Abstract
Purpose
While regurgitation is a common and often benign phenomenon in infants and younger children, it can also be a presenting symptom of gastroesophageal reflux disease (GERD). If untreated, GERD can lead to dangerous or lifelong complications. Clinical practice guidelines (CPGs) have been published to inform clinical diagnosis and management of pediatric GERD, but to date there has been no comprehensive review of guideline quality or methodological rigor.
Methods
A systematic literature search was performed, and a total of eight CPGs pertaining to pediatric GERD were identified. These CPGs were evaluated using the Appraisal of Guidelines for Research and Evaluation instrument.
Results
Three CPGs were found to be “high” quality, with 5 of 6 domains scoring >60%, one “average” quality, with 4 of 6 domains meeting that threshold, and the remaining four “low” quality.
Conclusion
Areas of strength among the CPGs included “Scope and Purpose” and “Clarity and Presentation,” as they tended to be well-written and easily understood. Areas in need of improvement were “Stakeholder Involvement,” “Rigor of Development,” and “Applicability,” suggesting these CPGs may not be appropriate for all patients or providers. This analysis found that while strong CPGs pertaining to the diagnosis and treatment of pediatric GERD exist, many published guidelines lack methodological rigor and broad applicability.
Keywords: Practice guidelines, Gastroesophageal reflux
INTRODUCTION
Gastroesophageal reflux (GER) is the passage of gastric contents into the esophagus or oropharynx. Though usually benign, these events can cause troublesome symptoms or complications in the case of gastroesophageal reflux disease (GERD) [1]. Knowing when and how to diagnose and treat reflux is challenging, particularly in pediatrics, where children often present with nonspecific symptoms [2,3].
The treatment of reflux in children has historically been characterized by overdiagnosis and over-prescription of medications [4,5]. Both pediatric GER and GERD are relatively common, and it is important for clinicians to know how to differentiate normal from pathological reflux. Effortless vomiting or regurgitation are characteristic of GER, while symptoms such as failure to thrive, feeding refusal, and Sandifer Syndrome, a stereotyped stretching and arching movement, all suggest GERD [6]. GER occurs daily in 50% of infants [7] and resolves in 95% of patients by 12-18 months of age [8]. Most of these cases are the result of physiological immaturity of the gastro-esophageal junction and do not require intervention [9]. However, if untreated, GERD, which is estimated to affect 26.9% of infants and as high as 10.1% of children older than 10 years of age [10], can lead to morbidities include dental erosion, reflux esophagitis, Barrett esophagus, and adenocarcinoma [11]. Treating GERD aggressively in young children can prevent lifelong symptoms and worrisome sequelae [12].
There are many possible treatments for pediatric GERD, ranging from nonpharmacological interventions such as thickened formula, sleep position change in older patients, and weight management, to pharmacological and surgical treatment [13]. Although recent studies show many advantages in non-pharmacological lifestyle changes, pediatricians often face intense pressure from parents to prescribe medications and invasive testing [14].
The challenges inherent in diagnosing and treating GERD in the pediatric population suggest the use of clinical practice guidelines (CPGs). CPGs are systematically developed statements that enable informed physician- and patient-decision making by providing explicit and evidence-based recommendations [9]. It is essential that CPGs are clear, practical, and free from bias [15], and the Appraisal of Guidelines for Research and Evaluation (AGREE II) collaboration has developed a system by which to evaluate the quality of CPGs. Reviewers assign CPGs numeric scores, evaluating the scope, developmental rigor, clarity, and applicability of the guidelines, among other criteria [16]. The AGREE instrument has been externally validated as being reliable and transparent and the best available CPG appraisal tool [17].
To the authors’ knowledge there has been no comprehensive review of CPGs relating to the care of pediatric patients with GERD. The goal of this paper is to assess and quantify the quality and developmental rigor of the existing practice guidelines for the diagnosis and clinical management of pediatric GERD using the AGREE II tool.
MATERIALS AND METHODS
Literature search
A systematic literature search was performed using the Scopus, PubMed, and Embase databases. The search terms were ((((“newborn” OR “infant” OR “neonate” OR “pediatric” OR “child”) AND (“Gastro-oesophageal AND Reflux” OR “gastric AND acid AND reflux” OR “gastroesophageal AND reflux AND disease” OR “gerd” OR “acid AND reflux” OR “heartburn” OR “regurgitation” OR “dyspepsia”) AND (“guideline” OR “consensus AND statement” OR “recommendation”)))) and all articles from database inception to February 1, 2021 were selected for initial review. An additional Google search was performed to identify other CPGs.
