Key Points
Question
What is the antigenic trigger in atypical granulomatous dermatitis in adults?
Findings
In this case series of 4 immunocompetent adult patients with atypical cutaneous granulomas, cutaneous granulomas harbored vaccine-derived and wild-type rubella virus (RuV), suggesting an association between RuV and a subset of granulomatous dermatitis previously deemed idiopathic.
Meaning
The study results suggest that clinicians should consider RuV etiology in adults with cutaneous granulomas regardless of immune status.
Abstract
Importance
Vaccine-derived and wild-type rubella virus (RuV) has been identified within granulomas in patients with inborn errors of immunity, but has not been described in granulomas of healthy adults.
Objective
To determine the association between RuV and atypical granulomatous inflammation in immune-competent adults.
Design, Setting, and Participants
This case series, conducted in US academic dermatology clinics from January 2019 to January 2021, investigated the presence of RuV in skin specimens using RuV immunofluorescent staining of paraffin-embedded tissue sections, real-time reverse-transcription polymerase chain reaction, whole-genome sequencing with phylogenetic analyses, and cell culture by the US Centers for Disease Control and Prevention. Rubella immunoglobulin G, immunoglobulin M enzyme-linked immunoassay, and viral neutralization assays were performed for the sera of immunocompetent individuals with treatment refractory cutaneous granulomas and histopathology demonstrating atypical palisaded and necrotizing granulomas. Clinical immune evaluation was performed.
Main Outcomes and Measures
Identification, genotyping, and culture of vaccine-derived and wild-type RuV within granulomatous dermatitis of otherwise clinically immune competent adults.
Results
Of the 4 total immunocompetent participants, 3 (75%) were women, and the mean (range) age was 61.5 (49.0-73.0) years. The RuV capsid protein was detected by immunohistochemistry in cutaneous granulomas. The presence of RuV RNA was confirmed by real-time reverse-transcription polymerase chain reaction in fresh-frozen skin biopsies and whole-genome sequencing. Phylogenetic analysis of the RuV sequences showed vaccine-derived RuV in 3 cases and wild-type RuV in 1. Live RuV was recovered from the affected skin in 2 participants. Immunology workup results demonstrated no primary immune deficiencies.
Conclusions and Relevance
The case series study results suggest that RuV (vaccine derived and wild type) can persist for years in cutaneous granulomas in clinically immunocompetent adults and is associated with atypical (palisaded and necrotizing type) chronic cutaneous granulomas. These findings represent a potential paradigm shift in the evaluation, workup, and management of atypical granulomatous dermatitis and raises questions regarding the potential transmissibility of persistent live RuV.
This case series study examines the association between rubella virus and atypical granulomatous inflammation in immune-competent adults.
Introduction
Granulomatous inflammation presents with a complex immunologic reaction pattern and a spectrum of clinical and histopathologic findings. Cutaneous granulomas represent a spectrum of disorders and have reported etiologies, including immunologic, drug induced, and infectious, although many are deemed idiopathic. Infectious agents have been implicated in triggering the formation of granulomas, including HIV, mycobacterium, syphilis, Epstein-Barr virus, hepatitis B virus, hepatitis C virus, and herpes zoster virus.1,2 To our knowledge, antigenic triggers for most cutaneous granulomas have not been identified.
Rubella virus (RuV) is an enveloped single-stranded RNA virus that can establish persistent infection in immune-privileged body sites, including the placenta and fetus. Persistence of RuV can be associated with congenital rubella syndrome, and rubella vaccines have been developed and used to prevent these complications. The variant RA27/3 vaccine strain, the rubella component of the measles, mumps, and rubella (MMR) vaccine, has been identified within granulomas in pediatric patients with several inborn errors of immunity and called immunodeficiency-related vaccine-derived rubella viruses (iVDRV).3,4 Rubella virus was found in M2 macrophages, and heritable defects in T-cell response appear to be important in the formation of granulomas surrounding iVDRV-infected macrophages.4,5,6 Recently, a case of wild-type (wt) RuV persistence within cutaneous granulomas was reported in an unvaccinated 77-year-old man with common variable immune deficiency.7
In this article, we describe RuV-associated granulomas in 4 immunocompetent adult patients with atypical cutaneous granulomas. Vaccine-derived and wt rubella virus were isolated from the skin. To our knowledge, this study provides the first evidence that wt RuV can be also associated with granulomas in otherwise healthy individuals.
