Abstract
The COVID-19 pandemic continues to have profound health, social, psychological, and economic ramifications. Infection by COVID-19 has been of concern in people who use opioids, as opioid use has been known to mediate immunosuppression and is associated with respiratory depression and end-organ damage. With differing modalities of opioid usage, the association between opioids and COVID-19 outcomes is not well understood.
We performed a comprehensive systematic search of seven health science databases, including PubMed, Embase, Cochrane Library, Web of Science, China National Knowledge Infrastructure (CNKI), and Wanfang Data, up to December 15, 2021. We identified a total of five related articles, which were included in this study.
The meta-analysis showed that opioids have a significant association with ICU admission for COVID-19 patients (OR = 5.41, 95%CI: 1.85 to 15.79, P = 0.002). Use of opioids was also associated with higher mortality among patients with COVID-19 compared to non-users (OR = 2.74, 95%CI: 1.34 to 5.62, P = 0.034), while use of opioids was not significantly associated with need for mechanical ventilation (OR = 3.68, 95%CI: 0.85 to 15.90, P = 0.081). Furthermore, the adjusted analysis indicated that COVID-19 patients with a history of opioid use were more likely to be admitted to the ICU (OR = 3.57, 95%CI: 3.05 to 4.17, P<0.001) and have higher mortality rates (OR = 1.72, 95%CI: 1.09 to 2.72, P = 0.02), while there was no significant association with need for mechanical ventilation (OR = 2.09, 95%CI: 0.77 to 5.64, P = 0.146). Significant heterogeneity existed across the included studies.
Patients using opioids with COVID-19 were at higher risk of ICU admission and mortality. Prospective studies are required to confirm these findings.
Keywords: COVID-19, Opioid, Severe, Mortality, meta-analysis
1. Introduction
As we reach two years since the discovery of coronavirus disease (COVID-19), the pandemic continues to rapidly evolve and have profound health, social, psychological, and economic ramifications [1,2]. Clinical presentation of COVID-19 is heterogeneous and involves multiple organ systems, with common symptoms including fever, cough, myalgia or fatigue, expectoration, and dyspnea [3,4]. Demographic features already identified as risk factors to severe illness or mortality from COVID-19 include age over 75, male sex, severe obesity and associated comorbidities, and presence of active cancer [5]. The omicron variant has been shown to be 10 times more infectious than the wild-type virus, and its vaccine-escape capability shown to be 14 times higher than that of the Delta variant [6]. Due to this, the discovery of the omicron variant has created additional pressures on healthcare systems and resource utilization. In the emergency department in particular, there is a crucial need to reduce the likelihood of overcrowding and have efficient methods of triage, furthering the need for an understanding of prognostic factors of COVID-19 infection [7].
The current opioid epidemic continues to be a profound public health concern with opioid-related deaths increasing and reaching over 68,000 in the United States in a single 12-month period [8]. Those seeking treatment for substance-use disorders are a vulnerable and marginalized population that depend on and benefit from in-person healthcare delivery [9,10]. The pandemic has exacerbated the opioid crisis by disrupting this care, further increasing numbers of overdose events as well as opioid-related hospitalizations and deaths [[11], [12], [13]]. Opioids are also known to have immunosuppressive effects; thus, long-term use of opioids is associated with increased risk of infections and mortality [14,15]. The use of prescribed opioids in active-phase cancer treatment, palliative care, and end-of-life care may therefore disproportionately affect the already immunocompromised patient.
In addition to opioid use disorder, opioids are also used perioperatively and in the treatment of chronic pain, with unclear associations with COVID-19 infection. In cases of moderate to severe pain caused by COVID-19 infections, opioids may be used for pain management; however, higher doses are required in those who use opioids, increasing the risk of toxicity and overdose [16]. Increased opioid usage is associated with a higher incidence of renal toxicity and acute kidney injury, the latter of which has been identified as a poor prognostic factor for COVID-19 [17]. Opioid-induced respiratory depression is also a negative indicator for COVID-19 prognosis [17].
