Abstract
Objectives
Gegen Qinlian decoction (GQD), a Chinese herbal compound, has been widely used in the treatment of ulcerative colitis (UC) in China. However, evidence from systematic reviews (SRs)/meta-analyses (MAs) of GQD in UC remains highly controversial. To collate, evaluate, and synthesize the current evidence, we carried out this study.
Methods
SRs/MAs of GQD for UC were obtained from eight databases. Methodological Quality of Systematic Reviews 2 (AMSTAR-2) was utilized to appraise the methodological quality, Preferred Reporting Item for Systematic Reviews and Meta-Analyses (PRISMA) for reporting quality, and Grading of Recommendations Assessment, Development, and Evaluation (GRADE) for evidence quality.
Results
Four eligible SRs/MAs were obtained. According to AMSTAR 2, all SRs/MAs were graded as critically low quality. According to PRISMA checklist, all SRs/MAs failed to report the information of protocol and registration. With GRADE, no outcome measure with high-quality evidence was found, and the evidence quality for outcome measures was in the moderate to critically low levels.
Conclusions
GQD with conventional medicine (CM) seems to be more effective in UC than CM alone. This finding provides a new alternative strategy for the treatment of UC. However, owing to the limitations of the evidence provided by the included SRs/MAs, this conclusion must be treated with caution.
1. Introduction
Ulcerative colitis (UC), a major form of inflammatory bowel disease, is characterized by remitting and relapsing mucosal inflammation that begins in the rectum and extends into the colon [1]. Abdominal pain and uncontrolled diarrhea mixed with blood are the main symptoms of UC, and the long-term maintenance of these symptoms causes serious distress to patients [2]. The incidence and prevalence of UC are steadily increasing, with 38 per 100,000 individuals per year in the United States [3] and 35 to 50 per 100,000 inhabitants in Northern Europe [4]. The mechanisms underlying UC are not fully defined; there is increasing evidence that environmental influences, microbiome imbalances, genetic variation, and disturbances in innate and adaptive immune responses are all associated with UC [5]. Although great progress has been made in the treatment of UC, there is still no single ideal therapy [6]. Most new drugs and action protocols could only control part of the UC symptoms with low efficacy [7]. Therefore, the search for effective therapeutic strategies is urgently needed.
As a Chinese herbal compound, Gegen Qinlian decoction (GQD) has been widely used in clinical treatment of UC. GQD contains a variety of effective active ingredients, such as berberine, baicalin, and puerarin [8]. Accumulating evidence suggests that these components are effective in improving UC symptoms in animal models [9]. Based on the theory of evidence-based medicine, systematic reviews (SRs)/meta-analyses (MAs) are considered the gold standard to appraise the benefits of clinical interventions. The initial search revealed several SRs/MAs on the treatment of UC with GQD that have been published. However, their quality varies and the results are highly controversial, which limits the use of evidence. Hence, to collate, evaluate, and integrate the results from these SRs/MAs, we performed this overview [10].
2. Methods
The method used for this overview follows the Cochrane Handbook, and the protocol has been registered on PROSPERO (CRD42021273358).
2.1. Search Strategy
SRs/MAs of GQD for UC were obtained from Cochrane Library, PubMed, Web of Science, Embase, China National Knowledge Infrastructure, Chinese Scientific Journal Database, Wanfang databases, and Chongqing VIP. Search period was from database establishment to October 2021. Ulcerative colitis, Chinese Medicine, Gegen Qinlian decoction, and systematic review were used as search keywords. Table 1 presents a search strategy for PubMed.
Table 1.
Search strategy for the PubMed database.
