Abstract
Objectives:
The objective of this study was to understand the functional impact of vestibular dysfunction on balance control in children with hearing loss. The vestibular system is an important contributor to maintaining balance. In adults, vestibular dysfunction is known to lead to unsteadiness and falls. Considerably less is known about the effects of vestibular dysfunction in children with hearing loss.
Design:
We conducted a systematic review in concordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines. We included articles on children with hearing loss who underwent vestibular and balance testing. The Downs and Black checklist was used to assess the risk of bias.
Results:
A total of 20 articles were included in this systematic review, of which, 17 reported an association between vestibular dysfunction and balance abnormalities in children with hearing loss. Bias (as measured by the Downs and Black Checklist) was a concern, as most studies were non-blinded cohort studies or case series selected through convenience sampling.
Conclusions:
Research to date has predominantly found that children with concomitant hearing loss and vestibular impairment tend to perform more poorly on balance measures than either children with hearing loss and normal vestibular function or children with both normal hearing and normal vestibular function. A standardized approach to assessing both vestibular function and balance would better characterize the impact of vestibular dysfunction in children with hearing loss at the population level.
Introduction:
Clinical services for vestibular dysfunction in the pediatric population are limited. Historically, children with vestibular dysfunction have been under-recognized and underserved because testing and treatment methodologies geared toward adults are not easily modified for children. However, this is changing. Emerging evidence suggests that 15–80% of children with hearing loss have concomitant vestibular impairment (Janky et al., 2018; Kimura et al., 2018; Kotait et al., 2019; Rine et al., 2000; Singh et al., 2012; Verbecque et al., 2017). While there is considerable variability in the reported prevalence due to methodological differences across studies, the rates of concomitant dysfunction may suggest that pediatric vestibular dysfunction may be an under-recognized clinical condition among children with hearing loss.
The lack of recognition for pediatric vestibular dysfunction may have negative consequences for balance and postural control as well as other downstream impacts. In adults, vestibular dysfunction often leads to complaints of unsteadiness and falls, in addition to the traditional reports of vertigo (Bamiou et al., 1999; Fina et al., 2003; Hillier & McDonnell, 2007; Popkirov et al., 2018). Most adults with peripheral vestibular disorders are able to compensate over time in order to reduce symptoms of vertigo and unsteadiness, and this process can be fostered by vestibular rehabilitation therapy when needed (Deveze et al., 2014; Dutia, 2010; Lacour et al., 2016; Strupp et al., 1998). Considerably less is known about the symptoms and functional impacts of vestibular dysfunction in children, especially congenital or early-onset vestibular dysfunction. Vestibular dysfunction in children has been reported to lead to developmental delays (Kimura et al., 2018; Masuda & Kaga, 2014), dizziness (Gioacchini et al., 2014; Wiener-Vacher et al., 2018), and even reading difficulties (Braswell & Rine, 2006; Sartori Franco & Panhoca, 2007). Some researchers hypothesize that there is a higher rate of cochlear implant failure in children with concomitant vestibular loss due to falls (Hänsel et al., 2018; Wolter et al., 2015). However, it remains unclear how well children compensate for vestibular dysfunction, which cases will require vestibular rehabilitation therapy, and the time window within which that intervention is effective.
There are many possible explanations for this lack of knowledge. One of the most challenging aspects of studying pediatric vestibular dysfunction stems from an imperfect understanding of how vestibular dysfunction presents in children. Young children may not be able to communicate symptoms of dizziness, vertigo, or imbalance, or may not even recognize them as abnormal. Similarly, parents and pediatricians also may recognize overall posturomotor developmental problems, but not necessarily attribute them to vestibular problems. Additionally, many of these symptoms are suggestive of but not specific to vestibular dysfunction because normal balance requires sensory integration from multiple sources (Wiener-Vacher, 2008). Our diagnostic capability for pediatric vestibular dysfunction is likely limited by access to routine vestibular laboratory testing which requires specialized equipment and training to perform. However, clinical balance testing can be conducted in a wider range of clinical settings and may be a suitable screening tool for vestibular dysfunction to further guide whether additional testing and/or intervention is warranted. Still, the extent to which vestibular problems in children with hearing loss manifest as balance problems is unclear. To better inform clinical practice, this systematic review sought to describe the impact of vestibular dysfunction on clinical balance performance in children with hearing loss.
Materials and Methods:
Systematic Literature Search
We conducted a systematic review in concordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (Moher et al., 2009). We searched the following databases: MEDLINE (PubMed), Embase (Elsevier), Web of Science (Clarivate), and the Cumulative Index of Nursing and Allied Health Literature (CINAHL) (EBSCO). The search was conducted by a professional medical librarian and included a mix of keywords and subject headings representing ‘hearing loss or impairment’ and ‘function or dysfunction’, as well as equipment-based and clinical/bedside ‘vestibular testing’ methods. No restrictions were placed by date. Results were filtered to include only pediatric studies. Editorials, letters, case reports, and comments were excluded, as were animal-only studies. Case reports were defined as single patient studies. We included case series of at least five patients in this systematic review. Reproducible search strategies in PubMed can be found online (Supplemental Digital Content 1), with analogous search strategies used in other databases.
All citations were imported into the online screening platform Covidence (Cochrane) via EndNote (Clarivate). Duplicate citations were automatically identified and removed by Covidence. Each article was screened by two reviewers who independently screened each reference by title and abstract and excluded irrelevant articles that did not align with screening criteria below. If studies did not have their abstract available, then they were moved to full text review. The full-text of articles were assessed by two independent reviewers. All discrepancies regarding abstract eligibility and full text were adjudicated by a third reviewer. Full-text articles meeting our initial criteria of having both measures of vestibular function and clinical balance assessments in children with hearing loss were identified. Article selection is presented by flowchart as per PRISMA guidelines (Figure 1).
