Abstract
The activities of a series of camptothecin and nitidine derivatives that might interact with topoisomerase I were compared against yeast and cancer cell lines. Our findings reveal that structural modifications to camptothecin derivatives have profound effects on the topoisomerase I-drug poison complex in cells. Although the water-soluble anticancer agents topotecan and irinotecan are less active than the original structure, camptothecin, other derivatives or analogs with substitutions that increase compound solubility have also increased antifungal activities. In fact, a water-soluble prodrug appears to penetrate into the cell and release its active form; the resulting effect in complex with Cryptococcus neoformans topoisomerase I is a fungicidal response and also potent antitumor activity. Some of the compounds that are not toxic to wild-type yeast cells are extremely toxic to the yeast cells when the C. neoformans topoisomerase I target is overexpressed. With the known antifungal mechanism of a camptothecin-topoisomerase I complex as a cellular poison, these findings indicate that drug entry may be extremely important for antifungal activity. Nitidine chloride exhibits antifungal activity against yeast cells through a mechanism(s) other than topoisomerase I and appears to be less active than camptothecin analogs against tumor cells. Finally, some camptothecin analogs exhibit synergistic antifungal activity against yeast cells in combination with amphotericin B in vitro. Our results suggest that camptothecin and/or nitidine derivatives can exhibit potent antifungal activity and that the activities of camptothecin derivatives with existing antifungal drugs may be synergistic against pathogenic fungi. These new compounds, which exhibit potent antitumor activities, will likely require further structural changes to find more selective activity against fungal versus mammalian cells to hold promise as a new class of antifungal agents.
The incidence of invasive fungal infections in immunocompromised patients has increased over the past two decades. These life-threatening mycoses have been treated with polyene and azole antifungal agents. Polyene antifungal agents, such as amphotericin B deoxycholate, are notorious for their host toxicities, but this toxicity profile has recently been improved with the use of new lipid formulations of amphotericin B. Although azoles are relatively safe to use in clinical practice and reasonably effective for management of some invasive fungal infections, the appearance of azole-resistant isolates of Candida albicans and Cryptococcus neoformans has increasingly been reported (1, 5, 19, 34). Moreover, the spectrum of azole antifungal activities remains limited, and these agents are not fungicidal. New antifungal agents with potent, broad-spectrum fungicidal activity and with a different mechanism(s) of action are needed for the effective management of life-threatening mycoses.
Topoisomerase I is a nuclear enzyme that catalyzes the breakage and rejoining of DNA and thus allows one strand to pass another. Topoisomerase I is required for DNA transcription, synthesis, and replication (27). Topoisomerase I is the sole target of camptothecin (CPT) (12). Other inhibitors, such as irinotecan (CPT-11) and topotecan (TPT), represent a new class of antineoplastic agents, derived from CPT, which have antitumor activities in patients with refractory solid cancers (20, 26). However, tumor cells vary in their sensitivities to these compounds, probably because the sensitivity is correlated with differences in topoisomerase I expression levels in various cancer cell lines. The mechanism for the antitumor and antifungal activities of CPT has been elucidated; a complex of topoisomerase I-CPT forms in the cell, creating a cellular poison. Therefore, in vitro antifungal activity studies for these classes of drugs in yeasts may be particularly variable because of reduced permeation of the drug into certain eukaryotic cells or reduced topoisomerase expression to produce the cellular poison complex (17). In fact, mutations that alter cell permeability, such as the Δerg6 or Δise2 mutations in Saccharomyces cerevisiae, can result in hypersensitivity to CPT killing of yeast through the ease of formation of this complex (6, 17, 18). Furthermore, overexpression of the topoisomerase I gene in S. cerevisiae increases cell killing by CPT (4, 6, 11, 17). Similarly, the overexpression of topoisomerase II in yeasts greatly increases sensitivity to amsacrine and etoposide (16), two anti-topoisomerase II agents (10). These findings suggest that the levels of topoisomerases I and II in cells may be critical for the treatment of a particular cancer (8, 21) or a fungal infection with these topoisomerase-specific drugs.
Nitidine is a benzophenanthridine alkaloid which has been shown to possess the ability to become a potent human topoisomerase I and II poison (7, 33). On the other hand, the fungal topoisomerase I of Aspergillus nidulans appears to be resistant to nitidine (9). These findings suggest that nitidine may have some selectivity for a target(s).
Recently, we isolated, cloned, and sequenced the C. neoformans topoisomerase I gene. Our findings reveal that, unlike S. cerevisiae, the topoisomerase I gene is essential for viability in C. neoformans (3). Moreover, the topoisomerase I enzymes of two major human pathogens, C. albicans (28) and C. neoformans, contain an amino acid insertion not found in the mammalian enzyme. This fungal insert is located in the linker domain. In human topoisomerase I the linker domain consists of 77 amino acids, while in the fungal topoisomerase I the linker domain contains 155 amino acids. Therefore, the essentiality of the C. neoformans topoisomerase I gene and its structural differences between the pathogenic fungal and mammalian enzymes should be further explored to design potential new selective antifungal agents.
In this study we determined whether CPT, nitidine, and several derivatives can efficiently target the C. neoformans topoisomerase I enzyme for antifungal activity. For instance, our previous studies have suggested that CPT can possess some direct activity against C. neoformans when cells are overexpressing topoisomerase I but not when wild-type cells are expressing normal levels (3). These findings suggest that CPT may have limited penetration into the cells and that the cellular poison complex can reach toxic amounts only in the presence of large amounts of the enzyme. Therefore, in this study we have used an S. cerevisiae strain with an Δerg6 mutation which allows better drug penetration (6) in order to test the direct antifungal activities of selected compounds or inhibitors that form complexes with DNA-topoisomerase I. To assay the antifungal activities of CPT, nitidine, and their derivatives, the C. neoformans topoisomerase I cDNA was cloned into an S. cerevisiae expression plasmid and introduced into an S. cerevisiae Δerg6 Δtop1 mutant yeast strain. The direct antifungal effects of a series of compounds with known structures were compared by standard in vitro susceptibility testing, so that structure-activity relationships could be ascertained. For an appreciation of their effects on mammalian cells, a comparison of the in vitro antifungal potencies of these compounds with their corresponding anticancer cell activities is also provided.
