Abstract
Aims
This international study aimed to assess: 1) the prevalence of preoperative and postoperative COVID-19 among patients with hip fracture, 2) the effect on 30-day mortality, and 3) clinical factors associated with the infection and with mortality in COVID-19-positive patients.
Methods
A multicentre collaboration among 112 centres in 14 countries collected data on all patients presenting with a hip fracture between 1st March-31st May 2020. Demographics, residence, place of injury, presentation blood tests, Nottingham Hip Fracture Score, time to surgery, management, ASA grade, length of stay, COVID-19 and 30-day mortality status were recorded.
Results
A total of 7090 patients were included, with a mean age of 82.2 (range 50–104) years and 4959 (69.9%) being female. Of 651 (9.2%) patients diagnosed with COVID-19, 225 (34.6%) were positive at presentation and 426 (65.4%) were positive postoperatively. Positive COVID-19 status was independently associated with male sex (odds ratio (OR) 1.38, p = 0.001), residential care (OR 2.15, p < 0.001), inpatient fall (OR 2.23, p = 0.003), cancer (OR 0.63, p = 0.009), ASA grades 4 (OR 1.59, p = 0.008) or 5 (OR 8.28, p < 0.001), and longer admission (OR 1.06 for each increasing day, p < 0.001). Patients with COVID-19 at any time had a significantly lower chance of 30-day survival versus those without COVID-19 (72.7% versus 92.6%, p < 0.001). COVID-19 was independently associated with an increased 30-day mortality risk (hazard ratio (HR) 2.83, p < 0.001). Increasing age (HR 1.03, p = 0.028), male sex (HR 2.35, p < 0.001), renal disease (HR 1.53, p = 0.017), and pulmonary disease (HR 1.45, p = 0.039) were independently associated with a higher 30-day mortality risk in patients with COVID-19 when adjusting for confounders.
Conclusion
The prevalence of COVID-19 in hip fracture patients during the first wave of the pandemic was 9%, and was independently associated with a three-fold increased 30-day mortality risk. Among COVID-19-positive patients, those who were older, male, with renal or pulmonary disease had a significantly higher 30-day mortality risk.
Keywords: Hip fracture, Frailty, Trauma, Orthopaedic, Geriatric, Risk, Prognosis, Outcomes, Reporting standards, COVID-19, Nosocomial, Communicable disease, Infection, Audit, Meta-audit
Introduction
The coronavirus disease 2019 (COVID-19) pandemic disrupted the delivery of Trauma and Orthopaedic (T&O) services, but despite a reduction in the incidence of activity-related trauma the incidence of fragility-related trauma was unchanged.[1], [2], [3] Developing COVID-19 in the perioperative period has been reported to double the background mortality risk following orthopaedic surgery, and the patients at greatest risk of mortality from COVID-19 are those who are older, comorbid and presenting with a fragility fracture.3 It is essential to have an understanding of the prevalence and patterns of SARS-CoV-2 infection within the hip fracture population, and to analyse the effects of the COVID-19 pandemic on this large and vulnerable patient group.
A recent systematic review and meta-analysis found that hip fracture patients with COVID-19 had a crude 30-day mortality of 35% and was seven times the risk of patients without COVID-19.4 However, in this same review less than half of the included studies reported patient age and sex and only two adjusted for confounding factors in their analysis.3 , 5 Two multicentre cohort studies by the International Multicentre Project Auditing COVID-19 in Trauma & Orthopaedics in Scotland (IMPACT-Scot) Group have reported that after adjusting for confounding factors the 30-day mortality risk in COVID-19-positive hip fracture patients was three times greater than in COVID-19-negative patients. Furthermore, the reports are from a single nation with a relatively homogenous population and a standardised approach to hip fracture services.[4], [6], [7]
The IMPACT Global Hip Fracture Audit aimed to determine factors associated with a positive COVID-19 diagnosis and the influence this has on outcome, with the inclusion of international data from a wider range of patients and healthcare providers from across the globe. The aims of this international multicentre audit were to examine the hip fracture population and assess the: 1) prevalence and clinical factors associated with a diagnosis of COVID-19 in the preoperative and postoperative periods; 2) the independent effect of COVID-19 on 30-day mortality, and 3) factors associated with mortality in COVID-19-positive patients.
Patients and methods
In March 2020 the International Multicentre Project Auditing COVID-19 in Trauma & Orthopaedics (IMPACT) was established in order to provide an emergency clinical audit response to the COVID-19 pandemic.8 , 9 It was recognised that investigation into the effects of COVID-19 on hip fracture patients and services was necessary and urgent. The IMPACT collaborative network gained support from the Scottish Hip Fracture Audit (SHFA), Scottish Government and the Scottish Committee for Orthopaedics & Trauma (SCOT). An international multicentre observational cohort study was subsequently established with data collected retrospectively from 112 hospitals in 14 nations, including: Australia, Argentina, Chile, Cyprus, England, India, Italy, Greece, Mexico, Northern Ireland, Scotland, Spain, Sudan, Wales. Centres were invited to participate through a recruitment process delivered through existing hospital networks and audit programmes, the Fragility Fracture Network (FFN) and the Royal College of Surgeons of England.
Data were collected in accordance with UK Caldicott guidance and equivalent principles in each nation, and no patient-identifiable information was transferred outside of local units or accessed by the IMPACT research team.10
Inclusion and exclusion criteria
All patients who were over 50 years of age and presenting with a hip fracture to any participating hospital in the study period (1st March 2020 to 31st May 2020) were included. The inclusion criteria were that of the SHFA and previous IMPACT reports: all intracapsular or extracapsular fractures of the femur proximal to and including the distal limit of the subtrochanteric region (defined as a point five centimetres distal to the lesser trochanter).11 Periprosthetic femur fractures and isolated fractures of the pubic rami, acetabulum, and greater trochanter were excluded.
Baseline data collection
Data collection was defined prior to the commencement of the audit, which was delivered by a team of data collectors (comprised of clinicians and trained auditors) who were local to each hospital. Patients were identified through retrospective review of local admission data throughout the study period, and these data were cross-referenced with patients’ medical records, surgical operating lists and discharge letters. Data were input into the IMPACT Hip Fracture Audit data collection tool, a database constructed with data-validated fields and automatically computed variable calculation mechanisms to ensure transcription accuracy, consistency, and completion, as well as to ensure intra- and inter-observer reliability.
Data on demographics, injury details, and surgical management were recorded and included: age; sex; pre-fracture residence (coded as: Home/Sheltered Housing; Care/Nursing Home, or ‘Hospital’); injury date; location where injury was sustained (coded as: Home/Indoor; Outdoor, or Hospital); admission date; date of surgery; surgical procedure; surgical delay status (defined as being surgery out with 36 h of admission), and reason for nonoperative management (if applicable).
Data concerning clinical patient factors were recorded and included: American Society of Anesthesiologists (ASA) classification, presence of major comorbidity (cardiovascular disease, renal disease, pulmonary disease, dementia, active cancer, or diabetes mellitus) and laboratory blood tests taken on admission (haemoglobin concentration, lymphocyte count, platelet count, serum sodium concentration, and serum albumin concentration).12 These laboratory blood tests were included on the basis of existing evidence that they may correlate with either disease severity in COVID-19 specifically, or with outcomes in hip fracture patients.[13], [14], [15], [16], [17] The Nottingham Hip Fracture Score (NHFS) was calculated from the variables included in the dataset.18
COVID-19 diagnosis
Data in relation to COVID-19 status in the preoperative and postoperative periods were collected independently and included whether patients demonstrated clinical features of COVID-19 infection, as well as any SARS-CoV-2 rt-PCR test result (positive or negative) obtained via the standard oropharyngeal and nasopharyngeal swab technique as part of the routine clinical management.
Outcomes
Data relevant to early patient outcome measures were collected and included: date and destination of discharge from acute admission (defined as the acute orthopaedic trauma admission, or the total acute hospital admission if a patient was transferred from an acute centre to another acute centre of comparable care level), date of death, and whether death occurred during the acute admission. Patients were followed up for a minimum of 30 days following presentation with hip fracture.
Statistical methods
Statistical analyses were performed using Statistical Product and Service Solutions version 17.0 (SPSS Inc. Released 2008. SPSS Statistics for Windows, Version 17.0. Chicago: SPSS Inc.). Parametric and non-parametric tests were used as appropriate to analyse continuous variables for significant differences between groups. Unpaired t-tests were used to compare values between groups for numerical variables that demonstrated a normal distribution. A Chi square test was used to assess dichotomous variables for differences between groups (Fisher's exact test was used if the frequency was 5 or less in any one cell). Kaplan–Meier methodology was used to investigate 30-day survival after hip fracture and Log rank was used to compare survival between patients who had a positive COVID-19 diagnosis with those with a negative COVID-19 diagnosis. Cox regression analysis was used to assess the independent association of COVID-19 status on 30-day mortality and factors associated with 30 day mortality in patients with COVID-19. Logistic regression analysis was used to assess the independence of predictors associated with a positive COVID-19 diagnosis. Receiver operating characteristic (ROC) curve analysis was used to identify a threshold values in the scalar variables that were identified as predictors associated with a positive COVID-19 diagnosis: i) on admission; ii) after admission, and iii) at any time. The area under the ROC curve (AUC) ranges from 0.5 (which indicates a test with no accuracy in distinguishing whether a patient is COVID-19-positive), to 1.0 (where the test accurately identifies all COVID-19-positive patients). The threshold value was defined as the point at which the sensitivity and specificity were maximal in predicting a COVID-19-positive patient. A p-value of <0.05 was defined as statistically significant.
Results
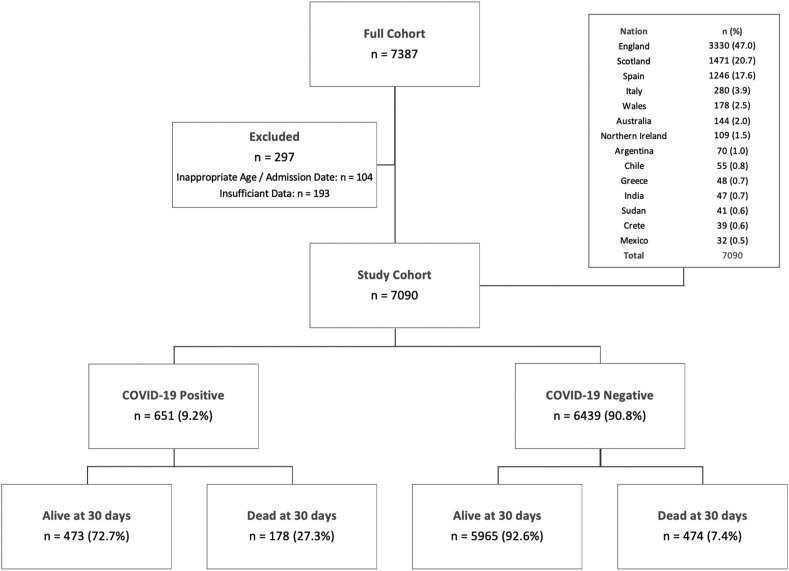
During the audit period data for 7387 patients with a hip fracture from 14 different countries were submitted. Data were excluded for 104 patients (1.4%) who were younger than 50 years of age or who presented outside the audit period. Another 193 patients (2.6%) did not have a COVID-19 status recorded and were excluded from further analysis (Fig. 1). The final cohort consisted of 7090 patients of whom 4959 (69.9%) were female and 2130 (30.0%) male (one patient did not have sex recorded). Mean age was 82.2 years (standard deviation (SD) 10.6, range 50–104) (Table 1 ).
Fig. 1.
Flow chart showing all patients, included and excluded patients, mortality outcomes according to COVID-19 status, and distribution of patients from participating nations.
Table 1.