Currently, more information is needed before prediction models for COVID-19 can be recommended for clinical guidance [18]. Proposed prognostic models to date have considered a combination of features derived from computed tomography scoring, lactate dehydrogenase, C reactive protein, lymphocyte count, comorbidity, age, and sex as predictors [18]. The significant biopsychosocial effects associated with opioid use warrant its investigation as a prognostic factor. However, with differing modalities of opioid usage and variations in preliminary findings, there remains a limited understanding of the association between opioid medications and COVID-19 outcomes. In this study, we perform the first systematic review and meta-analysis in the literature investigating the association between opioid usage and COVID-19 prognosis.
2. Methods
This meta-analysis was prepared following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [19]. To identify all relevant literature, a comprehensive search of PubMed, Embase, Cochrane Library, Web of Science, China National Knowledge Infrastructure (CNKI), and Wanfang Data from January 1, 2019, to December 15, 2021 was conducted. The following search terms were used, with no language restrictions: ((2019-nCoV) or (2019 novel coronavirus) or (coronavirus disease 2019) or (SARS-CoV-2) or (COVID-19) or (COVID-19[MeSH Terms]) or (coronavirus disease 2019[MeSH Terms]) or (2019-nCoV[MeSH Terms]) or (novel coronavirus[MeSH Terms])) AND ((opioid) or (opiate) or (morphine) or (oxycodone) or (methadone) or (fentanyl) or (tramadol) or (opioid[MeSH Terms]) or (opiate[MeSH Terms]) or (morphine[MeSH Terms]) or (oxycodone[MeSH Terms]) or (methadone[MeSH Terms]) or (fentanyl[MeSH Terms]) or (tramadol[MeSH Terms])). We also analyzed our listed references as well as the included articles of all eligible studies and performed a manual search of related articles to identify additional potential studies. The initial screening of titles and abstracts was done by two independent investigators (YW and GA) who retrieved full-length articles of all potential studies. Afterward, screening using the eligibility criteria was conducted, in which studies were only included if they (1) enrolled patients with diagnosis of COVID-19; (2) provided a comparison of the clinical outcomes of patients treated with opioids versus those who were not; (3) provided an odds ratio (OR) with 95% confidence interval (CI) for outcomes of interest, or data such as overall survival or relevant clinical events from which they could be calculated. Studies were excluded if they were reviews, editorials, abstracts, or conference presentations. All decisions regarding eligibility were made according to pre-specified selection criteria. Any discrepancies were resolved through consensus or discussion with a third investigator.
Relevant elements from each screened article were independently extracted by two reviewers (YW and JL). The following parameters were obtained from each study if available: first author's name, year of publication, number of participants, demographic information including age and gender, study design, country of origin, definition of opioid, adjusted variables, and outcomes of interest. The primary endpoint was the effect of opioid use on mortality and disease severity of COVID-19. The Jadad scale for randomized controlled studies was used to conduct a study quality assessment [20]. For non-randomized controlled studies, study quality was assessed using a nine-item Newcastle-Ottawa Scale (NOS) [21] by two independent investigators (YW and GA). Any discrepancies were resolved by re-evaluation and consensus among the authors.
Statistical analysis was performed using RevMan 5.3 (Cochrane Collaboration, London, UK) and Stata 12.0 (StataCorp, LLC, College Station, TX, USA). Unadjusted odds ratios (ORs) and adjusted ORs with 95% CIs were used as the summary statistic for dichotomous outcomes. Overall risk estimates for unadjusted and adjusted dichotomous data were calculated using the Mantel–Haenszel and inverse-variance methods, respectively. Statistical heterogeneity of all included studies was evaluated by Cochran's Q test and I2 statistic, where a Q-statistic I2 > 50% or a P < 0.05 suggested high heterogeneity. For cases where I2 > 50%, a random-effect model was used to assess the impact of an intervention. A fixed-effect model was implemented for cases where I2 < 50%. Sensitivity and subgroup analyses were done based on country of origin and other factors that may cause heterogeneity. A P value of <0.05 was considered statistically significant. This study is registered with PROSPERO, number CRD42022300217.