Methods
Clinical Samples
Patients were from the Medical College of Wisconsin (Milwaukee, Wisconsin) and University of Pennsylvania (Philadelphia, Pennsylvania) and had diagnoses of idiopathic chronic cutaneous granulomas. Formalin-fixed paraffin-embedded tissue sections of skin biopsies were submitted to the US Centers for Disease Control and Prevention (CDC) for testing. Fresh skin biopsies and nasopharyngeal swabs were collected and sent to CDC for molecular testing and cell culture. Serum (patients 2-4) or plasma (patient 1) specimens were submitted to CDC for serological testing. After diagnosis of rubella-associated granulomatous disease, patients received immunologic evaluations as clinically indicated.
Ethics Statement
Diagnostic samples were obtained from all patients as part of outpatient care with provision of oral informed consent. Molecular testing, virus culture, and rubella serology were performed as part of the reference and surveillance responsibilities of the CDC Rubella Laboratory. The work was reviewed by CDC, which provided institutional review board approval, and was conducted in a manner consistent with applicable federal law and CDC policy.
Histologic Immunofluorescent Staining and Rubella Molecular and Serological Analyses
Histological immunofluorescent staining of formalin-fixed paraffin-embedded tissue sections for RuV capsid protein was previously reported.4 Methods for RNA extraction from fresh skin samples and nasopharyngeal swabs, reverse-transcription polymerase chain reaction (RT-PCR), whole-genome sequencing, phylogenetic analyses, virus isolation by cell culture, rubella immunoglobulin (Ig) G and IgM enzyme-linked immunoassay, and rubella foci neutralization assays were described previously.4,8
Results
Case Presentations
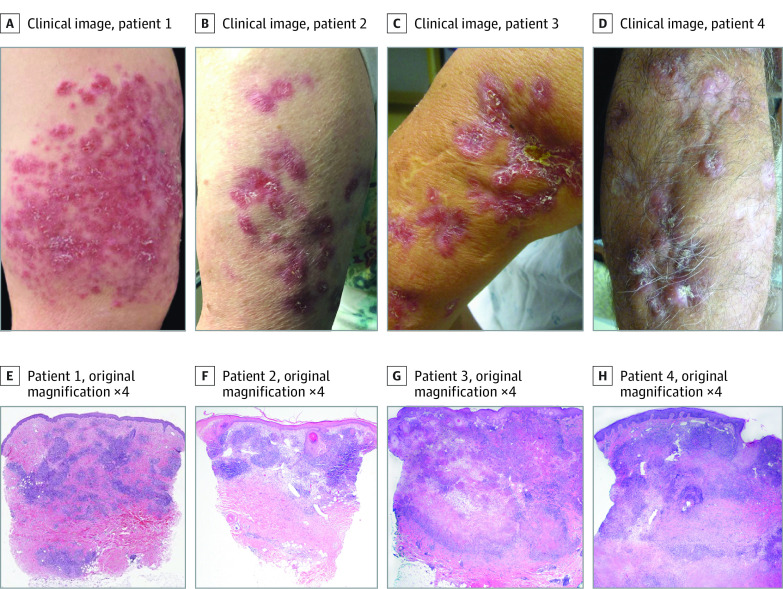
Four adult patients (3 women [75%]; 1 man [75%]; mean age, 61.5 years; range 49-73 years) presented with indurated pink to violaceous, arcuate plaques and nodules with focal pustules restricted to their left upper arms (Figure 1) that had been slowly growing for several years (mean, 11.25 years; range, 4-19 years). All patients denied having a history of recurrent or serious systemic infections. One patient had a diagnosis of multiple sclerosis that was treated with glatiramer acetate that preceded the appearance of her skin findings by approximately 7 years (Table 1 and Table 29). Patients 1, 2, and 3 had received MMR vaccination between 2 to more than 10 years before onset of their skin disease. Patient 4 denied prior vaccination with MMR and did not recall prior infection with RuV. All patients underwent multiple biopsies for diagnosis by histopathology and culture (average number, 3). Chest radiography was performed and produced normal results in all cases, excluding obvious intrathoracic granulomatous diseases. Anti-inflammatory treatments for presumed initial granulomatous dermatitis included methotrexate, 15 mg, weekly; bexarotene, 300 mg/m2 per day; hydroxychloroquine, 200 mg, twice daily, minocycline, 100 mg, twice daily; topical and intralesional corticosteroids; and topical imiquimod, all without benefit.
Figure 1. Clinical and Histopathological Images for the 4 Cases.
A-D, Clinically, all patients had pink to violaceous papules, nodules, and plaques restricted to the left upper arm. E-H, Histopathology (hematoxylin and eosin staining, original magnification ×4) demonstrated dermal palisaded granulomatous inflammation in cases 1 and 2 with caseating granulomatous inflammation in cases 3 and 4.
