Abstract
Background:
The direct-instillation nasal allergen challenge (NAC) and the environmental exposure chamber (EEC) are 2 methods of conducting controlled allergen provocations. The clinical and biological comparability of these methods has not been thoroughly investigated.
Objective:
We sought to compare clinical and immunologic responses to cat allergen in NAC versus EEC.
Methods:
Twenty-four participants were randomized to receive either NAC followed by a 2-day challenge in an EEC or a 2-day challenge in an EEC followed by NAC. Challenges were separated by 28-day washout periods. We measured total nasal symptom scores, peak nasal inspiratory flow, nasal (0–8 hours) and serum cytokines, serum antibodies, peripheral blood antigen-specific T lymphocytes, and gene expression in nasal scrapings. The primary outcome was the total nasal symptom score area under the curve for the first 3 hours after allergen exposure in NAC or after initiation of exposure in EEC.
Results:
Both challenges increased IL-5 and IL-13 in nasal fluids and serum and resulted in altered nasal cell expression of gene modules related to mucosal biology and transcriptional regulation. Changes in gene modules, more so than cytokine measurements, showed significant associations with total nasal symptom score and peak nasal inspiratory flow. Overall, EEC exposure generated larger responses and more early terminations compared with NAC. Although the 2 challenges did not correlate in symptom magnitude or temporality, striking correlations were observed in cytokine levels.
Conclusions:
Although clinical outcomes of NAC and EEC were temporally different and nonequivalent in magnitude, immunologic responses were similar. Selection of a particular allergen challenge method should depend on considerations of study objectives and cost.
Keywords: Nasal allergen challenge, environmental exposure chamber, Fel d1, cat allergy, total nasal symptom score, peak nasal inspiratory flow, cat dander, epithelium
The pathophysiology of allergic rhinitis and the response to therapies can be studied using experimental allergen provocations (challenges). Two types of challenges are commonly used: the nasal allergen challenge (NAC) and the environmental exposure chamber (EEC). In the NAC, a known amount of allergen is applied directly in the nasal cavity, whereas during an EEC exposure, the study participant inhales aerosolized allergen delivered into a temperature/pressure/humidity-controlled room. The NAC is performed readily in the clinic and requires minimal equipment but may not be fully reflective of allergen exposure in everyday life.1 The EEC represents a more naturalistic method of exposure, but requires a specialized chamber or facility to conduct the challenge and just as in everyday life, the exact amount of inhaled allergen is not easy to calculate.2 Direct comparisons of NAC and EEC have occurred in only 1 study; however, immunologic responses were not assessed.3
Various clinical and physiologic outcomes can be measured in the course of these challenges including standardized symptom scores, such as the total nasal symptom score (TNSS), and objective measures of nasal obstruction such as the peak nasal inspiratory flow (PNIF). The NAC has also been extensively used to conduct mechanistic research and has unveiled many aspects of the pathophysiology of allergic rhinitis including the acute release of mast cell mediators upon allergen exposure, the nasal late-phase reaction, the local influx of inflammatory cells, the production of type 2 and other cytokines and chemokines, and the development of nasal hyperrespnsiveness.4–11 In contrast, with the exception of recent studies by Ahuja et al,12,13 the EEC model has not been used in this type of research.
The goal of our study was to characterize clinical and mechanistic aspects of allergic responses during NAC and EEC using cat allergen and to identify similarities and differences between the 2 methodologies. For the mechanistic objectives, we focused on outcomes that have not been previously examined in either model or have been examined only in 1 of the 2 models: these included measurements of inflammatory cytokines in nasal secretions and in serum, examination of allergen-induced changes in allergen-specific peripheral blood T cells, and changes in the transcriptomic profiles of nasal cells obtained by nasal brushing.
METHODS
Study participants
This was a randomized open-label cross-over study conducted at Inflamax Research in Ontario, Canada (DBA Cliantha Research). Study participants were aged 18 to 65 years, with at least a 2-year history of moderate to severe allergic rhinitis with symptoms induced by cat exposure. After signing the informed consent form, participants were screened for eligibility. Participants were eligible if they had a positive skin prick test result to standardized cat extract. A wheal diameter greater than or equal to 5 mm larger than that achieved with the negative control was used to increase the probability that participants with moderate to severe cat allergy were enrolled. Exclusion criteria included a history of anaphylaxis to cat allergen, moderate to severe asthma, chronic obstructive pulmonary disease, chronic tobacco use, recurrent or chronic sinusitis, and a prebronchodilator FEV1 less than 80% of their predicted value. Individuals with other perennial or seasonal allergies that could not be avoided were also excluded. No participant owned a cat, and all participants were deemed to have low daily exposure to cat allergen. This study was approved by the Institutional Review Board Services protocol number 00000776. The study was conducted under Clinical Trial Application (CTA) number 179612 by Health Canada and was registered as NCT02163122 in ClinTrials.gov.
Study design
Participants were randomized into 2 groups using an opaque envelop system.14 Group A was assigned to undergo an NAC with measurement of nasal responses from 0 to 8 hours followed by a 2-day EEC with a 28-day washout period between the 2 challenges. Group B was assigned to undergo a 2-day EEC followed by an NAC with a 28-day washout period. An analysis of previous cat allergen challenge data demonstrated that EEC induced a priming effect and potentiated the clinical response on the last day of challenge. However, little increase in clinical response was observed after day 2. Therefore, EEC was performed over 2 days in our study.
Challenges
Participants underwent both an NAC and an EEC challenge. In the NAC, a single dose of cat allergen (Greer Laboratories, Lenoir, NC), which was 0.87 μg total Fel d1 and selected on the basis of a smaller dose-finding study (for additional details, see this article’s Online Repository at www.jacionline.org), was administered as a nasal spray with the use of a Bi-dose nasal applicator device (Aptar Pharma, Crystal Lake, Ill). TNSSs were captured on electronic tablets at 10, 30, 60, and 90 minutes and every 30 minutes thereafter for 8 hours total. PNIF was measured at 10, 30, and 60 minutes and every 60 minutes thereafter for 8 hours total. TNSS and PNIF recordings were made at the same time points during the NAC and EEC phases (Fig 1). During the EEC phase, participants stayed in a mobile temperature- and humidity-controlled room or EEC for 3 hours, with an exposure of 10 to 500 ng/m3 Fel d1 allergen and an estimated amount of 37.7 ng of Fel d1 inhaled on each day of the EEC (for additional details, see this article’s Online Repository).
FIG 1.
