Abstract
Background:
Recent advances in gene editing technology have enabled the production of multi-knockout (KO) and transgenic pigs in order to overcome immunologic barriers in xenotransplantation (XTx). However, the genetic manipulations required to produce these changes may have the unintended consequence of producing or revealing neoantigens reactive with natural antibodies present in baboons. In this study, we examined whether the neoantigens that develop in multi-transgenic (mTg) GalT, Cytidine monophospho-N-acetylneuraminic acid hydroxylase (CMAH), β-1,4-N-acetyl-galactosaminyl transferase 2 (B4) KO pigs can cause rejection of xenografts in baboons.
Methods:
Five baboons that had <35% cytotoxicity against GalT-KO peripheral blood mononuclear cells (PBMCs) in a pre-screening assay received pig kidneys and vascularized thymic grafts (VT + K) from multi-transgenic hCD47, human thrombomodulin (hTBM), human endothelial protein C receptor (EPCR) with/without hCD46 and hCD55 with GalT-KO/NeuGC-KO/B4-KO (mTg Tri-KO) swine. In order to further examine the effects of anti-donor non-Gal natural antibody (nAb), anti-pig preformed IgM and IgG nAb binding against the GalT-KO PBMCs was compared with the donor-type PBMCs using donor pretransplant sera as well as 5 additional naïve baboon sera by flow cytometric analysis.
Results:
Five baboons that received VT + K grafts had stable renal function in the first 11 days (serum creatinine < 1.5 mg/dL). Two of the five baboons had higher binding of preformed IgG to mTg Tri-KO PBMCs than to GalT-KO PBMCs (mTg Tri-KO > GalT-KO), and they rejected their grafts at POD 20. In contrast, the other three baboons demonstrated either mTg Tri-KO = GalT-KO or mTg Tri-KO < GalT-KO, and they maintained renal function 43, 52, and 154 days without rejection. Among 10 baboon sera, two had less antibody binding against PBMCs that were syngeneic to the mTg Tri-KO than against GalT-KO PBMCs (mTg Tri-KO < GalT-KO); three had similar binding to mTg Tri-KO and GalT-KO PBMCs (mTg Tri-KO = GalT-KO); and five had higher binding to m Tg Tri-KO than to GalT-KO PBMCs (mTg Tri-KO > GalT-KO).
Conclusions:
These data suggest that neoantigens associated with mTg Tri-KO promote acute xenograft rejection in a pig-to-baboon VT + K XTx model. The screening assays may be useful to select “safe” recipients to receive mTg Tri-KO kidneys.
Keywords: neoantigens, non-Gal natural antibodies, pig-to-baboon kidney transplantation, xenotransplantation
1 |. INTRODUCTION
Use of α-1,3-galactosyltransferase knockout (GalT-KO) pigs as donors for xenotransplantation has largely prevented hyperacute rejection.1–3 Our initial study demonstrated an 83-day survival of a baboon bearing a life-supporting GalT-KO pig kidney graft without rejection, when a vascularized donor pig thymus was co-transplanted from the same GalT-KO pig.4 In fact, survival of up to 193 days has been achieved with a modified immunosuppressive regimen that minimized the development of proteinuria, which is associated with activation of podocytes in kidney grafts during the induction period.5,6 Recently, after overcoming the early development of proteinuria in baboons,5,7 we have had multiple baboon recipients bearing GalT-KO kidney plus vascularized thymic grafts that maintained renal graft function longer than 4 months, even without complement inhibitors (Yamada et al manuscript in preparation). These baboons demonstrated no evidence of rejection histologically or by immunologic assays. They were ultimately euthanized due to either excessive organ growth or drug-associated complications (Yamada et al manuscript in preparation).
Our data suggest that our kidney plus vascularized thymus strategy facilitates long-term survival of GalT-KO kidney xenografts even without further transgenic modification in recipients with low levels of preformed anti-pig natural antibody.4,5 However, the risk of antibody-mediated rejection (AMR) persists if recipients have high levels of preformed anti-pig non-Gal antibody, akin to clinical ABO incompatible or HLA-sensitized Tx.8–11 Two major non-Gal antigens which are considered to induce xeno-reactive immune responses are N-glycolylneuraminic acid (Neu5Gc) and a Sda or Cad glycan antigens produced by β-1,4-N-acetyl-galactosaminyl transferase 2 (B4GalNT2).12–14 Recently, some groups have successfully produced pigs that were knockout for cytidine monophospho-N-acetylneuraminic acid hydroxylase (CMAH), β4GalNT2 and GalT,12,15 produced in the hope that these advanced KO pigs might reduce AMR associated with preformed anti-pig antibody. However, recent data have demonstrated that simultaneous inactivation of the GalT and CMAH genes increased non-human primate antibody binding when compared to cells lacking either GalT only or to those deficient in GalT-KO/CMAH-KO/B4-KO (Tri-KO).16,17
In this study, we examined preformed non-Gal Ab binding against mTg Tri-KO and GalT-KO in 10 baboons to assess whether neoantigens associated with mTg Tri-KO cause rejection of xenografts in baboons in our vascularized thymus and kidney xenotransplantation (VT + K XTx) model.
2 |. MATERIALS AND METHODS
2.1 |. Animals
All animal work was conducted in accordance with NIH and USDA guidelines and with approval from the Columbia University Institutional Animal Care and Use Committee.