Patient demographics, Nottingham hip fracture score, residence, place of injury, comorbidity, surgery within 36 h, ASA grade, surgical management, admission blood test and COVID status according to 30-day mortality.
| Demographic | Descriptive | 30-day Mortality |
Difference/Odds Ratio (95% CI) | p-valuea | |
|---|---|---|---|---|---|
| Alive (n = 6438) | Dead (n = 652) | ||||
| Age (years: mean, SD) | 81.8 (10.7) | 86.0 (9.0) | Diff 4.2 (3.3–5.0) | <0.001 | |
| Sex (n, % of group) | Female | 4602 (71.48) | 357 (54.75) | Reference | |
| Male | 1836 (28.52) | 294 (45.10) | 2.06 (1.75–2.43) | <0.001 | |
| Missing | 0 | 1 (0.15) | N/A | – | |
| Nottingham Hip Score (mean, SD) | 4.8 (2.4) | 6.0 (3.9) | Diff 1.2 (1.0–1.4) | <0.001 | |
| Residence (n, % of group) | Home/Sheltered | 4975 (77.27) | 390 (59.82) | Reference | |
| Care/Nursing home | 1166 (18.11) | 221 (56.67) | 2.42 (1.03–2.89) | <0.001 | |
| Hospital | 81 (1.26) | 22 (3.37) | 3.46 (2.14–5.61) | <0.001 | |
| Missing | 216 (3.34) | 19 (2.91) | 1.12 (0.69–1.81) | 0.639 | |
| Place of injury (n, % of group) | Home/Indoor | 5082 (78.94) | 552 (84.66) | Reference | |
| Outdoor | 919 (14.27) | 40 (6.13) | 0.40 (0.29–0.56) | <0.001 | |
| Hospital | 154 (2.39) | 37 (5.67) | 2.21 (1.53–3.20) | <0.001 | |
| Missing | 283 (4.40) | 23 (3.53) | 0.75 (0.48–1.15) | 0.188 | |
| Comorbiditya (n, % of group) | Not present | Reference | |||
| CVD | 4115 (63.92) | 486 (74.54) | 1.67 (1.39–2.01) | <0.001 | |
| Renal Disease | 1281 (19.90) | 209 (3.25) | 1.91 (1.60–2.27) | <0.001 | |
| Pulmonary Disease | 1362 (21.16) | 216 (3.36) | 1.85 (1.56–2.20) | <0.001 | |
| Dementia | 1868 (29.02) | 284 (4.41) | 1.90 (1.61–2.24) | <0.001 | |
| Cancer | 630 (9.79) | 109 (1.69) | 1.86 (1.49–2.32) | <0.001 | |
| Diabetes Mellitus | 1289 (20.02) | 126 (1.96) | 0.96 (0.78–1.18) | 0.696 | |
| Surgery <36 h (n, % of group) | Yes | 4043 (62.80) | 338 (5.25) | Reference | |
| No | 2253 (35.00) | 214 (3.32) | 1.14 (0.95–1.36) | 0.162 | |
| N/A | 110 (1.71) | 94 (1.46) | 10.22 (7.60–13.75) | <0.001 | |
| Missing | 32 (0.50) | 6 (0.09) | 2.24 (0.93–5.40) | 0.381 | |
| ASA grade (n, % of group) | 1 | 118 (0.02) | 4 (0.06) | 1.48 (0.52–4.26) | |
| 2 | 1400 (21.75) | 32 (0.50) | Reference | ||
| 3 | 3720 (57.78) | 354 (5.50) | 4.15 (2.88–5.99) | <0.001 | |
| 4 | 945 (14.68) | 219 (33.59) | 10.14 (6.93–14.8) | <0.001 | |
| 5 | 5 (0.08) | 16 (2.45) | 13.67 (4.72–39.60) | <0.001 | |
| Missing or N/A | 250 (3.88) | 27 (4.14) | 4.73 (2.78–8.02) | <0.001 | |
| Management (n, % of group) | Fixation | 3199 (49.69) | 292 (44.78) | Reference | |
| Arthroplasty | 3049 (47.36) | 255 (39.11) | 0.92 (0.77–1.09) | 0.327 | |
| Non-operative | 104 (1.62) | 91 (13.96) | 9.59 (7.06–13.01) | <0.001 | |
| Other | 35 (0.54) | 8 (1.23) | 2.50 (1.15–5.45) | ||
| Missing | 51 (0.79) | 6 (0.92) | 1.29 (0.55–3.03) | ||
| Admission Blood Tests (mean, SD) | |||||
| Haemoglobin Concentration (g/L) | n = 6435 vs 650 | 122.9 (18.0) | 118.9 (19.8) | 3.9 (2.5–5.4) | <0.001 |
| Lymphocyte Count (x 109/L) | n = 6430 vs 650 | 1.21 (0.73) | 1.09 (0.62) | 0.12 (0.06–0.18) | <0.001 |
| Platelet Count (x 109/L) | n = 6430 vs 648 | 245.8 (89.1) | 243.8 (98.6) | 2.0 (−5.2 to 9.3) | 0.582 |
| Sodium Concentration (mmol/L) | n = 6414 vs 648 | 137.6 (1.4) | 137.6 (4.8) | 0.0 (−0.3 to 0.4) | 0.879 |
| Albumin Concentration (g/L) | n = 6256 vs 641 | 36.6 (5.9) | 33.8 (6.2) | 2.8 (0.3–1.7) | 0.006 |
| COVID-19 status (n, % of group) | No | 5965 (92.65) | 474 (72.70) | Reference | |
| Yes | 473 (7.35) | 178 (27.30) | 4.74 (3.89–5.76) | <0.001 | |
| No | 5965 (92.65) | 474 (72.70) | Reference | ||
| On admission | 169 (2.62) | 56 (8.59) | 4.17 (3.04–5.72) | <0.001 | |
| Postoperative | 304 (4.72) | 122 (18.71) | 5.05 (4.01–6.36) | <0.001 | |
Data not available for four patients: two died within the 30 day follow up period.
The independent influence of COVID-19 on patient mortality
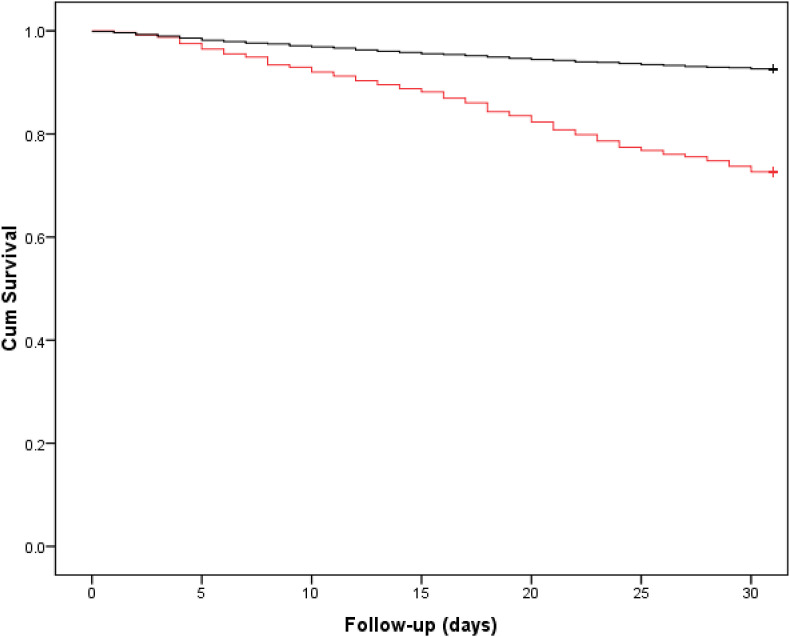
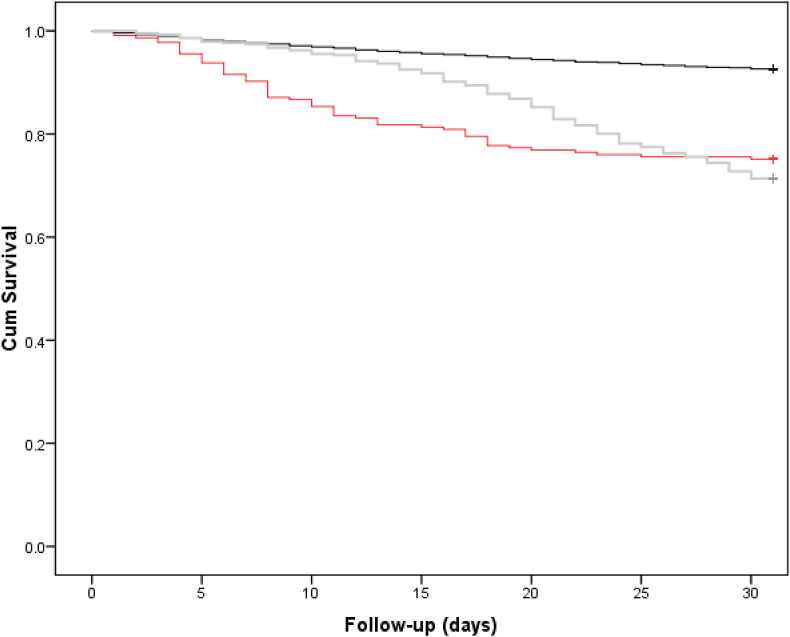
There were 651 (9.2%) patients who were assigned a diagnosis of COVID-19, of whom 225 (34.6%) were positive preoperatively and 426 (65.4%) positive postoperatively. In total 652 (9.2%) patients died within and including 30 days of presentation with a hip fracture, of whom 178/652 (27.3%) had been diagnosed with COVID-19. Patients diagnosed with COVID-19 at any timepoint had a significantly lower 30-day survival rate when compared to those without COVID-19 (72.7%, 95% Confidence Interval (CI) 69.4 to 76.0% versus 92.6%, 95% CI 92.4 to 92.8, Log rank p < 0.001, Fig. 2 ). There was no significant difference in 30-day survival (Log rank p = 0.661) when comparing those diagnosed with COVID-19 preoperatively (75.1%, 95% CI 69.4 to 80.8) and those diagnosed postoperatively (71.4%, 95% CI 67.1 to 75.7); survival was significantly lower for both groups (Log rank p < 0.001) than for patients without COVID-19 (Fig. 3 ).
Fig. 2.
Kaplan Meier curve for 30-day survival according to whether a patient was COVID negative (black) or COVID positive (red) within 30-days of admission. Log rank p < 0.001, 92.6% (95% CI 92.4 to 92.8) versus 72.7% (95% CI 69.4 to 76.0) at 30-days.
Fig. 3.
Kaplan Meier curve for 30-day survival according to whether a patient was COVID negative (black), COVID positive at admission (red) or COVID positive after admission (grey). Log rank p = 0.661, between COVID positive patients preoperatively (75.1%, 95% CI 69.4 to 80.8) versus postoperatively (71.4%, 95% CI 67.1 to 75.7) at 30-days.
Unadjusted analysis of factors associated with increased 30-day mortality were older age (p < 0.001), male sex (p < 0.001), a higher Nottingham Hip Fracture Score (p < 0.001), care/nursing home (p < 0.001) or hospital (p < 0.001) residence, hip fracture sustained indoors or in hospital (p < 0.001), cardiovascular disease (<0.001), renal disease (p < 0.001), pulmonary disease (p = 0.012), dementia (p = 0.004), active cancer (p = 0.039), higher ASA grades (4 or 5) (p < 0.001), and a positive COVID-19 status (p < 0.001) (Table 1 ). The significant influence of non-operative management (p < 0.001) and consequent ‘not applicable’ classification regarding surgery within 36 h of admission (p < 0.001) on mortality (Table 1) was thought to be a secondary marker of increased mortality risk due to frailty and was thus not included in the regression models. Cox regression analysis (Table 2 ) identified that a diagnosis of COVID-19 was associated with a significantly increased mortality rate in the 30-days following admission for a hip fracture after adjusting for confounding factors (Hazard ratio (HR) 2.83, 95% CI 2.33 to 3.42, p < 0.001). The associated HR was higher if COVID-19 was diagnosed after admission (3.09, 95% CI 2.48 to 3.85) compared to those diagnosed on admission (2.36, 95% 1.73 to 3.21), but this was not statistically different.
