Abstract
Purpose
To design a prospective study on endovascular closure of congenital portosystemic shunts. The primary endpoint was to assess the safety of endovascular closure. The secondary endpoint was to evaluate the clinical, analytical and imaging outcomes of treatment.
Methods
Fifteen patients (age range: 2 days to 21 years; 10 male) were referred to our center due to congenital portosystemic shunts. The following data were collected prior to treatment: age, sex, medical history, clinical and analytical data, urine trimethylaminuria, abdominal-US, and body-CT. The following data were collected at the time of intervention: anatomical and hemodynamic characteristics of the shunts, device used, and closure success. The following data were collected at various post-intervention time points: during hospital stay (to confirm shunt closure and detect complications) and at one year after (for clinical, analytical, and imaging purposes).
Results
The treatment was successful in 12 participants, migration of the device was observed in two, while acute splanchnic thrombosis was observed in one. Off-label devices were used in attempting to close the side-to-side shunts, and success was achieved using Amplatzer™ Ductus-Occluder and Amplatzer™ Muscular-Vascular-Septal-Defect-Occluder. The main changes were: increased prothrombin activity (p=0.043); decreased AST, ALT, GGT, and bilirubin (p=0.007, p=0.056, p=0.036, p=0.013); thrombocytopenia resolution (p=0.131); expansion of portal veins (p=0.005); normalization of Doppler portal flow (100%); regression of liver nodules (p=0.001); ammonia normalization (p=0.003); and disappearance of trimethylaminuria (p=0.285).
Conclusion
Endovascular closure is effective. Our results support the indication of endovascular closure for side-to-side shunts and for cases of congenital absence of portal vein.
Keywords: Portosystemic shunt, Portal vein, Ammonia, Trimethylaminuria
INTRODUCTION
Congenital portosystemic shunts are rare vascular communications between the portal and systemic veins that occur at birth because of an insult during embryological development of the portal venous system. As a result, total or partial deviation of portal blood from the liver to the systemic veins occurs. As a reslult, there is a consequent reduction of portal blood to the liver [1].
Congenital portosystemic shunts produce symptoms related to the depletion of portal blood to the liver. Examples of this include liver nodules [2,3,4,5,6,7,8,9], atrophy [10], and metabolic disorders, such as hepatopulmonary syndrome [11,12,13], portopulmonary hypertension [5,14,15], and encephalopathy [16].
Several congenital portosystemic shunts, including intrahepatic and distal shunts [17], and persistent ductus venosus [1] may resolve spontaneously. The prevalence of persistent congenital portosystemic shunts is 1 in every 30,000 births [18]. Furthermore, it can be symptomatic. While it is accepted that symptomatic shunts are treated [1], it remains controversial. Nevertheless, many authors recommend it to prevent complications [1,17,19].
Endovascular closure is a surgical treatment and the treatment of choice. It is indicated if endovascular closure is not possible. Liver transplant has been classically indicated in patients with congenital absence of the portal vein [20,21,22,23,24] and the consequent absence of portal blood flow to the liver; therefore, occlusion of their shunts can lead to catastrophic venous splanchnic congestion [1].
Prior to endovascular closure, a balloon occlusion test is performed. This provides information on whether to proceed with a one- or two-step endovascular closure. The balloon occlusion test is done by maintaining a balloon inflated in the shunt for 15 minutes, determining the maximum wedge pressure, and assessing the appearance of intrahepatic portal veins. Even if the portal veins are not demonstrated in conventional imaging or when wedge pressure is elevated, portal vein development is possible [10].
This study aimed to assess the clinical and radiological outcomes of the endovascular closure of congenital portosystemic shunts. The parameters that were investigated included the technical success of endovascular closure, liver-related outcomes, blood ammonia levels, and the presence of trimethylaminuria.
MATERIALS AND METHODS
Design of the study
This was an interventional prospective study in which patients of any sex and age with persistent congenital portosystemic shunts were enrolled from 2014 to 2017. The inclusion criteria were congenital portosystemic shunts, diagnosis of the aforementioned condition via ultrasound (US) and/or computed tomography (CT), and referral to our center. The exclusion criteria were the presence of uncorrectable coagulopathy and severe renal dysfunction.
Patients
Fifteen patients were enrolled; of whom, 10 were male subjects (66.7%) with a median age of 12.52 years (range: 0.005 to 22.19; standard deviation: 6.37). The most common findings in these patients were the occurrence of hyperammonaemia (93.3%), liver nodules (33.3%), trimethylaminuria (20%), and anomalies, such as atrial septal defects (40%) and Down syndrome (20%). Table 1 presents the symptoms and their associated anomalies.
Table 1. Sex, age, clinical symptoms, and anomalies in our series.
| ID | Sex/age | Clinical symptoms | Cardiac and vascular anomalies | Other |
|---|---|---|---|---|
| 1 | Male/1-day-old | Cholestasis | Tricuspid insufficiency | Microcephaly |
| Acute hepatic failure | ||||
| Neonatal acidosis | ||||
| Pulmonary hypertension | ||||
| Hyperammonemia | ||||
| 2 | Male/4.2-years-old | Hyperammonemia | Atrial septal defect | Down syndrome |
| 3 | Female/13.3 years-old | - | - | - |
| 4 | Female/18.2 years-old | Hyperammonemia | Persistent ductus arteriosus | Intrauterine growth restriction |
| Hepatic nodes | Bicuspid aortic valve | Silver Russell syndrome | ||
| Primary amenorrhea | Double IVC | Left vesicoureteral reflux | ||
| Suprahepatic veins draining directly into inferior vena cava | Right dysplastic kidney | |||
| 5 | Male/15.5-years-old | Hyperammonemia | Cor triatriatum | VACTERL association |
| Aortic coarctation | Nutcracker syndrome | |||
| Superior vena cava draining into coronary sinus | ||||
| Single umbilical artery | ||||
| 6 | Male/12.9-years-old | Hyperammonemia | Tricuspid insufficiency | - |
| Trimethylaminuria | Atrial septal defect | |||
| 7 | Male/13.7-years-old | Hyperammonemia | - | Medullary cystic kidney disease |
| Hepatic nodes | Bilateral retinopathy and optic nerve demyelination | |||
| 8 | Male/6.04-years-old | Hyperammonemia | - | Horseshoe kidney |
| 9 | Female/11.24-years-old | Hyperammonemia | Atrial septal defect and interventricular communications | Down syndrome |
| Aortic insufficiency | ||||