3. Results
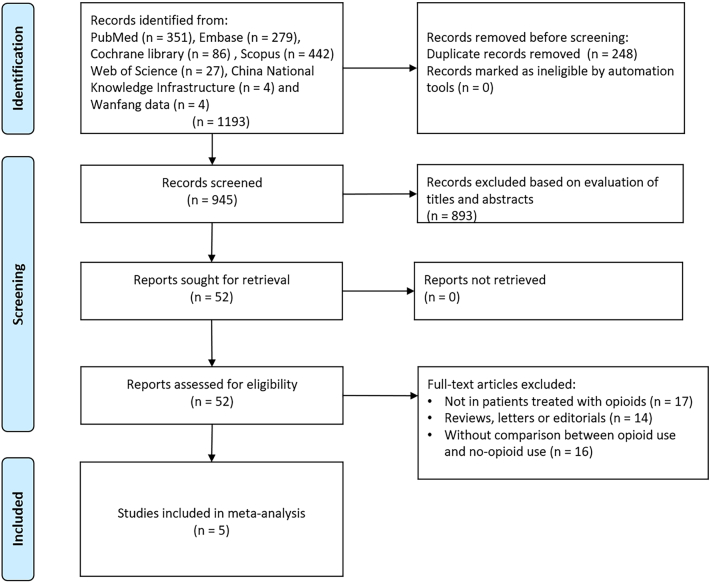
The electronic literature search identified 1193 potentially eligible articles. Among them, 248 studies were excluded due to repetition. Subsequently, 893 articles were considered irrelevant following evaluation of titles and abstracts. A full text review of the remaining 52 studies was then conducted, and 47 records were eliminated since they were reviews, abstract, letters, conferences, without comparison between opioid use and no-opioid use, or without clinical outcomes reported. Therefore, a total of 5 studies with 1,023,224 patients were included in the final analysis [[22], [23], [24], [25], [26]]. The process of study retrieval is shown in Fig. 1. All five studies were observational studies with four from the U.S.A and one from South Korea. The detailed characteristics of each study are shown in Table 1. Some studies adjusted for factors such as age, sex, and comorbidities. The details of quality assessment using the NOS tool are presented in Table 2. All studies included in our meta-analysis were of high quality. One study [18] categorized opioids into strong and weak opioids. Since the results of strong and weak opioids could not be combined, the results of strong opioids were used to best reflect the effects of opioids on clinical outcomes.
Fig. 1.
Flow diagram of literature search and study selection.
Table 1.
Characteristics of included studies.
| Study | Region | Opioid |
No opioid |
Study design | Sample size | Definition of opioid | Adjusted variables | NOS score | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | Male (%) | Age | Male (%) | |||||||
| Allen [22] 2020 | U.S.A | NR | NR | NR | NR | Retrospective cohort | 11,830 | ICD-10 codes F11 (opioid use disorder) | Sex, age, race and comorbidity | 8 |
| Oh [23] 2021 |
South Korea | NR | NR | NR | NR | Retrospective cohort | 7713 | All opiates except codeine, dihydrocodeine, hydrocodone, and tramadol. | Sex, age, race, annual income level in 2020 and comorbidity | 8 |
| Qeadan [24] 2021 | U.S.A | 60 (48–70) | 502 (49.6) | 53 (35–68) | 25,298 (49.3) | Retrospective cohort | 52,312 | Measured by past opioid overdose or opioid use disorders recorded in ICD-9 or ICD-10 codes | Age, gender, race/ethnicity, insurance, region, diabetes mellitus, asthma, hypertension, hydroxychloroquine, remdesivir, decadron or prednisone, aspirin and plavix | 8 |
| Tuan [25] 2021 | U.S.A | 52.1 ± 17.1 | 3764 (39.4) | 43.1 ± 17.6 | 199,947 (48.9) | Retrospective cohort | 418,216 | Individuals are prescribed with opioids in three or more consecutive months or at least 90 days at outpatient settings. | Age, sex, race/ethnicity and comorbidities (diabetes, essential hypertension, chronic pulmonary conditions, cardiovascular diseases, mental health disorders) | 8 |
| Wiener [26] 2021 | U.S.A | 71.8 ± 6.3 | 3382 (45.1) | 74.1 ± 7.3 | 245,186 (46.9) | Retrospective cohort | 533,153 | The unhealthful opioid use was defined with ICD-10_CM code F11 | Sex, race, nicotine dependence, socioeconomic status, body mass index, diabetes, and chronic lower respiratory disease | 8 |
NOS: Newcastle-Ottawa scale; ICD-10-CM: international classification of diseases, tenth revision, clinical modification; NR: not reported.