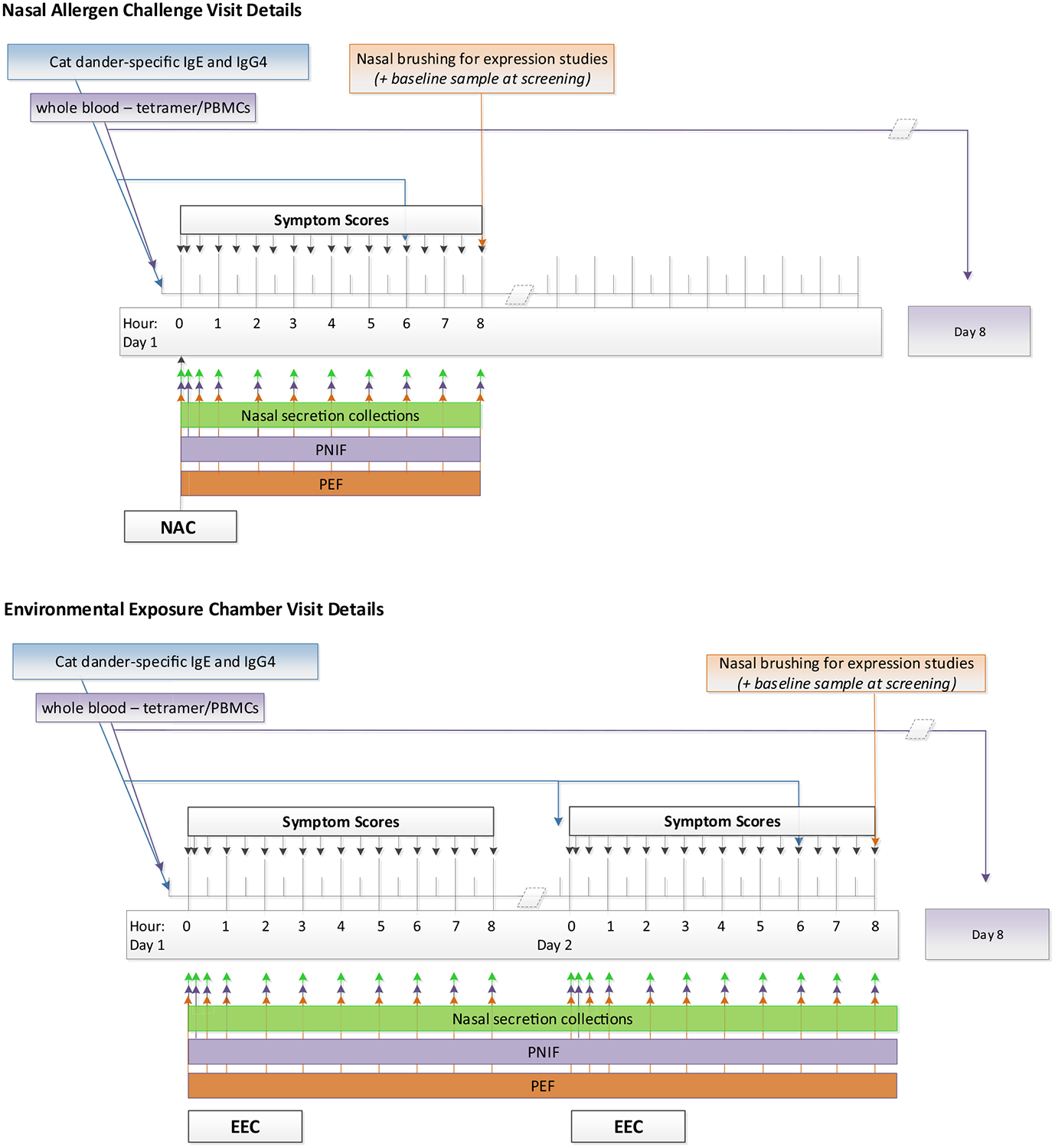
Timing of clinical assessments and mechanistic collections for NAC (top) and EEC (bottom). PEF, Peak expiratory flow.
Serum cytokine measurements
Blood was collected prechallenge and 6 hours postchallenge for NAC and pre–day 1, pre–day 2, and 6 hours postchallenge on day 2 of the EEC (Fig 1). Sera were frozen at −80°C until batched assays were performed. An ultrasensitive single-molecule digital immunoassay (Simoa HD-1 Analyzer, Quanterix, Billerica, Mass)15 was used to measure concentrations of IL-2, IL-4, IL-5, IL-10, and IL-13 in sera. The limits of detection for IL-2, IL-4, IL-5, IL-10, and IL-13 were 11.0, 4.6, 14.0, 3.8, and 5.5 fg/mL, respectively.
Nasal fluid collection and nasal cytokine measurements
Nasal secretions were collected as previously described16 and at time points indicated in Fig 1. Briefly, a single autoclave-sterilized sponge was inserted into each nostril of each participant for 2 minutes before removal. Each sponge was then placed in a cryovial with an indwelling cellulose acetate filter. The sample was eluted by adding 50 μL of Milliplex Buffer (Millipore, Burlington, Mass) per sponge and by centrifugation. The supernatants from both sponges (1 from each nostril) were combined, transferred to 0.5-mL cryovials, and stored at −80°C until cytokine assays were performed.
After thawing of nasal fluid supernatants, cytokines were measured in batches. Measurements of IL-4, IL-5, IL-6, IL-9, IL-13, and eotaxin 1 were performed using a human cytokine/chemokine magnetic bead panel 96-well plate assay (Milliplex Map Kit, Millipore) and a Luminex xMAP Magpix platform (Millipore), according to the manufacturer’s instructions. Twenty-five-microliter samples were analyzed in duplicate with standards and controls. The mean of the duplicate results was calculated and results below the lower limit of assay detection were given a value of 0.
Nasal brushing and RNA extraction
Nasal brushing was performed using a 3-mm cytology brush (Medical Packing Corporation, Camarillo, Calif) at study baseline (no allergen challenge), 8 hours after NAC, and 8 hours after the second day of the EEC challenge. Brushes were immediately placed in cryovials containing RLT buffer (Qiagen, Camarillo, Calif) and frozen at −80°C until RNA extractions were performed.
RNA sequencing
Whole-genome transcriptional profiling was performed on nasal brushing RNA samples. Methods for RNA quality control, library preparation, and RNA sequencing have been described previously17 and are presented in detail in this article’s Online Repository. Differential gene expression was performed using a weighted linear model (limma in R) appropriate for RNA-sequencing data and empirical Bayes method, and modular analysis using weighted gene correlation network analysis as previously described.18 Full details are given in this article’s Online Responsitory at www.jacionline.org.
Statistical methods used for analysis of clinical and cytokine data
The study’s sample size provided adequate statistical power to detect a correlation coefficient of 0.55 between NAC and EEC for the primary end point of TNSS area under the curve (AUC) 0 to 3 hours.
The AUC for the TNSS, adjusted for baseline, was computed from hour 0 to hour 3 of the NAC and from hour 0 to hour 3 on the second day of the EEC challenges. The trapezoidal rule was used to estimate the AUC of the TNSS; hour 0 was used as the base of the trapezoids in AUC calculations. The intraclass correlation coefficient was used to assess the equivalence of the 2 challenges in measuring TNSS AUC. To test the equivalence of the mean TNSS AUC between the EEC and the NAC, we computed a two one-sided test (TOST)19 using an equivalence margin of ±20%. The null hypothesis of TOST is that the challenges are not equivalent, so that rejecting the null leads to a conclusion of equivalence of the challenges.
For cytokine data, paired t tests were performed to compare prechallenge to postchallenge cytokine levels. Pearson correlation coefficients (r) and P values were calculated to determine whether levels of cytokines elicited by allergen were associated between the 2 challenges. The threshold for significance was P <.05 (2-sided). Other than analyses with RNA-sequencing data, mechanistic analyses were exploratory as described in the protocol and, therefore, were not adjusted for multiple comparisons.
All analyses were performed with SAS version 9.4 (SAS Institute Inc, Cary, NC) and R version 3.2.4 (R Foundation for Statistical Computing, Vienna, Austria).
For details on MHC class II tetramer analyses, antibody measurements, RT-PCR, along with additional details on other assays and statistics, please see this article’s Online Repository at www.jacionline.org.
Data sets for these analyses are accessible through TrialShare, a public Web site managed by the Immune Tolerance Network (https://www.itntrialshare.org/CATEEC_primary.url).
RESULTS
Participant disposition and safety
The 2 groups were similar in regard to age, sex, race, and reactivity to cat dander (see Table E2 in this article’s Online Repository at www.jacionline.org). Fig 2 shows the randomization and participant disposition. Of the 24 enrolled participants, 5 stopped the study early (3 were initially randomized to NAC and 2 were initially randomized to EEC). All early terminations occurred during the EEC phase. Participants stopped the study because of bronchospasm (1), upper respiratory tract infection (1), and asymptomatic reductions in peak expiratory flow from baseline during the EEC phase (3). All adverse events, including those that did not result in early withdrawal, are presented in Table E3 in this article’s Online Repository at www.jacionline.org.