The compiled literature was screened for guidelines that addressed the diagnosis or treatment of pediatric GERD. Articles were screened by title and then by abstract. The authors excluded primary studies, summaries, and non-English language publications. Guidelines that discussed pediatric GERD in the broader context of adult GERD were excluded. If the same society or group published multiple guidelines, only the most recent was used. Both national and international guidelines were included, as were those written for either general medical or specialized audiences. The selected articles were discussed by the authors (JH, KC, and KR) and any discrepancies in inclusion criteria were addressed.
Data extraction
From each CPG the following data were recorded: the development body, publication, publication year, publication country, development method, key developers, target users, number of references, and any reported relevant funding source.
Quality appraisal
All investigators completed the free, online training available on the AGREE website (www.agreetrust.org). Independent assessments of each selected CPG using the AGREE II tool were performed by four authors (JH, EB, KX, and AN). The AGREE II instrument measures a CPG on the following domains: (1) Scope and Purpose, (2) Stakeholder Involvement, (3) Rigor of Development, (4) Clarity of Presentation, (5) Applicability, and (6) Editorial Independence. Investigators assigned a score from 1-7 for each of the 23 criteria across these domains, with a score of 7 if the criterion was fully addressed and a score of 1 if not at all addressed. The line-item scores from the four authors were averaged, and then as per the guidelines in the AGREE II manual (AGREE Next Steps Consortium), domains were scored using the following formula:
Scaled domain score=([obtained score–minimum possible score] / [maximum possible score– minimum possible score]×100)
The AGREE II tool characterizes a scaled domain score of 60 or greater as high quality. Overall scores for each domain were calculated, and CPG quality was rated as “high” if 5 or more domains scored ≥60, “average” if 3–4 domains scored ≥60, and “low” if 2 or fewer domains scored ≥60.
Statistical analysis
An intraclass correlation coefficient (ICC) analysis was performed using RStudio to assess the consistency between the four reviewers. ICC was calculated as poor (<0.20), fair (0.21–0.41), moderate (0.41–0.60), good (0.61-0.80), and very good (0.81–1.00) according to previous literature [18,19].
RESULTS
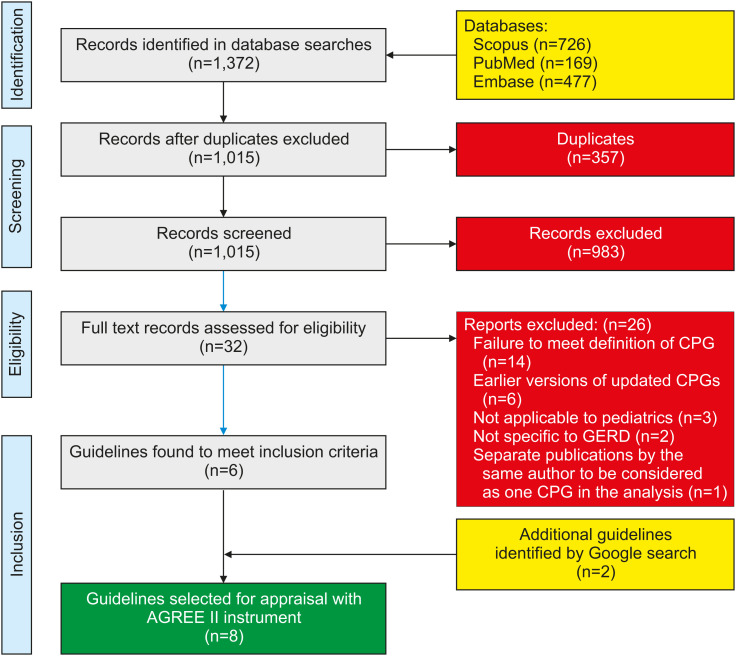
The initial database search yielded 1,015 non-duplicate results, which were then screened for exclusion criteria, leaving 25 articles for full review. Of these, six met the inclusion criteria described above. The Google search yielded an additional two CPGs, for a total of eight. This process is illustrated in Fig. 1.
Fig. 1. Flow diagram for identification of clinical practice guidelines (CPGs) and consensus statements.
GERD: gastroesophageal reflux disease, AGREE II: Appraisal of Guidelines for Research and Evaluation.
Guideline characteristics
Table 1 summarizes the development and methodology of the eight CPGs. Four guidelines were developed in the United States by the following institutions: the International Pediatric Endosurgery Group (IPEG) [20], the National Association of Pediatric Nurse Practitioners (NAPNAP) [21,22], the American College of Chest Physicians (CHEST) [23], and the Dell Children’s Medical Center (DCMC) [24]. Out of the remaining five guidelines, one was developed in Canada (University of Toronto) [25], one in the United Kingdom by the National Institute for Health and Care Excellence (NICE) [26], one in Australia by the Royal Children’s Hospital of Melbourne (RCHM) [27], and one that was a collaboration between the North American Society for Pediatric Gastroenterology, Hepatology & Nutrition (NASPGHAN) and the European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) [28]. The CPGs were published between 2008 and 2019, with one undated (DCMC). RCHM and DCMC published their guidelines on their hospital website and were identified by Google search. The other six CPGs were published in peer-reviewed journals.
