Abstract
Hydroxyurea has been shown to potentiate the anti-human immunodeficiency virus activities of 2′,3′-dideoxynucleoside analogs such as didanosine. We have now evaluated in vitro the effect of hydroxyurea on the antiherpesvirus activities of several nucleoside analogs (acyclovir [ACV], ganciclovir [GCV], penciclovir [PCV], lobucavir [LBV], (R)-9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine [H2G], and brivudin and nucleoside phosphonate analogs (cidofovir [CDV] and adefovir [ADV]). When evaluated in cytopathic effect (CPE) reduction assays, hydroxyurea by itself had little effect on CPE progression and potentiated in a subsynergistic (herpes simplex virus type 1 [HSV-1]) to synergistic (HSV-2) fashion the antiviral activities of ACV, GCV, PCV, LBV, H2G, ADV, and CDV. Hydroxyurea also caused marked increases in the activities of ACV, GCV, PCV, LBV, and H2G (compounds that depend for their activation on a virus-encoded thymidine kinase [TK]) against TK-deficient (TK−) HSV-1. In fact, in combination with hydroxyurea the 50% effective concentrations of these compounds for inhibition of TK− HSV-1-induced CPE decreased from values of 20 to ≥100 μg/ml (in the absence of hydroxyurea) to values of 1 to 5 μg/ml (in the presence of hydroxyurea at 25 to 100 μg/ml). When evaluated in a single-cycle virus yield reduction assay, hydroxyurea at a concentration of 100 μg/ml inhibited progeny virus production by 60 to 90% but had little effect on virus yield at a concentration of 25 μg/ml. Under these assay conditions hydroxyurea still elicited a marked potentiating effect on the antiherpesvirus activities of GCV and CDV, but this effect was less pronounced than that in the CPE reduction assay. It is conceivable that the potentiating effect of hydroxyurea stems from a depletion of the intracellular deoxynucleoside triphosphate pools, thus favoring the triphosphates of the nucleoside analogues (or the diphosphates of the nucleoside phosphonate analogues) in their competition with the natural nucleotides at the viral DNA polymerase level. The possible clinical implications of these findings are discussed.
Hydroxyurea is a drug that targets the cellular ribonucleotide reductase. This enzyme converts ribonucleotides to deoxyribonucleotides at the nucleoside 5′-diphosphate level, a rate-limiting step in (viral) DNA synthesis. Hydroxyurea has been used for many years for the treatment of a variety of neoplasms (10) and appears, in addition, to offer clinical benefit for the treatment of sickle cell anemia (4). Hydroxyurea has been shown to inhibit the replication of human immunodeficiency virus (HIV) type 1 (HIV-1) (16) and to potentiate the anti-HIV activities of several 2′,3′-dideoxynucleoside analogs, in particular, didanosine (ddI) (12–14, 18, 25), as well as those of the nucleoside phosphonate analogs adefovir [9-(2-phosphonylmethoxyethyl)adenine (PMEA)] and tenofovir [9-(2-phosphonymethoxypropyl)adenine (PMPA)] (26). The precise mechanism responsible for this potentiation has not been elucidated, but it is conceivable that the depletion of the intracellular 2′-deoxynucleoside 5′-triphosphate (dNTP) pools induced by hydroxyurea results in a decreased competition of the triphosphate metabolites of the antivirally active nucleoside analogs with the natural dNTP substrate at the level of the reverse transcriptase of HIV.
Clinical studies have confirmed that hydroxyurea is indeed able to improve the anti-HIV activity of ddI in HIV-infected individuals (3, 19, 30). HIV-infected patients may develop opportunistic herpesvirus infections for which they need treatment with antiherpesvirus agents. In patients who receive antiretroviral therapy regimens containing hydroxyurea, the latter compound may interact with drugs such as acyclovir (ACV), ganciclovir (GCV), penciclovir (PCV), or cidofovir (CDV) that are given concomitantly for an intercurrent herpesvirus infection. Since hydroxyurea results in a reduction of intracellular pools of dNTPs, it may well be plausible that the compound potentiates the antiviral activities of antiherpesvirus drugs.
We have recently demonstrated that the novel immunosuppressive agent mycophenolic acid (MPA) markedly potentiates the antiherpesvirus activities of nucleoside analogs such as ACV, GCV, PCV, lobucavir (LBV), and (R)-9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine (H2G) (21–24). MPA is a potent inhibitor of IMP dehydrogenase, the enzyme that converts IMP (via XMP) into GMP (27). Hence, MPA results in decreased intracellular concentrations of GTP and dGTP and, consequently, a marked increase in the antiherpesvirus activities of ACV, GCV, PCV, LBV, and H2G, molecules that in their triphosphate forms compete with dGTP at the level of the viral DNA polymerase (5).
Here we demonstrate that hydroxyurea also potentiates the antiherpesvirus activities of ACV, GCV, PCV, LBV, H2G, (E)-5-(2-bromovinyl)-2′-deoxyuridine (BVDU), ADV, and (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl) cytosine CDV. This information may be important when treating opportunistic herpesvirus infections with antiherpetic drugs in HIV-infected patients who are concomitantly receiving hydroxyurea as part of a combination therapy schedule.
MATERIALS AND METHODS
Cells and viruses.
The origins of herpes simplex virus (HSV) strains (HSV type 1 [HSV-1] KOS, HSV-2 G, and thymidine kinase [TK]-deficient [TK−] HSV-1 strain B2006) have been described before (6). Vero cells were propagated in minimal essential medium (MEM) supplemented with 10% fetal calf serum (FCS), 5 mM l-glutamine, and bicarbonate.
Compounds.
