Abstract
Purpose
The purpose of this study was to identify the risk factors for recurrent abdominal pain (RAP) in children who presented with nonorganic acute abdominal pain.
Methods
A retrospective, single study was conducted on 2–15-year-old children diagnosed with nonorganic acute abdominal pain at the pediatric outpatient department of Vajira Hospital, Nawamindradhiraj University, between January 2015 and December 2019. The potential risk factors were analyzed using univariate and multivariate analyses.
Results
Of the 367 patients with nonorganic acute abdominal pain, 94 (25.6%) experienced RAP within three months. In this group with RAP, 76 patients (80.8%) were diagnosed with functional gastrointestinal disorders, including functional dyspepsia, irritable bowel syndrome, functional abdominal pain-not otherwise specified, and functional constipation. History of gastrointestinal infection (p=0.011), mental health problems (p=0.022), abdominal pain lasting ≥7 days (p<0.001), and change in stool frequency (p=0.001) were the independent risk factors associated with RAP in children with nonorganic acute abdominal pain; their odds ratios and 95% confidence intervals were 3.364 (1.314–8.162), 3.052 (1.172–7.949), 3.706 (1.847–7.435), and 2.649 (1.477–4.750), respectively.
Conclusion
RAP is a common problem among children who first present with nonorganic acute abdominal pain. The identification of risk factors may provide proper management, especially follow-up plans for this group in the future.
Keywords: Abdominal pain, Recurrence, Pediatrics, Risk factors
INTRODUCTION
Abdominal pain (AP) is a common presentation in children in both emergency and outpatient settings and accounts for 9–12% of all hospital visits [1,2]. Although the cause of pain is usually a nonsurgical and self-limiting condition, such as constipation, gastroenteritis, viral infection, and dyspepsia, a large number of children have persistent or recurrent abdominal pain (RAP) [2,3,4].
RAP in children is defined as at least three episodes of AP that affect physical activity over 3 months [5]. The most common etiology is functional gastrointestinal disorder (FGID). A prospective study in Norway revealed that 87.7% of children with RAP met the ROME III criteria for the diagnosis of FGID, and the most common pain-related FGID or functional abdominal pain disorder (FAPD) was irritable bowel syndrome (IBS), followed by abdominal migraine, functional abdominal pain (FAP), and functional dyspepsia (FD) [6]. FAPD has a detrimental effect on children in terms of physical health, mental health, and academic performance [7]. Moreover, studies conducted in the United States and the Netherlands reported that a high proportion of healthcare expenditure is spent on investigations for FAPD, in both inpatient and outpatient care [8,9]. Therefore, determining the risk factors for RAP in children may help physicians to make an early diagnosis, and improve the prognosis of FGID [10].
The potential risk factors for RAP are of three main types: biological, psychosocial, and environmental factors [11]. Nevertheless, only a few studies have focused on children with AP; they reported some symptoms as independent risk factors for RAP, such as pain lasting for ≥7 days, pain causing night awakening, and epigastric pain [3,12]. However, some crucial risk factors have not been assessed, especially psychological factors that may greatly affect the clinical course of RAP. Thus, this study aimed to identify the risk factors for RAP in children who presented with nonorganic acute AP, including baseline characteristics, medical history, symptoms, and mental health problems.
MATERIALS AND METHODS
Patients and study design
This was a retrospective cohort study. We reviewed the medical records of children aged 2–15 years diagnosed with nonorganic acute AP who visited the pediatric outpatient department of Vajira Hospital, Nawamindradhiraj University, between January 2015 and December 2019. This age group was selected because chronic AP is a common problem among these individuals [13]. We identified patients using the Tenth Revision of International Classification of Diseases code related to nonorganic acute AP as follows: R10 (abdominal and pelvic pain without further specification), K29 (gastritis, unspecified, without hemorrhage), and K30 (dyspepsia).
