Abstract
Background: This report provided the effect of 15 preventable factors on the risk of breast cancer incidence.
Study design: A systematic review and meta-analysis.
Methods: A detailed research was conducted on PubMed, Web of Science, and Scopus databases in January 2020. Reference lists were also screened. Prospective cohort studies addressing the associations between breast cancer and 15 factors were analyzed. Between-study heterogeneity was investigated using the χ2, τ2, and I2 statistics. The probability of publication bias was explored using the Begg and Egger tests and trim-and-fill analysis. Effect sizes were expressed as risk ratios (RRs) with 95% confidence intervals (CIs) using a random-effects model.
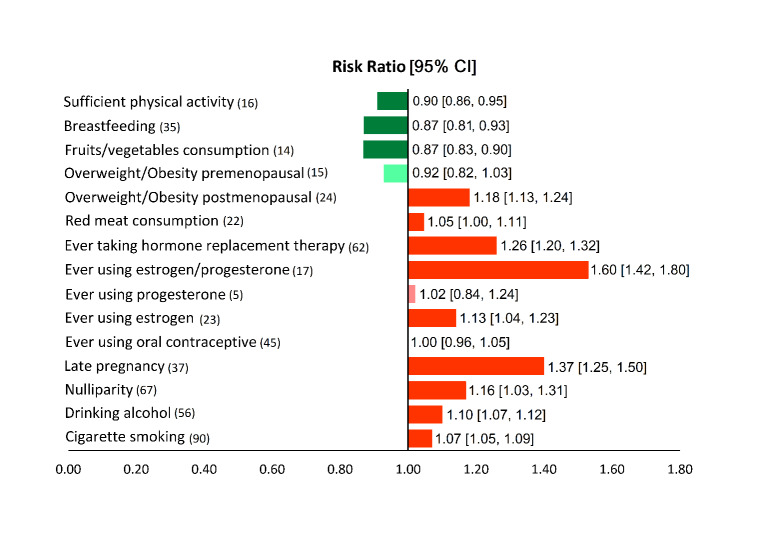
Results: Based on the results, out of 147,083 identified studies, 197 were eligible, including 19,413,702 participants. The RRs (95% CI) of factors associated with breast cancer were as follows: cigarette smoking 1.07 (1.05, 1.09); alcohol drinking 1.10 (1.07, 1.12); sufficient physical activity 0.90 (0.86, 0.95); overweight/obesity in premenopausal 0.92 (0.82, 1.03) and postmenopausal 1.18 (1.13, 1.24); nulliparity 1.16 (1.03, 1.31); late pregnancy 1.37 (1.25, 1.50); breastfeeding 0.87 (0.81, 0.93); ever using oral contraceptive 1.00 (0.96, 1.05); ever using estrogen 1.13 (1.04, 1.23); ever using progesterone 1.02 (0.84, 1.24); ever using estrogen/progesterone 1.60 (1.42, 1.80); ever taking hormone replacement therapy 1.26 (1.20, 1.32); red meat consumption 1.05 (1.00, 1.11); fruit/vegetable consumption 0.87 (0.83, 0.90); and history of radiation therapy, based on single study 1.31 (0.87, 1.98).
Conclusions: This meta-analysis provided a clear picture of several factors associated with the development of breast cancer. Moreover, the useful information in this study may be utilized for ranking and prioritizing preventable risk factors to implement effective prevention programs.
Keywords: Breast neoplasms, Risk factors, Behavior, Nutrition, Meta-analysis
Introduction
Breast cancer is the most commonly occurring cancer in women, regardless of race or ethnicity, impacting over 2 million women worldwide annually, responsible for over 600,000 deaths in 2018 1 . According to the biennial report of the American Cancer Society, the breast cancer incidence rate had increased slightly by 0.3% per year 2 . Several factors, such as genetic characteristics, lifestyle factors, and medical conditions, may play a role in the development of breast cancer. The risk factors for breast cancer can be divided into two categories, namely (a) fixed risk factors that may contribute to the development of breast cancer, however, cannot be changed, such as age, female gender, genetic characteristics 3 , immunologic biomarkers 4 , racial or ethnic characteristics 5 , family history 6 , and late menopause 7 ; and (b) modifiable risk factors that play a role in the development of breast cancer and can be changed, such as alcoholic beverage consumption 8 , cigarette smoking 9 , physical inactivity 10 , high body mass index (BMI) 11 , high dietary fat 12 , and dietary fiber intake 13 . These factors are largely modifiable and preventable, and therefore, can be considered when designing effective prevention programs.
Efforts to improve screening programs and the early detection and treatment of breast cancer are important; nevertheless, it is of priority to take action to address preventable factors that play an important role in the development of breast cancer. Some measures, such as ranking and prioritizing the risk factors that contribute to the development of breast cancer and implementing prevention programs, can reduce the incidence of breast cancer and prevent thousands of new cases each year. Effective intervention strategies and prevention programs need a comprehensive understanding and a clear picture of the contributing factors that promote breast cancer. To the best of our knowledge, no comprehensive systematic review has yet been conducted to address all the potential preventable factors playing a pivotal role in the development of breast cancer. This meta-analysis was performed to address the associations between breast cancer and 15 factors that might be potentially modifiable and preventable, and consequently, might provide an opportunity to be addressed in prevention programs aimed to reduce the incidence of breast cancer.
Methods
The eligibility criteria in this study were based on population, intervention, comparison, outcomes, and study design (PICOS). Accordingly, the women having any of the 15 preventable factors mentioned below were included in the exposure group and those without any of the 15 preventable factors mentioned below in the unexposed group. The outcome was considered breast cancer and the prospective cohort studies were reviewed.
Eligibility criteria
The outcome of interest was having pathologically confirmed breast cancer, of any type (i.e., ductal or lobular carcinomas), among the general population, regardless of age, gender, race, ethnicity, and geographical region. The exposures of interest are listed below:
Cigarette smoking (current/former smokers versus nonsmokers)
Drinking alcohol (current/former drinkers versus non-drinkers)
Physical activity (sufficient versus insufficient)
Body mass index (overweight/obese versus normal weight)
Parity (nulliparous versus primiparous/multiparous)
Late pregnancy (≥30 years versus <30 years)
Breastfeeding (ever versus never, or ≥6 months versus <6 months, or ≥12 months versus <12 months, or ≥24 months versus <24 months)
Ever using oral contraceptive (OCP) (yes versus no)
Ever using estrogen (yes versus no)
Ever using progesterone (yes versus no)
Ever using estrogen/progesterone (yes versus no)
Ever taking hormone replacement therapy (HRT) (yes versus no)
Intake of red meat (highest intake versus lowest intake)
Intake of fruit/vegetable (highest intake versus lowest intake)
History of radiation therapy (yes versus no)
A BMI of 18.5-24.9 kg/m2 was classified as normal weight, 25.0-29.9 kg/m2 as overweight, and ≥ 30.0 kg/m2 as obese. At least 30 minutes of moderate- to vigorous-intensity physical activity per day (or 150 minutes per week) was considered sufficient for adults 14 . Pregnancy over the age of 30 is considered high-risk 15 . Accordingly, reproductive ages of > 30 were considered late pregnancy in this study. The duration of breastfeeding is recommended for at least 6 months continued up to 2 years of age or longer 16 . Accordingly, various periods of breastfeeding, including ≥ 6, ≥ 12, and ≥ 24 months were considered in this research. The consumption of at least five total servings (400 grams) of fruit and vegetables per day is recommended 17 . However, the majority of the included studies did not report fruit and vegetable consumption according to the recommendations of the World Health Organization. Therefore, the highest intake versus the lowest intake of fruit and vegetables were compared in the present study. There is no universal recommendation for red meat consumption. In this respect, the highest intake versus the lowest intake of red meat was also compared in this research.
Prospective cohort studies addressing the association between breast cancer and any of the above factors were included in the meta-analysis, irrespective of their language and publication date and the participants' nationality, race, gender, and age. Wherever reported, full adjusted forms of risk ratio (RR) controlled was used for at least one or more potential confounding factors.
Information sources and search
A detailed search was conducted on PubMed, Web of Science, and Scopus databases in January 2020. The reference lists of the included studies were also explored. The search process was performed based on the following keywords: (Breast cancer or Breast neoplasms or Breast malignancy or Breast tumor) and (Smoking or Cigarette or Tobacco or Cigar or Alcohol or Ethanol or Exercise or Physical activity or Obese or Obesity or Overweight or Body mass index or BMI or Pregnancy or Breastfeeding or Contraceptive or Hormone or Estrogen or Progesterone or Fruit or Vegetables or Red meat or Radiation)
Study selection
The search results of all databases were combined using EndNote, and duplicates were deleted. Afterward, six authors (i.e., FH, FS, BZ, PA, FS, and FG) formed three two-person groups. Each group screened the titles and abstracts of one-third of the search results separately and independently and excluded ineligible studies. The full texts of potentially relevant studies were retrieved for further evaluation.
Data extraction
The data from the relevant studies were extracted by 6 authors (i.e., FH, FS, BZ, PA, FS, and FG) using an electronic data collection form prepared in Stata (StataCorp, College Station, TX, USA).
Methodological quality
The Newcastle-Ottawa Scale (NOS) 18 was used to assess the methodological quality of the included studies. Based on this scale, a maximum of 9 stars were assigned to each study. Studies that received 7 or more stars were labeled high-quality; otherwise, studies were classified as low-quality.
Heterogeneity and publication bias
The heterogeneity across studies was examined using the Chi-square (χ2) test 19 and tau-square (τ2) test and quantified by the I2 statistic 20 . According to the I2 value, heterogeneity was classified as low (<50%), moderate (50-74%), or high (≥75%). The possibility of publication bias was explored by the Egger 21 and Begg 22 tests and the trim-and-fill method 23 .
Summary measures
The effect measure of choice was the RR with 95% confidence intervals. The results were reported based on a random-effects model 24 . The data were analyzed at a significance level of 0.05 using Stata software (version 14.2; StataCorp, College Station, TX, USA) and Review Manager software (version 5.3).
Sensitivity analysis
If the between-study heterogeneity was moderate to high (I2≥50%), the source of heterogeneity was investigated using the MetaPlot Stata command based on the sequential algorithm 25-27 .