Figure 1:
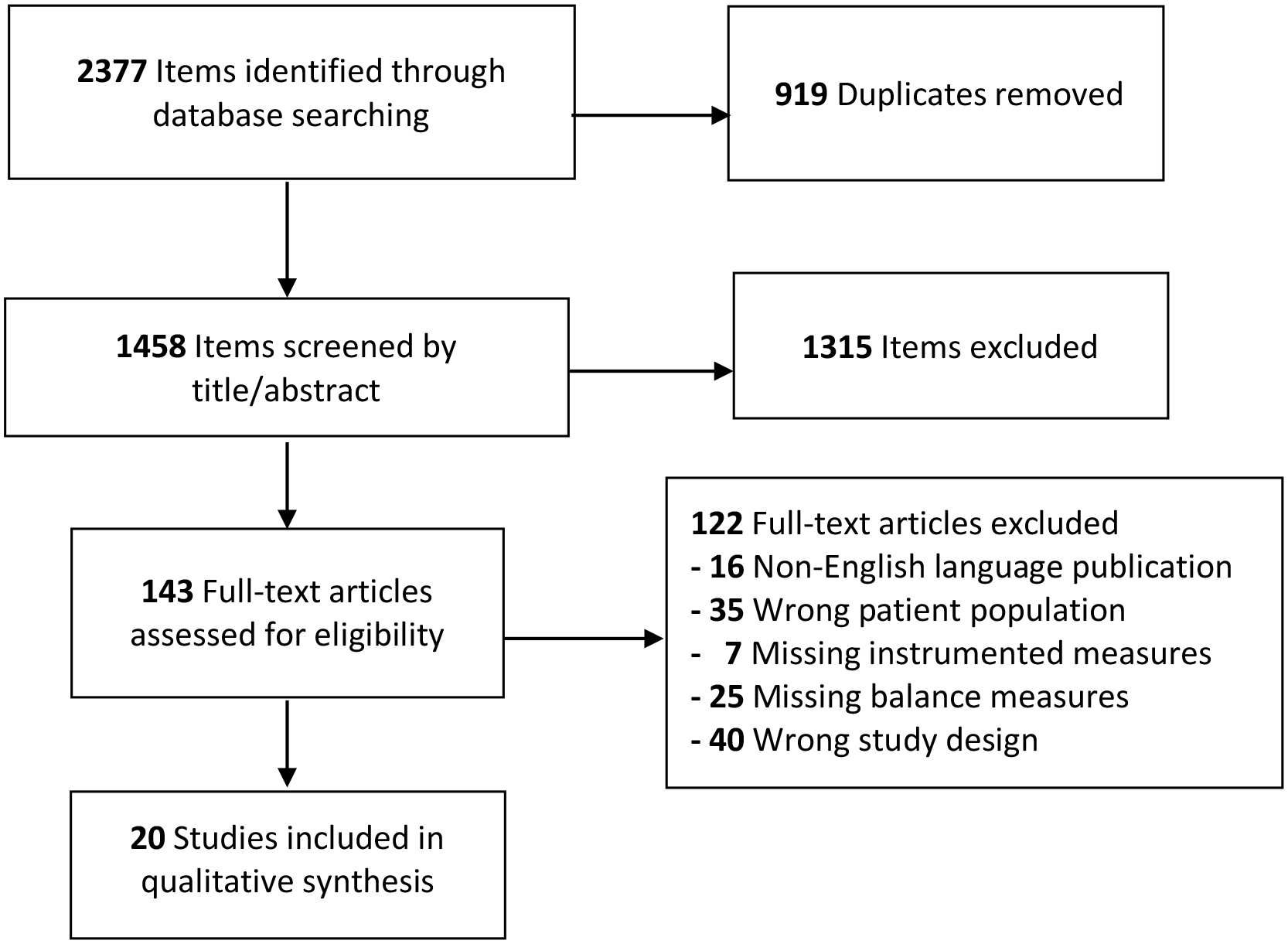
Study Inclusion Flow Chart
Inclusion/Exclusion Criteria
In order to be included in this systematic review, articles had to meet three inclusion criteria.
The patient population had to include children with sensorineural hearing loss. We defined children to be individuals up to and including the age of 21 years. If patients older than 21 years were included in a study, then the data had to be clearly distinguishable by age. The original article had to include participants with hearing loss, or the presence of audiologic testing. Participants may have been pre- or post-cochlear implantation (if applicable) and cochlear implant status was not used an exclusionary criterion.
All children had to have at least one laboratory test of vestibular function. Possible vestibular tests included electronystagmography (ENG), videonystagmography (VNG), caloric tests, video head impulse test (vHIT), vestibular evoked myogenic potential (VEMP), subjective visual vertical (SVV), instrumented dynamic visual acuity (DVA), and/or rotary chair (RC).
Children had to have an assessment of balance. This could be through an individual clinical test such as the Modified Clinical Test of Sensory Interaction in Balance (mCTSIB), or through a series of tests like the, Bruininks-Oseretsky Test of Motor Proficiency (BOT), or Movement Assessment Battery for Children (M ABC), or through posturography testing.
Articles were omitted if the patient population was limited exclusively to children with specific syndromes/diagnoses that have multiple sensory system involvement (e.g., Usher syndrome) or with known concomitant neurological problems. Articles were also excluded if written in a language other than English without a translation available.
Data Extraction/Risk of Bias Assessment
Data extracted included study characteristics, population, and results from each study. For study characteristics, these included design, country in which the study was conducted, and the overall goals of the study. Study population details included participant age, sex, number of participants, vestibular and balance tests conducted, and further information about the hearing loss of the participants. We extracted details about hearing loss including type, laterality, severity, etiology, and method of measurement. Information about control participants and inclusion of participants with cochlear implants was also extracted. Study results included laboratory vestibular testing methods and results as well as clinical balance testing methods and results. Greater than 80% of the information was extracted by two reviewers independently, with conflicts resolved between themselves through discussion, and a third reviewer available for adjudication if consensus could not be reached.
The Downs and Black (1998) checklist was used to assess the risk of bias of the articles. This 27-item checklist on methodological quality can be used for both randomized and non-randomized studies. It contains questions focusing on reporting, external validity, bias, confounding, and power. All studies were independently assessed by two reviewers, again with conflicts resolved between themselves. Reproducible strategies for risk of bias assessment can be found online (Supplemental Digital Content 2).
Results:
Literature Search Results
The database search was executed in March 2020 by a professional medical librarian (author JW). This yielded 2377 studies across databases. 919 duplicates were removed, leaving 1458 articles to be screened by title and abstract. A total of 143 full-text studies were subsequently reviewed for eligibility. Out of the 143 articles, 20 were identified as meeting inclusion/exclusion criteria, from which data were extracted. The PRISMA study inclusion chart is presented as Figure 1.
Study Characteristics
Of the 20 studies, four were published in the 1980s, (Crowe & Horak, 1988; Horak et al., 1988; Nishida et al., 1983; Potter & Silverman, 1984), zero in the 1990s, six between 2000 and 2009 (Abadie et al., 2000; Cushing et al., 2009; Cushing et al., 2008; Licameli et al., 2009; R. M. Rine et al., 2004; Shall, 2009) and ten between 2010 and 2020 (Apeksha et al., 2020; Birdane et al., 2016; Christy et al., 2014; De Kegel et al., 2012; De Kegel et al., 2015; Ionescu et al., 2020; Jafari & Asad Malayeri, 2011; Janky & Givens, 2015; Maes et al., 2014; Wolter et al., 2015). As seen in Table 1 many studies selected participants via convenience sampling. Most, although not all, of the studies had the goal of assessing the vestibular and/or balance or motor functions in children with sensorineural hearing loss.
