transferred per patient, N | 1.56±0.50 | 1.58±0.50 | >0.05 |
| High-quality embryos transferred per patient, N | 0.74±0.75 | 1.01±0.72 | >0.05 |
| Biochemical pregnancy rate, n (%) | 88/140 (62.86%) | 55/141 (39.01%) | <0.01 |
| Clinical pregnancy rate, n (%) | 70/140 (50.00%) | 35/141 (24.82%) | <0.01 |
| Implantation rate, n (%) | 91/218 (41.7%) | 42/223 (18.83%) | <0.01 |
| Ectopic pregnancy rate, n (%) | 0/88 (0.00%) | 0/55 (0.00%) | – |
| Biochemical miscarriage rate, n (%) | 18/88 (20.45%) | 18/55 (32.73%) | >0.05 |
| Clinical miscarriage rate, n (%) | 9/88 (10.23) | 9/55 (16.36%) | >0.05 |
| Cumulative transfers per patient, N | 1.13±0.31 | 1.42±0.70 | >0.05 |
| Cumulative embryos transferred per patient, N | 1.74±0.50 | 2.31±0.49 | >0.05 |
| Cumulative high-quality embryos transferred per patient, N | 0.87±0.76 | 1.37±0.73 | >0.05 |
| Cumulative biochemical pregnancy rate, n (%) | 99/140 (70.71%) | 68/141 (48.23%) | <0.01 |
| Cumulative clinical pregnancy rate, n (%) | 80/140 (57.14%) | 57/141 (40.43%) | <0.01 |
| Cumulative implantation rate, n (%) | 102/217 (42.21%) | 68/228 (29.82%) | <0.01 |
| Cumulative ectopic pregnancy rate, n (%) | 0/99 (0.00%) | 0/68 (0.00%) | – |
| Cumulative biochemical miscarriage rate, n (%) | 18/99 (18.18%) | 31/68 (30.88%) | <0.01 |
| Cumulative clinical miscarriage rate, n (%) | 10/99 (10.10%) | 11/68 (16.18%) | >0.05 |
Then, we compared the clinical outcomes in patients in the ERA and FET groups who transferred a single embryo, and no significant differences were detected, except that a higher biochemical pregnant rate was seen in the ERA group than in the FET group (Table 5).
Table 5.
Comparison of the clinical outcomes in patients in the endometrial receptivity analysis (ERA) and conventional frozen embryo transfer (FET) groups who transferred a single embryo.
| Clinical outcome | ERA group (n=140) | FET group (n=141) | P value |
|---|---|---|---|
| High-quality embryos transferred per patient, N | 0.35±0.09 | 0.73±0.14 | >0.05 |
| Biochemical pregnancy rate, n (%) | 32/62 (51.61%) | 20/59 (33.9%) | <0.05 |
| Clinical pregnancy rate, n (%) | 22/62 (35.48%) | 14/59 (23.73%) | >0.05 |
| Implantation rate, n (%) | 22/62 (35.48%) | 14/59 (23.73%) | >0.05 |
| Ectopic pregnancy rate, n (%) | 0/32 (0) | 0/20 (0) | – |
| Biochemical miscarriage rate, n (%) | 10/32 (31.25%) | 6/20 (30%) | >0.05 |
| Clinical miscarriage rate, n (%) | 3/32 (9.38) | 7/20 (35%) | >0.05 |
| Cumulative high-quality embryos transferred per patient, N | 0.44±0.11 | 0.39±0.14 | >0.05 |
| Cumulative biochemical pregnancy rate, n (%) | 38/63 (60.32%) | 25/54 (46.3%) | >0.05 |
| Cumulative clinical pregnancy rate, n (%) | 28/63 (57.14%) | 21/54 (38.89%) | >0.05 |
| Cumulative implantation rate, n (%) | 28/63 (57.14%) | 21/54 (38.89%) | >0.05 |
| Cumulative ectopic pregnancy rate, n (%) | 0/38 (0) | 0/25 (0) | – |
| Cumulative biochemical miscarriage rate, n (%) | 10/38 (26.32%) | 4/25 (16%) | >0.05 |
| Cumulative clinical miscarriage rate, n (%) | 5/38 (13.16%) | 6/25 (24%) | >0.05 |
Discussion
In this study, our findings showed higher pregnancy and implantation rates in Chinese women with RIF who underwent ERA than in those receiving FET (P<0.01). To the best of our knowledge, this is the first report to investigate and clarify the effectiveness of ERA for pET in a Chinese population with RIF.
