Abstract
Background
Influenza is a communicable acute respiratory infection which, during epidemics, can cause high morbidity and mortality rates. Traditional Chinese medicinal herbs, often administered following a particular Chinese medical theory, may be a potential treatment of choice.
Objectives
To assess the effect of Chinese medicinal herbs used to prevent and treat influenza and to estimate the frequency of adverse effects.
Search methods
We searched CENTRAL (2012, Issue 11), MEDLINE (January 1966 to November week 2, 2012), EMBASE (January 1988 to November 2012) and CNKI (January 1988 to 29 March 2012). We also searched reference lists of articles and the WHO ICTRP search portal (November 2012).
Selection criteria
Randomised controlled trials (RCTs) comparing traditional Chinese medicinal herbs with placebo, no treatment or conventional medicine normally used in preventing and treating uncomplicated influenza.
Data collection and analysis
Two review authors independently extracted data and assessed trial quality.
Main results
We included 18 studies involving 2521 participants. The methodological quality of 17 included studies was poor. Included RCTs separately compared medicinal herbs with different antiviral drugs, precluding any pooling of results. Only three indicated that compared with antiviral drugs, Chinese medicinal herbs may be effective in preventing influenza and alleviating influenza symptoms. 'Ganmao' capsules were found to be more effective than amantadine in decreasing influenza symptoms and speeding recovery in one study (in which adverse reactions were mentioned in the amantadine group although no data were reported). There were no significant differences between 'E Shu You' and ribavirin in treating influenza, nor in the occurrence of adverse reactions. Ten studies reported mild adverse reactions.
Authors' conclusions
Most Chinese medical herbs in the included studies showed similar effects to antiviral drugs in preventing or treating influenza. Few were shown to be superior to antiviral drugs. No obvious adverse events were reported in the included studies. However, current evidence remains weak due to methodological limitations of the trials. More high‐quality RCTs with larger numbers of participants and clear reporting are needed.
Plain language summary
Chinese medicinal herbs for influenza
Influenza is a viral respiratory infection that causes an acute febrile illness with myalgia, headache and cough, and can result in high morbidity and mortality rates during an epidemic. Annual epidemics are thought to result in between three and five million cases of severe influenza and between 250,000 and 500,000 deaths worldwide. Currently, annual vaccination is the primary strategy for preventing influenza, and four influenza antiviral agents (amantadine, rimantadine, zanamivir and oseltamivir) have been approved for treatment of influenza. However, high levels of drug resistance have been recorded. Many Chinese medicinal herbs are used to treat and prevent this condition.
This updated review assessed the therapeutic effects and safety of Chinese medicinal herbs as an alternative and adjunctive therapy to other commonly used drugs for influenza. Eighteen studies involving 2521 participants were included in the review. 'Ganmao' capsules were found to be more effective than amantadine in decreasing influenza symptoms and aiding recovery in one study (in which adverse reactions were mentioned in the amantadine group although no data were reported). There were no significant differences between 'E Shu You' and ribavirin in treating influenza, nor in the occurrence of adverse reactions. The remaining 17 Chinese herbal trials showed a similar effect to antiviral drugs in preventing or treating influenza. However, since these included studies were of poor quality, the evidence does not support or reject the use of any Chinese herbal preparations for influenza. High‐quality trials are required.
Background
Description of the condition
Influenza is an acute respiratory illness caused by a virus from the Orthomyxoviridae family, of which three serotypes are known (A, B and C). Influenza causes an acute febrile illness with myalgia, headache and cough. Uncomplicated influenza generally resolves over a two to five‐day period. However, in a significant minority, symptoms of weakness and malaise may persist for several weeks, particularly in the elderly. Complications of influenza include otitis media, pneumonia, exacerbation of chronic respiratory disease, croup and bronchiolitis. Additionally, influenza can cause a range of non‐respiratory complications including febrile convulsions, Reyes's syndrome and myocarditis (Wiselka 1994). The influenza virus is transmitted primarily via virus‐laden large droplets from sneezing, coughing or talking. Transmission may also occur by direct (for example, person‐to‐person) or indirect (person‐to‐fomite‐to person) contact (CDC 2007).
Influenza virus types (A, B or C) are based on antigenic characteristics of the nucleoproteins and matrix protein antigens. However, the influenza virus genome is segmented and there is a high frequency of re‐arrangements of the genes (Ahmed 1996; Alves Galvão 2012). A major factor in determining the severity and spread of influenza outbreaks is the level of immunity present in the population at risk. When an antigenically new influenza virus emerges in a community where few or no antibodies are present, extensive outbreaks may occur (Claas 1998; Fleming 1999). Annual epidemics are thought to result in between three and five million cases of severe influenza and between 250,000 and 500,000 deaths worldwide (WHO 2003a). The outbreak in humans of an H5N1 avian influenza virus in Hong Kong in 1997 has increased awareness of our vulnerability to a global pandemic. Since late 2003 the accelerated geographical spread of influenza A (H5N1) among birds has heightened concerns. Up until December 2012, 610 confirmed cases of human infection with influenza A (H5N1) and 360 death in 15 countries has been reported to the World Health Organization (WHO) (WHO 2012).
Description of the intervention
Annual vaccination is recommended as the primary strategy for preventing influenza (WHO 2003b). Over‐the‐counter (OTC) medications for controlling influenza symptoms may be recommended and antiviral medications can be prescribed. Four influenza antiviral agents (amantadine, rimantadine, zanamivir and oseltamivir) have been approved by the US Food and Drug Administration (FDA). Amantadine and rimantadine are effective against influenza A viruses (Jefferson 2012). However, high levels of drug resistance have been recorded and the Advisory Committee on Immunization Practices (ACIP) recommends that neither amantadine nor rimantadine be used for the treatment or chemoprophylaxis of influenza A in the USA until susceptibility to these antiviral medications has been re‐established. Zanamivir and oseltamivir are neuraminidase inhibitors effective against both influenza A and B viruses. Oseltamivir is approved for the treatment of people aged over one year and zanamivir for people aged over seven years. These medications should be taken within two days after the onset of symptoms and continued for five to seven days. They have been shown to lessen both the severity and duration of uncomplicated influenza (Smith 2006). Careful use of these products is encouraged because of the emergence of resistant influenza strains (Moscona 2005). Dosing and side effects vary depending on the drug, age, and hepatic and renal functions. The major side effects tend to affect the central nervous system (CNS) and the gastrointestinal tract. Other side effects include light‐headedness, nervousness, anxiety, difficulty in concentration, diarrhea and anorexia. Use of amantadine in people aged > 65 years, among whom some degree of renal impairment is common, particular attention should be paid to dosages (NACI 2006).
Traditional Chinese medicine (TCM) follows a particular theoretical and methodological pathway for assessing cause, diagnosis and treatment. Chinese medicinal herbs, the most important component of TCM, are derived from plants and usually incorporate one or more herbs as the basic drug(s) to treat the disease. Depending upon the different symptoms or causes, the herbs are selected and mixed together, following a particular process, to form the prescription.
How the intervention might work
In TCM the aim in treating influenza is not only to cure the respiratory symptoms but also to treat the whole body. In TCM, influenza is differentiated into two types: Wind‐cold Syndrome and Wind‐heat Syndrome (Table 1). The main symptoms of the Wind‐cold type are: severe cold, slight fever, absence of sweat, headache, aching pain of extremities, stuffy nose with nasal discharge, cough with thin sputum, thin, whitish coating on the tongue and a floating and tight pulse (Zhao 2001). Treatment of this type aims to relieve external symptoms with drugs which are pungent in flavour and warm in property, and to ventilate the lungs and expel the pathogenic cold. Herba Schizonepetae, Radix Ledebouriellae, Radix Bupleuri, Radix Platycodi and Rhizoma Zingiberis Recens are usually the main components of a prescription for Wind‐cold Syndrome. Moreover, supplementary drugs may be added when particular symptoms are present (Wang 2012).
1. TCM definitions.
| TCM term | Definition |
| Qi | In the theory of TCM, 'qi' is considered as a life force or energy in every body. 'Qi' must be kept balanced and flow freely to keep organs working well. When 'qi' is blocked in a certain part of the body, the organs involved get sick and people can have a pain there. For example, constrained 'gan qi' should be released to make 'qi' flow freely so that the liver can work well and 'qi' should be regulated to flow freely so that pain is relieved. Similarly, when the 'qi' of the lungs is not balanced, such as by being lost ascending out, people may cough; 'qi' must therefore be put down to maintain an adequate amount of 'qi' in the lungs |
| Wind‐cold type cold | If manifested by more severe chilliness, slight fever and a tongue with thin, white fur, then it belongs to the exterior syndrome caused by wind and cold and should be treated with strong perspiration drugs which are pungent in taste and warm in property, to dispel the wind and cold |
| Wind‐heat type cold | If manifested by more severe fever, milder chilliness and a tongue with thin, yellow fur, then it belongs to the exterior syndrome caused by wind and heat |
The main symptoms of Wind‐heat type are: a high fever, slight aversion to cold, headache, sore throat with congestion, expectoration of yellowish sputum, thirst, epistaxis, reddened tongue with a thin, yellowish coating and a floating and rapid pulse (Zhao 2001). Treatment of this type aims to: relieve external symptoms with drugs which are pungent in flavour and cool in property, and to promote the dispersing function of the lungs and clear up pathogenic heat. Flos Lonicerae, Fructus Forsythiae, Radix Isatidis, Radix Puerariae, Folium Mori, Flos Chrysanthemi, Fructus Arctii, Herba Lophatheri and Radix Platycodi are usually the main components of a prescription for Wind‐heat Syndrome. Supplementary drugs are sometimes added according to particular symptoms (Deng 1998; Hou 1995; Liu 2001; Ou 1992; Xu 1998; Zhang 1991) (Table 1; Table 2).
2. Medicinal herbs for influenza.
| Latin name | Common name | Properties, tastes | Function |
| Herba Schizonepetae | Schizonepeta | Pungent, slightly warm | 1. Expel wind, release the symptoms. 2. Promote the formation of eruption. 3. Stop bleeding and ablate boils. 4. Restrain and kill bacteria. 5. Tranquilliser, analgesic. 6. Anti‐inflammation, anti‐allergy |
| Radix Ledebouriellae | Ledebouriella root | Pungent, slightly warm | 1. Expel wind and relieve the symptoms. 2. Expel wind, dampness and alleviate pain. 3. Antipyretic, anti‐inflammatory, analgesic. 4. Relieve spasms. 5. Stop diarrhea |
| Radix Bupleuri | Bupleurum root | Pungent, bitter and slightly cold | 1. Reduce and disperse fever. 2. Relax constrained 'gan qi' and alleviate mental depression. 3. Improve immune function. 4. Regulate the flow of 'qi' to relieve pain. 5. Tranquillise the mind, stop coughing. 6. Anti‐inflammatory, anti‐influenza, anti‐mycobacterium, tuberculosis. 7. Reduce plasma cholesterol. 8. Strengthen body immunity |
| Radix Peucedani | Peucedanum root | Bitter, sour and slightly cold | 1. Descend 'qi' and expel phlegm. 2. Disperse wind heat. 3. Dilate coronary artery. 4. Inhibit influenza virus. 5. Relieve pain, tranquilliser |
| Radix Platycodi | Platycodon root | Bitter, sour, medium | 1. Promote the dispersing function of the lungs, relieve sore throat. 2. Expel phlegm and evacuate pus. 3. Relieve cough. 4. Anti‐inflammatory. 5. Tranquilliser, relieve pain and reduce fever. 6. Inhibit gastric juice secretion, anti‐gastric ulcer. 7. Reduce blood sugar. 8. Reduce blood lipid |
| Rhizoma Zingiberis Recens | Fresh ginger | Pungent, slightly warm | 1. Induce diaphoresis and relieve the symptoms. 2. Warm the mid section of the abdomen and alleviate vomiting. 3. Warm the lungs to arrest cough. 4. Reduce the poisonous effect of other herbs |
| Fructus Forsythiae | Forsythia fruit | Bitter, slightly cold | 1. Clear away pathogenic fever from the body. 2. Treat boils and resolve masses. 3. Control influenza virus. 4. Resist bacteria. 5. Reduce diuresis. 6. Resist hepatic injury. 7. Relieve vomiting |
| Radix Isatidis | Isatis root | Bitter, cold | 1. Clear away heat and toxic material. 2. Remove pathogenic heat from blood and relieve sore throat. 3. Resist virus. 4. Resist bacteria |
| Radix Puerariae | Pueraria root | Sweet, pungent and cool | 1. Reduce fever. 2. Stimulate the rash of measles to appear on the surface of the skin. 3. Control diarrhea. 4. Relieve spasms. 5. Invigorate vital function and promote the production of body fluid. 6. Reduce blood pressure. 7. Relieve coronary heart disease and angina pectoris. 8. Improve cerebral circulation |
| Folium Mori | Mulberry leaf | Bitter, sweet and cold | 1. Expel wind and clear heat from the lungs. 2. Clear the liver and the eyes. 3. Remove heat from blood to arrest bleeding. 4. Restrain and kill bacteria. 5. Lower blood pressure, reduce blood lipids |
| Flos Chrysanthemi | Chrysanthemum | Pungent, sweet, bitter and slightly cold | 1. Disperse wind and clear heat. 2. Clear away liver heat and brighten the eyes. 3. Restrain and kill bacteria, anti‐inflammation. 4. Increase volume of blood flow in coronary artery. 5. Increase oxygen consumption of heart. 6. Reduce blood pressure |
| Fructus Arctii | Chrysanthemum | Pungent, bitter and cold | 1. Disperse wind heat. 2. Reduce fever and relieve swelling. 3. Benefit the throat. 4. Stimulate rashes to appear on the surface of the skin |
Why it is important to do this review
A number of clinical trials of Chinese medicinal herbs for influenza have been conducted. The quality and effects of all these trials had not yet been assessed and systematically reviewed. Natural medicinal herbs are potential drug resources and the therapeutic and toxic effects of medicinal herbs need to be identified through a systematic review. Hundreds of millions of dollars are spent treating influenza annually in China, suggesting that a systematic review on the effectiveness of these medicinal herbs would be extremely useful in health policy planning.
This review summarises the existing evidence on the comparative effectiveness and safety of medicinal herbs for preventing and treating influenza, according to current clinical trials.
Objectives
To assess the effect of Chinese medicinal herbs used to prevent and treat influenza and to estimate the frequency of adverse effects.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) only. We confirmed authentic randomisation processes by telephoning the article authors.
Types of participants
People of all ages diagnosed with influenza by their clinical symptoms alone (for example, epidemic season, fever, myalgia, headache, cough, muscle aches and fatigue etc.), or with laboratory evidence (relatively elevated lymphatic cell count in routine blood tests, influenza antigen detected in the patients' secretions, serum antibody reaction or isolated influenza virus) were included.
In prophylaxis studies, healthy people of all ages in an influenza epidemic area were included.
Patients with influenza complications such as otitis media, pneumonia, secondary bacterial infection, exacerbation of chronic respiratory disease, croup and bronchiolitis, and non‐respiratory complications such as febrile convulsions, Reye's syndrome and myocarditis were excluded.
Types of interventions
Chinese medicinal herbs (including natural herbs and herbal products extracted from natural herbs) compared with placebo, no treatment or chemical drugs normally used in care. Co‐interventions were allowed if they were offered to both arms of the trial. Trials comparing different Chinese medicinal herbs were excluded.
Types of outcome measures
Primary outcomes
Rate of recovery: the symptoms and clinical manifestations were completely cleared and the body temperature returned to normal within one to three days after treatment. Or, time to symptom clearance, including fever, muscle pain, headache, cough, sore throat, stuffy nose, etc. This could be described as the rate by which symptoms cleared after treatment, for example, by day three. Or, rate of no improvement at a time point after treatment, for example, day three.
Mortality.
Incidence of influenza in prophylaxis studies.
Secondary outcomes
Length of hospital stay.
Marked improvement: most of the clinical symptoms had cleared and the body temperature returned to normal within one to three days. Partial improvement: some of the symptoms or manifestations of influenza neither improved nor did not worsen and the body temperature fell within three days.
Incidence of complications.
Adverse events: any adverse events such as malaise, nausea, fever, arthralgia, rash, headache and more generalised and serious signs resulting from the treatment that may be life‐threatening, cause a toxic response, anaphylaxis or discontinuation of treatment.