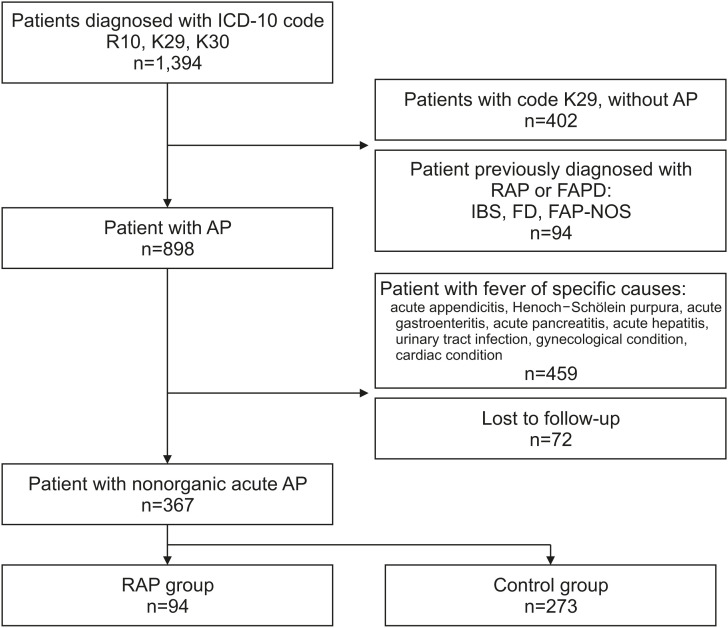
The electronic medical records were reviewed manually. For patients coded as K29 (gastritis), those without AP symptoms were excluded. The exclusion criteria were patients diagnosed with RAP or FAPD at the first visit, patients who either had fever or were diagnosed with specific causes during the follow-up period, and those who did not attend follow-up after the first diagnosis. RAP was defined as at least three episodes of AP that affected physical activity for 3 months [5]. According to this definition, patients were divided into two groups: the RAP group included those who had RAP within 3 months after the first visit, whereas the non-RAP group included those who did not meet the RAP definition (Fig. 1).
Fig. 1. Flow diagram of the study process and the number of patients in each group.
ICD-10: Tenth Revision of International Classification of Diseases, R10: ICD-10 code for abdominal and pelvic pain, K29: ICD-10 code for gastritis, unspecified, K30: ICD-10 code for dyspepsia, AP: abdominal pain, RAP: recurrent abdominal pain, FAPD: functional abdominal pain disorder, IBS: irritable bowel syndrome, FD: functional dyspepsia, FAP-NOS: functional abdominal pain, not otherwise specified.
We retrospectively collected data on independent variables from the electronic medical records of this cohort at the time they were first diagnosed with nonorganic acute AP, including the following: baseline characteristics such as sex, age, weight (kg), height (m), body mass index (BMI; kg/m2), and previous medical history within 1 year, including antibiotic use and gastrointestinal (GI) infection. For mental health problems, we identified children who were diagnosis with anxiety or major depressive disorder by psychiatrists based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition [14] within 1 year before AP and did not receive any medicine that contributed to AP. We included the following in the medical records: symptoms such as pain duration, change in stool frequency—defined as more than three bowel movements per day [15] or less than three bowel movements per week [16]—and site of AP. We used BMI to measure the degree of overweight and obesity, defined as >+1 and +2 standard deviations, respectively, for age and sex, and underweight, defined as <−2 standard deviations for age and sex, according to the World Health Organization’s child growth standards [17].
Outcome measurement
The primary outcomes were the risk factors for RAP. Outcomes were assessed by comparing baseline characteristics, previous medical history, and symptoms between children in the RAP and non-RAP groups.
Statistical analysis
Results are reported as numbers and percentages for categorical variables and means with ranges for continuous variables. The difference between the baseline characteristics of children with or without RAP was analyzed using the chi-square (χ2) test for sex and independent sample t-test for age. Univariate and multivariate logistic regression analyses were used to evaluate potential risk factors for RAP in children with nonorganic acute AP. Factors with a p-value <0.05 from univariate analysis were used in the multivariate analysis.