Table 2.
Study quality assessment using the Newcastle-Ottawa scale.
| Selection |
Outcome |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| First author, year of publication (reference) | Representativeness of exposed cohort | Selection of nonexposed cohort | Ascertainment of exposure | Outcome of interest absent at start of study | Comparability | Assessment of outcome | Follow-up long enough for outcomes to occur | Adequacy of follow-up | Total score |
| Allen | * | * | * | * | * * | * | … | … | 7 |
| Oh | * | * | * | * | * * | * | … | … | 7 |
| Qeadan | * | * | * | * | * * | * | … | … | 7 |
| Tuan | * | * | * | * | * * | * | … | … | 7 |
| Wiener | * | * | * | * | * * | * | … | … | 7 |
The meta-analysis of unadjusted results showed that opioid use has a significant association with ICU admission (OR = 5.41, 95%CI: 1.85 to 15.79, P = 0.002; I2 = 93.3%) (Fig. 2A). In addition, opioid use was associated with higher mortality among patients with COVID-19 compared to non-users (OR = 2.74, 95%CI: 1.34 to 5.62, P = 0.034; I2 = 98.6%) (Fig. 2B), while opioid use was not significantly associated with need for mechanical ventilation (OR = 3.68, 95%CI: 0.85 to 15.90, P = 0.081; I2 = 99.6%) (Fig. 2C). The adjusted analysis reinforced these results: COVID-19 patients with a history of opioid use were more likely to be admitted to the ICU (OR = 3.57, 95%CI: 3.05 to 4.17, P<0.001; I2 = 10.4%) (Fig. 3A) and have higher mortality rates (OR = 1.72, 95%CI: 1.09 to 2.72, P = 0.02; I2 = 93.4%) (Fig. 3B), while there was no significant association with need for mechanical ventilation (OR = 2.09, 95%CI: 0.77 to 5.64, P = 0.146; I2 = 98.4%) (Fig. 3E). There was substantial heterogeneity across the studies. Subgroup analysis based on countries (U.S.A versus South Korea) and sensitivity analysis by excluding one study [18] revealed non-significant results for mortality rate with an unadjusted OR = 1.853 (95% CI:0.875–3.925;P = 0.107;I2 = 98.9%) and adjusted OR = 1.306 (95% CI: 0.870–1.961; P = 0.197; I2 = 92.4%).
Fig. 2.

2A: meta-analysis of unadjusted results of association between opioid use and ICU admission; 2B: meta-analysis of unadjusted results of association between opioid use and mortality; 3C: meta-analysis of unadjusted results of association between opioid use and mechanical ventilation;
Fig. 3.

3A: meta-analysis of adjusted results of association between opioid use and ICU admission; 3B: meta-analysis of adjusted results of association between opioid use and mortality; 3C: meta-analysis of adjusted results of association between opioid use and mechanical ventilation.
