Abstract
Retinal damage has been associated with increased injection pressure during subretinal gene therapy delivery in various animal models, yet there are no human clinical data regarding the pressures required to initiate and propagate subretinal blebs. This study characterized the intraoperative pressure levels for subretinal gene therapy delivery across eight retinal conditions. A total of 116 patients with retinal degenerative diseases have been treated with subretinal gene therapy at OHSU-Casey Eye Institute as of June 2020; seventy patients (60.3%) were treated using a pneumatic-assisted subretinal delivery system. All retinal blebs were performed using a 41-gauge injection cannula, and use of a balanced salt solution (BSS) “pre-bleb” prior to gene therapy delivery was performed at the discretion of the surgeon. Patient age and intraoperative data for BSS and vector injections were analyzed in a masked fashion for all patients who received pneumatic-assisted subretinal gene therapy. The median age of the patients was 35 years (range 4–70). No significant differences in injection pressures were found across the eight retinal conditions. In this study, patient age was shown to affect maximum injection pressures required for bleb propagation, and the relationship between age and pressure varied based on retinal condition. These data have important implications in optimizing surgical protocols for subretinal injections.
Keywords: Ocular gene therapy, inherited retinal degenerations, retinal bleb, subretinal injection
Introduction
Despite their progressive nature, most inherited retinal degenerations have no treatment. There is currently only one FDA-approved prescription gene therapy, voretigene neparvovec-rzyl (Luxturna®), for patients with biallelic RPE65 mutations1. Numerous human gene therapy trials are ongoing to test the safety and efficacy of gene augmentation therapy across many disease states1–5. The most common route of delivery is injection into the subretinal compartment, which is ideal for the following reasons: 1) efficient transduction of the photoreceptor and/or retinal pigment epithelium (RPE) layer, 2) compartmentalization of the vector solution to allow for concentrated small volume dosing, and 3) minimization of inflammation risk due to the space being an active area of immune deviation. However, subretinal gene delivery techniques should be further refined to prevent adverse outcomes associated with tissue damage, which can negate the benefits of gene therapy. Such prioritization is important for all patients and is particularly important as we treat younger patients with less severe disease.
To create a subretinal bleb, the abnormally adherent retina of patients with outer retinal degenerations must be detached. Multiple pre-clinical studies have demonstrated that injection technique can affect outcomes. For example, faster injection speeds (1.8 ml/min vs 0.18 ml/min) using an automated syringe pump injector have been associated with significant loss of RPE in pig eyes compared to those injected at slower speeds7. Higher injection pressures in pounds per square inch (20 psi vs 6 psi) have led to RPE and photoreceptor damage in monkey eyes8. Injection pressures over 12 psi may provide continuous flow of fluid rather than a stream of droplets9, and elevated injection pressures may increase flow-related complications, such as overstretching or thinning of the retina, secondary macular hole, or excessive egress of the vector solution10. Avoidance of these adverse events is paramount to the success of gene therapy trials.
Intraoperative optical coherence tomography (OCT) and pneumatic-assisted pedal control allow real-time monitoring of retinal elevation during subretinal bleb formation while titrating infusion pressure by foot11, 12. This technology also allows the surgeon to record injection pressures during gene therapy delivery. Yet, there are scant real-world data from human gene therapy trials regarding intraoperative injection pressure levels.
This study critically evaluated all gene therapy surgeries performed at Oregon Health & Science University (OHSU)-Casey Eye Institute with characterization of the intraoperative injection pressure levels for subretinal bleb initiation and propagation across eight retinal degenerative conditions. Such data could aid in the prevention of intraoperative and post-operative complications, which can be devastating in patients with retinal dystrophies.
Materials and Methods
Subjects
Institutional Review Board approval at OHSU was obtained for review of all operative notes of patients receiving subretinal delivery of gene therapy. There were 116 patients with eight different retinal disease states who were identified as having received subretinal gene therapy as of June 2020. Forty-six patients (39.7%) had received gene therapy by manual injection and were excluded from this study. For 70 patients (60.3%) receiving pneumatic-assisted (i.e., foot pedal controlled) subretinal gene therapy at OHSU-Casey Eye Institute, there were eight retinal degenerative conditions represented (conditions A-H). Except for the surgeons (A.K.L and S.T.B.), the authors did not know condition type. This study included 1) participants of seven active clinical trials and 2) patients receiving pneumatic-assisted subretinal delivery of voretigene neparvovec-rzyl (Luxturna®; Spark Therapeutics, Inc., Philadelphia, PA).
Subretinal Injection Technique
All subjects were brought to the operating theatre and placed in supine position. General anesthesia was induced prior to performing 23-gauge pars plana vitrectomy in the study eye using the Constellation® Vision System (Alcon, Fort Worth, TX). All procedures were performed using sterile technique under an OPMI Lumera® 700 surgical operating microscope (Carl Zeiss Meditec AG, Jena, Germany) enhanced with intraoperative optical coherence tomography (OCT; Zeiss Rescan 7000, Carl Zeiss Meditec AG, Jena, Germany). Intraocular pressure (IOP) was maintained at the surgeon’s discretion during some of our earlier cases when the infusion pressure was not yet standardized. However, for most cases, the IOP was maintained at 10mmHg during bleb initiation and propagation. A posterior vitreous detachment was performed using the vitrector if the hyaloid was still attached. Triamcinolone acetonide was used, when indicated, to aid with or confirm hyaloid removal. The vitreous was trimmed in all clock hours in systemic fashion, and the periphery was inspected by scleral depression to identify retinal pathology prior to proceeding with subretinal injection.