Previous studies have demonstrated that embryo- and endometrium-related factors are primary causes of RIF [8–10]. A receptive endometrium is a prerequisite for the successful implantation of embryos [13], and the endometrium is found to be most receptive during the WOI [25]. If the WOI is accurately predicted and pET is performed during the WOI, the implantation rates of transferred embryos may be improved and successful pregnancies can be achieved.
There have been numerous attempts to predict the WOI through the assessment of endometrial receptivity. Ultrasound parameters, such as endometrial thickness, volume, pattern, and vascularization, have been employed to evaluate endometrial receptivity; however, none of these parameters alone are effective to predict the WOI [26–29]. The endometrial pinopode has been proposed as a potential clinical marker to assess endometrial receptivity [30]; nevertheless, pinopodes are detectable throughout the luteal phase of the menstrual cycle [31], making them inappropriate to determine the timeframe of endometrial receptivity (WOI) [32]. Detection of serum molecules (integrin, leukemia inhibitory factor, estrogen, progesterone and their receptors, calcitonin, matrix metalloproteinase) has also been employed as an attempt to correctly identify the endometrial receptive status; however, its poor ability to predict clinical pregnancy restricts further applications of this methodology in clinical practices [33]. Additionally, even though several genes that are involved in endometrial receptivity have been identified, their diagnostic performance and value for clinical practice still remain to be elucidated [34].
Recently, a genomic tool, which consists of a customized ERA and a bioinformatic predictor for endometrial dating, was developed to evaluate endometrial receptivity through the analysis of the transcriptomic profiles of 248 selected genes. This tool has shown a global accuracy of 0.88, sensitivity of 0.90, and specificity of 0.97 [35]. Results from a comparative prospective study showed that the concordance of histological dating and ERA related to LH, used as reference, was 0.922 (range of 0.815 to 1.0), the interobserver variability between pathologists assessed by the Kappa index was 0.622 (range of 0.435 to 0.839), and the reproducibility of the ERA test was 100% consistent [17]. In a Japanese study, the pregnancy rate was 35.3% in patients with a receptive endometrium and 50% in patients with a nonreceptive endometrium after the first personalized embryo transfer was guided by the ERA test [19]. In Canada, ERA-guided pET resulted in implantation and ongoing pregnancy rates that were higher than those obtained without pET (73.7% vs 54.2% and 63.2% vs 41.7%, respectively) [13]. Results from a prospective interventional multicenter clinical trial revealed that ERA-guided pET resulted in a 51.7% pregnancy rate and a 33.9% implantation rate in RIF patients with a receptive endometrium, and a 50% pregnancy rate and a 38.5% implantation rate in those with a nonreceptive endometrium [20]. In addition, a recent 5-year multicenter open-label randomized controlled trial showed that pET guided by ERA achieved higher cumulative pregnancy rates (93.6%, 79.7%, and 80.7%, P<0.01), higher live birth rates at the first embryo transfer (56.2%, 42.4%, and 45.7%, P>0.05), higher cumulative live birth rates after 12 months (71.2%, 55.4%, and 48.9%, P<0.05), higher pregnancy rates at the first embryo transfer (72.5%, 54.3%, and 58.5%, P<0.05), and higher implantation rates at the first embryo transfer (57.3%, 43.2%, and 38.6%, P<0.05) than FET and fresh embryo transfer in infertile patients undergoing IVF, while comparable obstetrical outcomes, types of delivery, and neonatal outcomes were seen among these 3 groups [21]. A recent study demonstrated that, among all patients undergoing ERA, significantly higher implantation rates (87.5% vs 56%, P=0.02) and ongoing pregnancy rates (75% vs 44%, P=0.03) were observed in the non-RIF group than in the RIF group [36]. However, the effectiveness of ERA for diagnosis of endometrial receptivity and its value in pET remain to be investigated in ethnically Chinese patients with RIF.