4. Discussion
With the rapid propagation of COVID-19 variants of concern demonstrating an increasing incidence of immune escape properties in vaccinated individuals, there have been considerable pressures on healthcare systems, highlighting the importance of having indicators for COVID-19 prognosis to inform resource utilization [27,28]. In this study, opioid use was shown to be strongly associated with poor outcomes following COVID-19 infection. Both unadjusted and adjusted analysis results indicated that opioid use was associated with an increased risk for overall mortality as well as an increased need for ICU admission. Mechanical ventilation was not found to be significantly associated with opioid use.
The association between opioid use and increased mortality and need for ICU admission following COVID-19 infection is likely multifactorial, involving both medication- and patient-specific factors. The immune modulation and suppressive properties associated with opioid use has been well documented in the literature. Opioid use has been found to alter the infiltration and cytotoxicity of immune cells when used for cancer pain and severely compromise the immune system when used chronically [29,30]. This impact on the immune response not only exposes patients using opioids to opportunistic infections but also contributes to the potential severity of disease. Furthermore, recent studies have identified individuals with substance use disorders as having higher risks of negative COVID-19 outcomes [[31], [32], [33]]. Individuals with opioid use disorder may also be more susceptible to primary COVID-19 infection due to the extensive public health measures implemented. Social distancing practices may contribute to reduced drug availability, disrupted access to health services and safe consumption sites, and isolation can negatively affect mental health and predispose opioid use [18].
Opioid use is known to induce respiratory depression through stimulation of μ-opioid receptors with risk of progressing to respiratory failure [34,35]. Given that COVID-19 infection may also cause respiratory depression, the additive respiratory impacts of opioid use and COVID-19 infection may worsen overall outcomes. Opioid use has also been documented in causing end-organ damage, an issue compounded further by the disease manifestations of COVID-19 [36,37]. Finally, individuals using opioid medication often have comorbidities including older age, cancer, chronic pain, psychiatric diagnoses, factors that predispose patients to worse outcomes and serve as risk factors for disease severity and death [[38], [39], [40]]. The synergistic consequences of opioid use and COVID-19 infection in addition to existing comorbidities increase the risk of poor prognosis, likely resulting in increased need for ICU admission and mortality following COVID-19 infection. Opioid use has a positive association with mechanical ventilation similar to that of ICU admission but is not statistically significant. It is possible that this is due to the small sample size of only two studies for mechanical ventilation. This may also be secondary to the concept that there are various factors necessitating ICU admission beyond mechanical ventilation and insults on the respiratory system, including sepsis and severe cardiovascular disease [41,42].
These prognostic factors provide an additional modality of informing the triage process for patients with COVID-19 in the emergency department, especially as the opioid epidemic continues to have considerable independently associated mortality and encompasses a large percentage of all emergency department visits [[43], [44], [45]].
Our study has several limitations and as such our results should be interpreted with caution. Our sample size was small with five included articles for the meta-analysis. Furthermore, while mortality was an outcome in all five, ICU admission and mechanical ventilator support were only outcomes in two studies each, thus having even smaller sample sizes. The heterogeneity across the included studies was significant, due to the broad definitions used surrounding opioid medication. As such, without more detailed information on opioid type, dose, course, duration, or clinical indication, subgroup analyses were not able to be conducted. In addition, while most included studies grouped all opioids, one study divided strong and weak opioids into separate categories, the results of which were not able to be combined notwithstanding the sample size of each group being inadequate. This may have created bias as the use of strong opioids may have more severe adverse outcomes compared to those of weak opioids. Future studies should consider analyzing differences between strong and weak opioids on COVID-19 outcomes. Despite these limitations, this study is the first systematic review and meta-analysis to investigate the association between opioid medication and COVID-19 outcomes. Additional research is needed to provide a larger sample size and a better understanding of patient circumstances, including opioid type, dose, course, duration, and clinical indication.