Table 1. Patient Characteristics.
| Case No. | Sex | Age of granuloma onset, y | Duration between MMR vaccination and disease presentation, y | Duration of granulomas at RuV at time of testing, y | Comorbidities | Cutaneous manifestations | Histopathology | Treatments |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 45 | 2 | 4 | Asthma, seasonal allergies | Solitary papule that increased to several papules coalescing into a plaque localized to the left arm | Atypical CD8+ lymphocytic infiltrate with associated granulomatous inflammation | Methotrexate, bexarotene, hydroxychloroquine, topical clobetasol, and topical imiquimod |
| 2 | F | 45 | 7 | 20 | Multiple sclerosis | Papules that grew to larger pink to violaceous plaque involving the left arm | Palisaded and foreign body granulomatous dermatitis with concern for follicular rupture, infection, or consideration of palisaded neutrophilic granulomatous dermatitis | Hydroxychloroquine |
| 3 | F | 55 | 13 | 10 | Hypertension, hyperlimidia | Papules and nodules that coalesce into larger plaque with focal pustules localized to the the left arm | Palisaded granulomatous dermatitis concerning for rheumatoid nodule or infection | Surgical excision, intralesional triamcinolone, and hydroxychloroquine |
| 4 | M | 56 | Unvaccinated | 8 | None | Papules and nodules coalescing into indurated plaques studded with pustules only on the left arm | Palisaded and caseating granulomatous inflammation concerning for infection | Minocycline and hydroxychloroquine |
Abbreviations: F, female; M, male; MMR, measles, mumps, and rubella; RuV, rubella virus.
Table 2. Patient Immunology Workup Results and RuV Serology.
| Case No. | IgG, IgA, and IgM levels, mg/dL | CD3+ T cells/mm3 |
CD3+ CD4+ T cells/mm3 |
CD3+ CD8+ T cells/mm3 |
Activated T cells HLA-DR+, % |
CD19+ B cells/mm3 |
CD56+ CD16+
NK cells/mm3 |
T-cell mitogen proliferation, % of control | Rubella IgG titer (IU/mL)a | RuV neutralization titer (NT50)b | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NA | 751-1560; 82-453; 46-304c | 700-2100c | 300-1400c | 210-820c | CD4+ (<15%)c | CD8+ (<25%)c | 100-500c | 90-800c | NA | ||
| 1 | 688/129/52 | 851.0 | 470.0 | 345.0 | NAd | NAd | 345.0 | 107.0 | PHA: 7 (low)e | 580.0 | 1280.0 |
| 2 | 754/244/68 | 2251.2 | 895.2 | 1382.8 (Elevated: reversed CD4:CD8 ratio) | 9.86 | 40.6% (Elevated) | 52.97 (Low) | 658.1 | PHA: 103 | 1171.0 | 5120.0 |
| 3 | 462/36/32 (Low) | 957.0 | 883.0 | 71 (Low) | NAd | NAd | 255.0 | 230.0 | NA | 1623.0 | 10 240.0 |
| 4 | 1012/356/132 | 598.9 (Low) | 492.6 | 95.3 (Low) | 10.52 | 15.52 | 33.8 (Low) | 58.2 (Low) | PHA: 93 | 1437.0 | 5120.0 |
Abbreviations: Ig, immunoglobulin; NA, not available; PHA, phytohemagglutinin; RuV, rubella virus.
Cutoff for rubella immunity: 15 IU/mL.
Inverse of the dilution, which achieved 50% reduction in RuV foci neutralization assay using the RA27/3 vaccine virus.
Immunoglobulin normal ranges for people older than 19 years.9
NA.
PHA is a mitogen used for the purpose of mitotic stimulation to human lymphocytes.
Histopathology features included dense granulomatous inflammation with variable lymphocytic infiltrate, and the overall granulomatous patterns were palisaded, sarcoidal, and suppurative with caseation (Figure 1). The histopathology results for patient 1 indicated lymphocytes with cellular atypia and increased CD8+ compared with CD4+ cells. Given the concern for granulomatous cutaneous T-cell lymphoma, T-cell gene rearrangement was performed and demonstrated a clonal abnormality that was suggestive of T-cell lymphoma. Gram, periodic acid–Schiff, and acid-fast bacteria stains as well as tissue cultures for bacteria, fungi, and mycobacteria yielded negative results in all cases. The pattern of atypical granulomatous inflammations was histologically similar to RuV-associated cutaneous granulomatous dermatitis in immunodeficient hosts, so further testing for RuV was performed. After diagnosis of RuV granulomatous disease, patient 1 also was treated with nitazoxanide, intralesional interferon α-2b, ribavirin and interferon α-2b, and most recently interleukin 2 with minimal improvement.
