Abstract
Background
Ultrarare Marshall–Smith and Malan syndromes, caused by changes of the gene nuclear factor I X (NFIX), are characterised by intellectual disability (ID) and behavioural problems, although questions remain. Here, development and behaviour are studied and compared in a cross-sectional study, and results are presented with genetic findings.
Methods
Behavioural phenotypes are compared of eight individuals with Marshall-Smith syndrome (three male individuals) and seven with Malan syndrome (four male individuals). Long-term follow-up assessment of cognition and adaptive behaviour was possible in three individuals with Marshall–Smith syndrome.
Results
Marshall–Smith syndrome individuals have more severe ID, less adaptive behaviour, more impaired speech and less reciprocal interaction compared with individuals with Malan syndrome. Sensory processing difficulties occur in both syndromes. Follow-up measurement of cognition and adaptive behaviour in Marshall–Smith syndrome shows different individual learning curves over time.
Conclusions
Results show significant between and within syndrome variability. Different NFIX variants underlie distinct clinical phenotypes leading to separate entities. Cognitive, adaptive and sensory impairments are common in both syndromes and increase the risk of challenging behaviour. This study highlights the value of considering behaviour within developmental and environmental context. To improve quality of life, adaptations to environment and treatment are suggested to create a better person-environment fit.
Keywords: adaptive behaviour, cognition, Malan syndrome, Marshall–Smith syndrome, NFIX variants, sensory processing
Introduction
Marshall-Smith syndrome (MIM# 164005) and Malan syndrome (MIM# 614753) are ultrarare disorders (Prevalence < 1/1 000 000; respectively about 57 patients with Marshall–Smith syndrome and 80 patients with Malan syndrome in literature to date) caused by changes of the gene nuclear factor I X (NFIX) (Orphanet 2020a; Orphanet 2020b; Priolo et al. 2018). Intellectual disability (ID), autistic features (e.g. communication difficulties and stereotypic behaviour), sensory processing difficulties (e.g. sensitivity to noise) and sensory impairments (vision and hearing) occur in both syndromes (Van Balkom et al. 2011; Priolo et al. 2018) and pose major demands on families and carers. Marshall–Smith syndrome is characterised by abnormal bone maturation (57/57 cases), prominent forehead (55/57 cases), proptosis (55/56 cases), airway obstructions (45/55 cases), growth problems (height in 38/39 cases < third centile), moderate to severe ID (57/57 cases) and communication difficulties (6/6 cases) (Marshall et al. 1971; Shaw et al. 2010; Van Balkom et al. 2011).
The hallmarks of Malan syndrome are ID (80/80 cases), autistic features (24/74 cases), anxieties (39/72 cases), hypotonia (56/74 cases) and overgrowth (45/78 cases) defined as ‘global or regional excess growth compared either to an equivalent body part or to the age-related peer group’ (Malan et al. 2010; Tatton-Brown and Weksberg 2013; Priolo et al. 2018). Phenotypical characteristics described above affect individual abilities, impede adequate interaction between individual and environment (person-environment fit), impair daily functioning and can lead to challenging behaviour (Lundqvist 2013; Huisman et al. 2017).
Most known data on development and behaviour in both syndromes originate from single case descriptions or small series. However, the dearth of validated instruments to assess cognitive functioning in individuals with severe ID (Carnaby 2009) and the fact that previous publications lack exact description of used instruments hampers interpretation and comparison.
In both syndromes, cognition has rarely been studied through direct in-person assessments (Van Balkom et al. 2011; Priolo et al. 2018). Although behavioural indicators of sensory difficulties are obvious in daily practice (e.g. getting anxious in loud or crowded places), sensory processing has never been studied in these syndromes.
We aim to investigate cognition, adaptive behaviour and sensory processing by (1) describing and comparing Marshall–Smith syndrome and Malan syndrome and (2) describing long-term follow-up of cognition and adaptive functioning in Marshall–Smith syndrome. We also list recommendations for clinical practice and future research.
Methods
Participants
This study followed approximately the methodology by Van Balkom et al. (2011); Priolo et al. (2018). Detailed genetic findings for Malan syndrome were described by Priolo et al. (2018).
All participants (n = 8) with Marshall–Smith syndrome were invited at international Marshall–Smith syndrome Family Events in the Netherlands (2015, 2017), participants from outside the Netherlands were also assessed during these events. Individuals with Malan syndrome known in the Netherlands (n = 8) were invited through their physicians.
Measures
Cognition, adaptive behaviour and sensory processing were assessed through direct in-person assessments, semi-structured interviews and additional questionnaires in individuals at different ages and developmental stages. Assessments took place within the context of participants’ daily environment and/or in presence of parent(s) or carer(s).
Test-battery included (1) Bayley-III – Special Needs Addition (Bayley-III – SNA; Ruiter et al. 2014) or Wechsler Preschool and Primary Intelligence Scale (WPPSI-III; Hendriksen and Hurks 2009), both were indicated as most suitable for these syndromes to assess level of development and/or cognition, based on a priori clinical impression (based on available literature indicating developmental delay and difficulties on several domains); (2) Vineland-2 Expanded Interview Form (Sparrow et al. 2008) to assess adaptive behaviour abilities and (3) Short Sensory Profile (SSP; Rietman 2013) to assess sensory processing. Please note that the use of differing cognitive measures impacts direct comparability and interpretation of results. In an effort to judge optimal individual capacity, adaptations of procedures and environment have included assessing within a familiar environment, allowing more time, closing curtains/dimming lights, using preferred toys and supporting instructions with gestures and pointing to objects.
Direct observations were performed by two experienced clinicians with extended expertise in diagnoses and management of individuals with (rare) genetic syndromes. The structured form used for direct observations and the psychometric properties of the instruments used are described in the supporting information.