Table 2.
Cox regression model identifying patient related factors associated with 30-day mortality following a hip fracture.
| Demographic | Descriptive | Hazard Ratio (95% CI) | p-value∗ |
|---|---|---|---|
| Age (for each increasing year) | 1.04 (1.03–1.05) | <0.001 | |
| Sex | Female | Reference | |
| Male | 1.93 (1.63–2.30) | <0.001 | |
| Nottingham Hip Score (for each increasing point) | 0.99 (0.96–1.01) | 0.331 | |
| Residence | Home/Sheltered | Reference | |
| Care/Nursing home | 1.44 (1.17–1.77) | 0.001 | |
| Hospital | 1.23 (0.67–2.26) | 0.507 | |
| Missing | 0.85 (0.52–1.40) | 0.854 | |
| Place of injury | Home/Indoor | Reference | |
| Outdoor | 0.65 (0.45–0.94) | 0.022 | |
| Hospital | 1.20 (0.75–1.91) | 0.452 | |
| Missing | 0.69 (0.40–1.18) | 0.174 | |
| Comorbidity∗ | Not present | ||
| CVD | 1.17 (0.96–1.42) | 0.129 | |
| Renal Disease | 1.23 (1.02–1.48) | 0.028 | |
| Pulmonary Disease | 1.45 (1.21–1.73) | <0.001 | |
| Dementia | 1.11 (0.91–1.35) | 0.299 | |
| Cancer | 1.46 (1.16–1.85) | 0.001 | |
| ASA grade | 1 | 3.06 (1.06–8.78) | 0.038 |
| 2 | Reference | ||
| 3 | 2.31 (1.55–3.45) | <0.001 | |
| 4 | 3.50 (2.30–5.32) | <0.001 | |
| 5 | 7.43 (3.65–15.12) | <0.001 | |
| Missing or N/A | 2.76 (1.58–4.81) | <0.001 | |
| Admission Blood Tests (for each increasing point) | Haemoglobin Concentration (g/L) | 1.00 (0.99–1.01) | 0.443 |
| Lymphocyte Count (x 109/L) | 0.94 (0.83–1.07) | 0.321 | |
| Albumin Concentration (g/L) | 0.96 (0.94–0.97) | <0.001 | |
| COVID-19 status | No | Reference | |
| Yes | 2.83 (2.33–3.42) | <0.001 | |
| Substituted in the model | |||
| No | Reference | ||
| On admission | 2.36 (1.73–3.21) | <0.001 | |
| Postoperative | 3.09 (2.48–3.85) | <0.001 | |
Predictors associated with having COVID-19 at any time
Factors associated with a positive COVID-19 status on unadjusted analysis were older age (p < 0.001), male sex (p = 0.012), a higher Nottingham Hip Fracture score (p = 0.001), place of residence (p = 0.001), place of injury (p = 0.001), cardiovascular disease (p = 0.001), renal disease (p = 0.039), pulmonary disease (p = 0.013), dementia (p = 0.001), active cancer (p = 0.046), increasing ASA grade (p < 0.001), lower lymphocyte count (p < 0.001), lower serum albumin concentration (p < 0.001) increased length of hospital stay (p < 0.001) (Table 3 ). Regression analysis demonstrated male sex, residence in a care/nursing home, place of injury, active cancer, ASA grade 4 and 5, and increased length of stay were independently associated with positive COVID-19 status (Table 4 ).
Table 3.
Patient demographics, Nottingham hip fracture score, admission blood results, residence, place of injury, comorbidity, time to surgery, ASA grade, management, admission blood tests, length of stay, and mortality according to COVID status.
| Demographic | Descriptive | COVID-19 Status |
Difference/Odds Ratio (95% CI) | p-valuea | |
|---|---|---|---|---|---|
| Negative (n = 6439) | Positive (n = 651) | ||||
| Age (years: mean, SD) | 82.0 (10.7) | 84.3 (9.0) | 2.3 (1.5–3.2) | <0.001 | |
| Sex (n, % of group) | Female | 4550 (70.66) | 409 (0.15) | Reference | |
| Male | 1888 (29.32) | 242 (37.17) | 1.43 (1.21–1.69) | <0.001 | |
| Missing | 1 (0.01) | 0 (0.00) | N/A | ||
| Nottingham HipFractureScore (mean, SD) | 4.8 (2.4) | 5.6 (4.0) | 0.8 (0.6–1.0) | <0.001 | |
| Residence (n, % of group) | Home/Sheltered | 5004 (77.71) | 361 (55.45) | Reference | |
| Care/Nursing home | 1160 (18.01) | 227 (34.87) | 2.71 (2.27–3.24) | <0.001 | |
| Hospital | 83 (1.29) | 20 (3.07) | 3.34 (2.03–5.51) | <0.001 | |
| Missing | 192 (2.98) | 43 (6.60) | 3.10 (2.19–4.39) | <0.001 | |
| Place of injury (n, % of group) | Home/Indoor | 5090 (79.05) | 544 (83.56) | Reference | |
| Outdoor | 916 (14.22) | 43 (6.60) | 0.44 (0.32–0.60) | <0.001 | |
| Hospital | 152 (2.36) | 39 (5.99) | 2.40 (1.67–3.45) | <0.001 | |
| Missing | 281 (4.36) | 25 3.84) | 0.83 (0.55–1.27) | 0.390 | |
| Comorbiditya (n, % of group) | Not present | ||||
| CVD | 4130 (64.14) | 471 (72.35) | 1.47 (1.23–1.76) | <0.001 | |
| Renal Disease | 1333 (20.70) | 157 (24.12) | 1.22 (1.01–1.48) | 0.039 | |
| Pulmonary Disease | 1408 (21.87) | 170 (26.11) | 1.26 (1.05–1.52) | 0.013 | |
| Dementia | 1865 (28.96) | 287 (44.09) | 1.94 (1.64–2.28) | <0.001 | |
| Cancer | 686 (10.65) | 53 (8.14) | 0.74 (0.56–1.0) | 0.046 | |
| Diabetes Mellitus | 1277 (19.83) | 138 (21.20) | 1.09 (0.89–1.33) | 0.398 | |
| Surgery <36 h (n, % of group) | Yes | 3991 (61.98) | 390 (59.91) | Reference | |
| No | 2246 (34.88) | 221 (33.95) | 1.01 (0.85–1.20) | 0.920 | |
| N/A | 167 (2.59) | 37 (5.68) | 2.27 (1.56–3.29) | <0.001 | |
| Missing | 35 (0.54) | 3 (0.46) | 0.88 (0.27–2.87) | ||
| ASA grade (n, % of group) | 1 | 119 (1.85) | 3 (0.46) | 0.50 (0.15–1.61) | 0.233 |
| 2 | 1363 (21.17) | 69 (10.60) | Reference | ||
| 3 | 3705 (57.55) | 369 (56.68) | 1.97 (1.51–2.56) | <0.001 | |
| 4 | 983 (15.27) | 181 (27.80) | 3.64 (2.72–4.85) | <0.001 | |
| 5 | 12 (0.19) | 9 (1.38) | 14.82 (6.04–36.35) | <0.001 | |
| Missing or N/A | 257 (3.99) | 20 (3.07) | 1.54 (0.92–2.57) | 0.100 | |
| Management (n, % of group) | Fixation | 3181 (49.40) | 310 (47.62) | Reference | |
| Arthroplasty | 3010 (46.75) | 294 (45.16) | 1.00 (0.86–1.16) | 0.999 | |
| Non-operative | 160 (2.48) | 35 (5.38) | 2.24 (1.52–3.29) | <0.001 | |
| Other | 37 (0.57) | 6 (0.92) | 1.66 (0.69–3.97) | ||
| Missing | 51 (0.79s) | 6 (0.92) | 1.20 (0.51–2.83) | 0.671 | |
| Admission Blood Tests (mean, SD) | |||||
| Haemoglobin Concentration (g/L) | n = 6434 vs 651 | 122.6 (18.3) | 121.5 (17.7) | 1.1 (−0.3 to 2.6) | 0.132 |
| Lymphocyte Count (x 109/L) | n = 6425 vs 651 | 1.21 (0.72) | 1.07 (0.68) | 0.14 (0.08–0.19) | <0.001 |
| Platelet Count (x 109/L) | n = 6427 vs 651 | 246.0 (90.0) | 241.8 (89.8) | 4.3 (−3.0 to 11.5) | 0.250 |
| Sodium Concentration (mmol/L) | n = 6411 vs 651 | 137.6 (4.4) | 137.6 (4.7) | 0.0 (−0.4 to 0.4) | 0.919 |
| Albumin Concentration (g/L) | n = 5546 vs 576 | 36.4 (6.0) | 35.3 (5.8) | 1.2 (0.7–1.7) | <0.001 |
| LOS (days: mean, SD) | 10.4 (7.7) | 17.2 (13.1) | 6.7 (6.0–7.4) | <0.001 | |
| 30-day mortality (n, % of group) | No | 5965 (92.64) | 473 (72.66) | Reference | |
| Yes | 474 (7.36) | 178 (27.34) | 4.74 (3.89–5.76) | <0.001 | |
∗∗chi square test.
Unpaired Students t-test unless otherwise stated.
Table 4.
Logistic regression model identifying patient related factors associated with COVID-19 positive patients and a hip fracture.