2.1.1 |. Pigs
We used two different lines of GalT-KO pigs for in vitro screening assays. One was the MHC inbred GalT-KO Sachs miniature swine without further genetic modification. The other was outbred domestic swine provided by Revivicor Inc that carried B4-KO, CMAH-KO with hCD46, hCD55, hCD47, human thrombomodulin (hTBM), human endothelial protein C receptor (EPCR) genetically modified GalT-KO (Tri-KO multi-Tg).18 The Tri-KO multi-Tg pigs were also used for VT + K XTx. Three baboons received grafts from Tri-KO with hCD46, hCD55, hCD47, hTBM, EPCR, HO-1 Tg (9GE) pigs and two received grafts from Tri-KO with hCD39, hTBM, EPCR, HO-1 Tg (7GE) pigs. All organ donors were porcine cytomegalovirous (PCMV) negative. (Table S1).
2.1.2 |. Baboons
Papio hamadryas were purchased from the Mannheimer Foundation.
2.2 |. Screening assays to assess preformed anti-pig antibody
Our pre-KXTx protocol includes pre-screening of baboon sera prior to purchase, using complement-dependent cytotoxicity (CDC) assays on GalT-KO PBMC in singletons. We selected baboons that have <35% cytotoxicity of preformed antibody, at 1:4 dilution of serum.7,19 In addition, since PBMC targets from human complement regulatory genes transgenic (CRP Tg) pigs interfere with killing activity in CDC (Figures S1 and S2), we included flow cytometric (FCM) ab binding assays to assess preformed non-Gal abs.
2.3 |. Complement-dependent cytotoxicity (CDC)
Complement-dependent cytotoxicity of baboon sera against porcine PBMC was measured using a modification of the technique in which detection of lysed cells was performed using the fluorescent viability stain 7-actinoaminomycin D (7-AAD, Sigma) in place of trypan blue. Analysis of cells stained with 7-AAD was performed on 2 × 105 cells per sample using a FCM (FACS CANTO II, BD Biosciences).20,21
2.3.1 |. Assessment of anti-non-Gal IgM and IgG antibodies (FCM binding assay)
Anti-non-Gal IgM and IgG serum antibodies were evaluated using GalT-KO PBMCs and mTg Tri-KO PBMCs5,19 (Table S1). Serum Ab binding to cells was detected by FCM (FACS CANTO II, BD Biosciences). FITC-conjugated goat anti-human IgM or IgG antibody (Invitrogen) were used as described.19 Data were expressed as median fluorescence intensity (MFI) “antibody index (Ab index)” which was calculated as follows: (Experimental median fluorescence intensity (MFI) – Negative control MFI)/(Positive control MFI – Negative Control MFI) × 100. Positive control was the recipient serum against Gal + pig PBMC, and negative control was secondary ab (IgM or IgG) with target cells without serum.
2.4 |. Surgery
Simultaneous VT + K XTxs were performed in the same manner as previously reported.4,20 Host thymectomy was performed 2 weeks prior to Tx as described.22 Central venous lines were placed for drug administration and blood sampling 1 week prior to Tx. Bilateral native kidney nephrectomies and splenectomy were performed at the time of Tx. Kidney graft function was assessed daily by serum creatinine (sCre) levels.
3 |. RESULTS
3.1 |. Vascularized thymus and kidney xenotransplantation (VT + K XTx)
Five cases of VT + K XTx were performed using either GalT-KO/CMAH-KO/B4-KO with hTBM, EPCR, hCD47, HO-1 transgenic (Tg) pigs (7GE) or GalT-KO/CMAH-KO/B4-KO with hTBM, EPCR, hCD47, HO-1, hCD46, hCD55, Tg pigs (9GE) (Table S1A). Pre-screening CDC assays using GalT-KO pig PBMCs showed all 5 baboon recipients had <35% cytotoxicity of preformed antibody (after % kilting of negative control was subtracted) at 1:4 ratio against GalT-KO targets (Figure 1). All baboons received anti-CD40 mAb-based immunosuppression (Figure S3). All baboons received cobra venom factor to maintain CH50 levels <1%. Four baboons received CVF daily for 6 weeks and one (15P46) received daily for 2 weeks, and then the interval was switched from daily to twice a week.
FIGURE 1.
