Abstract
Background:
Recent studies have established that C-C chemokine receptor type 2 (CCR2) marks pro-inflammatory subsets of monocytes, macrophages, and dendritic cells that contribute to adverse left ventricle (LV) remodeling and heart failure progression. Elucidation of the effector mechanisms that mediate adverse effects of CCR2+ monocytes, macrophages, and dendritic cells will yield important insights into therapeutic strategies to suppress myocardial inflammation.
Methods:
We utilized mouse models of reperfused myocardial infarction (MI), angiotensin II and phenylephrine (AngII/PE) infusion, and diphtheria toxin (DT) cardiomyocyte ablation to investigate C-C chemokine ligand 17 (CCL17). We employed Ccl17 knockout mice, flow cytometry, RNA sequencing, biochemical assays, cell trafficking studies, and in vivo cell depletion to identify the cell types that generate CCL17, define signaling pathways that controlled its expression, delineate the functional importance of CCL17 in adverse LV remodeling and heart failure progression, and determine the mechanistic basis by which CCL17 exerts its effects.
Results:
We demonstrated that CCL17 is expressed in CCR2+ macrophages and cluster of differentiation (CD)11b+ conventional dendritic cells following MI, AngII/PE infusion and DT cardiomyocyte ablation. We elucidated the transcriptional signature of CCL17+ macrophages and dendritic cells and identified granulocyte macrophage-colony stimulating factor (GM-CSF) signaling as a key regulator of CCL17 expression through cooperative activation of signal transducer and activator of transcription 5 (STAT5) and canonical nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling. Ccl17 deletion resulted in reduced LV remodeling, decreased myocardial fibrosis and cardiomyocyte hypertrophy, and improved LV systolic function following MI and AngII/PE infusion. We observed increased abundance of regulatory T cells (Tregs) in the myocardium of injured Ccl17 knockout mice. Mechanistically, CCL17 inhibited Treg recruitment through biased activation of CCR4. CCL17 activated Gq signaling and CCL22 activated both Gq and β-arrestin signaling downstream of CCR4. CCL17 competitively inhibited CCL22 stimulated β-arrestin signaling and Tregs migration. Finally, we provide evidence that Tregs mediated the protective effects of Ccl17 deletion on myocardial inflammation and adverse LV remodeling.
Conclusion:
Collectively, these findings identify CCL17 as a pro-inflammatory mediator of CCR2+ macrophages and dendritic cells and suggest that inhibition of CCL17 may serve as an effective strategy to promote Treg recruitment and suppress myocardial inflammation.
Keywords: chemokine CCL17, monocyte, macrophages, dendritic cells, receptor CCR4, Tregs recruitment, myocardial inflammation
Introduction
Myocardial inflammation is associated with heart failure incidence and progression in multiple patient populations.1, 2 Following MI peripheral monocyte abundance and expression of pro-inflammatory cytokines and chemokines predict adverse left ventricle (LV) remodeling and cardiovascular mortality. In the context of chronic heart failure, cytokine levels are associated with heart failure severity and mortality.3, 4 Despite extensive evidence that inflammation contributes to heart failure pathogenesis in pre-clinical models,5, 6 the therapeutic potential of targeting myocardial inflammation is yet to be realized.
Recent advances in cardiac immunology have reinvigorated efforts to target inflammation in the injured and failing heart.5, 7 High dimensional flow cytometry, genetic lineage tracing, and single cell RNA sequencing studies have identified populations of monocytes, macrophages and dendritic cells that generate pro-inflammatory cytokines, chemokines, and damaging oxidative products.8–10 These populations originate from monocytes recruited to the heart and are marked by the expression of C-C chemokine receptor 2 (CCR2).11, 12 Inhibition of monocyte recruitment is sufficient to prevent accumulation of pro-inflammatory CCR2+ monocytes, macrophages, and dendritic cells and suppress adverse LV remodeling across heart failure models.13–15 The effector mechanisms that mediate adverse effects of CCR2+ monocytes, macrophages, and dendritic cells remain poorly defined.
We previously identified C-C chemokine ligand 17 (CCL17/TARC) as a chemokine selectively expressed in mouse and human CCR2+ macrophages.11,16 Chemokines play an important role in heart inflammation by directing trafficking of infiltrating leukocytes, and thus, represent promising therapeutic targets.3, 17 CCL17 has previously been reported to influence helper T cell and Treg recruitment by activating the G protein-coupled C-C chemokine receptor type 4 (CCR4).18, 19 CCL17 is associated with inflammatory diseases including allergic asthma,20 dermatitis,21 colitis,22 arthritis,23 and atherosclerosis.24 CCL17 is yet to be investigated within the heart.
Herein, we employ mouse models of reperfused MI, angiotensin and phenylephrine (AngII/PE) infusion, and diphtheria toxin (DT) cardiomyocyte ablation to investigate the role of CCL17 in heart failure pathogenesis. We reveal that CCL17 is expressed in CCR2+ macrophages and CD11b+ conventional dendritic cells that infiltrate the heart following myocardial injury. We further define signaling pathways that regulate CCL17 expression and demonstrate that Ccl17 deficiency is sufficient to suppress adverse LV remodeling and heart failure progression. We provide evidence that CCL17 prevents Treg migration by biasing signaling events downstream of the CCR4 receptor and show that the protective effects of Ccl17 deficiency on myocardial inflammation and adverse LV remodeling are dependent on the presence of Tregs. These findings implicate CCL17 as a pro-inflammatory mediator of CCR2+ macrophages and dendritic cells and highlight the therapeutic potential of inhibiting CCL17 in the injured and/or failing heart.
Methods
Complete detailed methods are provided in the Data Supplement. Raw data that support the findings of this study are available from the corresponding author on reasonable request.
Animal Studies.
Mice were bred and maintained at the Washington University School of Medicine and all experimental procedures were done in accordance with the animal use oversight committee guidelines. Mouse strains utilized included Ccl17GFP25, (forkhead box P3) Foxp3-GFP/DTR26, (Zinc finger and BTB domain-containing protein 46) Zbtb46GFP27 and troponin T2 - diphtheria toxin receptor (Tnnt2-DTR)16. Tnnt2-DTR model: cardiomyocyte injury was induced by administering 20 ng diphtheria toxin via intraperitoneal injection. Closed-chest ischemia reperfusion (IR) injury: ischemia was induced for 90 minutes. Angiotensin II/phenylephrine infusion: Alzet minipumps containing either saline or Angiotensin II (1.5 μg/g/day) and Phenylephrine (50 μg/g/day) were implanted in a subcutaneous pocket.
Human Studies.
Myocardial tissue was obtained following left ventricular assist device implantation or heart transplantation. The study was approved by the Washington University Institutional Review Board (IRB# 201104172). All subjects provided informed consent before sample collection and the experiments were performed in accordance with the approved study protocol.
Statistical Analysis.
Comparisons between groups were calculated by t tests or one-way ANOVA or two-way ANOVA followed by suitable tests. Sample size, replicates, and statistical tests are documented in the figure legends.
Results
CCL17 is expressed following myocardial injury.
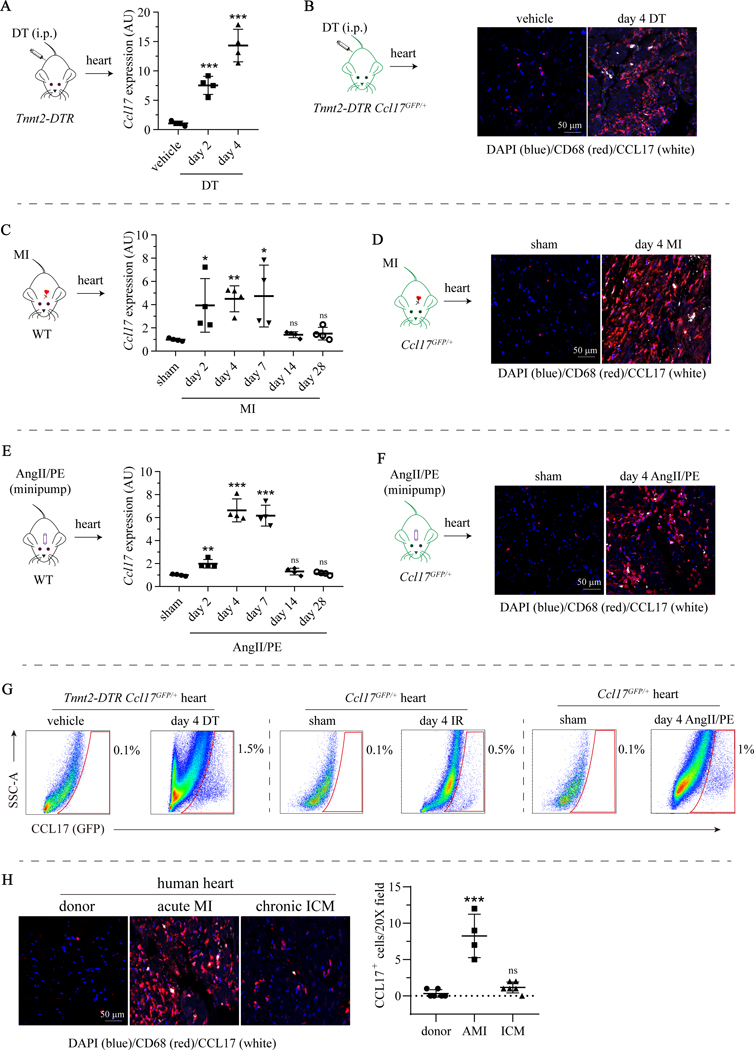
To evaluate the expression of Ccl17 in the healthy and injured heart, we examined mouse models of reperfused MI, AngII/PE infusion, and DT cardiomyocyte ablation. Reperfused MI was performed using a closed-chest model of IR injury (90 min. ischemia).16, 28 We utilized Ccl17GFP/+ mice to facilitate flow cytometry and immunostaining analyses. First, we examined Ccl17 expression in a DT cardiomyocyte ablation model that expresses the DT receptor (DTR) under the control of the Troponin T2 (Tnnt2) promoter. We have previously reported that DT injection specifically triggers the death of cardiomyocytes in Tnnt2-DTR transgenic mice.16, 29 We detected increased Ccl17 mRNA expression in the myocardium 2 and 4 days after DT administration compared to vehicle controls (Fig. 1A). Injection of DT into Tnnt2-DTR Ccl17GFP/+ mice revealed GFP expression in CD68+ cells (macrophages and dendritic cells) within the myocardium (Fig. 1B).
Figure 1. CCL17 is expressed following myocardial injury.
A, Quantitative RT-PCR measuring Ccl17 mRNA expression in hearts of Tnnt2-DTR mice 2 and 4 days after diphtheria toxin (DT, n=4) or normal saline (vehicle, n=4) administration. B, Immunostaining for CCL17-GFP (white), CD68 (red) and DAPI (4’,6-diamidino-2-phenylindole; blue) showing the spatial distribution of GFP+ CCL17 expressing cells in hearts of Tnnt2-DTR Ccl17GFP/+ mice 4 days after DT or saline administration. C, Quantitative RT-PCR measuring Ccl17 mRNA expression in hearts of wild type mice 2, 4, 7, 14 and 28 days after closed chest ischemia-reperfusion (IR, n=4) surgery or sham (n=4) surgery. D, Immunostaining for CCL17-GFP (white), CD68 (red) and DAPI (blue) showing the spatial distribution of GFP+ Ccl17 expressing cells in hearts of Ccl17GFP/+ mice 4 days after IR or sham surgery. E, Quantitative RT-PCR measuring Ccl17 mRNA expression in hearts of wild type mice 2, 4, 7, 14 and 28 days after implantation of osmotic minipump containing normal angiotensin II/phenylephrine (AngII/PE, n=4) or saline (sham, n=4). F, Immunostaining for CCL17-GFP (white), CD68 (red) and DAPI (blue) showing the spatial distribution of GFP+ CCL17 expressing cells in hearts of Ccl17GFP/+ mice 4 days after AngII/PE or saline minipump implantation. G, Flow cytometry revealing GFP+ (CCL17 expressing) immune cells (gated on CD45+ cells) 4 days after DT injection, IR surgery, AngII/PE infusion, and corresponding saline injection or sham treatments. H, Immunostaining for CCL17 (white), CD68 (red) and DAPI (blue) showing the spatial distribution of CCL17 expressing cells in healthy donor hearts (n=6), acute MI hearts (n=4) and chronic ischemic cardiomyopathy hearts (n=6). All data are mean ± SD and one-way ANOVA followed by Dunnett test or Dunnett T3 test was performed. *P< 0.05, **P< 0.01, ***P< 0.001.