Search methods for identification of studies
Electronic searches
For this 2012 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2012, Issue 11, part of The Cochrane Library, www.thecochranelibrary.com (accessed 27 November 2012), which includes the Cochrane Acute Respiratory Infections Review Group Specialised Register, MEDLINE (December 2006 to November week 2, 2012), EMBASE (December 2006 to November 2012) and CNKI (1988 to November 2012). See Appendix 1 for details of the previous search.
We used the following search strategy to search CENTRAL and MEDLINE. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We adapted the search strategy to search EMBASE (see Appendix 2) and CNKI (see Appendix 3). We did not use any publication or language restrictions.
MEDLINE (Ovid)
1 Influenza, Human/ 2 exp Influenzavirus A/ 3 exp Influenzavirus B/ 4 Influenzavirus C/ 5 (influenza or flu).tw. 6 or/1‐5 7 exp Medicine, Chinese Traditional/ 8 Medicine, East Asian Traditional/ 9 Drugs, Chinese Herbal/ 10 Plants, Medicinal/ 11 (Chinese adj4 (herb* or medic*)).tw. 12 (medic* adj2 herb*).tw. 13 Integrative Medicine/ 14 (integrat* adj2 medic*).tw. 15 or/7‐14 16 6 and 15
Searching other resources
We attempted to identify additional studies by searching the reference lists of relevant trials, reviews, conference proceedings and journals. In particular, with respect to journals, we searched those not indexed in the electronic databases. We also searched the WHO ICTRP search portal for ongoing trials (latest search 27 November 2012).
Data collection and analysis
Selection of studies
We scanned the titles, abstract sections and keywords of every record retrieved. We located full articles for further assessment when the information given suggested that the study: (1) included patients with uncomplicated influenza; (2) compared Chinese medicinal herbs with placebo or other active drugs; (3) assessed one or more relevant clinical outcome measure; (4) used random allocation for the comparison groups.
If there was any doubt regarding these criteria from the information given in the title and abstract, we retrieved the full article for clarification. We measured inter‐rater agreement for study selection using the kappa statistic (Cohen 1960). We resolved differences in opinion by discussion.
If random allocation was indicated in a trial but the randomisation procedure was not described, we telephoned the primary author to ask for detailed information regarding the randomisation procedure. We excluded the trial if the trial was a quasi‐RCT or falsely randomised (allocating patients by date of birth, date of admission, hospital number, alternation, or by the investigators' or patients' choosing, etc.). We excluded trials not reporting our stated outcome measures. We also excluded studies with a high percentage (more than 20%) of dropouts.
Data extraction and management
Two review authors (LHJ, TXW) independently extracted data concerning details of the study population, interventions and outcomes using a standard data extraction form, specifically designed for this review. We retrieved data on participants, interventions and outcomes, as described above. The data extraction form included the following items:
General information: published/unpublished, title, authors, reference/source, contact address, country, urban/rural etc., publication language, year of publication, duplicate publications, sponsor and setting.
Trial characteristics: design, duration of follow‐up, method of randomisation, allocation concealment, blinding (patients, people administering treatment, outcome assessors), checking of blinding.
Intervention(s): placebo included, interventions(s) (dose, route, timing), comparison intervention(s) (dose, route, timing), co‐medication(s) (contents, dose, route, timing).
Patients: sampling (random/convenience), exclusion criteria, total number and number in comparison groups, sex, age (children/adults), baseline characteristics, duration of influenza, diagnostic criteria, similarity of groups at baseline (including any co‐morbidity), assessment of compliance, withdrawals/losses to follow‐up (reasons/description), subgroups.
Outcomes: outcomes specified above, any other outcomes assessed, other events, length of follow‐up, quality of reporting of outcomes.
Results: for outcomes and times of assessment (including a measure of variation), if necessary converted to measures of effect specified below, intention‐to‐treat (ITT) analysis.
We resolved differences in data extraction by consensus and with reference to the original article. If necessary, we sought information from the authors of the primary studies. We managed to contact trial authors by letter or telephone regarding missing information and confusing points such as methods of randomisation and allocation concealment; separate information for certain patient subgroups; information about complications; and number of dropouts. We managed to contact manufacturers regarding the components of processed Chinese medicines if the components were unclear.
Two review authors (LHJ, TXW) independently extracted the original trial results. We resolved disagreements by discussion. For binary outcomes, we extracted the number of events and total number in each group. For continuous outcomes we extracted the mean, standard deviation and sample size from each group.
Assessment of risk of bias in included studies
We assessed the reporting quality of each trial, based largely on the quality criteria specified by Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). In particular, we studied the following factors.
Generation of the allocation sequence: an allocation sequence generated from a random numbers table, calculator or computer random‐number generator was considered as a real randomised RCT. Methods of allocating participants according to their date of birth, their hospital record number, the date to which they were invited to participate in the study and so on, were considered inadequate.
Allocation concealment: use of a central independent unit, opaque sealed envelopes, or similar, were considered adequate. Inadequate methods included those not described, an open table of random numbers or similar.
Double‐blinding: blinding of participants and investigators. Not performing double‐blinding or inconsistency in the delivery method (for example, tablets versus injections) were considered inadequate.
Follow‐up: number of and reasons for dropouts and withdrawals described was considered adequate; number of and reasons for dropouts and withdrawals not described was considered inadequate.
Based on these criteria, studies were broadly subdivided into the following three categories:
All quality criteria met: low risk of bias.
One or more of the quality criteria only partly met: moderate risk of bias.
One or more criteria not met: high risk of bias (Higgins 2011).
We used this classification as the basis for a sensitivity analysis. Additionally, we explored the influence of individual quality criteria in a sensitivity analysis. Two review authors (LHJ, TXW) independently assessed each trial. We calculated internal agreement using the kappa statistic and resolved disagreements by discussion. In cases of disagreement, one review author (KL) was consulted and a judgement was made, based on a consensus.
Measures of treatment effect
We intended to include data for some medicinal herbs in a meta‐analysis if possible.
We had decided in advance that a quantitative meta‐analysis should be performed when data for an outcome measure with a similar intervention (same herbal preparation or same main components of a herbal preparation) in more than two included studies were available. The data should be dichotomous or continuous and be expressed as risk ratio (RR) or mean difference (MD), respectively. The overall effect should be tested by using Z score with significance being set at P < 0.05.
Unit of analysis issues
The analysis was based on the individual participant.
Dealing with missing data
We obtained relevant missing data from trial authors by telephoning and carefully performing evaluation of important numerical data such as screened, randomised patients as well as the ITT, as‐treated and per‐protocol (PP) population. We investigated attrition rates, for example dropouts, losses to follow‐up and withdrawals, and critically appraised issues of missing data and imputation methods (for example, last observation carried forward (LOCF)).
Assessment of heterogeneity
In the event of substantial clinical, methodological or statistical heterogeneity we did not report study results as meta‐analytically pooled effect estimates. We identified heterogeneity by visual inspection of the forest plots, by using a standard Chi2 test and a significance level of α = 0.1, in view of the low power of this test. We specifically examined heterogeneity with the I2 statistic, quantifying inconsistency across studies to assess the impact of heterogeneity on the meta‐analysis, where an I2 statistic of 75% and more indicates a considerable level of inconsistency (Higgins 2011). When heterogeneity was found, we attempted to determine the potential causes by examining individual study and subgroup characteristics.
Assessment of reporting biases
We used funnel plots to assess the potential existence of small study bias.
Data synthesis
We will use a fixed‐effect model when the studies in the subgroup were sufficiently similar (P > 0.10, I2 statistic < 50%). We used a random‐effects model in the summary analysis when there was heterogeneity between the subgroups. We planned to test for publication bias by using a funnel plot or other corrective analytical methods, depending on the number of clinical trials included in the systematic review.
We did not find more than two studies using similar interventions in the treatment groups and consequently we did not use a meta‐analysis to calculate the pooled effect size. We analyzed data for each study and expressed the results a risk ratios (RR). We summarised the number of dropouts and the number of participants who were lost to follow‐up for each study, when available, using an ITT analysis. When different herbal preparations (as the intervention) in the treatment groups were considered as a whole and then compared to certain chemical drugs in the control groups, we assessed the therapeutic effect by a qualitative analysis.
Subgroup analysis and investigation of heterogeneity
Heterogeneity was to be tested for using the Chi2 test and I2statistic with significance being set at P < 0.1. Possible sources of heterogeneity were to be assessed by sensitivity and subgroup analyses as described below.
If suitable trials are found in the future, we will perform the following subgroup analyses in order to explore the effect size differences.
Adults versus children.
Intervention: different formulations between studies, administration routes (oral or intravenous) or doses (low and high, based on data).
Timing of outcome measures.
Sensitivity analysis
If suitable trials are found in the future, we will perform the following sensitivity analyses in order to explore the influence of the following factors on effect size.
Repeating the analysis excluding unpublished studies (if there are any).
Repeating the analysis taking into account study quality, as specified above.
Repeating the analysis excluding any very long or large studies to establish how much they dominate the results.
Repeating the analysis excluding studies using the following filters: diagnostic criteria, publication language, funding source (industry versus other) and country.
We will test the robustness of the results by using different measures of effect size (risk difference, odds ratio, etc.) and different statistical models (fixed‐ and random‐effects models), if necessary.
Results
Description of studies
Results of the search
In this updated review, 1487 hits were generated by searching CNKI. The updated searches of MEDLINE, EMBASE and CENTRAL yielded 54 records after duplicates were removed. Five records were identified in WHO ICTRP. We retrieved a total of 63 trials that claimed to be randomised. Of these trials, 42 were excluded, either because the trial authors misunderstood true random allocation or the trial reports were multiple version of same study. The authors of three studies were uncontactable by telephone and we allocated their trials into the Characteristics of studies awaiting classification section. Eighteen studies were identified as true RCTs and fulfilled our inclusion criteria (Chen 2010a; Chen 2010b; Jin 2010; Li 2009; Li 2010; Ouyang 2010; Qian 2011; Shi 2004; Tan 2010; Wang 2010; Wei 2010; Xie 2010; Xue 1999; Zhang 2010a; Zhang 2011; Zhao 2010; Zheng 2010b; Zhu 2010) (Figure 1).
1.
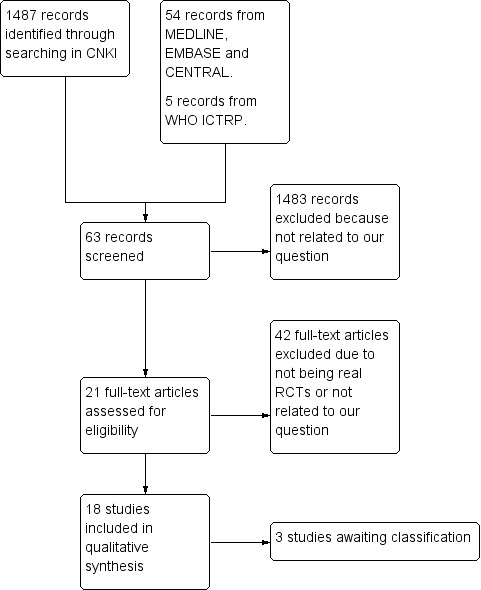
Study flow diagram.
Included studies
In the first version of this review (Chen 2005), two trials were identified as true RCTs and fulfilled our inclusion criteria (Shi 2004; Xue 1999). A total of 1012 participants were included in these two trials, with numbers of participants in each trial varying from 61 to 951. Of the included trials, one (Shi 2004) was a treatment trial and the other trial (Xue 1999) was a prophylaxis and treatment trial (Table 3). Information on the herbal preparations used in each trial, including excluded trials and trials awaiting assessment, is shown in Table 4.
3. Interpretation of the results in each study.
| Study ID | Interventions | Recovery | Marked improvement | Partial improvement | No improvement | Defervescence | Symptoms clearance | Adverse reaction | Interpretation |
| Xue 1999 | Ganmao capsule versus amantadine | RR 5.17, 95% CI 3.82 to 6.99 | Data not available | Data not available | Data not available | Data not available | Data not available | Adverse reaction in alimentary tract was mentioned in control group but data were not available | Ganmao capsule can improve recovery more than amantadine with statistical difference at the end of 2 days of treatment |
| Shi 2004 | E Shu You versus ribavirin | RR 2.18, 95% CI 0.87 to 5.43 | RR 1.02, 95% CI 0.45 to 2.29 | RR 0.91, 95% CI 0.36 to 2.27 | RR 0.40, 95% CI 0.14 to 1.17 | Data not available | Data not available | RR 0.58, 95% CI 0.09 to 3.73 | There were no significant differences between E Shu You and ribavirin for treating influenza in terms of effectiveness and adverse reactions |
CI: confidence interval RR: risk ratio
4. The composition of preparations of TCMs.
| Study ID | TCMs preparation | English TCM name | Pinyin TCM name |
| Xue 1999 | Ganmao capsule | Japanese honeysuckle stem, baical skullcap root, Platycodon root, bitter apricot seed, fine leaf Schizonepeta herb, divaricate Saposhicicovia root, fresh liquorice root | Rendongteng, Huangqi, Jiegeng, Xingren, Jingjie, Fangfeng, Shenggancao |
In this updated version of the review, 16 additional trials were identified as true RCTs and fulfilled our inclusion criteria (Chen 2010a; Chen 2010b; Jin 2010; Li 2009; Li 2010; Ouyang 2010; Qian 2011; Tan 2010; Wang 2010; Wei 2010; Xie 2010; Zhang 2010a; Zhang 2011; Zhao 2010; Zheng 2010b; Zhu 2010). A total of 1509 participants were included in the additional 16 trials, with numbers of participants in each trial varying from 48 to 174. The proportion of males to females was 1308 to 1021. All of them were Chinese and aged from seven months to 71 years old. All trials enrolled patients with influenza alone. Three trials (Li 2009; Wang 2010; Zhang 2011) mentioned that an "informed consent form" was signed by participants before they were included and one trial (Wang 2010) was reviewed and approved by the Medical Ethics Committee of the West China Hospital at Sichuan University. Two trials (Chen 2010a; Wang 2010) described withdrawals (2 and 15, respectively).
Details of the included studies are shown in the Characteristics of included studies table. All the included studies were parallel design RCTs.
The number of participants in the 18 included studies ranged from 46 to 951, totaling 2521 participants.
In Shi 2004, participants were children aged 6 to 10, clinically diagnosed with influenza B in an epidemic area. Disease duration was less than 17 hours. Laboratory tests excluded a bacterial infection.
In Xue 1999, participants were healthy people as well as participants clinically diagnosed with influenza A3/H3N2 within two days of disease onset in an epidemic area. The statistical analyses for prevention and treatment were performed separately. Those who subsequently developed influenza in the prevention study were eventually included in the treatment analyses.
Four trials (Chen 2010b; Jin 2010; Xie 2010; Xue 1999) did not report the age of participants included and three trials (Chen 2010b; Jin 2010; Tan 2010) did not mention the gender of the participants in the intervention and comparison groups.
In Chen 2010a, the average ages of both intervention and comparison groups were 19.87 and 20.68 years. The proportion of males to females was 18 to 13 in the intervention group and 9 to 13 in the comparison group.
In Li 2009, the average ages in both intervention and comparison groups were 19 and 18 years. The proportion of males to females was 11 to 14 in the intervention group and 9 to 16 in the comparison group.
In Li 2010, the average ages of both intervention and comparison groups were 31.35 and 30.77 years. The proportion of males to females was 28 to 27 in the intervention group and 32 to 23 in the comparison group.
In Ouyang 2010, the average ages of the three groups (intervention versus comparison I versus comparison II) were 19.23, 19.69 and 19.38 years, respectively. The proportion of males to females was 59 to 57 in the intervention group, 16 to 13 in comparison group I and 15 to 14 in comparison group II.
In Wang 2010, the average ages of both intervention and comparison groups were 37.3 and 35.9 years. The proportion of males to females was 72 to 105 in the intervention group and 23 to 25 in the comparison group.
In Wei 2010, the average ages of both intervention and comparison groups were 17.76 and 16.25 years. The proportion of males to females was 17 to 13 in the intervention group and 10 to 6 in the comparison group.
In Zhang 2010a trial, the average ages of both intervention and comparison groups were 17 and 14 years. The proportion of males to females was 19 to 9 in the intervention group and 15 to 13 in the comparison group.
In Zhang 2011, the average ages of both intervention and comparison groups were 22.77 and 23.37 years. The proportion of males to females was 17 to 13 in the intervention group and 16 to 14 in the comparison group.
In Zhao 2010, the average ages of both intervention and comparison groups were 18.20 and 16.27 years. The proportion of males to females was 18 to 12 in the intervention group and 16 to 14 in the comparison group.
