Abstract
Adequate nutrition in early life is proposed to shape a child’s future health by launching the growth trajectory in the proper direction, which helps to avoid negative metabolic programming effects. Protein intake during infancy and early childhood is of great importance, as it plays a key role in infant metabolic programming and the future risk of obesity. Breastfeeding provides the best nutrition in early life, with many benefits tailored for the baby, including the appropriate quantity and quality of proteins. Considering the high prevalence of childhood, and subsequent adult, obesity in the region, a virtual Middle East expert consensus meeting was held to discuss an effective approach for managing childhood obesity. Leading pediatric experts from Bahrain, Egypt, Kuwait, Oman, Qatar, Saudi Arabia, and the United Arab Emirates participated in the meeting. The experts discussed, debated, and agreed on certain directions, including the importance of educating parents, endorsing breastfeeding, and ensuring optimum quantity and quality intake of proteins in early life. This expert consensus may serve as the starting point for healthcare professionals in the region who are interested in shaping a healthy future for the generations to come.
Keywords: Pediatric obesity; Malnutrition; Non-communicable diseases; Middle East; Infant formula; Proteins; Milk, human
INTRODUCTION
In terms of human health, the early childhood period is of great opportunity and great vulnerability to the future health of individuals [1]. Optimum nutrition is key to maintaining children’s growth and development on the right track and help them learn, play, participate and contribute [2]. Growth is an important criterion that is universally used by healthcare experts and caregivers to judge how well babies and children are developing. Too little growth (failure to thrive) or too much (overweight/obesity) have been linked to adverse effects on an infant’s wellbeing and damage to developmental outcomes, both of which may have long-term health impacts [3]. Therefore, it is vital to invest our efforts and focus on this critical window to maximize the well-being of future generations.
Burden of malnutrition
Malnutrition refers to deficiencies or imbalances in a person’s intake of energy/nutrients. Malnutrition is not limited to undernutrition, but also includes overweight, obesity, and the resulting diet-related non-communicable diseases (NCDs) [4]. NCDs are chronic conditions, which are usually non-infectious, but long-lasting, with examples including cardiovascular disease, hypertension, cancer, and type 2 diabetes [5]. Globally, NCDs are the leading cause of mortality, and are responsible for more than 7 of every 10 deaths annually [6]. Many of the most common NCDs can be triggered, at least in part, by an unhealthy diet [7]. Here, we focus on the overweight and obesity facets of malnutrition.
According to World Health Organization (WHO) data, obesity rates have increased by nearly 200% since 1975. In 2016, more than 1.9 billion adults (≥18 years) were overweight or obese [8,9]. Most of the available epidemiology data hints at a possible connection between adult obesity and pediatric obesity [10,11,12,13], which is evident from the dramatically high prevalence rates observed in children. This prevalence increases exponentially with age; between 2016 and 2019, 38 million children below the age of 5 and 340 million children and young adults between the ages of 5 and 19 were considered as overweight or obese [9]. While this data is relatively recent, global health agencies have been aware of the issue for several decades; despite this, excess weight gain during childhood and adolescence remains one of the most important issues facing modern medicine [14]. The risk of developing class II/III obesity
in adulthood is particularly high for children with obesity and severe obesity (Fig. 1) [10]. Similar trends are observed in the Middle East region, where there has been a rapid rise in the number of NCDs, particularly obesity [14,15,16,17], which is probably linked to the low levels of physical activity in many Eastern Mediterranean region countries [17]. In fact, published data indicate that obesity rates have reached an alarming level across all age groups in the region, with prevalence rates of up to 21.9%, 45%, and 81.9% among preschool children (<5 years), school-age children, and adults respectively [17]. These data indicate that obesity is a major public health problem within the Middle East, and needs urgent action to prevent and control it becoming a greater issue [14,15,16,17]. Pre-obesity should also be considered, due to its increasing prevalence in many children [18].
Fig. 1. Children with obesity have a substantial risk of adult obesity [10].
BMI: body mass index.
The consequences of this epidemic go beyond the stigma of being overweight or obese. Individuals who are obese are more likely to experience psychiatric, psychological, and psychosocial disorders during childhood and have an increased risk of developing NCDs later in life [11]. Obesity in children and adolescents is also linked to a higher risk of mortality in early adulthood [19]. Other consequences of early-life obesity are lower self-esteem, increased risk of being bullied, reduced employment prospects as an adult, and lower- salaries than their non-obese peers [11,20,21]. Moreover, obese children typically perform less well at school, have reduced cognitive abilities and a poor basic working memory, which can extend into adulthood [21,22]. Thus, as the consequences of being overweight in childhood can influence the entire life span [14], this field warrants further investigation.
Pre- and post-natal influences affecting obesity
Obesity is a condition of complex, multifactorial etiology, which can be influenced by pre- and post-natal factors [18,23]. Prenatally, a mother’s pre-conception weight and her weight gain during pregnancy both influence how likely her child is to become obese [23,24,25]. Excessive weight gain and/or gestational diabetes both increase the risk of fetal and neonatal macrosomia, as well as infant adiposity, which may extend into adulthood [23,25,26,27,28,29,30]. Children born to mothers with excessive weight gain during pregnancy have been found to be four times more likely to be overweight by the age of 3 [31,32]. Data also suggest that maternal obesity may contribute to reduced cognitive ability and neurodevelopmental delays in offspring [33].
Additionally, post-natal influences, such as infant sleep-duration and weight-gain, may play an important role in determining the trajectory of later health [32,34,35]. The results of a cohort study that investigated 915 mother-infant pairs indicated that children who slept fewer than 12 hours a day were twice as likely to be overweight at 3 years old [32,36]. Additional data show that rapid growth and enhanced weight gain during the early years of life are associated with higher body mass index (BMI) and obesity in adulthood [29,30,37]. Infants who grow rapidly during their first year of life, as measured using standardized age-related percentiles, have a relative risk of between 1.06 and 5.70 of becoming obese later in life, which is significantly higher than the risk of children who grow at a slower rate [26,38]. For children aged between 6 and 11 years, an increase in body mass and fat mass of 100 g per month from birth to 8 months was shown to increase the risk of becoming overweight by 5-fold and obese by 8-fold [26,39].
How childhood nutrition affects obesity
Key nutritional factors that may alter the risk of childhood obesity include breastfeeding status and protein intake. Using infant formula rather than breastfeeding for the first 12 months and a high protein intake have both been shown to exacerbate the risk of childhood obesity [40,41]. Lifestyle habits, such as regularly eating high-calorie foods, consumption of sugar-sweetened drinks, and lack of physical activities, may amplify this problem [11,42].