| Query | Search term |
|---|---|
| #1 | Ulcerative colitis [Mesh] |
| #2 | Ulcerative colitis[Title/Abstract] OR idiopathic proctocolitis[Title/Abstract] OR ulcer colonitis[Title/Abstract] OR colitis gravis[Title/Abstract] OR inflammatory bowel disease[Title/Abstract] |
| #3 | #1 OR #2 |
| #4 | Traditional Chinese Medicine[Mesh] |
| #5 | Chinese Medicine[Title/Abstract] OR Gegen Qinlian[Title/Abstract] OR Gegen Qinlian decoction [Title/Abstract] OR herbal medicine[Title/Abstract] |
| #6 | #4 OR #5 |
| #7 | Meta-analysis as Topic[Mesh] |
| #8 | Systematic review[Title/Abstract] OR meta-analysis[Title/Abstract] OR meta analysis[Title/Abstract] OR meta-analyses OR metaanalysis[Title/Abstract] |
| #9 | #7 OR #8 |
| #10 | #3 AND #6 AND #9 |
2.2. Inclusion and Exclusion Criteria
SRs/MAs that conformed to the following criteria were involved: (1) participants: individuals diagnosed with UC according to appropriate diagnostic criteria; (2) type of design: SRs/MAs only enrolled randomized controlled trials; (3) intervention: GQD or in combination with conventional medication (CM) versus CM; (4) outcomes: effective rate, recurrence rate, level of serum inflammatory factor, ulcerative colitis endoscopic index of severity (UCEIS), and adverse events.
2.3. Data Extraction
Data extraction was performed by two independent reviewers. Literature screening was performed by two parts. Titles and abstracts were read for primary screening firstly, and full texts of initially eligible articles were further read to identify the final articles. The following items were included in the data extraction: (1) general information, (2) characteristics (sample size, intervention), and (3) results (outcomes, relative effect). Any disagreements were resolved by an experienced third reviewer.
2.4. Quality Assessment
Quality assessment was performed by two independent reviewers, and any disagreements were resolved by an experienced third reviewer. Methodological Quality of Systematic Reviews 2 (AMSTAR-2) [11] was utilized to appraise the methodological quality, Preferred Reporting Item for Systematic Reviews and Meta-Analyses (PRISMA) [12] for reporting quality, and Grading of Recommendations Assessment, Development, and Evaluation (GRADE) [13] for evidence quality.
3. Results
3.1. Literature Screening
As shown in Figure 1, 189 citations were obtained from the initial searches, and 50 duplicates were removed. In the first step of screening, 139 irrelevant citations were excluded by reading the titles and abstracts; in the second step of screening, 5 irrelevant citations were excluded by reading the full text. Ultimately, the remaining 4 articles [14–17] met the inclusion criteria for this study.
Figure 1.
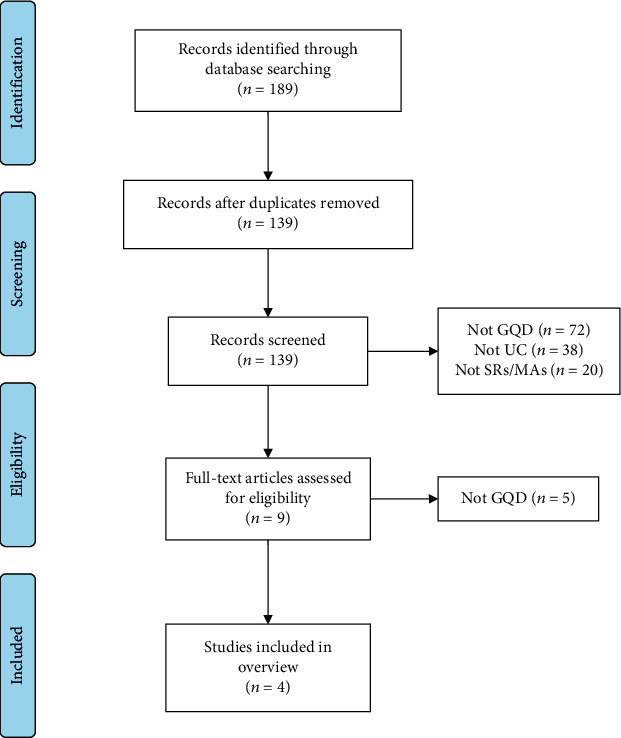
A flowchart of the literature selection process.
3.2. Study Characteristics
All included studies were conducted in China and published in recent years (2019-2021). Simple size ranged from 381 to 2028. GQD plus CM was applied as experimental intervention, while CM alone was applied as the control intervention in all studies. All reviews applied the Cochrane criteria tool for methodological quality assessment of included trails. Further details are presented in Table 2.
Table 2.