MATERIALS AND METHODS
Compounds.
A series of CPT, nitidine, and their derivatives were obtained as described previously (29, 31, 32). Stock solutions of 10 mg/ml were made in dimethyl sulfoxide, filter sterilized by passage through a 0.22-μm-pore-size Millex Durapore membrane filter, and stored at −70°C until use.
Isolation of C. neoformans TOP1 gene from cDNA of strain B3501 serotype D.
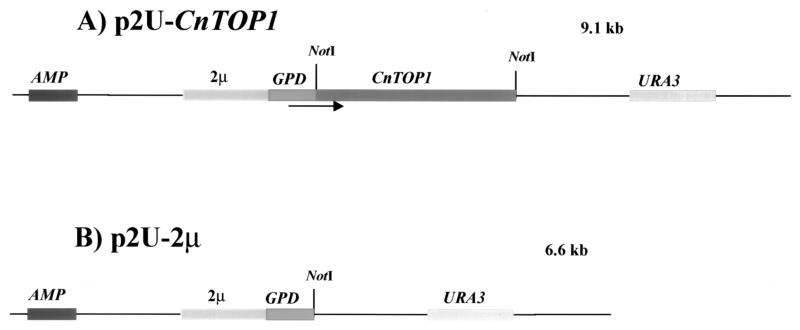
The topoisomerase I gene from C. neoformans genomic DNA serotype A strain H99 was cloned, sequenced, and described elsewhere (3). PCR was performed with C. neoformans cDNA as a template by using the following primers containing NotI sites (underlined): primer 9 (5′-TTAA GCGGCC GCA TGA GCG AGG ATG AGC AGC CTTTG-3′) and primer M2 (5′-GAGAGA GCGGCC GCC TAG AAA ACC CAG TAA GGT CCA-3′. PCR conditions were 95°C for 5 min (1 cycle); 93°C for 50 s, 50°C for 50 s, and 72°C for 80 s (25 cycles); and 72°C for 2 min (1 cycle). The amplification strategy produced a 2.5-kb fragment that was sequenced with the Sequenase, version 2.0, Sequencing Kit (U.S. Biochemicals, Life Science) (23). This 2.5-kb cDNA TOP1 fragment was digested with NotI and was cloned into the NotI site of a p2U-2μ vector, under control of the glyceraldehyde-3-phosphate dehydrogenase (GPD) promoter. This plasmid was named p2U-CnTOP1 (Fig. 1A). Plasmid p2U-2μ (Fig. 1B), which was made by insertion of the GPD promoter and the 2μm origin of replication into plasmid pRS306 (24, 25), was provided by Didier Picard (University of Geneva).
FIG. 1.
Map of p2U-CnTOP1 expression vector and the parent p2U-2μ plasmid. The cDNA topoisomerase I gene from C. neoformans B3501 was cloned into the NotI site of plasmid p2U-2μ, downstream of the 2μm replicon and GPD promoter, respectively (see Materials and Methods).
Test organisms.
Fungal isolates included two reference strains from the Duke University Mycology Research Unit, C. neoformans var. grubii H99 and C. albicans A39. The isogenic S. cerevisiae strain series consisting of the wild-type ERG6 TOP1 strain JHY6-1C (genotype, MATa ura3-52 leu2-3,112 his4 TRP1 rme1 HMLa), the Δerg6 mutant strain SMY73-3 (Δerg6::G418), and the Δerg6 Δtop1 mutant strain SMY75-1.4A (Δerg6::G418 Δtop1::G418) were constructed as follows. The ERG6 and TOP1 genes were disrupted by PCR-mediated single-step gene replacement in the isogenic MATa and MATα strains JHY6-1C and JHY6-1B, respectively, with PCR products spanning the G418 resistance gene and containing 40 bp of sequence homologous to the 5′ and 3′ ends of the targeted genes (13, 30). The resulting MATa Δerg6::G418 (SMY73-3) and MATα Δtop1::G418 (SMY74-3) mutant strains were mated, and the resulting diploid strain was sporulated and dissected. Tetrads in which the two G418 resistance genes cosegregated (two G418-resistant and 2 G418-sensitive segregants) were identified, and the presence of the Δerg6 and Δtop1 gene deletions was confirmed by PCR analysis of genomic DNA with flanking primers, to yield the Δerg6 Δtop1 double mutant strain SMY75-1.4A (Δerg6 Δtop1). The TRP1 prototrophic strains were used because the Δerg6 mutation is synthetically lethal with the trp1 auxotrophic mutation (6).
Transformation.
The expression vector p2U-CnTOP1 was transformed into the S. cerevisiae Δerg6 Δtop1 mutant strain as follows. Briefly, after preparing competent yeast cells in 1× Tris-EDTA (TE)-lithium acetate (TE-LiOAc), a transformation reaction was performed, as follows: 50 μl of competent cells, 10 μl of salmon sperm DNA (5 mg/ml), 0.5 μg of plasmid DNA, and 250 μl of 40% polyethylene glycol–1× TE-LiOAc were incubated at 30°C for 30 min. After heat shock at 42°C for 15 min, the reaction mixture was centrifuged for 25 s. The cells were suspended in 250 μl of 1× TE, plated on uracil dropout medium, and incubated at 30°C for 3 to 4 days. The strain obtained from transformation of p2U-CnTOP1 was named Δerg6 Δtop1+p2U-CnTOP1.