A retinal pre-bleb with injection of balanced salt solution (BSS®; Alcon, Fort Worth, TX) was performed at the discretion of the surgeon. To create a pre-bleb, a 41-gauge subretinal injection cannula (Dutch Ophthalmic, Inc., Exeter, NH, USA) and foot-pedal assisted control were used to inject BSS subretinally to create a small bleb. Using another 41-gauge subretinal injection cannula and foot-pedal assisted control, a pre-specified amount of vector solution was administered through the retinotomy site of the pre-bleb. The vector solution volume was dependent on the gene therapy administered and/or clinical trial specifications. The subretinal bleb was propagated in a controlled fashion for each patient; the target zone for treatment was selected prior to surgery to maximize functional benefit with care taken to avoid areas of suspected retinal fragility or increased retinal adherence. In certain cases, where bleb propagation did not extend to the treatment target zone or if the bleb failed to propagate, the surgeon initiated a bleb in a new area. Fluid-air exchange was performed based on the surgeon’s preference and/or as specified by the clinical trial. The trocars were removed, and the scleral and conjunctival wounds were closed with polyglactin or plain fast absorbing gut suture.
Intraoperative Data Analysis
The minimum and maximum intraoperative injection pressures, recorded in pounds per square inch (psi), for pre-bleb and retinal bleb propagation, the volumes of BSS and vector solution injected; the time (in minutes) required for vector delivery; the number of retinotomy sites created; and any complications were documented in a standardized operative note template used for all patients receiving subretinal gene therapy. The minimum pressure was recorded as the lowest injection pressure required to have propagation of the pre-bleb or bleb when initiating the bleb. The maximum pressure was the highest injection pressure recorded during pre-bleb or bleb propagation. The surgeons (A.K.L and S.T.B.) de-identified and compiled these operative data with patient age for review. To minimize biases and to avoid unmasking active clinical trial data, this study included only de-identified data with masking of patient retinal conditions, which were analyzed as conditions A through H. Operative videos, clinical reports, and ophthalmic images were not included in this study to avoid unmasking of condition.
Simulated Injection Pressure and Rate
Similar to the human surgeries, the Constellation® Vision System was set up for foot pedal-controlled injection using the viscous fluid injection mode. A MicroDose™ Injection Device (MedOne Surgical, Inc., Sarasota, FL) was filled with Balanced salt solution (BSS®; Alcon, Fort Worth, TX) and was attached to a 41-gauge subretinal injection cannula (Dutch Ophthalmic, Inc., Exeter, NH, USA). Using foot-pedal control, constant pressure delivery measured in pounds per square inch was simulated. The drip rate of ejected BSS from the cannula was measured in a 30-second timespan. The volume was also measured during this period. This procedure was performed in triplicate for each psi ranging from 1 to 20.
Statistical analyses
Statistical analyses were conducted using a two sample two-tailed Mann-Whitney U test, or Wilcoxon rank-sum test, where appropriate, to compare two groups. Kruskal–Wallis one-way analysis of variance (ANOVA) test with Dunn’s post-hoc analysis was used for comparison of three or more groups. The relationship between injection pressures and patient age was analyzed using linear regression with the best-fit line provided for all subjects and for individual conditions with >10 subjects. The deviation of the slope was reported as being significantly or not significantly greater than the zero with report of the p value and the ratio of the mean regression sum of squares divided by the mean error sum of squares (i.e., F value); the degrees of freedom and the total number of data points analyzed were included. Multiple linear regression models were performed to determine the relationship between injection pressure, patient age, and condition for aggregate data involving two or more conditions.
Significance was defined as p<0.05 for all analyses. All values of age, pressure, time, rate, and/or volume were reported as medians (upper and lower 95% confidence interval; 95% CI). Violin plots represented 25th to 75th percentiles with vertical bars providing range and horizontal bars representing median values. One-way ANOVA tests, linear regression, and best-fit curves with goodness of fit analyses were performed using GraphPad Prism 4.0b for Macintosh (GraphPad Software, San Diego, CA). Multiple linear regression models were performed using RStudio Version 1.2 (Boston, MA). GraphPad Prism 4.0b for Macintosh was used to generate figures.
Results
Seventy patients with eight different retinal degenerative conditions (A-H) received pneumatic-assisted (i.e., foot pedal controlled) subretinal gene therapy at OHSU-Casey Eye Institute as of June 2020. The subjects’ ages ranged from four to 70 with a median of 35 [33.3, 41.1] (Table 1).
Table 1.
Intraoperative Data for Pneumatic-Assisted Delivery of Subretinal Gene Therapy for Patients with Inherited Retinal Degenerations. Patient age, minimum and maximum injection pressure levels (in pounds per square inch, psi) for pre-bleb and bleb formation, total volumes of injected balanced salt solution (BSS) and vector solution, time for vector delivery, and rate of vector propagation were recorded for each subject.
| No. of Subjects* | Mean | Median | Range | St. Dev. | 95% CI** | |||
|---|---|---|---|---|---|---|---|---|
| Age of Subject(yrs) | Pneumatic-Assisted Subretinal Delivery | 70 | 37.2 | 35 | 4 – 70 | 16.4 | [33.3, 41.1] | |
| Pressure Level(psi) | BSS Pre-Bleb | Minimum | 36 | 5.8 | 5 | 1 – 12 | 2.9 | [4.8, 6.8] |
| Maximum | 63 | 8.8 | 8 | 3 – 20 | 4.0 | [7.8, 9.8] | ||
| Bleb Propagation with Vector | Minimum | 59 | 4.4 | 4 | 2 – 10 | 1.6 | [4.0, 4.8] | |
| Maximum | 68 | 7.6 | 6 | 4 – 16 | 2.6 | [6.9, 8.1] | ||
| Volume Injected (μL) | BSS Pre-Bleb | 60 | 37.0 | 30 | 10 – 100 | 22.8 | [31.1, 42.9] | |
| Bleb Propagation with Vector | 70 | 180.4 | 155 | 20 – 450 | 121.6 | [151.4, 209.4] | ||
| Time (min) | Duration of Subretinal Vector Delivery | 47 | 4.1 | 4 | 0.6 – 10 | 1.9 | [3.5, 4.7] | |
| Rate (μL/min) | Rate of Vector Delivery | 47 | 52.2 | 33 | 3 – 175 | 45.8 | [38.7, 65.6] | |
Number of subjects receiving pneumatic-assisted delivery of subretinal gene therapy who also had a documented entry for the intraoperative pressure level, injected volume, or time of vector delivery.