| Demographic | Descriptive | Odds Ratio (95% CI) | p-value∗ |
|---|---|---|---|
| Age (for each increasing year) | 1.00 (0.99–1.02) | 0.428 | |
| Sex | Female | Reference | |
| Male | 1.38 (1.13–1.69) | 0.001 | |
| Nottingham Hip Score (for each increasing point) | 1.03 (0.99–1.06) | 0.129 | |
| Residence | Home/Sheltered | Reference | |
| Care/Nursing home | 2.15 (1.69–2.73) | <0.001 | |
| Hospital | 1.31 (0.63–2.72) | 0.467 | |
| Missing | 2.57 (1.73–3.83) | <0.001 | |
| Place of injury | Home/Indoor | Reference | |
| Outdoor | 0.58 (0.40–0.84) | 0.004 | |
| Hospital | 2.23 (1.31–3.79) | 0.003 | |
| Missing | 1.22 (0.74–2.01) | 0.436 | |
| Comorbidity∗ | Not present | ||
| CVD | 1.24 (0.99–1.53) | 0.051 | |
| Renal Disease | 0.85 (0.68–1.07) | 0.165 | |
| Pulmonary Disease | 0.99 (0.79–1.23) | 0.917 | |
| Dementia | 1.18 (0.94–1.48) | 0.164 | |
| Cancer | 0.63 (0.44–0.89) | 0.009 | |
| ASA grade | 1 | 0.69 (0.21–2.31) | 0.548 |
| 2 | Reference | ||
| 3 | 1.16 (0.85–1.57) | 0.352 | |
| 4 | 1.59 (1.13–2.25) | 0.008 | |
| 5 | 8.28 (2.81–24.42) | <0.001 | |
| Missing or N/A | 0.68 (0.36–1.30) | 0.246 | |
| Admission Blood tests (for each point) | Lymphocyte Count (x 109/L) | 0.83 (0.71–0.98) | 0.023 |
| Albumin Concentration (g/L) | 0.99 (0.97–1.00) | 0.102 | |
| Length of stay (for each increasing day) | 1.06 (1.05–1.07) | <0.001 | |
Predictors associated with having COVID-19 on admission
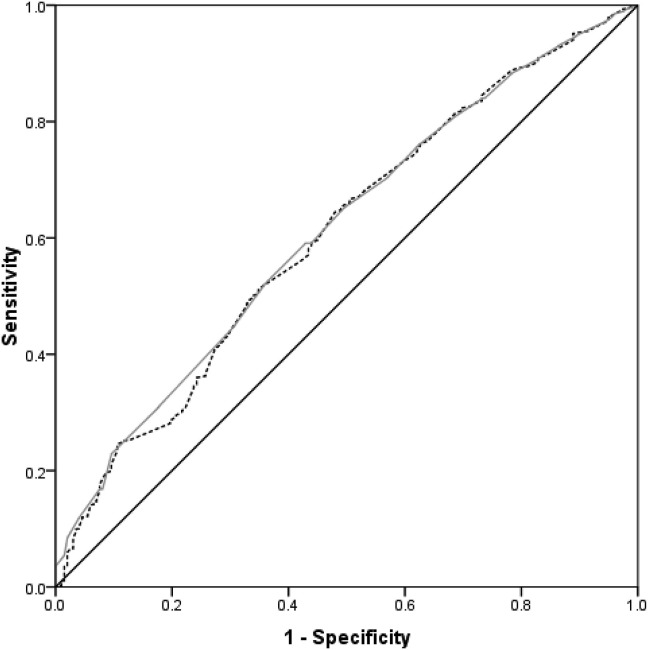
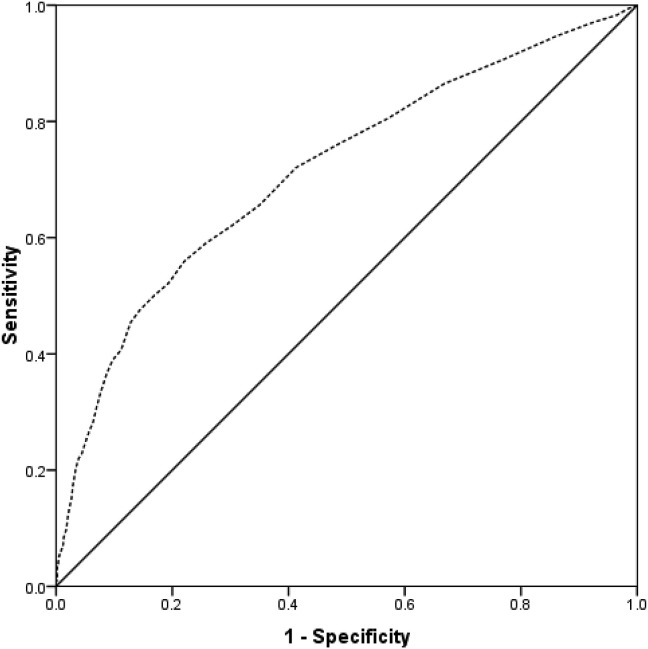
There were 225 patients who had COVID-19 at the time of presentation with hip fracture. Regression analysis demonstrated residence in a care/nursing home, in hospital fracture, ASA grade 5, lower lymphocyte count and albumin were all independently associated with a positive COVID-19 diagnosis on admission (Table 5 ). ROC curve analysis illustrated that a lymphocyte count at time of presentation of ≤0.93 and an albumin level of ≤36 g/dL were predictors of COVID-19 on admission (Fig. 4 ), but were poorly predictive, with an AUC of approximately 60%.
Table 5.
Logistic regression model identifying patient related factors associated with COVID-19 positive patients on admission with a hip fracture.
| Demographic | Descriptive | Odds Ratio (95% CI) | p-value∗ |
|---|---|---|---|
| Age (for each increasing year) | 1.00 (0.99–1.02 | 0.843 | |
| Sex | Female | Reference | |
| Male | 1.01 (0.71–1.50) | 0.941 | |
| Nottingham Hip Score (for each increasing point) | 0.98 (0.82–1.19) | 0.862 | |
| Residence | Home/Sheltered | Reference | |
| Care/Nursing home | 4.13 (2.78–6.13) | <0.001 | |
| Hospital | 0.85 (0.31–2.35) | 0.851 | |
| Missing | 0.54 (0.13–1.26) | 0.400 | |
| Place of injury | Home/Indoor | Reference | |
| Outdoor | 0.52 (0.25–1.09) | 0.085 | |
| Hospital | 4.98 (2.64–9.38) | <0.001 | |
| Missing | 0.71 (0.22–2.28) | 0.561 | |
| Comorbidity∗ | Not present | Reference | |
| CVD | 0.96 (0.69–1.33) | 0.800 | |
| Renal Disease | 0.78 (0.54–1.14) | 0.202 | |
| Pulmonary Disease | 0.87 (0.61–1.26) | 0.471 | |
| Dementia | 1.24 (0.81–1.92) | 0.324 | |
| Cancer | 0.61 (0.33–1.13) | 0.117 | |
| ASA grade | 1 | 1.43 (0.32–6.34) | 0.636 |
| 2 | Reference | ||
| 3 | 0.97 (0.60–1.57) | 0.902 | |
| 4 | 1.47 (0.86–2.51) | 0.159 | |
| 5 | 5.25 (1.30–21.31) | 0.020 | |
| Missing or N/A | 0.58 (0.23–1.49) | 0.258 | |
| Admission Blood Tests (for each point) | Lymphocyte Count (x 109/L) | 0.62 (0.46–0.83) | 0.001 |
| Albumin (g/L) | 0.95 (0.93 0.98) | <0.001 | |
Fig. 4.
ROC curve for lymphocyte count (grey) and albumin (black dashed) as a predictor of COVID-19 on admission. Lymphocyte: Area under the curve 60.7% (95% CI 56.7%–64.6%, p < 0.001). Threshold of 0.93 or less has 58.2% specificity and 56.6% sensitivity. Albumin: Area under the curve 61.3% (95% CI 57.5%–65.2%, p < 0.001). Threshold of 36 g/dL or less has 59.1% specificity and 57.1%sensitivity.
Predictors associated with having COVID-19 after admission
There were 426 patients diagnosed with positive COVID-19 after admission to hospital. Regression analysis demonstrated male sex, a fall indoor, cardiovascular disease, ASA grade 4 or 5, and longer duration of hospital stay were independently associated with a positive COVID-19 diagnosis on admission (Table 6). ROC curve analysis illustrated that length of stay of 10 or more days was a moderately reliable predictor of COVID-19 following admission (Fig. 5 ), with an AUC of 71.6%.
Table 6.
Logistic regression model identifying patient related factors associated with developing COVID-19 in hip fracture patients following admission.
| Demographic | Descriptive | Odds Ratio (95% CI) | p-value∗ |
|---|---|---|---|
| Age (for each increasing year) | 1.01 (0.99–1.02) | 0.480 | |
| Sex | Female | Reference | |
| Male | 1.56 (1.23–1.97) | <0.001 | |
| Nottingham Hip Score (for each increasing point) | 1.03 (0.99–1.06) | 0.110 | |
| Residence | Home/Sheltered | Reference | |
| Care/Nursing home | 1.22 (0.89–1.67) | 0.218 | |
| Hospital | 2.03 (0.81–5.11) | 0.133 | |
| Missing | 3.14 (2.07–4.77) | <0.001 | |
| Place of injury | Home/Indoor | Reference | |
| Outdoor | 0.56 (0.36–0.87) | 0.009 | |
| Hospital | 1.03 (0.79–2.36) | 0.942 | |
| Missing | 1.37 (0.79–2.36) | 0.263 | |
| Comorbidity∗ | Not present | ||
| CVD | 1.43 (1.09–1.86) | 0.009 | |
| Renal Disease | 0.90 (0.69–1.18) | 0.433 | |
| Pulmonary | 1.03 (0.79–1.34) | 0.850 | |
| Dementia | 1.18 (0.89–1.55) | 0.254 | |
| Cancer | 0.65 (0.43–0.98) | 0.041 | |
| ASA grade | 1 | 0.36 (0.05–2.69) | 0.317 |
| 2 | Reference | ||
| 3 | 1.35 (0.92–1.97) | 0.123 | |
| 4 | 1.79 (1.16–2.75) | 0.008 | |
| 5 | 10.84 (3.09–38.00) | <0.001 | |
| Missing or N/A | 0.69 (0.29–1.62) | 0.394 | |
| Admission Blood Tests (for each point) | Lymphocyte Count (x 109/L) | 0.92 (0.77–1.10) | 0.383 |
| Albumin (g/L) | 1.00 (0.99–1.08) | 0.681 | |
| Length of stay (for each increasing day) | 1.07 (1.06–1.08) | <0.001 | |
Fig. 5.
ROC curve for length of hospital stay (dashed line) as a predictor of developing COVID-19 following admission. Area under the curve 71.6% (95% CI 68.8%–74.4%, p < 0.001). Threshold of 10 days or more has 65% specificity and sensitivity.
Predictors associated with increased mortality in patients with COVID-19
Factors associated with increased risk of 30-day mortality on unadjusted analysis were older age, male sex, higher NHFS, injury sustained outdoors, renal disease, pulmonary disease, dementia, increasing ASA grade, nonoperative management, lower lymphocyte count, lower platelet count, and lower serum albumin concentration (Table 7 ). Regression analysis demonstrated that increasing age (HR 1.03, 95% CI 1.01–1.05, p = 0.028), male sex (HR 2.35, 95% CI 1.66–3.34, p < 0.001), renal disease (HR 1.53, 95% CI 1.08–2.18, p = 0.017), and pulmonary disease (HR 1.45, 95% CI 1.02–2.06, p = 0.039) were independently associated with an increased risk of 30-day mortality (Table 8 ).
Table 7.
Patient demographics, Nottingham hip fracture score, residence, place of injury, comorbidity, surgery within 36 h, ASA grade, surgical management, admission blood test according to 30-day mortality for COVID-19 positive patients only.
| Demographic | Descriptive | 30-day Mortality |
Difference/Odds Ratio (95% CI) | p-valuea | |
|---|---|---|---|---|---|
| Alive (n = 473) | Dead (n = 178) | ||||
| Age (years: mean, SD) | 83.7 (9.5) | 85.8 (7.5) | Diff 2.1 (0.5–3.7) | 0.008 | |
| Sex (n, % of group) | Female | 326 | 83 | Reference | |
| Male | 147 | 95 | OR 2.54 (1.78–3.61) | <0.001 | |
| Missing | 0 | 0 | |||
| Nottingham Hip Score (mean, SD) | 5.3 (1.6) | 6.5 (7.1) | Diff 1.2 (0.6–1.9) | <0.001 | |
| Residence (n, % of group) | Home/Sheltered | 270 | 91 | Reference | |
| Care/Nursing home | 154 | 73 | OR 1.41 (0.98–2.03) | 0.067 | |
| Hospital | 15 | 5 | OR 0.99 (0.45–2.16) | 0.999 | |
| Missing | 34 | 9 | OR 0.83 (0.45–1.52) | 0.537 | |
| Place of injury (n, % of group) | Home/Indoor | 385 | 159 | Reference | |
| Outdoor | 38 | 5 | OR 0.32 (0.12–0.82) | 0.013 | |
| Hospital | 30 | 9 | OR 0.73 (0.34–1.56) | 0.413 | |
| Missing | 20 | 5 | OR 0.61 (0.22–1.64) | 0.375 | |
| Comorbiditya (n, % of group) | Not present | Reference | Reference | ||
| CVD Disease | 335 | 136 | OR 1.33 (0.90–1.99) | 0.156 | |
| Renal Disease | 96 | 61 | OR 2.04 (1.39–2.99) | <0.001 | |
| Pulmonary Disease | 109 | 61 | OR 1.74 (1.20–2.54) | 0.004 | |
| Dementia | 196 | 91 | OR 1.48 (1.05–2.09) | 0.027 | |
| Cancer | 37 | 16 | OR 1.16 (0.63–2.15) | 0.628 | |
| Diabetes Mellitus | 104 | 34 | OR 0.83 (0.54–1.29) | 0.645 | |
| Surgery <36 h (n, % of group) | Yes | 288 | 102 | Reference | |
| No | 173 | 48 | OR 0.78 (0.53–1.16) | 0.221 | |
| N/A | 10 | 27 | OR 7.62 (3.57–16.30) | <0.001 | |
| Missing | 2 | 1 | OR 1.41 (0.13–15.74) | 0.999 | |
| ASA grade (n, % of group) | 1 | 2 | 1 | OR 6.40 (0.49–83.39) | 0.233 |
| 2 | 64 | 5 | Reference | ||
| 3 | 271 | 98 | OR 4.63 (1.81–11.84) | <0.001 | |
| 4 | 120 | 61 | OR 6.51 (2.49–17.01) | <0.001 | |
| 5 | 1 | 8 | OR 102.40 (10.59–990.6) | <0.001 | |
| Missing or N/A | 15 | 2 | OR (1.71 90.30 to 9.66) | 0.621 | |
| Management (n, % of group) | Fixation | 225 | 85 | Reference | |
| Arthroplasty | 227 | 67 | 0.78 (0.54–1.13) | 0.190 | |
| Non-operative | 10 | 25 | 6.62 (3.05–14.36) | <0.001 | |
| Other | 6 | 0 | – | 0.197 | |
| Missing | 5 | 1 | 0.53 (0.06–4.60) | 0.685 | |
| Admission Blood Tests (mean, SD) | |||||
| Haemoglobin | n = 473 vs 178 | 121.7 (17.4) | 120.8 (18.4) | 0.9 (−2.1 to 4.0) | 0.558 |
| Lymphocyte | n = 473 vs 178 | 1.11 (0.67) | 0.98 (0.70) | 0.13 (0.01–0.25) | 0.030 |
| Platelet | n = 473 vs 178 | 245.7 (91.5) | 231.3 (84.7) | 14.5 (−1.0 to 29.9) | 0.067 |
| Sodium | n = 473 vs 178 | 137.5 (4.7) | 138.0 (4.7) | 0.6 (−0.3 to 1.4) | 0.180 |
| Albumin | n = 419 vs 157 | 34.4 (5.7) | 35.6 (5.8) | 1.2 (0.1–2.3) | 0.027 |
| Time of COVID-19 Diagnosis (n, % of group) | Admission | 169 | 56 | Reference | |
| Following admission | 304 | 122 | 1.21 (0.84–1.75) | 0.307 | |
Data not available for four patients: two died within the 30 day follow up period.
