Summary
Background
WHO recommends that men who have sex with men (MSM) receive gonorrhoea and chlamydia testing, but many evidence-based preventive services are unaffordable. The pay-it-forward strategy offers an individual a gift (eg, a test for sexually transmitted diseases) and then asks whether they would like to give a gift (eg, a future test) to another person. This study examined the effectiveness of a pay-it-forward programme to increase gonorrhoea and chlamydia testing among MSM in China.
Methods
We did a randomised controlled superiority trial at three HIV testing sites run by MSM community-based organisations in Guangzhou and Beijing, China. We included MSM aged 16 years or older who were seeking HIV testing and met indications for gonorrhoea and chlamydia testing. Restricted randomisation was done using computer-generated permuted blocks. 30 groups were randomised into three arms (1:1:1): a pay-it-forward arm in which men were offered free gonorrhoea and chlamydia testing and then asked whether they would like to donate for testing of prospective participants, a pay-what-you-want arm in which men were offered free testing and given the option to pay any desired amount for the test, and a standard-of-care arm in which testing was offered at ¥150 (US$22). There was no masking to arm assignment. The primary outcome was gonorrhoea and chlamydia test uptake ascertained by administrative records. We used generalised estimating equations to estimate intervention effects with one-sided 95% CIs and a prespecified superiority margin of 20%. The trial is registered with ClinicalTrials.gov, NCT03741725.
Findings
Between Dec 8, 2018, and Jan 19, 2019, 301 men were recruited and included in the analysis. 101 were randomly assigned to the pay-it-forward group, 100 to the pay-what-you-want group, and 100 to the standard-of-care group. Test uptake for gonorrhoea and chlamydia was 56% (57 of 101 participants) in the pay-it-forward arm, 46% (46 of 100 participants) in the pay-what-you-want arm, and 18% (18 of 100 participants) in the standard-of-care arm. The estimated difference in test uptake between the pay-it-forward and standard-of-care group was 38·4% (95% CI lower bound 28·4%). Among men in the pay-it-forward arm, 54 of 57 (95%) chose to donate to support testing for others.
Interpretation
The pay-it-forward strategy can increase gonorrhoea and chlamydia testing uptake among Chinese MSM and could be a useful tool for scaling up preventive services that carry a mandatory fee.
Funding
US National Institute of Health; Special Programme for Research and Training in Tropical Diseases, sponsored by UNICEF, UNDP, World Bank, and WHO; the National Key Research and Development Program of China; Doris Duke Charitable Foundation; and Social Entrepreneurship to Spur Health.
Introduction
Many evidence-based preventive services are not affordable for individuals in resource-limited settings.1,2 Despite recommendations from WHO and others to make health care universally accessible,3 individuals routinely pay out-of-pocket fees for vaccines, drugs, and diagnostics.4 Mandatory fees decrease health service utilisation and reduce equitable access by disproportionately affecting the poor.5–7 Public sector programmes that subsidise preventive services are under increasing financial strain8 and altering prices is difficult.9 Programmes to reduce fees associated with preventive services have not been scaled up.4,10 Innovative strategies are needed to expand access to preventive services.
One novel strategy for promoting service uptake in health care is the pay-it-forward health services provision model.11 With pay-it-forward, individuals receive a gift and are then asked whether they would like to give a similar gift to another person.12 A single-city observational study11 used pay-it-forward to have men who have sex with men (MSM) receive a free gonorrhoea and chlamydia test. Then each participant decided whether to donate towards the test of the next person. This study found that the pay-it-forward approach substantially increased gonorrhoea and chlamydia testing among MSM.11 Pay-it-forward changes the conventional transactional exchange between buyer and seller to a social exchange between gift receivers and givers.13 This approach could increase the trust and community engagement in health services, which has been associated with sexually transmitted infection (STI) test uptake.14
Dual gonorrhoea and chlamydia tests are available in many Chinese hospitals for approximately US$22.15 Testing rates among Chinese MSM are low despite gonorrhoea and chlamydia infections being highly prevalent (12·5% for gonorrhoea and 18·1% for chlamydia, including urethral, rectal, and pharyngeal sites), often asymptomatic, and associated with an increased risk of HIV transmission and acquisition.16–18 Pay-it-forward could reduce financial barriers to testing while engaging local MSM communities.
The purpose of this multisite, three-arm, randomised controlled trial (RCT) is to evaluate the effectiveness of a pay-it-forward model for increasing dual gonorrhoea and chlamydia test uptake among Chinese MSM compared with a standard fee-based system. The primary outcome is uptake of dual gonorrhoea and chlamydia testing. The secondary objective of this study is to evaluate the cost-effectiveness of the interventions by comparison with the standard of care.