We also calculated variance inflation factors (VIF) to evaluate multicollinearity; a VIF <5 was considered an acceptable value. Binary logistic regression with the entering method for covariates was used to perform a multivariate analysis to assess the risk factors for chronic AP. All data were collected and analyzed using IBM SPSS Statistics for Windows, Version 21.0 (IBM Co., Armonk, NY, USA), and a p-value <0.05 was considered statistically significant.
Ethics statement
This study was approved by the Institutional Review Board of the Faculty of Medicine at Vajira Hospital (COA 015/2564).
RESULTS
Patient characteristics
Of the 367 patients with nonorganic acute AP who visited the pediatric outpatient department of Vajira Hospital, Nawamindradhiraj University, between January 2015 and December 2019, 94 (25.6%) experienced RAP within 3 months. The average patient age was 9.0±3.5 years, and 163 (44.4%) patients were male. No statistically significant difference was found between these two variables between the non-RAP and recurrent groups. In total, 112 eligible patients (30.5%) were overweight or obese, and the proportion of these patients in the RAP group (40.4%) was higher than that in the non-RAP group (26.7%). Similarly, the proportion of children who experienced AP for ≥7 days in the RAP group (27.2%) was greater than that in the non-RAP group (9.8%). Based on the site, epigastric pain was the most common (81.8%), followed by diffuse AP, lower quadrant pain, and upper quadrant pain. The most common associated symptoms were vomiting (46.5%), diarrhea (15.6%), and constipation (9.6%) (Table 1).
Table 1. Demographic and clinical characteristics of patients.
| Variable | RAP | Non-RAP | Total | p-value | ||
|---|---|---|---|---|---|---|
| Age (y) | 8.72±3.5 | 9.21±3.5 | 9.0±3.5 | 0.339 | ||
| Male sex | 36/94 (38.3) | 127/273 (46.5) | 163/367 (44.4) | 0.186 | ||
| Underweight | 15/94 (16.0) | 45/273 (16.5) | 60/367 (16.3) | 0.950 | ||
| Overweight | 38/94 (40.4) | 73/273 (26.7) | 112/367 (30.5) | 0.013 | ||
| Previous medical history | ||||||
| History of GI infection | 32/89 (36.0) | 67/242 (27.7) | 99/331 (29.9) | 0.005 | ||
| Prior antibiotic use | 28/89 (31.5) | 56/245 (22.9) | 84/334 (25.1) | 0.145 | ||
| Mental health problems | 11/89 (12.4) | 10/245 (4.1) | 21/334 (6.3) | 0.006 | ||
| Symptoms | ||||||
| Duration of pain (median, day) | 3 | 2 | 2 | |||
| Abdominal pain duration ≥7 days | 25/92 (27.2) | 26/265 (9.8) | 52/362 (14.4) | 0.001 | ||
| Site of pain | ||||||
| Epigastrium | ||||||
| Diffuse abdomen | 75/88 (85.2) | 202/253 (79.8) | 279/341 (81.8) | 0.265 | ||
| Left or right upper quadrant | 7/88 (8.0) | 17/253 (6.7) | 24/341 (7.0) | 0.234 | ||
| Left or right lower quadrant | 4/88 (4.5) | 11/253 (4.3) | 15/341 (4.4) | 0.965 | ||
| Change in stool frequency | ||||||
| Diarrhea | 12/88 (13.6) | 9/253 (3.6) | 21/341 (6.2) | 0.001 | ||
| Constipation | ||||||
| Vomiting | 36/93 (38.7) | 53/260 (20.4) | 89/353 (25.2) | 0.001 | ||
| 24/93 (25.8) | 31/260 (11.9) | 55/353 (15.6) | 0.002 | |||
| 12/93 (12.9) | 22/260 (8.5) | 34/353 (9.6) | 0.213 | |||
| 51/94 (54.3) | 116/265 (43.8) | 167/359 (46.5) | 0.080 | |||
Values are presented as mean±standard deviation, number (%), or median as appropriate.
RAP: recurrent abdominal pain, GI: gastrointestinal.