5. Conclusion
COVID-19 patients with opioid use were at higher risk of ICU admission and mortality. Prospective studies are required to confirm these findings.
CRediT authorship contribution statement
Guangyu Ao: Data curation, Project administration, Writing – original draft. Toni Li: Writing – review & editing, Writing – original draft, Formal analysis. Yushu Wang: Writing – review & editing, Software, Methodology. Jing Li: Data curation, Investigation, Methodology, Writing – original draft. Carolyn Tran: Data curation, Investigation. Min Chen: Funding acquisition, Writing – review & editing. Xin Qi: Supervision, Project administration, Conceptualization.
Declaration of Competing Interest
None of the authors have conflicts of interest to declare.
Acknowledgments
This study was supported by Xinglin Scholars Program of Chengdu University of Traditional Chinese Medicine (Grant number: YYZX2021119).
References
- 1.Malik P., Patel K., Pinto C., et al. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL) - a systematic review and meta-analysis. J Med Virol. 2022;94(1):253–262. doi: 10.1002/jmv.27309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaspal R., Breakwell G.M. Socio-economic inequalities in social network, loneliness and mental health during the COVID-19 pandemic. Int J Soc Psychiatry. 2022;68(1):155–165. doi: 10.1177/0020764020976694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 4.Li L.Q., Huang T., Wang Y.Q., et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(6):577–583. doi: 10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Booth A., Reed A.B., Ponzo S., et al. Population risk factors for severe disease and mortality in COVID-19: a global systematic review and meta-analysis. PLoS One. 2021;16(3) doi: 10.1371/journal.pone.0247461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J., Wang R., Gilby N.B., Wei G.W. Omicron variant (B.1.1.529): infectivity, vaccine breakthrough, and antibody resistance. J Chem Inf Model. 2022;62(2):412–422. doi: 10.1021/acs.jcim.1c01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinh M.M., Berendsen Russell S. Overcrowding kills: how COVID-19 could reshape emergency department patient flow in the new normal. Emerg Med Australas. 2020;33(1):175–177. [Google Scholar]
- 8.Chen Q., Sterner G., Segel J., Feng Z. Trends in opioid-related crime incidents and comparison with opioid overdose outcomes in the United States. Int J Drug Policy. 2022;101 doi: 10.1016/j.drugpo.2021.103555. [DOI] [PubMed] [Google Scholar]
- 9.Kang A.W., DeBritz A.A., Hoadley A., et al. Barriers and poor telephone counseling experiences among patients receiving medication for opioid use disorders. Patient Educ Couns. 2022;S0738-3991(22) doi: 10.1016/j.pec.2022.03.006. 00099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huskamp H.A., Riedel L., Uscher-Pines L., et al. Initiating opioid use disorder medication via telemedicine during COVID-19: implications for proposed reforms to the Ryan Haight act. J Gen Intern Med. 2022;37(1):162–167. doi: 10.1007/s11606-021-07174-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexander G.C., Stoller K.B., Haffajee R.L., Saloner B. An epidemic in the midst of a pandemic: opioid use disorder and COVID-19. Ann Intern Med. 2020;173(1):57–58. doi: 10.7326/M20-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodda L.N., West K.L., LeSaint K.T. Opioid overdose-related emergency department visits and accidental deaths during the COVID-19 pandemic. J Urban Health. 2020;97(6):808–813. doi: 10.1007/s11524-020-00486-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slavova S., Rock P., Bush H.M., Quesinberry D., Walsh S.L. Signal of increased opioid overdose during COVID-19 from emergency medical services data. Drug Alcohol Depend. 2020;214 doi: 10.1016/j.drugalcdep.2020.108176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y., Yan Y., Meng J., Girotra M., Ramakrishnan S., Roy S. Immune modulation mediated by extracellular vesicles of intestinal organoids is disrupted by opioids. Mucosal Immunol. 