Table 1. Characteristics of clinical practice guidelines (CPGs) included in study.
| Society | Publication year | Country | Development method | Developers | Target user | # of references | Funding source |
|---|---|---|---|---|---|---|---|
| IPEG | 2008 | USA | Systematic literature review, expert panel | Pediatric endosurgeons | Physicians | 32 | Not reported |
| University of Toronto | 2009 | Canada | Systematic literature review, expert opinion | Pediatric gastroenterologists | Development of future guidelines, clinical trials | 151 | INSINC Consulting, AstraZeneca Research |
| NICE | 2015 | UK | Systematic literature review, expert panel | Pediatric gastroenterologists; pediatricians; neonatologists; consultants in pediatric neurodisability; pediatric surgeons; GPs; pediatric NPs; pediatric dietitians; health visitors; relevant laypeople; experts in CPG methodology | Health and social care professionals, public health experts, commissioners or providers of health and social care services, and public | 8 | Not reported |
| NAPNAP | 2016 | USA | Not reported | Nurse, practitioners | Pediatrics | 24 | None |
| NASPGHAN & ESPGHAN | 2018 | North America & Europe | Systematic literature review, expert panel | Not reported | Pediatric gastroenterologists & primary care physicians | 302 | NASPGHAN & ESPGHAN |
| CHEST | 2019 | USA | Systematic literature review, expert opinion | Experts (unspecified), patients | Not reported | 26 | Not reported |
| RCHM | 2019 | Australia | Expert consensus, non-systematic literature review | Clinicians from general pediatrics, emergency medicine, and general practice | Clinicians working with young people | 10 | Not reported |
| DCMC | Not reported | USA | Expert consensus | Not reported | Primary care physicians | 0 | Not reported |
IPEG: International Pediatric Endosurgery Group, NICE: National Institute for Health and Care Excellence, NAPNAP: National Association of Pediatric Nurse Practitioners, NASPGHAN: North American Society for Pediatric Gastroenterology, Hepatology & Nutrition, ESPGHAN: European Society for Paediatric Gastroenterology Hepatology and Nutrition, CHEST: American College of Chest Physicians, RCHM: Royal Children’s Hospital Melbourne, DCMC: Dell Children’s Medical Center, GP: general practitioners, NP: nurse practitioners.
Five of the guidelines were developed based on both expert opinion and systematic literature reviews, with the other three based either on expert opinion alone (RCHM and DCMC) or did not specify (NAPNAP). The experts involved in the development of CPGs varied. In half of the CPGs, the experts consisted of pediatricians or gastroenterologists. Out of the remaining four, one CPG consisted of surgeon experts alone (IPEG), one CPG consisted of nurse practitioners (NAPNAP), and three consisted of a panel of experts but their professions were not specified. Target users in most instances were first-line medical caregivers such as pediatricians and nurses, though NICE targeted a wider range of public health and policy makers, and the University of Toronto targeted future CPG developers. Only three of the eight CPGs explicitly stated their funding sources (University of Toronto, NAPNAP, and NASPGHAN & ESPGHAN).
Guideline appraisal
Table 2 reports the domain scores for the CPGs. “Clarity and Presentation” and “Scope and Purpose” had the highest overall scaled scores, of 80.56 and 65.97 respectively. With a score of 27.86, “Applicability” scored the lowest. “Editorial Independence” had the greatest variability between CPGs, with a standard deviation of 44.14, while “Clarity and Presentation” had the least variability, with a standard deviation of 9.47. Three CPGs (University of Toronto, NICE, and NASPGHAN & ESPGHAN) were found to be high quality with 5/6 domains scoring ≥60. CHEST’s CPG was scored as average in quality, and the remaining four were deemed as low quality based on the domain scores.
Table 2. Domain scores of guidelines based on AGREE II analysis.