In total, 76 patients (80.8%) in the RAP group were diagnosed with FGIDs. FD was the most common diagnosis (53.9%), followed by IBS (23.7%), functional abdominal pain not otherwise specified (FAP-NOS, 17.1%), and functional constipation (5.3%). IBS was more often reported in adolescents (10–15 years), whereas FD and FAP-NOS were diagnosed more frequently in the younger group. The prevalence of IBS was higher in males than in females. The average age of the children with FGID seemed to be similar, ranging between 9.2 and 9.6 years, except for those diagnosed with FAP-NOS (7.4 years; Table 2).
Table 2. Characteristics of children with a functional gastrointestinal disorder (FGID).
| Characteristic | RAP (n=94) | ||||
|---|---|---|---|---|---|
| FGID (n=76) | Non FGID (n=18) | ||||
| Functional dyspepsia (n=41) | Irritable bowel syndrome (n=18) | Functional abdominal pain–not otherwise specified (n=13) | Functional constipation (n=4) | ||
| Age (y) | 9.6±3.1 | 9.2±2.9 | 7.5±3.4 | 9.3±2.5 | 7.3±4.4 |
| Adolescent | 19 (46.3) | 10 (55.6) | 4 (30.8) | 2 (50.0) | 7 (38.9) |
| Male sex | 12 (29.3) | 10 (55.6) | 5 (38.5) | 1 (25.0) | 10 (55.6) |
Values are presented as mean±standard deviation or number (%).
RAP: recurrent abdominal pain.
Risk factors for recurrent abdominal pain
Univariate logistic analysis revealed that being overweight or obese (p=0.013), a history of GI infection (p=0.007), mental health problems (p=0.009), duration of pain ≥7 days (p<0.001), and change in stool frequency (p=0.001) were risk factors associated with RAP in children with nonorganic acute AP. Odds ratios (ORs) and 95% confidence intervals (CIs) are shown in Table 3. All of these significant variables were assessed using multivariate logistic analysis. Four variables—history of GI infection (p=0.011), mental health problems (p=0.022), duration of pain ≥7 days (p<0.001), and change in stool frequency (p=0.001)—were identified as independent risk factors associated with RAP in children with nonorganic acute AP. The ORs and 95% CIs were 3.364 (1.314–8.162), 3.052 (1.172–7.949), 3.706 (1.847–7.435), and 2.649 (1.477–4.750), respectively (Table 4).
Table 3. Univariate analysis of risk factors for recurrent abdominal pain.
| Variable | OR | 95% IC | p-value | |
|---|---|---|---|---|
| Male sex | 1.401 | 0.868–2.263 | 0.167 | |
| Under 10 years old | 1.321 | 0.825–2.118 | 0.247 | |
| Overweight | 1.859 | 1.137–3.039 | 0.013 | |
| Previous medical history | ||||
| History of GI infection | 3.273 | 1.388–7.717 | 0.007 | |
| Prior antibiotic use | 1.549 | 0.905–2.652 | 0.111 | |
| Mental health problems | 3.314 | 1.356–8.101 | 0.009 | |
| Symptoms | ||||
| Pain duration ≥7 days | 3.430 | 1.860–6.327 | <0.001 | |
| Epigastric pain | 1.475 | 0.750–2.830 | 0.267 | |
| Change in stool frequency | 2.467 | 1.474–4.128 | 0.001 | |
OR: odds ratio, CI: confidence interval, GI: gastrointestinal.
Table 4. Multivariate analysis of risk factors for recurrent abdominal pain.
| Variable | OR | 95% IC | p-value |
|---|---|---|---|
| History of GI infection | 3.364 | 1.314–8.162 | 0.011 |
| Mental health problems | 3.052 | 1.172–7.949 | 0.022 |
| Pain duration ≥7 days | 3.706 | 1.847–7.435 | <0.001 |
| Change in stool frequency | 2.649 | 1.477–4.750 | 0.001 |
OR: odds ratio, CI: confidence interval, GI: gastrointestinal.
DISCUSSION
Our study found RAP in 25.6% of the children who presented with nonorganic acute AP, which is consistent with the previously reported prevalence of RAP (18–37%) [3,12]. Furthermore, 80.9% of patients in RAP group were diagnosed with FGID. This finding could support physicians in early diagnosis, reassure patients and their parents, help minimize investigations, and improve patient prognosis [10]. However, we did not find a significant difference in the distribution of FGIDs between each age group and sex.