Media.
Yeast-extract peptone dextrose agar medium was used for routine growth of S. cerevisiae Δerg6 and Δerg6 Δtop1 strains. Uracil dropout medium, which contains 6.7 g of yeast nitrogen broth without amino acids per liter, 1.3 g of an amino acid mixture minus uracil per liter, 10 g of glucose per liter, and 20 g of agar per liter, was used as the selective medium for transformations. Antifungal susceptibility testing was performed in RPMI 1640 medium (Sigma, St. Louis, Mo.) with glutamine and without sodium bicarbonate, buffered at pH 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS). In tests with the S. cerevisiae wild-type, Δerg6, and Δerg6 Δtop1 strains with the ura3 mutation, 40 mg of uracil was added to 1 liter of RPMI 1640 medium.
In vitro susceptibility testing.
The MIC for each fungal strain was determined by the broth macrodilution method recommended in document M27-A (15). Briefly, this method specifies an inoculum concentration from 0.5 × 103 to 2 × 103 CFU/ml, incubation at 35°C, and reading of the results at 48 h for all yeasts other than C. neoformans, for which the results are read at 72 h. The only difference was the drug dilutions, which were made from 100 to 0.09 μg/ml for all compounds tested except CPT-11, which was diluted 2,000 to 0.09 μg/ml, and TPT, which was diluted from 1,000 to 0.09 μg/ml. The MICs were defined as the lowest drug concentrations in tubes which showed a visual turbidity less than or equal to 80% inhibition compared with that produced by the growth control tube.
Minimum fungicidal concentration (MFC) experiments were performed as described by McGinnis (14). Briefly, 100-μl aliquots from drug dilution tubes with visual growth inhibition were plated onto Sabouraud agar medium. The lowest drug concentration that yielded three or fewer yeast colonies was recorded as the MFC. The MICs and MFCs reported here are the geometric means of three separate experiments.
Checkerboard broth microdilution method for synergy study.
Drug interactions were assessed by checkerboard titration by adhering to the recommendations of the National Committee for Clinical Laboratory Standards (15). Briefly, susceptibility testing was performed in RPMI 1640 medium (Sigma) that contained l-glutamine but not sodium bicarbonate and that was buffered to pH 7.0 with 0.165 M MOPS. Aliquots of 50 μl of each drug (and, for the single-drug control, 50 μl of that drug and 50 μl of sterile water) at a concentration of four times the target final concentration were dispensed into wells of a microtiter plate (96-well Cell Culture Cluster, flat-bottom; Constar, Cambridge, Mass.) to provide 77 drug combinations. Additional rows were used to determine the MIC of each agent alone and the MIC for the growth control well (drug-free wells). The yeast inoculum (100 μl), prepared by the proposed standard method (15), was added to each well, and the microtiter plates were incubated at 35°C without shaking. The readings were made at 48 h for C. albicans and wild-type S. cerevisiae and at 72 h for C. neoformans. Before making the readings, each plate was shaken for 5 min with an Easy-Shaker EAS 2/4 instrument (SLT, Lab-instruments, Grödig, Austria), and the optical density at 490 nm of each well was read on a microtiter reader (Titerek Multiskan MC; Flow Laboratories, Huntsville, Ala.). The MICs of both drugs, alone or in combination, were defined as the lowest drug concentration in a well which produced an absorbance that indicated ∼80% inhibition compared with that of the growth control well. Drug interactions were classified as synergistic, additive, autonomous, or antagonistic on the basis of the fractional inhibitory concentration (FIC) index. The FIC index is the sum of the FICs of each of the drugs, which in turn is defined as the MIC of each drug when used in combination divided by the MIC of the drug when used alone. The interaction was defined as synergistic if the FIC index was <1.0, additive if the FIC index was 1.0, autonomous if the FIC index was between 1.0 and 2.0, and antagonistic if the FIC index was >2.0.
Cell culture.
Cells of the murine B16 melanoma cell line were grown in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mM l-glutamine, 50 U of penicillin per ml, 50 μg of streptomycin per ml, 25 μg of gentamicin per ml, 0.75% sodium bicarbonate, 10 mM HEPES buffer (pH 7.4), and 0.06 mg of anti-pleuropneumonialike organism per ml. Cells of the MCF-7M human breast adenocarcinoma cell line were maintained in Eagle minimal essential medium (EMEM) supplemented with 5% non-heat-inactivated FBS and 1 nM insulin. Cells of the DU145 human prostate carcinoma cell line were maintained in EMEM supplemented with 10% non-heat-inactivated FBS and 2 mM l-glutamine. HS578T, a human ductal carcinoma cell line, was maintained in EMEM supplemented with 10% heat-inactivated FBS and 10−8 M insulin. Finally, cells of the MPC3 human prostate adenocarcinoma cell line were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated FBS and 2 mM l-glutamine.
In vitro growth inhibitory activities of tumor cell lines.