FIG 2.
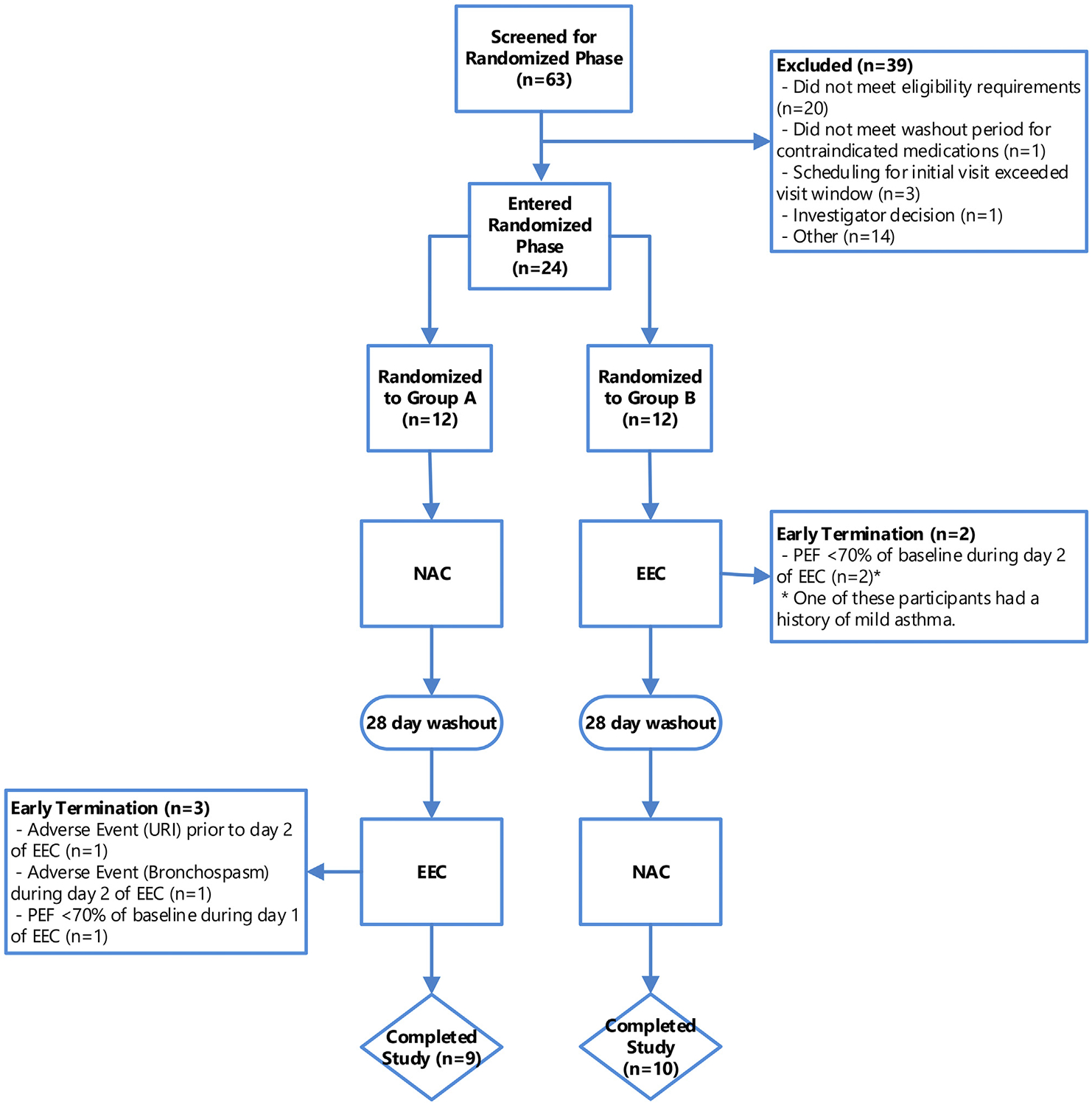
Consort diagram showing disposition of all participants screened and enrolled in the study. Group A underwent an NAC first followed by a 2-day EEC challenge after a 28-day washout period. Group B underwent the same challenges but started with the EEC challenge. Reasons for early withdrawal from the study and phase of study are also shown. PEF, Peak expiratory flow; URI, upper respiratory infection.
Symptom scores
TNSSs collected over 8 hours after the NAC and during and after the EEC showed distinct patterns, with symptoms peaking earlier in the NAC compared with either day 1 or day 2 of the EEC (Fig 3, A). Also, the 0- to 3-hour AUC of the TNSS was significantly greater with the EEC day 2 compared with the NAC (difference of least squares mean 3.4 with 95% CI 0.1–6.7). After adjusting for baseline TNSSs, the interclass correlation coefficient of the TNSS 0- to 3-hour AUC between the 2 challenges (NAC vs EEC day 2) was 0.304 (95% CI, −0.174 to 0.666). In general, the correlation was low between the 2 challenges for most AUC intervals or between peak symptom values and no specific interval could be identified where a strong correlation was observed (see Fig E1 in this article’s Online Repository at www.jacionline.org). The TOST of equivalence of the 0- to 3-hour TNSS AUC had a P value of .756, suggesting that the challenges were not equivalent in terms of this outcome. Notably, we found no difference in the 0- to 3-hour AUC between day 1 and day 2 of the EEC challenges.
FIG 3.
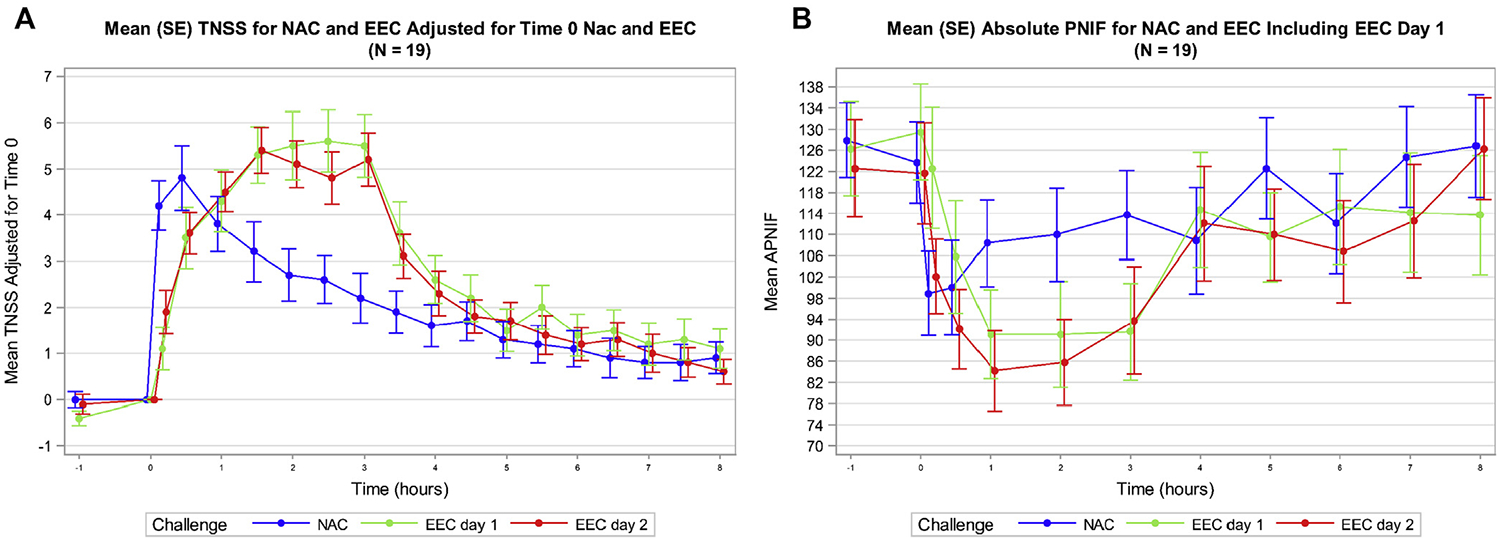
Clinical responses to NAC and EEC. A, Mean (SE) TNSSs shown over time for NAC, day 1 of EEC, and day 2 of EEC for all participants. TNSSs with NAC peaked earlier than EEC but have an overall reduced magnitude of response. B, Mean (SE) PNIF shown over time for NAC, day 1 of EEC, and day 2 of EEC for all participants. Similar to TNSSs, PNIF peaked earlier during NAC but EEC induced a greater magnitude of nasal air flow obstruction. APNIF, Absolute PNIF.
