Abstract
Background: The rapid increase in the spread of COVID-19 and the numbers of infected patients worldwide has highlighted the need for intensive care unit (ICU) beds and more advanced therapy. This need is more urgent in resource-constrained settings. The present study aimed to identify the predictors of ICU admission among hospitalized COVID-19 patients.
Study design: The current study was conducted based on a retrospective cohort design.
Methods: The participants included 665 definite cases of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) hospitalized in Imam Hossein Hospital from February 20 to May 14, 2020. The baseline characteristics of patients were assessed, and multivariate logistic regression analysis was utilized to determine the significant odds ratio (OR) for ICU admission.
Results: Participants were aged 59.52±16.72 years, and the majority (55.6%) of them were male. Compared to non-ICU patients (n=547), the ICU patients (n=118) were older, had more baseline comorbidities, and presented more often with dyspnea, convulsion, loss of consciousness, tachycardia, tachypnea, and hypoxia, and less often with myalgia. Significant OR (95% CI) of ICU admission was observed for the 60-80 age group (2.42, 95%CI: 1.01; 5.79), ≥80 age group (3.73, 95%CI: 1.44; 9.42), ≥3 comorbidities (2.07, 95%CI: 1.31; 3.80), loss of consciousness (6.70, 95%CI: 2.94, 15.24), tachypnea (1.79, 95%CI: 1.03, 3.11), and SpO2<90 (5.83, 95%CI: 2.74; 12.4). Abnormal laboratory results were more common among ICU-admitted patients; in this regard, leukocytosis (4.45, 95%CI: 1.49, 13.31), lymphopenia (2.39, 95%CI: 1.30; 4.39), elevated creatine phosphokinase (CPK) (1.99, 95%CI: 1.04; 3.83), and increased aspartate aminotransferase (AST) (2.25, 95%CI: 1.18-4.30) had a significant OR of ICU admission. Chest computer tomography (CT) revealed that consolidation (1.82, 95%CI: 1.02, 3.24), pleural effusion (3.19, 95%CI: 1.71, 5.95), and crazy paving pattern (8.36, 95%CI: 1.92, 36.48) had a significant OR of ICU admission.
Conclusion: As evidenced by the obtained results, the predictors of ICU admission were identified among epidemiological characteristics, presenting symptoms and signs, laboratory tests, and chest CT findings.
Keywords: SARS-CoV-2, COVID-19, Intensive Care Units, Risk factors, Critical care
Introduction
The coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first reported in Wuhan, China, in December 2019. It rapidly spread all over the world and was declared as a pandemic posing a major threat to public health 1 . By the end of January 2021, more than 101.5 million infected cases and 2.1 million deaths have been attributed to this disease across the globe 2 .
The rate of intensive care unit (ICU) admission among hospitalized infected patients has been reported to be up to 30% 3 . The rapid increase in disease spread and the numbers of infected patients worldwide has highlighted the need for ICUs and more advanced therapy. This need is more urgent in the resource-constrained settings which suffer from shortage in ICU and ventilator capacity 4,5 . Therefore, the identification of risk factors associated with disease severity may help better resource allocation. Moreover, due to rapid disease progression 6 , an awareness of the risk factors associated with poor outcomes would also help clinicians in early identification and better timely intervention of patients who would require advanced care during hospitalization. The risk factors associated with poor outcomes may also be used for the development of risk stratification models applicable in practice and also new standard thresholds for ICU admissions 7,8 .
Several clinical features have shown an association with ICU admission and severe outcomes among infected patients 9,10 . A systematic review study indicated that male gender among demographic data, dyspnea among signs and symptoms, as well as chronic obstructive pulmonary disease (COPD), cardiovascular disease (CVD), and hypertension (HTN) among comorbidities, were strongly associated with ICU admission 9 . Other clinical and laboratory predictive factors for ICU admission include older age, tachypnea, low pulse oxygen saturation, smoking history, low lymphocyte count, high lactate dehydrogenase, and procalcitonin levels greater than C-reactive protein (CRP) values 7,11 . Moreover, radiological examination, especially chest computer tomography (CT) plays a key role in the early detection of COVID-19. Furthermore, chest CT findings have prognostic utility for predicting the progression risk of patients at the time of admission and further need for ICU admission 12,13 .
Risk factors for ICU admission have not yet been validated in different populations, especially in low-income nations. These communities may be distinctive in terms of lifestyle, health-seeking behaviors, accessibility to high-quality health services, accurate symptom description, comorbid conditions, as well as ICU bed and ventilator capacity, which would affect ICU admission decision-making. Therefore, further investigation is required, particularly in Iran which is currently experiencing one of the highest mortality rates in the world 14 . The current study aimed to identify the key predictors of ICU admission among hospitalized COVID-19 patients in a large university hospital in Tehran, Iran.
Methods
Study participants
A total of 2643 suspected SARS-CoV-2 cases were admitted to Imam Hossein Hospital, a large university hospital located in Tehran, Iran, during the first peak of the COVID-19 surge in Iran from February 20 to May 14, 2020 15 . Hospital admission was based on the clinical judgment of an emergency physician. Definite hospitalized COVID-19 cases (based on positive reverse transcription-polymerase chain reaction [RT-PCR] assays) (n=691) were first enrolled. Finally, 665 cases were entered into this retrospective observational analytic study after the exclusion of cases aged<18 (n=7) and those with missing data (n=19).
Data collection
At the emergency department of the hospital, demographic data, as well as history and physical examination, were recorded, and blood samples were obtained and sent for laboratory tests. Nasopharyngeal swabs specimens were taken from suspected patients, followed by reverse transcription-polymerase chain reaction (RT-PCR). Upon admission, the patients underwent a low-dose chest CT scan in the supine position and at full inspiration without contrast medium injection. Chest CT scans were performed using a 16 detector CT scanner. Experienced radiologists evaluated and recorded imaging features, including the pattern of alternations (i.e. ground glass opacification [GGO], consolidation, nodule), distribution (peripheral/central, unilateral/bilateral, multifocal/unifocal), and associated findings (pleural and pericardial effusion, tree-in-bud and crazy paving pattern).