Methods
Study design and participants
We undertook this multisite RCT in two Chinese cities: Guangzhou (two sites in hospital-based STI clinics) and Beijing (in a community-based organisation). All sites offered free HIV testing and were run by MSM community-based organisations (Zhitong Guangzhou LGBT Center, Guangzhou; and Blued, Beijing) as a common service delivery mode in China.19 HIV testing was done using a third-generation HIV rapid test (InTec Products, Xiamen, China). We chose these sites because they already provided HIV testing services, included laypeople (ie, no physicians), and were affiliated with a local community-based organisation, which is common for HIV testing service delivery in China for key populations. These factors provide a strong foundation for the pay-it-forward strategy and makes our research findings more generalisable. More details about the study setting are available in the study protocol.20
We used a group-based RCT design on the basis of the following reasons: first, the intervention was framed as a group-based intervention in which donations from more than one MSM supported testing costs. Second, research studies in China suggest that peer influences on HIV test uptake are important,21,22 and that men are significantly more likely to receive HIV testing when accompanied by a partner than when being alone.23 In our study, we assigned partners to the same group, appreciating these social influences. Finally, our study was designed as a pragmatic trial to be relevant to other community-based sites that deliver HIV testing services. After discussions with our community partners, there was agreement that an individual-based RCT would interfere with normal clinical service provision and not be feasible in a real-world setting.
We recruited men who were seeking HIV testing at the study sites. Participants were eligible if they were born biologically male, were aged 16 years or older, ever had anal sex with a man, had not been tested for gonorrhoea and chlamydia in the past 12 months, and were willing to provide a cell phone number or WeChat ID for results notification. All participants provided written informed consent.
The study was approved by the ethics review committees of the Southern Medical University Dermatology Hospital (China), University of North Carolina at Chapel Hill (USA), and Yale University (USA). We reported our findings according to the CONSORT cluster-extension guidelines (appendix 2 pp 28–30).24
Randomisation and masking
TPZ, FY, WH, AL, and YW enrolled participants at the three sites and assigned participants on the basis of a predetermined allocation sequence. Groups of ten men were randomly assigned (1:1:1) to one of the three study arms: pay-it-forward, pay-what-you-want, or standard of care. A group was defined as a group of ten eligible men that arrived in order at the study site and agreed to participate. We chose a group size of ten on the basis of sample size calculation and implementation consideration (appendix 2 pp 24–26). All men in the same group were assigned to the same study arm. Men who presented together with their partners were assigned to the same group as their partners.
We generated the randomisation sequence using Stata (version 15) software before recruitment (appendix 2 p 27).25 Three groups, one from each arm, were bundled as a triplet and permutated within triplets to ensure balanced arms (1:1:1) at each site. After randomisation, sites with a high volume of men visiting to test for HIV received more triplets, so that the study period remained relatively the same across the sites. Study organisers and participants were not masked to arm assignment.
Procedures
The pay-it-forward programme was developed using crowdsourcing to solicit community input.11 Crowdsourcing is a practice in which a group solves a problem and then shares the solutions with the community.26 First, programme procedures were designed through an iterative consultation process with community partners (including staff members from community-based organisations, the co-authors WZ, DL, and GM, and volunteers) and were piloted at each of the three study sites. The pilot included a total of 43 men, and on the basis of its results, community partners and study staff optimised the standard operating procedure.20 Second, the name of the programme in Chinese was crowdsourced from the public using an open challenge contest.27 Third, postcards with hand-written notes from earlier participants were presented to subsequent participants in the pay-it-forward arm. Figure 1 shows the key concepts of the pay-it-forward and pay-what-you-want models applied in gonorrhoea and chlamydia testing.
Figure 1: Concepts of standard of care, pay-it-forward, and pay-what-you-want gonorrhoea and chlamydia testing.
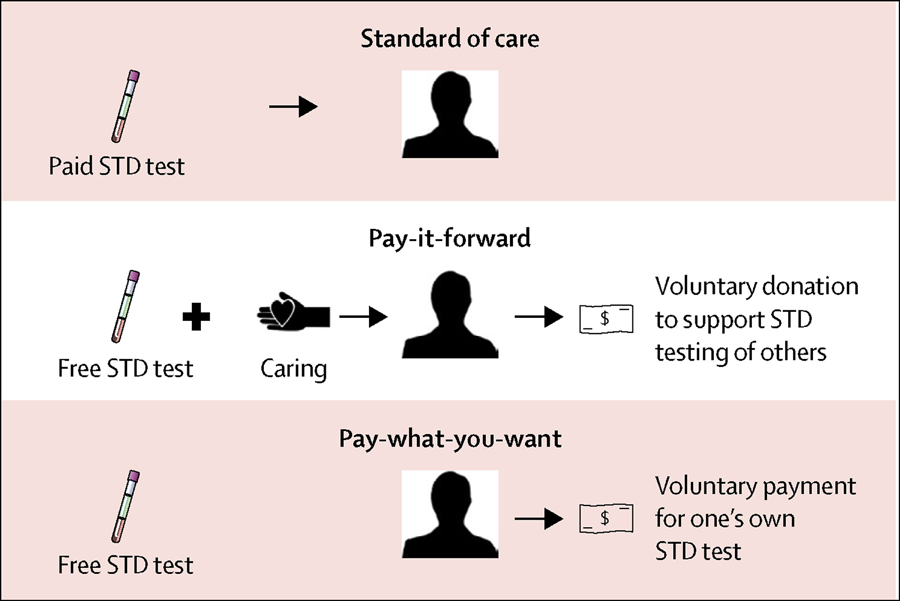
This schematic illustrates the trial arms from the perspective of a participant. In the standard-of-care arm, the participant was offered a test at a standard price (US$22). In the pay-it-forward arm, the participant was offered a free test, told that previous men donated to make this test possible, and shown postcards written by these previous men (“caring”). Then, the participant was asked whether they would donate toward testing for prospective patients (“voluntary donation”). In the pay-what-you-want arm, the participant was offered a free test. Then, the participant was told that they could pay any desired amount for their own test (“voluntary payment”). STD=sexually transmitted disease.