Results
Description of studies
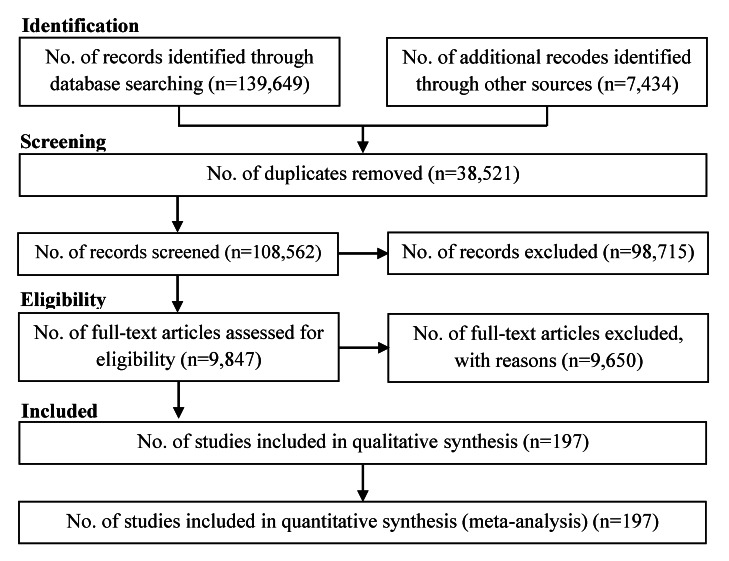
In total, 147,083 studies were identified, including 139,649 studies obtained by searching the electronic databases in January 2020 and 7,434 articles identified by searching the reference lists of the included studies. After excluding duplicates and ineligible studies, 197 studies with 19,413,702 participants (Table 1) were included in the meta-analysis (Figure 1).
Table 1. Characteristics of the included studies (sorted by authors’ names).
| Row | 1 St author, year | Country | Age (year) | Study design | Effect size | Adjustment | Sample Size | NOS Quality |
| 1 | Adebamowo, 2005 | USA | 20-46 | Prospective cohort | Rate Ratio | Adjusted | 90,638 | 8- High quality |
| 2 | Agurs-Collins, 2009 | USA | 21-69 | Prospective cohort | Rate Ratio | Adjusted | 50,778 | 9- High quality |
| 3 | Al-Ajmi, 2018 | UK | 56.3 | Prospective cohort | Risk Ratio | Adjusted | 273,467 | 9- High quality |
| 4 | Albrektsen, 2005 | Norway | 20-74 | Prospective cohort | Rate Ratio | Unadjusted | 1,700,000 | 6- Low quality |
| 5 | Al-Delaimy, 2004 | USA | 25-42 | Prospective cohort | Rate Ratio | Adjusted | 116,671 | 8- High quality |
| 6 | Alipour, 2019 | Iran | 40-75 | Nested case-control | Rate Ratio | Adjusted | 499 | 8- High quality |
| 7 | Anderson, 2018 | UK | 40-69 | Prospective cohort | Rate Ratio | Adjusted | 262,195 | 9- High quality |
| 8 | Arslan, 2014 | USA | 60.05 | Nested case-control | Rate Ratio | Adjusted | 998 | 7- High quality |
| 9 | Arthur, 2017 | USA | 21-85 | Nested case-control | Rate Ratio | Adjusted | 1,052 | 8- High quality |
| 10 | Azam, 2018 | Denmark | 50-69 | Prospective cohort | Hazard Ratio | Adjusted | 4,501 | 8- High quality |
| 11 | Baglietto, 2010 | Australia | 27-81 | Case-cohort | Risk Ratio | Unadjusted | 1,054 | 9- High quality |
| 12 | Baglietto, 2011 | Australia | 40-69 | Prospective cohort | Rate Ratio | Adjusted | 20,967 | 8- High quality |
| 13 | Bakken, 2011 | Europe | 58.1 | Prospective cohort | Risk Ratio | Adjusted | 133,744 | 9- High quality |
| 14 | Barlow, 2006 | USA | 35-84 | Prospective cohort | Risk Ratio | Adjusted | 2,392,998 | 9- High quality |
| 15 | Bassett, 2013 | Melbourne | 27-80 | Case-cohort | Risk Ratio | Adjusted | 20,756 | 8- High quality |
| 16 | Bellocco, 2016 | Sweden | 56.1 | Prospective cohort | Hazard Ratio | Adjusted | 19,196 | 9- High quality |
| 17 | Beral, 2011 | UK | 56.6 | Prospective cohort | Risk Ratio | Adjusted | 1,129,025 | 8- High quality |
| 18 | Bergkvist, 1989 | Sweden | ≥35 | Prospective cohort | Risk Ratio | Adjusted | 23,244 | 6- Low quality |
| 19 | Bjerkaas, 2013 | Norway | 44 | Prospective cohort | Hazard Ratio | Adjusted | 302,865 | 8- High quality |
| 20 | Bjørge, 2010 | Norway-Sweden-Austria | 44 | Prospective cohort | Risk Ratio | Adjusted | 287,320 | 8- High quality |
| 21 | Bravi, 2018 | Italy | 41-79 | Nested case-control | Rate Ratio | Adjusted | 13,212 | 8- High quality |
| 22 | Brinton, 2013 | Israel | 31.1 | Prospective cohort | Hazard Ratio | Adjusted | 87,403 | 9- High quality |
| 23 | Brinton, 2014 | USA | 50-71 | Prospective cohort | Hazard Ratio | Adjusted | 190,827 | 8- High quality |
| 24 | Buring, 1987 | USA | 30-55 | Prospective cohort | Risk Ratio | Adjusted | 33,335 | 6- Low quality |
| 25 | Butler, 2010 | Singapore | 45-74 | Prospective cohort | Rate Ratio | Adjusted | 34,028 | 8- High quality |
| 26 | Butt, 2014 | Sweden | 57.23 | Prospective cohort | Risk Ratio | Adjusted | 14,092 | 9- High quality |
| 27 | Campa, 2011 | USA-Europe | 62.39 | Nested case-control | Rate Ratio | Adjusted | 20,468 | 7- High quality |
| 28 | Catsburg, 2015 | Canada | 40-59 | Prospective cohort | Hazard Ratio | Adjusted | 89,835 | 9- High quality |
| 29 | Cerhan, 1998 | USA | 65-102 | Prospective cohort | Risk Ratio | Adjusted | 1,806 | 7- High quality |
| 30 | Chen, 2002 | USA | 50-74 | Nested case-control | Rate Ratio | Adjusted | 1,397 | 7- High quality |
| 31 | Chen, 2016 | Taiwan | ≥35 | Prospective cohort | Hazard Ratio | Adjusted | 1,393,985 | 9- High quality |
| 32 | Chlebowski, 2013 | USA | 50-79 | Prospective cohort | Hazard Ratio | Adjusted | 41,449 | 8- High quality |
| 33 | Cho, 2006 | USA | 26-46 | Prospective cohort | Risk Ratio | Adjusted | 90,659 | 9- High quality |
| 34 | Clavel-Chapelon, 2007 | France | 40-65 | Prospective cohort | Risk Ratio | Adjusted | 80,377 | 8- High quality |
| 35 | Cohen, 2013 | USA | 40-79 | Nested case-control | Rate Ratio | Adjusted | 2,730 | 7- High quality |
| 36 | Colditz, 2003 | USA | 25-42 | Prospective cohort | Risk Ratio | Adjusted | 116,671 | 9- High quality |
| 37 | Cottet, 2009 | France | 52.2 | Prospective cohort | Rate Ratio | Adjusted | 65,374 | 9- High quality |
| 38 | Couto, 2013 | Sweden | 30-49 | Prospective cohort | Risk Ratio | Adjusted | 49,258 | 9- High quality |
| 39 | Cross, 2007 | USA | 50-71 | Prospective cohort | Rate Ratio | Adjusted | 500,000 | 9- High quality |
| 40 | Cust, 2009 | Sweden | 50-69 | Nested case-control | Rate Ratio | Adjusted | 1,122 | 7- High quality |
| 41 | Dai, 2009 | China | 40-70 | Nested case-control | Rate Ratio | Adjusted | 1,288 | 8- High quality |
| 42 | Dallal, 2007 | USA | 20-79 | Prospective cohort | Risk Ratio | Adjusted | 110,599 | 9- High quality |
| 43 | Dartois, 2016 | France | 42-72 | Prospective cohort | Risk Ratio | Adjusted | 67,634 | 8- High quality |
| 44 | Diallo, 2018 | France | ≥35 | Prospective cohort | Rate Ratio | Adjusted | 61,476 | 8- High quality |
| 45 | Diergaarde, 2008 | USA | 50-76 | Nested case-control | Rate Ratio | Adjusted | 975 | 6- Low quality |
| 46 | Dorgan, 1994 | USA | 35-68 | Prospective cohort | Risk Ratio | Adjusted | 2,321 | 8- High quality |
| 47 | Dorgan, 2010 | Columbia | 31-56 | Nested case-control | Rate Ratio | Adjusted | 266 | 9- High quality |
| 48 | Dossus, 2014 | Europe | - | Prospective cohort | Hazard Ratio | Adjusted | 322,988 | 9- High quality |
| 49 | Dumeaux, 2004 | Norway | 30-70 | Prospective cohort | Risk Ratio | Adjusted | 86,948 | 9- High quality |
| 50 | Dumeaux, 2005 | France | 40-64 | Prospective cohort | Risk Ratio | Adjusted | 68,670 | 7- High quality |
| 51 | Egan, 2002 | USA | 30-55 | Prospective cohort | Risk Ratio | Adjusted | 78,206 | 8- High quality |
| 52 | Egeberg, 2008 | Denmark | 50-64 | Prospective cohort | Rate Ratio | Adjusted | 24,697 | 8- High quality |
| 53 | Eisen, 2008 | USA | 58.2 | Nested case-control | Rate Ratio | Adjusted | 472 | 6- Low quality |
| 54 | Elebro, 2014 | Sweden | - | Prospective cohort | Hazard Ratio | Adjusted | 17,035 | 9- High quality |
| 55 | Ellingjord-Dale, 2017 | Norway | 50-69 | Nested case-control | Rate Ratio | Adjusted | 29,162 | 9- High quality |
| 56 | Epplein, 2009 | USA | 45-75 | Nested case-control | Rate Ratio | Adjusted | 821 | 7- High quality |
| 57 | Fabre, 2007 | France | 51.8 | Prospective cohort | Risk Ratio | Adjusted | 73,664 | 8- High quality |
| 58 | Fagherazzi, 2015 | France | 40-65 | Prospective cohort | Hazard Ratio | Adjusted | 66,481 | 9- High quality |
| 59 | Falk, 2014 | USA | 55-74 | Prospective cohort | Hazard Ratio | Adjusted | 54,562 | 9- High quality |
| 60 | Farhat, 2011 | USA | 50-79 | Case-cohort | Risk Ratio | Adjusted | 903 | 9- High quality |
| 61 | Farvid, 2014 | USA | 26-45 | Prospective cohort | Rate Ratio | Adjusted | 88,804 | 9- High quality |
| 62 | Feigelson, 2004 | USA | 50-74 | Prospective cohort | Rate Ratio | Adjusted | 97,786 | 8- High quality |
| 63 | Ferrucci, 2009 | USA | 55-74 | Prospective cohort | Rate Ratio | Adjusted | 52,158 | 9- High quality |
| 64 | Fourkala, 2014 | UK | 64 | Prospective cohort | Hazard Ratio | Adjusted | 92,834 | 7- High quality |