Definitions
Confirmed infection was defined as a person with at least one positive nasopharyngeal SARS-CoV-2 RT-PCR test result 16 . The participants were assigned to two groups based on ICU admission during their hospitalization. Neutrophil count and lymphocyte count were calculated by multiplying the percentages of neutrophils and lymphocytes by the total white blood cell (WBC) count, respectively. Regarding radiologic characteristics, ground-glass opacity (GGO) was considered a hazy increased lung opacity area which does not obscure the underlying bronchial structures and vessels. Moreover, the location of the lesion was considered central if the lesion location was limited to the bronchi, trachea, or segmental bronchi; otherwise, it was considered peripheral 17 . The “tree-in-bud” pattern refers to a small soft-tissue centrilobular nodule linked to multiple branching linear structures of similar caliber originating from a single stalk 18 . “Crazy paving” was also considered the appearance of scattered GGO with superimposed interlobular and interlobular septal thickening 19 .
Statistical analysis
Data were expressed as mean ±standard deviation, median, and interquartile range (IQR) for quantitative variables and percentages for categorical variables. The comparison between ICU and non-ICU groups was made by independent t-test and Mann-Whitney U test for normally and non-normally distributed quantitative variables, respectively. Moreover, it was carried out by chi-square or two-tailed Fisher's exact test for categorical variables. A p-value less than 0.05 was considered statistically significant. The variables were categorized into four groups (namely demographic, clinical, laboratory, and radiologic). In each group, categorical variables with a significant difference between ICU and non-ICU were entered in univariate logistic regression analysis. Variables with significant between-group differences in univariate models entered in multivariate logistic regression analysis for the identification of demographic, clinical, laboratory, and radiologic risk factors associated with ICU admission. All the statistical analyses were performed in SPSS software (version 19.0).
Results
The present study was conducted on 665 subjects aged 59.52±16.72. In terms of gender, 44.4% of cases were female. They either ended up in admission to the general ward (n=547) or the ICU (n=118) (Table 1). Compared to patients admitted to the general ward, ICU-admitted patients were older (66.82 ±14.82 vs. 57.94 ±16.70). Most prevalent baseline comorbidities were hypertension (33.5%), diabetes mellitus (27.5%), and cardiovascular disease (CVD) (19.6%). The prevalence rates of underlying lung diseases were obtained at 3.9% and 2.0% for asthma and COPD. The ICU-admitted patients had a higher prevalence of the mean number of baseline comorbidities, compared to others (1.55±1.12 vs. 1.23±1.25). Further multivariate logistic regression analysis of variables revealed significant odds ratios for the need for ICU admission in those who were in the 60-80 age group (2.42, 95%CI: 1.01, 5.79), older than 80 (3.73, 95%CI: 1.44, 9.42), and those with three or more baseline comorbidities (2.07, 95%CI: 1.31, 3.80).
Table 1. Demographic and epidemiologic characteristics of hospitalized COVID-19 patients.
| Variables | All patients (n=665) | General ward (n=547) | ICU (n=118) | ||||
| Number | Percent | Number | Percent | Number | Percent | P -value | |
| Sex | 0.560 | ||||||
| Male | 370 | 55.6 | 295 | 53.9 | 75 | 63.6 | |
| Female | 295 | 44.4 | 252 | 46.1 | 43 | 36.4 | |
| Age (yr) | 0.001 | ||||||
| <40 | 89 | 13.4 | 82 | 15.0 | 7 | 5.9 | |
| 40-60 | 230 | 34.6 | 203 | 37.1 | 27 | 22.9 | |
| 60-80 | 257 | 38.6 | 200 | 36.6 | 57 | 48.3 | |
| ≥80 | 89 | 13.4 | 62 | 11.3 | 27 | 22.9 | |
| Body mass index (kg/m2) | 0.198 | ||||||
| Non-obese (<30) | 236 | 68.0 | 186 | 70.5 | 50 | 78.5 | |
| Obesity (≥30) | 92 | 28.0 | 78 | 29.5 | 14 | 21.5 | |
| Co-morbidities | |||||||
| Diabetes mellitus | 0.116 | ||||||
| No | 481 | 72.5 | 403 | 83.8 | 78 | 66.7 | |
| Yes | 182 | 27.5 | 143 | 26.2 | 39 | 33.3 | |
| Hypertension | 0.001 | ||||||
| No | 440 | 66.5 | 378 | 69.2 | 62 | 53.4 | |
| Yes | 222 | 33.5 | 168 | 30.8 | 54 | 46.6 | |
| Cardiovascular disease | 0.018 | ||||||
| No | 532 | 80.4 | 448 | 82.1 | 84 | 72.4 | |
| Yes | 130 | 19.6 | 98 | 17.9 | 32 | 27.6 | |
| Chronic kidney disease | 0.816 | ||||||
| No | 614 | 92.7 | 507 | 92.9 | 107 | 92.2 | |
| Yes | 48 | 7.3 | 39 | 7.1 | 9 | 7.8 | |
| Chronic obstructive pulmonary disease | 0.259 | ||||||
| No | 649 | 98.0 | 537 | 98.4 | 112 | 96.6 | |
| Yes | 13 | 2.0 | 9 | 1.6 | 4 | 3.4 | |
| Asthma | 0.193 | ||||||
| No | 636 | 96.1 | 527 | 96.5 | 109 | 94.0 | |
| Yes | 26 | 3.9 | 19 | 3.5 | 7 | 6.0 | |
| Malignancy | 0.628 | ||||||
| No | 609 | 92.0 | 501 | 91.8 | 108 | 93.1 | |
| Yes | 53 | 8.0 | 45 | 8.2 | 8 | 6.9 | |
| Immunosuppression | 0.161 | ||||||
| No | 606 | 91.5 | 496 | 90.8 | 110 | 94.8 | |
| Yes | 56 | 8.5 | 50 | 9.2 | 6 | 5.2 | |
| Cerebrovascular accident | 0.029 | ||||||
| No | 632 | 95.3 | 525 | 96.2 | 107 | 91.5 | |
| Yes | 31 | 4.7 | 21 | 3.8 | 10 | 8.5 | |
| Pregnancy | 0.223 | ||||||
| No | 652 | 98.5 | 536 | 98.2 | 116 | 100.0 | |
| Yes | 10 | 1.5 | 10 | 1.8 | 0 | 0.0 | |
| Numbers of co-morbidities | 0.001 | ||||||
| 0 | 222 | 33.4 | 201 | 36.7 | 21 | 17.8 | |
| 1 | 184 | 27.7 | 143 | 26.1 | 41 | 34.7 | |
| 2 | 150 | 22.6 | 119 | 21.8 | 31 | 26.3 | |
| ≥3 | 109 | 16.4 | 84 | 15.4 | 25 | 21.2 | |
The most prevalent presenting symptoms were reported as cough (62.6%), fever (55.9%), and dyspnea (52.6%) in all patients (Table 2). Dyspnea, convulsion, and loss of consciousness were more common in the ICU-admitted patients, whereas myalgia was more common in patients admitted to the general hospital ward. Regarding baseline vital signs, pulse rate (93.91 ±18.90 vs. 87.94 ±14.00) and respiratory rate (21.12 ±5.61 vs. 18.81 ±3.97) were higher in the ICU-admitted patients, compared to those reported in non-ICU patients. Nonetheless, ICU-admitted patients had lower SpO2 (84.35 ±10.96 vs. 91.11 ±5.60) and were more frequently presented with tachycardia, tachypnea, and hypoxia (SpO2 <90 and 90≤ SpO2 <95). Significant odds ratios for the need for ICU admission were observed for loss of consciousness (6.70, 95%CI: 2.94, 15.24), tachypnea (1.79, 95%CI: 1.03, 3.11), and SpO2 <90 (5.83, 95%CI: 2.74, 12.4). On the other hand, patients with myalgia as a presenting symptom had lower odds of ICU admission (0.52, 95%CI: 0.31, 0.87).