Ccl17 mRNA expression was also elevated within the LV myocardium 2, 4, and 7 days after IR injury compared to sham controls. Ccl17 expression returned to baseline levels on days 14 and 28 after IR injury (Fig. 1C), which was consistent with other inflammatory chemokines and cytokines (Supplemental Fig. IA). GFP and CD68 immunostaining of Ccl17GFP/+ hearts revealed that GFP was expressed in CD68+ cells within the infarct (Fig. 1D). Finally, AngII/PE infusion also induced myocardial Ccl17 expression compared to saline infused controls. Ccl17 mRNA expression was found to be elevated beginning 2 days after pump implantation with peak expression observed on days 4 and 7. Ccl17 mRNA expression returned to baseline values on days 14 and 28 (Fig. 1E). GFP and CD68 immunostaining of Ccl17GFP/+ hearts demonstrated the presence of GFP expressing CD68+ cells within the myocardium of mice infused with AngII/PE (Fig. 1F). Flow cytometric analysis indicated that 0.5–1.5% of CD45+ cells expressed GFP across injury models (Fig. 1G, Supplemental Fig. IB–D). Immunostaining of human myocardial samples obtained from donor controls or patients with acute MI or ischemic cardiomyopathy confirmed the presence of CCL17+ CD68+ cells following acute MI (Fig. 1H, Supplemental Table I). Collectively, these data indicate that CCL17 is expressed in CD68+ cells within the myocardium following cardiac injury.
CCL17 is expressed in subsets of monocyte-derived macrophages and conventional dendritic cells recruited to the injured heart.
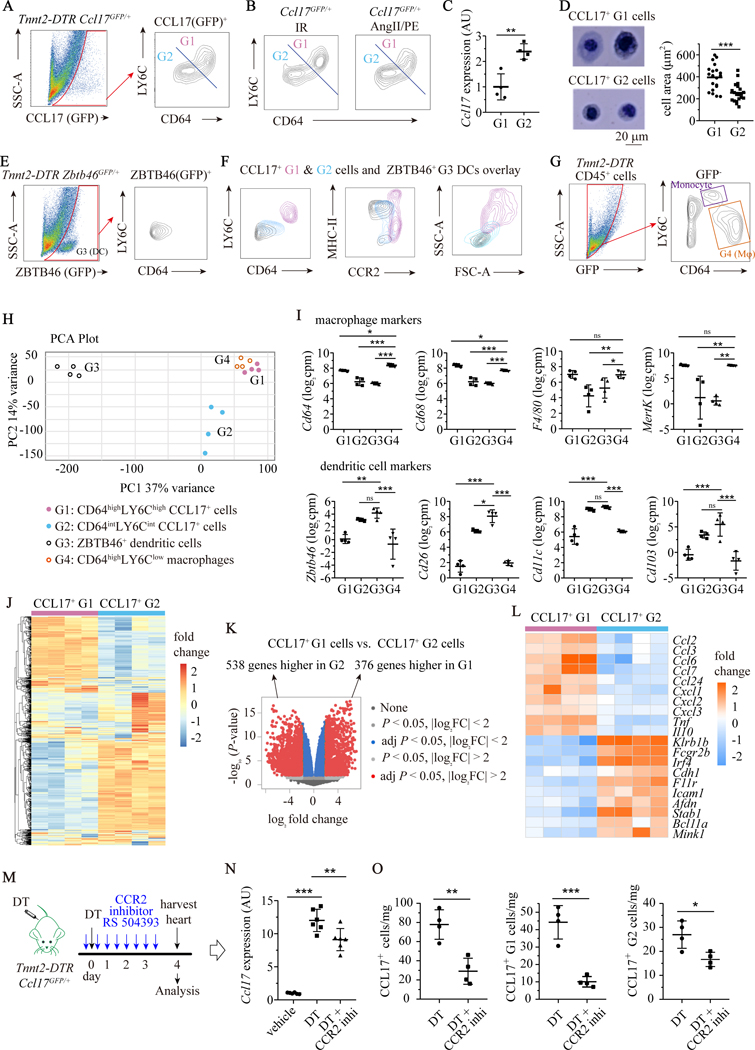
To define the precise immune cell types that express CCL17 within the injured heart, we performed flow cytometry and RNA sequencing using Tnnt2-DTR Ccl17GFP/+ mice. Flow cytometry 4 days after DT injection, revealed that GFP was expressed in cell populations marked by CD11b, CD64, CD11c and MHC-II. We did not detect GFP expression in T-cells (CD3), B-cells (CD19), natural killer cells (NK1.1), and neutrophils (LY6G). These data suggest that monocytes, macrophages, and/or dendritic cells express Ccl17 (Supplemental Fig. IIA). Flow cytometry further revealed two distinct populations of CCL17+ cells: CD64hiLY6Chi (Gate 1: G1) and CD64loLY6Clo (G2) cells (Fig. 2A). We identified similar populations of Ccl17 expressing CD64hiLY6Chi (G1) and CD64loLY6Clo (G2) cells in the hearts of mice subjected to IR injury and AngII/PE infusion (Fig. 2B). CD64hiLY6Chi (G1) and CD64loLY6Clo (G2) cells expressed different levels of Ccl17 (Fig. 2C) and displayed different sizes and morphologies (Fig. 2D), suggesting that they likely represent distinct cell types.
Figure 2. CCL17 is expressed in CCR2+ macrophages and conventional dendritic cells recruited to the injured heart.
A, Flow cytometry identified two populations of CCL17+ cells in hearts of Tnnt2-DTR Ccl17GFP/+ mice 4 days after DT administration: CCL17+CD64hiLY6Chi (G1) and CCL17+CD64loLy6Clo (G2). B, Flow cytometry demonstrating CCL17+CD64hiLY6Chi (G1) and CCL17+CD64loLY6Clo (G2) cells in hearts of CCL17GFP/+ mice 4 days after IR and AngII/PE injury. C, Quantitative RT-PCR comparing Ccl17 mRNA expression in CCL17+ G1 and G2 cells from DT injured hearts. n=4 per experimental group. D, Representative Hema-3 stained images of CCL17+ G1 (n=20) and G2 (n=20) cells from DT injured hearts (left panel) and quantification of cells area (right panel). E, Flow cytometry showing ZBTB46+CD64loLY6Clo dendritic cells (G3) in hearts of Tnnt2-DTR Zbtb46GFP/+ mice 4 days after DT administration. F, Flow cytometry overlay plots of CCL17+ G1 and G2 cells with ZBTB46+ dendritic cells (G3). G, Flow cytometry gating strategy for CD64hiLY6Clo macrophages (G4) in hearts of Tnnt2-DTR mice 4 days after DT administration. H, Principal component analysis (PCA) of RNA sequencing data obtained from CCL17+ G1 and G2 cells, ZBTB46+ dendritic cells (G3) and CD64hiLY6Clo macrophages (G4). n=4 per experimental group. I, Expression levels of characteristic macrophage markers (Cd64, Cd68, F4/80, MertK) and dendritic cell markers (Zbtb46, Cd26, Cd11c, Cd103). Plotted values are displayed as log2 counts per million (cpm). n=4 per experimental group. J, Hierarchical clustering of 1000 genes highlighting distinct signatures of CCL17+ G1 and G2 cells. K, Volcano plot showing genes differentially expressed between CCL17+ G1 and G2 cells. L, Heat map of genes associated with inflammation that were differentially expressed (P < 0.05) between G1 and G2 populations obtained from DT injured hearts. M, Schematic depicting the relative timing of DT injection, CCR2 inhibitor administrations, and flow cytometry analysis. N, Quantitative RT-PCR measuring Ccl17 mRNA expression in hearts of Tnnt2-DTR Ccl17GFP/+ mice treated with vehicle or RS 504393 4 days after DT administration. n=6 per experimental group. O, Numbers of total CCL17+ cells, G1, and G2 cells per mg of heart tissue from DT and RS 504393 treated mice, analyzed by flow cytometry. N=4 per experimental group and all data are mean ± SD. For comparisons between two groups (C, D and O), unpaired t test was performed. For multiple comparisons (I and N), one-way ANOVA followed by Tukey test or Games-Howell test was performed. *P< 0.05, **P< 0.01, ***P< 0.001, and ns indicates non-significance.
Previous studies have suggested that CCL17 is expressed in dendritic cells.25, 30 To determine whether CCL17+ G1 and G2 cells are macrophages or dendritic cells, we generated Tnnt2-DTR Zbtb46GFP/+ mice. ZBTB46 is specially expressed in dendritic cells and not macrophages or monocytes.31 Following DT injection, we observed accumulation of ZBTB46+ dendritic cells (G3) within the heart by flow cytometry (Fig. 2E, Supplemental Fig. IE). ZBTB46+ dendritic cells (G3) displayed low levels of CD64 and LY6C expression. Comparison of CD64 and LY6C expression between CCL17+CD64hiLY6Chi (G1), CCL17+CD64loLY6Clo (G2), and ZBTB46+ dendritic (G3) cells indicated similarities between CCL17+CD64loLY6Clo (G2) and ZBTB46+ dendritic (G3) cells. CCL17+CD64loLY6Clo (G2) and ZBTB46+ dendritic (G3) cells also displayed similar MHC-II and CCR2 expression and physical properties (forward and side scatter) (Fig. 2F).
We performed RNA sequencing to substantiate whether CCL17+CD64hiLY6Chi (G1) and CCL17+CD64loLY6Clo (G2) cells represent macrophages and dendritic cells, respectively. We compared CCL17+CD64hiLY6Chi (G1) and CCL17+CD64loLY6Clo (G2) cells to bona fide populations of ZBTB46-GFP+ dendritic cells (G3) and CD64+ macrophages (G4) (Fig. 2G) in the Tnnt2-DTR injury model. Principal component analysis revealed that CCL17+CD64hiLY6Chi (G1) cells clustered with CD64+ macrophages (G4) and were transcriptionally distinct from ZBTB46-GFP+ dendritic cells (G3). CCL17+CD64loLY6Clo (G2) cells clustered independent of the other populations (Fig. 2H). Quantification of genes associated with macrophage and dendritic cell identify demonstrated that CCL17+CD64hiLY6Chi (G1) cells expressed high levels of macrophage markers (Cd64, Cd68, F4/80, MertK) and low levels of dendritic cell markers (Zbtb46, Cd26, Cd11c, Cd103). In contrast, CCL17+CD64loLY6Clo (G2) cells expressed low levels of macrophage markers (Cd64, Cd68, F4/80, MertK) and high levels of dendritic cell markers (Zbtb46, Cd26, Cd11c, Cd103) (Fig. 2I). Collectively, these data suggest that CCL17+CD64hiLY6Chi (G1) cells are macrophages and CCL17+CD64loLY6Clo (G2) cells are dendritic cells. Based on robust expression of Cd11b, the phenotype of CCL17+CD64loLY6Clo cells is most consistent with cDC2 conventional dendritic cells.
Hierarchical clustering and differential gene expression analysis comparing CCL17+CD64hiLY6Chi (G1) macrophages and CCL17+CD64loLY6Clo (G2) dendritic cells confirmed that these populations were transcriptionally distinct and identified 914 differentially expressed genes (>2-fold change, FDR p<0.05) (Fig. 2J–K). CCL17+CD64hiLY6Chi (G1) macrophages expressed high levels of several chemokines (Ccl2, Ccl3, Ccl6, Ccl7, Ccl24, Cxcl1, Cxcl2, Cxcl3) and cytokines (TNF, Il10). CCL17+CD64loLY6Clo (G2) dendritic cells expressed higher levels of Klrb1b, Fcgr2b, Irf4, Cdh1, F11r, Icam1, Adfn, Stab1, Bcl11a, and Mink1 (Fig. 2L, Supplemental Fig. IIB).