In Zheng 2010b, the average ages of the intervention, comparison I and comparison II groups were 22.47, 19.79 and 23.53 years, respectively. The proportion of males to females was 12 to 7 in the intervention group, 7 to 7 in comparison group I and 9 to 6 in comparison group II.
In Zhu 2010, the average ages of both intervention and comparison groups were 35.12 and 34.23 years. The proportion of males to females was 25 to 13 in the intervention group and 22 to 10 in the comparison group.
In Shi 2004, the average ages of both intervention and comparison groups were 8.69 and 8.48 years. The proportion of males to females was 18 to 14 in the intervention group and 16 to 13 in the comparison group.
Interventions of included studies
The interventions in the 18 trials were Chinese medicinal herbs compared with antiviral drugs. Of those, 14 trials (Chen 2010a; Jin 2010;Li 2009; Li 2010; Ouyang 2010; Qian 2011; Tan 2010; Wei 2010; Xie 2010; Zhang 2010a; Zhang 2011; Zhao 2010 ; Zheng 2010b; Zhu 2010) compared TCM or TCM plus oseltamivir with oseltamivir as follows.
In Chen 2010a, the therapeutic effects of TCM versus oseltamivir were tested.
In Jin 2010, the therapeutic effects of TCM versus oseltamivir were tested.
In Li 2009, the therapeutic effects of Lianhua Qingwen capsule versus oseltamivir were compared.
In Li 2010, the therapeutic effects of Tanreqing injection plus oseltamivir versus oseltamivir were tested.
In Ouyang 2010, the therapeutic effects of Lianhua Qingwen capsule versus oseltamivir versus paracetamol were tested.
In Qian 2011, the therapeutic effects of Tanreqing injection plus oseltamivir versus oseltamivir were tested.
In Tan 2010, the therapeutic effects of TCM versus oseltamivir versus TCM plus oseltamivir versus no treatment were tested.
In Wei 2010, the therapeutic effects of Lianhua Qingwen capsule versus oseltamivir were tested.
In Xie 2010, the therapeutic effects of Tanreqing injection plus oseltamivir versus oseltamivir were tested.
In Zhang 2010a, the therapeutic effects of TCM versus TCM plus oseltamivir were tested.
In Zhang 2011, the therapeutic effects of TCM versus oseltamivir were tested.
In Zhao 2010, the therapeutic effects of TCM plus oseltamivir versus oseltamivir were tested.
In Zhu 2010, the therapeutic effects of Ge Geng decoction plus oseltamivir versus oseltamivir were tested.
In Zheng 2010b, the therapeutic effects of TCM versus oseltamivir versus TCM plus oseltamivir were tested.
The other four trials (Chen 2010b; Shi 2004; Wang 2010; Xue 1999) used the following comparisons.
In Chen 2010b, Fanggan granule was compared with conventional medicine to prevent and treat influenza.
In Shi 2004, volatile oil extracted from Zedoary was compared with ribavirin plus vitamin C for injection, used for three to five days, with the antibiotic erythromycin given to both arms for preventing secondary bacterial infection.
In Wang 2010, Antiwei was compared with placebo to test the treatment effect on influenza.
In Xue 1999, compound herbal preparations were compared with amantadine, both taken in capsular form for seven days, for either the prophylaxis or treatment study.
Outcome measures of included studies
Eleven trials (Chen 2010a; Chen 2010b; Li 2009; Li 2010; Qian 2011;Tan 2010; Wang 2010; Zhang 2010a; Zhang 2011; Zhao 2010; Zhu 2010) reported the duration of fever; seven trials (Chen 2010b; Li 2010; Ouyang 2010; Shi 2004; Xie 2010; Xue 1999; Zhang 2011) reported the total effective rate; five trials (Li 2010; Ouyang 2010; Shi 2004; Xue 1999; Zhang 2011) reported the events of cure; four trials (Chen 2010b; Li 2010; Zhang 2011; Zhu 2010) reported the duration of cough; two trials (Li 2009; Zhang 2011) reported the time to muscle pain remission; four trials (Chen 2010a; Chen 2010b; Zhao 2010; Zhu 2010) reported the time to symptom remission; one trial (Zhang 2011) reported sore throat remission; four trials (Chen 2010a; Qian 2011; Tan 2010; Zhang 2010a) reported length of hospital stay; eight trials (Chen 2010b; Li 2010; Ouyang 2010; Shi 2004; Wang 2010; Xie 2010; Xue 1999; Zhang 2011) reported recovery; seven trials (Chen 2010b; Li 2010; Ouyang 2010; Shi 2004; Xie 2010; Xue 1999; Zhang 2011) reported the outcome "no improvement"; and 10 trials (Chen 2010a; Chen 2010b; Jin 2010; Li 2009; Ouyang 2010; Shi 2004; Wang 2010; Wei 2010; Xie 2010; Zheng 2010b) reported adverse effects.
One trial (Shi 2004) assessed the rate of effectiveness at the end of day three, following treatment, as the outcome (recovery/marked improvement/partial improvement/no improvement), according to the defervescence period, the period and extent of symptoms alleviation. Adverse reactions in the gastrointestinal tract were reported in both trial arms.
The other trial (Xue 1999) assessed the incidence of influenza at the end of day seven following treatment, as the outcome for the prophylaxis study; and the rate of recovery and inefficacy at the end of day two after treatment, as the outcome for the treatment study. Inefficacy was defined as effectiveness other than recovery in this study, which covered marked improvement, partial improvement and no improvement as regulated in our review. Adverse reactions in the gastrointestinal tract were mentioned in the control group but no data were reported. Neither study used time to fever clearance or other symptom alleviation, or both, as outcome measures.
Excluded studies
A total of 57 trials claiming to be RCTs were retrieved. Of these, 41 trials were excluded for the following reasons (see Characteristics of excluded studies table): the interventions were one Chinese medicinal herb compared to another, with or without chemical drugs added in one arm in 17 trials (Chen 2010c; Dou 2010; Huang 2010b; Jiang 2003; Liu 2010; Wang 2001; Yang 2000b; Yang 2005a; Yang 2005b; Yu 2000; Zeng 2004; Zhang 2000; Zhang 2002; Zhang 2004; Zhang 2005; Zhao 2006; Zhong 2005); five trials did not provide the data to meet the outcome criteria (Hamazaki 2006; Hang 1998; Lindenmuth 2000; Lu 2004; Zhou 2010); participants in five trials experienced complications (Jin 1998; Li 2005; Liu 2002; Zeng 2004; Zheng 2010); and one trial used a Japanese herbal medicine as the intervention (Kubo 2007). We then conducted telephone interviews with the authors of the remaining 14 trials to obtain the information on the randomisation procedure and found that 13 trials were actually false or quasi‐RCTs (Du 1991; Hou 2002; Huang 2003; Huang 2010a; Li 2001; Qu 2005; Tang 2010; Xu 2001; Yang 2000a; Yao 2003; Yuan 2003; Xia 2010; Zhang 2010b). We failed to contact the authors of two trials, which are listed in the Studies awaiting classification section (Qiu 1997; Song 2002).
Risk of bias in included studies
The overall risk of bias is presented graphically in Figure 2 and summarised in Figure 3.
2.
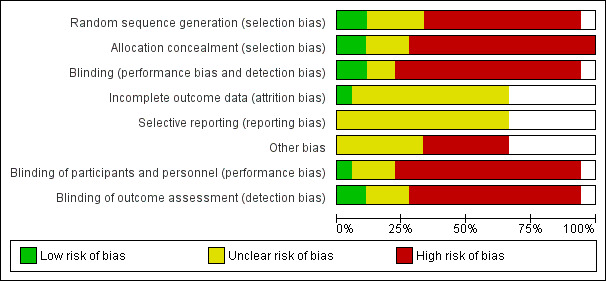
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
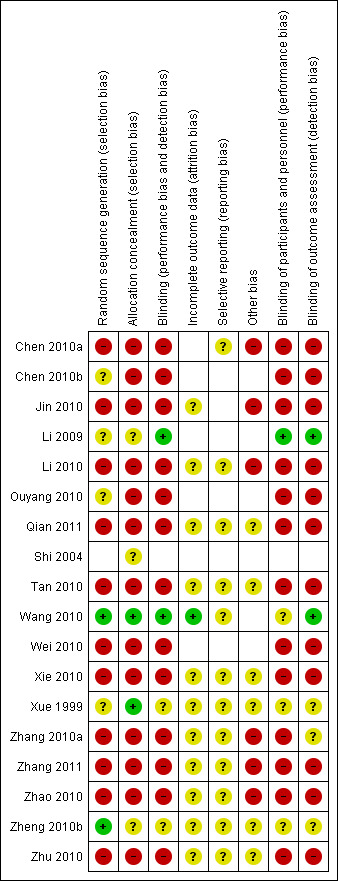
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Description of withdrawals and losses to follow‐up and intention‐to‐treat analysis
Two of the included studies (Chen 2010a; Wang 2010) mentioned dropouts but none of the included studies performed an intention‐to‐treat analysis.
In the Xue 1999 trial there were 519 participants in the intervention group and 432 in the control group. It is unclear whether the imbalance of participant numbers in the two arms was produced by inadequate randomisation or withdrawals during follow‐up, or for another reason. However, the trial author did not give us a satisfactory answer, as he could not remember the details. We considered both included studies at high risk for bias and graded them as category C.
Allocation
Nine trials reported on allocation sequence generation. Of these, two trials (Chen 2010b; Tan 2010) were based on a random numbers table; four trials used a computer to generate the allocate sequence (Chen 2010a; Shi 2004; Wang 2010; Xue 1999); and one trial was based on drawing lots. The other trials did not describe how participants were allocated. After conducting a telephone interview with the trial authors, we learned that the allocation sequence was generated by random number table.
Except for one included trial (Wang 2010), none of the studies mentioned allocation concealment. After conducting telephone interviews, we learned that allocation in four trials (Chen 2010a; Shi 2004; Wang 2010; Xue 1999) was generated by computer and allocation concealment was performed.
Blinding
With the exception of two studies (Wang 2010; Xue 1999), the trials did not mention blinding. Xue 1999 mentioned double‐blinding. Neither the participants nor the assessors knew which interventions were administered. The drugs in both arms were the same in appearance, route and schedule, to ensure blinding. Wang 2010 also mentioned double‐blinding and gave a very detailed description of how to make the dummy visually the same as Antiwei medicine.
Incomplete outcome data
Two trials (Chen 2010a; Wang 2010) recorded withdrawals as two and 34 respectively. However, none of the trials used ITT analysis on dropouts or withdrawals.
Selective reporting
The protocols for the included studies were unavailable. Eight trials (Li 2010; Qian 2011; Tan 2010; Xue 1999; Zhang 2010a; Zhang 2011; Zhao 2010; Zhu 2010) did not report adverse events.
Other potential sources of bias
The seriousness of influenza in each trial was different, which may have influenced the outcomes.
Effects of interventions
Due to clinical heterogeneity it was not possible to combine the results of the studies. Therefore, the results are presented as separate risk ratios (RR) for each study. We did not perform any of the planned subgroup/sensitivity analyses.
Primary outcomes
1. Rate of recovery
Trials showing statistically significant differences between the intervention and comparison
Lianhua Qingwen capsule showed a significantly better result than paracetamol within 24 hours after treatment (Ouyang 2010: risk ratio (RR) 1.91, 95% confidence interval (CI) 1.03 to 3.52) (Analysis 1.1).
Antiwei capsule showed a significantly better result than placebo within four days after treatment (Wang 2010: RR 3.80, 95% CI 1.23 to 11.72) (Analysis 1.1).
Ganmao capsule showed a significantly better result than amantadine for recovery within two days of treatment (Xue 1999: RR 5.17, 95% CI 3.82 to 6.99) (Analysis 1.1).
1.1. Analysis.
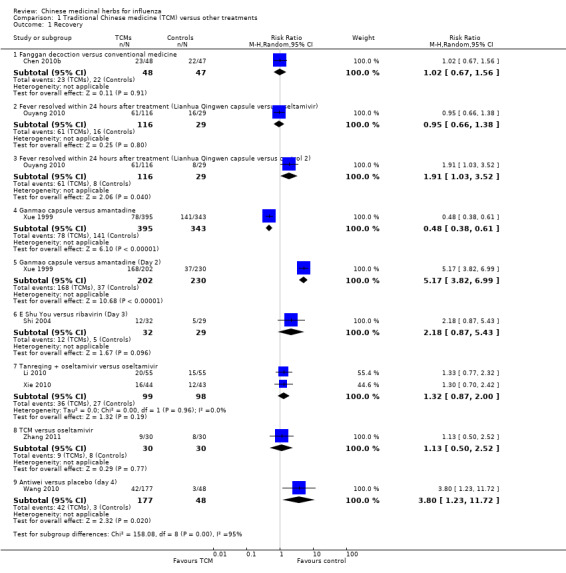
Comparison 1 Traditional Chinese medicine (TCM) versus other treatments, Outcome 1 Recovery.
Trials showing no statistically significant differences between the intervention and comparison
E Shu You (volatile oil extracted from Zedoary) showed a better result than ribavirin for recovery within three days of treatment, however the difference was not significant (Shi 2004: RR 2.17, 95% CI 0.87 to 5.43) (Analysis 1.1).
Fanggan decoction showed a better result than conventional medicines, however the difference was not significant (Chen 2010b: RR 1.02, 95% CI 0.67 to 1.56) (Analysis 1.1).
Lianhua Qingwen capsule showed a better result than oseltamivir, however the difference was not significant (Ouyang 2010: RR 0.95, 95% CI 0.66 to 1.38) (Analysis 1.1).
Ganmao capsule showed a better result than amantadine, however the difference was not significant (Ouyang 2010: RR 0.48, 95% CI 0.38 to 0.61) in influenza prevention (Analysis 1.1).
Tanreqing plus oseltamivir showed a better result than oseltamivir alone, however the difference was not significant (Li 2010 and Xie 2010: RR 1.32, 95% CI 0.87 to 2.00) (Analysis 1.1).
Traditional Chinese medicine (TCM) showed a better result than oseltamivir, however the difference was not significant (Zhang 2011: RR 1.13, 95% CI 0.50 to 2.52) (Analysis 1.1).
Time to fever clearance
Different TCM appeared different effect compared to oseltamivir (Analysis 1.2):
1.2. Analysis.
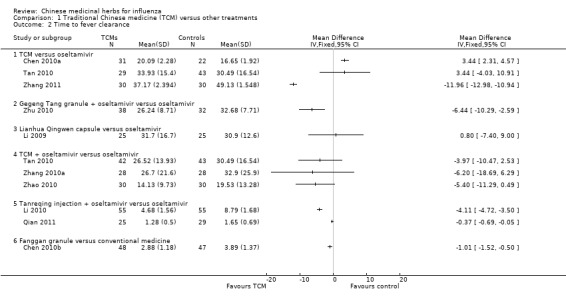
Comparison 1 Traditional Chinese medicine (TCM) versus other treatments, Outcome 2 Time to fever clearance.
Chen 2010a's TCM had longer clearance time than oseltamivir (MD 3.44, 95%CI 2.31 to 4.57); Tan 2010's TCM had a similar time of fever clearance, there was no statistical significant (MD 3.44, 95%CI ‐4.03 to 10.91).
Zhang 2011's TCM had a much shorter time of fever clearance with statistical significant (MD ‐11.96, 95%‐12.98 to ‐10.94).
Li 2009 reported Lianhua Qingwen capsule had a similar time of fever clearance (MD 0.8, 95%CI ‐7.40 to 9.00).
Dramatic results appeared in the comparison of integrated TCM's and oseltamivir did not appear superior than oseltamivir alone:
Tan 2010's TCM + oseltamivir versus oseltamivir: MD ‐3.97, 95%CI ‐10.47 to 2.53;
Zhang 2010a's TCM + oseltamivir versus oseltamivir: MD ‐6.2, 95%CI ‐18.69 to 6.29;
Zhao 2010's TCM + oseltamivir versus oseltamivir: MD ‐5.40, 95%CI ‐11.29 to 0.49.
Other three studies appeared TCM plus oseltamivir superior than oseltamivir alone:
Zhu 2010's Gegentang granule plus oseltamivir had a statistical significant shorter time than oseltamivir alone: MD ‐6.44, 95%CI ‐10.29 to ‐2.59;
Both Li 2010 and Qian 2011 used Tanreqing injection plus oseltamivir had a statistical significant shorter time than oseltamivir alone: MD ‐4.11, 95%CI ‐4.72 to ‐3.50 and MD ‐0.37, 95%CI ‐0.69 to ‐0.05, respectively, the combined MD‐1.18, 95%CI‐1.46 to ‐0.90.