| 65 | Fournier, 2014b | France | 59.68 | Prospective cohort | Hazard Ratio | Adjusted | 78,353 | 9- High quality |
| 66 | Fraser, 1997 | USA | 55 | Prospective cohort | Risk Ratio | Adjusted | 34,198 | 8- High quality |
| 67 | Friedenretch, 1993 | Canada | No data | Nested case-control | Rate Ratio | Adjusted | 1,701 | 8- High quality |
| 68 | Fuhrman, 2012 | UK | 55-74 | Nested case-control | Rate Ratio | Adjusted | 700 | 8- High quality |
| 69 | Fung, 2005 | USA | 30-55 | Prospective cohort | Risk Ratio | Adjusted | 11,700 | 8- High quality |
| 70 | Gapstur, 1999 | USA | 55-69 | Prospective cohort | Risk Ratio | Adjusted | 41,837 | 8- High quality |
| 71 | Garland, 1999 | USA | 25-42 | Prospective cohort | Rate Ratio | Adjusted | 116,671 | 8- High quality |
| 72 | Gaudet, 2014 | USA | No data | Prospective cohort | Risk Ratio | Adjusted | 28,965 | 9- High quality |
| 73 | Genkinger, 2013 | USA | 21-69 | Prospective cohort | Rate Ratio | Adjusted | 52,062 | 9- High quality |
| 74 | Gertig, 2006 | Australia | 40-69 | Prospective cohort | Hazard Ratio | Adjusted | 24,479 | 8- High quality |
| 75 | Goodman, 1997 | Japan | No data | Prospective cohort | Risk Ratio | Adjusted | 22,200 | 9- High quality |
| 76 | Gram, 2005 | Norway-Sweden | 30-50 | Prospective cohort | Risk Ratio | Adjusted | 102,098 | 9- High quality |
| 77 | Gram, 2015 | USA | 45-75 | Prospective cohort | Hazard Ratio | Adjusted | 83,300 | 9- High quality |
| 78 | Gram, 2016 | Norway | 34-70 | Prospective cohort | Hazard Ratio | Adjusted | 130,053 | 9- High quality |
| 79 | Ha, 2007 | USA | 22-92 | Prospective cohort | Hazard Ratio | Adjusted | 56,042 | 9- High quality |
| 80 | Hanaoka, 2005 | Japan | 40-59 | Prospective cohort | Risk Ratio | Adjusted | 27,398 | 9- High quality |
| 81 | Hankinson, 1997 | USA | 30-55 | Prospective cohort | Risk Ratio | Adjusted | 121,700 | 7- High quality |
| 82 | Hiatt, 1988 | USA | No data | Prospective cohort | Risk Ratio | Adjusted | 68,674 | 7- High quality |
| 83 | Holmberg, 1995 | Sweden | 40-75 | Nested case-control | Rate Ratio | Adjusted | 728 | 7- High quality |
| 84 | Holmes, 2003 | USA | 30-55 | Prospective cohort | Risk Ratio | Adjusted | 88,647 | 8- High quality |
| 85 | Horn, 2013 | Norway | 28-73 | Prospective cohort | Hazard Ratio | Adjusted | 58,426 | 9- High quality |
| 86 | Horn, 2014b | Norway | 48-64 | Prospective cohort | Hazard Ratio | Adjusted | 21,532 | 8- High quality |
| 87 | Horn-Ross, 2004 | USA | <85 | Prospective cohort | Risk Ratio | Adjusted | 103,460 | 9- High quality |
| 88 | Inoue-Choi, 2016 | USA | 24-43 | Prospective cohort | Rate Ratio | Adjusted | 193,742 | 9- High quality |
| 89 | Jick, 1980 | USA | 31-55 | Prospective cohort | Risk Ratio | Adjusted | 40,531 | 6- Low quality |
| 90 | Jones, 2017 | UK | 47 | Prospective cohort | Hazard Ratio | Adjusted | 102,927 | 8- High quality |
| 91 | Jordan, 2009 | Thailand | 28-51 | Nested case-control | Rate Ratio | Adjusted | 903 | 5- Low quality |
| 92 | Kabat, 2007 | USA | 40-59 | Prospective cohort | Rate Ratio | Adjusted | 49,654 | 9- High quality |
| 93 | Kabat, 2010 | USA-UK-Canada | No data | Nested case-control | Rate Ratio | Adjusted | 1,357 | 8- High quality |
| 94 | Kawai, 2010 | Japan | 40-64 | Prospective cohort | Hazard Ratio | Adjusted | 24,064 | 8- High quality |
| 95 | Kerlikowske, 2010 | USA | 56.4 | Prospective cohort | Risk Ratio | Adjusted | 587,369 | 8- High quality |
| 96 | Kim, 2012 | USA | 45-75 | Nested case-control | Rate Ratio | Adjusted | 1,426 | 7- High quality |
| 97 | Kim, 2017 | Korea | ≥30 | Prospective cohort | Risk Ratio | Adjusted | 5,046 | 9- High quality |
| 98 | Kojima, 2017 | Japan | 70-79 | Prospective cohort | Rate Ratio | Adjusted | 23,172 | 8- High quality |
| 99 | Komaroff, 2016 | USA | ≥50 | Nested case-control | Rate Ratio | Adjusted | 158 | 7- High quality |
| 100 | Kops, 2018 | Brazil | 40-69 | Nested case-control | Rate Ratio | Adjusted | 216 | 7- High quality |
| 101 | Kotsopoulos, 2010 | USA | 30-55 | Prospective cohort | Rate Ratio | Adjusted | 107,759 | 8- High quality |
| 102 | Krishnan, 2013 | Australia | 40-69 | Prospective cohort | Hazard Ratio | Adjusted | 14,441 | 8- High quality |
| 103 | Lahmann, 2007 | Europe | 20-80 | Prospective cohort | Hazard Ratio | Adjusted | 218,169 | 6- Low quality |
| 104 | Lambe, 1998 | Sweden | <65 | Nested case-control | Rate Ratio | Adjusted | 8,205 | 6- Low quality |
| 105 | Lando, 1999 | USA | 55.5 | Prospective cohort | Risk Ratio | Adjusted | 5,761 | 8- High quality |
| 106 | Larsen, 2010 | Denmark | 50-64 | Nested case-control | Rate Ratio | Adjusted | 1,618 | 7- High quality |
| 107 | Larsson, 2009 | Sweden | 60.8 | Prospective cohort | Rate Ratio | Adjusted | 61,433 | 9- High quality |
| 108 | Lecarpentier, 2012 | France | 40.4 | Prospective cohort | Risk Ratio | Adjusted | 1,337 | 8- High quality |
| 109 | Lee, 2006 | USA | 45-75 | Prospective cohort | Risk Ratio | Adjusted | 55,371 | 8- High quality |
| 110 | Lee, 2014 | Singapore | 45-74 | Nested case-control | Rate Ratio | Adjusted | 1,623 | 8- High quality |
| 111 | Leon, 1989 | UK | 16-59 | Prospective cohort | Rate Ratio | Adjusted | 113,263 | 7- High quality |
| 112 | Lew, 2009 | USA | 50-71 | Prospective cohort | Risk Ratio | Adjusted | 184,418 | 8- High quality |
| 113 | Lin, 2008 | Japan | 40-79 | Prospective cohort | Hazard Ratio | Adjusted | 34,401 | 9- High quality |
| 114 | Link, 2013 | USA | ≤84 | Prospective cohort | Rate Ratio | Adjusted | 91,779 | 9- High quality |
| 115 | Lipnick, 1986 | USA | 30-55 | Prospective cohort | Risk Ratio | Adjusted | 121,964 | 6- Low quality |
| 116 | Liu, 2013 | USA | 25-44 | Prospective cohort | Risk Ratio | Adjusted | 91,005 | 9- High quality |
| 117 | Liu, 2016 | Taiwan | 45-64 | Prospective cohort | Hazard Ratio | Adjusted | 15,863 | 8- High quality |
| 118 | London, 1989 | USA | 30-55 | Prospective cohort | Rate Ratio | Adjusted | 117,557 | 7- High quality |
| 119 | Lowery, 2011 | USA | >40 | Prospective cohort | Risk Ratio | Adjusted | 208,667 | 8- High quality |
| 120 | Lukanova, 2008 | Sweden | 30.96 | Nested case-control | Rate Ratio | Adjusted | 567 | 7- High quality |
| 121 | Luo, 2011 | USA | 50-79 | Prospective cohort | Hazard Ratio | Adjusted | 79,990 | 8- High quality |
| 122 | Ma, 2010 | USA | No data | Prospective cohort | Risk Ratio | Adjusted | 52,464 | 8- High quality |
| 123 | Manjer, 2000 | Sweden | No data | Prospective cohort | Risk Ratio | Adjusted | 10,902 | 8- High quality |
| 124 | Margolis, 2005 | Norway-Sweden | 30-49 | Prospective cohort | Rate Ratio | Adjusted | 99,504 | 8- High quality |
| 125 | Masala, 2017 | Italy | 35-64 | Nested case-control | Rate Ratio | Adjusted | 771 | 7- High quality |
| 126 | Mccarty, 2012 | USA | 55-74 | Nested case-control | Rate Ratio | Adjusted | 2,111 | 6- Low quality |
| 127 | Mertens, 2006 | USA | 45-64 | Prospective cohort | Hazard Ratio | Adjusted | 7,994 | 8- High quality |
| 128 | Michels, 1996 | USA | 30-55 | Prospective cohort | Rate Ratio | Adjusted | 121,701 | 9- High quality |
| 129 | Mills, 1989b | USA | 55.4 | Prospective cohort | Rate Ratio | Adjusted | 20,341 | 7- High quality |
| 130 | Missmer, 2002 | USA | 31-90 | Prospective cohort | Rate Ratio | Adjusted | 351,041 | 9- High quality |
| 131 | Moradi, 2002 | Sweden | 25-50 | Prospective cohort | Risk Ratio | Adjusted | 25,778 | 7- High quality |
| 132 | Morimoto, 2002 | USA | 50-79 | Prospective cohort | Risk Ratio | Adjusted | 85,917 | 8- High quality |
| 133 | Nitta, 2016 | Japan | 40-79 | Prospective cohort | Hazard Ratio | Adjusted | 38,610 | 8- High quality |
| 134 | Nyante, 2014 | USA | 50-71 | Prospective cohort | Hazard Ratio | Adjusted | 186,150 | 9- High quality |