Nasal obstruction
Similar to TNSS, maximum reduction in PNIF with NAC (20% after 10 minutes postchallenge) occurred earlier compared with either day 1 (30% at hour 1) or day 2 of the EEC (31% at hour 1) (Fig 3, B). However, the 0- to 3-hour AUC of the change in PNIF over baseline in the EEC was not significantly different compared with the NAC (EEC least squares mean = −97.1; NAC least squares mean = −57.4, P = .087). The TOST of equivalence of the 0- to 3-hour AUC of the change in PNIF over baseline had a P value of .856, suggesting that the 2 challenges were not equivalent in terms of PNIF. As with TNSS, no significant differences between day 1 and day 2 in the PNIF 0- to 3-hour AUC were detected in the EEC.
Effect of challenge sequence on clinical outcomes
We tested whether the order of the challenges could affect the clinical results of the NAC or the EEC by comparing challenge-specific TNSS AUC for 0 to 3 hours between the 2 treatment groups (ie, Group A NAC vs Group B NAC and Group A EEC vs Group B EEC). No significant differences between challenge-induced increases in TNSSs and reductions in PNIF were detected between Group A and Group B, indicating that the order of the challenges did not influence the clinical outcomes.
Local and systemic inflammatory and immunologic responses
Using RNA extracted from nasal brush samples, we performed RT-PCR to assess the expression levels of several type 1, type 2, and regulatory cytokine/chemokine genes (see Table E1 in this article’s Online Repository at www.jacionline.org) before and 8 hours after allergen challenge (NAC and EEC day 2). Both challenges led to significant increases in the transcription of the type 2 cytokines IL-5 (for NAC: P = .006 and for EEC: P = .0006; Fig 4, A) and IL-13 (for NAC: P = .023 and for EEC: P = .0002; Fig 4, B). There were no significant differences in the expression levels of IL-5 or IL-13 when NAC was compared with EEC. Overall, we observed increased expression of several type 2 cytokine genes, with small changes or decreased expression of type 1 and regulatory cytokines or alarmins (see Fig E2 in this article’s Online Repository at www.jacionline.org).
FIG 4.

Increases in local and systemic type 2 immune responses after NAC and EEC. Log10 fold increases in IL-5 (A) and IL-13 (B) gene expression from nasal brush samples collected at baseline and 8 hours after NAC (blue) or baseline and 8 hours after the second day of EEC (red). Levels of IL-5 (C) and IL-13 (D) in nasal fluid collected prechallenge and up to 8 hours after allergen challenge. Serum IL-5 and IL-13 concentrations prechallenge and 6 hours after NAC (E and F) or prechallenge day 1, prechallenge day 2, and 6 hours after EEC day 2 (G and H). NS, Not significant.*P < .05, **P < .01, ***P < .001, ****P < .0001.
In nasal fluids, we found significantly elevated levels of type 2 cytokines after both NAC and EEC over baseline (for IL-5: after NAC, P < .0001; after EEC day 1, P < .0001; after EEC day 2, P < .0001, Fig 4, C; for IL-13: after NAC, P < .0001; after EEC day 1, P <.0001; after EEC day 2, P < .0001, Fig 4, D; for other cytokines/chemokines, see Fig E3, A–D, in this article’s Online Repository at www.jacionline.org). The magnitude of IL-5 increase in nasal fluids was greater after EEC compared with NAC (NAC vs EEC day 1: P = .025 and NAC vs EEC day 2: P = .045). The increase in IL-13 after EEC was greater than NAC only on day 1 (P = .013).
NAC and EEC showed remarkable correlations in the allergen exposure-induced changes in IL-5 and IL-13 when expressed as 2- to 8-hour AUCs (Fig 5, A–D). After the first EEC exposure, nasal fluid IL-5 and IL-9, but not other cytokines, seemed to remain elevated for at least 24 hours because the EEC day 2 prechallenge levels were higher than the prechallenge levels on EEC day 1 (for IL-5, P = .001; for IL-9, P = .0071; see Fig 4, C, and Fig E3, C).
FIG 5.

Relationship between nasal fluid cytokines elicited by NAC and EEC. Pearson correlations of log10 2- to 8-hour AUC of NAC vs log10 2- to 8-hour AUC of EEC day 1 for IL-5 (A) and IL-13 (C) and log10 2- to 8-hour AUC of NAC vs log10 2- to 8-hour AUC of EEC day 2 for IL-5 (B) and IL-13 (D). Participants colored by order of challenge received first (blue = NAC followed by EEC and green = EEC followed by NAC).
To determine whether a systemic response was also elicited by allergen challenge, we used an ultrasensitive single-molecule digital immunoassay and detected significant increases in circulating IL-5 and IL-13 6 hours after both challenges (for IL-5: after NAC, P < .001; after EEC day 2, P < .01; for IL-13: after NAC, P < .0001; after EEC day 2, P < .0001, Fig 4, E–H). No prechallenge to postchallenge changes in serum IL-2, IL-4, or IL-10 could be detected for either challenge. The changes from baseline in the serum levels of IL-5 and IL-13 induced by NAC and EEC were significantly correlated (for IL-5, r = 0.71, P = .0007; for IL-13, r = 0.55, P = .014; see Fig E4, A and B, in this article’s Online Repository at www.jacionline.org) much like the nasal fluid cytokine data, albeit to a somewhat lesser extent.
Local and systemic type 2 cytokine responses did not correlate consistently with challenge-induced TNSS or PNIF in either challenge (see Table E4 in this article’s Online Repository at www.jacionline.org). For NAC, there was a modest inverse correlation between type 2 cytokines (IL-5 and IL-13 in nasal fluid and/or serum) and delta PNIF (r = −0.34 to −0.52 and P = .01 to .12).
In the context of the systemic effects of NAC, we tested peripheral blood for changes in the number of cat allergen-specific CD4 T cells using MHC class II tetramers for Fel d1, at prechallenge and 7 days after the NAC and EEC day 1 exposure. Of the 9 participants who could be analyzed by tetramers, 5 had virtually no tetramer-positive CD4 cells at baseline and after both types of allergen challenge. However, in participants with detectable tetramer-positive cells at baseline, we observed increases in cat allergen-specific CRTH21 CD4 cells after both NAC and EEC (see Fig E5 in this article’s Online Repository at www.jacionline.org). The magnitude of the increase appeared greater after EEC, but the difference did not reach significance, possibly due to the low number of cases.