Characteristics of the included reviews.
| Author, year | Country | Sample | Treatment intervention | Control intervention | Quality assessment | Diagnostic criteria | Outcomes | Conclusion summary |
|---|---|---|---|---|---|---|---|---|
| Tang et al. [14], 2021 | China | 15 (1323) | GQD + CM | CM | Cochrane criteria | Consensus on the diagnosis and treatment specifications for inflammatory bowel disease in China (2007) | ①, ②, ⑦ | Compared with WMs, GQD or combined with sulfasalazine appeared to be more effective for UC. This finding provides a new therapeutic option for the treatment of UC. Nevertheless, due to the limitations of the included studies, further studies with higher quality and more rigorous design are needed to confirm the results of this study. |
| Xing [15], 2021 | China | 10 (861) | GQD + CM | CM | Cochrane criteria | Consensus on the diagnosis and treatment specifications for inflammatory bowel disease in China (2007) | ⑤, ⑥ | GQD combined with CM can more effectively improve the effective rate of patients with UC and improve the level of serum inflammatory factors. However, further implementation of larger and higher quality clinical trials is necessary to verify the conclusion. |
| Qin et al. [16], 2019 | China | 5 (381) | GQD + CM | CM | Cochrane criteria | Consensus on the treatment of ulcerative colitis in Chinese medicine (2010) | ①, ③, ⑦ | GQD combined with CM can more effectively improve the effective rate of patients with UC. Nevertheless, due to the limitations of this study, high-quality studies are still needed to further strengthen the conclusions of this study. |
| Fan [17], 2019 | China | 22 (2028) | GQD + CM | CM | Cochrane criteria | Third European evidence-based consensus on diagnosis and management of UC | ①, ②, ④, ⑦ | The combination of GQD with CM has potential benefits in the treatment of UC. However, due to the poor methodological quality of the included studies, there is insufficient evidence to draw firm conclusions to support the role of GQD for UC. Large-sample studies with more rigorous designs should be conducted to further establish clinical evidence. |
①: effective rate; ②: recurrence rate; ③: mucosal improvement; ④: UCEIS score; ⑤: level of TNF-α; ⑥: level of IL-6; ⑦: adverse events.
3.3. Quality Assessment of the Included Studies
3.3.1. Methodological Quality
According to the results of AMSTAR-2, all included studies failed to meet the entry requirements and were therefore rated as critically low methodological quality. The main defects were concentrated in item 2 (no study provided protocol and registration), item 4 (only one study provided the search strategy), and item 7 (a list of excluded trails was missing in all studies). Further details are shown in Table 3.
Table 3.
Result of the AMSTAR-2 assessments.
| Reviews | AMSTAR-2 | Quality | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I1 | I2 | I3 | I4 | I5 | I6 | I7 | I8 | I9 | I10 | I11 | I12 | I13 | I14 | I15 | I16 | ||
| Tang et al. [14], 2021 | Y | PY | Y | PY | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | CL |
| Xing [15], 2021 | Y | PY | Y | PY | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | CL |
| Qin et al. [16], 2019 | Y | PY | Y | PY | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | CL |
| Fan [17], 2019 | Y | PY | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | CL |
Y: yes; PY: partial yes; N: no; CL: critically low; L: low; H: high.
3.3.2. Reporting Quality
According to the results of PRISMA, title, abstract, introductions, results, discussion, and funding were completely reported in all studies. However, in Methods, information of protocol and registration was missing in all studies. Furthermore, information of search strategy was missing 75% of the included studies. Further details are shown in Table 4.
Table 4.
Result of the PRISMA assessments.