To determine whether CCL17+ macrophages and dendritic cells originate from recruited populations of monocytes, we treated Tnnt2-DTR Ccl17GFP/+ mice with RS504393 (CCR2 inhibitor) (Fig. 2M). Following DT injection, we observed reduced CCL17 expression in the hearts of mice treated with RS504393 compared to vehicle treated controls (Fig. 2N). Flow cytometry revealed that the numbers of total CCL17+ cells, CCL17+CD64hiLY6Chi (G1) macrophages, and CCL17+CD64loLY6Clo (G2) dendritic cells within the myocardium was also reduced in RS504393 treated mice compared to vehicle treated controls (Fig. 2O). These results suggest that CCL17+CD64hiLY6Chi (G1) macrophages and CCL17+CD64loLY6Clo (G2) dendritic cells likely originate from monocytes or monocyte-like progenitors that infiltrate the injured heart.
GM-CSF signaling regulates CCL17 expression in cardiac macrophages and dendritic cells.
To identify upstream activators of CCL17 expression, we exposed mouse bone marrow-derived macrophages (BMDMs) to hypoxia, interleukin 1β (IL1β), interleukin 4 (IL4), polycytidylic acid (Poly I:C), lipopolysaccharides (LPS), CpG oligodeoxynucleotides, and granulocyte macrophage colony stimulating factor (GM-CSF). GM-CSF demonstrated the strongest effect on Ccl17 mRNA expression and secreted protein abundance (Supplemental Fig. IIIA–B). GM-CSF signaling activates several intracellular signaling pathways including phosphatidyl-inositol 3-kinase/protein kinase B (PI3K/AKT), mitogen-activated protein kinase (MAPK), canonical NF-κB and Janus kinase 2 (JAK2)/STAT5.32 To define the requirement for each pathway, we stimulated BMDMs with GM-CSF in the presence of the following inhibitors JAK1/2 inhibitor Ruxolitinib (Ruxo), IKK-2 inhibitor (TPCA), MAPK inhibitor (U0126), and AKT inhibitor Miltefosine (Milt). Ccl17 mRNA expression and secreted protein abundance was markedly reduced by TPCA and Ruxolitinib. U0126 and miltefosine only modestly reduced Ccl17 expression (Supplemental Fig. IIIC). Western blot and immunostaining demonstrated that GM-CSF promoted accumulation of NF-κB subunits p65 and p50 in the nucleus and phosphorylation of STAT5, which were prevented by TPCA and Ruxolitinib, respectively (Supplemental Fig. IIID–H). These results are consistent with predicted binding sites for NF-κB and STAT5 in the Ccl17 promotor and suggest that canonical NF-κB and STAT5 pathways are required for GM-CSF to induce Ccl17 expression (Supplemental Fig. IIII).
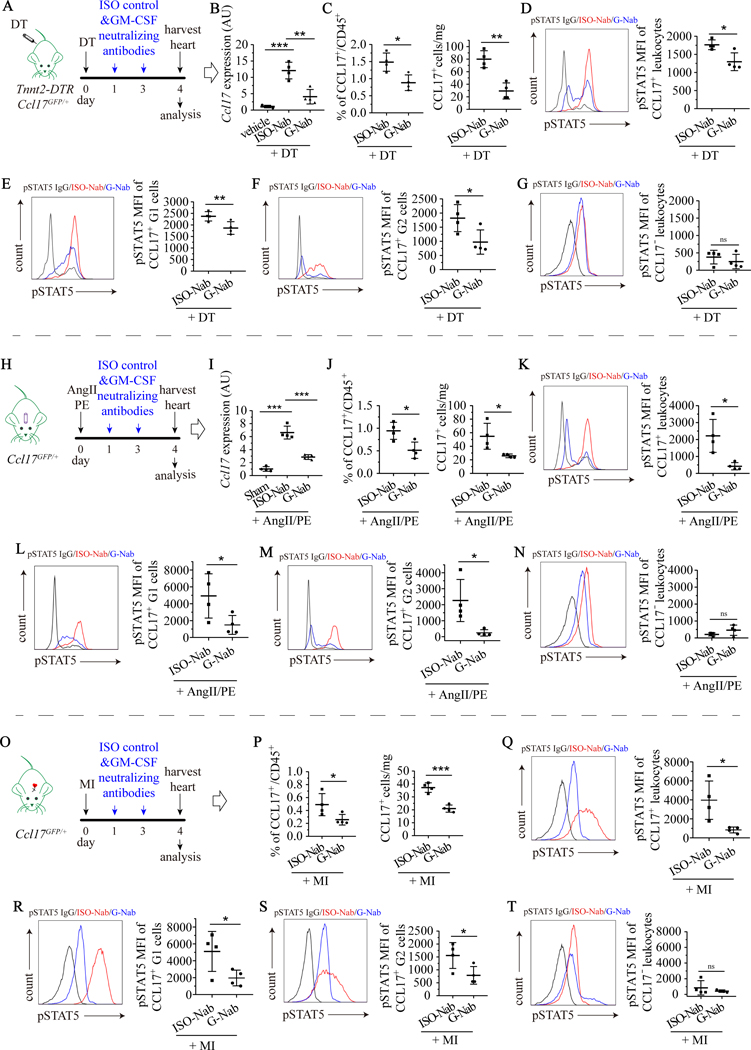
To decipher whether GM-CSF regulates Ccl17 in vivo, we treated Ccl17GFP/+ mice subjected to DT cardiomyocyte ablation, AngII/PE infusion, and myocardial IR injury with either an isotype control antibody (ISO-Nab) or GM-CSF neutralizing antibody (G-Nab). Reverse transcription polymerase chain reaction (RT-PCR) was utilized to measure myocardial Ccl17 mRNA expression. Flow cytometry was employed to quantify the percentage and absolute number of CCL17+ macrophages and dendritic cells as well as STAT5 phosphorylation. Compared to isotype control, the GM-CSF neutralizing antibody reduced myocardial Ccl17 mRNA expression, the total CCL17+ leukocyte number, the abundance of CCL17+ macrophages (G1) and dendritic cells (G2), and STAT5 phosphorylation in CCL17+ macrophages (G1) and dendritic cells (G2) following DT cardiomyocyte ablation (Fig. 3A–G) and AngII/PE infusion (Fig. 3H–N). Similarly, GM-CSF neutralizing antibody treatment decreased total CCL17+ leukocyte number, the abundance of CCL17+ macrophages (G1) and dendritic cells (G2), and STAT5 phosphorylation in CCL17+ macrophages (G1) and dendritic cells (G2) following myocardial IR injury (Fig. 3O–T). Across injury models, low levels of STAT5 phosphorylation was observed in CCL17− leukocytes and was not further reduced by GM-CSF neutralizing antibody treatment.
Figure 3. GM-CSF-STAT5 signaling is essential to specify CCL17+ cells.
A, Tnnt2-DTR Ccl17GFP/+ mice were treated with normal saline (vehicle) or DT on day 0, followed by isotype control (ISO-Nab) or GM-CSF neutralizing antibody (G-Nab) on days 1 and 3, and hearts harvested on day 4 for analysis. B, Quantitative RT-PCR measuring Ccl17 mRNA expression in hearts. C, Flow cytometry quantifying the percentage of CCL17+ immune cells (left panel) and the number of CCL17+ immune cells per mg heart tissue (right panel). D-G, Flow cytometry showing levels of phosphorylated STAT5 (pSTAT5) in all CCL17+ cells (D), CCL17+ G1 cells (E), CCL17+ G2 cells (F), and CD45+CCL17− cells (G) isolated from hearts. H, Ccl17GFP/+ mice were treated with saline (sham) or AngII/PE infusion via osmotic minipump on day 0 and administered ISO-Nab or GM-CSF neutralizing antibodies on days 1 and day 3. Hearts were harvested on day 4 for analysis. I, Quantitative RT-PCR measuring Ccl17 mRNA expression in hearts. J, Flow cytometry quantifying the percentage of CCL17+ immune cells (left panel) and the number of CCL17+ immune cells per mg heart tissue (right panel). K-N, Flow cytometry showing levels of pSTAT5 in all CCL17+ cells (K), CCL17+ G1 cells (L), CCL17+ G2 cells (M), and CD45+CCL17− cells (N) isolated from the hearts of mice treated with AngII/PE and ISO-Nab or G-Nab. O, Ccl17GFP/+ mice were subjected to closed-chest IR injury (90 minutes of ischemia) on day 0 and were administered ISO-Nab or G-Nab on days 1 and day 3. Hearts were harvested on day 4 for analysis. P, Flow cytometry quantifying the percentage of CCL17+ immune cells (left panel) and the number of CCL17+ immune cells per mg heart tissue (right panel). Q-T, Flow cytometry showing levels of pSTAT5 in all CCL17+ cells (Q), CCL17+ G1 cells (R), CCL17+ G2 cells (S), and CD45+CCL17− cells (T) isolated from hearts. D-G, K-N, Q-T, black: IgG control, red: pSTAT5 antibody and ISO control treatment, blue: pSTAT5 antibody and G-Nab treatment. For comparisons between two groups, unparied t tests were performed. For multiple comparisons, one-way ANOVA followed by Tukey test was performed. N=4 per experimental group and all data are mean ± SD. *P< 0.05, **P< 0.01, ***P< 0.001, and ns indicates non-significance.
Ccl17 deletion attenuates heart remodeling following reperfused MI and AngII/PE infusion.