Lower and upper 95% confidence interval (CI) of the mean
Subretinal bleb formation
Of the 70 patients included in this analysis, 65 patients received a pre-bleb with BSS (Fig. 1). Details of the minimum and maximum intraoperative pressure levels required for pre-bleb formation are found in Table 1. Not all operative notes had complete data; therefore, the number of subjects per documented entry is also provided in Table 1. In most cases, the maximum pressure in the viscous fluid injection mode was set at 16 psi. For two subjects, the pre-bleb maximum pressure was set at and reached 20 psi due to difficulty in bleb formation at lower pressures. The minimum pressures required to form pre-blebs were significantly higher than those used to propagate vector solution (5 v. 4 psi, p=0.01). The maximum pressure levels generated for pre-bleb formation trended higher than for vector propagation (8 v. 6 psi, p=0.09) (Fig. 1). Fourteen patients (22.2%) had elevated injection pressures (i.e., greater that 10 psi) during pre-bleb formation.
Figure 1.
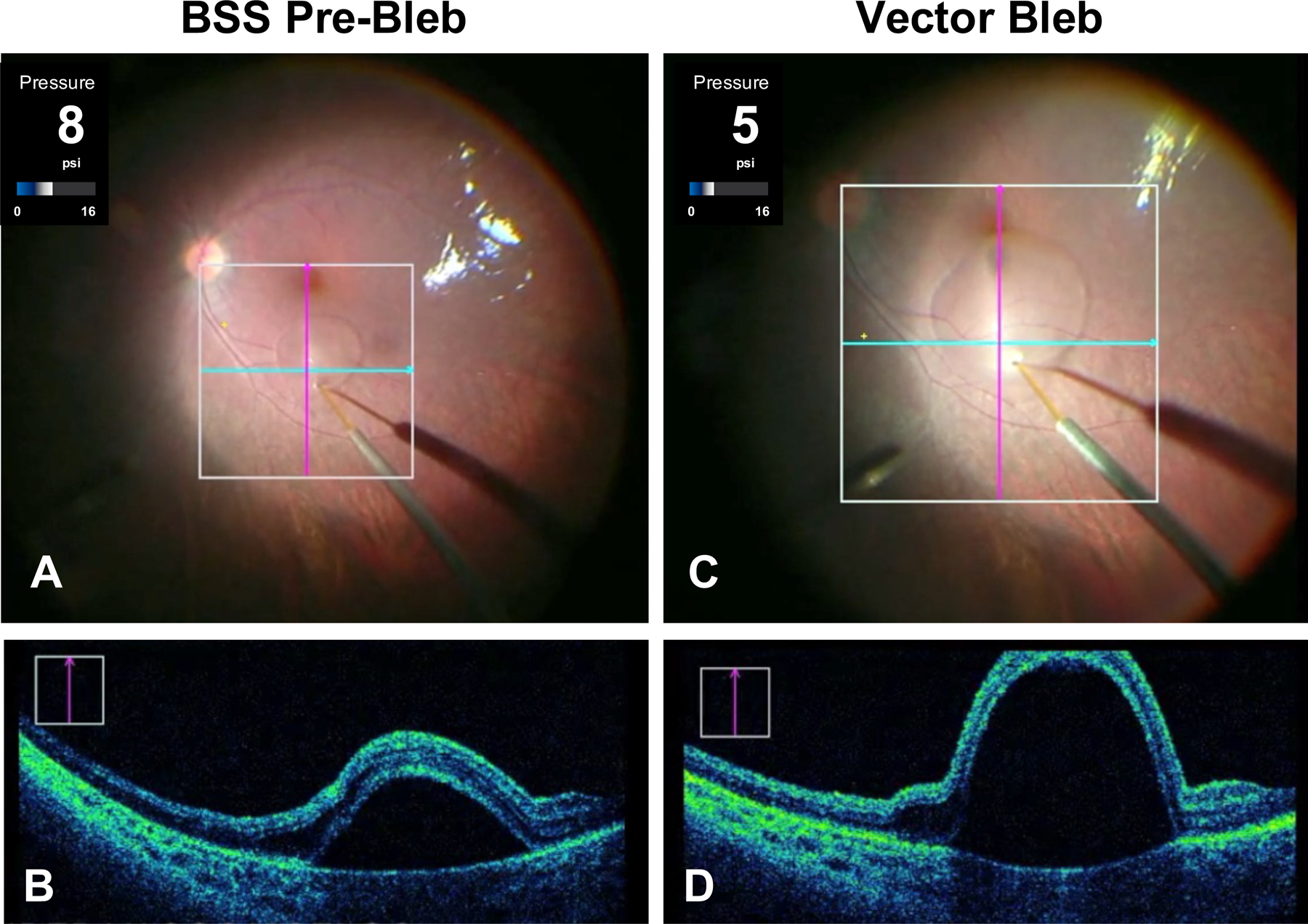
Subretinal bleb formation using intraoperative optical coherence tomography (OCT) and foot-pedal controlled delivery. (A) Intraoperative fundus photograph during initiation of a subretinal pre-bleb using a 41-gauge injection cannula and balanced salt solution (BSS). (B) A vertical scan from live OCT as seen intraoperatively by the surgeon. This cross-section is at the level of the purple vertical line in A. These OCT images demonstrate successful focal retinal detachment using BSS. (C) Intraoperative fundus photograph during vector propagation of a subretinal bleb approaching the fovea. (D) A vertical scan from live OCT is shown at the level of the purple vertical line in C. The intraoperative injection pressures in pounds per square inch (psi) were continuously monitored during bleb formation. Note that the pre-bleb injection pressure (8 psi) was higher than the pressure needed to propagate the vector (5 psi) at the time these images were captured.