Men in all three study arms were introduced to gonorrhoea and chlamydia testing and the study procedures of their respective study arm. Men in the pay-it-forward arm were told that the standard price of the gonorrhoea and chlamydia testing was ¥150 (US$22), and that previous participants who cared about them donated towards testing fees. Thus, men in the pay-it-forward arm received a free test, and then decided whether, and if so how much, to donate toward future testing for others. Participants were shown postcards and told that testing and donating were voluntary.
After being introduced to the testing and the study, men in the pay-what-you-want arm were told that the standard price of gonorrhoea and chlamydia testing was ¥150 (US$22), and that they would receive free testing and then decide the amount that they would like to pay for their own test.
Men in the standard-of-care arm received the same introduction to gonorrhoea and chlamydia testing than the men in the other two study arms. Then they were told that the standard price of gonorrhoea and chlamydia testing was ¥150 (US$22) and that they had to pay the full amount for their testing.
In all three study arms, men who decided to get tested were asked about their sexual practices and advised to consider urine, rectal, or both urine and rectal dual gonorrhoea and chlamydia testing. Their sample were immediately collected after they made the decision to do the test. All men were invited to complete a survey about their sexual history, testing history, and attitudes toward the testing programme, and towards the MSM community at each site (appendix 2 pp 2–7). Samples from all sites were transported to the Dermatology Hospital of the Southern Medical University laboratory in Guangzhou for nucleic acid amplification testing. We chose this test because of its great sensitivity and specificity, and its Chinese regulatory approval. Patients who tested positive were counselled and directed to the web page of the designated partner hospital in each city, where they would be able to make an appointment to receive treatment and follow up. Details on procedures for sample processing, payment method, and laboratory testing can be found elsewhere.20 The trial stopped once the predetermined sample size was reached.
Outcomes
The primary outcome was gonorrhoea and chlamydia test uptake immediately after the intervention, as assessed by administrative records. Secondary outcomes included incremental cost per test and incremental cost per diagnosis. We categorised costs into fixed and variable costs from a health-provider perspective and a time horizon within the trial. We first calculated the total economic cost for each study arm, then divided these costs by the number of men tested and by the number of new gonorrhoea or chlamydia cases detected in each study arm. We also report for each intervention the incremental cost-effectiveness ratios for cost per additional person tested and case identified. Details of the cost and cost-effectiveness analyses are summarised in the appendix 2 (pp 8–21). Other psychosocial outcomes investigated include community engagement,14 community connectedness,28,29 and social cohesion,30,31 measured using adapted scales that were piloted in the local context before the RCT.
Statistical analysis
We used descriptive analyses to examine sociodemographic characteristics of participants in each study arm. To account for potential correlations in outcomes within groups, generalised estimating equations modelling (GEE) was used to assess the population-averaged effect of the pay-it-forward and pay-what-you-want interventions on test uptake compared with standard of care. Correlation structure within groups was specified as equal correlation (ie, the exchangeable option on Stata). Additionally, the Huber–White Sandwich estimator of variance was used instead of the conventional variance estimator, so that the model estimates were robust even if the correlation structure was mis-specified. A binomial distribution was specified for test uptake with the identity link function to obtain the absolute difference in the proportions of test uptake. Key sociodemographic variables incorporated into the model as covariates included age as a continuous variable and study site as a nominal variable to account for potential confounding (for details, see appendix 2 p 23). A superiority margin of 0·2 (20% difference in probability of agreeing to test comparing interventions with standard of care) was prespecified as a clinically significant difference in gonorrhoea and chlamydia test uptake on the basis of what would be clinically relevant and a previous modelling study.32 The sample size has sufficient power (80%) to detect this difference (appendix 2 pp 24–26). For the test uptake proportion differences per study design using superiority by a margin test, one-sided 95% CIs were computed comparing pay-it-forward and pay-what-you-want respectively with standard of care, where the lower bounds were compared with the margin size of 0·2.
The costing analysis examined the full economic cost from a provider perspective using a micro-costing approach (appendix 2 p 7). The cost-effectiveness analysis used a decision tree model to compare the three study arms (appendix 2 p 7). This trial is registered with ClinicalTrials.gov, NCT03741725.
Role of funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Between Dec 8, 2018, and Jan 19, 2019, 431 men were screened for study eligibility. 15 men were deemed ineligible for having already participated in a pay-it-forward pilot study (n=6), having tested for gonorrhoea or chlamydia in the past 12 months (n=5), never having had anal sex with men (n=3), and not born biologically male (n=1). 115 eligible men declined to participate because of a lack of interest or a time conflict, resulting in a final sample of 301 men who were enrolled, assigned to arms, and included in analyses (101 men in the pay-it-forward group, 100 in the pay-what-you-want group, and 100 in the standard-of-care group). Figure 2 presents the study flow from recruitment to outcome assessment.
Figure 2: Study flow chart.