| 10 | Female/16.8-years-old | Hyperammonemia | Atrial septal defect, mitral valve prolapse and mitral insufficiency | Marfanoid phenotype |
| Hepatic nodes | Partial anomalous pulmonary venous return | Morgagni hernia | ||
| Thoracic scoliosis | ||||
| Bilateral hallux valgus | ||||
| Bilateral fifth-finger camptodactyly | ||||
| 11 | Male/12.3-years-old | Hyperammonemia | Left pulmonary artery stenosis | - |
| Hepatic nodes | Cutaneous hemangiomas | |||
| 12 | Male/4.4-years-old | Hyperammonemia | Atrial septal defect, patent foramen ovale, persistent ductus arteriosus | Down syndrome |
| Thoracic and lower limb telangiectasia | Apnea-hypopnea syndrome | |||
| 13 | Male/21.9-years-old | Hyperammonemia | Renal artery aneurysm | Cholelithiasis |
| Hepatic nodes | Absence of mid hepatic vein | Bilateral nephromegaly | ||
| 14 | Female/4.06-years-old | Hyperammonemia | Atrial septal defect, interventricular communications, persistent ductus arteriosus | Polysplenia related to heterotaxy |
| Trimethylaminuria | Pulmonary valve stenosis | Cholelithiasis | ||
| Pulmonary hypertension | Aortic insufficiency | Left hydronephrosis | ||
| Absence of the intrahepatic segment of inferior vena cava and azygos continuation of the inferior vena cava | Turner phenotype | |||
| Superior lip hemangioma and facial telangiectasia | ||||
| 15 | Male/3-years-old | Hyperammonemia | Patent foramen ovale | Niemann-Pick type C disease |
| Trimethylaminuria | Pulmonary valve stenosis | Noonan syndrome | ||
| Scapular venous malformation | Bilateral renal ectasia | |||
| Bilateral cryptorchidism | ||||
| Pontine low grade glioma |
A total of 93.3% of the patients developed clinical symptoms and anomalies. In addition, 80% of the patients had cardiac or vascular anomalies. Atrial septal defect (40%) and persistent ductus arteriosus (20%) are the most common among cardiac anomalies, while Down syndrome (20%) was also a common non-cardiac anomaly. Eleven of the cardiac and vascular anomalies were not previously related to congenital portosystemic shunts. These included the bicuspid aortic valve, cor triatriatum, suprahepatic veins draining directly into the inferior vena cava (IVC), absence of mid hepatic vein, superior vena cava draining into the coronary sinus, absence of the intrahepatic segment of IVC and azygos continuation, aortic insufficiency (n=2), partial anomalous pulmonary venous return, mitral valve prolapse, renal artery aneurysm, and scapular venous malformation. Among other anomalies, 11 of them were not previously reported. These included microcephaly, Silver Russell syndrome, VACTERL association, bilateral retinopathy, and optic nerve demyelination, Marfanoid phenotype, bilateral hallux valgus, apnea-hypopnea syndrome, cholelithiasis (n=2), bilateral nephromegaly, Niemann-Pick type C disease, and pontine low grade glioma.
Data collection
The following data were collected: sex, age, clinical symptoms, and associated anomalies. To assess liver-related outcomes prior to and at one year after the procedure, the following data were also collected: blood tests (prothrombin activity, liver transaminase levels, platelet count, and bilirubin), US findings (presence of Doppler flow characteristics of the main portal vein, diameters of the right and left portal veins and liver nodules, the pediatric Rosenburg value to assess the spleen size) [25], and CT liver volumetry using the three-dimensional image data (Aquarius; TeraRecon Inc., Durham, NC, USA), which was calculated. The pre-procedure CT was obtained in all patients. On the other hand, post-procedure CT was only ordered if the US findings were inconclusive. To determine whether encephalopathy is present, ammonia blood levels pre-procedure and at one-year follow-up were obtained. If symptoms of trimethylaminuria were present, urine tests, including trimethylamine and trimethylamine-n-oxide, were obtained pre-procedure and at one-year follow-up. Interventions were planned according to the results of the pre-procedure CT, and vascular access was chosen. The vascular access was established via the internal jugular vein or common femoral vein. All procedures were performed under general anesthesia in a suite with a Phillips-INTEGRIS-ALLURA-15&12-Monoplane (Phillips, Amsterdam, Netherlands) by the same two interventional radiologists. The patients were transferred to a pediatric critical care unit after the procedure.
During the intervention, the following data were collected: shunt anatomy, splanchnic blood pressure, minimum and maximum wedge pressure of other shunts, and selected occlusion device. To examine the shunt anatomy, the diameter, length, and afferent and efferent vessels of the shunt were obtained. They were calculated by calibrating a catheter using an angiograph software. The splanchnic blood pressure was determined through a deflated balloon. A 15-min balloon occlusion test and prophylactic endovascular heparin (10 IU/kg) were administered to prevent thrombosis. The balloons were chosen according to the shunt diameter (MustangTM; Boston Scientific, Marlborough, MA, XXLTM; Boston Scientific, and Reliant; Medtronic, Minneapolis, MN, USA). The appearance of intrahepatic portal veins was classified as minimum, moderate, or adequate. One-step endovascular closure was performed if the maximum wedge pressure was <30 mmHg, and if portal veins were present. If portal veins were present, the wedge pressure was ≥30 mmHg, and a two-step endovascular closure was planned. The technical success of the selection of an occlusion device was defined as adequate placement of the device and occlusion of the shunt. Perfusion of endovascular heparin (10 IU/kg/h) was administrated over 48 hours after endovascular closure, followed by prophylactic low-molecular-weight heparin, which was maintained until proper development of portal veins. Before discharge, US was performed to detect complications and confirm shunt closure.
There were different findings among the 15 patients.
1. Case 1
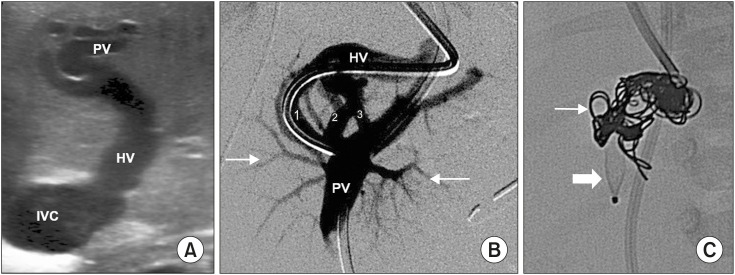
Pre-procedural US showed an inversion of the flow from the systemic to the portal system. The left hepatic vein (HV) was catheterized via the interjugular vein, confirming inversion and the adequacy of portal veins. Therefore, the balloon occlusion test was not necessary. The shunt was occluded via placement of a 6 mm AVP4 (AmplatzerTM Vascular Plug; Abbott, Abbott Park, IL, USA) and 3–8 mm coils (ConcertoTM Helix Detachable Coil; Medtronic) (Fig. 1).
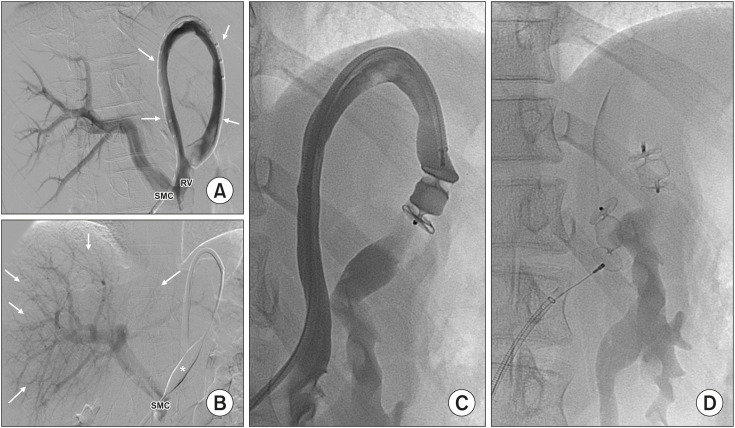
Fig. 1. (A) Congenital portosystemic shunts between left portal vein (PV) and left hepatic vein (HV) were observed in Case 1. (B) Venography showed three abnormal vessels (Case 1, 2, and 3) originating from the PV and draining into the HV. (C) It was closed using AmplatzerTM AVP4 (thick arrow) and detachable coils (thin arrow).