Patients were clinically immunocompetent without history of substantial, recurrent, or opportunistic infections or immune-related dysfunction. Humoral and cellular immunity were evaluated by measuring serum levels of immunoglobulins, flow cytometry of lymphocytes, and T-cell response to mitogens (Table 1 and Table 2). The results of HIV testing were negative in all patients. Patient 2 had a reversed CD4:CD8 ratio, which may have been associated with treatment with glatiramer acetate.10 Patients 3 and 4 had low CD8+ T-cell counts, and patient 3 had low immunoglobulin levels (IgG, IgA, IgM). These were interpreted as unknown clinical significance because of the lack of history of opportunistic infections or another immune-related dysfunction.
RuV Detection Within Granulomas
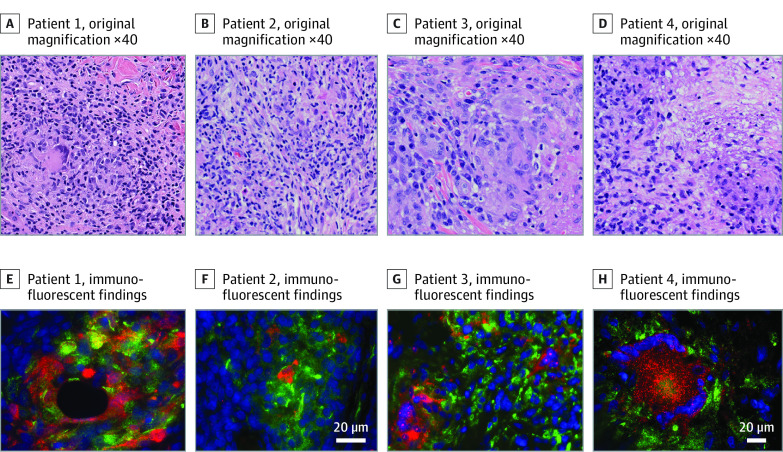
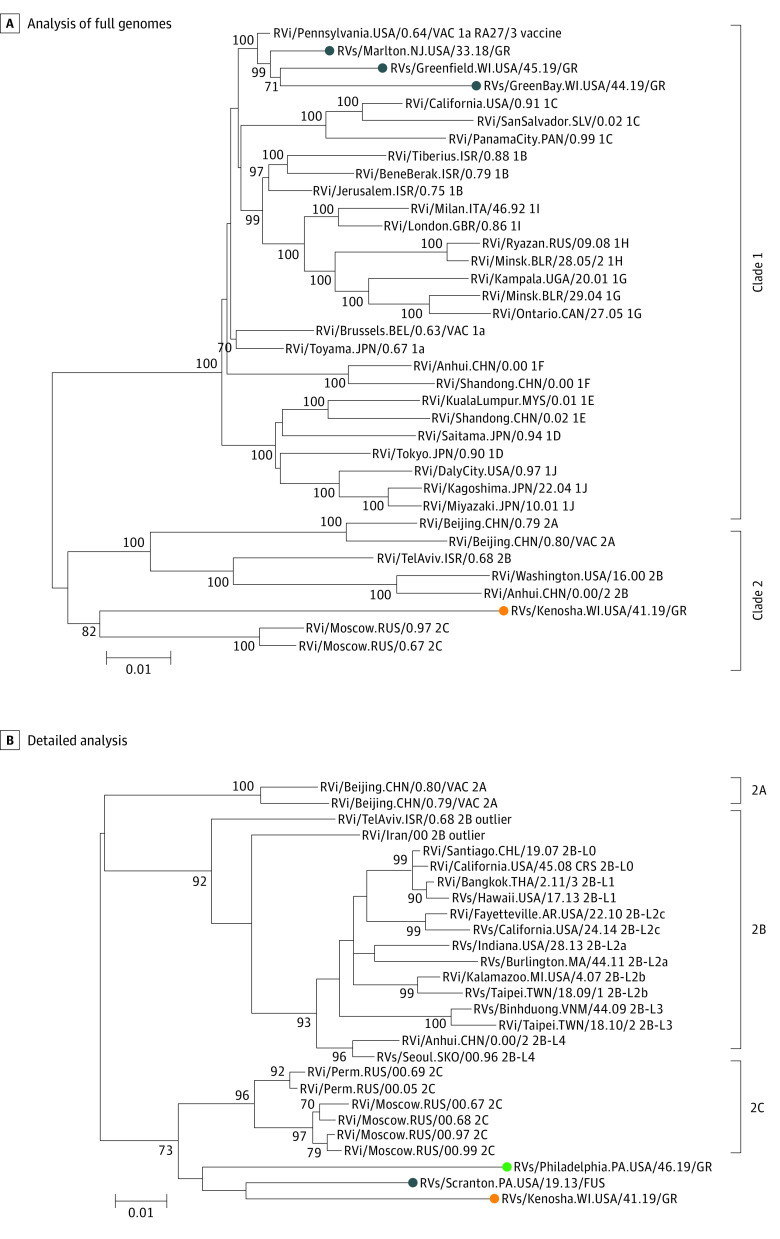
Immunofluorescence with an RuV capsid monoclonal antibody revealed the presence of RuV capsid in CD206+ M2 macrophages within the dermal granulomas in all cases, whereas no staining for measles nucleocapsid was observed in all granulomas and no RuV capsid staining was detected in normal human tissues (Figure 2; eFigures 1 in the Supplement). In all patients, nasopharyngeal swabs were negative for RuV by RT-PCR, and the virus was not detected in cell cultures. Rubella virus RNA was detected by RT-PCR in all 4 biopsies of affected granulomatous skin. Full genome sequences of these RuV RNA were obtained by Sanger sequencing of overlapping PCR fragments. The sequences were assigned names using an adaptation of the World Health Organization standard RuV naming convention: RVs/Marlton.NJ.USA/33.18/GR (patient 1), RVs/Greenfield.WI.USA/45.19/GR (patient 2), RVs/GreenBay.WI.USA/44.19/GR (patient 3), and RVs/Kenosha.WI.USA/41.19/GR (patient 4). Phylogenetic analysis of the full genome sequences from the granuloma samples and 32 RuV genomes of World Health Organization reference viruses showed that vaccine-derived rubella viruses (VDRVs) were present in skin biopsies for patients 1 to 3 (Figure 3; eFigure 2 in the Supplement) with 98% to 99% similarity to the RA23/7 sequence (genotype 1a, clade 1). The RuV from patient 4 was not vaccine derived but rather wt RuV and belonged to Clade 2 viruses with 90% similarity. Clades 1 and 2 reference the major phylogenetic groups of RuV with 8% to 10% nucleotide differences. Further phylogenetic analysis of an RuV 739-nt