Leading health organizations recommend lowering the risk of obesity by addressing and reducing the impact of known risk factors during the early years of life [43]. One way of doing this is to improve dietary habits in infancy; for example, by promoting breastfeeding, and where this is not possible, encouraging the use of suitable infant formulas, i.e., those with a low-protein content [44,45,46]. Early childhood is a time for establishing positive behaviors that can prevent excessive weight gain and improve overall health throughout life. Promoting healthy feeding practices, such as careful timing of weaning onto solids, feeding to appetite rather than over-feeding, and regular exposure to healthy dietary choices, with high nutritional value and fewer refined sugars, all contribute to a healthier relationship with food. Other steps that can be taken to reduce the risk of obesity include increasing physical activity, reducing sedentary behaviors, and improving parental understanding of normal growth patterns and satiety cues [43,44,45,46].
Metabolic programming is an important component of later life health
Genes play an important role in determining an individual’s risk of becoming obese; however, the environment is thought to play a more important role in determining overall health status than genetics. Genes can be held accountable for approximately 20% of health-related effects over the lifespan, meaning that 80% is attributable to lifestyle factors, such as nutrition and activity levels [47,48].
However, environmental factors, such as nutrition and hormones, can influence gene expression during sensitive development stages via epigenetic processes that selectively switch genes on and off - this is known as “Early Metabolic Programming” [49]. Indeed, many aspects of long-term health are thought to be “programmed” by environmental stimuli such as nutrition during the first 1,000 days of life [50]. Protein is one such nutrient thought to play an important role in this early life programming.
Optimal protein intake in early life is crucial
Healthy growth and development of infants and children depend on them receiving the correct nutrition. There are more than 50 macro- and micro-nutrients that should be incorporated at balanced levels into the diet, with protein being one of the most important [51]. In 2007, the Food and Agriculture Organization (FAO) and WHO updated their guidelines to recommend that the intake of protein for infants up to 6 months of age does not exceed 1.31 g per kg of body weight per day [52]; this was a reduction from the previous recommended daily intake of 1.65 g/kg/day for infants of this age from 1985 [53]. This updated recommendation aligns closely with other international guidelines produced during the early 2000s (Fig. 2) [52,53,54,55,56,57,58,59], suggesting a growing awareness of the importance of protein quantity. Monitoring daily intake is important because excessive dietary protein intake in the early years is thought to be associated with the development of obesity [26]. Children whose protein intake at 12 months and/or 18-24 months was considered high (14.8% and 13.8% of total energy intake, respectively), typically had a higher BMI by the age of 7, and their body fat percentage was also more likely to be above the 75th percentile for their age [26,60].
Fig. 2. Recommended daily protein intake [52,53,54,55,56,57,58,59]. (A) 6-12 months. (B) 12-24 months. (C) 24 months+.
PRI: population reference intake, FAO: Food and Agriculture Organization, WHO: World Health Organization, UNU: United Nations University, HCN: Health Council of the Netherlands, NNR: Nordic Nutrition Recommendations, IoM: U.S. Institute of Medicine, AFSSA: Agence Française de Sécurité Sanitaire des Aliments (French Food Safety Agency), D-A-CH: Deutschland-Austria-Confoederatio Helvetica, EFSA: European Food Safety Authority.
Dietary proteins also provide essential amino acids, which are required for protein synthesis, breakdown, and excretion [61]. The specific amino acid profile of a protein is an important determinant of its quality, with certain amino acids, such as valine, leucine, and isoleucine contributing to weight gain and increased insulin resistance, while others, such as tryptophan, influence the production of serotonin and melatonin, which modulate key physiological functions, including mood, appetite, immune responses, and behavior [62,63]. It is therefore reasonable to assume that the precise amino acid profile of a protein will have a significant impact on health status, and that protein quality, as determined by amino acid composition, is as important an overall measure of food standard, as the quantity of protein present.
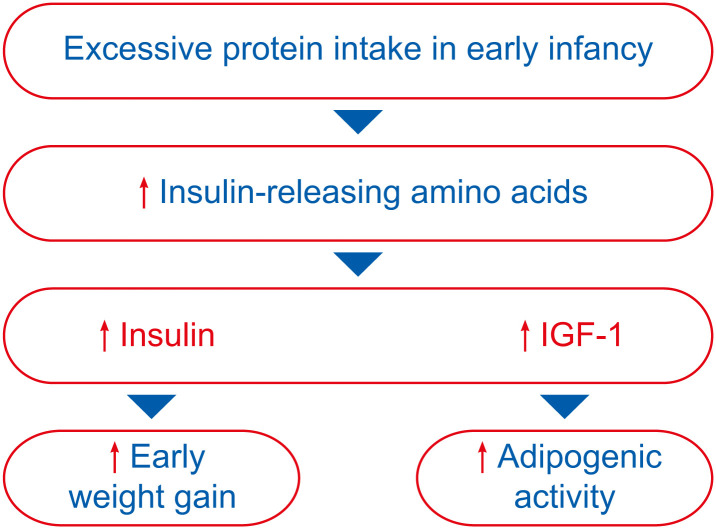
Oversupply of protein has also been linked to metabolic mal-programming and subsequent rapid growth and excessive weight gain [50,64]. According to the “Early Protein Hypothesis” proposed by Koletzko and colleagues [65,66], excessive protein intake in early life “programs” a tendency toward increased early weight gain and adipogenesis by increasing the levels of insulinogenic amino acids (e.g., valine, leucine, isoleucine, threonine), which in turn stimulate the secretion of insulin and insulin-like growth factor (IGF-1) (Fig. 3).
Fig. 3. The early protein hypothesis [65,66].
IGF-1: insulin-like growth factor.
Health consequences of high- and low-protein diets
The initial work to establish optimal daily intakes of protein focused on preventing deficiencies because the health impact of nutrient deficiency can be severe. Insufficient intake of protein and calories can trigger protein-energy malnutrition, which lowers immunity and increases susceptibility to infections [67,68]. Globally, malnutrition is the largest single cause of infant mortality [69]. Protein deficiencies can manifest clinically as kwashiorkor or marasmus, two life-threatening conditions that can have long-term health consequences [70]. Kwashiorkor is characterized by pitting edema and skin defects; it occurs when calorie intake is sufficient, but protein intake is low [68,70]. Marasmus is caused by a severe protein and calorie deficit, it causes growth retardation and muscle wastage, and affected children usually have a weight to height ratio that is at least 3 standard deviations below the average for their age [68,70]. Both conditions can increase infection susceptibility and may be a warning sign of other nutrient deficiencies [70].
Given the consequences of inadequate protein intake and malnutrition, recommended daily intakes of protein were calculated. However, over time, evidence emerged that too high a protein intake could also have damaging effects on an infant’s health, which is why between 1985 and 2007, the FAO and WHO reduced their recommendations for daily intake [52,53]. Ensuring a balanced intake of protein during childhood is important because, in addition to increased risk of obesity, excessive intake at these key developmental stages has been linked to reduced intellectual performance and damaging renal outcomes in later life [51,61]. Childhood obesity can also trigger other obesity-related conditions, such as cardiovascular disease, and can interfere with the timing of puberty [71,72].