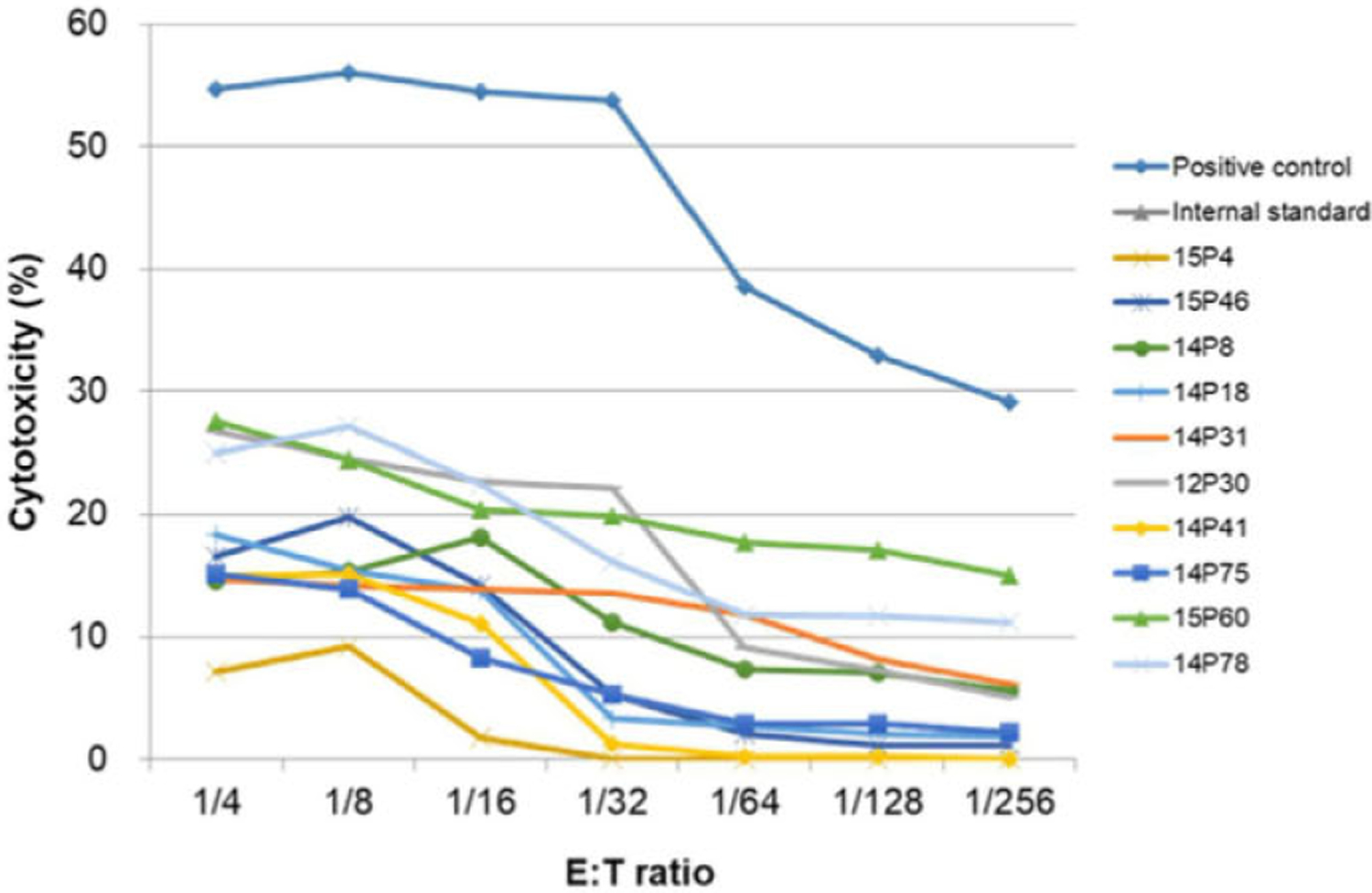
Pre-screening complement-dependent cytotoxicity (CDC) assays of 10 baboons to assess preformed antibody. GalT-KO pig PBMCs were used as targets. Positive control is a pooled baboon serum with confirmed high non-Gal CDC. Negative control is cells plus rabbit complement without serum. Internal standard is a pooled baboon serum that has confirmed CDC 10%−20% (following subtraction of the negative control). Along with these controls, sera from 10 baboons had <35% CDC following subtraction of negative control
We have had no cases of acute rejection during the first 4 weeks after GalT-KO VT + K XTx with CVF when we use baboons that had <35% cytotoxicity of preformed antibody in our previous studies.5 However, two of 5 baboons (14P41 with 7GE graft and 15P60 with 9GE graft) uniformly increased sCre from POD 17 and were euthanized due to rejection at POD 20 (Figures 1B and 2A).
FIGURE 2.
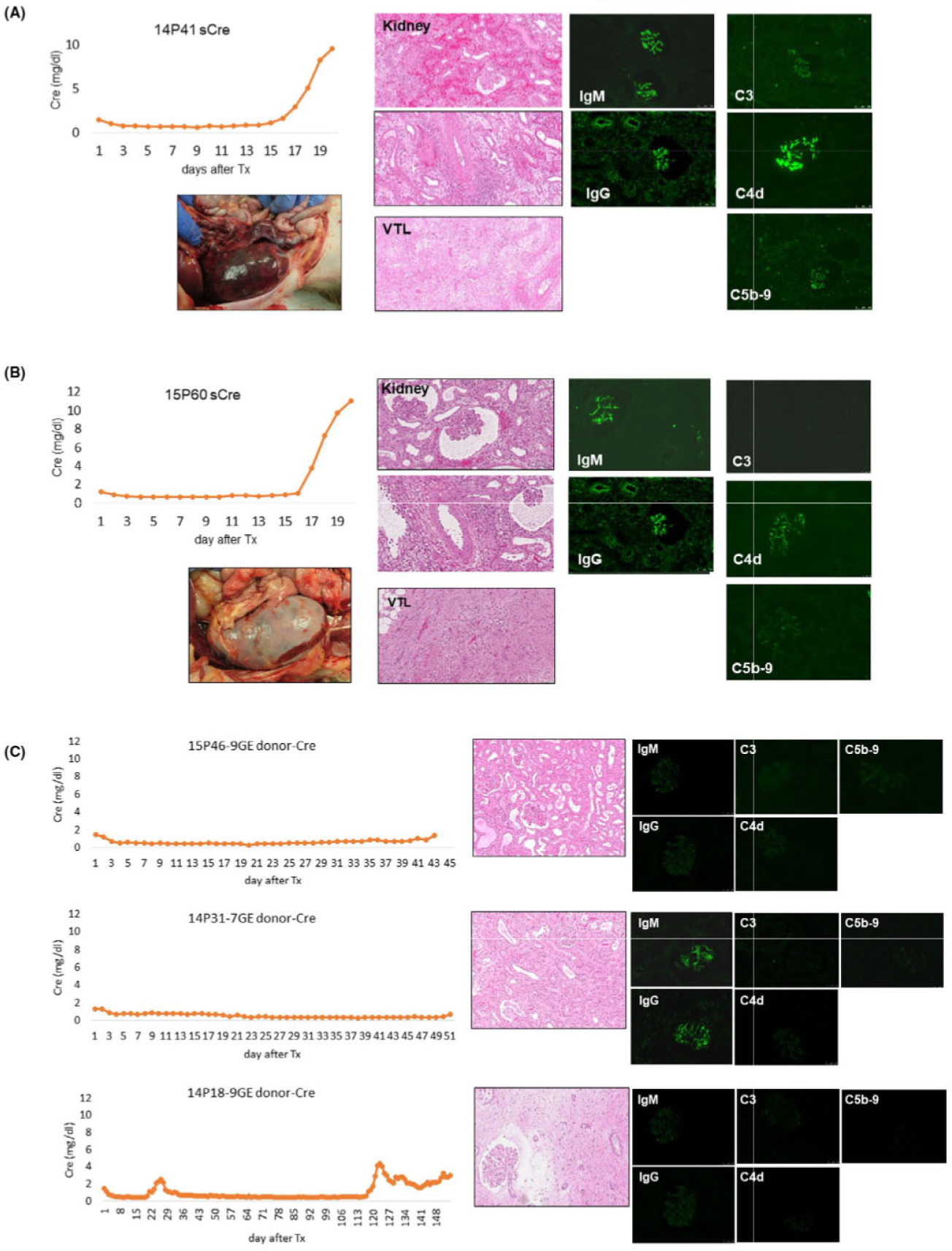
Serum creatinine levels following VT + K XTx and histological and gross findings of the excised kidneys. A, Samples from baboon #14P41. sCre levels following VT + K XTx (top left). Gross finding of hemorrhagic kidney and vascularized thymic grafts at POD 20 (bottom right). HE findings of excised kidney and vascularized thymic graft (middle column). Immunofluorescence pictures of IgM, IgG, C3, C4d, and C5t> (right column). B, Samples from baboon #15P60. sCre levels following VT + K XTx (top left). Gross finding of the excised kidney graft (bottom left). HE findings of excised kidney and vascularized thymic graft (middle column). Immunofluorescence pictures of IgM, IgG and C3 of the excised kidney graft (right column). C, Samples from baboons that did not reject kidneys. sCre levels following VT + K XTx of 15P46 (top left), 14P31 (middle left), and 14P18 (bottom left). HE and IF (IgM, IgG, C3, C4d, and C5b) findings of excised kidneys (middle and right columns). Baboons #15P46 and #14P31 were euthanized due to other causes (see main text). sCre of 14P18 showed increase in sCre from POD 120 due to cortical ischemia associated with increased graft volume. 9GE: GalT-KO/CMAH-KO/B4-KO with hCD46, hCD55, CD201, EPCR, hCD47, HO-1 Tg. 7GE: GalT-KO/CMAH-KO/B4-KO. CD201, EPCR, hCD47, HO-1 (neither hCD46 nor hCD55 Tg)