The microbiota–gut–brain axis represents the bidirectional interaction required to maintain homeostasis of the GI function. Disturbance of this axis plays a major role in the pathogenesis of FGID [18], which was previously reported to be the main cause of RAP [6]. GI infection and antibiotic usage may lead to dysbiosis and affect the microbiota–gut–brain axis. This supports our finding that children with a history of GI infection are 3.4 times more likely to develop RAP. Saps et al. [19] reported that IBS and FD were more commonly found in children who presented with bacterial diarrhea than in the control group. Moreover, in a multicenter prospective cohort study, Pensabene et al. [20] reported that the prevalence of AP-related FGID was significantly higher in children after acute infective diarrhea events than in the control group. Regarding antibiotic use, Uusijarvi et al. [21] reported an association between FGID incidence and exposure to multiple antibiotic courses in early life among girls aged 9–12 years in a large prospective cohort study. A large case-control study in adults also reported that the risk of developing FGID was nearly two-fold in patients who received antibiotic treatment for nonenteric infections; specifically, the risk of IBS was more than two-fold in this group [22]. However, we did not observe an association between antibiotic use and RAP in the present study. This may be due to the limitation of the retrospective study design that, for some patients, antibiotics received outside the hospital may not have been reported.
Psychological factors are crucial risk factors for FGID, which may be explained by the interaction between the brain, gut, and microbiota via the hypothalamic–pituitary–adrenal axis (HPA axis). Chronic stressors are associated with an increase in HPA axis activity and may therefore modify the gut microbiome, causing cortisol and pro-inflammatory cytokine release. Cortisol alters both intestinal permeability and the blood–brain barrier, resulting in ease of communication between the gut microbiota and the brain [23]. This finding is also supported by clinical research. Campo et al. [24] reported that the prevalence of anxiety and depression was significantly higher in children aged 8–15 years with RAP. Similarly, Yacob et al. [25] reported that children with symptoms of anxiety or major depression are more likely to have pain-predominant FGIDs than are healthy individuals. In a longitudinal study of children with FGID, Horst et al. [26] stated that extraintestinal somatic and depressive symptoms were a potential risk for predicting the development of FGID in adults. These findings are consistent with our results that children with depression or anxiety are approximately three times more likely to develop RAP than other individuals.
Clinical symptoms associated with RAP and FAP have been previously studied. In a prospective cohort study of 606 patients, El-Chammas et al. [27] compared the symptoms of children with FGID and Crohn’s disease. Children with FGID tend to complain of vomiting and headache, whereas children with Crohn’s disease experience hematochezia and weight loss. A prospective cohort study stated that the positive predictors for chronic AP were age, pain-causing night awakening, high levels of other somatic symptoms, and chronic AP at baseline, whereas epigastric pain was a negative predictor [12]. Furthermore, Wallis and Fiks [3] found that children who had persistent AP were likely to have AP for at least 7 days, and no association of persistent pain was detected between pain location and severity. Our study emphasized that children who had AP for ≥7 days often developed RAP, and that a change in stool frequency was highly associated with RAP. However, we did not find epigastric pain as a negative predictor, as in a previous study.
To our knowledge, previous studies have only recognized psychological problems and changes in stool frequency in children with RAP at baseline, but not in children with acute AP. Although we found several potential risk factors for RAP in children with nonorganic acute AP, the present study had certain limitations. Because we conducted the study at a single center, the results may not be representative of other healthcare settings or the general population. Our study employed a retrospective chart review; therefore, some data and regular follow-up data were missing. It is likely that patients in the non-RAP group subsequently developed RAP but sought treatment from other healthcare facilities. As we used K30 (dyspepsia) as one of the inclusion criteria, FD was found in most of our patients with FGID, whereas in other studies, IBS was usually detected in the highest proportion of patients with FGID. We also attempted to minimize selection bias by excluding children diagnosed with FGID at the first visit. Although we included psychological factors such as depression and anxiety, we could not explore other important factors, especially parental mental health problems and stressors in early life. Therefore, future research should be performed prospectively to include these potential risk factors and minimize limitations and biases.