Breast milk provides proteins of optimum quantity and quality
Breastfeeding is an unparalleled method for providing optimal nutrition to support the healthy growth and development of infants [73]. The quality and quantity of protein in human breast milk supports growth and improves short- and long-term health [74]. The protein content of breast milk evolves with time to match the infant’s needs, and thus avoids intake exceeding the metabolic requirements of the infant, protecting from overnutrition [75,76]. The metabolic system is programmed to recognize breast milk with its high protein quality, i.e., an amino acid profile that complements a growing infant’s exact requirements. This in turn optimizes metabolism and ensures long-term healthy functioning of the metabolic system [65,77].
What is an optimum infant formula according to these guidelines?
Although breast milk has multiple benefits and is the best option for infants during the first few months of life, sometimes breastfeeding is not possible. The next best option is to select an infant formula that is developed to mimic the composition of breast milk more closely, particularly regarding protein content. Replicating the quality and quantity of protein found in breast milk is challenging, not least because the endogenous levels of protein in breast milk differ as the infant grows, from 14 g/L immediately after birth to 8.3 g/L at 6 months of age [78]. However, with increasing evidence in support of the benefits of lower protein levels and technological advancements, many manufacturers are developing infant formulas functionally closer to breast milk [79].
The minimum protein content for infant formula authorized by European Commission (EC) and CODEX Standards is 1.8 g/100 kcal [80,81]. From a nutritional perspective, this covers the needs of almost all healthy term infants and there is no need to exceed these levels. Indeed, it may actually be damaging to do so, as nutrients which are not used or cannot be stored have to be excreted and this may compromise an infant’s immature metabolism [82]. To date, most attention has been focused on ensuring formulas reach the minimum protein levels; however, this can exceed the protein needs of infants over the age of 3 months by as much as 76% [83].
Clinical evidence on the influence of protein content
There is substantial evidence that high-protein infant formulas have a damaging effect on obesity levels and increase the risk of other NCDs [38,84]. Several clinical trials that compared the impact of low-protein versus high-protein infant formulas have shown that higher protein levels in infant formula increased the risk of childhood obesity led to a higher BMI, significant weight gain, and excess body fat [83,85,86,87,88,89,90]. A detailed description of these trials can be found in Table 1.
Table 1. Growth impact of protein content in infant formula.
| Study | Study design | Low protein intake | High protein intake | Age of intervention | Age at outcome | Outcome | Result | p-value |
|---|---|---|---|---|---|---|---|---|
| Koletzko et al., 2009 [87] | RCT | 1.77 g/100 kcal (infant formula) | 2.9 g/100 kcal (infant formula) | 0–12 mo | 24 mo | Weight for length (z score) | Higher protein: 0.20 greater | 0.005 |
| Multi-center | 2.2 g/100 kcal (follow-on formula) | 4.4 g/100 kcal (follow-on formula) | BMI (z score) | Higher protein: 0.23 greater | 0.001 | |||
| Singhal et al., 2010 [85] | 2 RCTs | 5–8 yr | ||||||
| Study 1: birth weight <10th centile | NA | 28% more protein (study 1)* | 0–9 mo (study 1) | Fat mass | Study 1: 38% higher with more protein | 0.009 | ||
| Study 2: birth weight <20th centile | NA | 43% more protein (study 2) | 0–6 mo (study 2) | Fat mass | Study 2: 18% higher with more protein | 0.040 | ||
| Weber et al., 2014 [89] | RCT | 1.25 g/dL (infant formula) | 2.05 g/dL (infant formula) | 0–12 mo | 6 yr | BMI | Higher protein: 0.51 greater | 0.009 |
| Multi-center | 1.6 g/dL (follow-on formula) | 3.2 g/dL (follow-on formula) | Risk of obesity | Higher protein: 2.43 times greater | 0.024 | |||
| Inostroza et al., 2014 [86] | RCT | 1.65 g/100 kcal† | 2.7 g/100 kcal | 3–12 mo | 3–6 mo | Weight gain | Higher protein: 1.77 g/day higher | 0.024 |
| Growth monitored for 2 yr | Weight and BMI | Higher protein: Remained higher after 2 yr | ||||||
| Ziegler et al., 2015 [83] | RCT | 1.61 g/100 kcal‡ | 2.15 g/100 kcal | 3–12 mo | 3–6 mo | Weight gain at 4–12 mo | Higher protein: Greater | 0.031 |
| Growth monitored for 12 mo | >85th percentile at 12 mo | Higher protein: Significantly more infants | 0.015 | |||||
| Oropeza-Ceja et al., 2018 [88] | RCT | 1 g/dL (IF1) | 1) 1.3 g/dL (IF2) | 1–4 mo | 4 mo | Weight gain | IF1 (lower protein): 25.8 g/day | |
| 2) 1.5 g/dL (IF3) | IF2: 32.3 g/day | 0.016 | ||||||
| IF3: 31.5 g/day | 0.006 | |||||||
| Weight–for–age (z score) | Lower for IF1 than IF2 | 0.031 | ||||||
| Lower for IF1 than IF3 | 0.014 | |||||||
| Totzauer et al., 2018 [90] | RCT | 1.25 g/dL (infant formula) | 2.05 g/dL (infant formula) | 0–12 mo | 6 yr | Excess body fat risk | Higher protein: doubled | 0.016 |
| Multi-center | 1.6 g/dL (follow-on formula) | 3.2 g/dL (follow-on formula) |
RCT: randomized control trial, BMI: body mass index, IF: infant formula, NA: not applicable.
*High-protein formula also contained higher energy levels; †Also contained probiotics; ‡Modified protein with caseinoglycomacropeptide removed.
Recent meta-analyses support the data from these original studies, with both Pimpin et al. [91] and Stokes et al. [92] confirming a positive association between high protein intake in the early years and increased weight gain/higher BMI in the months and years that follow. The first of these analyses compared the effects of high protein formula with low protein formula across 24 studies. The net result was a significant increase in weight (weighted mean difference) of 0.14 kg upon supplementation with high protein formula [91]. In the second meta-analysis, ten studies, representing six cohorts of formula-fed infants, reported a significant positive association (p<0.05) between total protein intake from birth to 2 years of age and BMI or BMI z score up to the age of 10 [92].
Considering all of these factors, a virtual online consensus meeting for the Middle East was held with the aim of gathering insights from local experts regarding the assessment and prevention of childhood obesity, and potentially its future health impacts.