Table 8.
Cox regression model identifying patient related factors associated with 30-day mortality following a hip fracture in patients for patients with COVID-19.
| Demographic | Descriptive | Hazard Ratio (95% CI) | p-value∗ |
|---|---|---|---|
| Age (for each increasing year) | 1.03 (1.01–1.05) | 0.028 | |
| Sex | Female | Reference | |
| Male | 2.35 (1.66–3.34) | <0.001 | |
| Nottingham Hip Score (for each increasing point) | 1.00 (0.97–1.03 | 0.825 | |
| Residence | Home/Sheltered | Reference | |
| Care/Nursing home | 1.32 (0.90–1.95) | 0.155 | |
| Hospital | 1.17 (0.30–4.45) | 0.823 | |
| Missing | 0.98 (0.46–2.12) | 0.982 | |
| Place of injury | Home/Indoor | Reference | |
| Outdoor | 0.35 (0.11–1.14) | 0.081 | |
| Hospital | 0.64 (0.24–1.72) | 0.374 | |
| Missing | 0.32 (0.06–1.56) | 0.158 | |
| Comorbidity | Not present | Reference | |
| Renal Disease | 1.53 (1.08–2.18) | 0.017 | |
| Pulmonary | 1.45 (1.02–2.06) | 0.039 | |
| Dementia | 1.24 (0.85–1.83) | 0.266 | |
| ASA grade | 1 | 8.69 (0.96–78.75) | 0.055 |
| 2 | Reference | ||
| 3 | 2.36 (0.94–5.88) | 0.066 | |
| 4 | 2.41 (0.94–6.14) | 0.066 | |
| 5 | 2.66 (0.78–9.02) | 0.117 | |
| Missing or N/A | 1.97 (0.46–8.44) | 0.358 | |
| Management | Fixation | Reference | |
| Arthroplasty | 0.75 (0.53–1.06) | 0.103 | |
| Non-operative | 2.59 (1.52–4.43) | <0.001 | |
| Other | – | ||
| Missing | 1.29 (0.13–12.38) | 0.824 | |
| Blood tests (for each increasing unit) | Lymphocyte | 0.83 (0.62–1.12) | 0.233 |
| Platelet | 1.00 (1.00–1.00) | 0.085 | |
| Albumin | 0.98 (0.95–1.01) | 0.132 | |
Discussion
This global multicentre audit reports the findings from 112 hospitals in 14 countries. A positive diagnosis of COVID-19 during an acute admission for hip fracture was independently associated with an approximate three-fold increase in 30-day mortality risk compared to patients without COVID-19, and it is likely that hip fracture patients are the single group of surgical admissions that account for the largest number of COVID-19-related deaths. Approximately two thirds of COVID-19 cases were diagnosed postoperatively, which supports findings from a previous study suggesting the major role of nosocomial transmission among this vulnerable patient group.19 For the first time, clinical factors that are associated with increased risk of death in hip fracture patients who have COVID-19 are reported and this may help to identify fragility trauma patients that could benefit from isolating or shielding. This study, which is understood to be the largest multicentre orthopaedic collaborative audit delivered, offers the only global data into hip fracture and COVID-19 from the pre-vaccination era and could be used to ensure better preparedness for future disease outbreaks, from seasonal influenza to emerging diseases.
The prevalence of COVID-19 in this study cohort was 9.2%. This is consistent with the existing literature from single-centre or regional studies, but was many times higher than the mean background prevalence in any of the participating nations throughout the study period (range 0·0-0·5%).5 The extreme vulnerability of this patient group may be under-recognised among healthcare professionals, and the major disruption to fragility trauma services experienced globally is likely to contribute to an enduring public health crisis. Although the study investigated only patients with hip fracture, these findings are likely to be generalisable to frail trauma patients, as well as to the wider frail inpatient population.20
The current data suggests that two-thirds of COVID-19 cases were diagnosed postoperatively, and IMPACT-Scot 2 demonstrated that approximately 60% of COVID-19 cases were likely to be hospital-acquired, with the majority of these nosocomial infections occurring in acute orthopaedic wards or following discharge to inpatient orthopaedic rehabilitation facilities.20 Nosocomial infection may be an important factor in the high rates of COVID-19 observed among vulnerable inpatients and this problem has significant implications for the spread of COVID-19 between hospitals, downstream bed facilities, residential care settings and the community. There remains little published evidence that demonstrates successful strategies for the mitigation of this phenomenon among frail orthogeriatric trauma patients.
The factors identified in the current study that were independently associated with a positive COVID-19 diagnosis (at any time) were consistent with the existing literature, although the current data identified differences depending on whether COVID-19 was identified at initial presentation or following admission, which is of particular relevance to clinical risk stratification and the isolation of at-risk patients.20 , 21 Factors predictive of having COVID-19 at admission were certain admission laboratory blood tests (lower blood albumin level and lymphocyte count), higher pre-fracture care demands (residential or inpatient care) and a high ASA grade. Male sex, pre-existing cardiovascular disease, high ASA grade, and a longer length of stay were predictive of COVID-19 diagnoses made postoperatively. Most of these factors are indicators of increasing frailty and may indicate vulnerability to infection. These findings may assist stratification of patients according to their risk of transmitting or acquiring COVID-19 in hospital, and facilitate deployment of clinical patient pathways for isolating, shielding, or ‘cohorting’ patients in COVID and non-COVID circuits – an approach which has been found to be effective in the management of hip fracture patients during the pandemic.21 The key modifiable risk factor identified was length of stay, which supports previous work in this area that underlines that safeguarding and prioritisation of fragility fracture services as essential to help protect this vulnerable patient group through early treatment and discharge planning.[22], [23] However, the causal relationship of increased length of stay on the likelihood of contracting COVID-19 is difficult to determine, since patients with COVID-19 are likely to require a longer hospital admission, and frailer patients (who are more vulnerable to acquiring COVID-19) typically require longer inpatient management prior to discharge.
Male sex was associated with a two-fold increased risk of 30-day mortality among patients diagnosed with COVID-19. This supports existing evidence from the general population that males with COVID-19 have a higher mortality rate than females.24 Various explanatory mechanisms have been suggested and include differences in expression of angiotensin-converting enzyme II, smoking status, obesity, and behavioural factors.[25], [26], [27], [28] The existence of underlying pulmonary disease was independently associated with a higher 30-day mortality risk, which is consistent with the known pathophysiology of COVID-19.28 The influence of renal disease on mortality is of particular importance in hip fracture patients given the relatively high prevalence of chronic kidney disease, acute kidney injury, or mixed acute kidney injury and chronic kidney disease, all of which have been shown to be associated with poorer outcomes in non-hip fracture groups with COVID-19.25 The identification of these clinical predictors in the hip fracture population is original and could guide clinical decision-making and prognosis.
The COVID-19 pandemic remains a dynamic situation subject to: further increases in the incidence of SARS-CoV-2 infection; new viral strains with higher transmissibility, mortality risk, and resistance to vaccinations; the need to reduce restrictions in order to meet the needs of the population, and challenges associated with achieving widespread and effective vaccination across the globe.26 , [29], [30], [31] This study will provide an important baseline against which to measure factors such as vaccine efficacy, strategies for the mitigation of viral transmission, and the effects of different viral strains on this vulnerable population.
Evidence from the IMPACT collaborative has demonstrated widespread disruption to orthopaedic services, with resources and staff being repurposed for non-orthopaedic patients and standard operating procedures being overhauled in favour of other services.20 Hip fracture patients were managed on open generalist wards by non-specialised staff, experienced delays to surgery and appropriate care, received less specialist multidisciplinary management, and were exposed to an increase in inter-departmental transit. These issues are known to increased risk of nosocomial infection, delirium, and longer duration of hospital stay.19 , 22 , 32 In future communicable disease outbreaks it would be prudent to ensure the protection of specialist multidisciplinary teams, clinical areas, and access to prompt surgical management in line with existing standards of care for this most vulnerable patient group, as well as robust strategies to minimise in-hospital transmission through the use of clinical pathways and closed circuits that have previously been described.19 , 21 , [33], [34], [35]
Early in the pandemic there was uncertainty about the infection prevention and control precautions required in the management of patients at risk of contracting SARS-CoV-2 infection. This caused disparities and frequent amendments to guidance about personal protective equipment, testing of patients and staff, the acceptability of risk relating to aerosol generating procedures such as cardiopulmonary resuscitation and anaesthetic procedures, and surgery.36 This led to confusion and delays to appropriate patient management and care ought to be taken to design procedures for the continuation of orthopaedic services in the context of future disease outbreaks. This is of relevance to unscheduled care and to urgent planned care, since the disruption has been to the detriment of patients attempting to access urgent elective care.[37], [38], [39]
The concerning finding of a high proportion of patients acquiring COVID-19 in the inpatient and downstream hospital settings raises questions regarding the efficacy of existing pathways and strategies for the prevention of infection transmission between healthcare services. The establishment of a robust and effective inpatient and post-discharge track and trace system could identify patients at risk of acquiring or transmitting infection, which has the potential to limit the harm from outbreaks and reduce the burden on rehabilitation and community health services.