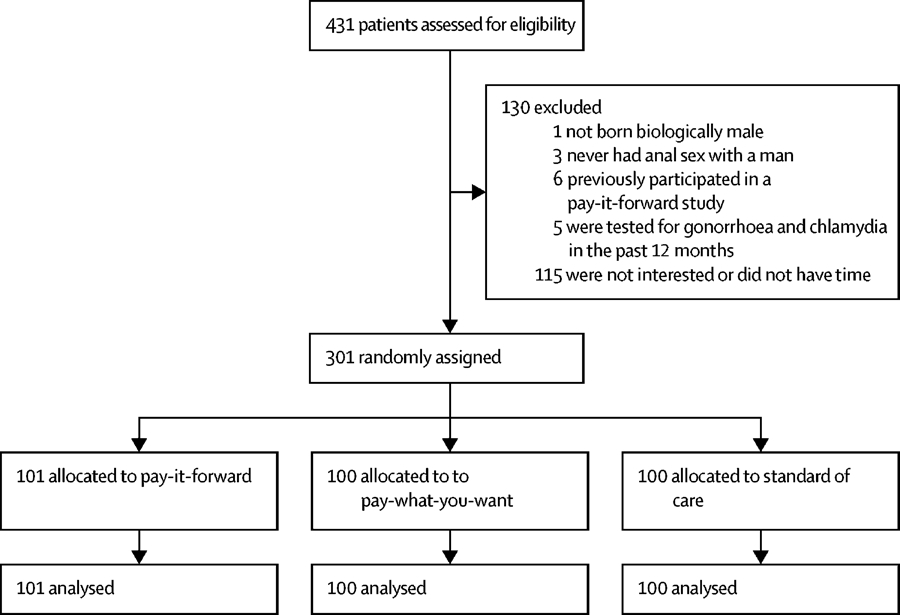
There is no loss to follow-up in this study; participants made decisions on whether or not to test immediately after being assigned to the study arms.
Table 1 presents the sociodemographic and sexual behaviour characteristics of participants by study arm. Overall, most men were 30 years old or younger, never married, and had a Bachelor or a higher educational degree (table 1). Their annual income varied, with 11·5% in the lowest category (<US$2680, converted from Chinese Yuan), and 38·2% in the highest category (>$14 294).
Table 1:
Sociodemographic and behavioural characteristics of patients*
| Total | Pay-it-forward | Pay-what-you-want | Standard of care | |
|---|---|---|---|---|
| Age (years) | ||||
| ≤30 >30 Mean (SD) |
206/288 (71.5) 82/288 (28.5) 281 (7.1) |
66/98 (67.3) 32/98 (32.7) 28.6 (7.8) |
74/93 (79.6) 19/93 (20.4) 26.6 (5.6) |
66/97 (68.0) 31/97 (32.0) 29.1 (7.5) |
| Marital status | ||||
| Never married Other† |
252/288 (87.5) 36/288 (12.5) |
87/98 (88.8) 11/98 (11.2) |
84/93 (90.3) 9/93 (9.7) |
81/97 (83.5) 16/9 (16.5) |
| Highest education | ||||
| Middle school or below High or vocational school College or above |
16/288 (5.6) 27/288 (9.4) 245/288 (85.0) |
5/98 (5.1) 8/98 (8.2) 85/98 (86.7) |
5/93 (5.4) 9/93 (9.7) 79/93 (84.9) |
6/97 (6.2) 10/97 (10.3) 81/97 (83.5) |
| Annual income (US$) | ||||
| <2680 2681–5360 5361–8934 8935–14294 –14294 |
33/288 (11.5) 26/288 (9.0) 43/288 (14.9) 76/288 (26.4) 110/288 (38.2) |
11/98 (11.2) 7/98 (7.1) 17/98 (17.4) 23/98 (23.5) 40/98 (40.8) |
13/93 (14.0) 9/93 (9.7) 15/93 (16.1) 23/93 (247) 33/93 (35.5) |
9/97 (9.3) 10/97 (10.3) 11/97 (11.3) 30/97 (30.9) 37/97 (38.2) |
| Number of sex partners in the past 3 months | ||||
| 0–1 Multiple |
136/278 (48.9) 142/278 (51.1) |
47/96 (49.0) 49/96 (51.0) |
47/91(51.6) 44/91 (48.4) |
42/91 (46.2) 49/91 (53.8) |
| Had anal sex in the past 3 months | ||||
| Yes No |
234/287 (81.5) 53/287 (18.5) |
34/98 (85.7) 14/98 (14.3) |
73/93 (78.5) 20/93 (21.5) |
77/96 (80.2) 19/96 (19.8) |
| Condom use frequency during anal sex in past 3 months ‡ | ||||
| Non-use Sometimes Often Always |
14/234 (6.0) 24/234 (10.2) 69/234 (29.5) 127/234 (54.3) |
5/84 (6.0) 6/84 (7.1) 21/84 (25.0) 52/84 (61.9) |
5/73 (6.9) 9/73 (12.3) 24/73 (32.9) 35/73 (47.9) |
4/77 (5.2) 9/77 (11.7) 24/77 (31.2) 40/77 (51.9) |
| HIV testing frequency in the past 2 years | ||||
| Never tested <Once every 2 years Once a year Every 6 months Every 3 months Monthly |
26/287 (9.1) 33/287 (11.5) 63/287 (21.9) 76/287 (26.5) 73/287 (25.4) 16/287 (5.6) |
6/98 (6.1) 15/98 (15.3) 23/98 (23.5) 26/98 (26.5) 21/98 (21.4) 7/98 (7.2) |
14/93 (15.0) 9/93 (9.7) 16/93 (17.2) 24/93 (25.8) 25/93 (26.9) 5/93 (5.4) |
6/96 (6.2) 9/96 (9.4) 24/96 (25.0) 26/96 (27.1) 27/96 (28.1) 4/96 (4.2) |
| Donation or payment amount (Chinese yuan) | ||||
| Median (IQR) | NA | 50 (20–60) | 20 (9–50) | NA |
Data are n/N (%), mean (SD), or median (IQR). NA=not applicable.