IVC: inferior vena cava.
2. Cases 2, 7, 9, 11, 12, and 13 (side-to-side portocaval shunts)
All interventions were performed via an internal jugular vein. A balloon occlusion test was not possible in one patient (case 13) as the shunt was wider (30 mm) than the balloons. Hence, attempting to perform the occlusion test during the procedure was not possible. Therefore, the necessary information for endovascular closure was not obtained, and the shunt was not treated.
The balloon occlusion test results indicated the conduct of a one-step endovascular closure in four cases (Cases 2, 7, 9, and 12) and two-step closure in one patient (Case 11). Vascular plugs (Amplatzer AVP or Amplatzer AVPII) were not suitable for this anatomy, but some cardiac plugs were.
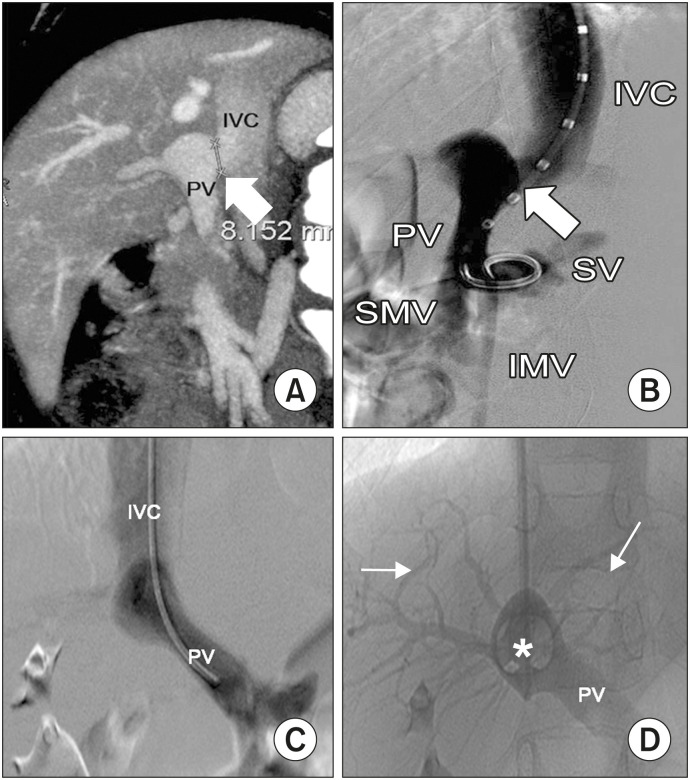
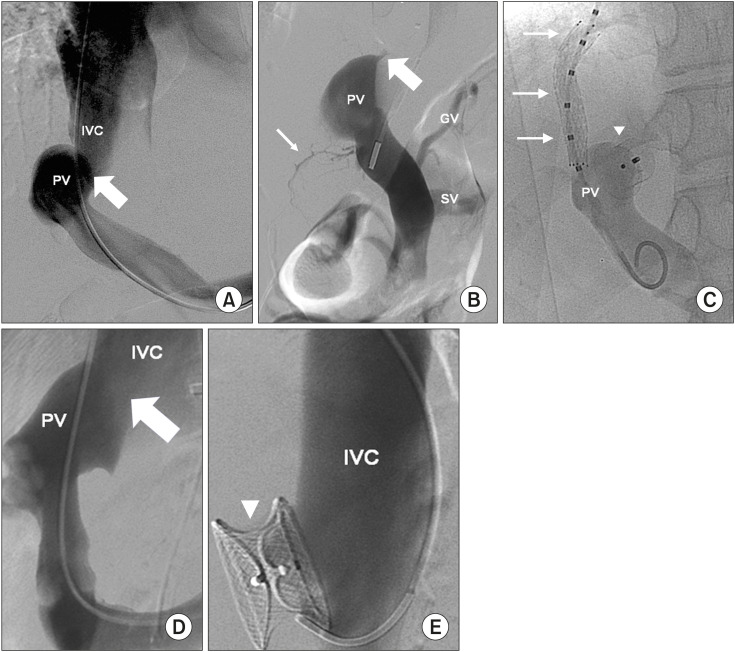
Our first choice was an AmplatzerTM-Duct-Occluder (Abbott) because it contained a retention skirt with diameters up to 22 mm. This remained on the portal side of the shunt with a cone with diameters of up to 14 mm, which were adjusted to the shunt diameter. Therefore, it was used when the shunt diameter was <14 mm, as was the case in three patients (Cases 2, 11, and 12; Fig. 2, Supplementary Fig. 1). In patient 11, prior to the placement of the AmplatzerTM Duct-Occluder, a transjugular intrahepatic portosystemic shunt (TIPS) was created between the mid HV and main portal vein using an 8×40 mm covered stent (EpicTM; Boston Scentific) due to the result of the balloon occlusion test, which recommended a two-step endovascular closure (Fig. 3A–C). The TIPS was planned to close the second step; however, it closed spontaneously. Case 7 had a shunt with a diameter of 24 mm; therefore, another cardiac plug with diameters of up to 26 mm was used (AmplatzerTM-Muscular-Ventricular-Septal-Defect-Occluder, Abbott) (Fig. 3D, E). Finally, Case 9 presented with a 28-mm shunt that was closed using an AmplatzerTM-Septal-Occluder (Abbott). However, migration of the shunt was observed 24 hours later.
Fig. 2. Side-to-side portocaval shunt in Case 2. Sagittal MPR reconstruction (A), oblique (B) and PA (C) venographies showing the shunt (arrow) between the main portal vein (PV) and the inferior vena cava (IVC). The superior mesenteric vein (SMV), inferior mesenteric vein (IMV), and splenic vein (SV) are also shown (B). Venography during balloon occlusion test (*) demonstrated normal intrahepatic portal branches (arrows) arranging from the PV (D).
MPR: multiplanar reconstruction, PA: posteroanterior.
Fig. 3. Side-to-side portocaval shunt (arrow) in patient 11. Portal vein (PV) and inferior vena cava (IVC) are shown (A). Balloon occlusion test (thick arrow) demonstrated minimum intrahepatic portal branches (thin arrow). Gastric vein (GV) and splenic vein (SV) are also shown (B). TIPS (arrows) was created before the portocaval shunt was closed with an Amplatzer™ Ductus-Occluder and Amplatzer™ (arrowhead). PV is shown (C). Portocaval shunt found in Case 7 (thick arrow), which was (D) treated with AmplatzerTM-Muscular-Ventricular-Septal-Defect-Occluder (E) (arrowhead). PV and IVC are also shown.