The median time required for subretinal vector injection was four minutes with six injections taking six minutes or longer (maximum 10 minutes) to complete the vector injection (Fig. 2A, Table 1). The rate for vector propagation ranged from 3 to 175 μL/min with a median rate of 33 μL/min (Fig. 2B, Table 1). None of those longer injection times were associated with multiple retinotomy sites or complications. Five (7.1%) of the total 70 cases analyzed required multiple retinotomies (2 sites, N=2; 3 sites, N=3); however, none of the cases with multiple retinotomies had documentation of the time spent performing bleb formation. In one of the cases, the development of an intraoperative macular hole necessitated a second bleb in order to treat the target zone.
Figure 2.
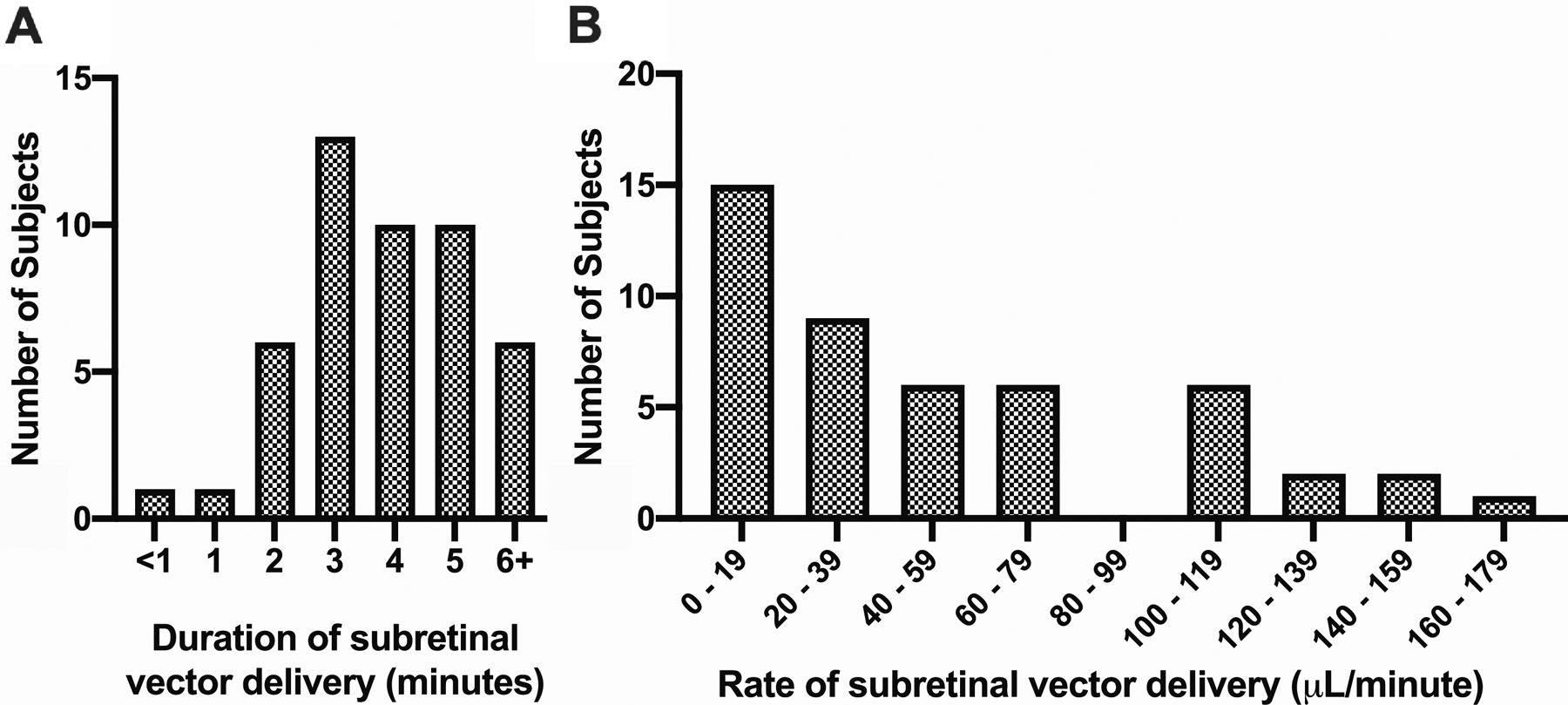
Subretinal Bleb Propagation Duration and Rate. The total duration (A) and rate (B) for subretinal vector injection was recorded for 47 (67.1%) out of 70 subjects; the mean, median, and ranges for the total duration and rate of injections are provided in Table 1. The vector volumes ranged from 20 to 450 μL depending on the clinical trial specifications and the individualized target area. Publication of these vector volumes per condition are not included so as not to unmask the data associated with active clinical trials.