Table 1:
Characteristics of studies included in the systematic review
| Study | Design | Patient Population | # Patients (Male) | Age, Mean (SD) | Controls | CI Patients Included (Y/N) | Hearing Loss Description & Range | Vestibular Measures | Balance Measures |
|---|---|---|---|---|---|---|---|---|---|
| Abadie et al., 2000 | Cohort | CHARGE syndrome | 17 (11) | none | none | N | none | RC | Examination & parental interview, Brunet-Lesine scale |
| Apeksha et al., 2020 | Cross-sectional | SNHL of various etiologies | 15 (8) | 7.8 years | 15, age- and sex-matched | N | Severe to profound | cVEMP, oVEMP | Romberg, Fukuda, tandem gait |
| Birdane et al., 2016 | Cohort | Unilateral HL of various etiologies | 33 (14) | 12.2 (3.7) years | 25, age-matched | N | Severe to profound SNHL | cVEMP, ENG | Romberg, Unterberger |
| Christy et al., 2014 | Case series | SNHL of various etiologies | 20 (14) | 8.9 (1.8) years | 23 NH children | Y, evaluated post-CI | Moderate to profound SNHL | cVEMP, RC, DVA, SOT, SVV | mCTSIB |
| Crowe et al., 1988 | Cohort | SNHL of various etiologies | 29 (11) | 9.8 years | 13, age-matched | N | > 30 dB HL in both ears | RC | BOT |
| Cushing & Horak, 2008 | Prospective cross-sectional with repeated measures | Children with CI | 40 (22) | 9.6 (4.6) years | None | Y, evaluated post-CI | Profound SNHL | Calorics, RC, cVEMP | BOT-2 |
| Cushing et al., 2009 | Prospective cohort | SNHL s/p bacterial meningitis | 9 (2) | 10.1 (4.6) years | None | Y, evaluated post-CI | Profound SNHL | Calorics, RC, cVEMP | BOT-2 |
| De Kegel et al., 2012 | Cross-sectional | Unilateral (n=9) or bilateral (n=39) HL with various etiologies | 48 (25) | 7.6 (2.5) years | 51 NH children | Y, evaluated post-CI | >40 dB HL | cVEMP, RC | M ABC-2, mCTSIB, balance beam walking, 1 leg-hopping |
| De Kegel et al., 2015 | Prospective cohort | CI candidates with various etiologies | 48 (23) | Tested at 18 and 24 months2 | 25 of 48 who were not CI candidates | At least 40 dB HL in one ear | Prior to study | cVEMP | Ghent Developmental Balance Test |
| Horak et al., 1988 | Cohort | HL acquired in first two years with various etiologies | 30 | 9.2 (1.8) years | 54 NH children, 15 with learning disabilities | N | >30 dB HL | RC | BOT |
| Ionescu et al., 2020 | Case series | Non-syndromic SNHL | 76 (48) | 9.3 (3.4) years | None | Not reported | Mild to profound SNHL | cVEMP | M ABC-2 |
| Jafari & Asad Malayeri, 2011 | Cohort | Congenital HL | 30 (16) | 6.93 (1.11) years | 30 age- and sex-matched children with NH | Not reported | Profound SNHL | cVEMP | BOT-2 |
| Janky & Givens, 2015 | Cohort | Children with CI | 11(7) | 13 years | 12, NH children; 15 adults | Y, evaluated post-CI | not described, all had CI | oVEMP, cVEMP, vHIT, RC | Dynamic gait index, single-leg stance (open/close), SOT |
| Licameli et al., 2009 | Cohort | Children with CI | 61 total Group 1: 42 (18), Group 2: 19 (6) | 9 (group1) 8 (group 2) | n/a | Y – either only post-CI (group 1) or both pre- and post-CI (group 2) | not described, all had CI | RC, VEMP | CDP |
| Maes et al., 2014 | Prospective cohort | SNHL of various etiology | 24 (11) | Group 1: 7 years 5 mo Group 2: 7 years 6 mo | 12 age & sex matched children with NH and normal vestibular function | Y, evaluated post-CI | Moderate to profound SNHL | RC, VEMP | Balance beam walking, one leg hopping, one leg stance with eyes closed |
| Nishida et al., 1983 | Case series | Congenital rubella syndrome | 80 (41) | 12 years | None | Not reported | Moderate to profound SNHL | Calorics | Righting Reflex1 |
| Potter & Silverman, 1984 | Cohort | Children at a school for Deaf | 34 (16) | 6.1 years (range 5.0–8.1 years) | 226 age-matched | Not reported | HL in better ear ranged from 55 to 120 dB HL | RC (SCPNT) | Balance on one foot |
| Rine et al., 2004 | Cohort | SNHL of various etiology | 21 (9) | 67.54 mo | 11, matched by age and age equiva-lent score on motor development testing | N | Bilateral moderate to profound HL | RC, posturography | Posturography and motor development sub-category |
| Shall et al., 2009 | Case series | SNHL of various etiology | 33 (20) | 66.1 (12.3) mo | None | Bilateral severe or profound SNHL | Prior to study | cVEMP | M ABC |
| Wolter et al., 2015 | Retrospective cohort | Children with and without CI failure | 187 (35 with CI failure, 130 with CI and no CI failure) | none | None | Y, evaluated post-CI | not described, all had CI | Calorics, vHIT, cVEMP | BOT-2 |
Note: BOT = Bruininks-Oseretsky Test of Motor Proficiency; CHARGE = coloboma, heart defects, atresia choanae, retardation, genital abnormalities, and ear abnormalities); CDP = computerized dynamic posturography; CI = cochlear implant; cVEMP = cervical vestibular evoked myogenic potential; dB = decibels; DVA = dynamic visual acuity; ENG = electronystagmography; HL = hearing loss; IQ = intelligence quotient; M ABC = Movement Assessment Battery for Children; mCTSIB = Modified Clinical Test of Sensory Interaction in Balance; NH=normal hearing, oVEMP = ocular vestibular evoked myogenic potential; RC = rotary chair; SCPNT = Southern California Postrotary Nystagmus Test; SD = standard deviation; SNHL = sensorineural hearing loss; SOT = sensory organization test;
SVV = subjective visual vertical; vHIT = video head impulse test
Righting Reflex abnormal defined an inability to remain erect when standing on both feet with eyes closed or inability to stand on one foot with eyes open or on one foot on either side with eyes closed
Data in Table 1 as extracted, not necessarily as presented in original study. While testing was completed around 6, 12, 18, and 24 months, we only extracted data from the 18- and 24-month test point, as this was when the Ghent Developmental Balance Test was utilized
Four studies focused on specific etiologies for their hearing loss including CHARGE (coloboma, heart defects, atresia choanae, retardation, genital abnormalities, and ear abnormalities) syndrome (Abadie et al., 2000), bacterial meningitis (Cushing et al., 2009), and congenital rubella syndrome (Nishida et al 1983). Two studies exclusively examined children with unilateral hearing loss of varying etiologies (Birdane et al., 2016; Cushing et al., 2008). A total of four studies were case series of at least five participants, three was cross-sectional, one was case-control study, and thirteen were cohort studies (Table 1). Sample sizes for the studies ranged from 9 to 187 participants. Fourteen of the twenty studies included control participants, mostly age-matched or age- and gender-matched children without hearing loss (Table 1).
All children included in this systematic review had hearing loss. While most participants had moderate to profound sensorineural hearing loss, hearing loss severity ranged from mild to profound. Hearing loss, when measured, was examined using age-appropriate testing methods.
Vestibular Function and Balance Testing
Vestibular testing was most often evaluated using the cervical vestibular evoked myogenic potential (cVEMP) test procedure (Apeksha et al., 2020; Birdane et al., 2016; De Kegel et al., 2015; Ionescu et al., 2020; Jafari & Asad Malayeri, 2011). Electronystagmography, (Birdane et al., 2016), caloric (Nishida et al., 1983), and rotary chair (RC) (Crowe & Horak, 1988; Horak et al., 1988; Potter & Silverman, 1984) testing were other methods utilized (Table 1). Ocular VEMP (oVEMP) test procedures were performed in limited studies (Apeksha et al., 2020; Janky & Givens, 2015).
A wide variety of clinical balance measures also were utilized, including tests such as the Romberg (Apeksha et al., 2020; Birdane et al., 2016) or the Fukuda or Unterberger stepping test, (Apeksha et al., 2020; Birdane et al., 2016) standardized clinical measures of balance such as the BOT (Horak et al., 1988) or M ABC, (Ionescu et al., 2020) or other clinical balance measures such as tandem gait (Apeksha et al., 2020) or standing on one foot (Jafari & Asad Malayeri, 2011; Potter & Silverman, 1984) (Table 1).
A summary of the numbers of normal and abnormal vestibular testing and balance results is presented in Supplemental Digital Content 3. This table also contains a qualitative summary the findings from each study regarding the association between vestibular testing results and balance abilities. As seen in Supplemental Digital Content 3 and Tables 2, most studies reported that those participants who demonstrated abnormal vestibular test results were more likely to perform poorly on balance testing (Abadie et al., 2000; Apeksha et al., 2020; Crowe & Horak, 1988; Cushing et al., 2009; Cushing et al., 2008; De Kegel et al., 2012; De Kegel et al., 2015; Horak et al., 1988; Ionescu et al., 2020; Jafari & Asad Malayeri, 2011; Janky & Givens, 2015; Maes et al., 2014; Nishida et al., 1983; Rose Marie Rine et al., 2004; Shall, 2009; Wolter et al., 2015). Two studies reported no association between vestibular function testing results and balance results (Birdane et al., 2016; Potter & Silverman, 1984). For example, Birdane and colleagues (2016) only considered children with unilateral hearing loss. Among those children with unilateral hearing loss, all their participants had either normal vestibular function or unilateral vestibular dysfunction. Even among those with unilateral vestibular dysfunction, they all performed normally on the Romberg and Unterberger stepping tests. Two additional studies did not collect information in a way to report an association between vestibular function testing and balance ability (Christy et al., 2014; Licameli et al., 2009). Seven studies presented balance results by degree of vestibular function abnormalities (Table 2). All seven studies show the general trend that balance performance is poorer as the degree of vestibular dysfunction increases.