In the present study, 281 patients with RIF were enrolled and assigned to the ERA or FET groups, and the baseline demographics and clinical characteristics were comparable between the 2 groups (P>0.05), including age, BMI, AMH in the fresh cycle, FSH in the fresh cycle, AFC in the fresh cycle, endometrial thickness on the day of embryo transfer, and number of attempts of ART treatment. In this study, ERA identified 35% of receptive results in the investigated patients, which was a lower percentage than that described in previous reports. Among 85 patients with RIF recruited from Spanish university-affiliated infertility and private clinics, ERA came back receptive for 74.1% of the patients [20]. Among 55 patients with a history of RIF recruited from 2 Japanese IVF centers, ERA diagnosed 76% of patients as receptive [19]. Results from a retrospective observational study involving 248 patients with unexplained RIF at an Indian tertiary infertility clinic showed that ERA diagnosed receptive endometria in 82.3% (204/248) of patients [37]. A recent retrospective review of 97 patients with a history of implantation failure showed that ERA diagnosed 48.5% of patients with a receptive endometrium [38]. The relatively lower rate of receptive endometria in the present study may be attributed to the patients’ individual clinical histories. Among the 140 patients who underwent ERA, there was a high prevalence of diagnosed fallopian tube diseases, ovarian disorders, and endometriosis, and these disorders of the female reproductive system can result in the detection of a nonreceptive endometrium [39,40]. In a previous retrospective observational study that recruited 248 Indian patients with unexplained RIF, vaginal micronized progesterone 400 mg twice a day was added if the serum progesterone level was <0.5 ng/mL, and the ERA test gave an 82.3% receptive endometria and 17.7% nonreceptive status, including a 61.4% pre-receptive and 38.6% post-receptive status [37]. Following transvaginal administration of micronized progesterone 200 mg 3 times daily, initial ERA results showed 48.5% receptive, 47.4% nonreceptive, and 2.01% insufficient endometria [41]. During the period from January 2016 to September 2018, 62 patients were administered intramuscularly 75 mg progesterone in oil once daily and 44 were administered 400 mg vaginal progesterone twice daily for approximately 5 full days for an HRT mock cycle, and the ERA test gave a combined receptivity rate of 71.6% (76/106), with 71.0% (44/62) receptive results in the intramuscular administration group and 72.7% (32/44) in the vaginal progesterone group; in addition, 27.4% (17/62) of patients in the intramuscular administration group were found to be pre-receptive and 1.6% (1/62) post-receptive, and 27.3% (12/44) of patients received a pre-receptive result in the vaginal progesterone group and no patients had a post-receptive result [41]. In that study, all patients were administered per vagina 300 mg utrogestan twice daily and orally administered 20 mg dydrogesterone twice a day in the HRT cycle. The variation in the receptive status may be attributed to various modes of HRT cycles [41].
In the present study, we observed no significant differences between patients with receptive and nonreceptive endometria diagnosed by ERA in terms of pregnancy, implantation, or miscarriages (P>0.05), which agreed with previous reports [37,38]. Patel et al reported comparable pregnancy rates (42.3% vs 31.4%, P=0.53), clinical pregnancy rates (55.4% vs 37.5%, P=0.35), implantation rates (44% vs 31%, P=0.3), abortion rates (18.6% vs 20%, P=0.93), ongoing pregnancy rates (48.5% vs 33.3%, P=0.39), and cumulative pregnancy rates (75.2% vs 58.3%, P=0.48) between RIF patients with a receptive ERA result who had a routine embryo transfer and those with a nonreceptive ERA result who underwent pET [37]. Cohen et al found that the clinical pregnancy rate was 26.7% in patients diagnosed with a receptive endometrium by ERA and 22.5% in nonreceptive patients after pET (P=0.66) [38]. In addition, results from a Japanese retrospective 2-center study showed comparable cumulative pregnancy rates (63.2% vs 66.7%, P=1.0), pregnancy rates (36.8% vs 66.7%, P=0.54), implantation rates (33.3% vs 75%, P=0.14), and miscarriage rates (25% vs 0, P=1.0) between receptive and nonreceptive groups diagnosed by ERA [19].