3. Congenital absence of portal vein (Cases 4, 5, 10, 14, 15)
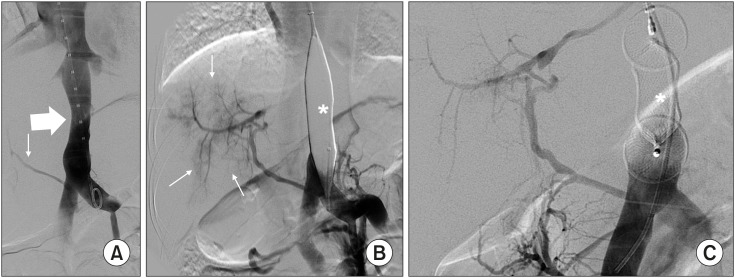
Five patients presented with a splanchnic venous return from a single vessel that flowed out directly into the systemic vasculature by end-to-side communication. Overall, the balloon occlusion test demonstrated a wedge pressure <30 mmHg and the presence of hypoplastic portal veins that CT had not previously shown. In these cases, a one-step endovascular closure was indicated. AmplatzerTM AVPII was successfully used in four patients (Cases 4, 5, 14, and 15; Fig. 4), and AmplatzerTM AVP in the remaining patient. However, it migrated immediately despite proper oversizing. In this patient, three intrahepatic congenital portosystemic shunts were identified and embolized using coils before the main shunt was closed.
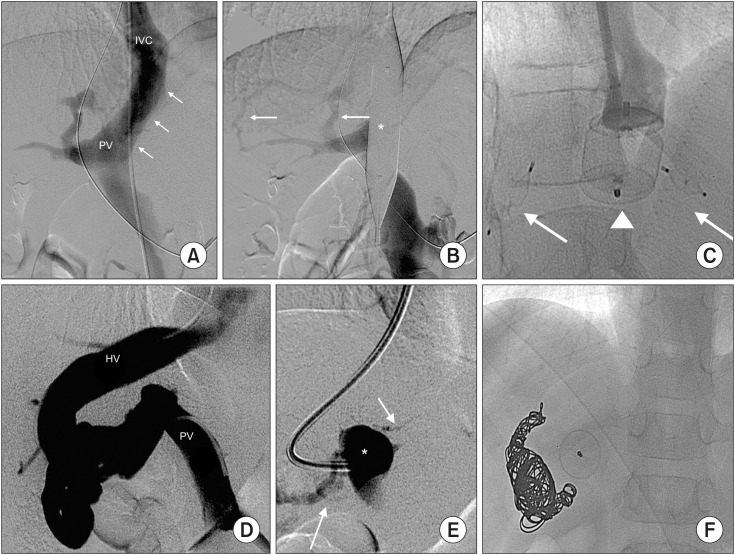
Fig. 4. Congenital absence of portal vein in Case 4. (A) All splanchnic venous return ran directly into right atrium (thick arrow), while a thin vessel ran into the liver (thin arrow). (B) Balloon occlusion test (*) demonstrated moderate intrahepatic portal branches (thin arrows) originating from the small vessel already shown during venography in “A” (thin arrow). (C) The shunt was closed using an AmplatzerTM AVPII (*).
4. Other presentations
Case 3 presented with a long vessel (160 mm) between the splenomesenteric confluence and left renal vein. The common femoral vein was catheterized for a balloon occlusion test, which showed adequate portal veins and a wedge pressure of <30 mmHg. The shunt was closed using a one-step endovascular closure with two Amplatzer AVPIs (Fig. 5).
Fig. 5. Shunt presented in Case 3. (A) A long and tortuous vessel (arrows) between the splenomesenteric confluence (SMC) and left renal vein (RV). (B) Balloon occlusion test (*) shows an adequate intrahepatic portal vein (arrows). (C, D) The shunt was subjected to a one-step closure using two AmplatzerTM AVPII.
Case 6 showed a 40-mm-long portocaval shunt. The internal jugular vein was catheterized, and an occlusion test was performed, which was oriented toward a one-step endovascular closure and showed two small intrahepatic shunts. The main shunt was embolized using an AmplatzerTM AVPII and intrahepatic with an AmplatzerTM AVP4 (Fig. 6A–C).
Fig. 6. (A) A long portocaval shunt (arrows) between the main portal vein (PV) and inferior vena cava (IVC) in Case 6. (B) Balloon occlusion test (*) showed two small intrahepatic shunts (arrows) and minimum portal branch- es. (C) The main shunt was embolized usiung an AmplatzerTM AVPII (arrowhead) and those intrahepatic with AmplatzerTM AVP4 (arrows). (D) The shunt found in Case 8 between the main PV and right hepatic vein (HV). (E) Balloon occlusion test (*) showed minimum portal branches. (F) The shunt was closed using AmplatzerTM AVP and detachable coils.
Finally, in Case 8, a vessel arising from the main portal vein gave rise to three smaller vessels flowing into the right HV. Via the intern jugular vein, the shunt was catheterized for a balloon occlusion test, which was oriented to a one-step endovascular closure. It was embolized using 16 mm-AmplatzerTM AVP and coils (Interlock-35; Boston Scientific) (Fig. 6D–F). Table 2 summarizes the characteristics of the shunts and the devices.
Table 2. Anatomic and hemodynamic findings and closure devices in our series.
| ID | Number of shunts | Diameter/length (mm) | Afferent/efferent vessels | Splanchnic blood pressure (mmHg) | Pressure min–max (mmHg) | Appearance intrahepatic portal veins during occlusion test | Closure device of the main shunt |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 6×16 | Left portal vein/left suprahepatic vein | - | - | - | 6 mm Amplatzer™ AVP4 and 3–8 mm coils |
| 2 | 1 | 8×<1 | Main portal vein/inferior vena cava | 8 | 19–24 | Adequate | 20 mm Amplatzer™-Duct-Occluder |
| 3 | 1 | 9×160 | Splenomesenteric confluence/left renal vein | 13 | 13–17 | Adequate | 12 mm Amplatzer™ AVPII (×2) |
| 4 | 1 | 15×80 | Splenomesenteric confluence/right atrium | 7 | 16–19 | Moderate | 22 mm Amplatzer™ AVPII |
| 5 | 1 | 10×100 | Splenomesenteric confluence/azygos | 8 | 17–17 | Adequate | 20 mm Amplatzer™ AVPII |
| 6 | 4 | 14×40 | Main portal vein/inferior vena cava | 12 | 14–14 | Minimum | 20 mm Amplatzer™ AVPII |
| 7 | 1 | 24×1–2 | Main portal vein/inferior vena cava | 7 | 14–15 | Minimum | 26 mm Amplatzer™-Muscular-Ventricular-Septal-Defect-Occluder |
| 8 | 1 | 11×10 | Main portal vein/right suprahepatic vein | 11 | 24–26 | Minimum | 16 mm Amplatzer™ AVPII |
| 9 | 1 | 28×10 | Main portal vein/inferior vena cava | 8 | 22–22 | Minimum | Amplatzer™-Septal-Occluder |
| 10 | 4 | 10×35 | Splenomesenteric confluence/right atrium | 9 | 22–26 | Minimum | 16 mm Amplatzer™ AVP |
| 11 | 1 | 12×1–2 | Main portal vein/inferior vena cava | 7 | 30–31 | Minimum | 20 mm Amplatzer™-Duct-Occluder |
| 12 | 1 | 12×<1 | Main portal vein/inferior vena cava | 8 | 22–25 | Moderate | 22 mm Amplatzer™-Duct-Occluder |
| 13 | 1 | 30×1–2 | Main portal vein/inferior vena cava | - | - | - | - |
| 14 | 1 | 15×45 | Superior mesenteric vein/left renal vein | 15 | 27–29 | Minimum | 18 and 20 mm Amplatzer™ AVPII |
| 15 | 1 | 8×10 | Splenomesenteric confluence/inferior vena cava | 10 | 25–28 | Minimum | 16 mm Amplatzer™ AVPII |
All patients but one presented with extrahepatic shunts. All patients with congenital absence of portal vein (Cases 4, 5, 10, 14, and 15) presented with endo-to-side shunts. Side-to-side portocaval shunts was present in six patients (Cases 2, 7, 9, 11, 12, and 13). Patient 6 also showed a portocaval shunt. However, in contrast to the others, a 40-mm-long vessel was communicating with the main portal vein and inferior vena cava. Hence, the chosen closure plugs differed from side-to-side portocaval shunts.