Table 2. Clinical characteristics of hospitalized COVID-19 patients.
| Variables | All patients (n=665) | General ward (n=547) | ICU (n=118) | P -value | |||
| Number | Percent | Number | Percent | Number | Percent | ||
| Symptoms | |||||||
| Cough | 0.236 | ||||||
| No | 248 | 37.4 | 199 | 36.4 | 49 | 19.8 | |
| Yes | 415 | 62.6 | 348 | 63.6 | 67 | 57.8 | |
| Fever | 0.391 | ||||||
| No | 292 | 44.1 | 245 | 44.9 | 47 | 40.5 | |
| Yes | 370 | 55.9 | 301 | 55.1 | 69 | 59.5 | |
| Dyspnea | 0.001 | ||||||
| No | 315 | 47.4 | 277 | 50.6 | 38 | 32.5 | |
| Yes | 349 | 52.6 | 270 | 49.4 | 79 | 67.5 | |
| Myalgia | 0.012 | ||||||
| No | 376 | 56.7 | 298 | 54.5 | 78 | 67.2 | |
| Yes | 287 | 43.3 | 249 | 45.5 | 38 | 32.8 | |
| Fatigue | 0.137 | ||||||
| No | 423 | 63.8 | 342 | 80.9 | 81 | 69.8 | |
| Yes | 240 | 36.2 | 205 | 37.5 | 35 | 30.2 | |
| Chest pain | 0.727 | ||||||
| No | 547 | 82.5 | 450 | 82.3 | 97 | 83.6 | |
| Yes | 116 | 17.5 | 97 | 17.7 | 19 | 16.4 | |
| Sweating | 0.239 | ||||||
| No | 607 | 91.6 | 504 | 92.1 | 103 | 88.8 | |
| Yes | 56 | 8.4 | 43 | 7.9 | 13 | 11.2 | |
| Anorexia | 0.698 | ||||||
| No | 511 | 77.1 | 420 | 76.8 | 91 | 78.4 | |
| Yes | 152 | 22.9 | 127 | 23.2 | 25 | 21.6 | |
| Headache | 0.517 | ||||||
| No | 601 | 90.6 | 494 | 90.3 | 107 | 92.2 | |
| Yes | 62 | 9.4 | 53 | 9.7 | 9 | 7.8 | |
| Sore throat | 1.000 | ||||||
| No | 653 | 98.5 | 538 | 98.4 | 115 | 99.1 | |
| Yes | 10 | 1.5 | 9 | 1.6 | 1 | 0.9 | |
| Diarrhea | 0.391 | ||||||
| No | 623 | 94.0 | 512 | 93.6 | 11 | 95.7 | |
| Yes | 40 | 6.0 | 35 | 6.4 | 5 | 4.3 | |
| Nausea/Vomiting | 0.355 | ||||||
| No | 540 | 81.4 | 442 | 80.8 | 98 | 84.5 | |
| Yes | 123 | 18.6 | 105 | 19.2 | 18 | 15.5 | |
| Abdominal pain | 0.392 | ||||||
| No | 610 | 92.0 | 501 | 91.6 | 109 | 94.0 | |
| Yes | 53 | 8.0 | 46 | 8.4 | 7 | 6.0 | |
| Dizziness | 0.336 | ||||||
| No | 634 | 95.6 | 525 | 96.0 | 109 | 94.0 | |
| Yes | 29 | 4.4 | 22 | 4.0 | 7 | 6.0 | |
| Convulsion | 0.001 | ||||||
| No | 653 | 98.5 | 544 | 99.5 | 109 | 94.0 | |
| Yes | 10 | 1.5 | 3 | 0.5 | 7 | 6.0 | |
| Loss of consciousness | 0.001 | ||||||
| No | 615 | 92.6 | 522 | 95.4 | 93 | 79.5 | |
| Yes | 49 | 7.4 | 25 | 4.6 | 24 | 20.5 | |
| Others | 0.187 | ||||||
| No | 584 | 88.1 | 486 | 88.8 | 98 | 84.5 | |
| Yes | 79 | 11.9 | 61 | 11.2 | 18 | 15.5 | |
| Vital signs | |||||||
| Tachycardia | 0.001 | ||||||
| No | 544 | 84.5 | 458 | 86.7 | 86 | 74.1 | |
| Yes | 100 | 15.5 | 70 | 13.3 | 30 | 25.9 | |
| Tachypnea | 0.001 | ||||||
| No | 455 | 79.3 | 386 | 82.3 | 69 | 65.7 | |
| Yes | 119 | 20.7 | 83 | 17.7 | 36 | 34.4 | |
| Temperature (≥38.5) | 0.807 | ||||||
| No | 527 | 83.0 | 434 | 82.8 | 93 | 83.8 | |
| Yes | 108 | 17.0 | 90 | 17.2 | 18 | 16.2 | |
| O2 saturation (Spo2) | 0.001 | ||||||
| ≥95 | 147 | 24.8 | 134 | 27.8 | 13 | 11.7 | |
| 90-94 | 246 | 41.5 | 219 | 45.4 | 27 | 24.3 | |
| <90 | 200 | 33.7 | 129 | 26.8 | 71 | 64.0 | |
Hematologic tests upon admission showed leukocytosis (16.3%), neutrophilia (20.9%), lymphopenia (34.5%), and anemia (32.2%) which were all higher in the ICU group, compared to the non-ICU group (P< 0.05). In terms of infection-related parameters, 85.5% of patients had increased CRP (higher in the ICU group), and 80.1% of cases had increased erythrocyte sedimentation rate (ESR). Coagulatory tests pointed to increased Prothrombin time (PT) (21.4%) and international normalized ratio (INR) (20.1%) which were more frequently observed in the ICU group. Venous blood gas (VBG) results confirmed lower pH in the ICU group, compared to the non-ICU patients. Other laboratory tests demonstrated a higher prevalence of abnormal results (increased urea, creatinine, sodium, Creatine phosphokinase [CPK], Aspartate aminotransferase [AST], alanine aminotransferase [ALT], Lactate dehydrogenase [LDH], and decreased sodium) in the ICU group, compared to the non-ICU patients (Table 3). Four abnormal tests at baseline were associated with significance odds of ICU admission: leukocytocis (4.45, 95%CI: 1.49, 13.31), lymphopenia (2.39, 95%CI: 1.30, 4.39), increased CPK (1.99, 95%CI: 1.04-3.83), and increased AST (2.25, 95%CI: 1.18, 4.30).