Data
Descriptive statistics illustrate development, adaptive behaviour and sensory processing. To compare outcomes on cognition and adaptive behaviour in the most appropriate way, comparison is based on age equivalents. For participants aged above 3 to 6 years who were assessed with the Bayley-III, only age equivalents could be derived. Age equivalents are also presented for participants who were assessed with the WPPS-III and from whom raw scores were computed to IQ-scores ≤55. To be able to differentiate between subtests, age equivalents were used. Differences between syndromes were explored through Mann–Whitney U tests, because of small sample sizes. Long-term follow-up data on cognition and adaptive behaviour in Marshall–Smith syndrome were compared with previous findings (Van Balkom et al. 2011). Parents received a report with results of assessments.
Ethics statement
The Marshall–Smith syndrome World Federation and parents supported this study. The medical ethics committee of Great Metropolitan Hospital Bianchi-Melacrino-Morelli in Reggio Calabria approved the study (approval No 200). Written informed consent was obtained prior to inclusion, and the study was conducted in accordance with ethical standards (Declaration of Helsinki and subsequent amendments).
Results
Eight individuals with Marshall–Smith syndrome and seven individuals with Malan syndrome were included (Table 1).
Table 1.
Participant characteristics
| Characteristic | Marshall–Smith syndrome (n = 8) |
Malan syndrome (n = 7) |
|---|---|---|
| Sex, Male (%) | 3 (37%) | 4 (57%) |
| Age (years) | ||
| Mean (SD) | 8.4 (5.8)** | 14.6 (6.7)** |
| Range | 2.3–20.0 | 5.8–25.1 |
| Median | 7.6 | 13.8 |
| IQR (first to third) | 8.3 (3.6–12.0) | 12.3 (8.8–21.1) |
| Hearing impairments | 3 (37%) | 2 (50%) |
| (%)* | ||
| Vision impairments (%)* | 4 (50%) | 4 (100%) |
| Epilepsy (%) | 0 (0%) | 1 (14%) |
| Speech | ||
| Absent (%) | 6 (75%) | 0 (0%) |
| Few words (%) | 2 (25%) | 3 (43%) |
| Sentences (%) | 0 (0%) | 4 (57%) |
| Cognitive age equivalent (months) | ||
| Mean (SD) | 15.9 (5.6) | 39.5 (5.0) |
| Range | 9–26 | 24–66 |
Hearing and vision were assessed during parental interviews. Speech abilities were based on Vineland-2 and direct assessments.
Available for four participants with Malan.
Significant difference; P < 0.05, based on Mann–Whitney U tests
The Marshall–Smith syndrome-group was significantly younger than the Malan syndrome-group (P < 0.05, Table 1). Male to female ratio was 3:5 in Marshall–Smith and 4:3 in Malan. Sensory impairments (vision and hearing) were present in both groups. Individuals with Marshall–Smith syndrome developed less expressive speech (few single words, n = 2) compared with individuals with Malan syndrome (words and sentences, n = 7).
Cognition was assessed with the Bayley-III-SNA (Marshall–Smith syndrome) and with the WPPSI-III (Malan syndrome). Outcomes on the cognitive assessments (Bayley-III-SNA and WPSSI-III-NL) were converted to age-equivalents in months, according to the manual (Hendriksen and Hurks 2009) to indicate the developmental age (Tables S1 and S2). Age equivalents of cognitive assessments are visualised in Figs 1 (Marshall–Smith) and 2 (Malan). Mean developmental age was 15.9 months (SD 5.6; range 9–26 months) and 39.5 months (SD 5.0; range 24–66 months) in Marshall–Smith syndrome and Malan syndrome, respectively.
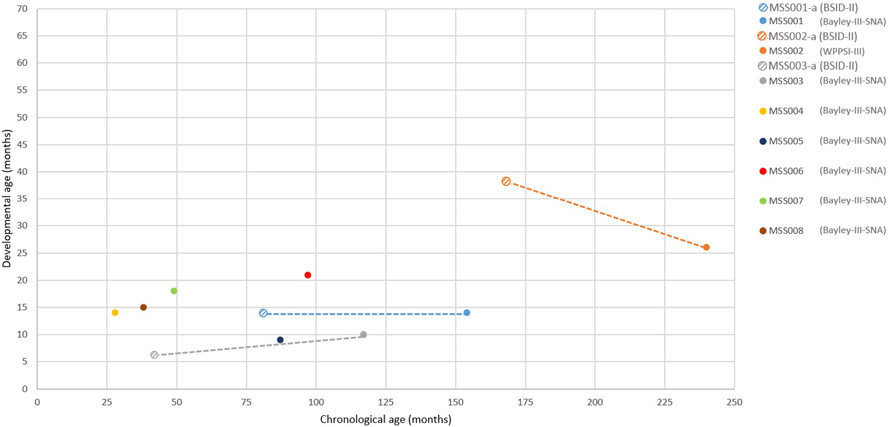
Figure 1.
Age equivalents of cognitive development in Marshall-Smith syndrome. Age equivalents of cognition in Marshall–Smith syndrome (Bayley-III-SNA). Previous findings in the same individuals are depicted with crossed circles in same colours as current findings. Instruments used are indicated between brackets after the participant-codes.
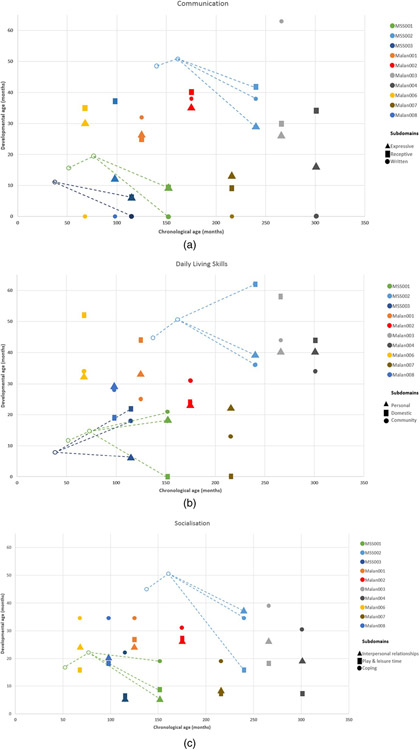
Adaptive functioning was assessed in three individuals with Marshall–Smith syndrome. Age equivalents ranged from 5 to 62 months, with mean age-equivalent scores of 21.7, 25.7 and 16.7 months on the domains Communication, Daily Living Skills and Socialisation, respectively. Percentile ranks on all domains were below <0.1 for all participants with Marshall–Smith syndrome (n = 3).