Previous studies have shown improvements in pregnancy and implantation rates following ERA-guided pET [21,36]. A recent multicenter open-label randomized controlled trial reported significantly higher pregnancy rates at the first embryo transfer (72.5% vs 54.3%, P=0.01), cumulative pregnancy rates (93.6% vs 79.7%, P=0.0005), cumulative live birth rates after 12 months (71.2% vs 55.4%, P=0.04), and implantation rates at first embryo transfer (57.3% vs 43.2%, P=0.03) in the group who underwent ERA than in the group who did not have the analysis [21]. A retrospective review of Canadian patients between 2014 and 2017 showed higher implantation (73.7% vs 54.2%) and ongoing pregnancy rates (63.2% vs 41.7%) in the ERA group than in the non-ERA group [36]. Similarly, our findings showed higher pregnancy and implantation rates in the ERA group than in the FET group (P<0.01). We observed comparable pregnancy outcomes among patients between the ERA and FET groups who transferred a single embryo, which may be attributed to the relatively small sample size. Recently, results from a prospective cohort study reported no significant differences between ERA and non-ERA groups in terms of live birth rate (56.5% vs 55.6%, P=0.89), clinical pregnancy rate (67.4% vs 65.4%, P=0.77), biochemical pregnancy rate (15.4% vs 14.8%, P=0.91), and miscarriage rate (15.2% vs 13.2%, P=0.75), suggesting that routine ERA does not improve pregnancy outcomes among patients undergoing their first autologous single euploid programmed embryo transfer [42]. Additionally, another recent study reported no evidence of clinical benefits from pET guided by ERA in patients with a history of implantation failure [38]. Since there is a dispute regarding the efficacy of ERA for pET among patients with RIF, further multicenter large-scale prospective randomized clinical trials are needed to validate the current evidence of its effectiveness.
The present study has potential limitations. First, this was a retrospective analysis, which introduces relatively more bias than prospective clinical trials. Second, the study sample was relatively small.
Conclusions
In summary, in this study of Chinese women with RIF undergoing IVF and embryo transfer, ERA-guided pET resulted in a significant improvement in pregnancy and implantation rates when compared with FET. As a novel diagnostic tool, ERA proved to be effective in guiding pET and improving the success rates of pregnancy in patients with RIF undergoing IVF and embryo transfer.
Footnotes
Conflict of interest: None declared
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors, who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: This study was supported by grants from the Sichuan Provincial Department of Science & Technology (grant no. 2019YFS0413), Sichuan Provincial Health of Commission (grant no. 19PJ185), and Chengdu Municipal Health Commission (grant no 2020184)
References
- 1.Inhorn MC, Patrizio P. Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 2015;21:411–26. doi: 10.1093/humupd/dmv016. [DOI] [PubMed] [Google Scholar]
- 2.Vander BM, Wyns C. Fertility and infertility: Definition and epidemiology. Clin Biochem. 2018;62:2–10. doi: 10.1016/j.clinbiochem.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 3.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun H, Gong TT, Jiang YT, et al. Global, regional, and national prevalence, and disability-adjusted life-years for infertility in 195 countries and territories, 1990–2017: Results from a global burden of disease study, 2017. Aging (Albany NY) 2019;11:10952–91. doi: 10.18632/aging.102497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Z, Zheng D, Wu H, et al. Epidemiology of infertility in China: A population-based study. BJOG. 2018;125:432–41. doi: 10.1111/1471-0528.14966. [DOI] [PubMed] [Google Scholar]
- 6.Tamrakar SR, Bastakoti R. Determinants of infertility in couples. J Nepal Health Res Counc. 2019;17:85–89. doi: 10.33314/jnhrc.1827. [DOI] [PubMed] [Google Scholar]