Exponentially growing cells (1 × 103 to 2 × 103 cells) in 0.1 ml of tissue culture medium were seeded on day 0 in a 96-well microtiter plate. On day 1, 0.1-ml aliquots of medium containing graded concentrations of test analogs were added in duplicate to the cell plates. After incubation at 37°C in a humidified incubator for 3 days (B16 cells) or 6 days (MCF-7M cells), the plates were centrifuged briefly and 100 μl of the growth medium was removed. Cell cultures were incubated with 50 μl of 3-(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide (MTT; 1 mg/ml in Dulbecco’s phosphate-buffered saline) for 4 h at 37°C. The resulting purple formazan precipitate was solubilized with 200 μl of 0.04 N HCl in isopropyl alcohol. The absorbance was monitored on a Bio-Rad model 3550 Microplate Reader at a test wavelength of 570 nm and a reference wavelength of 630 nm. The absorbance values were transferred to a computer and the 50% inhibitory concentrations (IC50s) were determined by a computer program (EX-ED50) that fits all of the data to the four-parameter logistic equation Y = (Amax − Amin)/[1 + (X/IC50)n] + Amin, where Amax is the absorbance of control cells, Amin is the absorbance of cells in the presence of the highest concentration of an agent, Y is the observed absorbance, X is the agent concentration, IC50 is the concentration of the agent that inhibits the cell growth by 50% of the growth of control cells (on the basis of the absorbance), and n is the slope of the curve.
RESULTS
The in vitro antifungal and antitumor activities of CPT, nitidine, and their derivatives were determined for (i) an isogenic series of S. cerevisiae strains including a wild-type strain, the Δerg6 permeable mutant, the Δerg6 Δtop1 double mutant, and the Δerg6 Δtop1 mutant strain expressing C. neoformans topoisomerase I, and (ii) a series of cancer cell lines: melanoma (B16 cells), breast cancer (MCF-7 and HS578T cells), and prostate cancer (DU145 and MPC3 cells) cells.
Table 1 displays the MICs of CPT, nitidine, and their derivatives for S. cerevisiae strains with and without the fungal topoisomerase I. Compounds 4, 5, 6, 7, 8, and 9 have very little or no activity. In contrast, compounds such as compounds 11, 12, and 15 are extremely active antifungal agents with MICs of ≤0.09 μg/ml when the strain contains the overexpressed cryptococcal topoisomerase I. When MFCs were determined, the MFCs were essentially the same as the MICs in Table 1 (data not shown). Nitidine and its derivatives had essentially the same MICs, irrespective of the topoisomerase I level.
TABLE 1.
MICs of CPT, nitidine, and their derivatives for S. cerevisiae wild type, Δerg6, Δerg6 Δtop1, and Δerg6 Δtop1 and C. neoformans topoisomerase Ia
Abbreviations: CnTOP1, C. neoformans topoisomerase 1; Me, methyl; MeO, methanol; Et, ethyl; gly, glycinate.
These compounds were also tested for their activities against rapidly growing mammalian tumor cell lines. In Table 2 the growth inhibition of melanoma, breast, and prostate cancer cell lines by CPT, nitidine, and derivatives is shown. These compounds inhibit tumor cell growth at a concentration lower than the concentration at which they exhibit fungicidal activity. There are also some differences in the potencies of the compounds, depending on the use of the tumor cell lines. Nitidine is generally not as potent as the CPT analogs for tumor cell inhibition.
TABLE 2.
Growth inhibition of melanoma, breast, and prostate cancer cell lines by various CPT analogs and nitidine
| Compound | IC50 (ng/ml)
|
||||
|---|---|---|---|---|---|
| B16a | MCF-7b | HS578Tb | DU145c | MPC3c | |
| 1 (CPT) | 8.35 | 0.69 | 4.14 | 3.09 | 2.54 |
| 3 (9-amino-CPT) | 19.23 | 1.08 | 2.87 | 2.61 | 0.72 |
| 4 (irinotican; CPT-11) | 945.44 | 217.7 | 273.6 | 988.9 | 8.70 |
| 5 (TPT) | 178.6 | 416.7 | 9.61 | ||
| 8 (10-hydroxy-7-ethyl-CPT) | 5.09 | 0.07 | 0.98 | 1.52 | 0.94 |
| 10 (10,11-MD-20 (R)-CPT) | 392.7 | 32.53 | 129.3 | 138.7 | 92.51 |
| 11 (10,11-MD-20 (S)-CPT) | 0.70 | 0.18 | 0.25 | 0.23 | 0.07 |
| 12 (10,11-MD-20 (S)-CPT-glycinate) | 5.09 | 0.16 | 0.45 | 0.47 | 0.33 |
| 13 (9-amino-10,11-MD-20 (S)-CPT) | 4.47 | 0.52 | 0.61 | 0.61 | 0.12 |
| 14 (9-amino-10,11-MD-20 (S)-CPT-glycinate) | 4.50 | 0.27 | 0.41 | 0.46 | 0.31 |
| 15 (7-chloromethyl-10,11-MD-(S)-CPT) | 1.98 | 0.36 | 0.57 | 0.21 | 0.39 |
| 17 (7-methyl-10,11-MD-20 (S)-CPT) | 2.15 | 0.06 | 0.10 | 0.07 | |
| 18 (nitidine chloride) | 370.0 | 40.59 | 145.0 | 2.64 | 78.89 |
Melanoma cell line.
Breast cancer cell line.
Prostate cancer cell line.
Selected groups of CPT analogs with both antifungal activity against S. cerevisiae (compounds 12 and 14) and no activity (compounds 13 and 17) were chosen to be tested for their potential synergistic activities with amphotericin B against C. albicans A39, C. neoformans H99, and wild-type S. cerevisiae. Only compound 12 had direct activity against a pathogenic yeast, with a MIC of 1.56 μg/ml for C. neoformans, which is similar to the in vitro antifungal activities of amphotericin B, with MICs of 0.5 μg/ml for A39 and H99, and fluconazole, with MICs of 0.25 μg/ml for A39 and 2.0 μg/ml for H99 (2). When these CPT analogs were added together with amphotericin B, synergistic activity was noted with compounds 12 and 13 for both C. albicans and C. neoformans (FIC indices, 0.51 to 0.56), and an additive effect was noted with the other derivatives and amphotericin B (Table 3).
TABLE 3.