Table 2:
Quantitative Balance Ability by Vestibular Function Results
| Study | Normal Vestibular Function | Unilateral Vestibular Dysfunction | Bilateral Vestibular Dysfunction |
|---|---|---|---|
| Abadie et al., 2000 | Head control (months): 6.6 ±
3.4 Stable sitting (months): 13.8 ± 2.9 Walking indoors (months): 27.5 ± 6.7 Walking outdoors (years): 2.75 ± 0.8 Running without falling (years): 3.6 ± 0.8 (n=8) |
Head control (months): 6.4
± 2.7 Stable sitting (months): 14.0 ± 3.9 Walking indoors (months): 33.6 ± 8.9 Walking outdoors (years): 3.75 ± 0.7 Running without falling (years): 4.5 ± 0.5 (n=8) |
|
| Christy et al., 2014 | mCTSIB total score: 116.0 ± 6.3 (n=11) | mCTSIB total score: 98.4 ± 20.4 (n=5) | mCTSIB total score: 84.4 ± 23.3 (n=3) |
| De Kegel et al., 2012 | M ABC-2 Manual Dexterity: 8.0 ±
2.4 M ABC-2 Ball Skills: 9.3 ± 2.8 M ABC-2 Balance: 7.6 ± 3.2 M ABC-2 Total: 7.5 ± 2.8 Balance Beam: 17.5 ± 15.4 One-leg Hopping: 24.0 ± 21.4 One-leg stance, EO: 63.1 ± 41.3 One-leg stance, EC: 18.7 ± 17.4 (n = 35) |
M ABC-2 Manual Dexterity: 8.3
± 2.0 M ABC-2 Ball Skills: 8.6 ± 4.5 M ABC-2 Balance: 3.6 ± 2.5 M ABC-2 Total: 5.4 ± 2.8 Balance Beam: 8.0 ± 10.2 One-leg Hopping: 18.7 ± 20.9 One-leg stance, EO: 52.0 ± 53.0 One-leg stance, EC: 4.6 ± 6.2 (n = 7) |
|
| Horak et al., 1988 | BOT Composite %ile: 44 ±
32 BOT Balance %ile: 14 ± 5 BOT Strength %ile: 15 ± 4 BOT Speed %ile: 13 ± 6 BOT Coordination %ile: 14 ± 4 (n = 7) |
BOT Composite %ile: 29
± 26 BOT Balance %ile: 6 ± 6 BOT Strength %ile: 14 ± 4 BOT Speed %ile: 12 ± 7 BOT Coordination %ile: 14 ± 4 (n = 20) |
|
| Ionescu et al., 2020 | M ABC-2 Balance: 25.5 ± 7.7 (n = 41) | M ABC-2 Balance: 16.0 ± 8.4 (n=18) | M ABC-2 Balance: 14.0 ± 11.4 (n=17) |
| Maes et al., 2014 | Balance beam walking (SS): 77.08 ±
10.01 One-leg hopping (SS): 85.92 ± 12.06 One-leg stance EC (z-score): −1.13 ± 0.91 (n = 12) |
Balance beam walking (SS):
63.17 ± 6.45 One-leg hopping (SS): 74.42 ± 12.98 One-leg stance EC (z-score): −2.00 ± 1.27 (n = 12) |
|
| Shall et al., 2009 | M ABC-2 Manual1: 5 M ABC-2 Ball Skills1: 3 M ABC-2 Balance1: 3 M ABC-2 Total1: 11 (n = 7) |
M ABC-2 Manual1: 7 M ABC-2 Ball Skills1: 4 M ABC-2 Balance1: 5 M ABC-2 Total1: 16 (n = 11) |
|
Note: BOT = Bruininks-Oseretsky Test of Motor Proficiency, mCTSIB: modified Clinical test of Sensory Interaction in Balance EC= eyes closed; EO = eyes open; M ABC = Movement Assessment Battery for Children
Shall et al., 2009 results estimated from their Figure 3
Methodological Quality Assessment
The Downs and Black risk of bias assessments for the articles included in this systematic review are presented in Table 3. There was wide variability in the risk of bias and methodological quality amongst the studies included. However, some assessment items were present in at least 75% of studies. These included clear study aims and outcome measures, clear descriptions of participant characteristics and findings, and appropriate use of statistical tests. Other checklist items were consistently absent across studies. Researchers were consistently not blinded to outcomes and only one study reported statistical methodology in sufficient detail to determine if sufficient power was obtained in studies (Table 3). Assessment items that were inconsistent across studies included adjusting for confounding variables; recruiting participants with hearing loss and control participants from the same population, setting, and timeframe; and having participants that represent the intended study population of children with hearing loss (Table 3). Overall, the risk of bias assessments demonstrated that studies were of variable and often low quality. As such, a meta-analysis was not undertaken.
Table 3:
Appraisal of Studies Using Downs and Black Risk of Bias Assessment1
| Abadie et al., 2000 | Apeksha et al., 2020 | Birdane et al., 2016 | Christie et al., 2014 | Crowe & Horak, 1988 | Cushing et al., 2008 | Cushing et al., 2009 | De Kegel et al., 2012 | Horak et al., 1988 | Ionescu et al., 2020 | Jafari & Asad Malayeri, 2011 | Janky & Givens, 2015 | Licameli et al., 2009 | Maes et al., 2014 | Nishida et al., 1983 | Potter & Silverman, 1984 | Rine et al., 2004 | Shall et al., 2009 | Wolter et al., 2015 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Clear hypothesis/aim/objective | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 2. Clear outcome measures | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | - | + |
| 3. Patient characteristics described | + | - | + | + | + | + | - | + | + | + | + | + | + | + | + | + | + | + | + |
| 4. Interventions clearly described | + | + | + | + | + | x | + | x | x | x | x | + | + | x | + | + | + | + | + |
| 5. Distributions of confounders described | - | + | + | - | + | + | + | + | ? | - | - | ? | ? | + | - | - | - | - | + |
| 6. Findings clearly described | - | + | + | - | - | + | + | + | + | + | + | - | + | + | + | + | + | - | + |
| 7. Estimates given of random variability | + | - | + | + | + | + | + | + | + | + | + | + | - | + | x | + | + | x | - |
| 8. Adverse events reported | x | - | - | - | - | x | - | x | x | x | x | x | x | x | x | x | - | x | x |
| 9. Patients lost to follow-up described | x | + | + | + | - | x | - | x | x | x | x | x | x | x | - | x | x | x | - |
| 10. Probability values reported | + | - | + | + | + | x | + | + | - | + | + | + | x | + | x | x | + | x | - |
| 11. Recruitment pool represents population | ? | - | ? | ? | ? | - | ? | + | + | ? | + | ? | ? | + | + | ? | - | + | + |
| 12. Participants represent population | ? | - | ? | ? | ? | - | ? | + | + | - | + | ? | ? | - | + | + | + | + | + |
| 13. Staff/places/facilities match standard treatment | ? | ? | ? | - | - | + | ? | + | + | ? | + | + | + | x | - | ? | ? | + | ? |
| 14. Participants blinded to intervention | - | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | - | x | x |
| 15. Those measuring outcomes blinded | - | x | ? | ? | + | x | + | x | x | x | x | x | x | x | x | ? | + | x | - |
| 16. Data dredging reported | x | + | + | + | + | + | + | + | + | + | ? | + | ? | + | ? | ? | - | ? | x |
| 17. Adjusted for different lengths of follow-up | x | x | - | + | x | x | - | x | x | x | x | x | x | x | x | x | x | - | x |
| 18. Appropriate statistical tests | ? | + | + | + | + | + | + | + | + | + | + | + | + | + | x | + | + | ? | + |
| 19. Reliable compliance with intervention | + | ? | + | - | + | + | + | x | x | x | x | + | - | x | ? | + | + | ? | ? |
Table format modified from Diment et al., 2018
Discussion:
This systematic review sought to describe the impact of vestibular dysfunction on balance abilities in children with hearing loss. Despite high rates of co-occurring hearing loss and vestibular loss, vestibular function is infrequently assessed in children with hearing loss. There is a lack of consensus regarding the need for assessment because little is known about the symptoms and functional impacts of vestibular dysfunction in children with hearing loss. In adults there is less ambiguity. Vestibular dysfunction can cause vertigo, dizziness, unsteadiness, and falls, (Bamiou et al., 1999; Fina et al., 2003; Hillier & McDonnell, 2007; Popkirov et al., 2018) which can lead to social withdrawal and occupational disability (Benecke et al., 2013; Celebisoy et al., 2009; Chang et al., 2006; Kollén et al., 2017; Lopez-Escamez et al., 2003; Skøien et al., 2008). These negative consequences are mitigated by vestibular compensation, either naturally or with the aid of vestibular rehabilitation (Deveze et al., 2014; Strupp et al., 1998). In children, the impact of vestibular dysfunction on balance abilities has not been well-characterized. Additionally, there is uncertainty on the effectiveness of vestibular compensation. Children with other neurodevelopmental problems such as cerebral palsy or stroke have been shown to have better motor rehabilitation and recovery of function than adults, due to neuroplasticity (Johnston, 2009; Reid et al., 2015), but we do not know if this is true in children with hearing loss. A prior systematic review by Melo et al. (2019) showed vestibular rehabilitation likely improved the balance and posturomotor control in children with sensorineural hearing loss, but Melo et al. reported that the current literature is of low quality. It is suspected that children can compensate more efficiently due to increased brain plasticity, leading to a lower prevalence of balance problems (Kolb & Gibb, 2011; Mundkur, 2005).