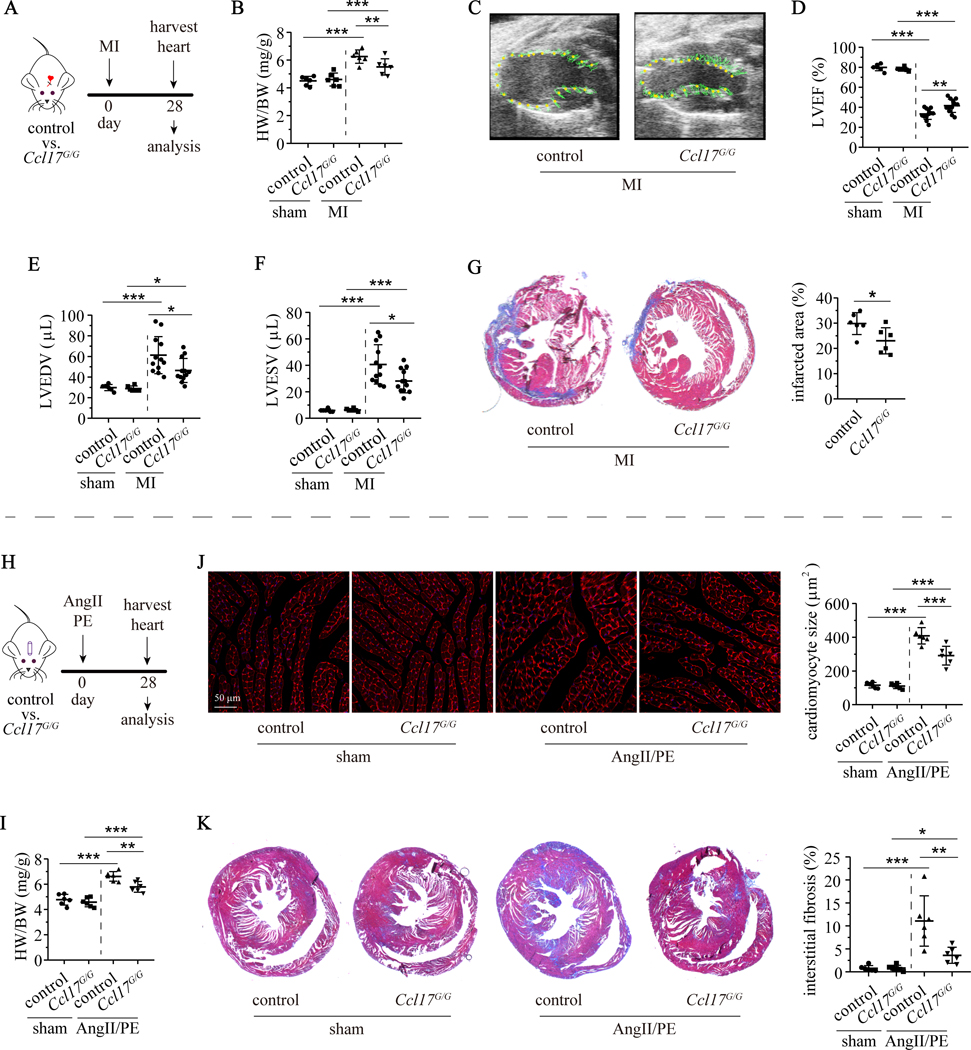
To delineate the functional relevance of Ccl17 in the context of myocardial injury, we investigated control (Ccl17+/+) and Ccl17 deficient (Ccl17GFP/GFP, abbreviated as Ccl17G/G) mice in clinically relevant models of reperfused MI and AngII/PE infusion. Compared to littermate controls, Ccl17G/G mice demonstrated attenuated left ventricle (LV) remodeling following 90 minutes of MI injury. Ccl17G/G mice showed reduced heart/body weight ratio compared with controls 28 days after reperfused MI injury. Echocardiography revealed that deletion of Ccl17 resulted in improved LV ejection fraction and smaller left ventricular volumes 28 days after myocardial IR injury. No differences were observed between control and Ccl17G/G mice that underwent the sham procedure (Fig. 4A–F). Wheat germ agglutinin (WGA) and trichrome staining revealed reduced cardiomyocyte cross-sectional area and interstitial fibrosis within the border zone of Ccl17G/G mice compared to controls 28 days after myocardial injury (Supplemental Fig. IVA–C). Ccl17G/G mice showed smaller sized infarcts 28 days after myocardial IR injury compared to control mice (Fig. 4G). The initial infarct area was equivalent between control and Ccl17G/G mice as measured by echocardiography 1 day and triphenyl tetrazolium chloride (TTC) staining 4 days after myocardial IR injury (Supplemental Fig. IVD–E), suggesting Ccl17 deletion influences infarction expansion.
Figure 4. Deficiency of Ccl17 attenuates LV remodeling following MI and AngII/PE infusion.
A, Control (Ccl17+/+) mice and Ccl17 knockout (Ccl17GFP/GFP, abbreviated as Ccl17G/G) mice were subjected to closed-chest IR injury or sham surgery on day 0. Hearts were harvested on day 28 after MI. B, Measurement of heart weight to body weight ratio (HW/BW) in control and Ccl17G/G mice following sham surgery (n=6) or MI (n=12). C, Representative end systolic echocardiographic images of control and Ccl17G/G hearts 28 days after MI. Regional LV displacement throughout the cardiac cycle is displayed. D-F, Quantification of LV ejection fraction (LVEF), LV end-diastolic volume (LVEDV) and LV end-systolic volume (LVESV) 28 days after IR surgery. G, Representative images of infarct area identified by trichrome staining (left panel). Quantification of infarct area (right panel). H, Control and Ccl17G/G mice were implanted with osmotic minipumps containing AngII/PE (n=6) or normal saline (sham, n=6) on day 0. Hearts were harvested on day 28 for analysis. I, Measurement of heart weight to body weight ratio (HW/BW) in control and Ccl17G/G mice implanted with osmotic minipumps containing AngII/PE or saline (sham). J, Represent WGA stained images (mid-LV) showing cardiomyocytes in cross-section (red, left panel) and quantification of cardiomyocyte cross-sectional area (right panel). K, Representative low magnification trichrome staining images to visualize interstitial fibrosis (left panel). Quantification of fibrotic area based on trichrome staining (right panel). All data are mean ± SD. For comparisons between two groups, unpaired t test was performed. For multiple comparisons, two-way ANOVA with Geisser-Greenhouse correction followed by Sidak test was performed. *P< 0.05, **P< 0.01, ***P< 0.001.
To investigate the role of Ccl17 in a non-ischemic injury model, we infused control and Ccl17G/G mice with either saline (sham) or AngII/PE (Fig. 4H). Quantification of heart/body weight ratio demonstrated reduced size of Ccl17G/G hearts compared to controls 28 days after AngII/PE infusion. No differences were observed between control and Ccl17G/G hearts infused with saline (Fig. 4I). WGA and trichrome staining further showed that Ccl17G/G hearts displayed blunted increases in cardiomyocyte cross-sectional area and interstitial fibrosis compared to controls following 28 days of AngII/PE infusion. Consistent with these findings, we observed reduced Nppa, Nppb, Col1a1, and Col3a1 mRNA expression in AngII/PE infused Ccl17G/G hearts compared to controls (Fig. 4J–K, Supplemental Fig. IVF–I). Taken together, these data indicate that Ccl17 deletion attenuates LV remodeling following myocardial injury.
Recruitment of cardiac Tregs in the absence of Ccl17.
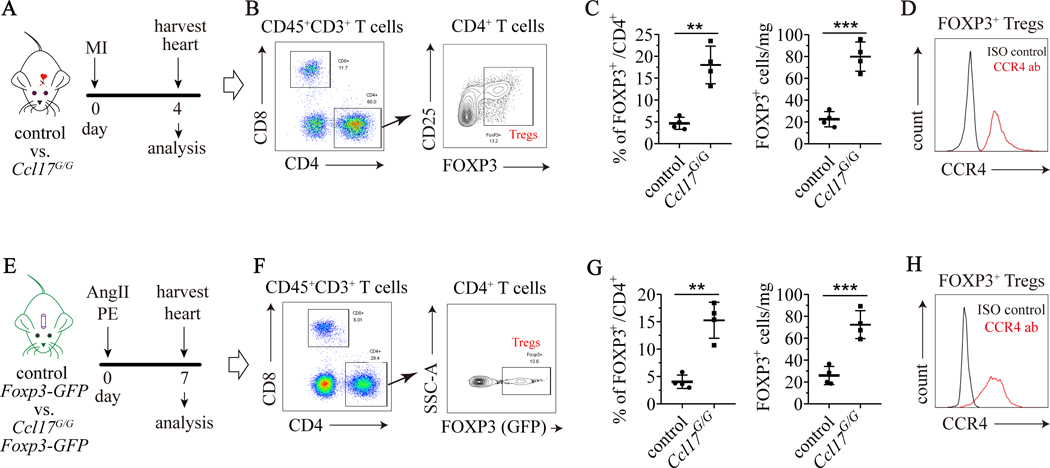
To determine the chemotactic function of Ccl17 in the heart, we first examined infiltration of innate immune cells. Flow cytometry and immunostaining did not reveal any differences in monocyte, macrophage, or neutrophil abundance within the heart between control and Ccl17G/G mice 4 days after reperfused MI or AngII/PE infusion (Supplemental Fig. VA–C, F–H). Ccl17 selectively binds to C-C chemokine receptor 4 (CCR4) which is expressed on T-cells.33, 34 We observed increased accumulation of CD4+ Tregs (CD3+CD4+ FOXP3+) in the hearts of Ccl17G/G mice subjected to reperfused MI or AngII/PE infusion compared to controls. FOXP3, a marker and regulator of Tregs,35 was detected by either intracellular flow cytometry (Fig. 5A–C) or by using Foxp3-GFP reporter mice (Fig. 5E–G). Flow cytometry confirmed that CCR4 was expressed on Tregs in each model of myocardial injury (Fig. 5D, H). We did not observe any change in the abundance of helper T cells (CD3+CD4+), cytotoxic T cells (CD3+CD8+), Th1 cells (CD3+CD4+CXCR3+), or Th17 cells (CD3+CD4+CCR6+) between control and Ccl17G/G mice 4 days after reperfused MI or AngII/PE infusion (Supplemental Fig. VD–E, I–J).
Figure 5. Ccl17 deficiency increases cardiac Treg abundance.
A, Control (Ccl17+/+) and Ccl17 knockout (Ccl17GFP/GFP, abbreviated as Ccl17G/G) mice were subjected to closed-chest IR injury on day 0 and hearts were harvested on day 4 for analysis. B, Flow cytometry gating scheme utilized to identify CD4+ FOXP3+ Tregs. C, Ccl17 deficiency led to expansion of Tregs expansion in hearts of IR injured mice. Flow cytometry showing the proportion of cardiac FOXP3+ Tregs among total CD4+ cells (left panel) and the number of FOXP3+ Tregs per mg heart tissue (right panel) after IR injury. D, Flow cytometry showing expression of CCR4 in cardiac Tregs from hearts of IR injured mice. Black: isotype control, red: CCR4 antibody. E, Control Foxp3-GFP mice and Ccl17G/G Foxp3-GFP were implanted with osmotic minipumps containing AngII/PE on day 0. Hearts were harvested on day 7 for analysis. F, Flow cytometry gating scheme utilized to identify FOXP3-GFP+ Tregs. G, Ccl17 deficiency increased Treg abundance in the hearts of AngII/PE infused mice. Flow cytometry demonstrating the proportion of cardiac FOXP3-GFP+ Tregs among total CD4+ cells (left panel) and the number of FOXP3-GFP+ Tregs per mg heart tissue (right panel) after AngII/PE infusion. H, Flow cytometry plots showing expression of CCR4 in Tregs from hearts of AngII/PE infused mice. Black, isotype control. Red, CCR4 antibody. N=4 per experimental group and all data are mean ± SD. Comparisons were calculated by unpaired t test. **P< 0.01, **P< 0.01.
CCL17 and CCL22 are competitive biased CCR4 ligands that differentially modulate Treg chemotaxis.
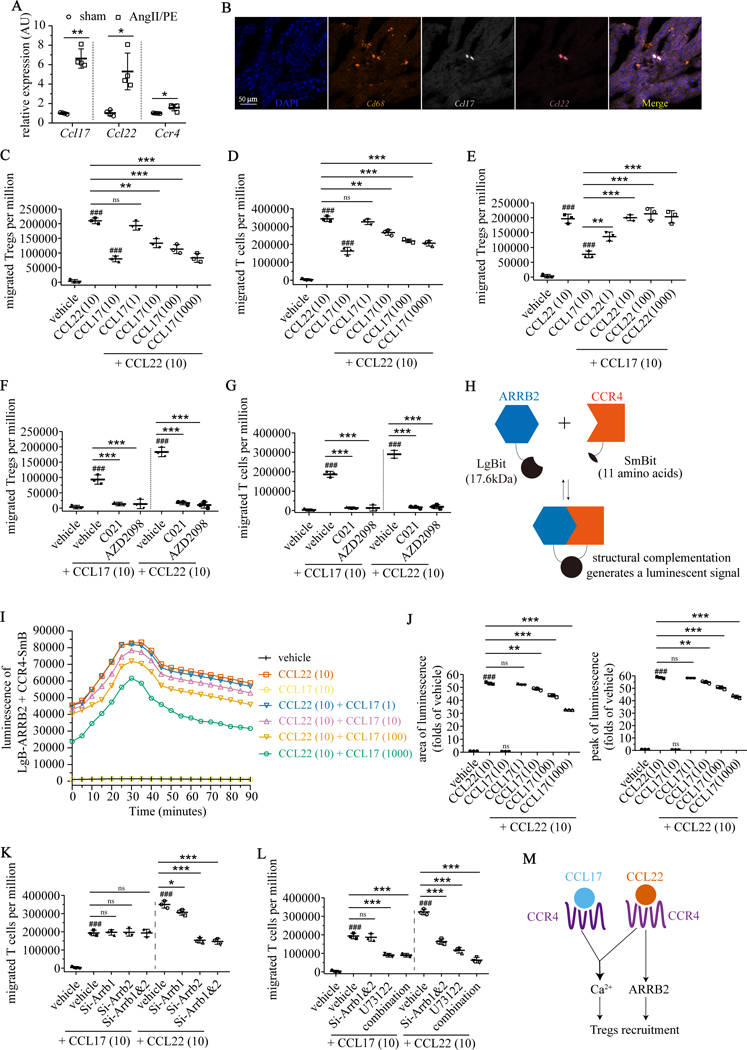
To assess mechanisms by which Ccl17 deficiency leads to Treg recruitment, we examined whether CCL17 influences Treg differentiation, proliferation, or chemotaxis. CCL17 had no impact on the differentiation of naïve CD4+ T-cells into Tregs in vitro (induced Tregs) following stimulation with IL-2, transforming growth factor-β (TGF-β), and anti-CD3/CD28 antibodies (Supplemental Fig. VIA). Flow cytometry revealed that Tregs displayed minimal proliferation rates in both control and Ccl17G/G hearts 7 days after AngII/PE infusion (Supplemental Fig. VIB). To assess potential effects on chemotaxis, we first sought to identify whether other CCR4 ligands are present within the injured heart. Quantitative RT-PCR revealed that Ccl22 was expressed in the myocardium after IR injury, AngII/PE infusion, and DT cardiomyocyte ablation (Fig. 6A and Supplemental Fig. VIC). RNA in situ hybridization showed co-staining of Ccl17 and Ccl22 mRNA in CD68+ macrophages (Fig. 6B).