Gross and histological findings of both kidney grafts at POD 20 showed antibody-mediated rejection (AMR). Graft histology of 15P60 showed focal interstitial hemorrhage in the kidney graft and dead cells in the vascularized thymus, while diffuse interstitial hemorrhages were seen both grossly and histologically in kidneys and thymic grafts that had neither hCD46 nor hCD55 Tg, in the 14P41 (Figure 2A vs 2B). Immunofluorescence staining of the kidney grafts showed both IgM and IgG deposition in glomeruli in both excised grafts. However, fluorescence intensity was much higher in the 14P41 graft. Although both received CVF, 14P41’s kidney graft also had C5b and C4d deposits while that of 15P60 did not.
In contrast, the other 3 baboons (14P31 with 7GE graft, and 14P180 and 15P46 with 9GE grafts) did not reject their grafts at this early time point (Figure 1C left column panels show stable sCre levels). Unfortunately, two of these three animals (15P46 and 14P31) were euthanized due to unexpected complications. One animal (15P46) developed anesthetic complications during sedation for a graft surveillance ultrasound on POD 42. The US images demonstrated good renal blood flow without hydronephrosis. The animal unfortunately developed respiratory arrest during the sedation and had to be euthanized. 14P31 was also euthanized due to respiratory dysfunction at POD 52. Both animals maintained good urinary output (2–3 mL/h) throughout the experimental period, and their kidney grafts were not rejected histologically. The animal euthanized at POD 42 showed no antibody or complement deposition (Figure 3 1 upper and middle panels) The 3rd animal (14P18) maintained stable renal function for over 4 months and was ultimately euthanized at POD 154 due to growth of the kidney graft5,23 (Figure 2C bottom row). The graft volume of this animal gradually increased and reached a graft index (ratio of kidney volume (cm3)/recipient’s body weight (kg)) of 25,5,23 resulting in the development of edema and fibrosis in the kidney graft without vasculitis, and compartment syndrome developed. Although sCre was slightly increased after POD 120, urinary output was >2 mL/kg/h until euthanasia at POD 154. Neither IgM nor IgG nor complement deposits were found in the excised kidney graft.