- 7.Coughlan C, Ledger W, Wang Q, et al. Recurrent implantation failure: Definition and management. Reprod Biomed Online. 2014;28:14–38. doi: 10.1016/j.rbmo.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Bashiri A, Halper KI, Orvieto R. Recurrent implantation failure-update overview on etiology, diagnosis, treatment, and future directions. Reprod Biol Endocrinol. 2018;16:121. doi: 10.1186/s12958-018-0414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laufer N, Simon A. Recurrent implantation failure: Current update and clinical approach to an ongoing challenge. Fertil Steril. 2012;97:1019–20. doi: 10.1016/j.fertnstert.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 10.Timeva T, Shterev A, Kyurkchiev S. Recurrent implantation failure: The role of the endometrium. J Reprod Infertil. 2014;15:173–83. [PMC free article] [PubMed] [Google Scholar]
- 11.Bellver J, Simón C. Implantation failure of endometrial origin: What is new? Curr Opin Obstet Gynecol. 2018;30:229–36. doi: 10.1097/GCO.0000000000000468. [DOI] [PubMed] [Google Scholar]
- 12.Kliman HJ, Frankfurter D. Clinical approach to recurrent implantation failure: Evidence-based evaluation of the endometrium. Fertil Steril. 2019;111:618–28. doi: 10.1016/j.fertnstert.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Teh WT, McBain J, Rogers P. What is the contribution of embryo-endometrial asynchrony to implantation failure? J Assist Reprod Genet. 2016;33:1419–30. doi: 10.1007/s10815-016-0773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Díaz-Gimeno P, Ruiz-Alonso M, Sebastian-Leon P, et al. Window of implantation transcriptomic stratification reveals different endometrial subsignatures associated with live birth and biochemical pregnancy. Fertil Steril. 2017;108:703–10e3. doi: 10.1016/j.fertnstert.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Lessey BA. Endometrial receptivity and the window of implantation. Best Pract Res Clin Obstet Gynaecol. 2000;14:775–88. doi: 10.1053/beog.2000.0118. [DOI] [PubMed] [Google Scholar]
- 16.Devyatova EA, Tsaturova KA, Vartanyan EV. Predicting of successful implantation at IVF cycles. Gynecol Endocrinol. 2016;32:27–29. doi: 10.1080/09513590.2016.1232803. [DOI] [PubMed] [Google Scholar]
- 17.Díaz-Gimeno P, Horcajadas JA, Martínez-Conejero JA, et al. A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil Steril. 2011;95:50–60. 60.e1–15. doi: 10.1016/j.fertnstert.2010.04.063. [DOI] [PubMed] [Google Scholar]
- 18.Díaz-Gimeno P, Ruiz-Alonso M, Blesa D, et al. The accuracy and reproducibility of the endometrial receptivity array is superior to histology as a diagnostic method for endometrial receptivity. Fertil Steril. 2013;99:508–17. doi: 10.1016/j.fertnstert.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto T, Koizumi M, Doshida M, et al. Efficacy of the endometrial receptivity array for repeated implantation failure in Japan: A retrospective, two-centers study. Reprod Med Biol. 2017;16:290–96. doi: 10.1002/rmb2.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruiz-Alonso M, Blesa D, Díaz-Gimeno P, et al. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril. 2013;100:818–24. doi: 10.1016/j.fertnstert.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Simón C, Gómez C, Cabanillas S, et al. A 5-year multicentre randomized controlled trial comparing personalized, frozen and fresh blastocyst transfer in IVF. Reprod Biomed Online. 2020;41:402–15. doi: 10.1016/j.rbmo.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Ruiz-Alonso M, Valbuena D, Gomez C, et al. Endometrial receptivity analysis (ERA): Data versus opinions. Hum Reprod Open. 2021;2021:hoab011. doi: 10.1093/hropen/hoab011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia Y, Sha YL, Qiu Z, et al. Endometrial receptivity analysis for personalized embryo transfer in patients with recurrent implantation failure: A retrospective analysis of a Chinese cohort. Human Reprod. 2021;36:deab130.312. [Google Scholar]
- 24.Gardner DK, Lane M, Stevens J, et al. Blastocyst score affects implantation and pregnancy outcome: Towards a single blastocyst transfer. Fertil Steril. 2000;73:1155–58. doi: 10.1016/s0015-0282(00)00518-5. [DOI] [PubMed] [Google Scholar]
- 25.Rincon A, Clemente-Ciscar M, Gomez E, et al. What is the real length of the window of implantation (WOI) in humans? Human Reproduction. England: Oxford Univ Press. 2018;33:360. [Google Scholar]