In Malan syndrome (n = 7) age-equivalent on adaptive functioning ranged from 7 to 63 months, with mean age-equivalent scores of 29.3, 32.0 and 23.3 months on Communication, Daily Living Skills and Socialisation. Percentile ranks were 0.4, 3.0 and 2.0 on the domains Communication, Daily Living Skills and Socialisation for Malan006. All other participants with Malan syndrome (n = 6) had percentile ranks of <0.1 on all domains.
The lowest mean age-equivalent for both groups (10 and 17 months in Marshall–Smith and Malan syndrome, respectively) was observed on the subdomain ‘Play and Leisure’. Adaptive behaviour scores for both syndromes are shown in Fig. 3a-c.
Figure 3.
(a) Vineland-2 scores for participants with Marshall–Smith syndrome and Malan syndrome on the Communication subdomains. Age-equivalents in months on the Y-axis. Participants who scored ‘zero’ on subdomain Written are all reported with an age-equivalent of <30 months, indicating no skills reported in this subdomain. Previous findings in the same individuals are depicted with circles in the same colours as current findings, connected by a striped trend line. Previous findings contain total domain scores only. (b) Vineland-2 scores for participants with Marshall–Smith syndrome and Malan syndrome on the Daily Living Skills subdomains. Previous findings in the same individuals are depicted with circles in the same colours as current findings, connected by a striped trend line. Previous findings contain total domain scores only. (c) Vineland-2 scores for participants with Marshall–Smith syndrome and Malan syndrome on the Socialisation subdomains. Previous findings in the same individuals are depicted with circles in the same colours as current findings, connected by a striped trend line. Previous findings contain total domain scores only.
Previous scores on level of development and adaptive behaviour in three individuals with Marshall–Smith syndrome (MSS001, MSS002 and MSS003) are also shown in Figs 1 and 3a-c and Table 2.
Table 2.
Previous and current age equivalents (years;months) on cognition and adaptive behaviour in three individuals with Marshall–Smith syndrome
| IndividualCalendar age | Previous outcomes* | Current outcomes* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cognition (BSID-II) | Adaptive behaviour (Vineland-1) |
Cognition | Adaptive behaviour (Vineland- 2) |
||||||
| Com | DLS | Soc | Calendar age | Com | DLS | Soc | |||
| MSS001 6;6 | 1;2 | 1;7 | 1;3 | 1;11 | 12;8 | 1;2 (Bayley-III-SNA) | 0;0–0;9 | 0;0–1;9 | 06;-1;7 |
| MSS002 13;10 | 3;2 | 4;3 | 4;3 | 4;3 | 20;0 | 2;2 (WPPSI-III) | 2;4–4;7 | 3;0–5;2 | 1;4–3;2 |
| MSS003 3;5 | 0;7 | 0;11 | 0;8 | NA | 9;7 | 0;10 (Bayley-III-SNA) | 0;0–0;7 | 0;7–1;10 | 0;6–1;11 |
Used instruments are mentioned between brackets.
Com, Communication; DLS, Daily Living Skills; NA, not available; Soc, Socialisation.
Comparison on adaptive behaviour scores over time (in MSS001, MSS002 and MSS003) showed a decrease in developmental level on all domains, except for some subdomains of Daily Living Skills.
Data on sensory processing were assessed in six participants with Marshall–Smith syndrome and seven participants with Malan syndrome. Participant MSS004 was excluded for comparison, because the SSP only goes down to 3 years. All participants showed sensory processing difficulties on several domains of the SSP. Most difficulties were reported on the domains Visual/Auditory Sensitivity, Low energy/weak, Underresponsive/seeking sensations and Tactile sensitivity. The latter sensitivity was more common in Malan syndrome, though grooming activities are more reported as stressful for Marshall–Smith syndrome. Table 3 shows scores on sections of the SSP for each syndrome. Supporting Information contains specific scores on all domains for each participant.
Table 3.
Scores on sections of the Short Sensory Profile for each syndrome
| Section | Marshall–Smith syndrome (n = 5) |
Malan syndrome (n = 7) |
||
|---|---|---|---|---|
| Probable difference* (n) | Definite difference** (n) | Probable difference* (n) | Definite difference** (n) | |
| Tactile sensitivity | – | 2 | 1 | 5 |
| Taste/Smell sensitivity | – | 1 | 1 | – |
| Movement sensitivity | – | 1 | 2 | 5 |
| Underresponsive/Seeks sensation | 1 | 2 | 4 | 2 |
| Auditory filtering | 2 | 1 | 4 | 2 |
| Low energy/Weak | – | 3 | 2 | 4 |
| Visual/Auditory sensitivity | – | 4 | 2 | 3 |
Probable difference: −1SD to −2 SD.
Definite difference: ≥ −2SD.
For Marshall–Smith syndrome, items reported by parents as frequently/always present were ‘Expresses distress during Grooming’ (n = 5), ‘Enjoys strange noises/seeks to make noises for noise’s sake’ (n = 4), ‘Is distracted or has trouble functioning if there is a lot of noise around’ (n = 5), ‘Seems to have weak muscles’ (n = 5) and ‘Is bothered by bright lights after others have adapted to the light’ (n = 4).
For Malan syndrome, items reported by parents as frequently/always present were ‘Expresses distress during Grooming’ (n = 5), ‘Fears of falling or heights’ (n = 5), ‘Touches people and objects’ (n = 4), ‘Is distracted or has trouble functioning if there is a lot of noise around’ (n = 7) and ‘Responds negatively to unexpected or loud noises’ (n = 6).