Interaction of amphotericin B with selected CPT derivatives in C. albicans A39, C. neoformans H99, and S. cerevisiae wild typea
| Strain | MIC of drug alone (μg/ml)
|
MICs of drugs combined (μg/ml)
|
FIC index
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12b | 13c | 14d | 17e | ABf | 12-AB | 13-AB | 14-AB | 17-AB | 12-AB | 13-AB | 14-AB | 17-AB | |
| A39 | >1.56 | >100 | >100 | >100 | 0.5 | 0.0048/0.25 | 12.5/0.25 | 6.25/0.5 | ≤0.09/0.5 | 0.53 | 0.56 | 1.00 | 1.00 |
| H99 | 1.56 | >100 | >100 | >100 | 0.5 | 0.0024/0.25 | ≤0.09/0.25 | 0.19/0.5 | ≤0.09/0.5 | 0.51 | 0.52 | 1.00 | 1.00 |
| Wild type | 3.12 | >100 | 6.25 | >100 | 0.25 | 1.56/0.125 | ≤0.09/0.25 | 0.39/0.25 | 0.78/0.25 | 1.00 | 1.00 | 1.00 | 1.00 |
For calculation purpose, an MIC of >100 μg/ml was assumed to be 200 μg/ml, and an MIC ≤0.09 μg/ml was assumed to be 0.04 μg/ml.
Compound 12, 10,11-MD-20(S)-CPT-20-glycinate; drug dilutions, 3.12 to 0.0024 μg/ml.
Compound 13, 9-amino-10,11-MD-20(S)-CPT; drug dilutions, 100 to 0.09 μg/ml.
Compound 14, 9-amino-10,11-MD-20(S)-CPT-20-glycinate; drug dilutions, 100 to 0.09 μg/ml.
Compound 17, 7-methyl-10,11-MD-20(S)-CPT; drug dilutions, 100 to 0.09 μg/ml.
AB, amphotericin B; drug dilutions, 2 to 0.03 μg/ml.
DISCUSSION
CPT, nitidine, and a series of derivatives have both potent antifungal and potent antitumor activities in vitro. In fact, these compounds, which are reported to target topoisomerase I, can be made to possess fungicidal activity. However, there are a series of structural and functional issues with these compounds, which have been recognized by this study.
Wild-type S. cerevisiae is resistant to CPT (MIC, >100 μg/ml), whereas an Δerg6 mutant strain is at least 10- to 100-fold more susceptible to CPT (MIC, 6.25 μg/ml). This finding for strains with the Δerg6 mutation, which are more permeable to compounds, supports the hypothesis that the antifungal activities of these compounds against yeasts are limited by drug penetration. This observation is further supported by overexpression of the C. neoformans target (TOP1) in S. cerevisiae, for which the MIC is even lower (≤0.09 μg/ml). These results corroborate our previous findings for C. neoformans, in which by directly overexpressing the target topoisomerase I in this pathogenic yeast, the MIC of >500 μg/ml for the wild-type strain can be reduced to 6.25 μg/ml for the isogenic strain when topoisomerase I has been overexpressed in the cell (3). These observations are consistent with the need for a critical intracellular amount of drug and topoisomerase I enzyme together to form enough cleavable complex toxin to produce yeast cytotoxicity. Furthermore, CPT is very toxic to the S. cerevisiae Δerg6 Δtop1 mutant strain that overexpresses the C. neoformans topoisomerase I, and this fact likely demonstrates that the basic CPT structure binds very efficiently to the cryptococcal DNA-topoisomerase I to form the ternary toxic complex. In addition, target specificity is confirmed by introduction in S. cerevisiae of a topoisomerase I mutation which renders the Δerg6 mutant strain completely resistant to CPT and its derivatives.
In the study of CPT derivatives, structure modifications were found to have significant effects on their antifungal activities. For instance, CPT-11 (compound 4) and TPT (compound 5) showed significantly reduced antifungal activities compared to that of CPT against the fungal topoisomerase I target. It appears that the structural modifications required to produce more soluble drugs (CPT-11 and TPT) reduce their ability to interact with the C. neoformans topoisomerase I. In fact, these compounds, which are now used to treat various cancers, represent some of the least potent CPT analogs against all the tumor cell lines. These results may be explained by the fact that the 10-carbamate ester of CPT-11 cannot be hydrolyzed under these in vitro conditions and that TPT is weakly active as a topoisomerase I poison.
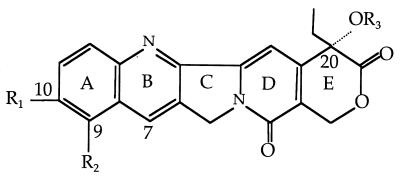
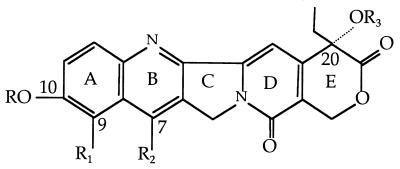
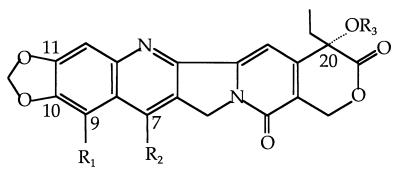
In the study of the structure-activity relationships with CPT and its derivatives, the presence of the α-hydroxy lactone in ring E is absolutely essential for CPT and its derivatives to interact with the cryptococcal topoisomerase I and produce a toxin. A further requirement is that the α-hydroxy group at C-20 is present and not protected as an ester (compounds 1 to 17). Various structural features have been found to be of great importance in topoisomerase I binding and subsequent fungicidal activity. An increase in fungal topoisomerase I interaction is conferred by various substituents, particularly in ring A and ring B. For example, in ring A, the presence of a 10,11-methylenedioxy (MD) (compounds 10 to 17) and/or a 9-amino group (compounds 3, 13, and 14) increases the level of interaction with topoisomerase I. In ring B, certain substitutions at the 7 position, such as ethyl, chloroethyl, or chloromethyl, greatly increase the retention time of the CPT-derivative complex in the cleavable complex (29), and this can help in antifungal potency. The glycinate ester at C-20 (compounds 7, 9, 12, 14, and 16) converts the water-insoluble parent drug into products with increased water solubility. In addition, the glycinate esters are hydrolyzed readily to the parent compound at physiological pH (pH 7.4). These compounds themselves are inactive as topoisomerase I poisons. However, once the glycinate ester prodrug penetrates the cell, it is readily hydrolyzed to the active form (31). For instance, the glycinate analogs of compounds 14 and 16 have fungicidal activity in wild-type S. cerevisiae (MICs, 6.25 and 3.12 μg/ml, respectively), but the parent derivatives (compounds 13 and 15) have no measurable antifungal activity. Furthermore, addition of the amino group at the 9 position of the 10,11-MD-20-CPT appears to decrease by ∼50-fold the potencies of the parent compounds against the C. neoformans topoisomerase I enzyme.