| Society/Institution | Domain 1 | Domain 2 | Domain 3 | Domain 4 | Domain 5 | Domain 6 | Domains ≥60/Total domains | Overall quality |
|---|---|---|---|---|---|---|---|---|
| Scope and Purpose (%) | Stakeholder Involvement (%) | Rigor of Development (%) | Clarity and Presentation (%) | Applicability (%) | Editorial Independence (%) | |||
| IPEG | 66.67 | 48.61 | 48.44 | 66.67 | 14.58 | 12.50 | 2/6 | Low |
| University of Toronto | 83.33 | 66.67 | 69.27 | 72.22 | 23.96 | 95.83 | 5/6 | High |
| NICE | 80.56 | 79.17 | 54.69 | 83.33 | 60.42 | 95.83 | 5/6 | High |
| NAPNAP | 54.17 | 19.44 | 13.54 | 84.72 | 26.04 | 35.42 | 1/6 | Low |
| NASPGHAN&ESPGHAN | 94.44 | 68.06 | 72.92 | 97.22 | 44.79 | 91.67 | 5/6 | High |
| CHEST | 84.72 | 55.56 | 68.23 | 83.33 | 25.00 | 83.33 | 4/6 | Average |
| RCHM | 20.83 | 9.72 | 4.17 | 73.61 | 20.83 | 0 | 1/6 | Low |
| DCMC | 43.06 | 31.94 | 0.52 | 83.33 | 7.29 | 0 | 1/6 | Low |
| Mean±SD | 65.97±25.00 | 47.40±24.83 | 41.47±30.58 | 80.56±9.47 | 27.86±16.10 | 51.82±44.14 |
AGREE II: Appraisal of Guidelines for Research and Evaluation, IPEG: International Pediatric Endosurgery Group, NICE: National Institute for Health and Care Excellence, NAPNAP: National Association of Pediatric Nurse Practitioners, NASPGHAN: North American Society for Pediatric Gastroenterology, Hepatology & Nutrition, ESPGHAN: European Society for Paediatric Gastroenterology Hepatology and Nutrition, CHEST: American College of Chest Physicians, RCHM: Royal Children’s Hospital Melbourne, DCMC: Dell Children’s Medical Center, SD: standard deviation.
Intraclass reliability
The ICCs for the six domains are presented in Table 3. The ICCs reflect the degree of consensus between the four reviewers (JH, EB, AN, and KX). “Scope and Practice”, “Stakeholder Involvement”, “Rigor of Development”, and “Editorial Independence” achieved “very good” intraclass reliability. “Clarity and Presentation” and “Applicability” achieved “good” intraclass reliability.
Table 3. Intraclass correlation coefficients for all domains.
| AGREE II domain | Intraclass correlation coefficient | 95% confidence interval |
|---|---|---|
| Scope and Purpose | 0.945 | 0.837 to 0.988 |
| Stakeholder Involvement | 0.929 | 0.789 to 0.984 |
| Rigor of Development | 0.958 | 0.876 to 0.991 |
| Clarity of Presentation | 0.601 | −0.184 to 0.910 |
| Applicability | 0.767 | 0.308 to 0.948 |
| Editorial Independence | 0.957 | 0.873 to 0.990 |
AGREE II: Appraisal of Guidelines for Research and Evaluation.
DISCUSSION
GERD can be difficult to diagnose, unpleasant and concerning for both patients and parents, and untreated can lead to long-term health morbidities. GER and GERD can be difficult to distinguish, and with many treatment options available, generating a diagnosis and treatment plan can be challenging for clinicians. CPGs, written by experts versed in the best available evidence, can help clinicians navigate challenging cases and provide standardized and cost-effective care [19]. Unfortunately, many published CPGs are of lower quality, contradictory, or outdated [29]. This study is the first to use the AGREE II tool to methodologically evaluate the quality of CPGs relating to the care of pediatric patients with GERD. Eight CPGs from around the world were identified and evaluated across the six AGREE II domains.
Scope and purpose
Domain 1, “Scope and Purpose,” asks whether a guideline clearly states its objectives, highlights the health questions, and describes the target population. Five out of the six CPGs scored highly in this domain. While almost all CPGs stated their objective, few specifically listed the health questions that they proposed to address. NASPGHAN & ESPGHAN, which was the highest scoring CPG in this domain, specifically stated each question they sought to answer. This type of organization allowed for a very clear understanding of the clinical decision making and practice recommendations.
Stakeholder involvement
Domain 2, “Stakeholder Involvement,” evaluates the authorship of the CPGs. Most CPGs performed poorly, suggesting that these development groups tended to show limited professional diversity. The three CPGs that cleared the high-quality threshold in this domain (University of Toronto, NICE, and NASPGHAN & ESPGHAN) included professionals from all relevant healthcare fields. This is necessary because each medical specialty manages GERD differently. A survey of gastroenterologists and otolaryngologists, for example, found differences between the two specialties with regards to diagnostic criteria, treatment dose and duration, and patient response for GERD [30]. Interestingly, general pediatricians were only involved in the development of half of the CPGs. This is unfortunate when considering that pediatricians are often the first clinician to see infants and children with GERD, and discuss reflux at one-quarter of infants’ routine 6-month visits [7].
Only one guideline (NICE) adequately sought public feedback on their recommendations before publishing. Patients’ expectations and experiences with health care are an important consideration for guideline development [16], and simple methods such as soliciting patient advocacy organizations or patients referred by clinicians can help patients’ voices be heard in the development process [31].