METHODS
Twelve leading experts from Bahrain, Egypt, Kuwait, Oman, Qatar, Saudi Arabia, and the United Arab Emirates convened in a virtual online meeting to discuss approaches for the management and prevention of childhood obesity. The meeting was held on April 22, 2020, under the guidance of an international expert. The collaboration platform Microsoft Teams was used to conduct this expert meeting, which lasted for a total of 3 hours. A structured quantitative method was used to aid the discussion and reach a consensus [93]. Draft outcome statements were prepared in collaboration with the experts, based on local clinical practice and published evidence. There was a dedicated session for discussion and debate of these outcome statements during the meeting, until the refined versions were developed and agreed upon by ≥75% of the participants, at which point all group members were asked to vote. All group members voted to say whether they ‘Agreed’ or ‘Disagreed’ with each refined statement. The votes from each expert were added to reach a percentage consensus for each outcome statement.
Consensus statements
This expert discussion focused on the management of early life obesity (Table 2). The experts agreed that childhood obesity was a growing challenge faced in their practice, despite a clear lack of formal figures on epidemiology within the region. It was agreed that a concerted effort is needed to estimate the burden of childhood obesity in the region. Experts stated that obesity is multifactorial and that various non-nutrition factors, including genetics, epigenetics, and maternal BMI, must also be taken into consideration, rather than simply focusing on the nutrition aspect. Preventative interventions in the first few years must focus on diet, not only because weight gain is highly dependent on food intake, but also because the diet can be modified relatively easily [94]. An infant’s diet primarily consists of breastmilk/formula for the first 6 months, with additional complementary foods from approximately 6 months of age. The experts from this panel were strongly in favor of promoting breastfeeding, but in cases where that is impossible, an infant formula containing low protein levels, especially in infants considered to be at higher risk of developing obesity, or its associated conditions, is recommended. The importance of considering the amino acid profile of proteins in infant formula, as a measure of protein quality, was also highlighted. As with any other childhood metabolic disorder, lifestyle intervention, parental and caregiver reassurance, counseling, support, increasing awareness, and the continual education of mothers and pediatricians on the correct formula feeding practices are integral aspects of the management of early life obesity [95,96].
Table 2. Expert consensus on early life obesity.
| No | Consensus statements based on expert opinion | Consensus* |
|---|---|---|
| 1 | Children from Middle East countries are at a high risk of obesity | 100% |
| 2 | All infants should be assessed for the risk of obesity | 100% |
| 2a | Parents should be educated about healthy growth and development | 100% |
| 3 | Maternal obesity plays a role in the risk of childhood obesity | 100% |
| 4 | Breastfeeding decreases the risk of developing obesity | 100% |
| 5 | If not breastfed, an infant should be given a formula closest to the quantity and quality of breast milk protein | 100% |
| 6 | High dairy protein intake during infancy plays a key role in the risk of obesity in childhood, as well as in lifelong health | 100% |
| 7 | A lower protein content in infant formula (1.8 g/100 Kcal) at 0–6 months provides adequate infant growth patterns compared to breastfed infants | 100% |
| 8 | A lower protein content in follow-on formula (1.6–1.8 g/100 Kcal) at 6–12 months provides adequate infant growth patterns compared to breastfed infants | 100% |
| 9 | Amino acid profile of the protein in an infant formula is an important consideration along with protein quantity | 100% |
100%=all 12 working group members were in complete agreement after discussion and refinement of the statements.
*Percentage of working group members who agreed with each statement.
Another important topic that the experts discussed was ‘metabolic programming’; the experts concluded that earlier intervention, starting from conception, leads to a better outcome and can have a long-term impact on health across the lifespan [66]. Experts added that ‘overfeeding’ is a key challenge that needs to be tackled; focusing particularly on working mothers, and infants who are combination fed (part breastfed and part formula-fed). Pediatricians who are already sensitized to this issue, should inform other pediatricians as well as the families of their patients, on the importance of healthy portion size.
Regular follow-up between the clinician and patient is important to support the prevention of adiposities in infants.
DISCUSSION
The first 1,000 days of life (9 months of pregnancy and the first 2 years of life) are a critical time for metabolic programming effects. The complementary feeding period is an additional important window in early life where an infant is exposed to food sources beyond just breast milk and formula. The food habits developed during these sensitive periods can alter long-term food preferences and dietary decision making. Evidence-based guidance on complementary feeding practices at this stage can not only help in preventing excess weight gain in the future but also ensure that life-long healthy dietary habits are established. Among all dietary variables, protein intake plays a particularly important role in weight regulation during childhood, based on both interventional and observational studies [94].
Evidence suggests that high protein intake during infancy can have a damaging effect on long-term health [65,97]. Several original studies indicated that high protein formula can result in accelerated weight gain, higher BMI, and increased body-fat, which in turn, exacerbate the risk of adulthood obesity [66,87,89,90,98,99]. The results of these studies have recently been validated by meta-analyses, which reached the same conclusion [91,92]. With rates of childhood obesity continuing to increase, early metabolic programming is becoming an area of interest for researchers in the field [66].
IGF-1 could provide the link between high protein intake in childhood and increased risk of obesity later in life. As discussed earlier, protein intake that is surplus to metabolic requirements during childhood and consists of high quantity, but poor-quality protein, increases the concentration of circulating amino acids. These amino acids stimulate the secretion of insulin and IGF-1, which increase adipogenesis and contribute to early weight gain [65,87,100,101].
Reducing the amount of protein in infant formulas could be one way to prevent excessive growth and minimize weight gain during childhood [89,102]. A meta-analysis of five randomized control trials showed that a diet of optimum quality and quantity protein formula leads to a growth pattern very close to the WHO growth standard at 4 months of age [103]. As well as increasing the risk of obesity, poor childhood nutrition and defective metabolic programming, have also been shown to trigger other NCDs, such as type 2 diabetes, hypertension, and cardiovascular disease, in later life [50,104,105,106]. Reducing the protein content of infant formula may result in healthier body composition in early childhood, support age-appropriate growth patterns in children, and contribute to improved health in later life [88,89,90,102].
However, producing an infant formula that is closer to breast milk and contains the optimum quality and quantity of proteins can be a challenging task for manufacturers. The amino acid profile of breast milk contains comparatively lower levels of threonine and higher levels of tryptophan [107]. Using different methods to reduce the levels of protein in infant formula has generally resulted in a poorer quality product. Therefore, research has focused on developing infant formulas with an amino acid profile that closely mimics that found in breast milk. Innovations in infant formula development have resulted in formulas with lower concentrations of protein and an improved amino acid profile. These new formulas support age-appropriate growth and may exert health benefits into adulthood [89,107,108,109].
CONCLUSION
Obesity is a major healthcare issue in the Middle East region and other parts of the world, having reached epidemic proportions. Scientific evidence indicates toward a strong influence of early life environmental factors, such as protein intake, on future risk of obesity. Although breastfeeding is the best nutritional option for infants, when it is not possible, clinicians should ensure optimal protein quantity and quality intake in early life, which may help to curb the exponential growth of obesity and its adverse health impacts in our region. With this expert consensus, we hope to highlight the importance of early life protein intake and generate more discussions on this topic of vital importance.