Generally, the anticancer and antifungal activities of CPT derivatives follow the same relative patterns of potency. However, a major difference between the cancer cell lines and the yeasts for structure-activity relationships is shown in the 10-hydroxy-CPT series (compounds 6, 7, 8, and 9). In the studies with cancer cell lines it was observed that the 10-hydroxy substituent produced a large increment in potency (31). For instance, in cancer cell lines, 10-hydroxy-7-ethyl-CPT (compound 8) is quite potent. In our yeast studies, the 10-hydroxy compounds are inactive even when the cryptococcal topoisomerase I is overexpressed. A possible explanation for differences is that the slightly acidic phenolic moiety in the 10-hydroxy-CPT analogs may inhibit yeast cell wall or membrane penetration of the compound. However, further studies will be required to understand this profound selectivity of the 10-hydroxy-CPT group for mammalian cells over yeast cells. On the other hand, most of the potent antifungal compounds are active against tumor cells, and the lack of selectivity may make issues of host toxicity very important with these compounds and in their development as antifungal agents.
The most active CPT derivatives for antifungal activity are the 10,11-MD-CPT group and their corresponding glycinate esters (compounds 12, 14, 15, and 16). These compounds are also very potent inhibitors of the growth of various cancer cell lines. In the case of fungal cells, except for 7-chloromethyl-10,11-MD-CPT (compound 15), only the glycinate ester prodrugs possess antifungal activities against both wild-type and Δerg6 strains. Furthermore, these active antifungal compounds are extremely potent when the C. neoformans topoisomerase I is overexpressed in the cells. These results indicate that the fungicidal activities of these compounds are limited by the availability of the topoisomerase I protein or that the cryptococcal enzyme is a more sensitive target than the S. cerevisiae enzyme. The addition of the chloromethyl group at the 7 position can increase the fungicidal activities of these derivatives, and in this case the glycinate ester does not further enhance the antifungal activities against yeast cells (compounds 15 and 16).
These results illustrate that structural modifications to CPT derivatives have profound effects on their activities both in cancer cell lines and in yeast cells. Specifically, these findings suggest the importance of certain structural features of CPT derivatives for potent antifungal interactions with the cryptococcal topoisomerase I complex. Furthermore, these active compounds appear to act via a classic CPT mechanism involving poisoning through enzyme-DNA cleavage complexes. The 10,11-MD-20-(S)-CPT-glycinate (compound 12) is even active against the C. neoformans topoisomerase I when the enzyme is not overexpressed in the wild-type strain (MIC, 1.56 μg/ml). This finding is probably attributable to its increased ability to penetrate the yeast cell wall or membrane and provides encouragement that one of the major problems with this class of compounds can be overcome.
Since reaching the drug target appears to be so limiting for some of these compounds, a series of the 10,11-MD-CPT analogs that have potent activities against yeast topoisomerase I complex were combined with amphotericin B to test for their synergistic activities against the S. cerevisiae wild-type strain, C. albicans A39, and C. neoformans H99. Ampthotericin B primarily acts by binding to sterols in the yeast cell membrane, with a resultant change in the permeability of the membrane to ions, and thus, we hypothesized that amphotericin B might aid in the passage of these compounds to their target. Interestingly, 10,11-MD-20-(S)-CPT-glycinate (compound 12) and 9-amino-10,11-MD-20-(S)-CPT (compound 13) each in combination with amphotericin B showed synergistic activities against C. albicans and C. neoformans and additive activity against wild-type S. cerevisiae (Table 3). The 9-amino-10,11-MD-20-(S)-CPT-glycinate (compound 14) and the 7-methyl-10,11-MD-20-(S)-CPT (compound 17) each in combination with amphotericin B exhibited only additive activities against all strains tested. Because a major action of amphotericin B is to bind to ergosterol to form pores in the fungal cell membrane, our findings of synergy and additive effects with this polyene provide further indirect evidence that one of the major obstacles for some CPT derivatives is entry into the cell. Also, these results demonstrate the potential value of combination therapies with these two classes of antifungal agents that possess different targets, which may be mutually beneficial to the fungicidal response.