Figure 3 shows the maximum injection pressure levels for bleb propagation. The maximum injection pressures for pre-bleb formation were determined for 63 (90%) out of the 70 patients receiving pneumatic-assisted subretinal gene therapy and compared across retinal degenerative conditions with no differences noted (Fig. 3A). The BSS volumes were determined by the surgeon, and there was no difference in BSS needed for pre-bleb formation across retinal conditions (Fig. 3B). A similar comparison was not performed for the vector volumes as these volumes were dependent on the exact gene therapy agent administered, ranging from 20 μL to 450 μL (Table 1); publication of these vector volumes per condition are not included so as not to unmask the data associated with active clinical trials. The median and mean volumes of total BSS and vector solution injected for pre-bleb and bleb formation, respectively, are documented in Table 1.
Figure 3.
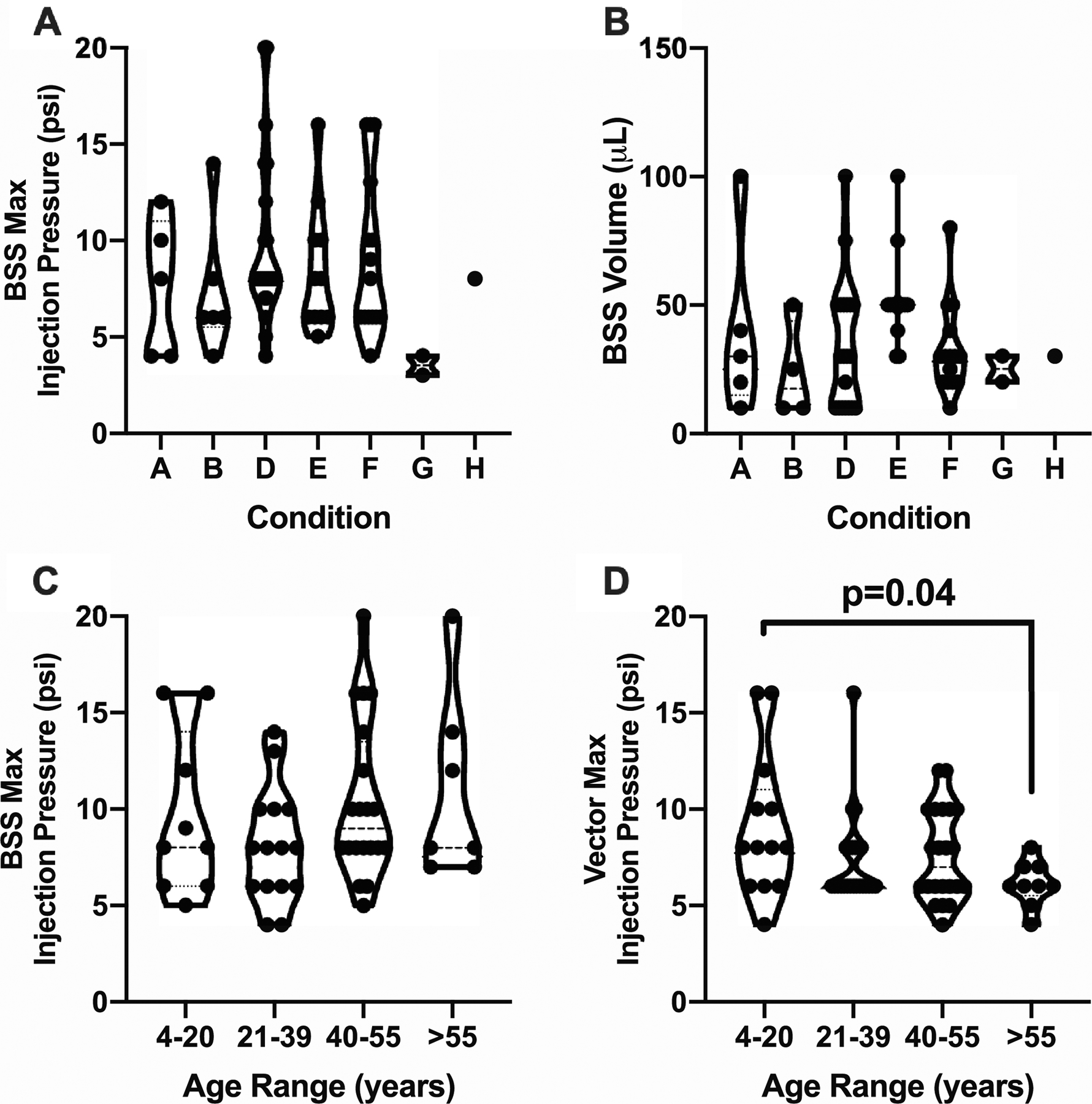
Intraoperative pressure levels for bleb propagation. (A) The maximum injection pressures, recorded in pounds per square inch (psi), for pre-bleb formation were determined for 63 (90%) out of the 70 patients receiving pneumatic-assisted subretinal gene therapy and compared across seven retinal degenerative conditions with no differences noted. (B) The volumes of balanced salt solution (BSS) injected were compared across conditions for 60 (85.7%) out of the 70 patients with no differences noted. The volumes used were determined by the surgeon. Note that no patients with condition C had BSS pre-blebs. (C) The maximum injection pressures for BSS pre-bleb formation (N=63) were then stratified by patient age. There was no difference across the four age groups. (D) Maximum vector propagation injection pressures were recorded for 68 (97.1%) of the 70 patients and stratified by age. The youngest patients (4–20 years) required significantly greater pressures for vector propagation than the oldest group (>55 years). One-way analysis of variance (ANOVA) testing was performed with post-hoc analysis for comparison across conditions (A-B) or age ranges (C-D). Significance was defined as p<0.05.