genotyping fragment from different lineages of Clade 2 viruses and viruses from case 4, a patient with Fuchs uveitis,11 and a patient with common variable immune deficiency–associated granuloma7 revealed that the 3 viruses formed a separate cluster with a 73% bootstrap value. A bootstrap value of greater than 70% is a threshold often used for good confidence that a cluster of viruses are from the same origin and associated (Figure 2). This cluster of viruses, from different patients each with persistent RuV infection and with evidence of initial infection for 2 of them between the late 1950s and early 1970s in the US, is associated with a previously undescribed lineage of RuVs.7,11 The points of initial RuV infections were estimated based on phylogenetic sequencing and similarity of those sequences to known viruses in circulation at different points. Most all virologic surveillance for RuVs has been performed after 1980. Live viruses were recovered from skin biopsies from patients 2 and 4 via cell culture, with vaccine-derived virus in patient 2 and wt virus in patient 4. To our knowledge, this is the first time that wt RuV was cultured from cutaneous granulomas, and it is surprising that it came from an adult immunocompetent patient.
Figure 2. Immunofluorescent Findings for the 4 Cases.
Higher magnification (hematoxylin and eosin staining, original magnification ×40) demonstrated multinucleated giant cells with associated inflammation (A-D). Immunofluorescence staining of the specimens with RuV capsid protein antibody (red) localized within M2 macrophages (green) with blue nuclei (E-H).
Figure 3. Molecular Phylogenetic Analysis by the Maximum Likelihood Method Demonstrating the Association Between the Viruses From 4 Cases.
A, Analysis of full genomes of the 4 patients’ viruses and World Health Organization (WHO) reference viruses. The blue circles indicate the RA27/3 vaccine-derived viruses from cases 1 to 3, the orange circle indicates the wild-type (wt) rubella virus (RuV) from case 4. B, Detailed analysis (739-nt genotyping fragment) of Clade 2 rubella viruses demonstrated that the virus from case 4 (orange circle) belongs to Clade 2 and is associated with RuVs from a patients with Fuchs disease (blue circle) and a patient with granuloma with common variable immune deficiency (green circle). This cluster of 3 viruses appears to be associated with a previously undetected wt genotype of RuV.