ACV [9-(2-hydroxyethoxymethyl)guanine] was from Glaxo Wellcome, GCV [9-(2-dihydroxypropoxymethyl)guanine] was from Sarva Syntex, LBV {[1R(1α,2β,3α)]-9-[2,3-bis(hydroxymethyl)cyclobutyl]-guanine} was obtained from Bristol-Myers Squibb (Wallingford, Conn.), PCV [9-4-(hydroxy-3-(hydroxymethyl)but-1-yl)guanine] was from Smith Kline Beecham, H2G was from Abbott Laboratories (Abbott Park, Ill.), CDV (HPMPC) and adefovir (PMEA) were from Gilead Sciences (Foster City, Calif.), and BVDU was from the Rega Institute. Hydroxyurea was purchased from Sigma (St. Louis, Mo.).
Antiviral assays.
Vero cells were grown to confluency in microtiter trays and were inoculated with one of the different HSV strains at 100 times the 50% cell culture infective dose. Following a 2-h incubation period the inoculum was removed and the compounds, either alone or in combination, were added. The virus-induced cytopathic effect (CPE) was recorded microscopically at 2 to 3 days postinfection. Drug combination effects were analyzed by the isobologram method as described previously (1).
Single-cycle virus yield assay.
Confluent cultures of Vero cells grown in 24-well trays were infected with HSV-1, HSV-2, and TK− HSV-1 at multiplicities of infection of 5, 1, and 1, respectively. Following a 1-h incubation period, the inoculum was removed and the compounds, either alone or in combination, were added. Twenty-four hours later, the cultures were frozen (−80°C) and thawed (two cycles), and cell debris was removed by centrifugation. Serial threefold dilutions were inoculated onto confluent Vero cell cultures. Virus titers were determined 2 days later.
Cell growth and cytotoxicity assays.
Inhibition of cell growth was assessed by counting the number of cells in the cell cultures with a Coulter counter. Briefly, Vero cells were seeded in microtiter trays at a density of 4,000 cells/well in MEM containing 20% FCS and were allowed to adhere to the plastic, after which the different drug combinations were added in MEM containing 2% FCS. The cultures were allowed to proliferate for 3 days, at which time they were trypsinized, the cells were counted, and the percent growth inhibition was calculated. The cytotoxic effects of the drug combination were analyzed with 1-day confluent Vero cell cultures by means of the Cell Titer 96 AQueas Non-Radioactive Cell Proliferation Assay (Promega, Leiden, The Netherlands). Briefly, cultures were incubated with the different drug(s) (combinations) for 3 days. Culture medium was removed and was replaced by fresh medium containing the tetrazolium salt 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) together with the coupling reagent phenazine methasulfate. Following the formation of the formazan product, the optical density was measured at 490 nm and the viability was expressed as the percentage of that of the untreated control cultures.
RESULTS
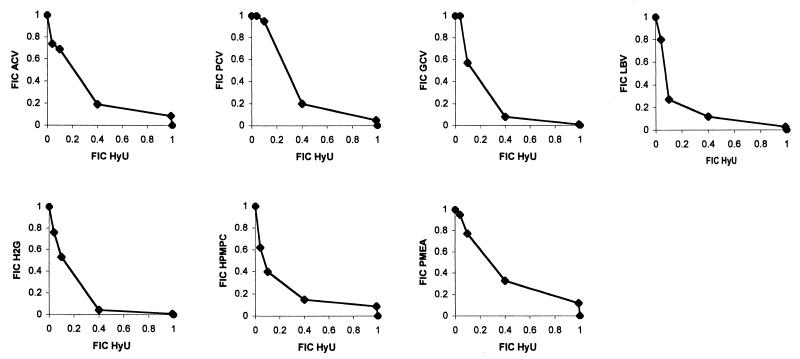
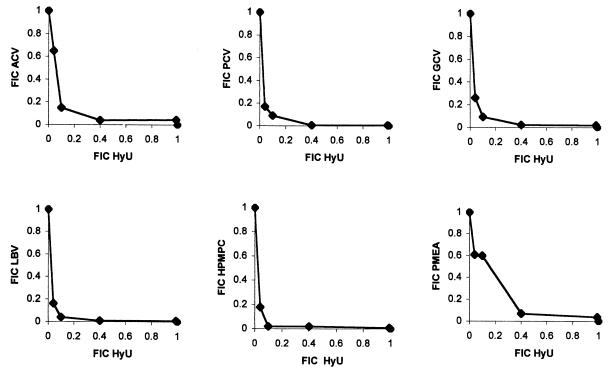
The effect of hydroxyurea on the antiherpesvirus activities of ACV, GCV, PCV, LBV, H2G, brivudin (BVDU), cidofovir (HPMPC), and adefovir (PMEA) was investigated in Vero cells. When the antiviral efficacy of the combination was assessed by means of a CPE reduction assay, the combined antiviral effect (on HSV-1 and HSV-2 replication) was analyzed by means of the isobologram method (Fig. 1 and 2). Hydroxyurea elicited a moderate synergistic effect on the anti-HSV-1 activities of the different nucleoside analogs. Minimum fractional inhibitory concentrations (FICmins) were 0.59, 0.6, 0.48, 0.37, 0.44, 0.5, and 0.73 for the combination of hydroxyurea with ACV, PCV, GCV, LBV, H2G, HPMPC, and PMEA, respectively (Fig. 1). The potentiating effect of hydroxyurea on the anti-HSV-2 activities of the different nucleoside analogs was more pronounced. FICmins were 0.25, 0.19, 0.19, 0.14, 0.12, 0.47 for the combination of hydroxyurea with ACV, PCV, GCV, LBV, HPMPC, and PMEA, respectively (Fig. 2). The fact that lower FICs were obtained with HSV-2 than with HSV-1 can partially be explained by the fact that HSV-2-induced CPE progression was more sensitive to hydroxyurea. Indeed, at a concentration of 100 μg/ml (the 50% effective concentration [EC50] for inhibition of HSV-2 CPE formation is even as high as 250 μg/ml), hydroxyurea still inhibited HSV-2-induced CPE formation by 30 to 40%, whereas at this concentration the compound had virtually no effect on HSV-1-induced CPE formation.