ACKNOWLEDGEMENTS
Medical writing support in the compilation of comments and development of this manuscript was provided by Gauri Rao of INSPIRE HealthScience, Dubai.
Footnotes
Funding: The consensus statements, algorithms, and supporting evidence presented in this study were discussed and formulated at an online virtual consensus meeting under the guidance of an international expert. All authors received support from the Nestlé Nutrition Institute to attend this meeting. The views expressed in this paper are purely those of the authors, without any influence from the Nestlé Nutrition Institute.
Conflict of Interest: Frank Jochum has received speakers' honoraria from Nestlé Nutrition and has received research support related to specific research projects from different dairy companies. None of the other authors have a conflict of interest.
References
- 1.UNICEF. Early childhood development [Internet] New York (NY): UNICEF; [cited 2020 Jun 11]. Available from: https://www.unicef.org/early-childhood-development. [Google Scholar]
- 2.WHO. Levels and trends in child malnutrition [Internet] Geneva: WHO; [cited 2022 Mar 9]. Available from: https://www.who.int/publications/i/item/9789240025257. [Google Scholar]
- 3.Lucas A, Makrides M, Ziegler EE. Importance of growth for health and development. Nestle Nutr Workshop Ser Pediatr Program. 2010;65 [Google Scholar]
- 4.WHO. Malnutrition [Internet] Geneva: WHO; 2021. Jun 09, [cited 2020 Jun 11]. Available from: https:// www.who.int/news-room/fact-sheets/detail/malnutrition. [Google Scholar]
- 5.Budreviciute A, Damiati S, Sabir DK, Onder K, Schuller-Goetzburg P, Plakys G, et al. Management and prevention strategies for non-communicable diseases (NCDs) and their risk factors. Front Public Health. 2020;8:574111. doi: 10.3389/fpubh.2020.574111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Division of Global Health Protection, Global Health, CDC. About Global NCDs [Internet] Atlanta (GA): CDC; [cited 2021 Oct 4]. Available from: https://www.cdc.gov/globalhealth/healthprotection/ncd/global-ncd-overview.html. [Google Scholar]
- 7.WHO. Noncommunicable diseases [Internet] Geneva: WHO; 2021. Apr 13, [cited 2021 Sep 20]. Available from: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases. [Google Scholar]
- 8.The Lancet Public Health. Tackling obesity seriously: the time has come. Lancet Public Health. 2018;3:e153. doi: 10.1016/S2468-2667(18)30053-7. [DOI] [PubMed] [Google Scholar]
- 9.WHO. Obesity and overweight [Internet] Geneva: WHO; 2021. Jun 09, [cited 2020 Jun 11]. Available from: https://www.who.int/news-room/fact-sheets. [Google Scholar]
- 10.Woo JG, Zhang N, Fenchel M, Jacobs DR, Jr, Hu T, Urbina EM, et al. Prediction of adult class II/III obesity from childhood BMI: the i3C consortium. Int J Obes (Lond) 2020;44:1164–1172. doi: 10.1038/s41366-019-0461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sahoo K, Sahoo B, Choudhury AK, Sofi NY, Kumar R, Bhadoria AS. Childhood obesity: causes and consequences. J Family Med Prim Care. 2015;4:187–192. doi: 10.4103/2249-4863.154628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biro FM, Wien M. Childhood obesity and adult morbidities. Am J Clin Nutr. 2010;91:1499S–505S. doi: 10.3945/ajcn.2010.28701B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lakshman R, Elks CE, Ong KK. Childhood obesity. Circulation. 2012;126:1770–1779. doi: 10.1161/CIRCULATIONAHA.111.047738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Cesare M, Sorić M, Bovet P, Miranda JJ, Bhutta Z, Stevens GA, et al. The epidemiological burden of obesity in childhood: a worldwide epidemic requiring urgent action. BMC Med. 2019;17:212. doi: 10.1186/s12916-019-1449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mirmiran P, Sherafat-Kazemzadeh R, Jalali-Farahani S, Azizi F. Childhood obesity in the Middle East: a review. East Mediterr Health J. 2010;16:1009–1017. [PubMed] [Google Scholar]
- 16.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627–2642. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musaiger AO. Overweight and obesity in eastern mediterranean region: prevalence and possible causes. J Obes. 2011;2011:407237. doi: 10.1155/2011/407237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO European Childhood Obesity Surveillance Initiative (COSI) Severe obesity among children aged 6-9 years [Internet] Copenhagen: WHO Regional Office for Europe; 2019. [cited 2020 Jun 11]. Available from: http://www.euro.who.int/__data/assets/pdf_file/0019/400654/COSI-Severe-Obesity-FS-ENG-LowRes.pdf. [Google Scholar]
- 19.Lindberg L, Danielsson P, Persson M, Marcus C, Hagman E. Association of childhood obesity with risk of early all-cause and cause-specific mortality: a Swedish prospective cohort study. PLoS Med. 2020;17:e1003078. doi: 10.1371/journal.pmed.1003078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO. Taking action on childhood obesity [Internet] Geneva: WHO; [cited 2022 Mar 9]. Available from: https://apps.who.int/iris/handle/10665/274792. [Google Scholar]
- 21.OECD. The heavy burden of obesity: the economics of prevention [Internet] Paris: OECD Publishing; 2019. [cited 2020 Jun 11]. Available from: [DOI] [Google Scholar]
- 22.Wu N, Chen Y, Yang J, Li F. Childhood obesity and academic performance: the role of working memory. Front Psychol. 2017;8:611. doi: 10.3389/fpsyg.2017.00611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trandafir LM, Temneanu OR. Pre and post-natal risk and determination of factors for child obesity. J Med Life. 2016;9:386–391. [PMC free article] [PubMed] [Google Scholar]
- 24.Institute of Medicine. Nutrition during pregnancy: part I: weight gain, part II: nutrient supplements. Washington, D.C.: National Academies Press; 1990. [DOI] [PubMed] [Google Scholar]
- 25.Rkhzay-Jaf J, O’Dowd JF, Stocker CJ. Maternal obesity and the fetal origins of the metabolic syndrome. Curr Cardiovasc Risk Rep. 2012;6:487–495. doi: 10.1007/s12170-012-0257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larqué E, Labayen I, Flodmark CE, Lissau I, Czernin S, Moreno LA, et al. From conception to infancy - early risk factors for childhood obesity. Nat Rev Endocrinol. 2019;15:456–478. doi: 10.1038/s41574-019-0219-1. [DOI] [PubMed] [Google Scholar]