Previous studies have demonstrated increases in allergen-specific antibody levels after allergen challenge.20 Therefore, we measured levels of cat dander–specific IgE and IgG4 in the serum before and 28 days after challenge. On average, the levels of IgE to cat dander increased after both NAC and EEC, but only the increase in IgE after EEC reached statistical significance (P = .007; see Fig E6, A, in this article’s Online Repository at www.jacionline.org). We also noted a significant increase in IgG4 after EEC (P = .04). Decreases in cat dander IgG4 to IgE ratios were also observed after both challenges, but only the decrease after NAC was significant (P = .037, Fig E6, B).
Effects of nasal challenges on nasal transcriptomics
Using nasal brush samples obtained at baseline and 8 hours after each challenge, we investigated changes in global gene expression attributable to each challenge procedure with an unbiased RNA-sequencing approach. The expression of 462 genes was significantly changed after EEC (Fig 6, A; see Table E5, A and B, in this article’s Online Repository at www.jacionline.org) and of 171 genes after NAC (Fig 6, B; see Table E5, A and B) (false discovery rate < 0.05). There was a high degree of overlap, with 102 genes from both lists showing significant changes (hypergeometric P < .001). Overall, differentially expressed genes showed a similar magnitude of change with each challenge and the changes were highly correlated (Fig 6, C; see Table E5, A and B).
FIG 6.
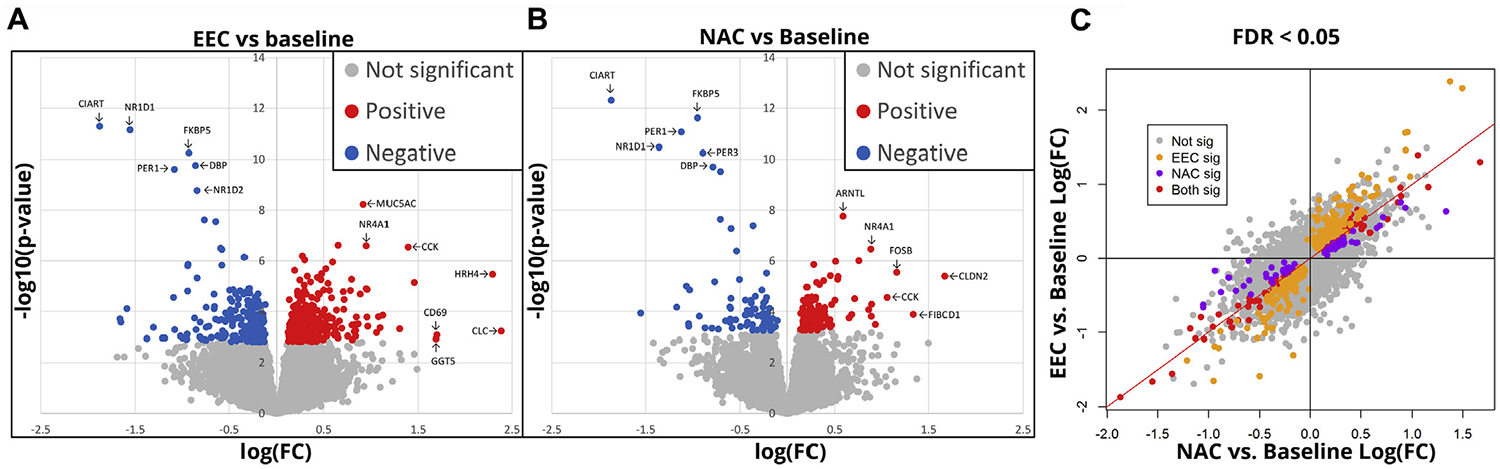
Similarities in gene expression after NAC and EEC. Volcano plots of nasal RNA-sequencing data showing genes with significant increases (red) or decreases (blue) in expression after (A) EEC or (B) NAC. C, Correlation in gene expression changes between NAC and EEC (FDR < 0.05). FC, Fold change; FDR, false discovery rate.
To determine the discrete biological pathways represented in these differentially expressed genes, we conducted a modular network analysis of the 531 total differentially expressed genes (102 genes common to EEC and NAC and 429 genes unique to either EEC or NAC). The genes clustered into 8 different coexpression modules representing multiple molecular pathways. Each of these modules was significantly differentially expressed after both EEC and NAC compared with baseline (false discovery rate < 0.001). Generally, the observed change was greater with EEC than with NAC, though this difference was not significant for any module after multiple testing correction (lowest false discovery rate = 0.18). A complete list of modules, their enriched biological pathways, and their constituent genes is provided in Table E6 in this article’s Online Repository at www.jacionline.org.
Of these modules, 2 (designated M3 and M4) were of particular interest because they also showed significant associations with both type 2 cytokine protein measurements and the clinical outcomes TNSS and PNIF. Specifically, the expression of M3, which contains 96 genes related to mucin and lysozyme production, prostaglandin synthesis, and mucosal healing (Fig 7, A), was increased after NAC (baseline to post-NAC expression change = 1.27; P < .001) and EEC (baseline to post-EEC expression change = 1.41; P <.001) (Fig 7, B), and the magnitude of change showed significant positive correlations with IL-5 and IL-13 levels in both nasal fluid and serum, as well as with TNSS, and significant inverse correlation with delta PNIF (Fig 7, C and D; see Table E7 in this article’s Online Repository at www.jacionline.org). Key hub genes in this network include mucin 5AC (MUC5AC) and lysozyme (LYZ), important secretory products of goblet and serous cells that likely reflect increased epithelial mucus production and hypersecretion in response to allergen. The network also includes several receptor genes involved in allergic inflammation, vasodilation, and tissue edema including HRH4, BDKRB2, and PTGER4, and the 3 trefoil factors (TFF1, TFF2, and TFF3), which are secretory proteins involved in mucosal healing.
FIG 7.