Statistical analysis
Statistical tests were performed using the SAS version 9.3 package (SAS Institute Inc., Cary, NC, USA). Pre- and post-intervention quantitative data were compared using the Wilcoxon signed-rank test, while qualitative data were compared using McNemar’s change test.
Statistical significance was set at p<0.05.
Ethics
The study protocol was approved by the ethics committee of Hospital Universitario La Paz (PI-2704). Procedural consent and consent for inclusion were obtained, and all the patients were enrolled.
RESULTS
The technical success and complications of the endovascular procedures
All patients who underwent the balloon occlusion test demonstrated hypoplastic portal veins with varying extent. Endovascular closure was thus attempted depending on the wedge pressure, as previously described.
All side-to-side portocaval shunts were approached via the internal jugular vein. If the shunts were <14 mm, they were successfully closed using an AmplatzerTM Ductus-Occluder. Two patients with diameters >14 mm (24 mm and 28 mm) were treated using an AmplatzerTM-Muscular-Ventricular-Septal-Defect-Occluder and AmplatzerTM-Septal-Occluder, respectively. Technical success was only obtained using the AmplatzerTM-Muscular-Ventricular-Septal-Defect-Occluder.
All five patients with congenital absence of the portal vein showed some extent of hypoplastic portal veins during the balloon occlusion test, and technical success was achieved in four patients using the AmplatzerTM AVPII. Migration was observed in one patient treated with the AmplatzerTM AVP.
The remaining four patients (Cases 1, 3, 6, and 8) presented with different shunt anatomy. Nevertheless, they shared enough length to enable the placement of vascular plugs or coils. Technical success was obtained in all of them.
Among the 14 patients in whom endovascular closure was intended, technical success was obtained in 12 (85.7%). Case 13 was not intended to be closed because the shunt was wider than expected. Migration of the device occurred in two cases (Cases 9 and 10), in which surgical retrieval was needed.
A thrombus was detected in the superior mesenteric vein of Case 4 48 hours post-treatment. It was resolved by perfusing urokinase (4,500 UI/kg/h) in the thrombus crossing through the AmplatzerTM AVPII via the internal jugular vein.
During follow-up, new shunts appeared in two patients. Case 4 developed an intrahepatic shunt that was closed using a 7 mm-AmplatzerTM AVP4, while Case 6 developed new portocaval communications, which was not yet treated.
Liver-related outcomes
The results in Case 1 were individually discussed due to the singularity of his presentation. It was a case of a newborn with acute hepatic failure (AST: 1,296 IU/L; ALT: 361 IU/L; prothrombin activity: 16%; bilirubin: 5.8 mg/dL; platelet count: 35×103 μL), metabolic acidosis, and hemodynamic instability that resolved after endovascular closure. The patient was discharged without symptoms at 3 weeks post-treatment (AST: 88 IU/L; ALT: 41 IU/L; prothrombin activity: 85%; platelet count: 312×103/μL; bilirubin: 0.5 mg/dL).
The changes observed in the remaining 11 patients in which technical success has been obtained is hereby discussed. The prothrombin activity of five patients showed a low percentage of prothrombin activity before treatment, which increased after treatment. In four patients, the values normalized (N: 70–120%). In one patient, the value was close to normal (68%). This increase was statistically significant (p=0.043). Additionally, all 11 patients showed a significant increase in prothrombin activity (p=0.003).
The transaminases and bilirubin of 11 patients were examined. Their AST levels (<95 IU/L) before and after treatment were normal. On the other hand, there was a significant downward trend in AST levels (p=0.007). A total of 36.4% of the patients had pre-procedure normal ALT (<35 IU/L), which increased to 72.7% after the procedure (p=0.125). The reduction in ALT levels was not statistically significant (p=0.056) after the treatment. A total of 54.5% of the patients showed pre-procedure normal GGT values (<38 IU/L), which increased to 90.9% after the treatment (p=0.125). After treatment, a significant downward trend in GGT levels was observed (p=0.036). Around 81.8% of the patients showed normal bilirubin concentrations (0.30-1.20 mg/dL) before treatment. After the treatment, 100% had normal bilirubin levels. In addition, a significant reduction in bilirubin concentration was observed (p=0.013).
Hypersplenism was assessed by observing the changes in spleen size and platelet count. Three patients developed splenomegaly after treatment; two had splenomegaly before, which resolved after; and six showed a normal spleen size before and after the treatment. The platelet counts were normal (N: 180–490×103/μL) in seven patients before and after the treatment, low in four patients before treatment, and initially low, which increased after treatment, in one patient. No association was observed between the endovascular closure and thrombocytopenia (p=0.131).
The portal vein in 10 patients showed abnormal pre-procedure Doppler flow. Changes were produced in response to treatment. If the portal flow was present, the portal flow changed from systemic to hepatopedal. If no portal veins could be identified, portal flow showed a normal pattern. The right and left portal vein diameters significantly increased in all patients (p=0.005).
A total of 15 liver nodules was detected in four patients (Cases 4, 6, 7, and 11). After treatment, 11 nodules disappeared (73.33%), 2 decreased in size (13.33%), and 2 remained unchanged (13.33%). These changes were statistically significant (p=0.001).
The liver volume was compared in three patients who underwent pre- and post-treatment CT. These patients showed a volume increase, although not significantly (p=0.109).
Ammonia
Ten of the 11 patients had hyperammonemia. After the intervention, nine had normal levels, while one had persistent hyperammonemia (Case 6). A significant reduction was observed (p=0.003).
Trimethylaminuria
Three patients showed symptoms of trimethylaminuria, which was confirmed via urinalysis. After treatment, the symptoms were resolved in two patients. Pre-procedure, all patients showed an elevated trimethylamine/trimethylamine-n-oxide ratio (N: 0.002–0.043), which normalized in two. The oxidizing ratio of trimethylamine-n-oxide (N: 98.4–99.8%) was reduced in all three patients before and after treatment. The values were normal in one patient and low in two. These changes were not significant (p=0.285).
Other changes
Patient 4, who had primary amenorrhea at the age of 18 years, began menses after treatment. Table 3 shows changes before and after treatment.