FIG. 1.
Anti-HSV-1 activities of the combination of hydroxyurea (HyU) with different nucleoside analogs.
FIG. 2.
Anti-HSV-2 activities of the combination of hydroxyurea with different nucleoside analogs.
Since GCV, ACV, PCV, LBV, H2G, and BVDU are all molecules that depend for their activation on the herpesvirus-encoded TK, they elicit little activity against TK− strains of HSV-1. It was of interest to study whether hydroxyurea is also able to potentiate the antiviral activities of different drugs on TK− HSV-1 replication. Because the EC50 for inhibition of viral replication by some of these compounds exceeded the highest concentration tested, it was not possible to calculate FICs and thus to depict these data in isobologram format. When combined with hydroxyurea, a marked increase in the antiviral activities of the different drugs was noted (Table 1). For example, for GCV and PCV, EC50s for inhibition of the replication of TK− HSV-1 decreased from concentrations that are not attainable in human plasma (40 to 100 μg/ml) to concentrations that can readily be reached in human plasma (1 to 3 μg/ml). However, no potentiating effect of hydroxyurea on the activity of BVDU against TK− HSV-1 was observed.
TABLE 1.
Effect of hydroxyurea on the anti-TK− HSV-1 activities of GCV, ACV, PCV, LBV, H2G, and BVDU in Vero cells
| Antiviral agent | Mean ± SD EC50 (μg/ml) of agent with hydroxyurea present ata:
|
||||
|---|---|---|---|---|---|
| 0 μg/ml | 250 μg/ml | 100 μg/ml | 25 μg/ml | 10 μg/ml | |
| GCV | 41 ± 12 | 0.19 ± 0.13 | 1.3 ± 0.8 | 5.4 ± 2.2 | 13 ± 12 |
| ACV | 46 ± 26 | 2.5 ± 0.49 | 5.1 ± 1.9 | 17 ± 11 | 44 ± 42 |
| PCV | >100 | 0.7 ± 0.06 | 3.6 ± 0.0 | 40 ± 16 | 75 ± 25 |
| LBV | 18 ± 4 | 0.15 ± 0.07 | 2.8 ± 2.2 | 4.9 ± 2.1 | 8.8 ± 4.9 |
| H2G | >10 | 0.67 ± 0.04 | 4.5 ± 1.4 | >10 | >10 |
| BVDU | >100 | >100 | >100 | >100 | >100 |
Data are mean values for at least three independent experiments.
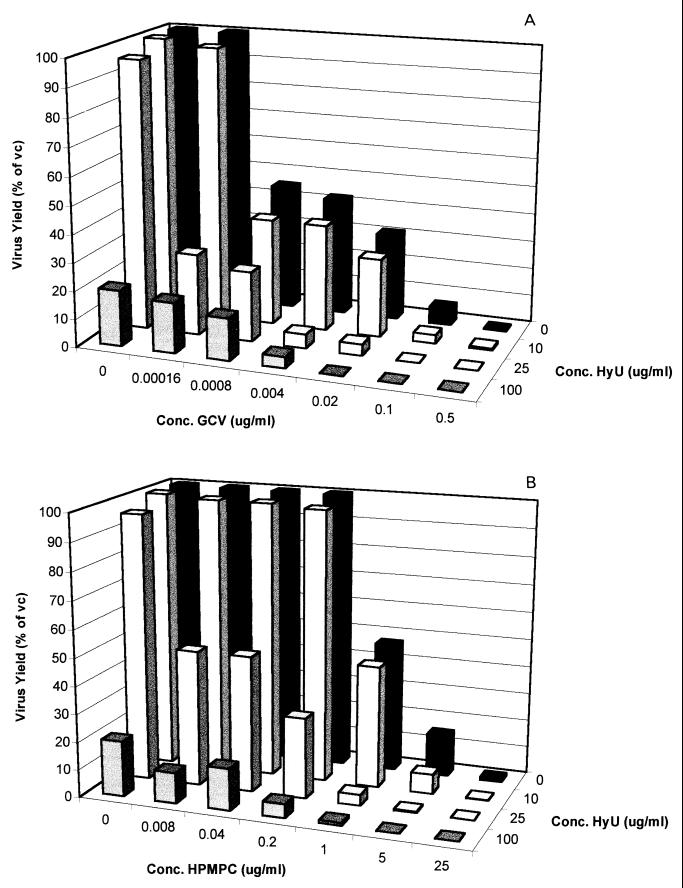
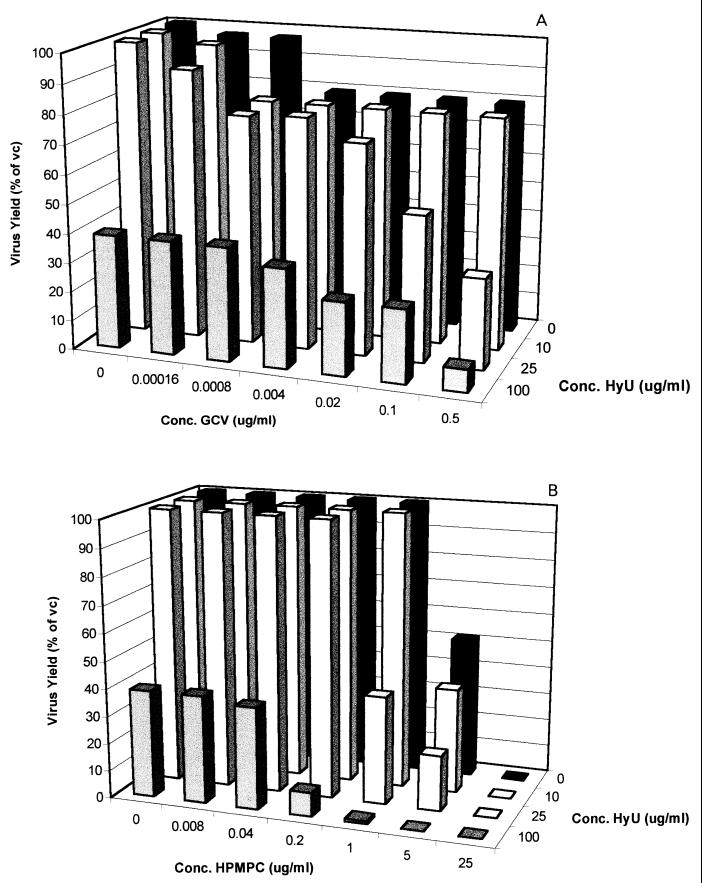
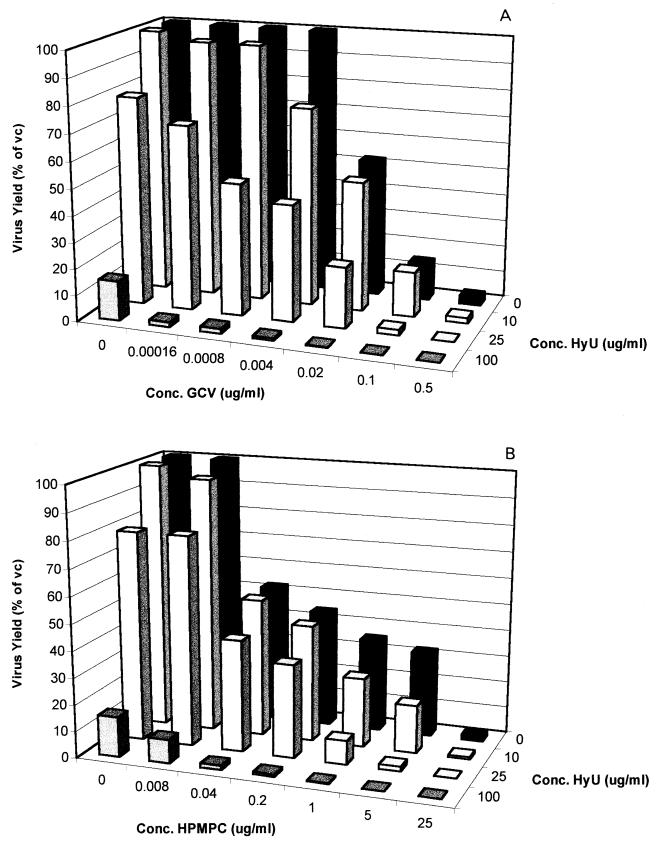
Although hydroxyurea had a limited effect on virus-induced CPE progression, at concentrations of ≥100 μg/ml the compound markedly reduced progeny virus production, as evaluated in a single-cycle virus yield reduction assay. Therefore, we also studied the effect of hydroxyurea on the anti-HSV-1, anti-HSV-2, and anti-TK− HSV-1 activities of a representative nucleoside analog (GCV) and a nucleoside phosphonate analog (CDV) in a single-cycle virus yield reduction assay. Although the potentiating effect of hydroxyurea on the