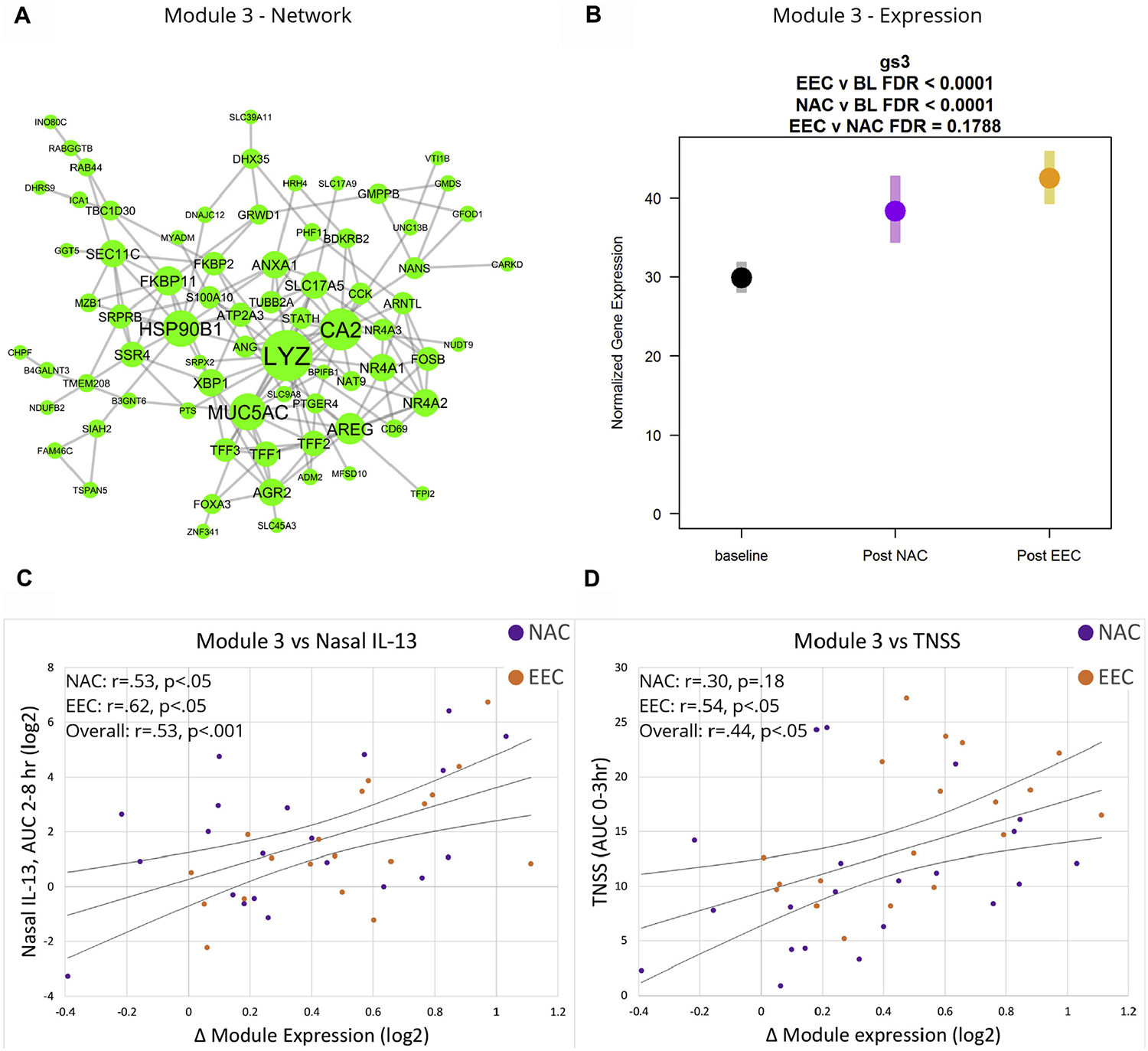
Gene network and module 3 expression. A, Gene-gene interaction network for module 3. B, Normalized gene expression of module 3 at baseline, post-NAC, and post-EEC. Correlation of module 3 expression with (C) nasal IL-13 levels and (D) TNSSs. BL, Baseline; FDR, false discovery rate.
M4, which contains genes related to the negative regulation of transcription (Fig 8, A), was decreased after NAC (baseline to post-NAC expression change = 0.76; P <.001) and EEC (baseline to post-EEC expression change = 0.74; P < .001) (Fig 8, B), and showed significant inverse correlations with IL-5 and IL-13 levels, as well as with TNSS, and significant positive correlation with delta PNIF (Fig 8, C and D; see Table E8 in this article’s Online Repository at www.jacionline.org). The module is a network of 61 genes composed predominantly of nuclear proteins including at least 11 genes involved in negatively regulating gene expression.
FIG 8.

Gene network and module 4 expression. A, Gene-gene interaction network for module 4. B, Normalized gene expression of module 3 at baseline, post-NAC, and post-EEC. Correlation of module 3 expression with (C) nasal IL-13 levels and (D) TNSSs. BL, Baseline; FDR, false discovery rate.
A third biologically interesting module was M1, which contains genes related to cilia production. The expression of this module was significantly increased with allergen exposure (baseline to post-NAC expression change = 1.17, P < .001, and baseline to post-EEC expression change = 1.27, P < .001; see Fig E7 in this article’s Online Repository at www.jacionline.org), but was not significantly associated with other mechanistic or clinical variables.
DISCUSSION
EEC and NAC are routinely used in allergic rhinitis research, but many outcomes have never been directly compared. Using a cross-over design, we examined clinical and immunologic outcomes aiming at assessing how interchangeable these methodologies are and at identifying differences, if present.
In designing this study, we also took the opportunity to investigate the suitability of some techniques for which relatively little experience is available in the context of NAC. This includes measurement of serum cytokines using ultrasensitive assays, assessment of allergen-induced changes in allergen-specific CD4+ T lymphocytes in peripheral blood, and nasal mucosal transcriptomics. Notably, transcriptomics post-EEC allergen exposure have been assessed in the recent work of Ahuja et al12 and our study offered a chance at replicating some of their findings.
Our study shows that the 2 types of allergen challenges, NAC and EEC, do not correlate with each other with respect to subjective clinical symptoms or the objective measure of nasal obstruction (PNIF) elicited by allergen exposure. Thus, the 2 methodologies cannot be used interchangeably when clinical oucomes are considered. Another study that compared clinical outcomes between NAC and EEC observed good agreement between both challenge modalities with respect to peak and mean symptom scores.3 It is unclear whether differences in the type/amount of allergen used in challenges, or simply the small number of participants used in experiments, may explain the lack of agreement with our data. Importantly, in our study, the effects of the 2 challenges on immunologic outcomes and on nasal mucosal transcriptomics show striking similarities and are highly correlated.
Contrasts between the 2 types of challenges in terms of the symptom and PNIF responses to allergen exposure are illustrated in Fig 3, A and B, and show both kinetic and magnitude differences. We were hoping that the differences in kinetics, which were expected, would be mitigated by expressing these outcomes as AUCs over the first 3 hours after allergen exposure in the NAC or the 3 hours of continuous allergen exposure in the EEC. However, the lack of correlation or agreement between the 2 challenges, even when AUC outcomes are considered, suggests that additional qualitative differences are present. These differences probably reflect the fact that, in NAC, a large amount of allergen is delivered within a few seconds to the nasal mucosa, resulting in a sudden, high local allergen concentration. In contrast, with EEC, allergen is inhaled at approximately the same rate for 3 hours, presumably resulting in different distribution along the nasal airways and probably different local concentrations. Variable distribution, concentration, and the timing of allergen exposure may translate into differential patterns of mast cell activation, as well as target organ stimulation. As an example, exposure of a wider mucosal surface will result in activation of a larger number of sensory nerve endings by mast cell products such as histamine and, given that neural responses mediate many rhinitis symptoms,21 result in qualitatively and quantitatively different symptoms within the same individual. Additional factors influencing the symptomatic response may include the size of the particles within which allergen is delivered (droplets of a solution primarily confined to the nose in NAC vs aerosolized dry particles capable of reaching the lower airways in EEC) and, perhaps, the total dose of allergen delivered by each challenge.
Although the dose of allergen delivered by the NAC is known, the dose delivered by the EEC is difficult to calculate. Based on approximations and assumptions of homogeneous concentration of allergen particles in the EEC air, continuous nasal breathing, normal respiratory rates, and average tidal volumes, we have calculated the average total dose of Fel d 1 inhaled through the nose in the EEC to be approximately 38 ng/d. Notwithstanding a degree of erroneous assumptions behind this calculation, the amount of allergen delivered by the EEC appears to be substantially lower than that delivered by the NAC, which was 872 ng (0.1 mL per nostril of a 4.36 μg/mL Fel d 1 solution). It is, therefore, surprising to observe the EEC generating significantly greater changes over baseline in the TNSS 0- to 3-hour AUC, compared with the NAC, and a similar trend with PNIF, albeit not reaching statistical significance. Also, differences in some immunologic outcomes and even in the magnitude of transcriptomic responses trended in the same direction. As discussed above, it is likely that the differences between the 2 challenges may reflect differential allergen distribution, local concentration, and mast cell activation and that the total dose of allergen delivered is of lesser importance.