Table 3. Changes after treatment.
| Parameters | Before treatment | After treatment | Average change (Wilcoxon) |
|---|---|---|---|
| Prothrombin activity (%) | Mean: 58.4 (SD: 7.40) | Mean: 78.2 (SD: 6.72) | 19.8 [95% CI: 12.80 to 26.79] |
| N: 5 (patients showing a low percentage of prothrombin activity before treatment) | Median: 59 | Median: 78 | p=0.043 |
| Prothrombin activity (%) | Mean: 68.45 (SD: 11.25) | Mean: 84.36 (SD: 9.36) | 15.90 [95% CI: 10.78 to 21.03] |
| N: 11 (all treated patients) | Median: 72 | Median: 83 | p=0.003 |
| AST (IU/L) | Mean: 48.64 (SD: 19.37) | Mean: 34.09 (SD: 14.46) | −14.54 [95% CI: −3.54 to −25.54] |
| N: 11 (all treated patients) | Median: 45 | Median: 34 | p=0.007 |
| ALT (IU/L) | Mean: 38.82 (SD: 16.87) | Mean: 28.64 (SD: 14.67) | −10.18 [95% CI: −0.38 to −19.98] |
| N: 11 (all treated patients) | Median: 35 | Median: 26 | p=0.056 |
| GGT (IU/L) | Mean: 47 (SD: 61.83) | Mean: 35.64 (SD: 51.29) | −11.36 [95% CI: 1.13 to 21.58] |
| N: 11 (all treated patients) | Median: 26 | Median: 19 | p=0.036 |
| Bilirubin (mg/dL) | Mean: 0.83 (SD: 0.60) | Mean: 0.50 (SD: 0.25) | −0.32 [95% CI: −0.07 to −0.58] |
| N: 11 (all treated patients) | Median: 0.5 | Median: 0.4 | p=0.013 |
| Platelet counts (×103/μL) | Mean: 210.82 (SD: 75.55) | Mean: 225.36 (SD: 93.35) | 14.54 [95% CI: 35 to 5.91] |
| N: 11 (all treated patients) | Median: 192 | Median: 204 | p=0.131 |
| Right portal vein (mm) | Mean: 0.9 (SD: 1.52) | Mean: 8.2 (SD: 1.68) | 7.8 [95% CI: 6.17 to 8.42] |
| N: 11 (all treated patients) | Median: 0 | Median: 8 | p=0.005 |
| Left portal vein (mm) | Mean: 2.2 (SD: 2.20) | Mean: 8.75 (SD: 1.90) | 6.55 [95% CI: 5.05 to 8.04] |
| N: 11 (all treated patients) | Median: 2.5 | Median: 8 | p=0.005 |
| Liver nodules (mm) | Mean: 28.47 (SD: 19.43) | Mean: 10.93 (SD: 24.13) | −17.53 [95% CI: −10.06 to −25] |
| N: 15 (number of nodules) | Median: 23 | Median: 0 | p=0.001 |
| Liver volume (mL) | Mean: 635.67 (SD: 183.5) | Mean: 1,097 (SD: 54.836) | 461.33 [95% CI: 110.58 to 812.08] |
| N: 3 (patients with CT before and after closure) | Median: 733 | Median: 1,090 | p=0.109 |
| Ammonia (μg/dL) | Mean: 99.30 (SD: 29.16) | Mean: 39.30 (SD: 26.36) | −60 [95% CI: −42.51 to −77.48] |
| N: 10 (patients showing hyperammonaemia before treatment) | Median: 94 | Median: 32 | p=0.003 |
| Ratio trimethylamine/trimethylamine-n-oxide | Mean: 0.350 (SD: 0.053) | Mean: 0.167 (SD: 0.265) | p=0.285 |
| N: 3 (patients with symptoms of trimethylaminuria) | Median: 0.367 | Median: 0.027 | |
| % trimethylamine-n-oxide | Mean: 74.13 (SD: 3.01) | Mean: 88.33 (SD: 17.73) | p=0.285 |
| N: 3 (patients with symptoms of trimethylaminuria) | Median: 73.2 | Median: 97.3 |
Statistical analysis. A low percentage of prothrombin activity was demonstrated in five patients prior to closure. This table shows a significant rising trend in the prothrombin activity not only in five patients (p=0.043), but also in all patients treated (p=0.003). No patient presented with abnormal level of AST. The percentage of the patients with normal levels of ALT before and after treatment changed from 36.4% to 72.7% (p=0.0125), and the reduction in ALT levels was also not significant (p=0.056). The percentage of the patients showed pre-procedure normal GGT values, which increased from 54.5% to 90.9% (p=0.125), and a significant downward trend in GGT levels was observed (p=0.036). The percentage of patients showing normal bilirubin concentrations changed from 81.8% to 100% after closure. A significant reduction in bilirubin concentrations was observed (p=0.013). No statistical association between endovascular closure and thrombocytopenia was observed (p=0.131). Right and left portal vein diameter significantly increased. In addition, the diameter of liver nodules significantly decreased. The volume increase of the liver was observed. However, it was not significant (p=0.285). A reduction in ammonia levels was significant (p=0.003). Changes in trimethylaminuria were observed, but they were not significant (both p=0.285). Symptoms resolved in two of three patients.
CT: computed tomography, SD: standard deviation, CI: confidence interval.
Table 3 shows the changes after
DISCUSSION
Discussion of the technique
The results indicated that endovascular closure of congenital portosystemic shunts was effective in 12 out of 14 patients in whom treatment was attempted. On the other hand, it was unsuccessful in two due to migration of the plug.
Side-to-side congenital portosystemic shunts were successfully treated in four patients using cardiac plugs (off-label for congenital portosystemic shunts). The authors of a previous study [17] referred to claimed that closure of the shunt was the only possible treatment; however, in this study, we managed to treat these patients using plugs designed for cases of patent ductus arteriosus (AmplatzerTM-Duct-Occluder) and interventricular communications (AmplatzerTM-Muscular-Ventricular-Septal-Defect-Occluder).
Although congenital portosystemic shunts are a different disease, these conditions share extremely short-wide communications. Our first choice was an AmplatzerTM-Duct-Ocsscluder, which had a retention skirt that fixed the plug on the portal side when delivered. However, the maximum diameter was limited to 14 mm. For wider shunts, success was achieved in one patient using the AmplatzerTM-Muscular-Ventricular-Septal-Defect-Occluder, and migration was observed in another patient treated with the AmplatzerTM-Septal-Occluder. The AmplatzerTM-Muscular-Ventricular-Septal-Defect-Occluder has two vessel fixation points on each side compared to the AmplatzerTM-Septal-Occluder, which has only one fixation point on each side. This was probably the reason for migration.
Congenital absence of the portal vein was only treatable with liver transplant. However, five of our patients showed this anatomy, and the balloon occlusion test demonstrated hypoplastic portal veins. Portal vein development was achieved in these cases at the one-year follow-up visit. This supports the idea that portal veins, although undetectable on CT, are not absent and would develop after endovascular closure. Thus, endovascular management of patients with side-to-side (portocaval) shunts and those with congenital absence of portal vein may be managed with surgery and liver transplant respectively.
There were several complications. Migration of the device occurred in two patients. These two patients, together with Case 13, which had a failure of shunt closure, were the oldest in our series. As shunts become wider, the occlusion armamentarium decreases. Thus, we recommend treating patients when they are still infants. Cases 9 and 13 would have been treated with the AmplatzerTM-Duct-Occluder if the diameter of their shunts was less than 14 mm. Case 10 had to be closed using an AmplatzerTM AVPII instead of an AmplatzerTM AVP. The first one provided a larger surface of contact with the vessel and more fixation points (three to one per side), so the risk of migration was lower. Recently, Case 10 was successfully re-treated by the placement of two parallel Amplatzer AVPII. In the first intervention, a single Amplatzer AVPII would probably have been sufficient, but the shunt had increased in size three years later.