Figure 6. CCL17 and CCL22 are competitive biased CCR4 ligands that differentially modulate Treg chemotaxis.
A, Quantitative RT-PCR measuring mRNA levels of Ccl17, Ccl22 and CCR4 in hearts of wild type mice 4 days after implantation of osmotic minipump containing AngII/PE (n=4) or normal saline (sham, n=4). B, Representative images of DAPI (blue), Cd68 (orange), Ccl17 (white) and Ccl22 (magenta) in hearts of AngII/PE infused mice by RNA in situ hybridization. C-D, Chemotaxis of primary induced-Tregs (C) and BW5147.3 T cells (D) in response to CCL22 (10 ng/mL) was suppressed by increasing concentrations of CCL17 (1, 10, 100, 1000 ng/mL). E, Chemotaxis of primary induced-Tregs in response to single dose CCL17 (10 ng/mL) was augmented by increasing concentrations of CCL22 (1, 10, 100, 1000 ng/mL). F-G, Chemotaxis of primary induced-Tregs (F) and BW5147.3 T cells (G) in response to CCL22 (10 ng/mL) and CCL17 (10 ng/mL) was abrogated by CCR4 antagonists (C021, AZD2098). H, Strategy to detect interactions between β-arrestin2 (ARRB2) and CCR4 using NanoBiT® Protein-Protein Interaction System. ARRB2 was fused to Large BiT (LgBit) and CCR4 was fused to Small BiT (SmBit). Constructs were expressed in BW5147.3 T cells. Interaction of fusion partners leads to structural complementation of LgBiT with SmBiT, generating a functional enzyme with a bright, luminescent signal. I, Luminescence intensity measured over 90 minutes in BW5147.3 T cells transfected with ARRB2-LgBit and CCR4-SmBit. Cells were treated with vehicle, CCL17, CCL22, and CCL22 + increasing concentrations of CCL17. J, Quantification of luminescence intensity measured by area under the curve (left panel) and peak intensity (right panel). K, Chemotaxis of BW5147.3 T cells in response to CCL22 (10 ng/mL), but not to CCL17 (10 ng/mL), was suppressed by small interfering RNAs targeting Arrb1 (Si-Arrb1), Arrb2 (Si-Arrb2), or Arrb1 and Arrb2 (Si-Arrb1&2). L, Effects of Si-Arrb1&2 and phospholipase C (PLC) inhibitor U73122 (10 μM) on the chemotaxis of BW5147.3 T cells in response to CCL22 (10 ng/mL) and CCL17 (10 ng/mL). Chemotaxis in response to CCL17 (10 ng/mL) was suppressed by U73122. Chemotaxis in response to CCL22 (10 ng/mL) was synergistically suppressed by U73122 with Si-Arrb1&2. M, Schematic of the proposed mechanism by which CCL17 and CCL22 differentially regulate Treg recruitment. All data are mean ± SD. Unpaired t test was performed in Figure A. For Figure B-L, n=3 per experimental group and one-way ANOVA followed by Tukey test was performed. ###P< 0.001, *P< 0.05, **P< 0.01, ***P< 0.001, and ns indicates non-significance.
Based on these findings, we examined the relative impact of CCL17 and CCL22 on Treg chemotaxis. Transwell cell migration assays revealed that CCL17 inhibited CCL22-induced chemotaxis of induced Tregs and CCR4 expressing BW5147.3 T cells36 in a concentration-dependent manner (Fig. 6C–D). Increasing doses of CCL22 were sufficient to augment Treg chemotaxis induced by CCL17 in T induced Tregs (Fig. 6E) and BW5147.3 T cells (Supplemental Fig. VIE). These findings suggest that CCL17 and CCL22 are competitive agonists and that CCL22 has a greater capacity to elicit chemotaxis, consistent with prior findings regarding a competitive relationship between CCL17 and CCL22.37 Both induced Tregs and BW5147.3 T cells expressed CCR4 on their cell surface and did not display evidence of internalization following CCL17 treatment (supplemental Fig. VID, K). CCR4 antagonists (C021, AZD2098) abrogated Treg migration toward CCL17 and CCL22 (Fig. 6F, G). These data suggest that the relative balance of CCL17 and CCL22 is an important determinant of Treg chemotactic capacity.
To investigate the mechanistic basis by which CCL17 and CCL22 competitively regulate Treg chemotaxis, we examined major second messenger signals downstream of CCR4 including Gαa (cyclic adenosine monophosphate, cAMP), Gαq (intracellular Ca2+), and β-arrestin. Compared to vehicle control, CCL17 and CCL22 each increased intracellular calcium levels in induced Tregs and BW5147.3 cells. The combination of CCL17 and CCL22 had a synergistic effect (Supplemental Fig. VIF, H). Neither CCL17 nor CCL22 increased cAMP levels compared to vehicle control in induced Tregs or BW5147.3 cells (Supplemental Fig. VIG, I). To examine β-arrestin (ARRB) signaling, we focused on BW5147.3 cells given their ability to be transfected. To measure physical interactions between CCR4 and ARRB, we used the NanoBiT system, which assesses intracellular protein-protein interactions by structural complementation of luciferase (Fig. 6H). BW5147.3 T cells were transiently transfected with CCR4-SmBit and ARRB2-LgBiT. Compared to vehicle control, CCL17 did not lead to binding of ARRB2 to CCR4. In contrast, robust luminescence was detected following application of CCL22 indicating binding of ARRB2 to CCR4. Intriguingly, CCL17 abrogated CCR4-ARRB2 binding in a dose dependent fashion (Fig. 6I–J).
To decipher the relative requirement for ARRB signaling in Treg chemotaxis, we transfected BW5147.3 T cells with ARRB1 (Si-Arrb1) and ARRB2 (Si-Arrb2) siRNAs. Quantitative RT-PCR and western blot analysis confirmed the knockdown efficiency of each siRNA (Supplemental Fig. VIL). ARRB1 and ARRB2 siRNAs inhibited chemotaxis of BW5147.3 T cells induced by CCL22 but not CCL17 (Fig. 6K). Application of U73122 (phospholipase C inhibitor), which blocks agonist-induced Ca2+ efflux from endoplasmic reticulum, suppressed accumulation of intracellular calcium and BW5147.3 T-cell chemotaxis induced by either CCL17 or CCL22 (Fig. 6L, Supplemental Fig. VIM). Collectively, these findings suggest that CCL22 promotes Treg chemotaxis through concomitant ARRB and Ca2+ signaling. The presence of CCL17 dampens Treg chemotaxis by inhibiting ARRB signaling (Fig. 6M). This concept is consistent with previous reports that β-arrestin2 deletion reduces the number of CD4+FOXP3+ Tregs in experimental autoimmune encephalitis38 and that CCL22 selectively induces concentration-dependent coupling of CCR4 to β-arrestin 2.39
Ccl17 deficiency suppresses heart remodeling through a Treg dependent mechanism.
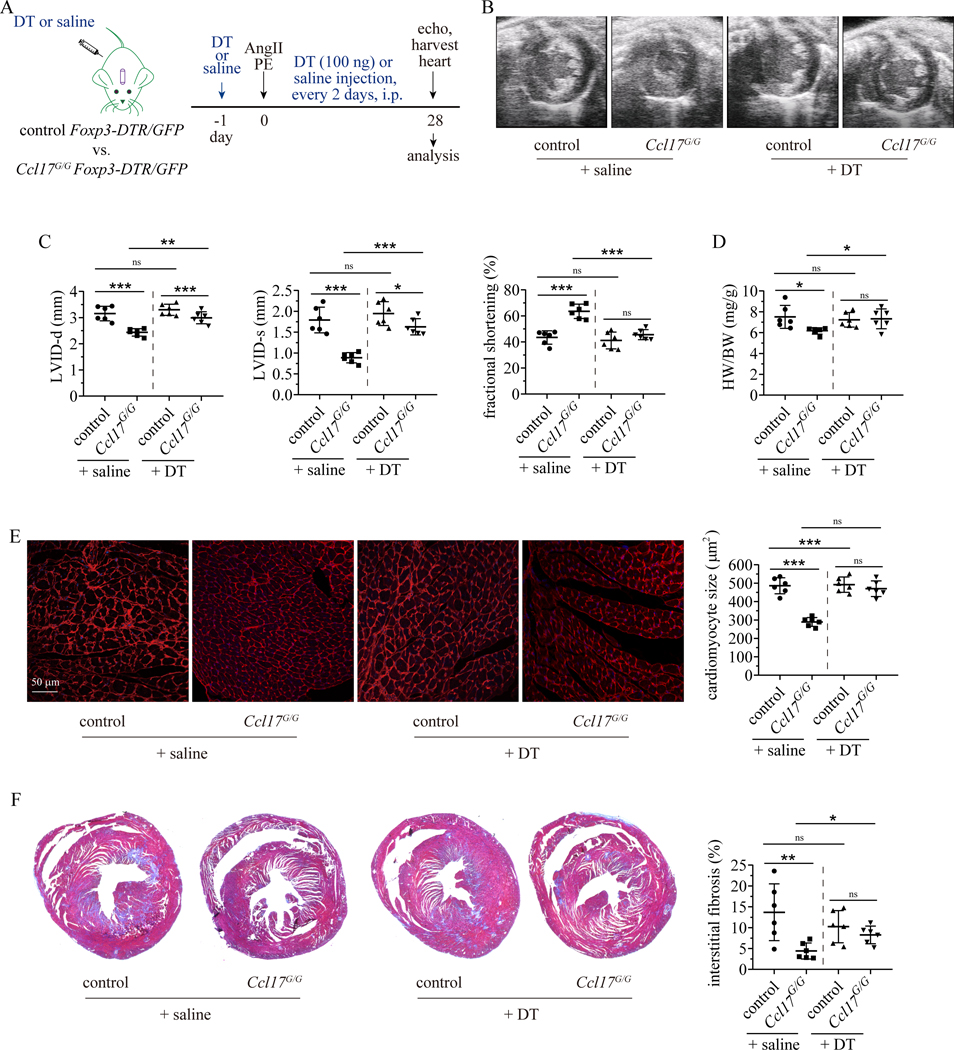
To determine whether Treg recruitment is responsible for the protective effect of Ccl17 deficiency on heart remodeling, we depleted Tregs from control and Ccl17G/G mice. To selectively deplete Tregs, we crossed control and Ccl17G/G mice to Foxp3-DTR/GFP mice. Flow cytometry confirmed efficient depletion of Tregs in the resultant progeny mice after DT (100 ng/mouse, i.p. every 2 days) treatment (Supplemental Fig. VIIA–B). For these experiments, we chose to focus on the AngII/PE model. We implanted osmotic minipumps containing AngII/PE into control and Ccl17G/G Foxp3-DTR/GFP mice and either saline or DT was injected to deplete Tregs (Fig. 7A). Echocardiography performed 28 days after AngII/PE infusion revealed that compared to control Foxp3-DTR/GFP (control + saline) mice, Ccl17G/G Foxp3-DTR/GFP (Ccl17G/G + saline) mice displayed smaller LV chamber dimensions, increased LV fractional shortening, and reduced heart weight to body weight ratio. Depletion of Tregs from Ccl17G/G Foxp3-DTR/GFP (Ccl17G/G + DT) mice led to increased LV chamber dimensions, decreased fractional shortening, and increased heart weight to body weight ratios compared to Ccl17G/G Foxp3-DTR/GFP (Ccl17G/G + saline) mice. We did not observe any significant difference between Foxp3-DTR/GFP mice treated with saline or DT (Fig. 7B–D, Supplemental Table II). WGA and trichrome staining demonstrated reduced cardiomyocyte cross-sectional area and interstitial fibrosis in Ccl17G/G Foxp3-DTR/GFP compared to Foxp3-DTR/GFP hearts treated with saline. Depletion of Tregs from Ccl17G/G Foxp3-DTR/GFP mice led to greater cardiomyocyte cross-sectional area and increased interstitial fibrosis compared to Ccl17G/G Foxp3-DTR/GFP mice that did not undergo Treg depletion. No significant differences were observed between Foxp3-DTR/GFP hearts treated with saline or DT (Fig. 7E–F, Supplemental Fig. VIIC–D). These data indicate that Ccl17 deficiency attenuates LV remodeling in a Treg dependent manner.