Among all 301 study participants, 288 chose to fill out the survey questionnaire.
Includes engaged, married, divorced, or separated.
Questions asked only to participants who reported having had anal sex in the past 3 months.
142 men (51·1%) reported that they had multiple sexual partners in the past 3 months, and 234 (81·5%) men reported having had anal sex in the past 3 months. Among these, 127 (54·3%) reported consistent condom use during anal sex in the past 3 months.
57 (56%) men in the pay-it-forward arm agreed to receive the gonorrhoea and chlamydia test, 46 (46%) in the pay-what-you-want arm, and 18 (18%) in the standard-of-care arm (table 2). The GEE output suggested that the pay-it-forward arm was associated with a 38% increase in test uptake probability when compared with the standard-of-care arm. This effect estimate comes with a one-sided 95% CI with a lower bound of 28%, which is greater than the 20% superiority margin (table 2), suggesting superiority of pay-it-forward over standard of care. After adjusting for participant age and site, this finding remained unchanged (probability difference 0·39, one-sided 95% CI lower bound 0·28).
Table 2:
Study arm participation and dual test uptake
| n/N (%) | Number of groups | Intraclass correlation | Probability difference* | 95% CI† | Adjusted probability difference‡ | Adjusted 95% CI†‡ | |
|---|---|---|---|---|---|---|---|
| Pay-it-forward | 57/101 (56%) | 10 | <0.0001 | 0.384 | 0.284 | 0.390 | 0.283 |
| Pay-what-you-want | 46/100 (46%) | 10 | 0.028 | 0.280 | 0.163 | 0.278 | 0.153 |
| Standard of care | 18/100 (18%) | 10 | 0.050 | NA | NA | NA | NA |
NA=not applicable.
The probability difference between the intervention arms (pay-it-forward or pay-what-you-want) and standard of care.
The lower bound one-sided 95% CI.
Adjusted for age and site.
Compared with standard of care, the pay-what-you-want intervention was associated with a 28% absolute increase in the proportion of men receiving a gonorrhoea and chlamydia test, with a lower bound one-sided 95% CI of 16% (table 2), which is less than the 20% superiority margin but still greater than 0. After adjusting for participant age and site, this finding also remained unchanged (risk difference 0·28, one-sided 95% CI lower bound 0·15). As in the pay-it-forward arm, age and testing site location were not significantly associated with the primary outcome (data not shown). Alternative multivariable models adjusting for additional covariates were tested and yielded similar results (appendix 2 p 23).
Among the 121 participants who tested for gonorrhoea and chlamydia (40·2%), five (4%) men had a gonorrhoea infection and 19 (16%) men had a chlamydia infection. Among all 301 men, seven (2·3%) had a positive test for HIV infection.
A complete cost and cost-effectiveness analysis is provided in the appendix 2 (pp 8–21). In summary, the total health provider economic cost (including start-up, test kits, staff time, overheads) for pay-it-forward ($1125) and for pay-what-you-want ($967) were higher than that of the standard of care ($612). Of the 57 men who received testing through the pay-it-forward arm, 54 (94·6%) chose to donate some amount toward testing of future participants. Among the 46 men who were tested through the pay-what-you-want approach, 42 (91%) paid some amount for the tests they received. The incremental cost-effectiveness ratio using economic costs per additional person tested was $12·68 for the pay-what-you-want approach compared with standard of care, and $14·27 for the pay-it-forward approach compared with the pay-what-you-want approach. The incremental cost-effectiveness ratio using economic costs was $12·96 per additional person tested for the pay-it-forward approach compared with standard of care. This finding underlines the benefits of pay-it-foward from a cost-effectiveness standpoint. The total donation amount was $473 and the average donation amount was $7·57 (SD $6·71). Key study procedures and findings were summarised in a video (appendix 2 p 22).
Discussion
The goal of this study was to assess the superiority of a pay-it-forward strategy to standard of care in promoting STI testing among MSM in China. We found that a pay-it-forward strategy increased STI testing and generated a substantial portion of costs associated with testing. This study extends the literature by using an RCT and suggests that pay-it-forward strategies might increase the uptake of screening services that would otherwise be associated with fees.
We found that men in the pay-it-forward arm had higher gonorrhoea and chlamydia test uptake. This finding is consistent with one observational study11 and some literature supporting pay-it-forward outside of health.12,13,33 This effect of pay-it-forward might be related to free testing or the specific context in which men knew that other men from their community cared about them, or both. The high rates of test uptake in the pay-what-you-want arm suggests that free testing itself might be responsible for a substantial portion of the test uptake effect. However, the specific context of receiving a generous gift is likely to facilitate implementation and build trust in the service.