Rigor of development
Domain 3, “Rigor of Development,” is considered the strongest predictor for overall guideline quality, as it quantifies the evidentiary basis for published guidelines [32]. Only three CPGs (University of Toronto, NASPGHAN & ESPGHAN, and CHEST) scored as high quality in this domain. These three groups performed systematic literature reviews (including multiple databases, specific search terms, and clearly defined exclusion and inclusion criteria) and reported the quality of the evidence for each individual recommendation using the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) method, a well-validated tool used to appraise a body of evidence [33]. Weaker scoring CPGs relied exclusively on expert opinion or non-systematic literature reviews. While there is a role for expert opinion in CPGs [34], a systematic review ensures the literature has been appropriately consulted. A review of AGREE II analyses since the tool’s inception noted consistently low scores for “Rigor of Development” [35]. This again suggests the need for a multidisciplinary development team: research librarians can bring expertise in systematic literature reviews and can ensure proper documentation of search strategies [36].
Other areas where guidelines fell short were in review by outside experts (only 2/8 CPGs) and explicit procedures for updates (2/8 CPGs). These are relatively simple areas for improvement that could greatly improve guideline quality.
Clarity and presentation
Domain 4, “Clarity and Presentation,” was the domain in which the CPGs performed the best, with all eight rated as high quality. This domain evaluates the language, structure, and format of the guidelines. A survey of pediatricians’ attitudes and practices regarding CPGs found simplicity to be the greatest independent predictor of guideline use [37], further validating the importance of this domain. Strategies employed by the appraised CPGs include listing key recommendations at the beginning of the document [21,22,23,26] and providing flow charts that walk clinicians through diagnostic and treatment decisions [21,22,25,26,27,28].
Applicability
Domain 5, “Applicability,” reflects the extent to which the guidelines are valid in settings with different resources and barriers to implementation, and acknowledged, for example, the challenges faced in applying these guidelines with underinsured or otherwise disadvantaged populations. This is the domain in which the CPGs performed the worst, with only one guideline (NICE) achieving a high-quality rating. No CPG was found to adequately address the resource implications of their recommendations, and only a few provided tools or advice on how their guidelines could be put into practice [26,27,28]. For example, multiple guidelines called for long-term monitoring of symptoms either as a diagnostic criterion or to determine treatment but did not provide tools for clinicians when patients are likely to be lost to follow up [26,27,28]. Additionally, the CPGs did not account for minority populations or populations with language barriers or lower socioeconomic class.
The combination of scores from this domain and the “Stakeholder Development” domain reveal a lack of diversity both in the developers and in the populations to which these guidelines might apply. The NICE guideline was the only CPG to score highly on both these domains. The NICE CPG included in its developing committee not only representatives from many fields of medicine but also experts in policy and social work as well as members of the public. They also specifically articulated the importance of cost-effective interventions and provided tools for calculating monthly costs for patients on different therapies. Emphasizing affordability is critical, as high cost is one of the primary reasons pediatricians do not adhere to CPGs [38].
Editorial independence
The greatest variability was noted in Domain 6, “Editorial Independence,” a measure of transparency in research funding. Three guidelines scored >90 points, while the guidelines overall averaged a score of 51.82 with a standard deviation of 44.14. This phenomenon has been seen in other AGREE II analyses [18,19], perhaps because some societies report funding information on their websites and not in the guidelines themselves. All other academic publications and presentations require disclosures and CPGs should be held to the same standards. Directly reporting this information in the guidelines is less ambiguous and ensures that users have this information when making clinical decisions.
Recommendations
Three CPGs were validated by this AGREE II analysis: those developed by the University of Toronto, NICE, and NASPGHAN & ESPGHAN. All scored “high quality” on 5 of the 6 domains and were thus found to be “high quality” guidelines. A summary of the key recommendations for the diagnosis and treatment of GERD from these three “high quality” guidelines is presented in Table 4. These recommendations emphasize the importance of differentiating GER from GERD, ruling out other diagnoses, understanding the variability in presenting symptoms at different ages, and the roles for various interventions.
Table 4. Key recommendations from the quality “High-” clinical practice guidelines.