Structured behavioural observations during direct assessments showed that participants with Marshall–Smith syndrome had a friendly demeanour, few reciprocal responses and no eye-to-eye gaze. All focused on physical contact (touching the researcher with their hands, seeking physical proximity without clear reciprocal intention). Speech and language were limited, and some used a few single words. Body movement (restlessness) intensified with increasing tension and effort. There was a clear focus on some favourite objects and persistent behaviour to obtain it. Participants with Marshall–Smith syndrome quickly built up routines of behaviour, which inhibited ability to shift between tasks. Sensory issues were evident with manual tactile exploration of materials. Environmental stimuli (visual and auditory) were easily distracting, and attention span was commonly shorter in Marshall–Smith syndrome than in Malan syndrome. Participants with Marshall–Smith showed a positive mood and no overt anxiety.
Individuals with Malan syndrome needed some time to adjust to the researcher, evident in initial reserve in interaction. Reciprocity was commonly present and social interaction pleasant, although demanding attention and energy (e.g. ‘freeze’ during social interaction and turning head away). Expressive language difficulties, especially pronunciation, were common. Stereotypic finger mannerisms were seen with increased tension (e.g. hyperextending of fingers) and most presented repetitive verbalisations (repeating subjects of high interest). All individuals with Malan syndrome showed difficulties with information retrieval and often used or needed supportive associations or gestures for successful retrieval. All individuals needed longer processing time, but shifting between tasks was not problematic. Most were easily distracted by environmental stimuli (visual and auditory) and had a high frustration tolerance (the ability to persevere through difficulties). All participants had a positive mood with no signs of anxiety.
Details of assessments of all participants are presented in Tables S1 and S2.
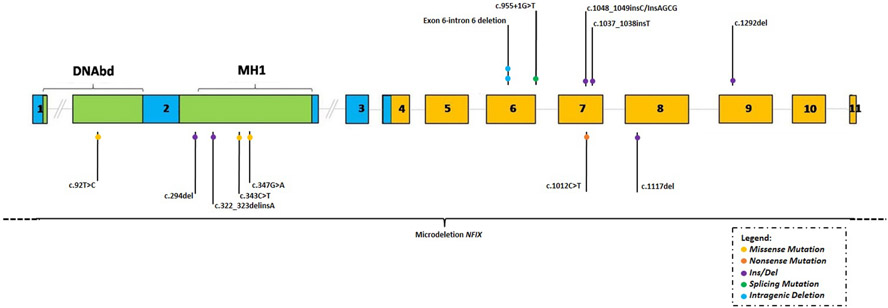
Participants with Marshall–Smith syndrome had mutations in exon 6 (n = 3), exon 7 (n = 2) and exon 9 (n = 1), and those with Malan syndrome had mutations in exons 1 and 2 (n = 5), exon 7 (n = 2), exon 8 (n = 1) and a microdeletion of NFIX (n = 1) (Fig. 4). Table 4 presents a summary of phenotypic differences between syndromes.
Figure 4.
NFIX variants of Marshall–Smith and Malan syndrome. NFIX figure adapted from Priolo et al. (2018) with variants in Marshall–Smith syndrome (above the gene) and Malan syndrome (underneath the gene). The colour legend shows missense, nonsense, ins/dels, splicing and intragenic deletions. Recurring variants are reported with additional circles.
Table 4.
Key phenotypic characteristics in Marshall–Smith syndrome and Malan syndrome in current study population
| Characteristic | Marshall–Smith syndrome | Malan syndrome |
|---|---|---|
| Sample age | Mean (SD): 8.4 (5.8) years | Mean (SD): 14.6 (6.7) years |
| Cognition | Mean age-equivalent: 15.8 months | Mean age-equivalent: 39.5 months |
| Adaptive behaviour skills | Range age-equivalents: 32 months | 10–Range age-equivalents: 17–36 months |
| Sensory processing (>50% of sample with DefiniteLow energy/Weak; Visual/Auditory sensitivity Tactile sensitivity; movement sensitivity; Low Difference) | ||
| Speech/language | Present in 2/8 participants | Present in 7/7 participants |
| Social | Self-absorbed, seeking physical contact | Reciprocal interaction |
| Other characteristics | Rigidity, stereotypic behaviour | Retrieval problems |
Discussion
We compared developmental and behavioural phenotypes of Marshall–Smith syndrome and Malan syndrome, both caused by changes of NFIX, but with clear differences in consequences of variants causing one syndrome or the other (Priolo et al. 2018). We studied (long-term) cognition, adaptive behaviour and sensory processing, finding significant differences between syndromes. Participants with Marshall–Smith syndrome show more severe ID, less adaptive behaviour skills, more impaired speech and language, and less reciprocal social interaction when compared with participants with Malan syndrome. Follow-up measurements on cognition and adaptive functioning in Marshall–Smith syndrome (n = 3) revealed considerable variance in learning curves over time. Here, we discuss five areas of interest: cognition (Bayley-III-SNA and WPSSI-III), adaptive behaviour (Vineland-2), sensory processing (SSP-NL), behavioural observations and genetic findings.
Cognition
Results on cognition confirm previous studies on level of ID (Van Balkom et al. 2011; Klaassens et al. 2015) and provide additional insight into cognitive development in Marshall–Smith syndrome and cognitive strengths and weaknesses within Malan syndrome. Earlier reports described a moderate-profound level of ID in Marshall–Smith syndrome (Shaw et al. 2010) and cognitive developmental ages between 7 and 31 months (Van Balkom et al. 2011). Current findings (Fig. 1) display cognitive developmental progression over time in one participant (MSS003), one (MSS001) with same level of cognitive functioning despite increased age and one (MSS002) with a decrease in cognitive developmental age. The decrease in developmental age of participant MSS002 is possibly due to measuring with the WPSSI-III vs. the Bayley Scales of Infant Development-II (BSID-II) during previous measurement. The BSID-II uses attractive, playful materials and preschool tasks, in contrast to the more school-task oriented WPPSI-III. Earlier studies (Van Balkom et al. 2011) reported the preference for toys and/or materials, which stimulate several senses at the same time. It is likely that attractive materials improve motivation, leading to better results. Furthermore, the BSID-II makes less use of spoken language than the WPPSI-III, possibly relevant when taking severe speech and language difficulties in Marshall–Smith syndrome into account.