Table 3. Baseline laboratory values of hospitalized COVID-19 patients.
| Variables |
All patients
(n=665) |
General ward
(n=547) |
ICU
(n=118) |
P -value | |||
| Number | Percent | Number | Percent | Number | Percent | ||
| Leukocytes (×109/L) | 0.001 | ||||||
| >11.20 | 105 | 16.3 | 71 | 13.4 | 34 | 29.1 | |
| 4.20-11.20 | 454 | 70.4 | 384 | 72.7 | 70 | 59.8 | |
| <4.20 | 86 | 13.3 | 73 | 13.8 | 13 | 11.1 | |
| Neutrophil count (×109/L) | 0.001 | ||||||
| >7.70 | 134 | 20.9 | 96 | 18.3 | 38 | 32.5 | |
| 1.5-7.7 | 495 | 77.4 | 418 | 80.0 | 77 | 65.8 | |
| <1.50 | 11 | 1.7 | 9 | 1.7 | 2 | 1.7 | |
| Lymphocyte count (×109/L) | 0.001 | ||||||
| >4.00 | 10 | 1.6 | 8 | 1.5 | 2 | 1.7 | |
| 1.00-4.00 | 404 | 63.9 | 350 | 67.8 | 54 | 47.0 | |
| <1.00 | 221 | 34.5 | 161 | 30.7 | 60 | 51.3 | |
| Platelets (×109/L) | 0.253 | ||||||
| >450.0 | 176 | 27.3 | 137 | 25.9 | 39 | 33.3 | |
| 150.0-450.0 | 454 | 70.4 | 379 | 71.8 | 75 | 64.1 | |
| <150.0 | 15 | 2.3 | 12 | 2.3 | 3 | 2.6 | |
| Hemoglobin (g/dL) | 0.475 | ||||||
| ≥12 | 437 | 67.8 | 361 | 68.4 | 76 | 65.0 | |
| <12 | 208 | 32.2 | 167 | 31.6 | 41 | 35.0 | |
| CRP (mg/L) | 0.268 | ||||||
| >10 | 511 | 85.0 | 412 | 84.3 | 99 | 88.4 | |
| ≤10 | 90 | 15.0 | 77 | 15.7 | 13 | 11.6 | |
| ESR (mm/h) | 0.975 | ||||||
| >20 | 330 | 80.1 | 253 | 80.1 | 77 | 80.2 | |
| ≤20 | 82 | 19.9 | 63 | 19.9 | 19 | 19.8 | |
| INR | 0.027 | ||||||
| >1.26 | 82 | 20.1 | 54 | 17.6 | 28 | 27.7 | |
| ≤1.26 | 326 | 79.9 | 253 | 82.4 | 73 | 72.3 | |
| Prothrombin time (s) | 0.032 | ||||||
| >13.6 | 87 | 21.4 | 58 | 18.9 | 29 | 29.0 | |
| ≤13.6 | 320 | 78.6 | 249 | 81.1 | 71 | 71.1 | |
| Partial thrombin time (s) | 0.596 | ||||||
| >40 | 20 | 5.0 | 14 | 4.6 | 6 | 5.9 | |
| ≤40 | 384 | 95.5 | 289 | 95.4 | 05 | 94.1 | |
| PH | 0.001 | ||||||
| >7.41 | 328 | 53.9 | 269 | 54.7 | 59 | 50.4 | |
| 7.31-7.41 | 242 | 29.7 | 202 | 33.2 | 40 | 34.1 | |
| <7.31 | 39 | 6.4 | 21 | 4.3 | 18 | 15.5 | |
| PCO2 (mmHg) | 0.633 | ||||||
| >52.0 | 76 | 12.5 | 60 | 12.2 | 16 | 13.7 | |
| 40.0-52.0 | 326 | 53.5 | 268 | 54.5 | 58 | 49.6 | |
| <40.0 | 207 | 34.0 | 164 | 33.3 | 43 | 36.8 | |
| HCO3 (mEq/L) | 0.254 | ||||||
| >27.00 | 306 | 50.2 | 251 | 51.0 | 55 | 47.0 | |
| 22.0-27.0 | 231 | 37.9 | 188 | 38.2 | 43 | 36.8 | |
| <22.00 | 72 | 11.8 | 53 | 10.8 | 19 | 16.2 | |
| Urea (mg/dl) | 0.001 | ||||||
| >45.0 | 202 | 31.7 | 149 | 28.7 | 53 | 45.3 | |
| ≤45.0 | 435 | 68.3 | 371 | 85.3 | 64 | 54.7 | |
| Serum creatinine (mg/dl) | 0.001 | ||||||
| >1.5 | 128 | 20.1 | 92 | 17.7 | 36 | 31.0 | |
| ≤1.5 | 509 | 79.9 | 429 | 82.3 | 80 | 69.0 | |
| Serum sodium (mmol/L) | 0.001 | ||||||
| >146.00 | 5 | 0.8 | 1 | 0.2 | 4 | 3.4 | |
| 133.0-146.0 | 528 | 86.0 | 438 | 72.8 | 90 | 77.6 | |
| <133.00 | 81 | 13.2 | 59 | 11.8 | 22 | 19.0 | |
| Serum potassium (mmol/L) | 0.482 | ||||||
| >5.00 | 49 | 8.0 | 38 | 7.6 | 11 | 9.5 | |
| 3.80-5.00 | 446 | 72.8 | 359 | 72.2 | 87 | 75.0 | |
| <3.80 | 118 | 19.2 | 100 | 20.1 | 18 | 15.5 | |
| CPK (IU/L) | 0.001 | ||||||
| >195.0 | 173 | 32.1 | 124 | 28.4 | 49 | 48.0 | |
| ≤195.0 | 366 | 67.9 | 313 | 71.6 | 53 | 52.0 | |
| AST (U/L) | 0.001 | ||||||
| >40.0 | 137 | 36.6 | 92 | 31.6 | 45 | 54.2 | |
| ≤40.0 | 238 | 63.4 | 200 | 68.4 | 38 | 45.8 | |
| ALT (U/L) | 0.050 | ||||||
| >50.0 | 60 | 16.1 | 41 | 14.1 | 19 | 23.2 | |
| ≤50.0 | 312 | 83.9 | 249 | 85.9 | 63 | 76.8 | |
| LDH (U/L) | 0.031 | ||||||
| >460.0 | 168 | 66.7 | 119 | 63.0 | 49 | 77.8 | |
| ≤460.0 | 84 | 33.3 | 70 | 37.0 | 12 | 22.2 | |
Normal CT scan findings were observed only in the non-ICU patients (6.2%; Table 4). The most prevalent radiologic lesion was GGO (observed in 80.3% of patients), and the distribution of lesions were mainly bilateral (84.2%), peripheral (74.3%), and multifocal (72.5%). Findings which were more frequently observed in the ICU patients included consolidation, pleural effusion, cardiomegaly, aortocoronary calcification, and crazy paving pattern. The most prevalent combination of findings were GOO + consolidation (10.4%), GOO + pleural effusion (9.0%), and consolidation + pleural effusion (5.0%) respectively. The results of multivariate logistic regression analysis showed significant odds of ICU admission for consolidation (1.82, 95%CI: 1.02, 3.24), pleural effusion (3.19, 95%CI: 1.71, 5.95), and crazy paving pattern (8.36, 95%CI: 1.92, 36.48) (Figure 1).