We found that nearly all men who were offered pay-it-forward voluntarily chose to donate to testing for future patients. This pay-it-forward donation covered 42% ($473 of $1125) of the total economic cost for implementing pay-it-forward. The high donation rate and associated cost reduction suggest that pay-it-forward could help to extend existing preventive services. This cost reduction is particularly relevant to China and other low-income and middle-income countries, where few resources have been allocated to non-HIV STI prevention and related services.15,18 Donations from a pay-it-forward programme could allow more individuals to receive free or subsidised STI testing services. Pay-it-forward could be relevant in other settings in which groups of individuals pay mandatory fees for preventive services.
Our study has several limitations. First, the study was done in two metropolitan cities in China and making inferences to other settings should be done with caution. At the same time, there are many low-income and middle-income settings in which well-defined populations pay fees for preventive health services. Several aspects of the trial were designed to enhance generalisability to other community-based HIV testing sites: no doctors were involved in implementation, protocols were streamlined into routine services, and messaging was simplified. Future studies should investigate the transferability of using pay-it-forward to promote preventive service uptake in other resource-constrained settings. Although there are other examples of MSM community financing for health services,34 the potential for this approach to be integrated into existing health systems has not been explored.
A second limitation is that we evaluated this approach in a research context. We did not examine whether pay-it-forward might work in practice, although an earlier pragmatic study suggests that it could be implemented outside of research settings.11 Our cost-effectiveness analysis used a short-term time horizon and did not calculate the disability-adjusted life-years averted or quality-adjusted life-years gained. Therefore, our results are a conservative estimate of the probable benefit from the interventions, because earlier diagnosis and treatment of STIs could also reduce onward transmission of the STI to other sexual partners and reduce the morbidity from the STI. There is currently no consensus on the willingness to pay per additional person tested for chlamydia or gonorrhoea, or for an additional person diagnosed with chlamydia or gonorrhoea. However, one study of cost-utility of screening for chlamydia and gonorrhoea among MSM reported potential cost-effectiveness for screening.35
This study has implications for research and policy. From a research perspective, this study expands the scarce existing trial data examining the effectiveness of interventions related to behavioural economics and social innovation. Further RCTs and qualitative research studies will be important to understand the pay-it-forward mechanism of action and scalability. Our study might have generated a rewarding positive feeling from helping others,36 which seemed to inspire both participants and research staff, although this effect was not captured in our prespecified outcomes. In this study, men could donate money for tests of subsequent participants or write a simple postcard for other community members. Given that MSM are a marginalised population in China and many other settings in low-income and middle-income countries, programmes spurring social engagement, such as pay-it-forward, could potentially build collective agency and social cohesion. From a policy perspective, this intervention is not meant to replace public provision of STI testing services for subpopulations. However, this type of programme could be useful as a temporary measure to generate testing demand and build trust in new services, before the introduction of more comprehensive public-funded programmes.
In conclusion, pay-it-forward can increase gonorrhoea and chlamydia testing among Chinese MSM. Our study offers an innovative solution to supplementing testing services by using the power of the local community. Pay-it-forward could be a useful tool for the scale-up of preventative services that have out-of-pocket fees.
Supplementary Material
Research in context.
Evidence before this study
Gonorrhoea and chlamydia are common sexual transmitted infections among men who have sex with men (MSM) in many low-income and middle-income countries. However, there are few interventions focused on increasing gonorrhoea and chlamydia test uptake. We did a PubMed and Google Scholar search for studies reporting gonorrhoea and chlamydia testing in Chinese MSM that were published up to Nov 19, 2019, with the search terms “MSM”, “China”, and “testing” or “screening” or “intervention.” No language restrictions were applied. We identified three studies showing that gonorrhoea and chlamydia testing uptake are low among MSM in China. We found one observational study evaluating the effect of a pay-it-forward approach to increase gonorrhoea and chlamydia testing. We did not find any randomised controlled trials or costing studies that evaluate a pay-it-forward approach.
Added value of this study
This study examined a pay-it-forward model for gonorrhoea and chlamydia testing among Chinese MSM. We found that the pay-it-forward model had a higher test uptake than standard of care. The programme generated donations from local MSM and was cost effective. This study expands the literature by formally evaluating the pay-it-forward strategy using a randomised controlled trial.
Implications of all the available evidence
Our research study found that the pay-it-forward model increased gonorrhoea and chlamydia test uptake. The high rates of donating suggest substantial generosity, independent of income level. Pay-it-forward appears to be a promising strategy for integrating HIV and STI testing.