The maximum intraoperative pressures for BSS pre-bleb formation were stratified by patient age with no difference across the four age groups (Fig. 3C). Maximum vector propagation injection pressures were recorded for 68 (97.1%) of the 70 patients and stratified by age (Fig. 3D). The youngest patients (4–20 years) required significantly greater pressures for vector propagation than the oldest group (>55 years) (Fig. 3D).
Age and condition affect intraoperative pressure levels during bleb propagation
The ages of all subjects were stratified by condition (A-H), and there were significant differences in the ages of the patients with condition D compared to those with condition E (p<0.0001) and condition F (p<0.01) (Fig. 4B). When comparing all conditions, there was no significant difference in maximum pressure levels for vector propagation when comparing any two conditions (Fig. 4A); however, conditions A, B, C, G, and H had few subjects.
Figure 4.
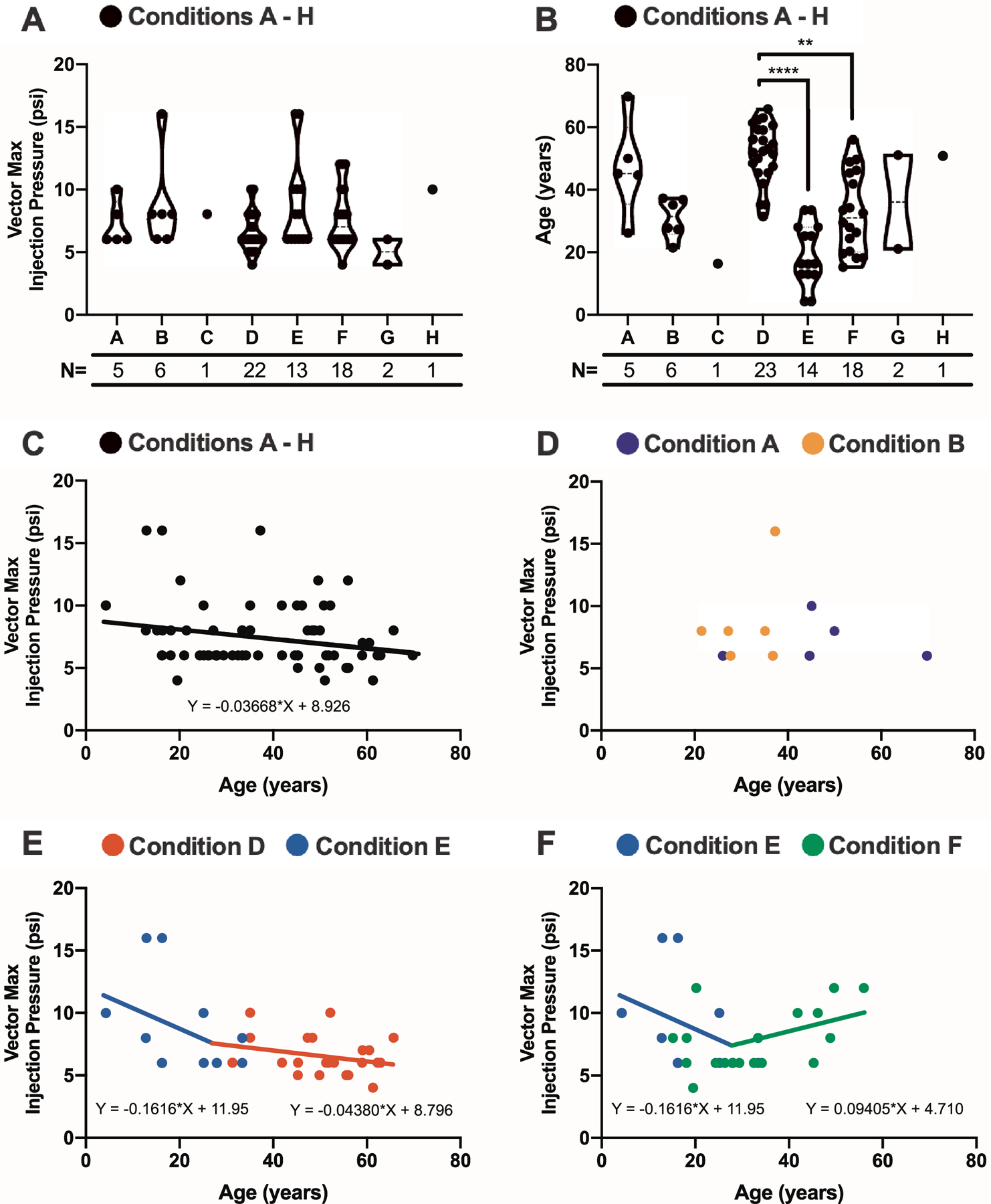
Patient age and retinal condition affect intraoperative pressure levels required for bleb propagation. (A) The maximum intraoperative pressures, recorded in pounds per square inch (psi), for vector propagation were determined for 68 (97.1%) out of the 70 patients receiving pneumatic-assisted subretinal gene therapy and compared across eight retinal degenerative conditions A – H with no differences noted. (B) The ages of 70 patients were compared across retinal conditions with significant differences noted between condition D and conditions E (p<0.0001) and F (p<0.01). The number of subjects analyzed per condition are included for A and B. (N = number of patients in each disease group). (C) Maximum injection pressures for vector propagation (N=68) trended higher with decreased patient age as determined linear regression (p=0.06). (D) Maximum vector propagation injection pressures and patient ages were recorded for condition A (purple circles) and condition B (orange circles) with no trend noted. (E-F) Maximum vector propagation injection pressures and patient ages were plotted for condition D (red circles), condition E (blue circles), and condition F (green circles). Best-fit lines and equations are included. (E) Maximum intraoperative pressures were higher with decreased patient age when collectively evaluating conditions D and E (p=0.02); only trending significance remained (p=0.1–0.2) when comparing these conditions separately. (F) In contrast, there were significantly higher pressures with increased age for condition F (p=0.04). Significance was defined as p<0.05. Note that conditions C, G, and H had inadequate numbers for plotting.