In conclusion, we identified potential risk factors, including history of GI infection, mental health problems, AP lasting ≥7 days, and changes in stool frequency. These risk factors may help physicians identify children who are likely to develop RAP; thus, they could provide proper management, especially follow-up plans for this group.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
References
- 1.Loening-Baucke V, Swidsinski A. Constipation as cause of acute abdominal pain in children. J Pediatr. 2007;151:666–669. doi: 10.1016/j.jpeds.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Magnúsdóttir MB, Róbertsson V, Þorgrímsson S, Rósmundsson Þ, Agnarsson Ú, Haraldsson Á. Abdominal pain is a common and recurring problem in paediatric emergency departments. Acta Paediatr. 2019;108:1905–1910. doi: 10.1111/apa.14782. [DOI] [PubMed] [Google Scholar]
- 3.Wallis EM, Fiks AG. Nonspecific abdominal pain in pediatric primary care: evaluation and outcomes. Acad Pediatr. 2015;15:333–339. doi: 10.1016/j.acap.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Lisman-van Leeuwen Y, Spee LA, Benninga MA, Bierma-Zeinstra SM, Berger MY. Prognosis of abdominal pain in children in primary care--a prospective cohort study. Ann Fam Med. 2013;11:238–244. doi: 10.1370/afm.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Apley J, Naish N. Recurrent abdominal pains: a field survey of 1,000 school children. Arch Dis Child. 1958;33:165–170. doi: 10.1136/adc.33.168.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helgeland H, Flagstad G, Grøtta J, Vandvik PO, Kristensen H, Markestad T. Diagnosing pediatric functional abdominal pain in children (4-15 years old) according to the Rome III Criteria: results from a Norwegian prospective study. J Pediatr Gastroenterol Nutr. 2009;49:309–315. doi: 10.1097/MPG.0b013e31818de3ab. [DOI] [PubMed] [Google Scholar]
- 7.Devanarayana NM, Rajindrajith S, Benninga MA. Quality of life and health care consultation in 13 to 18 year olds with abdominal pain predominant functional gastrointestinal diseases. BMC Gastroenterol. 2014;14:150. doi: 10.1186/1471-230X-14-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhroove G, Chogle A, Saps M. A million-dollar work-up for abdominal pain: is it worth it? J Pediatr Gastroenterol Nutr. 2010;51:579–583. doi: 10.1097/MPG.0b013e3181de0639. [DOI] [PubMed] [Google Scholar]
- 9.Hoekman DR, Rutten JM, Vlieger AM, Benninga MA, Dijkgraaf MG. Annual costs of care for pediatric irritable bowel syndrome, functional abdominal pain, and functional abdominal pain syndrome. J Pediatr. 2015;167:1103–8.e2. doi: 10.1016/j.jpeds.2015.07.058. [DOI] [PubMed] [Google Scholar]
- 10.Trivić I, Hojsak I. Initial diagnosis of functional gastrointestinal disorders in children increases a chance for resolution of symptoms. Pediatr Gastroenterol Hepatol Nutr. 2018;21:264–270. doi: 10.5223/pghn.2018.21.4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huguet A, Olthuis J, McGrath PJ, Tougas ME, Hayden JA, Stinson JN, et al. Systematic review of childhood and adolescent risk and prognostic factors for persistent abdominal pain. Acta Paediatr. 2017;106:545–553. doi: 10.1111/apa.13736. [DOI] [PubMed] [Google Scholar]
- 12.Spee LA, Lisman-van Leeuwen Y, Benninga MA, Bierma-Zeinstra SM, Kollen BJ, Berger MY. Predictors of chronic abdominal pain affecting the well-being of children in primary care. Ann Fam Med. 2015;13:158–163. doi: 10.1370/afm.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu T, Lingam R, Lycett K, Mensah FK, Muller J, Hiscock H, et al. Parent-reported prevalence and persistence of 19 common child health conditions. Arch Dis Child. 2018;103:548–556. doi: 10.1136/archdischild-2017-313191. [DOI] [PubMed] [Google Scholar]