FIGURE 3.
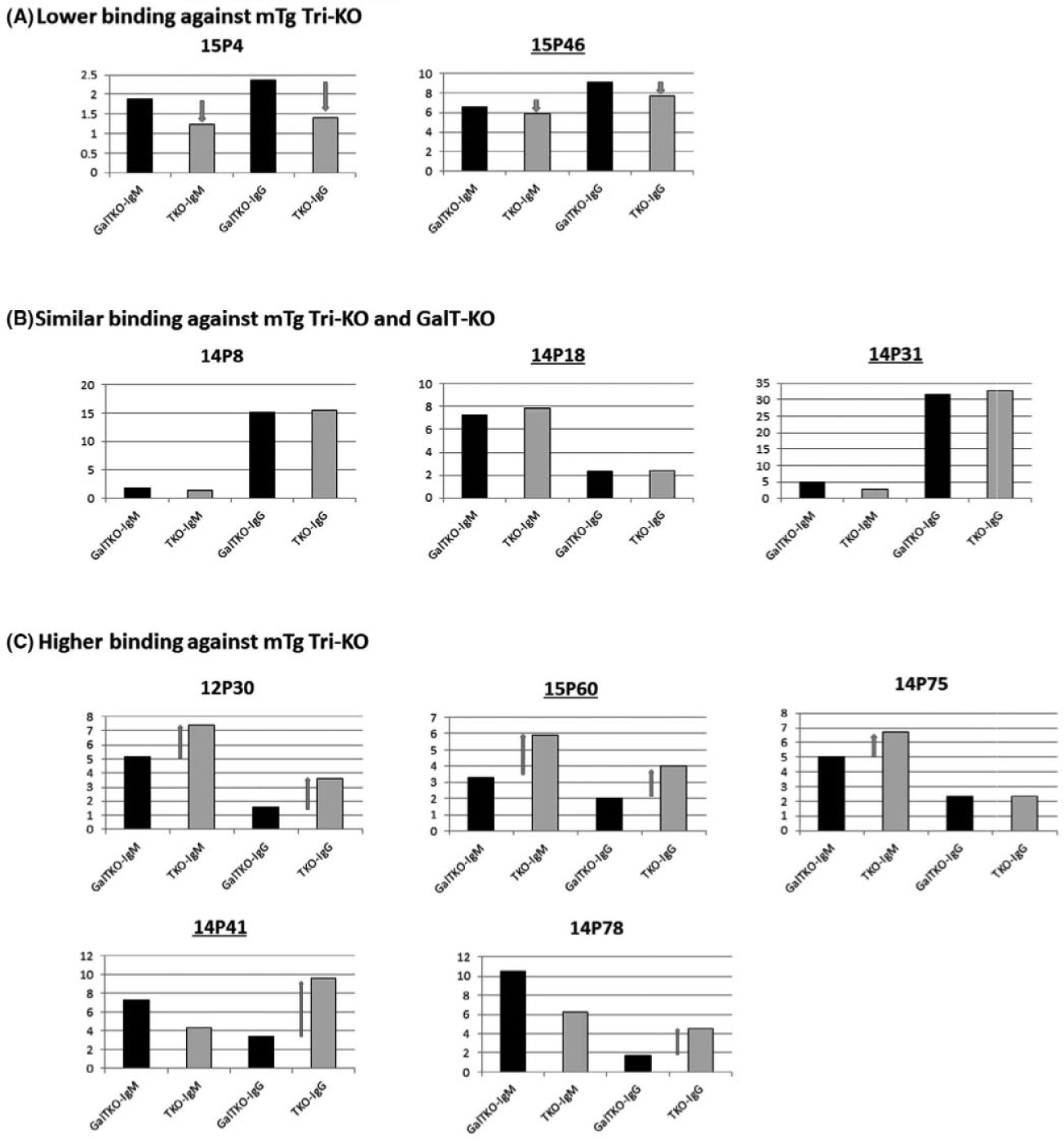
Flow cytometric analysis of preformed antibody binding against GalT-KO pig PBMC (black bars) and mTg Tri-KO PBMC (gray bars). Preformed antibody was assessed using sera from 10 baboons that were confirmed to have <35% killing at 1:4 T:E ratio. Data were expressed as “antibody index (Ab index).” A, Sera from two baboons showed lower MFI Ab index of both IgM and IgG against mTg Tri-KO than GalT-KO PBMC. B, Sera from three baboons showed similar levels of MFI Ab index of both IgM and IgG against mTg Tri-KO and GalT-KO PBMC. C: Sera from five baboons showed higher MFI Ab index against mTg Tri-KO than GalT-KO PBMC. Baboon ID with underline indicates that baboons received kidney grafts shown in this paper
3.2 |. Assays to evaluate preformed Abs specific to donors using syngeneic 9GE PBMC in vitro
In order to evaluate whether preformed Abs specific to donors promotes the early rejection, we tested sera from 10 baboons, including the 5 recipients that were used for CDC screening assays for Ab binding flow cytometric analysis. We used the GalT-KO PBMCs that were identical to those used in the CDC assays and mTg Tri-KO PBMCs that were syngeneic to the 7GE and 9GE VT + K donors. Data were expressed as median fluorescence intensity (MFI) Ab index (Figure 3). Two baboons had lower MFI Ab indexes of IgM and IgG against the mTg Tri-KO than the GalT-KO PBMCs (mTg Tri-KO < GalT-KO) (Figure 3A). Three baboons had Ab binding to the mTg Tri-KO equal to the GalT-KO (Figure 3B). Despite all of tested sera had < 35% CDC against GalT-KO PBMC (Figure 1), the remaining 5 showed higher MFI Ab indexes against the mTg Tri-KO than the GalT-KO (mTg Tri-KO > GalT-KO) (Figure 3C). Among these 5 baboons, two baboons, 12P30 and 15P60, had both IgM and IgG with higher MFI Ab indexes against the mTg Tri-KO than the GalT-KO. Two (14P41 and 14P78) had higher MFI Ab indexes of IgG with lower MFI of IgM against the mTg Tri-KO compared to the GalT-KO. One (14P75) of five had higher IgM MFI Ab index against the mTg Tri-KO than the GalT-KO but IgG MFI Ab index of the mTg Tri-KO binding was similar to the GalT-KO.