Acknowledgments
This study received support from the US National Institutes of Health (NIAID K24AI143471, NIA P30 AG034420, UL1TR002489, NIAID 5P30AI050410); the Special Programme for Research and Training in Tropical Diseases sponsored by UNICEF, UNDP, World Bank, and WHO; the National Key Research and Development Program of China (2017YFE0103800); Doris Duke Charitable Foundation; and the Social Entrepreneurship to Spur Health Global. The content is solely the responsibility of the authors and does not necessarily represent the official views of the US National Institute of Health. We thank all study participants, community-based organisation staff and volunteers, and staff members from the Social Entrepreneurship to Spur Health Global, Zhitong Guangzhou LGBT Center, BlueD, and Southern Medical University Guangdong Provincial Center for Skin Diseases and STI Control, who contributed to this study. We thank Huanyu Bao, Anne Sung, and Aoxiang Chang for their help with figure 1 and the video (appendix 2 p 22), and Guangyu Ji for his help with recruitment and coordination at the Blued testing site in Beijing, China, during the study implementation.
Footnotes
Declaration of interests
We declare no competing interests.
Data sharing
All deidentified data, survey instruments, and informed consent documents will be available to researchers on the Social Entrepreneurship to Spur Health website upon publication.
Contributor Information
Fan Yang, Dermatology Hospital of Southern Medical University, Guangzhou, China; University of North Carolina at Chapel Hill, Project-China, Guangzhou, China.
Tiange P Zhang, University of North Carolina at Chapel Hill, Project-China, Guangzhou, China; Loyola University Chicago Stritch School of Medicine, Maywood, IL, USA.
Weiming Tang, Dermatology Hospital of Southern Medical University, Guangzhou, China; University of North Carolina at Chapel Hill, Project-China, Guangzhou, China.
Jason J Ong, Central Clinical School, Monash University, Melbourne, VIC, Australia; London School of Hygiene and Tropical Medicine, London, UK.
Marcus Alexander, Yale Institute for Network Science, Yale University, New Haven, CT, USA.
Laura Forastiere, Yale Institute for Network Science, Yale University, New Haven, CT, USA; Department of Biostatistics, Yale School of Public Health,New Haven, CT, USA.
Navin Kumar, Yale Institute for Network Science, Yale University, New Haven, CT, USA.
Katherine T Li, University of North Carolina at Chapel Hill, Project-China, Guangzhou, China; Massachusetts General Hospital, Boston, MA, USA.
Prof Fei Zou, Department of Biostatistics, University of North Carolina at Chapel Hill, NC, USA.
Ligang Yang, Dermatology Hospital of Southern Medical University, Guangzhou, China.
Guodong Mi, Blued, Beijing, China.
Yehua Wang, University of North Carolina at Chapel Hill, Project-China, Guangzhou, China.
Wenting Huang, University of North Carolina at Chapel Hill, Project-China, Guangzhou, China.
Amy Lee, University of North Carolina at Chapel Hill, Project-China, Guangzhou, China.
Weizan Zhu, Zhitong Guangzhou LGBT Center, Guangzhou, China.
Danyang Luo, Zhitong Guangzhou LGBT Center, Guangzhou, China.
Prof Peter Vickerman, School of Social and Community Medicine, University of Bristol, Bristol, UK.
Dan Wu, University of North Carolina at Chapel Hill, Project-China, Guangzhou, China; London School of Hygiene and Tropical Medicine, London, UK.
Prof Bin Yang, Dermatology Hospital of Southern Medical University, Guangzhou, China.
Prof Nicholas A Christakis, Yale Institute for Network Science, Yale University, New Haven, CT, USA.
Joseph D Tucker, University of North Carolina at Chapel Hill, Project-China, Guangzhou, China; London School of Hygiene and Tropical Medicine, London, UK.
References
- 1.WHO. The World Health Report 2010. Health systems financing: the path to universal coverage. https://www.who.int/whr/2010/10_summary_en.pdf?ua=1 (accessed March 9, 2020).
- 2.Our common interest: report of the Commission for Africa. London: Commission for Africa, 2005. [Google Scholar]
- 3.Robert E, Ridde V. Global health actors no longer in favor of user fees: a documentary study. Global Health 2013; 9: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lagarde M, Palmer N. The impact of user fees on health service utilization in low- and middle-income countries: how strong is the evidence? Bull World Health Organ 2008; 86: 839–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pariyo GW, Ekirapa-Kiracho E, Okui O, et al. Changes in utilization of health services among poor and rural residents in Uganda: are reforms benefitting the poor? Int J Equity Health 2009; 8: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masiye F, Chitah BM, McIntyre D. From targeted exemptions to user fee abolition in health care: experience from rural Zambia. Soc Sci Med 2010; 71: 743–50. [DOI] [PubMed] [Google Scholar]
- 7.Ridde V, Haddad S, Heinmüller R. Improving equity by removing healthcare fees for children in Burkina Faso. J Epidemiol Community Health 2013; 67: 751–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee GM, Santoli JM, Hannan C, et al. Gaps in vaccine financing for underinsured children in the United States. JAMA 2007; 298: 638–43. [DOI] [PubMed] [Google Scholar]
- 9.Healthcare Financial Management Association. Strategies for reconfiguring cost structure. https://www.hfma.org/content/dam/hfma/Documents/PDFs/Strategies%20for%20Reconfiguring%20Cost%20Structure.pdf (accessed March 9, 2020). [PubMed]
- 10.Yates R Universal health care and the removal of user fees. Lancet 2009; 373: 2078–81. [DOI] [PubMed] [Google Scholar]