RuV Antibody Responses
All sera were negative for RuV IgM. Rubella IgG titers (average, 1202 IU/mL; range, 580-1623 IU/mL) were significantly elevated, with values much higher than those observed after immunization in immunologically typical individuals. For example, IgG titers in individuals age 15 years after receiving 2 doses of MMR vaccine averaged about 40 IU/mL (95% CI, 37-41 IU/mL), although 6% of individuals had titers greater than 120 IU/mL.12 Further, RuV neutralization titers (NT50 range, 1280-10240) were also significantly elevated in all 4 patients, which parallels the immunologic profiling observed in children with inborn errors of immunity and chronic granulomas and confirms ongoing antigenic stimulation and are much higher than IgG and neutralization titers of immunologically typical, vaccinated individuals without granulomas.8
Discussion
This case series study found chronic cutaneous granulomas that harbored RuV in clinically immunocompetent adults. Further, we identified the persistence of vaccine-derived virus and wt RuV in this population, strengthening the possible association between RuV and cutaneous granulomas and expanding the clinicopathologic phenotype of RuV-associated granulomas.3,5,13,14 Granulomas developed on the left upper arms of patients, a common site for administering vaccines, which resulted in the similar anatomic location of skin involvement seen in patients 1 to 3. For patient 4, wt RuV was identified in an individual with no history of vaccination or substantial trauma to the site. The initial investigation of RuV within immunocompetent patients was identified solely based on the location of the granuloma on the upper arms (at the site of the vaccine inoculation) because many of the cases of iVDRV began at the inoculation site.
The localization of RuV antigens within the central focus of cutaneous granulomas, identification of viral genomic RNA in all lesions, and isolation of infectious viruses from lesions suggest but do not confirm a causal role for RuV in granuloma formation in these patients. In applying Bradford Hill criteria for causation, this investigation further suggests a role of RuV in this disease process. The clinical morphology of RuV-associated granulomas was consistent and specific across the cases (Figure 1), and the histopathology shared overlapping features, including focal necrosis and palisaded architecture. Location of the virus demonstrated within the granulomas and culture of the live virus from the skin biopsies provided further biologic plausibility. Nearly 60% of idiopathic granulomas in patients with inborn errors of immunity have been associated with the RuV vaccine (strength of association), and MMR vaccination has always preceded granuloma development (temporality).15 However, further studies, including experimental evaluation in animal models, are required to further support causation in terms of coherence, experimental, and biological gradient criteria. The delay between vaccination or infection and presentation of the cutaneous granulomas must be explained. The nasopharyngeal swab results were negative for RuV, suggesting that RuV was not actively being shed via the respiratory route at the time of specimen collection. However, wt RuV was cultured from patient 4, and the implications of the transmissibility of this virus are unknown, although no RuV outbreaks were noted in the area. The possibility that RuV is shed intermittently or via other routes, such as the urine, also needs to be further evaluated. Finding high titers for RuV IgG and RuV neutralizing antibody in the patients’ sera was expected, as it was previously reported for patients with inborn errors of immunity with granulomas.8
Detecting wt RuV in granuloma in patient 4 in addition to VDRVs in patients 1 to 3 suggests the capability of the virus itself to persist subclinically in clinically immunocompetent individuals after vaccination and natural infection. In these patients, cutaneous lesions with VDRVs initially developed anywhere from 2 to 10 years or longer after last vaccination with MMR, and the plaques continued to grow despite standard treatments used for granulomatous inflammation. Persistence is a fundamental property of RuV that is associated with its pathogenesis in rare cases. Rubella virus can persist in cell culture16 and human tissues, including synovial joint fluid, vitreous fluid, and brain, which is followed by arthritis, uveitis, and encephalitis.11,17,18,19 The mechanisms of RuV persistence in the presence of antiviral immune responses and whether a possible viral reservoir exists are not well understood, representing some of the major obstacles in treating persistent RuV.8 There is a hypothesis that RuV persists within neutrophils within bone marrow and later emerges in sites of ongoing tissue inflammation (perhaps from trauma), either being followed by chronic granulomas or exacerbating local inflammation.20
The subclinical persistence of RuV and identification of the granulomas years after suspected exposure suggests a possible progressing or acquired immune system dysfunction in these 4 patients. A waning immune system, as explained by immunosenescence, evolving immune defects associated with CD8+ cells, acquired anticytokine autoantibodies, or other cutaneous local immunity defects, might allow silent RuV to emerge from a persistent reservoir and produce sufficient amounts of viral antigen to trigger granulomatous inflammation years after initial RuV inoculation. The 3 patients with vaccine-derived RuV within granulomas received MMR boosters as adults for waning immunity as determined by titers, and this repeated antigenic exposure in these patients may have served as a triggering event. The patients described in this article were clinically immunocompetent; while they did not meet standard thresholds for immunocompromise, all cases had isolated laboratory abnormalities, including (1) low numbers of CD8+ T cells, (2) low serum immunoglobin levels, or (3) low T-cell mitogen responses or a reversed ratio of CD8 to CD4 counts.