Figure 7. Ccl17 deficiency suppresses LV remodeling through a Treg dependent mechanism.
A, Control (Ccl17+/+) and Ccl17G/G mice were crossed with Foxp3-DTR/GFP to generate control Foxp3-DTR/GFP and Ccl17G/G Foxp3-DTR/GFP mice in which Tregs are deleted after diphtheria toxin (DT) administration. Osmotic minipumps containing AngII/PE were implanted into control Foxp3-DTR/GFP and Ccl17G/G Foxp3-DTR/GFP mice. Mice were treated with DT or normal saline every two days. Echocardiography was performed on day 28 and hearts were harvested thereafter. B, Representative echocardiographic images of control + saline, control + DT, Ccl17G/G + saline, Ccl17G/G + DT hearts 28 days after AngII/PE infusion. C, Quantification of left ventricular internal diameter at end-diastole (left panel) and at end-systole (center panel) and left ventricular fractional shortening (right panel) 28 days after AngII/PE infusion. D, Quantification of heart weight to body weight ratio (HW/BW). E, WGA staining (red) examining the effects of Treg depletion on cardiomyocyte hypertrophy of control mice and Ccl17G/G mice 28 days after AngII/PE infusion (left panel). Quantification of cardiomyocyte cross-sectional area based on WGA staining (right panel). F, Low magnification trichrome stained images (left panel) examining the effects of Treg depletion on myocardial interstitial fibrosis in control and Ccl17G/G mice 28 days after AngII/PE injury. Quantification of fibrotic area based on trichrome staining (right panel). N=6 per group and all data are mean ± SD. Two-way ANOVA with Geisser-Greenhouse correction followed by Sidak test was performed. *P< 0.05, **P< 0.01, ***P< 0.001, and ns indicates non-significance.
Treg recruitment suppresses macrophages associated inflammation.
To determine the mechanistic basis by which Treg recruitment confers protection in Ccl17 deficient mice, we first performed RNA sequencing on CD4+FOXP3+ cells isolated by FACS from control and Ccl17G/G hearts 7 days after AngII/PE infusion. Principle component analysis (PCA) and differential gene expression analysis did not demonstrate any significant differences between Tregs isolated from the hearts of control and Ccl17G/G hearts (Supplemental Fig. VIIIA–C). These data suggest that Ccl17 deficiency influences the relative number of Tregs in the heart as opposed to stimulating their activation.
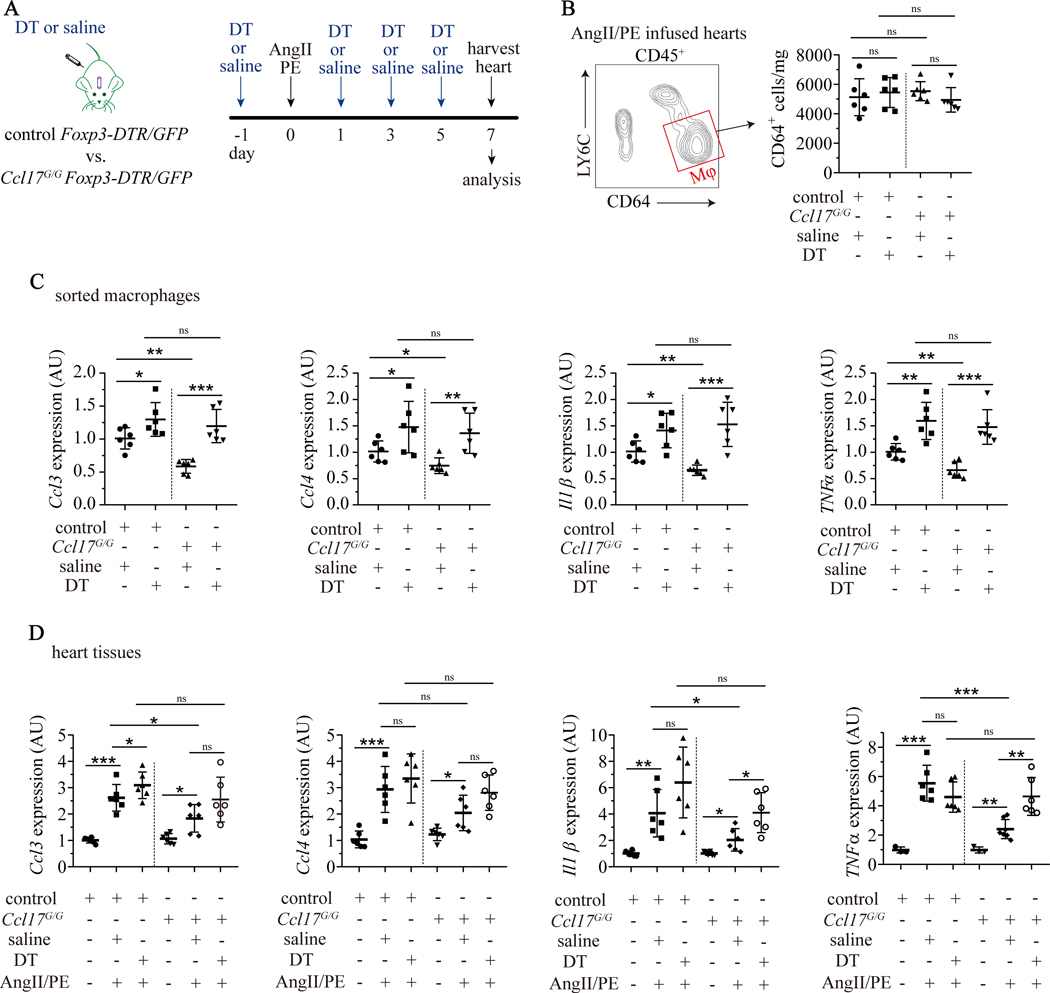
Tregs are potent negative regulators of inflammation in various biological contexts and inhibit pro-inflammatory properties of macrophages.40 To examine this possibility, we quantified macrophage abundance and pro-inflammatory gene expression 7 days after AngII/PE infusion in control and Ccl17G/G Foxp3-DTR/GFP mice treated with either saline or DT (Fig. 8A). We did not detect any significant differences in macrophages abundance between experimental groups (Fig. 8B). Next, we isolated cardiac macrophages (CD64+LY6C−) by FACS and measured the expression of pro-inflammatory cytokines and chemokines in macrophages. Quantitative RT-PCR demonstrated that compared to Foxp3-DTR/GFP mice treated with saline, macrophages isolated from Ccl17G/G Foxp3-DTR/GFP hearts treated with saline displayed reduced Ccl3, Ccl4, Il1β, and TNFα mRNA expression. Depletion of Tregs resulted in upregulation of Ccl3, Ccl4, Il1β, and TNFα mRNA expression in macrophages isolated from Foxp3-DTR/GFP and Ccl17G/G Foxp3-DTR/GFP hearts treated with DT. No significant differences were observed in Ccl17G/G Foxp3-DTR/GFP mice hearts treating with saline or DT (Fig. 8C).
Figure 8. Ccl17 deficiency suppresses macrophages associated inflammation through a Treg dependent mechanism.
A, Schematic of Treg depletion in control (Ccl17+/+) mice and Ccl17 deficient (Ccl17GFP/GFP, abbreviated as Ccl17G/G) mice infused with AngII/PE. Control Foxp3-DTR/GFP and Ccl17G/G Foxp3-DTR/GFP mice were implanted with minipumps containing AngII/PE and injected with DT (abbreviated as control + DT and Ccl17G/G + DT) or normal saline (abbreviated as control + saline and Ccl17G/G + saline) every two days. Hearts were harvested on day 7 for analysis. B, Effect of Treg deletion on cardiac macrophage abundance following AngII/PE infusion. Strategy to isolate CD45+LY6C−CD64+ macrophages by FACS from AngII/PE infused hearts (left panel). Quantification of CD64+ macrophage number per mg heart tissue (right panel). C, Quantitative RT-PCR measuring mRNA levels of chemokine (C-C) ligand 3 (Ccl3), Ccl4, Interleukin 1β (Il1β) and tumor necrosis factor α (TNFα) in sorted macrophages from AngII/PE injured hearts. D, Quantitative RT-PCR measuring mRNA levels of Ccl3, Ccl4, Il1β and TNFα in heart tissue from saline and AngII/PE infused mice. N=6 per group and all data are mean ± SD. Two-way ANOVA with Geisser-Greenhouse correction followed by Sidak test was performed. *P< 0.05, **P< 0.01, ***P< 0.001 and ns indicates non-significance.
We then assessed the expression of Ccl3, Ccl4, Il1β, and TNFα in the myocardium of Foxp3-DTR/GFP mice and Ccl17G/G Foxp3-DTR/GFP mice treated with saline or DT. Each of these pro-inflammatory mediators was increased by AngII/PE infusion. Compared to saline treated Foxp3-DTR/GFP mice infused with AngII/PE, saline treated Ccl17G/G Foxp3-DTR/GFP treated mice infused with AngII/PE exhibited blunted expression of Ccl3, Ccl4, Il1β, and TNFα mRNA in the myocardium. Depletion of Tregs reversed the protective effects of Ccl17 deficiency, resulting in expression levels of Ccl3, Ccl4, Il1β, and TNFα that were equivalent to control mice infused with AngII/PE (Fig. 8D). Gm-csf, Il6, and Ifnγ were modestly impacted by Ccl17 deletion and no effects were observed with respect to Ccl9 expression (Supplemental Fig. VIIID–E). Together, these findings demonstrate that Tregs suppress the expression of macrophage-derived pro-inflammatory mediators in Ccl17 deficient mice.
Discussion
Within the mouse and human heart, CCR2 expression identifies monocytes, macrophages, and dendritic cells with robust inflammatory potential.11, 12, 41–46 The relevant effector molecules by which CCR2+ monocytes, macrophages, and dendritic cells orchestrate cardiac inflammation remains to be elucidated. In this manuscript, we investigated the functional significance of CCL17, a chemokine specifically expressed in CCR2+ macrophages and dendritic cells. We report that Ccl17 is expressed in CCR2+ macrophages and type II conventional dendritic cells recruited to the injured heart. Within these populations, GM-CSF signaling induced CCL17 expression. Deletion of Ccl17 was sufficient to improve LV systolic function and attenuate key components of LV remodeling including LV dilation, cardiomyocyte hypertrophy, and fibrosis in response to MI and AngII/PE infusion. Ccl17 deficiency conferred protection through enhanced Treg cell recruitment and subsequent attenuation of inflammatory macrophage gene expression. Mechanistically, we identified CCL17 and CCL22 as biased CCR4 ligands with opposing effects on Treg migration. These data establish CCL17 as a pro-inflammatory mediator of CCR2+ macrophages and dendritic cells and suggest that inhibition of CCL17 may serve as an effective strategy to promote Treg recruitment and suppress damaging myocardial inflammation.