- 26.Järvelä IY, Sladkevicius P, Kelly S, et al. Evaluation of endometrial receptivity during in-vitro fertilization using three-dimensional power Doppler ultrasound. Ultrasound Obstet Gynecol. 2005;26:765–69. doi: 10.1002/uog.2628. [DOI] [PubMed] [Google Scholar]
- 27.Kupesic S, Bekavac I, Bjelos D, et al. Assessment of endometrial receptivity by transvaginal color Doppler and three-dimensional power Doppler ultrasonography in patients undergoing in vitro fertilization procedures. J Ultrasound Med. 2001;20:125–34. doi: 10.7863/jum.2001.20.2.125. [DOI] [PubMed] [Google Scholar]
- 28.Ng EH, Chan CC, Tang OS, et al. The role of endometrial and subendometrial vascularity measured by three-dimensional power Doppler ultrasound in the prediction of pregnancy during frozen-thawed embryo transfer cycles. Hum Reprod. 2006;21:1612–17. doi: 10.1093/humrep/dei502. [DOI] [PubMed] [Google Scholar]
- 29.Mercé LT, Barco MJ, Bau S, et al. Are endometrial parameters by three-dimensional ultrasound and power Doppler angiography related to in vitro fertilization/embryo transfer outcome? Fertil Steril. 2008;89:111–17. doi: 10.1016/j.fertnstert.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 30.Nikas G. Endometrial receptivity: Changes in cell-surface morphology. Semin Reprod Med. 2000;18:229–35. doi: 10.1055/s-2000-12561. [DOI] [PubMed] [Google Scholar]
- 31.Quinn C, Ryan E, Claessens EA, et al. The presence of pinopodes in the human endometrium does not delineate the implantation window. Fertil Steril. 2007;87:1015–21. doi: 10.1016/j.fertnstert.2006.08.101. [DOI] [PubMed] [Google Scholar]
- 32.Quinn CE, Casper RF. Pinopodes: A questionable role in endometrial receptivity. Hum Reprod Update. 2009;15:229–36. doi: 10.1093/humupd/dmn052. [DOI] [PubMed] [Google Scholar]
- 33.Craciunas L, Gallos I, Chu J, Coomarasamy A. Conventional and modern markers of endometrial receptivity: A systematic review and meta-analysis. Hum Reprod Update. 2019;25:202–23. doi: 10.1093/humupd/dmy044. [DOI] [PubMed] [Google Scholar]
- 34.Altmäe S, Koel M, Võsa U, et al. Meta-signature of human endometrial receptivity: A meta-analysis and validation study of transcriptomic biomarkers. Sci Rep. 2017;7:10077. doi: 10.1038/s41598-017-10098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clemente-Ciscar M, Ruiz-Alonso M, Blesa D, et al. Endometrial receptivity analysis (ERA) using a next generation sequencing (NGS) predictor improves reproductive outcome in recurrent implantation failure (RIF) patients when compared to ERA arrays. Hum Reprod. 2018;33:8. [Google Scholar]
- 36.Tan J, Kan A, Hitkari J, et al. The role of the endometrial receptivity array (ERA) in patients who have failed euploid embryo transfers. J Assist Reprod Genet. 2018;35:683–92. doi: 10.1007/s10815-017-1112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel JA, Patel AJ, Banker JM, et al. Personalized embryo transfer helps in improving in vitro fertilization/ICSI outcomes in patients with recurrent implantation failure. J Hum Reprod Sci. 2019;12:59–66. doi: 10.4103/jhrs.JHRS_74_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen AM, Ye XY, Colgan TJ, et al. Comparing endometrial receptivity array to histologic dating of the endometrium in women with a history of implantation failure. Syst Biol Reprod Med. 2020;66:347–54. doi: 10.1080/19396368.2020.1824032. [DOI] [PubMed] [Google Scholar]
- 39.Li HH, Liu S, Li Y. Research progress of factors affecting the endometrial receptivity. Reprod Controcep. 2016;36:833–38. [Google Scholar]
- 40.Kuroda K, Horikawa T, Moriyama A, et al. Impact of chronic endometritis on endometrial receptivity analysis results and pregnancy outcomes. Immun Inflamm Dis. 2020;8:650–58. doi: 10.1002/iid3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stankewicz T, Adaniya G, Cinnioglu C, et al. Intramuscular versus vaginal progesterone: Can we expect differences in endometrial receptivity? Fertil Steril. 2019;111:E28. [Google Scholar]
- 42.Riestenberg C, Kroener L, Quinn M, et al. Routine endometrial receptivity array in first embryo transfer cycles does not improve live birth rate. Fertil Steril. 2021;115:1001–6. doi: 10.1016/j.fertnstert.2020.09.140. [DOI] [PubMed] [Google Scholar]