Both of 15P60 and 14P41 which rejected their kidney grafts at POD 20 had higher preformed MFI Ab indexes against the 9GE than GalT-KO. Preformed antibody in 15P60 showed higher MFI Ab index of both IgM and IgG against the mTg Tri-KO than GalT-KO while 14P41 MFI Ab index showed higher MFI of IgG but lower MFI of IgM against the mTg Tri-KO compared to those against the GalT-KO.
In contrast, none of the other 3 baboons which did not reject their kidney grafts in the early time points had higher preformed abs against the mTg Tri-KO than the GalT-KO. Two of these baboons, 14P18 and 14P31, had similar MFI against the mTg Tri-KO to that against the GalT-KO. 15P46 had lower MFI against the mTg Tri-KO than the GalT-KO (Figure 2). It is notable that 14P31, which had a high MFI Ab index of preformed IgG against the GalT-KO PBMCs compared to the other 9 baboons in the same assay and in which IgM and IgG deposits were found in glomeruli of the excised graft, maintained stable sCre levels throughout the experimental period despite the absence of human CRP Tg in the graft (Figure 2C middle row). No evidence of activated complement deposition was found in this graft (Figure 2C middle row).
4 |. DISCUSSION
Although recent data have shown long-term acceptance of solid organs in pig-to-baboon models,24,25 the risk of antibody-mediated rejection (AMR) persists. 7GE and 9GE pigs are mTg Tri-KO pigs that were produced to minimize AMR associated with preformed anti-pig non-Gal antibodies following XTx by adding B4-KO and CMAH-KO.16,18,26–28 Our data demonstrate three different types of baboon anti-pig antibody binding in 10 tested baboons. Five of ten animals had higher preformed antibody binding against the mTg Tri-KO PBMCs when compared to GalT-KO. Although the difference in IgG MFI of preformed antibody between the mTg Tri-KO and the GalT-KO was not large, both baboons that had preformed nAb IgG MFI against the mTg Tri-KO PBMCs higher than against the GalT-KO PBMCs had almost identical clinical courses and rejected their grafts at POD 20 (Figure 2A and B). Moreover, one baboon (14P41) which had lower IgM antibody binding against the mTg Tri-KO than IgM antibody binding GalT-KO still rejected its graft due to AMR. Additionally, baboon 14P31 which had a higher MFI of preformed IgG against GalT-KO when compared to the other 9 baboons in the same assay did not reject its kidney.
Some reports have demonstrated that, although B4-KO/GalT-KO and CMAH-KO/GalT-KO showed reduced human IgM and IgG binding compared to GalT-KO cells, CMAFI-KO/GalT-KO pig PBMCs showed increased non-human primate antibody binding as well as cytotoxicity against erythrocytes when compared to GalT-KO.12,17,26 Taken together, our data suggest that neoantigens may be produced or revealed during the production of CMAH-KO pigs. Although it remains unclear if these neoantigens would be sufficiently immunogenic as to cause rejection of porcine kidneys, our findings also suggest that preformed IgG rather IgM against neoantigens associated with additional KO plays an essential role in acute graft rejection. In the antigen-binding screening assay, we used the same mTg Tri-KO PBMC that are syngeneic to the donors. In the Ab binding assay, background that were responses against positive and negative controls are different in each assay and each animal. We are aware that the variability of IgG and IgM between individual animals may make it difficult to compare the MFI between animals. Therefore, it is important to be able to quantify and compare this in some way. All baboons have anti-Gal Ab. Although not without its limitations, percent binding against non-Gal Abs compared to anti-Gal Ab is one way to help standardize assay results. Based on this thought, we developed the assessment to be standardized. We developed “Ab index” and used the assessment in this study. This assessment will be used for future assays as well because this standardized assessment allows us to compare results from assays that were set up on different days.