Table 4. Radiologic findings of hospitalized COVID-19 patients.
| Variables | All patients (n=480) | General ward (n=390) | ICU (n=90) | P -value | |||
| Number | Percent | Number | Percent | Number | Percent | ||
| Normal CT finding | 0.013 | ||||||
| No | 456 | 95.0 | 366 | 93.8 | 90 | 100.0 | |
| Yes | 24 | 5.0 | 24 | 6.2 | 0 | 0.0 | |
| Ground glass opacification (GGO) | 0.702 | ||||||
| No | 94 | 19.7 | 75 | 19.3 | 19 | 21.1 | |
| Yes | 384 | 80.3 | 313 | 80.7 | 71 | 78.9 | |
| Consolidation | 0.001 | ||||||
| No | 392 | 81.8 | 330 | 84.4 | 62 | 68.9 | |
| Yes | 87 | 18.2 | 59 | 15.2 | 28 | 31.1 | |
| Nodules | 0.800 | ||||||
| No | 452 | 94.6 | 366 | 94.3 | 86 | 95.6 | |
| Yes | 26 | 5.4 | 22 | 5.7 | 4 | 4.4 | |
| Bilateral lesion distribution | 0.179 | ||||||
| No | 75 | 15.8 | 65 | 16.8 | 10 | 11.1 | |
| Yes | 401 | 84.2 | 321 | 83.2 | 80 | 88.9 | |
| Peripheral lesion distribution | 0.965 | ||||||
| No | 122 | 25.7 | 99 | 25.8 | 23 | 25.6 | |
| Yes | 352 | 74.3 | 285 | 74.2 | 67 | 74.4 | |
| Multifocal lesion distribution | 0.740 | ||||||
| No | 130 | 27.5 | 104 | 27.2 | 26 | 28.9 | |
| Yes | 343 | 72.5 | 279 | 72.8 | 64 | 71.1 | |
| Pleural effusion | <0.001 | ||||||
| No | 418 | 87.4 | 354 | 91.2 | 64 | 71.1 | |
| Yes | 60 | 12.6 | 34 | 8.8 | 26 | 28.9 | |
| Pericardial effusion | 0.238 | ||||||
| No | 473 | 99.0 | 385 | 99.2 | 88 | 97.8 | |
| Yes | 5 | 1.0 | 3 | 0.8 | 2 | 2.2 | |
| Cardiomegaly | 0.001 | ||||||
| No | 413 | 86.4 | 345 | 88.9 | 68 | 75.6 | |
| Yes | 65 | 13.6 | 43 | 11.1 | 22 | 24.4 | |
| Lymphadenopathy | 0.839 | ||||||
| No | 425 | 89.3 | 345 | 89.1 | 80 | 89.9 | |
| Yes | 51 | 10.7 | 42 | 10.9 | 9 | 10.1 | |
| Aortocoronary calcification | 0.018 | ||||||
| No | 369 | 77.7 | 308 | 79.8 | 61 | 68.2 | |
| Yes | 106 | 22.3 | 78 | 20.2 | 28 | 31.8 | |
| Tree in bud | 1.000 | ||||||
| No | 461 | 96.6 | 374 | 96.6 | 87 | 96.7 | |
| Yes | 16 | 3.4 | 13 | 3.4 | 3 | 3.3 | |
| Crazy paving | 0.002 | ||||||
| No | 467 | 98.1 | 384 | 99.2 | 83 | 93.3 | |
| Yes | 9 | 1.9 | 3 | 0.8 | 6 | 6.7 | |
| GGO + Consolidation | 0.077 | ||||||
| No | 430 | 89.6 | 355 | 90.8 | 75 | 84.4 | |
| Yes | 50 | 10.4 | 36 | 9.2 | 14 | 15.6 | |
| GGO + Pleural effusion | 0.001 | ||||||
| No | 435 | 91.0 | 363 | 93.8 | 72 | 78.9 | |
| Yes | 43 | 9.0 | 24 | 6.2 | 19 | 21.1 | |
| Consolidation + Pleural effusion | 0.001 | ||||||
| No | 456 | 95.0 | 381 | 96.7 | 75 | 87.8 | |
| Yes | 24 | 5.0 | 13 | 3.3 | 11 | 12.2 | |
| Bilateral GGO | 0.946 | ||||||
| No | 116 | 24.2 | 94 | 24.1 | 22 | 24.4 | |
| Yes | 364 | 75.8 | 296 | 75.9 | 68 | 75.6 | |
| Bilateral Consolidation | 0.005 | ||||||
| No | 408 | 85.0 | 341 | 87.2 | 67 | 75.4 | |
| Yes | 72 | 15.0 | 50 | 12.8 | 22 | 24.6 | |
| Bilateral Nodules | 0.748 | ||||||
| No | 469 | 96.9 | 381 | 96.7 | 88 | 97.8 | |
| Yes | 15 | 3.1 | 13 | 3.3 | 2 | 2.2 | |
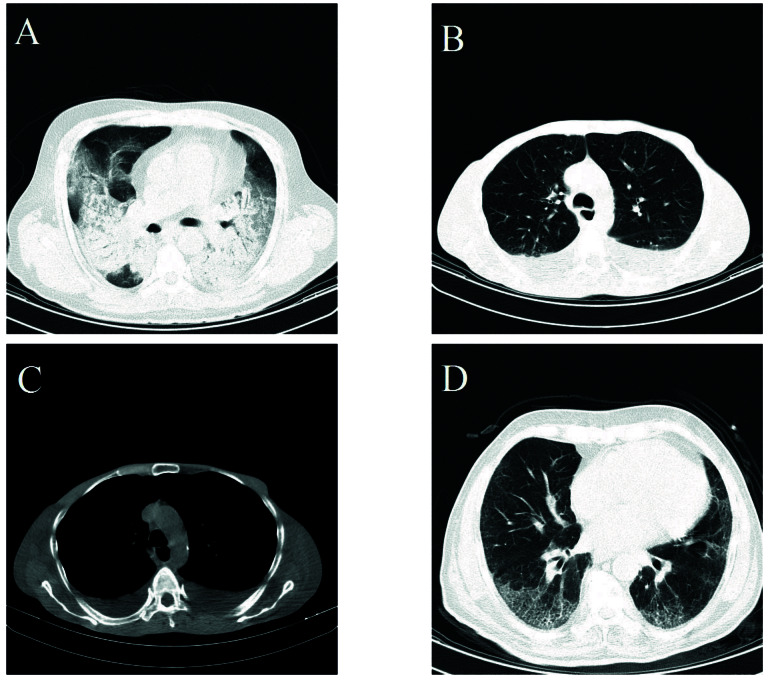
Figure 1.
Spiral chest CT scan of SARS-CoV2 infected patients. A 40- year-old woman with bilateral GGO and consolidation (A). A 56-year-old man with bilateral pleural effusion apparent at lung view (B) and mediastinal view (C). A 75-year-old man with bilateral ground glass opacity combined with interlobular septal thickening making a Crazy Paving appearance (D).
The overall mortality rate was 19.1% (76.3% in the ICU group and 6.3% in the non-ICU group; P<0.001). Compared to general ward patients, ICU-admitted patients had a longer duration of hospitalization (9.73 ±7.49 vs. 5.27 ±4.71; P<0.001), a higher rate of invasive mechanical ventilation (IMV) (67.8% vs. 3.7%; P<0.001), non-invasive mechanical ventilation (NIMV) (21.4% vs. 2.6%, P<0.001), dialysis, tracheostomy, and other procedures (Supplementary Table 1).
Discussion