Nitidine has about the same order of activity as CPT in the inhibition of topoisomerase I function, but unlike CPT and its derivatives, nitidine also inhibits topoisomerase II (7, 30a, 33). However, nitidine and its derivatives (32) are substantially less potent than CPT and its active derivatives against cancer cell lines. In yeasts, nitidine chloride and its derivatives appear to possess antifungal activities against all yeast strains tested, including the Δerg6 Δtop1 mutant strain. This indicates that topoisomerase I is not the only target for this class of compounds, and similar to Aspergillus nidulans, topoisomerase I is probably not the target in yeasts. There also appears to be fewer problems with drug reaching its target, since the MIC for the Δerg6 mutant was the same as that for the wild-type strain. These results also suggest that this class of drugs may have more selective potencies between mammalian and fungal cell targets. In comparison to CPT and its derivatives, nitidine is less potent against tumor cells but retains a reasonable amount of antifungal activity.
In summary, the 10,11-MD moiety in CPT derivatives conveys greater potency to the biological activities of these compounds against both cancer and fungal cells. In particular, the 20-glycinate esters of the 10,11-MD-CPT can penetrate yeast cells more readily and release their active forms and thus produce a potent fungicidal effect. The glycinate esters may be particularly useful for in vivo studies, since they can be administered orally, parenterally, or subcutaneously and since they have a superior therapeutic index ratio compared to those of the corresponding parent compounds.
Topoisomerase I is an established target for antineoplastic compounds, and our studies show that it can be considered a potential target for antifungal agents. However, the penetration of the CPT class of compounds and possible host toxicity need further studies. On the other hand, the CPT-topoisomerase I interaction has now been solved (22), and this may help in the development of this target and drug class for new antifungal and anticancer agents. It should be mentioned that drugs in this class, CPT-11 and TPT, have been given to humans, and although toxicities do occur, they are not general poisons for normal human cells. Nitidine and its derivatives appear to possess fungicidal activity against yeast cells, and thus, they may also have potential as an antifungal class for development if selective toxicity issues are resolved.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants AI 28388, AI41937, and AI-94-014 from the National Institute of Allergy and Infectious Diseases and in part by a grant (grant POAI 44975-01) from the Duke University Mycology Research Unit research program. Joseph Heitman is a Burroughs Wellcome Scholar in Molecular Pathogenic Mycology and an associate investigator of the Howard Hughes Medical Institute.
We are grateful to Mary Ann Howard for assistance with manuscript preparation.
REFERENCES
- 1.Cameron M L, Schell W A, Bruch S, Bartlett J A, Waskin H A, Perfect J R. Correlation of in vitro fluconazole resistance of Candida isolates in relation to therapy and symptoms of individuals seropositive for human immunodeficiency virus type 1. Antimicrob Agents Chemother. 1993;37:2449–2453. doi: 10.1128/aac.37.11.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Poeta M, Schell W A, Dykstra C C, Jones S K, Tidwell R R, Kumar A, Boykin D W, Perfect J R. In vitro antifungal activity of a series of dicationic substitute carbazoles, furans and benzimidazoles. Antimicrob Agents Chemother. 1998;42:2503–2510. doi: 10.1128/aac.42.10.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Poeta M, Toffaletti D L, Rude T H, Dykstra C C, Heitman J, Perfect J R. Topoisomerase 1 is essential in Cryptococcus neoformans: role in pathobiology and as an antifungal target. Genetics. 1999;152:167–178. doi: 10.1093/genetics/152.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eng W K, Faucette R, Johnson K, Sternglanz R. Evidence that topoisomerase 1 is necessary for the cytotoxic effects of camptothecin. Mol Pharmacol. 1988;34:755–760. [PubMed] [Google Scholar]
- 5.Fox R, Neal K R, Leen C L S, Ellis M E, Mandal B K. Fluconazole resistant candida in AIDS. J Infect. 1991;22:201–203. doi: 10.1016/0163-4453(91)91767-r. [DOI] [PubMed] [Google Scholar]
- 6.Gaber R F, Copple D M, Kennedy B K, Vidal M, Bard M. The yeast gene ERG6 is required for normal membrane function but is not essential for biosynthesis of the cell-cycle-sparking sterol. Mol Cell Biol. 1989;9:3447–3456. doi: 10.1128/mcb.9.8.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gatto B, Sanders M M, Yu C, Wu H-Y, Makhey D, La Voie E J, Liu L F. Identification of topoisomerase I as the cytotoxic target of the protoberberise alkaloid coralyne. Cancer Res. 1996;56:2795–2800. [PubMed] [Google Scholar]
- 8.Giovanella B C, Stehlin J S, Wall M E, Wani M C, Nichols A W, Liu L F, Silber R S, Potmesil M. DNA topoisomerase 1-targeted chemotherapy of human colon cancer in xenografts. Science. 1989;246:1046–1048. doi: 10.1126/science.2555920. [DOI] [PubMed] [Google Scholar]
- 9.Goldman G H, Yu C, Wu H-Y, Sanders M M, La Voie E J, Liu L F. Differential poisoning of human and Aspergillus nidulans DNA topoisomerase 1 by bi- and terbenzimidazoles. Biochemistry. 1997;36:6494. doi: 10.1021/bi963033t. [DOI] [PubMed] [Google Scholar]