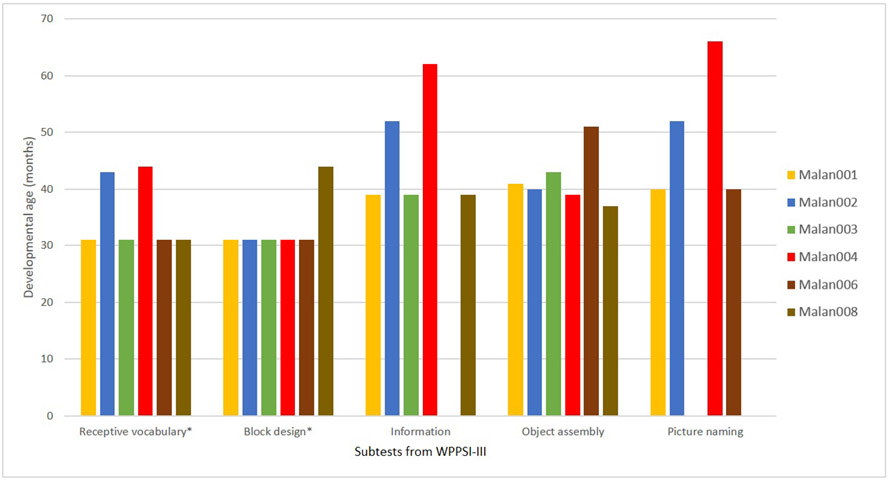
Current results on cognitive functioning in Malan syndrome concur with previously reported levels of mild to severe ID (Klaassens et al. 2015; Priolo et al. 2018). Our findings (Fig. 2) show considerable differences between subtests of the WPPSI-III. Results on subtest Receptive Vocabulary are lower compared with the subtest Picture Naming. It is possible that results on Receptive Vocabulary (e.g. auditory memory, auditory and visual discrimination) are lowered by the high demand placed on simultaneous processing (discrimination and integration) of verbal and visual input and working memory in contrast to less complex tasks on Picture Naming (e.g. expressive language skills, the ability to connect visual stimuli with language) (Hendriksen and Hurks 2009). Both subtests include concepts of perceptual learning (such as visual and auditory discrimination). Perceptual learning enables us to make sense of what we see, hear, feel, taste or smell (Gold and Watanabe 2010). Difficulties in perceptual learning affect complex cognitive processes such as language acquisition (Gervain and Mehler 2010), which may explain reported language difficulties (Malan et al. 2010). Individuals with Malan syndrome need an environment, which provides clear (augmentative) communication to support understanding and enhance daily functioning. Other marked differences are the lower results on the subtest Block design compared with Object assembly. Block design measures visuospatial perception and visuomotor coordination of abstract and meaningless visual information, while Object assembly measures visual-perceptual organisation of meaningful stimuli (Hendriksen and Hurks 2009). Visual motor integration is the ability to well-coordinate visual perception and finger-hand movements (Beery and Beery 2010). Visual perception, defined as the total process for reception and cognition of visual stimuli, facilitates extracting, structuring and interpreting visual stimuli, giving meaning to what is seen. Individuals with Malan syndrome may benefit from the use of occupational therapy directed to use all senses, adaptation and presentation of materials in an organised way and linking them to what they already know (zone of proximal development; Vygotsky 1978). This would reduce activity limitations and enhance participation in everyday activities (Schneck 2001).
Figure 2.
Age equivalents of cognitive development in Malan syndrome. Age equivalents of cognitive development in Malan syndrome assessed with the WPPSI-III. Asterisks (*) denote that both subtests have a minimum score of 31 months according the manual. All participants with a score of 31 months have an age-equivalent < 31.
Adaptive behaviour
Results on adaptive behaviour show an overall lower level of functioning in Marshal–Smith syndrome
(n = 8) than in Malan syndrome (n = 7), corresponding with expectations based on cognitive development. The Communication domain was the most affected for both syndromes. Daily Living Skills are relatively well-developed in both syndromes, in contrast to an earlier study in which Daily Living Skills was reported as the weakest domain in Marshall–Smith syndrome (Van Balkom et al. 2011). Slight improvement in several subdomains of Daily Living Skills over time (n = 3) suggests development follows an individual learning curve. This may also be the case in Malan syndrome and could be explored with follow-up assessments.
The apparent decrease in communication abilities in Marshall–Smith syndrome (n = 3) over time is notable. Van Balkom et al. (2011) reported improvement at follow-up measurement after 24 months, whereas in this study, 78 months later, a decline in communicative functioning seems present. Impaired results on the Communication domain might be explained by higher (social) demands from the environment during aging and increased change and unpredictability in life during adolescence (as was the case in MSS001 and MSS002). It might be possible that these changes during aging influence development, daily functioning and communicative abilities. Future follow-up may further delineate and elucidate possible age-dependent vulnerability in development as reported in studies on age-related adaptive and executive functioning in Cornelia de Lange syndrome (Srivastava et al. 2014; Reid et al. 2017).
Participants with Malan syndrome showed expressive language difficulties during direct in-person assessment. They sometimes seemed to know the answer but appeared unable to retrieve the right information from their long-term (verbal) memory based on verbal cues only and also had difficulties pronouncing words. Supportive gestures or showing pictures resulted in increased correct answers, suggesting that augmentative and alternative communication might be helpful. Receptive language skills in Malan syndrome may depend on the way verbal information is offered, understanding may increase by combining verbal and visual stimuli. We think assessing level of sense-making in communication (Maljaars et al. 2012) in both syndromes is helpful, for example with the ComFor-2 (Noens et al. 2006), to be able to adequately meet individual needs for augmentative and alternative communication to support perceptual learning (Mulder et al. 2016).