antiherpesvirus activities of GCV and CDV was less pronounced when it was assessed by virus yield reduction than when it was assessed by CPE reduction assays, a marked potentiation was still observed (Fig. 3 to 5). This was most pronounced at a hydroxyurea concentration of 25 μg/ml (which, as such, has little effect on HSV-2 yield and virtually no effect on HSV-1 and TK− HSV-1 yields). Thus, although the observed potentiating effect of hydroxyurea appears to be less pronounced when it is assessed by means of a virus yield reduction assay than when it is assessed by a CPE reduction assay, subsynergistic to synergistic antiviral activities were observed in both assay systems.
FIG. 3.
Effect of the combination of hydroxyurea (HyU) with either GCV (A) or CDV (B) on HSV-1 progeny formation in Vero cells in a single-cycle virus yield reduction assay.
FIG. 5.
Effect of the combination of hydroxyurea (HyU) with either GCV (A) or CDV (B) on TK− HSV-1 progeny formation in Vero cells in a single-cycle virus yield reduction assay.
Next we studied the effect of hydroxyurea on the cytotoxic (Table 2) and cytostatic (Table 3) effects of the different nucleoside analogs. At a concentration of 250 μg/ml, hydroxyurea reduced the viability of 1-day confluent Vero cells by 35%; cell viability was reduced by 12% at a hydroxyurea concentration of 25 μg/ml. None of the nucleoside analogs had, at the highest concentration tested (100 μg/ml), a marked effect on cell viability. Consequently, when combined with hydroxyurea, the reduction in cell viability was solely attributable to hydroxyurea. Indeed, when Vero cell cultures were treated with the nucleoside analogs at a concentration of 100 or 20 μg/ml together with the different concentrations of hydroxyurea, the viabilities of the cultures did not deviate much (in either a negative or a positive sense) from the viabilities of the cultures that had been treated only with hydroxyurea. In a second experiment we determined the cytostatic action (in growing Vero cell cultures) of hydroxyurea when it was combined with the different nucleoside analogs. Hydroxyurea had, by itself, a marked effect on cell growth (50% cell culture inhibitory concentration, 6.3 μg/ml). The 50% cell culture inhibitory concentrations for inhibition of Vero cell growth were 43 μg/ml for GCV, 92 μg/ml for ACV, 10 μg/ml for PCV, 3.2 μg/ml for LBV, 68 μg/ml for H2G, 34 μg/ml for BVDU, 14 μg/ml for HPMPC, and 67 μg/ml for PMEA. As can be derived from Table 3, the cytostatic effects of the drug combinations mainly appeared to be of an additive nature.