Chen 2010b's Fanggan granule had a shorter time of fever clearance than conventional medicine with statistical significant (MD‐1.01, 95%CI‐1.52 to ‐0.5).
Duration of cough
Zhu 2010's Gegeng Tang granule did not show any benefit when combined use with oseltamivir versus oseltamivir alone (MD ‐0.56, 95%CI ‐29.71 to 28.59).
Other three studies showed that use of TCM had a statistical significant shorter duration than oseltamivir alone on cough:
Chen 2010b's Fanggan decoction shorter than conventional medicine (MD ‐3.04, 95%CI ‐4.27 to ‐1.81);
Li 2010 used Tanreqing injection plus oseltamivir had a shorter duration than oseltamivir alone (MD ‐3.87, 95%CI ‐4.77 to ‐2.97)
Zhang 2011's TCM had a shorter duration than oseltamivir (MD‐18.73, 95%CI ‐19.7 to ‐17.76).
Time to remission of muscle pain
Two studies reported time to remission of muscle pain:
Li 2009 reported Lianhuan Qingwen capsule similar with oseltamivir (MD ‐5.90, 95%CI ‐14.01 to 2.21);
Zhang 2011's TCM had a statisitically significant shorter time than oseltamivir to remission of muscle pain (MD ‐22.83, 95%CI ‐25.15 to ‐20.51).
Time to symptom remission
Four studies reported this outcome:
Chen 2010a's TCM had a similar time to symptom remission (MD ‐2.64, 95%CI ‐16.52 to 11.24);
Chen 2010b's Fanggan granule had a significantly shorter time than conventional medicine (MD ‐1.24, 95%CI ‐1.71 to ‐0.77);
Zhu 2010's Gegeng Tang granule plus oseltamivir had a significantly shorter time than oseltamivir alone (MD ‐14.11, 95%CI ‐18.35 to ‐9.87);
Zhao 2010's TCM plus oseltamivir had a significantly shorter time than oseltamivir alone (MD ‐13.33, 95%CI ‐24.28 to ‐2.38).
Time to remission of sore throat
Zhang 2011's TCM had a significant shorter time of remission the sore throat than oseltamivir (MD ‐15.60, 95%CI ‐17.87 to ‐13.33).
No improvement
Six trials reported the outcome "no improvement" and none of them showed statistical significance.
Shi 2004: E Shu You showed a lower rate of no improvement than ribavirin in the treatment of influenza, without a significant difference (RR 0.40, 95% CI 0.14 to 1.17) (Analysis 1.8).
1.8. Analysis.
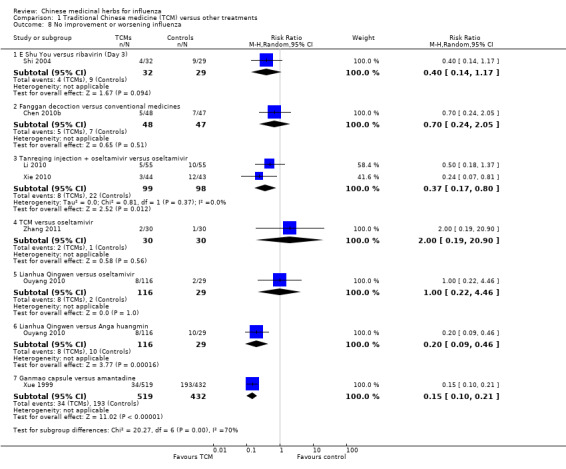
Comparison 1 Traditional Chinese medicine (TCM) versus other treatments, Outcome 8 No improvement or worsening influenza.
Chen 2010b: Fanggan granule versus conventional medicine: RR 0.70, 95% CI 0.24 to 2.05 (Analysis 1.8).
Li 2010 and Xie 2010: Tanreqing plus oseltamivir versus oseltamivir: RR 0.37, 95% CI 0.17 to 0.80 (Analysis 1.8).
Zhang 2011: TCM versus oseltamivir: RR 2.00, 95% CI 0.19 to 20.90 (Analysis 1.8).
Ouyang 2010: Lianhua Qingwen capsule versus oseltamivir: RR 1.00, 95% CI 0.22 to 4.46 (Analysis 1.8).
Ouyang 2010: Lianhua Qingwen capsule versus paracetamol: RR 0.20, 95% CI 0.09 to 0.46 (Analysis 1.8).
Xue 1999: Ganmao capsule versus amantadine: RR 0.15, 95% CI 0.10 to 0.21 (Analysis 1.8).
2. Mortality
No study reported mortality.
3. Incidence of influenza in prophylaxis studies
In the prophylaxis study (Xue 1999) the incidence of influenza was statistically significantly lower in the Ganmao capsule group than in the amantadine group, within seven days of treatment (RR 0.48, 95% CI 0.38 to 0.61).
Secondary outcomes
1. Length of hospital stay
Four studies reported length of hospital stay, except one study (Qian 2011), other three studies showed no difference with control remedies:
Chen 2010a's TCM similar as oseltamivir (MD ‐0.04, 95%CI ‐0.71 to 0.63);
Tan 2010's TCM similar as oseltamivir (MD 0.26, 95%CI ‐0.43 to 0.95);
Tan 2010's TCM plus oseltamivir versus oseltamivir alone (MD ‐0.57, 95%CI ‐1.38 to 0.24);
Tan 2010's TCM versus placebo (MD ‐0.62, 95%CI ‐1.92 to 0.68);
Zhang 2010a's TCM plus oseltamivir versus oseltamivir (MD‐0.20, 95%CI ‐1.46 to 1.06);
Qian 2011 reported Tanreqing injection plus oseltamivir had significant shorter time than oseltamivir alone (MD ‐1.01, 95%CI ‐2.00 to ‐0.02).
2. Marked improvement
Only one study (Shi 2004) provided data for marked improvement for analysis with no significant difference between E Shu You and ribavirin in the treatment of influenza (RR 1.02, 95% CI 0.45 to 2.29).
Partial improvement
Data for partial improvement were available for analysis in six trials (Chen 2010b; Li 2010; Ouyang 2010; Shi 2004; Xie 2010; Zhang 2011). Chen 2010b, Li 2010, Ouyang 2010, Shi 2004, Xie 2010 and Zhang 2011 found no significant difference between treatment and comparison groups in the treatment of influenza, with the data as follows.
Chen 2010b: Fanggan granule versus conventional medicine: RR 1.09, 95% CI 0.66 to 1.78 (Analysis 1.9).
Li 2010: Tanreqing injection plus oseltamivir versus oseltamivir: RR 0.60, 95% CI 0.23 to 1.54 (Analysis 1.9).
Ouyang 2010: Lianhua Qingwen capsule versus oseltamivir: RR 1.2, 95% CI 0.50 to 2.87 (Analysis 1.9).
Ouyang 2010: Lianhua Qingwen capsule versus paracetamol: RR 1.2, 95% CI 0.50 to 2.87 (Analysis 1.9).
Xie 2010: Tanreqing plus oseltamivir versus oseltamivir: RR 1.29, 95% CI 0.84 to 1.96 (Analysis 1.9).
Zhang 2011: TCM versus oseltamivir: RR 0.44, 95% CI 0.15 to 1.29 (Analysis 1.9).
Shi 2004: E Shu You versus ribavirin: RR 0.91, 95% CI 0.36 to 2.27 (Analysis 1.9).
1.9. Analysis.
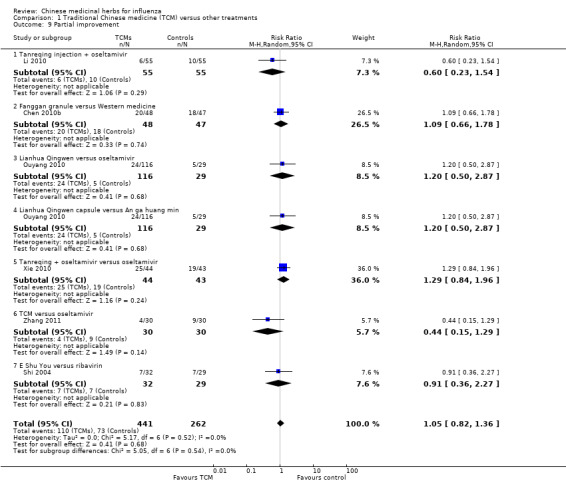
Comparison 1 Traditional Chinese medicine (TCM) versus other treatments, Outcome 9 Partial improvement.
3. Incidence of complications
No studies reported this outcome.
4. Adverse events
Data on adverse reactions were available in 10 studies, but none of the recorded adverse reactions fulfilled the definition in this review and were slight. Two trials (Wang 2010; Xie 2010) reported that none of the participants experienced adverse reactions. The other eight trials reported as follows.
TCM versus oseltamivir (Chen 2010a; Jin 2010; Zheng 2010b): RR 1.12, 95% CI 0.04 to 32.35 (Analysis 1.10).
Lianhua Qingwen versus oseltamivir (Li 2009; Ouyang 2010; Wei 2010): RR 0.34, 95% CI 0.11 to 1.02 (Analysis 1.10).
Fanggan granule versus conventional medicine (Chen 2010b): RR 0.98, 95% CI 0.14 to 6.67 (Analysis 1.10).
E Shu You versus ribavirin (Shi 2004): RR 0.60, 95% CI 0.11 to 3.36 (Analysis 1.10).
Lianhua Qingwen versus An ga huang min (Ouyang 2010): RR 0.73, 95% CI 0.08 to 6.78 (Analysis 1.10).
1.10. Analysis.
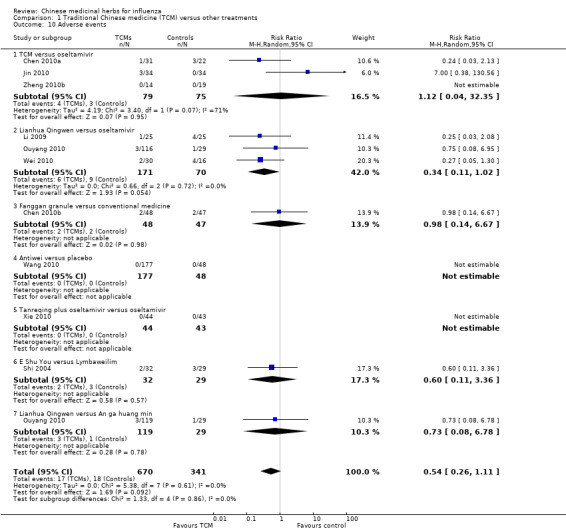
Comparison 1 Traditional Chinese medicine (TCM) versus other treatments, Outcome 10 Adverse events.
Discussion
Summary of main results
Due to clinical heterogeneity, we did not perform meta‐analyses. Of the 18 included studies, only three (Ouyang 2010; Wang 2010; Xue 1999) indicated that compared with antiviral drugs, Chinese medicinal herbs showed a superior effect in preventing influenza and alleviating influenza symptoms. The remain 15 studies (Chen 2010a; Chen 2010b; Jin 2010; Li 2009; Li 2010; Qian 2011; Shi 2004; Tan 2010; Wei 2010; Xie 2010; Zhang 2010a; Zhang 2011; Zhao 2010; Zheng 2010b; Zhu 2010) had a similar effect to antiviral drugs. No obvious adverse events were reported in the included studies. However, the small number of participants and studies, together with the poor quality of these studies, does not allow us to draw reliable conclusions.
Overall completeness and applicability of evidence
Definitive conclusions could not be reached as differences between the traditional Chinese medicine (TCM) formulations in the included studies lower the generalisability of the results regarding the effectiveness of TCM for patients with influenza.
The applicability of the included studies was limited, since their inclusion criteria, interventions, durations and outcome measures were different. More well‐designed trials are required.
Quality of the evidence
We rated the quality of the evidence from the included studies as very low to low, and the reasons for this are listed below.
Most of the retrieved studies did not give adequate descriptions of the methodology used, which may have misled us if we had not clarified the details, for example, inclusion of non‐randomised trials and classification the trials into category B rather than C. It was an exhausting but necessary process to interview every primary trial author before deciding whether to include these trials, when the methodological details were not reported. Contacting authors by telephone was more effective than writing to them because of a higher response rate. However, even after confirmation of true randomisation, we found that the methodological quality of the studies remained poor.
Allocation concealment is an important marker of trial quality. However, very few potential articles considered for our review reported or performed allocation concealment; the included trials failed to perform allocation concealment, leading to high risk of selection and confounding bias.
In one of the included trials, no blinding was used for either the participants or the investigators, which led to a high risk of performance bias. None of the studies mentioned blinding to the outcome assessors, which promotes suspicion of detection bias. Publication bias may exist as all the included studies were published in Chinese and no primary articles reporting negative results were found. The huge difference in the number of participants between the two arms raises suspicion of inadequate randomisation or a significant number of withdrawals, which may have led to high selection or attrition bias in one study (Xue 1999).
During the process of interviewing the trial authors, we understood that it was difficult for them to perform double‐blinding because of certain features associated with Chinese medicinal herbs, for example, aroma and appearance. Capsules were used in one study (Xue 1999). Other methods included extracts from herbal medicines administered by injection by using an opaque cover around the fluid bag if the herb was of a particular colour. Many trials are conducted to assess the efficacy of a plant before making the expensive decision to produce it as a patented medicine and double‐blinding is almost impossible.
All the patients in the included studies were diagnosed by epidemiology, clinical symptoms and routine tests. It is possible that participants with other acute respiratory infections not caused by the influenza virus, such as the common cold, may have been misdiagnosed as having influenza and were included in the trials. The disease duration on entry varied between the potential studies we retrieved for inclusion. Secondary bacterial infection or other complications that complicate influenza treatment may have been present, even if the trial authors did not find or report them.
In the practice of TCM, herbal preparations should match the type of 'zheng' which equates to a diagnosis. Trial authors are encouraged to explain each 'zheng' by using conventional medical terms, therefore making it more convenient for physicians and consumers to choose an appropriate preparation.
Regarding interventions, we considered the commonly used antiviral and antipyretic‐analgesic drugs to be acceptable controls. However, there is potential for bias. If the trial author knows that a 'positive' drug was used and the study was an 'equal effect test' study, there is a potential risk that the outcome detectors will consider similar results for the two groups. In this case, even double‐blinding is useless. If it is a 'superior effect test', the trial authors tend to overestimate the effect in the treatment group if allocation concealment and blinding were inadequate. When a Chinese herbal medicine combined with a supposed 'positive' intervention is found to be more effective than the 'positive' drug alone for influenza, this herbal medicine is considered effective. An alternative would be to compare Chinese medicinal herbs to a placebo (it is also recommended to compare first to placebo to test effectiveness and subsequently to compare to another treatment that was tested against placebo and proved as effective), with another 'positive' drug given to both arms.
For superior trial design, one of the key techniques for avoiding performance and detection bias is blinding. As most TCMs have particular characteristics, blinding is rather difficult. The TCM industry should develop a simulation agent when designing trials.
Although Chinese herbal medicines as a treatment for influenza and the method of manufacturing these medicines are widely accepted in China, most of the constituents of the pharmacologically prepared drugs used in trials cannot be specified. This is in marked contrast to the pharmacological agents used in conventional medicine, for which the chemical constituents, their quantities and the percentage of any impurities or contaminants are precisely known. In addition, the variation between different production batches of conventional medicines is kept within specified limits. In contrast, variation between formulations and batches of pharmacological agents is inevitable in TCM, although the Chinese government specifies the acceptable limits of variation. This variation is a factor that may contribute to any heterogeneity between different study results. The application of TCM signs is also limited as not everyone is familiar with them. However, one must accept that the overall treatment concept for TCM is different to that used in conventional medicines.
Ten included studies reported slight adverse reactions. This suggests that the TCMs used in the included studies are safe.
The definition and timing of outcome measures varied between studies. The outcome measures, defined in the primary version of this review, were based on a subjective assessment of defervescence and symptom withdrawal using dichotomous data. We may have missed additional information from studies which did not use the outcome measures stated in our original review. In this updated review, we added continuous data for duration of defervescence and symptom withdrawal, as well as influenza incidence in the prophylaxis studies. In one of the included studies (Shi 2004) ibuprofen was added temporally to patients with high fever, whereas no data were provided about how many participants in each group received the extra drug. This may have influenced the results.
TCM signs are important outcome measures in traditional practice. We will consider including TCM signs as a secondary or an additional outcome in the next update of this review. However, it is difficult to compare or quantify TCM signs as they have subjective outcomes. For example, 'mai xiang' equates to pulse presentations. Diagnosing 'mai xiang' in TCM is a complex and difficult technique, dependent on the TCM physician's experience. TCM researchers and physicians should find a gold standard method which is repeatable and easy to practice when measuring TCM signs.