| Section/topic | Items | Tang et al., 2021 | Xing, 2021 | Qin et al., 2019 | Fan, 2019 | Compliance (%) |
|---|---|---|---|---|---|---|
| Title | (Q1) Title | Y | Y | Y | Y | 100% |
|
| ||||||
| Abstract | (Q2) Structured summary | Y | Y | Y | Y | 100% |
|
| ||||||
| Introduction | (Q3) Rationale | Y | Y | Y | Y | 100% |
| (Q4) Objectives | Y | Y | Y | Y | 100% | |
|
| ||||||
| Methods | (Q5) Protocol and registration | N | N | N | N | 0% |
| (Q6) Eligibility criteria | Y | Y | Y | Y | 100% | |
| (Q7) Information sources | Y | Y | Y | Y | 100% | |
| (Q8) Search | PY | PY | PY | Y | 25% | |
| (Q9) Study selection | Y | Y | Y | Y | 100% | |
| (Q10) Data collection process | Y | Y | Y | Y | 100% | |
| (Q11) Data items | Y | Y | Y | Y | 100% | |
| (Q12) Risk of bias in individual studies | Y | Y | Y | Y | 100% | |
| (Q13) Summary measures | Y | Y | Y | Y | 100% | |
| (Q14) Synthesis of results | Y | Y | Y | Y | 100% | |
| (Q15) Risk of bias across studies | Y | Y | Y | Y | 100% | |
| (Q16) Additional analyses | Y | Y | Y | Y | 100% | |
|
| ||||||
| Results | (Q17) Study selection | Y | Y | Y | Y | 100% |
| (Q18) Study characteristics | Y | Y | Y | Y | 100% | |
| (Q19) Risk of bias within studies | Y | Y | Y | Y | 100% | |
| (Q20) Results of individual studies | Y | Y | Y | Y | 100% | |
| (Q21) Synthesis of results | Y | Y | Y | Y | 100% | |
| (Q22) Risk of bias across studies | Y | Y | Y | Y | 100% | |
| (Q23) Additional analysis | Y | Y | Y | Y | 100% | |
|
| ||||||
| Discussion | (Q24) Summary of evidence | Y | Y | Y | Y | 100% |
| (Q25) Limitations | Y | Y | Y | Y | 100% | |
| (Q26) Conclusions | Y | Y | Y | Y | 100% | |
|
| ||||||
| Funding | (Q27) Funding | Y | Y | Y | Y | 100% |
Y: yes; PY: partial yes; N: no.
3.3.3. Evidence Quality
According to GRADE, 12 outcome indicators were appraised, of which 5 were of moderate quality, 5 were of low quality, and 2 were of critical low quality. The factors affecting the evidence quality were risk of bias, imprecision, publication bias, and inconsistency. Further details are shown in Table 5.
Table 5.
Certainty of evidence quality.
| Author, year | Outcomes | Limitations | Inconsistency | Indirectness | Imprecision | Publication bias | Relative effect (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|
| Tang et al. [14], 2021 | Effective rate | -1 | 0 | 0 | 0 | 0 | OR 3.77 (2.61, 5.45) | M |
| Adverse events | -1 | 0 | 0 | 0 | 0 | OR 0.43 (0.21, 0.90) | M | |
| Recurrence rate | -1 | 0 | 0 | -1 | 0 | OR 0.16 (0.05, 0.50) | L | |
|
| ||||||||
| Xing [15], 2021 | Level of TNF-α | -1 | 0 | 0 | -1 | -1 | SMD -0.81 (-1.07, -0.54) | CL |
| Level of IL-6 | -1 | -1 | 0 | -1 | -1 | SMD -1.20 (-2.00, -0.41) | CL | |
|
| ||||||||
| Qin et al. [16], 2019 | Effective rate | -1 | 0 | 0 | 0 | 0 | RR 1.18 (1.06, 1.30) | M |
| Adverse events | -1 | 0 | 0 | -1 | 0 | RR O.11 (0.041, 1.92) | L | |
| Mucosal improvement | -1 | 0 | 0 | -1 | 0 | RR 1.13 (0.95, 1.88) | L | |
|
| ||||||||
| Fan [17], 2019 | Effective rate | -1 | 0 | 0 | 0 | 0 | RR 1.21 (1.16, 1.27) | M |
| Recurrence rate | -1 | 0 | 0 | -1 | 0 | RR 0.18 (0.06, 0.61) | L | |
| Adverse events | -1 | 0 | 0 | 0 | 0 | RR 0.37 (0.15, 0.90) | M | |
| UCEIS score | -1 | -1 | 0 | 0 | 0 | MD -0.63 (-1.26, -0.01) | L | |
3.4. Descriptive Analysis
3.4.1. Description of Efficacy
Relative effects associated with efficacy of GQD in UC are shown in Table 5. Effective rate was utilized in three studies [14, 16, 17] to evaluate the effect of GQD for UC; the pooled results suggested that the GQD group was super to CM. Recurrence rate was utilized in two studies [14, 17]; the pooled results suggested that the GQD group was super to CM. Levels of TNF-α and IL-6 were reported in one review [15]; results revealed that GQD plus CM had an advantage over the CM group. One review [16] compared mucosal improvement in the GQD and CM groups; the results suggested no statistical difference between these two groups. One review [17] reported the results of the UCEIS score; the pooled results suggested that the GQD group was super to the CM group.