The relationship of maximum injection pressure and patient age was investigated for all subjects (Fig. 4C), and a linear regression model demonstrated a trend that younger patients required higher pressures for bleb propagation (p=0.06; F(1,66)=3.539). This relationship was similarly studied for individual conditions D (N=22), E (N=13), and F (N=18) to control for condition as a confounding variable (Fig. 4, E & F); all other conditions had too few subjects to perform similar analyses. There were trending significance that younger patients required higher pressures for condition D (p=0.2; F(1,20)=1.55) and condition E (0.1, F(1,11)=2.69) (Fig. 4E). When studied collectively, maximum intraoperative pressures were significantly higher for younger patients with conditions D and E (p=0.04, F(2,32)=5.64) (Fig. 4E). In contrast, there were significantly higher pressures required for older patients for condition F (p=0.04, F(1,16)=4.86; Fig. 4F).
Simulated Injection Pressure, Volume, and Drip Rate
Injections were simulated outside of the eye. The volume delivered over time was determined for each injection pressure ranging from psi of 1 to 20; the number of drops per minute were also correlated with injection pressure of the same range. Best-fit curves were best modeled to sigmoidal data, and Supplementary Figure 1 shows these relationships. The flow of BSS from the cannula reached a steady stream at a psi of 21.
Discussion
Pressure readings during BSS pre-bleb formation and vector propagation were evaluated for all patients who had pneumatic-assisted subretinal delivery of gene therapy. Our intraoperative data demonstrated that pre-blebs generally require greater injection pressures compared to the propagation of the retinal bleb. Vitreoretinal surgeons should be aware of this inclination as more than one-fifth of patients in this study had pressure levels greater than 10 psi during the pre-bleb step. Similarly, with vector propagation, the injection pressure should be tightly controlled. Our team achieved relatively low injection pressures for vector propagation likely by prolonging the injection time to allow for gentle dissection of the adherent retina; the median time for bleb propagation was four minutes for a median volume of 155 μL. The median rate was 33 μL/min. We found that multiple blebs were sometimes needed to achieve detachment of the target zone and/or to avoid overstretching the retina where the bleb stopped propagating. Fluid-air exchange may help coalesce multiple blebs to treat the target zone.
Gange, et al. recently reported perifoveal chorioretinal atrophy following subretinal injection of voretigene neparvovec-rzyl in 18 eyes of 10 patients13. Of these patients, the mean age was 11.6 (range 5–20), and the authors are unsure what factors predispose to this complication13. Our data show that younger patients require higher injection pressures, which may lead to development of atrophy within the bleb region. Although the youngest patient cohort in this study (condition E) had minimal age overlap with the oldest patient cohort (condition D), they both showed trends of higher injection pressures needed for younger patients. The relationship was stronger in the younger cohort. Although analysis of conditions with larger sample size found that age may affect maximum pressure for propagation differently across conditions, masking of conditions limits clinical correlation with these findings. Further, subgroup analysis of age based on condition was limited by sample size for some conditions and limited age distribution within conditions.
The overall relationship of injection pressures with age can help determine expected parameters for treatment as we continue to treat younger patients. The finding that condition type may affect this relationship requires more study once these clinical trials are closed. As was noted in condition F, it is conceivable that retinal adherence may have a component that is disease specific. Clustering of injection pressures according to disease type may also be a function of a certain degree of degeneration that is defined by study entry criteria. As gene therapy becomes available for commercial use and younger patients are treated to avoid or limit the effect of deprivational amblyopia, a better understanding of patient age and condition and their relationship to injection pressures will help us treat patients more safely for a treatment that promises to have a lifelong effect.
We encourage vitreoretinal surgeons to perform subretinal injections using a foot-pedal control system to closely monitor the pressure readings throughout gene therapy delivery in an attempt to prevent photoreceptor or RPE shearing, vector solution egress that may stimulate intraocular inflammation, and/or retinal thinning that could lead to secondary macular hole formation. Setting a maximum injection pressure limit at the level that creates a continuous flow is advisable to prevent these complications; this degree of control and prevention is not feasible when performing subretinal injections manually.
Supplementary Material
Acknowledgements
The authors acknowledge the support of NIH P30EY010572, The Heed Foundation, and an unrestricted grant to the Casey Eye Institute Department of Ophthalmology from Research to Prevent Blindness, Inc. New York, NY. The authors would like to thank Sheila Markwardt, MPH for her expertise and assistance in statistical analysis.
Grants and Funding:
The authors acknowledge the support of NIH P30EY010572, NIH K08EY026650 (P.Y.), Foundation Fighting Blindness Career Development Award CD-NMT-0714-0648 (P.Y.), The Heed Foundation (B.A.S.), and an unrestricted grant to the Casey Eye Institute Department of Ophthalmology from Research to Prevent Blindness, Inc. New York, NY.