In addition to the methodological limitations, the imprecision of the results is a common problem in each included study. The confidence intervals for the effects were wide in most of the results. Another problem is that most Chinese medicines were tested in one study only.
Potential biases in the review process
Most of the trials did not adequately report their methodology in the original publications, so we obtained this information by telephone communication with the authors. The studies were conducted several years ago and may be influenced by recall bias.
Agreements and disagreements with other studies or reviews
The results of well‐designed randomised controlled trials (RCTs) with large sample sizes in the future may confirm or refute our current conclusions. There is no other known systematic review of TCM for influenza.
Authors' conclusions
Implications for practice.
The current evidence is too weak to draw a conclusion which supports or rejects the use of any Chinese medicinal herbs for preventing or treating uncomplicated influenza.
Implications for research.
More studies, performed worldwide, with high methodological quality, large numbers of participants and good reporting are required to provide stronger evidence. Information on the conduct of trials should be reported in detail according to CONSORT (Moher 2010). The intervention in the control group should be a placebo, no treatment or the commonly used antiviral and antipyretic‐analgesic drugs, but not herbal medicines or a combination of drugs plus herbal medicines, until proved to be effective for influenza. Co‐interventions given equally to both arms are acceptable. The disease duration on entry should be restricted and if economics permit, laboratory tests (routine blood tests, serum tests or pathogenic examinations) and chest X‐rays should be conducted to define inclusion and exclusion criteria. Attention should also be paid to the definition of outcome measures and the incidence of adverse reactions.
What's new
| Date | Event | Description |
|---|---|---|
| 27 November 2012 | New citation required but conclusions have not changed | The conclusions remain unchanged due to the low quality of the trials. Two new review authors joined the team to update this review. |
| 27 November 2012 | New search has been performed | Searches were updated. Sixteen new studies were included (Chen 2010a; Chen 2010b; Jin 2010; Li 2009; Li 2010; Ouyang 2010; Qian 2011; Tan 2010; Wang 2010; Wei 2010; Xie 2010; Zhang 2010a; Zhang 2011; Zhao 2010; Zheng 2010b; Zhu 2010). Eleven new studies were excluded (Chen 2010c; Dou 2010; Han 2010; Han 2010; Huang 2010a; Huang 2010b; Liu 2010; Tang 2010; Zhang 2010b; Zheng 2010; Zhou 2010). |
History
Protocol first published: Issue 1, 2004 Review first published: Issue 1, 2005
| Date | Event | Description |
|---|---|---|
| 4 July 2008 | Amended | Converted to new review format. |
| 22 March 2007 | New citation required and conclusions have changed | In this 2007 updated review we added "to assess the effectiveness of Chinese medicinal herbs in preventing cases of influenza" in the Objectives section because Chinese medicinal herbs are also commonly used for preventing influenza during epidemic periods. We excluded quasi‐RCTs. We interviewed the trial authors and excluded any supposed RCTs which we discovered were in fact not randomised controlled trials. We excluded comparisons of one herbal medicine with another herbal medicine as we were uncertain of the control herb's efficacy. Accordingly, the references to studies were changed and new trials were found. We also changed the types of outcome measures because we added prophylactic studies and continuous data for analyses. As a result, the 'Description of studies', 'Risk of bias in included studies', 'Results' and 'Discussion' sections were amended. |
| 28 October 2004 | New search has been performed | Searches conducted. |
Acknowledgements
The authors thank Elizabeth Dooley for assistance; Drs. Leonard Leibovici, Bob Douglas, Alyson Huntley and Amy Kathleen Godfrey Arkle for helpful comments and advice for the primary version; and Ruth Foxlee for useful advice in constructing the search strategy. We finally wish to thank Anca Zalmanovici, Shirley Manknell, George Lewith and Mark Jones for helpful comments and advice for this updated version. We also thank the previous authors for their contributions: Guanjian Liu who was responsible for quality assessment of the trials, data extraction and data analysis; Jieqi Qiao, Xin Duan, Juan Ni, Likun Zhou, Qin Wang, Jie Zheng and Jaifu Wei, who were responsible for searching for trials.
Appendices
Appendix 1. Previous search
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2007, Issue 1), which includes the Cochrane Acute Respiratory Infections Review Group Specialised Register; MEDLINE (January 1966 to January 2007); EMBASE (January 1988 to January 2007); CBM (Chinese Biomedical Database) (January 1980 to January 2007); and the Chinese Cochrane Center's Controlled Trials Register (up to January 2007). A comprehensive and exhaustive search strategy was formulated in an attempt to identify all relevant studies regardless of language or publication status (published, unpublished, in press and in progress) using the following terms in combination with the search strategy defined by the Cochrane Collaboration and detailed in Appendix 5c of the Cochrane Reviewers' Handbook (Edition 4.0) (Alderson 2004). The search string was adapted for other databases.
MEDLINE (OVID) 1 exp INFLUENZA/ 2 influenza.mp. 3 or/1‐2 4 exp Medicine, Chinese Traditional/ 5 exp Medicine, Oriental Traditional/ 6 exp Drugs, Chinese Herbal/ 7 exp Plants, Medicinal/ 8 chinese herb$.mp. 9 (chinese adj medic$).mp. 10 (medicin$ adj herb$).mp. 11 or/4‐10 12 3 and 11
After scanning the full articles, we excluded studies which were not RCTs or clinical trials.
We also searched databases of ongoing trials: Current Controlled Trials (www.controlled‐trials.com); and The National Research Register (http://www.update‐software.com/National/). We attempted to identify additional studies by searching the reference lists of relevant trials, reviews, conference proceedings and journals. In particular, with respect to journals, we searched those not indexed in the electronic databases.
Organisations (including the WHO), individual researchers working in the field and medicinal herbal manufacturers were contacted in order to obtain additional references, unpublished trials, ongoing trials, confidential reports and raw data for published trials.
2 Embase.com search strategy
17. #12 AND #16 16. #13 OR #14 OR #15 15. random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross‐over':ab,ti OR 'cross over':ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti OR ((singl* OR doubl*) NEAR/2 (mask* OR blind*)):ab,ti 14. 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp 13. 'randomized controlled trial'/exp 12. #4 AND #11 11. #5 OR #6 OR #7 OR #8 OR #9 OR #10 10. (medic* NEAR/2 herb*):ab,ti 9. (chinese NEAR/4 (herb* OR medic*)):ab,ti 8. 'medicinal plant'/exp 7. 'herbal medicine'/exp 6. 'oriental medicine'/exp 5. 'chinese medicine'/exp 4. #1 OR #2 OR #3 3. influenza:ab,ti OR flu:ab,ti 2. 'influenza virus'/exp 1. 'influenza'/exp
Appendix 2. EMBASE.com search strategy
#21 #12 AND #20 #20 #15 NOT #19 #19 #16 NOT #18 #18 #16 AND #17 #17 'human'/de #16 'animal'/de OR 'nonhuman'/de OR 'animal experiment'/de #15 #13 OR #14 #14 random*:ab,ti OR placebo*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR allocat*:ab,ti OR trial:ti OR (doubl* NEXT/1 blind*):ab,ti #13 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp #12 #3 AND #11 #11 #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 #10 (integrat* NEAR/2 medic*):ab,ti #9 'integrative medicine'/de #8 (chinese NEAR/4 (herb* OR medic*)):ab,ti OR (medic* NEAR/2 (herb* OR plant*)):ab,ti #7 'medicinal plant'/exp #6 'herbaceous agent'/de OR 'herbal medicine'/de #5 'traditional medicine'/de #4 'chinese medicine'/exp OR 'oriental medicine'/de #3 #1 OR #2 #2 influenza*:ab,ti OR flu:ab,ti #1 'influenza'/exp OR 'influenza virus'/exp
Appendix 3. CNKI database search
Search results in the Chinese database CNKI up to November 2012
#1 流感:11519 hits
#2 甲流: 1459 hits
#3 H1N1: 3352 hits
#4 禽流感: 1665 hits
#5 中药: 104873 hits
#6 中草药: 92658 hits
#7. (or/#1˜#4) AND (#5 or #6): 1487 hits
Data and analyses
Comparison 1. Traditional Chinese medicine (TCM) versus other treatments.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recovery | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Fanggan decoction versus conventional medicine | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.67, 1.56] |
| 1.2 Fever resolved within 24 hours after treatment (Lianhua Qingwen capsule versus oseltamivir) | 1 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.66, 1.38] |
| 1.3 Fever resolved within 24 hours after treatment (Lianhua Qingwen capsule versus control 2) | 1 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [1.03, 3.52] |
| 1.4 Ganmao capsule versus amantadine | 1 | 738 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.38, 0.61] |
| 1.5 Ganmao capsule versus amantadine (Day 2) | 1 | 432 | Risk Ratio (M‐H, Random, 95% CI) | 5.17 [3.82, 6.99] |
| 1.6 E Shu You versus ribavirin (Day 3) | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 2.18 [0.87, 5.43] |
| 1.7 Tanreqing + oseltamivir versus oseltamivir | 2 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.87, 2.00] |
| 1.8 TCM versus oseltamivir | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.50, 2.52] |
| 1.9 Antiwei versus placebo (day 4) | 1 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 3.80 [1.23, 11.72] |
| 2 Time to fever clearance | 10 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 TCM versus oseltamivir | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Gegeng Tang granule + oseltamivir versus oseltamivir | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Lianhua Qingwen capsule versus oseltamivir | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 TCM + oseltamivir versus oseltamivir | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Tanreqing injection + oseltamivir versus oseltamivir | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Fanggan granule versus conventional medicine | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Duration of cough | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Fanggan decoction versus conventional medicine | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Ge Geng Tang granule + oseltamivir versus oseltamivir | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Tanreqing injection + oseltamivir versus oseltamivir | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 TCM versus oseltamivir | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Time to remission of muscle pain | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Lianhua Qingwen capsule | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 TCM versus oseltamivir | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Time to symptom remission | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 TCM versus oseltamivir | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Fanggan granule versus conventional medicine | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Gegeng Tang granule + oseltamivir versus oseltamivir (body pain) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 TCM + oseltamivir versus oseltamivir | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Time to remission of sore throat | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 TCM versus oseltamivir | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐15.60 [‐17.87, ‐13.33] |
| 7 Length of hospital stay | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 TCM versus oseltamivir | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Tanreqing injection + oseltamivir versus oseltamivir | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 TCM plus oseltamivir versus oseltamivir | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 TCM versus placebo | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 No improvement or worsening influenza | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 E Shu You versus ribavirin (Day 3) | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.14, 1.17] |
| 8.2 Fanggan decoction versus conventional medicines | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.24, 2.05] |
| 8.3 Tanreqing injection + oseltamivir versus oseltamivir | 2 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.17, 0.80] |
| 8.4 TCM versus oseltamivir | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.19, 20.90] |
| 8.5 Lianhua Qingwen versus oseltamivir | 1 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.22, 4.46] |
| 8.6 Lianhua Qingwen versus Anga huangmin | 1 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.09, 0.46] |
| 8.7 Ganmao capsule versus amantadine | 1 | 951 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.10, 0.21] |
| 9 Partial improvement | 6 | 703 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.82, 1.36] |
| 9.1 Tanreqing injection + oseltamivir | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.6 [0.23, 1.54] |
| 9.2 Fanggan granule versus Western medicine | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.66, 1.78] |
| 9.3 Lianhua Qingwen versus oseltamivir | 1 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 1.2 [0.50, 2.87] |
| 9.4 Lianhua Qingwen capsule versus An ga huang min | 1 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 1.2 [0.50, 2.87] |
| 9.5 Tanreqing + oseltamivir versus oseltamivir | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.84, 1.96] |
| 9.6 TCM versus oseltamivir | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.15, 1.29] |
| 9.7 E Shu You versus ribavirin | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.36, 2.27] |
| 10 Adverse events | 10 | 1011 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.26, 1.11] |
| 10.1 TCM versus oseltamivir | 3 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.04, 32.35] |
| 10.2 Lianhua Qingwen versus oseltamivir | 3 | 241 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.11, 1.02] |
| 10.3 Fanggan granule versus conventional medicine | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.14, 6.67] |
| 10.4 Antiwei versus placebo | 1 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.5 Tanreqing plus oseltamivir versus oseltamivir | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.6 E Shu You versus Lymbaweilim | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.11, 3.36] |
| 10.7 Lianhua Qingwen versus An ga huang min | 1 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.08, 6.78] |
1.3. Analysis.
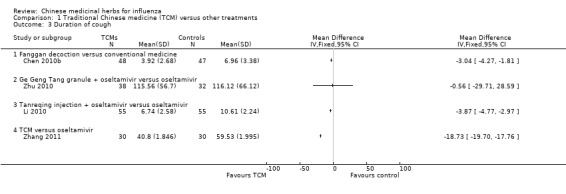
Comparison 1 Traditional Chinese medicine (TCM) versus other treatments, Outcome 3 Duration of cough.
1.4. Analysis.
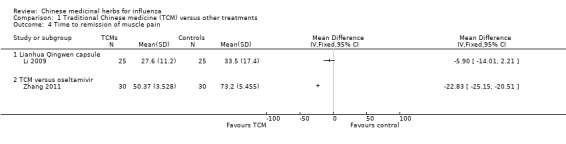
Comparison 1 Traditional Chinese medicine (TCM) versus other treatments, Outcome 4 Time to remission of muscle pain.
1.5. Analysis.
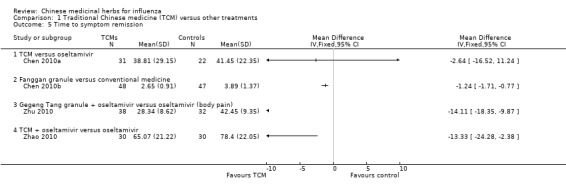
Comparison 1 Traditional Chinese medicine (TCM) versus other treatments, Outcome 5 Time to symptom remission.
1.6. Analysis.
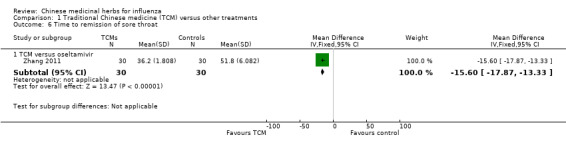
Comparison 1 Traditional Chinese medicine (TCM) versus other treatments, Outcome 6 Time to remission of sore throat.