3.4.2. Description of Safety
Three reviews [14, 16, 17] reported on the outcome of adverse events. Two of which [14, 17] showed that the use of GQD in combination with CM reduced the incidence of adverse events, while the other [16] showed no statistical difference in the incidence of adverse events between the combination group and the CM group, which may be attributed to the small sample size.
4. Discussion
SRs/MAs are considered the gold standard for evaluating health interventions. However, evidence provided by high-quality SRs/MAs is credible, while low-quality evidence may mislead clinical decisions [18]. Thus, there is a gap between the use of evidence and its practical implementation in real-world dynamics. In response to this issue, the method of overview of SRs/MA is brought up by evidence-based medicine experts [19], with the purposes of evaluation and synthesis of evidence on the same topic [19]. In China, GQD has been widely used for the clinical treatment of UC. However, the published SRs/MAs emphasize that this therapy is still not fully implemented in a real-world context. To collate, appraise, and synthesize the current evidence, we therefore carried out this study.
In this study, methodological quality, reporting quality, and evidence quality of the included reviews were appraised. Our results suggest that the use of GQD in combination with CM is beneficial in patients with UC, with improved effective rate and UCEIS scores and reduced relapse rates, serum inflammatory markers, and adverse events. However, these findings must be considered cautiously owing to the limitations of the enrolled reviews. Notably, almost all included SRs/MAs suggested that GQD plus CM appeared to have a significant benefit in the treatment of UC; nevertheless, most authors did not wish to draw firm conclusions owing to the small sample size or low methodological quality of the included trials. With AMSTAR-2 results, neither I2 (protocol and registration) nor I7 (list of excluded trials) were followed, which is likely to increase the risk of bias and weaken the reliability of the results. With PRISMA results, information of I5 (protocol and registration) and I8 (search) was severely missing, which seriously undermines the rigor of SRs/MAs. For GRADE results, no high-quality evidence was found, suggesting that the results from the included reviews may differ from the real results and cannot provide reliable available evidence. Although quality from the included SRs/MAs is generally low and defects are frequent, this also means that there is much room for progress in the SR/MA process. Our study highlights areas of methodology that need to be improved, which have directionally guiding value for rapidly improving the quality of evidence in the future.
GQD consists of four Chinese herbal medicines, Radix Puerariae, licorice, Coptidis Rhizoma, and Scutellariae Radix. Based on traditional Chinese medicine theory, “dampness-heat” is the core of UC. Intestinal damp-heat can stimulate qi stagnation and blood stasis to damage the intestinal mucosa, so that patients will have diarrhea, pus, and bloody stools [20]. Therefore, the use of methods to remove damp-heat may contribute to the healing of the diseased intestinal mucosa [21]. In addition, experimental studies have also preliminarily revealed the pharmacological effects of GQD in the treatment of UC. It has been reported that GQD can inhibit Toll-like receptor 4/nuclear factor-kB signaling, which in turn relieves UC symptoms and repairs the intestinal epithelial barrier [22]. Moreover, GQD was observed to regulate Th17/Treg cell homeostasis by inhibiting IL-6/JAK2/STAT3 signaling in DSS-induced UC mice, which in turn alleviated symptoms [9]. Network pharmacology has also found that GQD can reduce the degree of inflammation in ulcerative colitis by downregulating the EGFR/PI3K/AKT signaling pathway and inhibiting the release of proinflammatory cytokines [23]. Baicalin, puerarin, baicalin, berberine, and glycyrrhizic acid, as the main components of GQD, have also been found to have antiviral and antidiarrheal effects and may be beneficial in improving the symptoms of UC [24]. Additionally, the combination of Radix Puerariae, Radix Glycyrrhizae, and Rhizoma Coptidis has been observed to drive the repair of colonic mucosa according to the internal meridian [25]. Given the current findings, the mechanism of GQD in UC involves multiple components and multiple targets and may be a promising therapeutic strategy.
This is the first study to evaluate and synthesize the evidence of GQD in combination with CM for UC, which may provide evidence reference for the treatment decision of UC. Moreover, our study highlights areas of methodology that need to be improved, which may help guide future high-quality SRs/MAs. Nevertheless, limitations should be acknowledged, as quality evaluation is based on subjective assessment tools, and the assessment results may vary from reviewer to reviewer.