Author Disclosures:
A.K.L. has received consulting fees or funds in support of clinical research from AGTC, Allergan, Atsena, Biogen, Cambridge Consulting, Genentech, IvericBio, Oxford BioMedic, Regenxbio, TeamedOn. M.E.P. has received consulting fees from Adverum, AGTC, Allergan, Astellas Pharmaceuticals, Biogen, BlueRock, Editas, Iveric Bio, Novartis, Ora, RegenexBio, Roche, Viewpoint Therapeutics. M.E.P. serves on the scientific advisor boards for Atsena, DTx Therapeutics, Endogena, Eyevensys, Horama, Nayan, Nacuity Pharmaceuticals, Ocugen, Sparing Vision, and Vedere. P.Y. has received consulting fees from Adverum, AGTC, Nanoscope Therapeutics. All other authors have no conflicts of interest.
Competing Interests
Dr. Lauer has received consulting fees or funds in support of clinical research from AGTC, Allergan, Atsena, Biogen, Cambridge Consulting, Genentech, IvericBio, Oxford BioMedic, Regenxbio, TeamedOn. Dr. Pennesi has received consulting fees from Adverum, AGTC, Allergan, Astellas Pharmaceuticals, Biogen, BlueRock, Editas, Iveric Bio, Novartis, Ora, RegenexBio, Roche, Viewpoint Therapeutics. Dr. Pennesi serves on the scientific advisor boards for Atsena, DTx Therapeutics, Endogena, Eyevensys, Horama, Nayan, Nacuity Pharmaceuticals, Ocugen, Sparing Vision, and Vedere. Dr. Yang has received consulting fees from Adverum, AGTC, Nanoscope Therapeutics. All other authors declare no potential competing interests.
Footnotes
Meeting Presentation: Virtual presentation at the Retina Society Annual Meeting in September 2020
Ethical Approval
Institutional Review Board approval at OHSU was obtained for review of all operative notes of patients receiving subretinal delivery of gene therapy.
Data Availability
The data generated or analysed during this study can be found within the published article and its supplementary files.
References
- 1.Russell S, Bennett J, Wellman J, Chung D, Yu Z, Tillman A, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390(10097):849–860. doi: 10.1016/S0140-6736(17)31868-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cehajic-Kapetanovic J, Xue K, Martinez-Fernandez de la Camara C, Nanda A, Davies A, Wood L, et al. Initial results from a first-in-human gene therapy trial on X-linked retinitis pigmentosa caused by mutations in RPGR. Nat Med. 2020;26(3):354–359. doi: 10.1038/s41591-020-0763-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fischer M, Ochakovski G, Beier B, Seitz I, Vaheb Y, Kortuem C, et al. Efficacy and Safety of Retinal Gene Therapy Using Adeno-Associated Virus Vector for Patients With Choroideremia: A Randomized Clinical Trial. JAMA Ophthalmol. 2019;137(11):1247–1254. doi: 10.1001/jamaophthalmol.2019.3278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cukras C, Wiley H, Jeffrey B, Sen H, Turriff A, Zeng Y, et al. Retinal AAV8-RS1 Gene Therapy for X-Linked Retinoschisis: Initial Findings from a Phase I/IIa Trial by Intravitreal Delivery. Mol Ther. 2018;26(9):2282–2294. doi: 10.1016/j.ymthe.2018.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacLaren R, Groppe M, Barnard A, Cottriall C, Tolmachova T, Seymour L, et al. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 2014;383(9923):1129–37. doi: 10.1016/S0140-6736(13)62117-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis J, Gregori N, MacLaren R, Lam B. Surgical Technique for Subretinal Gene Therapy in Humans with Inherited Retinal Degeneration. Retina. 2019;39 Suppl 1:S2–S8. doi: 10.1097/IAE.0000000000002609 [DOI] [PubMed] [Google Scholar]
- 7.Scruggs B, Jiao C, Cranston C, Kaalberg E, Wang K, Russell S, et al. Optimizing Donor Cellular Dissociation and Subretinal Injection Parameters for Stem Cell-Based Treatments. Stem Cells Transl Med. 2019;8(8):797–809. doi: 10.1002/sctm.18-0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi K, Morizane Y, Hisatomi T, Tachibana T, Kimura S, Hosokawa M, et al. The influence of subretinal injection pressure on the microstructure of the monkey retina. PLoS One. 2018;13(12):e0209996. doi: 10.1371/journal.pone.0209996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xue K, Groppe M, Salvetti A, MacLaren R. Technique of retinal gene therapy: delivery of viral vector into the subretinal space. Eye (Lond). 2017;31(9):1308–1316. doi: 10.1038/eye.2017.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng Y, Tang L, Zhou Y. Subretinal Injection: A Review on the Novel Route of Therapeutic Delivery for Vitreoretinal Diseases. Ophthalmic Res. 2017;58(4):217–226. doi: 10.1159/000479157 [DOI] [PubMed] [Google Scholar]
- 11.Vasconcelos H, Lujan B, Pennesi M, Yang P, Lauer A. Intraoperative optical coherence tomographic findings in patients undergoing subretinal gene therapy surgery. Int J Retina Vitreous. 2020;6:13. doi: 10.1186/s40942-020-00216-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gregori N, Lam B, Davis J. Intraoperative Use of Microscope-Integrated Optical Coherence Tomography for Subretinal Gene Therapy Delivery. Retina. 2019;39 Suppl 1:S9–S12. doi: 10.1097/IAE.0000000000001646 [DOI] [PubMed] [Google Scholar]
- 13.Gange W, Sisk R, Besirli C, Lee T, Havunjian M, Schwartz H, et al. Perifoveal Chorioretinal Atrophy following Subretinal Voretigene Neparvovec-rzyl for RPE65-mediated Leber Congenital Amaurosis. Ophthalmol Retina. 2021;S2468–6530(21)00106–8. doi: 10.1016/j.oret.2021.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated or analysed during this study can be found within the published article and its supplementary files.