To the best of our knowledge, this is the first study to assess the predictors of ICU admission in a sample of hospitalized COVID-19 patients in a developing country. In the present study, 17.7% of patients were admitted to ICU, and they faced a high mortality rate (76.3%), indicating the severity and highly progressed state of their disease course. Multivariate logistic regression analysis revealed the best predictors of ICU admission among epidemiological characteristics (age of over 80 years, age of over 60 years, and having more than three baseline co-morbidities). The best predictors of ICU admission among presenting signs and symptoms are loss of consciousness, SpO2 <90, and tachypnea. Leukocytosis, lymphopenia, increased AST, and elevated creatine phosphokinase (CPK), as well as radiologic findings of chest CT scan (including crazy paving pattern, pleural effusion, and consolidation), can also predict ICU admission.
Medical history of diabetes mellitus (DM), HTN, CVD, cerebrovascular accident (CVA), and COPD have been cited as predictive factors for severe outcomes in COVID-19 patients 9,20 . The present study also found significantly higher rates of HTN, CVD, and, CVA in ICU rather than non-ICU patients. Nevertheless, none of the underlying diseases had an independent significant association with ICU admission in multivariate analysis in the current study. However, having multiple comorbidities was found to predict ICU admission (more than three underlying comorbidities were two times more associated with ICU admission, irrespective of the underlying disease nature). The findings of the current study are in line with those reported by Carlino et al. who showed that none of the underlying medical conditions alone could predict ICU admission with a good accuracy 21 .
The well-established association of age with severe outcomes, such as ICU admission in COVID-19 infected patients, had been attributed to the increased number of comorbidities in older people 7,11 . However, the present study suggested that patients aged 60-80 years (OR= 2.42, 95%CI 1.01-5.79) and over 80 years (OR=3.73, 95%CI 1.44-9.42) are at higher risk of disease deterioration and ICU admission independently from other cofounders, such as underlying comorbidities. It has been proposed that underlying mechanisms associated with impaired cell-mediated and humoral immune systems, as well as dysfunctional pro-inflammatory responses, might play a role in consequent severe outcomes, such as ICU admission in older adults 22 .
In accordance with current knowledge, those with higher respiratory rates at presentation were at increased risk for severe outcomes 11,21 . Moreover, in line with the previous literature, SpO2<90% was the most strong predictive vital sign for ICU admission (OR=5.83, 95% CI: 2.74; 12.4) 7,23 . This finding confirms the notion that lung involvement in the form of pneumonia (interstitium inflammation and alteration of the alveolar ventilation) and consequent hypoxemia is the main pathophysiological mechanism of the disease in critical patients 24 and is present in the ICU group since their admission to the emergency department.
Dyspnea as a symptom of lung involvement was significantly more common among ICU-admitted patients; however, it did not display an independent significant association with ICU admission in multivariate analysis in the current study. It could be explained by ‘silent hypoxemia’ which had been suggested in previous studies as the presence of hypoxemia without experiencing difficulty in breathing 25,26 . Moreover, the rapid deterioration of the disease in cases of severe hypoxemia and consequent loss of consciousness was among the most significant predictors of ICU admission and severe outcomes in the present study. It can be regarded as an explanation for the observed finding since dyspnea is a purely subjective symptom 27 . On the other hand, it was observed that myalgia is a protective factor for ICU admission (OR=0.52, 95%CI: 0.31, 0.87) probably due to the fact that patients complaining about a mild symptom, such as myalgia, are not struggling with a severe or progressive disease course.