TABLE 2.
Combined cytotoxicities of hydroxyurea and various nucleoside (or nucleotide) analogs in Vero cells
| Hydroxyurea concn (μg/ml) | % Viabilitya
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 μg/ml
|
20 μg/ml
|
|||||||||||||||
| GCV | ACV | PCV | LBV | H2G | BVDU | HPMPC | PMEA | GCV | ACV | PCV | LBV | H2G | BVDU | HPMPC | PMEA | |
| 250 | 67 | 74 | 62 | 60 | 58 | 65 | 50 | 67 | 75 | 78 | 63 | 61 | 59 | 63 | 55 | 63 |
| 100 | 72 | 76 | 65 | 61 | 67 | 66 | 58 | 68 | 76 | 81 | 63 | 66 | 68 | 67 | 64 | 70 |
| 25 | 103 | 93 | 75 | 78 | 92 | 83 | 86 | 87 | 99 | 103 | 83 | 84 | 90 | 83 | 95 | 83 |
| 10 | 98 | 99 | 79 | 78 | 93 | 92 | 103 | 96 | 99 | 107 | 85 | 87 | 95 | 87 | 97 | 89 |
| 0 | 104 | 110 | 90 | 86 | 91 | 93 | 103 | 106 | 104 | 113 | 90 | 91 | 89 | 92 | 107 | 98 |
| % Viabilitya
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 μg/ml
|
0.8 μg/ml
|
0 μg/ml | ||||||||||||||
| GCV | ACV | PCV | LBV | H2G | BVDU | HPMPC | PMEA | GCV | ACV | PCV | LBV | H2G | BVDU | HPMPC | PMEA | |
| 77 | 80 | 67 | 66 | 59 | 60 | 55 | 65 | 70 | 82 | 65 | 65 | 59 | 60 | 57 | 65 | 65 |
| 78 | 80 | 65 | 68 | 70 | 60 | 68 | 65 | 72 | 77 | 69 | 70 | 71 | 63 | 72 | 68 | 70 |
| 101 | 100 | 80 | 100 | 90 | 83 | 97 | 91 | 99 | 97 | 77 | 100 | 98 | 85 | 96 | 87 | 88 |
| 101 | 102 | 82 | 88 | 97 | 87 | 94 | 90 | 100 | 100 | 80 | 91 | 95 | 90 | 107 | 97 | 90 |
| 99 | 108 | 92 | 90 | 90 | 98 | 110 | 100 | 102 | 106 | 95 | 97 | 92 | 98 | 112 | 97 | 99 |
Values are expressed as percent viability compared to that for the untreated controls and are mean values for two determinations (standard deviation, ≤10%). One-day confluent Vero cells were incubated for 3 days in the presence of the drug(s) (combinations). Cell viability was determined by means of the MTS method.
TABLE 3.
Combined cytostatic effect of hydroxyurea and various nucleoside analogs in Vero cells
| Hydroxyurea concn (μg/ml) | % Cell growtha
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 μg/ml
|
20 μg/ml
|
|||||||||||||||
| GCV | ACV | PCV | LBV | H2G | BVDU | HPMPC | PMEA | GCV | ACV | PCV | LBV | H2G | BVDU | HPMPC | PMEA | |
| 50 | 3 | 6 | 3 | 2 | 9 | 3 | 4 | 6 | 6 | 5 | 2 | 4 | 8 | 5 | 4 | |
| 25 | 10 | 17 | 9 | 10 | 12 | 6 | 4 | 16 | 17 | 13 | 5 | 8 | 17 | 8 | 5 | |
| 10 | 17 | 41 | 9 | 34 | 13 | 11 | 8 | 35 | 40 | 41 | 5 | 34 | 26 | 14 | 15 | |
| 2.5 | 30 | 49 | 15 | 44 | 13 | 10 | 11 | 66 | 78 | 40 | 7 | 38 | 71 | 16 | 36 | |
| 0 | 31 | 48 | 34 | 42 | 19 | 10 | 29 | 67 | 92 | 38 | 13 | 76 | 66 | 35 | 67 | |
| % Cell growtha
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 μg/ml
|
0.8 μg/ml
|
0 μg/ml | ||||||||||||||
| GCV | ACV | PCV | LBV | H2G | BVDU | HPMPC | PMEA | GCV | ACV | PCV | LBV | H2G | BVDU | HPMPC | PMEA | |
| 6 | 6 | 4 | 5 | 4 | 9 | 6 | 4 | 5 | 8 | 5 | 5 | 3 | 14 | 7 | 4 | 7 |
| 15 | 15 | 12 | 5 | 14 | 20 | 15 | 7 | 14 | 21 | 16 | 8 | 9 | 28 | 22 | 14 | 16 |
| 39 | 30 | 40 | 8 | 35 | 29 | 36 | 35 | 40 | 34 | 38 | 21 | 36 | 52 | 52 | 53 | 36 |
| 71 | 65 | 95 | 16 | 44 | 75 | 56 | 54 | 83 | 78 | 100 | 35 | 63 | 77 | 102 | 89 | 80 |
| 78 | 90 | 68 | 43 | 56 | 78 | 99 | 68 | 83 | 88 | 80 | 93 | 83 | 100 | 102 | 94 | 100 |
Values are expressed as percent cell growth compared to that for the untreated controls and are mean values for two determinations (standard deviation, ≤10%). Cells were allowed to proliferate for 3 days in the presence of the drug(s) (combinations), after which the number of cells was determined with a Coulter counter.