The splanchnic thrombosis was caused by the administration of heparin. It is fundamental to administer prophylactic endovascular heparin after endovascular closure and to maintain prophylactic low-molecular-weight heparin until the development of portal veins.
New shunts were identified during the follow-up. We postulated that if we had performed balloon-occlusion suprahepatic venography, we would have identified them during the procedure, and no re-interventions would have been needed.
The final technical aspect concerns the balloon occlusion test. The portal pressure increased during the first five minutes, which then stabilized. Accordingly, rather than conducting a standard 15-min balloon occlusion test [10,17], it is better to wait for the pressure to increase and remain stable. Thus, by reducing the balloon occlusion test time, we can lower the risk of thrombosis and intervention time.
Finally, although it was not included in the protocol study, the shunt size during the embolization procedure generally differed from the preoperative CT measurement; therefore, the real diameter was larger than expected.
Discussion of the clinical outcomes
The results must be contextualized in the short-term outcome (one year) and require further follow-up. Since the patients are infants, US is the best option. Periodic blood tests, including liver parameters and ammonia levels, should be obtained.
Liver-related outcomes
No patient showed liver-related symptoms, except for Case 1 who had acute failure. However, the results demonstrated an increased percentage of patients with normal liver laboratory data, a rising trend in prothrombin activity, and a downward trend in transaminase levels after endovascular closure.
Furthermore, no association between endovascular closure and thrombocytopenia was observed, but three patients developed splenomegaly at the one-year visit. Hence, further follow-up must be performed. Unexpected results included the splenomegaly in two patients. Splenomegaly was detected before endovascular closure. Nevertheless, it resolved after. Moreover, four presented with thrombocytopenia before treatment, which resolved in one patient.
A joint analysis of these data suggests that non-treated patients have some extent of hepatic dysfunction and portal hypertension. The acute hepatic failure observed in Case 1 and the presence of elevated splanchnic venous pressure (balloon deflated) in four patients support this hypothesis.
Portal plasticity [26] and the relationship between congenital portosystemic shunts and hyperplastic nodules [2,3,27,28] have been established. Our results strongly support both and point to an increased volume after treatment, although without significance, as only three patients have been analyzed.
Ammonia
Our results suggest that ammonia levels return to normal after endovascular closure [16,29,30]. Clinical improvement was felt by the patients’ parents in terms of school records and a better quality of life.
Trimethylaminuria
Three patients had symptoms of trimethylaminuria, which was characterized by a foul odor due to the presence of trimethylamine. Trimethylamine is absorbed in the intestine and transported to the liver, where it is converted into odorless trimethylamine-n-oxide. Healthy individuals excrete >92% of trimethylamine-n-oxide [31]. After treatment, two patients showed urine trimethylamine-n-oxide levels >92% and reported the disappearance of the foul odor. The third patient (Case 6) showed no improvement in clinical symptoms and urine measurements. These findings support the idea that congenital portosystemic shunts can cause secondary trimethylaminuria [32], which treatment can resolve.
Other changes
Hyperandrogenism is related to the congenital portosystemic shunts in a few cases due to liver bypass of dehydroepiandostendione [33,34]. One patient experienced primary amenorrhea, in whom menses commenced in response to treatment.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
SUPPLEMENTARY MATERIAL
Delivery of an Amplatzer™ Duct-Occluder in a latero-lateral portocaval shunt present in Case 2.
References
- 1.Guérin F, Blanc T, Gauthier F, Abella SF, Branchereau S. Congenital portosystemic vascular malformations. Semin Pediatr Surg. 2012;21:233–234. doi: 10.1053/j.sempedsurg.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Hao Y, Hong X, Zhao X. Congenital absence of the portal vein associated with focal nodular hyperplasia of the liver and congenital heart disease (Abernethy malformation): a case report and literature review. Oncol Lett. 2015;9:695–700. doi: 10.3892/ol.2014.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turkbey B, Karcaaltincaba M, Demir H, Akcoren Z, Yuce A, Haliloglu M. Multiple hyperplastic nodules in the liver with congenital absence of portal vein: MRI findings. Pediatr Radiol. 2006;36:445–448. doi: 10.1007/s00247-005-0103-0. [DOI] [PubMed] [Google Scholar]
- 4.Pupulim LF, Vullierme MP, Paradis V, Valla D, Terraz S, Vilgrain V. Congenital portosystemic shunts associated with liver tumours. Clin Radiol. 2013;68:e362–e369. doi: 10.1016/j.crad.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 5.Peker A, Ucar T, Kuloglu Z, Ceyhan K, Tutar E, Fitoz S. Congenital absence of portal vein associated with nodular regenerative hyperplasia of the liver and pulmonary hypertension. Clin Imaging. 2009;33:322–325. doi: 10.1016/j.clinimag.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Lautz TB, Shah SA, Superina RA. Hepatoblastoma in children with congenital portosystemic shunts. J Pediatr Gastroenterol Nutr. 2016;62:542–545. doi: 10.1097/MPG.0000000000001012. [DOI] [PubMed] [Google Scholar]
- 7.Correa C, Luengas JP, Howard SC, Veintemilla G. Hepatoblastoma and Abernethy malformation type I: case report. J Pediatr Hematol Oncol. 2017;39:e79–e81. doi: 10.1097/MPH.0000000000000650. [DOI] [PubMed] [Google Scholar]
- 8.Barton JW, 3rd, Keller MS. Liver transplantation for hepatoblastoma in a child with congenital absence of the portal vein. Pediatr Radiol. 1989;20:113–114. doi: 10.1007/BF02010653. [DOI] [PubMed] [Google Scholar]
- 9.Kawano S, Hasegawa S, Urushihara N, Okazaki T, Yoshida A, Kusafuka J, et al. Hepatoblastoma with congenital absence of the portal vein - a case report. Eur J Pediatr Surg. 2007;17:292–294. doi: 10.1055/s-2007-965448. [DOI] [PubMed] [Google Scholar]