2021;14(4):887–898. doi: 10.1038/s41385-021-00392-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malafoglia V., Ilari S., Vitiello L., et al. The interplay between chronic pain, opioids, and the immune system. Neuroscientist. 2021 doi: 10.1177/10738584211030493. 10738584211030493, online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Athanasos P., Smith C.S., White J.M., Somogyi A.A., Bochner F., Ling W. Methadone maintenance patients are cross-tolerant to the antinociceptive effects of very high plasma morphine concentrations. Pain. 2006;120(3):267–275. doi: 10.1016/j.pain.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Althobaiti Y.S., Alzahrani M.A., Alsharif N.A., Alrobaie N.S., Alsaab H.O., Uddin M.N. The possible relationship between the abuse of tobacco, opioid, or alcohol with COVID-19. Healthcare (Basel) 2020;9(1):2. doi: 10.3390/healthcare9010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mellis A.M., Kelly B.C., Potenza M.N., Hulsey J.N. Factors associated with drug overdoses during the COVID-19 pandemic. J Addict Med. 2022;16(1):e67–e69. doi: 10.1097/ADM.0000000000000816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. 2021 Jun;134:178–189. doi: 10.1016/j.jclinepi.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Jadad A.R., Moore R.A., Carroll D., et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–17. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 21.Wells G.A., Shea B., O’Connell D., et al. Dept of Epidemiology and Community Medicine, University of Ottawa; Ottawa: 2021. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-Analyses.http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm Accessed on December 1st, 2021. [Google Scholar]
- 22.Allen B., El Shahawy O., Rogers E.S., Hochman S., Khan M.R., Krawczyk N. Association of substance use disorders and drug overdose with adverse COVID-19 outcomes in new York City: January-October 2020. J Public Health (Oxf) 2021;43(3):462–465. doi: 10.1093/pubmed/fdaa241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh T.K., Song I.A., Lee J., Eom W., Jeon Y.T. Musculoskeletal disorders, pain medication, and in-hospital mortality among patients with COVID-19 in South Korea: a population-based cohort study. Int J Environ Res Public Health. 2021;18(13):6804. doi: 10.3390/ijerph18136804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qeadan F., Tingey B., Bern R., et al. Opioid use disorder and health service utilization among COVID-19 patients in the US: a nationwide cohort from the Cerner real-world data. EClinicalMedicine. 2021;37 doi: 10.1016/j.eclinm.2021.100938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuan W.J., Spotts H., Zgierska A.E., Lennon R.P. COVID-19 outcomes among adult patients treated with long-term opioid therapy for chronic non-cancer pain in the USA: a retrospective cohort study. BMJ Open. 2021;11(11) doi: 10.1136/bmjopen-2021-056436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiener R.C. Unhealthy opioid use and COVID-19 mortality incidence in older adults: a multicenter research network study. Subst Use Misuse. 2021;56(13):2044–2048. doi: 10.1080/10826084.2021.1967988. [DOI] [PubMed] [Google Scholar]
- 27.CDC COVID-19 Response Team SARS-CoV-2 B.1.1.529 (Omicron) variant - United States, December 1–8, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(50):1731–1734. doi: 10.15585/mmwr.mm7050e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y., Zhang L., Li Q., et al. The significant immune escape of pseudotyped SARS-CoV-2 variant omicron. Emerg Microbes Infect. 2022;11(1):1–5. doi: 10.1080/22221751.2021.2017757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boland J.W., Pockley A.G. Influence of opioids on immune function in patients with cancer pain: from bench to bedside. Br J Pharmacol. 