1.7. Analysis.
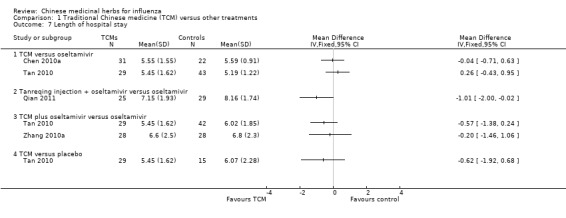
Comparison 1 Traditional Chinese medicine (TCM) versus other treatments, Outcome 7 Length of hospital stay.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chen 2010a.
| Methods | That "computer randomisation form was used to allocate the participants" was mentioned in the text. Traditional Chinese medicine (TCM) plus placebo group versus oseltamivir group | |
| Participants | Participants were confirmed with influenza A H1N1 according to the Diagnostic Standard by Ministry of Health, China (Second edition, 2009). Ages ranged from 5 to 65 years and temperature was above 37.5 degrees Celsius. Patients had influenza symptoms. Patients with serious diseases of any origin, for example, kidney, heart, lung, blood vessel, nervous system, metabolic diseases, immunodeficiency diseases, tumours, hepatitis or cirrhosis, pregnant woman, or accepting hormone or immune inhibitor therapy, were excluded. In total 55 participants were included. 31 (male 18, female 13) were allocated to the TCM plus placebo group, 22 (male 9, female 13) to the control group | |
| Interventions | Participants in the TCM group were given modified Yin Qiao Shan or Huo Bo Xia Lin Tang or Pu Ji Xiao Du Yin for the acute phase of the influenza and placebo (simulation agent of oseltamivir) 75 mg, twice a day for 5 days. In the convalescent phase, participants were given 150 ml Shang Ju Yin in the morning and evening in the TCM group. Oseltamivir was given to participants in the control group (75 mg) twice a day | |
| Outcomes | Primary outcome: 1. Length of disease (time to symptom clearance) Secondary outcomes: 1. Length of hospitalisation 2. Rate of complications 3. Adverse events |
|
| Notes | Adverse reaction mentioned: 1 participant had diarrhea in the TCM group, 1 case of rash and 1 case of vomiting in the control group. There was no statistically significant difference between the 2 groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Who generated the randomisation sequence and which software was used was not mentioned; the numbers of participants in the 2 arms were not balanced (31:22). This suggests that the randomisation is questionable |
| Allocation concealment (selection bias) | High risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | No TCM placebo was used |
| Selective reporting (reporting bias) | Unclear risk | There was potential selective reporting bias due to the imbalanced numbers of participants in the 2 arms 2 participants withdrew due to allergic reactions or adverse reactions |
| Other bias | High risk | The prescriptions were made by the authors |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Although a placebo was used, the participants still knew which intervention was the TCM |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome assessors knew who took the TCM |
Chen 2010b.
| Methods | Parallel‐group design; a random number table was used to allocate the participants | |
| Participants | The participants were diagnosed by laboratory tests 48 participants were allocated to the TCM group and 47 to the control group. The temperatures were higher than 38.5 degrees Celsius |
|
| Interventions | TCM group used Fanggan granule, 2 times a day for 3 to 5 days. Conventional medicine group used anti‐symptom drugs; participants with high fever were given anti‐fever treatment. No detailed information about the drugs | |
| Outcomes | 1. Effect or no effect (recovery or no improvement), judged by changes in the symptoms 2. Chest X‐ray 3. Liver and renal function 4. Throat swabs test |
|
| Notes | The time point of assessment of outcome recovery/no improvement was not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random number table was used to "allocate" the participants. The description is not adequate (should be "used to generate the allocation sequence") |
| Allocation concealment (selection bias) | High risk | Not used |
| Blinding (performance bias and detection bias) All outcomes | High risk | Could not be blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | |
Jin 2010.
| Methods | "Randomly allocated patients" mentioned but the randomisation method used was not described. Parallel‐group design. No blinding. The study was conducted in Hebei Province TCM hospital, China | |
| Participants | 68 participants were randomly allocated to 2 groups; 34 in each group | |
| Interventions | Group 1 was given the doctor‐prepared Qinfei Jiedu decoction Group 2 was given oseltamivir |
|
| Outcomes | 1. Change in sore throat 2. Change in cough, sputum |
|
| Notes | The study data were not analyzed because unfeasible methods were used to assess the effects and the reporting was unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not mentioned |
| Allocation concealment (selection bias) | High risk | Not used |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned |
| Other bias | High risk | The decoction was self prepared by the trialist, so there was high risk of conflict of interest |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not used |
Li 2009.
| Methods | "Randomly allocated the patients, signed consent inform" mentioned but lack of information of what method used for generation of allocation sequence. Blinding, parallel‐group design, conducted in the Kaifeng City Infectious Diseases Hospital, Henan, China | |
| Participants | 50 participants with H1N1 type influenza were included, 25 in each group | |
| Interventions | Vitamin B Complex ‐ Squibb 1 pill/day was administrated in each group. Addition to this, Lianhua Qingwen capsule was given to the experimental group orally 4 grains/time, 3 times a day and the oseltamivir simulation agent capsule. In the control group, oral administration oseltamivir capsule 75 mg, twice a day and Lianhua Qingwen capsule simulation agent capsule | |
| Outcomes | 1. Time to fever clearance 2. Normalisation of virus nucleic acid 3. Time to influenza symptom improvement 4. Adverse events |
|
| Notes | We did not identify the randomisation procedure by contacting the trialist | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly allocate the patients" mentioned but the method was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Simulation agents were used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Simulation agents were used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Simulation agents were used |
Li 2010.
| Methods | "Randomly allocated the patients" was mentioned but the randomisation method used was not described. The study was conducted in Yantai City Infectious Diseases Hospital, China. No blinding | |
| Participants | 110 participants were included, 55 in each group | |
| Interventions | Oseltamivir was given in both groups; additionally the experimental group was given Tanreqing injection 20 ml/day, intravenous infusion; treatment duration 7 to 14 days | |
| Outcomes | 1. Change of symptoms 2. Recovery, improvement, no improvement |
|
| Notes | The method used to assess the effects was not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Generation of allocation sequence not mentioned |
| Allocation concealment (selection bias) | High risk | Not used |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear |
| Selective reporting (reporting bias) | Unclear risk | Not clear |
| Other bias | High risk | |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not used |
Ouyang 2010.
| Methods | Parallel groups. Recruiting duration from September 2009 to March 2010 in 2 tertiary hospitals. Blinding was not used | |
| Participants | Participants with fever (temperature higher than 38.0 degrees) and with typical symptoms of influenza, diagnosed by the positive findings of real‐time Reverse Transcription Polymerase Chain Reaction (RT‐PCR) test in throat swabs. The eligible participants had no history of administrating any medicine in the past 48 hours. Participants were allocated by the ratio of 4:1:1 into 3 groups: 116 in the Lianhua Qingwen capsule group, 29 in the oseltamivir group and 29 in the paracetamol, caffeine, artificial cow‐bezoar and chlorphenamine maleate capsules group | |
| Interventions | Group 1: Lianhua Qingwen capsule 1.4 G/time for adults, 0.35 to 0.70 G/time for children, 3 times a day Group 2: oseltamivir 75 mg for adults, 2 mg/kg for children, 2 times a day Group 3: paracetamol, caffeine, artificial cow‐bezoar and chlorphenamine maleate capsules 2 pieces once for adults and half or 1 piece once for children, 3 times a day Total of 5 days |
|
| Outcomes | 1. Effect of fever clearance; efficacy was defined as the fever abated within 24 hours of treatment 2. No improvement in fever clearance was defined as the temperature down less than 0.5 degree 3. Time to fever clearance 4. Effect on symptoms improvement |
|
| Notes | We decided not to include the "efficacy on changing of symptoms" data for analysis, because the time point was unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly allocated patients" was mentioned but no description of the method of randomisation |
| Allocation concealment (selection bias) | High risk | Not used |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not used |
Qian 2011.
| Methods | "Randomly allocated patients" was mentioned, but the randomisation method was not described. Parallel groups, not blinded. The study was conducted in the Third Hospital of Nantong University, Jiangsu, China | |
| Participants | All 57 participants were diagnosed by the test of nucleic acid of H1N1 influenza and complicated with pneumonia. 25 participants (11 male/14 female) in the experimental group, 29 (16 male/13 female) in the control group | |
| Interventions | Supportive therapy included breath oxygen and infusion, alfa‐1 thymosin muscle injection and oseltamivir capsule 75 mg, 2 times a day was given in both groups. Antibiotics were given when anyone had a bacterial infection. In additional to this, Tanreqing injection 20 ml was added to 250 ml 0.9% NaCI for intravenous infusion per day | |
| Outcomes | 1. Time to fever clearance 2. Normalisation of virus test 3. Hospitalisation |
|
| Notes | The number of participants in the 2 groups was not balanced. Loss to follow‐up was not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Whether there was an allocation sequence was not clear and the method used to generate the allocation sequence was not mentioned |
| Allocation concealment (selection bias) | High risk | Allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not used |
Shi 2004.
| Methods | Trial design: randomised, controlled, parallel study Randomisation procedure: random number generated by New Drug Statistical Treatment statistical software Blinding: no blinding | |
| Participants | Country: China Setting: Hangzhou, Zhejiang province 61 children with type B influenza (32 cases in the therapy group, 29 cases in the control group) Diagnostic criteria: (1) sudden fever; (2) accompanied by respiratory catarrh symptoms or alimentary tract symptoms such as abdominal pain, vomiting, diarrhea. Examination of stool sample and vomitus under microscope was negative; (3) may be accompanied with headache and myalgia; (4) physical examination found diffused congestion of pharyngeal cavity or hyperplasia of lymph follicle in the pharyngeal posterior wall; (5) over 5 people who had been in contact had similar symptoms; (6) WBC count of routine blood test was normal or decreased, neutrophil cell count was normal or lymphatic cell count was high Baseline: gender, age, disease duration and severity of disease were similar in the 2 groups (P > 0.05) Withdrawal: not reported | |
| Interventions | 1. TCM group: E Shu You glucose injection containing 0.1g E Shu You and 12.5 G glucose per 250 ml injection (10 mg/kg/day intravenous injection 4 times a day) 2. Control group: ribavirin injection (10 to 15 mg/kg/day ) + vitamin C (50 mg/kg/d) + 10% glucose 500 ml: 10% normal saline 10 ml intravenous injection 4 times a day Erythromycin capsule 30 mg to 50 mg orally 3 times a day was given to both groups. Ibuprofen was given temporarily to patients with high fever Treatment duration was 3 to 5 days | |
| Outcomes | Recovery: temperature falls to normal within 72 hours, symptoms and physical signs had improved by more than 90% Marked improvement: temperature falls to normal within 72 hours, symptoms and physical signs had improved by more than 70% General improvement: temperature falls but not to normal within 72 hours, symptoms and physical signs had improved by more than 30% No improvement: temperature does not fall or even increases within 72 hours, symptoms and physical signs improve by less than 30% | |
| Notes | Influenza virus B was isolated by CDC in Hangzhou city in this local epidemic | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not used |
Tan 2010.
| Methods | 4 parallel groups design. "Random number table method was used to allocate patients" was mentioned, but who and how was not mentioned in detail. The study was conducted in the Eighth Hospital of Guangzhou and the Third Hospital of Shenzhen city, China. No blinding | |
| Participants | Total 129 participants were included, 29 in TCM group, 43 in oseltamivir group, 42 in TCM + oseltamivir group, 15 in supportive treatment group | |
| Interventions | TCM versus oseltamivir versus TCM + oseltamivir versus support treatment. Anyone suffering bacterial infection was given antibiotics | |
| Outcomes | 1. Normalisation of virus test 2. Fever clearance time |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Unbalanced number of participants suggests that this may not be a RCT |
| Allocation concealment (selection bias) | High risk | Not used |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not used |
Wang 2010.
| Methods | Parallel groups, double‐blinding, placebo control. The study was conducted in 8 Chinese centres led by the West China Hospital, Sichuan University. The allocation sequence was generated by computer software SAS and the allocation details were sealed in an envelope unknown both to investigators and participants. The placebo granule was composed of starch and bitter agents, but was visually indistinguishable from the Antiwei in appearance, colour, size and packaging | |
| Participants | 225 participants were confirmed with influenza from 480 adults with influenza‐like symptoms. 177 participants were allocated to the intervention group and 48 to the control group | |
| Interventions | Chinese herbal medicine Antiwei was given 6 G twice daily in the intervention group and placebo in the control | |
| Outcomes | Primary outcomes: 1. Recovery: all symptoms abated after 3 days of treatment 2. Reduction of severity of illness measured by the mean symptom scores Secondary outcomes: 3. Time to resolution of fever 4. The severity of each symptom 5. The rate of influenza virus‐positive conversion to negative 6. Adverse effects: the participants were required to record any unexpected signs |
|
| Notes | The calculation of sample size was explained as: a sample size of 135 for the Antiwei group and 45 for the placebo group, at a ratio of 3:1, calculated according to published data to have a power of 80% or greater to detect a difference of 10% in recovery rate, assuming a significance level of 0.05 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible randomisation |
| Allocation concealment (selection bias) | Low risk | Eligible concealment of allocation procedure |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinding performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Free to withdraw from the study at any time" was mentioned |
| Selective reporting (reporting bias) | Unclear risk | We checked the registered data: lack of reporting of the rate of influenza virus‐positive conversion to negative and length of time to alleviation of fever within the first 24 hours as described in the protocol. Some results were reported by percentages but not in detail, such as improved symptom score for fever, cough and expectoration. We were unable to abstract the data for analysis |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blinding performed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinding performed |
Wei 2010.
| Methods | Parallel groups. "Randomly allocated patients" mentioned, but lack of description of the randomisation method. No blinding | |
| Participants | 30 participants with mild type A influenza (H1N1) in the group 1 and 16 in group 2 | |
| Interventions | Group 1: Lianhua Qingwen capsule 4 pieces, 3 times a day, 3 to 5 days Group 2: oseltamivir 75 mg, 2 times a day, 3 to 5 days |
|
| Outcomes | 1. Time to fever clearance 2. Time to symptoms clearance |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Randomly allocated patients" mentioned, but lack of description of the method |
| Allocation concealment (selection bias) | High risk | Not used |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | |
Xie 2010.
| Methods | "Randomly allocated patients" was mentioned, but no any information about whether there was an allocation sequence and what method was used to generate the sequence. Parallel groups, no blinding. The study was conducted in Fangcheng City, Guangxi, China | |
| Participants | Total of 87 participants were included, 44 in the experimental group, 43 in the control group | |
| Interventions | Supportive treatment and oseltamivir were given in both groups, the participants with high fever were given Tylenol. In addition to this, Tanreqing was given by intravenous infusion | |
| Outcomes | 1. Obvious improvement: the temperature normalised 12 to 48 hours after treatment, the symptoms disappeared or decreased 2. Improvement: the temperature normalised 48 to 72 hours after treatment, the symptoms disappeared or were much better 3. No improvement: the temperature normalised more than 72 hours after treatment, no change in symptoms |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Although "randomly allocated patients" was mentioned, no information about how and who performed the allocation |
| Allocation concealment (selection bias) | High risk | Not used |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
Xue 1999.
| Methods | Trial design: randomised, controlled, parallel study Randomisation procedure: the allocation sequence was generated by computer software Blinding: double‐blinding | |
| Participants | Country: China Setting: influenza epidemic area 951 healthy participants and participants with influenza were recruited in this trial (519 cases in the therapy group with 124 influenza participants, male/female 316/203; 432 in control group with 89 influenza participants male/female 263/169) Data from healthy participants at entry were used for analyses of the prevention study. Data from those with influenza at entry and who subsequently developed influenza from the prevention study were used in the treatment analyses All the participants had similar typical influenza symptoms and disease duration within 48 hours In the treatment study: 202 participants were in the therapy group and 230 participants were in the control group Withdrawals: not reported | |
| Interventions | 1. TCM group (trial group): Ganmao capsule (3.5 G, 3 times a day orally) 2. Control group: amantadine capsule (0.07 G, 3 times a day, orally) Therapy duration was 7 days for both the prevention and treatment studies | |
| Outcomes | Influenza morbidity within 7 days of treatment Recovery: the systemic symptoms and local typical symptoms clear within 24 hours to 48 hours after administration Ineffectiveness: other than those who achieved recovery the rest of patients were defined as inefficacy Patients using other drugs during the study were not included in the effectiveness statistics | |
| Notes | Influenza virus A3 was isolated by the Center of Disease Prevention and Control (CDC) in Tianjin city in local epidemics | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
Zhang 2010a.
| Methods | Parallel groups. "Randomly allocated patients" mentioned, but lack of description of the method of randomisation. No blinding | |
| Participants | 56 participants were inpatients, 28 in each group. The rates of males/females in the 2 groups were: 19/9 in the experimental group and 15/13 in the control group | |
| Interventions | Same basic treatment in 2 groups Treatment group: Ju Lan Qing Du decoction 2 to 3 times a day, with oseltamivir 75 mg twice a day Control group: Ju Lan Qing Du decoction alone, 2 to 3 times a day |
|
| Outcomes | 1. Time to fever clearance 2. Length of hospital stay |
|
| Notes | The Ju Lan Qing Du decoction was prepared by the author's hospital | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Randomly allocate the patients" mentioned, but the method of randomisation unclear |
| Allocation concealment (selection bias) | High risk | Not used |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear |
| Selective reporting (reporting bias) | Unclear risk | Not clear |
| Other bias | High risk | Potential conflict of interest |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not used |
Zhang 2011.
| Methods | "Parallel group, randomised control" was mentioned, but lacked detailed description of the method. No blinding. The study was conducted in the Chengdu City Infectious Diseases Hospital, but the authors' institution is Chengdu TCM University Hospital | |
| Participants | Total of 60 participants included, 30 in each group | |
| Interventions | Experimental group: the preparation recommended by Sichuan Province TCM Administration Control group: oseltamivir 75 mg, 2 times a day for 5 days |
|
| Outcomes | 1. Change in symptom scores 2. Time to fever clearance 3. Remission time of cough 4. Remission time of sore throat 5. Remission time of muscle pain |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Random" was just mentioned, but the randomisation procedure was not described in detail |
| Allocation concealment (selection bias) | High risk | Not used |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | High risk | The decoction was made by the hospital |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not used |
Zhao 2010.