Balance Testing Results in Children with Hearing Loss
This systematic review assessed the association between vestibular function and balance ability in children with hearing loss. Across the twenty studies identified through a systematic literature review, nineteen were focused on determining the association between vestibular function and balance ability. Of these nineteen studies, the majority, seventeen, reported an association between poor performance on measures of vestibular function and poor performance on balance testing but did not necessarily determine whether these were statistically significant differences. These findings need to be considered in light of potential publication bias, in that studies that report an association between vestibular dysfunction and balance problems may be more likely to be published than studies that find no association between vestibular dysfunction and balance issues. Several of these previously published studies employed descriptive analysis in their studies and reported the qualitative finding that abnormal results on vestibular testing more often resulted in abnormal results on clinical balance testing. In other words, the authors pointed out that those who had abnormal vestibular testing results often had a greater frequency of abnormal balance results compared to control participants with normal hearing and normal vestibular results (Apeksha et al., 2020; Crowe & Horak, 1988; Nishida et al., 1983). Little data are available on balance function when examining varying levels of vestibular function. Only Ionescue et al. (2020), Christy et al. (2014), and Janky & Givens (2015) examined whether there was any dose-effect (i.e., greater vestibular impairment leads to greater balance difficulty). Janky & Givens (2015) reported that when characterizing degree of impairment (based on 1–10 scale relating to the number of vestibular end-organs impaired) those with greater residual function generally performed better on measures of balance and gait. While the scaled approach provides a unique and helpful way of examining gross residual vestibular function, the scale does not intuitively provide the number of unilateral versus bilateral impairments.
Ionescu et al. (2020) found balance performance was significantly worse in the groups with either unilateral or bilateral vestibular dysfunction compared to group with normal vestibular function. However, they did not find that balance performance was significantly different between children with unilateral and bilateral vestibular dysfunction. In contrast, Birdane et al. (2016) found that children with unilateral vestibular dysfunction had normal results on balance tests (n = 33). This suggests that children are able to effectively employ vestibular compensation such that the peripheral vestibular input from one ear may be sufficient for normal balance ability. As a result, it is difficult to determine the extent to which unilateral vestibular dysfunction leads to functional balance problems in children with hearing loss. Perhaps functional deficits are only realized with more challenging balance tasks.
While the majority of studies report some association between vestibular testing results and balance function, two studies (Potter & Silverman, 1984; Birdane et al., 2016) came to the conclusion that there was no such association. In both instances, either the measure used to quantify vestibular function (in the case of Potter & Silverman, 1984) or the balance outcome measure (in Birdane et al., 2016) are generally not well accepted measures due to their test performance. Potter and Silverman (1984) which was amongst the first published in this area, used the Southern California Postrotary Nystagmus Test as a measure of vestibular function. This method was not used in any other study and generally speaking the measure is no longer widely used due to concerns validity of the measure (Cohen, 1989; Polatajko, 1983). Second, Birdane et al. (2016) reported no association between unilateral vestibular dysfunction and balance measures in 2016. This study utilized cVEMP and ENG testing, as well as both the Romberg and Unterberger (Fukuda) stepping tests, and all children had normal balance testing results (Table 2) (Birdane et al., 2016). Similar to concerns that were raised regarding the validity of the Southern California Postrotary Nystagmus Test, the Unterberger (Fukuda) stepping test has been reported to be neither sensitive nor specific for vestibular dysfunction (sensitivity = 0.43–0.50, specificity=0.61–0.65) (Honaker et al., 2009). The use of this measure may have impacted the interpretation of the results.
More recently, VEMP testing has been developed that allows for assessment of otolith function and otolith-mediated pathways. These new techniques may provide important insight into the impacts of certain patterns of vestibular impairment as prior research has suggested that otolith organs are a larger contributor to balance and postural control than the canal inputs (Allum & Shepard, 1999; Markham, 1987; Wilson & Peterson, 1978). This is in part supported by the fact that canal inputs primarily terminate in the cervical region whereas, otolith organs innervate motor neurons along the full spinal pathway (cervical through sacral region) (Wilson & Peterson, 1978). As a result, assessment methods specific for otolith damage may be more sensitive in detecting balance difficulties. The results of our systematic review make it difficult to tease apart canal versus otolith contributions based on the reporting.
Limitations within Existing Literature
Overall, there appears to be an association between vestibular loss and clinical balance performance. However, it is difficult to quantify the association given the limitations in methodology identified in the Downs and Black risk-of-bias assessment (Table 3). While studies often had good reporting measures, only one (Potter & Silverman, 1984) reported sufficient detail regarding power analyses to discern if the study was adequately powered to detect differences. Additionally, bias is a concern, as most studies were cohort studies or case series selected through convenience sampling, with no blinding. Convenience sampling as a form of non-random sampling can be problematic as patients may not be representative of all children with hearing loss (Etikan, 2016; Farrokhi & Mahmoudi, 2012). For example, if patients are chosen from an otolaryngology clinic for further vestibular or balancing testing, then they may be more likely to display more severe vestibular or balance symptoms than those not chosen for further testing. Without blinding, researchers may have expected to see worse balance outcomes in children with hearing loss and concomitant known vestibular dysfunction. This may have influenced their test interpretation, especially given the subjective nature of clinical balance measurements. Most studies included control groups that were age-matched or age- and gender-matched, but did not include explicit controls for other potential confounders such as medical co-morbidities and medication use that may affect balance.