In this study, GalT-KO PBMCs were obtained from Sachs MHC inbred miniature swine (SLAhh) and the mTg Tri-KO pig PBMCs were obtained from domestic swine provided by Revivicor Inc Although mTg Tri-KO pigs are domestic pigs, VT + K XTx donors were clone pigs and were syngeneic. One concern that may rise is a difference in the immunogenicity between Sachs MHC inbred miniature swine. We have recently obtained GalT-KO pigs without further gene transfer for VT + KTx. Although further studies are required, thus far, we have not seen notable differences between Sachs GalT-KO pigs and domestic GalT-KO pigs provided by Revicor Inc No differences in screening FCM assays as well as in vivo survivals were observed. We have experimental baboons with survival times of >6 months that have received VT + K grafts from Sachs GalT-KO-pig or Revivicor GalT-KO pigs similarly treated (Yamada et al manuscript in preparation).
We have had no cases of acute rejection during the induction period with CVF in our previous studies of GalT-KO VT + K XTx.5 Strategy of VT XTx is to tolerize new or naïve T cells that potentially respond to pig antigens. However, this strategy is unlikely to inhibit or tolerize sensitized T-cell responses. Therefore, our results also suggest that those recipients with higher IgG against mTg Tri-KO than against GalT-KO may have been exposed to antigens with cross-reactivity to the neoantigens. Timing of the rise in sCre from POD 17 in rejectors corresponded to a small increase in effector T memory cells, which may have contributed to AMR (Figure S4). Further studies to assess Tmem cells in previous and future cases will help to clarify if sensitized T-cell responses are involved in such the early acute rejection seen in this model.
Our recent data indicated that hCD47 expression on porcine endothelial cells or glomerular podocytes inhibited phagocytosis by baboon or human phagocytes.6 None of the baboons that received mTg GalT-KO kidneys including hCD47 Tg developed proteinuria, including these two rejectors. This also indicates that the loss of these two baboons was not nephrotic syndrome and acute rejection in this study did not cause proteinuria. The effect of hCD47 Tg on development of proteinuria will be reported separately (Yamada et al manuscript in preparation). CMAH-KO-specific antigens may not be an issue in pig-to-human XTx based upon previous data demonstrating that non-human primate antibody binding against simultaneous inactivation of the GalT and CMAH genes increased while human antibody binding did not12,17 However, a similar risk of developing neoantigens against any knockout may occur. Therefore, it is important to identify potential risk factors for rejection by utilizing pre-screening assays, such as FCM binding cross-match and CDCs. We recognize that our study has some limitations. The number of transplants was relatively small, and two donor genotypes were used. Our study design did not allow us to conclusively identify the gene modifications) responsible for generation of the putative neoantigen(s), although previous work implicates CMAH-KO. Nevertheless, we believe that our results provide valuable insights to those in the field. Further studies to determine which gene modification would be responsible for rejection in order to address single antigen analysis for pathological and immunologic roles will be performed. Our present study suggests that a screening assay to assess KO-specific preformed IgG antibody using both GalT-KO and mTg Tri-KO cells may be useful to select “safe” recipients to receive gene edited multi-KO kidneys. However, larger groups of animals of each type will help to confirm the utility of this approach.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr Masayuki Tasaki for his critical review of the manuscript. Dr Yuichi Ariyoshi received an ATS Young Investigator award for this study. We thank Genzyme for providing rabbit ATG and Genentech for Rituximab. This research was supported by NIH grant, P01AI45897, and sponsored research funding from Lung Biotechnology PBC. FCM analysis in this article was performed in the CCTI flow cytometry core, supported in part by the Office of the Director, NIH, under awards S10RR027050 and S10OD020056. All animal work was conducted in accordance with NIH and USDA guidelines and with approval from the Columbia University Institutional Animal Care and Use Committee.
Funding information
Supported by NIAID P01AI045897.
Abbreviations:
- AMR
antibody-mediated rejection
- B4-KO
β-l,4-N-acetyl-galaetosaminyl transferase2
- CDC
Complement-DependentCytotoxicity
- CMAH
Cytidine monophospho-N-acetylneuraminic acid hydroxylase
- CRP
complement regulatory genes
- EC
endothelial cell
- FBS
fetal bovine serum
- FCM
flow cytometric
- GalT-KO
galactosyl-α1-3-galactosyl transferase gene knockout
- GE
genetic engineering
- KO
knockout
- KXTx
kidney xenotransplantation
- MFI
median fluorescence intensity
- mTgTri-KO
multi-transgenic triple knockout
- NeuSGc
N-glycolylneuraminic acid
- PBMC
Peripheral Blood Mononuclear Cells
- PCMV
porcine cytomegalovirous
- POD
post-operative day
- sCre
serum creatinine
- Tg
transgenic
- VT + K XTx
vascularized thymus and kidney xenotransplantation
- XTx
xenotransplantation
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Kolber-Simonds D, Lai L, Watt SR, et al. Production of a-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci USA. 2004;101:7335–7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dai Y, Vaught TD, Boone J, et al. Targeted disruption of the alpha1,3-galactosyltransferase gene in cloned pigs. Nat Biotechnol. 2002;20:251–255. [DOI] [PubMed] [Google Scholar]