| 135 | Olsson, 2003 | Sweden | 25-65 | Prospective cohort | Hazard Ratio | Adjusted | 28,378 | 9- High quality |
| 136 | Opatrny, 2008 | UK | 50-75 | Nested case-control | Rate Ratio | Adjusted | 37,863 | 7- High quality |
| 137 | Ozmen, 2008 | Turkey | 18-70 | Nested case-control | Rate Ratio | Adjusted | 3,659 | 5- Low quality |
| 138 | Pala, 2009 | Italy | 25-70 | Prospective cohort | Rate Ratio | Adjusted | 319,826 | 9- High quality |
| 139 | Park, 2014 | USA | 45-75 | Prospective cohort | Hazard Ratio | Adjusted | 85,089 | 9- High quality |
| 140 | Persson, 1999 | Sweden | No data | Prospective cohort | Risk Ratio | Adjusted | 10,472 | 9- High quality |
| 141 | Phipps, 2012 | USA | 40-84 | Prospective cohort | Hazard Ratio | Adjusted | 1,054,466 | 8- High quality |
| 142 | Pijpe, 2010 | Netherlands | 44.5 | Prospective cohort | Risk Ratio | Adjusted | 725 | 8- High quality |
| 143 | Pijpe, 2012 | France-UK-Netherlands | >18 | Prospective cohort | Risk Ratio | Adjusted | 1,993 | 6- Low quality |
| 144 | Poosari, 2014 | Thailand | 30-69 | Prospective cohort | Hazard Ratio | Adjusted | 11,414 | 9- High quality |
| 145 | Pouchieu, 2014 | France | 48.15 | Prospective cohort | Rate Ratio | Adjusted | 4,684 | 7- High quality |
| 146 | Reynolds, 2004 | USA | No data | Prospective cohort | Hazard Ratio | Adjusted | 116,544 | 9- High quality |
| 147 | Rice, 2016 | USA | 32-70 | Nested case-control | Rate Ratio | Adjusted | 4,712 | 7- High quality |
| 148 | Rintala, 2003 | Finland | >25 | Prospective cohort | Rate Ratio | Adjusted | 10,049 | 8- High quality |
| 149 | Risch, 1994 | Canada | 43-49 | Prospective cohort | Risk Ratio | Adjusted | 33,003 | 7- High quality |
| 150 | Rockhill, 1999 | USA | 30-55 | Prospective cohort | Risk Ratio | Adjusted | 85,364 | 8- High quality |
| 151 | Rod, 2009 | Denmark | 62 | Prospective cohort | Hazard Ratio | Adjusted | 5,054 | 9- High quality |
| 152 | Rohan, 2000 | Canada | 40-59 | Case-cohort | Risk Ratio | Adjusted | 56,837 | 8- High quality |
| 153 | Romieu, 1989 | USA | 30-55 | Prospective cohort | Risk Ratio | Adjusted | 118,273 | 6- Low quality |
| 154 | Saxena, 2010 | USA | 60.82 | Prospective cohort | Rate Ratio | Adjusted | 56,867 | 8- High quality |
| 155 | Schairer, 1994 | USA | 57.4 | Prospective cohort | Rate Ratio | Adjusted | 49,017 | 7- High quality |
| 156 | Schatzkin, 1987 | USA | 25-74 | Prospective cohort | Risk Ratio | Adjusted | 7,188 | 9- High quality |
| 157 | Schoemaker, 2014 | UK | No data | Nested case-control | Rate Ratio | Adjusted | 608 | 7- High quality |
| 158 | Schuurman, 1995 | Netherlands | 55-69 | Prospective cohort | Rate Ratio | Adjusted | 62,573 | 7- High quality |
| 159 | Sellers, 1992 | USA | 55-69 | Prospective cohort | Risk Ratio | Adjusted | 37,105 | 7- High quality |
| 160 | Setiawan, 2009 | USA | 45-75 | Prospective cohort | Risk Ratio | Adjusted | 84,427 | 8- High quality |
| 161 | Shannon, 2005 | China | 50-64 | Prospective cohort | Rate Ratio | Adjusted | 1,070 | 8- High quality |
| 162 | Shin, 2016 | Japan | 50-70 | Prospective cohort | Rate Ratio | Adjusted | 49,552 | 9- High quality |
| 163 | Shore, 2008 | USA | 35-65 | Nested case-control | Rate Ratio | Adjusted | 1,224 | 7- High quality |
| 164 | Sieri, 2009 | Italy | 35-69 | Nested case-control | Rate Ratio | Adjusted | 837 | 8- High quality |
| 165 | Simon, 1991 | USA | ≥21 | Prospective cohort | Risk Ratio | Adjusted | 1,954 | 9- High quality |
| 166 | Sonestedt, 2008 | Sweden | 46-75 | Prospective cohort | Risk Ratio | Adjusted | 15,773 | 8- High quality |
| 167 | Sonnenschein, 1999 | USA | 35-65 | Prospective cohort | Risk Ratio | Adjusted | 8,157 | 8- High quality |
| 168 | Stahlberg, 2004 | Denmark | >44 | Prospective cohort | Risk Ratio | Adjusted | 10,874 | 6- Low quality |
| 169 | Stahr, 2019 | USA | 18-100 | Prospective cohort | Risk Ratio | Adjusted | 21,931 | 9- High quality |
| 170 | Stuebe, 2009 | USA | 25-42 | Prospective cohort | Hazard Ratio | Adjusted | 60,075 | 8- High quality |
| 171 | Suzuki, 2006 | Sweden | 64.6 | Prospective cohort | Rate Ratio | Adjusted | 51,823 | 8- High quality |
| 172 | Taylor, 2007 | UK | 35-69 | Prospective cohort | Rate Ratio | Adjusted | 35,372 | 9- High quality |
| 173 | Tehard, 2006 | France | 45-70 | Prospective cohort | Rate Ratio | Adjusted | 69,116 | 8- High quality |
| 174 | Terry, 2001 | Sweden | 40-76 | Prospective cohort | Rate Ratio | Adjusted | 61,463 | 9- High quality |
| 175 | Thomas, 2001 | Iceland | 20-81 | Nested case-control | Rate Ratio | Adjusted | 10,422 | 7- High quality |
| 176 | Thorbjarnardottir, 2014 | Iceland | 59.2 | Prospective cohort | Hazard Ratio | Adjusted | 16,928 | 9- High quality |
| 177 | Thune, 1997 | Norway | 20-54 | Prospective cohort | Risk Ratio | Adjusted | 25,624 | 9- High quality |
| 178 | Tikk, 2015 | Europe | 54.8 | Nested case-control | Rate Ratio | Adjusted | 614 | 7- High quality |
| 179 | Tjønneland, 2004b | Denmark | 50-64 | Prospective cohort | Rate Ratio | Adjusted | 23,618 | 7- High quality |
| 180 | Trapido, 1981 | USA | 25-57 | Prospective cohort | Risk Ratio | Adjusted | 95,519 | 7- High quality |
| 181 | Trieu, 2017 | Vietnam | 48.09 | Nested case-control | Rate Ratio | Adjusted | 788 | 6- Low quality |
| 182 | Tryggvadottir, 1997 | Iceland | 18-43 | Nested case-control | Rate Ratio | Adjusted | 1,387 | 8- High quality |
| 183 | Tulinius, 1990 | Iceland | No data | Prospective cohort | Risk Ratio | Adjusted | 61,040 | 5- Low quality |
| 184 | van den Brandt, 2017 | Netherland | 55-69 | Case-cohort | Risk Ratio | Adjusted | 62,573 | 8- High quality |
| 185 | van der Hel, 2004 | Netherlands | 20-59 | Prospective cohort | Rate Ratio | Adjusted | 493 | 7- High quality |
| 186 | Vatten, 1992 | Norway | 20-49 | Prospective cohort | Risk Ratio | Adjusted | 29,981 | 9- High quality |
| 187 | Velie, 2005 | USA | 40-91 | Prospective cohort | Rate Ratio | Adjusted | 40,559 | 8- High quality |
| 188 | Voorrips, 2002 | Netherlands | 55-69 | Prospective cohort | Rate Ratio | Adjusted | 62,573 | 8- High quality |
| 189 | Wada, 2015 | Japan | 54.15 | Prospective cohort | Hazard Ratio | Adjusted | 15,719 | 9- High quality |
| 190 | Wang, 2015a | China | 35.55 | Nested case-control | Rate Ratio | Adjusted | 129 | 7- High quality |
| 191 | Wang, 2015b | USA | 30-55 | Prospective cohort | Hazard Ratio | Adjusted | 106,037 | 8- High quality |
| 192 | Ward, 2008 | UK | 45-75 | Nested case-control | Rate Ratio | Adjusted | 1,189 | 8- High quality |
| 193 | Weiderpass, 2004 | Norway | 30-49 | Prospective cohort | Risk Ratio | Adjusted | 99,717 | 8- High quality |
| 194 | White, 2017b | USA | 35-74 | Prospective cohort | Hazard Ratio | Adjusted | 50,884 | 8- High quality |
| 195 | Willett, 1987 | USA | 34-59 | Prospective cohort | Risk Ratio | Adjusted | 121,700 | 8- High quality |
| 196 | Zeleniuch-jacquotte, 2012 | USA | 34-65 | Nested case-control | Rate Ratio | Adjusted | 1,039 | 7- High quality |
| 197 | Zhang, 1999 | USA | 12-62 | Prospective cohort | Rate Ratio | Adjusted | 5,048 | 8- High quality |
NOS: Newcastle Ottawa Scale, HRT: Hormone replacement therapy, OCP: Oral contraceptive pill, PA: Physical activity
Figure 1.
Flow of information through the various phases of the systematic review
Synthesis of results
Cigarette smoking — Based on 90 studies (Supplementary File 1), the overall RR for smokers versus nonsmokers was 1.07 (95% CI, 1.05, 1.09). The overall effect measure showed that smoking significantly increased the risk of breast cancer by 7% (P <0.001). Between-study heterogeneity was moderate (I2=54%). The overall effect became a bit stronger (RR, 1.08; 95% CI, 1.06, 1.10; I2=42%) after performing a sensitivity analysis (Table 2).