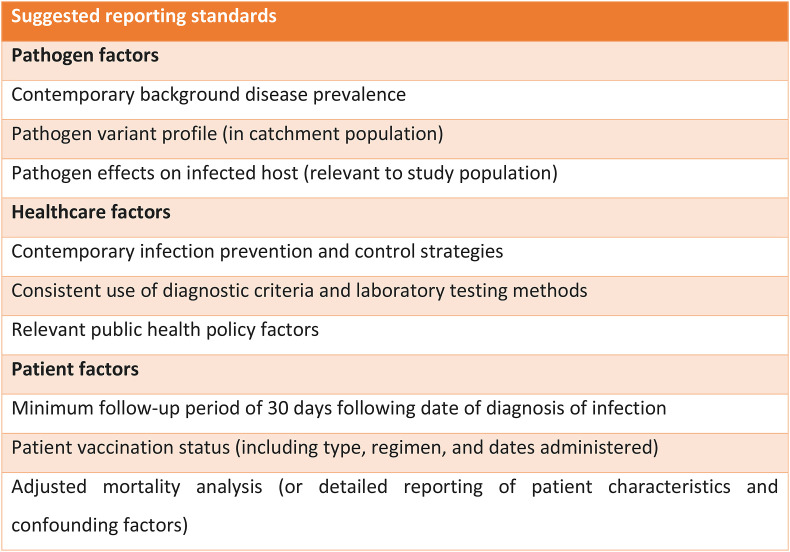
This international study was conducted within the context of a rapidly-developing global pandemic. As a result, there are limitations inherent in the natural variation between nations relating to the background COVID-19 prevalence, which ranged from 0.003 to 0.294% during the study period. There was no standardised diagnostic protocol, such as routine regular testing of all patients, and the availability of laboratory testing may have varied between regions; the prevalence of COVID-19 may therefore have been underestimated. Furthermore, as routine clinical testing was not in place in most countries during the first wave of the pandemic, the mortality associated with undiagnosed COVID-19 was not quantifiable, and because the precise dates of COVID-19 diagnoses are not known the distinction between community- and hospital-acquired SARS-CoV-2 infections cannot be determined with certainty. This reflects real-world uncertainty around clinical criteria for diagnosing COVID-19 and variation in the approaches to population screening and symptomatic testing, and highlights the need to establish early consensus on these matters early in an outbreak in order to facilitate effective research and audit. There was variation in the approach to the provision of hip fracture services, though this could be considered a strength due to increased generalisability across the range of nations affected by the disease. Clinical audit in future outbreaks should strive for even greater coverage of geographical and health-economic context.[40], [41] Follow-up period was limited to 30 days post-presentation with hip fracture, which may underestimate mortality especially in patients who developed COVID-19 later in the admission. This limited follow-up is common amongst studies reporting the mortality associated with COVID-19.4 However, the current study controlled for this issue by reporting subgroups of patients with COVID-19 confirmed at initial presentation in the preoperative period versus later in the admission following surgical management. Variation in the systems available to clinicians to follow up patients after discharge may underestimate mortality rates in regions that don't have, for example, a unified healthcare system with patients linked by a universally-applied unique community identifier. This ought to be considered in the methodology of future studies. There remains a lack of evidence pertaining to the indirect effects of the pandemic on COVID-19-negative hip fracture, or the effect that mass population vaccination will have on prevalence, transmissibility, and mortality. There was heterogeneity in the literature reporting investigations in COVID-19 in hip fracture, with many studies being limited by a lack of robust diagnostic criteria, insufficient follow-up durations, unadjusted mortality analyses, and a lack of relevant information pertaining to background prevalence, pathogen variant profiles, and infection prevention and control measures in the catchment population.5 Adoption of shared reporting standards may improve the quality of evidence available to clinicians and researchers (Fig. 6 ).
Fig. 6.
Suggested reporting standards for studies investigating COVID-19 in hip fracture patients.
The strengths of the study include the large number of patients and the unique international nature that has provided an analysis across a range of hospitals, hip fracture services, healthcare systems, ethnicities and reporting processes. This diversity would suggest that the findings are generalisable globally. The findings pertaining to COVID-19 prevalence, mortality risk, and predictors of infection support existing evidence and provide insight into clinical factors associated with COVID-19 and outcome. The high levels of participation in the UK and Spain in particular, ensured extensive coverage across these geographical areas, which may have helped account for regional variations in clinical practice, patient demographics and COVID-19 prevalence. Furthermore, the size of the COVID-19 positive cohort was large and afforded the first opportunity to perform subgroup regression analyses to identify factors associated with acquiring the infection and the mortality associated with it. The lessons learned from this study of the COVID-19 pandemic are applicable to future disease outbreaks and may facilitate better preparedness for other transmissible diseases such as seasonal influenza, emerging strains of existing pathogens, or novel communicable diseases.
Conclusion
The prevalence of COVID-19 in the hip fracture population was at least ten times higher than the background prevalence and was independently associated with a three-fold increase in 30-day mortality. Thus, hip fracture patients may be the cohort of hospital admissions that account for the largest number of COVID-19-related deaths. It is likely that nosocomial transmission of this disease was responsible for a significant proportion of infections, and the development of robust infection prevention and control strategies are likely to improve the management of future outbreaks. The IMPACT collaborative has demonstrated important lessons in the conduct of rapid clinical audit in order to guide the evidence-based response to emerging diseases, and a number of strategies are suggested that can be applied prospectively to ensure better preparedness for future health crises.
Funding
None.
Previous presentation of findings
This work was conducted in the context of an evolving global pandemic and the need for timely dissemination of information was critical. To this end a limited number of findings from the current study have been presented as abstracts at the British Orthopaedic Association Annual Congress 2021 (Free Paper Session: Infection & COVID-19), and the Scottish Committee for Orthopaedics and Trauma (SCOT) 2021 Meeting (Free Paper Session).42 , 43
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
The authors would like to acknowledge Karen Adam (Scottish Government) who made rapid collaboration possible, as well as the contributions of all co-authors acknowledged herein. The authors also wish to thank all collaborators involved in the delivery of the International Multicentre Project Auditing COVID-19 in Trauma & Orthopaedics (IMPACT) Global Hip Fracture Audit.
Contributor Information
IMPACT-Global Group:
Hani Abdul-Jabar, Rashid Abu-Rajab, Ahmed Abugarja, Karen Adam, Héctor J. Aguado Hernández, Gedeón Améstica Lazcano, Sarah Anderson, Mahmood Ansar, Jonathan Antrobus, Esteban Javier Aragón Achig, Maheswaran Archunan, Mirentxu Arrieta Salinas, Sarah Ashford–Wilson, Cristina Assens Gibert, Katerina Athanasopoulou, Mohamed Awadelkarim, Stuart Baird, Stefan Bajada, Shobana Balakrishnan, Sathishkumar Balasubramanian, James A. Ballantyne, Leopoldo Bárcena Goitiandia, Benjamin Barkham, Christina Barmpagianni, Mariano Barres-Carsi, Sarah Barrett, Dinnish Baskaran, Jean Bell, Katrina Bell, Stuart Bell, Giuseppe Bellelli, Javier Alberto Benchimol, Bruno Rafael Boietti, Sally Boswell, Adriano Braile, Caitlin Brennan, Louise Brent, Ben Brooke, Gaetano Bruno, Abdus Burahee, Shirley Burns, Giampiero Calabrò, Lucy Campbell, Guido Sebastian Carabelli, Carol Carnegie, Guillermo Carretero Cristobal, Ethan Caruana, M.a Concepción Cassinello Ogea, Juan Castellanos Robles, Pablo Castillon, Anil Chakrabarti, Antonio Benedetto Cecere, Ping Chen, Jon V. Clarke, Grace Collins, Jorge E. Corrales Cardenal, Maurizio Corsi, Gara María Cózar Adelantado, Simon Craxford, Melissa Crooks, Javier Cuarental-García, Rory Cuthbert, Graham Dall, Ioannis Daskalakis, Annalisa De Cicco, Diana de la Fuente de Dios, Pablo Demaria, John Dereix, Julian Díaz Jiménez, José Luis Dinamarca Montecinos, Ha Phuong Do Le, Juan Pablo Donoso Coppa, Georgios Drosos, Andrew Duffy, Jamie East, Deborah Eastwood, Hassan Elbahari, Carmen Elias de Molins Peña, Mamoun Elmamoun, Ben Emmerson, Daniel Escobar Sánchez, Martina Faimali, Maria Victòria Farré-Mercadé, Luke Farrow, Almari Fayez, Adam Fell, Christopher Fenner, David Ferguson, Louise Finlayson, Aldo Flores Gómez, Nicholas Freeman, Jonathan French, Santiago Gabardo Calvo, Nicola Gagliardo, Joan Garcia Albiñana, Guillermo García Cruz, Unai García de Cortázar Antolín, Virginia García Virto, Sophie Gealy, Sandra Marcela Gil Caballero, Moneet Gill, María Soledad González González, Rajesh Gopireddy, Diane Guntley, Binay Gurung, Guadalupe Guzmán Rosales, Nedaa Haddad, Mahum Hafeez, Petra Haller, Emer Halligan, John Hardie, Imogen Hawker, Amr Helal, Mariana Herrera Cruz, Ruben Herreros Ruiz-Valdepeñas, James Horton, Sean Howells, Alan Howieson, Luke Hughes, Flavia Lorena Hünicken Torrez, Ana Hurtado Ortega, Peter Huxley, Hytham K.S. Hamid, Nida Ilahi, Alexis Iliadis, Dominic Inman, Piyush Jadhao, Rajan Jandoo, Lucy Jawad, Malwattage Lara Tania Jayatilaka, Paul J. Jenkins, Rathan Jeyapalan, David Johnson, Andrew Johnston, Sarah Joseph, Siddhant Kapoor, Georgios Karagiannidis, Krishna Saga Karanam, Freddy Kattakayam, Alastair Konarski, Georgios Kontakis, Gregorio Labrador Hernández, Victoria Lancaster, Giovanni Landi, Brian Le, Ignatius Liew, Kartik Logishetty, Andrew Carlomaria Daniel Lopez Marquez, Judit Lopez, Joann Lum, Gavin J. Macpherson, Suvira Madan, Sabreena Mahroof, Khalid Malik-Tabassum, Ravi Mallina, Afnan Maqsood, Ben Marson, M. José Martin Legorburo, Encarna Martin-Perez, Tania Martínez Jiménez, Javier Martinez Martin, Alistair Mayne, Amy Mayor, Gavan McAlinden, Lucille McLean, Lorna McDonald, Joshua McIntyre, Pamela McKay, Greg McKean, Heather McShane, Antonio Medici, Chelsea Meeke, Evonne Meldrum, Mijail Mendez, Scott Mercer, Josu Merino Perez, María-Pilar Mesa-Lampré, Shuna Mighton, Kirsty Milne, Muhammed Mohamed Yaseen, Iain Moppett, Jesus Mora, Sira Morales-Zumel, Irene Blanca Moreno Fenoll, Adham Mousa, Alastair W. Murray, Elspeth V. Murray, Radhika Nair, Fiona Neary, Giacomo Negri, Oliver Negus, Fiona Newham-Harvey, Nigel Ng, Jess Nightingale, Sumiya Noor Mohamed Anver, Perrico Nunag, Matthew O'Hare, Ben Ollivere, Raquel Ortés Gómez, AnneMarie Owens, Siobhan Page, Valentina Palloni, Andreas Panagiotopoulos, Elias Panagiotopoulos, Paul Panesar, Antonios Papadopoulos, Papagiannis Spyridon, Teresa Pareja Sierra, Chang Park, Hammad Parwaiz, Paul Paterson-Byrne, Sam Patton, Jack Pearce, Marina Porter, Achille Pellegrino, Arturo Pèrez Cuellar, Raffaele Pezzella, Ashish Phadnis, Charlotte Pinder, Danielle Piper, Matilda Powell-Bowns, Rocío Prieto Martín, Annabel Probert, Ashwanth Ramesh, Manuel Vicente Mejía Ramírez de Arellano, Duncan Renton, Stephen Rickman, Alastair Robertson, Adrian Roche Albero, José Alberto Rodrigo Verguizas, Myriam Rodríguez Couso, Joanna Rooney, Pilar Sáez-López, Andres Saldaña-Díaz, Adriano Santulli, Marta Isabel Sanz Pérez, Khaled M. Sarraf, Christine Scarsbrook, Chloe E.H. Scott, Jennifer Scott, Sachi Shah, Sharief Sharaf, Sidharth Sharma, Denise Shirley, Antonio Siano, James Simpson, Abhinav Singh, Amit Singh, Tim Sinnett, Gurudatt Sisodia, Philomena Smith, Eugenia Sophena Bert, Michael Steel, Avril Stewart, Claire Stewart, Kapil Sugand, Niall Sullivan, Lauren Sweeting, Michael Symes, Dylan Jun Hao Tan, Francesco Tancredi, Irini Tatani, Philip Thomas, Fraser Thomson, Niamh S. Toner, Anna Tong, Antonio Toro, Theodoros Tosounidis, Stylianos Tottas, Andrea Trinidad Leo, Damien Tucker, Krishna Vemulapalli, Diego Ventura Garces, Olivia Katherine Vernon, Juan Carlos Viveros Garcia, Alex Ward, Kirsty Ward, Kate Watson, Thisara Weerasuriya, Udara Wickramanayake, Hannah Wilkinson, Joseph Windley, Janet Wood, William Wynell-Mayow, Giovanni Zatti, Moez Zeiton, and Miriam Zurrón Lobato
Appendix.