DISCUSSION
ACV, GCV, PCV, LBV, and H2G are guanosine nucleoside analogs with selective activity against herpesviruses (and hepadnaviruses, i.e., hepatitis B virus [HBV] [PCV, LBV]). ACV and PCV are being used for the treatment of infections caused by HSV-1, HSV-2, and varicella-zoster virus (VZV), and GCV is being used for the treatment of life- and sight-threatening infections with cytomegalovirus (CMV) (5). Lobucavir is not only an inhibitor of the replication of herpesviruses but is also effective against HBV and HIV. Clinical trials with this compound for the treatment of HBV and CMV infections have, however, recently been suspended (11, 29). H2G has particularly good activity against VZV (17) and has entered clinical trials for the treatment of VZV infections. The mode of the antiherpesvirus activity of each of these molecules is based on a selective phosphorylation by the virus (HSV-1, HSV-2, or VZV)-encoded TK or by the CMV UL-97-encoded protein phosphokinase to the 5′-monophosphate form. After this step cellular kinases further convert this metabolite to the corresponding triphosphate derivative. The latter serves as a selective inhibitor of the viral DNA polymerase and does so in competition with the natural substrate dGTP (5). Brivudin (BVDU) is a potent inhibitor of the replication of several herpesviruses (including HSV-1, VZV, and Epstein-Barr virus). Brivudin is selectively phosphorylated to its 5′-mono- and diphosphate derivatives by the viral TK and then further on by cellular kinases to BVDU triphosphate. The latter is a selective competitive inhibitor of herpetic DNA polymerases with respect to dTTP (5).
Cidofovir and adefovir are nucleoside phosphonate analogs. These compounds are able to bypass the first phosphorylation step of nucleoside analogs by the viral TKs (7, 8). CDV is a potent and broad-spectrum inhibitor of the replication of various DNA viruses. The compound is effective in the treatment of a variety of viral infections (for a review, see reference 20) and has been approved for the treatment of CMV retinitis. Cidofovir is phosphorylated intracellularly to its diphosphorylated metabolite (HPMPCpp), which is a selective inhibitor of the DNA polymerase activities of DNA viruses and which inhibits the viral DNA polymerase in competition with dCTP (31, 32). Adefovir is a potent inhibitor of the replication of HIV-1, HIV-2, and HBV and is also effective against herpesviruses. The compound (in its oral prodrug form, adefovir dipivoxil) has proved to be effective clinically against HIV infections (9). Adefovir (PMEA) is phosphorylated to its diphosphate metabolite PMEApp, either directly by phosphoribosylpyrophosphate synthetase or in two subsequent steps by AMP kinase and nucleoside diphosphate kinase (2). PMEApp selectively inhibits the reverse transcriptase (RT) of HIV, the RT/DNA polymerase of HBV, and the DNA polymerase of herpesviruses and does so in competition with dATP (for a review, see reference 20).
All the nucleoside analogs discussed here compete in their triphosphate form (in the case of ACV, PCV, GCV, LBV, H2G, and BVDU) or their diphosphate form (in the case of HPMPC and PMEA) with the natural dNTPs (either dGTP [ACV, GCV, PCV, LBV, H2G], dCTP [HPMPC], or dATP [PMEA]). As an inhibitor of ribonucleotide reductase, hydroxyurea brings about a decrease in the levels of the intracellular pools of the different dNTPs. It is conceivable that a decrease in the levels of the intracellular dNTP pools may lead to a decreased competition with the antivirally active metabolites at the level of the DNA polymerase and, thus, enhanced inhibition of viral replication. We have recently made similar observations for the combination of MPA with ACV, PCV, GCV, LBV, and H2G (21–24).
Of all dNTP pools, dATP levels are most efficiently depleted by hydroxyurea (15). Adefovir (PMEA) is the only molecule in the series studied that competes (as its metabolite PMEApp) with dATP. However, the potentiating effect of hydroxyurea on the antiherpesvirus activity of PMEA was not more pronounced than it was for most of the other compounds. In fact, for HSV-1, the potentiating effect of hydroxyurea on PMEA was relatively weak. In a recent study, Palmer and colleagues (26) demonstrated that PMEA and hydroxyurea synergistically inhibit the replication of wild-type and drug-resistant HIV. We do not yet have an explanation of why the antiherpesvirus activity of PMEA is less potentiated by hydroxyurea than those of other nucleoside analogs, the active metabolites of which do not, unlike PMEA, compete with dATP in the (viral) DNA polymerization process.
Hydroxyurea has been shown to increase the intracellular TK and deoxycytidine kinase activities (14), but it is unlikely that this mechanism contributes to the potentiating effect of hydroxyurea on the activities of the antiherpesvirus agents studied here. Indeed, none of the molecules is a (good) substrate for either one of these two enzymes. For zidovudine and dideoxycytidine the improvements in their anti-HIV activities when they are combined with hydroxyurea have mainly been ascribed to the increased activities of these salvage enzymes (14).
Although there are only a limited number of published clinical data on the use of hydroxyurea in combination with ddI (and stavudine), the combination of these dideoxynucleosides with hydroxyurea has become one of the most frequently prescribed antiretroviral drug combinations (28). The observations presented here may therefore be of clinical relevance. Indeed, HIV-infected individuals may develop opportunistic herpesvirus infections for which treatment with antiherpetic agents is needed. If these individuals receive an antiretroviral therapy regimen that includes hydroxyurea, the latter may be administered concomitantly, albeit inadvertently, with drugs such as ACV, GCV, PCV, and CDV, thus resulting in increased antiviral efficacy.