| Methods | "Randomly allocated patients" was mentioned, but no detailed information about the randomisation procedure. No blinding. The study was conducted in The Second Hospital of Yinchuan City, Ningxia, China | |
| Participants | Total of 60 participants included, 30 in each group. The diagnosis of influenza A H1N1 for 9 participants in the experimental group and 11 in the control group was confirmed | |
| Interventions | Oseltamivir 75 mg 2 times a day for adults and 45 mg 2 times a day for children for all participants in the 2 groups. Antibiotics were given for bacterial infections In addition to this, the participants in the experimental group were given self made Qingwen Tuire decoction |
|
| Outcomes | 1. Time to fever clearance 2. Time to main symptom remission |
|
| Notes | There was no definition of change of symptoms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The randomisation procedure was not described |
| Allocation concealment (selection bias) | High risk | Not used |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | High risk | Conflict of interest |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not used |
Zheng 2010b.
| Methods | Participants were allocated by drawing lots, but no detailed information about blinding given | |
| Participants | Participants with fever (temperature higher than 37.8 degrees) and with typical symptoms of influenza, diagnosed by a positive finding of real‐time RT‐PCR test in throat swabs. The eligible participants had no history of administration of any medicine in past 48 hours. The symptoms of influenza had lasted over 48 hours and within 72 hours Participants were allocated into 3 groups: 19 in the oseltamivir group, 14 in the TCM treatment group and 15 in the oseltamivir and TCM treatment group |
|
| Interventions | TCM versus oseltamivir versus TCM combined with oseltamivir | |
| Outcomes | 1. Total course of disease 2. Duration of flu symptoms (except cough) 3. Time until A/H1N1 virus disappeared 4. Symptom remission time of fever 5. Symptom remission time of cough |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocated patients by drawing lots |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
Zhu 2010.
| Methods | Parallel groups, no blinding. "Out‐patients" or "in‐patients" were included. "Randomly allocated patients" was mentioned, but no description of what method was used | |
| Participants | 70 participants were included, 38 in experimental group (25 males, 13 females), 32 in the control group (22 males, 10 females) | |
| Interventions | Oseltamivir 75 mg, twice a day orally in both groups. Also, in the experimental group, Ge Geng Tang granule 4 G each, 3 times a day, was given. Duration of treatment was 5 days, follow‐up 2 days | |
| Outcomes | 1. Time to fever clearance 2. Time to symptom abatement, including cough and sore throat |
|
| Notes | Did not stated who provided the Ge Geng Tang granule | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The numbers of participants in the 2 groups were not balanced, while the method of randomisation was not described, therefore the randomisation is questionable |
| Allocation concealment (selection bias) | High risk | Not used |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not used |
b.i.d.: twice a day CDC: Center for Disease Control and Prevention h: hours i.v.: intravenous M/F: male/female NDST: New Drug Statistical Treatment q.d.: once a day RT‐PCR: reverse transcription polymerase chain reaction TCM: traditional Chinese medicine t.i.d: three times a day WBC: white blood cell
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chen 2010c | "Randomly allocated the patients" mentioned but the patients were allocated optionally by the doctor |
| Dou 2010 | "Randomly allocated patients" was mentioned in the abstract, but in the text the author stated "we retrospectively analysed the data". Therefore, we judged it not to be a RCT |
| Du 1991 | It was claimed to be a "RCT". We telephone interviewed the trial author and learned that it was not a RCT |
| Hamazaki 2006 | Outcome measures were haemagglutinin titers and natural killer (NK) activity which did not match our outcome measures as defined in this review |
| Han 2010 | "Randomly allocated the patients" mentioned but the patients were allocated optionally by the doctor |
| Hang 1998 | Observation duration (3 to 5 days) exceeded the criteria for observational span specified in this review |
| Hou 2002 | It was claimed to be a "RCT". We telephone interviewed the trial author and learned that it was a quasi‐RCT using alternative allocation |
| Huang 2003 | A quasi‐RCT. Participants were allocated according to the odd/even entry days |
| Huang 2010a | "Randomly allocated the patients" mentioned but the patients were allocated optionally by the doctor |
| Huang 2010b | "Randomly allocated patients" was mentioned. The first author was telephoned to identify the randomisation procedure. The first author said "no any random method was used, the patients were allocated according to their symptom". Therefore, this was not a RCT |
| Jiang 2003 | One TCM was compared to another TCM |
| Jin 1998 | Complications of influenza were included in this study |
| Kubo 2007 | The drug used was a Japanese herbal medicine |
| Li 2001 | Claimed to be a "RCT". We telephone interviewed the trial author and learned that it was not a RCT |
| Li 2005 | Participants had severe influenza with complications |
| Lindenmuth 2000 | Participants had severe influenza with complications and the common cold and influenza were not analyzed separately |
| Liu 2002 | The participants had severe influenza with pneumonia complications ‐ found by lab tests and chest radiographs |
| Liu 2010 | Compared one TCM versus other TCM |
| Lu 2004 | Participants with common cold and influenza were included and data for influenza were not separately reported |
| Qu 2005 | Claimed to be a "RCT". We telephone interviewed the trial author and learned that it was a quasi‐RCT using alternative allocation. Participants were allocated according to odd/even entry days |
| SRCG 1981 | This was a prevention and not a treatment study. Baihua Baijiang (Whiteflower Patrinia Herb) was used to prevent influenza |
| Tang 2010 | The data were reported briefly, but could not be analyzed |
| Wang 2001 | Herbal medicine was compared with chemical medicine plus another Chinese patent medicine |
| Xia 2010 | "Randomly allocated the patients" mentioned, but the patients were actually allocated optionally by the doctor |
| Xu 2001 | Claimed to be a "RCT". We telephone interviewed the trial author and learned that it was a quasi‐RCT using alternative allocation |
| Yang 2000a | Claimed to be a "RCT". We telephone interviewed the trial author and learned that it was a quasi‐RCT using alternative allocation |
| Yang 2000b | Herbal medicines were compared with chemical medicines plus another Chinese patented medicine |
| Yang 2005a | One TCM was compared to another TCM |
| Yang 2005b | One TCM was compared to another TCM |
| Yao 2003 | Claimed to be a "RCT". We telephone interviewed the trial author and learned that it was a quasi‐RCT using alternative allocation |
| Yu 2000 | One TCM was compared to a chemical medicine plus another TCM |
| Yuan 2003 | Claimed to be a "RCT". We telephone interviewed the trial author and learned that it was not a RCT |
| Zeng 2004 | Herbal medicines were compared with chemical medicines plus another Chinese patented medicine. The patients had complications of pneumonia, bronchitis and tonsillitis |
| Zhang 2000 | The patients had the complication of pneumonia, found by lab tests and chest radiographs |
| Zhang 2002 | One TCM was compared to another TCM |
| Zhang 2004 | One TCM was compared to another TCM plus an antiviral drug |
| Zhang 2005 | One TCM was compared to another TCM |
| Zhang 2010b | "Randomly allocated the patients" mentioned but the patients were allocated optionally by the doctor |
| Zhao 2006 | One TCM was compared to another TCM |
| Zheng 2010 | "Randomly allocated patients by ballot" was mentioned, but the number of participants in the 3 groups had an odd rate. We therefore judged that this was not a RCT |
| Zhong 2005 | One herbal medicine was compared to another Chinese patented medicine |
| Zhou 2010 | "Randomly allocated the patients" mentioned but the patients were allocated optionally by the doctor |
RCT: randomised controlled trial TCM: traditional Chinese medicine
Characteristics of studies awaiting assessment [ordered by study ID]
Qiu 1997.
| Methods | "Randomly allocated patients" was mentioned but no information on the randomisation |
| Participants | Total of 170 influenza participants with fever |
| Interventions | Chinese herbal medicine and conventional medicine versus conventional medicine |
| Outcomes | Efficacy |
| Notes |
Song 2002.
| Methods | "Randomly allocated patients" was mentioned but no information about the randomisation procedure in detail |
| Participants | 395 participants were included |
| Interventions | Subiao Jiedu decoction versus Subiao Jiedu decoction + conventional medicine versus conventional medicine |
| Outcomes | Marked efficacy, efficacy, no efficacy |
| Notes | Only 1 author to conduct this study including 395 participants is questionable |
Wang 2011.
| Methods | Prospective, non‐blinded, randomised, controlled trial (ClinicalTrials.gov registration number: NCT00935194) |
| Participants | Setting: 11 hospitals from 4 provinces in China 410 people [corrected] aged 15 to 69 [corrected] years with laboratory‐confirmed H1N1 influenza |
| Interventions | Oseltamivir, 75 mg twice daily, maxingshiman‐yinqiaosan decoction (composed of 12 Chinese herbal medicines, including honeyfried herba Ephedrae), 200 ml 4 times daily; oseltamivir plus maxingshigan‐yinqiaosan; or no intervention (control). Interventions and control were given for 5 days |
| Outcomes | Primary outcome was time to fever resolution. Secondary outcomes included symptom scores and viral shedding determined by using real‐time reverse transcriptase polymerase chain reaction |
| Notes |
Contributions of authors
Lanhui Jiang (JLH), Linyu Deng (DLY) and Wu Taixiang (WTX) were responsible for developing the protocol, searching for trials, quality assessment of the trials, data extraction, data analysis, review development and updating this 2012 version.
Sources of support
Internal sources
Chinese Cochrane Center, West China Hospital of Sichuan University, China.
External sources
Chinese Medical Board of New York (CMB), USA.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Chen 2010a {published data only}
- Chen H, Zeng YL, Liu DF, Ye Q, Li LH, Li Y, et al. The stochastically control investigation of oseltamivir and traditional Chinese medicine in treating influenza A H1N1. Sichuan Medical Journal 2010;31(8):1050‐1. [Google Scholar]
Chen 2010b {published data only}
- Chen F, Song K, Zhu XH, Chen H. The clinical study of the H1N1 influenza virus with fanggan decoction. Journal of Zhejiang University of Traditional Chinese Medicine 2010;34(5):658‐60. [Google Scholar]
Jin 2010 {published data only}
- Jin F, Zhang JH, Jin PL. Observation for effect of Qingfei Jiedu therapy in the treatment of patients with suspected H1N1 type influenza with "Redu Xifei combined sore throat". Journal of Sichuan of Traditional Chinese Medicine 2010;28(8):81‐2. [Google Scholar]
Li 2009 {published data only}
- Li BF, Zhang CQ, Fu M, Bai W, Liu BH, Li SS. Clinical study for Lianhua Qingwen capsule in the treatment of H1N1 influenza. Journal of Medical Forum 2009;30(23):91‐2. [Google Scholar]
Li 2010 {published data only}
- Li G. Observation of Tanreqing injection combined with oseltamivir in influenza A (H1N1). Journal of Emergency of Traditional Chinese Medicine 2010;19(10):1681‐2. [Google Scholar]
Ouyang 2010 {published data only}
- Ouyang HX, Tang QY, Chen YH, Wei Y, Li GS. Clinical observation of Lianhua Qingwen emergency department capsules in treatment of the influenza A/H1N1. China Medical Herald 2010;7(30):6‐8. [Google Scholar]
Qian 2011 {published data only}
- Qian J, Xu JR, Shi LQ. Observation for the effect of Tanreqing injection combined with oseltamivir in the treatment of type A/H1N1 influenza. Jilin Medicine 2011;32(2):266‐7. [Google Scholar]
Shi 2004 {published data only}
- Shi XX, Chen MM. Clinical effect of E Shu You glucose injection in the treatment of type B influenza in children. Zhong Guo Lin Chuang Yao Xue Za Zhi [Chinese Journal of Clinical Pharmacy] 2004;13(1):38‐40. [Google Scholar]
Tan 2010 {published data only}
- Tan XH, Liu YX, Xia Z, Li HJ, Meng J, Fu JP, et al. Clinical study of integrated Chinese‐Western therapy for a new type of swine‐origin influenza A. Journal of Guangzhou University of Traditional Chinese Medicine 2010;27(5):441‐4. [Google Scholar]
Wang 2010 {published data only}
- Wang L, Zhang RM, Wei BL, Wang Y, Cai HY, Li FS, et al. Chinese herbs in treatment of influenza: a randomised, double‐blind, placebo‐controlled trial. Respiratory Medicine 2010;104(9):1362‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wei 2010 {published data only}
- Wei Q, Luo H. Observation for the effect of Lianhua Qingwen capsule and oseltamivir in the treatment of mild type A H1N1 influenza. Chinese Journal of Guang Ming Chinese Medicine 2010;25(12):2318‐9. [Google Scholar]
Xie 2010 {published data only}
- Xie Y. Observation for the effect of Tanreqing combined with oseltamivir in the treatment of influenza H1N1. China Modern Doctor 2010;48(18):47‐8. [Google Scholar]
Xue 1999 {published data only}
- Xue EB, Dong Z. Clinical observation of 519 influenza patients prevented and treated with Ganmao Jiaonang. Tianjin Zhong Yi [Tianjin Journal of Traditional Chinese Medicine] 1999;16(4):13‐4. [Google Scholar]
Zhang 2010a {published data only}
- Zhang ZQ, Zheng HJ, Liu HD, Li JS, Ying FR, Li L. Modified Ju Lan Qing Du decoction combined with Western medicine in the treatment of 28 patients with type A H1N1. Hebei Journal of Traditional Chinese Medicine 2010;32(8):1192‐3. [Google Scholar]
Zhang 2011 {published data only}
- Zhang WS, He CS, Wang YL. A clinical study of the preparation recommended by Sichuan province TCM administration in the treatment of influenza A H1N1. Gansu Journal of TCM 2011;24(1):20‐2. [Google Scholar]
Zhao 2010 {published data only}
- Zhao ZL, Liu HY, Wang YZ, Lei ZY. Observation for the effect of self‐prepared Qingwen Tuire decoction in treatment of 30 patients with influenza A H1N1. Ningxia Medical Journal 2010;32(4):376‐7. [Google Scholar]
Zheng 2010b {published data only}
- Zheng HP, Yang Z, Zhang FC, Zhang AM, Wang J, Hong WX. Study on therapeutic effects of TCM on swine‐origin influenza A (H1N1) virus patients. Chinese Journal of Traditional Chinese Medicine and Pharmacology (Zhong Hua Zhong Yi Yao Za Zhi) 2010;25(5):780‐2. [Google Scholar]
Zhu 2010 {published data only}
- Zhu YH, Tian L, Xu N. Ge Gen Tang granule combined with Tamiflu in the treatment of 38 cases with type A H1N1. Shandong Journal of Traditional Chinese Medicine 2010;29(8):535‐6. [Google Scholar]
References to studies excluded from this review
Chen 2010c {published data only}
- Chen JD, Sun XD, Yan H, Wang J, Mao YS. Impact of Xingnaojing injection on acute phase proteins and its efficacy in patients of H1N1 influenza A. Chinese Archives of Traditional Chinese Medicine 2010;28(9):2015‐6. [Google Scholar]
Dou 2010 {published data only}
- Dou AH, Li TZ, Liu YZ, Yu F, Ou Yang Y, Zhang C, et al. Curative effect observation of Tamiflu and traditional Chinese medicine on type A H1N1 influenza. Modern Journal of Integrated Traditional Chinese and Western Medicine 2010;19(26):3289‐90. [Google Scholar]
Du 1991 {published data only}
- Du YL, Ma WM. 100 cases with influenza fever treated with Bo Hao Sanhua Yin. Hebei Zhong Yi [Hebei Journal of Traditional Chinese Medicine] 1991;13(5):6. [Google Scholar]
Hamazaki 2006 {unpublished data only}
- Hamazaki K, Sawazaki S, Itomura M, Huan M, Shibahara N, Kawakita T, et al. No effect of a traditional Chinese medicine, Hochu‐ekki‐to, on antibody titre after influenza vaccination in men: a randomised, placebo‐controlled, double‐blind trial. Phytomedicine 2006;24:Epub ahead of print. [DOI] [PubMed] [Google Scholar]
Han 2010 {published data only}
- Han F. Clinical observation for Shangyuyin decoction combined with Yanhuning decoction in the treatment of 144 patients with H1N1 type influenza. Clinical Journal of Traditional Chinese Medicine 2010;22(5):417‐8. [Google Scholar]
Hang 1998 {published data only}
- Hang JZ. 96 children with influenza treated with Yuxingcao Koufu Ye Compound. Zhejiang Zhong Yi Za Zhi [Zhejiang Journal of Traditional Chinese Medicine] 1998;33(8):381. [Google Scholar]
Hou 2002 {published data only}
- Hou YJ. Clinical observation of treating winter influenza with Traditional Chinese Medicine 'Shuang Jie Tang'. Beijing Zhong Yi [Journal of Beijing University of Traditional Chinese Medicine] 2002;21(4):231‐2. [Google Scholar]
Huang 2003 {published data only}
- Huang ZP. Effect analysis of Yu Xing Cao in the treatment of 46 cases with influenza. Zhongguo Xin Yi Yao [China New Medicine] 2003;2(3):63‐4. [Google Scholar]
Huang 2010a {published data only}
- Huang SJ, Xie JY. Clinical observation on influenza H1N1 A treated by Qingwen Baidusan plus acupuncture. Zhongguo Zhongyi Jizheng 2010;19(6):925‐6. [Google Scholar]
Huang 2010b {published data only}
- Huang M, Jiang Y. Clinical analysis for Chansu injection in the treatment of suspended type A influenza H1N1 patients with high fever. Chinese Journal of Misdiagnosis 2010;10(9):2079‐80. [Google Scholar]
Jiang 2003 {published data only}
- Jiang M, Xiong LL, Qi ZQ, Zou JD. Clinical trial for treating wind‐heat syndrome of upper respiratory infection and influenza with Yinhua Jiedu Granule. Zhong Yao Xin Yao Yu Lin Chuang Yao Li [Traditional Chinese Drug Research & Clinical Pharmacology] 2003;14(4):270‐2. [Google Scholar]
Jin 1998 {published data only}
- Jin Y. Clinical observation of treating influenza fever with Shouqi Jiedu Fa. Xinjiang Zhong Yi Yao [Xinjiang Journal of Traditional Chinese Medicine] 1998;16(1):18. [Google Scholar]
Kubo 2007 {published data only}
- Kubo T, Nishimura H. Antipyretic effect of Mao‐to, a Japanese herbal medicine, for treatment of type A influenza infection in children. Phytomedicine 2007;14(2‐3):96‐101. [DOI] [PubMed] [Google Scholar]
Li 2001 {published data only}
- Li YJ. Effect analysis of treating influenza fever with Chinese medicine and Western medicine. Xian Dai Zhong Xi Yi Jie He Za Zhi [Modern Journal of Integrated Traditional Chinese and Western Medicine] 2001;10(3):214. [Google Scholar]
Li 2005 {published data only}
- Li HG. Curative observation on summer severe influenza treated with Chuan Hu Ning injection plus San Shi decoction modified. Zhong Guo Zhong Yi Ji Zheng [Journal of Emergency in Traditional Chinese Medicine] 2005;14(6):543‐5. [Google Scholar]
Lindenmuth 2000 {published data only}
- Lindenmuth GF, Lindenmuth EB. The efficacy of echinacea compound herbal tea preparation on the severity and duration of upper respiratory and flu symptoms: a randomized, double‐blind, placebo‐controlled study. Journal of Alternative and Complementary Medicine 2000;6(4):327‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Liu 2002 {published data only}
- Liu JL, Wang XY. 80 cases of treating influenza pneumonia with Yuxingcao injection. Liaoning Zhong Yi Za Zhi [Liaoning Journal of Traditional Chinese Medicine] 2002;29:502. [Google Scholar]
Liu 2010 {published data only}
- Liu XF, Xia XL. Ganmao Xiaoyanpian in the treatment of children with influenza A H1N1: a randomised controlled trial. Master degree thesis, Kunming Medical University 2010.