2018;175(14):2726–2736. doi: 10.1111/bph.13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roy S., Ninkovic J., Banerjee S., et al. Opioid drug abuse and modulation of immune function: consequences in the susceptibility to opportunistic infections. J Neuroimmune Pharmacol. 2011;6(4):442–465. doi: 10.1007/s11481-011-9292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baillargeon J., Polychronopoulou E., Kuo Y.F., Raji M.A. The impact of substance use disorder on COVID-19 outcomes. Psychiatr Serv. 2021;72(5):578–581. doi: 10.1176/appi.ps.202000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Q.Q., Kaelber D.C., Xu R., Volkow N.D. COVID-19 risk and outcomes in patients with substance use disorders: analyses from electronic health records in the United States. Mol Psychiatry. 2021;26(1):30–39. doi: 10.1038/s41380-020-00880-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun Y., Bao Y., Kosten T., Strang J., Shi J., Lu L. Editorial: challenges to opioid use disorders during COVID-19. Am J Addict. 2020;29(3):174–175. doi: 10.1111/ajad.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dahan A., Aarts L., Smith T.W. Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology. 2010;112(1):226–238. doi: 10.1097/ALN.0b013e3181c38c25. [DOI] [PubMed] [Google Scholar]
- 35.Overdyk F., Dahan A., Roozekrans M., van der Schrier R., Aarts L., Niesters M. Opioid-induced respiratory depression in the acute care setting: a compendium of case reports. Pain Manag. 2014;4(4):317–325. doi: 10.2217/pmt.14.19. [DOI] [PubMed] [Google Scholar]
- 36.Ataei M., Shirazi F.M., Lamarine R.J., Nakhaee S., Mehrpour O. A double-edged sword of using opioids and COVID-19: a toxicological view. Subst Abuse Treat Prev Policy. 2020;15(1):91. doi: 10.1186/s13011-020-00333-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porubsky S., Kuppe C., Maier T., et al. Renal lipidosis in patients enrolled in a methadone substitution program. Arch Pathol Lab Med. 2014;138(5):689–693. doi: 10.5858/arpa.2013-0075-CR. [DOI] [PubMed] [Google Scholar]
- 38.Namba R.S., Singh A., Paxton E.W., Inacio M.C.S. Patient factors associated with prolonged postoperative opioid use after total knee arthroplasty. J Arthroplasty. 2018;33(8):2449–2454. doi: 10.1016/j.arth.2018.03.068. [DOI] [PubMed] [Google Scholar]
- 39.Kobus A.M., Smith D.H., Morasco B.J., et al. Correlates of higher-dose opioid medication use for low back pain in primary care. J Pain. 2012;13(11):1131–1138. doi: 10.1016/j.jpain.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buckeridge D., Huang A., Hanley J., et al. Risk of injury associated with opioid use in older adults. J Am Geriatr Soc. 2010;58(9):1664–1670. doi: 10.1111/j.1532-5415.2010.03015.x. [DOI] [PubMed] [Google Scholar]
- 41.Kimball S.L., Levy M.M. Sepsis and the opioid crisis: integrating treatment for two public health emergencies. Crit Care Med. 2021;49(12):2151–2153. doi: 10.1097/CCM.0000000000005152. [DOI] [PubMed] [Google Scholar]
- 42.Chow S.L., Sasson C., Benjamin I.J., et al. Opioid use and its relationship to cardiovascular disease and brain health: a presidential advisory from the American Heart Association. Circulation. 2021;144(13):e218–e232. doi: 10.1161/CIR.0000000000001007. [DOI] [PubMed] [Google Scholar]
- 43.Compton W.M., Valentino R.J., DuPont R.L. Polysubstance use in the U.S. opioid crisis. Mol Psychiatry. 2021;26(1):41–50. doi: 10.1038/s41380-020-00949-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soares W.E., 3rd, Melnick E.R., Nath B., et al. Emergency department visits for nonfatal opioid overdose during the COVID-19 pandemic across six US health care systems. Ann Emerg Med. 2022;79(2):158–167. doi: 10.1016/j.annemergmed.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Langabeer J.R., Stotts A.L., Bobrow B.J., et al. Prevalence and charges of opioid-related visits to U.S. emergency departments. Drug Alcohol Depend. 2021;221:108568. doi: 10.1016/j.drugalcdep.2021.108568. [DOI] [PubMed] [Google Scholar]