| Clinical topic | Key takeaways | |
|---|---|---|
| Defining GER and GERD | GERD should be diagnosed only when symptoms become troublesome or lead to potentially dangerous or long-term complications [25,26,28] | |
| Treatment of GER | Effortless vomiting is common among infants, and appropriate treatment should emphasize empathetic parental reassurance. The more aggressive treatments indicated for GERD should be avoided [26,28] | |
| Signs & symptoms of GERD | Presenting symptoms vary between infants, younger children, and older children. Older children tend to present with heartburn and epigastric pain as is associated with adult GERD, while infants and younger children present with more variable symptoms, such as emesis, arching of the back, crying, and irritability [25,28] | |
| Red flag signs & symptoms | Red flag symptoms that suggest an alternate diagnosis include projectile vomiting, weight loss, nocturnal vomiting, systemic symptoms, or onset of vomiting at >6 months of age, among many others [26,28]. These signs and symptoms indicate the need for a referral to a relevant specialist [26]. | |
| Non-pharmacological treatment of GERD | Infants | |
| • Indicated: thickened formula [26,28] | ||
| • Not indicated: positional therapy [26,28] | ||
| Children | ||
| • Indicated: patient/caregiver education [26,28]; inform patient/caregiver that excess weight is associated with GERD symptoms [28] | ||
| • Not indicated: prebiotics, probiotics, or herbal medication [28] | ||
| Pharmacological treatment of GERD | • Indicated: proton pump inhibitors (PPIs, first-line), H2 antihistamines (H2RAs, second-line) [28], or select between PPIs and H2RAs based on practicality [26] | |
| • Not indicated: any medication for otherwise healthy patients with isolated overt regurgitation; metoclopramide, domperidone, or erythromycin [26,28] | ||
| Surgical treatment of GERD | Consider anti-reflux surgery such as fundification in infants or children: | |
| • Only if other conditions have been ruled out [28] | ||
| • If symptoms are refractory to lifestyle changes and medication [26,28] | ||
| • If there is a need for chronic pharmacotherapy [28] | ||
| • In cases where a chronic condition places patient at serious risk for a GERD-related complication [28] | ||
| Refractory GERD in primary-care settings | Referral to pediatric gastroenterologist [26,28] | |
GER: gastroesophageal reflux, GERD: gastroesophageal reflux disease.
AGREE II and pediatrics
The publication of a CPG is not itself enough to improve clinical care. Following the publication of the 2009 NASPGHAN & ESPGHAN reflux guidelines, it was found that fewer than 2% of pediatricians followed the guidelines strictly, and proton pump inhibitors continued to be overprescribed at rates exceeding 80% [39]. An analysis of AGREE II papers within pediatrics showed that the quality of pediatric CPGs are generally mediocre, particularly in “Applicability” and “Editorial Independence” [40]. Improving this body of literature is essential, and the AGREE II tool can act as a guide for CPG developers. It is notable that the CPG by NASPGHAN & ESPGHAN, which was the CPG with the highest total score, performed an AGREE II analysis as part of their literature search; familiarity with the format and methodology suggested by the AGREE collaboration can lead to more rigorous and applicable guidelines.
Limitations
This study has several limitations. The AGREE II tool evaluates the presentation and methodological rigor of CPGs but not the accuracy of the medical information they contain. It is possible that well-developed and clear CPGs could present inaccurate information, or that the AGREE II analysis could fail to identify CPGs that provide helpful and relevant guidance. More specific investigation is needed to confirm that the guidelines rated “high quality” are in fact indicated. Secondly, the AGREE II tool weighs all domains equally, despite evidence that “Rigor of Development” and “Editorial Independence” are more strongly associated with effective clinical guidelines. Thirdly, the AGREE II tool relies on subjective ratings from the reviewers; although statistical techniques were used to generate consensus ratings, these numbers reflect the opinions of four authors. Lastly, the literature search could have missed applicable guidelines, particularly those in non-English languages, despite the potential significance of these internationally.
In conclusion, CPGs can facilitate evidence-based clinical decision making. However, it is important that they are methodologically rigorous and offer high-quality guidance. Based on our analysis using the AGREE II instrument, only three of the eight (37.5%) identified CPGs pertaining to pediatric reflux are high quality. Areas for improvement include the domains of “Stakeholder Involvement,” “Applicability,” and “Editorial Independence.”
Footnotes
Funding: AM reports a grant from the Parker B. Francis Foundation and a research grant from 2R25-HL126140, outside the submitted work. All other authors declare no competing interests.
Conflict of Interest: The authors have no financial conflicts of interest.