Another reason limiting strong conclusions in this review is that almost all studies had different assessment methods or batteries of tests for assessing both vestibular function and balance ability, making it difficult to compare results. While there is a discrete number of available vestibular laboratory tests, there is a nearly infinite number of ways to assess balance performance. Some of these clinical balance assessments have been found to be more or less useful than others (Honaker et al., 2009). Additionally, studies had different criteria for the definition of abnormal. For example, among studies that utilized Romberg testing, some researchers considered a test to be abnormal if the patient became unsteady and fell immediately upon closing his or her eyes (Koyuncu et al., 1999), whereas others considered a Romberg test abnormal if the patient showed significant unsteadiness even without a fall (Camarda et al., 1981). In our systematic review, at least one article lacked sufficient detail to determine how an abnormality on Romberg testing was defined (Birdane et al., 2016).
Future Research
While the quality of research is this area has improved over time, there continues to be a need for higher quality studies. This field would benefit from more rigorous sampling methods to better represent all children with hearing loss. In particular, random sampling could help control for potential confounders and variability in hearing loss etiology. Similarly, blinding researchers would minimize bias. Ideally, researchers should perform studies adequately powered to detect statistically significant associations between vestibular dysfunction and balance testing abnormalities in children with hearing loss and include a range of both hearing and vestibular losses in the study. With the limited current data available, it is difficult to fully understand what would be the most appropriate battery to recommend; however, we believe that the ideal situation would be to have a consensus from an expert panel develop an interim minimum test battery that would be used in future studies and data from future high-quality studies that specifically target this question would ultimately determine the standard test battery needed. This would lead to an increased ability to make stronger conclusions about vestibular dysfunction and balance in children with hearing loss. Further work examining unilateral and bilateral vestibular dysfunction separately is also needed to characterize the effects of each on balance ability in children with hearing loss. Similarly, little is known about differences between congenital and early-onset vestibular dysfunction. A further area of research would be to consider the effects of both the severity and the type of vestibular loss (i.e. canal vs otolith). Moreover, natural history studies could be helpful to determine if vestibular dysfunction and balance problems affect early development of children with hearing loss in a meaningful way, such as limiting the ability to socialize with other children or participate in organized sports. This could inform the need, or lack thereof, for targeted intervention like vestibular rehabilitation.
Conclusion
Vestibular dysfunction is known to cause balance problems in adults, but less is known about the impact of vestibular dysfunction on children. This systematic review identified 20 studies on vestibular function and balance measures in children with hearing loss. To date, the majority of studies, but not all, have reported an association between the presence of vestibular dysfunction and poorer outcomes on balance testing. Methodological quality concerns, variety in assessment methods, and different definitions of normal versus abnormal testing results somewhat attenuate the ability to draw strong conclusions on the effect of vestibular dysfunction on balance testing measures in children with hearing loss. Nonetheless, this systematic review demonstrates that the research to date has predominantly found the children with concomitant hearing loss and vestibular dysfunction tend to perform more poorly on balance testing than children without vestibular dysfunction and either normal hearing or hearing loss.
Supplementary Material
Supplemental Digital Content 1 .docx: Description of systematic review search strategy
Supplemental Digital Content 2 .docx: Downs and Black Quality Assessment Tool Form
Supplemental Digital Content 3. Docx: Description of Vestibular Function and Balance Measures Results for all studies
Acknowledgements
A Singh contributed to the conception and design of the study, acquisition of the data, the data analysis and interpretation, the manuscript drafting, and the critical revision of the manuscript.
H Heet contributed to the design of the study, acquisition of the data, the data analysis and interpretation, and the critical revision of the manuscript.
DS Guggenheim contributed to the, acquisition of the data, the data analysis, the manuscript drafting, and the critical revision of the manuscript.
M Lim contributed to the, acquisition of the data, the data analysis, the manuscript drafting, and the critical revision of the manuscript.
B Garg contributed to the, acquisition of the data, the data analysis, the manuscript drafting, and the critical revision of the manuscript.
M Bao contributed to the, acquisition of the data, the data analysis, the manuscript drafting, and the critical revision of the manuscript.
SL Smith contributed to the conception and design of the study, acquisition of the data, the data analysis and interpretation, the manuscript drafting, and the critical revision of the manuscript.
D Garrison contributed to the design of the study, acquisition of the data, the data analysis and interpretation, and the critical revision of the manuscript.
EM Raynor contributed to the conception and design of the study, acquisition of the data, the data analysis and interpretation, and the critical revision of the manuscript.
JW Lee contributed to the conception and design of the study, acquisition of the data, the data analysis and interpretation, and the critical revision of the manuscript.
J Wrigley contributed to the conception and design of the study, acquisition of the data, and the critical revision of the manuscript.
KM Riska contributed to the conception and design of the study, acquisition of the data, data analysis and interpretation, the manuscript drafting, the critical revision of the manuscript, and the supervision.
We thank David M. Kaylie, MD, and Leila Ledbetter, MLIS, for their guidance and technical assistance on systematic review practices.
Conflicts of Interest and Source of Funding:
No authors have any conflicts of interest to report. Research reported in this publication was supported by National Institute for Deafness and Other Communication Disorders (NIDCD) of the National Institutes of Health under award number 1R21DC018616. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
References:
- Abadie V, Wiener-Vacher S, Morisseau-Durand MP, Porée C, Amiel J, Amanou L, Peigné C, Lyonnet S, & Manac’h Y (2000). Vestibular anomalies in CHARGE syndrome: investigations on and consequences for postural development. Eur J Pediatr, 159(8), 569–574. 10.1007/s004319900409 [DOI] [PubMed] [Google Scholar]
- Allum JH, & Shepard NT (1999). An overview of the clinical use of dynamic posturography in the differential diagnosis of balance disorders. J Vestib Res, 9(4), 223–252. [PubMed] [Google Scholar]