- 27.Catalano PM. The impact of gestational diabetes and maternal obesity on the mother and her offspring. J Dev Orig Health Dis. 2010;1:208–215. doi: 10.1017/S2040174410000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA. Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics. 2003;111:e221–e226. doi: 10.1542/peds.111.3.e221. [DOI] [PubMed] [Google Scholar]
- 29.Parsons TJ, Power C, Logan S, Summerbell CD. Childhood predictors of adult obesity: a systematic review. Int J Obes Relat Metab Disord. 1999;23(Suppl 8):S1–S107. [PubMed] [Google Scholar]
- 30.Rogers I. The influence of birthweight and intrauterine environment on adiposity and fat distribution in later life. Int J Obes Relat Metab Disord. 2003;27:755–777. doi: 10.1038/sj.ijo.0802316. [DOI] [PubMed] [Google Scholar]
- 31.Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol. 2007;196:322.e1–322.e8. doi: 10.1016/j.ajog.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harvard T.H. Chan School of Public Health. Obesity prevention source [Internet] Boston (MA): Harvard T.H. Chan School of Public Health; [cited 2020 Jun 14]. Available from: https://www.hsph.harvard.edu/obesity-prevention-source/ [Google Scholar]
- 33.Godfrey KM, Reynolds RM, Prescott SL, Nyirenda M, Jaddoe VW, Eriksson JG, et al. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017;5:53–64. doi: 10.1016/S2213-8587(16)30107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel SR. Reduced sleep as an obesity risk factor. Obes Rev. 2009;10(Suppl 2):61–68. doi: 10.1111/j.1467-789X.2009.00664.x. [DOI] [PubMed] [Google Scholar]
- 35.Reilly JJ, Armstrong J, Dorosty AR, Emmett PM, Ness A, Rogers I, et al. Early life risk factors for obesity in childhood: cohort study. BMJ. 2005;330:1357. doi: 10.1136/bmj.38470.670903.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taveras EM, Rifas-Shiman SL, Oken E, Gunderson EP, Gillman MW. Short sleep duration in infancy and risk of childhood overweight. Arch Pediatr Adolesc Med. 2008;162:305–311. doi: 10.1001/archpedi.162.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng M, Lamb KE, Grimes C, Laws R, Bolton K, Ong KK, et al. Rapid weight gain during infancy and subsequent adiposity: a systematic review and meta-analysis of evidence. Obes Rev. 2018;19:321–332. doi: 10.1111/obr.12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ. 2005;331:929. doi: 10.1136/bmj.38586.411273.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koontz MB, Gunzler DD, Presley L, Catalano PM. Longitudinal changes in infant body composition: association with childhood obesity. Pediatr Obes. 2014;9:e141–e144. doi: 10.1111/ijpo.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brands B, Demmelmair H, Koletzko B. How growth due to infant nutrition influences obesity and later disease risk. Acta Paediatr. 2014;103:578–585. doi: 10.1111/apa.12593. [DOI] [PubMed] [Google Scholar]
- 41.Uwaezuoke SN, Eneh CI, Ndu IK. Relationship between exclusive breastfeeding and lower risk of childhood obesity: a narrative review of published evidence. Clin Med Insights Pediatr. 2017;11:1179556517690196. doi: 10.1177/1179556517690196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mayo Clinic. Childhood obesity [Internet] Rochester (MN): Mayo Clinic; [cited 2020 Jun date]. Available from: https://www.mayoclinic.org/diseases-conditions/childhood-obesity/symptoms-causes/syc-20354827. [Google Scholar]
- 43.Hassink SG. Early child care and education: a key component of obesity prevention in infancy. Pediatrics. 2017;140:e20172846. doi: 10.1542/peds.2017-2846. [DOI] [PubMed] [Google Scholar]
- 44.Lumeng JC, Taveras EM, Birch L, Yanovski SZ. Prevention of obesity in infancy and early childhood: a National Institutes of Health workshop. JAMA Pediatr. 2015;169:484–490. doi: 10.1001/jamapediatrics.2014.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Denney-Wilson E, Laws R, Russell CG, Ong KL, Taki S, Elliot R, et al. Preventing obesity in infants: the Growing healthy feasibility trial protocol. BMJ Open. 2015;5:e009258. doi: 10.1136/bmjopen-2015-009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paul IM, Bartok CJ, Downs DS, Stifter CA, Ventura AK, Birch LL. Opportunities for the primary prevention of obesity during infancy. Adv Pediatr. 2009;56:107–133. doi: 10.1016/j.yapd.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Godfrey KM, Lillycrop KA, Burdge GC, Gluckman PD, Hanson MA. Epigenetic mechanisms and the mismatch concept of the developmental origins of health and disease. Pediatr Res. 2007;61(5 Pt 2):5R–10R. doi: 10.1203/pdr.0b013e318045bedb. [DOI] [PubMed] [Google Scholar]
- 48.Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 49.Koletzko B. Early nutrition and its later consequences: new opportunities. Adv Exp Med Biol. 2005;569:1–12. doi: 10.1007/1-4020-3535-7_1. [DOI] [PubMed] [Google Scholar]
- 50.Koletzko B, Brands B, Demmelmair H. The Early Nutrition Programming Project (EARNEST): 5 y of successful multidisciplinary collaborative research. Am J Clin Nutr. 2011;94(6 Suppl):1749S–53S. doi: 10.3945/ajcn.110.000471. [DOI] [PubMed] [Google Scholar]
- 51.Raiha NCR. Protein metabolism during infancy. Nestle Nutr Workshop Ser. 1994;33 [Google Scholar]
- 52.Joint FAO/WHO/UNU Expert Consultation on Protein and Amino Acid Requirements in Human Nutrition. Protein and amino acid requirements in human nutrition: report of a joint FAO/WHO/UNU expert consultation [Internet] Geneva: WHO; 2007. [cited 2021 Sep 29]. Available from: https://apps.who.int/iris/handle/10665/43411. [Google Scholar]
- 53.Energy and protein requirements. Report of a joint FAO/WHO/UNU Expert Consultation. World Health Organ Tech Rep Ser. 1985;724:1–206. [PubMed] [Google Scholar]
- 54.EFSA NDA Panel. (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2012. Scientific Opinion on Dietary Reference Values for protein. EFSA J. 2012;10:2557. [Google Scholar]
- 55.Health Council of the Netherlands. Dietary reference intakes: energy, proteins, fats, and digestible carbohydrates. The Hague: Health Council of the Netherlands; 2001. [Google Scholar]
- 56.Deutsche Gesellschaft für Ernährung (DGE) Protein [Internet] Bonn: DGE; 2008. [cited 2021 Oct 4]. Available from: https://www.dge.de/wissenschaft/referenzwerte/protein/ [Google Scholar]