References
- 1.Vakil N. Disease definition, clinical manifestations, epidemiology and natural history of GERD. Best Pract Res Clin Gastroenterol. 2010;24:759–764. doi: 10.1016/j.bpg.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs KH, Babic B, Breithaupt W, Dallemagne B, Fingerhut A, Furnee E, et al. EAES recommendations for the management of gastroesophageal reflux disease. Surg Endosc. 2014;28:1753–1773. doi: 10.1007/s00464-014-3431-z. [DOI] [PubMed] [Google Scholar]
- 3.Baird DC, Harker DJ, Karmes AS. Diagnosis and treatment of gastroesophageal reflux in infants and children. Am Fam Physician. 2015;92:705–714. [PubMed] [Google Scholar]
- 4.Barron JJ, Tan H, Spalding J, Bakst AW, Singer J. Proton pump inhibitor utilization patterns in infants. J Pediatr Gastroenterol Nutr. 2007;45:421–427. doi: 10.1097/MPG.0b013e31812e0149. [DOI] [PubMed] [Google Scholar]
- 5.Scherer LD, Zikmund-Fisher BJ, Fagerlin A, Tarini BA. Influence of “GERD” label on parents’ decision to medicate infants. Pediatrics. 2013;131:839–845. doi: 10.1542/peds.2012-3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michail S. Gastroesophageal reflux. Pediatr Rev. 2007;28:101–110. doi: 10.1542/pir.28-3-101. [DOI] [PubMed] [Google Scholar]
- 7.Lightdale JR, Gremse DA. Gastroesophageal reflux: management guidance for the pediatrician. Pediatrics. 2013;131:e1684–e1695. doi: 10.1542/peds.2013-0421. [DOI] [PubMed] [Google Scholar]
- 8.Leung AK, Hon KL. Gastroesophageal reflux in children: an updated review. Drugs Context. 2019;8:212591. doi: 10.7573/dic.212591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rybak A, Pesce M, Thapar N, Borrelli O. Gastro-esophageal reflux in children. Int J Mol Sci. 2017;18:1671. doi: 10.3390/ijms18081671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singendonk M, Goudswaard E, Langendam M, van Wijk M, van Etten-Jamaludin F, Benninga M, et al. Prevalence of gastroesophageal reflux disease symptoms in infants and children: a systematic review. J Pediatr Gastroenterol Nutr. 2019;68:811–817. doi: 10.1097/MPG.0000000000002280. [DOI] [PubMed] [Google Scholar]
- 11.Farahmand F, Sabbaghian M, Ghodousi S, Seddighoraee N, Abbasi M. Gastroesophageal reflux disease and tooth erosion: a cross-sectional observational study. Gut Liver. 2013;7:278–281. doi: 10.5009/gnl.2013.7.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gremse DA. GERD in the pediatric patient: management considerations. MedGenMed. 2004;6:13. [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman C, Sarantos G, Katz S, Geisler S. Understanding gastroesophageal reflux disease in children. JAAPA. 2021;34:12–18. doi: 10.1097/01.JAA.0000731488.99461.39. [DOI] [PubMed] [Google Scholar]
- 14.Bingham SM, Muniyappa P. Pediatric gastroesophageal reflux disease in primary care: evaluation and care update. Curr Probl Pediatr Adolesc Health Care. 2020;50:100784. doi: 10.1016/j.cppeds.2020.100784. [DOI] [PubMed] [Google Scholar]
- 15.Murad MH. Clinical practice guidelines: a primer on development and dissemination. Mayo Clin Proc. 2017;92:423–433. doi: 10.1016/j.mayocp.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182:E839–E842. doi: 10.1503/cmaj.090449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vlayen J, Aertgeerts B, Hannes K, Sermeus W, Ramaekers D. A systematic review of appraisal tools for clinical practice guidelines: multiple similarities and one common deficit. Int J Qual Health Care. 2005;17:235–242. doi: 10.1093/intqhc/mzi027. [DOI] [PubMed] [Google Scholar]
- 18.Luu NN, Chorath KT, May BR, Bhuiyan N, Moreira AG, Rajasekaran K. Clinical practice guidelines in idiopathic facial paralysis: systematic review using the appraisal of guidelines for research and evaluation (AGREE II) instrument. J Neurol. 2021;268:1847–1856. doi: 10.1007/s00415-020-10345-0. [DOI] [PubMed] [Google Scholar]
- 19.Chorath K, Garza L, Tarriela A, Luu N, Rajasekaran K, Moreira A. Clinical practice guidelines on newborn hearing screening: a systematic quality appraisal using the AGREE II instrument. Int J Pediatr Otorhinolaryngol. 2021;141:110504. doi: 10.1016/j.ijporl.2020.110504. [DOI] [PubMed] [Google Scholar]
- 20.International Pediatric Endosurgery Group (IPEG) IPEG guidelines for the surgical treatment of pediatric gastroesophageal reflux disease (GERD) J Laparoendosc Adv Surg Tech A. 2009;19(Suppl 1):x–xxiii. doi: 10.1089/lap.2009.9982.supp. [DOI] [PubMed] [Google Scholar]