IMPACT Global Group
| Surname | Forename |
| Abdul-Jabar | Hani |
| Abu-Rajab | Rashid |
| Abugarja | Ahmed |
| Adam | Karen |
| Aguado Hernández | Héctor J. |
| Améstica Lazcano | Gedeón |
| Anderson | Sarah |
| Ansar | Mahmood |
| Antrobus | Jonathan |
| Aragón Achig | Esteban Javier |
| Archunan | Maheswaran |
| Arrieta Salinas | Mirentxu |
| Ashford-Wilson | Sarah |
| Assens Gibert | Cristina |
| Athanasopoulou | Katerina |
| Awadelkarim | Mohamed |
| Baird | Stuart |
| Bajada | Stefan |
| Balakrishnan | Shobana |
| Balasubramanian | Sathishkumar |
| Ballantyne | James A. |
| Bárcena Goitiandia | Leopoldo |
| Barkham | Benjamin |
| Barmpagianni | Christina |
| Barres-Carsi | Mariano |
| Barrett | Sarah |
| Baskaran | Dinnish |
| Bell | Jean |
| Bell | Katrina |
| Bell | Stuart |
| Bellelli | Giuseppe |
| Benchimol | Javier Alberto |
| Boietti | Bruno Rafael |
| Boswell | Sally |
| Braile | Adriano |
| Brennan | Caitlin |
| Brent | Louise |
| Brooke | Ben |
| Bruno | Gaetano |
| Burahee | Abdus |
| Burns | Shirley |
| Calabrò | Giampiero |
| Campbell | Lucy |
| Carabelli | Guido Sebastian |
| Carnegie | Carol |
| Carretero Cristobal | Guillermo |
| Caruana | Ethan |
| Cassinello Ogea | M.ª Concepción |
| Castellanos Robles | Juan |
| Castillon | Pablo |
| Chakrabarti | Anil |
| Cecere | Antonio Benedetto |
| Chen | Ping |
| Clarke | Jon V. |
| Collins | Grace |
| Corrales Cardenal | Jorge E. |
| Corsi | Maurizio |
| Cózar Adelantado | Gara María |
| Craxford | Simon |
| Crooks | Melissa |
| Cuarental-García | Javier |
| Cuthbert | Rory |
| Dall | Graham |
| Daskalakis | Ioannis |
| De Cicco | Annalisa |
| de la Fuente de Dios | Diana |
| Demaria | Pablo |
| Dereix | John |
| Díaz Jiménez | Julian |
| Dinamarca Montecinos | José Luis |
| Do Le | Ha Phuong |
| Donoso Coppa | Juan Pablo |
| Drosos | Georgios |
| Duffy | Andrew |
| East | Jamie |
| Eastwood | Deborah |
| Elbahari | Hassan |
| Elias de Molins Peña | Carmen |
| Elmamoun | Mamoun |
| Emmerson | Ben |
| Escobar Sánchez | Daniel |
| Faimali | Martina |
| Farré-Mercadé | Maria Victòria |
| Farrow | Luke |
| Fayez | Almari |
| Fell | Adam |
| Fenner | Christopher |
| Ferguson | David |
| Finlayson | Louise |
| Flores Gómez | Aldo |
| Freeman | Nicholas |
| French | Jonathan |
| Gabardo Calvo | Santiago |
| Gagliardo | Nicola |
| Garcia Albiñana | Joan |
| García Cruz | Guillermo |
| García de Cortázar Antolín | Unai |
| García Virto | Virginia |
| Gealy | Sophie |
| Gil Caballero | Sandra Marcela |
| Gill | Moneet |
| González González | María Soledad |
| Gopireddy | Rajesh |
| Guntley | Diane |
| Gurung | Binay |
| Guzmán Rosales | Guadalupe |
| Haddad | Nedaa |
| Hafeez | Mahum |
| Haller | Petra |
| Halligan | Emer |
| Hardie | John |
| Hawker | Imogen |
| Helal | Amr |
| Herrera Cruz | Mariana |
| Herreros Ruiz-Valdepeñas | Ruben |
| Horton | James |
| Howells | Sean |
| Howieson | Alan |
| Hughes | Luke |
| Hünicken Torrez | Flavia Lorena |
| Hurtado Ortega | Ana |
| Huxley | Peter |
| Hamid | Hytham K. S. |
| Ilahi | Nida |
| Iliadis | Alexis |
| Inman | Dominic |
| Jadhao | Piyush |
| Jandoo | Rajan |
| Jawad | Lucy |
| Jayatilaka | Malwattage Lara Tania |
| Jenkins | Paul J. |
| Jeyapalan | Rathan |
| Johnson | David |
| Johnston | Andrew |
| Joseph | Sarah |
| Kapoor | Siddhant |
| Karagiannidis | Georgios |
| Karanam | Krishna Saga |
| Kattakayam | Freddy |
| Konarski | Alastair |
| Kontakis | Georgios |
| Labrador Hernández | Gregorio |
| Lancaster | Victoria |
| Landi | Giovanni |
| Le | Brian |
| Liew | Ignatius |
| Logishetty | Kartik |
| Lopez Marquez | Andrew Carlomaria Daniel |
| Lopez | Judit |
| Lum | Joann |
| Macpherson | Gavin J. |
| Madan | Suvira |
| Mahroof | Sabreena |
| Malik-Tabassum | Khalid |
| Mallina | Ravi |
| Maqsood | Afnan |
| Marson | Ben |
| Martin Legorburo | M José |
| Martin-Perez | Encarna |
| Martínez Jiménez | Tania |
| Martinez Martin | Javier |
| Mayne | Alistair |
| Mayor | Amy |
| McAlinden | Gavan |
| McLean | Lucille |
| McDonald | Lorna |
| McIntyre | Joshua |
| McKay | Pamela |
| McKean | Greg |
| McShane | Heather |
| Medici | Antonio |
| Meeke | Chelsea |
| Meldrum | Evonne |
| Mendez | Mijail |
| Mercer | Scott |
| Merino Perez | Josu |
| Mesa-Lampré | María-Pilar |
| Mighton | Shuna |
| Milne | Kirsty |
| Mohamed Yaseen | Muhammed |
| Moppett | Iain |
| Mora | Jesus |
| Morales-Zumel | Sira |
| Moreno Fenoll | Irene Blanca |
| Mousa | Adham |
| Murray | Alastair W. |
| Murray | Elspeth V. |
| Nair | Radhika |
| Neary | Fiona |
| Negri | Giacomo |
| Negus | Oliver |
| Newham-Harvey | Fiona |
| Ng | Nigel |
| Nightingale | Jess |
| Noor Mohamed Anver | Sumiya |
| Nunag | Perrico |
| OHare | Matthew |
| Ollivere | Ben |
| Ortés Gómez | Raquel |
| Owens | AnneMarie |
| Page | Siobhan |
| Palloni | Valentina |
| Panagiotopoulos | Andreas |
| Panagiotopoulos | Elias |
| Panesar | Paul |
| Papadopoulos | Antonios |
| Spyridon | Papagiannis |
| Pareja Sierra | Teresa |
| Park | Chang |
| Parwaiz | Hammad |
| Paterson-Byrne | Paul |
| Patton | Sam |
| Pearce | Jack |
| Porter | Marina |
| Pellegrino | Achille |
| Pèrez Cuellar | Arturo |
| Pezzella | Raffaele |
| Phadnis | Ashish |
| Pinder | Charlotte |
| Piper | Danielle |
| Powell-Bowns | Matilda |
| Prieto Martín | Rocío |
| Probert | Annabel |
| Ramesh | Ashwanth |
| Ramírez de Arellano | Manuel Vicente Mejía |
| Renton | Duncan |
| Rickman | Stephen |
| Robertson | Alastair |
| Roche Albero | Adrian |
| Rodrigo Verguizas | José Alberto |
| Rodríguez Couso | Myriam |
| Rooney | Joanna |
| Sáez-López | Pilar |
| Saldaña-Díaz | Andres |
| Santulli | Adriano |
| Sanz Pérez | Marta Isabel |
| Sarraf | Khaled M. |
| Scarsbrook | Christine |
| Scott | Chloe E. H. |
| Scott | Jennifer |
| Shah | Sachi |
| Sharaf | Sharief |
| Sharma | Sidharth |
| Shirley | Denise |
| Siano | Antonio |
| Simpson | James |
| Singh | Abhinav |
| Singh | Amit |
| Sinnett | Tim |
| Sisodia | Gurudatt |
| Smith | Philomena |
| Sophena Bert | Eugenia |
| Steel | Michael |
| Stewart | Avril |
| Stewart | Claire |
| Sugand | Kapil |
| Sullivan | Niall |
| Sweeting | Lauren |
| Symes | Michael |
| Tan | Dylan Jun Hao |
| Tancredi | Francesco |
| Tatani | Irini |
| Thomas | Philip |
| Thomson | Fraser |
| Toner | Niamh S. |
| Tong | Anna |
| Toro | Antonio |
| Tosounidis | Theodoros |
| Tottas | Stylianos |
| Trinidad Leo | Andrea |
| Tucker | Damien |
| Vemulapalli | Krishna |
| Ventura Garces | Diego |
| Vernon | Olivia Katherine |
| Viveros Garcia | Juan Carlos |
| Ward | Alex |
| Ward | Kirsty |
| Watson | Kate |
| Weerasuriya | Thisara |
| Wickramanayake | Udara |
| Wilkinson | Hannah |
| Windley | Joseph |
| Wood | Janet |
| Wynell-Mayow | William |
| Zatti | Giovanni |
| Zeiton | Moez |
| Zurrón Lobato | Miriam |
References
- 1.Jenkins P.J. British Orthopaedic Association (BOA); 2020. The early effect of COVID-19 on trauma and elective orthopaedic surgery.https://www.boa.ac.uk/resources/knowledge-hub/the-early-effect-of-covid-19-on-trauma-and-elective-orthopaedic-surgery.html [Internet]. BOA. [cited 2022 Jan 14]. Available from: [Google Scholar]
- 2.Scott C.E., Holland G., Powell-Bowns M.F., Brennan C.M., Gillespie M., Mackenzie S.P., et al. Population mobility and adult orthopaedic trauma services during the COVID-19 pandemic: fragility fracture provision remains a priority. Bone Jt Open. 2020;1(6) doi: 10.1302/2633-1462.16.BJO-2020-0043.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luceri F., Morelli I., Accetta R., Mangiavini L., Maffulli N., Peretti G.M. Italy and COVID-19: the changing patient flow in an orthopedic trauma center emergency department. J Orthop Surg Res. 2020;15(1):323. doi: 10.1186/s13018-020-01816-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clement N.D., Hall A.J., Makaram N.S., Robinson P.G., Patton R.F.L., Moran M., et al. IMPACT-Restart: the influence of COVID-19 on postoperative mortality and risk factors associated with SARS-CoV-2 infection after orthopaedic and trauma surgery. Bone Joint J. 2020;102-B(12):1774–1781. doi: 10.1302/0301-620X.102B12.BJJ-2020-1395.R2. [DOI] [PubMed] [Google Scholar]
- 5.Clement N.D., Ng N., Simpson C.J., Patton R.F.L., Hall A.J., Simpson A.H.R.W., et al. The prevalence, mortality, and associated risk factors for developing COVID-19 in hip fracture patients: a systematic review and meta-analysis. Bone Jt Res. 2020;9(12):873–883. doi: 10.1302/2046-3758.912.BJR-2020-0473.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall A.J., Clement N.D., Farrow L., MacLullich A.M.J., Dall G.F., Scott C.E.H., et al. IMPACT-Scot report on COVID-19 and hip fractures: a multicentre study assessing mortality, predictors of early SARS-CoV-2 infection, and the effects of social lockdown on epidemiology. Bone Jt J. 2020;102-B(9):1219–1228. doi: 10.1302/0301-620X.102B9.BJJ-2020-1100.R1. [DOI] [PubMed] [Google Scholar]
- 7.Scottish Hip Fracture Audit . Public Health Scotland; 2019. Scottish Standards of Care for Hip Fracture Patients 2019.https://www.shfa.scot.nhs.uk/_docs/2019/Scottish-standards-of-care-for-hip-fracture-patients-2019.pdf [Internet] [cited 2020 Sep 25]. Available from: [Google Scholar]
- 8.Hall A.J., Scotland P.H. 2021. Scotland makes an IMPACT on global clinical audit.https://www.shfa.scot.nhs.uk/Reports/_docs/2021/shfa-annual-report-2021-scotland-makes-an-impact.pdf [Internet]. Edinburgh. Available from: [Google Scholar]
- 9.Hall A.J. IMPACT: international multicentre Project auditing COVID-19 in trauma & orthopaedics. 2020. https://www.trauma.co.uk/impact [Internet] Available from: [DOI] [PMC free article] [PubMed]
- 10.Caldicott F. The Caldicott report. IHRIM. 1999;40(2):17–19. [PubMed] [Google Scholar]
- 11.Scottish Hip Fracture Audit. Hip fracture. The Scottish Hip Fracture Audit [Internet]. Available from: https://www.shfa.scot.nhs.uk.