The observed differences in the magnitude of the clinical outcomes (TNSS and PNIF) between the 2 challenge methods might have relevance to their use in clinical trials. To examine the implication of the larger clinical signal in the EEC, we conducted sample size calculations for a hypothetical clinical trial with the objective to detect a 30% difference in 0- to 3-hour AUC TNSS between an active agent and placebo with 80% statistical power. The number of study participants needed was lower for EEC day 1 (N = 102) and EEC day 2 (N = 62) compared with NAC (N = 168). Because there were no statistically significant differences in the cat allergen-induced symptoms and the reduction in PNIF between EEC day 1 and 2, an observation that may only apply to perennial allergens, the smaller number of participants needed with EEC day 2 outcomes was largely due to reduced SD. This analysis highlights the fact that either NAC or EEC can be used to test a potential therapeutic, but choosing the best challenge method for a study ultimately depends on available resources and specific study objectives. For example, the EEC, besides mimicking natural allergen exposure more closely than the NAC, shows an advantage for sample size, but this should be balanced by other factors such as time, cost, and organizational complexity, where the NAC approach may be more advantageous. It is also important to note that all 5 participants who were either removed or dropped out of the study were in the EEC phase and the reasons for withdrawal were related to lower or upper respiratory tract symptoms or reduction in lung function.
The differences in clinical outcomes between NAC and EEC might be expected to yield different immunologic responses, but our data suggest that although the magnitude of immunologic responses differs slightly, both challenges elicit fundamentally the same local and systemic type 2 responses. This was supported by various methodologies ranging from local expression (RT-PCR) and production (protein assays) of cytokines/chemokines, to changes in systemic cytokine levels, changes in antigen-specific T cells, and to the overall patterns of gene expression in nasal brushings assessed by RNA sequencing.
Measurements of nasal cytokine mRNA through RT-PCR showed a clear type 2 immune response including IL-5, IL-13, CCL26, and CCL2 (Fig E2). Not surprisingly, type 1 cytokine signal was unchanged or downregulated. This was consistent after both types of allergen challenges. Cytokine protein measurements confirmed the same response pattern and, except for the occasional elevation of a non–type 2 cytokine (such as IL-6), both challenges were concordant. Furthermore, for IL-5 and IL-13, strong correlations between the 2 challenges were observed in the allergen-induced changes in nasal, as well as in serum, cytokines (Figs 5 and E4). It is important to note that the use of an ultrasensitive assay methodology for the first time in an allergen challenge setting allowed us to detect changes in serum type 2 cytokines, which could be a useful biomarker to monitor the response to inhaled allergens. Interestingly, in the nasal RT-PCR measurements, we failed to detect an increase in thymic stromal lymphopoietin and IL-33 expression after challenge, epithelial products also strongly associated with type 2 immune responses. Increases in alarmin expression have been observed after bronchial allergen challenge in at least 1 other human study.22 Location, or timing of sampling, or different allergens used may explain the inconsistency in outcomes.
Nasal brushing transcriptomics yielded biologically insightful results that were associated with clinical responses. Specifically, we identified 2 relevant networks of genes that showed opposite change patterns in response to the allergen challenges and were significantly associated with both clinical measurements (ie, TNSS and PNIF) and local and systemic type 2 cytokine measurements. We saw upregulation of a gene module (M3) characteristic of multiple aspects of allergic inflammation including mucin and lysozyme production, upregulation of histamine, bradykinin, and prostaglandin receptors, and mucosal healing. The direct association of this gene network to clinical symptoms and changes in nasal patency is consistent with these biologic pathways having a direct role in the severity of the allergic response. Furthermore, this association directly links the clinical response with local and systemic type 2 inflammation, specifically IL-5 and IL-13 production. As an inverse finding, we observed a decrease in a gene module (M4) that is related to negative regulation of transcription. This suggests that, in addition to induction of type 2 immune response genes, induction of the allergic immune response requires a disinhibition of transcriptional regulators of epithelial cell function and offers an opportunity for more precise mechanistic outcomes and potential biomarkers that can be tested in future allergen challenge and interventional studies. Because the prechallenge nasal RNA was collected at a different time of day than the postchallenge sampling, we cannot rule out diurnal variation playing a role in the gene expression differences we observed. However, given that the M3 results are biologically relevant and directly linked to physiologic changes, it is unlikely that diurnal variation was a major contributor to our observations.
Because the analysis was based on nasal brushings, we assumed that most cells were nasal epithelial cells. Therefore, it was expected that many of the differentially expressed genes represent epithelial functions, such as the upregulation of ciliary genes (M1), and barrier secretory molecules such as MUC5AC and LYZ (M3). However, we also observed changes in the expression of many genes typically associated with leukocytes rather than epithelial cells. In particular, in M3, several genes including NR4A1, NR4A2, NR4A3, GFOD1, FOSB, and CD69 are known to be expressed by mast cells, eosinophils, and/or basophils, suggesting that part of the observed response derives from intraepithelial inflammatory cells relevant to the allergic response. All these cell types are known to undergo transepithelial migraton during symptomatic allergic rhinitis, whereas T cells tend to compartmentalize in tissues. It may be that a higher proportion of such cells in the epithelium plays a direct role linking the observed epithelial response with type 2 cytokine levels and the magnitude of clinical response.
Our findings are consistent with and expand upon previous work. Ahuja et al12 previously found that expression levels of 10 individual genes positively associate with symptom scores collected during EEC with house-dust mite allergen.12 Two of the genes they described, CD69 and CLC, are components that we found coexpressed within a larger module (M3) that represents the multifaceted epithelial inflammatory response. Furthermore, our work demonstrates the link between this immune response at the epithelium and local and systemic type 2 inflammation.
Several limitations ought to be mentioned: first, the 2 challenges differed with respect to the total dose of allergen delivered to the nose; to match the 2 challenges would require technology not practical to implement. Most importantly, our intention was to compare the 2 challenge methodologies in the manner they are regularly applied in research protocols. Second, our study was only conducted with cat allergen and one should be cautious in extrapolating the results to other allergens given potentially different direct effects on the epithelium and on the innate immune system. Third, the participants/staff were not blinded to the allergen challenge that they received/administered, something that would have required 2 additional sham challenges; the consequences of this limitation are unknown. Fourth, the study was not powered to detect all immunologic differences between the 2 types of challenges. It is, therefore, possible, that, with higher numbers of participants, more differences between the NAC and the EEC outcomes would have been detected. However, the strong similarities that were observed between the 2 challenges in immunologic outcomes and nasal transcriptomics offer substantial certainty to our conclusions.
In summary, our study demonstrates that although the different methodologies of allergen challenge yield different clinical responses, immunologic responses are very similar. In addition, molecular analysis of local tissue responses correlates well with clinical symptoms and local and systemic immunologic measurements. Furthermore, the data presented in this article can be used to guide the choice of allergen challenge methodology in future trials and in vivo mechanistic studies of respiratory allergy.
Supplementary Material
Key messages.
NAC and EEC did not correlate in regard to symptom magnitude or temporality.
Immunologic responses correlated well between challenges, indicating similar biological pathways are elicited by both allergen challenge methodologies.
Gene expression changes of local tissues correlated well with clinical and immunologic responses.