- 21.Papachrisanthou MM, Davis RL. Clinical practice guidelines for the management of gastroesophageal reflux and gastroesophageal reflux disease: 1 year to 18 years of age. J Pediatr Health Care. 2016;30:289–294. doi: 10.1016/j.pedhc.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Papachrisanthou MM, Davis RL. Clinical practice guidelines for the management of gastroesophageal reflux and gastroesophageal reflux disease: birth to 1 year of age. J Pediatr Health Care. 2015;29:558–564. doi: 10.1016/j.pedhc.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Chang AB, Oppenheimer JJ, Kahrilas PJ, Kantar A, Rubin BK, Weinberger M, et al. Chronic cough and gastroesophageal reflux in children: CHEST guideline and expert panel report. Chest. 2019;156:131–140. doi: 10.1016/j.chest.2019.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dell Children’s Medical Center of Central Texas. Endocrinology management and referral guidelines. Austin: Dell Children’s Medical Center of Central Texas; 2016. [Google Scholar]
- 25.Sherman PM, Hassall E, Fagundes-Neto U, Gold BD, Kato S, Koletzko S, et al. A global, evidence-based consensus on the definition of gastroesophageal reflux disease in the pediatric population. Am J Gastroenterol. 2009;104:1278–1295. doi: 10.1038/ajg.2009.129. quiz 1296. [DOI] [PubMed] [Google Scholar]
- 26.Davies I, Burman-Roy S, Murphy MS. Gastro-oesophageal reflux disease in children: NICE guidance. BMJ. 2015;350:g7703. doi: 10.1136/bmj.g7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The Royal Children’s Hospital Melbourne. Gastrooesophageal reflux disease in infants. Melbourne: Royal Children’s Hospital Melbourne; 2019. [Google Scholar]
- 28.Rosen R, Vandenplas Y, Singendonk M, Cabana M, DiLorenzo C, Gottrand F, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66:516–554. doi: 10.1097/MPG.0000000000001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiffman RN, Shekelle P, Overhage JM, Slutsky J, Grimshaw J, Deshpande AM. Standardized reporting of clinical practice guidelines: a proposal from the Conference on Guideline Standardization. Ann Intern Med. 2003;139:493–498. doi: 10.7326/0003-4819-139-6-200309160-00013. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed TF, Khandwala F, Abelson TI, Hicks DM, Richter JE, Milstein C, et al. Chronic laryngitis associated with gastroesophageal reflux: prospective assessment of differences in practice patterns between gastroenterologists and ENT physicians. Am J Gastroenterol. 2006;101:470–478. doi: 10.1111/j.1572-0241.2006.00502.x. [DOI] [PubMed] [Google Scholar]
- 31.Légaré F, Boivin A, van der Weijden T, Pakenham C, Burgers J, Légaré J, et al. Patient and public involvement in clinical practice guidelines: a knowledge synthesis of existing programs. Med Decis Making. 2011;31:E45–E74. doi: 10.1177/0272989X11424401. [DOI] [PubMed] [Google Scholar]
- 32.Hoffmann-Eßer W, Siering U, Neugebauer EAM, Brockhaus AC, McGauran N, Eikermann M. Guideline appraisal with AGREE II: online survey of the potential influence of AGREE II items on overall assessment of guideline quality and recommendation for use. BMC Health Serv Res. 2018;18:143. doi: 10.1186/s12913-018-2954-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 34.Eibling D, Fried M, Blitzer A, Postma G. Commentary on the role of expert opinion in developing evidence-based guidelines. Laryngoscope. 2014;124:355–357. doi: 10.1002/lary.24175. [DOI] [PubMed] [Google Scholar]
- 35.Alonso-Coello P, Irfan A, Solà I, Gich I, Delgado-Noguera M, Rigau D, et al. The quality of clinical practice guidelines over the last two decades: a systematic review of guideline appraisal studies. Qual Saf Health Care. 2010;19:e58. doi: 10.1136/qshc.2010.042077. [DOI] [PubMed] [Google Scholar]
- 36.Cooper ID, Crum JA. New activities and changing roles of health sciences librarians: a systematic review, 1990-2012. J Med Libr Assoc. 2013;101:268–277. doi: 10.3163/1536-5050.101.4.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flores G, Lee M, Bauchner H, Kastner B. Pediatricians’ attitudes, beliefs, and practices regarding clinical practice guidelines: a national survey. Pediatrics. 2000;105(3 Pt 1):496–501. doi: 10.1542/peds.105.3.496. [DOI] [PubMed] [Google Scholar]
- 38.Hendaus MA, Alhammadi AH, Razig EA, Alnaimi L. Pediatricians’ perceptions of clinical practice guidelines. J Multidiscip Healthc. 2014;7:349–354. doi: 10.2147/JMDH.S66147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quitadamo P, Papadopoulou A, Wenzl T, Urbonas V, Kneepkens CM, Roman E, et al. European pediatricians’ approach to children with GER symptoms: survey of the implementation of 2009 NASPGHAN-ESPGHAN guidelines. J Pediatr Gastroenterol Nutr. 2014;58:505–509. doi: 10.1097/MPG.0b013e3182a69912. [DOI] [PubMed] [Google Scholar]
- 40.Isaac A, Saginur M, Hartling L, Robinson JL. Quality of reporting and evidence in American Academy of Pediatrics guidelines. Pediatrics. 2013;131:732–738. doi: 10.1542/peds.2012-2027. [DOI] [PubMed] [Google Scholar]