5. Conclusion
GQD with conventional medicine (CM) seems to be more effective in UC than CM alone. This finding provides a new alternative strategy for the treatment of UC. However, owing to the limitations of the evidence provided by the included SRs/MAs, this conclusion must be treated with caution.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81830118), China Academy of Chinese Medical Sciences Innovation Fund (No. CI 2021A01012), China Academy of Chinese Medical Sciences Excellent Young Talent Cultivation Fund (No. ZZ 15-YQ-002), and Administration of Traditional Chinese Medicine Digestive Refractory Disease Inheritance and Innovation Team Project (No. ZYYCXTD-C-202010).
Abbreviations
- GQD:
Gegen Qinlian decoction
- UC:
Ulcerative colitis (UC)
- SR:
Systematic review
- MA:
Meta-analysis
- AMSTAR-2:
Assessing the Methodological Quality of Systematic Reviews 2
- UCEIS:
Ulcerative colitis endoscopic index of severity
- GRADE:
Grading of Recommendations, Assessment, Development, and Evaluation
- PRISMA:
Preferred Reporting Item for Systematic Reviews and Meta-Analyses
- CM:
Conventional medicine.
Contributor Information
Fengyun Wang, Email: wfy811@163.com.
Xudong Tang, Email: txdly@sina.com.
Data Availability
All analyses were based on previously published studies; thus, no data is required.
Conflicts of Interest
All authors declare that there is no conflict of interest.
Authors' Contributions
Jinke Huang and Jiaqi Zhang initiated the study design. Jinke Huang drafted the manuscript. Yifan Wang, Jing Ma, Xuefei Yang, Mi Lv, Xiaoxue Guo, Jinxin Ma, and Yijun Zheng helped with implementation to this work. Fengyun Wang and Xudong Tang contributed to methodology, review, and editing. All authors read and approved the final manuscript. Jinke Huang and Jiaqi Zhang are co-first authors.
References
- 1.Ungaro R., Mehandru S., Allen P. B., Peyrin-Biroulet L., Colombel J. F. Ulcerative colitis. Lancet . 2017;389(10080):1756–1770. doi: 10.1016/S0140-6736(16)32126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armuzzi A., Liguori G. Quality of life in patients with moderate to severe ulcerative colitis and the impact of treatment: a narrative review. Digestive and Liver Disease . 2021;53(7):803–808. doi: 10.1016/j.dld.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Kappelman M. D., Rifas–Shiman S. L., Kleinman K., et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clinical Gastroenterology and Hepatology . 2007;5:1424–1429. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Annese V., Latiano A., Andriulli A. Genetics of inflammatory bowel disease: the beginning of the end or the end of the beginning? Digestive and Liver Disease . 2003;35(6):442–449. doi: 10.1016/S1590-8658(03)00213-5. [DOI] [PubMed] [Google Scholar]
- 5.Schirbel A., Fiocchi C. Inflammatory bowel disease: established and evolving considerations on its etiopathogenesis and therapy. Journal of Digestive Diseases . 2010;11(5):266–276. doi: 10.1111/j.1751-2980.2010.00449.x. [DOI] [PubMed] [Google Scholar]
- 6.Sicilia B., García-López S., González-Lama Y., Zabana Y., Hinojosa J., Gomollón F. GETECCU 2020 guidelines for the treatment of ulcerative colitis. Developed using the GRADE approach. Gastroenterología y Hepatología . 2020;43:1–57. doi: 10.1016/j.gastrohep.2020.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Kucharzik T., Koletzko S., Kannengießer K., Dignaß A. Ulcerative colitis-diagnostic and therapeutic algorithms. Deutsches Ärzteblatt International . 2020;117:564–574. doi: 10.3238/arztebl.2020.0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei M., Li H., Li Q., et al. Based on network pharmacology to explore the molecular targets and mechanisms of Gegen Qinlian decoction for the treatment of ulcerative colitis. BioMed Research International . 2020;2020:18. doi: 10.1155/2020/5217405.5217405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Y., Luan H., Jiang H., et al. Gegen Qinlian decoction relieved DSS-induced ulcerative colitis in mice by modulating Th17/Treg cell homeostasis via suppressing IL-6/JAK2/STAT3 signaling. Phytomedicine . 2021;84, article 153519 doi: 10.1016/j.phymed.2021.153519. [DOI] [PubMed] [Google Scholar]