- 3.Chen G, Qian H, Starzl T, et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat Med. 2005;11:1295–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamada K, Yazawa K, Shimizu A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11:32–34. [DOI] [PubMed] [Google Scholar]
- 5.Rivard CJ, Tanabe T, Lanaspa MA, et al. Upregulation of CD80 on glomerular podocytes plays an important role in development of proteinuria following pig-to-baboon xeno-renal transplantation - an experimental study Transpl Int. 2018;31:1164–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nomura S, Ariyoshi Y, Watanabe H, et al. Transgenic expression of human CD47 reduces phagocytosis of porcine endothelial cells and podocytes by baboon and human macrophages. Xenotransplantation. 2020;27(1):e12549. 10.1111/xen.12549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tasaki M, Shimizu A, Hanekamp I, Torabi R, Villani V, Yamada K. Rituximab treatment prevents the early development of proteinuria following pig-to-baboon xeno-kidney transplantation. J Am Soc Nephrol. 2014;25:737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tasaki M, Saito K, Nakagawa Y, et al. Analysis of the prevalence of systemic de novo thrombotic microangiopathy after ABO-incompatible kidney transplantation and the associated risk factors. Int J Urol. 2019;26:1128–1137. [DOI] [PubMed] [Google Scholar]
- 9.Ishida H, Koyama I, Sawada T, et al. Anti-AB titer changes in patients with ABO incompatibility after living related kidney transplantations: survey of 101 cases to determine whether splenectomies are necessary for successful transplantation. Transplantation. 2000;70:681–685. [DOI] [PubMed] [Google Scholar]
- 10.Salvade I, Aubert V, Venetz JP, et al. Clinically-relevant threshold of preformed donor-specific anti-HLA antibodies in kidney transplantation. Hum Immunol. 2016;77:483–489. [DOI] [PubMed] [Google Scholar]
- 11.Ziemann M, Altermann W, Angert K, et al. Preformed donor-Specific HLA antibodies in living and deceased donor transplantation: a multicenter study. Clin J Am Soc Nephrol. 2019;14:1056–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butler JR, Martens GR, Estrada JL, et al. Silencing porcine genes significantly reduces human-anti-pig cytotoxicity profiles: an alternative to direct complement regulation. Transgenic Res. 2016;25:751–759. [DOI] [PubMed] [Google Scholar]
- 13.Byrne G, Ahmad-Villiers S, Du Z, McGregor C. B4GALNT2 and xenotransplantation: A newly appreciated xenogeneic antigen. Xenotransplantation. 2018;25:e12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao C, Cooper DKC, Dai Y, Hara H, Cai Z, Mou L. The Sda and Cad glycan antigens and their glycosyltransferase, beta1,4GalNAcT-II, in xenotransplantation. Xenotransplantation. 2018;25:e12386. [DOI] [PubMed] [Google Scholar]
- 15.Wang RG, Ruan M, Zhang RJ, et al. Antigenicity of tissues and organs from GGTA1/CMAH/beta4GalNT2 triple gene knockout pigs. J Biomed Res. 2018;33(4):235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams AB, Kim SC, Martens GR, et al. Xenoantigen deletion and chemical immunosuppression can prolong renal xenograft survival. Ann Surg. 2018;268:564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang ZY, Burlak C, Estrada JL, Li P, Tector MF, Tector AJ. Erythrocytes from GGTA1/CMAH knockout pigs: implications for xenotransfusion and testing in non-human primates. Xenotransplantation. 2014;21:376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper DKC, Hara H, Iwase H, et al. Justification of specific genetic modifications in pigs for clinical organ xenotransplantation. Xenotransplantation. 2019;26:e12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tasaki M, Wamala I, Tena A, et al. High incidence of xenogenic bone marrow engraftment in pig-to-baboon intra-bone bone marrow transplantation. Am J Transplant. 2015;15:974–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamada K, Scalea J. Thymic transplantation in pig-to-nonhuman primates for the induction of tolerance across xenogeneic barriers. Methods Mol Biol. 2012;885:191–212. [DOI] [PubMed] [Google Scholar]
- 21.Yamada K, Ariyoshi Y, Pomposelli T, Sekijima M. Co-transplantation of vascularized thymic graft with kidney in pig-to-nonhuman primates for the induction of tolerance across xenogeneic barriers. Methods Mol Biol. 2020;2110:151–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griesemer AD, Hirakata A, Shimizu A, et al. Results of gal-knock-out porcine thymokidney xenografts. Am J Transplant. 2009;9(12):2669–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanabe T, Watanabe H, Shah JA, et al. Role of intrinsic (Graft) versus extrinsic (Host) factors in the growth of transplanted organs following allogeneic and xenogeneic transplantation. Am J Transplant. 2017;17:1778–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohiuddin MM, Singh AK, Corcoran PC, et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat Commun. 2016;7:11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwase H, Liu H, Wijkstrom M, et al. Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date. Xenotransplantation. 2015;22:302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lutz AJ, Li P, Estrada JL, et al. Double knockout pigs deficient in N-glycolylneuraminic acid and galactose alpha-1,3-galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation. 2013;20:27–35. [DOI] [PubMed] [Google Scholar]
- 27.Burlak C, Bern M, Brito AE, et al. N-linked glycan profiling of GGTA1/CMAH knockout pigs identifies new potential carbohydrate xenoantigens. Xenotransplantation. 2013;20:277–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perota A, Galli C. N-Glycolylneuraminic Acid (Neu5Gc) null large animals by targeting the CMP-Neu5Gc Hydroxylase (CMAH). Front Immunol. 2019;10:2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