FIG. 4.
Effect of the combination of hydroxyurea (HyU) with either GCV (A) or CDV (B) on HSV-2 progeny formation in Vero cells in a single-cycle virus yield reduction assay.
ACKNOWLEDGMENTS
We thank Miette Stuyck for excellent technical assistance and Christiane Callebaut, Dominique Brabants, and Inge Aerts for fine editorial help.
This work was supported by grants from the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen and the Geconcerteerde Onderzoeksacties-Vlaamse Gemeenschap. J. Neyts is a postdoctoral research assistant from the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen.
REFERENCES
- 1.Baba M, Pauwels R, Balzarini J, Herdewijn P, De Clercq E, Desmyter J. Ribavirin antagonizes inhibitory effects of pyrimidine 2′,3′-dideoxynucleosides but enhances inhibitory effects of purine 2′,3′-dideoxynucleosides on replication of human immunodeficiency virus in vitro. Antimicrob Agents Chemother. 1987;31:1613–1617. doi: 10.1128/aac.31.10.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balzarini J, De Clercq E. 5-Phosphoribosyl 1-pyrophosphate synthetase converts the acyclic nucleoside phosphonates 9-(3-hydroxy-2-phosphonylmethoxypropyl)adenine and 9-(2-phosphonylmethoxyethyl)adenine directly to their antivirally active diphosphate derivatives. J Biol Chem. 1991;266:8686–8689. [PubMed] [Google Scholar]
- 3.Biron F, Lucht F, Peyramond D, Fresard A, Vallet T, Nugier F, Grange J, Malley S, Hamedi-Sangsari F, Vila J. Anti-HIV activity of the combination of didanosine and hydroxyurea in HIV-1-infected individuals. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:36–40. [PubMed] [Google Scholar]
- 4.Charache S. Mechanism of action of hydroxyurea in the management of sickle cell anemia in adults. Semin Hematol. 1997;34:15–21. [PubMed] [Google Scholar]
- 5.De Clercq E. Trends in the development of new antiviral agents for the chemotherapy of infections caused by herpesviruses and retroviruses. Rev Med Virol. 1995;5:149–164. [Google Scholar]
- 6.De Clercq E, Descamps J, Verhelst G, Walker R T, Jones A S, Torrence P F, Shugar D. Comparative efficacy of different antiherpes drugs against different strains of herpes simplex virus. J Infect Dis. 1980;141:563–574. doi: 10.1093/infdis/141.5.563. [DOI] [PubMed] [Google Scholar]
- 7.De Clercq E, Holý A, Rosenberg I, Sakuma T, Balzarini J, Maudgal P C. A novel selective broad-spectrum anti-DNA virus agent. Nature. 1986;323:464–467. doi: 10.1038/323464a0. [DOI] [PubMed] [Google Scholar]
- 8.De Clercq E, Sakuma T, Baba M, Pauwels R, Balzarini J, Rosenberg I, Holý A. Antiviral activity of phosphonylmethoxyalkyl derivatives of purine and pyrimidines. Antivir Res. 1987;8:261–272. doi: 10.1016/s0166-3542(87)80004-9. [DOI] [PubMed] [Google Scholar]
- 9.Deeks S G, Collier A, Lalezari J, Pavia A, Rodrigue D, Drew W L, Toole J, Jaffe H S, Mulato A S, Lamy P D, Li W, Cherrington J M, Hellmann N, Kahn J. The safety and efficacy of adefovir dipivoxil, a novel anti-human immunodeficiency virus (HIV) therapy, in HIV-infected adults: a randomized, double-blind, placebo-controlled trial. J Infect Dis. 1997;176:1517–1523. doi: 10.1086/514150. [DOI] [PubMed] [Google Scholar]
- 10.Donehowever R C. An overview of the clinical experience with hydroxyurea. Semin Oncol. 1992;19(Suppl. 9):11–19. [PubMed] [Google Scholar]
- 11.Dunkle L M. Lobucavir: new broad-spectrum antiviral agent. In: Sapienza D M, editor. Antivirals, latest preclinical and clinical developments for infectious diseases. Southborough, Mass: International Business Communications, Inc.; 1998. pp. 1–5. [Google Scholar]
- 12.Gao W-Y, Johns D G, Mitsuya H. Anti-human immunodeficiency virus type 1 activity of hydroxyurea in combination with 2′,3′-dideoxynucleosides. Mol Pharmacol. 1994;46:767–772. [PubMed] [Google Scholar]
- 13.Gao W-Y, Johns D G, Chokekijchai S, Mitsuya H. Disparate actions of hydroxyurea in potentiation of purine and pyrimidine 2′,3′-dideoxynucleoside activities against replication of human immunodeficiency virus. Proc Natl Acad Sci USA. 1995;92:8333–8337. doi: 10.1073/pnas.92.18.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao W-Y, Mitsuya H, Driscoll J S, Johns D G. Enhancement by hydroxyurea of the anti-human immunodeficiency virus type 1 potency of 2′-β-fluoro-2′,3′-dideoxyadenosine in peripheral blood mononuclear cells. Biochem Pharmacol. 1995;50:274–276. doi: 10.1016/0006-2952(95)00106-a. [DOI] [PubMed] [Google Scholar]
- 15.Johns D G, Gao W-Y. Selective depletion of DNA precursors. An evolving strategy for potentiation of dideoxynucleoside activity against human immunodeficiency virus. Biochem Pharmacol. 1998;55:1551–1556. doi: 10.1016/s0006-2952(97)00664-3. [DOI] [PubMed] [Google Scholar]
- 16.Lori F, Malykh A, Caran A, Sun D, Weinstein J N, Lisziewicz J, Gallo R C. Hydroxyurea as an inhibitor of human immunodeficiency virus-type 1 replication. Science. 1994;266:801–805. doi: 10.1126/science.7973634. [DOI] [PubMed] [Google Scholar]