Table 2. Results of sensitivity analysis.
| Variables | Before the sensitivity analysis | After the sensitivity analysis | ||||||
| Studies | χ 2 | I 2 | RR (95% CI) | Studies | χ 2 | I 2 | RR (95% CI) | |
| Cigarette smoking | 90 | 0.001 | 54% | 1.07 (1.05, 1.09) | 84 | 0.002 | 42% | 1.08 (1.06, 1.10) |
| Alcohol drinking | 56 | 0.001 | 63% | 1.10 (1.07, 1.12) | 53 | 0.001 | 49% | 1.08 (1.06, 1.11) |
| Sufficient physical activity | 16 | 0.001 | 63% | 0.90 (0.86, 0.95) | 15 | 0.030 | 45% | 0.89 (0.85, 0.94) |
| Overweight/obesity | 52 | 0.001 | 76% | 1.10 (1.05, 1.14) | 45 | 0.001 | 45% | 1.11 (1.08, 1.15) |
| Nulliparity | 67 | 0.001 | 97% | 1.16 (1.03, 1.31) | 60 | 0.001 | 44% | 1.22 (1.18, 1.27) |
| Late pregnancy | 37 | 0.001 | 90% | 1.37 (1.25, 1.50) | 36 | 0.002 | 41% | 1.29 (1.23, 1.35) |
| Breastfeeding | 35 | 0.001 | 82% | 0.87 (0.81, 0.93) | 32 | 0.450 | 1% | 0.93 (0.91, 0.96) |
| Ever using oral contraceptive | 45 | 0.001 | 64% | 1.00 (0.96, 1.05) | 42 | 0.008 | 38% | 1.04 (1.01, 1.08) |
| Ever using estrogen | 23 | 0.001 | 88% | 1.13 (1.04, 1.23) | 18 | 0.010 | 46% | 1.09 (1.03, 1.16) |
| Ever using progesterone | 5 | 0.020 | 67% | 1.02 (0.84, 1.24) | 3 | 0.910 | 0% | 1.01 (0.94, 1.10) |
| Ever using estrogen/progesterone | 17 | 0.001 | 95% | 1.60 (1.42, 1.80) | 8 | 0.050 | 50% | 1.47 (1.37, 1.59) |
| Ever taking hormone replacement therapy | 62 | 0.001 | 88% | 1.26 (1.20, 1.32) | 42 | 0.001 | 50% | 1.27 (1.23, 1.32) |
| Red meat consumption | 22 | 0.003 | 52% | 1.05 (1.00, 1.11) | 21 | 0.010 | 47% | 1.06 (1.01, 1.12) |
| Fruit/vegetable consumption | 14 | 0.550 | 0% | 0.87 (0.83, 0.90) | A sensitivity analysis was not necessary. | |||
Based on 48 studies, the overall RR for current smokers versus never smokers was 1.06 (95% CI, 1.03, 1.10). The overall effect measure showed that current smoking significantly increased the risk of breast cancer by 6% (P<0.001). Between-study heterogeneity was moderate (I2=65%). The overall effect became a bit stronger (RR, 1.09; 95% CI, 1.05, 1.13; I2=49%) after performing a sensitivity analysis.
Based on 42 studies, the overall RR for former smokers versus never smokers was 1.07 (95% CI, 1.05, 1.10). The overall effect measure showed that former smoking significantly increased the risk of breast cancer by 7% (P<0.001). Between-study heterogeneity was low (I2=29%). There was no evidence of publication bias (P=0.222 and P=0.965 based on the Begg and Egger tests, respectively)
Drinking alcohol — Based on 56 studies (Supplementary File 2), the overall RR for drinkers versus nondrinkers was 1.10 (95% CI, 1.07, 1.12). The overall effect measure showed that drinking significantly increased the risk of breast cancer by 10% (P<0.001). Between-study heterogeneity was moderate (I2=63%). The overall effect became slightly weaker (RR, 1.08; 95% CI, 1.06, 1.11; I2=49%) after performing a sensitivity analysis (Table 2).
Based on 46 studies, the overall RR for current drinkers versus never drinkers was 1.09 (95% CI, 1.06, 1.12). The overall effect measure showed that current drinking significantly increased the risk of breast cancer by 9% (P<0.001). Between-study heterogeneity was moderate (I2=66%). The overall effect became slightly weaker (RR, 1.08; 95% CI, 1.05, 1.10; I2=50%) after performing a sensitivity analysis.
Based on 10 studies the overall RR for former drinkers versus never drinkers was 1.22 (95% CI, 1.07, 1.39). The overall effect measure showed that former drinking significantly increased the risk of breast cancer by 22% (P<0.001). Between-study heterogeneity was low (I2=43%). There was no evidence of publication bias (P=0.997 and P=0.211 based on the Begg and Egger tests, respectively).
Sufficient physical activity — Based on 16 studies (Supplementary File 3), the overall RR for sufficient versus insufficient physical activity was 0.90 (95% CI, 0.86, 0.95). The overall effect measure showed that physical activity reduced significantly the risk of breast cancer by 9% (P<0.001). Between-study heterogeneity was moderate (I2=63%). The overall effect became slightly stronger (RR, 0.89; 95% CI, 0.85, 0.94; I2=45%) after performing a sensitivity analysis. There was no evidence of publication bias (P=0.677 and P=0.136 based on the Begg and Egger tests, respectively).
Body mass index — Based on 52 studies (Supplementary File 4), the overall RR for overweight/obesity versus normal weight was 1.10 (95% CI, 1.05, 1.14). The overall effect measure showed that overweight/obesity significantly increased the risk of breast cancer by 10% (P<0.001). Between-study heterogeneity was high (I2=76%). The overall effect became slightly stronger (RR, 1.11; 95% CI, 1.08, 1.14; I2=49%) after performing a sensitivity analysis (Table 2). There was no evidence of publication bias (P=0.917 and P=0.105 based on the Begg and Egger tests, respectively).
The effect of body mass index on the incidence risk of breast cancer was evaluated in pre- and post-menopausal periods separately. Based on 15 studies (Supplementary File 5), the overall RR for overweight/obesity versus normal weight in the premenopausal period was 0.92 (95% CI, 0.82, 1.03). The overall effect measure showed that overweight/obesity had no significant effect on the risk of breast cancer (P=0.140). Between-study heterogeneity was low (I2=50%). On the other hand, based on 24 studies (Supplementary File 6), the overall RR for overweight/obesity versus normal weight during the postmenopausal period was 1.18 (95% CI, 1.13, 1.24). The overall effect measure showed that overweight/obesity significantly increased the risk of breast cancer by 18% (P<0.001).
Parity — Based on 67 studies (Supplementary File 7), the overall RR for nulliparous versus primiparous/multiparous was 1.16 (95% CI, 1.03, 1.31). The overall effect measure showed that nulliparity significantly increased the risk of breast cancer by 16% (P<0.001). Between-study heterogeneity was high (I2=97%). The overall effect became slightly stronger (RR, 1.22; 95% CI, 1.18, 1.27; I2=44%) after performing a sensitivity analysis (Table 2).
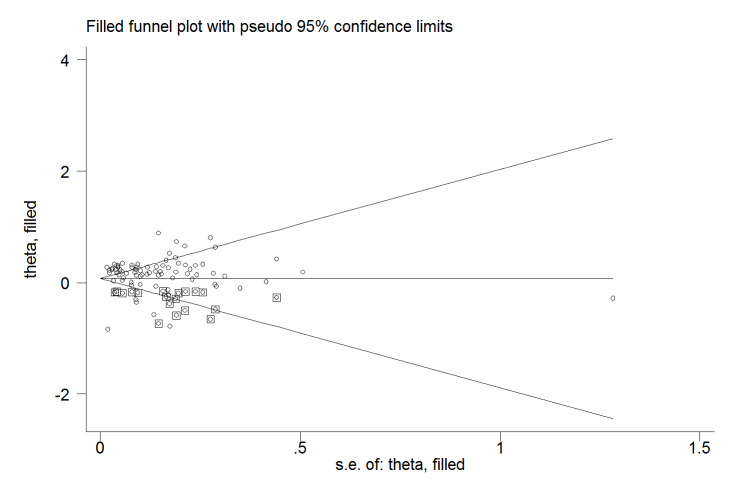
The Egger test revealed no evidence of publication bias (P=0.182); however, the Begg test did indicate evidence of publication bias (P=0.001). Trim-and-fill analysis estimated 19 missing studies (Figure 2) and the overall effect became slightly weaker (RR, 1.08; 95% CI, 0.99, 1.17).
Figure 2.
Trim-and-fill analysis estimating the number of possible missing studies for the association between breast cancer and nulliparity
The squares represent the possible missing studies.
Late pregnancy — Based on 37 studies (Supplementary File 8), the overall RR for late pregnancy of ≥30 years versus normal pregnancy of <30 years was 1.37 (95% CI, 1.25, 1.50). The overall effect measure showed that late pregnancy significantly increased the risk of breast cancer by 37% (P<0.001). Between-study heterogeneity was high (I2=90%). The overall effect became slightly weaker (RR, 1.29; 95% CI, 1.23, 1.35; I2=41%) after performing a sensitivity analysis (Table 2).
The Egger test revealed no evidence of publication bias (P=0.150); nevertheless, the Begg test did indicate evidence of publication bias (P=0.001); however, the trim-and-fill analysis estimated no missing studies.
Breastfeeding — Based on 35 studies Supplementary File 9, the overall RR for breastfeeding versus no breastfeeding was 0.87 (95% CI, 0.81, 0.93). The overall effect measure showed that breastfeeding reduced significantly the risk of breast cancer by 13% (P<0.001). Between-study heterogeneity was high (I2=82%). The overall effect became slightly weaker (RR, 0.93; 95% CI, 0.91, 0.96; I2=1%) after performing a sensitivity analysis (Table 2). There was no evidence of publication bias (P=0.178 and P=0.249 based on the Begg and Egger tests, respectively).
Ever using OCP — Based on 45 studies (Supplementary File 10), the overall RR for using OCP versus not using OCP was 1.00 (95% CI, 0.96, 1.05). Using OCP did not affect breast cancer (P=0.870). Between-study heterogeneity was moderate (I2=64%). The overall effect became slightly stronger and significant (RR, 1.04; 95% CI, 1.01, 1.08; I2=38%) after performing a sensitivity analysis (Table 2). There was no evidence of publication bias (P=0.417 and P=0.588 based on the Begg and Egger tests, respectively).
Ever using estrogen — Based on 23 studies (Supplementary File 11), the overall RR for using estrogen versus not using estrogen was 1.13 (95% CI, 1.04, 1.23). The overall effect measure showed that using estrogen significantly increased the risk of breast cancer by 13% (P<0.001). Between-study heterogeneity was high (I2=88%). The overall effect became slightly weaker (RR, 1.09; 95% CI, 1.03, 1.16; I2=46%) after performing a sensitivity analysis (Table 2). There was no evidence of publication bias (P=0.464 and P=0.913 based on the Begg and Egger tests, respectively).