CCL17 expression is increased in various inflammatory diseases including atopic dermatitis,47 bronchial asthma,20 arthritis,23 colitis22 and atherosclerosis.24, 48 Consistent with these findings, we showed that CCL17 expression is increased following MI, AngII/PE infusion and DT cardiomyocyte ablation. We demonstrated that CCL17 is expressed in CCR2+ macrophages and type II conventional dendritic cells, in agreement with our prior finding that human CCR2+ macrophages express CCL17 in ischemic cardiomyopathy.11 Using a combination of in vitro and in vivo experiments we identified GM-CSF signaling as an essential regulator of CCL17 expression within the heart, consistent with prior studies examining CCL17 expression in the spleen and cultured macrophages.49, 50 GM-CSFR is a hematopoietic stem cell growth factor that is expressed on monocytes, macrophages and dendritic cells51, 52. GM-CSFR signaling activates several intracellular signaling pathways including JAK/STAT, MAPK, PI3K, and canonical NF-κB.51, 53 Through mechanistic studies we showed that independent activation of STAT5 and canonical NF-κB signaling is required for CCL17 expression. Inhibition of CCL17 may be favorable in several cardiovascular and inflammatory diseases. Ccl17 deficiency reduces atherosclerosis,24 colitis,22 and hypochlorite induced peritoneal fibrosis.54 We add to this argument by showing that Ccl17 deletion suppressed myocardial inflammation leading to improved LV systolic function, reduced LV remodeling, cardiomyocyte hypertrophy, and myocardial fibrosis in a model of heart failure (reperfused MI) and a model of myocardial remodeling (AngII/PE infusion).
CCL17 signals through CCR4 expressed on CD4+ helper T cells.55 We and others observed that Ccl17 deletion selectively increases Treg abundance in the diseased heart, aorta, and colon.22, 24, 56 Tregs attenuate inflammation through effects on macrophages, effector T cells, and fibroblasts.57 In each of these scenarios, Treg depletion was sufficient to reverse the protective effects of Ccl17 deficiency. Within the injured heart, Ccl17 deletion reduced pro-inflammatory chemokine and cytokine expression in macrophages in a Treg-dependent manner. Intriguingly, RNA sequencing of Tregs isolated from injured control and Ccl17−/− hearts did not reveal evidence of altered gene expression or function indicating that Ccl17 deletion impacted Treg recruitment and not activation. Together, these findings suggest a generalized role for CCL17 in regulating Treg trafficking and identify Ccl17 as a therapeutic target to expand Tregs, limit tissue inflammation, and improve organ function.
Little is understood regarding the mechanisms by which Tregs are recruited to the heart and no effective strategies exist to expand Tregs within the injured myocardium. We provide new insights into the mechanism by which CCR4 ligands (CCL17, CCL22) regulate Treg recruitment. CCL17 and CCL22 are co-expressed following myocardial injury. However, their relative concentration within the infarct are not known. Using primary Tregs and a Treg cell line, we show that CCL17 and CCL22 are competitive biased ligands for CCR4. CCL17 selectively activated Gq signaling, while CCL22 simultaneously activated Gq and β-arrestin signaling downstream of CCR4. We further demonstrated that CCL17 competitively inhibited CCL22 stimulated β-arrestin signaling and Treg chemotaxis. We cannot exclude the possibility that CCL17 inhibits CCL22 through additional mechanisms that are independent of CCR4 in vivo. Indeed, CCL22 promotes interactions between dendritic and regulatory T-cells within lymph nodes and regulates immunity.58 These findings provide an explanation for how CCL17 might inhibit Treg recruitment in the injured heart and highlight the possible use of biased CCR4 agonists59 as therapeutics to increase Treg recruitment and suppress inflammation.
This study is not without limitations. While we exclusively detected CCL17 expression in macrophages and dendritic cells within the heart, we cannot exclude the possibility that CCL17 expressing cells outside of the heart contributed to the observed protection following cardiac injury. Conditional CCL17 knockout mice, cardiac macrophage, and dendritic specific Cre recombinases are not currently available. Furthermore, while we included multiple models of cardiac injury, it is possible that CCL17 deletion may not be protective in all cardiac pathologies. Finally, future studies are needed to delineate the potential benefit of altering the balance of CCL17 and CCL22 signaling in vivo and assess the therapeutic window of CCL17 inhibition.
In conclusion, our findings identify CCL17 as a pro-inflammatory mediator of CCR2+ macrophages and dendritic cells and suggest that inhibition of CCL17 may serve as an effective strategy to promote Treg recruitment, suppress myocardial inflammation, and attenuate LV remodeling.
Supplementary Material
Clinical Perspective.
What is new?
C-C chemokine ligand 17 is an inflammatory mediator expressed in CCR2+ macrophages and CD11b+ conventional dendritic cells recruited to the heart following myocardial injury.
GM-CSF signaling is a key regulator of CCL17 expression through cooperative activation of STAT5 and canonical NF-κB signaling.
Ccl17 deletion attenuates inflammatory cytokine/chemokine expression, left ventricular remodeling and myocardial fibrosis in mouse models of reperfused MI and hypertrophy through increased recruitment of regulatory T-cells to the injured heart.
What are the clinical implications?
Identification of effector molecules that mediate adverse effects of CCR2+ monocytes, macrophages, and dendritic cells provided new insights into therapeutic strategies to suppress myocardial inflammation
Inhibition of CCL17 expression or neutralization of CCL17 signaling may serve as an effective strategy to promote regulatory T-cell recruitment and suppress myocardial inflammation.
Acknowledgments
We thank the Genome Technology Access Center at Washington University School of Medicine. The Center is partially supported by NCI Cancer Center Support Grant #P30 CA91842 to the Siteman Cancer Center and by ICTS/CTSA Grant# UL1TR002345 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. We recognize the mouse cardiovascular phenotyping core for performing echocardiography.
G.F. performed the immunostaining, cell culture, TTC, histology, RNA sequencing, flow cytometry, and cell culture experiments. G.B. and A.B. assisted with the flow cytometry experiments. P.A., A.K. and L.L. assisted with the RNAseq analyses. I.L. assisted with animal treatments, breeding, and harvests. I.F. provided the Ccl17GFP mice. D.K. provided Foxp3-GFP/DTR mice and assisted with experimental design and critical review of the manuscript. K.L. is responsible for all aspects of this manuscript including experimental design, data analysis, and manuscript production.
Sources of Funding
KL is supported by the National Institutes of Health [R01 HL138466, R01 HL139714, R01 HL151078, R35 HL161185], Leducq Foundation Network (#20CVD02), Burroughs Welcome Fund (1014782), Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital (CH-II-2015–462, CH-II-2017–628, PM-LI-2019–829), Amgen, and Foundation of Barnes-Jewish Hospital (8038–88).
Non-standard Abbreviations and Acronyms
- AngII
Angiotensin II
- AKT
protein kinase B
- ARRB
β-arrestin
- BMDMs
bone marrow-derived macrophages
- cAMP
cyclic adenosine monophosphate
- CCL
C-C chemokine ligand
- CCR
C-C chemokine receptor type
- CD
cluster of differentiation
- CXCL
C-X-C chemokine ligand
- DT
diphtheria toxin
- DTR
diphtheria toxin receptor
- FACS
fluorescence activated cell sorting
- FOXP3
forkhead box P3
- GFP
green fluorescent protein
- GM-CSF
granulocyte-macrophage colony stimulating factor
- IKK-2
inhibitor of nuclear factor kappa-B kinase subunit β
- IL
interleukin
- IR
ischemia reperfusion
- JAK
Janus kinase
- LPS
lipopolysaccharides
- LV
left ventricle
- LY6C
lymphocyte antigen 6 complex, locus C
- LY6G
lymphocyte antigen 6 complex, locus G6D
- MAPK
mitogen-activated protein kinase
- MertK
MER proto-oncogene tyrosine kinase
- MHCII
major histocompatibility complex II
- MI
myocardial infarction
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PE
phenylephrine
- PI3K
phosphatidyl-inositol 3-kinase
- pSTAT5
phosphorylated signal transducer and activator of transcription 5
- Poly I:C
polycytidylic acid
- RT-PCR
reverse transcription polymerase chain reaction
- STAT5
signal transducer and activator of transcription 5
- TGF-β
transforming growth factor-β
- Tnnt2
troponin T2
- Treg
regulatory T cell
- ZBTB46
Zinc finger and BTB domain-containing protein 46
Footnotes
Disclosures
No conflicts
REFERENCES
- 1.Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, Von Schlippenbach J, Skurk C, Steendijk P, Riad A. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circulation: Heart Failure. 2011;4:44–52. [DOI] [PubMed] [Google Scholar]
- 2.Marchant DJ, Boyd JH, Lin DC, Granville DJ, Garmaroudi FS, McManus BM. Inflammation in myocardial diseases. Circulation research. 2012;110:126–144. [DOI] [PubMed] [Google Scholar]
- 3.Hanna A, Frangogiannis NG. Inflammatory Cytokines and Chemokines as Therapeutic Targets in Heart Failure. Cardiovascular Drugs and Therapy. 2020;34:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gullestad L, Ueland T, Vinge LE, Finsen A, Yndestad A, Aukrust P. Inflammatory cytokines in heart failure: mediators and markers. Cardiology. 2012;122:23–35. [DOI] [PubMed] [Google Scholar]
- 5.Adamo L, Rocha-Resende C, Prabhu SD, Mann DL. Reappraising the role of inflammation in heart failure. Nature Reviews Cardiology. 2020;17:269–285. [DOI] [PubMed] [Google Scholar]
- 6.Shirazi LF, Bissett J, Romeo F, Mehta JL. Role of inflammation in heart failure. Current atherosclerosis reports. 2017;19:27. [DOI] [PubMed] [Google Scholar]
- 7.Oikonomou E, Tousoulis D, Siasos G, Zaromitidou M, Papavassiliou AG, Stefanadis C. The role of inflammation in heart failure: new therapeutic approaches. Hellenic J Cardiol. 2011;52:30–40. [PubMed] [Google Scholar]
- 8.Nahrendorf M, Swirski FK. Monocyte and macrophage heterogeneity in the heart. Circulation research. 2013;112:1624–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annual review of immunology. 2013;31:563–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxidants & redox signaling. 2014;20:1126–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bajpai G, Schneider C, Wong N, Bredemeyer A, Hulsmans M, Nahrendorf M, Epelman S, Kreisel D, Liu Y, Itoh A. The human heart contains distinct macrophage subsets with divergent origins and functions. Nature medicine. 2018;24:1234–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen B, Brickshawana A, Frangogiannis NG. The Functional Heterogeneity of Resident Cardiac Macrophages in Myocardial Injury: CCR2+ Cells Promote Inflammation, Whereas CCR2− Cells Protect. 2019;124:183–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel B, Bansal SS, Ismahil MA, Hamid T, Rokosh G, Mack M, Prabhu SD. CCR2+ monocyte-derived infiltrating macrophages are required for adverse cardiac remodeling during pressure overload. JACC: Basic to Translational Science. 2018;3:230–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frangogiannis NG, Dewald O, Xia Y, Ren G, Haudek S, Leucker T, Kraemer D, Taffet G, Rollins BJ, Entman ML. Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation. 2007;115:584–592. [DOI] [PubMed] [Google Scholar]