- 14.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Arlington: American Psychiatric Association; 2013. [Google Scholar]
- 15.Benninga MA, Faure C, Hyman PE, St James Roberts I, Schechter NL, Nurko S. Childhood functional gastrointestinal disorders: neonate/toddler. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.02.016. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Hyams JS, Di Lorenzo C, Saps M, Shulman RJ, Staiano A, van Tilburg M. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2016;150:1456–68.e2. [Google Scholar]
- 17.de Onis M, Lobstein T. Defining obesity risk status in the general childhood population: which cut-offs should we use? Int J Pediatr Obes. 2010;5:458–460. doi: 10.3109/17477161003615583. [DOI] [PubMed] [Google Scholar]
- 18.Thapar N, Benninga MA, Crowell MD, Di Lorenzo C, Mack I, Nurko S, et al. Paediatric functional abdominal pain disorders. Nat Rev Dis Primers. 2020;6:89. doi: 10.1038/s41572-020-00222-5. [DOI] [PubMed] [Google Scholar]
- 19.Saps M, Pensabene L, Di Martino L, Staiano A, Wechsler J, Zheng X, et al. Post-infectious functional gastrointestinal disorders in children. J Pediatr. 2008;152:812–816. 816.e1. doi: 10.1016/j.jpeds.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 20.Pensabene L, Talarico V, Concolino D, Ciliberto D, Campanozzi A, Gentile T, et al. Postinfectious functional gastrointestinal disorders in children: a multicenter prospective study. J Pediatr. 2015;166:903–7.e1. doi: 10.1016/j.jpeds.2014.12.050. [DOI] [PubMed] [Google Scholar]
- 21.Uusijärvi A, Bergström A, Simrén M, Ludvigsson JF, Kull I, Wickman M, et al. Use of antibiotics in infancy and childhood and risk of recurrent abdominal pain--a Swedish birth cohort study. Neurogastroenterol Motil. 2014;26:841–850. doi: 10.1111/nmo.12340. [DOI] [PubMed] [Google Scholar]
- 22.Paula H, Grover M, Halder SL, Locke GR, 3rd, Schleck CD, Zinsmeister AR, et al. Non-enteric infections, antibiotic use, and risk of development of functional gastrointestinal disorders. Neurogastroenterol Motil. 2015;27:1580–1586. doi: 10.1111/nmo.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang TT, Lai JB, Du YL, Xu Y, Ruan LM, Hu SH. Current understanding of gut microbiota in mood disorders: an update of human studies. Front Genet. 2019;10:98. doi: 10.3389/fgene.2019.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campo JV, Bridge J, Ehmann M, Altman S, Lucas A, Birmaher B, et al. Recurrent abdominal pain, anxiety, and depression in primary care. Pediatrics. 2004;113:817–824. doi: 10.1542/peds.113.4.817. [DOI] [PubMed] [Google Scholar]
- 25.Yacob D, Di Lorenzo C, Bridge JA, Rosenstein PF, Onorato M, Bravender T, et al. Prevalence of pain-predominant functional gastrointestinal disorders and somatic symptoms in patients with anxiety or depressive disorders. J Pediatr. 2013;163:767–770. doi: 10.1016/j.jpeds.2013.02.033. [DOI] [PubMed] [Google Scholar]
- 26.Horst S, Shelby G, Anderson J, Acra S, Polk DB, Saville BR, et al. Predicting persistence of functional abdominal pain from childhood into young adulthood. Clin Gastroenterol Hepatol. 2014;12:2026–2032. doi: 10.1016/j.cgh.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Chammas K, Majeskie A, Simpson P, Sood M, Miranda A. Red flags in children with chronic abdominal pain and Crohn’s disease-a single center experience. J Pediatr. 2013;162:783–787. doi: 10.1016/j.jpeds.2012.09.014. [DOI] [PubMed] [Google Scholar]