Ever using progesterone — Based on 5 studies (Supplementary File 12), the overall RR for using progesterone versus not using progesterone was 1.02 (95% CI, 0.84, 1.24). The overall effect measure showed that using progesterone had no significant effect on breast cancer (P=0.820). Between-study heterogeneity was moderate (I2=67%). The overall effect became slightly weaker (RR, 1.01; 95% CI, 0.94, 1.10; I2=0%) after performing a sensitivity analysis (Table 2). There was no evidence of publication bias (P=0.293 and P=0.211 based on the Begg and Egger tests, respectively).
Ever using estrogen/progesterone — Based on 17 studies (Supplementary File 13), the overall RR for using estrogen/progesterone versus not using estrogen/progesterone was 1.60 (95% CI, 1.42, 1.80). The overall effect measure showed that using estrogen/progesterone significantly increased the risk of breast cancer by 60% (P<0.001). Between-study heterogeneity was high (I2=95%). The overall effect became slightly weaker (RR, 1.47; 95% CI, 1.37, 1.59; I2=50%) after performing a sensitivity analysis (Table 2). There was no evidence of publication bias (P=0.537 and P=0.528 based on the Begg and Egger tests, respectively).
Ever taking hormone replacement therapy — Based on 62 studies (Supplementary File 14), the overall RR for taking HRT versus not taking HRT was 1.26 (95% CI, 1.20, 1.32). The overall effect measure showed that taking HRT significantly increased the risk of breast cancer by 26% (P<0.001). Between-study heterogeneity was high (I2=88%). The overall effect became slightly stronger (RR, 1.27; 95% CI, 1.23, 1.32; I2=50%) after performing a sensitivity analysis (Table 2). There was no evidence of publication bias (P=0.775 and P=0.440 based on the Begg and Egger tests, respectively).
Red meat consumption — Based on 22 studies (Supplementary File 15), the overall RR for the highest intake versus the lowest intake of red meat was 1.05 (95% CI, 1.00, 1.11). The overall effect measure showed that the consumption of red meat had no significant effect on breast cancer (P=0.030). Between-study heterogeneity was moderate (I2=52%). The overall effect became slightly stronger (RR, 1.06; 95% CI, 1.01, 1.12; I2=47%) after performing a sensitivity analysis (Table 2). The Begg test revealed no evidence of publication bias (P=0.108), while the Egger test did indicate evidence of publication bias (P=0.022). However, the trim-and-fill analysis estimated no missing studies.
Fruit/vegetable consumption — Based on 14 studies (Supplementary File 16), the RR for the highest intake versus the lowest intake of fruit/vegetables infrequently was 0.87 (95% CI, 0.83, 0.90). The overall effect measure showed that fruit/vegetable consumption significantly reduced the risk of breast cancer by 23% (P=0.001). Between-study heterogeneity was low (I2=0%). There was no evidence of publication bias (P=0.412 and P=0.536 based on the Begg and Egger tests, respectively).
History of radiation therapy — Only one prospective cohort study 28 was found that investigated the effect of previous radiation therapy on the incidence of breast cancer. Based on the results of this study, the RR for ever-exposing to radiation therapy versus never-exposing to radiation therapy was 1.31 (0.87, 1.98). The effect measure showed that exposure to radiation therapy had no significant effect on breast cancer.
Unified overview
Figure 3 presents a unified overview of the associations between breast cancer and all nutritional and behavioral factors. As shown in this figure, taking HRT, using estrogen/progesterone, using estrogen, having late pregnancy, being nulliparous, consuming red meat, and being overweight/obese in the postmenopausal period were found to significantly increase the risk of breast cancer. In contrast, sufficient physical activity, fruit/vegetable consumption, and breastfeeding reduced significantly the risk of breast cancer. Meanwhile, exposure to ionizing radiation, using progesterone, using OCP, and being overweight/obese in the premenopausal period had no statistically significant effects on the risk of breast cancer.
Figure 3.
Associations (95% CIs) between breast cancer and nutritional and behavioral factors in a single view
Protective factors are shown in green (dark green, significant) and risk factors are shown in red (dark red, significant; light red, non-significant). The figures in parenthesis show the number of included studies.
Discussion
According to our findings, estrogen/progesterone uptake and late pregnancy were the first and second most powerful risk factors for breast cancer, respectively, whereas, sufficient fruit/vegetable consumption and sufficient physical activity were the first and second most powerful protective factors against breast cancer, respectively. The magnitudes of the effect measures reported in this systematic review may be used for ranking and prioritizing the relative importance of risk and protective factors. However, it should be kept in mind that these factors vary in terms of their physiological modus operandi and their units of exposure; therefore, direct comparisons are often unwarranted 29 . In other words, the mere fact that the RRs of some risk factors for breast cancer are higher than the RRs of other risk factors is not a sufficient basis for ranking and prioritizing risk factors. Instead, the prevalence of risk factors in the community is an essential criterion that needs to be taken into account when ranking and prioritizing risk factors. When the effect of a particular risk factor on the outcome of interest is strong (a high RR), however, the prevalence of that risk factor is low in the community, the overall impact of the risk factor on the disease burden in the community is low. In contrast, when a particular risk factor is common in the community, the overall impact of the factor on the outcome of interest may be tremendous even if the association between the risk factor and the outcome is not very strong (a low RR). Therefore, ranking and prioritizing the behavioral and nutritional factors affecting the risk of breast cancer depend on both the strength of the associations (the magnitude of RRs) and the prevalence of the factors in the community. Furthermore, it is impossible to consider risk or protective factors as separate elements, rather, they should be considered a collection. Risk factors facilitate the occurrence of diseases, while protective factors inhibit their occurrence. When a balance exists between risk and protective factors or when protective factors overcome risk factors, the disease will not occur. In contrast, the disease will occur when risk factors are stronger than protective factors 30 .
Our results revealed a positive association between cigarette smoking and the development of breast cancer. Ambrosone et al. conducted a meta-analysis of observational studies in 2008 and reported the effect of cigarette packs/years on breast cancer risk. They reported a dose-dependent fashion RR=1.44 (95% CI: 1.23, 1.68 for ≥20 pack/years versus never smokers) 31 . Cigarette smoke contains over 7,000 toxic chemical compounds, including human carcinogens 32 . These toxins and carcinogens can result in direct DNA damage. Since DNA controls the normal growth and function of cells, damage to DNA can alter the growth patterns of cells. These abnormal gastric epithelial cells with DNA damage can turn into cancer 33,34 .
This meta-analysis indicated that drinking alcohol increased the risk of developing breast cancer. Acetaldehyde, the first and most toxic metabolite of ethanol, is a human carcinogen and can induce DNA lesions by inhibiting DNA methylation and by interacting with retinoid metabolism 35 . DNA lesions can cause cell mutations, which can convert a normal cell into cancer 36 . Moreover, alcohol can act as an irritant and lead to mucosal damage. The damaged cells may try to repair themselves, which may cause DNA changes that can be a step toward cancer 37 .
According to our results, the risk of breast cancer of former drinkers was higher than that of current drinkers. One possible explanation for this finding is that former drinkers might be heavy drinkers who had drunk alcohol for many years; however, they were forced to quit drinking alcohol because of severe hepatobiliary and gastrointestinal complications 38 . Consistent with our findings, Key et al. performed a meta-analysis of studies addressing the association between alcohol and breast cancer in 2006. They concluded that excess risk associated with alcohol drinking was 22% (95% CI: 9%, 37%); each additional 10 g ethanol/day was associated with a risk increase by 10% (95% CI: 5%, 15%) 39 . In addition, Bagnardi et al. 40 conducted a dose-response meta-analysis to address the effect of alcohol consumption and site-specific cancer risk. Based on the results of the mentioned meta-analysis, the relative risk of female breast cancer was reported to be 1.04 (95%: 1.01, 1.07), 1.23 (95% CI: 1.19, 1.28), and 1.61 (95% CI: 1.33, 1.94) for light, moderate, and heavy drinking, respectively. Although the approach of this meta-analysis to address the effect of drinking alcohol on breast cancer risk was different from ours, its results were consistent with ours confirming that drinking alcohol can increase the risk of breast cancer.
Our results showed a positive and significant causal relationship between breast cancer and overweight and obesity as a whole. However, the effect of BMI on the incidence risk of breast cancer was different in pre- and post-menopausal periods separately. It was revealed that overweight/obesity had no significant effect on the risk of breast cancer in the premenopausal period (P<0.170); nonetheless, it had a significant impact on the postmenopausal period (P<0.001). These findings were consistent with the results of a previous meta-analysis conducted by Cheraghi et al. 11 in 2012. They reported that overweight and obesity had no significant effect on the incidence of breast cancer during the premenopausal period, whereas it might increase the postmenopausal risk of breast cancer. Evidence, based on the meta-analyses of observational studies, indicated that excess BMI not only increased the postmenopausal risk of breast cancer but also heightened the risk of gynecologic cancer in women, such as endometrial cancer 41 , cervical cancer 42 , and ovarian cancer 43 .
Based on our findings, using OCP, estrogen, progesterone, a combination of estrogen/progesterone, and HRT significantly increased the risk of breast cancer. The results of several previously conducted meta-analyses approved our findings. Steinberg et al. conducted a meta-analysis of case-control studies using community controls that analyzed the effect of conjugated equine estrogens on breast cancer. They reported that the risk of breast cancer after 10 years of estrogen use increased by at least 15% and up to 29% 44 . Based on the findings of another meta-analysis conducted by Steinberg et al., hormone replacement therapy using estradiol (with or without progestin) was associated with an increased risk of breast cancer RR=2.2 (95% CI, 1.4, 3.4) after 15 years 45 . Several mechanisms have been suggested to explain the association by which HRT increases the risk of breast cancer. The results of experimental studies showed that rigorous cell proliferation occurs upon hormonal exposure in patients with hormone receptor-positive breast cancer. Zghair et al. indicated that breast cancer type 1 susceptibility protein (BRCA1) was the predominant marker gene responsible for estrogen regulation. They reported that exposure to high levels of estrogen, as well as exposure to high levels of iron during the postmenstrual period, exerted synergistic effects on cellular proliferation in BRCA1-linked hormone-responsive breast cancer 46 . Additionally, both in vivo and in vitro investigations have been demonstrated that combination therapy with estradiol and estrogen/norethisterone increases the overexpression of proliferation of progesterone receptor membrane component 1 in breast cancer cells 47 . Furthermore, Wiebe et al. reported that progesterone metabolite 5α-pregnane stimulated breast cell proliferation and detachment, and therefore, played an important role in the development of breast cancer 48 .