As acknowledged in previously conducted studies, CPK level was significantly higher among the ICU group 28 , representing an early sign of tissue injury and was associated with nearly twice-fold increased risk for ICU admission. Lactate dehydrogenase (LDH) level was also significantly higher among the ICU group; however, we failed to enter it in the multivariate analysis due to the high rate of missing data among LDH levels. Consistent with the present study, increased leukocyte count and neutrophil count were associated with ICU admission and severe outcomes in previous studies 21 . Moreover, in the current study increased leukocyte count was among the top predictors of ICU admission (OR=4.45, 95%CI: 1.49; 13.31). Furthermore, among laboratory features, decreased lymphocyte count was also an independent predictor of ICU admission. The observed increased lymphocyte and neutrophil count, as well as decreased lymphocyte count, in the ICU group, might be an indicator of the higher systemic inflammatory response induced by the body’s cytokines 29 . In addition, bacterial co-infections are higher among these patients and results in the aggravation of their respiratory condition. Moreover, it has been reported that lung infiltration of neutrophils and Neutrophil Extracellular Traps (NETs) observed in an autopsy specimen from a COVID-19 patient may play a role in patient deterioration and severe outcomes 30 . Higher AST levels were also associated with a greater risk of ICU admission (OR=2.25, 95% CI: 1.18; 4.30). Although the causality between COVID-19 and liver damage is still not fully understood, the association of liver injury with severe COVID-19 infection and outcomes has been supported 31 .
The majority of the patients had bilateral, multifocal, and peripheral lesions with a GGO pattern resembling the radiographic features related to pneumonia caused by COVID-19, and this finding was not significantly different between ICU and non-ICU patients 32,33 . Nevertheless, it has been suggested that CT scan features upon hospital admission could predict further outcomes 34 . In the current study, 24 patients (5.0%) had a clear chest CT, and none of them were admitted to the ICU during their hospitalization, suggesting that normal CT upon presentation is associated with a good prognosis. Moreover, three radiologic patterns (crazy-paving pattern, pleural effusion, and consolidation) were associated with ICU admission (and consequently, poor prognosis). Furthermore, the ICU-admitted patients were more likely to have a combination of CT scan findings, indicating more severe disease. Consolidation was found to be associated with severe disease and an indicator of poor outcome in the present study, as well as some previous studies 35 . The increased rate of consolidations, along with increasing percentages of lung involvement in patients is associated with disease progression and could partially explain the observed association 36,37 . Although crazy-paving pattern, a lesion indicative of extensive lung involvement, diffuse alveolar edema, and interstitial inflammation, is accounted as an uncommon finding even in ICU patients, it has been shown by multivariate analysis as the most strong predictors of ICU admission. However, the results of previous studies are inconsistent. Some of them pointed to an increased frequency of crazy paving patterns, along with disease progression 37 in ICU-admitted patients, while some others did not show any differences between different clinical groups in this regard 38 . Pleural effusion (bilateral in 86.7% of cases), another uncommon finding, was also associated with ICU admission and poor prognosis in the present study, as well as previous studies 13 , and it could be an indicator of bacterial co-infection. About 30% of patients with pleural effusion had underlying heart disease. The frequency of aortic calcification and cardiomegaly was found to be significantly higher in the ICU group; nonetheless, it did not display a significant OR for ICU admission.
Regarding the notable limitations of the present study, one can refer to limited generalizability of the results since it was a retrospective study based on a single institution and it did not include patients with mild to moderate symptoms. Moreover, the obtained result might have been biased toward the overestimation of mortality, especially in the ICU-admitted group, since our hospital had a relatively high patient load and limited resources leading to restricted ICU admission criteria. Another important limitation was the high proportion of missing data in some laboratory tests (i.e. LDH which was excluded from multivariate logistic regression analysis) and the CT scan of patients. On the other hand, the strengths of the study lie in its considerable sample size and the performance of multivariate logistic regression analysis which allowed the precise identification of disease severity and ICU admission predictors.
Conclusion
The risk factors for critically ill COVID-19 patients requiring ICU admission must be identified to allow better recognition of the most vulnerable target group of the disease, especially in the developing countries facing limited ICU beds and more complex resource allocation problems. The present study determined the best epidemiologic, as well as clinical and paraclinical predictors of ICU admission of hospitalized COVID-19 patients, facilitating decision making of frontline physicians to stratify high-risk patients in need of intensive care.
Acknowledgements
The authors' deepest appreciation goes to the staff and patients of Imam Hossein Hospital who took part in this research project. Our sincere gratitude is also extended to Professor Amir Kavousi for his guidance in statistical analysis and Mrs. Soheila Rahavard who participated in the data entry process. This study was extracted from an MPH dissertation submitted by Shayan Aryannezhad to the School of Public Health and Safety, Shahid Beheshti University of Medical Sciences (IR.SBMU.PNHS.REC.1399.028).
Conflict of interest
The authors declare that they have no conflict of interest regarding the publication of the current article.
Funding
The research team did not receive any grant from organizations in the public or private sectors.
Highlights
Developing countries challenged by COVID-19 are facing limited intensive care unit (ICU) beds and resources.
Older age and having more than three comorbidities predict ICU admission.
Loss of consciousness, SpO2 <90, and tachypnea predict ICU admission.
Leukocytosis, lymphopenia, increased aspartate aminotransferase, and elevated creatine phosphokinase can predict ICU admission.
Crazy paving pattern, pleural effusion, and consolidation predict ICU admission.
Citation: Hatami H, Soleimantabar H, Ghasemian M, Delbari N, Aryannezhad S. Predictors of Intensive Care Unit Admission among Hospitalized COVID-19 Patients in a Large University Hospital in Tehran, Iran. J Res Health Sci. 2021; 21(1): e00510.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J. et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–33. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO Coronavirus Disease (COVID-19) Dashboard. WHO website; 2021 [cited 31 Jan 2021] Available from: https://covid19.who.int.