Social adaptive behaviour skills are less well developed compared with communication and daily living skills. This contrasts with earlier findings in Marshall–Smith syndrome (n = 3) (Van Balkom et al. 2011) and could possibly indicate that the learning curve of socialisation flattens and reaches its peak, while the curve of Daily Living Skills gradually progresses over a longer time-period. Continuous investment in social interaction and social play seems of great importance for overall development in both syndromes. Play stimulates cognitive, social, communicative and emotional development of children (Vygotsky 1978; Jordan 2003; Pellegrini 2009). During direct in-person assessments, we noted that attractive, playful materials supported joint attention and (re)directed attention towards the tasks.
Follow-up measurement in Marshall–Smith syndrome revealed some preliminary notable trends in adaptive development. Several hypotheses might explain these findings. First, we used an updated, more extensive version (Vineland-2) of the Vineland Adaptive Behaviour Scales compared with earlier assessment (Van Balkom et al. 2011). Adaptive skills are divided into smaller developmental steps, and the scoring system is more precise (current five-points Likert-scale compared to a three-points Likert scale used previously), which may lead to lower results, although probably also indicating more precisely the zone of proximal development. The more precise indication of level of development enhances the possibility to adapt (expectations from) the environment and set more feasible goals for further development. Second, biological changes (especially during puberty/adolescence) may impede development due to concomitant physical and/or psychological problems. Changes in cognitive functioning, mood and increased anxiety possibly related to biological changes with aging were previously discussed in Cornelia de Lange syndrome (Nelson et al. 2014; Reid et al. 2017), which might also be the case in Marshall–Smith syndrome. Third, the lack of development and difficulties in acquiring and using daily skills may have become more apparent through the years, possibly influencing the parents’ perspective on the development of their child, leading to the current lower scores on the Vineland-2.
Sensory processing
Sensory processing difficulties (e.g. sensitivity in visual and auditory stimuli and tactile sensitivity) are present in both syndromes. Behavioural responses to sensory stimuli were previously reported (Shaw et al. 2010; Priolo et al. 2018). Here, a difference between syndromes was tactile sensitivity.
Participants with Marshall–Smith syndrome showed for example more tactile exploration of materials (e.g. putting object against mouth). Environmental stimuli (such as noises and movement) often disrupted task-performance; recapturing attention was easier in individuals with Malan syndrome. Sensory difficulties hamper adequate adaptive responses to environment and participation in daily activities, applying environmental adaptations might prevent sensory overstimulation or understimulation (Baker et al. 2008; Schaaf et al. 2011). We applied some adaptations in direct in-person assessments to meet sensory needs of participants in an effort to assess their best abilities (e.g. assessment in familiar environment, close curtains and use of preferred toys). Studying sensory processing as part of the individual’s developmental profile following a dedicated assessment battery is important (Mulder et al. 2016, 2019). Addressing individual sensory needs (e.g. activation by use of colourful materials or reducing environmental stimuli) prevents overstimulation or understimulation, thereby enhancing participation, learning and daily functioning (Engel-Yeger et al. 2011).
Behavioural observations
Behavioural observations showed marked different behavioural features in social interaction in Marshall–Smith syndrome compared with Malan syndrome. In contrast, earlier reports described socialisation as a relative strength in Marshall–Smith syndrome when compared with other adaptive abilities, although also noting prominent deficits in communication and social interaction during direct in-person assessments (Van Balkom et al. 2011). No participants showed overt anxiety, although anxiety was previously observed in Malan syndrome (Priolo et al. 2018). Nevertheless, parents and carers did report restlessness and excitement in participants before assessments, possibly due to anticipation stress. Once assessments started, participants became more at ease. Allowing some time to get used to the situation, proximity of a familiar person, and quick clarity on proceedings proved helpful.
Strengths and limitations
The present study has several strengths and limitations. A major strength is the direct in-person assessment of cognition in each participant. Second, by applying some adaptations in test procedures to increase the individual’s task-oriented behaviour, we improved the possibility of measuring the optimal abilities of the participant. Third, we compared development and behaviour of both syndromes on group level and provided individual descriptions. These case descriptions revealed different needs and preferences within syndromes, requiring tailored assessment, care and support. Fourth, parents received a report of the assessments to discuss with their own healthcare professionals. Detailed description of the individual developmental profile also enables adjustment of parental expectations when necessary, improves understanding of environmental influences and essential adjustments to fit abilities and needs. Fifth, the description of development and behaviour presented with genetic findings increases understanding of developmental and behavioural similarities and differences of individuals with NFIX variants on group level (Table 4), with specific information provided on individual level (Figs 1-4). Results support previous proposition to consider Marshall–Smith syndrome and Malan syndrome as two separate entities (Malan et al. 2010; Priolo et al. 2018); however, future studies of new variants of NFIX might reveal overlapping phenotypic features resembling both entities (Priolo et al. 2018).