Lu 2004 {published data only}
- Lu ZQ. 246 cases of clinical observation on Shuanghuanglian Kou Fu Ye for cold. Shenzhen Zhong Xi Yi Jie He Za Zhi [Shenzhen Journal of Integrated Traditional Chinese and Western Medicine] 2004;14(6):368‐70. [Google Scholar]
Qu 2005 {published data only}
- Qu JL, Gao X, Zhou SF, Xu SP, Yu Y. Clinical effect of Tan Re Qing injection in the treatment of upper respiratory tract infection caused by type‐A influenza. Zhong Guo Zhong Yi Ji Zheng [Journal of Emergency in Traditional Chinese Medicine] 2005;14(1):26‐7. [Google Scholar]
SRCG 1981 {published data only}
- Scientific Research Collaboration Group (SRCG) for preventing and treating influenza in Yichun Area in Jiangxi Province. Effect observation of 401 influenza patients treated with Baihua Baijiang. Zhong Ji Yi Kan [Chinese Journal of Medicine] 1981;31(3):39‐40. [Google Scholar]
Tang 2010 {published data only}
- Tang CB, Pu YN. Xiyanping combined with western medicine in the treatment of 30 patients with influenza H1N1 with high fever. Chinese Community Doctor 2010;12(20):141. [Google Scholar]
Wang 2001 {published data only}
- Wang SY. 100 children with influenza treated with Jiang Bang Pu Bo Tang he Chai Ping Tang. Fujian Zhong Yi Yao [Fujian Journal of Traditional Chinese Medicine] 2001;32(2):49‐50. [Google Scholar]
Xia 2010 {published data only}
- Xia BL, Shi J, Jia N, Wang HX, Zhang X. Observation on the efficacy of the treatment on influenza H1N1 A by Ganmao Qingre granule. People's Military Surgeon 2010;53(9):645‐6. [Google Scholar]
Xu 2001 {published data only}
- Xu J. Clinical observation of Da Qing Long Tang treating influenza fever. Changchun Zhong Yi Xue Yuan Xue Bao [Academic Periodical of Changchun College of Traditional Chinese Medicine] 2001;17(2):29‐30. [Google Scholar]
Yang 2000a {published data only}
- Yang CX, Yan TY. Clinical research on Redu Jing treating influenza‐caused upper respiratory tract infection. Beijing Zhong Yi [Journal of Beijing University of Traditional Chinese Medicine] 2000;19(4):17. [Google Scholar]
Yang 2000b {published data only}
- Yang H. 101 influenza patients treated with Chai Ge Jieji Tang Jiawei. Zhong Guo Zhong Yi Ji Zheng [Journal of Emergency in Traditional Chinese Medicine] 2000;9(3):132. [Google Scholar]
Yang 2005a {published data only}
- Yang LB, Ji ZH, Wang BQ. Clinical observation of the therapeutic effect of Lianhuaqingwen capsule on 280 cases of influenza. Yi Nan Bing Za Zhi [Journal of Difficult and Complicated Cases] 2005;4(5):276‐8. [Google Scholar]
Yang 2005b {published data only}
- Yang LB, Ji ZH, Gao XD, Gu CH. Phase 2 clinical study of Lianhua Qingwen capsule for influenza. Zhong Yao Xin Yao Yu Lin Chuang Yao Li [Traditional Chinese Drug Research & Clinical Pharmacology] 2005;16(4):290‐3. [Google Scholar]
Yao 2003 {published data only}
- Yao WH, Zhou AG, Qu JH, Han X. Clinical analysis of treating influenza fever with modified Gan Lu Xiao Du Dan. Yi Xue Yan Jiu Tong Xun [Bulletin of Medical Research] 2003;32(5):64‐5. [Google Scholar]
Yu 2000 {published data only}
- Yu DC, Meng XF. 98 influenza patients treated with Liugan Heji. Xian Dai Zhong Xi Yi Jie He Za Zhi [Modern Journal of Integrated Traditional Chinese and Western Medicine] 2000;9(8):736. [Google Scholar]
Yuan 2003 {published data only}
- Yuan XH, Liu B. 120 cases of treating winter influenza with Jing Fang Yin Qiao Tang. Shi Yong Zhong Yi Nei Ke Za Zhi [Journal of Practical Traditional Chinese Internal Medicine] 2003;17(3):191. [Google Scholar]
Zeng 2004 {published data only}
- Zeng QX, Hu DZ. Clinical study on Yinma mixture in treating fever of Wenre influenza. Zhongguo Zhong Xi Yi Jie He Ji Jiu Za Zhi [Chinese Journal of Integrated Traditional and Western Medicine in Intensive Critical Care] 2004;11(3):176‐8. [Google Scholar]
Zhang 2000 {published data only}
- Zhang RW, Liu FL, Peng XJ, Gao GL. Treating influenza with Yiqi Qingjie Fa. Shandong Zhong Yi Za Zhi [Shandong Journal of Traditional Chinese Medicine] 2000;19(8):460‐1. [Google Scholar]
Zhang 2002 {published data only}
- Zhang XM, Jiang LD, Shang XZ, Zhang YS. Comparison of therapeutic effect between Chaihu Guizhi Tang Jiawei Fang Tangji and Chaihu Guizhi Tang Jiawei Fang Keliji. Zhong Guo Zhong Yi JI Zheng [Journal of Emergency in Traditional Chinese Medicine] 2002;11(3):174‐5. [Google Scholar]
Zhang 2004 {published data only}
- Zhang DN, Che SQ, Xu Y, et al. Self‐composed herbal preparation of Influenza No.1 for 960 cases of influenza. Shanxi Zhong Yi Za Zhi [Shanxi Journal of Traditional Chinese Medicine] 2004;25(8):722‐3. [Google Scholar]
Zhang 2005 {published data only}
- Zhang SX, Chai JL. Gan Qing Dai Paoji for treating 60 cases of influenza. Qilu Yao Shi [Qilu Pharmaceutical Affairs] 2005;24(12):751‐2. [Google Scholar]
Zhang 2010b {published data only}
- Zhang M. Clinical observation on influenza H1N1 A treated by OTC medicine. Modern Preventive Medicine 2010;37(17):3349‐50. [Google Scholar]
Zhao 2006 {published data only}
- Zhao DY. Clinical study on kang liu gan he ji for influenza. Zhejiang Zhong Yi Za Zhi [Zhejiang Journal of Traditional Chinese Medicine] 2006;41(6):326‐7. [Google Scholar]
Zheng 2010 {published data only}
- Zheng HP, Yang Z, Zhang FC, Zhang AM, Wang J, Hong WX, et al. Study on therapeutic effects of TCM on swine‐origin influenza A (H1N1) virus patients. Chinese Journal of Traditional Chinese Medicine (CJTCMP) 2010;25(5):780‐2. [Google Scholar]
Zhong 2005 {published data only}
- Zhong Q, Zhou HF, Lin K, Pang ZW, Chen N, Ning DX, et al. Clinical analysis of Lu Qing granule in the treatment of influenza. Liaonng Zhong Yi Za Zhi [Liaoning Journal of Traditional Chinese Medicine] 2005;32(7):628. [Google Scholar]
Zhou 2010 {published data only}
- Zhou H, Huang HQ, Zhang ZD, Tao LT. Clinical observation on influenza H1N1 A for 2015 cases treated by traditional Chinese medicine. Journal of New Chinese Medicine 2011;43(1):24‐6. [Google Scholar]
References to studies awaiting assessment
Qiu 1997 {published data only}
- Qiu LY. Effect of integrated traditional Chinese medicine and Western medicine in the treatment of influenza fever in spring and summer. Shi Yong Zhong Xi Yi Jie He Za Zhi [The Practical Journal of integrating Chinese with Modern Medicine] 1997;10(8):786‐7. [Google Scholar]
Song 2002 {published data only}
- Song GL. Clinical effect of Shubiao Jiedu Yin in the treatment of influenza. Sichuan Zhong Yi [Journal of Sichuan Traditional Chinese Medicine] 2002;20(10):28‐9. [Google Scholar]
Wang 2011 {published data only}
- Wang C, Cao B, Liu QQ, Zou ZQ, Liang ZA, Gu L, et al. Oseltamivir compared with the Chinese traditional therapy Maxinshigan‐Yinqiaosan in the treatment of H1N1 influenza: a randomized trial. Annals of Internal Medicine 2011;155(4):217‐25. [DOI] [PubMed] [Google Scholar]
Additional references
Ahmed 1996
- Ahmed AH, Nicholson K. The efficacy of influenza vaccine. Review in Medical Microbiology 1996;7(1):23‐30. [Google Scholar]
Alves Galvão 2012
- Alves Galvão MG, Rocha Crispino Santos MA, Alves da Cunha AJL. Amantadine and rimantadine for influenza A in children and the elderly. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD002745.pub2] [DOI] [PubMed] [Google Scholar]
CDC 2007
- CDC. Influenza: The Disease. http://www.cdc.gov/flu/about/disease.htm 2007 (accessed March 2007).
Claas 1998
- Class EC, Jong JC, Beek R, Rimmelzwaan GF, Osterhaus AD. Human influenza virus, A/Hong Kong/156/97(H5N1) infection. Vaccine 1998;16(9‐10):977‐8. [DOI] [PubMed] [Google Scholar]
Cohen 1960
- Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement 1960;20:37‐46. [Google Scholar]
Deng 1998
- Deng WL. In: Shen YJ editor(s). Pharmacology of Traditional Chinese Medicine. 1st Edition. Beijing: People Health Publishing House, 1998. [Google Scholar]
Fleming 1999
- Fleming D, Zambon M, Waston J. Management of influenza. National Prescribing Center 1999.
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Chichester, UK: Wiley‐Blackwell, 2011. [Google Scholar]
Hou 1995
- Hou JY. Contemporary Pharmacology of Traditional Chinese Medicine. 1st Edition. Tianjin: Tianjin Scientific and Technological Publishing House, 1995. [Google Scholar]
Jefferson 2012
- Jefferson T, Jones MA, Doshi P, Mar CB, Heneghan CJ, Hama R, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD008965.pub3] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from www.cochrane‐handbook.org. Chichester, UK: Wiley‐Blackwell, 2011. [Google Scholar]
Liu 2001
- Liu GW. Chinese Herbal Medicine. 1st Edition. Beijing: Hua Xia Publishing House, 2001. [Google Scholar]
Moher 2010
- Moher D, Schulz KF, Altman D. CONSORT Statement 2010. www.CONSORT.org 2010.
Moscona 2005
- Moscona A. Oseltamivir resistance ‐ disabling our influenza defences. New England Journal of Medicine 2005;353(25):2633‐6. [DOI] [PubMed] [Google Scholar]
NACI 2006
- National Advisory Committee on Immunization. Statement on influenza vaccination for the 2006‐2007 season. http://www.phac‐aspc.gc.ca/publicat/ccdr‐rmtc/06vol32/acs‐07/index‐eng.php 2006.
Ou 1992
- Ou M. Chinese‐English Manual of Commonly Used in Traditional Chinese Medicine. 1st Edition. Guangdong: Guangdong Scientific and Technological Publishing House, 1992. [Google Scholar]
Smith 2006
- Smith NM, Bresee JS, Shay DK, Uyeki TM, Cox NJ, Strikas RA. Prevention and Control of Influenza ‐ Recommendations of the Advisory Committee on Immunization Practices (ACIP). Recommendations and Reports (http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5510a1.htm) 2006. [PubMed]
Wang 2012
- Wang K, Shun‐Shin M, Gill P, Perera R, Harnden A. Neuraminidase inhibitors for preventing and treating influenza in children (published trials only). Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD002744.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2003a
- World Health Organization. Influenza. http://www.who.int/mediacentre/factsheets/fs211/en/ 2003 (accessed 2006).
WHO 2003b
- World Health Organization. Influenza vaccination for the 2003‐04 season recommendations in the context of concern about SARS. www.who.int/influenza/vaccines/vaccinerecommendations1/en/index13.html 2003.
WHO 2012
- World Health Organization. WHO guidelines for investigation of human cases of avian influenza A (H5N1). http://www.who.int/influenza/human_animal_interface/EN_GIP_20121217CumulativeNumberH5N1cases.pdf 2012 (accessed in 15 Jan, 2013).
Wiselka 1994
- Wiselka M. Influenza: diagnosis, management and prophylaxis. BMJ 1994;308:1341‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xu 1998
- Xu QP, Zhao L. Pharmacology of Traditional Chinese Medicine. 1st Edition. Beijing: People Health Publishing House, 1998. [Google Scholar]
Zhang 1991
- Zhang EQ. The Chinese Materia Medica. 1st Edition. Shanghai: Publishing House of Shanghai College of Traditional Chinese Medicine, 1991. [Google Scholar]
Zhao 2001
- Zhao EJ. Pulse Diagnosis in Chinese Medicine. 2nd Edition. Tianjin: Tianjin Scientific and Technologic Publishing House, 2001. [Google Scholar]
References to other published versions of this review
Chen 2005
- Chen XY, Wu TX, Liu GJ, Wang Q, Zheng J, Wei J, et al. Chinese medicinal herbs for influenza. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD004559.pub3] [DOI] [PubMed] [Google Scholar]
Chen 2007
- Chen XY, Wu TX, Liu GJ, Wang Q, Zheng J, Wei J, et al. Chinese medicinal herbs for influenza. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD004559.pub3] [DOI] [PubMed] [Google Scholar]