- 3.Abate SM, Ahmed Ali S, Mantfardo B, Basu B. Rate of intensive care unit admission and outcomes among patients with coronavirus: A systematic review and Meta-analysis. PloS One. 2020;15(7):e0235653. doi: 10.1371/journal.pone.0235653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global coalition to accelerate COVID-19 clinical research in resource-limited settings. Lancet. 2020;395(10233):1322–5. doi: 10.1016/S0140-6736(20)30798-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dondorp AM, Hayat M, Aryal D, Beane A, Schultz MJ. Respiratory support in COVID-19 patients, with a focus on resource-limited settings. Am J Trop Med Hyg. 2020;102(6):1191–7. doi: 10.4269/ajtmh.20-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J. et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Z, Chen A, Hou W, Graham JM, Li H, Richman PS. et al. Prediction model and risk scores of ICU admission and mortality in COVID-19. PloS One. 2020;15(7):e0236618. doi: 10.1371/journal.pone.0236618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wollenstein-Betech S, Cassandras CG, Paschalidis IC. Personalized predictive models for symptomatic COVID-19 patients using basic preconditions: Hospitalizations, mortality, and the need for an ICU or ventilator. Int J Med Inform. 2020;142:104258. doi: 10.1016/j.ijmedinf.2020.104258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain V, Yuan JM. Predictive symptoms and comorbidities for severe COVID-19 and intensive care unit admission: a systematic review and meta-analysis. Int J Public Health. 2020;65(5):533–46. doi: 10.1007/s00038-020-01390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A. et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–81. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He F, Quan Y, Lei M, Liu R, Qin S, Zeng J. et al. Clinical features and risk factors for ICU admission in COVID-19 patients with cardiovascular diseases. Aging Dis. 2020;11(4):763–9. doi: 10.14336/AD.2020.0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng Z, Yu Q, Yao S, Luo L, Zhou W, Mao X. et al. Early prediction of disease progression in COVID-19 pneumonia patients with chest CT and clinical characteristics. Nat Commun. 2020;11(1):4968. doi: 10.1038/s41467-020-18786-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bao C, Liu X, Zhang H, Li Y, Liu J. Coronavirus disease 2019 (COVID-19) CT findings: a systematic review and meta-analysis. J Am Coll Radiol. 2020;17(6):701–9. doi: 10.1016/j.jacr.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johns Hopkins Coronavirus Resource Center - Mortality Analysis. Johns Hopkins University of Medicine Website; 2021 [cited 31 Jan 2021] Available from: https://coronavirus.jhu.edu/data/mortality.
- 15.Doosti-Irani A, Haghdoost AA, Najafi F, Eybpoosh S, Moradi G, Bagheri Amiri F. et al. How can the epidemic curve of COVID-19 in Iran be interpreted? J Res Health Sci. 2020;20(3):e00491. doi: 10.34172/jrhs.2020.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. WHO COVID-19 Case definition. WHO website; 2021 [cited 6 Nov 2020] Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-Surveillance_Case_Definition-2020.1.
- 17.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 18.Rossi SE, Franquet T, Volpacchio M, Giménez A, Aguilar G. Tree-in-bud pattern at thin-section CT of the lungs: radiologic-pathologic overview. Radiographics. 2005;25(3):789–801. doi: 10.1148/rg.253045115. [DOI] [PubMed] [Google Scholar]
- 19.Rossi SE, Erasmus JJ, Volpacchio M, Franquet T, Castiglioni T, McAdams HP. "Crazy-paving" pattern at thin-section CT of the lungs: radiologic-pathologic overview. Radiographics. 2003;23(6):1509–19. doi: 10.1148/rg.236035101. [DOI] [PubMed] [Google Scholar]
- 20.Williamson EJ, Walker AJ. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–6. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlino M V, Valenti N, Cesaro F. Predictors of intensive care unit admission in patients with coronavirus disease 2019 (COVID-19) Monaldi Arch chest Dis. 2020;90(1410):430–6. doi: 10.4081/monaldi.2020.1410. [DOI] [PubMed] [Google Scholar]
- 22.Opal SM, Girard TD, Ely EW. The immunopathogenesis of sepsis in elderly patients. Clin Infect Dis. 2005;41(Suppl 7):S504–12. doi: 10.1086/432007. [DOI] [PubMed] [Google Scholar]
- 23.Turcotte JJ, Meisenberg BR, MacDonald JH, Menon N, Fowler MB, West M. et al. Risk factors for severe illness in hospitalized Covid-19 patients at a regional hospital. PloS One. 2020;15(8):e0237558. doi: 10.1371/journal.pone.0237558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tobin MJ, Laghi F, Jubran A. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020;202(3):356–60. doi: 10.1164/rccm.202006-2157CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fung ML. Expressions of angiotensin and cytokine receptors in the paracrine signaling of the carotid body in hypoxia and sleep apnea. Respir Physiol Neurobiol. 2015;209:6–12. doi: 10.1016/j.resp.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, Crawford JM. et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med. 2020;217(6):e20200652. doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chandra A, Chakraborty U, Pal J, Karmakar P. Silent hypoxia: a frequently overlooked clinical entity in patients with COVID-19. BMJ Case Rep. 2020;13:e237207. doi: 10.1136/bcr-2020-237207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Coronavirus disease 2019 (COVID-19)-Prognosis. BMJ Best Practice website; 2021 [cited 6 Nov 2020] Available from: https://bestpractice.bmj.com/topics/en-us/3000168/prognosis.
- 29.Sun Z, Zhang N, Li Y, Xu X. A systematic review of chest imaging findings in COVID-19. Quant Imaging Med Surg. 2020;10(5):1058–79. doi: 10.21037/qims-20-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Li S, Liu J, Liang B, Wang X, Wang H. et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ali N. Relationship between COVID-19 infection and liver injury: a review of recent data. Front Med (Lausanne) 2020;7:458. doi: 10.3389/fmed.2020.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernheim A, Mei X. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology. 2020;295(3):200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan F, Ye T. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295(3):715–21. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu F, Zhang Q, Huang C, Shi C, Wang L, Shi N. et al. CT quantification of pneumonia lesions in early days predicts progression to severe illness in a cohort of COVID-19 patients. Theranostics. 2020;10(12):5613–22. doi: 10.7150/thno.45985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan M, Yin W, Tao Z, Tan W, Hu Y. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PloS One. 2020;15(3):e0230548. doi: 10.1371/journal.pone.0230548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruch Y, Kaeuffer C, Ohana M, Labani A, Fabacher T, Bilbault P. et al. CT lung lesions as predictors of early death or ICU admission in COVID-19 patients. Clin Microbiol Infect. 2020;26(10):1417. doi: 10.1016/j.cmi.2020.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanne JP, Little BP. Essentials for radiologists on COVID-19: an update-radiology scientific expert panel. Radiology. 2020;296(2):E113–e4. doi: 10.1148/radiol.2020200527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou S, Wang Y, Zhu T, Xia L. CT features of coronavirus disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. AJR Am J Roentgenol. 2020;214(6):1287–94. doi: 10.2214/AJR.20.22975. [DOI] [PubMed] [Google Scholar]