Autoimmune phenocopies of inborn errors of immunity have been described in aging individuals. These individuals develop antibodies against key cytokines that block or suppress the cytokine’s biological function. Patients with anticytokine autoantibodies present with a similar clinical phenotype as the associated inborn errors of immunity. Further exploration into the underlying immune system in these patients would be necessary to understand any complex interactions. Accumulation of immune escape viral variants also could provide a potential explanation for delayed lesion occurrence.
The current rubella vaccine (RA27/3) is one of the most effective live-attenuated virus vaccines, which has allowed substantial reduction in congenital rubella syndrome worldwide. On March 21, 2005, rubella was formally declared eliminated in the US.21 Adverse effects of the live-attenuated RA27/3 vaccine are rare, but because vaccine-derived RuVs have been demonstrated to persist in a small number of vaccinated individuals, it may be prudent to start examining the possibility of an inactive subunit vaccine, such as was done with polio.22 Until then, continued vaccination to maintain the elimination of rubella and congenital rubella syndrome in the US is important because these diseases have not been eliminated globally.
Currently, to our knowledge, there is no effective antiviral therapy for RuV infection, leaving vaccination as the only control measure. Nitazoxanide had been shown to be effective against RuV in vitro but had limited efficacy in patients with inborn errors of immunity and RuV-induced cutaneous granulomas.23 In another study, RuV-cutaneous granulomas improved with treatment with ribavirin in 1 patient, but therapy was limited by anemia.7 Hematopoietic stem cell transplant has improved cutaneous and visceral granulomas in patients with combined immunodeficiencies.14 With a growing evidence suggesting an association between RuV and a chronic, sometimes debilitating, disease that can occur in patients with or without primary immunodeficiencies, response to antivirals would further support a causative role of RuV in cutaneous granulomas.
Limitations
Our observations are limited by the low number of patients that presented at 2 institutions.
Conclusions
This case series study highlights the fact that vaccine-derived and wt RuV can be associated with cutaneous granulomas in clinically immunocompetent adults and strengthens the evidence that RuV can persist and may be an etiologic factor for a subset of granulomatous dermatitis previously deemed idiopathic. Future studies include a large, retrospective cohort study that evaluates cutaneous granulomas of unknown origin from across the US to further define the role of RuV in idiopathic granulomas and identify potential shedding of RuV. Advancing the understanding of the etiology and mechanisms of cutaneous granuloma formation may lead to the development of effective therapeutic approaches for patients.
eFigure 1. Controls for immunofluorescent IHC staining
eFigure 2. Genetic groupings of rubella viruses (WHO nomenclature)
References
- 1.Song JE, Krunic AL. A rare variant of generalized granuloma annulare presenting with chronic Epstein-Barr virus infection: coincidence or association? Acta Dermatovenerol Alp Pannonica Adriat. 2011;20(4):207-211. [PubMed] [Google Scholar]
- 2.Ma HJ, Zhu WY, Yue XZ. Generalized granuloma annulare associated with chronic hepatitis B virus infection. J Eur Acad Dermatol Venereol. 2006;20(2):186-189. doi: 10.1111/j.1468-3083.2005.01366.x [DOI] [PubMed] [Google Scholar]
- 3.Bodemer C, Sauvage V, Mahlaoui N, et al. Live rubella virus vaccine long-term persistence as an antigenic trigger of cutaneous granulomas in patients with primary immunodeficiency. Clin Microbiol Infect. 2014;20(10):O656-O663. doi: 10.1111/1469-0691.12573 [DOI] [PubMed] [Google Scholar]
- 4.Perelygina L, Plotkin S, Russo P, et al. Rubella persistence in epidermal keratinocytes and granuloma M2 macrophages in patients with primary immunodeficiencies. J Allergy Clin Immunol. 2016;138(5):1436-1439.e11. doi: 10.1016/j.jaci.2016.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchbinder D, Hauck F, Albert MH, et al. Rubella virus–associated cutaneous granulomatous disease: a unique complication in immune-deficient patients, not limited to DNA repair disorders. J Clin Immunol. 2019;39(1):81-89. doi: 10.1007/s10875-018-0581-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gross M, Speckmann C, May A, et al. Rubella vaccine-induced granulomas are a novel phenotype with incomplete penetrance of genetic defects in cytotoxicity. J Allergy Clin Immunol. 2022;149(1):388-399.e4. [DOI] [PubMed] [Google Scholar]