The results of the present study indicated that breastfeeding decreased the risk of breast cancer by 13%, while late pregnancy significantly increased the risk of breast cancer by 40%. Consistent with our findings, Unar-Munguía recently conducted a meta-analysis in 2017 and showed that the relative risk for breast cancer in women who had breastfed exclusively was 0.72 (95% CI: 0.58, 0.90), compared to women who had never breastfed 49 . On the other hand, Namiranian et al. showed in a meta-analysis that the age of first pregnancy after 30 years was associated with an increased risk of breast cancer odds ratio=1.52 (95% CI: 1.30, 1.77) 50 . Pregnancy is associated with extensive changes to the breasts, making breast cells less likely to multiply and develop tumors. This issue explains the protective effect of pregnancy on younger women. However, after the age of 35 years, breast tissue is more likely to have accumulated cells carrying cancer-causing mutations, or clusters of abnormal cells with the potential to become cancerous. However, the important question is why the first pregnancy after age 35 increases the risk of breast cancer. The answer to this question lies in a signaling pathway called the JAK-STAT5 pathway. During pregnancy, pre-existing precancerous cells activate the PRLR-Jak2-STAT5 signaling pathway, accelerating their progression to fully cancerous cells. Blocking Jak2-STAT5 activity can reduce breast cancer risk associated with late-age pregnancy. This pathway can be blocked by various molecules, including Ruxolitinib, AG490, and C188-9 51 .
Our results indicated that sufficient physical activity significantly reduced the risk of breast cancer. Based on the findings of a meta-analysis conducted by Chen et al., there was an inverse association between physical activity and risk of breast cancer OR=0.87 (95% CI: 0.84, 0.90) 52 . Another meta-analysis conducted by Wu et al. reported a dose-response inversed relationship between physical activity and breast cancer risk. According to the results of this meta-analysis, the risk of breast cancer decreased by 2% for every 25 metabolic equivalents (MET)-h/week increment in non-occupational physical activity, 3% for every 10 MET-h/week increments in a recreational activity, and 5% for every 2 h/week increments in moderate plus vigorous recreational activity 53 . The mechanism by which physical activity reduces the risk of breast cancer is controversial. The results of empirical studies proposed that exercise-induced transient systemic acidosis will alter the in situ tumor microenvironment and delay tumor adaptation to regional hypoxia and acidosis in the later stages of carcinogenesis. Smallbone et al. demonstrated that repeated episodes of transient systemic acidosis would interrupt critical evolutionary steps in the later stages of carcinogenesis resulting in a substantial delay in the evolution of the invasive phenotype. They suggested that transient systemic acidosis might mediate the observed reduction in cancer risk associated with increased physical activity 54 .
Based on our findings, the intake of fruit and vegetable had a significant protective effect against breast cancer. The results of a meta-analysis recently conducted by Zhang et al. showed that the intake of vegetable-fruit-soybean dietary patterns could reduce the risk of breast cancer RR=0.87 (95% CI: 0.82, 0.91) 55 . Another meta-analysis conducted by Gandini et al. reported similar results. Based on the results of the mentioned meta-analysis, the relative risk of breast cancer for those who consumed vegetables was 0.75 (95% CI: 0.66, 0.85), and for those who consumed fruit was 0.94 (95% CI: 0.79, 1.11) 56 . It has been postulated that the anti-carcinogenic effects of fruits and vegetables may be attributed to the antioxidant effect of their vitamin content, especially vitamin C and beta-carotene. Antioxidants neutralize reactive oxygen free radicals, which cause DNA damage 57,58 , which in turn may result in genetic modifications and carcinogenesis ,34 .
Based on our findings, red meat consumption had a weak, yet, significant positive association with breast cancer. Farvid et al. 59 conducted a meta-analysis to address the effect of red and processed meat consumption on breast cancer incidence. They concluded that red meat consumption was associated with a 6% higher breast cancer risk (RR=1.06; 95% CI: 0.99, 1.14). The findings of another meta-analysis conducted by Guo et al. showed similar results. They reported that the relative risk of breast cancer for the highest versus the lowest consumption of red meat was 1.10 (95% CI: 1.02, 1.19) 60 . Current evidence recommends consuming no more than moderate amounts of red meat, such as beef, pork, and lamb, and eat little, if any, processed meat. The recommendation is to limit consumption to no more than about three portions per week, which are equivalent to about 350-500 grams (about 12-18 ounces) cooked weight of red meat 61 . There is strong evidence that the intake of either red or processed meat is the cause of colorectal, stomach, and breast cancers 38,62 .
This review had a few limitations and potential biases. There were some studies, mostly old, that seemed potentially eligible to be included in this meta-analysis; nevertheless, neither their full texts nor their corresponding authors were accessible. This issue might have introduced a selection bias in our results. Furthermore, some epidemiological studies that addressed the associations between breast cancer and some risk factors were excluded from the meta-analysis since they were not consistent with the inclusion criteria defined for this review. This issue might also have raised the possibility of selection bias.
Despite its limitations, this meta-analysis had three priorities over the previously conducted ones. First, many of the previous meta-analyses were carried out several years ago and needed to be updated based on current evidence. Second, in this study, 15 modifiable risk factors were examined, for some of which, no meta-analysis has been conducted before. Third, only the results of prospective cohort studies were employed that were the gold standard for observational studies with higher credibility.
Conclusion
This meta-analysis provided a clear picture of several factors playing pivotal roles in the development of breast cancer. These results are helpful and may be utilized for ranking and prioritizing preventable risk factors to implement effective interventions and community-based prevention programs. It is reemphasized that both the strength of associations and the prevalence of factors in the community should be taken into account when ranking and prioritizing breast cancer-associated factors.
Acknowledgments
These results were obtained as a part of an MSc thesis in Epidemiology. The authors would like to appreciate the Vice-Chancellor for Research and Technology of the Hamadan University of Medical Sciences, Hamadan, Iran, for approval and financial support of this study.
Conflict of interests
The authors have no conflict of interest to declare.
Funding
The Vice-Chancellor of Research and Technology, Hamadan University of Medical Sciences, funded this study (No. 9611247562). The funder had a role in the study design, data collection, analysis, publication decision, or manuscript preparation.
Highlights
Using estrogen/progesterone and late pregnancy are the first and second most powerful risk factors for breast cancer, respectively.
Sufficient fruit/vegetable consumption and sufficient physical activity were the first and second most powerful protective factors against breast cancer, respectively.
Ranking and prioritizing risk factors are essential for prevention programs.
Both the strength of association and the prevalence of risk factors are important for ranking.
Citation: Poorolajal J, Heidarimoghis F, Karami M, Cheraghi Z, Gohari-Ensaf F, Shahbazi F, Zareie B, Ameri P, Sahraei F Factors for the Primary Prevention of Breast Cancer: A Meta-Analysis of Prospective Cohort Studies. J Res Health Sci. 2021; 21(3): e00520.
References
- 1. World Health Organization. Breast cancer. Geneva: WHO; 2020 [cited 15 Feb 2020]; Available from: https://www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/en/.
- 2.DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A. et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69:438–51. doi: 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- 3.Sifri R, Gangadharappa S, Acheson LS. Identifying and testing for hereditary susceptibility to common cancers. CA Cancer J Clin. 2004;54:309–26. doi: 10.3322/canjclin.54.6.309. [DOI] [PubMed] [Google Scholar]
- 4.Poorolajal J, Nafissi N, Akbari ME, Mahjub H, Esmailnasab N, Babaee E. Breast cancer survival analysis based on immunohistochemistry subtypes (ER/PR/HER2): a retrospective cohort study. Arch Intern Med. 2016;19:680–6. [PubMed] [Google Scholar]
- 5.DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69:211–33. doi: 10.3322/caac.21555. [DOI] [PubMed] [Google Scholar]
- 6.Eccles DM, Pichert G. Familial non-BRCA1/BRCA2-associated breast cancer. Lancet Oncol. 2005;6:705–11. doi: 10.1016/S1470-2045(05)70318-1. [DOI] [PubMed] [Google Scholar]
- 7.Poorolajal J, Akbari ME, Ziaee F, Karami M, Ghoncheh M. Breast cancer screening (BCS) chart: a basic and preliminary model for making screening mammography more productive and efficient. J Public Health (Oxf) 2018;40:e118–25. doi: 10.1093/pubmed/fdx052. [DOI] [PubMed] [Google Scholar]
- 8.Shield KD, Soerjomataram I, Rehm J. Alcohol Use and Breast Cancer: A Critical Review. Alcohol Clin Exp Res. 2016;40:1166–81. doi: 10.1111/acer.13071. [DOI] [PubMed] [Google Scholar]
- 9.Johnson KC, Miller AB, Collishaw NE, Palmer JR, Hammond SK, Salmon AG. et al. Active smoking and secondhand smoke increase breast cancer risk: the report of the Canadian Expert Panel on Tobacco Smoke and Breast Cancer Risk (2009) Tob Control. 2011;20:e2. doi: 10.1136/tc.2010.035931. [DOI] [PubMed] [Google Scholar]
- 10.Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380:219–29. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheraghi Z, Poorolajal J, Hashem T, Esmailnasab N, Doosti Irani A. Effect of body mass index on breast cancer during premenopausal and postmenopausal periods: a meta-analysis. Plos One. 2012;7:e51446. doi: 10.1371/journal.pone.0051446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blackburn GL, Wang KA. Dietary fat reduction and breast cancer outcome: results from the Women's Intervention Nutrition Study (WINS) Am J Clin Nutr. 2007;86:s878–81. doi: 10.1093/ajcn/86.3.878S. [DOI] [PubMed] [Google Scholar]
- 13.Mourouti N, Kontogianni MD, Papavagelis C, Panagiotakos DB. Diet and breast cancer: a systematic review. Int J Food Sci Nutr. 2015;66:1–42. doi: 10.3109/09637486.2014.950207. [DOI] [PubMed] [Google Scholar]
- 14. World Health Organization. Global strategy on diet, physical activity and health: Fifty -seventh World Health Assembly WHA 57.17. Geneva: WHO; 2004.
- 15. Health Encyclopedia. Risks of pregnancy over age 30. University of Rochester Medical Center; 2020 [cited July 09, 2020]; Available from: https://www.urmc.rochester.edu/encyclopedia/content.aspx?contenttypeid=90&contentid=P02481.