First limitation is the small sample in absolute terms, although it is relatively large given the rarity of these syndromes. Second is the lack of appropriate instruments to directly measure cognition in severe ID. We considered Bayley-III-SNA and WPPSI-III to be the most suitable instruments based on a priori clinical impression (based on available literature indicating developmental delay and difficulties on several domains) and applied some adaptations appropriate to individual needs. Included adaptations are allowing more time for a task, using materials of interest instead of prescribed materials (e.g. shimmering vs. non-shimmering ball) to increase motivation and attention, perform assessments in a for the participant familiar environment (e.g. at home or day-care centre) and use extra non-verbal communication (e.g. gestures) to support instructions. We are aware that deviation from standard procedures has consequences for interpretation of results and follow-up measurements, though they yielded important additional information necessary for motivation, task oriented behaviour and interaction. Third, comparisons of Vineland results were problematic. No reports of adaptive behaviour in Malan syndrome exist. Because of the use of the updated version of the instrument, we could only compare current findings on domain-level in Marshall–Smith syndrome as subdomain scores are not available in previous results (Van Balkom et al. 2011). Future follow-up measures with the same instrument may clarify learning curves and possible age-dependent vulnerability in development as previously seen in adaptive behaviour and executive functioning in Cornelia de Lange syndrome (Srivastava et al. 2014; Reid et al. 2017). Finally, while not designed to study sensory processing in severe ID, we found the SSP useful to measure and describe sensory difficulties. Continued use of this instrument in future studies of individuals with (severe) ID will improve comparability and understanding of the development of sensory processing and inform necessary (environmental) adaptations during the lifespan.
Recommendations
The results of our study indicate the following five recommendations:
Usual measures to assess cognitive and developmental functioning have clear limitations when used in syndromes with (severe) multiple disabilities. However, adapting environment and procedure to enhance motivation and task-orientedness can likely lead to more realistic outcomes of individual capacities.
Adaptive functioning may progress very slowly and continued investment in development of adaptive skills by using a predictable, step-by-step method with attractive materials and playful activities is important.
Future studies should reassess adaptive behaviour skills over time in order to understand developmental trajectories within syndromes and identify what might be necessary and beneficial to encourage development and daily functioning.
Understanding issues in sensory processing is key to inform parents/carers to address sensory needs and adapt the environment to optimise daily functioning.
Future research should consider publishing detailed case descriptions of performed assessments.
This increases awareness of developmental and behavioural variability within syndromes and demonstrates the need for tailored approaches. Offering parents/carers separate individual reports with results of the assessments can highlight helpful approaches for care and support, so that participants and their families may also benefit directly from participating in scientific studies.
Conclusion
Comparison of developmental and behavioural phenotypes and presenting results with genetic findings in Marshall–Smith and Malan syndrome, both caused by NFIX changes, shows significant between and within syndrome variability. This supports the hypothesis that different NFIX variants underlie distinct clinical phenotypes leading to separate entities. Cognitive, adaptive and sensory impairments are common in both syndromes and these hamper development, social participation, and increase the risk for emergence of challenging behaviour. This study highlights the importance of direct in-person assessments and the need to consider behaviour within one’s own developmental and environmental context. Use of a dedicated standard of instruments improves comparability over time. The methodology used in this study can be applied cross-syndromic, and results are indicative of essential information to be found in other syndromes. We suggest adaptations to environment, support and treatment to create a better person-environment fit and improve quality of life.
Supplementary Material
Data S1.Psychometric properties of test-battery
Table S1 Developmental and Behavioural Characteristics in Individuals with Marshall-Smith syndrome
Table S2 Developmental and Behavioural Characteristics in Dutch Individuals with Malan syndrome.
Acknowledgements
We thank the participants, their families and the Marshall-Smith Syndrome World Federation (for recruitment) who have made this research possible.
Source of Funding
GM was supported by National Institute of Neurological Disorders and Stroke (NINDS) (K08NS092898).
Footnotes
Conflict of Interest
W. Dunn is the author of the Sensory Profile and receives royalties for its sale. Pearson Publishing owns the copyright for this assessment.
Supporting Information
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- Baker AEZ, Lane A, Angley MT & Young RL (2008) The relationship between sensory processing patterns and behavioural responsiveness in autistic disorder: a pilot study. Journal of Autism and Developmental Disorders 38, 867–75. [DOI] [PubMed] [Google Scholar]
- Beery KE & Beery NA (2010) The Beery-Buktenica Developmental Test of Visual-Motor Integration (Beery VMI): Administration, Scoring, and Teaching Manual, 6th edn, pp. 11–20. NCS Pearson, Minneapolis, MN. [Google Scholar]
- Carnaby S (ed) (2009) Clinical assessment of people with profound intellectual and multiple disabilities. In: Profound Intellectual and Multiple Disabilities: Nursing Complex Needs (eds. Pawlyn J & Carnaby S), pp. 98–110. Blackwell Publishing, Oxford, United Kingdom. [Google Scholar]
- Engel-Yeger B, Hardal-Nasser R & Gal E (2011) Sensory processing dysfunctions as expressed among children with different severities of intellectual developmental disabilities. Research in Developmental Disabilities 32, 1770–5. [DOI] [PubMed] [Google Scholar]
- Gervain J & Mehler J (2010) Speech perception and language acquisition in the first year of life. Annual Review of Psychology 61, 191–218. [DOI] [PubMed] [Google Scholar]