- 16.Bajpai G, Bredemeyer A, Li W, Zaitsev K, Koenig AL, Lokshina I, Mohan J, Ivey B, Hsiao HM, Weinheimer C, et al. Tissue Resident CCR2- and CCR2+ Cardiac Macrophages Differentially Orchestrate Monocyte Recruitment and Fate Specification Following Myocardial Injury. Circ Res. 2019;124:263–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen B, Frangogiannis NG. Chemokines in myocardial infarction. Journal of cardiovascular translational research. 2020:1–18. [DOI] [PubMed] [Google Scholar]
- 18.Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, Sugai H, Fujii H. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. International journal of cancer. 2008;122:2286–2293. [DOI] [PubMed] [Google Scholar]
- 19.Tsunemi Y, Saeki H, Nakamura K, Nagakubo D, Nakayama T, Yoshie O, Kagami S, Shimazu K, Kadono T, Sugaya M. CCL17 transgenic mice show an enhanced Th2-type response to both allergic and non-allergic stimuli. European journal of immunology. 2006;36:2116–2127. [DOI] [PubMed] [Google Scholar]
- 20.Staples KJ, Hinks TS, Ward JA, Gunn V, Smith C, Djukanović R. Phenotypic characterization of lung macrophages in asthmatic patients: overexpression of CCL17. Journal of allergy and clinical immunology. 2012;130:1404–1412. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riis JL, Johansen C, Vestergaard C, Bech R, Kragballe K, Iversen L. Kinetics and differential expression of the skin-related chemokines CCL27 and CCL17 in psoriasis, atopic dermatitis and allergic contact dermatitis. Experimental dermatology. 2011;20:789–794. [DOI] [PubMed] [Google Scholar]
- 22.Heiseke AF, Faul AC, Lehr HA, Förster I, Schmid RM, Krug AB, Reindl W. CCL17 Promotes Intestinal Inflammation in Mice and Counteracts Regulatory T Cell–Mediated Protection From Colitis. Gastroenterology. 2012;142:335–345. [DOI] [PubMed] [Google Scholar]
- 23.Radstake TR, van der Voort R, ten Brummelhuis M, de Waal Malefijt M, Looman M, Figdor C, van den Berg W, Barrera P, Adema G. Increased expression of CCL18, CCL19, and CCL17 by dendritic cells from patients with rheumatoid arthritis, and regulation by Fc gamma receptors. Annals of the rheumatic diseases. 2005;64:359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber C, Meiler S, Döring Y, Koch M, Drechsler M, Megens RT, Rowinska Z, Bidzhekov K, Fecher C, Ribechini E. CCL17-expressing dendritic cells drive atherosclerosis by restraining regulatory T cell homeostasis in mice. The Journal of clinical investigation. 2011;121:2898–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alferink J, Lieberam I, Reindl W, Behrens A, Weiß S, Hüser N, Gerauer K, Ross R, Reske-Kunz AB, Ahmad-Nejad P. Compartmentalized production of CCL17 in vivo: strong inducibility in peripheral dendritic cells contrasts selective absence from the spleen. The Journal of experimental medicine. 2003;197:585–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nature immunology. 2007;8:191–197. [DOI] [PubMed] [Google Scholar]
- 27.Satpathy AT, Kc W, Albring JC, Edelson BT, Kretzer NM, Bhattacharya D, Murphy TL, Murphy KM. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. Journal of Experimental Medicine. 2012;209:1135–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nossuli TO, Frangogiannis NG, Knuefermann P, Lakshminarayanan V, Dewald O, Evans AJ, Peschon J, Mann DL, Michael LH, Entman ML. Brief murine myocardial I/R induces chemokines in a TNF-alpha-independent manner: role of oxygen radicals. Am J Physiol Heart Circ Physiol. 2001;281:H2549–58. [DOI] [PubMed] [Google Scholar]
- 29.Heo GS, Kopecky B, Sultan D, Ou M, Feng G, Bajpai G, Zhang X, Luehmann H, Detering L, Su Y, et al. Molecular Imaging Visualizes Recruitment of Inflammatory Monocytes and Macrophages to the Injured Heart. Circ Res. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruland C, Renken H, Kuzmanov I, Mehr AF, Schwarte K, Cerina M, Herrmann A, Otte D-M, Zimmer A, Schwab N. Chemokine CCL17 is expressed by dendritic cells in the CNS during experimental autoimmune encephalomyelitis and promotes pathogenesis of disease. Brain, behavior, and immunity. 2017;66:382–393. [DOI] [PubMed] [Google Scholar]
- 31.Satpathy AT, Kc W, Albring JC, Edelson BT, Kretzer NM, Bhattacharya D, Murphy TL, Murphy KM. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J Exp Med. 2012;209:1135–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van de Laar L, Coffer PJ, Woltman AM. Regulation of dendritic cell development by GM-CSF: molecular control and implications for immune homeostasis and therapy. Blood. 2012;119:3383–93. [DOI] [PubMed] [Google Scholar]
- 33.Yuan Q, Bromley SK, Means TK, Jones KJ, Hayashi F, Bhan AK, Luster AD. CCR4-dependent regulatory T cell function in inflammatory bowel disease. The Journal of experimental medicine. 2007;204:1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugiyama D, Nishikawa H, Maeda Y, Nishioka M, Tanemura A, Katayama I, Ezoe S, Kanakura Y, Sato E, Fukumori Y. Anti-CCR4 mAb selectively depletes effector-type FoxP3+ CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proceedings of the National Academy of Sciences. 2013;110:17945–17950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu L, Barbi J, Pan F. The regulation of immune tolerance by FOXP3. Nat Rev Immunol. 2017;17:703–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fülle L, Steiner N, Funke M, Gondorf F, Pfeiffer F, Siegl J, Opitz FV, Haßel SK, Erazo AB, Schanz O. RNA aptamers recognizing murine CCL17 inhibit T cell chemotaxis and reduce contact hypersensitivity in vivo. Molecular Therapy. 2018;26:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mariani M, Lang R, Binda E, Panina-Bordignon P, D’Ambrosio D. Dominance of CCL22 over CCL17 in induction of chemokine receptor CCR4 desensitization and internalization on human Th2 cells. European journal of immunology. 2004;34:231–240. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Liu C, Wei B, Pei G. Loss of beta-arrestin 2 exacerbates experimental autoimmune encephalomyelitis with reduced number of Foxp3+ CD4+ regulatory T cells. Immunology. 2013;140:430–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ajram L, Begg M, Slack R, Cryan J, Hall D, Hodgson S, Ford A, Barnes A, Swieboda D, Mousnier A, et al. Internalization of the chemokine receptor CCR4 can be evoked by orthosteric and allosteric receptor antagonists. Eur J Pharmacol. 2014;729:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin J, Li M, Wang Z, He S, Ma X, Li D. The role of CD4+CD25+ regulatory T cells in macrophage-derived foam-cell formation. J Lipid Res. 2010;51:1208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA. Revisiting cardiac cellular composition. Circulation research. 2016;118:400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Willenborg S, Lucas T, Van Loo G, Knipper JA, Krieg T, Haase I, Brachvogel B, Hammerschmidt M, Nagy A, Ferrara N. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood, The Journal of the American Society of Hematology. 2012;120:613–625. [DOI] [PubMed] [Google Scholar]
- 43.Lee JS, Jeong S-J, Kim S, Chalifour L, Yun TJ, Miah MA, Li B, Majdoubi A, Sabourin A, Keler T. Conventional dendritic cells impair recovery after myocardial infarction. The Journal of Immunology. 2018;201:1784–1798. [DOI] [PubMed] [Google Scholar]
- 44.Lavine KJ, Pinto AR, Epelman S, Kopecky BJ, Clemente-Casares X, Godwin J, Rosenthal N, Kovacic JC. The macrophage in cardiac homeostasis and disease: JACC macrophage in CVD series (Part 4). Journal of the American College of Cardiology. 2018;72:2213–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dick SA, Macklin JA, Nejat S, Momen A, Clemente-Casares X, Althagafi MG, Chen J, Kantores C, Hosseinzadeh S, Aronoff L. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nature immunology. 2019;20:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clemente-Casares X, Hosseinzadeh S, Barbu I, Dick SA, Macklin JA, Wang Y, Momen A, Kantores C, Aronoff L, Farno M. A CD103+ conventional dendritic cell surveillance system prevents development of overt heart failure during subclinical viral myocarditis. Immunity. 2017;47:974–989. e8. [DOI] [PubMed] [Google Scholar]
- 47.Thymus Kataoka Y. and activation-regulated chemokine as a clinical biomarker in atopic dermatitis. The Journal of dermatology. 2014;41:221–229. [DOI] [PubMed] [Google Scholar]
- 48.Ye Y, Yang X, Zhao X, Chen L, Xie H, Zeng Y, Shen Z, Fan Z, Liu Z, Zhang S. Serum chemokine CCL17/thymus activation and regulated chemokine is correlated with coronary artery diseases. Atherosclerosis. 2015;238:365–369. [DOI] [PubMed] [Google Scholar]
- 49.Globisch T, Steiner N, Fulle L, Lukacs-Kornek V, Degrandi D, Dresing P, Alferink J, Lang R, Pfeffer K, Beyer M, et al. Cytokine-dependent regulation of dendritic cell differentiation in the splenic microenvironment. Eur J Immunol. 2014;44:500–10. [DOI] [PubMed] [Google Scholar]
- 50.Achuthan A, Cook AD, Lee MC, Saleh R, Khiew HW, Chang MW, Louis C, Fleetwood AJ, Lacey DC, Christensen AD, et al. Granulocyte macrophage colony-stimulating factor induces CCL17 production via IRF4 to mediate inflammation. J Clin Invest. 2016;126:3453–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van de Laar L, Coffer PJ, Woltman AM. Regulation of dendritic cell development by GM-CSF: molecular control and implications for immune homeostasis and therapy. Blood. 2012;119:3383–3393. [DOI] [PubMed] [Google Scholar]
- 52.Rosas M, Gordon S, Taylor PR. Characterisation of the expression and function of the GM-CSF receptor α-chain in mice. European journal of immunology. 2007;37:2518–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ju SM, Song HY, Lee SJ, Seo WY, Sin DH, Goh AR, Kang Y-H, Kang I-J, Won M-H, Yi J-S. Suppression of thymus-and activation-regulated chemokine (TARC/CCL17) production by 1, 2, 3, 4, 6-penta-O-galloyl-β-d-glucose via blockade of NF-κB and STAT1 activation in the HaCaT cells. Biochemical and biophysical research communications. 2009;387:115–120. [DOI] [PubMed] [Google Scholar]
- 54.Chen YT, Hsu H, Lin CC, Pan SY, Liu SY, Wu CF, Tsai PZ, Liao CT, Cheng HT, Chiang WC. Inflammatory macrophages switch to CCL17-expressing phenotype and promote peritoneal fibrosis. The Journal of pathology. 2020;250:55–66. [DOI] [PubMed] [Google Scholar]
- 55.Andrew DP, Ruffing N, Kim CH, Miao W, Heath H, Li Y, Murphy K, Campbell JJ, Butcher EC, Wu L. CC chemokine receptor 4 expression defines a major subset of circulating nonintestinal memory T cells of both Th1 and Th2 potential. The Journal of immunology. 2001;166:103–111. [DOI] [PubMed] [Google Scholar]
- 56.Weber C, Meiler S, Doring Y, Koch M, Drechsler M, Megens RT, Rowinska Z, Bidzhekov K, Fecher C, Ribechini E, et al. CCL17-expressing dendritic cells drive atherosclerosis by restraining regulatory T cell homeostasis in mice. J Clin Invest. 2011;121:2898–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH 17 and regulatory T cells. Nature. 2006;441:235–238. [DOI] [PubMed] [Google Scholar]
- 58.Rapp M, Wintergerst MWM, Kunz WG, Vetter VK, Knott MML, Lisowski D, Haubner S, Moder S, Thaler R, Eiber S, et al. CCL22 controls immunity by promoting regulatory T cell communication with dendritic cells in lymph nodes. J Exp Med. 2019;216:1170–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anderson CA, Solari R, Pease JE. Biased agonism at chemokine receptors: obstacles or opportunities for drug discovery? J Leukoc Biol. 2016;99:901–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.