- 16. Center for Chronic Disease Prevention and Health Promotion. What are the benefits of breastfeeding? Atlanta: CDC; 2020 [updated May 28, 2020; cited July 9, 2020]; Available from: https://www.cdc.gov/breastfeeding/faq/index.htm.
- 17. World Health Organization. Global action plan for the prevention and control of noncommunicable diseases 2013-2020. Geneva: WHO; 2013.
- 18. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ontario: Ottawa Hospital Research Institute; 2009 [cited 12 November 2018]; Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 19. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.0.0. Oxford: The Cochrane Collaboration; 2008.
- 20.Higgins JPT, Thompson SG, Deeks JJ, Altman D. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 23.Duval S, Tweedie R. A nonparametric "trim and fill" method of accounting for publication bias in metaanalysis. J Am Stat Assoc. 2000;95:89–98. [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 25.Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37:1148–57. doi: 10.1093/ije/dyn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poorolajal J, Mahmoodi M, Majdzadeh R, Fotouhi A. MetaPlot: a novel Stata graph for assessing heterogeneity at a glance. Iran J Public Health. 2010;39:102–4. [PMC free article] [PubMed] [Google Scholar]
- 27.Poorolajal J, Noornejad S. Metaplot: A new Stata module for assessing heterogeneity in a meta-analysis. Plos One. 2021;16:e0253341. doi: 10.1371/journal.pone.0253341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodman MT, Cologne JB, Moriwaki H, Vaeth M, Mabuchi K. Risk factors for primary breast cancer in Japan: 8-year follow-up of atomic bomb survivors. Prev Med. 1997;26:144–53. doi: 10.1006/pmed.1996.9979. [DOI] [PubMed] [Google Scholar]
- 29. Szklo M, Nieto FJ. Epidemiology, beyond the basics. Burlington: Jones & Bartlett Learning; 2019.
- 30.Poorolajal J. Equivalence model: a new graphical model for causal inference. Epidemiol Health. 2020;42:e2020024. doi: 10.4178/epih.e2020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ambrosone CB, Kropp S, Yang J, Yao S, Shields PG, Chang-Claude J. Cigarette smoking, N-acetyltransferase 2 genotypes, and breast cancer risk: pooled analysis and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2008;17:15–26. doi: 10.1158/1055-9965.EPI-07-0598. [DOI] [PubMed] [Google Scholar]
- 32. World Health Organization. World No Tobacco Day 2017: Beating tobacco for health, prosperity, the environment and national development. Geneva: WHO; 2017 [cited 1 June 2017]; Available from: http://www.who.int/mediacentre/news/releases/2017/no-tobacco-day/en/.
- 33.Dyke GW, Craven JL, Hall R, Garner RC. Smoking-related DNA adducts in human gastric cancers. Int J Cancer. 1992;52:847–50. doi: 10.1002/ijc.2910520602. [DOI] [PubMed] [Google Scholar]
- 34.Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, Hainaut P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene. 2002;21:7435–51. doi: 10.1038/sj.onc.1205803. [DOI] [PubMed] [Google Scholar]
- 35.Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer. 2007;7:599–612. doi: 10.1038/nrc2191. [DOI] [PubMed] [Google Scholar]
- 36.Deman J, Van Larebeke N. Carcinogenesis: mutations and mutagens. Tumour Biol. 2001;22:191–202. doi: 10.1159/000050615. [DOI] [PubMed] [Google Scholar]
- 37.Huh K, Kwon TH, Shin US, Kim WB, Ahn BO, Oh TY. et al. Inhibitory effects of DA-9601 on ethanol-induced gastrohemorrhagic lesions and gastric xanthine oxidase activity in rats. J Ethnopharmacol. 2003;88:269–73. doi: 10.1016/s0378-8741(03)00235-6. [DOI] [PubMed] [Google Scholar]
- 38.Poorolajal J, Moradi L, Mohammadi Y, Cheraghi Z, Gohari-Ensaf F. Risk factors for stomach cancer: a systematic review and meta-analysis. Epidemiol Health. 2020;42:e2020004. doi: 10.4178/epih.e2020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Key J, Hodgson S, Omar RZ, Jensen TK, Thompson SG, Boobis AR. et al. Meta-analysis of studies of alcohol and breast cancer with consideration of the methodological issues. Cancer Causes Control. 2006;17:759–70. doi: 10.1007/s10552-006-0011-0. [DOI] [PubMed] [Google Scholar]
- 40.Bagnardi V, Rota M, Botteri E, Tramacere Tramacere, Islami F, Fedirko V. et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose–response meta-analysis. Br J Cancer. 2015;112:280–593. doi: 10.1038/bjc.2014.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jenabi E, Poorolajal J. The effect of body mass index on endometrial cancer: a meta-analysis. Public Health. 2015;129:872–80. doi: 10.1016/j.puhe.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 42.Poorolajal J, Jenabi E. The association between BMI and cervical cancer risk: a meta-analysis. Eur J Cancer Prev. 2016;25:232–8. doi: 10.1097/CEJ.0000000000000164. [DOI] [PubMed] [Google Scholar]
- 43.Poorolajal J, Jenabi E, Masoumi SZ. Body Mass Index Effects on Risk of Ovarian Cancer: A Meta-Analysis. Asian Pac J Cancer Prev. 2014;15:7665–71. doi: 10.7314/apjcp.2014.15.18.7665. [DOI] [PubMed] [Google Scholar]
- 44.Steinberg KK, Smith SJ, Thacker SB, Stroup DF. Breast cancer risk and duration of estrogen use: the role of study design in meta-analysis. Epidemiology. 1994;5:415–21. doi: 10.1097/00001648-199407000-00007. [DOI] [PubMed] [Google Scholar]
- 45.Steinberg KK, Thacker SB, Smith SJ, Stroup DF, Zack MM, Flanders WD. et al. A meta-analysis of the effect of estrogen replacement therapy on the risk of breast cancer. JAMA. 1991;265:1985–90. [PubMed] [Google Scholar]
- 46.Zghair AN, Sharma R, Sharma AK. Hormone responsive breast cancer and BRCA1 mutation: mechanism, regulation and iron-mediated effects. Curr Pharm Biotechnol. 2014;15:1113–24. doi: 10.2174/1389201015666141126120725. [DOI] [PubMed] [Google Scholar]
- 47.Neubauer H, Ruan X, Schneck H, Seeger H, Cahill MA, Liang Y. et al. Overexpression of progesterone receptor membrane component 1: possible mechanism for increased breast cancer risk with norethisterone in hormone therapy. Menopause. 2013;20:504–10. doi: 10.1097/GME.0b013e3182755c97. [DOI] [PubMed] [Google Scholar]
- 48.Wiebe JP, Pawlak KJ, Kwok A. Mechanism of action of the breast cancer-promoter hormone, 5alpha-dihydroprogesterone (5alphaP), involves plasma membrane-associated receptors and MAPK activation. J Steroid Biochem Mol Biol. 2016;155:166–76. doi: 10.1016/j.jsbmb.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 49.Unar-Munguia M, Torres-Mejia G, Colchero MA, Gonzalez de Cosio T. Breastfeeding Mode and Risk of Breast Cancer: A Dose-Response Meta-Analysis. J Hum Lact. 2017;33:422–34. doi: 10.1177/0890334416683676. [DOI] [PubMed] [Google Scholar]
- 50.Namiranian N, Moradi-Lakeh M, Razavi-Ratki SK, Doayie M, Nojomi M. Risk factors of breast cancer in the Eastern Mediterranean Region: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2014;15:9535–41. doi: 10.7314/apjcp.2014.15.21.9535. [DOI] [PubMed] [Google Scholar]
- 51.Chakravarthi BV, Varambally S. Targeting the link between late pregnancy and breast cancer. Elife. 2013;2:e01926. doi: 10.7554/eLife.01926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen X, Wang Q, Zhang Y, Xie Q, Tan X. Physical Activity and Risk of Breast Cancer: A Meta-Analysis of 38 Cohort Studies in 45 Study Reports. Value Health. 2019;22:104–28. doi: 10.1016/j.jval.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 53.Wu Y, Zhang D, Kang S. Physical activity and risk of breast cancer: a meta-analysis of prospective studies. Breast Cancer Res Treat. 2013;137:869–82. doi: 10.1007/s10549-012-2396-7. [DOI] [PubMed] [Google Scholar]
- 54.Smallbone K, Maini PK, Gatenby RA. Episodic, transient systemic acidosis delays evolution of the malignant phenotype: Possible mechanism for cancer prevention by increased physical activity. Biol Direct. 2010;5:22. doi: 10.1186/1745-6150-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang L, Huang S, Cao L, Ge M, Li Y, Shao J. Vegetable-Fruit-Soybean Dietary Pattern and Breast Cancer: A Meta-Analysis of Observational Studies. J Nutr Sci Vitaminol (Tokyo) 2019;65:375–82. doi: 10.3177/jnsv.65.375. [DOI] [PubMed] [Google Scholar]
- 56.Gandini S, Merzenich H, Robertson C, Boyle P. Meta-analysis of studies on breast cancer risk and diet: the role of fruit and vegetable consumption and the intake of associated micronutrients. Eur J Cancer. 2000;36:636–46. doi: 10.1016/s0959-8049(00)00022-8. [DOI] [PubMed] [Google Scholar]
- 57.Akyon Y. Effect of antioxidants on the immune response of Helicobacter pylori. Clin Microbiol Infect. 2002;8:438–41. doi: 10.1046/j.1469-0691.2002.00426.x. [DOI] [PubMed] [Google Scholar]
- 58.Drake IM, Davies MJ, Mapstone NP, Dixon MF, Schorah CJ, White KL. et al. Ascorbic acid may protect against human gastric cancer by scavenging mucosal oxygen radicals. Carcinogenesis. 1996;17:559–62. doi: 10.1093/carcin/17.3.559. [DOI] [PubMed] [Google Scholar]
- 59.Farvid MS, Stern MC, Norat T, Sasazuki S, Vineis P, Weijenberg MP. et al. Consumption of red and processed meat and breast cancer incidence: A systematic review and meta-analysis of prospective studies. Int J Cancer. 2018;143:2787–99. doi: 10.1002/ijc.31848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo J, Wei W, Zhan L. Red and processed meat intake and risk of breast cancer: a meta-analysis of prospective studies. Breast Cancer Res Treat. 2015;151:191–8. doi: 10.1007/s10549-015-3380-9. [DOI] [PubMed] [Google Scholar]
- 61. World Cancer Research Fund Network. Recommendations and public health and policy implications: WCRFN; 2018.
- 62. World Cancer Research Fund Network. Diet, nutrition, physical activity and colorectal cancer: WCRFN; 2017.