- 12.American Society Of Anesthesiologists . American Society of Anesthesiologists; 2019. Standards and Guidelines: ASA Physical Status Classification System.https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system [Google Scholar]
- 13.Yombi J.C., Putineanu D.C., Cornu O., Lavand’homme P., Cornette P., Castanares-Zapatero D. Low haemoglobin at admission is associated with mortality after hip fractures in elderly patients. Bone Jt J. 2019;101-B(9):1122–1128. doi: 10.1302/0301-620X.101B9.BJJ-2019-0526.R1. [DOI] [PubMed] [Google Scholar]
- 14.Aldebeyan S., Nooh A., Aoude A., Weber M.H., Harvey E.J. Hypoalbuminaemia—a marker of malnutrition and predictor of postoperative complications and mortality after hip fractures. Injury. 2017;48(2):436–440. doi: 10.1016/j.injury.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 15.He Z., Zhao C., Dong Q., Zhuang H., Song S., Peng G., et al. Effects of severe acute respiratory syndrome (SARS) coronavirus infection on peripheral blood lymphocytes and their subsets. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2005;9(6):323–330. doi: 10.1016/j.ijid.2004.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bomhof G., Mutsaers P.G.N.J., Leebeek F.W.G., Boekhorst P.A.W., Hofland J., Croles F.N., et al. COVID-19-associated immune thrombocytopenia. Br J Haematol. 2020;190(2) doi: 10.1111/bjh.16850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagino T., Ochiai S., Watanabe Y., Senga S., Saito M., Takayama Y., et al. Hyponatremia at admission is associated with in-hospital death in patients with hip fracture. Arch Orthop trauma surgery. 2013;133(4):507–511. doi: 10.1007/s00402-013-1693-x. [DOI] [PubMed] [Google Scholar]
- 18.Wiles M.D., Moran C.G., Sahota O., Moppett I.K. Nottingham Hip Fracture Score as a predictor of one year mortality in patients undergoing surgical repair of fractured neck of femur. Br J anaesthesia. 2011;106(4):501–504. doi: 10.1093/bja/aeq405. [DOI] [PubMed] [Google Scholar]
- 19.Hall A.J., Clement N.D., MacLullich A.M.J., White T.O., Duckworth A.D. IMPACT-Scot 2 report on COVID-19 in hip fracture patients: a nationwide study of mortality, risk factors for community and hospital acquired COVID-19, and suggested care pathways. Bone Joint J. 2021;103(5):1–10. doi: 10.1302/0301-620X.103B.BJJ-2020-2027.R1. [DOI] [PubMed] [Google Scholar]
- 20.Hall A.J., Clement N.D., MacLullich A.M.J., Ojeda-Thies C., Hoefer C., Brent L., et al. IMPACT of COVID-19 on hip fracture services: a global survey by the international multicentre Project auditing COVID-19 in trauma & orthopaedics. Surgeon. 2021 May 24;ePub ahead of print doi: 10.1016/j.surge.2021.04.007. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8141714/ [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ojeda-Thies C., Cuarental-García J., García-Gómez E., Salazar-Zamorano C.H., Alberti-Maroño J., Ramos Pascua L.R. Hip fracture care and mortality among patients treated in dedicated COVID-19 and non-COVID-19 circuits. Eur Geriatr Med. 2021;12(4):749–757. doi: 10.1007/s41999-021-00455-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farrow L., Hall A., Wood A.D., Smith R., James K., Holt G., et al. Quality of care in Hip Fracture Patients: the relationship between adherence to national standards and improved outcomes. J Bone Jt Surg - Am. 2018 May;100(9):751–757. doi: 10.2106/JBJS.17.00884. [DOI] [PubMed] [Google Scholar]
- 23.Farrow L., Hall A., Aucott L., Holt G., Myint P.K. Does quality of care in hip fracture vary by day of admission? Arch Osteoporos. 2020 Mar 20;15(1) doi: 10.1007/s11657-020-00725-4. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bwire G.M. Coronavirus: why men are more vulnerable to covid-19 than women? SN Compr Clin Med. 2020;2(7):874–876. doi: 10.1007/s42399-020-00341-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gasparini M, Khan S, Patel JM, Parekh D, Bangash MN, Stϋmpfle R, et al. Renal impairment and its impact on clinical outcomes in patients who are critically ill with COVID-19: a multicentre observational study. Anaesthesia. 2021;76(3):320–326. doi: 10.1111/anae.15293. [DOI] [PubMed] [Google Scholar]
- 26.Wang P., Nair M.S., Liu L., Iketani S., Luo Y., Guo Y., et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593(7857):130–135. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- 27.Simpson A.H.R., Simpson C.J., Frost H., Welburn S.C. COVID-19: obesity, deprivation and death. J Glob Health. 2020 Dec;10(2) doi: 10.7189/jogh.10.020389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehra M.R., Desai S.S., Kuy S., Henry T.D., Patel A.N. Cardiovascular disease, drug therapy, and mortality in covid-19. N Engl J Med. 2020;382(25) doi: 10.1056/NEJMoa2007621. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Public Health England . Sage; 2021. SARS-CoV-2 variants of concern and variants under investigation in England; pp. 1–50. April. [Google Scholar]
- 30.European Centre for Disease Control . Emergence of SARS-CoV-2 B . 1 . 617 variants in India and situation in the EU/EEA Event background Epidemiology. ECDC; 2021. pp. 1–12.https://www.ecdc.europa.eu/en/publications-data/threat-assessment-emergence-sars-cov-2-b1617-variants [cited 2022 Jan 14]. Available from: [Google Scholar]
- 31.Sheikh A., McMenamin J., Taylor B., Robertson C. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397(10293):2461–2462. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis D.H.J., Kreisel S.H., Terrera G.M., Hall A.J., Morandi A., Boustani M., et al. The epidemiology of delirium: challenges and opportunities for population studies. Am J Geriatr Psychiatr. 2013;21(12):1173–1189. doi: 10.1177/2047487315610663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morelli I., Luceri F., Giorgino R., Accetta R., Perazzo P., Mangiavini L., et al. COVID-19: not a contraindication for surgery in patients with proximal femur fragility fractures. J Orthop Surg Res. 2020;15(1):285. doi: 10.1186/s13018-020-01800-9. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jannelli E., Castelli A., Ferranti Calderoni E., Annunziata S., Maccario G., Ivone A., et al. Fractures in patients with COVID-19 infection: early prognosis and management. A case series of 20 patients in a single institution in lombardy, Northern Italy. J Orthop Trauma. 2020;34(10) doi: 10.1097/BOT.0000000000001905. https://journals.lww.com/jorthotrauma/Fulltext/2020/10000/Fractures_in_Patients_With_COVID_19_Infection_.17.aspx [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 35.Farrow L., Hall A.J., Ablett A.D., Myint P.K., Johansen A. The influence of hospital-level variables on hip fracture outcomes. Bone Jt J. 2021 doi: 10.1302/0301-620X.103B10.BJJ-2021-0461.R1. In press. [DOI] [PubMed] [Google Scholar]
- 36.Simpson A.H.R.W., Dall G., Haas J.G. vol. 9. Bone & joint research; 2020. pp. 200–201. (COVID-19: potential transmission through aerosols in surgical procedures and blood products). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clement N.D., Scott C.E.H., Murray J.R.D., Howie C.R., Deehan D.J. The number of patients “worse than death” while waiting for a hip or knee arthroplasty has nearly doubled during the COVID-19 pandemic. Bone Jt J. 2021;103-B(4):672–680. doi: 10.1302/0301-620X.103B.BJJ-2021-0104.R1. [DOI] [PubMed] [Google Scholar]
- 38.Clement N.D., Hall A.J., Kader N., Ollivere B., Oussedik S., Kader D.F., et al. The rate of COVID-19 and associated mortality after elective hip and knee arthroplasty prior to cessation of elective services in UK. Bone Jt J. 2021;103-B(4):681–688. doi: 10.1302/0301-620X.103B.BJJ-2020-1776.R1. [DOI] [PubMed] [Google Scholar]
- 39.Hall A.J., Dunstan E. Day-case total hip arthroplasty: a safe and sustainable approach to improve satisfaction and productivity, and meet the needs of the orthopaedic population. Orthop Trauma. 2022;36(1):14–21. doi: 10.1016/j.mporth.2021.11.003. [DOI] [Google Scholar]
- 40.Hall A.J., Duckworth A.D., MacLullich A.M.J., Farrow L., White T.O., Clement N.D. Meta-audit: a novel approach to healthcare improvement clinical audit and health data. Bone Jt 360. 2021;10(6):42–44. doi: 10.1302/2048-0105.106.360913. [DOI] [Google Scholar]
- 41.Johansen A., Ojeda-Thies C., Poacher A., Hall A.J., Ahern E., Costa M. Developing a Minimum Common Dataset for Hip Fracture Audit to help counties set up national audits that can support international comparisons. Bone and Joint Journal. 2022 doi: 10.1302/0301-620X.104B6.BJJ-2022-0080.R1. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hall A.J., Clement N.D., Impact-Global G., Ojeda-Thies C., MacLullich A.M.J., Toro G., et al. In: BOA Congress. BOA, editor. Orthopaedic Proceedings; 2021. 296 - IMPACT-global: prevalence, clinical predictors and mortality associated with COVID-19 in hip fracture patients. An international multicentre study of 7,090 patients. [Google Scholar]
- 43.Hall A.J., Clement N.D., Impact-Global, Ojeda-Thies C., MacLullich A.M.J., Toro G., et al. In: Scottish Committee for Orthopaedics and Trauma 2021 Meeting, Dunblane. SCOT, editor. The British Editorial Society of Bone & Joint Surgery; 2021. IMPACT-global: prevalence, clinical predictors and mortality associated with COVID-19 in hip fracture patients. An international multicentre study of 7,090 patients; p. 2. [Google Scholar]