- Apeksha K, Singh S, Rathnamala M, Varalakshmi S, Preethu DJ, Kavya V, Sowndarya DS, Arpitha S, Milana K, Navya S, & Thejasvi MA (2020). Balance Assessment of Children with Sensorineural Hearing Loss. Indian Journal of Otolaryngology and Head & Neck Surgery. 10.1007/s12070-020-01797-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamiou DE, Davies RA, McKee M, & Luxon LM (1999). The effect of severity of unilateral vestibular dysfunction on symptoms, disabilities and handicap in vertiginous patients. Clin Otolaryngol Allied Sci, 24(1), 31–38. 10.1046/j.1365-2273.1999.00203.x [DOI] [PubMed] [Google Scholar]
- Benecke H, Agus S, Kuessner D, Goodall G, & Strupp M (2013). The Burden and Impact of Vertigo: Findings from the REVERT Patient Registry. Front Neurol, 4, 136. 10.3389/fneur.2013.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdane L, Incesulu A, Ozudogru E, Cingi C, Cakli H, Gurbuz MK, & Adapinar B (2016). Evaluation of the Vestibular System and Etiology in Children with Unilateral Sensorineural Hearing Loss. J Int Adv Otol, 12(2), 161–165. 10.5152/iao.2016.2439 [DOI] [PubMed] [Google Scholar]
- Braswell J, & Rine RM (2006). Evidence that vestibular hypofunction affects reading acuity in children. International journal of pediatric otorhinolaryngology, 70(11), 1957–1965. https://pdf.sciencedirectassets.com/271188/1-s2.0-S0165587606X02148/1-s2.0-S0165587606002424/ [DOI] [PubMed] [Google Scholar]
- Camarda V, Moreno AM, Boschi V, Di Carlo A, Spaziani G, & Saponara M (1981). Vestibular ototoxicity in children: a retrospective study of 52 cases. Int J Pediatr Otorhinolaryngol, 3(3), 195–198. 10.1016/0165-5876(81)90002-1 [DOI] [PubMed] [Google Scholar]
- Celebisoy N, Bayam E, Güleç F, Köse T, & Akyürekli O (2009). Balance in posterior and horizontal canal type benign paroxysmal positional vertigo before and after canalith repositioning maneuvers. Gait Posture, 29(3), 520–523. 10.1016/j.gaitpost.2008.12.002 [DOI] [PubMed] [Google Scholar]
- Chang WC, Hsu LC, Yang YR, & Wang RY (2006). Balance ability in patients with benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg, 135(4), 534–540. 10.1016/j.otohns.2005.10.001 [DOI] [PubMed] [Google Scholar]
- Christy JB, Payne J, Azuero A, & Formby C (2014). Reliability and diagnostic accuracy of clinical tests of vestibular function for children. Pediatr Phys Ther, 26(2), 180–189. 10.1097/PEP.0000000000000039 [DOI] [PubMed] [Google Scholar]
- Cohen H (1989). Testing vestibular function: problems with the Southern California Postrotary Nystagmus Test. Am J Occup Ther, 43(7), 475–477. 10.5014/ajot.43.7.475 [DOI] [PubMed] [Google Scholar]
- Crowe TK, & Horak FB (1988). Motor proficiency associated with vestibular deficits in children with hearing impairments. Phys Ther, 68(10), 1493–1499. [PubMed] [Google Scholar]
- Cushing SL, Papsin BC, Rutka JA, James AL, Blaser SL, & Gordon KA (2009). Vestibular end-organ and balance deficits after meningitis and cochlear implantation in children correlate poorly with functional outcome. Otol Neurotol, 30(4), 488–495. 10.1097/MAO.0b013e31819bd7c8 [DOI] [PubMed] [Google Scholar]
- Cushing SL, Papsin BC, Rutka JA, James AL, & Gordon KA (2008). Evidence of vestibular and balance dysfunction in children with profound sensorineural hearing loss using cochlear implants. Laryngoscope, 118(10), 1814–1823. 10.1097/MLG.0b013e31817fadfa [DOI] [PubMed] [Google Scholar]
- De Kegel A, Maes L, Baetens T, Dhooge I, & Van Waelvelde H (2012). The influence of a vestibular dysfunction on the motor development of hearing‐impaired children. The Laryngoscope, 122(12), 2837–2843. https://onlinelibrary.wiley.com/doi/full/10.1002/lary.23529 [DOI] [PubMed] [Google Scholar]
- De Kegel A, Maes L, Van Waelvelde H, & Dhooge I (2015). Examining the impact of cochlear implantation on the early gross motor development of children with a hearing loss. Ear Hear, 36(3), e113–121. 10.1097/aud.0000000000000133 [DOI] [PubMed] [Google Scholar]
- Deveze A, Bernard-Demanze L, Xavier F, Lavieille JP, & Elziere M (2014). Vestibular compensation and vestibular rehabilitation. Current concepts and new trends. Neurophysiol Clin, 44(1), 49–57. 10.1016/j.neucli.2013.10.138 [DOI] [PubMed] [Google Scholar]
- Dutia MB (2010). Mechanisms of vestibular compensation: recent advances. Curr Opin Otolaryngol Head Neck Surg, 18(5), 420–424. 10.1097/MOO.0b013e32833de71f [DOI] [PubMed] [Google Scholar]
- Etikan I (2016). Comparison of Convenience Sampling and Purposive Sampling. American Journal of Theoretical and Applied Statistics, 5, 1. 10.11648/j.ajtas.20160501.11 [DOI] [Google Scholar]
- Farrokhi F, & Mahmoudi A (2012). Rethinking Convenience Sampling: Defining Quality Criteria. Theory and Practice in Language Studies, 2. 10.4304/tpls.2.4.784-792 [DOI] [Google Scholar]
- Fina M, Skinner M, Goebel JA, Piccirillo JF, Neely JG, & Black O (2003). Vestibular dysfunction after cochlear implantation. Otol Neurotol, 24(2), 234–242; discussion 242. 10.1097/00129492-200303000-00018 [DOI] [PubMed] [Google Scholar]
- Gioacchini FM, Alicandri-Ciufelli M, Kaleci S, Magliulo G, & Re M (2014). Prevalence and diagnosis of vestibular disorders in children: a review. Int J Pediatr Otorhinolaryngol, 78(5), 718–724. 10.1016/j.ijporl.2014.02.009 [DOI] [PubMed] [Google Scholar]
- Hänsel T, Gauger U, Bernhard N, Behzadi N, Romo Ventura ME, Hofmann V, Olze H, Knopke S, Todt I, & Coordes A (2018). Meta-analysis of subjective complaints of vertigo and vestibular tests after cochlear implantation. Laryngoscope, 128(9), 2110–2123. 10.1002/lary.27071 [DOI] [PubMed] [Google Scholar]
- Hillier SL, & McDonnell M (2007). Vestibular rehabilitation for unilateral peripheral vestibular dysfunction. Cochrane Database of Systematic Reviews(4). 10.1002/14651858.CD005397.pub2 [DOI] [PubMed] [Google Scholar]
- Honaker JA, Boismier TE, Shepard NP, & Shepard NT (2009). Fukuda stepping test: sensitivity and specificity. J Am Acad Audiol, 20(5), 311–314; quiz 335. 10.3766/jaaa.20.5.4 [DOI] [PubMed] [Google Scholar]
- Horak FB, Shumway-Cook A, Crowe TK, & Black FO (1988). Vestibular function and motor proficiency of children with impaired hearing, or with learning disability and motor impairments. Dev Med Child Neurol, 30(1), 64–79. 10.1111/j.1469-8749.1988.tb04727.x [DOI] [PubMed] [Google Scholar]
- Ionescu E, Reynard P, Gouleme N, Becaud C, Spruyt K, Ortega-Solis J, & Thai-Van H (2020). How sacculo-collic function assessed by cervical vestibular evoked myogenic Potentials correlates with the quality of postural control in hearing impaired children? Int J Pediatr Otorhinolaryngol, 130, 109840. 10.1016/j.ijporl.2019.109840 [DOI] [PubMed] [Google Scholar]
- Jafari Z, & Asad Malayeri S (2011). The effect of saccular function on static balance ability of profound hearing-impaired children. Int J Pediatr Otorhinolaryngol, 75(7), 919–924. 10.1016/j.ijporl.2011.04.006 [DOI] [PubMed] [Google Scholar]
- Janky KL, & Givens D (2015). Vestibular, Visual Acuity, and Balance Outcomes in Children With Cochlear Implants: A Preliminary Report. Ear Hear, 36(6), e364–372. 10.1097/aud.0000000000000194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janky KL, Thomas MLA, High RR, Schmid KK, & Ogun OA (2018). Predictive Factors for Vestibular Loss in Children With Hearing Loss. Am J Audiol, 27(1), 137–146. 10.1044/2017_AJA-17-0058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston MV (2009). Plasticity in the developing brain: implications for rehabilitation. Dev Disabil Res Rev, 15(2), 94–101. 10.1002/ddrr.64 [DOI] [PubMed] [Google Scholar]