- 11.Li KT, Tang W, Wu D, et al. Pay-it-forward strategy to enhance uptake of dual gonorrhea and chlamydia testing among men who have sex with men in China: a pragmatic, quasi-experimental study. Lancet Infect Dis 2018; 19: 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung MH, Nelson LD, Gneezy A, Gneezy U. Paying more when paying for others. J Pers Soc Psychol 2014; 107: 414–31. [DOI] [PubMed] [Google Scholar]
- 13.Gray K, Ward AF, Norton MI. Paying it forward: generalized reciprocity and the limits of generosity. J Exp Psychol Gen 2014; 143: 247–54. [DOI] [PubMed] [Google Scholar]
- 14.Zhang TP, Liu C, Han L, et al. Community engagement in sexual health and uptake of HIV testing and syphilis testing among MSM in China: a cross-sectional online survey. J Int AIDS Soc 2017; 20: 21372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu D, Li KT, Tang W, et al. Low chlamydia and gonorrhea testing rates among men who have sex with men in Guangdong and Shandong Provinces, China. Sexually transmitted diseases. Sex Transm Dis 2019; 46: 260–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernstein KT, Marcus JL, Nieri G, Philip SS, Klausner JD. Rectal gonorrhea and chlamydia reinfection is associated with increased risk of HIV seroconversion. J Acquir Immune Defic Syndr 2010; 53: 537–43. [DOI] [PubMed] [Google Scholar]
- 17.Cohen MS, Hoffman IF, Royce RA, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet 1997; 349: 1868–73. [DOI] [PubMed] [Google Scholar]
- 18.Yang LG, Zhang XH, Zhao PZ, et al. Gonorrhea and chlamydia prevalence in different anatomical sites among men who have sex with men: a cross-sectional study in Guangzhou, China. BMC Infect Dis 2018; 18: 675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang F, Janamnuaysook R, Boyd MA, Phanuphak N, Tucker JD. Key populations and power: people-centred social innovation in Asian HIV services. Lancet HIV 2020; 7: e69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang TP, Yang F, Tang W, et al. Pay-it-forward gonorrhea and chlamydia testing among men who have sex with men in China: a study protocol for a three-arm cluster randomized controlled trial. Infect Dis Poverty 2019; 8: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei C, Muessig KE, Bien C, et al. Strategies for promoting HIV testing uptake: willingness to receive couple-based and collective HIV testing among a cross-sectional online sample of men who have sex with men in China. Sex Transm Infect 2014; 90: 469–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hesketh T, Duo L, Li H, Tomkins AM. Attitudes to HIV and HIV testing in high prevalence areas of China: informing the introduction of voluntary counselling and testing programmes. Sex Transm Infect 2005; 81: 108–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tucker JD, Yang LG, Yang B, et al. A twin response to twin epidemics: integrated HIV/syphilis testing at STI clinics in South China. J Acquir Immune Defic Syndr 2011; 57: e106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell MK, Elbourne DR, Altman DG. CONSORT statement: extension to cluster randomised trials. BMJ 2004; 328: 702–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.StataCorp. Stata Statistical Software: release 15. College Station, TX: StataCorp LLC; 2017. [Google Scholar]
- 26.Tucker JD, Day S, Tang W, Bayus B. Crowdsourcing in medical research: concepts and applications. PeerJ 2019; 7: e6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO, Social Entrepreneurship to Spur Health. Crowdsourcing in health and health research: a practical guide. Geneva: World Health Organisation, 2018. [Google Scholar]
- 28.Frost DM, Meyer IH. Measuring community connectedness among diverse sexual minority populations. J Sex Res 2012; 49: 36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swann WB Jr, Gómez A, Seyle DC, Morales JF, Huici C. Identity fusion: the interplay of personal and social identities in extreme group behavior. J Pers Soc Psychol 2009; 96: 995–1011. [DOI] [PubMed] [Google Scholar]
- 30.Grover E, Grosso A, Ketende S, et al. Social cohesion, social participation and HIV testing among men who have sex with men in Swaziland. AIDS Care 2016; 28: 795–804. [DOI] [PubMed] [Google Scholar]
- 31.Lippman SA, Donini A, Díaz J, Chinaglia M, Reingold A, Kerrigan D. Social-environmental factors and protective sexual behavior among sex workers: the Encontros intervention in Brazil. Am J Public Health 2010; 100 (suppl 1): S216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiridou M, Vriend HJ, Lugner AK, et al. Modelling the impact of chlamydia screening on the transmission of HIV among men who have sex with men. BMC Infect Dis 2013; 13: 436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fowler JH, Christakis NA. Cooperative behavior cascades in human social networks. Proc Natl Acad Sci USA 2010; 107: 5334–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tucker JD, Muessig KE, Cui R, et al. Organizational characteristics of HIV/syphilis testing services for men who have sex with men in South China: a social entrepreneurship analysis and implications for creating sustainable service models. BMC Infect Dis 2014; 14: 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chesson HW, Bernstein KT, Gift TL, Marcus JL, Pipkin S, Kent CK. The cost-effectiveness of screening men who have sex with men for rectal chlamydial and gonococcal infection to prevent HIV Infection. Sex Transm Dis 2013; 40: 366–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andreoni J Giving with impure altruism: Applications to charity and Ricardian equivalence. J Polit Econ 1989; 97: 1447–58. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