- 7.Shields BE, Perelygina L, Samimi S, et al. Granulomatous dermatitis associated with rubella virus infection in an adult with immunodeficiency. JAMA Dermatol. 2021;157(7):842-847. doi: 10.1001/jamadermatol.2021.1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perelygina L, Chen MH, Suppiah S, et al. Infectious vaccine-derived rubella viruses emerge, persist, and evolve in cutaneous granulomas of children with primary immunodeficiencies. PLoS Pathog. 2019;15(10):e1008080. doi: 10.1371/journal.ppat.1008080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pagana KD, Pagana TJ, Pagana TN. Mosby’s Diagnostic and Laboratory Test Reference. 14th ed. Elsevier; 2019. [Google Scholar]
- 10.Karandikar NJ, Crawford MP, Yan X, et al. Glatiramer acetate (Copaxone) therapy induces CD8(+) T cell responses in patients with multiple sclerosis. J Clin Invest. 2002;109(5):641-649. doi: 10.1172/JCI200214380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abernathy E, Peairs RR, Chen MH, Icenogle J, Namdari H. Genomic characterization of a persistent rubella virus from a case of Fuchs uveitis syndrome in a 73 year old man. J Clin Virol. 2015;69:104-109. doi: 10.1016/j.jcv.2015.06.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLean HQ, Fiebelkorn AP, Ogee-Nwankwo A, et al. Rubella virus neutralizing antibody response after a third dose of measles-mumps-rubella vaccine in young adults. Vaccine. 2018;36(38):5732-5737. doi: 10.1016/j.vaccine.2018.08.010 [DOI] [PubMed] [Google Scholar]
- 13.Neven B, Pérot P, Bruneau J, et al. Cutaneous and visceral chronic granulomatous disease triggered by a rubella virus vaccine strain in children with primary immunodeficiencies. Clin Infect Dis. 2017;64(1):83-86. doi: 10.1093/cid/ciw675 [DOI] [PubMed] [Google Scholar]
- 14.Leclerc-Mercier S, Moshous D, Neven B, et al. Cutaneous granulomas with primary immunodeficiency in children: a report of 17 new patients and a review of the literature. J Eur Acad Dermatol Venereol. 2019;33(7):1412-1420. doi: 10.1111/jdv.15568 [DOI] [PubMed] [Google Scholar]
- 15.Perelygina L, Icenogle J, Sullivan KE. Rubella virus-associated chronic inflammation in primary immunodeficiency diseases. Curr Opin Allergy Clin Immunol. 2020;20(6):574-581. doi: 10.1097/ACI.0000000000000694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perelygina L, Hautala T, Seppänen M, Adebayo A, Sullivan KE, Icenogle J. Inhibition of rubella virus replication by the broad-spectrum drug nitazoxanide in cell culture and in a patient with a primary immune deficiency. Antiviral Res. 2017;147:58-66. doi: 10.1016/j.antiviral.2017.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraser JR, Cunningham AL, Hayes K, Leach R, Lunt R. Rubella arthritis in adults: isolation of virus, cytology and other aspects of the synovial reaction. Clin Exp Rheumatol. 1983;1(4):287-293. [PubMed] [Google Scholar]
- 18.Groen-Hakan F, van de Laar S, van der Eijk-Baltissen AA, Ten Dam-van Loon N, de Boer J, Rothova A. Clinical manifestations, prognosis, and vaccination status of patients with rubella virus–associated uveitis. Am J Ophthalmol. 2019;202:37-46. doi: 10.1016/j.ajo.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 19.Frey TK. Neurological aspects of rubella virus infection. Intervirology. 1997;40(2-3):167-175. doi: 10.1159/000150543 [DOI] [PubMed] [Google Scholar]
- 20.Perelygina L, Faisthalab R, Abernathy E, et al. Rubella virus infected macrophages and neutrophils define patterns of granulomatous inflammation in inborn and acquired errors of immunity. Front Immunol. 2021;12(5452):796065. doi: 10.3389/fimmu.2021.796065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.US Centers for Disease Control and Prevention . Rubella no longer major public health threat in the United States. Accessed January 15, 2020. https://www.cdc.gov/media/pressrel/r050321.htm
- 22.Polio vaccines: WHO position paper, January 2014. Wkly Epidemiol Rec. 2014;89(9):73-92. [PubMed] [Google Scholar]
- 23.Perelygina L, Buchbinder D, Dorsey MJ, et al. Outcomes for nitazoxanide treatment in a case series of patients with primary immunodeficiencies and rubella virus–associated granuloma. J Clin Immunol. 2019;39(1):112-117. doi: 10.1007/s10875-019-0589-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Controls for immunofluorescent IHC staining
eFigure 2. Genetic groupings of rubella viruses (WHO nomenclature)