- 10.Jannatipour M, Liu Y X, Nitiss J L. The top 2-5 mutant of yeast topoisomerase Ii encodes an enzyme resistant to etoposide and amsacrine. J Biol Chem. 1993;268:18586–18592. [PubMed] [Google Scholar]
- 11.Knab A M, Fertala J, Bjornsti M A. Mechanism of camptothecin resistance in yeast DNA topoisomerase 1 mutants. J Biol Chem. 1993;268:22322–22330. [PubMed] [Google Scholar]
- 12.Liu L F, Duann P, Lin C T, D’Arpa P, Wu J. Mechanism of action of camptothecin. Ann N Y Acad Sci. 1996;13:803–844. doi: 10.1111/j.1749-6632.1996.tb26375.x. [DOI] [PubMed] [Google Scholar]
- 13.Lorenz M C, Muir R S, Lim E, McElver J, Weber S C, Heitman J. Gene disruption with PCR products in Saccharomyces cerevisiae. Gene. 1995;158:113–117. doi: 10.1016/0378-1119(95)00144-u. [DOI] [PubMed] [Google Scholar]
- 14.McGinnis M R. Laboratory handbook of medical mycology. New York, N.Y: Academic Press, Inc.; 1980. Susceptibility testing and bioassay procedure; p. 431. [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. Reference method for broth dilution susceptibility testing of yeasts. Approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 16.Nitiss J L, Liu Y X, Harbury P, Jannatipour M, Wasserman R, Wang J C. Amsacrine and etoposide hypersensitivity of yeast cells overexpressing DNA topoisomerase II. Cancer Res. 1992;52:4467–4472. [PubMed] [Google Scholar]
- 17.Nitiss J L, Rose A, Sykes K C, Harris J, Zhou J. Using yeast to understand drugs that target topoisomerases. Ann N Y Acad Sci. 1996;803:32–43. doi: 10.1111/j.1749-6632.1996.tb26374.x. [DOI] [PubMed] [Google Scholar]
- 18.Nitiss J L, Wang J C. DNA topoisomerase-targeting antitumor drugs can be studied in yeast. Proc Natl Acad Sci USA. 1988;85:7501–7505. doi: 10.1073/pnas.85.20.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfaller M A, Rhine-Chalberg J, Redding S W, Smith J, Farinacci G, Fathergill A W, Rinaldi M G. Variations in fluconazole susceptibility and electrophoretic horyotype among oral isolates of Candida albicans from patients with AIDS and oral candidiasis. J Clin Microbiol. 1994;32:59–64. doi: 10.1128/jcm.32.1.59-64.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Potmesil M. Camptothecins: from the laboratory to the bedside. Cancer Res. 1994;54:431–439. [Google Scholar]
- 21.Potmesil M, Hsiang Y-X, Liu L F, Knowles D, Kirschenbaum S, Forlenza T J, Penziner A, Kanganis D, Traganos F, Silber R. Resistance of human leukemic and normal lymphocytes to drug induced DNA cleavage and low levels of topoisomerase II. Cancer Res. 1988;48:3537–3543. [PubMed] [Google Scholar]
- 22.Redinbo M R, Stewart L, Kuhn P, Champoux J J, Hol W G J. Crystal structure of human topoisomerase 1 in covalent and noncovalent complexes with DNA. Science. 1998;279:1504–1513. doi: 10.1126/science.279.5356.1504. [DOI] [PubMed] [Google Scholar]
- 23.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schena M, Picard D, Yamamoto K R. Vectors for constitutive and inducible gene expression in yeast. Methods Enzymol. 1991;194:389–398. doi: 10.1016/0076-6879(91)94029-c. [DOI] [PubMed] [Google Scholar]
- 25.Sikorski R S, Hieter P A. System of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slichenmyer W J, Rowunsky E K, Dohehower R C, Kaufmann S H. The current status of camptothecin analogues as antitumor agents. J Natl Cancer Inst. 1993;85:271–291. doi: 10.1093/jnci/85.4.271. [DOI] [PubMed] [Google Scholar]
- 27.Stewart L, Redinbo M R, Qiu X, Hol W G J, Champoux J J. A model for the mechanism of human topoisomerase 1. Science. 1998;279:1534–1541. doi: 10.1126/science.279.5356.1534. [DOI] [PubMed] [Google Scholar]
- 28.Taylor A, Giles K, Sarthy A V, McGinical T, Fostel J. Identification of the gene encoding DNA topoisomerase I from Candida albicans. FEMS Microbiol Lett. 1996;138:113–121. doi: 10.1111/j.1574-6968.1996.tb08143.x. [DOI] [PubMed] [Google Scholar]
- 29.Valenti M, Nieves-Neira W, Kohlhagen G, Kohn K W, Wall M E, Wani M C, Prommier Y. Novel 7-alkyl methylenedioxy-camptothecin derivatives exhibit increased cytotoxicity and induce persistent cleavable complexes both with purified mammalian topoisomerase 1 and human colon carcinoma SW620 cells. Mol Pharmacol. 1997;52:82–87. doi: 10.1124/mol.52.1.82. [DOI] [PubMed] [Google Scholar]
- 30.Wach A, Brachat A, Pohlmann R, Philippsen P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast. 1994;10:1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- 30a.Wall, M. E. Unpublished data.
- 31.Wall M E, Wani M C, Nicholas A W, Manikumar G, Tele C, Moore L, Truesdale A, Leitner P, Besterman J M. Plant antitumor agents. 30. Synthesis and structure activity of novel camptothecin analogs. J Med Chem. 1993;36:2689–2700. doi: 10.1021/jm00070a013. [DOI] [PubMed] [Google Scholar]
- 32.Wall M E, Wani M C, Taylor H. Plant antitumor agents. 27. Isolation, structure and structure activity relationships of alkaloids from Fagara macrophylla. J Nat Prod. 1987;50:1095–1099. doi: 10.1021/np50054a014. [DOI] [PubMed] [Google Scholar]
- 33.Wang L K, Johnson R K, Hecht S M. Inhibition of topoisomerase I function by nitidine and fagaronise. Chem Res Toxicol. 1993;6:813–818. doi: 10.1021/tx00036a010. [DOI] [PubMed] [Google Scholar]
- 34.Willocks L, Leen C L S, Brettle R P, Urquhart D, Russel T B, Milne L J R. Fluconazole resistance in AIDS patients. J Antimicrob Chemother. 1991;28:937–939. doi: 10.1093/jac/28.6.937. [DOI] [PubMed] [Google Scholar]