- 10.Hunt H., Pollock A., Campbell P., Estcourt L., Brunton G. An introduction to overviews of reviews: planning a relevant research question and objective for an overview. Systematic Reviews . 2018;7(1):p. 39. doi: 10.1186/s13643-018-0695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shea B. J., Reeves B. C., Wells G., et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ . 2017;358, article j4008 doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liberati A., Altman D. G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Journal of Clinical Epidemiology . 2009;339, article b2700 doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The GRADE Working Group, Atkins D., Eccles M., et al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches the GRADE Working Group. BMC Health Services Research . 2004;4(1):p. 38. doi: 10.1186/1472-6963-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang S. N., Zhao D. S., Cao S. Q., Liu Z. J., Li Y. Y. Meta-analysis on the clinical efficacy and safety of Gegen Qinlian decoction combined with western medicine in the treatment of ulcerative colitis. Hunan Journal of Traditional Chinese Medicine . 2021;37(8):142–146. [Google Scholar]
- 15.Xing X. X. Evidence-based medicine analysis of classic prescription in treating ulcerative colitis patients with dampness-heat syndrome . Beijing University of Chinese Medicine; 2021. [Google Scholar]
- 16.Qin K. J., Cui C., Huang X. S., Liang R. Y., Lou T. Z., Wu J. A meta-analysis of Gegen-Qinlian decoction for ulcerative colitis. International Journal of Traditional Chinese Medicine . 2019;1:99–103. [Google Scholar]
- 17.Fan Y., Yi W., Huang H., Mei Z., Feng Z. Efficacy of herbal medicine (Gegen Qinlian Decoction) on ulcerative colitis. Medicine . 2019;98(52, article e18512) doi: 10.1097/MD.0000000000018512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang J., Qin X., Shen M., Xu Y., Huang Y. The effects of Tai Chi exercise among adults with chronic heart failure: an overview of systematic review and meta-analysis. Frontiers in Cardiovascular Medicine . 2021;8, article 589267 doi: 10.3389/fcvm.2021.589267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bougioukas K. I., Liakos A., Tsapas A., Ntzani E., Haidich A. B. Preferred reporting items for overviews of systematic reviews including harms checklist: a pilot tool to be used for balanced reporting of benefits and harms. Journal of Clinical Epidemiology . 2018;93:9–24. doi: 10.1016/j.jclinepi.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Wu P. T. What should be kept in mind in the TCM differential treatment for ulcerative colitis? Journal of Traditional Chinese Medicine . 2008;28:308–309. doi: 10.1016/s0254-6272(09)60018-2. [DOI] [PubMed] [Google Scholar]
- 21.Ye B., Shen H., Lu Y., Wang Y. Q. Clinical Observations on 100 Cases of Ulcerative Colitis Treated with the Method of Clearing Away Heat, Expelling Dampness, Promoting Blood Circulation and Healing Ulcer. Journal of Traditional Chinese Medicine . 2010;30(2):98–102. doi: 10.1016/S0254-6272(10)60022-2. [DOI] [PubMed] [Google Scholar]
- 22.Li R., Chen Y., Shi M., et al. Gegen Qinlian decoction alleviates experimental colitis via suppressing TLR4/NF-κB signaling and enhancing antioxidant effect. Phytomedicine . 2016;23(10):1012–1020. doi: 10.1016/j.phymed.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Liu X., Fan Y., Du L., Mei Z., Fu Y. In silico and in vivo studies on the mechanisms of Chinese medicine formula (Gegen Qinlian Decoction) in the treatment of ulcerative colitis. Frontiers in Pharmacology . 2021;12, article 665102 doi: 10.3389/fphar.2021.665102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu B., Zhang G., Ji Y. Active components alignment of _Gegenqinlian_ decoction protects ulcerative colitis by attenuating inflammatory and oxidative stress. Journal of Ethnopharmacology . 2015;162:253–260. doi: 10.1016/j.jep.2014.12.042. [DOI] [PubMed] [Google Scholar]
- 25.Wen Y. Effects of Gegen Qinlian decoction and its disassembled on P selectin and colonic mucosal ultrastructure in rats ulcerative colitis. Lishizhen Medicine and Materia Medica Research . 2012;23:635–636. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All analyses were based on previously published studies; thus, no data is required.