- 57.L’Agence française de sécurité sanitaire des aliments (Afssa) Apport en protéines: consommation, qualité, besoins et recommandations [Internet] Maisons-Alfort: Afssa; 2007. [cited 2021 Oct 4]. Available from: https://www.anses.fr/fr/system/files/NUT-Ra-Proteines.pdf. [Google Scholar]
- 58.Nordic Council of Ministers. Nordic nutrition recommendations 2004: integrating nutrition and physical activity. 4th ed. Copenhagen: Nordic Council of Ministers; 2004. [Google Scholar]
- 59.Institute of Medicine. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: National Academies Press; 2005. [Google Scholar]
- 60.Günther AL, Buyken AE, Kroke A. Protein intake during the period of complementary feeding and early childhood and the association with body mass index and percentage body fat at 7 y of age. Am J Clin Nutr. 2007;85:1626–1633. doi: 10.1093/ajcn/85.6.1626. [DOI] [PubMed] [Google Scholar]
- 61.Raiha NCR. Protein requirement of healthy term infants during the first four months of life. Nestle Nutr Workshop Ser. 1994;33:153–164. [Google Scholar]
- 62.O’Rourke L, Clarke G, Nolan A, Watkins C, Dinan TG, Stanton C, et al. Tryptophan metabolic profile in term and preterm breast milk: implications for health. J Nutr Sci. 2018;7:e13. doi: 10.1017/jns.2017.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. Erratum in: Cell Metab 2009;9:565-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lucas A. Growth and later health: a general perspective. Nestle Nutr Workshop Ser Pediatr Program. 2010;65:1–9. doi: 10.1159/000281107. discussion 9-11. [DOI] [PubMed] [Google Scholar]
- 65.Koletzko B, von Kries R, Closa R, Escribano J, Scaglioni S, Giovannini M, et al. Can infant feeding choices modulate later obesity risk? Am J Clin Nutr. 2009;89:1502S–8S. doi: 10.3945/ajcn.2009.27113D. Erratum in: Am J Clin Nutr 2009;90:248. [DOI] [PubMed] [Google Scholar]
- 66.Koletzko B, Brands B, Poston L, Godfrey K, Demmelmair H. Early nutrition programming of long-term health. Proc Nutr Soc. 2012;71:371–378. doi: 10.1017/S0029665112000596. [DOI] [PubMed] [Google Scholar]
- 67.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ibrahim MK, Zambruni M, Melby CL, Melby PC. Impact of childhood malnutrition on host defense and infection. Clin Microbiol Rev. 2017;30:919–971. doi: 10.1128/CMR.00119-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grover Z, Ee LC. Protein energy malnutrition. Pediatr Clin North Am. 2009;56:1055–1068. doi: 10.1016/j.pcl.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 70.Titi-Lartey OA, Gupta V. Marasmus [Internet] Treasure Island (FL): StatPearls Publishing; 2021. [cited 2021 Nov 30]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559224/ [Google Scholar]
- 71.Marcovecchio ML, Chiarelli F. Obesity and growth during childhood and puberty. World Rev Nutr Diet. 2013;106:135–141. doi: 10.1159/000342545. [DOI] [PubMed] [Google Scholar]
- 72.Nadeau KJ, Maahs DM, Daniels SR, Eckel RH. Childhood obesity and cardiovascular disease: links and prevention strategies. Nat Rev Cardiol. 2011;8:513–525. doi: 10.1038/nrcardio.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.WHO. Breastfeeding [Internet] Geneva: WHO; 2013. [cited 2020 Jun]. Available from: https://www.who.int/health-topics/breastfeeding#tab=tab_1. [Google Scholar]
- 74.Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics. 2005;115:1367–1377. doi: 10.1542/peds.2004-1176. [DOI] [PubMed] [Google Scholar]
- 75.van’t Hof MA, Haschke F, Darvay S. Euro-Growth references on increments in length, weight, and head and arm circumferences during the first 3 years of life. Euro-Growth Study Group. J Pediatr Gastroenterol Nutr. 2000;31(Suppl 1):S39–S47. doi: 10.1097/00005176-200007001-00004. [DOI] [PubMed] [Google Scholar]
- 76.Hernell O. Human milk vs. cow’s milk and the evolution of infant formulas. Nestle Nutr Workshop Ser Pediatr Program. 2011;67:17–28. doi: 10.1159/000325572. [DOI] [PubMed] [Google Scholar]
- 77.Lönnerdal B. Nutritional and physiologic significance of human milk proteins. Am J Clin Nutr. 2003;77:1537S–43S. doi: 10.1093/ajcn/77.6.1537S. [DOI] [PubMed] [Google Scholar]
- 78.Fomon SJ. Requirements and recommended dietary intakes of protein during infancy. Pediatr Res. 1991;30:391–395. doi: 10.1203/00006450-199111000-00001. Erratum in: Pediatr Res 1992;31:21. [DOI] [PubMed] [Google Scholar]
- 79.Hardwick J, Sidnell A. Infant nutrition - diet between 6 and 24 months, implications for paediatric growth, overweight and obesity. Nutr Bull. 2014;39:354–363. [Google Scholar]
- 80.European Commission. Commission Directive 2006/141/EC of 22 December 2006 on infant formulae and follow-onformulae and amending Directive 1999/21/EC. Off J. Eur Union. 2006;49:1–33. [Google Scholar]
- 81.Codex Alimentarius. Standard for infant formula and formulas for special medical purposes intended for infants. CXS 72-1981. Formerly CAC/RS 72-1972. Adopted as a worldwide Standard in 1981. Amended in 1983, 1985, 1987, 2011, 2015, 2016, 2020. Revised in 2007. Rome: FAO; 2020. [Google Scholar]
- 82.EFSA NDA Panel. (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2014. Scientific Opinion on the essential composition of infant and follow-on formulae. EFSA J. 2014;12:3760. [Google Scholar]
- 83.Ziegler EE, Fields DA, Chernausek SD, Steenhout P, Grathwohl D, Jeter JM, et al. Adequacy of infant formula with protein content of 1.6 g/100 kcal for infants between 3 and 12 months. J Pediatr Gastroenterol Nutr. 2015;61:596–603. doi: 10.1097/MPG.0000000000000881. [DOI] [PubMed] [Google Scholar]
- 84.Michaelsen KF, Greer FR. Protein needs early in life and long-term health. Am J Clin Nutr. 2014;99:718S–22S. doi: 10.3945/ajcn.113.072603. [DOI] [PubMed] [Google Scholar]
- 85.Singhal A, Kennedy K, Lanigan J, Fewtrell M, Cole TJ, Stephenson T, et al. Nutrition in infancy and long-term risk of obesity: evidence from 2 randomized controlled trials. Am J Clin Nutr. 2010;92:1133–1144. doi: 10.3945/ajcn.2010.29302. [DOI] [PubMed] [Google Scholar]