Acknowledgments
This research was performed as a project of the Immune Tolerance Network, an international clinical research consortium headquartered at the Benaroya Research Institute. This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Numbers UM1AI109565, UM2AI117870, and HHSN272200800029C. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure of potential conflict of interest: P. Patel reports grants/pending grants and fees related to participation in review activities (such as data monitoring boards, statistical analysis, and end point committees) paid to his institution to support an element of the current study. A. M. Salapatek and P. Couroux report fees paid to their institution to support an element of the current study. S. Sanda reports grants/pending grants to his institution from the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases/National Institute of Allergy and Infectious Diseases (NIAID) during the conduct of the study. M. H. Shamji reports payments for lectures from ALK, ASIT Biotechsa, and Allergpharma outside of the submitted work; grants/pending grants from ALK, Regeneron, Merck, ASIT Biotech.sa, and the Immune Tolerance Network; and serving as a consultant for ASIT Biotech.sa outside of the submitted work. A. Lelic reports fees paid by Inflamax Research to support an element of the current study. A. Pina, W. W. Kwok, J. L. Johnson, and M. L. Sever report grants from the NIH/NIAID during the conduct of the study. E. James reports a grant/pending grant from Janssen outside of the submitted work. M. Larché reports a grant from the Immune Tolerance Network to support an element of the current study; is also a member of the scientific advisory board of Aravax Pty; has served as a consultant for Aravax Pty, Back Bay Life Sciences, Circassia Ltd, and Adiga Life Sciences outside of the submitted work; reports stock/stock options from Adiga Life Sciences and Circassia Pharmaceutials PLC and travel/meeting expenses from Nestlé outside of the submitted work; and is also listed on patents (pending or issued) with Adiga Life Sciences outside of the submitted work. M. C. Altman reports a grant from the Immune Tolerance Network to support an element of the current study. The rest of the authors declare that they have no relevant conflicts of interest.
We acknowledge other Cat EEC vs NAC study contributors: Rebecca Parkin, Jorge Pardo, Judith Evind, Kristina Harris, and Peter Sayre. We also thank the patients for their participation in the study.
Abbreviations used
- AUC
Area under the curve
- EEC
Environmental exposure chamber
- NAC
Nasal allergen challenge
- PNIF
Peak nasal inspiratory flow
- TNSS
Total nasal symptom score
- TOST
Two one-sided test
REFERENCES
- 1.Soliman M, North ML, Steacy LM, Thiele J, Adams DE, Ellis AK. Nasal allergen challenge studies of allergic rhinitis: a guide for the practicing clinician. Ann Allergy Asthma Immunol 2014;113:250–6. [DOI] [PubMed] [Google Scholar]
- 2.Ellis AK, North ML, Walker T, Steacy LM. Environmental exposure unit: a sensitive, specific, and reproducible methodology for allergen challenge. Ann Allergy Asthma Immunol 2013;111:323–8. [DOI] [PubMed] [Google Scholar]
- 3.Tenn MW, Steacy LM, Adams DE, Walker TJ, Ellis AK. Comparison of allergic rhinitis outcomes of the environmental exposure unit and nasal allergen challenge model. Ann Allergy Asthma Immunol 2019;123:105–6.e1. [DOI] [PubMed] [Google Scholar]
- 4.Iliopoulos O, Proud D, Adkinson NF Jr, Creticos PS, Norman PS, Kagey-Sobotka A, et al. Effects of immunotherapy on the early, late, and rechallenge nasal reaction to provocation with allergen: changes in inflammatory mediators and cells. J Allergy Clin Immunol 1991;87:855–66. [DOI] [PubMed] [Google Scholar]
- 5.Naclerio RM, Proud D, Togias AG, Adkinson NF Jr, Meyers DA, Kagey-Sobotka A, et al. Inflammatory mediators in late antigen-induced rhinitis. N Engl J Med 1985;313:65–70. [DOI] [PubMed] [Google Scholar]
- 6.Scadding GW, Calderon MA, Bellido V, Koed GK, Nielsen NC, Lund K, et al. Optimisation of grass pollen nasal allergen challenge for assessment of clinical and immunological outcomes. J Immunol Methods 2012;384:25–32. [DOI] [PubMed] [Google Scholar]
- 7.Scadding GW, Calderon MA, Shamji MH, Eifan AO, Penagos M, Dumitru F, et al. Effect of 2 years of treatment with sublingual grass pollen immunotherapy on nasal response to allergen challenge at 3 years among patients with moderate to severe seasonal allergic rhinitis: the GRASS randomized clinical trial. JAMA 2017;317: 615–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scadding GW, Eifan A, Penagos M, Dumitru A, Switzer A, McMahon O, et al. Local and systemic effects of cat allergen nasal provocation. Clin Exp Allergy 2015;45:613–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scadding GW, Eifan AO, Lao-Araya M, Penagos M, Poon SY, Steveling E, et al. Effect of grass pollen immunotherapy on clinical and local immune response to nasal allergen challenge. Allergy 2015;70:689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shamji MH, Bellido V, Scadding GW, Layhadi JA, Cheung DK, Calderon MA, et al. Effector cell signature in peripheral blood following nasal allergen challenge in grass pollen allergic individuals. Allergy 2015;70:171–9. [DOI] [PubMed] [Google Scholar]
- 11.Togias A, Naclerio RM, Proud D, Baumgarten C, Peters S, Creticos PS, et al. Mediator release during nasal provocation: a model to investigate the pathophysiology of rhinitis. Am J Med 1985;79:26–33. [DOI] [PubMed] [Google Scholar]
- 12.Ahuja SK, Manoharan MS, Harper NL, Jimenez F, Hobson BD, Martinez H, et al. Preservation of epithelial cell barrier function and muted inflammation in resistance to allergic rhinoconjunctivitis from house dust mite challenge. J Allergy Clin Immunol 2017;139:844–54. [DOI] [PubMed] [Google Scholar]
- 13.He W, Jimenez F, Martinez H, Harper NL, Manoharan MS, Carrillo A, et al. Cockroach sensitization mitigates allergic rhinoconjunctivitis symptom severity in patients allergic to house dust mites and pollen. J Allergy Clin Immunol 2015; 136:658–66. [DOI] [PubMed] [Google Scholar]
- 14.Doig GS, Simpson F. Randomization and allocation concealment: a practical guide for researchers. J Crit Care 2005;20:187–91, discussion 91–3. [DOI] [PubMed] [Google Scholar]
- 15.Yeung D, Ciotti S, Purushothama S, Gharakhani E, Kuesters G, Schlain B, et al. Evaluation of highly sensitive immunoassay technologies for quantitative measurements of sub-pg/mL levels of cytokines in human serum. J Immunol Methods 2016;437:53–63. [DOI] [PubMed] [Google Scholar]
- 16.Renand A, Shamji MH, Harris KM, Qin T, Wambre E, Scadding GW, et al. Synchronous immune alterations mirror clinical response during allergen immunotherapy. J Allergy Clin Immunol 2018;141: 1750–60.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altman MC, Whalen E, Togias A, O’Connor GT, Bacharier LB, Bloomberg GR, et al. Allergen-induced activation of natural killer cells represents an early-life immune response in the development of allergic asthma. J Allergy Clin Immunol 2018;142:1856–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altman MC, Gill MA, Whalen E, Babineau DC, Shao B, Liu AH, et al. Transcriptome networks identify mechanisms of viral and nonviral asthma exacerbations in children. Nat Immunol 2019;20:637–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuirmann DJ. A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharmacokinet Biopharm 1987;15:657–80. [DOI] [PubMed] [Google Scholar]
- 20.Naclerio RM, Adkinson NF Jr, Moylan B, Baroody FM, Proud D, Kagey-Sobotka A, et al. Nasal provocation with allergen induces a secondary serum IgE antibody response. J Allergy Clin Immunol 1997;100:505–10. [DOI] [PubMed] [Google Scholar]
- 21.Sarin S, Undem B, Sanico A, Togias A. The role of the nervous system in rhinitis. J Allergy Clin Immunol 2006;118:999–1016. [DOI] [PubMed] [Google Scholar]
- 22.Wang W, Li Y, Lv Z, Chen Y, Li Y, Huang K, et al. Bronchial allergen challenge of patients with atopic asthma triggers an alarmin (IL-33, TSLP, and IL-25) response in the airways epithelium and submucosa. J Immunol 2018;201: 2221–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