- Gold JI & Watanabe T (2010) Perceptual learning. Current Biology 20, 46–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriksen JGM & Hurks PPM (2009) Technische handleiding WPPSI-III-NL. Pearson Assessment and Information B.V, Amsterdam. [Google Scholar]
- Huisman SA, Mulder PA, Redeker E, Bader I, Bisgaard AM, Brooks A et al. (2017) Phenotypes and genotypes in individuals with SMC1A variants. American Journal of Medical Genetics. Part A 173A, 2108–25. [DOI] [PubMed] [Google Scholar]
- Jordan R (2003) Social play and autistic spectrum disorders: a perspective on theory, implications and educational approaches. Autism 7, 347–60. [DOI] [PubMed] [Google Scholar]
- Klaassens M, Morrogh D, Rosser EM, Jaffer F, Vreeburg M, Bok LA et al. (2015) Malan syndrome: Sotos-like overgrowth with de novo NFIX sequence variants and deletions in six new patients and a review of the literature. European Journal of Human Genetics 23, 610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist L (2013) Prevalence and risk markers of behavior problems among adults with intellectual disabilities: a total population study in Orebro county, Sweden. Research in Developmental Disabilities 34, 1346–56. [DOI] [PubMed] [Google Scholar]
- Malan V, Rajan D, Thomas S, Shaw AC, Louis Dit Picard H, Layet V et al. (2010) Distinct effects of allelic NFIX mutations on nonsense-mediated mRNA decay engender either a Sotos-like or a Marshall-Smith syndrome. American Journal of Human Genetics 87, 189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maljaars J, Noens I, Scholte E & van Berckelaer-Onnes I (2012) Level of sense-making in children with autistic disorder and intellectual disability: patterns of delay and deviance in development. Research in Autism Spectrum Disorders 6, 806–14. [Google Scholar]
- Marshall RE, Graham CB, Scott CR & Smith DW (1971) Syndrome of accelerated skeletal maturation and relative failure to thrive: a newly recognized clinical growth disorder. Journal of Pediatrics 78, 95–101. [DOI] [PubMed] [Google Scholar]
- Mulder PA, Huisman S, Landlust AM, Moss J, SMC1A Consortium, Piening S et al. (2019) Development, behaviour and autism in Individuals with SMC1A Variants. Journal of Child Psychology and Psychiatry 60, 305–13. [DOI] [PubMed] [Google Scholar]
- Mulder PA, Huisman SA, Hennekam RC, Oliver C, van Balkom IDC & Piening S (2016) Behaviour in Cornelia de Lange syndrome: a systematic review. Developmental Medicine and Child Neurology 59, 361–6. [DOI] [PubMed] [Google Scholar]
- Nelson L, Moss J & Oliver C (2014) A longitudinal follow-up study of affect in children and adults with Cornelia de Lange syndrome. American Journal on Intellectual and Developmental Disabilities 119, 235–52. [DOI] [PubMed] [Google Scholar]
- Noens I, Van Berckelaer-Onnes I, Verpoorten R & Van Duijn G (2006) The ComFor: an instrument for the indication of augmentative communication. Journal of Intellectual Disability Research 50, 621–32. [DOI] [PubMed] [Google Scholar]
- Orphanet (2020a) Malan overgrowth syndrome. Orphanet ecyclopedia. Available at: https://www.orpha.net/consor/cgi-bin/Disease_Search.php?lng=EN%26data_id=23101%26Disease%20name=Malan-overgrowth-syndrome%26search=Disease_Search_Simple%26title=Malan%20overgrowth%20syndrome (retrieved February 2020). [Google Scholar]
- Orphanet (2020b) Marshall-Smith syndrome. Orphanet encyclopedia. Available at: https://www.orpha.net/consor/cgi-bin/Disease_Search.php?lng=EN%26data_id=2279%26Disease_Disease_Search_diseaseGroup=Marshall-Smith%26Disease_Disease_Search_diseaseType=Pat%26Disease(s)/group%20of%20diseases=Marshall-Smith-syndrome%26title=Marshall-Smith%20syndrome%26search=Disease_Search_Simple (retrieved February 2020). [Google Scholar]
- Pellegrini AD (2009) The Role of Play in Human Development. Oxford University Press, New York. [Google Scholar]
- Priolo M, Schanze D, Tatton-Brown K, Mulder PA, Tenorio T, Kooblall K et al. (2018) Further delineation of Malan syndrome. Human Mutation 39, 1226–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid D, Moss J, Nelson L, Groves L & Oliver C (2017) Executive functioning in Cornelia de Lange syndrome: domain asynchrony and age-related performance. Journal of Neurodevelopmental Disorders 9, 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietman A (2013) Sensory Profile-NL. Handleiding. Pearson Assessment and Information, Amsterdam. [Google Scholar]
- Ruiter SAJ, Visser L, van der Meulen BF & Timmerman ME (2014) Bayley Scales of Infant and Toddler Development – Derde Editie, Nederlandstalige bewerking, Special Needs Addition. Pearson Assessment and Information B.V, Amsterdam. [Google Scholar]
- Schaaf RC, Toth-Cohen S, Johnson SL, Outten G & Benevides TW (2011) The everyday routines of families of children with autism: examining the impact of sensory processing difficulties on the family. Autism: The International Journal of Research and Practice 15, 373–89. [DOI] [PubMed] [Google Scholar]
- Schneck CM (2001) In: Visual Perception. Occupational Therapy for Children (ed. Case-Smith J), 4th edn, pp. 382–412. Mosby, New South Wales, Australia. [Google Scholar]
- Shaw AC, Van Balkom ID, Bauer M, Cole TR, Delrue MA, Van Haeringen A et al. (2010) Phenotype and natural history in Marshall–Smith syndrome. American Journal of Medical Genetics 152A, 2714–26. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti VD & Balla AD (2008) Vineland Adaptive Behaviour Scales, 2nd edn. American Guidance Service; Circle Pines, Circle Pines, MN. [Google Scholar]
- Srivastava S, Landy-Schmitt C, Clark B, Kline AD, Specht M & Grados MA (2014) Autism traits in children and adolescents with Cornelia de Lange syndrome. American Journal of Medical Genetics. Part A 321A, 1400–10. [DOI] [PubMed] [Google Scholar]
- Tatton-Brown K & Weksberg R (2013) Molecular mechanisms of childhood overgrowth syndromes. American Journal of Medical Genetics Part C Seminars in Medical Genetics 163C, 71–5. [DOI] [PubMed] [Google Scholar]
- Van Balkom ID, Shaw A, Vuijk PJ, Franssens M, Hoek HW & Hennekam RC (2011) Development and behaviour in Marshall-Smith syndrome: an exploratory study of cognition, phenotype and autism. Journal of Intellectual Disability Research 55, 973–87. [DOI] [PubMed] [Google Scholar]
- Vygotsky LS (1978) Mind in Society: The Development of Higher Psychological Processes. Harvard University Press, Harvard. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.Psychometric properties of test-battery
Table S1 Developmental and Behavioural Characteristics in Individuals with Marshall-Smith syndrome
Table S2 Developmental and Behavioural Characteristics in Dutch Individuals with Malan syndrome.