- 86.Inostroza J, Haschke F, Steenhout P, Grathwohl D, Nelson SE, Ziegler EE. Low-protein formula slows weight gain in infants of overweight mothers. J Pediatr Gastroenterol Nutr. 2014;59:70–77. doi: 10.1097/MPG.0000000000000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koletzko B, von Kries R, Closa R, Escribano J, Scaglioni S, Giovannini M, et al. Lower protein in infant formula is associated with lower weight up to age 2 y: a randomized clinical trial. Am J Clin Nutr. 2009;89:1836–1845. doi: 10.3945/ajcn.2008.27091. [DOI] [PubMed] [Google Scholar]
- 88.Oropeza-Ceja LG, Rosado JL, Ronquillo D, García OP, Caamaño MDC, García-Ugalde C, et al. Lower protein intake supports normal growth of full-term infants fed formula: a randomized controlled trial. Nutrients. 2018;10:886. doi: 10.3390/nu10070886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weber M, Grote V, Closa-Monasterolo R, Escribano J, Langhendries JP, Dain E, et al. Lower protein content in infant formula reduces BMI and obesity risk at school age: follow-up of a randomized trial. Am J Clin Nutr. 2014;99:1041–1051. doi: 10.3945/ajcn.113.064071. [DOI] [PubMed] [Google Scholar]
- 90.Totzauer M, Luque V, Escribano J, Closa-Monasterolo R, Verduci E, ReDionigi A, et al. Effect of lower versus higher protein content in infant formula through the first year on body composition from 1 to 6 years: follow-up of a randomized clinical trial. Obesity (Silver Spring) 2018;26:1203–1210. doi: 10.1002/oby.22203. [DOI] [PubMed] [Google Scholar]
- 91.Pimpin L, Kranz S, Liu E, Shulkin M, Karageorgou D, Miller V, et al. Effects of animal protein supplementation of mothers, preterm infants, and term infants on growth outcomes in childhood: a systematic review and meta-analysis of randomized trials. Am J Clin Nutr. 2019;110:410–429. doi: 10.1093/ajcn/nqy348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stokes A, Campbell KJ, Yu HJ, Szymlek-Gay EA, Abbott G, He QQ, et al. Protein intake from birth to 2 years and obesity outcomes in later childhood and adolescence: a systematic review of prospective cohort Studies. Adv Nutr. 2021;12:1863–1876. doi: 10.1093/advances/nmab034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.CDC Evaluation Research Team. Gaining consensus among stakeholders through the nominal group technique [Internet] Atlanta (GA): CDC; 2018. [cited 2019 Mar 13]. Available from: https://www.cdc.gov/healthyyouth/evaluation/pdf/brief7.pdf. [Google Scholar]
- 94.Tang M. Protein intake during the first two years of life and its association with growth and risk of overweight. Int J Environ Res Public Health. 2018;15:1742. doi: 10.3390/ijerph15081742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dattilo AM, Birch L, Krebs NF, Lake A, Taveras EM, Saavedra JM. Need for early interventions in the prevention of pediatric overweight: a review and upcoming directions. J Obes. 2012;2012:123023. doi: 10.1155/2012/123023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Appleton J, Laws R, Russell CG, Fowler C, Campbell KJ, Denney-Wilson E. Infant formula feeding practices and the role of advice and support: an exploratory qualitative study. BMC Pediatr. 2018;18:12. doi: 10.1186/s12887-017-0977-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Escribano J, Luque V, Ferre N, Zaragoza-Jordana M, Grote V, Koletzko B, et al. Increased protein intake augments kidney volume and function in healthy infants. Kidney Int. 2011;79:783–790. doi: 10.1038/ki.2010.499. [DOI] [PubMed] [Google Scholar]
- 98.Agostoni C, Grandi F, Giannì ML, Silano M, Torcoletti M, Giovannini M, et al. Growth patterns of breast fed and formula fed infants in the first 12 months of life: an Italian study. Arch Dis Child. 1999;81:395–399. doi: 10.1136/adc.81.5.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Escribano J, Luque V, Ferre N, Mendez-Riera G, Koletzko B, Grote V, et al. Effect of protein intake and weight gain velocity on body fat mass at 6 months of age: the EU Childhood Obesity Programme. Int J Obes (Lond) 2012;36:548–553. doi: 10.1038/ijo.2011.276. [DOI] [PubMed] [Google Scholar]
- 100.Kirchberg FF, Harder U, Weber M, Grote V, Demmelmair H, Peissner W, et al. Dietary protein intake affects amino acid and acylcarnitine metabolism in infants aged 6 months. J Clin Endocrinol Metab. 2015;100:149–158. doi: 10.1210/jc.2014-3157. [DOI] [PubMed] [Google Scholar]
- 101.Martin RM, Holly JM, Smith GD, Ness AR, Emmett P, Rogers I, et al. Could associations between breastfeeding and insulin-like growth factors underlie associations of breastfeeding with adult chronic disease? The Avon Longitudinal Study of Parents and Children. Clin Endocrinol (Oxf) 2005;62:728–737. doi: 10.1111/j.1365-2265.2005.02287.x. [DOI] [PubMed] [Google Scholar]
- 102.Haschke F, Grathwohl D, Detzel P, Steenhout P, Wagemans N, Erdmann P. Postnatal high protein intake can contribute to accelerated weight gain of infants and increased obesity risk. Nestle Nutr Inst Workshop Ser. 2016;85:101–109. doi: 10.1159/000439492. [DOI] [PubMed] [Google Scholar]
- 103.Grathwohl D, Macé K, Fichot MC, Spivey-Krobath E, Steenhout P. 1216 Growth of infants fed with NAN is in good agreement with the WHO growth standard: a meta-analysis. Pediatr Res. 2010;68:602. [Google Scholar]
- 104.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Godfrey KM, Gluckman PD, Hanson MA. Developmental origins of metabolic disease: life course and intergenerational perspectives. Trends Endocrinol Metab. 2010;21:199–205. doi: 10.1016/j.tem.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 106.Ruemmele FM. Early programming effects of nutrition - life-long consequences? Ann Nutr Metab. 2011;58(Suppl 2):5–6. doi: 10.1159/000329574. [DOI] [PubMed] [Google Scholar]
- 107.Csapó J, Salamon S. Composition of the mother’s milk I. Protein contents, amino acid composition, biological value. A review. Acta Univ Sapientiae Aliment. 2009;2:174–195. [Google Scholar]
- 108.Sidnell A, Greenstreet E. Infant nutrition - protein and its influence on growth rate. Nutr Bull. 2009;34:395–400. [Google Scholar]
- 109.Räihä NC, Fazzolari-Nesci A, Cajozzo C, Puccio G, Monestier A, Moro G, et al. Whey predominant, whey modified infant formula with protein/energy ratio of 1.8 g/100 kcal: adequate and safe for term infants from birth to four months. J Pediatr Gastroenterol Nutr. 2002;35:275–281. doi: 10.1097/00005176-200209000-00008. [DOI] [PubMed] [Google Scholar]