- Kimura Y, Masuda T, & Kaga K (2018). Vestibular Function and Gross Motor Development in 195 Children With Congenital Hearing Loss-Assessment of Inner Ear Malformations. Otol Neurotol, 39(2), 196–205. 10.1097/MAO.0000000000001685 [DOI] [PubMed] [Google Scholar]
- Kolb B, & Gibb R (2011). Brain plasticity and behaviour in the developing brain. Journal of the Canadian Academy of Child and Adolescent Psychiatry = Journal de l’Academie canadienne de psychiatrie de l’enfant et de l’adolescent, 20(4), 265–276. https://pubmed.ncbi.nlm.nih.gov/22114608 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3222570/ [PMC free article] [PubMed] [Google Scholar]
- Kollén L, Hörder H, Möller C, & Frändin K (2017). Physical functioning in older persons with dizziness: a population-based study. Aging Clin Exp Res, 29(2), 197–205. 10.1007/s40520-016-0567-9 [DOI] [PubMed] [Google Scholar]
- Kotait MA, Moaty AS, & Gabr TA (2019). Vestibular testing in children with severe-to-profound hearing loss. Int J Pediatr Otorhinolaryngol, 125, 201–205. 10.1016/j.ijporl.2019.07.015 [DOI] [PubMed] [Google Scholar]
- Koyuncu M, Saka MM, Tanyeri Y, Seşen T, Unal R, Tekat A, & Yilmaz F (1999). Effects of otitis media with effusion on the vestibular system in children. Otolaryngol Head Neck Surg, 120(1), 117–121. 10.1016/s0194-5998(99)70381-5 [DOI] [PubMed] [Google Scholar]
- Lacour M, Helmchen C, & Vidal PP (2016). Vestibular compensation: the neuro-otologist’s best friend. J Neurol, 263 Suppl 1, S54–64. 10.1007/s00415-015-7903-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licameli G, Zhou G, & Kenna MA (2009). Disturbance of vestibular function attributable to cochlear implantation in children. Laryngoscope, 119(4), 740–745. 10.1002/lary.20121 [DOI] [PubMed] [Google Scholar]
- Lopez-Escamez JA, Gamiz MJ, Fernandez-Perez A, Gomez-Fiñana M, & Sanchez-Canet I (2003). Impact of treatment on health-related quality of life in patients with posterior canal benign paroxysmal positional vertigo. Otol Neurotol, 24(4), 637–641. 10.1097/00129492-200307000-00018 [DOI] [PubMed] [Google Scholar]
- Maes L, De Kegel A, Van Waelvelde H, & Dhooge I (2014). Association between vestibular function and motor performance in hearing-impaired children. Otol Neurotol, 35(10), e343–347. 10.1097/mao.0000000000000597 [DOI] [PubMed] [Google Scholar]
- Markham CH (1987). Vestibular control of muscular tone and posture. Can J Neurol Sci, 14(3 Suppl), 493–496. 10.1017/s0317167100037975 [DOI] [PubMed] [Google Scholar]
- Masuda T, & Kaga K (2014). Relationship between acquisition of motor function and vestibular function in children with bilateral severe hearing loss. Acta Otolaryngol, 134(7), 672–678. 10.3109/00016489.2014.890290 [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, & Altman DG (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med, 6(7), e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundkur N (2005). Neuroplasticity in children. Indian J Pediatr, 72(10), 855–857. 10.1007/bf02731115 [DOI] [PubMed] [Google Scholar]
- Nishida Y, Ueda K, & Fung KC (1983). Congenital rubella syndrome: function of equilibrium of 80 cases with deafness. Laryngoscope, 93(7), 938–940. 10.1288/00005537-198307000-00018 [DOI] [PubMed] [Google Scholar]
- Polatajko HJ (1983). The Southern California Postrotary Nystagmus Test: A Validity Study. Canadian Journal of Occupational Therapy, 50(4), 119–123. 10.1177/000841748305000404 [DOI] [Google Scholar]
- Popkirov S, Staab JP, & Stone J (2018). Persistent postural-perceptual dizziness (PPPD): a common, characteristic and treatable cause of chronic dizziness. Pract Neurol, 18(1), 5–13. 10.1136/practneurol-2017-001809 [DOI] [PubMed] [Google Scholar]
- Potter CN, & Silverman LN (1984). Characteristics of vestibular function and static balance skills in deaf children. Phys Ther, 64(7), 1071–1075. 10.1093/ptj/64.7.1071 [DOI] [PubMed] [Google Scholar]
- Reid LB, Rose SE, & Boyd RN (2015). Rehabilitation and neuroplasticity in children with unilateral cerebral palsy. Nat Rev Neurol, 11(7), 390–400. 10.1038/nrneurol.2015.97 [DOI] [PubMed] [Google Scholar]
- Rine RM, Braswell J, Fisher D, Joyce K, Kalar K, & Shaffer M (2004). Improvement of motor development and postural control following intervention in children with sensorineural hearing loss and vestibular impairment. International journal of pediatric otorhinolaryngology, 68(9), 1141–1148. https://pdf.sciencedirectassets.com/271188/1-s2.0-S0165587600X01930/1-s2.0-S0165587604001181/ [DOI] [PubMed] [Google Scholar]
- Rine RM, Braswell J, Fisher D, Joyce K, Kalar K, & Shaffer M (2004). Improvement of motor development and postural control following intervention in children with sensorineural hearing loss and vestibular impairment. Int J Pediatr Otorhinolaryngol, 68(9), 1141–1148. 10.1016/j.ijporl.2004.04.007 [DOI] [PubMed] [Google Scholar]
- Rine RM, Cornwall G, Gan K, LoCascio C, O’Hare T, Robinson E, & Rice M (2000). Evidence of progressive delay of motor development in children with sensorineural hearing loss and concurrent vestibular dysfunction. Percept Mot Skills, 90(3 Pt 2), 1101–1112. 10.2466/pms.2000.90.3c.1101 [DOI] [PubMed] [Google Scholar]
- Sartori Franco E, & Panhoca I (2007). Otoneurologic evaluation in children with school difficulties: vestibular function investigation. Braz J Otorhinolaryngol, 73(6), 803–815. 10.1016/s1808-8694(15)31177-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shall MS (2009). The importance of saccular function to motor development in children with hearing impairments. Int J Otolaryngol, 2009, 972565. 10.1155/2009/972565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Gupta RK, & Kumar P (2012). Vestibular evoked myogenic potentials in children with sensorineural hearing loss. Int J Pediatr Otorhinolaryngol, 76(9), 1308–1311. 10.1016/j.ijporl.2012.05.025 [DOI] [PubMed] [Google Scholar]
- Skøien AK, Wilhemsen K, & Gjesdal S (2008). Occupational disability caused by dizziness and vertigo: a register-based prospective study. Br J Gen Pract, 58(554), 619–623. 10.3399/bjgp08X330744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strupp M, Arbusow V, Maag KP, Gall C, & Brandt T (1998). Vestibular exercises improve central vestibulospinal compensation after vestibular neuritis. Neurology, 51(3), 838–844. 10.1212/wnl.51.3.838 [DOI] [PubMed] [Google Scholar]
- Verbecque E, Marijnissen T, De Belder N, Van Rompaey V, Boudewyns A, Van de Heyning P, Vereeck L, & Hallemans A (2017). Vestibular (dys)function in children with sensorineural hearing loss: a systematic review. Int J Audiol, 56(6), 361–381. 10.1080/14992027.2017.1281444 [DOI] [PubMed] [Google Scholar]
- Wiener-Vacher SR (2008). Vestibular disorders in children. Int J Audiol, 47(9), 578–583. 10.1080/14992020802334358 [DOI] [PubMed] [Google Scholar]
- Wiener-Vacher SR, Quarez J, & Priol AL (2018). Epidemiology of Vestibular Impairments in a Pediatric Population. Semin Hear, 39(3), 229–242. 10.1055/s-0038-1666815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson VJ, & Peterson BW (1978). Peripheral and central substrates of vestibulospinal reflexes. Physiol Rev, 58(1), 80–105. 10.1152/physrev.1978.58.1.80 [DOI] [PubMed] [Google Scholar]
- Wolter NE, Gordon KA, Papsin BC, & Cushing SL (2015). Vestibular and Balance Impairment Contributes to Cochlear Implant Failure in Children. Otol Neurotol, 36(6), 1029–1034. 10.1097/mao.0000000000000751 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1 .docx: Description of systematic review search strategy
Supplemental Digital Content 2 .docx: Downs and Black Quality Assessment Tool Form
Supplemental Digital Content 3. Docx: Description of Vestibular Function and Balance Measures Results for all studies