- 10.Kanazawa H, Nosaka S, Miyazaki O, Sakamoto S, Fukuda A, Shigeta T, et al. The classification based on intrahepatic portal system for congenital portosystemic shunts. J Pediatr Surg. 2015;50:688–695. doi: 10.1016/j.jpedsurg.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Morikawa N, Honna T, Kuroda T, Kitano Y, Fuchimoto Y, Kawashima N, et al. Resolution of hepatopulmonary syndrome after ligation of a portosystemic shunt in a pediatric patient with an Abernethy malformation. J Pediatr Surg. 2008;43:e35–e38. doi: 10.1016/j.jpedsurg.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Osorio MJ, Bonow A, Bond GJ, Rivera MR, Vaughan KG, Shah A, et al. Abernethy malformation complicated by hepatopulmonary syndrome and a liver mass successfully treated by liver transplantation. Pediatr Transplant. 2011;15:E149–E151. doi: 10.1111/j.1399-3046.2010.01337.x. [DOI] [PubMed] [Google Scholar]
- 13.Fu L, Wang Q, Wu J, Guo Y, Huang M, Liu T, et al. Congenital extrahepatic portosystemic shunt: an underdiagnosed but treatable cause of hepatopulmonary syndrome. Eur J Pediatr. 2016;175:195–201. doi: 10.1007/s00431-015-2623-4. [DOI] [PubMed] [Google Scholar]
- 14.Iida T, Ogura Y, Doi H, Yagi S, Kanazawa H, Imai H, et al. Successful treatment of pulmonary hypertension secondary to congenital extrahepatic portocaval shunts (Abernethy type 2) by living donor liver transplantation after surgical shunt ligation. Transpl Int. 2010;23:105–109. doi: 10.1111/j.1432-2277.2009.00964.x. [DOI] [PubMed] [Google Scholar]
- 15.Ersch J, Bänziger O, Braegger C, Arbenz U, Stallmach T. An infant with pulmonary hypertension due to a congenital porto-caval shunt. Eur J Pediatr. 2002;161:660–662. doi: 10.1007/s00431-002-1096-4. [DOI] [PubMed] [Google Scholar]
- 16.Eroglu Y, Donaldson J, Sorensen LG, Vogelzang RL, Melin-Aldana H, Andersen J, et al. Improved neurocognitive function after radiologic closure of congenital portosystemic shunts. J Pediatr Gastroenterol Nutr. 2004;39:410–417. doi: 10.1097/00005176-200410000-00019. [DOI] [PubMed] [Google Scholar]
- 17.Bernard O, Franchi-Abella S, Branchereau S, Pariente D, Gauthier F, Jacquemin E. Congenital portosystemic shunts in children: recognition, evaluation, and management. Semin Liver Dis. 2012;32:273–287. doi: 10.1055/s-0032-1329896. [DOI] [PubMed] [Google Scholar]
- 18.Ono H, Mawatari H, Mizoguchi N, Eguchi T, Sakura N. Clinical features and outcome of eight infants with intrahepatic porto-venous shunts detected in neonatal screening for galactosaemia. Acta Paediatr. 1998;87:631–634. doi: 10.1080/080352598750014021. [DOI] [PubMed] [Google Scholar]
- 19.Franchi-Abella S, Branchereau S, Lambert V, Fabre M, Steimberg C, Losay J, et al. Complications of congenital portosystemic shunts in children: therapeutic options and outcomes. J Pediatr Gastroenterol Nutr. 2010;51:322–330. doi: 10.1097/MPG.0b013e3181d9cb92. [DOI] [PubMed] [Google Scholar]
- 20.Brasoveanu V, Ionescu MI, Grigorie R, Mihaila M, Bacalbasa N, Dumitru R, et al. Living donor liver transplantation for unresectable liver adenomatosis associated with congenital absence of portal vein: a case report and literature review. Am J Case Rep. 2015;16:637–644. doi: 10.12659/AJCR.895235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasahara M, Nakagawa A, Sakamoto S, Tanaka H, Shigeta T, Fukuda A, et al. Living donor liver transplantation for congenital absence of the portal vein with situs inversus. Liver Transpl. 2009;15:1641–1643. doi: 10.1002/lt.21839. [DOI] [PubMed] [Google Scholar]
- 22.Uchida H, Sakamoto S, Shigeta T, Hamano I, Kanazawa H, Fukuda A, et al. Living donor liver transplantation with renoportal anastomosis for a patient with congenital absence of the portal vein. Case Rep Surg. 2012;2012:670289. doi: 10.1155/2012/670289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuura T, Soejima Y, Taguchi T. Auxiliary partial orthotopic living donor liver transplantation with a small-for-size graft for congenital absence of the portal vein. Liver Transpl. 2010;16:1437–1439. doi: 10.1002/lt.22179. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto N, Matsusaki T, Hiroi K, Kaku R, Yoshida R, Umeda Y, et al. Pediatric living donor liver transplantation for congenital absence of the portal vein with pulmonary hypertension: a case report. Transplant Proc. 2020;52:630–633. doi: 10.1016/j.transproceed.2019.11.032. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg HK, Markowitz RI, Kolberg H, Park C, Hubbard A, Bellah RD. Normal splenic size in infants and children: sonographic measurements. AJR Am J Roentgenol. 1991;157:119–121. doi: 10.2214/ajr.157.1.2048509. [DOI] [PubMed] [Google Scholar]
- 26.Bruckheimer E, Dagan T, Atar E, Schwartz M, Kachko L, Superina R, et al. Staged transcatheter treatment of portal hypoplasia and congenital portosystemic shunts in children. Cardiovasc Intervent Radiol. 2013;36:1580–1585. doi: 10.1007/s00270-013-0581-7. [DOI] [PubMed] [Google Scholar]
- 27.Gülşen Z, Yiğit H, Demir P. Multiple regenerative nodular hyperplasia in the left infrarenal vena cava accompanied by Abernethy malformation. Surg Radiol Anat. 2016;38:373–378. doi: 10.1007/s00276-015-1460-5. [DOI] [PubMed] [Google Scholar]
- 28.Chandler TM, Heran MK, Chang SD, Parvez A, Harris AC. Multiple focal nodular hyperplasia lesions of the liver associated with congenital absence of the portal vein. Magn Reson Imaging. 2011;29:881–886. doi: 10.1016/j.mri.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Yamagami T, Yoshimatsu R, Matsumoto T, Terayama K, Nishiumra A, Maeda Y, et al. Successful embolization using interlocking detachable coils for a congenital extrahepatic portosystemic venous shunt in a child. J Pediatr Surg. 2007;42:1949–1952. doi: 10.1016/j.jpedsurg.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 30.Grimaldi C, Monti L, Falappa P, d’Ambrosio G, Manca A, de Ville de Goyet J. Congenital intrahepatic portohepatic shunt managed by interventional radiologic occlusion: a case report and literature review. J Pediatr Surg. 2012;47:e27–e31. doi: 10.1016/j.jpedsurg.2011.10.079. [DOI] [PubMed] [Google Scholar]
- 31.Messenger J, Clark S, Massick S, Bechtel M. A review of trimethylaminuria: (fish odor syndrome) J Clin Aesthet Dermatol. 2013;6:45–48. [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandez MS, Gutierrez C, Vila JJ, Lopez A, Ibanez V, Sanguesa C, et al. Congenital intrahepatic portocaval shunt associated with trimethylaminuria. Pediatr Surg Int. 1997;12:196–197. [PubMed] [Google Scholar]
- 33.Bas S, Guran T, Atay Z, Haliloglu B, Abalı S, Turan S, et al. Premature pubarche, hyperinsulinemia and hypothyroxinemia: novel manifestations of congenital portosystemic shunts (Abernethy malformation) in children. Horm Res Paediatr. 2015;83:282–287. doi: 10.1159/000369395. [DOI] [PubMed] [Google Scholar]
- 34.Satoh M, Yokoya S, Hachiya Y, Hachiya M, Fujisawa T, Hoshino K, et al. Two hyperandrogenic adolescent girls with congenital portosystemic shunt. Eur J Pediatr. 2001;160:307–311. doi: 10.1007/s004310000539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Delivery of an Amplatzer™ Duct-Occluder in a latero-lateral portocaval shunt present in Case 2.