- 17.Lowe D M, Alderton W K, Ellis M R, Parmar V, Miller W H, Roberts G B, Fyfe J A, Gaillard R, Ertl P, Snowden W, Littler E. Mode of action of (R)-9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine against herpesviruses. Antimicrob Agents Chemother. 1995;39:1802–1808. doi: 10.1128/aac.39.8.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malley S D, Grange J M, Hamedi Sangsari F, Vila J R. Synergistic anti-human immunodeficiency virus type 1 effect of hydroxamate compounds with 2′,3′-dideoxyinosine in infected resting human lymphocytes. Proc Natl Acad Sci USA. 1994;91:11017–11021. doi: 10.1073/pnas.91.23.11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montaner J S G, Zala C, Conway B, Raboud J, Patenaude P, Rae S, O’Shaughnessy M V, Schechter M T. A pilot study of hydroxyurea among patients with advanced human immunodeficiency virus (HIV) disease receiving chronic didanosine therapy: Canadian HIV trials network protocol 080. J Infect Dis. 1997;175:801–806. doi: 10.1086/513974. [DOI] [PubMed] [Google Scholar]
- 20.Naesens L, Snoeck R, Andrei G, Balzarini J, Neyts J, De Clercq E. HPMPC (cidofovir), PMEA (adefovir) and related acyclic nucleoside phosphonate analogues: a review of their pharmacology and clinical potential in the treatment of viral infections. Antivir Chem Chemother. 1997;8:1–23. [Google Scholar]
- 21.Neyts J, De Clercq E. Mycophenolate mofetil strongly potentiates the antiherpesvirus activity of acyclovir. Antivir Res. 1998;40:53–56. doi: 10.1016/s0166-3542(98)00047-3. [DOI] [PubMed] [Google Scholar]
- 22.Neyts J, De Clercq E. The immunosuppressive agent mycophenolate mofetil markedly potentiates the activity of Lobucavir [1R(1α,2β,3α)]-9-[2,3-bis(hydroxymethyl)-cyclobutyl]guanine against different herpesviruses. Transplantation. 1999;67:760–764. doi: 10.1097/00007890-199903150-00022. [DOI] [PubMed] [Google Scholar]
- 23.Neyts J, Andrei G, De Clercq E. The novel immunosuppressive agent mycophenolate mofetil markedly potentiates the antiherpesvirus activities of acyclovir, ganciclovir, and penciclovir in vitro and in vivo. Antimicrob Agents Chemother. 1998;42:216–222. doi: 10.1128/aac.42.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neyts J, Andrei G, De Clercq E. The antiherpesvirus activity of H2G [(R)-9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine] is markedly enhanced by the novel immunosuppressive agent mycophenolate mofetil. Antimicrob Agents Chemother. 1998;42:3285–3289. doi: 10.1128/aac.42.12.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmer S, Cox S. Increased activation of the combination of 3′-azido-3′-deoxythymidine and 2′-deoxy-3′-thiacytidine in the presence of hydroxyurea. Antimicrob Agents Chemother. 1997;41:460–464. doi: 10.1128/aac.41.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmer S, Shafter R W, Merigan T C. Hydroxyurea enhances the activities of didanosine, 9-[2-(phosphonylmethoxy)ethyl]adenine, and 9-[2-(phosphonylmethoxy)propyl]adenine against drug-susceptible and drug-resistant human immunodeficiency virus isolates. Antimicrob Agents Chemother. 1999;43:2046–2050. doi: 10.1128/aac.43.8.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ransom J T. Mechanism of action of mycophenolate mofetil. Ther Drug Monit. 1995;17:681–684. doi: 10.1097/00007691-199512000-00023. [DOI] [PubMed] [Google Scholar]
- 28.Rutschmann O T, Opravil M, Iten A, Malinverni R, Vernazza P L, Bucher H C, Bernasconi E, Sudre P, Leduc D, Yerly S, Perrin L H, Hirschel B the Swiss HIV Cohort Study. A placebo-controlled trial of didanosine plus stavudine, with and without hydroxyurea, for HIV infection. AIDS. 1998;12:F71–F77. doi: 10.1097/00002030-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Tenney D J, Yamanaka G, Voss S M, Cianci C W, Tuomari A V, Sheaffer A K, Alam M, Colonno R J. Lobucavir is phosphorylated in human cytomegalovirus-infected and -uninfected cells and inhibits the viral DNA polymerase. Antimicrob Agents Chemother. 1997;41:2680–2685. doi: 10.1128/aac.41.12.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vila J, Nugier F, Barguès G, et al. Absence of viral rebound after treatment of HIV-infected patients with didanosine and hydroxycarbamide. Lancet. 1997;348:203–204. doi: 10.1016/S0140-6736(97)24035-3. [DOI] [PubMed] [Google Scholar]
- 31.Xiong X, Smith J L, Kim C, Huang E S, Chen M S. Kinetic analysis of the interaction of cidofovir diphosphate with human cytomegalovirus DNA polymerase. Biochem Pharmacol. 1996;51:1563–1567. doi: 10.1016/0006-2952(96)00100-1. [DOI] [PubMed] [Google Scholar]
- 32.Xiong X, Smith J L, Chen M S. Effect of incorporation of cidofovir into DNA by human cytomegalovirus DNA polymerase on DNA elongation. Antimicrob Agents Chemother. 1997;41:594–599. doi: 10.1128/aac.41.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]